Abstract
Background
Management of chronic obstructive pulmonary disease (COPD) commonly involves a combination of long‐acting bronchodilators including beta2‐agonists (LABA) and muscarinic antagonists (LAMA). LABA and LAMA bronchodilators are now available in single‐combination inhalers. In individuals with persistent symptoms or frequent exacerbations, inhaled corticosteroids (ICS) are also used with combination LABA and LAMA inhalers. However, the benefits and risks of adding ICS to combination LABA/LAMA inhalers as a triple therapy remain unclear.
Objectives
To assess the effects of adding an ICS to combination LABA/LAMA inhalers for the treatment of stable COPD.
Search methods
We searched the Cochrane Airways Group Register of Trials, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Embase up to 30 November 2022. We also searched ClinicalTrials.gov and the WHO ICTRPup to 30 November 2022.
Selection criteria
We included parallel‐group randomised controlled trials of three weeks' duration or longer that compared the treatment of stable COPD with ICS in addition to combination LABA/LAMA inhalers against combination LABA/LAMA inhalers alone.
Data collection and analysis
We used standard Cochrane methodological procedures. The primary outcomes were acute exacerbations of COPD, respiratory health‐related quality of life, pneumonia and other serious adverse events. The secondary outcomes were symptom scores, lung function, physical capacity, and mortality. We used GRADE to assess certainty of evidence for studies that contributed data to our prespecified outcomes.
Main results
Four studies with a total of 15,412 participants met the inclusion criteria. The mean age of study participants ranged from 64.4 to 65.3 years; the proportion of female participants ranged from 28% to 40%. Most participants had symptomatic COPD (COPD Assessment Test Score ≥ 10) with severe to very severe airflow limitation (forced expiratory volume in one second (FEV1) < 50% predicted) and one or more moderate‐to‐severe COPD exacerbations in the last 12 months. Trial medications differed amongst studies. The duration of follow‐up was 52 weeks in three studies and 24 weeks in one study. We assessed the risk of selection, performance, and detection bias to be low in the included studies; one study was at high risk of attrition bias, and one study was at high risk of reporting bias.
Triple therapy may reduce rates of moderate‐to‐severe COPD exacerbations compared to combination LABA/LAMA inhalers (rate ratio (RR) 0.74, 95% confidence interval (CI) 0.67 to 0.81; n = 15,397; low‐certainty evidence). Subgroup analysis stratifying by blood eosinophil counts showed there may be a greater reduction in rate of moderate‐to‐severe COPD exacerbations with triple therapy in participants with high‐eosinophils (RR 0.67, 95% CI 0.60 to 0.75) compared to low‐eosinophils (RR 0.87, 95% CI 0.81 to 0.93) (test for subgroup differences: P < 0.01) (high/low cut‐offs: 150 eosinophils/µL in three studies; 200 eosinophils/µL in one study). However, moderate‐to‐substantial heterogeneity was observed in both high‐ and low‐eosinophil subgroups. These subgroup analyses are observational by nature and thus results should be interpreted with caution. Triple therapy may be associated with reduced rates of severe COPD exacerbations (RR 0.75, 95% CI 0.67 to 0.84; n = 14,131; low‐certainty evidence).
Triple therapy improved health‐related quality of life assessed using the St George's Respiratory Questionnaire (SGRQ) by the minimal clinically important difference (MCID) threshold (4‐point decrease) (35.3% versus 42.4%, odds ratio (OR) 1.35, 95% CI 1.26 to 1.45; n = 14,070; high‐certainty evidence). Triple therapy may result in fewer symptoms measured using the Transition Dyspnoea Index (TDI) (OR 1.33, 95% CI 1.13 to 1.57; n = 3044; moderate‐certainty evidence) and improved lung function as measured by change in trough FEV1 (mean difference 38.68 mL, 95% CI 22.58 to 54.77; n = 11,352; low‐certainty evidence). However, these benefits fell below MCID thresholds for TDI (1‐unit decrease) and trough FEV1 (100 mL), respectively.
Triple therapy is probably associated with a higher risk of pneumonia as a serious adverse event compared to combination LABA/LAMA inhalers (3.3% versus 1.9%, OR 1.74, 95% CI 1.39 to 2.18; n = 15,412; moderate‐certainty evidence). In contrast, all‐cause serious adverse events may be similar between groups (19.7% versus 19.7%, OR 0.95, 95% CI 0.87 to 1.03; n = 15,412; low‐certainty evidence). All‐cause mortality may be lower with triple therapy (1.4% versus 2.0%, OR 0.70, 95% CI 0.54 to 0.90; n = 15,397; low‐certainty evidence).
Authors' conclusions
The available evidence suggests that triple therapy may reduce rates of COPD exacerbations (low‐certainty evidence) and results in an improvement in health‐related quality of life (high‐certainty evidence) compared to combination LABA/LAMA inhalers, but probably confers an increased pneumonia risk as a serious adverse event (moderate‐certainty evidence). Triple therapy probably improves respiratory symptoms and may improve lung function (moderate‐ and low‐certainty evidence, respectively); however, these benefits do not appear to be clinically significant. Triple therapy may reduce the risk of all‐cause mortality compared to combination LABA/LAMA inhalers (low‐certainty evidence). The certainty of the evidence was downgraded most frequently for inconsistency or indirectness. Across the four included studies, there were important differences in inclusion criteria, trial medications, and duration of follow‐up. Investigation of heterogeneity was limited due to the small number of included studies. We found limited data on the effects of triple therapy compared to combination LABA/LAMA inhalers in patients with mild‐moderate COPD and those without a recent exacerbation history.
Plain language summary
In stable COPD, should inhaled corticosteroids be used with combination long‐acting beta2‐agonist/long‐acting muscarinic antagonist inhalers?
Key messages
• We are confident that quality of life is improved with triple therapy (inhaled corticosteroids (ICS) plus long‐acting beta2‐agonists (LABA) plus long‐acting muscarinic antagonists (LAMA)) compared to combination bronchodilator inhalers (LABA plus LAMA).
• We are moderately confident that compared to combination bronchodilator inhalers, triple therapy results in reduced symptoms and an increased risk of pneumonia.
• We have little confidence that triple therapy lowers rates of chronic obstructive pulmonary disease (COPD) flare‐ups and the risk of death or improves lung function when compared to combination bronchodilator inhalers.
Why is this question important?
COPD is a common long‐term lung disease that includes chronic bronchitis and emphysema. It is often associated with smoking. People with COPD experience persistent symptoms including breathlessness, cough, and phlegm production, and may suffer from COPD flare‐ups where their symptoms become worse.
COPD can be treated with inhalers. Inhalers can contain one, two, or three different medications. These medications include long‐acting bronchodilators (LABA and LAMA), which open (dilate) the airways; and ICS, which reduce inflammation in the airways. In some people who have persistent symptoms or frequent flare‐ups, all three medications may be taken.
LABA and LAMA can be taken together in one inhaler. This is called a 'combination inhaler'. Combination inhalers have been found to improve adherence to treatment and patient outcomes. LABA and LAMA and ICS can also be taken together, which is known as 'triple therapy'.
We wanted to find out if triple therapy is better than combination therapy in people with stable COPD. Specifically, we wanted to know whether triple therapy improved patient outcomes and what side effects there might be.
How did we answer this question?
We looked for all randomised controlled trials (a type of study where participants are randomly assigned to one of two or more treatment groups) comparing treatment with triple therapy or combination inhaler treatment in people with stable COPD.
What did we find?
We found four studies including a total of 15,412 people with COPD. The studies compared triple therapy ICS/LABA/LAMA inhalers to combination LABA/LAMA inhalers. Most people in the studies were in their mid‐sixties, had severe or very severe symptoms of COPD, and had had at least one recent COPD flare‐up; there were more men than women in the included studies.
We are confident that quality of life is improved with triple therapy compared to combination inhalers. We are moderately confident that symptoms are reduced by triple therapy, and that people treated with triple therapy have an increased risk of pneumonia compared to those treated with combination inhalers. We have little confidence in the findings that triple therapy may lower rates of COPD flare‐ups compared to combination inhalers; that there may be a lower risk of death with triple therapy compared to combination inhalers; or that triple therapy may reduce adverse events or improve lung function.
More research is needed, especially involving people with less severe COPD and those without recent COPD flare‐ups.
How up‐to‐date is the evidence?
The evidence is current to 30 November 2022.
Summary of findings
Summary of findings 1. Triple therapy compared to LABA/LAMA combination for COPD.
| Triple therapy compared to LABA/LAMA combination for COPD | ||||||
| Patient or population: people with stable COPD Setting: outpatient Intervention: LABA/LAMA/ICS combination (triple therapy) Comparison: LABA/LAMA combination | ||||||
|
Outcomes (weighted mean follow‐up duration 52 weeks) |
Anticipated absolute effects (95% CI)* | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with LABA/LAMA combination | Risk with triple therapy | |||||
| Moderate‐to‐severe exacerbations (rate) | Mean rate ranged from 0.59 to 1.42 moderate‐to‐severe exacerbations per year. | Mean rate ranged from 0.46 to 1.08 moderate‐to‐severe exacerbations per year. | RR 0.74 (0.67 to 0.81) | 15,397 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | |
| Severe exacerbations (rate) | Mean rate ranged from 0.09 to 0.19 severe exacerbations per year. | Mean rate ranged from 0.07 to 0.14 severe exacerbations per year. | RR 0.75 (0.67 to 0.84) | 14,131 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 |
|
| Respiratory HRQoL measured by SGRQ, ≥ 4‐point decrease from baseline (lower scores indicate better HRQoL) | 1741/4934 (35.3%) | 3878/9136 (42.4%) | OR 1.35 (1.26 to 1.45) | 14,070 (3 RCTs) | ⊕⊕⊕⊕ HIGH | Translates to 71 more responders per 1000 treated with triple therapy (54 more to 89 more). MCID: 4 points |
| Pneumonia (labelled as an SAE) | 105/5590 (1.9%) | 328/9822 (3.3%) | OR 1.74 (1.39 to 2.18) | 15,412 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | Translates to 13 more pneumonia SAEs per 1000 treated with triple therapy (7 more to 21 more) |
| All‐cause SAEs | 1101/5590 (19.7%) | 1938/9822 (19.7%) | OR 0.95 (0.87 to 1.03) | 15,412 (4 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | Translates to 8 fewer all‐cause SAEs per 1000 treated with triple therapy (21 fewer to 5 more) |
| Symptom score TDI, ≥ 1‐unit increase (lower scores indicate worse dyspnoea) |
300 per 1000 | 363 per 1000 (326 to 402) | OR 1.33 (1.13 to 1.57) | 3044 (1 RCT) | ⊕⊕⊕⊝ MODERATE 5 | Translates to 63 more responders per 1000 treated with triple therapy (26 more to 102 more). MCID: 1 point |
| Lung function, change in trough FEV1 (mL) | Mean change in trough FEV1 in the LABA/LAMA group was 36.92 mL. | Mean change in trough FEV1 in the triple therapy group was 85.16 mL. | MD 38.68 (22.58 to 54.77) | 11,352 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 3 | MCID: 100 mL |
| Mortality (all‐cause) | 112/5585 (2.0%) | 139/9812 (1.4%) | OR 0.70 (0.54 to 0.90) | 15,397 (4 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | Translates to 6 fewer deaths per 1000 treated with triple therapy (9 fewer to 2 fewer) |
| *The risk (and 95% CI) in the triple therapy groups is based on the assumed risk in the LABA/LAMA group and the relative effect (and 95% CI) of triple therapy. CI: confidence interval; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1 second; HRQoL: health‐related quality of life; LABA: long‐acting beta2‐agonists; LAMA: long‐acting muscarinic antagonists; MCID: minimal clinically important difference; MD: mean difference; OR: odds ratio; RR: rate ratio; SAE: serious adverse events; SGRQ: St George's Respiratory Questionnaire; TDI: Transition Dyspnoea Index | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded for inconsistency because of moderate‐substantial heterogeneity (I² > 30%). 2Downgraded for indirectness because an included study had a significantly higher proportion of participants with significant reversibility/without a history of recent exacerbations (exacerbation outcomes only). 3Downgraded for indirectness because an included study used different LABA agents (non‐intervention medication) in the treatment arms (applied if outcome not otherwise downgraded for indirectness). 4Downgraded for imprecision because the 95% CI includes both an important benefit/harm and line of no effect. 5Downgraded for imprecision because of small number of events/optimal information size not met (applied if outcome not otherwise downgraded for imprecision).
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is a common respiratory condition associated with substantial morbidity and mortality (GOLD 2023). It is currently the fourth leading cause of death worldwide (WHO 2012). In the Burden of Obstructive Lung Disease (BOLD) initiative, a large international study of COPD, the prevalence of stage II or higher COPD was reported to be 10.1% using the Global Initiative for Obstructive Lung Disease (GOLD) definition of disease, but considerably more common in the elderly (Buist 2007; GOLD 2023).
The most common cause of COPD is tobacco smoke exposure. However, occupational dusts and air pollution have been identified as strong independent risk factors (GOLD 2023). Lumenal airway inflammation in COPD, caused by inhalation of noxious agents, causes reactive oxygen species (ROS) epithelial activation, small airways fibrosis and obliteration and parenchymal destruction (Baraldo 2012). These changes impair expiratory flow and result in both resting and dynamic hyperinflation of the lungs (Bateman 2014). The characteristic symptoms of COPD include persistent breathlessness, cough, and sputum production, all of which are usually progressive in nature.
The natural progression of COPD is characterised by episodes of acute or subacute clinical deterioration, termed exacerbations, which involve both increased airway and systemic inflammation (Wedzicha 2013). COPD exacerbations are associated with an increased risk of mortality, poorer quality of life, increased hyperinflation, and long‐term decline in lung function (Rennard 2004; van Geffen 2018; Wang 2005). These effects are greater in those who experience frequent exacerbations (Halpin 2012; Vestbo 2011). Pharmacological and medical treatment of COPD is therefore aimed at relieving symptoms, improving quality of life, and preventing exacerbations. Treating specific traits (e.g. hyperinflation) might also improve quality of life and relieve symptoms (GOLD 2023; van Geffen 2019).
Description of the intervention
Bronchodilators are a key aspect of the pharmacological management of COPD, usually following a stepwise approach in which short‐acting agents are used initially to control intermittent acute symptoms (COPDX 2019; GOLD 2023; NICE 2019). In individuals with persistent symptoms, combined bronchodilators of different classes are recommended to maximise bronchodilation. Inhaled long‐acting beta2‐agonists (LABA) and long‐acting muscarinic antagonists (LAMA) are now most commonly used. LABA and LAMA can be taken together in one inhaler, called a 'combination inhaler'. Combination inhalers have been found to improve adherence to treatment and patient outcomes. LABA and LAMA and inhaled corticosteroids (ICS) can also be taken together, known as 'triple therapy'.
The mechanism of bronchodilation by LABA and LAMA agents is achieved by different pathways. Beta2‐agonists stimulate adenylyl cyclase activity and directly cause smooth muscle relaxation, whilst muscarinic antagonists inhibit the action of acetylcholine on airway muscarinic receptors and cause smooth muscle relaxation (de Miguel‐Díez 2014; Montuschi 2013). Drugs in both classes of bronchodilator, LABA and LAMA, are beneficial for quality of life and lung function and reduce hyperinflation in COPD both individually and when given together (Kew 2014a).
Although dual bronchodilation with LABA and LAMA agents have conventionally been administered via separate inhalers, new combination inhalers have been developed. These combination inhalers may promote adherence to treatment and improve patient‐centred outcomes (van der Molen 2012). Moreover, combination LABA/LAMA inhalers have demonstrated superior efficacy compared to the individual components, with similar safety profiles (Farne 2015; Montuschi 2015; Rodrigo 2014). Current combinations include:
aclidinium/formoterol;
glycopyrronium/indacaterol;
umeclidinium/vilanterol; and
tiotropium/olodaterol.
GOLD guidelines recommend triple therapy with ICS, LABA, and LAMA in patients with persistent symptoms and a high risk of exacerbations (Group D) who are unresponsive to ICS/LABA or LAMA therapy alone (GOLD 2023).
