Abstract
Although proton pump inhibitors (PPIs) are widely used in the treatment of various acid-related disorders, observational studies have raised concern about an association between PPI use and the risk of gastrointestinal cancer. The present study aimed to investigate the association between them using a meta-analysis of cohort studies. PubMed and Excerpta Medica dataBASE were searched from inception to December 2022 to identify relevant cohort studies. The primary outcome was the risk of gastrointestinal cancer among PPI users, expressed as a pooled odds ratio (OR), relative risk (RR) or hazard ratio (HR) and its 95% CI based on a random-effects model. A total of 25 cohort studies from 23 articles were included in the final analysis. In the meta-analysis of all studies, an increased risk of gastrointestinal cancer following the use of PPIs was observed (OR/RR/HR, 2.09; 95% CI, 1.78–2.46). Subgroup analyses by type of cancer also revealed an association between PPI use and the risk of esophageal, gastric, liver and pancreatic cancer, whereas there was no association for colorectal cancer. The increased risk of gastrointestinal cancer was also observed in individuals who had used PPIs for <1 year (OR/RR/HR, 5.23; 95% CI, 2.96–9.24) as well as individuals who had used PPIs for up to 3 years. The present meta-analysis revealed that the use of PPIs was associated with an increased risk of gastrointestinal cancer.
Keywords: proton pump inhibitors, gastrointestinal cancer, cohort study, meta-analysis, systematic review
Introduction
Since the first approval of proton pump inhibitors (PPIs) in 1989, they have proven to be an effective first-line treatment for gastrointestinal disorders including symptomatic peptic ulcer disease, gastresophageal reflux disease, and Zollinger-Ellison syndrome, as well as for the prevention of gastrointestinal bleeding in patients receiving antiplatelet therapy (1–7). PPIs are also one of the standard treatments for Helicobacter pylori infection, along with antibiotics (1). Their popularity has steadily grown, and they are now one of the most prescribed drug classes worldwide, both in the outpatient and inpatient clinical settings (8). In the United States, the consumption of PPIs in non-hospitalized patients doubled from 1999 to 2012 (9). In England, over 50 million prescriptions that contained PPIs were prescribed in 2015 (10). Due to the effectiveness of PPIs in prevention and treatment of gastrointestinal disorders, physicians tend to prescribe PPIs in the long term for specific conditions such as Barrett's esophagus, chronic use of non-steroidal anti-inflammatory drugs (NSAIDs) with high to moderate bleeding risk, severe oesophagitis, and Zollinger-Ellison syndrome, and patients usually take these medications for longer than needed (11–14). In England, amongst new users of PPIs in a cohort study from 1990 to 2014, 26.7% of them continued taking PPIs for more than one year.15 Sixty percent of the long-term PPI users did not make an attempt to step down or discontinue PPI therapy (15), and approximately 30% of the PPI users were not appropriately prescribed for the long-term treatment (16,17).
The long-term use of PPIs has raised concerns about infection (18,19), dementia (20), osteoporosis, fracture (21), and cancer (22). Specifically, the risk of gastrointestinal cancers has been a major concern to both patients and physicians. Previous laboratory and animal studies have reported that PPIs can suppress gastric acid secretion and interfere with bacterial growth and nitrosamine formation (23,24). Furthermore, PPIs have been linked to hypergastrinemia, which has been identified as a possible risk factor for cancer progression (25,26).
Meanwhile, observational epidemiological studies have reported inconsistent findings on whether PPIs increase the risk of gastrointestinal cancers (27–49). Fourteen cohort studies reported a significant increased risk of gastrointestinal cancers by the use of PPIs (29,30,32–37,40,42–45,47), while 10 cohort studies did no association between them (27,28,31,38,39,41,46,48,49).
Several meta-analyses of retrospective cohort studies and case-control studies have reported the associations between the use of PPIs and a specific type of gastrointestinal cancers such as gastric cancer, colorectal cancer, and pancreatic cancer (50–54). However, no comprehensive meta-analysis of cohort studies for all types of gastrointestinal cancers including esophageal cancer, liver cancer, and biliary cancer has been reported up to date.
Thus, the current study aimed to investigate the association between the use of PPIs and the risk of gastrointestinal cancers using a comprehensive meta-analysis of cohort studies.
Materials and methods
Literature search strategy
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (55). A literature search in both PubMed and Excerpta Medica dataBASE (EMBASE) databases was conducted up to December 2022. This search used a combination of the National Library of Medicine (NLM) Medical Subject Headings (MeSH) terms with a wide range of free-text terms as search terms to identify as many relevant articles as possible. A PICO framework was used to determine search terms related with the topic of this study as follows: P for population is ‘general population’; I for intervention (exposure in this study) is ‘use of PPIs’; C for comparison is ‘no use of PPIs’; and O for outcome is ‘incidence of cancer’. Study design of included studies was restricted to cohort study for the current meta-analysis. Thus, using Boolean operators for all the determined MeSH and free-text terms, a combination of search terms was created as follows: (proton pump inhibitors or omeprazole or esomeprazole or pantoprazole or lansoprazole or dexlansoprazole or rabeprazole) and cancer and cohort study. Data S1 shows the final search strategy for the PubMed example. Additionally, the reference lists of the identified articles were examined to identify relevant studies that were not detected through the initial search strategy.
Eligibility criteria
Observational epidemiological studies were included in the final meta-analysis based on the following criteria: i) an original prospective or retrospective cohort study; ii) investigated the association between the use of PPIs and any types of gastrointestinal cancers; iii) reported outcome measures with an adjusted relative risk (RR), odds ratio (OR) or hazard ratios (HR) and its 95% confidence intervals (CI); iv) publication in English. If data were reported in multiple publications from the same study, the study presenting the most comprehensive data was included. Studies that were not published in peer-reviewed journals or only presented in academic conferences were excluded.
Selection of relevant studies
Two authors (Tran HT and Trinh TKT) independently selected all studies retrieved from the databases. Discrepancies in study selection were resolved by reaching a consensus with a senior author (Myung SK). The extraction process encompassed the collection of year of publication and first author's name, type of study, country, year of the enrollment of participants, population (number of participants, gender, and baseline age range), type of cancer, definition of PPI exposure and a control group, adjusted OR/RR/HR with 95% CI, and adjusted variables.
Assessment of methodological quality
The methodological quality of the included studies was assessed using the Newcastle-Ottawa Scale (NOS) for assessing the quality of cohort studies in the meta-analyses (56). The NOS score system ranges from 0 to 9 representing the three subscales of the study quality dimensions: study selection, comparability, and exposure assessment. Given the absence of established cutoff criteria for designating a study as high- or low-quality, studies scoring above the average were categorized as high-quality.
Main and subgroup analyses
The main meta-analysis investigated the association between the use of PPIs and the risk of gastrointestinal cancers. Subsequently, subgroup meta-analyses were conducted, categorized by type of cancer (esophageal, gastric, pancreatic, colorectal, liver, gallbladder, or bile duct cancer), sex (male or female), age (over 50 years old), obesity (yes or no), smoking status (yes or no), type of PPIs (omeprazole, lansoprazole, esomeprazole, pantoprazole, or rabeprazole), duration of PPI use (within 1 year, 1–3 years, 3–5 years, or over 5 years), concurrent medications (aspirin or statins), geographical region, study design (retrospective or prospective cohort study), and methodological quality of study (high or low quality).
