Abstract
Background
Aortic valve disease is a common condition easily treatable with cardiac surgery. This is conventionally performed by opening the sternum ('median sternotomy') and replacing the valve under cardiopulmonary bypass. Median sternotomy is well tolerated, but as less invasive options become available, the efficacy of limited incisions has been called into question. In particular, the effects of reducing the visibility and surgical access have raised safety concerns with regard to the placement of cannulae, venting of the heart, epicardial wire placement, and de‐airing of the heart at the end of the procedure. These difficulties may increase operating times, affecting outcome. The benefits of smaller incisions are thought to include decreased pain; improved respiratory mechanics; reductions in wound infections, bleeding, and need for transfusion; shorter intensive care stay; better cosmesis; and a quicker return to normal activity. This is an update of a Cochrane review first published in 2017, with seven new studies.
Objectives
To assess the effects of minimally invasive aortic valve replacement via a limited sternotomy versus conventional aortic valve replacement via median sternotomy in people with aortic valve disease requiring surgical replacement.
Search methods
We performed searches of CENTRAL, MEDLINE and Embase from inception to August 2021, with no language limitations. We also searched two clinical trials registries and manufacturers' websites. We reviewed references of primary studies to identify any further studies of relevance.
Selection criteria
We included randomised controlled trials comparing aortic valve replacement via a median sternotomy versus aortic valve replacement via a limited sternotomy. We excluded trials that performed other minimally invasive incisions such as mini‐thoracotomies, port access, transapical, transfemoral or robotic procedures. Although some well‐conducted prospective and retrospective case‐control and cohort studies exist, these were not included in this review.
Data collection and analysis
Two review authors independently assessed trial papers to extract data, assess quality, and identify risk of bias. A third review author provided arbitration where required. We determined the certainty of evidence using the GRADE methodology and summarised results of patient‐relevant outcomes in a summary of findings table.
Main results
The review included 14 trials with 1395 participants. Most studies had at least two domains at high risk of bias. We analysed 14 outcomes investigating the effects of minimally invasive limited upper hemi‐sternotomy on aortic valve replacement as compared to surgery performed via full median sternotomy.
Upper hemi‐sternotomy may have little to no effect on mortality versus full median sternotomy (risk ratio (RR) 0.93, 95% confidence interval (CI) 0.45 to 1.94; 10 studies, 985 participants; low‐certainty evidence). Upper hemi‐sternotomy for aortic valve replacement may increase cardiopulmonary bypass time slightly, although the evidence is very uncertain (mean difference (MD) 10.63 minutes, 95% CI 3.39 to 17.88; 10 studies, 1043 participants; very low‐certainty evidence) and may increase aortic cross‐clamp time slightly (MD 6.07 minutes, 95% CI 0.79 to 11.35; 12 studies, 1235 participants; very low‐certainty evidence), although the evidence is very uncertain. Most studies had at least two domains at high risk of bias.
Postoperative blood loss was probably lower in the upper hemi‐sternotomy group (MD ‐153 mL, 95% CI ‐246 to ‐60; 8 studies, 767 participants; moderate‐certainty evidence). Low‐certainty evidence suggested that there may be no change in pain scores by upper hemi‐sternotomy (standardised mean difference (SMD) ‐0.19, 95% CI ‐0.43 to 0.04; 5 studies, 649 participants). Upper hemi‐sternotomy may result in little to no difference in quality of life (MD 0.03 higher, 95% CI 0 to 0.06 higher; 4 studies, 624 participants; low‐certainty evidence). Two studies reporting index admission costs concluded that limited sternotomy may be more costly at index admission in the UK National Health Service (MD 1190 GBP more, 95% CI 420 GBP to 1970 GBP, 2 studies, 492 participants; low‐certainty evidence).
Authors' conclusions
The evidence was of very low to moderate certainty. Sample sizes were small and underpowered to demonstrate differences in some outcomes. Clinical heterogeneity was also noted.
Considering these limitations, there may be little to no effect on mortality. Differences in extracorporeal support times are uncertain, comparing upper hemi‐sternotomy to full sternotomy for aortic valve replacement.
Before widespread adoption of the minimally invasive approach can be recommended, there is a need for a well‐designed and adequately powered prospective randomised controlled trial. Such a study would benefit from also performing a robust cost analysis. Growing patient preference for minimally invasive techniques merits thorough quality of life analyses to be included as end points, as well as quantitative measures of physiological reserve.
Keywords: Humans, Aortic Valve, Aortic Valve/surgery, Aortic Valve Disease, Pain, Prospective Studies, Quality of Life, Randomized Controlled Trials as Topic, Retrospective Studies, State Medicine, Sternotomy, Sternotomy/adverse effects, Surgical Wound
Plain language summary
Heart surgery for aortic valve replacement through a small incision versus the standard full incision at the front of the chest
Key messages
‐ We did not find enough high‐certainty evidence to answer whether the best way to undertake aortic valve replacement was through the conventional full‐size incision in the breastbone or a smaller incision at the top of the breastbone.
‐ None of the important problems that occur after heart surgery were more common in either group.
What is aortic valve replacement?
Aortic valve replacement is a common operation performed to replace one of the valves of the heart. The reasons for needing this include valves that do not open properly or do not close properly, which can happen with ageing. People with aortic valve disease can experience chest pain, breathlessness, collapse or sudden death.
How can aortic valve replacement be performed?
The most common way of performing the operation is by opening the whole length of the breastbone. Another method involves a smaller 'keyhole'‐type cut that only divides a small part of the breastbone. Doing it this way makes the scar smaller, but can also make the operation more challenging because it is more difficult to see and reach the heart. This might make the operation longer and less safe, even though it looks smaller from the outside.
What did we want to find out?
We wanted to find out if the smaller 'keyhole'‐type cut (limited sternotomy) was better than the usual full cut down the breastbone (full sternotomy) when performing aortic valve replacement surgery in adults. We wanted to see if both were as safe and effective as each other.
What did we do?
We updated a review that we had previously written on the topic. We searched for studies that compared limited sternotomy with full sternotomy in adults undergoing aortic valve replacement. We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as the study methods and sizes.
What did we find?
We found 14 studies with 1395 participants from Europe, Russia, and North Africa. There was a mixture of different conditions needing aortic valve replacement. Most of these people were 60 to 70 years old and approximately half were male. The participants in each group were similar.
There may be no difference between the groups in the number of people who died as a result of having surgery. If 25 out of every 1000 people who had the full‐size cut in their breastbone died after the operation, around 23 (somewhere between 11 and 48) in every 1000 would die using the 'keyhole' operation. Because that range goes from two times less to two times more, it is difficult to say whether the operation is definitely better or worse.
The amount of time that surgeons needed to use a heart‐lung machine to support the heart while doing the 'keyhole' operation may have been on average around 11 minutes longer – not a large amount. The amount of time that the heart was completely stopped to do the 'keyhole' operation may be six minutes longer on average, though we were not confident in the evidence.
None of the important problems that occur after heart surgery were more common in either group (infections around the heart, irregular heart rhythms or the need for an urgent reoperation because of bleeding), although again it was uncertain if the evidence was robust enough. Participants probably bled slightly less after having minimally invasive surgery. In the operation with the smaller cut, the average blood loss was 153 mL less. There may be no change in pain and quality of life may not have been any different between the two groups.
Limited sternotomy possibly costs more per operation to perform, by about 1190 pounds sterling.
What are the limitations of the evidence?
We were not very confident in the evidence. One of the main problems with the studies was that they were small and may not have picked up subtle differences between the groups. Because problems after heart surgery are rare, we need to assess lots of people having operations in order to spot small changes. Another problem is that surgeons tend to have lots of slightly different ways in which they do operations. There were also differences in practice, meaning that measurements might not have been taken at the same time, in the same way. We need to be careful about making conclusions about which differences in the groups in this review were due to the smaller incision and which were due to these other factors.
How up to date is this evidence?
This review updates our previous review. The evidence is up to date to August 2021.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Limited sternotomy compared to full sternotomy for aortic valve replacement.
| Limited sternotomy compared to full sternotomy for aortic valve replacement | ||||||
| Patient or population: adults undergoing aortic valve replacement Setting: hospital in‐patients Intervention: limited sternotomy Comparison: full sternotomy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with full sternotomy | Risk with limited sternotomy | |||||
| Mortality assessed with: in‐hospital mortality | 25 per 1000 | 23 per 1000 (11 to 48) | RR 0.93 (0.45 to 1.94) | 985 (10 RCTs) | ⊕⊕⊝⊝ Lowa,b | Limited sternotomy may result in little to no difference in mortality. |
| Cardiopulmonary bypass time (minutes) | The mean cardiopulmonary bypass time (minutes) was 80 minutes | MD 10.63 minutes higher (3.39 higher to 17.88 higher) | ‐ | 1043 (10 RCTs) | ⊕⊝⊝⊝ Very lowa,c,d | The evidence is very uncertain about the effect of limited sternotomy on cardiopulmonary bypass time. |
| Aortic cross‐clamp time (minutes) | The mean aortic cross‐clamp time (minutes) was ~50 minutes | MD 6.07 minutes higher (0.79 higher to 11.35 higher) | ‐ | 1235 (12 RCTs) | ⊕⊝⊝⊝ Very lowa,c,d | The evidence is very uncertain about the effect of limited sternotomy on aortic cross‐clamp time. |
| Postoperative blood loss (mL) | The mean postoperative blood loss (mL) was ~400 mL | MD 153.04 mL lower (245.96 lower to 60.12 lower) | ‐ | 767 (8 RCTs) | ⊕⊕⊕⊝ Moderatee | Limited sternotomy likely reduces postoperative blood loss slightly. |
| Pain scores, measured in various ways at a median of 2 days' follow‐up (range: 12 hours to 3 months) | ‐ | SMD0.19 lower (0.43 lower to 0.04 higher) | ‐ | 649 (5 RCTs) | ⊕⊕⊝⊝ Lowe,f,g | Limited sternotomy may result in little to no difference in pain scores. |
| Quality of life, measured with EQ‐5D at 1 to 3 months (higher scores are better) | The mean quality of life was 0.75 points | MD 0.03 higher (0 to 0.06 higher) | ‐ | 624 (4 RCTs) | ⊕⊕⊝⊝ Lowb,f | Limited sternotomy may result in little to no difference in quality of life. |
| Index admission costs | The mean index admission cost was 8000 GBP | MD 1190 GBP higher (420 higher to 1970 higher) | ‐ | 492 (2 RCTs) | ⊕⊕⊝⊝ Lowh,i,j | Limited sternotomy may increase Index Admission Costs slightly. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EQ‐5D: EuroQoL 5D; GBP: pounds sterling; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded for high risk of bias: 50% (or more) of included studies had two or more domains considered high risk of bias. bDowngraded for imprecision: sample sizes did not meet optimal information size criteria and 95% confidence intervals overlapped no effect. Optimal information size estimated at 4600 (to determine 1% difference using α 0.05, β 0.80). The majority of studies had fewer than 100 participants. cDowngraded twice for inconsistency: differences in surgical technique between studies and, in one study, use of rapid deployment valves in one arm only, created significant heterogeneity. dDowngraded for risk of publication bias: indicated by funnel plot asymmetry. eDowngraded for inconsistency: variations in surgical or anaesthetic management might affect outcome. fDowngraded for high risk of bias: outcome measure sensitive to lack of blinding in study. gNot downgraded but note that the different measures of pain across studies required standardised mean differences to synthesise. Cohen's effect size therefore used to interpret effect (i.e. 0.2 is low, 0.5 moderate and 0.8 a large effect). hNot downgraded as Hancock 2019 was at low risk of bias in all domains and contributed 85.9% of weight. iDowngraded for indirectness: UK admission costs only. jDowngraded for imprecision: sample size did not meet optimal information size criteria.
Background
Aortic valve disease affects approximately 1% of the adult population in the USA and comprises a range of pathologies, including senile degeneration and functional regurgitation (Nkomo 2006). Of the 20 million people worldwide estimated to have rheumatic heart disease (Kumar 2013), aortic valve involvement accounts for nearly one‐third of cases (Manjunath 2014). These conditions, spanning stenosis or incompetence of the aortic valve, tend to be progressive, causing angina, breathlessness, and eventually precipitating heart failure and death. Attempts at medical management of the conditions underlying aortic valve disease have not proved fruitful (Coffey 2014; Freeman 2005; Kumar 2013; Scheuble 2005); surgical intervention remains, therefore, the gold standard in treating the condition. Aortic valve surgery has evolved significantly since its inception, such that it can be performed with relatively low mortality; attention is now directed at reducing morbidity.
Description of the condition
Since the mid‐1980s, rheumatic fever, the leading cause of valvular heart disease, has been on the decline in high‐income countries (Rose 1986). In the rest of the world, rheumatic heart disease continues to have a high burden of mortality and morbidity (Carapetis 2005). While it is relatively uncommon in North America (Dare 1993), rheumatic heart disease still represents 22% of valvular heart disease in Europe (Iung 2014). In industrialised nations, senile or degenerative aortic disease typified by aortic stenosis predominates, the incidence of which is increasing in an ageing population. The prevalence of aortic stenosis rises exponentially from the age of 50 years, affecting more than 1 in 50 adults over the age of 75 years (Thaden 2014). Aortic valve disease represents over half of the valvular heart disease in Europe (Iung 2003).
Severe aortic valve disease necessitates surgical intervention for symptomatic relief or prognostic benefit, or both. Previously it was believed that people with severe aortic stenosis maintained a long asymptomatic period with low risk of death (Ross 1968). However, even where symptoms are absent, the outlook is poor for people with severe stenosis; the majority will develop symptoms within five years (Pellikka 2005), and event‐free survival is as low as 21% at two years (Otto 1997). In the SEAS (Simvastatin and Ezetimibe in Aortic Stenosis) study from 2001 to 2004, even people with mild or moderate aortic stenosis, 10% and 38%, respectively, progressed to surgically significant disease within five years (Gohlke‐Bärwolf 2013). It is thought that the burden of valvular heart disease will continue to increase and that indications for surgery will become broader; at present half of diagnoses of aortic stenosis are made postmortem (d'Arcy 2011).
Description of the intervention
The first total aortic valve replacement was performed in 1958 in a person in whom an attempt at repair caused disintegration of the cusps (Lillehei 1962). In the intervening half‐century, aortic valve repair has grown less common, with replacement with tissue or mechanical prosthetic valves now representing 99% of surgical management of aortic valve disease in the Euro Heart Survey (Iung 2003). It is the second‐most common cardiac surgical procedure in North America (Lee 2011). The prognostic benefit of this operation has been known for many years (Schwarz 1982), and since the early 1980s, the mortality from isolated, uncomplicated aortic valve replacement has dropped more than five‐fold to less than 1% (Carabello 2013). The long‐term freedom from serious complications is similar, even with mechanical valves requiring warfarinisation (Braunwald 2000).
Worldwide, aortic valve replacement is most commonly performed via median sternotomy, an incision that extends from the sternal notch to the xiphisternum and divides the entire sternum longitudinally.
Rao and Kumar were the first to describe an aortic valve replacement through a right anterior thoracotomy (Rao 1993). The group used central cannulation and an oblique aortotomy. Subsequently, Cosgrove and Sabik used the term "minimally invasive" to describe an aortic valve procedure via a 10‐cm right anterior thoracotomy, excising the second and third costal cartilages, and employing femoral cannulation to establish cardiopulmonary bypass (CPB) (Cosgrove 1996). Various modifications have since been described, including limited upper hemi‐sternotomy in a J‐shape (Liu 1999a), inverted T‐ or Y‐shape (Cohn 1997), or lazy S (Autschbach 1998). These techniques variably allow access to cannulate the ascending aortic and right atrium – as in open surgery – to establish CPB. Due to the limited access, CPB and aortic cross clamp times may be longer, with theoretical effects on neurological and renal morbidity. Other modifications to the open technique may also be necessary, warranting investment in additional equipment and training (Malaisrie 2014; Walther 2006).
How the intervention might work
Median sternotomy is generally well tolerated due to fixation of the sternum on closure (Lee 2004), but the disruption can nonetheless cause pain, affect respiratory dynamics, reduce mobility, and is thought to necessitate restriction of upper body weight‐bearing (Walther 1999a). Minimally invasive surgery, by virtue of preserving the integrity of the thoracic cage, aims to improve pain, mobility and return to normal activities following discharge (Cohn 1997). These benefits are thought to offset any increase in operative time as a result of reduced surgical access, and therefore potentially reduce cost by up to 20% in all but the people at the highest risk (Cohn 1998). However, these benefits are not guaranteed, as disruption of the intercostal nerves with some approaches might paradoxically cause more pain than that associated with sternotomy (Walther 1999a), and additional port sites or groin cannulation may offset the cosmetic advantage, quality of life or satisfaction (Detter 2002a). Where people have any doubt about the efficacy of a minimally invasive approach, many choose full sternotomy (Ehrlich 2000).
Why it is important to do this review
Aortic valve replacement via full sternotomy is well tolerated and demonstrates excellent long‐term event‐free survival and quality of life. At present, few cardiac surgeons offer minimally invasive aortic valve replacement via limited sternotomy as there is uncertainty whether it offers advantages over conventional aortic valve replacement and there is clinical equipoise. If equivalence in key measures of mortality and morbidity, along with evidence of reduced pain, immobility, length of stay, and overall cost could be demonstrated, there would be momentum to make minimally invasive aortic valve replacement the gold standard. This review sought to evaluate the effect of aortic valve replacement through limited upper hemi‐sternotomy on 30‐day mortality, morbidity, health‐related quality of life, and cost compared with conventional aortic valve replacement through a full median sternotomy in people undergoing aortic valve replacement.
At present, there are no guidelines to either recommend or discourage surgeons from using minimally invasive approaches to aortic valve surgery. Neither the American guidelines (Nishimura 2017) nor the European guidelines (Baumgartner 2017) on valvular heart disease make any reference to its use. The Society of Thoracic Surgeons Aortic Valve Guidelines for Management and Quality Measures refers to potential and future benefits of minimally invasive surgery, but makes no specific recommendations (Svensson 2013). The International Society for Minimally Invasive Cardiothoracic Surgery has no consensus guidelines on the subject of minimally invasive aortic valve replacement. As these approaches have been used for nearly two decades, however, it is likely that a dearth of strong evidence influences the decision not to offer recommendations.
Previous meta‐analyses have addressed this subject (Brown 2009; Khoshbin 2011; Murtuza 2008; Phan 2014), and this is an update of our previous Cochrane review (Kirmani 2017), but the results of two recent well‐known randomised controlled trials (Hancock 2019; Nair 2019) prompted a contemporary review, including an updated literature search. In total, seven new studies with 887 additional participants were identified in this version of the Cochrane review.
Objectives
To assess the effects of minimally invasive aortic valve replacement via a limited sternotomy versus conventional aortic valve replacement via median sternotomy in people with aortic valve disease requiring surgical replacement.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials and excluded cluster‐randomised trials. We included studies reported as full text, those published as abstract only, and unpublished data.
Types of participants
We included adults (aged 18 years or greater) with a diagnosis of isolated aortic valve disease requiring aortic valve replacement with no aortovascular intervention (e.g. root replacement or ascending aortic replacement). Where trials included a subset of eligible participants, we aimed to obtain the trial data for the subset of interest from the trialist. If subset data were not available, we included trials if the number of ineligible participants did not exceed 10% of the trial population, with a plan to explore the impact of these with a sensitivity analysis. No characteristics or comorbidities were excluded.
Types of interventions
We included trials comparing minimally invasive aortic valve surgery through any form of partial‐sternotomy with conventional, isolated aortic valve surgery via median sternotomy. We did not consider transapical or transfemoral aortic valve replacement, or any minimally invasive procedures performed through thoracotomies, video‐assisted thoracoscopic surgery, or other access not through a partial sternotomy. We considered any modifications to the surgical technique to facilitate this form of access, including femoral cannulation, transvenous pacing, and rapid deployment/sutureless valves.
Types of outcome measures
The following were the outcome measures of interest for this study. We did not exclude studies that did not report any of the outcomes of interest, but we did comment on them, in narrative form, in the Discussion section where the trial authors were unable to provide unreported data (or the data were in an unusable format).
Primary outcomes
Mortality (i.e. all‐cause mortality) at 30 days (or in‐hospital if not reported as 30 days)
-
Extracorporeal support times (intraoperative)
cardiopulmonary bypass (CPB) (minutes)
aortic cross‐clamp (minutes)
Secondary outcomes
Organ failure requiring support (including respiratory, renal, gastrointestinal, or multi‐organ failure) in hospital.
Length of hospital stay (days)
Postoperative blood loss at 12 hours (mL).
Deep sternal wound infection in hospital.
Pain scores (as measured by visual analogue scale) in hospital.
Quality of life (as measured by EuroQoL 5D (EQ‐5D)) or any other validated scale) at circa 12 weeks
Index admission costs
Intensive care unit stay (days)
Postoperative pulmonary function tests in hospital
Re‐exploration in hospital
Postoperative atrial fibrillation in hospital
Postoperative ventilation times
Search methods for identification of studies
Electronic searches
We identified trials through systematic searches of the following bibliographic databases on 8 August 2021:
Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 7 of 12, 2021);
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily, and MEDLINE (Ovid, 1946 to 8 August 2021);
Embase (Ovid, 1980 to week 27, 2021).
We adapted the preliminary search strategy for MEDLINE (Ovid) for use in the other databases (Appendix 1). We applied the Cochrane sensitivity‐maximising randomised controlled trial filter to MEDLINE (Ovid) and for Embase we applied terms as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011).
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (apps.who.int/trialsearch/), on 15 September 2021.
We searched all databases from their inception and imposed no restrictions on the language of publication.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for trial information (performed in July 2015 and updated in January 2021):
St Jude Medical (now Abbott: www.cardiovascular.abbott/us/en/hcp.html);
Edwards Lifesciences (www.edwards.com/healthcare-professionals/products-services/surgical-heart);
Medtronic (www.medtronic.com/for-healthcare-professionals/products-therapies/cardiovascular/index.htm);
On‐X (www.onxlti.com/);
Sorin/LivaNova (www.livanova.com/en-us).
Where the information from initial screening of papers identified studies with uncertain value for this review, we contacted authors to gain access to missing data.
Data collection and analysis
Selection of studies
Two review authors (BHK, SGJ) independently screened titles and abstracts for inclusion of all the potential studies we identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. In case of disagreement (or conflict of interest as BHK was a co‐author on one screened study), we asked a third review author (SCM or ADM) to arbitrate. We retrieved the full‐text study reports/publication, and two review authors (BHK, SGJ and/or ADM where BHK was a co‐author on a study paper) independently screened the full text and identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion or, if required, by consulting a third review author (SCM, ADM or EFA). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table.
Data extraction and management