However, evidence on the efficacy of triple therapy compared to dual bronchodilator therapy is limited to a small number of clinical trials (Gaebel 2011). Findings from the WISDOM (Withdrawal of Inhaled Glucocorticoids and Exacerbations of COPD) trial suggest that the beneficial effect of ICS therapy may be limited to achieving an initial phase of clinical stability in people with stable COPD. Even so, the long‐term role of ICS therapy in COPD management remains unclear (Magnussen 2014). In particular, little is known specifically about the efficacy of ICS used in addition to new combination LABA/LAMA inhalers. This topic will be the focus of this review.
How the intervention might work
As individual classes of treatment in COPD, LABA and LAMA are both effective in improving health‐related quality of life, improving lung function outcomes (Geake 2015), and reducing exacerbations (Kew 2013). A slightly larger improvement in health‐related quality of life and lung function is seen when tiotropium (LAMA) is added to a LABA, compared with either agent alone (Farne 2015; Karner 2012). A network meta‐analysis of treatment options specifically for people with severe COPD (forced expiratory volume (FEV) predicted 40% to 50%) who required additional treatment to short‐acting bronchodilators, found that combination ICS/LABA was the highest‐ranked intervention for improving quality of life compared to placebo at six and 12 months (Kew 2014a). LAMA and LABA therapy were independently ranked second and third, and ICS alone was ranked fourth at six months, although class differences between LABA, LAMA, and ICS were less prominent at 12 months.
ICS are anti‐inflammatory drugs that reduce the rate of exacerbation and improve quality of life in COPD, although they do not have a significant effect on overall mortality or long‐term decline in forced expiratory volume in one second (FEV1) (Yang 2012). Responsiveness to ICS varies (Miravitlles 2011), which may be related to the presence/absence of an airway luminal eosinophilic inflammatory profile (Miravitlles 2011; Siva 2007), an obstructive‐dominant rather than an emphysema‐dominant phenotype (Lee 2009), or bronchial hyperresponsiveness (BHR) (Leuppi 2005). However, ICS delivered alone or in combination with a LABA is also associated with an increased risk of pneumonia (Kew 2014b; Spencer 2011). There is currently insufficient evidence to confirm an overall benefit of adding ICS to combination LABA/LAMA inhalers in COPD (GOLD 2023; Karner 2011).
Why it is important to do this review
The combined data of the large trials assessing triple therapy for the treatment of COPD are yet to be assessed. The latest GOLD report includes a pooled analysis of three trials (GOLD 2023), but not all trials have been systematically combined; therefore, the effects of adding ICS to combination LABA/LAMA inhalers for COPD remains unclear. This review is an update of a previously published review (Tan 2016).
Objectives
To assess the effects of adding an inhaled corticosteroid (ICS) to combination long‐acting beta2‐agonist (LABA)/long‐acting muscarinic antagonist (LAMA) inhalers for the treatment of stable chronic obstructive pulmonary disease (COPD).
Methods
Criteria for considering studies for this review
Types of studies
We included parallel‐group randomised controlled trials (RCTs) that were reported as full‐text articles, abstract only, or as unpublished data. We excluded studies of less than three weeks' duration due to the expected delayed onset of ICS activity. Quasi‐randomised trials were not eligible for inclusion in the review.
Types of participants
We included all participants with a diagnosis of stable COPD and recorded the study authors' definition of stable COPD and its severity. We did not exclude participants with significant comorbidities. Studies with only a subset of eligible participants were included if data for the eligible subset were reported separately. In such cases, only data for participants meeting our inclusion criteria were extracted.
Types of interventions
We included studies comparing combination LABA/LAMA inhalers plus an ICS versus combination LABA/LAMA combination inhalers without an ICS (with or without placebo control).
Types of outcome measures
Primary outcomes
Acute exacerbation of COPD (AECOPD): defined as needing treatment with oral steroids, antibiotics, or hospital attendance, or some combination of such management, for a COPD exacerbation. We also accepted exacerbation events based on standardised patient‐reported outcome tools for measuring changes in symptoms.
Respiratory health‐related quality of life (HRQoL): measured by the Chronic Respiratory Questionnaire (CRQ) or St George's Respiratory Questionnaire (SGRQ).
Pneumonia and other serious adverse events: defined as requiring treatment or hospital admission for pneumonia or other (all‐cause) serious adverse events.
Secondary outcomes
Symptom score: measures of breathlessness, cough, wheeze, and sputum production, preferably using validated scales.
Lung function: pre‐bronchodilator (BD) and post‐BD measures, including FEV and forced vital capacity (FVC).
Physical capacity: measures including timed walking tests, endurance tests.
Mortality
Reporting one or more of the outcomes listed here was not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Information Specialist for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED), and PsycINFO, and through handsearching of respiratory journals and meeting abstracts (see Appendix 1). We searched all records in the CAGR using the search strategy presented in Appendix 2 up to 30 November 2022.
We also searched the following databases on 30 November 2022:
CENTRAL (2022, Issue 11; the Cochrane Library);
MEDLINE via PubMed (1946 to 30 November 2022);
Embase via Embase.com (1980 to 2022 week 48).
See Appendix 3 for further details. We imposed no restriction on the language of publication.
Searching other resources
We screened the reference lists of all primary studies and review articles for additional references. We also conducted a search of ClinicalTrials.gov (clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/) up to 30 November 2022.
Data collection and analysis
Selection of studies
Two review authors (DT, WVG) independently screened the titles and abstracts of all records identified as a result of the search. We retrieved the full texts of eligible or potentially eligible records, and two review authors (DT, WVG) independently screened the full texts to identify studies for inclusion and recorded the reasons for exclusion of excluded studies. Any disagreements were resolved through discussion, or in consultation with a third review author (JW) if required. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process as a PRISMA flow diagram.
Data extraction and management
Two review authors (DT, WVG) independently extracted outcome data from the included studies using a data collection form. We extracted the following data.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, withdrawals, and date of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected and time points reported.
Other: funding for study and notable conflicts of interest of study authors.
When disagreements occurred, a third review author (JW) was consulted as arbiter. One review author (DT) transferred data into Review Manager 5 (Review Manager 2020), and the data entry was double‐checked by a member of the Cochrane Airways Group editorial team (CC). Results presented in graphical form were extracted using PlotDigitizer software (PlotDigitizer).
Assessment of risk of bias in included studies
Two review authors (DT, WVG) independently assessed risk of bias for each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any areas of disagreement were discussed with a third review author (JW). We assessed risk of bias based on the following risk of bias domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other biases.
We graded each domain as low, high, or unclear and provided a quote from the study report along with a justification for our judgement in a risk of bias table. We summarised the risk of bias judgements across different studies for each domain listed.
Measures of treatment effect
We analysed dichotomous data as odds ratios (ORs) and continuous data as mean differences (MDs) or standardised mean differences (SMDs) with the respective 95% CIs, and narratively described skewed data reported as medians and interquartile ranges. We undertook meta‐analyses only of studies with sufficient similarity (e.g. treatments, participants, clinical question) to derive meaningful pooled estimates.
Unit of analysis issues
We used participants, rather than events, as the unit of analysis to avoid counting participants more than once.
Where multiple arms were reported in a single trial, we only included relevant study arms. Where two comparisons were combined in the same meta‐analysis, we halved the control group to avoid double‐counting; we used this approach to assess data from the Rabe 2020 study. We used the methods described by Rücker 2017 to meta‐analyse rate ratio (RR) and hazard ratio (HR) outcomes in Rabe 2020 by multiplying the standard error with √1.5. We did not include cluster‐RCTs, as this study design was not viable to addressing the review question. We also did not include cross‐over RCTs due to the expected carryover effects of ICS.
Dealing with missing data
We contacted investigators to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was identified as an abstract only). If this information was not available, and the study authors did not report the true intention‐to‐treat (ITT) data, we would perform an available‐case analysis by including data for all participants for whom data were collected (whether or not participants completed the trial) and conduct sensitivity analyses to explore the impact of including such studies in the overall assessment of results.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity amongst the studies in each analysis. We interpreted statistical heterogeneity as follows (Higgins 2022):
0% to 40%: might not be important;
30% to 60%: moderate heterogeneity;
50% to 90%: substantial heterogeneity;
75% to 100%: considerable heterogeneity.
When we found substantial heterogeneity, we reported this and explored possible causes by performing prespecified subgroup analysis.
Assessment of reporting biases
We planned to create standard funnel plots to explore possible small‐study and publication biases if we pooled more than 10 studies. In future updates, we will assess reporting bias by visual inspection of the funnel plots and the formal Egger test for funnel plot asymmetry, according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022).
Data synthesis
In the absence of moderate‐substantial heterogeneity, we combined study results using a fixed‐effect model. Where we judged heterogeneity to be important (I² > 40%), we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We intended to explore potential causes of heterogeneity by performing the following subgroup analyses.
Different LAMA/LABA combinations.
Baseline COPD severity (severe to very severe versus mixed population) as defined by the individual studies.
Blood or sputum eosinophilia (eosinophilia versus no eosinophilia).
Length of follow‐up (less than six months versus six months or longer).
Baseline ICS use (participants with baseline ICS use versus participants without baseline ICS use).
However, due to the small number of included studies, an informative subgroup analysis could only be performed for high versus low blood eosinophil counts.
Sensitivity analysis
We intended to carry out the following sensitivity analyses.
A comparison based on our risk of bias assessments.
A comparison of results from fixed‐effect models versus results from random‐effects models.
A comparison of available‐case analyses versus true ITT analyses, when the ITT analyses are imputed with best‐case and worse‐case outcome data.
We performed a sensitivity analysis for all exacerbation outcomes in which data from Ferguson 2018 were excluded. This study had a much higher proportion of participants with significant reversibility and participants without a history of recent exacerbations compared to the other included studies. Ferguson 2018 also had a shorter duration of follow‐up (24 weeks versus 52 weeks in the other studies).
We performed an additional sensitivity analysis for all primary and secondary outcomes in which data from Papi 2018 were excluded. This study used different LABA agents in the comparison arms (LABA/LAMA: indacaterol/glycopyrronium; triple therapy: beclometasone/formoterol/glycopyrronium). Papi 2018 also used different inhaler devices and drug particle sizes in the comparison arms (LABA/LAMA: single‐dose dry powder inhaler; triple therapy: pressurised metred dose inhaler with extrafine formulation). All primary and secondary outcomes were examined due to these important differences between comparison arms.
Summary of findings and assessment of the certainty of the evidence
We included the following outcomes in the summary of findings table: rate of moderate‐to‐severe exacerbations, rate of severe exacerbations, respiratory HRQoL, pneumonia as a serious adverse event, all‐cause serious adverse events, symptom score, lung function, and mortality.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for studies that contributed data to our prespecified outcomes. We used GRADEpro GDT software to develop the summary of findings table (GRADEpro GDT). We justified all decisions to upgrade or downgrade the certainty of evidence using footnotes and made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
In the original 2016 search, 586 records were identified, and no studies met the inclusion criteria. With the 2022 search, 2840 records were identified; after duplicates were removed, 1628 records were screened, of which we assessed 1571 as clearly irrelevant based on their titles or abstracts, or both (Figure 1). We assessed the remaining 57 records in full text and identified four studies as suitable for inclusion in the review (Ferguson 2018; Lipson 2018; Papi 2018; Rabe 2020).
1.
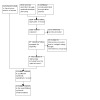
PRISMA study flow diagram.
Included studies
The four included studies, Ferguson 2018 (KRONOS), Lipson 2018 (IMPACT), Papi 2018 (TRIBUTE), and Rabe 2020 (ETHOS), were multinational trials based in outpatient settings that recruited symptomatic COPD patients with COPD Assessment Test (CAT) scores ≥ 10. COPD was defined according to international GOLD or American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines in all included studies (Characteristics of included studies; Table 2; Table 3).
1. Baseline characteristics of participants in the included studies.
| Study ID | Mean age (years)* | Male (%) | Current smoker (%) | Pre‐bronchodilator FEV1 L* | Significant bronchodilator reversibility (%) | CAT score | History of exacerbations prior to study entry (%) | Baseline eosinophil counts (cells/µL)* | Use of ICS at screening |
| Ferguson 2018 | 65.0 (7.7) | 70% | 41% | NR | 44% | 18.4 (6.3) | 25% | Counts NR (48% had blood eosinophils < 150 cells/µL) |
78% |
| Lipson 2018 | 65.3 (8.2) | 66% | 35% | 1.17 (0.47) | 18% (Significant reversibility cut‐off: ≥ 12% and ≥ 200 mL from baseline) |
20.1 (6.1) | 100% | Counts NR (43% had blood eosinophils < 150 cells/µL) |
72% |
| Papi 2018 | 64.4 (7.7) | 72% | 45% | 1.07 (0.31) | 33% (Significant reversibility cut‐off: > 12% from baseline) |
NR | 100% | 235 (200) | 65% |
| Rabe 2020 | 64.6 (7.6) | 60% | 41% | NR | 31% | 19.6 (6.6) | 100% | Counts NR, estimated median 167 | 80% |
*Data presented as mean (SD).
Abbreviations: CAT: Chronic Obstructive Pulmonary Disease Assessment Test; FEV1: forced expiratory volume in 1 second; ICS: inhaled corticosteroids; NR: not reported; SD: standard deviation.
2. Additional baseline characteristics of included studies.
| Study ID | Number of participants | Study duration (weeks) | Experimental interventions, dose in µg, dosing schedule | Comparator interventions, dose in µg, dosing schedule |
| Ferguson 2018 | 1267 | 24 | BUD/GLY/FM 320/18/9.6, 2 inhalations, twice‐daily | GLY/FM 18/9.6, 2 inhalations, twice‐daily |
| Lipson 2018 | 6221 | 52 | FF/UMEC/VI 100/62.5/25, 1 inhalation, once‐daily | UMEC/VI 62.5/25, 1 inhalation, once‐daily |
| Papi 2018 | 1532 | 52 | Extrafine BDP/FM/GLY 67/5/92, 2 inhalations, twice‐daily | IND/GLY 85/43, 1 inhalation, once‐daily |
| Rabe 2020 | 6438 | 52 | BUD/GLY/FM 320/18/9.6, 1 inhalation, once‐daily; BUD/GLY/FM 160/18/9.6, 1 inhalation, once‐daily |
GLY/FM 18/9.6, 1 inhalation, once‐daily |
BDP: beclometasone; BUD: budesonide; EF: extrafine; FF: fluticasone furoate; FM: formoterol; GLY: glycopyrronium; IND: indacaterol; UMEC: umeclidinium; VI: vilanterol
Two studies recruited participants with severe‐to‐very severe COPD (FEV1 < 50% predicted) and at least one moderate‐to‐severe exacerbation in the last 12 months (Lipson 2018; Papi 2018); Lipson 2018 also included participants with moderate COPD (FEV1 50% to 80%) who had two or more moderate‐to‐severe exacerbations in the last 12 months. Rabe 2020 included patients with an FEV1 between 25% and 65% of predicted and at least one moderate‐to‐severe exacerbation in the last 12 months. In comparison, Ferguson 2018 recruited participants with moderate‐to‐very severe COPD (FEV1 25% to 80%), irrespective of exacerbation history. Notably, only 25% of participants in Ferguson 2018 had a history of moderate‐to‐severe exacerbations in the preceding 12 months, as compared to 100% in Lipson 2018 and Papi 2018. Three studies excluded participants with a concurrent diagnosis of asthma (Ferguson 2018; Papi 2018; Rabe 2020); however, the proportion of participants with significant bronchodilator reversibility at baseline was higher in these studies (44% in Ferguson 2018; 33% in Papi 2018; 31% in Rabe 2020) compared to Lipson 2018, which did not exclude participants with a history of asthma (18%).