Statistical analysis
A pooled OR/RR/HR with its 95% CI was calculated using the adjusted OR/RR/HR and its respective 95% CI from each study reporting the association between the use of PPIs and the risk of gastrointestinal cancers. Additionally, an evaluation of heterogeneity across the studies was performed using Higgins I2, which measures the percentage of total variation across the studies (57). The I2 value is calculated as follows:
I2 = 100% × (Q - df)/Q,
where Q is Cochran's heterogeneity statistic, and df indicates the degrees of freedom. Negative values of the I2 were set at zero; the I2 ranges from 0% (no observed heterogeneity) to 100% (maximal heterogeneity) (57). An I2 value greater than 50% indicates substantial heterogeneity (57).
The pooled estimate was computed using the DerSimonian and Laird method (58). A random-effects model was used due to the diverse geographical contexts and varying populations in which the identified studies were conducted.
Publication bias was assessed utilizing the Begg's funnel plot and Egger's test (59). Publication bias exists when the Begg's funnel plot shows asymmetry or when the P-value of the Egger's test is less than 0.05 (59). Further, sensitivity analyses were conducted to explore the influence of each study on the pooled estimate by omitting a study one by one and re-analyzing. Stata SE version 16.1 statistical software package (StataCorp, College Station, Texas, USA) was used for all the meta-analyses.
Results
Identification of relevant studies
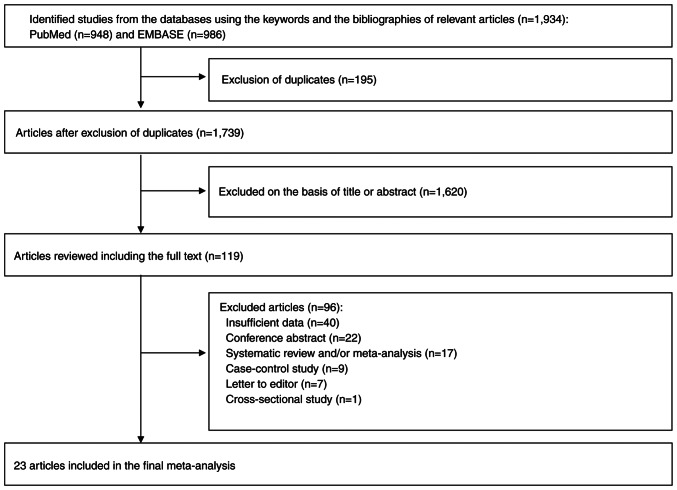
Fig. 1 shows a flow diagram of the selection process for the current study. A total of 1,934 articles were identified by searching two electronic databases, PubMed and EMBASE. After removing 195 duplicate articles, an additional 1,620 articles were excluded based on the predetermined selection criteria. A thorough review was conducted on the remaining 119 articles. Among these, 96 articles were excluded for the following reasons: insufficient data (n=40); conference abstract (n=22); systematic review or/and meta-analysis (n=17); case-control studies (n=9); letters to editor (n=7); and cross-sectional studies (n=1). The remaining 23 articles (27–49) were included in the final analysis. The result of the assessment with Cohen's kappa in the selecting studies was 0.97, suggesting an almost perfect agreement between the two authors.
Figure 1.
Flow diagram of identification of relevant studies.
Characteristics of studies included in the final meta-analysis
This meta-analysis included 25 cohort studies from 23 articles that had a total of 10,309,227 participants. Table I shows the general characteristics of the studies included in the final meta-analysis. Types of cancers were as follows: esophageal, gastric, pancreatic, liver, colorectal, gallbladder, and bile duct cancer. Of the 23 articles, 13 articles are prospective cohort studies, and 10 articles are retrospective cohort studies. Publication dates ranged from 2009 to 2022. Eleven studies were conducted in Europe, nine studies in Asia, and three studies in North America.
Table I.
Characteristics of the studies included in the final meta-analysis (n=23).
| First author/s, year | Type of study | Country | Study period | Population (sex and age) | Type of cancer | Definition of PPI exposure | OR/RR/HR (95% CI) | Adjusted variables | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|
| Nguyen et al, 2009 | Retrospective cohort study | USA | 1982-2005 | 344 individuals (men and women; mean age, 61 years) | Esophageal cancer | Received a dispensed prescription for PPIs vs. non-users | 0.40 (0.16–0.97) | Sex, age, Barrett's esophagus length and NSAIDs/COX-2/aspirin | (27) |
| Poulsen et al, 2009 | Retrospective cohort study | Denmark | 1990-2003 | 18,790 individuals | Gastric cancer | Patients who received ≥2 PPI prescriptions during the study period vs. non-users | 1.20 (0.80–2.00) | Calendar period, sex, age, history of H. pylori eradication therapy, gastroscopy, COPD, alcohol-related admission or therapy and ever using NSAIDs | (28) |
| Boursi et al, 2017 | Retrospective cohort study | UK | 1995-2013 | 19,146 individuals | Pancreatic cancer | PPI users vs. non-users | 1.89 (1.52–2.36) | Age, smoking, insulin, oral hypoglycemics, metformin, HbA1C, Hb, total cholesterol, creatinine and alkaline phosphatase | (29) |
| Brusselaers et al, 2017 | Prospective cohort study | Sweden | 2005-2012 | 797,067 individuals | Gastric cancer | PPI users vs. non-users | 3.38 (3.25–3.53) | Age, sex, calendar and categories | (30) |
| Hwang et al, 2017 | Prospective cohort study | South Korea | 2002-2013 | 451,284 individuals (men and women aged ≥40 years) | Colorectal cancer | Patients who consumed >60 DDDs of PPIs vs. non-users | 0.98 (0.78–1.24) | Age, male sex, obesity, current smoking, frequent drinking, low physical activity, comorbid conditions (including type 2 diabetes), concurrent drug use, (aspirin, metformin, statin) and low socioeconomic status | (31) |
| Wennerström et al, 2017 | Retrospective cohort study | Denmark | 1995-2011 | 1,563,860 individuals | Gastric cancer | PPI users vs. non-users | 2.51 (2.26–2.79) | Age, sex and municipality | (32) |
| Cheung et al, 2018 | Retrospective cohort study | Hong Kong | 2003-2012 | 63,397 individuals (≥18-year-old male and female patients) | Gastric cancer | PPI users vs. non-users | 2.44 (1.42–4.20) | Age of receiving H. pylori eradication therapy, sex, smoking, alcohol use, comorbidities and concomitant medications | (33) |
| Hwang et al, 2018 | Prospective cohort study | South Korea | 2002-2013 | 453,655 individuals (men and women aged ≥40 years) | Pancreatic cancer | Patients who consumed >60 DDDs of PPIs vs. non-users | 1.32 (1.03–1.70) | Age, male sex, obesity, current smoking, frequent drinking, low physical activity, comorbid conditions (type 2 diabetes, chronic pancreatitis), Charlson Comorbidity Index score and low socioeconomic status | (34) |