We used a data collection form for study characteristics and outcome data that had been piloted on at least one study in the review. Two review authors (BHK, SGJ and/or ADM) extracted study characteristics from included studies. We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: n, mean age, age range, gender, pathophysiology of aortic disease (stenotic or regurgitant), severity of condition, EuroSCORE or Society of Thoracic Surgeons score, left ventricular ejection fraction, prevalence of diabetes, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention including mode of access and modifications to cannulation strategy, comparison group, CPB time, and aortic cross‐clamp time.
Outcomes: primary and secondary outcomes as specified and collected, and time points reported.
Notes: funding for trial and notable conflicts of interest of trial authors.
Two review authors (BHK, SGJ and/or ADM) independently extracted outcome data from included studies. We resolved any disagreements by consensus or by involving a third review author (DC, RJNNW, SCM, ADM or EFA). One review author (BHK) transferred data into Review Manager 5 in the original review (RevMan 2014), and RevMan Web for the review update (RevMan Web 2022). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (SGJ) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (BHK, SGJ and/or ADM) independently assessed risk of bias for each study using the Cochrane RoB 1 tool and the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). Where one study included co‐authors who were also involved in this Cochrane review (Hancock 2019, BHK and EFA), these review authors did not participate in the data extraction or risk of bias assessments and these were undertaken by another author (ADM). We resolved any disagreements by discussion or by involving another review author (SCM). We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias (e.g. small‐study bias).
We graded each potential source of bias as high, low, or unclear risk of bias and provided a quote from the study report together with a justification for our judgement in the risk of bias table. We summarised the risk of bias judgements across different studies for each of the domains listed. Where there were different risks of bias from blinding, depending on outcome, within a study (e.g. pain scores are subjective and considered at risk from non‐blinding, whereas estimated blood loss is objective and unlikely to be affected by non‐blinding), we differentiated between risks of bias from blinding of outcome assessment for objective and subjective outcomes in the risk of bias tables. Mortality, postoperative blood loss, deep sternal wound infection, re‐exploration, and postoperative atrial fibrillation rates were considered objective and independent of blinding as these were less easily influenced by participant or assessor. Cardiopulmonary bypass times, aortic cross clamp times, length of hospital stay, pain scores, quality of life, intensive care unit stays, postoperative lung function tests, postoperative ventilation time and costs were considered prone to bias if the participant or assessor knew the treatment arm. For example, a surgeon might perceive a limited sternotomy as technically challenging and spend longer in the procedure for this reason. Similarly, assessments for extubation, discharge from the intensive care unit or hospital discharge might be influenced by participant or staff perceptions about the speed of recovery with minimally invasive methods. Pain, quality of life and respiratory effort, affecting lung function tests, might also be skewed by participant perceptions of the size of their incision. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the risk of bias table. Industry funding was considered a risk of bias in the original Cochrane review (Kirmani 2017) but, following further guidance from Cochrane, this was removed for this update.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Measures of treatment effect
We analysed dichotomous data as risk ratios (RR) with 95% confidence intervals (CI). The reason we chose RRs in preference to odds ratios was because they are considered easier to interpret (Higgins 2020), and uniformly presenting data with a consistent presentation would allow simpler comparison of effects on complications or risks of surgery. We analysed continuous data as mean difference (MD) (or standardised mean difference (SMD) if different scales were used for measurement of the same outcome measure) with 95% CIs. We considered, in particular, the challenges in interpretation of SMD and considered the Cochrane Handbook for Systematic Reviews of Interventions guidance (Section 15.5; Higgins 2020) on alternatives. In the absence of familiar measures, options for dichotomisation, large effects, or strong evidence for minimal important differences, we presented SMD. To interpret the estimate of effect, we used Cohen's Effect Size for SMD, where 0.2 was a small effect size, 0.5 a moderate effect and 0.8 a large effect (Cochrane Handbook for Systematic Reviews of Interventions Section 15.5.3.1; Higgins 2020). We entered data presented as a scale with a consistent direction of effect. Where the standardised mean difference was used as a measure of an effect, the trial population and standard deviation of each different scale of measure were assessed for clinical correlation and variations reported.
We narratively described skewed data reported as medians and interquartile ranges (IQR).
Unit of analysis issues
Outcome reporting at multiple time points was dealt with by considering data reported at the time point most frequently reported. Where required, we chose time points that were comparable between studies and then made an assessment as to which time point was of the greatest clinical importance. We did not anticipate any other unit of analysis issues as we expected all trials to be parallel design.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results through a sensitivity analysis.
Assessment of heterogeneity
We used visual assessment of the study data, supplemented by use of the I2 statistic to measure heterogeneity among the trials in each analysis. Where we identified substantial heterogeneity (widely distributed study findings or an I2 greater than 50%, or both), we reported it and explored possible causes.
Assessment of reporting biases
Where we were able to pool more than 10 trials, we planned to create and examine a funnel plot to explore possible small‐study biases for the primary outcomes.
Data synthesis
We undertook meta‐analysis only where this was meaningful: that is, if we considered the treatments, participants, and the underlying clinical question to be similar enough for pooling to make sense.
We used a fixed‐effect model on the assumption that surgical techniques for aortic valve replacement were sufficiently standardised in the key components of the procedure to be comparable. If there was substantial heterogeneity (either in the visual inspection of the forest plots, clear heterogeneity from the study designs or I2 greater than 50%), we used a random‐effects model for pooling, to account for the small but cumulative differences in surgical technique and aftercare that exist between surgeons and units.
Subgroup analysis and investigation of heterogeneity
We did not anticipate performing any subgroup analyses.
Sensitivity analysis
We planned to carry out the following sensitivity analysis: only including studies with a low risk of bias (no domains in the summary of bias table considered high risk). As only one of the included studies was at overall low risk of bias, our final sensitivity analyses were performed by excluding studies evaluated to be at high risk of bias (more than two domains in the summary of bias table judged as high risk). We also performed a separate sensitivity analysis excluding studies where rapid‐deployment valves were utilised.
Summary of findings and assessment of the certainty of the evidence
We created a summary of findings table for the main comparison (limited sternotomy versus full sternotomy for aortic valve replacement) and seven of the most important outcomes: mortality, extracorporeal support times (cardiopulmonary bypass time and aortic cross‐clamp time), postoperative blood loss, pain scores, quality of life, and index admission costs. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence as it related to the studies which contributed data to the meta‐analyses for the prespecified outcomes, including for outcomes not included in the final summary of findings table. We used methods and recommendations described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020), using GRADEpro software. We justified all decisions to downgrade the certainty of the evidence using footnotes, and we made comments to aid readers' understanding of the review where necessary.
Results
Description of studies
Results of the search
In our original review (Kirmani 2017), we retrieved 203 references through electronic searching of CENTRAL, MEDLINE, and Embase, following de‐duplication. After review of titles and abstracts, we screened out 151 references as they were not relevant. From the remaining 52 references, we excluded 45 studies following full‐text review.
The review update performed in August 2021 identified an additional 274 references, 244 new references after de‐duplication. Of these, we assessed 17 in full text, of which seven (from 11 references) were relevant to the study question and included in the full‐text review, giving a total of 14 included studies (Figure 1).
1.
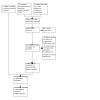
Study flow diagram
We checked the status of 17 trials that were listed as ongoing in 2017. Ten were not relevant to the current review, one had been terminated before completion (NCT00221663), one was a proposed long‐term registry (NCT02278666 (SATURNO)), and one had recently completed but not been published, so it is still listed as ongoing (NCT02272621), with authors contacted but preferring to publish their works before inclusion in this meta‐analysis. Of those that were ongoing or completed without any published results at the time of the original 2017 review, four have since been published and are included in this version of the review (Dalén 2018; Hancock 2019; Nair 2018; Rodríguez‐Caulo 2021). One study had been identified in conference proceedings, but was excluded as it had not been prospectively randomised. This review update identified and included as an ongoing trial one new trial listed as ongoing in 2021 that has recently been completed but not yet been published (NCT04012060 (LIAR)). No other additional ongoing trials were identified for the updated review.
Included studies
Following the search, screening, and exclusion of irrelevant studies, we identified 14 studies that met the inclusion criteria. These 14 randomised controlled trials represented 1395 participants in studies of between 40 and 270 participants, performed between 1999 and 2019. The studies were performed in Austria (Mächler 1999), Czech Republic (Gofus 2020), Spain (Aris 1999a; Rodríguez‐Caulo 2021), Italy (Bonacchi 2002), Germany (Borger 2015; Dogan 2003), France (Calderon 2009), Egypt (Moustafa 2007), Russia (Shneider 2020), Sweden (Dalén 2018), Serbia (Vukovic 2019) and the UK (Hancock 2019; Nair 2018). All were undertaken in cardiothoracic surgical settings and only one was a multicentre trial, conducted by 12 surgeons across five German centres (Borger 2015).
Three of the studies reported power calculations (Calderon 2009; Hancock 2019; Nair 2018), and seven cited the outcome measures a priori in the methods or published the protocol (Aris 1999a; Borger 2015; Calderon 2009; Dalén 2018; Dogan 2003; Hancock 2019; Nair 2018). All sought institutional ethical approval prior to conduct of the study.
Participants
All 14 trials included participants undergoing elective, isolated aortic valve replacement. The majority of studies included both aortic stenosis and aortic regurgitation pathologies except one which excluded participants with pure aortic regurgitation (Borger 2015). Acute pathology of the aortic valve (i.e. endocarditis), calcified ascending aorta, and other recent potential confounding comorbidities (e.g. myocardial infarction, cerebrovascular accident, significant neurological impairment) were variably described as exclusion criteria, but by definition of the inclusion criteria, all studies were likely to have excluded such participants empirically.
Variations in the participant population may have existed as three studies excluded people with very poor left ventricular ejection fraction under 25% (Bonacchi 2002; Borger 2015; Moustafa 2007). Two studies excluded participants with moderate left ventricular function under 40% to 45% and participants with chronic airway disease or renal impairment (Calderon 2009; Nair 2018). As the primary outcome measure in Hancock 2019 was bleeding following surgery, this trial excluded patients with preoperative anaemia or coagulopathies. The study from Egypt included a much younger patient population (Moustafa 2007), presumably with more rheumatic heart disease than the European studies, which appear to have older participants with degenerative heart valve sclerosis.
Interventions
All but one study used reversed L‐shaped/J‐shaped upper hemi‐sternotomy as the limited sternotomy; one study used a reversed C‐shaped incision according to anatomy (Bonacchi 2002). For clarity, we will refer to the minimally invasive incision as a limited upper hemi‐sternotomy for the remainder of this review. The surgical technique remained similar between studies, with all employing aortic arterial cannulation and either right atrial or femoral venous cannulation, to institute normothermic or mild hypothermic CPB. Cross‐clamping was exclusively across the ascending aorta and cardioplegia techniques varied in terms of delivery and type. All studies used antegrade, both as root and ostial cardioplegia, but some also gave retrograde cardioplegia for open cases. The choice of cardioplegia solution included blood and crystalloid (either St Thomas', del Nido or Bretschneider's solutions).
The choice of prostheses varied across studies. Some studies used exclusively mechanical valves (Aris 1999a; Moustafa 2007, although the former had a single participant exception), whilst others varied the valve choice dependent on participant age. The valve insertion technique was not stipulated in the majority of cases (e.g. interrupted, pledgeted, semi‐continuous, etc.) except for one study which compared rapid deployment balloon expandable stented valves for the mini‐sternotomy arm (Borger 2015). Venting strategies also varied between studies with pulmonary vein, pulmonary artery, aortic root, or no venting used or described.
Outcomes
Of the primary outcome measures, all studies reported perioperative mortality (as either in‐hospital or 30‐day mortality). Bonacchi 2002 did not provide data for CPB time and Mächler 1999 and Gofus 2020 reported this as median with IQR, precluding them from inclusion in the quantitative analysis. All studies reported aortic cross‐clamp times, but again two studies reported data as median and ranges (Gofus 2020; Mächler 1999), which we therefore excluded from meta‐analysis. Only one of the studies reported major adverse outcomes as a composite (Rodríguez‐Caulo 2021), but all reported major complications individually. None of the studies described long‐term follow‐up beyond 12 months.
The secondary outcome measures for the meta‐analysis were also variably reported. Studies frequently documented organ failure requiring support, but not universally in the outcome tables. All but two studies reported total hospital stay (Borger 2015; Mächler 1999), both from Germany where length of stay is not considered a quality marker. Blood loss was described by several different methods, which were not universally comparable. Four studies measured quality of life, all with the EuroQoL 5‐D measure (Borger 2015; Hancock 2019; Nair 2018; Rodríguez‐Caulo 2021). Two trials reported a cost analysis (Hancock 2019; Nair 2018), but the former presented findings as the results of cost‐effectiveness per quality adjusted life‐year simulation and was therefore not directly comparable with the other study. Both showed costs for index admission. Pulmonary function tests included forced expiratory volume in one second (FEV1) as a percentage of predicted values (based on the participant's age, height and weight) in all studies that reported this outcome.
Two of the studies described their criteria and protocols for transfusion and discharge from hospital (i.e. time until fit for discharge if different from time until discharge) (Hancock 2019; Nair 2018), but no studies indicated protocols for return to theatre or discharge from the intensive care unit.
This information is summarised in the Characteristics of included studies tables.
Ongoing studies
Two studies registered on clinicaltrials.org (between them randomising 260 participants to full median sternotomy or limited upper hemi‐sternotomy) completed recruiting in 2020 (NCT02272621; NCT04012060 (LIAR)). The former had previously been under the name of a different responsible party with different primary outcomes and a smaller sample size, but had undergone substantial changes in design. The named lead for this trial is now the same as for another, published, study which is included in this meta‐analysis, registered with a separate NCT number (Dalén 2018). The second trial, NCT04012060 (LIAR), was complete and a manuscript had been submitted for publication, which the lead author preferred to be the source of data for this review rather than unpublished or preprint results.
Excluded studies
The Characteristics of excluded studies table effectively summarises the reasons for excluding the 47 studies not included in the meta‐analysis. Six studies were not randomised, 37 were observational, and four were not via partial sternotomies (two via thoracotomy, one port access, and one robotic).
Risk of bias in included studies
The risk of bias is summarised in the risk of bias graph (Figure 2) and risk of bias summary table (Figure 3). We made overall judgements on the risk of bias per study based on the number of domains assessed as high risk. Due to the nature of studies on surgical incisions, nearly all included studies were at high risk of bias for lack of blinding, but this will have affected the various outcome measures inconsistently (e.g. pain was likely to have been highly influenced by lack of blinding whereas deep sternal wound infection was not). For studies with a high risk of bias related to non‐blinding, we considered them to be at overall high risk of bias and excluded them from the sensitivity analyses.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Eight studies used computer‐generated random allocation sequence generation (Aris 1999a; Bonacchi 2002; Calderon 2009; Gofus 2020; Hancock 2019; Nair 2018; Rodríguez‐Caulo 2021; Vukovic 2019); one used sealed envelopes (Dalén 2018), and the method was unclear in five studies (Borger 2015; Dogan 2003; Mächler 1999; Moustafa 2007; Shneider 2020).
Three studies achieved allocation concealment by the use of sealed envelopes opened at the time of surgery (Aris 1999a; Calderon 2009; Moustafa 2007); one used a telephone system concealing allocation until surgery (Nair 2018), one used a web‐based concealment system (Hancock 2019), and two used independent staff responsible for allocation concealment (Rodríguez‐Caulo 2021; Vukovic 2019). This was unspecified for the other studies. One study performed surgery the day after randomisation with allocation by an unspecified random numbering method (Gofus 2020), and had a statistically significant difference in the bodyweight of the two randomised groups (76 kg versus 91 kg for limited versus full sternotomy, respectively).
Blinding
Although blinding of the participants following minimally invasive surgery has previously been described by the use of standardised dressings, only one of the trials included here employed participant blinding and only for the first two days of the study (Hancock 2019). The surgeons were not blinded in any trial, and it was not clear who the outcome assessors were in most trials. Bonacchi 2002 employed blinded outcome assessors for pain score measurements, but not for any of the remaining outcomes. Nonetheless, for several quantifiable and objective outcome measures (e.g. postoperative blood loss) there will have been no effect from non‐blinding. We noted that some outcome measures (noted in Figure 3 as subjective outcome measures) could be influenced by knowledge of the treatment allocation: patients might take deeper breaths if they knew they had a smaller scar, affecting lung function tests; time to discharge from the intensive care unit or hospital might also be affected by assumptions about the time to recovery from minimally invasive surgery, etc.
Incomplete outcome data
The majority of studies reported outcomes on all randomised participants (Aris 1999a; Bonacchi 2002; Calderon 2009; Dogan 2003; Gofus 2020; Mächler 1999; Moustafa 2007; Shneider 2020). In one study, there were six withdrawals from the study after randomisation (five in the limited sternotomy and one in the full sternotomy group), and the data were reported for participants who underwent treatment as intended (Borger 2015). Three of the participants in this study were withdrawn because participants randomised to minimally invasive surgery "eventually received a conventional valve because of problems with their anatomy". As such, these participants would have constituted a failure to proceed with intended surgery because of the intervention and would contribute to attrition bias. One study reporting outcomes to 12 months had loss to follow‐up of 31 patients (Nair 2018), whereas another study with the same length of follow‐up reported on 100% of participants (Hancock 2019). Rodríguez‐Caulo 2021 reported in their CONSORT diagram that 103 participants were randomised, but only reported on 94 of those.
Selective reporting
The majority of studies had not widely published a trial protocol citing their intended outcome measures. Aris 1999a had a protocol approved by their Departmental Research Committee but did not register it with an international registry. Two studies did not describe having a protocol prior to starting the trial and were not registered on international registries (Bonacchi 2002; Vukovic 2019). Borger 2015 published a protocol (CADENCE-MIS), but did not report on four prespecified secondary outcome measures (velocity‐time index, left ventricular outflow tract diameter, annular size, or septal thickness). Calderon 2009 had published a protocol with similar characteristics to the published study (NCT00221663), but this was updated as "Terminated ‐ due to slow recruitment". Only one proposed outcome measure from the retracted protocol was not included (cytokine levels from tracheal aspirates). The published study described approval from the local ethics committee. Four studies were approved by the institutional ethics committees, but the protocols were not published a priori (Dalén 2018; Dogan 2003; Gofus 2020; Shneider 2020), although Dalén 2018 was registered. Mächler 1999 did not describe a prestudy protocol, and Moustafa 2007 stated that their study had received approval from the protocol research committee at their institution, but did not have a published protocol in a registry. We considered the study by Dalén 2018 to be at high risk of selective reporting bias as the treatment arms of deceased patients were not clear in the per‐protocol analysis. No protocol, flow diagram or sample size statistics were shown for Shneider 2020. Three studies published full trial protocols along with the trial registration (Hancock 2019; Nair 2018; Rodríguez‐Caulo 2021).
Whilst specific outcomes may have had variable reporting within studies, we adopted an approach of assessing selective reporting bias at a study‐level, in accordance with recommendations from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We considered which outcome measures for aortic valve replacement were important and could be reasonably expected to be reported, and found that all studies had included information about the most important measures. We did not downgrade the judgement for the Borger 2015 study on the basis of the missing variables as we did not consider these to be important clinical measures.
Other potential sources of bias
The minor differences in the surgical techniques between studies were not thought to have contributed a significant risk of bias, although they may have introduced some explicable heterogeneity.
Within studies, one trial had a significant confounding factor in the methodology in that the limited upper hemi‐sternotomy group also received rapid deployment balloon‐expandable valves, whereas the full‐sternotomy group received conventional surgically implanted stented prostheses (Borger 2015). This study was also funded by the manufacturer of the expanding valve.
Four studies did not report detailed demographic differences between the two groups at baseline (Aris 1999a; Bonacchi 2002; Calderon 2009; Dogan 2003), and it is unclear if this may have introduced bias.
We downgraded Dalén 2018 for undertaking a per‐protocol analysis and Vukovic 2019 and Gofus 2020 for not defining a statistical analysis plan a priori. The study by Nair 2018 was downgraded for a high rate (12%) of conversion from limited to full sternotomy, suggesting that the surgeons involved may have been inexperienced and still learning the procedure.
Effects of interventions
See: Table 1
Table 1 provides an overview of the aggregated results of the studies. In the original review we included all outcomes, but for this update we were more closely adherent to the Cochrane Handbook, which encouraged limiting this to seven outcomes (Higgins 2020). The methodology was still utilised, however, to provide a GRADE rating for all outcomes before the summary of findings table was reduced to show only the most important outcomes.
Primary outcomes
Mortality
All trials reported mortality either as in‐hospital or 30‐day mortality, but the effect was not estimable for four studies that had zero events in both arms (Dogan 2003; Gofus 2020; Hancock 2019; Moustafa 2007). We deemed the evidence to be of low certainty because of the number of domains at high risk of bias and the imprecision of results. The overall effect estimate for 873 participants in the remaining 10 studies suggested there may be no difference between limited and full sternotomy on perioperative mortality (RR 0.93, 95% CI 0.45 to 1.94; 10 studies, 985 participants; low‐certainty evidence; Analysis 1.1). The 95% confidence intervals, however, spanned both possible benefit and possible harm, and the low certainty of evidence would suggest taking a cautious approach to this important effect estimate. A sensitivity analysis, removing studies at high risk of bias (Borger 2015; Dalén 2018; Rodríguez‐Caulo 2021; Vukovic 2019), did not change this outcome. There did not appear to be any evidence of publication bias in the funnel plot (Figure 4).
1.1. Analysis.

