Abstract
Objectives. To describe RDS in neonatal deaths at the CHAMPS-Kenya site between 2017 and 2021. Methods. We included 165 neonatal deaths whose their Causes of death (COD) were determined by a panel of experts using data from post-mortem conducted through minimally invasive tissue specimen testing, clinical records, and verbal autopsy. Results. Twenty-six percent (43/165) of neonatal deaths were attributable to RDS. Most cases occurred in low birthweight and preterm neonates. From these cases, less than half of the hospitalizations were diagnosed with RDS before death, and essential diagnostic tests were not performed in most cases. Most cases received suboptimal levels of supplemental oxygen, and critical interventions like surfactant replacement therapy and mechanical ventilation were not adequately utilized when available. Conclusion. The study highlights the urgent need for improved diagnosis and management of RDS, emphasizing the importance of increasing clinical suspicion and enhancing training in its clinical management to reduce mortality rates.
Keywords: respiratory distress syndrome, neonatal mortality, histopathological presentation, low and middle-income countries
Introduction
Respiratory distress syndrome (RDS), or hyaline membrane disease is an acute lung disease caused by inadequate amounts of surfactant due to both synthesis and secretion impairement. 1 Decreased surfactant results in increased surface tension in the alveolus during expiration, leading to atelectasis, decreased gas exchange, severe hypoxia, and acidosis. 2 The contribution of RDS to neonatal mortality in low and middle-income countries is nearly 3 times that of high-income countries.3-5 In Kenya, the contribution of RDS to neonatal mortality is unknown.
RDS is the most common respiratory disease in preterm infants, and its severity and incidence are inversely related to birth weight and gestational age.5,6 In addition to prematurity, other risk factors for developing RDS include a poor Apgar score, antepartum bleeding, multiple pregnancy, cesarean section, and maternal conditions such as gestational diabetes mellitus and hypertension.7,8 Clinical diagnosis for RDS is made in preterm infants with respiratory difficulty: tachypnea, retractions, grunting respirations, nasal flaring, and a need for an increased fraction of inspired oxygen. Several studies have also shown the ability to diagnose RDS based on pathological changes in the lungs.9,10
RDS typically worsens over the first 48 to 72 hours; early diagnosis and treatment are important for improving outcomes. The goal of RDS management is to reduce its severity using antenatal corticosteroids for mothers at risk of preterm delivery, followed by optimal management of the disease after birth, such as the use of CPAP, mechanical ventilation, oxygen administration, and surfactant replacement therapy. 11 More than 90% of babies with RDS survive under close medical supervision world wide. 12 There is little or no recent literature describing the pathology of RDS in the tissues among children aged below 1 year. In addition, immunohistochemical testing is also limited in most developing countries. The interaction of the molecules with tissues in the microenvironment of the alveoli lining plays a key role in the outcome of RDS; therefore, understanding its pathology will facilitate improved diagnosis and treatment. 13
The burden of RDS in Kenya has not been well characterized, and its contribution to child morbidity and mortality is unknown. In this study, we characterize the burden of RDS among neonatal deaths enrolled in the Child Health and Mortality Prevention Surveillance (CHAMPS) site in Kenya and compare the antemortem versus post-mortem diagnosis of RDS.