| Li et al, 2018 | Prospective cohort study | USA | 2001-2015 | 11,526 individuals (men and women; median age, 53 years) | Liver cancer | PPI users vs. non-users | 2.01 (1.50–2.70) | Age, sex, ethnicity, smoking history, alcohol abuse history, body mass index, diabetes, baseline FIB-4 score, gastroesophageal reflux disease, HCV genotype, past completed anti-HCV treatment and attainment of SVR | (35) |
| Tran et al, 2018 | Prospective cohort study | UK | 1991-2004 | 475,768 individuals | Liver cancer | PPI users vs. non-users | 1.99 (1.34–2.94) | Age, sex, deprivation, BMI, alcohol, smoking, comorbidities (including GORD, peptic ulcer disease, cirrhosis, hepatitis and diabetes) and other medication use (statins, aspirin) | (36) |
| Brusselaers et al, 2019 | Prospective cohort study | Sweden | 2005-2012 | 796,492 individuals (≥18-year-old male and female patients) | Gastric cancer; esophageal cancer | ≥180 days of accumulated use of PPIs vs. non-users | 2.97 (2.83–3.10); 3.93 (3.63–4.24) | Age and calendar categories | (37) |
| Kao et al, 2019 | Retrospective cohort study | Taiwan | 2003-2013 | 14,984 individuals | Liver cancer | PPI users vs. non-users | HBV cohort, 1.25 (0.90–1.73) HCV cohort, 1.19 (0.88–1.61) | Age, sex, year of cohort entry, comorbidities (cirrhosis, nonalcoholic liver disease, alcoholic liver disease, hypertension, chronic kidney disease, hyperlipidemia, diabetes) and concomitant medication (interferon/nucleos(t)ides, nonaspirin NSAIDs, histamine 2 receptor antagonist, aspirin, statin, fibrate, insulin, metformin) | (38) |
| Babic et al, 2020 | Prospective cohort study | USA | 1988-2015 | 175,871 individuals (female nurses aged 25–55 years; male health professionals aged 40–75 years) | Colorectal cancer | PPI users vs. non-users | 1.12 (0.78–1.59) | Age, BMI, physical activity, family history of colorectal cancer, alcohol intake, pack-years of smoking, history of lower endoscopy, caloric intake, vitamin D, calcium intake, regular aspirin use, folate intake, menopausal hormone therapy use and red meat as main dish | (39) |
| Brusselaers et al, 2020 | Prospective cohort study | Sweden | 2005-2012 | 796,492 individuals (≥18-year-old male and female patients) | Pancreatic cancer | PPI users vs. non-users | 2.22 (2.12–2.32) | Age, sex and calendar period | (40) |
| Liu et al, 2020 | Prospective cohort study | UK | 2006-2014 | 471,779 individuals | Gastric cancer | PPI users vs. non-users | 1.28 (0.86–1.90) | Age, sex, socioeconomic status, alcohol, smoking, BMI, comorbidities (diabetes, GORD, oesophagitis and peptic ulcer) and other medication uses (statins and aspirin) | (41) |
| Kamal et al, 2021 | Prospective cohort study | Sweden | 2005-2012 | 738,881 individuals | Gallbladder cancer; extrahepatic bile ducts cancer; intrahepatic bile ducts cancer | PPI users vs. non-users | 1.58 (1.37–1.81); 1.77 (1.56–2.00); 1.88 (1.57–2.23) | Sex, age group and calendar period | (42) |
| Lei et al, 2021 | Retrospective cohort study | Taiwan | 1999-2011 | 90,764 individuals (men and women) non-users | Colorectal cancer | Patients who used PPIs ≥30 days vs. CVD, CAD, COPD, | 2.03 (1.56–2.63) | Age, sex, comorbidities (hypertension, diabetes, dyslipidemia, liver cirrhosis), and baseline medication (aspirin, NSAIDs, statin and metformin) | (43) |
| Ng et al, 2021 | Retrospective cohort study | Hong Kong | 2004-2017 | 13,476 individuals (men and women) | Gastric cancer | Patients who used PPIs for ≥30 days vs. non-users (exposed to PPIs <14 days) | 2.38 (1.20–4.76) | Age, sex, comorbidities and baseline medication | (44) |
| Seo et al, 2021 | Retrospective cohort study | South Korea | 2002-2013 | 23,482 individuals (men and women; age ≥19 years) | Gastric cancer | Patients who used PPIs for ≥30 consecutive days vs. non-users | General population cohort, 2.44 (1.17–5.16) Post H. pylori eradication cohort, 2.22 (1.05–4.67) | Age, sex, smoking, alcohol, comorbidities and baseline medication | (45) |
| Shin et al, 2021 | Retrospective cohort study | South Korea | 2004-2015 | 39,799 individuals (men and women; age ≥40 years) | Gastric cancer | PPI users vs. H2RA users | 1.01 (0.88–1.16) | Age, sex, calendar period of prescription, time from medication start to 180 cDDD-days (months), socioeconomic characteristics (income, smoking and alcohol use), indication for drug use (gastresophageal reflux disease or peptic ulcer), Charlson Comorbidity Index, Helicobacter pylori eradication and use of other medications (aspirin, metformin and statin) | (46) |
| Abrahami et al, 2022 | Prospective cohort study | UK | 1990-2018 | 973,281 individuals (men and women; mean age, 60.4 years) | Gastric cancer | PPI users vs. H2RA users | 1.45 (1.06–1.98) | Age, sex, alcohol-related disorders, smoking status, BMI, comorbidities and baseline medication | (47) |
| Abrahami et al, 2022 | Prospective cohort study | UK | 1990-2018 | 1,293,749 individuals (men and women; mean age, 52.6 years) | Colorectal cancer | PPI users vs. H2RA users | 1.02 (0.92–1.14) | Age, sex, alcohol-related disorders smoking status, BMI, comorbidities, baseline medication, mammographic screening, prostate-specific antigen testing, colorectal cancer screening and influenza vaccination | (48) |
| Gong et al, 2022 | Prospective cohort study | South Korea | 2002-2013 | 1,025,340 individuals (men and women; age ≥20 years) | Gastric cancer | PPI users vs. H2RA users | 1.30 (0.75–2.27) | Age, sex, residential area, household income and comorbidities | (49) |
NSAID, Non-steroidal anti-inflammatory drug; COX-2, cyclooxygenase-2; H2RAs, H-2 receptor antagonists; COPD, chronic obstructive pulmonary disease; GORD, gastro-esophageal reflux disease; OR, odds ratio; RR, relative ratio; CVD, cerebrovascular disease; CAD, coronary artery disease; HBV, hepatitis B virus; HCV, hepatitis C virus; SVR, sustained virologic response; FIB-4, fibrosis-4; cDDD, cumulative defined daily dose; DDD, defined daily dose; PPI, proton pump inhibitor; HR, hazard ratio; HbA1C, hemoglobin A1C; Hb, hemoglobin.