Comparison 1: Limited versus full sternotomy for aortic valve replacement, Outcome 1: Mortality
4.

Funnel plot for studies reporting mortality
Cardiopulmonary bypass time
Ten studies reported CPB times in formats that could be pooled. One study did not cite CPB times but noted that there was no statistically significant difference between the two groups (Bonacchi 2002). Two studies only provided median CPB times, with IQR, that were assumed to be skewed and therefore not amenable to meta‐analysis (Gofus 2020; Mächler 1999).
The remainder of the studies showed significant heterogeneity, likely to represent the cumulative effects of intraoperative differences between surgeons, hospitals, and countries. It is unlikely that this clinical heterogeneity can be corrected for by trial methodology, and we therefore elected to use a random‐effects model to mitigate these differences to some degree. As CPB time is such an important surrogate marker of clinical outcome following cardiac surgery, we chose not to exclude this outcome measure completely from quantitative meta‐analysis, but downgraded the certainty level of evidence. The overall effect was that there may be an increase in the limited sternotomy group, although the evidence was uncertain and the clinical significance of this difference was minimal (MD 10.63 minutes, 95% CI 3.39 to 17.88; I2 = 92%; 10 studies, 1043 participants; very low‐certainty evidence; Analysis 1.2). Sensitivity analysis performed by exclusion of the study using rapid‐deployment valves in the limited sternotomy arm of the study did not change the results (Borger 2015). Additionally, excluding the single study with inexplicably shorter CPB time in the limited sternotomy arm did not change the findings either (Moustafa 2007). We downgraded the GRADE rating for high risk of bias. We also downgraded the GRADE rating twice for inconsistency as aspects of surgical technique varied between studies, including cannulation technique and use of rapid deployment valves in one arm of one study. Additionally, the funnel plot (Figure 5) was asymmetric, suggesting a non‐reporting publication bias in the absence of other plausible explanations. However, methodological biases in the form of experienced‐operators for limited sternotomy aortic valve replacement (AVR) and usual‐operators for full sternotomy may have also contributed to this asymmetry.
1.2. Analysis.

Comparison 1: Limited versus full sternotomy for aortic valve replacement, Outcome 2: Cardiopulmonary bypass time (minutes)
5.

Funnel plot for studies reporting cardiopulmonary bypass times
Aortic cross‐clamp time
We excluded two studies in the analysis of aortic cross‐clamp times, as data were presented as median and IQR (Gofus 2020; Mächler 1999). The estimate of effect for the remaining studies suggested there may be a small but probably not clinically significant benefit in the outcome favouring full sternotomy, but the evidence was uncertain (MD 6.07 minutes, 95% CI 0.79 to 11.35; I2 = 94%; 12 studies, 1235 participants; very low‐certainty evidence; Analysis 1.3). Several explanations might exist for the heterogeneity in these studies. Because of variations in the type of aortic pathology across studies, some aortic annuli may have required more extensive decalcification than others. The use of rapid deployment valves in one arm of one study (when these devices could be used in either arm) may also have affected the clinical heterogeneity (Borger 2015). Borger and colleagues used Edwards Intuity rapid deployment valves, which do not require aortic decalcification (unlike some other rapid deployment valves), and this will also have contributed to the reduction in aortic cross‐clamp time. As with our meta‐analysis of CPB times, we felt that the clinical importance of this measure warranted quantification with appropriate consideration of reasons for differences across studies. Sensitivity analysis, removing the study by Borger 2015 that may have been biased by the use of rapid‐deployment valves in the minimally invasive group, did not substantially change the overall effect. We downgraded the GRADE rating once for high risk of bias, twice for inconsistency due to high heterogeneity, and once for possible publication bias. The funnel plot (Figure 6), as for cardiopulmonary bypass, indicated publication bias with an asymmetric appearance.
1.3. Analysis.

Comparison 1: Limited versus full sternotomy for aortic valve replacement, Outcome 3: Aortic cross‐clamp time (minutes)
6.

Funnel plot for studies reporting aortic cross clamp time
Secondary outcomes
Organ failure requiring support
No studies reported organ failure requiring support.
Length of hospital stay
Ten studies assessed length of hospital stay following aortic valve replacement via either full or upper hemi‐sternotomy. Aris 1999a, Calderon 2009, Nair 2018 and Dogan 2003 had results that clustered around the point of equipoise, with Bonacchi 2002 demonstrating a 95% CI that just fell in favour of surgery via limited hemi‐sternotomy. Length of stay was presented as median and IQR in Gofus 2020 and therefore excluded from meta‐analysis, but was not different in the two arms. The study from Egypt showed a much greater advantage of upper hemi‐sternotomy, though the length of stay in the full sternotomy group was substantially higher than other studies (mean stay more than two weeks), suggesting methodological biases (Moustafa 2007). Hancock 2019 was the only study to show a significant disadvantage to length of stay for people undergoing limited sternotomy. As the discharge criteria for institutions can vary and the mean stay in this study was likely to have been affected by a long‐staying outlier, this may explain the high heterogeneity. The overall estimate of effect favoured limited sternotomy, but there was significant uncertainty about the findings (MD ‐1.09 days, 95% CI ‐1.90 to ‐0.28; I2 = 83%; 11 studies, 1141 participants; very low‐certainty evidence; Analysis 1.4). We downgraded the GRADE rating for high risk of bias from non‐blinding, inconsistency in discharge criteria, imprecision from not meeting Optimal Information Size and indirectness. The funnel plot (Figure 7) was asymmetric and causes for this may have included non‐reporting biases or methodological issues with studies that did not prespecify a discharge protocol, producing larger effect estimates.
1.4. Analysis.

Comparison 1: Limited versus full sternotomy for aortic valve replacement, Outcome 4: Length of hospital stay (days)
7.

Funnel plot for studies reporting length of hospital stay
Postoperative blood loss
There was substantial heterogeneity in the results of the studies that assessed blood loss in the postoperative period, so we employed a random‐effects model. We excluded two studies reporting this outcome as median and IQR (Dalén 2018; Gofus 2020). We considered the reasons for the heterogeneity and weighed the benefits of performing a quantitative meta‐analysis. As the total measured blood loss may vary across studies as a result of the type of drainage tubes, haemostatic protocols, and postoperative thromboprophylaxis measures employed, we considered this outcome measure to be at high risk of clinical heterogeneity across cardiac surgical units. However, minimally invasive procedures are more susceptible to field flooding with small amounts of bleeding, and more meticulous haemostasis is required during dissection, which may have reduced the overall bleeding in this group. In addition, the use of transpleural drains in people undergoing upper hemi‐sternotomies (due to the sub‐xiphoid site being difficult to reach, depending on the length of the partial sternotomy) may have allowed some pericardial bleeding to evacuate into the pleura, thereby reducing the estimated blood loss in this group. All but two studies demonstrated an advantage in this domain for minimally invasive surgery via limited sternotomy and the cumulative effect was that upper hemi‐sternotomy probably reduces postoperative bleeding (MD ‐153.04 millilitres, 95% CI ‐245.96 to ‐60.12; I2 = 89%; 8 studies; 767 participants; moderate‐certainty evidence; Analysis 1.5). A sensitivity analysis for this outcome measure that excluded studies at high risk of bias found no change in the effect. We downgraded the GRADE rating for inconsistency due to variations in the surgical or anaesthetic management between studies.
1.5. Analysis.

Comparison 1: Limited versus full sternotomy for aortic valve replacement, Outcome 5: Postoperative blood loss (mL)
Deep sternal wound infection
Eight of the studies that reported deep sternal wound infections had events to allow comparison (Bonacchi 2002; Borger 2015; Gofus 2020; Mächler 1999; Nair 2018; Rodríguez‐Caulo 2021; Shneider 2020; Vukovic 2019), suggesting that low event rates would lead to imprecision. The estimate of effect suggested there may be no differences between full or limited sternotomy (RR 0.75, 95% CI 0.32 to 1.76; I2 = 51%; 8 studies, 868 participants; low‐certainty evidence; Analysis 1.6), but the wide variation in the effects both within and between studies implied that these were not powered to identify a difference. Sensitivity analyses had no effect on the estimate. We downgraded the GRADE rating for imprecision as the sample size did not meet optimal information size criteria and again for imprecision as the confidence intervals crossed the line of null effect and included both appreciable harm and benefit.
1.6. Analysis.

Comparison 1: Limited versus full sternotomy for aortic valve replacement, Outcome 6: Deep sternal wound infection
Pain scores
We used SMD for this outcome measure in the absence of a unified mode of measurement and no alternative options to rationalise, as per the methods section.
Five studies described pain scores between the two groups, and one study (Gofus 2020) reported pain only within the parameters of the Short‐Form 36 Health Related Quality of Life (SF‐36) questionnaire at three months. We graded the overall certainty of the evidence as low because we considered this outcome measure to be particularly susceptible to non‐blinding (i.e. participants might consider a smaller incision to be less painful) and the majority of studies did not blind participants, apart from Hancock 2019; in addition, the studies did not define their analgesic protocols in most cases, and this may have also been a source of bias. Bonacchi 2002 used self‐reported pain scores at one and 12 hours, measured by nurses blinded to the treatment groups. The data for pain scores at 12 hours were compared here. Participants experiencing moderate pain were treated with morphine and non‐steroidal anti‐inflammatory medications. Calderon 2009 employed a 40‐mm visual analogue scale for pain measurements at two days postoperatively. All participants were given paracetamol 1 g every six hours and a morphine patient‐controlled analgesia device to deliver 1‐mg boluses up to every seven minutes. Non‐steroidal analgesia was added to this regimen if participants were still in pain. Unlike the other studies that reported pain levels, participants in this study reported more pain in the limited sternotomy group than in the full sternotomy group (not reaching statistical significance), but the analgesia usage was also lower in the upper hemi‐sternotomy group. The effects of non‐blinding may have been responsible for this disparity as participants with limited upper hemi‐sternotomy surgery may have felt that they should not require as much analgesia and therefore ended up with higher pain scores. The study by Dogan 2003 also utilised a visual analogue scale to measure pain on the second postoperative day. These were repeated on day five but not included for comparison. Hancock 2019 undertook pain assessments on days 2, 3 and 4 and again at week 6 and week 12. The day 2 assessments were used for meta‐analysis. There were no differences in pain or analgesia use between the two groups at any time point. Nair 2018 undertook daily pain scores for the first 10 days and although the mean scores were lower in the limited sternotomy group, the 95% confidence intervals crossed over substantially, with no statistically significant difference. The day 2 values were used for the meta‐analysis. The overall estimate of effect using a random‐effects model for the heterogenous data suggests there may not be any advantage to surgery via limited upper hemi‐sternotomy, falling below the Cochrane Handbook suggested threshold for Cohen's effect size of 0.2 for standardised mean difference (SMD ‐0.19, 95% CI ‐0.43 to 0.04; I2 = 50%; 5 studies, 649 participants; low‐certainty evidence; Analysis 1.7). Sensitivity analyses had no effect on the estimate.
1.7. Analysis.

Comparison 1: Limited versus full sternotomy for aortic valve replacement, Outcome 7: Pain scores
Quality of life
Five studies examined quality of life using a validated tool, four of which used EQ‐5D (Borger 2015; Hancock 2019; Nair 2018; Rodríguez‐Caulo 2021), and one of which used the SF‐36 (Gofus 2020). Because the two measures are not related linearly and the SF‐36 is reported in eight domains which cannot be simply summated, we did not include the data from Gofus 2020 in the quantitative meta‐analysis. Borger 2015 undertook quality of life measures at three months following surgery; Hancock 2019 at baseline, two days, six weeks and 12 weeks; Nair 2018 at multiple time points, including six weeks and six months; Rodríguez‐Caulo 2021 at one, six and 12 months; and Gofus 2020 at 90 days. We compared the closest time points (six weeks for Nair 2018 and Hancock 2019, three months for Borger 2015 and one month for Rodríguez‐Caulo 2021). There may be no difference between full and upper hemi‐sternotomy groups (MD 0.03, 95% CI 0.00 to 0.06; I2 = 58%; 4 studies, 624 participants; low‐certainty evidence; Analysis 1.8). Only Rodríguez‐Caulo 2021 had been powered specifically to report a 'minimal important difference' in quality of life between the two groups of 0.10, but both other studies had the same or a larger sample size. The findings of the study by Gofus 2020 correlated with the synthesis of the other data, suggesting no difference at 90 days in any measures of health‐related quality of life. We downgraded the GRADE rating for imprecision due to the small sample sizes and for the high risk of bias in this outcome from non‐blinding.
1.8. Analysis.

Comparison 1: Limited versus full sternotomy for aortic valve replacement, Outcome 8: Quality of life
Index admission costs
Two studies reported economic analyses (Hancock 2019; Nair 2018), both from the UK: Nair 2018 reported absolute costs in hospital and at 12 months; Hancock 2019 provided additional unpublished data on costs in hospital and at 12 weeks. We therefore used the in‐hospital costs of these two studies for the data synthesis and demonstrated that there may be higher index admission costs for limited sternotomy compared to full sternotomy (MD 1190 GBP, 95% CI 420 GBP to 1970 GBP; I2 = 0%; 2 studies; 492 participants; low‐certainty of evidence). Hancock's published results included a health‐economics analysis as a simulated model to assess cost‐effectiveness per quality adjusted life year (QALY). This was not comparable in a meta‐analysis but indicated that although cost and quality of life were no different between the two groups, in a bivariate analysis, conventional full sternotomy was more cost‐effective per QALY. There was only a 5.8% probability of limited sternotomy being cost‐effective at a willingness to pay threshold of 20,000 GBP per QALY. At this threshold, Nair 2018 estimated a 3.7% probability of cost‐effectiveness. At a willingness to pay threshold of 30,000 GBP per QALY, the probability was 5.1%. Both these studies therefore concluded that limited sternotomy for aortic valve replacement in the UK National Health Service was not cost‐effective. We downgraded the GRADE rating for indirectness as both studies showed only UK admission costs and for imprecision as the sample sizes did not meet optimal information size criteria.
Intensive care unit stay
Ten papers described intensive care stay; Dalén 2018 reported median and IQR and was therefore excluded, and Calderon 2009 reported one standard deviation of zero, making it inestimable for synthesis. Therefore, the analysis was only based on the remaining eight studies. There was no difference in the effect estimate, but the evidence for this was very uncertain (MD ‐0.22 days, 95% CI ‐0.58 to 0.15; I² = 83%; 9 studies, 624 participants; very low‐certainty evidence; Analysis 1.10). Lack of blinding was thought to have a greater influence on intensive care length of stay than some other outcome measures: trial participants undergoing limited sternotomy were likely to have been promoted for discharge from the critical care area in order to facilitate their mobilisation and recovery. In addition, clinical heterogeneity will have been influenced by the differing practices of monitoring and discharge from intensive care across surgical departments, and the sample sizes required to identify a difference in time to discharge were not met. We downgraded the GRADE rating for risk of bias from non‐blinding, inconsistency in discharge criteria and imprecision. Prespecified sensitivity analysis that removed studies at high risk of bias changed the effect estimate to a possible small benefit from limited sternotomy (MD ‐0.45, 95% CI ‐0.84 to ‐0.06; 5 studies, 490 participants).
1.10. Analysis.

Comparison 1: Limited versus full sternotomy for aortic valve replacement, Outcome 10: Intensive care unit stay (days)
Postoperative pulmonary function tests
Four studies assessed the effects of aortic valve replacement through limited upper hemi‐sternotomy on lung function, although FEV1 (either as an absolute measurement or as a percentage of predicted) was the only common parameter assessed in all of them. As lung function tests are height and weight dependent, we excluded studies that provided only absolute measurements (i.e. not indexed against nomograms for body measurements as a percentage of the predicted lung function). Aris 1999a performed lung function tests preoperatively and again at discharge, finding a statistically significant drop in lung function following surgery, but no difference in the drop between full and upper hemi‐sternotomy groups. Bonacchi 2002 performed lung function tests at five days postoperatively and again at one to two months. The figures for the fifth postoperative day were included in this comparison. Baseline reference pulmonary function tests were not described. The study by Calderon 2009 included preoperative baseline lung function tests and again at 24 hours, 48 hours, and seven days postoperatively. We used the data for day seven in the analysis. Moustafa 2007 also performed lung function tests at baseline (preoperative), one week, and one month. The data were not clearly annotated; we assumed the variability was the standard error (rather than standard deviation) due to the small differences and converted it accordingly. We used the figures for FEV1 at one week in the analysis. Gofus 2020 undertook pulmonary function tests at baseline, day 7 and day 90, finding FEV1, FEV1/FVC ratio, and FEV1/VC were all still worse in the full median sternotomy groups at day 90. We used the day 7 values in the analysis.
Despite the differences in time of measurement, there was relatively little heterogeneity in the studies included, and the overall effect was that there may be a small but not clinically significant increase in FEV1 postoperatively in participants undergoing upper hemi‐sternotomy compared to full sternotomy (MD 2.08, 95% CI 0.74 to 3.41; I² = 20%; 5 studies, 297 participants; low‐certainty evidence; Analysis 1.11). Data from Nair 2018 was presented as absolute lung volumes rather than as percentage of predicted values, and was therefore not included in the meta‐analysis, but demonstrated a reduction in FEV1 at discharge in both groups with a return to baseline at six weeks in both arms. Similarly, data from Hancock 2019 was presented as absolute values and showed a significant drop in FEV1 immediately postoperatively, improving (although not quite to baseline) at six weeks, with a better improvement in the limited sternotomy group. We downgraded the GRADE rating due to inconsistency in time point of measurement, baseline starting values and risk of bias from non‐blinding. Sensitivity analysis had no effect on the estimates.
1.11. Analysis.

Comparison 1: Limited versus full sternotomy for aortic valve replacement, Outcome 11: Postoperative pulmonary function tests (% FEV1)
Re‐exploration
All studies described re‐exploration for bleeding, although one had no events in either group and the effect was therefore not estimable in the analysis (Aris 1999a). The confidence intervals for each of the studies crossed over the line of no effect; therefore, the net effect was of no difference between full and limited upper hemi‐sternotomy (RR 0.82, 95% CI 0.50 to 1.33; I² = 0%; 13 studies, 1355 participants; very low‐certainty evidence; Analysis 1.12), although the 95% confidence interval included possible benefit and possible harm. The effects of a sensitivity analysis (removing the studies at high risk of bias) did not change this outcome. We downgraded the GRADE rating once for high risk of bias and twice for imprecision: once for not meeting the optimal information size criteria and again as the confidence intervals crossed the line of null effect including both appreciable harm and benefit. A funnel plot showed no evidence of publication non‐reporting bias (Figure 8).
1.12. Analysis.

