Abstract
Physical therapies such as electroconvulsive therapy (ECT) may result in higher response and recovery rates, especially in patients who have treatment-resistant depression. Various studies have reported different changes in heart rate variability (HRV) parameters before and after depression treatment with ECT. Therefore, the present study reviews systematically the evidence describing changes in HRV parameters and the cardiac autonomic nervous system associated with ECT. Scopus, Web of Science, PubMed, and Embase electronic databases were searched for papers published up to September 8, 2022, without any restriction on the year and language of the study. A total of 895 articles were reviewed by two independent groups and nine articles that met the inclusion criteria were selected. Time-domain and frequency-domain HRV parameters were assessed. In conclusion, the results of our systematic review provided limited evidence for the influence of ECT on HRV parameters. Despite studies suggesting depression results in a decrease in parasympathetic activity and ECT results in an increase in cardiac vagal activity, ECT seems to have no consistent effect on HRV parameters.
Keywords: Autonomic nervous system, depression, electroconvulsive therapy, heart rate variability
INTRODUCTION
Throughout the world, 280 million people suffer from depression 5.0% of whom are adults and 5.7% older than 60.[1] Furthermore, about a third of depression patients have treatment-resistant depression (TRD), occurring when adequate medications do not eradicate the condition or the patient relapses. Depressive disorders can be effectively treated with medications and psychotherapy,[2,3] However, when conventional pharmacological approaches fail with TRD patients, ECT could be an important treatment option.[4,5] It is recommended to conduct ECT with general anesthesia, muscle relaxants, appropriate respiratory management, oxygen supplementation, and monitoring of blood circulation dynamically.[6,7] Evidence indicates that depression may be a major risk factor for adverse cardiovascular events and ANS dysfunction is thought to be responsible. Research has shown that the therapeutic outcomes of ECT are related to the recovery of parasympathetic nerve activity.[8,9] According to the studies, an abrupt pattern of change in cardiac autonomic nervous activity during the ECT sessions occurs. Repetition of ECT sessions appears to initiate parasympathetic nervous activity earlier, which may contribute to the therapeutic effects of ECT. According to the studies, an abrupt pattern of change occurs in cardiac autonomic nervous activity during ECT sessions. Repetition of such sessions appears to expedite the initiation of parasympathetic nervous activity, which may in turn contribute to the therapeutic effects of ECT.
A non-invasive method for assessing the performance of the autonomic nervous system, impaired by depression, is the assessment of HRV.[10] HRV, the variability of normal sinus beat intervals could also predict- adverse outcomes, such as arrhythmias and sudden cardiac death.[11] Generally, parasympathetic nervous system activity increases HRV and decreases HR, while sympathetic nervous system activity has the opposite effect on HRV, equivalent to the variability of normal sinus beat intervals, and could also predict adverse outcomes such as arrhythmias, sudden cardiac death, etc., Generally, parasympathetic nervous system activity increases HRV and decreases HR, while sympathetic nervous system activity has the opposite effect.
HRV can be interpreted through frequency-domain indices, time-domain indices, and nonlinear measurements.[12] Low-frequency (LF), high-frequency (HF), and LF/HF ratios are measured in the frequency domain. An average of two minutes is required to record the LF band (0.04–0.15 Hz).[13] LF power is produced by both PNS and SNS, and regulation of blood pressure through baroreceptors[14–17] occurs primarily through the PNS,[18] or exclusively through baroreflex activity.[19] It is conventional to record the HF or respiratory band (0.15–0.40 Hz) over a minimum period of one minute.[20] The HF band is called the respiratory band because it corresponds to the HR variations associated with the respiratory cycle and reflects parasympathetic activity. These phasic HR changes may not serve as a reliable indicator of cardiac vagal control.[21] To estimate SNS and PNS activity, the LF/HF ratio is used.[13] The LF/HF ratio is calculated on the assumption that SNS produces LF power while PNS produces HF power. Based on this model, parasympathetic dominance is indicated by a low LF/HF ratio, while sympathetic dominance is indicated by a high LF/HF ratio.