Methods
Study Design and Overview
CHAMPS is a multi-country, long-term surveillance program that systematically collects and tests post-mortem tissue specimens and fluids from deceased children under the age of 5 years (<5s) in a well-defined health and demographic surveillance site. Its objectives are to document all causes of under-five mortality, prompt immediate public health actions, and develop interventions designed to reduce similar future child mortality. It also provides information on the cause of death, which, along with clinical, maternal, verbal autopsy, and demographic data, forms the substrate for conducting mortality reviews of children under the age of 5. CHAMPS methods have been described in detail elsewhere. 14
Overview of Data Collection and Study Sites
Between May 2017 and December 2021, study staff contacted parents within 24 hours of a child’s death, and consent was obtained to minimally invasive tissue sampling (MITS) procedure for microbiologic, molecular, and histopathological testing, medical records for the deceased child, and a verbal autopsy. 14 Data were collected (abstracted) from a review of medical records, verbal autopsy, diagnostic and pathology reports (attained from MITS procedures) for neonates from Manyatta and Karemo Health Demographic Surveillance System (HDSS) located in Kisumu and Siaya Counties, in Kenya, respectively. The diagnostic report includes microbiology/culture, molecular diagnostics (TaqMan Array), human immunodeficiency virus, tuberculosis, malaria testing, pathology local report, and case photos, while the International pathology report was obtained from the central pathology laboratory (CPL) based in Atlanta, which included further histopathologic, immunohistochemistry and PCR diagnosis testing. 15
The final cause(s) of death was assigned and classified using the WHO ICD-10 by the Determination of Cause of Death (DeCoDe) panel. The DeCoDe panel is a group of experts that composed of neonatologist, pediatrician, microbiologist, pathologist, epidemiologist, and Obstetrician. 16 This panel of experts reviews complete cases to assign the cause of death and identify various public health interventions that may have contributed to death. Each review concludes with a “Data to Action” discussion, where interventions are recommended by the panel to reduce the probability of similar deaths occurring in the future. 17 The determination of cause of death in cases of RDS was based on histological evidence of the hyaline membrane in the lung tissue and clinical presentation before death. The histological evidence of a hyaline membrane in the lung tissue is marked by presence of cellular debris and proteinaceous exudate mixed with fibrin in the walls of alveoli. However, in some cases, hyaline membranes were not prominent histologically, but the diagnosis of RDS was based on clinical presentation. In such cases, the certainty level for the diagnosis based on the diagnostic criteria in DeCoDe was low. The standard diagnostic criteria for assigning the cause of death are classified into 3 categories: Level 1: strong histological evidence of hyaline membranes in the lung tissue Level 2: medical documentation of birth below 37 weeks and clinical and diagnostic criteria for RDS Level 3; verbal autopsy report of birth more than 1 month early with more signs of respiratory distress.
In addition, Manyatta and Karemo HDSS have about 44 public health facilities, but only one main facility in the region (Jaramogi Oginga Odinga Teaching and Referral Hospital) that serves Manyatta HDSS population has neonatal intensive care unit (NICU).
Study Samples and Data Analysis
We described RDS for neonates (children less than 28 days of age) enrolled in CHAMPS by examining various characteristics: gestational age, low birth weight, poor Apgar score, antepartum bleeding, multiple pregnancy, Cesarean section, and maternal conditions such as gestational diabetes, maternal HIV, and hypertension. The basic description included the prevalence, clinical presentation, management, and histopathological patterns of RDS among neonates in CHAMPS. The histopathological features of RDS were described by fibrin and necrotic tissue lined within the alveolar duct.
We calculated frequencies and proportions for various characteristics: gestational age at delivery, low birth weight, poor Apgar score, antepartum bleeding, multiple pregnancy, Cesarean section, and maternal conditions such as gestational diabetes, maternal HIV and hypertension for deceased neonates diagnosed with RDS. We then categorized and calculated frequencies and proportions for clinical presentations, management, and histopathological patterns of RDS among the decedents and explored the immediate, morbid/intervening, and underlying conditions by calculating their proportions. Stata version 16.1 was used for the analysis.
Eligibility Criteria
The cases were newborn babies with less than 28 days who were enrolled in Kenya CHAMPS program and were residents of Karemo and Manyatta HDSS in Siaya and Kisumu, respectively. That had undergone the process of DeCoDe and hade their cause of death determined between 2017 and 2021. We excluded all the stillbirths and children above 28 days of age that were enrolled and DeCoDe during the same period.
Ethical Consideration
The study is part of the CHAMPS protocol and the informed consent documents were reviewed and approved by the Kenya Medical Research Institute (KEMRI) Scientific Ethics Review Unit (SERU), and Jaramogi Oginga Odinga Teaching Referral Hospital ethics review committee (ERC) Protocol number #3308. U.S. Center for Disease Control (CDC) institution review board relied on SERU approval. Every parent provided written, informed consent, and all information gathered from them was treated with strict confidentiality. Neither the case files nor the data were used for any other reason.
Results
Of the 165 neonatal deaths included in CHAMPS between May 2017 and December 2021, 43 (26.1%) had RDS in the causal chain. Forty-one (95.3%), among those with RDS, were early neonatal deaths (<7 days), and 2 were late neonatal deaths. Of these early neonatal deaths, 30 (73.2%) were within 24 hours, while 11 (26.8%) neonatal deaths occurred between 1 and 6 days (Figure 1).
Figure 1.