Comparison 1: Limited versus full sternotomy for aortic valve replacement, Outcome 12: Re‐exploration
8.

Funnel plot for studies reporting re‐exploration
Postoperative atrial fibrillation
Nine studies included data on rates of postoperative atrial fibrillation. Management of atrial fibrillation varies considerably from surgeon to surgeon, with some adopting a prophylactic approach, most treating at onset, and pharmacological options for treatment being quite wide. Despite this, there was low heterogeneity and only one study had a markedly different outcome to the other studies (Mächler 1999), which appeared to be a reporting bias due to the remarkably low rate of events (which are normally expected in around a third of patients), but this did not affect the outcomes in a sensitivity analysis. There may be little to no difference in atrial fibrillation by minimally invasive aortic valve replacement through limited hemi‐sternotomy (RR 1.12, 95% CI 0.84 to 1.51; I² = 36%; 9 studies, 1012 participants; low‐certainty evidence; Analysis 1.13). A sensitivity analysis made no change to this overall effect. We downgraded the GRADE rating for inconsistency in management of arrhythmia prophylaxis between centres and again for imprecision as the confidence intervals crossed the line of null effect, including both appreciable harm and benefit.
1.13. Analysis.

Comparison 1: Limited versus full sternotomy for aortic valve replacement, Outcome 13: Postoperative atrial fibrillation
Postoperative ventilation time
All but one study (Borger 2015) reported the length of invasive ventilation postoperatively. The studies by Dalén 2018, Gofus 2020 and Mächler 1999 presented this as median and IQR, and we did not include them in the quantitative comparison. Dalén 2018 and Gofus 2020 found no difference in ventilation time. Mächler 1999 found a statistically significant reduction in postoperative ventilation time in limited compared to full sternotomy (median 7 hours (IQR 5.3 to 11) with limited versus 10 hours (IQR 8.5 to 12) with full; P < 0.0001). The data from the remaining studies were highly heterogeneous and this is likely to have been due to clinical differences in extubation protocols between units. Only one study described their criteria for extubation (Bonacchi 2002).
The overall estimate of effect was that limited sternotomy may have little to no reduction in postoperative ventilation time, although the 95% confidence interval spanned a reduction and an increase and the evidence was low‐certainty (MD ‐0.53 hours, 95% CI ‐2.30 to 1.24; I² = 96%; 9 studies, 989 participants; low‐certainty evidence; Analysis 1.14). Making the assumption that the studies that had presented data as median and IQRs were doing so for normally distributed data and making an approximated conversion did not change the overall effect (Section 6.5.2.9, Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2020). The prespecified sensitivity analysis had no effect.
1.14. Analysis.

Comparison 1: Limited versus full sternotomy for aortic valve replacement, Outcome 14: Postoperative ventilation time (hours)
Discussion
Summary of main results
This systematic review assessed the effects of full versus limited sternotomy on mortality, CPB time, aortic cross‐clamp time, length of hospital and intensive care unit stay, postoperative blood loss, deep sternal wound infection, pain scores, index admission costs, quality of life measures, pulmonary function tests, re‐exploration for bleeding, postoperative atrial fibrillation, and ventilation times. We found 14 randomised controlled trials with 1395 participants that answered the study question. Evidence was generally of very low to moderate certainty. The high risks of bias associated with the inherent difficulties of blinding surgical access, relatively small study sizes (with corresponding failure to meet optimal information size criteria), and clinically heterogeneous populations were the main reasons for downgrading the certainty of evidence.
All the identified studies used upper hemi‐sternotomy as the mode of limited sternotomy. Meta‐analysis found there may be no survival benefit, or increase in risk, with minimally invasive surgery via limited upper hemi‐sternotomy, although the 95% confidence interval spanned risk and benefit. This correlates with other literature (Phan 2014). The wide confidence intervals of the studies, including null events in some of the studies, indicate that few of these studies were powered to demonstrate differences in perioperative mortality; the aggregate sample size did not cross the optimal information size criteria either.
There is very uncertain evidence that there may have been small treatment effects for extracorporeal support and ischaemic times between the two groups: amounting to a mean difference of possibly around 11 minutes more on cardiopulmonary bypass and six minutes more with the cross‐clamp with limited sternotomy. These would be unlikely to have any clinical impact. In our initial 2017 review we noted that "the oldest study showed the largest difference between full and minimally invasive surgery with a significant disadvantage to performing limited sternotomy (Aris 1999a), but this was less apparent in subsequent trials, presumably as a result of the technique being refined" (Kirmani 2017). However, this effect disappeared with the newer trials, confirming that limited access does indeed seem to prolong the procedure. In one trial, the use of rapid deployment valves meant that the disadvantage of limited sternotomy on operative times was negated (Borger 2015). In fact, the advantage conferred by these valves meant that operative times were explicably shorter for the limited sternotomy cohort in this trial. This may have confounded the comparison between the two groups, and the authors acknowledged this in their discussion. Removing the study for sensitivity analysis, however, did not change the estimate of effect, which remained equivalent between the two groups. Another study found shorter CPB and cross‐clamp times with limited incisions (Moustafa 2007), which could not be explained.
Length of hospital stay may be shorter with minimally invasive surgery via limited sternotomy, although the evidence was very uncertain. This was not seen in our original review. The advantage of limited sternotomy on intensive care stay was negated by the additional studies identified for this updated review, as the evidence became more uncertain. It was unclear why this occurred, however, as there were differences in surgical times, respiratory function, and bleeding rates between the two groups.
Economic analyses were undertaken in two UK trials that showed that limited sternotomy may be more expensive at index admission. These studies also showed that limited sternotomy was unlikely to be cost‐effective at a willingness to pay threshold of 20,000 GBP per QALY. Equally, there may be no quality of life improvement with limited sternotomy.
Overall completeness and applicability of evidence
All the studies included in this systematic review directly addressed the review question and allowed meta‐analyses on a variety of outcome measures. The trial populations seemed to be representative of the people who might undergo aortic valve replacement, including people with aortic regurgitation and aortic stenosis. People at high risk from cardiac surgery were typically excluded (e.g. people with a need for multiple or complex procedures or people with left ventricular impairment) and this may well reflect the typical patient selection in a real‐world setting (where concomitant procedures require open surgery and high risk procedures are considered for transcatheter treatments). The surgical techniques appeared to be consistent with current practice in aortic valve implantation, with a combination of mechanical and tissue prosthetic valves implanted. One study used aprotinin routinely (Aris 1999a), which was withdrawn by the manufacturers in the interim because of an increased risk of mortality. This suspension was lifted in 2012. Most studies employed aorto‐atrial CPB unless exposure dictated femoral cannulation to be necessary. As such, the techniques employed appear to be relevant to modern practice, although clinically heterogeneous.
One small study had an overall mortality of 10% for uncomplicated isolated aortic valve replacement via either approach, due to two deaths in each arm of 20 participants (Aris 1999a). The remainder of the studies appeared to have mortality rates consistent with the expected rates for selected participants. CPB times and aortic cross‐clamp times appeared to be consistent with expected operating times. One trial had a cross‐over rate of 12% from limited sternotomy to full sternotomy (Nair 2018), which the authors noted was a limitation: there was a possibility that the surgeons were still developing confidence in undertaking the procedure. In ideal circumstances, the conversion rate would be lower. The remainder of the outcome measures in the conventional approach (full sternotomy) group appeared to correlate with equivalent data in the literature, suggesting that in all studies, the operating surgeons had already passed their learning curves for the procedures.
Quality of the evidence
This meta‐analysis represented 1395 participants in 14 randomised controlled studies. Most of the studies were underpowered to identify differences in the outcome measures cited. The overall certainty of the evidence was very low to moderate. Table 1 describes the main factors affecting certainty within and between studies.
Limitations in study design and implementation
Nine studies described adequate control of sequence generation, of which seven had robust means of allocation concealment. Blinding was not performed in any study, except for pain scores in the paper by Bonacchi 2002 and for 48 hours for pain and quality of life measures by Hancock 2019. There was no evidence of selective outcome reporting of important outcome measures, but only five studies had a pretrial protocol published in an international registry (Borger 2015; Dalén 2018; Hancock 2019; Nair 2018; Rodríguez‐Caulo 2021). Two studies presented data as per‐protocol analyses rather than intention‐to‐treat (Borger 2015; Dalén 2018). Participants who dropped out of particular arms of the study could represent failure of the procedure in that case (especially for minimally invasive approaches where the intended valve could not be deployed). The follow‐up was complete in all cases.
Indirectness of evidence
In general, there were no serious concerns of systematic bias as a result of indirectness. We considered length of stay to be a poor surrogate marker of surgical outcome, as different healthcare systems have different philosophies on discharging from hospital: some consider an expedited discharge an indication of participant well‐being, whereas others do not construe early discharge in this way.
Unexplained heterogeneity or inconsistency of results
Seven of the outcome measures that were amenable to quantitative analysis showed substantial heterogeneity. The individual reasons for these have been explored in the discussions for each outcome, but can broadly be attributed to the array of surgical and postoperative differences in practice across departments. Many of these cumulative, minor differences in practice were not protocolised or described in the studies and will have contributed to the clinical heterogeneity in this study. In addition, the use of a novel rapid deployment valve in the minimally invasive arm of one study introduced further heterogeneity in the results (Borger 2015), as this will have shortened operative time for one arm only. Inclusion and exclusion criteria were broadly similar, but the variation in aortic valve pathology across studies may have introduced differences in operating times (due to the need for annular decalcification in stenotic valves) and different risk profiles (absence of calcium deposition in the aortic root may reduce the risk of neurological complications). The absence of clear protocols for transfusion in the majority of studies, discharge from the intensive care unit, discharge from hospital, and return to theatre may also have caused differences in outcome measures, but this should have been standardised between groups within studies. One study had within‐study differences in pain control according to which measure was used (visual analogue scale of self‐reported pain versus total dose of morphine delivered via patient‐controlled analgesia), confirming that surrogate markers may not always be reliable indicators (Calderon 2009).
Imprecision of results
The studies were all underpowered according to optimal information size to measure mortality, deep sternal wound infection, and re‐exploration for bleeding. The details of this calculation are outlined in the footnotes of the Table 1.
Publication bias
A number of randomised controlled trials that have been registered but not completed may reflect attempts to perform aortic valve replacement via minimally invasive approaches that were deemed unsuccessful. There may, therefore, be some potential for publication bias if centres that have demonstrated poor results have terminated their programmes or failed to publish their results.
Potential biases in the review process
Four of the review authors have practices that include minimally invasive aortic valve replacement and one of the review authors consults for a manufacturer of minimally invasive surgical equipment. However, the literature search, review and analysis has been performed in a transparent and reproducible manner. This should have reduced any risk of bias in this review.
We had a number of postprotocol changes to the review methodology, which might indicate a bias in the process resulting from prior knowledge of the findings. Several outcome measures were added following aggregation of data: these were not known to us at the time of writing the protocol, and we do not believe this could have been foreseen.
Agreements and disagreements with other studies or reviews
One large meta‐analysis incorporating randomised controlled trials, propensity matched studies, and observational studies, found similar outcomes to this review (Phan 2014). They found a significant reduction in perioperative mortality for minimally invasive aortic valve replacement (including but not exclusive to upper hemi‐sternotomy), but in subgroup analysis, this difference was only evident in the non‐randomised studies. However, the differences in cross‐clamp and CPB times were significant only when randomised trials were excluded – which was our finding in the original 2017 review (Kirmani 2017). With more recent studies, both mortality and operative times came closer to each other in both groups, suggesting an early learning curve for minimally invasive surgery. For other outcome measures, Phan and colleagues aggregated mini‐sternotomy and mini‐thoracotomy approaches to aortic valve replacement and the comparisons are therefore not applicable for a comparison of limited versus full sternotomy.
Our findings correlated with the trend in the literature: that minimally invasive aortic valve surgery via limited upper hemi‐sternotomy may be performed at least as safely as conventional surgery via a full sternotomy, although the evidence was uncertain for some outcomes. The only outcome that showed a likely benefit of limited hemi‐sternotomy was postoperative blood loss.
Authors' conclusions
Implications for practice.
Our review demonstrates there may be no increase in mortality or serious morbidity with minimally invasive aortic valve replacement through limited upper hemi‐sternotomy, although the evidence for some of the complications was very uncertain. It was uncertain whether limited hemi‐sternotomy had different cardiopulmonary bypass or aortic cross‐clamp times compared to full sternotomy. Concerns about the effects of longer cardiopulmonary bypass (CPB) and aortic cross‐clamp times appear to be unfounded as most of the sequelae of these (i.e. the other outcomes of this study) may not be any different, although the evidence was very uncertain for some of these outcomes. Increasing experience in limited sternotomy has brought CPB and aortic cross‐clamp times within equivalent clinical margins of extracorporeal support times in full sternotomy. There may be benefits in postoperative bleeding rates, postoperative lung function, and hospital length of stay. Although index admission costs may be 1190 GBP higher, studies that reported economic analysis did not consider it to be cost‐effective at a 20,000 GBP per quality adjusted life year threshold. Benefits in pain relief and deep sternal wound infection rate were not realised. This would suggest that limited‐sternotomy access may be a comparable, if not cost‐effective, approach for aortic valve replacement, although some of the evidence is very uncertain.
Implications for research.
One significant potential improvement for future research in this field might include blinding of participants and outcome assessors using standardised dressings and a postoperative care team blinded to the approach. In this way, while the operating team would know what surgery had been performed, those making outcome assessments would not.
In addition, future trials would benefit from performing a priori sample size calculations and considering follow‐up of participants beyond discharge. Bypass and cross‐clamp times are reliable indices of the complexity of an isolated aortic valve procedure and correlate well with clinical outcomes and learning curves. In minimally invasive procedures, however, the surgical time from skin incision to skin closure can also be increased, as developing access and maintaining meticulous haemostasis are more crucial. Recording this surgical skin‐to‐skin time in future studies may, therefore, provide a reliable index of the progress of the procedures.
Finally, the use of practitioners with prestated expertise criteria (e.g. 100 cases performed prior to trial participation with < 5% conversion rate) might reduce the uncertainty associated with the learning curve that affected outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 6 December 2023 | New search has been performed | Identification of at least two new high‐profile trials on minimally invasive aortic valve replacement prompted a review update.
|
| 6 December 2023 | New citation required and conclusions have changed | Seven new studies identified following updated literature search. |
History
Protocol first published: Issue 8, 2015 Review first published: Issue 4, 2017
Acknowledgements
Cochrane Heart supported the authors in the development of this systematic review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Professor Rui Providencia, University College London, UK
Managing Editors (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Ghazaleh Aali, University College London, UK and Luisa Fernandez Mauleffinch, Cochrane Central Editorial Service, UK
Copy Editor (copy‐editing and production): Andrea Takeda, Cochrane Central Production Service, UK
We are indebted to Nicole Martin, former Managing Editor, and the Cochrane Heart Information Specialist, for invaluable advice in formulating the search strategy and providing timely and pragmatic advice on all aspects of the review process.
We are also most grateful to the anonymous peer reviewer, Cochrane Heart editorial team, and Liz Bickerdicke, Senior Managing Editor, Central Editorial Service Editorial Team, whose insights and guidance were most welcome in bringing the updated review in line with current Cochrane recommendations.
Appendices
Appendix 1. Search strategies
CENTRAL
#1 MeSH descriptor: [Aortic Valve] this term only
#2 (aortic valve* near/3 (operation* or replace* or surgery)):ti,ab,kw
#3 #1 or #2
#4 MeSH descriptor: [Minimally Invasive Surgical Procedures] this term only
#5 MeSH descriptor: [Robotics] this term only
#6 MeSH descriptor: [Thoracoscopy] explode all trees
#7 MeSH descriptor: [Sternotomy] this term only
#8 minim* invasiv*:ti,ab,kw
#9 ((surgical or surgery or surgeries or replacement* or operation*) near/3 minim*):ti,ab,kw
#10 ((surgery or surgeries or surgical) near/3 (keyhole or percutaneous or robot‐assisted)):ti,ab,kw
#11 thoracoscop*:ti,ab,kw
#12 pleuroscop*:ti,ab,kw
#13 ("hemi‐sternotomy" or "mini‐sternotomy"):ti,ab,kw
#14 #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13
#15 #3 and #14
MEDLINE Ovid
1. Aortic Valve/
2. (aortic valve* adj3 (operation* or replace* or surgery)).tw.
3. 1 or 2
4. Surgical Procedures, Minimally Invasive/
5. Robotics/
6. Thoracoscopy/
7. Sternotomy/
8. minim* invasiv*.tw.
9. ((surgical or surgery or surgeries or replacement* or operation*) adj3 minim*).tw.
10. ((surgery or surgeries or surgical) adj3 (keyhole or percutaneous or robot‐assisted)).tw.
11. thoracoscop*.tw.
12. pleuroscop*.tw.
13. ("hemi‐sternotomy" or "mini‐sternotomy").tw.
14. or/4‐13
15. 3 and 14
16. exp animals/ not humans.sh.
17. 15 not 16
18. randomized controlled trial.pt.
19. controlled clinical trial.pt.
20. randomized.ab.
21. placebo.ab.
22. drug therapy.fs.
23. randomly.ab.
24. trial.ab.
25. groups.ab.
26. 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25
27. exp animals/ not humans.sh.
28. 26 not 27
29. 17 and 28
Embase Ovid
1. Aorta Valve/
2. (aortic valve* adj3 (operation* or replace* or surgery)).tw.
3. 1 or 2
4. Minimally Invasive Surgery/
5. Robotics/
6. Thoracoscopy/
7. Sternotomy/
8. minim* invasiv*.tw.
9. ((surgical or surgery or surgeries or replacement* or operation*) adj3 minim*).tw.
10. ((surgery or surgeries or surgical) adj3 (keyhole or percutaneous or robot‐assisted)).tw.
11. thoracoscop*.tw.
12. pleuroscop*.tw.
13. ("hemi‐sternotomy" or "mini‐sternotomy").tw.
14. or/4‐13
15. 3 and 14
16. random$.tw.
17. factorial$.tw.
18. crossover$.tw.
19. cross over$.tw.
20. cross‐over$.tw.
21. placebo$.tw.
22. (doubl$ adj blind$).tw.
23. (singl$ adj blind$).tw.
24. assign$.tw.
25. allocat$.tw.
26. volunteer$.tw.
27. crossover procedure/
28. double blind procedure/
29. randomized controlled trial/
30. single blind procedure/
31. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30
32. (animal/ or nonhuman/) not human/
33. 31 not 32
34. 15 and 33
Clinicaltrials.gov
"aortic valve" AND ("minimally invasive" OR "hemi‐sternotomy" OR "mini‐sternotomy")
WHO International Clinical Trials Registry Platform
"aortic valve" AND ("minimally invasive" OR "hemi‐sternotomy" OR "mini‐sternotomy")
Data and analyses
Comparison 1. Limited versus full sternotomy for aortic valve replacement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mortality | 10 | 985 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.94] |
| 1.2 Cardiopulmonary bypass time (minutes) | 10 | 1043 | Mean Difference (IV, Random, 95% CI) | 10.63 [3.39, 17.88] |
| 1.3 Aortic cross‐clamp time (minutes) | 12 | 1235 | Mean Difference (IV, Random, 95% CI) | 6.07 [0.79, 11.35] |
| 1.4 Length of hospital stay (days) | 11 | 1141 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.90, ‐0.28] |
| 1.5 Postoperative blood loss (mL) | 8 | 767 | Mean Difference (IV, Random, 95% CI) | ‐153.04 [‐245.96, ‐60.12] |
| 1.6 Deep sternal wound infection | 8 | 868 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.32, 1.76] |
| 1.7 Pain scores | 5 | 649 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.43, 0.04] |
| 1.8 Quality of life | 4 | 624 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [0.00, 0.06] |
| 1.9 Index admission costs | 2 | 492 | Mean Difference (IV, Fixed, 95% CI) | 1.19 [0.42, 1.97] |
| 1.10 Intensive care unit stay (days) | 9 | 1023 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.58, 0.15] |
| 1.11 Postoperative pulmonary function tests (% FEV1) | 5 | 297 | Mean Difference (IV, Fixed, 95% CI) | 2.08 [0.74, 3.41] |
| 1.12 Re‐exploration | 13 | 1355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.50, 1.33] |
| 1.13 Postoperative atrial fibrillation | 9 | 1012 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.84, 1.51] |
| 1.14 Postoperative ventilation time (hours) | 9 | 989 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐2.30, 1.24] |
1.9. Analysis.