Nevertheless, SNS and PNS have a complex relationship (both linear and non-linear). When PNS activity increases, SNS activity may decrease, increase, or remain unchanged. Therefore, the ratio of LF to HF power does not necessarily reflect autonomic balance.
A time-domain index of HRV, such as SDNN or RMSSD, evaluates the amount of HRV observed during monitoring periods ranging from a few seconds up to 24 h. The SDNN is considered the “golden standard” in the medical stratification of cardiac risks when they are recorded over 24 hours.[14] Both SNS and PNS activities contributed to SDNN by calculating each successive difference in time between heartbeats in ms. The root mean square of successive differences (RMSSD) between normal heartbeats is calculated by squaring the value of each successive difference and the result is averaged before the square root is calculated. HF power is correlated with RMSSD[22] and PNS has a greater influence on RMSSD than SDNN. A 2 min epoch is also required to calculate the percentage of adjacent NN intervals that differ from each other by more than 50 ms (pNN50). A period of 60 s has been proposed by researchers.[23] There is a strong correlation between pNN50 and PNS activity,[24] as well as between pNN50 and RMSSD and HF power.
Various studies have reported different changes in HRV parameters before and after depression treatment with ECT. For example, some studies reported a decrease in HRV values,[25,26,27] while others reported an increase.[28,29,30] In addition, some studies reported a decrease in LF/HF ratio,[28,31,32] while others reported an increase.[27,29] Therefore, the present study reviews the evidence describing changes in HRV parameters and the cardiac autonomic nervous system associated with ECT.
MATERIALS AND METHODS
This review followed the referred Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[33] Four electronic databases [PubMed (n = 61), Scopus (n = 554), Web of Science (n = 147), and Embase (n = 131)] were searched for relevant papers published up to September 8, 2022, without any restriction on the year and language of the study. The search terms used were “(“Heart rate variability” OR “HVR” OR “Heart rate change” OR “Cycle length variability” OR “Cycle length variability”) AND (“Electroconvulsive therapy” OR “Electro convulsive therapy” OR “electroconvulsive” OR “electroshock”)”.
This systematic review included all studies that reported HRV parameters before and after ECT treatment. Also, studies that were non-original studies, animal studies, case report studies, or no full-text were excluded from the study.
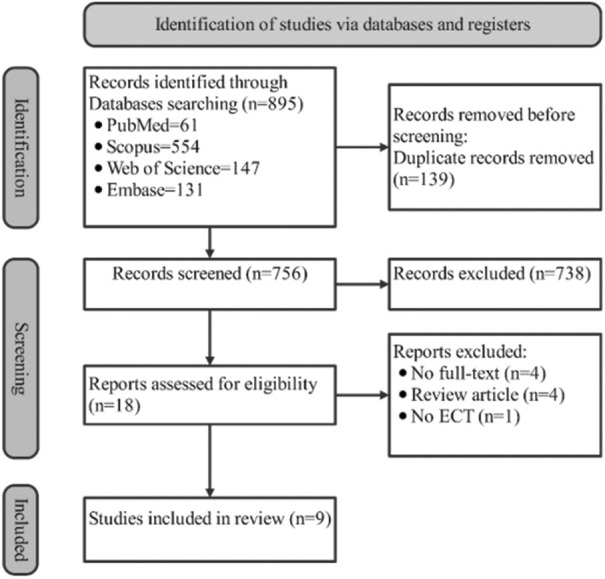
The flowchart in Figure 1 illustrates the search process and its results based on PRISMA. Four authors in two pairs executed article screening and examined the results based on the inclusion and exclusion criteria. After searching the databases and removing 139 duplicates, 756 documents were identified. In the first phase, after reviewing the title and abstract of these 756 documents, 738 were excluded. In the second step, based on reading the full text, 9 articles were excluded due to no full-text (n = 4), review (n = 4), and no ECT (n = 1), thus 9 articles that met the inclusion criteria were accepted.
Figure 1.
Process flowchart for study selection. ECT: electro-convulsive therapy
RESULTS
Prisma flowcharts (1) provide a visual representation of the search process and its results [Figure 1]. The inclusion criteria were met by nine articles in total.[9,25,26,27,28,29,30,31,32] The selected studies are summarized in Table 1 with information concerning the study, year, number of participants (gender), age range, anesthetic medication, number of ECT sessions, and time to measure HRV parameters.