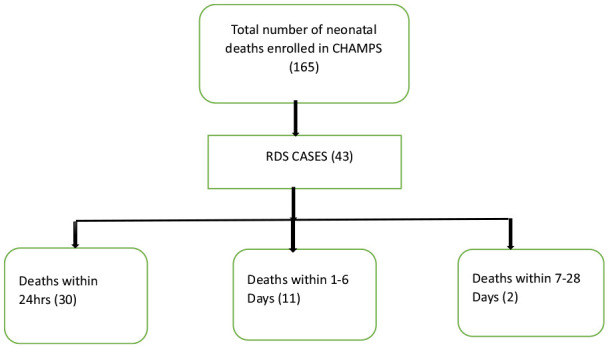
Total number of RDS cases among neonatal deaths enrolled in Kenya CHAMPS.
Thirty-one (72.1%) of the 43 cases had RDS as immediate COD and 12 (27.9%) as other morbid COD. Other immediate causes of death among the remaining 12 cases were intrauterine hypoxia (3), neonatal sepsis (6), congenital pneumonia (1), birth asphyxia (1), and aspiration pneumonia (1). Six cases of neonatal deaths due to RDS had other infections considered to have contributed to death: HIV disease (1, 16.7%), cytomegalovirus infection (1, 16.7%), Escherichia coli sepsis (2, 33.3%), and congenital pneumonia (2, 33.3%). Two of the RDS cases also had aspiration pneumonia, while 1 had meconium aspiration syndrome as other fetal cause of death.
Ninety-three percent of cases were hospital births (Table 1). An antemortem diagnosis of RDS was made in 22 (55%) of all hospitalized cases and was based on a clinical presentation during admission. One neonate was admitted to the NICU before dying. Thirty-eight (88.4%) cases were managed on oxygen via nasal prongs, and the amount of oxygen administered was 0.5 L/min. Two-thirds of cases (65.1%) were also managed on 10% dextrose for hypoglycemia, and none of the neonates received proper care (CPAP, surfactant). Most cases had prematurity (33, 76.7%) and low birth weight (39, 90.6%) while 27 cases had an Apgar score of >5 in 5 minutes. Multiple pregnancies accounted for 32% of cases. Five mothers were HIV positive, and 11.6% had pre-eclampsia during pregnancy.
Table 1.
Clinical Management, Child, and Maternal Characteristics in RDS Among Neonatal Deaths Enrolled in Kenya CHAMPS.
| Characteristic | n = 43(%) | |
|---|---|---|
| Neonatal and maternal characteristics | ||
| Sex | ||
| Female | 23(53.5) | |
| Male | 20(46.5) | |
| Birth weight, g | <1000 | 16 (37.2) |
| 1000-1499 | 17 (39.5) | |
| 1500-2499 | 6 (14.0) | |
| Above 2500 | 4 (9) | |
| Average gestational age | 29 weeks | |
| Gestational age, weeks | <28 | 13 (30.2) |
| 28-32 | 15 (34.9) | |
| 32-36 | 5 (11.6) | |
| >36 | 4 (9.3) | |
| Undetermined | 6 (13.9) | |
| Type of pregnancy | Multiple | 14 (32.6) |
| Single | 29 (67.4) | |
| Apgar Score at 5 minutes | <5 | 8 (18.6) |
| >5 | 27 (62.8) | |
| Undetermined | 8 (18.6) | |
| Average maternal age | 25 years | |
| Maternal HIV | Yes | 5 (11.6) |
| No | 38 (88.4) | |
| Maternal hypertension | Yes | 5 (11.6) |
| No | 38 (88.4) | |
| Diagnostic investigation | ||
| Hospitalized | 40 (93.0) | |
| Yes | 3 (7.0) | |
| No | ||
| Abdominal ultrasound | 0 (0) | |
| X ray | 0 (0) | |
| ECG | 0 (0) | |
| Blood gases | 0 (0) | |
| Antemortem diagnosis | ||
| Yes | 22 (55.0) | |
| No | 18 (45.0) | |
| Treatment administration | ||
| Dextrose | 28 (65.1) | |
| Oxygen | 36 (83.7) | |
| Antibiotic | 24 (55.8) | |
| CPAP | 0 (0) | |
| Mechanical ventilation | 0 (0) | |
| Surfactant replacement therapy | 0 (0) | |
Histopathological changes in the lungs revealed a variety of changes, including hyaline membranes (30, 69.8%), preterm lung (27, 62.8%), squamous cells (19, 44.2%), focal fibrin (18, 41.9%), intra-alveolar hemorrhage (10, 23.3%), intra-alveolar macrophages (6, 14.0%), congestion (6, 14.0%), meconium ball (3, 7.0%), bronchopneumonia (3, 7.0%), interstitial pneumonitis (1, 2.3%), pneumonia (1, 2.3%), neutrophilic leucocytosis (2, 4.7%), patchy hemorrhage (1, 2.3%), bacterial rods (2, 4.7%), interstitial neutrophilic cells (2, 4.7%), atelectasis (4, 9.3%), and apoptotic debris (1, 2.3%). Some of the findings were not associated with RDS but with other morbid conditions (eg, bacterial rods) (Figure 2).