Comparison 1: Limited versus full sternotomy for aortic valve replacement, Outcome 9: Index admission costs
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aris 1999a.
| Study characteristics | ||
| Methods |
Study design: prospective randomised controlled study Duration: 4 months No. of centres: single Location: Spain Setting: cardiac surgical centre Withdrawals: none Dates: not stated |
|
| Participants | 40 consecutive participants undergoing first‐time elective isolated aortic valve replacement Exclusion criteria: none Demographics[limited / full sternotomy] Number of participants: 40 [20 / 20] Mean age (± SD) (range): 64 ± 11 years (26 to 76 years) Gender: not stated Pathophysiology: 31 AS, 9 AR Severity of disease: not stated Mean risk score: [11.6 ± 5.0 / 11.4 ± 5.5] Mean left ventricular ejection fraction: [62.3 ± 11 / 64.9 ± 13] Diabetes mellitus: not stated Preoperative lung function % predicted FEV1: [79 ± 14 / 81 ± 21] Preoperative lung function % predicted FVC: [79 ± 14 / 80 ± 20] Smoking status: not stated |
|
| Interventions |
Limited sternotomy: reversed L‐ or reversed J‐shaped mini‐sternotomy Modifications from full sternotomy: none stated |
|
| Outcomes |
Primary outcomes: cross‐clamp and pump times, time to extubation, chest drainage (24 hours), number of blood transfusions, ICU stay, and total postoperative length of stay Secondary outcomes: pain scores (daily) and cosmetic evaluation (discharge) Other reported outcomes: none |
|
| Standard care | Standard care was aortic and right atrial cannulation, aprotinin, antegrade cold blood cardioplegia (through coronary ostia), and no left ventricular vent. Mechanical prostheses in most participants. No transoesophageal echocardiography | |
| Notes | No funding or conflict of interests declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Envelope opened at time of surgery |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Low risk from non‐blinding (objective measures) | Low risk | Mortality, blood loss, deep sternal wound infection, re‐exploration, and postoperative atrial fibrillation rates were unlikely to be affected by absence of blinding. |
| Blinding of outcome assessment (detection bias) At risk from non‐blinding (subjective measures) | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Relevant outcome measures reported |
| Other bias | Unclear risk | Limited description of preoperative participant demographics |
Bonacchi 2002.
| Study characteristics | ||
| Methods |
Study design: prospective randomised controlled study Duration: 2 years No. of centres: single Location: Italy Setting: cardiac surgery centre Withdrawals: none Dates: January 1999 to July 2001 |
|
| Participants | 80 consecutive participants with aortic valve pathology undergoing elective aortic valve replacement. Exclusion criteria: emergent surgery, concomitant coronary revascularisation, left ventricular ejection fraction < 25% or heavily calcified aorta Demographics[limited / full sternotomy] Number of participants: 80 [40/40] Mean age (± SD): [62.6 ± 9.5 years / 64 ± 12.4 years] Gender: not stated Pathophysiology (AS:AR:mixed): [12:8:20 / 10:7:23] Severity of disease (NYHA status): [2.7 ± 0.9 / 2.5 ± 0.7] Mean risk score: not stated Mean left ventricular ejection fraction: [57 ± 12 / 56 ± 13] Diabetes mellitus: not stated Preoperative lung function: not stated Smoking status: not stated |
|
| Interventions |
Limited sternotomy: reversed C‐ or reversed L‐shaped sternal incision with < 10 cm skin incision Modifications from full sternotomy: none stated |
|
| Outcomes |
Primary outcomes: not stated Secondary outcomes: not stated Other reported outcomes: in‐hospital death, re‐exploration for bleeding, mean mediastinal drainage or bleeding > 800 mL, blood transfusion, atrial fibrillation, atelectasis, respiratory insufficiency, sternal wound infection, sternal instability, mechanical ventilation time, oxygen requirements (pre‐ and postextubation), pain scores (1 and 12 hours), analgesia requirements, ICU stay, hospital stay, spirometry (5 days and 1 to 2 months) (follow‐up time in parentheses) |
|
| Standard care | Standard care was normothermic CPB and aortic cross‐clamping with aortic and right atrial 2‐stage venous cannulation. Retrograde and ostial antegrade cold blood cardioplegia were given. A right superior pulmonary vent was used in all cases. Transverse or oblique aortotomies were utilised depending on valve choice rather than surgical approach. Transoesophageal echocardiography was employed in all cases | |
| Notes | No funding or conflict of interests declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) Low risk from non‐blinding (objective measures) | Low risk | Mortality, blood loss, deep sternal wound infection, re‐exploration, and postoperative atrial fibrillation rates are unlikely to be affected by absence of blinding. |
| Blinding of outcome assessment (detection bias) At risk from non‐blinding (subjective measures) | Low risk | Participants and staff blinded to surgical incision |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants reported on |
| Selective reporting (reporting bias) | Low risk | Relevant outcome measures reported |
| Other bias | Unclear risk | Limited description of preoperative participant characteristics |
Borger 2015.
| Study characteristics | ||
| Methods |
Study design: prospective randomised controlled study Duration: 9 months No. of centres: 5 centres Location: Germany Setting: cardiac surgical centres Withdrawals: 6 (5 in minimally invasive group, 1 in full sternotomy group) Dates: May 2012 to February 2013 |
|
| Participants | 100 participants with AS in 5 German centres Inclusion criteria: logistic EuroSCORE < 20, NYHA ≥ 2 Exclusion criteria: pure AR, previous cardiac surgery, congenital true bicuspid valve (Sievers type 0), emergency surgery, left ventricular ejection fraction < 25%, recent myocardial infarction (≤ 90 days), or stroke or TIA ≤ 6 months Demographics[limited / full sternotomy] Number of participants: 100 randomised [46 / 48]; 6 dropouts: 1 randomised to full sternotomy withdrew; 5 randomised to minimally invasive surgery were unable to have the procedure Mean age (± SD): [73.0 ± 5.3 years / 74.2 ± 5.0 years] Male gender: [27 (58.7%) / 21 (43.7%)] Pathophysiology: AS with or without aortic insufficiency Severity of disease (NYHA ≥ III): [31 (67.4%) / 29 (60.4%)] Mean STS risk score: [1.6 ± 0.7 / 1.7 ± 0.6] Mean left ventricular ejection fraction: not stated Diabetes mellitus: [15 (32.6%) / 11 (22.9%)] Preoperative COPD: [6 (13.0%) / 7 (14.9%)] Smoking status: [22 (47.8%) / 12 (25.5%)] |
|
| Interventions |
Limited sternotomy: upper hemi‐sternotomy into third or fourth intercostal space Modifications from full sternotomy: percutaneous femoral venous cannulation if right atrial cannulation not possible. Use of rapid deployment aortic valve prosthesis ‐ Edwards Intuity valve (a stented, trileaflet bovine pericardial bioprosthesis with a balloon‐expandable cloth covered skirt frame) |
|
| Outcomes |
Primary outcomes: cross‐clamp and CPB time Secondary outcomes: haemodynamic performance, quality of life (EQ‐5D), NYHA class Safety outcomes: cardiac reoperation, thromboembolism, renal failure, paravalvular leak, permanent pacemaker insertion, resternotomy, major bleeding events, endocarditis, myocardial infarction, deep sternal wound infection, cerebrovascular accident, respiratory failure |
|
| Standard care | Standard care was full sternotomy with ascending aortic and right atrial cannulation. Normothermic or mild hypothermic CPB with antegrade crystalloid, cold or warm blood cardioplegia was given. Transverse aortotomies were employed in all cases. CO2 field flooding was used. In all full‐sternotomy participants, the valve choices were conventional stented valves | |
| Notes | Disclosure: sponsored by Edwards Lifesciences LLC. Manuscript facilitated by Edwards Lifesciences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) Low risk from non‐blinding (objective measures) | Low risk | Mortality, blood loss, deep sternal wound infection, re‐exploration, and postoperative atrial fibrillation rates were unlikely to be affected by absence of blinding. |
| Blinding of outcome assessment (detection bias) At risk from non‐blinding (subjective measures) | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: "three [patients] who were randomized to MIS‐RADVR [minimally invasive surgical rapid‐deployment aortic valve replacement] eventually received a conventional valve because of problems with their anatomy". Comment: these participants appeared to have been excluded following randomisation and an intention‐to‐treat analysis may have identified difficulty with the minimally invasive approach. |
| Selective reporting (reporting bias) | Low risk | Relevant outcome measures reported. 4 secondary outcome measures described in pretrial protocol were not described in the final study publication, but these were not considered clinically important measures. |
| Other bias | High risk | Significant confounder as mini‐sternotomy utilised rapid‐deployment valve and full‐sternotomy employed standard surgical valves. (Study funded by manufacturer although this was not reason for downgrading) |
Calderon 2009.
| Study characteristics | ||
| Methods |
Study design: prospective randomised controlled study Duration: 4 years No. of centres: single Location: France Setting: university hospital Withdrawals: 1 from full sternotomy group Dates: 2003 to 2007 |
|
| Participants | 78 participants undergoing aortic valve replacement for stenotic, regurgitant, or mixed aortic valve disease by a single surgeon Inclusion criteria: adults, ASA grade ≤ 3, informed consent, left ventricular ejection fraction > 40% Exclusion criteria: redo, combined surgery, ASA ≥ 4, acute pulmonary oedema, COPD, endocarditis, chronic renal failure, antiplatelet use < 7 days before surgery, haemostatic abnormality Demographics[limited / full sternotomy] Number of participants: 78 randomised [38 / 39] Mean age (± SD): [70.9 ± 11.4 years / 70.8 ± 10.2 years] Male gender: [23 (60.5%) / 27 (69.2%)] Pathophysiology: 75% AS, 24% AR, 1% mixed Severity of disease: not stated Mean risk score: [5.4 ± 1.9 / 5.2 ± 1.8] Left ventricular ejection fraction > 50%: [36 (94.7%) / 34 (87.2%)] Diabetes mellitus: not stated Preoperative % predicted FEV1: [73.9 ± 18.2 / 78.8 ± 21] Preoperative % predicted FVC: [81.1 ± 16.1 / 83.6 ± 19.4] Smoking status: not stated |
|
| Interventions |
Limited sternotomy: minimal sternotomy access via 6 to 10 cm mid‐line skin incision and reversed L sternal incision Modifications from full sternotomy: none |
|
| Outcomes |
Primary outcomes: respiratory parameters Secondary outcomes: bleeding, transfusion, and pain status Other reported outcomes: intraoperative and postoperative blood loss, transfusion rates, CPB and cross‐clamp times, operation time, mechanical ventilation time, ICU stay, hospital stay, systemic inflammatory response syndrome, re‐exploration for bleeding, death, spirometry (1, 2, and 7 days), pain scores, cardiac output studies |
|
| Standard care | Standard care included routine anaesthesia, aprotinin prophylaxis, right atrial appendage and ascending aortic cannulation, and Bretschneider's cardioplegia solution. Aortic root vent only was employed. | |
| Notes | Funding: French Ministry of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 computer‐generated 6‐per‐block randomisation, designed by a statistician. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Low risk from non‐blinding (objective measures) | Low risk | Mortality, blood loss, deep sternal wound infection, re‐exploration, and postoperative atrial fibrillation rates are unlikely to be affected by absence of blinding. |
| Blinding of outcome assessment (detection bias) At risk from non‐blinding (subjective measures) | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants reported |
| Selective reporting (reporting bias) | Low risk | All relevant outcome measures reported |
| Other bias | Unclear risk | Limited description of preoperative participant characteristics |
Dalén 2018.
| Study characteristics | ||
| Methods |
Study design: prospective randomised controlled study Duration: 2 years No. of centres: single Location: Sweden Setting: university hospital Withdrawals: 3 ‐ 1 conversion from minimally invasive to open sternotomy; 2 deaths in sternotomy group Dates: 2013 to 2015 |
|
| Participants | 40 participants undergoing aortic valve replacement for severe aortic stenosis, in sinus rhythm Inclusion criteria: adults, severe symptomatic aortic stenosis, sinus rhythm, able to provide consent Exclusion criteria: participation in other trials, LVEF < 45%, coexisting severe valvular dysfunction, previous cardiac surgery, urgent surgery Demographics[limited / full sternotomy] Number of participants: 40 randomised [20 / 20] but analysis performed as per‐protocol with one patient cross‐over intra‐operatively [19/21] Mean age (± SD): [67 ± 9.0 years / 70 ± 7.9 years] Male gender: [12 (63%) / 13 (62%)] Pathophysiology: 100% AS with 10% concomitant moderate AR Severity of disease: 100% severe Mean risk score: EuroSCORE II [1.26 ± 0.65 / 1.44 ± 0.90] Left ventricular ejection fraction > 50%: 100% Diabetes mellitus: [4 (21%) / 6 (29%)] Preoperative % predicted FEV1: not stated Preoperative % predicted FVC: not stated Smoking status: not stated |
|
| Interventions |
Limited sternotomy: minimal sternotomy access via 6 cm mid‐line skin incision and partial J sternal incision to third intercostal space. Partial pericardial incision anterior to ascending aorta, not extending over right ventricle. Pericardium closed at end of procedure. Central arterial and peripheral venous cannulation. Antegrade Custodial cardioplegia solution Modifications in full sternotomy: full length pericardial incision; pericardium not closed in full sternotomy cases; antegrade and retrograde cold blood cardioplegia |
|
| Outcomes |
Primary outcomes: TAPSE; RV pulsed‐wave tissue Doppler velocity; RV fractional area change; basal and mid‐RV transversal diameters (all on day 4 postsurgery) Secondary outcomes: none stated Other reported outcomes: invasive ventilation time, respiratory insufficiency, pneumonia, new‐onset atrial fibrillation, stroke, TIA, postoperative bleeding, re‐operation for bleeding, pericardiocentesis, postoperative dialysis, packed red blood cell transfusion, re‐operation for paravalvular leak, de novo pacemaker insertion, ICU stay, total hospital stay |
|
| Standard care | Full sternotomy, with complete pericardial incision left open at end of procedure. Antegrade and/or retrograde cold blood cardioplegia used. | |
| Notes | 1 patient randomised to minimally invasive surgery had intraoperative conversion to sternotomy and was analysed in this group as a per‐protocol analysis rather than intention‐to‐treat. Full study protocol was never published. No funding or conflict of interests declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A blinded envelope system using sequentially numbered containers was used to randomize patients to intervention without blocking |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Low risk from non‐blinding (objective measures) | Low risk | Some outcomes low risk of bias even without blinding |
| Blinding of outcome assessment (detection bias) At risk from non‐blinding (subjective measures) | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Protocol not published: all outcomes may not have been published but expected outcomes are presented |
| Selective reporting (reporting bias) | High risk | Some data presented ambiguous: e.g. which arm deceased patients belonged to |
| Other bias | High risk | Per‐protocol analysis |
Dogan 2003.
| Study characteristics | ||
| Methods |
Study design: prospective randomised controlled study Duration: not stated No. of centres: single Location: Germany Setting: university hospital Withdrawals: none Dates: not stated |
|
| Participants | 40 consecutive participants scheduled for elective aortic valve replacement Exclusion criteria: stentless valves or pulmonary autograft, carotid stenosis > 50%, severe ascending aortic calcification, history of TIA or stroke, Alzheimer's or Parkinson's disease Demographics[limited / full sternotomy] Number of participants: 40 [20 / 20] Mean age (± SD): [65.7 ± 1.9 years / 64.3 ± 2.9 years] Male gender: [9 (45%) / 11 (55%)] Pathophysiology (AS:AR:mixed): [8:3:9 / 6:1:13] Severity of disease mean gradient: [57 ± 14 / 63 ± 15] Mean risk score: not stated Mean left ventricular ejection fraction: [64 ± 3 / 65 ± 2] Diabetes mellitus: [4(20%) / 3(15%)] Preoperative FEV1: [2.3 ± 0.9 / 2.6 ± 0.8] Preoperative FVC: [3.0 ± 1.0 / 3.2 ± 1.0] Smoking status: not stated |
|
| Interventions |
Limited sternotomy: limited median skin incision (7 to 9 cm) and reversed L‐shaped upper partial sternotomy into fourth or fifth right intercostal space Modifications from full sternotomy: the venting and cardioplegia strategies in the minimally invasive cases were different. Different surgeons performed minimally invasive and full‐sternotomy operations. |
|
| Outcomes |
Primary outcomes: operative time, CPB and cross‐clamp time, postoperative ventilation, 24‐hour chest tube drainage, ICU stay, and hospital stay Secondary outcomes: spirometry (postoperative day 6 or 7), pain scores (days 2 to 3 and 6 to 7), neuropsychological and biochemical tests Other reported outcomes: none (follow‐up time in parentheses) |
|
| Standard care | Standard care was propofol anaesthesia, ascending aorta and right atrial cannulation, apical left ventricular vent, antegrade and retrograde cold blood cardioplegia. Right temporary pacing wires | |
| Notes | No conflict of interest or funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Low risk from non‐blinding (objective measures) | Low risk | Mortality, blood loss, deep sternal wound infection, re‐exploration, and postoperative atrial fibrillation rates are unlikely to be affected by absence of blinding. |
| Blinding of outcome assessment (detection bias) At risk from non‐blinding (subjective measures) | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All relevant outcome measures reported |
| Other bias | Unclear risk | Some confounding aspects of surgical techniques differing between 2 groups (vent and cardioplegia techniques) |
Gofus 2020.
| Study characteristics | ||
| Methods |
Study design: prospective randomised controlled study No. of centres: single Location: Czech Republic Setting: university hospital Withdrawals: none Dates: May 2017 to September 2019 |
|
| Participants | 40 consecutive participants scheduled for elective biological aortic valve replacement suitable for either upper hemi‐sternotomy or median sternotomy based on chest x‐ray and aortography Exclusion criteria: redo surgery or concomitant surgery Demographics[limited / full sternotomy] Number of participants: 40 [20 / 20] Mean age (± SD): not specified Male gender: [11 (55%) / 16 (80%)] Pathophysiology (AS:AR:mixed): [15:1:5 / 14:1:5] Severity of disease mean gradient: not specified Median risk score (EuroSCORE II): [1.5 (1‐1.9) / 1.1 (0.8 ‐ 1.6)] Median left ventricular ejection fraction (IQR): [65 (61.3 ‐ 65) / 65 (60 ‐ 66.8)] Diabetes mellitus: [9(45%) / 11(55%)] Preoperative FEV1 (% predicted median (IQR): [99.5 (88.5 ‐ 110) / 80 (75.3 ‐ 91)] Preoperative FVC (% predicted median (IQR): [97 (92.3; 101.8)/ 91.5 (85; 100.8)] Ex‐Smoking status: [2 (10%) / 8 (40%)] |
|
| Interventions |
Limited sternotomy: J‐shape splitting of the sternum from the jugular notch to the level of third or fourth intercostal space with central cannula‐ tion of the ascending aorta and the superior vena cava Modifications from full sternotomy: "FS group was performed in a standard fashion" but not specified. Presumed right atrial cannulation. A stented bioprosthesis was implanted in all the patients in a supra‐annular fashion using double‐pledgeted interrupted stitches. |
|
| Outcomes |
Primary outcomes: pulmonary function and health‐related quality of life Secondary outcomes: none stated Other reported outcomes: operative times, CPB times, aortic cross‐clamp times, ventilation times, 24h blood loss, transfusions, ICU stay, hospital length of stay, revision for bleeding, infections, respiratory complications, wound complications, neurological complications, atrial fibrillation, conduction block |
|
| Standard care | Not specified | |
| Notes | No conflict of interest or funding declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator |
| Allocation concealment (selection bias) | High risk | Not specified how allocation concealment was performed. Allocation occurred 24h after randomisation by an unspecified random numbering method. Significant differences in patient groups noted: limited sternotomy group vs full sternotomy group bodyweights were 76 kg versus 91 kg (P = 0.02). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) Low risk from non‐blinding (objective measures) | Low risk | Mortality, blood loss, transfusion, infection and AF unlikely to be affected by non‐blinding |
| Blinding of outcome assessment (detection bias) At risk from non‐blinding (subjective measures) | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete specification of data |