Methodological quality of studies
The quality scores by the NOS for the individual studies ranged from 6 to 9; the average score was 8.4. In this meta-analysis, a study scored 9 was considered to possess high level of quality. Thus thirteen studies were rated as high-quality studies (Table II).
Table II.
Methodological quality of studies included in the final analysis based on the Newcastle-Ottawa Scalea for assessing the quality of cohort studies (n=23).
| Selection | Comparability | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| First author/s, year (n=23) | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome of interest was not present at start of study | Control for important factor or additional factor | Assessment of outcome | Follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | Total | (Refs.) |
| Nguyen et al, 2009 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 7 | (27) |
| Poulsen et al, 2009 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (28) |
| Boursi et al, 2017 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | (29) |
| Brusselaers et al, 2017 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (30) |
| Hwang et al, 2017 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (31) |
| Wennerström et al, 2017 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (32) |
| Cheung et al, 2018 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (33) |
| Hwang et al, 2018 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (34) |
| Li et al, 2018 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | (35) |
| Tran et al, 2018 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (36) |
| Brusselaers et al, 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (37) |
| Kao et al, 2019 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | (38) |
| Babic et al, 2020 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | (39) |
| Brusselaers et al, 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (40) |
| Liu et al, 2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (41) |
| Kamal et al, 2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | (42) |
| Lei et al, 2021 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (43) |
| Ng et al, 2021 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (44) |
| Seo et al, 2021 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (45) |
| Shin et al, 2021 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (46) |
| Abrahami et al, 2022 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (47) |
| Abrahami et al, 2022 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (48) |
| Gong et al, 2022 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (49) |
Each study can be awarded a maximum of one point for each item within the selection and exposure categories, while a maximum of two points can be given for the comparability category. Mean score, 8.4.
Use of PPIs and risk of gastrointestinal cancers
As shown in Fig. 2, PPI use was significantly associated with a significantly increased risk of gastrointestinal cancer (OR/RR/HR=2.09; 95% CI 1.78–2.46). In the subgroup meta-analyses by type of cancer, the use of PPIs was associated with a significantly increased risk of esophageal cancer (OR/RR/HR=2.44; 95% CI 1.61–3.70; n=2), gastric cancer (OR/RR/HR=2.88; 95% CI 2.29–3.61; n=11), pancreatic cancer (OR/RR/HR=1.80; 95% CI 1.34–2.42; n=3), and liver cancer (OR/RR/HR=1.55; 95% CI 1.17–2.06; n=3), while no association was found in the risk of colorectal cancer (OR/RR/HR=1.15; 95% CI 0.85–1.54; n=4) (Table III).
Figure 2.
Association between proton pump inhibitor use and risk of gastrointestinal cancer in a random-effects model meta-analysis of cohort studies. aRandom-effects model. OR, odds ratio; RR, relative risk; HR, hazard ratio; AC, adenocarcinoma; SCC, squamous cell carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus.
Table III.
Association between PPIs and risk of gastrointestinal cancer in subgroup meta-analyses using a random-effects model.
| Factors | No. of studies | Summary OR/RR/HR (95% CI) | Heterogeneity, I2 (%) | (Refs.) |
|---|---|---|---|---|
| All studies | 23 | 2.09 (1.78–2.46) | 98.0 | (27–49) |
| Type of cancer | ||||
| Esophageal cancer | 2 | 2.44 (1.61–3.70) | 49.6 | (27,37) |
| Gastric cancer | 11 | 2.88 (2.29–3.61) | 97.4 | (28,30,32,33,37,41,44–47,49) |
| Pancreatic cancer | 3 | 1.80 (1.34–2.42) | 88.6 | (34,40) |
| Colorectal cancer | 4 | 1.15 (0.85–1.54) | 88.9 | (31,39,43,48) |
| Liver cancer | 3 | 1.55 (1.17–2.06) | 67.2 | (35,36,38) |
| Gallbladder cancer | 1 | 1.58 (1.37–1.81) | N/A | (42) |
| Extrahepatic bile ducts cancer | 1 | 1.17 (1.56–2.00) | N/A | (42) |
| Intrahepatic bile ducts cancer | 1 | 1.88 (1.57–2.33) | N/A | (42) |
| Sex | ||||
| Male | 11 | 1.70 (1.36–2.12) | 98.1 | (30,31,34,35,38,40,41,42,46–48) |
| Female | 10 | 1.84 (1.55–2.19) | 96.1 | (30,31,34,38,40,41,42,46–48) |
| Age (≥50 years) | 7 | 1.76 (1.41–2.20) | 97.7 | (30,31,34,38,40,42,46) |
| Obesity | 3 | 1.14 (1.01–1.27) | 0 | (31,34,35) |
| Smoking | 4 | 1.11 (0.98–1.27) | 65.8 | (31,34,35,47) |
| Type of PPIs | ||||
| Omeprazole | 4 | 1.32 (0.96–1.80) | 69.4 | (41,43,47,48) |
| Lansoprazole | 4 | 1.42 (0.99–2.06) | 81.1 | (41,43,47,48) |
| Pantoprazole | 3 | 1.08 (0.91–1.28) | 0 | (43,47,48) |
| Esomeprazole | 3 | 1.17 (0.70–1.96) | 72.6 | (43,47,48) |
| Rabeprazole | 3 | 1.09 (0.77–1.56) | 51.6 | (43,47,48) |
| Duration of PPI use | ||||
| ≤1 year | 4 | 5.23 (2.96–9.24) | 99.6 | (30,37,40,42) |
| 1-3 years | 10 | 1.72 (1.44–2.07) | 86.8 | (30,33,37,40,42,43–45,47,48) |
| 3-5 years | 6 | 1.17 (0.96–1.43) | 79.5 | (27,30,33,37,40,43) |
| >5 years | 4 | 1.16 (0.74–1.84) | 96.6 | (30,37,40,42) |
| Concurrent medication | ||||
| Aspirin | 3 | 1.09 (1.01–1.18) | 0 | (31,38,47) |
| Statins | 4 | 0.85 (0.69–1.06) | 56.5 | (31,35,38,47) |
| Region | ||||
| America | 3 | 1.09 (0.55–2.17) | 86.3 | (27,35,39) |
| Asia | 9 | 1.45 (1.17–1.80) | 79.8 | (31,33,34,38,43–46,49) |
| Europe | 11 | 1.93 (1.51–2.45) | 98.3 | (28–30,32,36,37,40–42,47,48) |
| Study design | ||||
| Retrospective cohort | 10 | 2.60 (1.88–3.60) | 97.6 | (27,28,30,32,33,38,43–46) |
| Prospective cohort | 13 | 1.71 (1.41–2.08) | 97.9 | (29,31,34–37,39–42,47–49) |
| Methodological quality | ||||
| High quality | 13 | 1.43 (1.20–1.70) | 78.2 | (28,31,33,34,36,41,43–49) |
| Low quality | 10 | 2.61 (2.20–3.09) | 98.0 | (27,29,30,32,35,37–40,42) |
PPI, proton pump inhibitor; OR, odds ratio; RR, relative risk; HR, hazard ratio.