Figure 2.
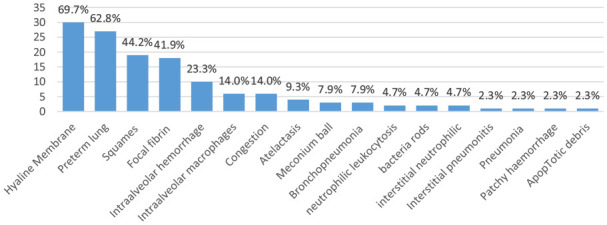
Post-mortem histopathological findings among neonatal deaths enrolled in Kenya CHAMPS.
Figure 3 shows changes in the lung tissues associated with respiratory distress syndrome. The alveolar duct is diffusely lined with fibrin and necrotic tissue, typical of a hyaline membrane; the hyaline membrane and increased alveolar macrophages are scattered along the alveolar duct. The air spaces are lined with granular eosinophilic materials, intra-alveolar damage, squames, focal fibrin, pneumonitis, and neutrophilic leucocytosis, which shows inflammation. The vascular endothelium and alveolar endothelium are injured and necrotic.
Figure 3.
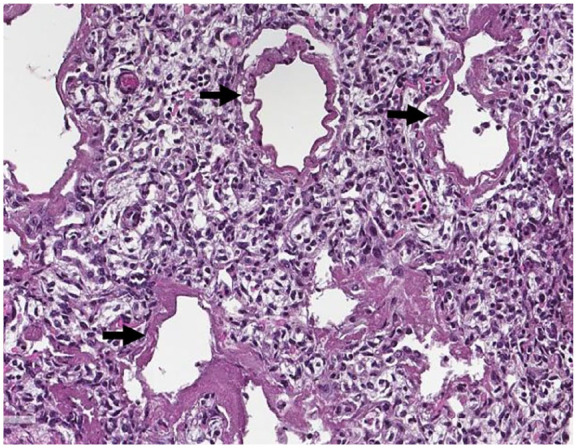
Pictorial presentation of the lung: Arrows indicate hyaline membranes.
Most public health interventions recommended by the panel of experts for cases of RDS were facility-based, such as using laboratory and radiological support in patient diagnosis and treatment (41.8%), proper dosage and methods of oxygen administration, fluid administration, and antibiotic use (41.8%), proper documentation, history taking, and examination (37.2%), adequate refresher training of staffs and CMEs (capacity building) (30.2%), proper monitoring through partographing and management of labor (26.8%), and appropriate referrals (23.3%). In addition, the dangers of inadequate antenatal clinic attendance (30.2%), early detection and response to danger signs by family members and delay in seeking care (16.3%), were identified as community factors (Table 2).
Table 2.
Data to Action Challenges and Activities Recommended by the Panel of Experts Among Neonatal Deaths Enrolled in Kenya CHAMPS.