We included a total of 15,412 participants from the four studies. Individual study sizes ranged from 1266 to 8509 participants. The mean age of study participants ranged from 64.4 to 65.3 years; the proportion of female participants ranged from 28% to 40%. Each study used a different LABA/LAMA/ICS combination. Lipson 2018 compared fluticasone furoate/umeclidinium/vilanterol (100 µg/62.5 µg/25 µg, 1 inhalation, once‐daily) to umeclidinium/vilanterol (62.5 µg/25 µg, 1 inhalation, once‐daily); Papi 2018 compared extrafine beclometasone/formoterol/glycopyrronium (87 µg/5 µg/9 µg, 2 inhalations, twice‐daily) to indacaterol/glycopyrronium (85 µg/43 µg, 1 inhalation, once‐daily); and Ferguson 2018 compared budesonide/glycopyrronium/formoterol (320 µg/18 µg/9.6 µg, 2 inhalations, twice‐daily) to glycopyrronium/formoterol (18 µg/9.6 µg, 2 inhalations, twice daily). Run‐in protocols also differed between studies. In Rabe 2020 four arms were used, of which this review included three (the budesonide/formoterol arm was excluded). The included arms used budesonide (80 μg or 160 μg per inhaler actuation; analysed as Rabe 2020 (160 μg budesonide) and Rabe 2020 (320 μg budesonide) in Data and analyses, respectively), a LAMA glycopyrronium (9 μg per actuation), and a LABA formoterol fumarate (4.8 μg per actuation) twice a day for 52 weeks. It is worth noting the different formulations/delivery mechanisms of inhalers across the included studies. Fluticasone furoate/umeclidinium/vilanterol is a dry powder delivered through a dry‐powder inhaler (DPI). Beclometasone dipropionate/formoterol/glycopyrronium is delivered through a pressurised metred‐dose inhaler (pMDI); budesonide/formoterol fumarate/glycopyrronium is also delivered through a pMDI.
In Lipson 2018, participants continued regular medications for two weeks; in Papi 2018, participants used indacaterol/glycopyrronium for two weeks; in Ferguson 2018, participants continued regular ICS as monotherapy, and long‐acting bronchodilators were swapped to regular ipratropium during a screening period of one to two weeks.
Excluded studies
We excluded a total of 49 studies after full‐text assessment. Eighteen studies were post hoc or subanalyses of included studies; six studies were reviews; and three studies were withdrawal studies. We excluded nine studies due to ineligible study design features. We excluded several studies based on issues related to the interventions/comparators: three trials because they compared a triple combination with another triple combination; seven trials because they used separate inhalers instead of a single inhaler; and four trials because they used a LABA + ICS (long‐acting beta‐agonist + inhaled corticosteroid) combination as the comparator, which did not align with the desired comparison. For details, see Characteristics of excluded studies.
Risk of bias in included studies
Risk of bias for the included studies is summarised in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary figure presenting all judgements in a cross‐tabulation of study by entry.
Allocation
We assessed randomisation procedure, sequence generation, and allocation concealment as being adequate in all studies (Ferguson 2018; Lipson 2018; Papi 2018; Rabe 2020).
Blinding
All studies were double‐blinded for participants, personnel, and outcome assessors (Ferguson 2018; Lipson 2018; Papi 2018; Rabe 2020).
Incomplete outcome data
Three studies adequately addressed incomplete data (Ferguson 2018; Papi 2018; Rabe 2020). We judged risk of bias as high in one study because a higher proportion of participants discontinued treatment prematurely with combination LABA/LAMA inhalers (27% total; 8% due to lack of efficacy) compared to triple therapy (18% total; 4% due to lack of efficacy) (Lipson 2018).
Selective reporting
We assessed two studies as free of selective reporting (Lipson 2018; Papi 2018). We also judged risk of bias for selective reporting as low in Rabe 2020, in which all outcomes were reported, although Transition Dyspnoea Index (TDI) outcomes were only reported at 24 weeks (52‐week outcome not reported). We judged risk of bias as high in one study that made changes to ranking of outcomes (primary or secondary) and reduced the non‐inferiority cut‐off point for TDI from 1.0 to 0.75 after trial commencement (Ferguson 2018).
Other potential sources of bias
We judged Ferguson 2018 to be at high risk of bias because two different LABA agents were used in the control and intervention arm (indacaterol in the LABA/LAMA arm and formoterol in the triple therapy arm).
Effects of interventions
See: Table 1
See: Table 1 for the main comparison: triple therapy compared to combination LABA/LAMA inhalers for chronic obstructive pulmonary disease.
1. Primary outcomes
1.1. Acute exacerbations of COPD (AECOPD)
AECOPD was defined as "an acute worsening of COPD symptoms above normal day‐to‐day variation" in all four studies. Severity was rated according to the level of treatment: mild (short‐acting bronchodilators), moderate (systemic glucocorticoids or antibiotics), or severe (hospitalisation or death, or both).
Triple therapy may reduce rates of moderate‐to‐severe COPD exacerbations compared to combination LABA/LAMA inhalers (rate ratio (RR) 0.74, 95% confidence interval (CI) 0.67 to 0.81; n = 15,397; low‐certainty evidence; Analysis 1.1). Time to next moderate‐to‐severe COPD exacerbation may be longer in those treated with triple therapy (hazard ratio (HR) 0.84, 95% CI 0.77 to 0.92; n = 15,397; low‐certainty evidence; Analysis 1.2). There was substantial heterogeneity across studies for both outcomes (I2 = 68% and 52%, respectively). Triple therapy may reduce the odds of developing ≥ 1 moderate‐to‐severe COPD exacerbations (odds ratio (OR) 0.80, 95% CI 0.64 to 0.99; n = 6109; low‐certainty evidence; Analysis 1.3). Triple therapy may be associated with a reduced rate of severe COPD exacerbations (RR 0.75, 95% CI 0.67 to 0.84; n = 14,131; low‐certainty evidence; Analysis 1.6) and longer time to next severe COPD exacerbation (HR 0.77, 95% CI 0.67 to 0.88; n = 7753; low‐certainty evidence; Analysis 1.7). Based on data from Lipson 2018, the rate of all COPD exacerbations may be reduced by triple therapy compared to combination LABA/LAMA inhalers (RR 0.75, 95% CI 0.70 to 0.81; n = 4151; Analysis 1.8).
1.1. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 1: Moderate‐to‐severe exacerbations (Rate)
1.2. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 2: Moderate‐to‐severe exacerbations (Time‐to‐first)
1.3. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 3: Moderate‐to‐severe exacerbations (≥ 1 exacerbation)
1.6. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 6: Severe exacerbations (Rate)
1.7. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 7: Severe exacerbations (Time‐to‐first)
1.8. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 8: All exacerbations (Rate)
When stratified by blood eosinophil counts, the reduction in rate of moderate‐to‐severe COPD exacerbations with triple therapy may be greater in those with high‐eosinophils (RR 0.67, 95% CI 0.60 to 0.75) compared to low‐eosinophils (RR 0.87, 95% CI 0.81 to 0.93) (test for subgroup differences: P < 0.01; Analysis 1.4) (high/low cut‐offs: 150 eosinophils/µL in Lipson 2018, Ferguson 2018, Rabe 2020; 200 eosinophils/µL in Papi 2018). However, as we observed moderate‐to‐substantial heterogeneity in both high‐ and low‐eosinophil subgroups, and the test for subgroup differences was borderline, we assessed the evidence for this subgroup difference to be of low certainty. Stratification by baseline ICS usage showed no difference in the reduction of moderate‐to‐severe COPD exacerbations (participants with baseline ICS use: RR 0.75, 95% CI 0.69 to 0.82; without baseline ICS use: RR 0.75, 95% CI 0.62 to 0.91; test for subgroup differences: P = 1.00; Analysis 1.5).
1.4. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 4: Moderate‐to‐severe exacerbations (Rate, stratified by eosinophil count)
1.5. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 5: Moderate‐to‐severe exacerbations (Rate, stratified by baseline ICS use)
1.2. Respiratory health‐related quality of life (HRQoL)
All four studies measured HRQoL using St George's Respiratory Questionnaire (SGRQ) (range 0 to 100, lower scores indicate better HRQoL; minimal clinically important difference (MCID): 4 points). Triple therapy improved SGRQ over the duration of the studies compared to combination LABA/LAMA inhalers (mean difference (MD) −1.65, 95% CI −2.15 to −1.15; n = 13,879; high‐certainty evidence; Analysis 1.9). Three studies also measured SGRQ as a categorical positive outcome (≥ 4‐point decrease). The proportion of participants who met this threshold was higher amongst those treated with triple therapy (OR 1.35, 95% CI 1.26 to 1.45; n = 14,070; high‐certainty evidence; Analysis 1.10). Lipson 2018 reported improved HRQoL measured by the SGRQ at end of study (MD −1.80, 95% CI −2.59 to −1.01; Analysis 1.11).
1.9. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 9: Change in SGRQ
1.10. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 10: SGRQ, ≥ 4 decrease from baseline
1.11. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 11: SGRQ, end of study
1.3. Pneumonia
All four included studies reported pneumonia. The studies reported two different outcomes: diagnosed/confirmed pneumonia and pneumonia labelled as serious adverse event. The definition of pneumonia as a serious adverse event was provided by regulatory bodies and included pneumonia leading to hospitalisation, death, or life‐threatening pneumonia. As the severity and incidence of pneumonia varied between the different two outcomes reported in the included studies, we decided to report them separately.
Four studies reported diagnosed/confirmed pneumonia. Our meta‐analysis shows that risk of diagnosed/confirmed pneumonia was probably higher in those treated with triple therapy compared to combination LABA/LAMA inhalers (OR 1.59, 95% CI 1.33 to 1.89; n = 15,412; moderate‐certainty evidence; Analysis 1.12). This equated to an absolute effect of additional 18 participants diagnosed with pneumonia (95% CI 10 to 27) per 1000 treated with triple therapy. Time to first pneumonia was probably also shorter with triple therapy (moderate‐certainty evidence; Analysis 1.13).
1.12. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 12: Pneumonia
1.13. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 13: Pneumonia (Time‐to‐first)
Four studies reported pneumonia labelled as a serious adverse event. The risk of pneumonia as a serious adverse event was probably higher with triple therapy compared to combination LABA/LAMA inhalers (OR 1.74, 95% CI 1.39 to 2.18; n = 15,412; moderate‐certainty evidence; Analysis 1.14). This equated to an absolute effect of an additional 13 participants developing pneumonia as a serious adverse event (95% CI 7 to 21) per 1000 treated with triple therapy.
1.14. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 14: Serious adverse events, pneumonia
1.4. All‐cause serious adverse events
All four studies reported all‐cause serious adverse events, and these may be similar between the triple therapy group and the combinaton LABA/LAMA group (OR 0.95, 95% CI 0.87 to 1.03; n = 15,412; low‐certainty evidence; Analysis 1.15). This equated to 8 fewer participants experiencing serious adverse events (95% CI 21 fewer to 5 more) per 1000 treated with triple therapy.
1.15. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 15: Serious adverse event, all‐cause
2. Secondary outcomes
2.1. Symptom score
Two studies measured symptom scores using the Evaluating Respiratory Symptoms in COPD scale (E‐RS: range 0 to 40, lower scores indicate less severe symptoms). Triple therapy may reduce E‐RS over the duration of the studies compared to combination LABA/LAMA inhalers (MD −0.30, 95% CI −0.59 to −0.00; n = 2798; low‐certainty evidence; Analysis 1.16). This improvement fell below the E‐RS MCID threshold of 3 units.
1.16. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 16: Change in E‐RS
Dyspnoea was measured in three studies using the Transition Dyspnoea Index (TDI: range −9 to +9, lower scores indicate worse dyspnoea; MCID: 1 unit). The proportion of participants who achieved the MCID cut‐off for TDI (≥ 1‐unit increase) was probably higher in those treated with triple therapy (OR 1.33, 95% CI 1.13 to 1.57; n = 3044; moderate‐certainty evidence; Analysis 1.17). This equated to 300 responders per 1000 treated with LABA/LAMA combination compared to 363 (95% CI 326 to 402) per 1000 treated with triple therapy. In contrast, there may be a trivial difference in TDI measured at the end of one study between triple therapy and combination LABA/LAMA inhalers (MD 0.18, 95% CI −0.07 to 0.43; n = 1266; low‐certainty evidence; Analysis 1.18) (Ferguson 2018). However, one other study did show a slightly larger difference between groups (MD 0.40, 95% CI 0.20 to 0.60; low‐certainty evidence; Analysis 1.18) (Rabe 2020).
1.17. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 17: TDI, ≥ 1‐unit increase
1.18. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 18: TDI (Focal score), over 24 weeks
2.2. Lung function
All four included studies measured change in trough FEV1. Triple therapy may improve trough FEV1 over the duration of the studies compared to combination LABA/LAMA inhalers (MD 38.68 mL, 95% CI 22.58 to 54.77; n = 11,352; low‐certainty evidence; Analysis 1.19). However, this improvement fell below the MCID threshold of 100 mL, and there was substantial heterogeneity for this outcome (I2 = 62%). Trough FEV1 measured at the end of one study was probably higher with triple therapy (MD 54.00 mL, 95% CI 39.29 to 68.71; n = 6221; moderate‐certainty evidence; Analysis 1.20) (Lipson 2018). In comparison, FEV1 area under the curve from 0 to 4 hours did not appear to differ between triple therapy and combination LABA/LAMA inhalers (MD 17.00 mL, 95% CI −6.42 to 40.42; n = 1266; low‐certainty evidence; Analysis 1.21). Of note, time to action is an outcome providing information regarding the time to action of inhaled bronchodilation and is often reported as FEV1 area under the curve (taken from several serial time points pre‐ and post‐dose) (Beeh 2021; van Geffen 2016; van Geffen 2020).
1.19. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 19: Change in trough FEV1 (mL)
1.20. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 20: Trough FEV1, end of study (mL)
1.21. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 21: FEV1 AUC 0 to 4 (mL), end of study
Change in trough FVC did not appear to differ between triple therapy and combination LABA/LAMA inhalers (MD 24.41 mL, 95% CI −21.68 to 70.50; n = 1532; low‐certainty evidence; Analysis 1.22).
1.22. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 22: Change in trough FVC (mL)
2.3. Physical capacity
None of the included studies reported physical capacity.
2.4. Mortality
The four included studies reported all‐cause mortality, and this may be lower amongst those treated with triple therapy compared to those treated with combination LABA/LAMA inhalers (OR 0.70, 95% CI 0.54 to 0.90; n = 15,397; low‐certainty evidence; Analysis 1.23). This equated to 6 fewer deaths (95% CI 9 to 2 fewer) per 1000 treated with triple therapy. Time to death may be longer with triple therapy in three studies (low‐certainty evidence; Analysis 1.24).
1.23. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 23: Death, all causes
1.24. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 24: Death, all causes (Time‐to‐event)
Three studies reported cause‐specific mortality. Triple therapy may reduce cardiovascular mortality (OR 0.51, 95% CI 0.34 to 0.77; n = 13,865; Analysis 1.25) but not respiratory mortality (n = 11,539; Analysis 1.26), and may reduce COPD‐related mortality (OR 0.60, 95% CI 0.30 to 1.19; n = 6221; Analysis 1.27). However, the evidence for these outcomes was of very low certainty due to wide CIs that included both important potential benefits and no effect.
1.25. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 25: Death, cardiovascular causes
1.26. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 26: Death, respiratory causes
1.27. Analysis.

Comparison 1: Triple therapy versus LABA/LAMA combination, Outcome 27: Death, COPD‐related
3. Sensitivity analysis
In the sensitivity analysis excluding Ferguson 2018 for all exacerbation outcomes, a small reduction in the magnitude of the effect of triple therapy on rate of moderate‐to‐severe exacerbations (RR 0.76, 95% CI 0.72 to 0.82) and time to first moderate‐to‐severe exacerbation (HR 0.86, 95% CI 0.82 to 0.91) was observed. There was no evidence of statistical heterogeneity in these analyses (I2 = 0% and 0%, respectively). Of note, for the aforementioned eosinophil stratified analysis (Analysis 1.4), exclusion of Ferguson 2018 may have resulted in a similar reduction in the effect of triple therapy on moderate‐to‐severe exacerbations in both high‐ (RR 0.69, 95% CI 0.65 to 0.72) and low‐eosinophil (RR 0.78, 95% CI 0.70 to 0.86) subgroups (test for subgroup differences: P < 0.01).