Use of PPIs and risk of gastrointestinal cancers by various factors
Table III shows findings from the subgroup meta-analyses stratified by baseline characteristics (sex, age over 50 years old, obesity, and smoking status), type of PPIs, duration of PPI use, concurrent medications, geographical region of studies, study design, and methodological quality of study. In the subgroup meta-analyses by duration of PPI use, a significantly increased risk was observed in people using PPIs within 1 year and from 1 to 3 years. No significant association was found in the subgroup meta-analyses by type of PPIs.
Heterogeneity, publication bias, and sensitivity analysis
Statistical heterogeneity was observed (I2=85.7%) in the meta-analysis of all the studies. Publication bias was not observed in both the Begg's funnel plot (Fig. 3) and Egger's test (P=0.105). Sensitivity analysis to discern the influence of each study did not show any substantial change in the pooled estimate of the effect size and statistical significance (data not shown in figure).
Figure 3.
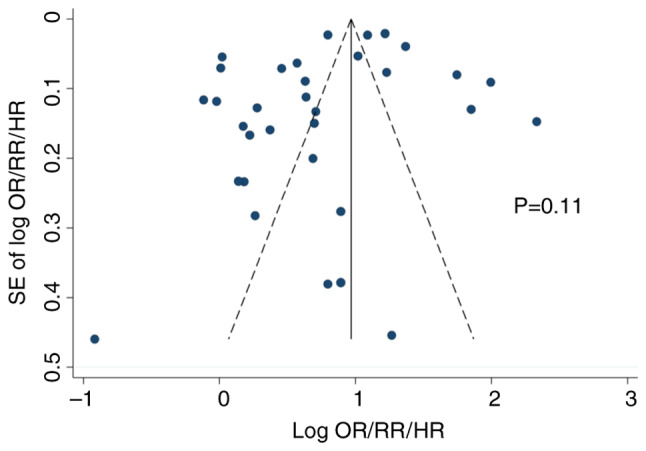
Begg's funnel plot and Egger's test to identify publication bias in the meta-analysis of cohort studies. OR, odds ratio; RR, relative risk; HR, hazard ratio; SE, standard error.
Discussion
In this meta-analysis of cohort studies, a significant association was observed between the use of PPIs and an increased risk of gastrointestinal cancers. In the subgroup meta-analysis by type of gastrointestinal cancers, the use of PPIs was significantly associated with the increased risk of gastric cancer, liver cancer, pancreatic cancer, and esophageal cancer, whereas there was no association for colorectal cancer. The increased risk of gastrointestinal cancers was also observed in people who had used PPIs within 1 year as well as up to 3 years.
Possible biological mechanisms for the increased risk of gastrointestinal cancers by the use of PPIs can be explained by previous in vitro and in vivo studies (60–62). First, PPIs reduce gastric acid secretion by blocking the H+/K+ ATPase of parietal cells (63), which can induce an increase of gastrin secretion from G-cells (64). Gastrin has long been suspected to be a potential risk factor of gastric cancer by causing hypergastrinemia (25). Hypergastrinemia also could lead to the development of gastrointestinal cancers, including esophagus, stomach, pancreatic, and liver cancers (25). Second, PPIs might contribute to increased bacterial colonisation and a larger number of bacteria that are able to produce nitrosamines (23,24). Nitrosamines and gut microbiome alterations could lead to an increased risk of gastrointestinal cancers (23,65,66). Third, PPIs might increase the production of enterochromaffin-like cells (ECL cells) by inducing hypoacidity (67). ECL cells are the key target cells of gastrin in the oxyntic mucosa and are associated with the expression of cholecystokinin-2 (CCK-2) receptors, which might consequently lead to the formation of neuroendocrine tumors (NETs), such as pancreatic cancer (67).
Some preclinical studies have reported a preventive effect of PPIs against the development of colorectal cancer (68,69). Pantoprazole was identified to be a potential T-cell-originated protein kinase (TOPK) inhibitors and blocked the anchorage-independent proliferation of colorectal cancer cells with high TOPK levels in an in vitro cancer cell line study and an in vivo mouse study (68). Also, a rat azoxymethane (AOM) model study showed that omeprazole suppressed the proliferation and carcinogenesis of colon cancer cell lines (69). On the other hand, a transgenic APC genes (APCMin-/+) mouse model study reported that omeprazole-induced hypergastrinemia lead to a significant increase in the proliferation of colorectal adenomas. Thus, there are some inconsistencies regarding the effect of PPI use on the colorectal cancer risk.
The findings of this meta-analysis are in line with previous meta-analyses that investigated the association between the use of PPIs and the risk of gastric cancer (50,51,70). Jiang et al (71) found that long-term use of PPIs may possibly increase the risk of gastric cancer (OR 2.50; 95% CI: 1.74–3.85). Nevertheless, they were unable to assess publication bias due to the small number of studies, and all studies were retrospective in design. Tran-Duy et al (51) assessed the effects of PPI therapy on the risk of gastric cancer by including a cohort and 3 case-control studies. They concluded that PPI therapy was positively associated with an increased risk of gastric cancer (51). Nonetheless, there were too few studies included in their analysis to confirm the association. Segna et al (50) conducted a meta-analysis including 5 retrospective cohort and 8 case-control studies. They found that PPI use had a 1.94-fold higher risk of gastric cancer compared with the non-PPI group (50). They also included retrospective studies only.
Regarding the risk of pancreatic cancer, the finding of this meta-analysis is consistent with two previous meta-analyses, which also included only a few studies. Laoveeravat et al (53) and Alkhushaym et al (54) included one and two cohort studies, respectively. They also found that PPIs use could significantly increase the risk of pancreatic cancer. Regarding the risk of colorectal cancer, the finding of this meta-analysis is consistent with that of Ma et al's (52) meta-analysis of 3 cohort studies, which reported that there was no statistically significant association between PPI use and the risk of colorectal cancer.
To the best of current knowledge, this is the most comprehensive meta-analysis of cohort studies on this topic. Although a recent meta-analysis of observational epidemiological studies regarding this topic was published in 2021 (50), it included only case-control and retrospective cohort studies and revealed no clear duration-dependent risk increase among PPI users. This meta-analysis included a total of 23 cohort studies with 13 prospective cohort studies as well as 10 retrospective studies and reported the evidence of the gastrointestinal cancers risk with long-term use of PPIs. In addition, this meta-analysis provided information regarding the types of PPI and the risk of gastrointestinal cancers. This meta-analysis also assessed the risk of gastrointestinal cancers by providing various subgroup analyses that might help to minimize potential confounding factors.