| Public health challenges | n = 43(%) |
|---|---|
| Community | |
| Dangers of inadequate antenatal clinic attendance (ANC) | 13 (30.2) |
| Early detection and response to danger signs by family members and delay in seeking care | 7 (16.3) |
| Dangers of unskilled deliveries (Traditional birth attendants) | 4 (9.3) |
| Use of family planning | 3 (7.0) |
| Dangers of Teenage pregnancy | 3 (7.0) |
| Facility | |
| Using of laboratory and radiological support in patient diagnosis and treatment | 18 (41.8) |
| Proper dosage and methods of oxygen administration, fluid, use of antibiotic | 18 (41.8) |
| Proper documentation, history taking and examination | 16 (37.2) |
| Adequate refresher trainings of staffs/CMEs (Capacity building) | 13 (30.2) |
| Proper monitoring (Partographing) and management of labour | 11 (26.8) |
| Appropriate referral system | 10 (23.3) |
| Need for staff change of attitude towards patients and caregivers and communication of clinical procedure | 6 (14.0) |
| Early detection and response to danger signs by health personnel while in hospital | 5 (11.6) |
| Contingency measures during health care workers strikes | 4 (9.3) |
| Adherence to National treatment guidelines | 4 (9.3) |
Discussion
The prevalence of RDS was high in this study, besides significant antemortem gaps in the diagnosis and clinical management of cases of RDS. The high prevalence of RDS among neonatal deaths is consistent with findings from studies conducted in Refs.4,6,18,19 Furthermore, this study showed that the majority of RDS deaths occurred within the first 24 hours of life. These findings differ from the literature by Sudeep et al, which states that the severity of the disease worsens within 48 to 72 hours in cases of untreatment. 20 However, our cohort consists mostly of very low birth weight infants who had severe RDS, making them more likely to die early without proper care. In addition, the disease occurred together with other conditions such as intrauterine hypoxia, preterm birth, neonatal sepsis of the newborn and congenital pneumonia, consistent with findings from the region. 21
In this cohort, the antemortem diagnosis of RDS was missed in almost half of the cases, and the diagnosis was based on initial clinical presentations (nasal flaring, grunting, use of accessory muscles, and lower chest wall indrawing) and the clinical course. In these cases, prematurity, low birth weight, and birth asphyxia were the most common clinical diagnoses given. The inadequate investigation of RDS using universally recommended procedures such as chest radiographs and blood gases were not practiced, which may be due to the insufficient availability of diagnostic tools and technical capacity in the study setting. 22 In addition, treatment of RDS is still challenging, with nearly all the cases receiving suboptimal oxygen administration (0.5 L/min via nasal canula) instead of >2 L/min used in management of RDS. 23 Surfactant replacement therapy, CPAP, and mechanical ventilation, all of which have proved to improve RDS management across the world, were unavailable and unutilized.24-26 Although CPAP was available in 3 major public hospitals, it was not used. CPAP, when used early, prevents progression to severity as it increases the functional residual capacity as it recruits alveoli. 27 Inadequate management of RDS may explain the high mortality caused by RDS in our study.
Our study revealed that low birthweight, low gestational age, and multiple pregnancy were the most observed factors in the development of RDS. These findings were consistent with the results obtained by other studies.28,29 However, measures to reduce the preterm birth rate through good prenatal care combined with good and quality obstetric care during labor, such as monitoring the active phase of labor with a partograph, prevent preterm delivery. Administration of dexamethasone in the early stages of pre-term labor and delivery in higher facilities with neonatal intensive care units would decrease the prevalence of RDS and improve the management of RDS, thereby preventing and reducing further RDS.6,30,31 Continual health education to identify the early danger signs during pregnancy coupled with early seeking of health care will improve patient management during the antenatal period, thereby reducing the rate of preterm birth, which has been significantly associated with RDS.
Hyaline membranes were observed in lung histopathology in nearly 3-quarters of cases; fibrin and necrotic tissue were lined within the alveolar duct, typical of respiratory distress syndrome. Of significance were acute inflammation and injury to the vascular and alveolar endothelium, the inflammatory responses manifested by scattered granular eosinophilic materials, intra-alveolar damage, squamous cells focal fibrin, pneumonitis, and neutrophilic leucocytosis commonly associated with RDS associated with infectious diseases such as sepsis. The histological differences in tissue and cell changes due to incomplete lung development and pathological features associated with RDS may help to better understand the pathogenesis of hyaline membrane deposition in neonates. Therefore, using MITS technique to conduct autopsies will allow practitioners to better understand the clinical history or presentation of the disease and improve the management of the disease.
Our study was limited to deceased neonates and therefore may have underestimated the number of RDS in the general population. This study was largely a descriptive analysis based on a convenience sample of neonates enrolled in the CHAMPS study; hence, the power analysis for sample size calculation was not done, limiting its generalizability. In addition, MITS can miss a focal pathologic finding, hence the underdiagnosis of cases. Furthermore, gaps in the documentation of relevant clinical data and data from a single location may influence the diagnosis of RDS. The strength of this study is that it provides an insight into gaps in the diagnosis and management of RDS, coupled with increasing knowledge of lung tissue histology, and highlighted opportunities for clinical care improvement.