We conducted an additional sensitivity analysis by excluding data from one trial that used different LAMA/LABA combinations in the triple therapy and comparator groups (Papi 2018). The findings of this sensitivity analysis did not differ materially from the main analysis for any of our primary or secondary outcomes (Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5; Analysis 2.6; Analysis 2.7; Analysis 2.8; Analysis 2.9; Analysis 2.10; Analysis 2.11; Analysis 2.12; Analysis 2.13; Analysis 2.14; Analysis 2.15; Analysis 2.16; Analysis 2.17; Analysis 2.18; Analysis 2.19; Analysis 2.20; Analysis 2.21; Analysis 2.22; Analysis 2.23; Analysis 2.24; Analysis 2.25; Analysis 2.26; Analysis 2.27). Of note, because the certainty of evidence was downgraded for indirectness in analyses including Papi 2018, its exclusion raised the certainty of evidence for most outcomes. These changes included all‐cause serious adverse events (OR 0.96, 95% CI 0.88 to 1.04; n = 13,880; high‐certainty evidence; Analysis 2.15); change in trough FEV1 (MD +42.70 mL, 95% CI 25.01 to 60.38; n = 9820; moderate‐certainty evidence; Analysis 2.19); and all‐cause mortality (OR 0.69, 95% CI 0.53 to 0.91; n = 13,865; moderate‐certainty evidence; Analysis 2.23).
2.1. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 1: Moderate‐to‐severe exacerbations (Rate)
2.2. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 2: Moderate‐to‐severe exacerbations (Time‐to‐first)
2.3. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 3: Moderate‐to‐severe exacerbations (≥ 1 exacerbation)
2.4. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 4: Moderate‐to‐severe exacerbations (Rate, stratified by eosinophil count)
2.5. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 5: Moderate‐to‐severe exacerbations (Rate, stratified by baseline ICS use)
2.6. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 6: Severe exacerbations (Rate)
2.7. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 7: Severe exacerbations (Time‐to‐first)
2.8. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 8: All exacerbations (Rate)
2.9. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 9: Change in SGRQ
2.10. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 10: SGRQ, ≥ 4 decrease from baseline
2.11. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 11: SGRQ, end of study
2.12. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 12: Pneumonia
2.13. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 13: Pneumonia (Time‐to‐first)
2.14. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 14: Serious adverse events, pneumonia
2.15. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 15: Serious adverse event, all‐cause
2.16. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 16: Change in E‐RS
2.17. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 17: TDI, ≥ 1‐unit increase
2.18. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 18: TDI (Focal score), over 24 weeks
2.19. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 19: Change in trough FEV1 (mL)
2.20. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 20: Trough FEV1, end of study (mL)
2.21. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 21: FEV1 AUC 0 to 4 (mL), end of study
2.22. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 22: Change in trough FVC (mL)
2.23. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 23: Death, all causes
2.24. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 24: Death, all causes (Time‐to‐event)
2.25. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 25: Death, cardiovascular causes
2.26. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 26: Death, respiratory causes
2.27. Analysis.

Comparison 2: Triple therapy versus LABA/LAMA combination – Exclude Papi 2018, Outcome 27: Death, COPD‐related
Discussion
Summary of main results
For this update of a previous review (Tan 2016), we identified four eligible RCTs (Ferguson 2018 (KRONOS); Lipson 2018 (IMPACT); Papi 2018 (TRIBUTE); Rabe 2020 (ETHOS)) that included 15,412 participants with moderate‐to‐very severe, symptomatic COPD. Compared to combination LABA/LAMA inhalers, triple therapy may reduce rates of both moderate‐to‐severe exacerbations and severe exacerbations (low‐certainty evidence). The reduction in rate of moderate‐to‐severe exacerbations may be greater in those with high‐eosinophil counts compared to those with low‐eosinophil counts. Triple therapy results in an improvement in HRQoL as measured using the SGRQ MCID threshold (≥ 4‐point decrease) compared to LABA/LAMA inhalers (high‐certainty evidence). Triple therapy probably improves symptoms measured using the TDI score (moderate‐certainty evidence), and may improve lung function as measured by trough FEV1 (low‐certainty evidence); however, these benefits fell below MCID thresholds (TDI 1‐unit decrease; FEV1 100 mL increase). The risk of developing pneumonia as a serious adverse event is likely higher with triple therapy (moderate‐certainty evidence). In contrast, all‐cause serious adverse events may be similar between groups (low‐certainty evidence), and all‐cause mortality may be lower amongst people with COPD treated with triple therapy.
Overall completeness and applicability of evidence
The four new studies provided data on the benefit‐to‐risk profile of new triple therapy inhalers in stable COPD. However, there were multiple methodological differences between the included studies which limit the generalisability of our pooled analysis. Important baseline differences included the proportions of participants with a history of recent exacerbations (25% in Ferguson 2018 compared to 100% in Papi 2018, Rabe 2020, and Lipson 2018), bronchodilator reversibility (44% in Ferguson 2018, 33% in Papi 2018, 31% in Rabe 2020, 18% in Lipson 2018), and comorbid asthma (excluded in Ferguson 2018, Rabe 2020, and Papi 2018, but not in Lipson 2018). ICS medications also differed between the studies (fluticasone furoate in Lipson 2018; extrafine beclometasone in Papi 2018; budesonide in Ferguson 2018 and Rabe 2020), as did formulations of combination LABA/LAMA inhalers. We did not pursue a separate analysis for each individual ICS; however, potential differences between formulations may play a role in the overall effect estimate of pneumonia (Montuschi 2016). We plan to further explore the difference between fluticasone furoate, beclometasone dipropionate, and budesonide in terms of risk of pneumonia in future updates with sufficient study data.
The duration of follow‐up also differed between studies (52 weeks in Lipson 2018, Rabe 2020, Papi 2018 compared to 24 weeks in Ferguson 2018). One study, Papi 2018, also used different LABA agents in the two study arms (indacaterol in the dual arm and formoterol in the triple arm), potentially confounding the effect of the ICS. One study, Rabe 2020, compared four arms, of which only three could be included in our analysis. Furthermore, we observed differences in ICS dosing and formulations/delivery modes of different inhalers (DPI versus pMDI). These methodological differences may explain in part the moderate‐to‐substantial heterogeneity observed for the exacerbations outcomes. Details/results of the individual studies may be more informative than the pooled estimates for these outcomes. Despite these limitations, the available evidence was mostly from patients with symptomatic (CAT score ≥ 10), severe‐to‐very severe COPD (FEV1 ≤ 50%) and ≥ 1 moderate‐to‐severe exacerbation in the last year. Conversely, we found limited data on triple therapy compared to combination LABA/LAMA inhalers in patients with mild‐moderate COPD and those without recent exacerbations.
Treatment decisions in COPD should be personalised, based on individual benefit‐risk profiles, phenotypes, and patient preferences. Benefits of triple therapy over combination LABA/LAMA inhalers included a reduced rate of COPD exacerbations and improved HRQoL. In contrast, adverse effects included an increased risk of pneumonia serious adverse events, although this was not reflected in increased mortality. There is growing interest in the use of blood eosinophils counts as a predictive biomarker of ICS responsiveness in COPD. All trials reported rates of moderate‐to‐severe COPD exacerbations stratified by blood eosinophils. There was some evidence that high blood eosinophil counts was associated with a greater reduction in rate of moderate‐to‐severe exacerbations with triple therapy compared to low blood eosinophils (high/low cut‐offs: 150 eosinophils/µL in three studies; 200 eosinophils/µL in one study). However, because moderate‐to‐substantial heterogeneity was observed in both high‐ and low‐eosinophil subgroups, and because the test for subgroup differences was borderline, we assessed the evidence as being of low certainty. Notably, Ferguson 2018 also showed that the effect of triple therapy on rate of moderate‐to‐severe exacerbations increased concordantly with blood eosinophil counts. This finding has also been replicated in a post hoc analysis of IMPACT (Pascoe 2019), which showed that below a blood eosinophil count of 100 cells/µL, triple therapy was no more effective than LABA/LAMA in reducing rates of moderate‐to‐severe COPD exacerbations, whilst the greatest benefit was observed in those with blood eosinophil counts ≥ 300 cells/µL; this was also suggested by the results of the WISDOM trial (Watz 2016). For reference, mean blood eosinophil counts in healthy adults aged over 40 years have been estimated to be approximately 107 eosinophils/µL (Hartl 2020). Taken together, these studies suggest that blood eosinophil counts may be useful in identifying a subset of patients who are more likely to benefit from ICS treatment (GOLD 2023).
Quality of the evidence
The number of included trials was small, but the total number of participants was large, with each study contributing between 1266 and 6438 participants to our review. The studies used COPD definitions consistent with international guidelines and measured relevant outcomes. The certainty of evidence for our outcomes ranged from very low to high, as shown in Table 1. We downgraded the certainty of the evidence most often due to inconsistency related to moderate‐to‐substantial heterogeneity which could not be fully explored due to the small number of included studies. The numerous methodological differences between the included studies may be contributing factors.
All trials were industry‐sponsored. IMPACT was funded, run, and analysed by GlaxoSmithKline; TRIBUTE similarly by Chiesi Farmaceutici; and ETHOS and KRONOS by AstraZeneca. However, all trials were registered prospectively, and all prespecified outcomes were reported. We judged the risk of selection, performance, and detection bias to be low in the included studies. We assessed the risk of attrition bias and reporting bias as being high in one study each.
Potential biases in the review process
We made every effort to identify all relevant published and unpublished studies. We searched drug company databases, clinical trial registration sites, and reference lists of related articles in addition to the comprehensive searches of the main electronic databases. We contracted trials authors for additional data where they had been measured but not reported. All review authors adhered to a published protocol incorporating best practice guidelines for study selection, resolution of disagreements, and data extraction and analysis to reduce bias and error. Subjective decisions have been avoided, but may have occurred during the review process regarding the cut‐off value of the high‐ and low‐eosinophil groups as discussed in the Overall completeness and applicability of evidence section.
In the original review published in 2016 (Tan 2016), we adopted the method of choosing a random‐effects or fixed‐effect model based on statistical heterogeneity (the I2 statistic), which was a common approach at the time. We recognise that Cochrane methods have evolved since then and the limitation of this historical approach, as the selection of fixed‐effect or random‐effects meta‐analysis should not be made on the basis of a statistical test for heterogeneity (Higgins 2022). We are keen to ensure consistency between versions of the review and have thus maintained this method, but will revisit the issue in future updates of the review.
Agreements and disagreements with other studies or reviews
To date, several systematic reviews of triple therapy in COPD have been published (e.g. Lai 2019; Zayed 2018; Zheng 2018). However, none has included severe COPD exacerbations as an outcome. Zheng 2018 focused on rate of moderate‐to‐severe exacerbations and preceded KRONOS, which was published at a later date. Zayed 2018 did not include mortality or assess subgroup differences by blood eosinophil counts. Lai 2019 also did not assess subgroup differences by blood eosinophil counts. The findings of our review and those of the existing reviews were consistent for overlapping outcomes.
There is some evidence that stepwise withdrawal of ICS after achieving a period of clinical stability may be non‐inferior to long‐term triple therapy (with ongoing ICS) with respect to exacerbation frequency in patients with severe but stable COPD (Magnussen 2014). In this study (WISDOM trial), daily ICS (fluticasone propionate) was periodically reduced every 6 weeks from a starting dose of 1000 μg (1000 μg to 500 μg to 200 μg to 0 μg), with a total study duration of 52 weeks (Magnussen 2014). In contrast, an abrupt withdrawal of ICS has been associated with increased exacerbation frequencies and reduced HRQoL (van der Valk 2002). The evidence from these studies should be considered when determining the optimal long‐term role of ICS added to combination LABA/LAMA inhalers.
Authors' conclusions
Implications for practice.
This Cochrane Review update identified new evidence that was unavailable at the time of the previous review (Tan 2016). The evidence from the four newly included studies suggests that triple therapy may reduce rates of chronic obstructive pulmonary disease (COPD) exacerbations and results in improved health‐related quality of life compared to combination long‐acting beta2‐agonists (LABA)/long‐acting muscarinic antagonists (LAMA) inhalers. Triple treatment likely increases the risk of diagnosed/confirmed pneumonia (any severity) and the risk of pneumonia as a serious adverse event, and may decrease mortality. There is some evidence that blood eosinophil counts may be useful for predicting responsiveness to triple therapy and may be considered when weighing the benefits and risks of triple therapy compared to combination LABA/LAMA inhalers; however, the optimal cut‐off has yet to be determined, and given the observational nature of subgroup analysis, these findings should be interpreted with caution. Most of the evidence in this review was of low or moderate certainty, downgraded for inconsistency or indirectness. Further research may have an impact on the certainty of evidence or may change the estimates.
Whilst the findings of this review appear to be most generalisable to symptomatic COPD patients with severe‐to‐very severe airflow limitation and one or more moderate‐to‐severe COPD exacerbations in the last year, there were important methodological differences between the included studies, therefore the details/results of the individual studies may be more informative than the pooled estimates for some outcomes, such as exacerbation rates. Treatment decisions in COPD should be personalised, based on individual benefit‐risk profiles, phenotypes, and patient preferences.
Implications for research.
The studies in this updated Cochrane Review included COPD patients with mostly severe‐to‐very severe airflow obstruction and a history of recent COPD exacerbations. We found limited data on triple therapy compared to combination LABA/LAMA inhalers in patients with mild‐moderate COPD and those without a recent exacerbation history, and further studies in people with less severe COPD are now required. Because the included studies used different inhaled corticosteroids/LABA/LAMA agents, it is unknown if outcomes differ meaningfully between these different combinations. It also remains unclear whether treatment effects differ based on the presence or absence of comorbidities.
What's new
| Date | Event | Description |
|---|---|---|
| 8 February 2024 | Amended | Fixing typographical error in review Abstract |
History
Protocol first published: Issue 4, 2015 Review first published: Issue 11, 2016
| Date | Event | Description |
|---|---|---|
| 6 December 2023 | New citation required and conclusions have changed | Since the previous review (0 studies included), we found four new studies with a total of 15,412 participants. The latest review findings indicate that triple therapy may reduce rates of COPD exacerbations and improves health‐related quality of life as compared to combination LABA/LAMA inhalers. |
| 6 December 2023 | New search has been performed | Literature search was updated in November 2022, four new studies were identified and included in this update version. |
| 17 June 2020 | Amended | New statement about conflicts of interest added to Published notes. |
| 28 January 2020 | Amended | This review was not compliant with the conflict of interest policy at the time of publication. An explanation has been added to Published notes. |
Acknowledgements
We thank David Lipson for providing additional data from the IMPACT trial. We thank Information Specialist Alies van der Wal for running the search originally developed by Cochrane Airways.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways. Cochrane Airways supported the authors in the development of this review update. Cochrane Airways liaised directly with the following peer reviewers for feedback: David JW Evans, formerly Lancaster University, UK; Amir Sharafkhaneh, Baylor College of Medicine and Michael E DeBakey VA Medical Center, USA; Paolo Montuschi, Catholic University of the Sacred Heart, Italy. Wouter van Geffen was a member of Cochrane Airways but was not involved in the editorial process or decision‐making for this review update.
Upon content transfer from Cochrane Airways to Cochrane Central Editorial Service, the following people conducted the editorial process (managed by Cochrane Central Editorial Service) for this review update.