This study has several limitations. This meta-analysis only included cohort studies. In terms of evidence-based medicine, it is important to emphasize that while randomised controlled trials (RCTs) offer a higher level of evidence compared to cohort studies, they pose ethical and practical challenges when investigating the association between PPI use and cancer risk. Nevertheless, it would be possible to conduct a meta-analysis of RCTs using secondary outcomes from the original trials. Second, the stratification of gastrointestinal cancer risk based on PPI dosage was hindered by the limited availability of relevant data from individual studies. Lastly, it was not feasible to confirm the effect of PPI use on the risk of gallbladder and bile duct cancers because only one study for each type of cancer was included in the current study. Further studies are warranted.
This meta-analysis of cohort studies suggested a significant association between the use of PPIs and the increased risk of gastrointestinal cancers. This finding was observed in people using PPIs for less than 1 year as well as up to 3 years. These findings should be confirmed by RCTs that provide a higher level of evidence than cohort studies.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable requests.
Authors' contributions
SKM and THT conceptualized the present study, conducted the investigation, and were involved in data curation. THT and TTKT analyzed and interpreted data. THT wrote the original draft. SKM, THT, and TTKT wrote, reviewed and edited the manuscript. THT and SKM confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Agrawal NM, Campbell DR, Safdi MA, Lukasik NL, Huang B, Haber MM. Superiority of lansoprazole vs ranitidine in healing nonsteroidal anti-inflammatory drug-associated gastric ulcers: Results of a double-blind, randomized, multicenter study. NSAID-associated gastric Ulcer study group. Arch Intern Med. 2000;160:1455–1461. doi: 10.1001/archinte.160.10.1455. [DOI] [PubMed] [Google Scholar]
- 2.Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastresophageal reflux disease: A meta-analysis. Gastroenterology. 1997;112:1798–1810. doi: 10.1053/gast.1997.v112.pm9178669. [DOI] [PubMed] [Google Scholar]
- 3.Dekkers CP, Beker JA, Thjodleifsson B, Gabryelewicz A, Bell NE, Humphries TJ. Comparison of rabeprazole 20 mg versus omeprazole 20 mg in the treatment of active duodenal ulcer: A European multicentre study. Aliment Pharmacol Ther. 1999;13:179–186. doi: 10.1046/j.1365-2036.1999.00449.x. [DOI] [PubMed] [Google Scholar]
- 4.Farley A, Wruble LD, Humphries TJ. Rabeprazole versus ranitidine for the treatment of erosive gastresophageal reflux disease: A double-blind, randomized clinical trial. Raberprazole study group. Am J Gastroenterol. 2000;95:1894–1899. doi: 10.1111/j.1572-0241.2000.02233.x. [DOI] [PubMed] [Google Scholar]
- 5.Huang JQ, Hunt RH. pH, healing rate and symptom relief in acid-related diseases. Yale J Biol Med. 1996;69:159–174. [PMC free article] [PubMed] [Google Scholar]
- 6.Lew EA, Pisegna JR, Starr JA, Soffer EF, Forsmark C, Modlin IM, Walsh JH, Beg M, Bochenek W, Metz DC. Intravenous pantoprazole rapidly controls gastric acid hypersecretion in patients with Zollinger-Ellison syndrome. Gastroenterology. 2000;118:696–704. doi: 10.1016/S0016-5085(00)70139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norton JA, Fraker DL, Alexander HR, Venzon DJ, Doppman JL, Serrano J, Goebel SU, Peghini PL, Roy PK, Gibril F, Jensen RT. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;34:635–644. doi: 10.1056/NEJM199908263410902. [DOI] [PubMed] [Google Scholar]
- 8.Farrell B, Lass E, Moayyedi P, Ward D, Thompson W. Reduce unnecessary use of proton pump inhibitors. BMJ. 2022;379:e069211. doi: 10.1136/bmj-2021-069211. [DOI] [PubMed] [Google Scholar]
- 9.Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ. 2008;336:2–3. doi: 10.1136/bmj.39406.449456.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connelly D. The development and safety of proton pump inhibitors. The Pharm J. 2016;296:7890. [Google Scholar]
- 11.Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728–738. doi: 10.14309/00000434-200903000-00035. [DOI] [PubMed] [Google Scholar]
- 12.Targownik LE, Fisher DA, Saini SD. AGA clinical practice update on de-prescribing of proton pump inhibitors: Expert review. Gastroenterol. 2022;162:1334–1342. doi: 10.1053/j.gastro.2021.12.247. [DOI] [PubMed] [Google Scholar]
- 13.Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449–1461. doi: 10.1016/S0140-6736(09)60938-7. [DOI] [PubMed] [Google Scholar]
- 14.Jankowski JAZ, de Caestecker J, Love SB, Reilly G, Watson P, Sanders S, Ang Y, Morris D, Bhandari P, Brooks C, et al. Esomeprazole and aspirin in Barrett's esophagus (AspECT): A randomised factorial trial. Lancet. 2018;392:400–408. doi: 10.1016/S0140-6736(18)31388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Othman F, Card TR, Crooks CJ. Proton pump inhibitor prescribing patterns in the UK: A primary care database study. Pharmacoepidemiol Drug Saf. 2016;25:1079–1087. doi: 10.1002/pds.4043. [DOI] [PubMed] [Google Scholar]
- 16.Blackett JW, Faye AS, Phipps M, Li J, Lebwohl B, Freedberg DE. Prevalence and risk factors for inappropriate continuation of proton pump inhibitors after discharge from the intensive care unit. Mayo Clin Proc. 2021;96:2550–2560. doi: 10.1016/j.mayocp.2020.07.038. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen PV, Tamaz R. Inappropriate prescription of proton pump inhibitors in a community setting. Can J Hosp Pharm. 2018;71:267–271. [PMC free article] [PubMed] [Google Scholar]
- 18.Cao F, Chen CX, Wang M, Liao HR, Wang MX, Hua SZ, Huang B, Xiong Y, Zhang JY, Xu YL. Updated meta-analysis of controlled observational studies: Proton-pump inhibitors and risk of Clostridium difficile infection. J Hosp Infect. 2018;98:4–13. doi: 10.1016/j.jhin.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Wang CH, Li CH, Hsieh R, Fan CY, Hsu TC, Chang WC, Hsu WT, Lin YY, Lee CC. Proton pump inhibitors therapy and the risk of pneumonia: A systematic review and meta-analysis of randomized controlled trials and observational studies. Expert Opin Drug Saf. 2019;18:163–172. doi: 10.1080/14740338.2019.1577820. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Luo Z, Yu S, Tang Z. Proton pump inhibitor use and risk of dementia: Systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e14422. doi: 10.1097/MD.0000000000014422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Li X, Fan L, Yang J, Wang J, Sun J, Wang Z. Proton pump inhibitors therapy and risk of bone diseases: An update meta-analysis. Life Sci. 2019;218:213–223. doi: 10.1016/j.lfs.2018.12.058. [DOI] [PubMed] [Google Scholar]
