History
As microsurgical techniques are refined, autologous breast reconstruction will accordingly increase in popularity. Advances made in autologous breast reconstruction have reduced operative time, length of stay, and morbidity.1,2 In the realm of breast reconstruction, abdominal-based flaps remain the gold-standard tissue source.3–5 However, when an abdominal donor site is unavailable, alternate flaps, including thigh-based tissue, should be explored. Owing to the rich vascular supply of the thigh, a multitude of flaps have been designed from this anatomic region for reconstruction of both locoregional and distant defects. A gluteal thigh flap was first described in 1980 in the setting of coverage and reconstruction of chronic wounds of the perineal and sacral area.6 In 1984, Song et al. described the use of a posterior thigh free flap based on perforators arising off the profunda artery.7 In their series of 15 lower extremity-based flaps, all were used for reconstruction of burn contractures of the head and neck. Only two of the 15 flaps were harvested as profunda artery perforator (PAP) flaps, while the rest were anterolateral or anteromedial thigh flaps. In 2001, Angrigiani and colleagues expanded on the posterior thigh flap and described the technical aspects of PAP flap harvest and perforator reliability using latex injection in cadaveric specimens.8 They translated this into 25 live patients: 14 flaps were transferred as pedicled flaps for reconstruction of the ischial/perineal region, while the remaining 11 patients had transfer of free flaps for resurfacing of burn contractures in the head and neck or for reconstruction of lower extremity defects.
In 2012, Allen was the first to expand the use of PAP flaps for autologous breast reconstruction.9 In his landmark paper, he detailed the harvest and transfer of 27 PAP flaps, including descriptions on the use of preoperative imaging, surgical technique and method of harvest, postoperative recovery, and complications. This study heralded a massive shift in the potential reconstructive options for breast cancer patients. With increasing familiarity and surgeon experience, the PAP flap has emerged as a popular alternate donor site when abdominal flaps are contraindicated or undesired.
The PAP flap addresses the disadvantages of gracilis flap variants. By nature of being a perforator flap, the PAP flap spares sacrifice of any muscle. In comparison to gracilis flaps, the PAP flap is larger in volume, has a longer and greater caliber pedicle, and its skin paddle design can be larger.10 Additionally, when compared to transverse upper gracilis (TUG) flaps, the dissection is more distant from draining lymphatics and avoids key structures in the femoral triangle, thus preventing potential dead space, and decreasing the subsequent risk of lymphedema/seroma and the potential associated morbidity.10,11 The donor site scar for a PAP flap can be conveniently hidden in the inferior gluteal crease to create an aesthetic donor site. Further, the tissue of the posterior thigh is favorable for creating a youthful breast, as it tends to contain firmer, yet moldable fat. The elliptical skin paddle design also allows for insetting and shaping into a natural, youthful breast mound. These factors likely contribute to the increasingly common utilization of PAP flaps as an alternative to abdominal-based tissue.
Indications and Patient Selection
PAP flaps are preferred for breast reconstruction in patients with contraindications to an abdominal-based donor site. This includes patients with scant abdominal tissue, prior failed abdominal flap/history of abdominoplasty, significant prior abdominal surgeries, or patients who are reluctant to have abdominal scarring. Further, young patients who desire future pregnancy may wish to avoid abdominal donor site harvest. We recommend avoiding PAP flap harvest in patients with significant burns/scars of the thigh region or those with significant iliofemoral vascular occlusive disease.
It has been shown that in patients with a low body mass index (BMI) and/or minimal abdominal tissue, there is often adequate volume of potential donor tissue in the posterior thigh region.12–14 Several studies have been performed analyzing both patient and PAP flap characteristics.10,12–20 The average PAP flap weight across these studies ranged from 242 to 425 grams (g) (minimum 132 g, maximum 815 g), while the average patient BMI ranged from 23–27 kg/m2. Table 1 provides a detailed review of patient and PAP flap characteristics across studies specific to breast reconstruction.10,12–20 PAP flaps can also be combined with a second free flap (e.g., deep inferior epigastric perforator [DIEP] flap) in a “stacked” flap fashion, or augmented with implants and/or fat grafting when a larger breast size is desired than what the posterior thigh donor site can provide.21 Nonetheless, similar to abdominal-based flaps, PAP flaps provide sufficient volume to reconstruct a wide volumetric range of mastectomy defects.22
Table 1.
Review of patient and PAP flap characteristics across 10 studies.
| Study | Year | Study Type | Mean Age (years) | Number of Patients/Number of Flaps | Mean BMI (kg/m2) | Mean Flap Weight Range (grams) | Mean Pedicle Length (cm) |
|---|---|---|---|---|---|---|---|
| Hunter et al | 2015 | PC | - | 13/22 | 21.6 | 242 (132–455) | - |
| Haddad et al | 2016 | PC | - | 30/30 | - | 301 (195–700) | 9.88 |
| Ito et al | 2016 | RR | 41.6 | 5/7 | 23.5 | 257.1 (200–350) | 9.4 |
| Hupkens et al | 2016 | PC | 44 | 30/40 | 23.3 | 372.4 (250–470) | 11 |
| Allen et al | 2016 | RR | 48 | 96/164 | 22.5 | 367.4 (225–739) | 10.2 |
| Haddock et al | 2017 | RR | - | 56/101 | 26.4 | 425 (170–815) | 10.3 |
| Fosseprez et al | 2017 | RR | - | 15/17 | - | - | - |
| Haddock et al | 2019 | RR | 51.5 | 20/40 | 27.3 | 398.5 (170–600) | 12.9 |
| Haddock et al | 2020 | RR | - | 138/265 | 26.5 | 403 (190–800) | 11.2 |
| Atzeni et al | 2021 | PC | 47.56 | 86/116 | 24.72 | 251.3 (152–455) | - |
RR: Retrospective Review; PC: Prospective Cohort Study; BMI: Body Mass Index; cm: centimeters; min: minutes
PAP Flap Anatomy
The PAP flap is based on perforators branching off the profunda femoris (deep femoral) artery. The profunda femoris arises 3–4 cm distal to the inguinal ligament on the posterolateral aspect of the common femoral artery. The vessel then runs posterior to the femur in a lateral to medial direction. Coursing between the adductor magnus and semitendinosus muscles, it gives off several musculocutaneous and septocutaneous perforators supplying the fat and skin of the posteromedial thigh.23 The first perforating vessel supplies the adductor magnus and gracilis muscles. The second branch supplies the semimembranosus, biceps femoris, and vastus lateralis muscles.24 Typically, the perforator(s) selected for microvascular anastomosis arise 5–7 cm caudal to the inferior gluteal crease. The profunda artery consistently supplies at least two usable perforators, with some patients having 5 perforators in a single thigh.24 These vessels have been shown to be of adequate caliber and size for microsurgical transfer and postoperative flap perfusion.25–30 The arterial perforator averages 2.3 mm in diameter, with its associated vena comitans averaging 2.8 mm in diameter.23 Pedicle length measures an average length of 11–13 cm across several studies.15,17,20,23,24
Preoperative Imaging
In the era of advanced imaging, modalities including magnetic resonance angiography (MRA) and computed tomography angiography (CTA) are increasingly used in the preoperative setting for assessment and confirmation of perforators. (Figure 1). We prefer preoperative MRA for the detailed anatomy and 3-D reprocessing techniques that it provides. This high-resolution imaging allows the surgeon to carefully plan the incisions sites and anticipated skin paddle. Unlike abdominal-based flap harvest, the inconsistent location of a dominant perforator in PAP flaps has many surgeons relying on preoperative imaging. However, newer modifications of the PAP, including a diagonal (dPAP) and vertical (vPAP) design to be discussed later in the text, allow for capture of more perforators along the course of the profunda artery, compared to flaps with a classical transverse (tPAP) orientation. Therefore, imaging may not be mandatory for diagonal and vertical flap designs.