Sign‐off Editor (final editorial decision): Ian Yang, Faculty of Medicine, University of Queensland
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Joey Kwong, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks and supported editorial team): Lisa Wydrzynski, Cochrane Central Editorial Service
Copy Editor (copy‐editing and production): Lisa Winer, Cochrane Central Production Service
Peer reviewers (provided comments and recommended an editorial decision): Elizabeth K Berger, COPD Foundation (consumer review), Iain Crossingham, East Lancashire Hospitals NHS Trust (clinical/content review), Nuala Livingstone, Cochrane Evidence Production and Methods Directorate (methods review), Jo Platt, Cochrane GNOC (search review)
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (The Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. CAGR search strategy
| #1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All |
| #2 MeSH DESCRIPTOR Bronchitis, Chronic |
| #3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*) |
| #4 COPD:MISC1 |
| #5 (COPD OR COAD OR COBD):TI,AB,KW |
| #6 #1 OR #2 OR #3 OR #4 OR #5 |
| #7 LAMA |
| #8 MeSH DESCRIPTOR Muscarinic Antagonists |
| #9 tiotropium |
| #10 aclidinium |
| #11 glycopyrronium |
| #12 umeclidinium |
| #13 darotropium OR GSK233705 |
| #14 #7 OR #8 or #9 or #10 or #11 or #12 or #13 |
| #15 LABA |
| #16 MeSH DESCRIPTOR Adrenergic beta‐2 Receptor Agonists |
| #17 salmeterol |
| #18 formoterol |
| #19 bambuterol |
| #20 vilanterol |
| #21 indacaterol |
| #22 olodaterol |
| #23 carmoterol |
| #24 abediterol or LAS100977 |
| #25 PF‐610355 |
| #26 #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 |
| #27 QVA149 |
| #28 (#14 and #26) OR #27 |
| #29 ICS:TI,AB |
| #30 inhal* NEAR2 (corticosteroid* or steroid*) |
| #31 fluticasone |
| #32 budesonide |
| #33 beclomethasone |
| #34 triamcinolone |
| #35 flunisolide |
| #36 #29 or #30 or #31 or #32 or #33 or #34 or #35 |
| #37 #6 AND #28 and #36 |
Appendix 3. CENTRAL, MEDLINE (via PubMed), and Embase search strategies
CENTRAL
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All
#2 MeSH DESCRIPTOR Bronchitis, Chronic
#3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)
#4 COPD:MISC1
#5 (COPD OR COAD OR COBD):TI,AB,KW
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 LAMA
#8 MeSH DESCRIPTOR Muscarinic Antagonists
#9 tiotropium
#10 aclidinium
#11 glycopyrronium
#12 umeclidinium
#13 darotropium OR GSK233705
#14 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13
#15 LABA
#16 MeSH DESCRIPTOR Adrenergic beta‐2 Receptor Agonists
#17 salmeterol
#18 formoterol
#19 bambuterol
#20 vilanterol
#21 indacaterol
#22 olodaterol
#23 carmoterol
#24 abediterol OR LAS100977
#25 PF‐610355
#26 #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25
#27 QVA149
#28 (#14 and #26) OR #27
#29 ICS:TI,AB
#30 inhal* NEAR2 (corticosteroid* or steroid*)
#31 fluticasone
#32 budesonide
#33 beclomethasone
#34 triamcinolone
#35 flunisolide
#36 #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35
#37 #6 AND #28 AND #36
MEDLINE
| 1. exp Pulmonary Disease, Chronic Obstructive/ |
| 2. (obstruct* adj3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)).ti,ab. |
| 3. COPD.ti,ab. |
| 4. or/1‐3 |
| 5. LAMA.ti,ab. |
| 6. (long‐acting or long acting).ti,ab. and Muscarinic Antagonists/ |
| 7. tiotropium.tw. |
| 8. aclidinium.tw. |
| 9. glycopyrronium.tw. |
| 10. umeclidinium.tw. |
| 11. (darotropium or GSK233705).tw. |
| 12. or/5‐11 |
| 13. LABA.ti,ab. |
| 14. exp Adrenergic beta‐2 Receptor Agonists/ and (long‐acting or long acting).ti,ab. |
| 15. salmeterol.tw. |
| 16. formoterol.tw. |
| 17. bambuterol.tw. |
| 18. vilanterol.tw. |
| 19. indacaterol.tw. |
| 20. olodaterol.tw. |
| 21. carmoterol.tw. |
| 22. (abediterol or LAS100977).tw. |
| 23. PF‐610355.tw. |
| 24. or/13‐23 |
| 25. (inhaled adj3 (steroid$ or corticosteroid$)).ti,ab. |
| 26. ICS.ti,ab. |
| 27. fluticasone.tw. |
| 28. budesonide.tw. |
| 29. beclomethasone.tw. |
| 30. triamcinolone.tw. |
| 31. flunisolide.tw. |
| 32. or/25‐31 |
| 33. (controlled clinical trial or randomized controlled trial).pt. |
| 34. (randomized or randomised).ab,ti. |
| 35. placebo.ab,ti. |
| 36. dt.fs. |
| 37. randomly.ab,ti. |
| 38. trial.ab,ti. |
| 39. groups.ab,ti. |
| 40. or/33‐39 |
| 41. Animals/ |
| 42. Humans/ |
| 43. 41 not (41 and 42) |
| 44. 40 not 43 |
| 45. 4 and 12 and 24 and 32 and 44 |
Embase
| 1. exp Pulmonary Disease, Chronic Obstructive/ |
| 2. (obstruct* adj3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)).ti,ab. |
| 3. COPD.ti,ab. |
| 4. or/1‐3 |
| 5. LAMA.ti,ab. |
| 6. (long‐acting or long acting).ti,ab. and Muscarinic Antagonists/ |
| 7. tiotropium.tw. |
| 8. aclidinium.tw. |
| 9. glycopyrronium.tw. |
| 10. umeclidinium.tw. |
| 11. (darotropium or GSK233705).tw. |
| 12. or/5‐11 |
| 13. LABA.ti,ab. |
| 14. exp Adrenergic beta‐2 Receptor Agonists/ and (long‐acting or long acting).ti,ab. |
| 15. salmeterol.tw. |
| 16. formoterol.tw. |
| 17. bambuterol.tw. |
| 18. vilanterol.tw. |
| 19. indacaterol.tw. |
| 20. olodaterol.tw. |
| 21. carmoterol.tw. |
| 22. (abediterol or LAS100977).tw. |
| 23. PF‐610355.tw. |
| 24. or/13‐23 |
| 25. (inhaled adj3 (steroid$ or corticosteroid$)).ti,ab. |
| 26. ICS.ti,ab. |
| 27. fluticasone.tw. |
| 28. budesonide.tw. |
| 29. beclomethasone.tw. |
| 30. triamcinolone.tw. |
| 31. flunisolide.tw. |
| 32. or/25‐31 |
| 33. Randomized Controlled Trial/ |
| 34. randomization/ |
| 35. controlled clinical trial/ |
| 36. Double Blind Procedure/ |
| 37. Single Blind Procedure/ |
| 38. Crossover Procedure/ |
| 39. (clinica$ adj3 trial$).tw. |
| 40. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. |
| 41. exp Placebo/ |
| 42. placebo$.ti,ab. |
| 43. random$.ti,ab. |
| 44. ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. |
| 45. (crossover$ or cross‐over$).ti,ab. |
| 46. or/33‐45 |
| 47. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ |
| 48. human/ or normal human/ or human cell/ |
| 49. 47 and 48 |
| 50. 47 not 49 |
| 51. 46 not 50 |
| 52. 4 and 12 and 24 and 32 and 51 |
Data and analyses
Comparison 1. Triple therapy versus LABA/LAMA combination.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Moderate‐to‐severe exacerbations (Rate) | 5 | 15397 | Rate Ratio (IV, Random, 95% CI) | 0.74 [0.67, 0.81] |
| 1.2 Moderate‐to‐severe exacerbations (Time‐to‐first) | 5 | 15397 | Hazard Ratio (IV, Random, 95% CI) | 0.84 [0.77, 0.92] |
| 1.3 Moderate‐to‐severe exacerbations (≥ 1 exacerbation) | 3 | 6109 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.64, 0.99] |
| 1.4 Moderate‐to‐severe exacerbations (Rate, stratified by eosinophil count) | 5 | Rate Ratio (IV, Random, 95% CI) | 0.74 [0.67, 0.83] | |
| 1.4.1 High Eosinophils | 5 | Rate Ratio (IV, Random, 95% CI) | 0.67 [0.60, 0.75] | |
| 1.4.2 Low Eosinophils | 5 | Rate Ratio (IV, Random, 95% CI) | 0.87 [0.81, 0.93] | |
| 1.5 Moderate‐to‐severe exacerbations (Rate, stratified by baseline ICS use) | 2 | Rate Ratio (IV, Random, 95% CI) | 0.75 [0.70, 0.82] | |
| 1.5.1 Baseline ICS Use | 2 | Rate Ratio (IV, Random, 95% CI) | 0.75 [0.69, 0.82] | |
| 1.5.2 No Baseline ICS Use | 2 | Rate Ratio (IV, Random, 95% CI) | 0.75 [0.62, 0.91] | |
| 1.6 Severe exacerbations (Rate) | 4 | 14131 | Rate Ratio (IV, Fixed, 95% CI) | 0.75 [0.67, 0.84] |
| 1.7 Severe exacerbations (Time‐to‐first) | 2 | 7753 | Hazard Ratio (IV, Random, 95% CI) | 0.77 [0.67, 0.88] |
| 1.8 All exacerbations (Rate) | 1 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 1.9 Change in SGRQ | 5 | 13879 | Mean Difference (IV, Fixed, 95% CI) | ‐1.65 [‐2.15, ‐1.15] |
| 1.10 SGRQ, ≥ 4 decrease from baseline | 4 | 14070 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [1.26, 1.45] |
| 1.11 SGRQ, end of study | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.12 Pneumonia | 5 | 15412 | Odds Ratio (M‐H, Random, 95% CI) | 1.59 [1.33, 1.89] |
| 1.13 Pneumonia (Time‐to‐first) | 3 | Hazard Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 1.14 Serious adverse events, pneumonia | 5 | 15412 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.39, 2.18] |
| 1.15 Serious adverse event, all‐cause | 5 | 15412 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.87, 1.03] |
| 1.16 Change in E‐RS | 2 | 2798 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.59, ‐0.00] |
| 1.17 TDI, ≥ 1‐unit increase | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 1.18 TDI (Focal score), over 24 weeks | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.19 Change in trough FEV1 (mL) | 5 | 11352 | Mean Difference (IV, Random, 95% CI) | 38.68 [22.58, 54.77] |
| 1.20 Trough FEV1, end of study (mL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.21 FEV1 AUC 0 to 4 (mL), end of study | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.22 Change in trough FVC (mL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.23 Death, all causes | 5 | 15397 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.54, 0.90] |
| 1.24 Death, all causes (Time‐to‐event) | 3 | Hazard Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 1.25 Death, cardiovascular causes | 4 | 13865 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.34, 0.77] |
| 1.26 Death, respiratory causes | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.27 Death, COPD‐related | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Triple therapy versus LABA/LAMA combination – Exclude Papi 2018.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Moderate‐to‐severe exacerbations (Rate) | 4 | Rate Ratio (IV, Random, 95% CI) | 0.72 [0.64, 0.80] | |
| 2.2 Moderate‐to‐severe exacerbations (Time‐to‐first) | 4 | Hazard Ratio (IV, Random, 95% CI) | 0.83 [0.75, 0.91] | |
| 2.3 Moderate‐to‐severe exacerbations (≥ 1 exacerbation) | 2 | 5199 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.52, 1.06] |
| 2.4 Moderate‐to‐severe exacerbations (Rate, stratified by eosinophil count) | 4 | Rate Ratio (IV, Random, 95% CI) | 0.72 [0.64, 0.82] | |
| 2.4.1 High Eosinophils | 4 | Rate Ratio (IV, Random, 95% CI) | 0.65 [0.58, 0.73] | |
| 2.4.2 Low Eosinophils | 4 | Rate Ratio (IV, Random, 95% CI) | 0.87 [0.80, 0.93] | |
| 2.5 Moderate‐to‐severe exacerbations (Rate, stratified by baseline ICS use) | 2 | Rate Ratio (IV, Random, 95% CI) | 0.75 [0.70, 0.82] | |
| 2.5.1 Baseline ICS Use | 2 | Rate Ratio (IV, Random, 95% CI) | 0.75 [0.69, 0.82] | |
| 2.5.2 No Baseline ICS Use | 2 | Rate Ratio (IV, Random, 95% CI) | 0.75 [0.62, 0.91] | |
| 2.6 Severe exacerbations (Rate) | 3 | Rate Ratio (IV, Fixed, 95% CI) | 0.75 [0.66, 0.84] | |
| 2.7 Severe exacerbations (Time‐to‐first) | 1 | Hazard Ratio (IV, Random, 95% CI) | 0.75 [0.64, 0.87] | |
| 2.8 All exacerbations (Rate) | 1 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.9 Change in SGRQ | 4 | 12347 | Mean Difference (IV, Fixed, 95% CI) | ‐1.66 [‐2.20, ‐1.11] |
| 2.10 SGRQ, ≥ 4 decrease from baseline | 3 | 12538 | Odds Ratio (M‐H, Random, 95% CI) | 1.37 [1.27, 1.48] |
| 2.11 SGRQ, end of study | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.12 Pneumonia | 4 | 13880 | Odds Ratio (M‐H, Random, 95% CI) | 1.67 [1.39, 2.01] |
| 2.13 Pneumonia (Time‐to‐first) | 3 | Hazard Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.14 Serious adverse events, pneumonia | 4 | 13880 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.45, 2.34] |
| 2.15 Serious adverse event, all‐cause | 4 | 13880 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.04] |
| 2.16 Change in E‐RS | 1 | 1266 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.77, ‐0.03] |
| 2.17 TDI, ≥ 1‐unit increase | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.18 TDI (Focal score), over 24 weeks | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.19 Change in trough FEV1 (mL) | 4 | 9820 | Mean Difference (IV, Random, 95% CI) | 42.70 [25.01, 60.38] |
| 2.20 Trough FEV1, end of study (mL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.21 FEV1 AUC 0 to 4 (mL), end of study | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.22 Change in trough FVC (mL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.23 Death, all causes | 4 | 13865 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.53, 0.91] |
| 2.24 Death, all causes (Time‐to‐event) | 3 | Hazard Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2.25 Death, cardiovascular causes | 4 | 13865 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.34, 0.77] |
| 2.26 Death, respiratory causes | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.27 Death, COPD‐related | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ferguson 2018.
| Study characteristics | ||
| Methods | Study design: follow‐up: RCT, parallel group Dates conducted: August 2018 to January 2018 Location, no. of centres: 215 sites over 4 countries (Canada, China, Japan, USA) |
|
| Participants | Inclusion criteria:
Exclusion criteria:
Baseline imbalances: no major baseline imbalance across treatment groups N Screened: 3047 N Randomised: 1902 N Triple therapy: 640 N LABA/LAMA: 627 N Completed: 1090 |
|
| Interventions | Treatment group: triple therapy (budesonide (320 µg) + glycopyrronium (18 µg) + formoterol (9.6 µg)), 2 inhalations, twice daily via MDI Control group: LABA/LAMA (glycopyrronium (18 µg) + formoterol (9.6 µg)), 2 inhalations, twice daily via MDI Run‐in period: no run‐in period Co‐interventions: rescue salbutamol as needed |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | KRONOS (NCT02497001) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “We randomly assigned patients using an interactive web response system” Comment: Probably done. |
| Allocation concealment (selection bias) | Low risk | Quote: “Study site personnel randomly assigned patients using an interactive web response system to treatment”; “randomization was stratified by reversibility to salbutamol, country and disease severity” Comment: Probably done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Double‐blind”; “Patients, study site personnel, and the study sponsor were masked to the treatment assignment”; “all were administered matching MDIs” Comment: Probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Double‐blind”; “Patients, study site personnel, and the study sponsor were masked to the treatment assignment”; “all were administered matching MDIs” Quote "Attributions to tolerability or lack of efficacy were determined programmatically in a blinded manner prior to database lock" Comment: Probably done for patients, personnel and outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24 weeks: 74 (11%) and 101 (16%) prematurely discontinued in the intervention and control arms, respectively. Reasons for discontinuation provided and roughly equal between groups. |
| Selective reporting (reporting bias) | High risk | Several changes to made to ranking of outcomes after trial commencement. Cut‐off for non‐inferiority margin for TDI decreased from 1.0 to 0.75. Clinical trials registration available. All outcomes reported. Protocol available. |
Lipson 2018.