- 22.Zhang ML, Fan YX, Meng R, Cai WK, Yin SJ, Zhou T, Huang YH, Wang P, Jiang FF, Yang M, He GH. Proton pump inhibitors and cancer risk: An umbrella review and meta-analysis of observational studies. Am J Clin Oncol. 2022;45:475–485. doi: 10.1097/COC.0000000000000949. [DOI] [PubMed] [Google Scholar]
- 23.Verdu E, Viani F, Armstrong D, Fraser R, Siegrist HH, Pignatelli B, Idström JP, Cederberg C, Blum AL, Fried M. Effect of omeprazole on intragastric bacterial counts, nitrates, nitrites, and N-nitroso compounds. Gut. 1994;35:455–460. doi: 10.1136/gut.35.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imhann F, Bonder MJ, Vila AV, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJ, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundell L, Vieth M, Gibson F, Nagy P, Kahrilas PJ. Systematic review: The effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment Pharmacol Ther. 2015;42:649–663. doi: 10.1111/apt.13324. [DOI] [PubMed] [Google Scholar]
- 26.Feng J, Petersen CD, Coy DH, Jiang JK, Thomas CJ, Pollak MR, Wank SA. Calcium-sensing receptor is a physiologic multimodal chemosensor regulating gastric G-cell growth and gastrin secretion. Proc Natl Acad Sci USA. 2010;107:17791–17796. doi: 10.1073/pnas.1009078107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen DM, El-Serag HB, Henderson L, Stein D, Bhattacharyya A, Sampliner RE. Medication usage and the risk of neoplasia in patients with Barrett's esophagus. Clin Gastroenterol Hepatol. 2009;7:1299–1304. doi: 10.1016/j.cgh.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poulsen AH, Christensen S, McLaughlin JK, Thomsen RW, Sørensen HT, Olsen JH, Friis S. Proton pump inhibitors and risk of gastric cancer: A population-based cohort study. Br J Cancer. 2009;100:1503–1507. doi: 10.1038/sj.bjc.6605024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boursi B, Finkelman B, Giantonio BJ, Haynes K, Rustgi AK, Rhim AD, Mamtani R, Yang YX. A clinical prediction model to assess risk for pancreatic cancer among patients with new-onset diabetes. Gastroenterology. 2017;152:840–850.e3. doi: 10.1016/S0016-5085(17)31215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brusselaers N, Wahlin K, Engstrand L, Lagergren J. Maintenance therapy with proton pump inhibitors and risk of gastric cancer: A nationwide population-based cohort study in Sweden. BMJ Open. 2017;7:e017739. doi: 10.1136/bmjopen-2017-017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang IC, Chang J, Park SM. Emerging hazard effects of proton pump inhibitor on the risk of colorectal cancer in low-risk populations: A Korean nationwide prospective cohort study. PLoS One. 2017;12:e0189114. doi: 10.1371/journal.pone.0189114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wennerström ECM, Simonsen J, Camargo MC, Rabkin CS. Acid-suppressing therapies and subsite-specific risk of stomach cancer. Br J Cancer. 2017;116:1234–1238. doi: 10.1038/bjc.2017.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: A population-based study. Gut. 2018;67:28–35. doi: 10.1136/gutjnl-2017-314605. [DOI] [PubMed] [Google Scholar]
- 34.Hwang IC, Chang J, Park SM. Association between proton pump inhibitor use and the risk of pancreatic cancer: A Korean nationwide cohort study. PLoS One. 2018;13:e0203918. doi: 10.1371/journal.pone.0203918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li DK, Yan P, Abou-Samra AB, Chung RT, Butt AA. Proton pump inhibitors are associated with accelerated development of cirrhosis, hepatic decompensation and hepatocellular carcinoma in noncirrhotic patients with chronic hepatitis C infection: Results from ERCHIVES. Aliment Pharmacol Ther. 2018;47:246–258. doi: 10.1111/apt.14391. [DOI] [PubMed] [Google Scholar]
- 36.Tran KT, McMenamin ÚC, Hicks B, Murchie P, Thrift AP, Coleman HG, Iversen L, Johnston BT, Lee AJ, Cardwell CR. Proton pump inhibitor and histamine-2 receptor antagonist use and risk of liver cancer in two population-based studies. Aliment Pharmacol Ther. 2018;48:55–64. doi: 10.1111/apt.14796. [DOI] [PubMed] [Google Scholar]
- 37.Brusselaers N, Lagergren J, Engstrand L. Duration of use of proton pump inhibitors and the risk of gastric and esophageal cancer. Cancer Epidemiol. 2019;62:101585. doi: 10.1016/j.canep.2019.101585. [DOI] [PubMed] [Google Scholar]
- 38.Kao WY, Su CW, Chia-Hui Tan E, Lee PC, Chen PH, Tang JH, Huang YH, Huo TI, Chang CC, Hou MC, et al. Proton pump inhibitors and risk of hepatocellular carcinoma in patients with chronic hepatitis B or C. Hepatology. 2019;69:1151–1164. doi: 10.1002/hep.30247. [DOI] [PubMed] [Google Scholar]
- 39.Babic A, Zhang X, Morales-Oyarvide V, Yuan C, Khalaf N, Khalili H, Lochhead P, Chan AT, Ogino S, Wolpin BM, et al. Acid-suppressive medications and risk of colorectal cancer: Results from three large prospective cohort studies. Br J Cancer. 2020;123:844–851. doi: 10.1038/s41416-020-0939-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brusselaers N, Sadr-Azodi O, Engstrand L. Long-term proton pump inhibitor usage and the association with pancreatic cancer in Sweden. J Gastroenterol. 2020;55:453–461. doi: 10.1007/s00535-019-01652-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu P, McMenamin ÚC, Johnston BT, Murchie P, Iversen L, Lee AJ, Vissers PAJ, Cardwell CR. Use of proton pump inhibitors and histamine-2 receptor antagonists and risk of gastric cancer in two population-based studies. Br J Cancer. 2020;123:307–315. doi: 10.1038/s41416-020-0860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamal H, Sadr-Azodi O, Engstrand L, Brusselaers N. Association between proton pump inhibitor use and biliary tract cancer risk: A Swedish population-based cohort study. Hepatology. 2021;74:2021–2031. doi: 10.1002/hep.31914. [DOI] [PubMed] [Google Scholar]
- 43.Lei WY, Wang JH, Yi CH, Liu TT, Hung JS, Wong MW, Bair MJ, Vaezi MF, Orr WC, Chen CL. Association between use of proton pump inhibitors and colorectal cancer: A nationwide population-based study. Clin Res Hepatol Gastroenterol. 2021;45:101397. doi: 10.1016/j.clinre.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 44.Ng AK, Ng PY, Ip A, Cheung KS, Siu CW. Association between proton pump inhibitors after percutaneous coronary intervention and risk of gastric cancer. BMJ Open Gastroenterol. 2021;8:e000719. doi: 10.1136/bmjgast-2021-000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seo SI, Park CH, You SC, Kim JY, Lee KJ, Kim J, Kim Y, Yoo JJ, Seo WW, Lee HS, Shin WG. Association between proton pump inhibitor use and gastric cancer: A population-based cohort study using two different types of nationwide databases in Korea. Gut. 2021;70:2066–2075. doi: 10.1136/gutjnl-2020-323845. [DOI] [PubMed] [Google Scholar]