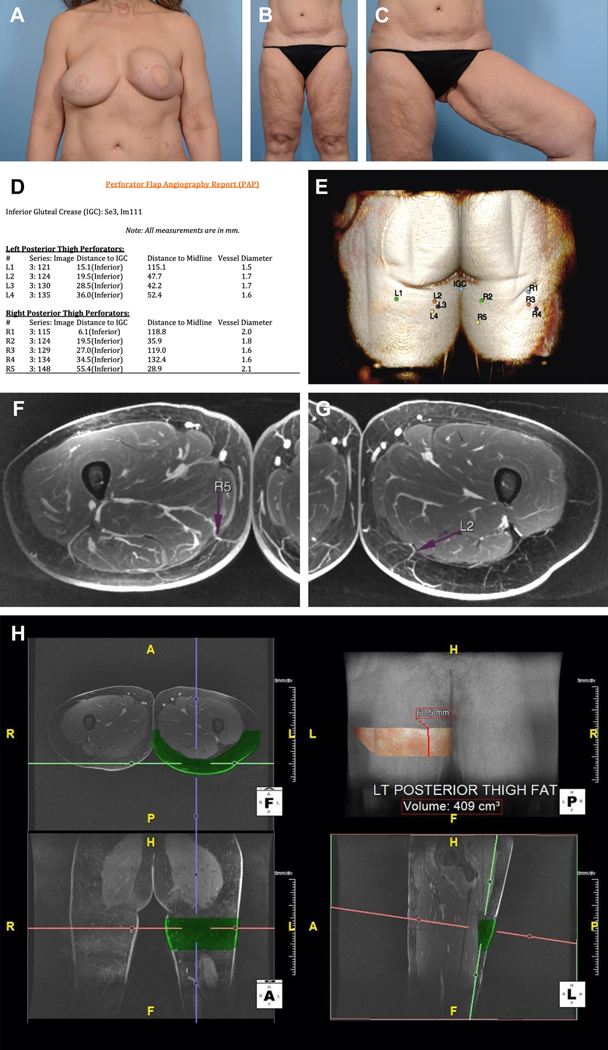
Figure 1.
Preoperative images of the (A) breasts and (B, C) lower extremities. Patient had a history of right breast cancer and underwent mastectomy with DIEP flap reconstruction. The left breast subsequently developed cancer and was reconstructed with a pedicled latissimus dorsi flap and implant. Note severe capsular contracture of left breast a well as laxity of tissue in the posteromedial thighs. Magnetic resonance angiography (MRA) of the bilateral lower extremities with and without intravenous contrast and 3-dimensional (3-D) postprocessing was performed. (D) Perforator flap angiography report detailing vessel caliber as well as location. IGC=Inferior Gluteal Crease. (E) 3-D perforator map showing perforator location on posterior thigh. (F) Axial view of the thigh demonstrating a favorable R5 perforator located 55.4 mm inferior to the IGC, 28.9 mm to the right of midline, and 6.9 mm posterior to the posterior margin of gracilis. Vessel diameter is 2.1 mm. It travels 185.2 mm with an intramuscular course before joining the inferior gluteal artery. (G) Axial view of the thigh demonstrating a favorable L2 perforator located 19.5 mm inferior to the IGC, 47.7 mm to the left of midline, and 39.5 mm posterior to the posterior margin of gracilis. Vessel diameter is 1.7 mm. It travels 185.2 mm with an intramuscular course before joining the inferior gluteal artery. (H) Fat volume of a 22×6 cm flap on posterior left thigh is 409.0cc
Operative Technique
When possible, we prefer a split-leg bed or lithotomy for patient positioning and adequate exposure of the inner thigh (Figure 2). When positioning patients in lithotomy, one should be cognizant to pad pressure points and properly position the lower extremities to avoid potential nerve entrapment/compression. Both split-leg and lithotomy allow the surgeon to be situated in-between the patient’s thighs and approach the operation from multiple angles, facilitating efficient and safe flap harvest. Additionally, the nature and location of the PAP flap permits a two-team approach, allowing the mastectomy and recipient site to be prepared in conjunction with flap harvest. In stacked DIEP-PAP flap cases, the DIEP flap dissection can commence at the same time as the PAP flap. Similar to abdominal-based breast reconstruction, PAP flaps can be performed in an immediate or delayed fashion. In fact, single-stage breast reconstruction with PAP flaps offers comparable results to delayed reconstruction.31,32
Figure 2.