| Study characteristics | ||
| Methods | Study design: RCT, parallel group Follow‐up: 52 weeks Dates conducted: June 2014 to July 2017 Location, no. of centres: multicentre trial performed in 37 countries |
|
| Participants | Inclusion criteria:
Exclusion criteria: No specified exclusion criteria Baseline imbalances: none reported. Treatment group: age (65.3 ± 8.2 years); female (1385 (33%)) Control group: age (65.2 ± 8.3 years); female (714 (34%)) N Screened: 13,906 N Randomised: 10,355 N Triple therapy: 4151 N LABA/LAMA: 2070 N Completed: 9087 (88%) |
|
| Interventions | Treatment group: triple therapy (fluticasone furoate (100 µg) + umeclidinium (62.5 µg) + vilanterol (25 µg)), once daily, administered in a single dry‐powder inhaler (Ellipta, GSK) Control group: LABA/LAMA (umeclidinium (62.5 µg) + vilanterol (25 µg)), once daily, administered in a single dry‐powder inhaler (Ellipta, GSK) Run‐in period: 2 weeks, participants continued to take their own medications. Co‐interventions: none reported. |
|
| Outcomes | Primary: Annual rate of moderate to severe exacerbations, stratified effect estimates provided by peripheral eosinophil levels (< 150 cells/µL vs ≥ 150 cells/µL) Secondary:
|
|
| Notes | IMPACT (NCT02164513) Study did not exclude patients with a history of asthma. Study run‐in period continued previous inhaled medications. Participants taking ICS who were randomised to LABA/LAMA therapy would have had an abrupt cessation of ICS therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “patients who underwent randomization” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: “randomization code will be generated by GSK using a validated computerized system. Subjects will be randomized using an Interactive Voice Response System (IVRS‐RAMOS)” Comment: Probably done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “double‐blind, parallel‐group”; “an independent data monitoring committee reviewed unblinded safety information at regular intervals” Comment: Probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “double‐blind, parallel‐group”; “an independent data monitoring committee reviewed unblinded safety information at regular intervals” Comment: Probably done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 52 weeks: 758 (18%) prematurely discontinued in intervention groups, 163 (4%) due to ‘lack of efficacy’; 566 (27%) prematurely discontinued in control group, 172 (8%) due to ‘lack of efficacy’ |
| Selective reporting (reporting bias) | Low risk | Protocol available. All outcomes reported. |
Papi 2018.
| Study characteristics | ||
| Methods | Study design: RCT, parallel group Follow‐up: 52 weeks Dates conducted: May 2015 to July 2017 Location, no. of centres: 187 sites across 17 countries |
|
| Participants | Inclusion criteria:
Exclusion criteria:
Baseline imbalances: no comparative statistics reported in table of baseline characteristics. However, no obvious major differences in demographics. N Screened: 2103 N Randomised: 1532 N Triple therapy: 764 N LABA/LAMA: 768 N Completed: 1314 |
|
| Interventions | Treatment group: triple therapy (extrafine: beclometasone (87 µg), formoterol (5 µg), glycopyrronium (9 µg)), 2 inhalations, twice daily, administered in a single pressurised MDI Control group: LABA/LAMA (indacaterol (85 µg), glycopyrronium (43 µg)), once daily, administered via a single dose DPI (Ultibro Breezhaler, Novartis) Run‐in period: 2 weeks, all participants received 1 inhalation of indacaterol/glycopyrronium (85/43 µg) per day via a single dose DPI (Ultibro Breezhaler, Novartis) Co‐interventions: participants were permitted to use salbutamol via MDI or terbutaline via DPI as rescue medication. |
|
| Outcomes | Primary: Rate of moderate‐to‐severe COPD exacerbations Secondary:
|
|
| Notes | TRIBUTE (NCT02164513) Study did not include patients who were already on triple therapy in the 2 months prior to screening. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Via an interactive response technology system” Comment: Probably done. |
| Allocation concealment (selection bias) | Low risk | Quote: “Patients were randomly assigned to treatment groups by central randomization stratified by country and severity of airflow limitation…in accordance with a randomization list generated by the interactive response technology provider” Comment: Probably done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Double‐blind, double dummy”; “Patients, investigators, site staff and sponsor personnel were masked to treatment assignment for the duration of the study by use of a double‐dummy approach, with each patient using both a pressurized MDI…and a single dose dry‐powder inhaler” Comment: Probably done for patients, personnel and outcome assessors. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Double‐blind, double dummy”; “Patients, investigators, site staff and sponsor personnel were masked to treatment assignment for the duration of the study by use of a double‐dummy approach, with each patient using both a pressurized MDI…and a single dose dry‐powder inhaler” Comment: Probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 52 weeks: 98 (13%) and 120 (16%) prematurely discontinued in the intervention and control arms, respectively. Reasons for discontinuation provided and roughly equal between groups. |
| Selective reporting (reporting bias) | Low risk | Clinical trials registration available. All outcomes reported. No protocol available. |
Rabe 2020.
| Study characteristics | ||
| Methods | Study design: RCT, parallel group Follow‐up: 52 weeks Dates conducted: July 2015 to July 2019 Location, no. of centres: multicentre trial performed in 26 countries |
|
| Participants | Inclusion criteria:
Exclusion criteria:
Baseline imbalances: none reported. Treatment group: age (64.6 ± 7.6 years); female (1700 (40%)) Control group: age (64.8 ± 7.6 years); female (876 (41%)) N Screened: 16,033 N Randomised: 8588 N Triple therapy: 4295 (DT1) N LABA/LAMA: 2143 N Completed: 5000 (78%) |
|
| Interventions | Treatment group: triple therapy (budesonide (320 or 160 µg) + glycopyrronium (18 µg) + formoterol (9.6 µg)), once daily, administered in a single DPI (Aerosphere, AstraZeneca) Control group: LABA/LAMA (glycopyrronium (18 µg) + formoterol (9.6 µg)), once daily, administered in a single DPI (Aerosphere, AstraZeneca) Run‐in period: 1 to 4 weeks, participants continued inhaled glucocorticoids (if used before screening) and commenced regular ipratropium and as‐needed albuterol Co‐interventions: none reported. |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ETHOS (NCT02465567) Study run‐in period involved continuation of previous inhaled medications. Participants taking ICS who were randomised to LABA/LAMA therapy would have had an abrupt cessation of ICS therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Eligible patients were randomly assigned and Obtain subject randomization number and treatment assignment information from IWRS” Comment: Probably done. |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization will be centralized through the use of an IWRS” Comment: Probably done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Double‐blinded, parallel group trial”, Details on investigator and participant blinding provided in protocol. Quote: "(protocol): A pre‐specified interim efficacy analysis was conducted following the blinded sample size re‐estimation, at which an independent Data Monitoring Committee reviewed unblinded efficacy data " Comment: Probably done for participants and personnel and outcome assessors. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Double‐blinded, parallel group trial”, Details on investigator and participant blinding provided in protocol. Comment: Probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 52 weeks: 849 (20%) prematurely discontinued in intervention groups, 232 (5%) due to adverse event, 198 (5%) withdrew from trial and 205 (5%) due to ‘lack of efficiency’. 544 (25%) withdrew from control group, 171 (8%) due to ‘lack of efficiency’, 147 (7%) due to adverse event, 123 (6%) withdrew from trial. Approximately equal in both arms. |
| Selective reporting (reporting bias) | Low risk | Protocol available. TDI outcomes only reported at 24 weeks (52 week outcome not reported). All outcomes reported. |
Rabe 2020 (160 μg budesonide).
| Study characteristics | ||
| Methods | Study design: RCT, parallel group Follow‐up: 52 weeks Dates conducted: July 2015 to July 2019 Location, no. of centres: multicentre trial performed in 26 countries |
|
| Participants | Inclusion criteria:
Exclusion criteria:
Baseline imbalances: none reported. Treatment group: age (64.6 ± 7.6 years); female (1700 (40%)) Control group: age (64.8 ± 7.6 years); female (876 (41%)) N Screened: 16,033 N Randomised: 8588 N Triple therapy: 4295 (DT1) N LABA/LAMA: 2143 N Completed: 5000 (78%) |
|
| Interventions | Treatment group: triple therapy (budesonide (320 or 160 µg) + glycopyrronium (18 µg) + formoterol (9.6 µg)), once daily, administered in a single DPI (Aerosphere, AstraZeneca) Control group: LABA/LAMA (glycopyrronium (18 µg) + formoterol (9.6 µg)), once daily, administered in a single DPI (Aerosphere, AstraZeneca) Run‐in period: 1 to 4 weeks, participants continued inhaled glucocorticoids (if used before screening) and commenced regular ipratropium and as‐needed albuterol Co‐interventions: none reported. |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ETHOS (NCT02465567) Study run‐in period involved continuation of previous inhaled medications. Participants taking ICS who were randomised to LABA/LAMA therapy would have had an abrupt cessation of ICS therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Eligible patients were randomly assigned and obtain subject randomization number and treatment assignment information from IWRS” Comment: Probably done. |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization will be centralized through the use of an IWRS” Comment: Probably done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "(protocol): A pre‐specified interim efficacy analysis was conducted following the blinded sample size re‐estimation, at which an independent Data Monitoring Committee reviewed unblinded efficacy data " Comment: Probably done for participants and personnel and outcome assessors. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Double‐blinded, parallel group trial”, Details on investigator and participant blinding provided in protocol. Comment: Probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 52 weeks: 849 (20%) prematurely discontinued in intervention groups, 232 (5%) due to adverse event, 198 (5%) withdrew from trial and 205 (5%) due to ‘lack of efficiency’. 544 (25%) withdrew from control group, 171 (8%) due to ‘lack of efficiency’, 147 (7%) due to adverse event, 123 (6%) withdrew from trial. Approximately equal in both arms. |
| Selective reporting (reporting bias) | Low risk | Protocol available. TDI outcomes only reported at 24 weeks (52 week outcome not reported). All outcomes reported. |
Rabe 2020 (320 μg budesonide).
| Study characteristics | ||
| Methods | Study design: RCT, parallel group Follow‐up: 52 weeks Dates conducted: July 2015 to July 2019 Location, no. of centres: multicentre trial performed in 26 countries |
|
| Participants | Inclusion criteria:
Exclusion criteria:
Baseline imbalances: none reported. Treatment group: age (64.6 ± 7.6 years); female (1700 (40%)) Control group: age (64.8 ± 7.6 years); female (876 (41%)) N Screened: 16,033 N Randomised: 8588 N Triple therapy: 4295 (DT1) N LABA/LAMA: 2143 N Completed: 5000 (78%) |
|
| Interventions | Treatment group: triple therapy (budesonide (320 or 160 µg) + glycopyrronium (18 µg) + formoterol (9.6 µg)), once daily, administered in a single DPI (Aerosphere, AstraZeneca) Control group: LABA/LAMA (glycopyrronium (18 µg) + formoterol (9.6 µg)), once daily, administered in a single DPI (Aerosphere, AstraZeneca) Run‐in period: 1 to 4 weeks, participants continued inhaled glucocorticoids (if used before screening) and commenced regular ipratropium and as‐needed albuterol Co‐interventions: none reported. |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | ETHOS (NCT02465567) Study run‐in period involved continuation of previous inhaled medications. Participants taking ICS who were randomised to LABA/LAMA therapy would have had an abrupt cessation of ICS therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Eligible patients were randomly assigned and obtain subject randomization number and treatment assignment information from IWRS” Comment: Probably done. |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization will be centralized through the use of an IWRS” Comment: Probably done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Double‐blinded, parallel group trial”, Details on investigator and participant blinding provided in protocol. Comment: Probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "(protocol): A pre‐specified interim efficacy analysis was conducted following the blinded sample size re‐estimation, at which an independent Data Monitoring Committee reviewed unblinded efficacy data " Comment: Probably done for participants and personnel and outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 52 weeks: 849 (20%) prematurely discontinued in intervention groups, 232 (5%) due to adverse event, 198 (5%) withdrew from trial and 205 (5%) due to ‘lack of efficiency’. 544 (25%) withdrew from control group, 171 (8%) due to ‘lack of efficiency’, 147 (7%) due to adverse event, 123 (6%) withdrew from trial. Approximately equal in both arms. |
| Selective reporting (reporting bias) | Low risk | Protocol available. TDI outcomes only reported at 24 weeks (52 week outcome not reported). All outcomes reported. |
ATS: American Thoracic Society AUC: area under curve CAT: COPD Assessment Test COPD: chronic obstructive pulmonary disease DPI: dry‐powder inhaler E‐RS: Evaluating Respiratory Symptoms in COPD scale ERS: European Respiratory Society FEV1: forced expiratory volume in 1 second FVC: forced vital capacity HRQoL: health‐related quality of life ICS: inhaled corticosteroids LABA: long‐acting beta2‐agonist LAMA: long‐acting muscarinic antagonist MDI: metred‐dose inhaler RCT: randomised controlled trial SGRQ: St George's Respiratory Questionnaire TDI: Transition Dyspnoea Index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aaron 2007 | Intervention arm used 2 separate devices (ICS/LABA + LAMA) instead of a single combination inhaler |
| Anonymous 2017 | Review article |
| Antohe 2018 | Review article |
| Bateman 2015 | Comparison of LABA/LAMA versus monocomponents (abstract only) |
| Bishwakarma 2017 | Observational study of LABA +/‐ ICS |
| Bölükbas 2011 | Comparison of LAMA + LABA/ICS versus LAMA + LABA (separate inhalers) |
| Chapman 2018a | Comparison of triple therapy versus withdrawal of ICS (SUNSET study) |
| Chapman 2018b | Comparison of triple therapy versus withdrawal of ICS (SUNSET study) |
| Dransfield 2020 | Ineligible study design – post hoc analysis of IMPACT |
| EUCTR2017‐004369‐29‐NL | Comparison of multiple triple therapy inhaler regimens (trial protocol) |
| EUCTR2018‐001704‐10‐NL | Cross‐over RCT with < 3 weeks per treatment period |
| EUCTR2019‐003351‐11‐NL | Cross‐over RCT and incorrect intervention (did not use combination triple therapy inhaler) |
| Ferguson 2020 | Ineligible study type – pulmonary function test substudy of ETHOS evaluating lung function decline |
| Gosden 2018 | Cost‐benefit analysis using data from TRIBUTE |
| Halpin 2019 | Ineligible study type – post hoc analysis of IMPACT |
| Halpin 2020 | Ineligible study type – post hoc analysis of IMPACT |
| Han 2020 | Ineligible study design – post hoc analysis of IMPACT |
| Hara 2007 | Ineligible intervention: comparison of LAMA versus LABA/ICS |
| Harada 2020 | Ineligible study design – insufficient duration |
| Hovelmann 2018 | Observational study of combination triple therapy versus multiple inhaler triple therapy (abstract) |
| Ichinose 2019 | Subgroup analysis of the KRONOS study (Japanese participant data already included) |
| James 2013 | Incorrect intervention: comparison of LABA + LAMA (separate inhalers) versus LABA + LAMA + ICS (separate inhalers). The corresponding author contacted: the authors were blinded to the specific inhalers used. They were unable to provide further details. |
| Kato 2019 | Subgroup analysis of the IMPACT study (Japanese participant data already included) |
| Kerwin 2019 | Ineligible study type: extension study involving a subset of patients who participated in KRONOS to examine the effects on bone mineral density and ocular safety |
| Khan 2018 | Observational study of LABA/ICS versus LABA/LAMA/ICS |
| Krishnan 2018 | Commentary on IMPACT |
| Lipari 2017 | Systematic review |
| Lipson 2019a | Subanalysis of IMPACT on cardiovascular outcomes |
| Lipson 2019b | Subanalysis of IMPACT by mixed models repeated measures |
| Magnussen 2014 | WISDOM study – comparison of LABA + LAMA + ICS (separate inhalers) versus LABA + LAMA + stepwise withdrawal of ICS (separate inhalers) |
| Mammen 2021 | Post hoc analysis of IMPACT on mortality |
| Martinez 2020 | Post hoc analysis of ETHOS: additional analyses evaluating the reduction in all‐cause mortality; performed after the collection of additional vital status information after trial completion for patients with incomplete vital status at the end of the study |
| Masayuki 2018 | LABA/LAMA/ICS without comparator group (abstract) |
| Mehta 2018 | Pharmacokinetic study of triple versus dual therapy in COPD (abstract) |
| Montuschi 2016 | Review article |
| NCT01911364 | Combination LABA/LAMA/ICS versus LAMA versus LABA/ICS + LAMA (trial protocol) |
| NCT02729051 | Combination LABA/LAMA/ICS versus LABA/ICS + LAMA (trial protocol) |
| NCT02731846 | Combination LABA/LAMA/ICS versus LABA/ICS + LAMA (trial protocol) |
| NCT03478683 | Combination LABA/LAMA/ICS versus LAMA (trial protocol) |
| NCT04320342 | Triple therapy versus ICS/LABA |
| NCT04675463 | Non‐randomised study |
| NCT04923347 | Ineligible study design: single group assignment |
| Palli 2019 | Cost‐benefit analysis using an observational dataset |
| Scuri 2018 | Post hoc analysis of the TRILOGY, TRINITY, and TRIBUTE studies (abstract) |
| Singh 2016 | TRILOGY study – comparison of triple therapy (separate inhalers) to dual bronchodilator therapy (separate inhalers) |
| Singh 2018 | Review article |
| Stiegler 2019 | Subanalysis of IMPACT to assess the relationship between COPD Assessment Test (CAT) score and risk of exacerbations |
| van den Berge 2020 | Cross‐over RCT |
| van den Berge 2021 | Cross‐over RCT |
COPD: chronic obstructive pulmonary disease ICS: inhaled corticosteroids LABA: long‐acting beta2‐agonists LAMA: long‐acting muscarinic antagonists RCT: randomised controlled trial
Differences between protocol and review
We amended the cut‐offs for what we considered to be not important, moderate, and substantial heterogeneity. We performed a sensitivity analysis for exacerbation outcomes in which we compared including versus excluding data from one study, Ferguson 2018, that had a higher proportion of participants with bronchodilator reversibility and lower proportion of participants with recent exacerbations compared to the other included studies. We performed an additional sensitivity analysis for all primary and secondary outcomes in which we compared including versus excluding data from Papi 2018, which used different LABA agents, different inhaler devices, and different drug particle sizes in the two comparison arms.