| Selective reporting (reporting bias) | Unclear risk | No prespecified plan of reporting |
| Other bias | Unclear risk | Not registered in advance |
Hancock 2019.
| Study characteristics | ||
| Methods |
Study design: single‐centre, single‐blind, prospective, randomised, controlled superiority trial Duration: 28 months No. of centres: single Location: UK Setting: university hospital Withdrawals: 1 (randomised in error and did not receive any surgery) Dates: March 2014 to July 2016 |
|
| Participants | 270 participants scheduled for elective, isolated aortic valve replacement Exclusion criteria: concomitant cardiac procedures, redo surgery, only suitable for median sternotomy, haemoglobin < 90g/L, pregnant, in another interventional trial, previous cardiac surgery, unable to stop anticoagulant treatment, history of thrombophilia, thrombocytopaenia, or other haematological conditions that would affect participation (as assessed by any of the participating surgeons), infective endocarditis, prevented from having blood products according to a system of beliefs, any other medical, psychiatric or social reason to preclude participation Demographics[limited / full sternotomy] Number of participants: 270 [135 / 135] Mean age (± SD): [69.3 ± 9.3 years / 68.7 ± 8.4 years] Male gender: [78 (58%) / 87 (64%)] Pathophysiology (AS:AR:mixed): [(132:3:0) / (127:8:0)] Severity of disease mean gradient: not stated Mean risk score: EuroSCORE II [1.5 ± 1.1 / 1.5 ± 1.2] and Logistic EuroSCORE I [5.2 ± 3.5 / 5.1 ± 3.5] Diabetes mellitus: not stated Preoperative FEV1: [2.1 ± 0.7 / 2.2 ± 0.7] Preoperative FVC: [2.9 ± 0.9 / 2.9 ± 1.0] Smoking status: not stated |
|
| Interventions |
Limited sternotomy: limited median skin incision (5 to 7 cm) and manubrium‐limited mini‐sternotomy to 1 cm below manubrio‐sternal junction. Modifications from full sternotomy: percutaneous femoral venous cannulation instead of 2‐stage right atrio‐caval cannula |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Standard care | Patients are given lorazepam as a pre‐medication, followed by anaesthesia with propofol, fentanyl, rocuronium bromide and morphine. All patients are given a total dose of tranexamic acid (TXA) at 30 mg/kg. Where patients have a presurgical creatinine >200 mmol/L, the dose of TXA is halved to 15 mg/kg. Prior to cardiopulmonary bypass, systemic anticoagulation is achieved with heparin given at a dose that achieves an activated clotting time (ACT) of greater than 400 seconds. Fresh frozen plasma (FFP) is administered if the target ACT is not reached. During cardiopulmonary bypass, haemoglobin (Hb) is kept at 60 g/L or above. Haemofiltration followed by RBC transfusion may be required to achieve this. Following cardiopulmonary bypass (CPB), protamine administered to reverse heparin, according to the dose of heparin given. Blood products may be used intra‐operatively in the presence of excessive blood loss. Cell salvage will be used in all patients All patients have the new aortic valve assessed at the end of surgery using a transoesophageal echocardiogram (TOE) |
|
| Notes | 16 patients crossed over from limited sternotomy to conventional median sternotomy. Data analysed as intention‐to‐treat Funded by National Institute for Health and Care Research (NIHR) (United Kingdom). 1 author declared competing interests with funding from the British Heart Foundation and NIHR, and grant funding from Zimmer Biomet. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by designated and trained members of the research team using a 24‐hour, central, secure, web‐based randomization system with concealed allocation, managed by Durham Clinical Trials Unit. Eligible patients were randomised in a 1:1 ratio between the intervention under study and usual care. Randomisation stratified by baseline logistic EuroSCORE and preoperative Haemoglobin (Hb). |
| Allocation concealment (selection bias) | Low risk | Web‐based randomisation with concealed allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All patients were blinded to the type of sternotomy they received until after they completed their day 2 quality of life and pain assessments. All patients had trial‐specific opaque dressings applied to their sternal wound, and to their groin. Clinical teams were informed of surgical allocation. |
| Blinding of outcome assessment (detection bias) Low risk from non‐blinding (objective measures) | Low risk | Blinding used for some outcomes up to 2 days |
| Blinding of outcome assessment (detection bias) At risk from non‐blinding (subjective measures) | Low risk | Other outcomes unlikely to be influenced by blinding (e.g. bleeding) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants reported |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Low risk | No other risks identified |
Moustafa 2007.
| Study characteristics | ||
| Methods |
Study design: prospective randomised controlled study Duration: not stated No. of centres: single Location: Egypt Setting: university hospital Withdrawals: none Dates: not stated |
|
| Participants | 60 consecutive participants undergoing first‐time elective aortic valve replacement for either AS or AR Exclusion criteria: emergency surgery, left ventricular ejection fraction < 25%, heavily calcified ascending aorta, redo valve surgery, other associated valve lesions Demographics[limited / full sternotomy] Number of participants: 60 [30 / 30] Mean age (± SD): [22.9 ± 2.4 / 23.8 ± 3.5] Male gender: [16 / 15] Pathophysiology (AS:AR): [15:15 / 15:15] Severity of disease: not stated Mean risk score: not stated Mean left ventricular ejection fraction: [56 ± 2.3 / 55 ± 2.6] Diabetes mellitus: not stated Preoperative lung function: not stated Smoking status: not stated |
|
| Interventions |
Limited sternotomy: reversed L‐shaped mini‐sternotomy to the third intercostal space Other modifications from full sternotomy: venous drainage not specified in methods but noted to be different for mini‐sternotomy group |
|
| Outcomes |
Primary outcomes: not stated Secondary outcomes: not stated Other reported outcomes: pulmonary function tests (1 week and 1 month post), length of incision, operating time, CPB time, ventilation time, chest drainage at 24 hours, blood transfusions, ICU stay, total hospital stay, participant survey of cosmetic effect, analgesia use (follow‐up time in parentheses) |
|
| Standard care | Standard care was aortic and right atrial cannulation, coronary ostial and root antegrade cold blood cardioplegia, main pulmonary artery or left atrial appendage venting. All participants received a St Jude Medical mechanical bileaflet prosthesis | |
| Notes | No funding or conflict of interests declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Closed envelope method |
| Allocation concealment (selection bias) | Low risk | Closed envelope method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Low risk from non‐blinding (objective measures) | Low risk | Mortality, blood loss, deep sternal wound infection, re‐exploration, and postoperative atrial fibrillation rates are unlikely to be affected by absence of blinding. |
| Blinding of outcome assessment (detection bias) At risk from non‐blinding (subjective measures) | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All cited and relevant outcome measures reported |
| Other bias | Low risk | No other risks identified |
Mächler 1999.
| Study characteristics | ||
| Methods |
Study design: prospective randomised controlled study Duration: 18 months No. of centres: single Location: Austria Setting: university hospital Withdrawals: none Dates: July 1996 to December 1997 |
|
| Participants | 120 adults requiring aortic valve procedures Exclusion criteria: acute endocarditis, concomitant procedures, reoperation Demographics[limited / full sternotomy] Number of participants: 120 [60 / 60] Median age (IQR): [65 (56 to 70) years / 65 (55 to 72) years] Male gender: [35 / 36] Pathophysiology (AS:AR): [55:5 / 54:6] Severity of disease AVA (IQR): [0.6 (0.5 to 0.7) / 0.6 (0.5 to 0.8)] Mean risk score: not stated Median left ventricular ejection fraction (IQR): [67 (60 to 71) / 63 (48 to 70)] Diabetes mellitus: not stated Preoperative lung function: not stated Smoking status: not stated |
|
| Interventions |
Limited sternotomy: mid‐line 8 to 10 cm incision, L‐shaped sternotomy to third or fourth right intercostal space Other modifications from full sternotomy: none |
|
| Outcomes |
Primary outcomes: not stated Secondary outcomes: not stated Other reported outcomes: cross‐clamp time, CPB time, operation time, postoperative ejection fraction, duration of ventilation, chest tube drainage at 24 hour, reoperation requirements, pericardial effusions, conversion to full sternotomy, arrhythmias, strokes, wound infection, sternal instability, sternal pain |
|
| Standard care | Standard care was isoflurane anaesthesia with bolus fentanyl, ascending and right atrial cannulation, 30 to 32 °C hypothermia on CPB, right superior pulmonary vein or pulmonary artery venting, ostial antegrade St. Thomas' cardioplegia and transvenous pacing wires if required only | |
| Notes | Only the first 10 participants had echocardiography No funding or conflict of interests declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignation to surgeons, but no clear randomisation |
| Allocation concealment (selection bias) | Unclear risk | Concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) Low risk from non‐blinding (objective measures) | Low risk | Mortality, blood loss, deep sternal wound infection, re‐exploration, and postoperative atrial fibrillation rates are unlikely to be affected by absence of blinding. |
| Blinding of outcome assessment (detection bias) At risk from non‐blinding (subjective measures) | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants reported |
| Selective reporting (reporting bias) | Low risk | All relevant outcome measures reported |
| Other bias | Low risk | No other risks identified |
Nair 2018.
| Study characteristics | ||
| Methods |
Study design: prospective, pragmatic, open‐label 1:1 randomised controlled study Duration: 5 years No. of centres: 2 Location: UK Setting: cardiac surgical centres Withdrawals: 9 following randomisation (8 limited sternotomy, 1 full sternotomy); 31 at 12 months (13 limited sternotomy and 18 full sternotomy) Dates: January 2010 to April 2015 |
|
| Participants | 222 participants undergoing first‐time elective aortic valve replacement Exclusion criteria: emergency AVR, left ventricular ejection fraction ≤ 30%, chest wall deformities, severe chronic obstructive pulmonary disease (forced expiratory volume in 1 second or transfer factor of the lung for carbon monoxide < 40% of predicted), body mass index > 35 kg/m2, concomitant cardiac surgery, redo surgery, and inability to perform transoesophageal echocardiography Demographics[limited / full sternotomy] Number of participants: 222 [118 / 104] Mean age (± SD): [71.3 ± 12.3 / 72.1 ± 10.9] Male gender: [65 (55%) / 47 (45%)] Pathophysiology (AS:AR): not stated Severity of disease: not stated Mean risk score: EuroSCORE [5.9 ± 2.1 / 6.1 ± 2.1] Mean left ventricular ejection fraction: not stated Diabetes mellitus: not stated Preoperative lung function: not stated Smoking status: not stated |
|
| Interventions |
Limited sternotomy: 8 cm skin incision, upper midline hemi‐sternotomy to fourth right intercostal space Other modifications from full sternotomy: venous drainage with flat venous cannula |
|
| Outcomes |
Primary outcomes: length of postoperative hospital stay and time from surgery to patient being medically fit for discharge Secondary outcomes: duration of surgery, total theatre time, aortic cross‐clamp and cardiopulmonary bypass (CPB) times, blood loss in the first 12 hours after surgery, transfusion of blood and clotting products in the first 48 hours (the blood transfusion trigger was a haemoglobin level < 80 g/L), frequency of reintubation, time to initial extubation, mediastinal drain removal and first independent mobilisation, daily pain scores at rest and on deep breathing (over the first 10 days or until hospital discharge) on a scale of 0 to 10, left ventricular ejection fraction, severity of paraprosthetic regurgitation at hospital discharge and at 6 months, and time to all‐cause death. Non‐clinical secondary endpoints including health related quality of life and health care resource use Other reported outcomes: time to independent mobilisation, re‐operations for bleeding and tamponade, conversions for other reasons |
|
| Standard care | Antegrade cold blood cardioplegia, oblique or transverse aortotomy, semi‐continuous or mattress valve sutures (according to surgeon preferences), atrial and ventricular pacing wires | |
| Notes | Funded by the National Institute for Health and Care Research (NIHR) (United Kingdom). No conflict of interests. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation (1:1) used random permuted blocks of variable lengths (6 or 8), stratified by surgeon and valve prosthesis (bioprosthetic or mechanical) |
| Allocation concealment (selection bias) | Low risk | Random allocations were pregenerated, held in secure files by the Papworth Trials Unit. Retrieved after induction of anaesthesia in theatre. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) Low risk from non‐blinding (objective measures) | Low risk | Objective measures (e.g. bleeding, intubation time etc) unlikely to be affected by non‐blinding |
| Blinding of outcome assessment (detection bias) At risk from non‐blinding (subjective measures) | High risk | Primary outcome (time to discharge) susceptible to non‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Up to 10% missing data at 12 months (but little attrition at discharge) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes measured |
| Other bias | High risk | High rate of cross‐over in limited sternotomy group (12%) suggestive of learning curve effect. Expert surgeons may have had different results. |
Rodríguez‐Caulo 2021.
| Study characteristics | ||
| Methods |
Study design: independent (not industry supported), single‐blind (patient), single‐centre, randomised clinical trial Duration: 2 years No. of centres: single Location: Spain Setting: university hospital Withdrawals: unclear ‐ CONSORT diagram states no losses to follow‐up and analysis as intention‐to‐treat but 103 patients randomised and only 94 analysed. Appears to have had 9 lost to follow‐up, including 1 withdrawal, 3 cross‐overs) Dates: January 2010 to April 2015 |
|
| Participants | 103 participants undergoing aortic valve replacement for severe aortic stenosis (or mixed aortic valve disease with predominant stenosis) Inclusion criteria: severe aortic stenosis, NYHA ≥ 2, angina or syncope, age ≥ 18y, capacity to consent Exclusion criteria: moderate LVEF impairment, prior heart surgery, emergency surgery, infectious endocarditis, COPD greater than moderate severity, need for concomitant surgery (except Morrow) Demographics[limited / full sternotomy] Number of participants: 103 randomised, 100 analysed [50 / 50] Mean age (± SD): [66.2 ± 11.2 / 67.6 ± 7.5] Male gender: [27 (54%) / 30 (60%)] Pathophysiology (AS:AR): not stated (all had severe AS) Severity of disease: mean gradient [53.6 ± 12.4 / 53.3 ± 11.5] Mean risk score: Logistic EuroSCORE [5.2 ± 4.2 / 4.3 ± 2.1] Mean left ventricular ejection fraction: [64.2 ± 6.9 / 66.4 ± 8.1] Diabetes mellitus: [17 (34%) / 15 (30%)] COPD: [17 (34%) / 15 (30%)] |
|
| Interventions |
Limited sternotomy: partial upper hemi‐sternotomy extended into a J‐shape into the right fourth intercostal space irrespective of the skin incision (usually 10 cm in length) Other modifications from full sternotomy: single Blake drain rather than 2 Blake drains |
|
| Outcomes |
Primary outcomes: change from baseline of EQ‐5D‐5 L Index at 1, 6 and 12 months following surgery Secondary outcomes:
Other reported outcomes: time to independent mobilisation, re‐operations for bleeding and tamponade, conversions for other reasons |
|
| Standard care | Ascending aorta and right atrium cannulation for cardiopulmonary bypass. Right superior pulmonary vein vent. Antegrade cold blood cardioplegia intermittently via the aortic root or coronary ostia every 20 minutes. Transverse aortotomy, valve extraction and decalcification. 2/0 Ti‐Cron pledgeted sutures for valve and 4/0 polypropylene aortic closure. Transitional pacemaker | |
| Notes | This work was supported by grants from Spanish Cardiovascular Research Network co‐founded by Fondo Europeo de Desarrollo Regional (FEDER). No conflict of interests declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer |
| Allocation concealment (selection bias) | Low risk | Administrative officer maintaining custody of randomisation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patient blinded initially, but not for duration of study. Personnel not blinded |
| Blinding of outcome assessment (detection bias) Low risk from non‐blinding (objective measures) | Low risk | Objective measures (e.g. bleeding, intubation time) unlikely to be affected by non‐blinding |
| Blinding of outcome assessment (detection bias) At risk from non‐blinding (subjective measures) | High risk | Primary outcome susceptible to detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear from CONSORT diagram where and why incomplete reporting |
| Selective reporting (reporting bias) | Low risk | All relevent clinical outcomes reported |
| Other bias | Low risk | Appears to have been well‐designed |
Shneider 2020.
| Study characteristics | ||
| Methods |
Study design: single‐centre, prospective randomised clinical trial Duration: 32 months No. of centres: single Location: Russia Setting: Federal Centre for High Medical Technologies Withdrawals: 3 patients from limited sternotomy group crossed over; analysed as intention to treat Dates: January 2012 to December 2017 |
|
| Participants | 112 participants aged 18 to 85 years undergoing isolated aortic valve replacement Inclusion criteria: indications for isolated aortic valve replacement Exclusion criteria: aortic dimensions > 42mm or computerised tomography demonstrating unsuitable for partial upper sternotomy Demographics[limited / full sternotomy] Number of participants: 112 [56/56] Mean age (± SD): [53.1 ± 14.9 / 56.1 ± 14.3] Male gender: [24 (43%) / 25 (45%)] Pathophysiology (AS:AR): not stated Severity of disease: mean gradient [102.8 ± 25.3 / 106.2 ± 23.9] Mean risk score: Logistic EuroSCORE II [2.3 ± 0.7 / 2.6 ± 0.5] Mean left ventricular ejection fraction: [58.3 ± 5.6 / 58.5 ± 5.1] Diabetes mellitus: [10 (18%) / 12 (21%)] COPD: [15 (27%) / 6 (11%)] |
|
| Interventions |
Limited sternotomy: J‐shaped partial upper sternotomy made up to the 3rd or the 4th intercostal spaces depending on CT data; central cannulation Other modifications from full sternotomy: none stated. Del Nido cardioplegia used in both cases with On‐X mechanical and Edwards Perimount biological valves in either arm |
|
| Outcomes |
Primary outcomes: mortality and freedom from thromboembolic complications at 32 months Secondary outcomes
Other reported outcomes: blood transfusion, sternal wound infection, |
|
| Standard care | Del Nido cardioplegia and central cannulation used for both techniques. On‐X mechanical valves and Perimount stented bioprostheses for tissue valves. | |
| Notes | No funding or conflict of interests declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation technique not stated |
| Allocation concealment (selection bias) | High risk | No allocation concealment stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) Low risk from non‐blinding (objective measures) | Low risk | Some outcomes (e.g. wound infection, acute MI and stroke) not subject to bias from non‐blinding |
| Blinding of outcome assessment (detection bias) At risk from non‐blinding (subjective measures) | High risk | Some outcomes (e.g. length of hospital stay) liable to bias from non‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No protocol, flow diagrams or sample size calculations |
| Selective reporting (reporting bias) | High risk | No protocol, flow diagrams or sample size calculations |
| Other bias | Low risk | No other clear sources of bias |
Vukovic 2019.
| Study characteristics | ||
| Methods |
Study design: prospective randomised, controlled trial Duration: 22 months No. of centres: single Location: Serbia Setting: cardiovascular institute Withdrawals: not stated Dates: February 2016 to November 2017 |
|
| Participants | 100 participants undergoing aortic valve replacement Inclusion criteria: not stated Exclusion criteria: concomitant procedures, urgent surgery Demographics[limited / full sternotomy] Number of participants: 100 randomised [50 / 50] Mean age (± SD): [65 ± 8.9 / 67.8 ± 8.7] Male gender: [22 (44%) / 28 (56%)] Pathophysiology (AS:AR): not stated Severity of disease: mean gradient [65 ± 19 / 62 ± 17] Mean risk score: EuroSCORE II [1.87 ± 1.03 / 1.98 ± 1.8] Mean left ventricular ejection fraction: [53.1 ± 10.6 / 50.8 ± 11] Diabetes mellitus: [12 (24%) / 13 (26%)] COPD: not stated |
|
| Interventions |