Table 1.
A summary of the studies included (n=9)
| Study, year | Study design | Number of participants (gender) | Range age (mean age±SD) years | Anesthetic medications | Number of ECT sessions | Time to measure HRV parameters |
|---|---|---|---|---|---|---|
| Schultz et al.,[25] 1997 | USA | 9 (NR females, NR males) | (42.2±12.2) | Methohexital (0.75 mg/kg), succinylcholine (0.6 mg/kg), and glycopyrrolate 0.2 mg | Three per week | 24 to 36 h after the last ECT |
| Nahshoni et al.,[31]2001 | Israel | 11 (8 females, 3 males) | 60 to 84 (70±7) | Methohexital sodium (1.0 mg/kg) and succinylcholine (0.5 mg/kg) | Twice weekly for a maximum of 12, if needed | 72 h after the last ECT |
| Nahshoni et al.,[28]2004 | Israel | 10 (7 females, 3 males) | 60 to 84 (70±7) | Methohexital sodium (1.0 mg/kg) and succinylcholine (0.5 mg/kg) | Twice weekly for a maximum of 12, if needed | 48 h after the last ECT |
| Karpyak et al.,[32] 2004 | USA (Rochester, MN.) | 11 (8 females, 3 males) | 25 to 70 (45.5±12.4) | 0.2 mg IV glycopyrrolate, 1.5–2.0 mg/kg IV thiopental, and 1.0 mg/kg succinylcholine | Three per week | Between 1:45 hours to more than 72 h |
| Takada et al.,[26] 2005 | Brasil | 38 (20 females, 18 males) | 50 to 83 (64.7±8.6) | Etomidate (0.2–0.3 mg/kg) | NR | Seven 4 min intervals before the shock, a 4 min interval during the shock, and seven 4 min intervals after the shock |
| Ebert et al.,[9]2010 | Germany | 24 (21 females, 3 males) | 18 to 72 (61.1±16.8) | Etomidate and succinylcholine (according to in-house standards) | Six | Data were collected less than one week before the first ECT, and 24-h after the third and sixth ECT sessions. |
| Royster et al.,[29]2012 | USA | 21 (17 females, 4 M males) | 18 to 90 (48±18) | NR | Six (between ECT treatments was 48 to 72 h) | Just before ECT-1 and ECT-6 |
| Bozkurt et al.,[27] 2013 | Turkey | 14 (0 female, 14 males) | 30 to 54 (37.78±6.12) | thiopental (5 mg/kg) and succinylcholine (0.5 mg/kg) | Nine (three per week) | Holter monitoring, 24 h, was performed before treatment and at the end of the first, third, and three weeks after the last ECT. |
| Suzuki et al.,[30]2015 | Japan | 8 (6 females, 2 males) | (66.5±7.1) | Intravenous thiopental (2–4 mg/kg) | One to three per week (for one week) | From 4 min before ECT onset to 4 min after |
NR=not reported
Populations of the study
A total of 155 individuals participating in these studies comprised an average sample size of 17 (standard deviation: 9.28) individuals. In one study,[27] only males were included; however, in other studies,[9,25,26,28,29,32] both males and females were included [Table 1]. There were three studies[25,29,32] conducted in the United States of America, two studies[28,31] in Israel, and one study each in Brazil,[26] Germany,[9] Turkey,[27] and Japan.[30] It should be noted that the DSM-IV criteria[34] were used in eight studies[9,25,27,28,29,30,31,32] to diagnose major depression. However, in one study,[26] all participants selected for the study were over 50 years of age and due to their major depressive disorder and resistance to drug therapy were referred to an institution for ECT.
ECT and HRV devices
To measure HRV parameters, five studies used ECG,[9,25,28,30,31] and four studies Holter monitoring.[26,27,29,32] ECT was performed in three studies using the MECTA device,[9,25,27] in four studies using the Thymatron device,[28,30,31,32] and in one study using both the Thymatron and MECTA devices.[26] In one study, the type of ECT device was not reported.[29] It should be noted that five studies used bilateral stimulation,[25,27,29,30,32] while four studies used unilateral stimulation.[9,26,28,31] A description of the HRV and ECT devices and the location of the stimulating electrodes can be found in Table 2.