Contributions of authors
Both WVG and DT contributed equally to this review update. WVG, DT, and JW authored the first draft of the review update. HW commented, edited, and contributed to revisions.
Sources of support
Internal sources
-
Breathe Well NHMRC Centre of Research Excellence, University of Tasmania, Australia
Office and logistic support to all authors
External sources
-
Commonwealth Department of Health and Ageing, Australia
Funding to JAEW Coordination support, Cochrane Airways Australia
-
Asthma Foundation Tasmania, Australia
Cochrane Airways Australia Scholarship to DT and CW
-
Daniel Tan, Australia
funded by a National Health and Medical Research Council (NHMRC) Postgraduate Scholarship and Royal Australasian College of Physicians (RACP) Woolcock Scholarship.
Declarations of interest
Wouter H van Geffen: Fiduciary Officer, Long Range Planning Committee ERS Assembly 11 – Thoracic Oncology, European Respiratory Society (ERS); Board Member, Dutch Society of Respiratory Physicians (NVALT); Consultant Respiratory Physician, Medical Centre Leeuwarden; affiliated to ERS and NVALT; member, Editorial Board of Cochrane Airways (closed in March 2023) but was not involved in the editorial decision‐making of this Cochrane Review update.
Daniel J Tan: no relevant interests; General Medicine Registrar at the Royal Melbourne Hospital, Melbourne, Victoria, Australia.
Julia AE Walters: none known.
E Haydn Walters: no relevant interests; Acute Physician, Alfred Hospital Melbourne.
These authors should be considered joint first author
Edited (no change to conclusions)
References
References to studies included in this review
Ferguson 2018 {published data only}
- Ferguson GT, Rabe KF, Martinez FJ, Fabbri LM, Wang C, Ichinose M, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respiratory Medicine 2018;6(10):747-58. [DOI] [PubMed] [Google Scholar]
Lipson 2018 {published and unpublished data}
- Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. New England Journal of Medicine 2018;378(18):1671-80. [DOI] [PubMed] [Google Scholar]
- Pascoe SJ, Lipson DA, Locantore N, Barnacle H, Brealey N, Mohindra R, et al. A phase III randomised controlled trial of single-dose triple therapy in COPD: the IMPACT protocol. European Respiratory Journal 2016;48(2):320-30. [DOI] [PubMed] [Google Scholar]
Papi 2018 {published data only}
- Papi A, Vestbo J, Fabbri L, Corradi M, Prunier H, Cohuet G, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet 2018;391(10125):1076-84. [DOI] [PubMed] [Google Scholar]
Rabe 2020 {published data only}
- Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, et al. A phase III study of triple therapy with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler 320/18/9.6mug and 160/18/9.6mug using co-suspension delivery technology in moderate-to-very severe COPD: the ETHOS study protocol. Respiratory Medicine 2019;158:59-66. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. New England Journal of Medicine 2020;383:35-48. [DOI] [PubMed] [Google Scholar]
Rabe 2020 (160 μg budesonide) {published data only}
- Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, et al. A phase III study of triple therapy with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler 320/18/9.6mug and 160/18/9.6mug using co-suspension delivery technology in moderate-to-very severe COPD: the ETHOS study protocol. Respiratory Medicine 2019;158:59-66. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. New England Journal of Medicine 2020;383:35-48. [DOI] [PubMed] [Google Scholar]
Rabe 2020 (320 μg budesonide) {published data only}
- Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, et al. A phase III study of triple therapy with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler 320/18/9.6mug and 160/18/9.6mug using co-suspension delivery technology in moderate-to-very severe COPD: the ETHOS study protocol. Respiratory Medicine 2019;158:59-66. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. New England Journal of Medicine 2020;383:35-48. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aaron 2007 {published data only}
- Aaron SD, Vandemhee KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine 2007;146(8):545-55. [DOI] [PubMed] [Google Scholar]
Anonymous 2017 {published data only}
Antohe 2018 {published data only}
- Antohe I, Antoniu SA, Gavrilovici C. Triple fixed inhaled therapy in frequent chronic obstructive pulmonary disease exacerbators: potential advantages for various degrees of airways obstruction. Expert Opinion on Pharmacotherapy 2018;19(3):287-9. [DOI] [PubMed] [Google Scholar]
Bateman 2015 {published data only}
- Bateman ED, Chapman KR, Rennard SI, Lakkis H, Moya M, Garcia Gil E. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combination in patients with moderate to severe COPD, stratified by inhaled corticosteroid use. American Journal of Respiratory and Critical Care Medicine 2015;191:A5782. [Google Scholar]
Bishwakarma 2017 {published data only}
- Bishwakarma R, Zhang W, Kuo YF, Sharma G. Long-acting bronchodilators with or without inhaled corticosteroids and 30-day readmission in patients hospitalized for COPD. International Journal of Chronic Obstructive Pulmonary Disease 2017;12:477-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bölükbas 2011 {published data only}
- Bölükbas S, Eberlein M, Eckhoff J, Schirren J. Short-term effects of inhalative tiotropium/formoterol/budesonide versus tiotropium/formoterol in patients with newly diagnosed chronic obstructive pulmonary disease requiring surgery for lung cancer: a prospective randomised trial. European Journal of Cardio-Thoracic Surgery 2011;39(6):995-1000. [DOI] [PubMed] [Google Scholar]
Chapman 2018a {published data only}
- Chapman KR, Hurst J, Frent S, Larbig M, Fogel R, Guerin T, et al. Withdrawal of inhaled corticosteroids from COPD patients inhaling long-term triple therapy: the SUNSET study. American Journal of Respiratory and Critical Care Medicine 2018;197:A1009. [DOI] [PubMed] [Google Scholar]
Chapman 2018b {published data only}
- Chapman KR, Hurst JR, Frent S-M, Larbig M, Fogel R, Guerin T, et al. Long-term triple therapy de-escalation to indacaterol/ glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. American Journal of Respiratory and Critical Care Medicine 2018;198(3):329-39. [DOI] [PubMed] [Google Scholar]
Dransfield 2020 {published data only}
- Dransfield M, Halpin D, Han ML, Hartley B, Jones CE, Kalhan R, et al. Time-dependent risk of cardiovascular events following an exacerbation in patients with COPD: post hoc analysis from the impact trial. Chest 2020;158(4 Suppl):A1722-A1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
EUCTR2017‐004369‐29‐NL {published data only}
- EUCTR2017-004369-29-NL. Single inhaler triple therapy (fluticasone furoate + vilanterol trifenatate + umeclidinium bromide) (inhaled) – COPD. https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-004369-29/results 2018.
EUCTR2018‐001704‐10‐NL {published data only}
- EUCTR2018-001704-10-NL. Phase 3b study to evaluate the effects of budesonide/glycopyrronium/formoterol fuumarate and glycopyrronium/formoterol fumarate on specific image based airway volumes and resistance in subjects with chronic obstructive pulmonary disease (COPD). https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02068001/full 2018.
EUCTR2019‐003351‐11‐NL {published data only}
- EUCTR2019-003351-11-NL. Study to the effects of using "triple therapy" on the health status of COPD patients with characteristics of asthma. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2019-003351-11 2019.
Ferguson 2020 {published data only}
- Ferguson G, Martinez F, Rabe K, Ballal S, Darken P, Aurivillius M, et al. Benefits of budesonide-containing therapies on reducing lung function decline in patients with COPD in the ETHOS study. Chest 2020;158(4 Suppl):A1656-A1659. [Google Scholar]
Gosden 2018 {published data only}
- Gosden TB, Pitcher A, Nour N, Madoni A, Friggi E. A cost-effectiveness analysis of a first-in-class, triple fixed dose combination therapy against LABA/LAMA therapy in moderate-severe COPD. Value in Health 2018;21(Suppl 1):S235. [Google Scholar]
Halpin 2019 {published data only}
- Halpin DMG, Bardsley S, Criner G, Dransfield M, Han MK, Jones CE, et al. The IMPACT trial: single inhaler triple therapy fluticasone furoate/umeclidinium/vilanterol versus fluticasone furoate/vilanterol and umeclidinium/vilanterol in patients with COPD: analysis according to smoking status. American Journal of Respiratory and Critical Care Medicine 2019;199(9):A3339. [Google Scholar]
Halpin 2020 {published data only}
- Halpin DMG, Dransfield MT, Han MK, Jones CE, Kilbride S, Lange P, Lipson DA, Lomas DA, Martinez FJ, Pascoe S, Singh D, Wise R, Criner GJ. The effect of exacerbation history on outcomes in the IMPACT trial. European Respiratory Journal 2020;55(6):1901921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2020 {published data only}
- Han MK, Criner GJ, Dransfield MT, Halpin DMG, Jones CE, Kilbride S, et al. The effect of inhaled corticosteroid withdrawal and baseline inhaled treatment on exacerbations in the IMPACT study. A randomized, double-blind, multicenter clinical trial. American Journal of Respiratory and Critical Care Medicine 2020;202(9):1237-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hara 2007 {published data only}
- Hara K, Kurashima K, Tokunaga D, Ueno M, Aoyagi K, Isobe Z, et al. Single blind comparison of tiotropium and salmeterol plus fluticasone propionate of treatment in patients with chronic obstructive pulmonary disease (COPD) [Abstract]. In: American Thoracic Society International Conference; 2007 May 18-23; San Francisco. San Francisco, California, USA, 2007:Poster #A1.
Harada 2020 {published data only}
- Harada N, Otsuka K, Horiuchi A, Shinka Y, Yamamura H, Miyao N. Triple therapy with fluticasone furoate/umeclidinium/vilanterol compared with dual bronchodilation or triple therapy with inhaled corticosteroids/dual bronchodilation in patients with chronic obstructive pulmonary disease. European Respiratory Journal 2020;56(suppl 64):3276. [Google Scholar]
Hovelmann 2018 {published data only}
- Hovelmann R, Gessner C. TriOptimize: a prospective non-interventional trial to document potential optimization of health related quality of life in COPD patients prescribed a fixed vs. free LAMA/LABA/ICS triple therapy. Pneumologie 2018;72:S01. [DOI: 10.1055/s-0037-1619366] [DOI] [Google Scholar]
Ichinose 2019 {published data only}
- Ichinose M, Fukushima Y, Inoue Y, Hataji O, Ferguson GT, Rabe KF, et al. Long-term safety and efficacy of budesonide/ glycopyrrolate/formoterol fumarate metered dose inhaler formulated using co-suspension delivery technology in Japanese patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease 2019;14:2993-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
James 2013 {published data only (unpublished sought but not used)}
- James SP, Paul D, Prabhu D, Venugopal KP. A comparative study on inhaled corticosteroids versus placebo in management of chronic obstructive pulmonary disease. Lung India 2013;30(Suppl 1):S29. [Google Scholar]
Kato 2019 {published data only}
- Kato M, Tomii K, Hashimoto K, Nezu Y, Ishii T, Jones CE, et al. The IMPACT study – single inhaler triple therapy (FF/UMEC/VI) versus FF/VI and UMEC/VI in patients with COPD: efficacy and safety in a Japanese population. International Journal of Chronic Obstructive Pulmonary Disease 2019;14:2849-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerwin 2019 {published data only}
- Kerwin EM, Ferguson GT, Mo M, DeAngelis K, Dorinsky P. Bone mineral density and ocular safety after 52 weeks' treatment with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler (BGF MDI) using co-suspension delivery technology in COPD. American Journal of Respiratory and Critical Care Medicine 2019;199:A3319. [Google Scholar]
Khan 2018 {published data only}
- Khan PA, Sujala A, Sarah Nousheen BB, Fatima AF, Ala HT, Pallavi Reddy ABT. A comparative evaluation of the efficacy of triple drug therapy with dual drug therapy in COPD patients. International Journal of Pharmacy and Pharmaceutical Sciences 2018;10(4):105-9. [Google Scholar]
Krishnan 2018 {published data only}
- Krishnan J, Zappetti D. IMPACT Trial: triple versus dual therapy for COPD results in decreased rate of exacerbations. Clinical Pulmonary Medicine 2018;25(5):195-6. [Google Scholar]
Lipari 2017 {published data only}
- Lipari M, Wilhelm S, Kale-Pradhan P. Triple versus dual therapy in COPD: a meta-analysis. Pharmacotherapy 2017;32(12):e236. [DOI] [PubMed] [Google Scholar]
Lipson 2019a {published data only}
- Lipson DA, Criner G, Dransfield MT, Halpin DMG, Han MK, Jones CE, et al. The IMPACT trial: single inhaler triple therapy fluticasone furoate/umeclidinium/vilanterol versus fluticasone furoate/vilanterol and umeclidinium/vilanterol in patients with COPD: results on cardiovascular safety. American Journal of Respiratory and Critical Care Medicine 2019;199:A3334. [Google Scholar]
Lipson 2019b {published data only}
- Lipson DA, Criner G, Dransfield M, Halpin D, Han M, Elaine Jones C, et al. The IMPACT Trial: single inhaler triple therapy vs dual therapies: efficacy across multiple COPD endpoints over time. European Respiratory Journal 2019;54:PA2482. [DOI: 10.1183/13993003.congress-2019.PA2482] [DOI] [Google Scholar]
Magnussen 2014 {published data only}
- Magnussen H, Disse B, Rodriguez-Roisin R, Kirsten A, Watz H, Tetzlaff K, et al. Exacerbation risk is not worse when ICS are withdrawn in a stepwise manner in severe to very severe COPD patients receiving LAMA+LABA: The WISDOM study. Chest 2014;146(4):63A. [Google Scholar]
- Magnussen H, Disse B, Rodriguez-Roisin R, Kirsten A, Watz H, Tetzlaff K, et al. The impact of stepwise withdrawal of ICS on FEV1, mMRC, and SQRQ in severe to very severe COPD patients treated with LAMA+LABA: The WISDOM study. Chest 2014;146(4):76A. [Google Scholar]
- Magnussen H, Disse B, Rodriguez-Roisin R, Kirsten A, Watz H, Tetzlaff K, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. New England Journal of Medicine 2014;371(14):1285-94. [DOI] [PubMed] [Google Scholar]
Mammen 2021 {published data only}
- Mammen MJ, Carr TF, Criner GJ, Dransfield MT, Halpin DMG, Han MK, et al. All-cause mortality by subgroup in patients with chronic obstructive pulmonary disease: post hoc analysis of the impact trial. American Journal of Respiratory and Critical Care Medicine 2021;203:A2241. [Google Scholar]
Martinez 2020 {published data only}
- Martinez FJ, Rabe KF, Ferguson GT, Wedzicha JA, Singh D, Wang C, et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for COPD: a randomized, double-blind, multi-center parallel-group study. American Journal of Respiratory and Critical Care Medicine 2020;203(5):553-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Masayuki 2018 {published data only}
- Masayuki H. Clinical usefulness of once-daily triple therapy, ciclesonide and tiotropium bromide/olodaterol combination inhaler for COPD patients. American Journal of Respiratory and Critical Care Medicine 2018;197:A4770. [Google Scholar]
Mehta 2018 {published data only}
- Mehta R, Farrell C, Kilbride S, Zhu C, Brooks J, Barnhart F, et al. Population pharmacokinetic analysis of single inhaler triple therapy (ICS/LAMA/LABA) versus dual therapy (LAMA/LABA AND ICS/LABA) in patients with symptomatic COPD: combined results from three phase iii trials. American Journal of Respiratory and Critical Care Medicine 2018;197:A3029. [Google Scholar]
Montuschi 2016 {published data only}
- Montuschi P, Malerba M, Macis G, Mores N, Santini G. Triple inhaled therapy for chronic obstructive pulmonary disease. Drug Discovery Today 2016;21(11):1820-7. [DOI] [PubMed] [Google Scholar]
NCT01911364 {published data only}
- NCT01911364. Efficacy of fixed combination of beclometasone + formoterol + glycopyrrolate in chronic obstructive pulmonary disease [A 52-wk randomized double blind parallel trial: combination of beclometasone+formoterol+glycopyrrolate vs tiotropium and vs combination of beclometasone+formoterol and tiotropium in patients with chronic obstructive pulmonary disease]. clinicaltrials.gov/ct2/show/NCT01911364 (first received 30 July 2013).