Conclusion
The prevalence of RDS is high among the Neonatal deaths enrolled in Kenya CHAMPS program, with significant antemortem gaps in diagnosis and clinical management of cases identified. Given the complexity of diagnosing and managing children with RDS, a high index of clinical suspicion coupled with training on optimal management can be considered to reduce RDS-associated neonatal deaths.
Acknowledgments
We thank all the Kenya Medical Research Institute (KEMRI) CHAMPS staff involved in the data and specimen collection, processing, and analysis. We also thank all the study participants, without whom these data would not have been available. This article is published with the permission of the Director General of KEMRI.
Footnotes
Authors’ Note: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Author Contributions: All participating authors qualify for authorship and have critically reviewed the manuscript for scholarly content and approved the final manuscript for submission. They have accepted responsibility for all aspects of the work.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Bill & Melinda Gates Foundation
ORCID iD: Harun O. Owuor  https://orcid.org/0009-0007-4811-8138
https://orcid.org/0009-0007-4811-8138
References
- 1. Reynolds EO. Effect of alterations in mechanical ventilator settings on pulmonary gas exchange in hyaline membrane disease. Arch Dis Child. 1971;46(246):152-159. doi: 10.1136/adc.46.246.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ainsworth SB. Pathophysiology of neonatal respiratory distress syndrome: implications for early treatment strategies. Treat Respir Med. 2005;4(6):423-437. doi: 10.2165/00151829-200504060-00006 [DOI] [PubMed] [Google Scholar]
- 3. Dyer J. Neonatal respiratory distress syndrome: tackling a worldwide problem. P&T. 2019;44(1):12-14. doi: 10.1159/000458466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muhe LM, McClure EM, Nigussie AK, et al. Major causes of death in preterm infants in selected hospitals in Ethiopia (SIP): a prospective, cross-sectional, observational study. Lancet Glob Health. 2019;7(8):e1130-e1138. doi: 10.1016/S2214-109X(19)30220-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chanie ES, Alemu AY, Mekonen DK, et al. Impact of respiratory distress syndrome and birth asphyxia exposure on the survival of preterm neonates in East Africa continent: systematic review and meta-analysis. Heliyon. 2021;7(6). doi: 10.1016/j.heliyon.2021.e07256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qari SA, Alsufyani AA, Muathin SH. Prevalence of respiratory distress syndrome in neonates. Egypt J Hosp Med. 2018;70(2):257-264. doi: 10.12816/0043086 [DOI] [Google Scholar]
- 7. Ersch J, Roth-Kleiner M, Baeckert P, Bucher HU. Increasing incidence of respiratory distress in neonates. Acta Paediatr. 2007;96(11):1577-1581. doi: 10.1111/j.1651-2227.2007.00440.x [DOI] [PubMed] [Google Scholar]
- 8. Sastroasmoro S. Risk factors for the development of hyaline membrane disease in preterm infants. Paediatr Indones. 2017;38:243. [Google Scholar]
- 9. Locci G, Fanos V, Gerosa C, Faa G. Hyaline membrane disease (HMD): the role of the perinatal pathologist. J Pediatr Neonatal Individ Med. 2014;3(2). doi: 10.7363/030255 [DOI] [Google Scholar]
- 10. Reynolds EO. Hyaline membrane disease. Am J Obstet Gynecol. 1970;106:780-797. [DOI] [PubMed] [Google Scholar]
- 11. Ekhaguere OA, Okonkwo IR, Batra M, Hedstrom AB. Respiratory distress syndrome management in resource limited settings - current evidence and opportunities in 2022. Front Pediatr. 2022;10:961509. doi: 10.3389/fped.2022.961509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mihaylova A, Gueorguiev S, Parahuleva N, et al. Frequency of hyaline membrane disease in preterm infants after prenatal corticosteroid prophylaxis. Biomed Res. 2018;29(6):1115-1119. [Google Scholar]