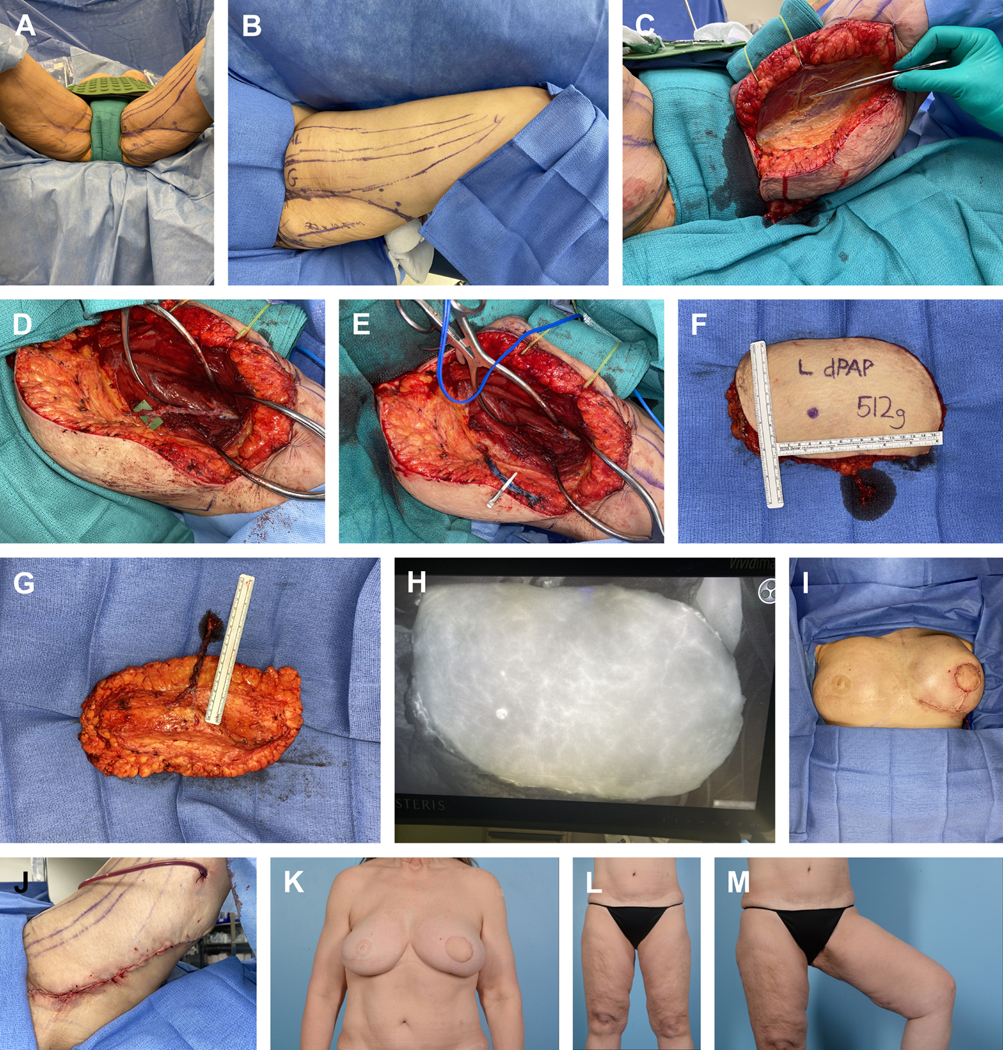
Preoperative surgical site markings and patient positioning for a planned diagonal PAP. (A) Patient positioning in lithotomy. (B) Preoperative marking delineating the adductor longus (AL) muscle, gracilis (G) muscle, and a 22×10.5 cm anticipated diagonal skin paddle. (C) The anterior skin incision is made and carried down through the fascia investing the gracilis, at which point the gracilis is retracted anteriorly. Forceps identify the gracilis pedicle. (D) The investing fascia overlying the adductor magnus is incised and subfascial dissection proceeds. Meticulous posterior dissection of the adductor magnus fascia reveals a large perforator with 2 cutaneous branches (E) A microvascular bulldog was then placed on the main perforator and its associated vena comitans, at which point the vessels were clipped and divided. (F, anterior; G, posterior) The posterior incision was completed to allow for flap harvest and weighing (512g). Flap preparation proceeded on the back table and revealed a pedicle length of 10.5 cm. The flap was then transferred to the left mastectomy defect and microvascular anastomoses were performed in a standard fashion to the internal mammary system. (H) SPY-PHI (Stryker Corp., Kalamazoo, MI; manufactured by Medical London LP, London, Ontario, Canada) angiography of the free flap reveals excellent tissue perfusion (I) The flap was then selectively de-epithelialized and inset with absorbable sutures to recreate a youthful breast mound and the wound was closed over a drain (J) Layered closure of the donor site over a closed-suction drain. (K) Postoperative appearance of the breast and (L, M) lower extremities. Note improved contour of the left upper thigh with an inconspicuous donor site scar.
After visualization of perforator status with advanced imaging modalities, the donor vessel(s) are confirmed with a Doppler probe and are marked for intraoperative reference (Figure 2). The adductor longus and gracilis muscles are highlighted as important landmarks, as is the inferior gluteal crease (Figure 2). The skin paddle is designed using the pinch test, such that the width of the flap is an estimate of the amount of tissue that can be excised while still allowing for a tension-free primary closure of the donor site. In the most classic orientation (tPAP), skin markings are typically made with the patient upright, to ensure the incision remains hidden in the inferior gluteal crease. The anterior incision is made and taken through the subcutaneous tissue. Electrocautery is used to proceed with dissection from anterior to posterior. The fascia investing the gracilis is entered and the muscle retracted anteriorly. The adductor magnus fascia is then exposed, incised, and dissected proximally in a subfascial manner until the perforators are identified. Intramuscular dissection of the perforators then proceeds in a standard fashion until adequate pedicle length is achieved or the profunda artery is encountered (Figure 2). When pedicle length and vessel caliber are deemed suitable for microsurgical transfer, the flap is divided and transferred to reconstruct the mastectomy defect. If additional time is needed for preparation of the recipient site, the perforating flap vessels can still be ligated given the fact that perfusion remains intact via posterior skin attachments and musculocutaneous perforators. When ready, the posterior attachments are divided, and the flap is harvested, weighed, and prepared on the back table for transfer.