NCT02729051 {published data only}
- NCT02729051. Comparative study of fluticasone furoate(FF)/umeclidinium bromide (UMEC)/ vilanterol (VI) closed therapy versus FF/VI plus UMEC open therapy in subjects with chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT02729051 (first received 6 April 2016).
NCT02731846 {published data only}
- NCT02731846. A study comparing the closed triple therapy, open triple therapy and a dual therapy for effect on lung function in subjects with chronic obstructive pulmonary disease (COPD) [A phase III, 4-week, randomized, double-blind study to compare 'closed' triple therapy (FF/UMEC/VI), 'open' triple therapy (FF/VI + UMEC) and dual therapy (FF/VI) in subjects with chronic obstructive pulmonary disease (COPD)]. clinicaltrials.gov/ct2/show/NCT02731846 (first received 8 April 2016).
NCT03478683 {published data only}
- NCT03478683. A randomized study, comparing fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) single inhaler triple therapy, versus multiple inhaler therapy (budesonide/formoterol plus tiotropium) in subjects with chronic obstructive pulmonary disease (COPD) [A phase IV, 12-week, randomised, double-blind, triple dummy study to compare single inhaler triple therapy, fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) with multiple inhaler therapy (budesonide/formoterol plus tiotropium) based on lung function and symptoms in participants with chronic obstructive pulmonary disease]. clinicaltrials.gov/ct2/show/NCT03478683 (first received 27 March 2018).
NCT04320342 {published data only}
- NCT04320342. A phase III study comparing efficacy, safety and tolerability of the fixed dose triple combination CHF 5993 with the fixed dose dual combination CHF 1535 in subjects With COPD. https://clinicaltrials.gov/ct2/show/NCT04320342 (first received 23 March 2020).
NCT04675463 {published data only}
- NCT04675463. The effects of inhaled budesonide-formoterol-glycopyrronium in moderate-to-severe COPD. https://clinicaltrials.gov/ct2/show/NCT04675463 (first received 14 December 2020).
NCT04923347 {published data only}
- NCT04923347. A study to evaluate the safety and efficacy of fluticasone furoate (FF)/umeclidinium(UMEC)/vilanterol (VI) in participants with chronic obstructive pulmonary disease (COPD). https://clinicaltrials.gov/ct2/show/NCT04923347 (first received 6 June 2021).
Palli 2019 {published data only}
- Palli SR, Buikema AR, DuCharme M, Frazer M, Kaila S, Juday T. Costs, exacerbations and pneumonia after initiating combination tiotropium olodaterol versus triple therapy for chronic obstructive pulmonary disease. Journal of Comparative Effectiveness Research 2019;8(15):1299-316. [DOI] [PubMed] [Google Scholar]
Scuri 2018 {published data only}
- Scuri M, Fabbri LM, Singh D, Roche N, Corradi M, Alessandro G, et al. Reduction in fatal events with ICS-containing medications: results of safety pooled analysis from the TRILOGY, TRINITY and TRIBUTE studies. American Journal of Respiratory and Critical Care Medicine 2018;197:A7725. [Google Scholar]
Singh 2016 {published data only}
- Singh D, Papi A, Corradi M, Pavlisova I, Montagna I, Francisco C, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting beta2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet 2016;388(10048):963-73. [DOI] [PubMed] [Google Scholar]
Singh 2018 {published data only}
- Singh D. Single inhaler triple therapy with extrafine beclomethasone, formoterol, and glycopyrronium for the treatment of chronic obstructive pulmonary disease. Expert Opinion on Pharmacotherapy 2018;19(11):1-9. [DOI] [PubMed] [Google Scholar]
Stiegler 2019 {published data only}
- Stiegler MA, Thomashow B, Criner GJ, Dransfield MT, Halpin DMG, Han MK, et al. COPD assessment test (CAT) score is associated with risk of future exacerbation: an analysis from the IMPACT trial. American Journal of Respiratory and Critical Care Medicine 2019;199:A1591. [Google Scholar]
van den Berge 2020 {published data only}
- den Berge M, De Backer J, Van Holsbeke C, De Backer W, Trivedi R, Jenkins M, et al. Functional respiratory imaging assessment of budesonide/glycopyrrolate/formoterol fumarate and glycopyrrolate/formoterol fumarate metered dose inhalers in patients with COPD. Chest 2020;158(4 Suppl):A1782-A1783. [Google Scholar]
van den Berge 2021 {published data only}
- den Berge M, De Backer J, Van Holsbeke C, De Backer W, Trivedi R, Jenkins M, et al. Functional respiratory imaging assessment of budesonide/glycopyrrolate/formoterol fumarate and glycopyrrolate/formoterol fumarate metered dose inhalers in patients with COPD: the value of inhaled corticosteroids. Respiratory Research 2021;22(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Baraldo 2012
- Baraldo S, Turato G, Saetta M. Pathophysiology of the small airways in chronic obstructive pulmonary disease. Respiration 2012;84:89-97. [DOI] [PubMed] [Google Scholar]
Bateman 2014
- Bateman ED, Mahler DA, Vogelmeier CF, Wedzicha JA, Patalano F, Banerji D. Recent advances in COPD disease management with fixed-dose long-acting combination therapies. Expert Review of Respiratory Medicine 2014;8(3):357-79. [DOI] [PubMed] [Google Scholar]
Beeh 2021
- Beeh KM, Kuna P, Corradi M, Viaud I, Guasconi A, Georges G. Comparison of dry-powder inhaler and pressurized metered-dose inhaler formulations of extrafine beclomethasone dipropionate/formoterol fumarate/glycopyrronium in patients with COPD: The TRI-D randomized controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2021;16:79-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buist 2007
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD study): a population-based prevalence study. Lancet 2007;370(9589):741-50. [DOI] [PubMed] [Google Scholar]
COPDX 2019
- Yang IA, Brown JL, George J, Jenkins S, McDonald CF, McDonald V, et al. Australian and New Zealand Guidelines for the Management of Chronic Obstructive Pulmonary Disease 2019 Version 2.58, June 2019. copdx.org.au/wp-content/uploads/2019/09/COPDX-V2-58-June-2019-FINAL2.pdf (accessed 3 August 2016).
de Miguel‐Díez 2014
- Miguel-Díez J, Jiménez-García R. Considerations for new dual-acting bronchodilator treatments for chronic obstructive pulmonary disease. Expert Opinion on Investigational Drugs 2014;23(4):453-6. [DOI] [PubMed] [Google Scholar]
Farne 2015
- Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD008989. [DOI: 10.1002/14651858.CD008989.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaebel 2011
- Gaebel K, McIvor RA, Xie F, Blackhouse G, Robertson D, Assasi N, et al. Triple therapy for the management of COPD: a review. COPD 2011;8(3):206-43. [DOI: 10.3109/15412555.2011.560131] [DOI] [PubMed] [Google Scholar]
Geake 2015
- Geake JB, Dabscheck EJ, Wood-Baker R, Cates CJ. Indacaterol, a once-daily beta2-agonist, versus twice-daily beta2-agonists or placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No: CD010139. [DOI: 10.1002/14651858.CD010139.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
GOLD 2023
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (2023 report). https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf 2023.
Halpin 2012
- Halpin DMG, Decramer M, Celli B, Kesten S, Liu D, Tashkin DP. Exacerbation frequency and course of COPD. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:653-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartl 2020
- Hartl S, Breyer MK, Burghuber OC, Ofenheimer A, Schrott A, Urban MH, et al. Blood eosinophil count in the general population: typical values and potential confounders. European Respiratory Journal 2020;14(55):1901874. [DOI: 10.1183/13993003.01874-2019] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2022
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
Karner 2011
- Karner C, Cates CJ. The effect of adding inhaled corticosteroids to tiotropium and long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 9. Art. No: CD009039. [DOI: 10.1002/14651858.CD009039.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karner 2012
- Karner C, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 4. Art. No: CD008989. [DOI: 10.1002/14651858.CD008989.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kew 2013
- Kew KM, Mavergames C, Walters JAE. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD010177. [DOI: 10.1002/14651858.CD010177.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kew 2014a
- Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD010844. [DOI: 10.1002/14651858.CD010844.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kew 2014b
- Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD010115. [DOI: 10.1002/14651858.CD010115.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 2019
- Lai CC, Chen CH, Lin CYH, Wang CY, Wang YH. The effects of single inhaler triple therapy vs single inhaler dual therapy or separate triple therapy for the management of chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized controlled trials. International Journal of COPD 2019;14:1539-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2009
- Lee J-H, Lee YK, Kim E-K, Kim T-H, Huh JW, Kim WJ, et al. Responses to inhaled long-acting beta-agonist and corticosteroid according to COPD subtype. Respiratory Medicine 2009;104(4):542-9. [DOI] [PubMed] [Google Scholar]
Leuppi 2005
- Leuppi JD, Tandjung R, Anderson SD, Stolz D, Brutsche MH, Bingisser R, et al. Prediction of treatment-response to inhaled corticosteroids by mannitol-challenge test in COPD. A proof of concept. Pulmonary Pharmacology and Therapeutics 2005;18(2):83-8. [DOI] [PubMed] [Google Scholar]
Miravitlles 2011
- Miravitlles M. Arguments in favor of inhaled corticosteroids in COPD by phenotype instead of by severity. Archivos de Bronconeumologia 2011;47(6):271-3. [DOI] [PubMed] [Google Scholar]
Montuschi 2013
- Montuschi P, Macagno F, Valente S, Fuso L. Inhaled muscarinic acetylcholine receptor antagonists for treatment of COPD. Current Medicinal Chemistry 2013;20(12):1464-1476. [DOI] [PubMed] [Google Scholar]
Montuschi 2015
- Montuschi P, Ciabattoni G. Bronchodilating drugs for chronic obstructive pulmonary disease: current status and future trends. Journal of Medicinal Chemistry 2015;58(10):4131-4164. [DOI] [PubMed] [Google Scholar]
Montuschi 2016
- Montuschi P, Malerba M, Macis G, Mores N, Santini G. Triple inhaled therapy for chronic obstructive pulmonary disease. Drug Discovery Today 2016;21(11):1820-1827. [DOI] [PubMed] [Google Scholar]
NICE 2019
- National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management NICE guideline [NG115]. www.nice.org.uk/guidance/ng115 (accessed prior to 8 October 2019). [PubMed]
Pascoe 2019
- Pascoe S, Barnes N, Brusselle G, Compton C, Criner GJ, Dransfield MT, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respiratory Medicine 2019;7(9):745-56. [DOI: 10.1016/S2213-2600(19)30190-0] [DOI] [PubMed] [Google Scholar]
PlotDigitizer [Computer program]
- PlotDigitizer. Porbital, 2022. Available at plotdigitizer.com.
Rennard 2004
- Rennard SI, Farmer SG. Exacerbations and progression of disease in asthma and chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2004;1(2):88-92. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rodrigo 2014
- Rodrigo GJ, Plaza V. Efficacy and safety of a fixed-dose combination of indacaterol and glycopyrronium for the treatment of COPD: a systematic review. Chest 2014;146(2):309-17. [DOI] [PubMed] [Google Scholar]
Rücker 2017
- Rücker G, Cates CJ, Schwarzer G. Methods for including information from multi-arm trials in pairwise meta-analysis. Research Synthesis Methods 2017;8:392-403. [DOI: 10.1002/jrsm.1259] [DOI] [PubMed] [Google Scholar]
Siva 2007
- Siva R, Green RH, Brightling CE, Shelley M, Hargadon B, McKenna S, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. European Respiratory Journal 2007;29(5):906-13. [DOI] [PubMed] [Google Scholar]
Spencer 2011
- Spencer S, Karner C, Cates CJ, Evans DJ. Inhaled corticosteroids versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 12. Art. No: CD007033. [DOI: 10.1002/14651858.CD007033.pub2] [DOI] [PubMed] [Google Scholar]
van der Molen 2012
- Molen T, Cazzola M. Beyond lung function in COPD management: effectiveness of LABA/LAMA combination therapy on patient-centred outcomes. Primary Care Respiratory Journal 2012;21(1):101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Valk 2002
- Valk P, Monninkhof E, Palen J, Zielhuis G, Herwaarden C. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. American Journal of Respiratory and Critical Care Medicine 2002;166(10):1358-63. [DOI] [PubMed] [Google Scholar]
van Geffen 2016
- Geffen WH, Douma WR, Slebos DJ, Kerstjens HAM. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD011826. [DOI: 10.1002/14651858.CD011826.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Geffen 2018
- Geffen WH, Kerstjens HAM. Static and dynamic hyperinflation during severe acute exacerbations of chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease 2018;13:1269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Geffen 2019
- Geffen WH, Slebos DJ, Herth FJ, Kemp SV, Weder W, Shah PL. Surgical and endoscopic interventions that reduce lung volume for emphysema: a systemic review and meta-analysis. The Lancet. Respiratory Medicine 2019;7(4):313-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
van Geffen 2020
- Geffen WH, Carpaij OA, Westbroek LF, Seigers D, Niemeijer A, Vonk JM, et al. Long-acting dual bronchodilator therapy (indacaterol/glycopyrronium) versus nebulized short-acting dual bronchodilator (salbutamol/ipratropium) in chronic obstructive pulmonary disease: a double-blind, randomized, placebo-controlled trial. Respiratory Medicine 2020;171:106064. [DOI] [PubMed] [Google Scholar]
Vestbo 2011
- Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. Changes in forced expiratory volume in 1 second over time in COPD. New England Journal of Medicine 2011;365(13):1184-92. [DOI] [PubMed] [Google Scholar]
Wang 2005
- Wang Q, Bourbeau J. Outcomes and health-related quality of life following hospitalisation for an acute exacerbation of COPD. Respirology 2005;10(3):334-40. [DOI] [PubMed] [Google Scholar]
Watz 2016
- Watz H, Tetzlaff K, Wouters EFM, Kirsten A, Magnussen H, Rodriguez-Roisin R, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respiratory Medicine 2016;4(5):390-398. [DOI] [PubMed] [Google Scholar]
Wedzicha 2013
- Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Medicine 2013;11:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. WHO Global Report: mortality attributable to tobacco. whqlibdoc.who.int/publications/2012/9789241564434_eng.pdf (accessed 11 December 2014).
Yang 2012
- Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD002991. [DOI: 10.1002/14651858.CD002991.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zayed 2018
- Zayed Y, Barbarawi M, Kheiri B, Haykal T, Chahine A, Rashdan L, et al. Triple versus dual inhaler therapy in moderate-to-severe COPD: a systematic review and meta-analysis of randomized controlled trials. Clinical Respiratory Journal 2018;13(7):413-28. [DOI] [PubMed] [Google Scholar]
Zheng 2018
- Zheng Y, Zhu J, Liu Y, Lai W, Lin C, Qiu K, et al. Triple therapy in the management of chronic obstructive pulmonary disease: systematic review and meta-analysis. BMJ 2018;363(8176):k4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Tan 2016
- Tan DJ, White CJ, Walters JAE, Walters EH. Inhaled corticosteroids with combination inhaled long‐acting beta2‐agonists and long‐acting muscarinic antagonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No: CD011600. [DOI: 10.1002/14651858.CD011600.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