- 13. Okello F, Egiru E, Ikiror J, et al. Reducing preterm mortality in eastern Uganda: the impact of introducing low-cost bubble CPAP on neonates <1500 g< fibrio-root>. BMC Pediatr. 2019;19(1):311. doi: 10.1186/s12887-019-1698-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salzberg NT, Sivalogan K, Bassat Q, et al.; Child Health and Mortality Prevention Surveillance (CHAMPS) Methods Consortium. Mortality surveillance methods to identify and characterize deaths in child health and mortality prevention surveillance network sites. Clin Infect Dis. 2019;69(Supplement_4):S262-S273. doi: 10.1093/cid/ciz599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rakislova N, Fernandes F, Lovane L, et al. Standardization of minimally invasive tissue sampling specimen collection and pathology training for the child health and mortality prevention surveillance network. Clin Infect Dis. 2019;69(Suppl 4):S302-S310. doi: 10.1093/cid/ciz565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blau DM, Caneer JP, Philipsborn RP, et al. Overview and development of the child health and mortality prevention surveillance determination of cause of death (DeCoDe) process and DeCoDe diagnosis standards. Clin Infect Dis. 2019;69(Suppl 4):S333-S341. doi: 10.1093/cid/ciz572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dowell SF, Zaidi A, Heaton P. Why child health and mortality prevention surveillance? Clin Infect Dis. 2019;69(Suppl 4):S260-S261. doi: 10.1093/cid/ciz542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yismaw AE, Gelagay AA, Sisay MM. Survival and predictors among preterm neonates admitted at University of Gondar comprehensive specialized hospital neonatal intensive care unit, Northwest Ethiopia. Ital J Pediatr. 2019;45(1):4-11. doi: 10.1186/s13052-018-0597-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yismaw AE, Tarekegn AA. Proportion and factors of death among preterm neonates admitted in University of Gondar comprehensive specialized hospital neonatal intensive care unit, Northwest Ethiopia. BMC Res Notes. 2018;11(1):867-7. doi: 10.1186/s13104-018-3970-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sudeep Y, Lee B, Kamity R. Neonatal Respiratory Distress Syndrome. StatPearls Publishing; 2022. [Google Scholar]
- 21. Tewabe T, Mohammed S, Tilahun Y, et al. Clinical outcome and risk factors of neonatal sepsis among neonates in Felege Hiwot referral Hospital, Bahir Dar, Amhara Regional State, North West Ethiopia 2016: a retrospective chart review. BMC Res Notes. 2017;10(1):265-267. doi: 10.1186/s13104-017-2573-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vidyasagar D, Velaphi S, Bhat VB. Surfactant replacement therapy in developing countries. Neonatol. 2011;99(4):355-366. doi: 10.1159/000326628 [DOI] [PubMed] [Google Scholar]
- 23. Ministry of Health. Basic Paediatric Protocols. Ministry of Health; 2016:25. [Google Scholar]
- 24. Sweet D, Carnielli V, Greisen G, et al. European consensus guidelines on the management of respiratory distress syndrome - 2019 update. Neonatol. 2019;115(4):432-450. doi: 10.1159/000499361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kamath BD, MacGuire ER, McClure EM, Goldenberg RL, Jobe AH. Neonatal mortality from respiratory distress syndrome: lessons for low-resource countries Pediatrics. 2014;127:1139-1146. doi: 10.1542/peds.2010-3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu J, Liu G, Wu H, Li Z. Efficacy study of pulmonary surfactant combined with assisted ventilation for acute respiratory distress syndrome management of term neonates. Exp Ther Med. 2017;14:2608-2612. doi: 10.3892/etm.2017.4839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tochie J, Sibetcheu A, Nkeck J, Temgoua M. The epidemiology, risk factors, mortality rate, diagnosis, etiologies and treatment of neonatal respiratory distress: a scoping review. Published online 2020. [Google Scholar]
- 28. Saboute M, Kashaki M, Bordbar A, Khalessi N, Farahani Z. The incidence of respiratory distress syndrome among preterm infants admitted to neonatal intensive care unit: a retrospective study. Open J Pediatr. 2015;05(04):285-289. doi: 10.4236/ojped.2015.54043 [DOI] [Google Scholar]
- 29. Swarnkar K, Swarnkar M. Prevalence of hyaline membrane disease with special reference to significance of gastric shake test in preterm infants. Asian J Biomed Pharm Sci. 2015. [Google Scholar]
- 30. Albasri S, Shouib G, Bajouh O, et al. Maternal and neonatal outcomes in twin and triplet gestations in Western Saudi Arabia. Saudi Med J. 2017;38:657-661. doi: 10.15537/smj.2017.6.17699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ondoa-Onama C, Tumwine JK. Immediate outcome of babies with low Apgar score in Mulago Hospital, Uganda. East Afr Med J. 2003;80(1):22-29. doi: 10.4314/eamj.v80i1.8662 [DOI] [PubMed] [Google Scholar]