Microsurgical anastomosis is then performed in a standard fashion, most often antegrade to the internal mammary artery and vein. Alternatively, the retrograde mammary or thoracodorsal vessels can be utilized as backup options.33,34 We use SPY-PHY (Stryker Corp., Kalamazoo, MI; manufactured by Medical London LP, London, Ontario, Canada) angiography to visualize and confirm flap perfusion following microvascular anastomosis, and any tissue with questionable viability is excised. The flap is then inset with absorbable sutures. One should take time to ensure sufficient inferomedial pole fullness, as these areas tend to be difficult to augment during subsequent revisional procedures.35 (Figure 2) Selective de-epithelialization is then carried out while maintaining an adequate skin paddle for postoperative monitoring. The skin paddle can be easily removed at the time of revision or staged nipple reconstruction.36 The skin is approximated in layers over a closed suction drain placed in the mastectomy space.
Given the muscle-sparing nature inherent to the PAP flap, there is a decreased chance of dead space in the donor site. Nonetheless, care must be taken to ensure a tension-free, multilayered closure. This is achieved with careful elevation of the inferior skin flap off the investing thigh fascia. Quilting sutures can further facilitate closure and decrease tension on the wound. The donor site is approximated in layers over a closed-suction drain (Figure 2). The lower extremity is placed in a compression garment, and patients are instructed to avoid strenuous activity for at least 6 weeks, at which time activities can be liberalized as indicated.
Variations in PAP Flap Design: Transverse, Vertical, and Diagonal
Various derivations and modifications have been made to the original PAP flap used in breast reconstruction, which was designed with a transverse skin paddle (tPAP).9 The tPAP has several limitations. Namely the width of the skin paddle is limited in size to about 6–8 cm. Additionally, there is a great degree of tension on the donor site, increasing the risk of wound complications. The incision is also compressed when patients are in the sitting position, a factor which may potentiate wound complications and/or chronic posterior thigh paresthesia secondary to damage to the posterior cutaneous nerves.37
Hupkens and colleagues described a geometric modification of the tPAP flap, in which dissection was extended cranially to include additional tissue from the inferior gluteal area, thus increasing the volume available for harvest.17 Similarly, there has been description of a vertical PAP (vPAP) flap in which a longitudinal skin paddle was delineated to maximize the number of larger, distal perforators that are harvested.38,39 Tielemans and colleagues then expanded on the “extended” PAP flap in 2021, citing similar complication profiles to “standard” PAP flaps and a 97.8% flap survival rate.40 Also, the vertical incision avoids dissection near lower extremity nerves and lymphatics, and distributes tension in a circumferential manner around the thigh, thus decreasing the risk of potential wound complications/scar migration. The donor site remains well-concealed with a vPAP and resembles an inner thighplasty scar.
Dayan and Allen Jr. described the harvest of a diagonal PAP (dPAP) oriented along Langer’s lines, which allows for a wider skin paddle and decreased tension on the donor site seen with traditional tPAP flaps.37 Additionally, the diagonal design captures more fat from the posteromedial thigh that is ideal for breast reconstruction. Similar to a vPAP, the incision with dPAPs is not compressed in a sitting position, thus decreasing potential wound sequalae and/or thigh paresthesia.
Lastly, a fleur-de-lis modification of the PAP flap, in which both vertical and horizontal limbs are included, allows for harvest of the entire angiosome of perforators and maximizes flap volume from a single donor site.41–43
Specialized PAP Flaps: Expanded Use and Modifications
Since its introduction by Allen in the realm of autologous breast reconstruction, the PAP flap has been refined and its use extended. Several techniques have been utilized that increase the potential reconstructive aspects of the PAP flap. For example, Mayo et al. described the utilization of the PAP flap for non-breast reconstruction in the setting of lower extremity and head and neck defects.44
A PAP flap can be harvested from both thighs and used in a “stacked” fashion for unilateral or bilateral breast reconstruction. The most common combination of stacked flaps used for reconstruction is a DIEP flap combined with a PAP flap.20,45–49 This remains a viable option for patients with scant donor tissue who wish to avoid staged revision with implants or repeated attempts at fat grafting. The placement of a second flap into a mastectomy defect provides additional volume for body-specific reconstruction with acceptable complication rates.46 However, utilization of a second free flap necessitates an additional set of microvascular anastomoses, thus increasing operative time, technical difficulty, surgeon fatigue, and potential complications. However, recipient vessel options in this scenario are plentiful, and include the antegrade and retrograde internal mammary systems, thoracodorsal, thoracoacromial, and branching vessels off of the primary flap pedicle.34 Haddock et al. noted an increase in the rate of flap loss when the retrograde internal mammary system is used,50 and for this reason they advocate for the use of intra-flap vessels for secondary recipient anastomoses. That being said, the retrograde system is our preferred second option if the antegrade mammary vessels are of poor quality.
The topic of sensate flap creation for breast reconstruction remains a topic of debate.51,52 Nonetheless, Dayan and Allen Jr. first described the successful transfer of a sensate PAP flap. In this case, neurorrhaphy was achieved by anastomosing the anterior branch of the obturator nerve to the lateral branch of the T4 intercostal nerve. In a recent study utilizing cadaveric thigh dissection, Song and colleagues described the feasibility and location of the posterior femoral cutaneous nerve and its potential for the transfer and creation of a sensate PAP flap.53
Postoperative Care
Postoperatively, routine flap monitoring is carried out at the recipient site, typically with pencil Doppler assessment of the skin paddle. Patients are instructed to wear lower body compression garments for 3 weeks to help decrease swelling and prevent potential scar widening/hypertrophy or seroma formation. Drain output is carefully monitored for volume and consistency. Patients are typically admitted for 1–3 days. When criteria for discharge are met, patients should be educated on drain care and have reinforcement of activity restrictions until they follow up in clinic. Additionally, one should attempt to avoid pressure on the donor incision site.