- 46.Shin GY, Park JM, Hong J, Cho YK, Yim HW, Choi MG. Use of proton pump inhibitors vs histamine 2 receptor antagonists for the risk of gastric cancer: Population-based cohort study. Am J Gastroenterol. 2021;116:1211–1219. doi: 10.14309/ajg.0000000000001167. [DOI] [PubMed] [Google Scholar]
- 47.Abrahami D, McDonald EG, Schnitzer ME, Barkun AN, Suissa S, Azoulay L. Proton pump inhibitors and risk of gastric cancer: population-based cohort study. Gut. 2022;71:16–24. doi: 10.1136/gutjnl-2021-325096. [DOI] [PubMed] [Google Scholar]
- 48.Abrahami D, McDonald EG, Schnitzer ME, Barkun AN, Suissa S, Azoulay L. Proton pump inhibitors and risk of colorectal cancer. Gut. 2022;71:111–118. doi: 10.1136/gutjnl-2021-325096. [DOI] [PubMed] [Google Scholar]
- 49.Gong EJ, Bang CS, Kim DK, Lee JJ, Baik GH. Use of proton pump inhibitors and the risk for the development of gastric cancers: A nationwide population-based cohort study using balanced operational definitions. Cancers. 2022;14:5172. doi: 10.3390/cancers14205172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Segna D, Brusselaers N, Glaus D, Krupka N, Misselwitz B. Association between proton-pump inhibitors and the risk of gastric cancer: A systematic review with meta-analysis. Therap Adv Gastroenterol. 2021;14:17562848211051463. doi: 10.1177/17562848211051463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tran-Duy A, Spaetgens B, Hoes AW, de Wit NJ, Stehouwer CD. Use of proton pump inhibitors and risks of fundic gland polyps and gastric cancer: Systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1706–1719.e5. doi: 10.1016/j.cgh.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 52.Ma T, Wu M, Jia S, Yang L. Proton pump inhibitors and the risk of colorectal cancer: A systematic review and meta-analysis of observational studies. Int J Colorectal Dis. 2020;35:2157–2169. doi: 10.1007/s00384-020-03717-5. [DOI] [PubMed] [Google Scholar]
- 53.Laoveeravat P, Thavaraputta S, Vutthikraivit W, Suchartlikitwong S, Mingbunjerdsuk T, Motes A, Nugent K, Rakvit A, Islam E, Islam S. Proton pump inhibitors and histamine-2 receptor antagonists on the risk of pancreatic cancer: A systematic review and meta-analysis. QJM. 2020;113:100–107. doi: 10.1093/qjmed/hcz234. [DOI] [PubMed] [Google Scholar]
- 54.Alkhushaym N, Almutairi AR, Althagafi A, Fallatah SB, Oh M, Martin JR, Babiker HM, McBride A, Abraham I. Exposure to proton pump inhibitors and risk of pancreatic cancer: A meta-analysis. Expert Opin Drug Saf. 2020;19:327–334. doi: 10.1080/14740338.2020.1715939. [DOI] [PubMed] [Google Scholar]
- 55.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wells GA, Shea B, O'Connell D, et al. Ottawa Hospital Research Institute. Ottawa; ON, Canada: 2000. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Google Scholar]
- 57.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 58.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: An update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med. 2001;20:641–654. doi: 10.1002/sim.698. [DOI] [PubMed] [Google Scholar]
- 60.Lee Y, Urbanska AM, Hayakawa Y, Wang H, Au AS, Luna AM, Chang W, Jin G, Bhagat G, Abrams JA, et al. Gastrin stimulates a cholecystokinin-2-receptor-expressing cardia progenitor cell and promotes progression of Barrett's-like esophagus. Oncotarget. 2016;8:203–214. doi: 10.18632/oncotarget.10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Betton GR, Dormer CS, Wells T, Pert P, Price CA, Buckley P. Gastric ECL-cell hyperplasia and carcinoids in rodents following chronic administration of H2-antagonists SK&F 93479 and oxmetidine and omeprazole. Toxicol Pathol. 1988;16:288–298. doi: 10.1177/019262338801600222. [DOI] [PubMed] [Google Scholar]
- 62.Kidd M, Tang LH, Modlin IM, Zhang T, Chin K, Holt PR, Moss SF. Gastrin-mediated alterations in gastric epithelial apoptosis and proliferation in a mastomys rodent model of gastric neoplasia. Digestion. 2000;62:143–151. doi: 10.1159/000007806. [DOI] [PubMed] [Google Scholar]
- 63.Joo MK, Park JJ, Chun HJ. Proton pump inhibitor: The dual role in gastric cancer. World J Gastroenterol. 2019;25:2058–2070. doi: 10.3748/wjg.v25.i17.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Henwood M, Clarke P, Smith AM, Watson SA. Expression of gastrin in developing gastric adenocarcinoma. Br J Surg. 2001;88:564–568. doi: 10.1046/j.1365-2168.2001.01716.x. [DOI] [PubMed] [Google Scholar]
- 65.Wroblewski LE, Peek RM, Coburn LA. The role of the microbiome in gastrointestinal cancer. Gastroenterol Clin North Am. 2016;45:543–556. doi: 10.1016/j.gtc.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weitsman S, Celly S, Leite G, Mathur R, Sedighi R, Barlow GM, Morales W, Sanchez M, Parodi G, Villanueva-Millan MJ, et al. Effects of proton pump inhibitors on the small bowel and stool microbiomes. Dig Dis Sci. 2022;67:224–232. doi: 10.1007/s10620-021-06857-y. [DOI] [PubMed] [Google Scholar]
- 67.Waldum HL, Sørdal Ø, Fossmark R. Proton pump inhibitors (PPIs) may cause gastric cancer-clinical consequences. Scand J Gastroenterol. 2018;53:639–642. doi: 10.1080/00365521.2018.1450442. [DOI] [PubMed] [Google Scholar]
- 68.Zeng X, Liu L, Zheng M, Sun H, Xiao J, Lu T, Huang G, Chen P, Zhang J, Zhu F, et al. Pantoprazole, an FDA-approved proton-pump inhibitor, suppresses colorectal cancer growth by targeting T-cell-originated protein kinase. Oncotarget. 2016;7:22460–22473. doi: 10.18632/oncotarget.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patlolla JM, Zhang Y, Li Q, Steele VE, Rao CV. Anti-carcinogenic properties of omeprazole against human colon cancer cells and azoxymethane-induced colonic aberrant crypt foci formation in rats. Int J Oncol. 2012;40:170–175. doi: 10.3892/ijo.2011.1214. [DOI] [PubMed] [Google Scholar]
- 70.Watson SA, Smith AM. Hypergastrinemia promotes adenoma progression in the APC(Min-/+) mouse model of familial adenomatous polyposis. Cancer Res. 2001;61:625–631. [PubMed] [Google Scholar]
- 71.Jiang K, Jiang X, Wen Y, Liao L, Liu FB. Relationship between long-term use of proton pump inhibitors and risk of gastric cancer: A systematic analysis. J Gastroenterol Hepatol. 2019;34:1898–1905. doi: 10.1111/jgh.14759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable requests.