Limited sternotomy: midline 6 to 10 cm skin incision from the second rib down to the fourth rib. A reverse J‐shaped upper mini‐sternotomy was performed from the sternal notch to the third or fourth intercostal space. Other modifications from full sternotomy: none |
|
| Outcomes |
Primary outcomes: composite of 30‐day mortality, myocardial infarction, stroke, and surgical site infection Secondary outcomes: individual components of the primary endpoint, lengths of ICU and hospital stays, and the time period to reach full physical recovery after surgery. The time point of full recovery was defined as the period needed to return to regular physical activity. Other reported outcomes: none |
|
| Standard care | Central arterial cannulation, antegrade cold blood cardioplegia, right upper pulmonary vein vent, de‐airing without lifting the heart | |
| Notes | No funding or conflict of interests declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Independent investigator generated the allocation scheme and randomisation did not influence the surgeon selection. The attending surgeon was informed about the type of treatment at the induction of anaesthesia. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) Low risk from non‐blinding (objective measures) | Low risk | Objective measures (e.g. bleeding) unlikely to have been affected by non‐blinding |
| Blinding of outcome assessment (detection bias) At risk from non‐blinding (subjective measures) | High risk | No blinding described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No unexplained loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported |
| Other bias | High risk | No a priori statistical hypothesis |
AR: aortic regurgitation; AS: aortic stenosis; ASA: American Society of Anesthesiologists; AVA: aortic valve area; AVR: aortic valve replacement; CPB: cardiopulmonary bypass; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; ICU: intensive care unit; LVEF: left ventricular ejection fraction; IQR: interquartile range; NYHA: New York Heart Association; RV: right ventricle; SD: standard deviation; TAPSE: tricuspid annular plane systolic excursion; TIA: transient ischaemic attack.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abjigtova 2021 | Not randomised |
| Aris 1999b | Not randomised |
| Bakir 2014 | Not randomised |
| Baumbach 2010 | Not randomised |
| Borrero 2020 | Not randomised controlled trial |
| Bruce 2014 | Intervention group was robotic surgery |
| Canosa 1999 | Observational study |
| Chang 1999 | Observational study |
| Christiansen 1999 | Observational study |
| Concistre 2013 | Observational study |
| Corbi 2003 | Observational study |
| Dalen 2015 | Observational study (propensity matched) |
| Detter 2002b | Observational study |
| Doll 2002 | Observational study |
| Fareed 2018 | Apparently randomised data collection not prospective randomisation of patients ‐ authors contacted for clarification and did not respond. |
| Farhat 2003 | Prospective but not randomised |
| Ferdinand 2001 | Observational study |
| Foghsgaard 2009 | Prospective but not randomised |
| Frazier 1998 | Observational study |
| Gilmanov 2013 | Observational study |
| Glauber 2013 | Observational study |
| Glower 2014 | Observational study |
| Hamano 2001 | Observational study |
| Hiraoka 2011 | Observational study |
| Johnston 2012 | Observational study (propensity matched) |
| Korach 2010 | Observational study |
| Leshnower 2006 | Observational study |
| Liu 1999b | Observational study |
| Mahesh 2011 | Observational study |
| Masiello 2002 | Observational study |
| Mihos 2013 | Observational study |
| Mikus 2013 | Observational study Redo surgeries |
| Ruttmann 2010 | Observational study |
| Sansone 2012 | Mini‐thoracotomy |
| Santarpino 2012 | Observational study |
| Sener 2001 | Mini‐thoracotomy |
| Sharony 2003 | Observational study |
| Sharony 2004 | Observational study (propensity matched) |
| Sidiropolous 1999 | Observational study |
| Stamou 2003 | Observational study (propensity matched) |
| Suenaga 2004 | Observational study |
| Svensson 1998 | Observational study |
| Vanoverbeke 2004 | Observational study |
| Walther 1999b | Observational study |
| Wheatley 2004 | Port access |
| Yon 2014 | Observational study |
| You 2012 | Observational study |
Characteristics of ongoing studies [ordered by study ID]
NCT02272621.
| Study name | Bleeding in Partial Upper Hemisternotomy Versus Full Sternotomy Aortic Valve Replacement (original title was "Surgical Trauma After Partial Upper Hemisternotomy Versus Full Sternotomy Aortic Valve Replacement") |
| Methods |
Study design: open‐label randomised controlled trial Duration: 20 months No. of centres: single Location: Sweden Setting: cardiac surgical centre Dates: April 2014 to December 2016 |
| Participants | Originally planned to recruit 40 participants scheduled for aortic valve replacement. Has since been amended to state 100 patients, 50 to each arm Inclusion criteria: aged ≥ 18 years; severe aortic stenosis defined as aortic valve area of < 1 cm2 or index area of 0.6 cm2/m2 by echocardiography; referred for medically indicated aortic valve replacement; sinus rhythm; provide written informed consent Exclusion criteria: left ventricular ejection fraction < 0.45; presence of any coexisting severe valvular disorder; previous cardiac surgery; urgent or emergent surgery |
| Interventions | Partial upper hemi‐sternotomy |
| Outcomes |
Original primary outcomes: interleukin‐6; interleukin‐8; interleukin‐10; tumour necrosis factor‐alpha. All postoperatively at 0 to 3 days Revised primary outcome: universal definition of perioperative bleeding in adult cardiac surgery within 3 days from surgery |
| Starting date | April 2015 |
| Contact information | Originally Peter Svenarud, MD, PhD +46 (0) 8 517 708 12 peter.svenarud@karolinska.se Now Magnus Dalen but no contact information on clinicaltrials.gov |
| Notes | Study closed in August 2018 with no published results. Contact made with original responsible party and current responsible clinical lead |
NCT04012060 (LIAR).
| Study name | LIAR |
| Methods | Single‐centre, single blind randomised controlled clinical trial, comparing 2 arms of 80 patients undergoing limited access surgical aortic valve replacement via J‐shaped upper hemi‐sternotomy (UHS) or conventional through median sternotomy. In all randomised patients, the diseased native aortic valve is planned to be replaced with a rapid deployment stented bioprosthesis |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Mini‐aortic valve replacement (mini‐AVR)
Conventional surgical aortic valve replacement (SAVR)
Patients participating in the prospective registry will undergo an isolated SAVR through full median sternotomy. The choice of valve to be implanted is at the surgeon's discretion. The choice of either a mechanical or biological valve will be decided in consultation with the patient. |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 13 June 2016 |
| Contact information | Idserd Klop |
| Notes | Trial complete June 2020. Results not yet published, but author contacted to acquire data for this review |
Differences between protocol and review
Changes to outcome measures
Major adverse cardiovascular and cerebrovascular outcomes was removed from our list of primary outcome measures. It had not been seen as frequently in the trials as we had anticipated. Although, as a clinical measure, it would have been useful to see the results of this reported, it became apparent that composite outcomes were subject to even greater variability in definition and were therefore unreliable. We have therefore removed it for the August 2021 search date update, along with composite multi‐organ failure.
We amended one of our secondary outcome measures from 'Blood loss and transfusion requirements' to 'Postoperative blood loss' as transfusion requirements were typically not stated, and transfusion protocols differed between studies. However, we identified three outcome measures of interest that were reported in several studies and which we proceeded to perform analysis on: intensive care unit stay, lung function tests, and re‐exploration for bleeding. Following review, we also added incidence of postoperative atrial fibrillation and ventilation time. In the updated review, we responded to our acquired knowledge of quality of life reporting in these trials, which were clustered around 6 to 12 weeks postsurgery. This made sense, rather than the longest follow‐up, as peak differences in quality of life can be clinically expected at this time.
We renamed several outcome measures according to the wording most commonly used in the included studies, in order to bring our review in line with the accepted nomenclature. Although the clinical outcomes did not change, this allowed for standardisation across our review. We changed all‐cause mortality to mortality; total hospital stay to length of hospital stay; sternal wound infection to deep sternal wound infection; intensive care unit length of stay to intensive care unit stay; postoperative lung function tests to postoperative pulmonary function tests; and costs of surgery to index admission costs.
The original review had presented all our outcomes in the summary of findings table, but in this version of the review, we followed newer Cochrane Handbook and editorial guidance more closely and limited the outcomes presented to the most clinically‐relevant ones.
Contributions of authors
BHK: guarantor of the review and conceived, designed, and co‐ordinated the review and update.
BHK, SGJ, ADM: data collection, including search strategy design, searching, screening, retrieval, appraisal, and data extraction.
BHK, SGJ: additional data from papers, unpublished data, and industry evidence.
DAC, RJNNW, SCM, ADM, EFA: review and arbitration of papers.
BHK: data entry.
All review authors: analysis and interpretation of data.
BHK, SGJ: writing the review.
DAC, RJNNW, SCM, ADM, EFA: expert advice on content of review.
Sources of support
Internal sources
-
Liverpool Heart and Chest Hospital, UK
Financial support in retrieving relevant papers
External sources
-
NIHR, UK
This project was supported by the National Institute for Health and Care Research, via Cochrane Infrastructure funding to the Heart Group until 31 March 2023. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, NIHR, NHS or the Department of Health and Social Care
Declarations of interest
BHK: recipient of an unrestricted grant from Edwards LifeSciences in 2017 to undertake a minimally invasive cardiac surgery fellowship. This was paid to the institution to cover salary only and was limited to a six‐month period with no other conditions or financial benefit. Edwards manufactures the rapid‐deployment valve that was utilised in one arm of one study (Borger 2015) but can be used in either minimally invasive or full sternotomy aortic valve replacement (i.e. either interventions in the review), and would not therefore gain financially regardless of the outcome of the review. BHK was a co‐author on an included study (Hancock 2019) and therefore had no involvement in the screening, data extraction or risk of bias assessment for this study.
SGJ: none known.
ADM: has a practice that includes minimally invasive aortic valve replacement.
SCM consults for Edwards Lifesciences, a manufacturer of equipment for minimal incision surgery. He is on the Advisory Board for Minimally Invasive Surgery, as well as being a member of the Speaker's Bureau. He is also an investigator on the PARTNER II trial, a prospective, multicentre, open‐label trial of people undergoing aortic valve surgery for severe aortic stenosis that includes transcatheter valve implantation strategies. He received personal honoraria from Edwards LifeSciences, Medtronic and Cryolife from 2019 to 2020, who manufacture valves that may have been included in the review. As a result, he was not involved in screening of titles or abstracts, did not assess full‐texts or contribute to data extraction (except for arbitration) and did not engage in assessing risk of bias or certainty of evidence assessments.
DC: has a practice that includes minimally invasive aortic valve replacement.
RJNNW: has a practice that includes minimally invasive aortic valve replacement.
EFA: has a practice that includes minimally invasive aortic valve replacement. EFA was a co‐author on an included study (Hancock 2019) and therefore had no involvement in the data extraction or risk of bias assessment for this study.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Aris 1999a {published data only}
- Aris A, Camara ML, Montiel J, Delgado LJ, Galan J, Litvan H. Ministernotomy versus median sternotomy for aortic valve replacement: a prospective, randomized study. Annals of Thoracic Surgery 1999;67:1583-7. [DOI] [PubMed] [Google Scholar]
Bonacchi 2002 {published data only}
- Bonacchi M, Prifti E, Giunti G, Frati G, Sani G. Does ministernotomy improve postoperative outcome in aortic valve operation? A prospective randomized study. Annals of Thoracic Surgery 2002;73:460-5. [DOI] [PubMed] [Google Scholar]
Borger 2015 {published data only}
- Borger MA, Dohmen PM, Knosalla C, Hammerschmidt R, Merk DR, Richter M, et al. Haemodynamic benefits of rapid deployment aortic valve replacement via a minimally invasive approach: 1-year results of a prospective multicentre randomized controlled trial. European Journal of Cardio-thoracic Surgery 2016;50(4):713-20. [DOI] [PubMed] [Google Scholar]
- Borger MA, Moustafine V, Conradi L, Knosalla C, Richter M, Merk DR, et al. A randomized multicenter trial of minimally invasive rapid deployment versus conventional full sternotomy aortic valve replacement. Annals of Thoracic Surgery 2015;99:17-25. [DOI] [PubMed] [Google Scholar]
Calderon 2009 {published data only}
- Calderon J, Richebe P, Guibaud JP, Coiffic A, Branchard O, Asselineau J, et al. Prospective randomized study of early pulmonary evaluation of patients scheduled for aortic valve surgery performed by ministernotomy or total median sternotomy. Journal of Cardiothoracic and Vascular Anesthesia 2009;23:795-801. [DOI] [PubMed] [Google Scholar]
Dalén 2018 {published data only}
- Dalén M, Da Silva CO, Sartipy U, Winter R, Franco-Cereceda A, Barimani J, et al. Comparison of right ventricular function after ministernotomy and full sternotomy aortic valve replacement: a randomized study. Interactive CardioVascular and Thoracic Surgery 2018;26:790-7. [DOI: 10.1093/icvts/ivx422] [DOI] [PubMed] [Google Scholar]
- Hashemi N, Johnsson J, Gomez A, Alam M, Winter R. The impact of right ventricular function from aortic valve replacement: a randomised study comparing minimally invasive aortic valve surgery and conventional open heart surgery. European Heart Journal Cardiovascular Imaging 2016;17(ii):130. [Google Scholar]
- Hashemi N, Johnson J, Brodin LA, Gomes-Bernardes A, Sartipy U, Svenarud P, et al. Right ventricular mechanics and contractility after aortic valve replacement surgery: a randomised study comparing minimally invasive versus conventional approach. Open Heart 2018;5:e000842. [DOI: 10.1136/openhrt-2018-000842] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dogan 2003 {published data only}
- Dogan S, Dzemali O, Wimmer-Greinecker G, Derra P, Doss M, Khan MF, et al. Minimally invasive versus conventional aortic valve replacement: a prospective randomized trial. Journal of Heart Valve Disease 2003;12:76-80. [PubMed] [Google Scholar]
Gofus 2020 {published data only}
- Gofus J, Vobornik M, Koblizek V, Smolak P, Myjavec A, Vojacek J, et al. Pulmonary function and quality of life after aortic valve replacement through ministernotomy: a prospective randomized study. Kardiologia Polska (Polish Heart Journal) 2020;78(12):1278-80. [DOI: 10.33963/KP.15668] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hancock 2019 {published and unpublished data}
- Hancock HC, Maier RH, Kasim A, Mason J, Murphy G, A Goodwin A, et al. Mini-sternotomy versus conventional sternotomy for aortic valve replacement: a randomised controlled trial. BMJ Open 2021;11(1):e041398. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock HC, Maier RH, Kasim AS, Mason JM, Murphy GJ, Goodwin AT, et al. Mini-sternotomy versus conventional sternotomy for aortic valve replacement. Journal of the American College of Cardiology 2019;73(19):2491-92. [DOI: 10.1016/j.jacc.2019.03.462] [DOI] [PubMed] [Google Scholar]
Mächler 1999 {published data only}
- Mächler HE, Bergmann P, Anelli-Monti M, Dacar D, Rehak P, Knez I, et al. Minimally invasive versus conventional aortic valve operations: a prospective study in 120 patients. Annals of Thoracic Surgery 1999;67:1001-5. [DOI] [PubMed] [Google Scholar]
Moustafa 2007 {published data only}
- Moustafa MA, Abdelsamad AA, Zakaria G, Omarah MM. Minimal vs median sternotomy for aortic valve replacement. Asian Cardiovascular Thoracic Annals 2007;15:472-5. [DOI] [PubMed] [Google Scholar]
Nair 2018 {published data only}
- Nair SK, Sudarshan CD, Thorpe BS, Singh J, Pillay T, Catarino P, et al. Mini-Stern trial: a randomized trial comparing mini-sternotomy to full median sternotomy for aortic valve replacement. Journal of Thoracic and Cardiovascular Surgery 2018;156(6):2124-32. [DOI: 10.1016/j.jtcvs.2018.05.057] [DOI] [PubMed] [Google Scholar]
Rodríguez‐Caulo 2021 {published data only}
- Rodríguez-Caulo EA, Guijarro-Contreras A, Guzón A, Otero-Forero J, Mataró MJ, Sánchez-Espín G, et al. Quality of life, satisfaction and outcomes after ministernotomy versus full sternotomy aortic valve replacement (QUALITY-AVR). Seminars in Thoracic and Cardiovascular Surgery 2021;33(2):328-334. [DOI: 10.1053/j.semtcvs.2020.07.013] [DOI] [PubMed] [Google Scholar]
Shneider 2020 {published data only}
- Shneider YA, Tsoi MD, Fomenko MS, Pavlov AA, Shilenko PA. Aortic valve replacement via J-shaped partial upper sternotomy: randomized trial, mid-term results [Effektivnost' i bezopasnost' protezirovaniya aortal'nogo klapana cherez <<mini-J>> sternotomiyu: randomizirovannoe issledovanie, sredne-otdalennye rezul'taty]. Khirurgiia (Mosk) 2020;7:25-30. [DOI: 10.17116/hirurgia202007125] [DOI] [PubMed] [Google Scholar]
Vukovic 2019 {published data only}
- Vukovic PM, Milojevic P, Stojanovic I, Micovic S, Zivkovic I, Peric M, et al. The role of ministernotomy in aortic valve surgery—a prospective randomized study. Journal of Cardiac Surgery 2019;34:435-9. [DOI: 10.1111/jocs.14053] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abjigtova 2021 {published data only}
- Abjigitova D, Veen KM, Mokhles MM, Bekkers JA, Oei FB, Bogers AJ. Initial clinical experience with minimally invasive surgical aortic valve replacement. Journal of Cardiovascular Surgery 2021;62(3):268-77. [DOI] [PubMed] [Google Scholar]
Aris 1999b {published data only}
- Aris A, Camara ML, Casan P, Litvan H. Pulmonary function following aortic valve replacement: a comparison between ministernotomy and median sternotomy. Journal of Heart Valve Disease 1999;8(6):605-8. [PubMed] [Google Scholar]
Bakir 2014 {published data only}
- Bakir I, Casselman FP, Onan B, Van Praet F, Vermeulen Y, Degrieck I. Does a minimally invasive approach increase the incidence of patient-prosthesis mismatch in aortic valve replacement? Journal of Heart Valve Disease 2014;23(2):161-7. [PubMed] [Google Scholar]
Baumbach 2010 {published data only}
- Baumbach H, Burger JH, Nagib R, Albert M, Ursulescu A, Franke U. Minimal invasive access-the beneficial impact on quality of life for patients undergoing isolated aortic valve replacement. Thoracic and Cardiovascular Surgeon 2010;58:n. pag.. [Google Scholar]
Borrero 2020 {published data only}
- Borrero A, Samboni TJ, Prado N, Carrillo-Gomez DC, Giraldo-Gonzalez GC, Florez-Elvira L, et al. J-Shaped upper mini-sternotomy versus full sternotomy for aortic valve replacement: a comparative study. Heart Surgery Forum 2020;23(4):E411-5. [DOI] [PubMed] [Google Scholar]
Bruce 2014 {published data only}
- Bruce KM, Yelland GW, Almeida AA, Smith JA, Robinson SR. Effects on cognition of conventional and robotically assisted cardiac valve operation. Annals of Thoracic Surgery 2014;97(1):48-55. [DOI] [PubMed] [Google Scholar]
Canosa 1999 {published data only}
- Canosa C, Mariani MA, Grandjean JG, Alessandrini F, Boonstra PW, De Filippo CM, et al. Aortic valve replacement via ministernotomy: early results of a two-center study. Cardiologia 1999;44(10):925-7. [PubMed] [Google Scholar]
Chang 1999 {published data only}
- Chang YS, Lin PJ, Chang CH, Chu JJ, Tan PP. "I" ministernotomy for aortic valve replacement. Annals of Thoracic Surgery 1999;68(1):40-5. [DOI] [PubMed] [Google Scholar]
Christiansen 1999 {published data only}
- Christiansen S, Stypmann J, Tjan TD, Wichter T, Van Aken H, Scheld HH, et al. Minimally-invasive versus conventional aortic valve replacement - perioperative course and mid-term results. European Journal of Cardio-thoracic Surgery 1999;16(6):647-52. [DOI] [PubMed] [Google Scholar]
Concistre 2013 {published data only}
- Concistre G, Santarpino G, Pfeiffer S, Farneti P, Miceli A, Chiaramonti F, et al. Two alternative sutureless strategies for aortic valve replacement: a two-center experience. Innovations: Technology & Techniques in Cardiothoracic & Vascular Surgery 2013;8(4):253-7. [DOI] [PubMed] [Google Scholar]
Corbi 2003 {published data only}