Outcomes and Complications
Complications following PAP flap surgery occur at low, acceptable rates at both the recipient and donor sites. Donor site complications include seroma, hematoma, wound dehiscence, wound infection/cellulitis, and pain.19 In 2016, Allen and colleagues reviewed 164 total flaps used in 96 patients.19 They noted a recipient complication profile of hematoma (1.9%), seroma (6%), fat necrosis (7%), and one instance of flap loss. Haddock et al. published the results of their PAP flap experience in 2017 and 2020.12,13 Of 101 PAP flaps, there were 2 total flap losses (2%) and one case of fat necrosis. In 2020 they expanded their investigation to 265 PAP flaps and noted 8 flap losses (3%). A prospective review of 30 PAP flaps by Haddad and colleagues noted 2 cases of flap loss (6.7%).15
Patients should also be educated on the nature of donor site complications, which are typically managed nonoperatively. Cho et al. showed that donor site morbidity and complications occurred at a higher rate as BMI increased, with all of their complications localizing to the medial thigh.54 Cited donor complication rates by Haddock and colleagues included seroma (4.5%), hematoma (2.6%), infection (4.9%), and significant wound issues (6.8%).13 Significant wounds were defined as those necessitating procedural intervention or negative pressure therapy. In 2019, Qian and colleagues performed a systematic review of 12 studies that included 516 PAP flaps in 327 patients.55 The pooled success rate was 99%. Pooled donor complications included wound dehiscence (6%), seroma (2%), hematoma (1%). The rate of partial flap loss was 2%, while total flap loss occurred at a pooled incidence of 1%. Table 2 notes the rates of donor complications as well as total flap loss across the 10 studies that were cited in Table 1.
Table 2.
Review of donor site complications across 10 studies.
| Study | Year | Study Type | Hematoma | Wound Dehiscence | Wound Infection | Seroma | Total Flap Loss |
|---|---|---|---|---|---|---|---|
| Hunter et al | 2015 | PC | - | 4.50% | - | 4.50% | 9.09% |
| Haddad et al | 2016 | PC | - | - | - | 6.67% | 6.67% |
| Ito et al | 2016 | RR | - | - | - | - | - |
| Hupkens et al | 2016 | PC | - | 10% | - | - | - |
| Allen et al | 2016 | RR | 1.90% | 3.60% | - | 6% | 0.61% |
| Haddock et al | 2017 | RR | - | 10.89% | 5.90% | - | 1.98% |
| Fosseprez et al | 2017 | RR | 5.8%* | - | - | 35.30% | 11.80% |
| Haddock et al | 2019 | RR | - | 3% | - | - | - |
| Haddock et al | 2020 | RR | 2.60% | 6.80% | 4.90% | 4.50% | 3% |
| Atzeni et al | 2021 | PC | 1.70% | 2.60% | - | 2.60% | - |
RR: Retrospective Review; PC: Prospective Cohort Study; * denotes recipient site complication
Enhanced recovery after surgery (ERAS) protocols exist across several surgical specialties and have been shown to be effective in breast reconstruction with abdominal-based tissue.56 This has been translated into PAP flap-based breast reconstruction, and has proven to lower hospital length of stay, operative time, and postoperative opioid consumption.57 Additionally, patient reported outcome measures (PROMs) have been assessed for patients undergoing breast reconstruction with PAP flaps, and indicate high scores in all BREAST-Q domains.13,14,58
Case Example
We present a case of a 53-year-old female with a history of bilateral breast cancer. She had right breast reconstruction with a DIEP flap and subsequently developed left breast cancer. The left side was reconstructed with a pedicled latissimus flap and silicone implant. Unfortunately, the patient developed capsular contracture of the left breast and desired implant removal and autologous reconstruction. The implant was removed, and an ipsilateral diagonal PAP flap was harvested for left breast reconstruction (Figure 1, 2 and Video 1).
Conclusions
PAP flaps have emerged as a leading alternative for autologous breast reconstruction when abdominal-based tissue is contraindicated or undesired. Several studies have cited excellent surgical and patient-reported outcomes with low, predictable recipient and donor site morbidity. The aforementioned modifications of the original PAP flap (e.g., vPAP, dPAP, fleur-de-lis PAP) increase the potential reconstructive capacity of this donor tissue. As techniques are refined and surgeon experience improves, we expect the clinical application and utilization of PAP flaps to expand accordingly.
Supplementary Material
Synopsis:
Autologous free flap breast reconstruction allows for natural-appearing breasts, while avoiding the risks associated with implants, including exposure, rupture, and capsular contracture. However, this is offset by a much higher technical challenge. The abdomen remains the most common tissue source for autologous breast reconstruction. However, in patients with scant abdominal tissue, prior abdominal surgery, or a desire to avoid scarring in this region, thigh-based flaps remain a viable alternative. The profunda artery perforator (PAP) flap has emerged as a preferred alternative tissue source, due to excellent aesthetic outcomes and low donor-site morbidity.
Key Points:
Abdominal-based tissue is the gold standard for autologous free flap breast reconstruction.
The profunda artery perforator (PAP) flap may be an option for those who are not candidates for abdominal tissue transfer.
The PAP flap is based off perforators from the profunda artery, supplying the skin and subcutaneous tissue of the posteromedial thigh.
Due to low donor-site morbidity, reliability of the vascular supply, and excellent cosmetic outcomes, the PAP flap has emerged as a preferred alternate source.
Clinical Care Points:
Abdominal-based tissue is the gold standard for autologous free flap breast reconstruction.
The profunda artery perforator (PAP) flap may be an option in those who are not candidates for abdominal tissue transfer.
The PAP flap is based off perforators from the profunda artery, supplying the skin and subcutaneous tissue of the posteromedial thigh.
Due to low donor-site morbidity, reliability of the vascular supply, and excellent cosmetic outcomes, the PAP flap has emerged as a preferred alternate source.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Zack Cohen, Memorial Sloan Kettering Cancer Center/Maimonides Medical Center, 166 Elizabeth St. #2A, New York, NY 10012.
Saïd C. Azoury, Memorial Sloan Kettering Cancer Center, 321 East 61st Street, New York, NY 10065.
Evan Matros, Memorial Sloan Kettering Cancer Center, 321 East 61st Street, New York, NY 10065.
Jonas A. Nelson, Memorial Sloan Kettering Cancer Center, 321 East 61st Street, New York, NY 10065.
Robert J. Allen, Jr., Memorial Sloan Kettering Cancer Center, 321 East 61st Street, New York, NY 10065.
References
- 1.Bonde C, Khorasani H, Eriksen K, Wolthers M, Kehlet H, Elberg J. Introducing the fast track surgery principles can reduce length of stay after autologous breast reconstruction using free flaps: A case control study. Journal of Plastic Surgery and Hand Surgery. 2015/11/02 2015;49(6):367–371. doi: 10.3109/2000656X.2015.1062387 [DOI] [PubMed] [Google Scholar]
- 2.Rozen WM, Ashton MW. Improving Outcomes in Autologous Breast Reconstruction. Aesthetic Plastic Surgery. 2009/05/01 2009;33(3):327–335. doi: 10.1007/s00266-008-9272-1 [DOI] [PubMed] [Google Scholar]
- 3.Tachi M, Yamada A. Choice of flaps for breast reconstruction. International Journal of Clinical Oncology. 2005/10/01 2005;10(5):289–297. doi: 10.1007/s10147-005-0527-4 [DOI] [PubMed] [Google Scholar]
- 4.Gurunluoglu R, Gurunluoglu A, Williams SA, Tebockhorst S. Current Trends in Breast Reconstruction: Survey of American Society of Plastic Surgeons 2010. Annals of Plastic Surgery. 2013;70(1):103–110. doi: 10.1097/SAP.0b013e31822ed5ce [DOI] [PubMed] [Google Scholar]
- 5.Pien I, Caccavale S, Cheung MC, et al. Evolving Trends in Autologous Breast Reconstruction: Is the Deep Inferior Epigastric Artery Perforator Flap Taking Over? Annals of Plastic Surgery. 2016;76(5):489–493. doi: 10.1097/sap.0000000000000339 [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz DJ, Walton RL. Closure of chronic wounds of the perineal and sacral regions using the gluteal thigh flap. Ann Plast Surg. May 1982;8(5):375–86. doi: 10.1097/00000637-198205000-00004 [DOI] [PubMed] [Google Scholar]
- 7.Song YG, Chen GZ, Song YL. The free thigh flap: a new free flap concept based on the septocutaneous artery. Br J Plast Surg. Apr 1984;37(2):149–59. doi: 10.1016/0007-1226(84)90002-x [DOI] [PubMed] [Google Scholar]
- 8.Angrigiani C, Grilli D, Thorne CH. The adductor flap: a new method for transferring posterior and medial thigh skin. Plast Reconstr Surg. Jun 2001;107(7):1725–31. doi: 10.1097/00006534-200106000-00013 [DOI] [PubMed] [Google Scholar]
- 9.Allen RJ, Haddock NT, Ahn CY, Sadeghi A. Breast reconstruction with the profunda artery perforator flap. Plast Reconstr Surg. Jan 2012;129(1):16e–23e. doi: 10.1097/PRS.0b013e3182363d9f [DOI] [PubMed] [Google Scholar]
- 10.Hunter JE, Lardi AM, Dower DR, Farhadi J. Evolution from the TUG to PAP flap for breast reconstruction: Comparison and refinements of technique. J Plast Reconstr Aesthet Surg. Jul 2015;68(7):960–5. doi: 10.1016/j.bjps.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 11.Dayan JH, Allen RJ Jr. Lower Extremity Free Flaps for Breast Reconstruction. Plast Reconstr Surg. Nov 2017;140(5S Advances in Breast Reconstruction):77S–86S. doi: 10.1097/PRS.0000000000003944 [DOI] [PubMed] [Google Scholar]
- 12.Haddock NT, Gassman A, Cho MJ, Teotia SS. 101 Consecutive Profunda Artery Perforator Flaps in Breast Reconstruction: Lessons Learned with Our Early Experience. Plast Reconstr Surg. Aug 2017;140(2):229–239. doi: 10.1097/PRS.0000000000003553 [DOI] [PubMed] [Google Scholar]
- 13.Haddock NT, Teotia SS. Consecutive 265 Profunda Artery Perforator Flaps: Refinements, Satisfaction, and Functional Outcomes. Plast Reconstr Surg Glob Open. Apr 2020;8(4):e2682. doi: 10.1097/GOX.0000000000002682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atzeni M, Salzillo R, Haywood R, Persichetti P, Figus A. Breast reconstruction using the profunda artery perforator (PAP) flap: Technical refinements and evolution, outcomes, and patient satisfaction based on 116 consecutive flaps. J Plast Reconstr Aesthet Surg. Dec 1 2021;doi: 10.1016/j.bjps.2021.11.085 [DOI] [PubMed] [Google Scholar]
- 15.Haddad K, Hunsinger V, Obadia D, Hivelin M, Lantieri L. [Breast reconstruction with profunda artery perforator flap: A prospective study of 30 consecutive cases]. Ann Chir Plast Esthet. 2016/06//2016;61(3):169–176. doi: 10.1016/j.anplas.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 16.Ito R, Huang JJ, Wu JC, Lin MC, Cheng MH. The versatility of profunda femoral artery perforator flap for oncological reconstruction after cancer resection-Clinical cases and review of literature. J Surg Oncol. Aug 2016;114(2):193–201. doi: 10.1002/jso.24294 [DOI] [PubMed] [Google Scholar]
- 17.Hupkens P, Hameeteman M, Westland PB, Slater NJ, Vasilic D, Ulrich DJ. Breast reconstruction using the geometrically modified profunda artery perforator flap from the posteromedial thigh region: combining the benefits of its predecessors. Annals of Plastic Surgery. 2016;77(4):438–444. [DOI] [PubMed] [Google Scholar]
- 18.Fosseprez P, Gerdom A, Servaes M, Deconinck C, Pirson G, Berners A. [Profunda artery perforator flap: Reliable secondary option for breast reconstruction?]. Ann Chir Plast Esthet. Dec 2017;62(6):637–645. Profunda artery perforator flap : quelle place dans la reconstruction mammaire par lambeau autologue ? doi: 10.1016/j.anplas.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 19.Allen RJ Jr., Lee ZH, Mayo JL, Levine J, Ahn C, Allen RJ, Sr. The Profunda Artery Perforator Flap Experience for Breast Reconstruction. Plast Reconstr Surg. Nov 2016;138(5):968–975. doi: 10.1097/PRS.0000000000002619 [DOI] [PubMed] [Google Scholar]