- Corbi P, Rahmati M, Donal E, Lanquetot H, Jayle C, Menu P, et al. Prospective comparison of minimally invasive and standard techniques for aortic valve replacement: initial experience in the first hundred patients. Journal of Cardiac Surgery 2003;18(2):133-9. [DOI] [PubMed] [Google Scholar]
Dalen 2015 {published data only}
- Dalen M, Biancari F, Rubino AS, Santarpino G, De Praetere H, Kasama K, et al. Ministernotomy versus full sternotomy aortic valve replacement with a sutureless bioprosthesis: a multicenter study. Annals of Thoracic Surgery 2015;99(2):524-30. [DOI] [PubMed] [Google Scholar]
Detter 2002b {published data only}
- Detter C, Deuse T, Boehm DH, Reichenspurner H, Reichart B. Midterm results and quality of life after minimally invasive vs. conventional aortic valve replacement. Thoracic and Cardiovascular Surgeon 2002;50(6):337-41. [DOI] [PubMed] [Google Scholar]
Doll 2002 {published data only}
- Doll N, Borger MA, Hain J, Bucerius J, Walther T, Gummert JF, et al. Minimal access aortic valve replacement: effects on morbidity and resource utilization. Annals of Thoracic Surgery 2002;74(4):S1318-22. [DOI] [PubMed] [Google Scholar]
Fareed 2018 {published and unpublished data}
- Fareed S, Bassiony A. Early outcome of mini aortic valve replacement surgery. Journal of the Egyptian Society of Cardio-thoracic Surgery 2018;26(1):1-7. [Google Scholar]
Farhat 2003 {published data only}
- Farhat F, Lu Z, Lefevre M, Montagna P, Mikaeloff P, Jegaden O. Prospective comparison between total sternotomy and ministernotomy for aortic valve replacement. Journal of Cardiac Surgery 2003;18(5):396-401; discussion 402-3. [DOI] [PubMed] [Google Scholar]
Ferdinand 2001 {published data only}
- Ferdinand FD, Sutter FP, Goldman SM. Clinical use of stentless aortic valves with standard and minimally invasive surgical techniques. Seminars in Thoracic and Cardiovascular Surgery 2001;13(3):283-90. [DOI] [PubMed] [Google Scholar]
Foghsgaard 2009 {published data only}
- Foghsgaard S, Schmidt TA, Kjaergard HK. Minimally invasive aortic valve replacement: late conversion to full sternotomy doubles operative time. Texas Heart Institute Journal 2009;36(4):293-7. [PMC free article] [PubMed] [Google Scholar]
Frazier 1998 {published data only}
- Frazier BL, Derrick MJ, Purewal SS, Sowka LR, Johna S. Minimally invasive aortic valve replacement. European Journal of Cardio-thoracic Surgery 1998;14 Suppl 1:S122-5. [DOI] [PubMed] [Google Scholar]
Gilmanov 2013 {published data only}
- Gilmanov D, Bevilacqua S, Murzi M, Cerillo AG, Gasbarri T, Kallushi E, et al. Minimally invasive and conventional aortic valve replacement: a propensity score analysis. Annals of Thoracic Surgery 2013;96(3):837-43. [DOI] [PubMed] [Google Scholar]
Glauber 2013 {published data only}
- Glauber M, Miceli A, Gilmanov D, Ferrarini M, Bevilacqua S, Farneti PA, et al. Right anterior minithoracotomy versus conventional aortic valve replacement: a propensity score matched study. Journal of Thoracic & Cardiovascular Surgery 2013;145(5):1222-6. [DOI] [PubMed] [Google Scholar]
Glower 2014 {published data only}
- Glower DD, Desai BS, Hughes GC, Milano CA, Gaca JG. Aortic valve replacement via right minithoracotomy versus median sternotomy: a propensity score analysis. Innovations: Technology & Techniques in Cardiothoracic & Vascular Surgery 2014;9(2):75-81; discussion 81. [DOI] [PubMed] [Google Scholar]
Hamano 2001 {published data only}
- Hamano K, Kawamura T, Gohra H, Katoh T, Fujimura Y, Zempo N, et al. Stress caused by minimally invasive cardiac surgery versus conventional cardiac surgery: incidence of systemic inflammatory response syndrome. World Journal of Surgery 2001;25(2):117-21. [DOI] [PubMed] [Google Scholar]
Hiraoka 2011 {published data only}
- Hiraoka A, Kuinose M, Chikazawa G, Totsugawa T, Katayama K, Yoshitaka H. Minimally invasive aortic valve replacement surgery: comparison of port-access and conventional standard approach. Circulation Journal 2011;75(7):1656-60. [DOI] [PubMed] [Google Scholar]
Johnston 2012 {published data only}
- Johnston DR, Atik FA, Rajeswaran J, Blackstone EH, Nowicki ER, Sabik JF 3rd, et al. Outcomes of less invasive J-incision approach to aortic valve surgery. Journal of Thoracic & Cardiovascular Surgery 2012;144(4):852-8.e3. [DOI] [PubMed] [Google Scholar]
Korach 2010 {published data only}
- Korach A, Shemin RJ, Hunter CT, Bao Y, Shapira OM. Minimally invasive versus conventional aortic valve replacement: a 10-year experience. Journal of Cardiovascular Surgery 2010;51(3):417-21. [PubMed] [Google Scholar]
Leshnower 2006 {published data only}
- Leshnower BG, Trace CS, Boova RS. Port-access-assisted aortic valve replacement: a comparison of minimally invasive and conventional techniques. Heart Surgery Forum 2006;9(2):E560-4; discussion E564. [DOI] [PubMed] [Google Scholar]
Liu 1999b {published data only}
- Liu J, Sidiropoulos A, Konertz W. Minimally invasive aortic valve replacement (AVR) compared to standard AVR. European Journal of Cardio-thoracic Surgery 1999;16 Suppl 2:S80-3. [PubMed] [Google Scholar]
Mahesh 2011 {published data only}
- Mahesh B, Navaratnarajah M, Mensah K, Ilsley C, Amrani M. Mini-sternotomy aortic valve replacement: is it safe and effective? Comparison with standard techniques. Journal of Heart Valve Disease 2011;20(6):650-6. [PubMed] [Google Scholar]
Masiello 2002 {published data only}
- Masiello P, Coscioni E, Panza A, Triumbari F, Preziosi G, Di Benedetto G. Surgical results of aortic valve replacement via partial upper sternotomy: comparison with median sternotomy. Cardiovascular Surgery 2002;10(4):333-8. [DOI] [PubMed] [Google Scholar]
Mihos 2013 {published data only}
- Mihos CG, Santana O, Lamas GA, Lamelas J. Incidence of postoperative atrial fibrillation in patients undergoing minimally invasive versus median sternotomy valve surgery. Journal of Thoracic & Cardiovascular Surgery 2013;146(6):1436-41. [DOI] [PubMed] [Google Scholar]
Mikus 2013 {published data only}
- Mikus E, Calvi S, Tripodi A, Lamarra M, Del Giglio M. Upper 'J' ministernotomy versus full sternotomy: an easier approach for aortic valve reoperation. Journal of Heart Valve Disease 2013;22(3):295-300. [PubMed] [Google Scholar]
Ruttmann 2010 {published data only}
- Ruttmann E, Gilhofer TS, Ulmer H, Chevtchik O, Kocher A, Schistek R, et al. Propensity score-matched analysis of aortic valve replacement by mini-thoracotomy. Journal of Heart Valve Disease 2010;19(5):606-14. [PubMed] [Google Scholar]
Sansone 2012 {published data only}
- Sansone F, Punta G, Parisi F, Dato GM, Zingarelli E, Flocco R, et al. Right minithoracotomy versus full sternotomy for the aortic valve replacement: preliminary results. Heart, Lung & Circulation 2012;21(3):169-73. [DOI] [PubMed] [Google Scholar]
Santarpino 2012 {published data only}
- Santarpino G, Pfeiffer S, Schmidt J, Concistre G, Fischlein T. Sutureless aortic valve replacement: first-year single-center experience. Annals of Thoracic Surgery 2012;94(2):504-8; discussion 508-9. [DOI] [PubMed] [Google Scholar]
Sener 2001 {published data only}
- Sener T, Gercekoglu H, Evrenkaya S, Aydin NB, Cimen S, Demirtas M, et al. Comparison of minithoracotomy with conventional sternotomy methods in valve surgery. Heart Surgery Forum 2001;4(1):26-30. [PubMed] [Google Scholar]
Sharony 2003 {published data only}
- Sharony R, Grossi EA, Saunders PC, Schwartz CF, Ribakove GH, Culliford AT, et al. Minimally invasive aortic valve surgery in the elderly: a case-control study. Circulation 2003;108 Suppl 1:II43-7. [DOI] [PubMed] [Google Scholar]
Sharony 2004 {published data only}
- Sharony R, Grossi EA, Saunders PC, Schwartz CF, Ribakove GH, Baumann FG, et al. Propensity score analysis of a six-year experience with minimally invasive isolated aortic valve replacement. Journal of Heart Valve Disease 2004;13(6):887-93. [PubMed] [Google Scholar]
Sidiropolous 1999 {published data only}
- Sidiropolous A, Liu J, Konertz W. Minimally invasive access for aortic valve replacement (AVR). Zeitschrift fur Kardiologie 1999;88(S4):30-4. [Google Scholar]
Stamou 2003 {published data only}
- Stamou SC, Kapetanakis EI, Lowery R, Jablonski KA, Frankel TL, Corso PJ. Allogeneic blood transfusion requirements after minimally invasive versus conventional aortic valve replacement: a risk-adjusted analysis. Annals of Thoracic Surgery 2003;76(4):1101-6. [DOI] [PubMed] [Google Scholar]
Suenaga 2004 {published data only}
- Suenaga E, Suda H, Katayama Y, Sato M, Fujita H, Yoshizumi K, et al. Comparison of limited and full sternotomy in aortic valve replacement. Japanese Journal of Thoracic & Cardiovascular Surgery 2004;52(6):286-91. [DOI] [PubMed] [Google Scholar]
Svensson 1998 {published data only}
- Svensson LG, D'Agostino RS. Minimal-access aortic and valvular operations, including the "J/j" incision. Annals of Thoracic Surgery 1998;66(2):431-5. [DOI] [PubMed] [Google Scholar]
Vanoverbeke 2004 {published data only}
- Vanoverbeke H, Van Belleghem Y, Francois K, Caes F, Bove T, Van Nooten G. Operative outcome of minimal access aortic valve replacement versus standard procedure. Acta Chirurgica Belgica 2004;104(4):440-4. [PubMed] [Google Scholar]
Walther 1999b {published data only}
- Walther T, Falk V, Metz S, Diegeler A, Battellini R, Autschbach R, et al. Pain and quality of life after minimally invasive versus conventional cardiac surgery. Annals of Thoracic Surgery 1999;67(6):1643-7. [DOI] [PubMed] [Google Scholar]
Wheatley 2004 {published data only}
- Wheatley GH 3rd, Prince SL, Herbert MA, Ryan WH. Port-access aortic valve surgery: a technique in evolution. Heart Surgery Forum 2004;7(6):E628-31. [DOI] [PubMed] [Google Scholar]
Yon 2014 {published data only}
- Yon LC, Totaro P, Mazzola A. Ministernotomy versus median sternotomy for isolated aortic valve replacement: is it time to define a new gold standard? Innovations: Technology and Techniques in Cardiothoracic and Vascular Surgery 2014;9(3):241-2. [Google Scholar]
You 2012 {published data only}
- You B, Gao F, Li P, Xu Y, Xu LL, Liu S, et al. Clinical study of minimally invasive versus conventional sternotomy for aortic valve replacement. Chung-Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2012;92(40):2859-61. [PubMed] [Google Scholar]
References to ongoing studies
NCT02272621 {published data only (unpublished sought but not used)}
- NCT02272621. Surgical trauma after partial upper hemisternotomy versus full sternotomy aortic valve replacement. classic.clinicaltrials.gov/ct2/history/NCT02272621?V_3=View (first received 21 October 2014).
NCT04012060 (LIAR) {published data only (unpublished sought but not used)}
- Klop ID, Putte BP, Kloppenburg GT, Sprangers MA, Nieuwkerk PT, Klein P. Comparing quality of life and postoperative pain after limited access and conventional aortic valve replacement: design and rationale of the limited access aortic valve replacement (LIAR) trial. Contemporary Clinical Trials Communications 2021;21:100700. [DOI: 10.1016/j.conctc.2021.100700] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04012060. Patient reported health-related quality of life after limited access and conventional aortic valve replacement (LIAR) [Patient reported health-related quality of life after limited access and conventional aortic valve replacement: design and rationale of the LIAR-Trial]. clinicaltrials.gov/study/NCT04012060 (first received 2 July 2019).
Additional references
Autschbach 1998
- Autschbach R, Walther T, Falk V, Diegeler A, Metz S, Mohr FW. S-shaped in comparison to L-shaped partial sternotomy for less invasive aortic valve replacement. European Journal of Cardio-thoracic Surgery 1998;14 Suppl 1:S117-21. [DOI] [PubMed] [Google Scholar]
Baumgartner 2017
- Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. European Heart Journal 2017;38(36):2739-91. [DOI: 10.1093/eurheartj/ehx391] [DOI] [PubMed] [Google Scholar]
Braunwald 2000
- Braunwald E. Aortic valve replacement: an update at the turn of the millennium. European Heart Journal 2000;21(13):1032-3. [DOI] [PubMed] [Google Scholar]
Brown 2009
- Brown ML, McKellar SH, Sundt TM, Schaff HV. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. Journal of Thoracic and Cardiovascular Surgery 2009;137(3):670-9.e5. [DOI] [PubMed] [Google Scholar]
Carabello 2013
- Carabello BA. Introduction to aortic stenosis. Circulation Research 2013;113(2):179-85. [DOI] [PubMed] [Google Scholar]
Carapetis 2005
- Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infectious Diseases 2005;5(11):685-94. [DOI] [PubMed] [Google Scholar]
Coffey 2014
- Coffey S, Cox B, Williams MJ. The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. Journal of the American College of Cardiology 2014;63(25 Pt A):2852-61. [DOI] [PubMed] [Google Scholar]
Cohn 1997
- Cohn LH, Adams DH, Couper GS, Bichell DP, Rosborough DM, Sears SP, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Annals of Surgery 1997;226(4):421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohn 1998
- Cohn LH. Minimally invasive aortic valve surgery: technical considerations and results with the parasternal approach. Journal of Cardiac Surgery 1998;13(4):302-5. [DOI] [PubMed] [Google Scholar]
Cosgrove 1996
- Cosgrove DM, Sabik JF. Minimally invasive approach for aortic valve operations. Annals of Thoracic Surgery 1996;62(2):596-7. [PubMed] [Google Scholar]
d'Arcy 2011
- d'Arcy JL, Prendergast BD, Chambers JB, Ray SG, Bridgewater B. Valvular heart disease: the next cardiac epidemic. Heart 2011;97(2):91-3. [DOI] [PubMed] [Google Scholar]
Dare 1993
- Dare AJ, Veinot JP, Edwards WD, Tazelaar HD, Schaff HV. New observations on the etiology of aortic valve disease: a surgical pathologic study of 236 cases from 1990. Human Pathology 1993;24(12):1330-8. [DOI] [PubMed] [Google Scholar]
Detter 2002a
- Detter C, Deuse T, Boehm DH, Reichenspurner H, Reichart B. Midterm results and quality of life after minimally invasive vs. conventional aortic valve replacement. Thoracic and Cardiovascular Surgeon 2002;50(6):337-41. [DOI] [PubMed] [Google Scholar]
Ehrlich 2000
- Ehrlich W, Skwara W, Klavekorn WP, Roth M, Bauer EP. Do patients want minimally invasive aortic valve replacement? European Journal of Cardio-thoracic Surgery 2000;17(6):714-7. [DOI] [PubMed] [Google Scholar]
Freeman 2005
- Freeman RV, Otto CM. Spectrum of calcific aortic valve disease pathogenesis, disease progression, and treatment strategies. Circulation 2005;111(24):3316-26. [DOI] [PubMed] [Google Scholar]
Gohlke‐Bärwolf 2013
- Gohlke-Bärwolf C, Minners J, Jander N, Gerdts E, Wachtell K, Ray S, et al. Natural history of mild and of moderate aortic stenosis. New insights from a large prospective European study. Current Problems in Cardiology 2013;38(9):365-409. [DOI: 10.1016/j.cpcardiol.2013.06.003] [DOI] [PubMed] [Google Scholar]
Hancock 2019
- Hancock HC, Maier RH, Kasim AS, Mason JM, Murphy GJ, Goodwin AT, et al. Mini-sternotomy versus conventional sternotomy for aortic valve replacement. Journal of the American College of Cardiology 2019;73(19):2491-92. [DOI: ] [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1/.
Iung 2003
- Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. European Heart Journal 2003;24(13):1231-43. [DOI] [PubMed] [Google Scholar]
Iung 2014
- Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Canadian Journal of Cardiology 2014;30(9):962-70. [DOI] [PubMed] [Google Scholar]
Khoshbin 2011
- Khoshbin E, Prayaga S, Kinsella J, Sutherland FW. Mini-sternotomy for aortic valve replacement reduces the length of stay in the cardiac intensive care unit: meta-analysis of randomised controlled trials. BMJ Open 2011;1(2):e000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2013
- Kumar RK, Tandon R. Rheumatic fever and rheumatic heart disease: the last 50 years. Indian Journal of Medical Research 2013;137(4):643-58. [PMC free article] [PubMed] [Google Scholar]
Lee 2004
- Lee BY, Gleason TG, Sonnad SS. Quality of life after aortic valve replacement. Expert Review of Pharmacoeconomics and Outcomes Research 2004;4(3):265-75. [DOI] [PubMed] [Google Scholar]
Lee 2011
- Lee R, Li S, Rankin JS, O'Brien SM, Gammie JS, Peterson ED, et al. Fifteen-year outcome trends for valve surgery in North America. Annals of Thoracic Surgery 2011;91(3):677-84. [DOI: 10.1016/j.athoracsur.2010.11.009] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lillehei 1962
- Lillehei CW, Levy MJ, Varco RL, Wang Y, Adams P, Anderson RC. Surgical treatment of congenital aortic stenosis by cardiopulmonary bypass including methods for preoperative diagnosis of types. Circulation 1962;26(5):856-72. [DOI] [PubMed] [Google Scholar]
Liu 1999a
- Liu J, Sidiropoulos A, Konertz W. Minimally invasive aortic valve replacement (AVR) compared to standard AVR. European Journal of Cardio-thoracic Surgery 1999;16(Suppl 2):S80-3. [PubMed] [Google Scholar]
Malaisrie 2014
- Malaisrie SC, Barnhart GR, Farivar RS, Mehall J, Hummel B, Rodriguez E, et al. Current era minimally invasive aortic valve replacement: techniques and practice. Journal of Thoracic and Cardiovascular Surgery 2014;147(1):6-14. [DOI] [PubMed] [Google Scholar]
Manjunath 2014
- Manjunath CN, Srinivas P, Ravindranath KS, Dhanalakshmi C. Incidence and patterns of valvular heart disease in a tertiary care high-volume cardiac center: a single center experience. Indian Heart Journal 2014;66(3):320-6. [DOI: 10.1016/j.ihj.2014.03.010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murtuza 2008
- Murtuza B, Pepper JR, Stanbridge RD, Jones C, Rao C, Darzi A, et al. Minimal access aortic valve replacement: is it worth it? Annals of Thoracic Surgery 2008;85(3):1121-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nair 2019
- Nair SK, Sudarshan CD, Thorpe BS, Singh J, Pillay T, Catarino P, et al. Mini-SternTrial: a randomized trial comparing mini-sternotomy to fullmedian sternotomy for aortic valve replacement. Journal of Thoracic and Cardiovascular Surgery 2018;156(6):2124-32. [DOI] [PubMed] [Google Scholar]
Nishimura 2017
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin 3rd JP, Fleisher LA, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2017;135(25):e1159-e1195. [DOI: 10.1161/CIR.0000000000000503] [DOI] [PubMed] [Google Scholar]
Nkomo 2006
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368(9540):1005-11. [DOI] [PubMed] [Google Scholar]
Otto 1997
- Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, et al. Prospective study of asymptomatic valvular aortic stenosis clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997;95(9):2262-70. [DOI] [PubMed] [Google Scholar]
Pellikka 2005
- Pellikka PA, Sarano ME, Nishimura RA, Malouf JF, Bailey KR, Scott CG, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005;111(24):3290-5. [DOI] [PubMed] [Google Scholar]
Phan 2014
- Phan K, Xie A, Di EM, Yan TD. A meta-analysis of minimally invasive versus conventional sternotomy for aortic valve replacement. Annals of Thoracic Surgery 2014;98(4):1499-511. [DOI] [PubMed] [Google Scholar]
Rao 1993
- Rao PN, Kumar AS. Aortic valve replacement through right thoracotomy. Texas Heart Institute Journal 1993;20(4):307-8. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rose 1986
- Rose AG. Etiology of acquired valvular heart disease in adults. A survey of 18,132 autopsies and 100 consecutive valve-replacement operations. Archives of Pathology & Laboratory Medicine 1986;110(5):385-8. [PubMed] [Google Scholar]
Ross 1968
- Ross J, Braunwald E. Aortic stenosis. Circulation 1968;38(1 Suppl):61-7. [DOI] [PubMed] [Google Scholar]
Scheuble 2005
- Scheuble A, Vahanian A. Aortic insufficiency. American Journal of Cardiovascular Drugs 2005;5(2):113-20. [DOI] [PubMed] [Google Scholar]
Schwarz 1982
- Schwarz F, Baumann P, Manthey J, Hoffmann M, Schuler G, Mehmel HC, et al. The effect of aortic valve replacement on survival. Circulation 1982;66(5):1105-10. [DOI] [PubMed] [Google Scholar]
Svensson 2013
- Svensson LG, Adams DH, Bonow RO, Kouchoukos NT, Miller DC, O'Gara PT, et al. Aortic valve and ascending aorta guidelines for management and quality measures: executive summary. Annals of Thoracic Surgery 2013;95(4):1491-505. [DOI] [PubMed] [Google Scholar]
Thaden 2014
- Thaden JJ, Nkomo VT, Enriquez-Sarano M. The global burden of aortic stenosis. Progress in Cardiovascular Diseases 2014;56(6):565-71. [DOI] [PubMed] [Google Scholar]
Walther 1999a
- Walther T, Falk V, Metz S, Diegeler A, Battellini R, Autschbach R, et al. Pain and quality of life after minimally invasive versus conventional cardiac surgery. Annals of Thoracic Surgery 1999;67(6):1643-7. [DOI] [PubMed] [Google Scholar]
Walther 2006
- Walther T, Falk V, Mohr FW. Minimally invasive surgery for valve disease. Current Problems in Cardiology 2006;31(6):399-437. [DOI: 10.1016/j.cpcardiol.2006.02.002] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kirmani 2015
- Kirmani BH, Jones SG, Chung DA, Williams RJ, Malaisrie SC. Limited versus full sternotomy for aortic valve replacement. Cochrane Database of Systematic Reviews 2015, Issue 8. Art. No: CD011793. [DOI: 10.1002/14651858.CD011793] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirmani 2017
- Kirmani BH, Jones SG, Malaisrie SC, Chung DA, Williams RJ. Limited versus full sternotomy for aortic valve replacement. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD011793. [DOI: 10.1002/14651858.CD011793.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