- 20.Haddock NT, Cho MJ, Gassman A, Teotia SS. Stacked Profunda Artery Perforator Flap for Breast Reconstruction in Failed or Unavailable Deep Inferior Epigastric Perforator Flap. Plast Reconstr Surg. Mar 2019;143(3):488e–494e. doi: 10.1097/PRS.0000000000005375 [DOI] [PubMed] [Google Scholar]
- 21.Weichman KE, Broer PN, Tanna N, et al. The role of autologous fat grafting in secondary microsurgical breast reconstruction. Ann Plast Surg. Jul 2013;71(1):24–30. doi: 10.1097/SAP.0b013e3182920ad0 [DOI] [PubMed] [Google Scholar]
- 22.Jo T, Jeon DN, Han HH. The PAP Flap Breast Reconstruction: A Practical Option for Slim Patients. J Reconstr Microsurg. Jan 2022;38(1):27–33. doi: 10.1055/s-0041-1727200 [DOI] [PubMed] [Google Scholar]
- 23.Saad A, Sadeghi A, Allen RJ. The anatomic basis of the profunda femoris artery perforator flap: a new option for autologous breast reconstruction--a cadaveric and computer tomography angiogram study. J Reconstr Microsurg. Jul 2012;28(6):381–6. doi: 10.1055/s-0032-1313773 [DOI] [PubMed] [Google Scholar]
- 24.Ahmadzadeh R, Bergeron L, Tang M, Geddes CR, Morris SF. The posterior thigh perforator flap or profunda femoris artery perforator flap. Plast Reconstr Surg. Jan 2007;119(1):194–200. doi: 10.1097/01.prs.0000244848.10434.5f [DOI] [PubMed] [Google Scholar]
- 25.DeLong MR, Hughes DB, Bond JE, Thomas SM, Boll DT, Zenn MR. A detailed evaluation of the anatomical variations of the profunda artery perforator flap using computed tomographic angiograms. Plast Reconstr Surg. Aug 2014;134(2):186e–192e. doi: 10.1097/PRS.0000000000000320 [DOI] [PubMed] [Google Scholar]
- 26.Wong C, Nagarkar P, Teotia S, Haddock NT. The Profunda Artery Perforator Flap: Investigating the Perforasome Using Three-Dimensional Computed Tomographic Angiography. Plast Reconstr Surg. Nov 2015;136(5):915–919. doi: 10.1097/PRS.0000000000001713 [DOI] [PubMed] [Google Scholar]
- 27.Haddock NT, Greaney P, Otterburn D, Levine S, Allen RJ. Predicting perforator location on preoperative imaging for the profunda artery perforator flap. Microsurgery. Oct 2012;32(7):507–11. doi: 10.1002/micr.21980 [DOI] [PubMed] [Google Scholar]
- 28.Agrawal MD, Thimmappa ND, Vasile JV, et al. Autologous breast reconstruction: preoperative magnetic resonance angiography for perforator flap vessel mapping. J Reconstr Microsurg. Jan 2015;31(1):1–11. doi: 10.1055/s-0034-1372475 [DOI] [PubMed] [Google Scholar]
- 29.Largo RD, Chu CK, Chang EI, et al. Perforator Mapping of the Profunda Artery Perforator Flap: Anatomy and Clinical Experience. Plastic and Reconstructive Surgery. // 2020;146(5):1135–1145. doi: 10.1097/PRS.0000000000007262 [DOI] [PubMed] [Google Scholar]
- 30.DeLong MR, Hughes DB, Bond JE, Thomas SM, Boll DT, Zenn MR. A Detailed Evaluation of the Anatomical Variations of the Profunda Artery Perforator Flap Using Computed Tomographic Angiograms. Plastic and Reconstructive Surgery. 2014;134(2):186e–192e. doi: 10.1097/prs.0000000000000320 [DOI] [PubMed] [Google Scholar]
- 31.Levine SM, Snider C, Gerald G, et al. Buried Flap Reconstruction after Nipple-Sparing Mastectomy: Advancing toward Single-Stage Breast Reconstruction. Plastic and Reconstructive Surgery. 2013;132(4):489e–497e. doi: 10.1097/PRS.0b013e3182a00e79 [DOI] [PubMed] [Google Scholar]
- 32.Tanna N, Broer PN, Weichman KE, et al. Microsurgical breast reconstruction for nipple-sparing mastectomy. Plast Reconstr Surg. Feb 2013;131(2):139e–147e. doi: 10.1097/PRS.0b013e3182789b51 [DOI] [PubMed] [Google Scholar]
- 33.Teotia SS, Cho MJ, Haddock NT. Salvaging Breast Reconstruction: Profunda Artery Perforator Flaps Using Thoracodorsal Vessels. Plast Reconstr Surg Glob Open. Sep 2018;6(9):e1837. doi: 10.1097/GOX.0000000000001837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stalder MW, Lam J, Allen RJ, Sadeghi A. Using the Retrograde Internal Mammary System for Stacked Perforator Flap Breast Reconstruction: 71 Breast Reconstructions in 53 Consecutive Patients. Plast Reconstr Surg. Feb 2016;137(2):265e–277e. doi: 10.1097/01.prs.0000475743.08559.b6 [DOI] [PubMed] [Google Scholar]
- 35.Allen RJ, Mehrara BJ. 36 - Breast Reconstruction. In: Farhadieh RD, Bulstrode NW, Mehrara BJ, Cugno S, eds. Plastic Surgery - Principles and Practice. Elsevier; 2022:535–564. [Google Scholar]
- 36.Frey JD, Stranix JT, Chiodo MV, et al. Evolution in Monitoring of Free Flap Autologous Breast Reconstruction after Nipple-Sparing Mastectomy: Is There a Best Way? Plast Reconstr Surg. May 2018;141(5):1086–1093. doi: 10.1097/PRS.0000000000004271 [DOI] [PubMed] [Google Scholar]
- 37.Dayan JH, Allen RJ Jr. Neurotized Diagonal Profunda Artery Perforator Flaps for Breast Reconstruction. Plast Reconstr Surg Glob Open. Oct 2019;7(10):e2463. doi: 10.1097/GOX.0000000000002463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivera-Serrano CM, Aljaaly HA, Wu J, Cheng M-H. Vertical PAP Flap: Simultaneous Longitudinal Profunda Artery Perforator Flaps for Bilateral Breast Reconstructions. Plastic and Reconstructive Surgery – Global Open. 2017;5(2):e1189. doi: 10.1097/gox.0000000000001189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scaglioni MF, Chen YC, Lindenblatt N, Giovanoli P. The vertical posteromedial thigh (vPMT) flap for autologous breast reconstruction: A novel flap design. Microsurgery. Jul 2017;37(5):371–376. doi: 10.1002/micr.30074 [DOI] [PubMed] [Google Scholar]
- 40.Tielemans HJP, van Kuppenveld PIP, Winters H, Hupkens P, Ulrich DJO, Hummelink S. Breast reconstruction with the extended profunda artery perforator flap. J Plast Reconstr Aesthet Surg. Feb 2021;74(2):300–306. doi: 10.1016/j.bjps.2020.08.109 [DOI] [PubMed] [Google Scholar]
- 41.Bourn L, Torabi R, Stalder MW, et al. Mosaic Fleur-de-Profunda Artery Perforator Flap for Autologous Breast Reconstruction. Plast Reconstr Surg Glob Open. Mar 2019;7(3):e2166. doi: 10.1097/GOX.0000000000002166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saussy K, Stalder MW, Delatte SJ, Allen RJ, St Hilaire H. The Fleur-de-PAP Flap for Bilateral Breast Reconstruction. J Reconstr Microsurg Open. //08.02.2017 2017;02(01):e1–e3. [Google Scholar]
- 43.Hunsinger V, Lhuaire M, Haddad K, et al. Medium-and large-sized autologous breast reconstruction using a fleur-de-lys profunda femoris artery perforator flap design: a report comparing results with the horizontal profunda femoris artery perforator flap. Journal of Reconstructive Microsurgery. 2019;35(01):008–014. [DOI] [PubMed] [Google Scholar]
- 44.Mayo JL, Canizares O, Torabi R, Allen RJ Sr., Hilaire HS. Expanding the Applications of the Profunda Artery Perforator Flap. Plast Reconstr Surg. Feb 2016;137(2):663–669. doi: 10.1097/01.prs.0000475776.22020.b6 [DOI] [PubMed] [Google Scholar]
- 45.Blechman KM, Broer PN, Tanna N, Ireton JE, Ahn CY, Allen RJ. Stacked profunda artery perforator flaps for unilateral breast reconstruction: a case report. J Reconstr Microsurg. Nov 2013;29(9):631–4. doi: 10.1055/s-0033-1348065 [DOI] [PubMed] [Google Scholar]
- 46.Mayo JL, Allen RJ, Sadeghi A. Four-flap Breast Reconstruction: Bilateral Stacked DIEP and PAP Flaps. Plast Reconstr Surg Glob Open. May 2015;3(5):e383. doi: 10.1097/GOX.0000000000000353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haddock N, Nagarkar P, Teotia SS. Versatility of the Profunda Artery Perforator Flap: Creative Uses in Breast Reconstruction. Plast Reconstr Surg. Mar 2017;139(3):606e–612e. doi: 10.1097/PRS.0000000000003053 [DOI] [PubMed] [Google Scholar]
- 48.Haddock NT, Cho MJ, Teotia SS. Comparative Analysis of Single versus Stacked Free Flap Breast Reconstruction: A Single-Center Experience. Plast Reconstr Surg. Sep 2019;144(3):369e–377e. doi: 10.1097/PRS.0000000000005906 [DOI] [PubMed] [Google Scholar]
- 49.Haddock NT, Suszynski TM, Teotia SS. Consecutive Bilateral Breast Reconstruction Using Stacked Abdominally Based and Posterior Thigh Free Flaps. Plast Reconstr Surg. Feb 1 2021;147(2):294–303. doi: 10.1097/PRS.0000000000007548 [DOI] [PubMed] [Google Scholar]
- 50.Teotia SS, Dumestre DO, Jayaraman AP, Sanniec KJ, Haddock NT. Revisiting Anastomosis to the Retrograde Internal Mammary System in Stacked Free Flap Breast Reconstruction: An Algorithmic Approach to Recipient-Site Selection. Plast Reconstr Surg. Apr 2020;145(4):880–887. doi: 10.1097/PRS.0000000000006712 [DOI] [PubMed] [Google Scholar]
- 51.Weissler JM, Koltz PF, Carney MJ, Serletti JM, Wu LC. Sifting through the evidence: a comprehensive review and analysis of neurotization in breast reconstruction. Plastic and Reconstructive Surgery. 2018;141(3):550–565. [DOI] [PubMed] [Google Scholar]
- 52.Liew S, Hunt J, Pennington D. Sensory recovery following free TRAM flap breast reconstruction. Br J Plast Surg. Jun 1996;49(4):210–3. doi: 10.1016/s0007-1226(96)90052-1 [DOI] [PubMed] [Google Scholar]
- 53.Song B, Kumbla PA, Boyd C, de la Torre JI, Fix J. The Feasibility of a Sensate Profunda Artery Perforator Flap in Autologous Breast Reconstruction: An Anatomic Study for Clinical Application. Annals of Plastic Surgery. 2020;84(6S):S451–S454. doi: 10.1097/sap.0000000000002275 [DOI] [PubMed] [Google Scholar]
- 54.Cho MJ, Teotia SS, Haddock NT. Classification and Management of Donor-Site Wound Complications in the Profunda Artery Perforator Flap for Breast Reconstruction. J Reconstr Microsurg. Feb 2020;36(2):110–115. doi: 10.1055/s-0039-1697903 [DOI] [PubMed] [Google Scholar]
- 55.Qian B, Xiong L, Li J, et al. A Systematic Review and Meta-Analysis on Microsurgical Safety and Efficacy of Profunda Artery Perforator Flap in Breast Reconstruction. Journal of Oncology. 2019/07/29 2019;2019:9506720. doi: 10.1155/2019/9506720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Temple-Oberle C, Shea-Budgell MA, Tan M, et al. Consensus Review of Optimal Perioperative Care in Breast Reconstruction: Enhanced Recovery after Surgery (ERAS) Society Recommendations. Plast Reconstr Surg. May 2017;139(5):1056e–1071e. doi: 10.1097/PRS.0000000000003242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho MJ, Garza R, Teotia SS, Haddock NT. Utility of ERAS Pathway in Nonabdominal-Based Microsurgical Breast Reconstruction: Efficacy in PAP Flap Reconstruction? J Reconstr Microsurg. Aug 28 2021;doi: 10.1055/s-0041-1733993 [DOI] [PubMed] [Google Scholar]
- 58.Dickey RM, Garza R, Liu Y, Cho MJ, Teotia SS, Haddock NT. 4: Four-flap Breast Reconstruction: Assessing Breast-q And Donor Site Morbidity In Bilateral Stacked Autologous Breast Reconstruction. Plastic and Reconstructive Surgery – Global Open. 2021;9(7S):41. doi: 10.1097/01.Gox.0000770152.46344.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.