Table 2.
A description of the heart rate variability (HRV) and electroconvulsive therapy (ECT) devices and the location of the stimulating electrodes
| Study, year | HRV recording device | ECT device | The location of the stimulus electrodes |
|---|---|---|---|
| Schultz et al.,[25] 1997 | A continuous ECG using standard chest leads (Biotach and Pneumotrace, Gould Electronics, Valley View, OH) | Constant current bidirectional square wave stimulus device (MECTA Model SR-1) (MECTA Corporation) | Bilaterally in the standard bifrontotemporal position |
| Nahshoni et al.,[31] 2001 | One unfiltered ECG limb lead (LII) (Hipec analyzer HA-200/Aerotel Computerized Systems, Ramat Gan, Israel) | Constant-current, brief bidirectional square-wave device (Thymatron DGx; Somatics Inc.) | Right or left unilateral (d’Elia placement) ECT in all patients except one, whose bilateral (frontotemporal) ECT |
| Nahshoni et al.,[28] 2004 | One unfiltered ECG limb lead (LII) (Hipec analyzer HA-200/Aerotel Computerized Systems, Ramat Gan, Israel) | Constant current, brief bidirectional square-wave device (Thymatron DGx, Somatics, Lake Bluff, Illinois) | Right or left unilateral (d’Elia placement) ECT in all patients except one, who bilateral (frontotemporal) ECT |
| Karpyak et al.,[32] 2004 | Holter monitoring | Thymatron System IV ECT device (Somatics, Inc., Lake Bluff, IL) | In seven patients, bitemporal electrodes and in four patients, a combination of bitemporal and bifrontal electrodes |
| Takada et al.,[26] 2005 | ECG Holter monitoring with Marquette 800 portable amplitude-modulated two-channel devices (Marquette 9428; Marquette Medical Systems Milwaukee, WI, USA) | Mecta SR-2 (Lake Oswego, OR, USA) and Thymatron DGx (Lake Bluff, IL, USA) | Temporal areas |
| Ebert et al.,[9] 2010 | A high-resolution ECG (1000 Hz) from two separate adhesive monitoring electrodes using the Task Force Monitor-device (CNSystems, Medizin-Technik GmbH, Austria) | Spectrum 5000Q-device of unilateral stimulation (MECTA, Portland, Oregon, USA) | Unilateral stimulation |
| Royster et al.,[29] 2012 | Marquette Series 8500 Holter monitor (GE Marquette Medical Systems, Milwaukee, WI, USA) | NR | Bilateral stimulation |
| Bozkurt et al.,[27] 2013 | Twenty-four-hour Holter monitoring | Bilateral ECT device Mecta Spectrum 5000Q | Bilateral stimulation |
| Suzuki et al.,[30] 2015 | ECG from two separate adhesive monitoring electrodes, which were placed on the positive input of V5 (12-lead ECG) and under the right clavicle as the reference with LRR-03 (GMS) | Pulse wave machine (Thymatron System IV; Somatics LLC, Lake Bluff, IL) | Bilateral frontal poles |
NR=not reported
HRV parameters
The measured HRV parameters and their relationship with SNS and PNS functions are summarized in Table 3. HRV parameters in the frequency domain were reported more frequently than HRV parameters in the time domain. Six[25–30] and four[26,27,28,29] studies reported HF and LF power in ms2, respectively, while two studies reported HF and LF power in normalized units.[9,31] In addition, four studies reported the ratio of LF/HF power.[26,27,29,31] In the time domain, three[9,27,32] and two[27,29] studies reported SDNN and RMSDD, respectively, in ms. Furthermore, two studies[27,32] reported pNN50.
Table 3.
An overview of the heart rate variability (HRV) measures in frequency-domain and time-domain and their relationship to the function of the sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS)
| HRV Parameter | Units | Description | Association to SNS and PNS function | Studies reported | |
|---|---|---|---|---|---|
| Frequency-domain parameter | HF power | ms2 | High-frequency power (between 0.15 and 0.4 Hz) (synchronous with respiration); Parasympathetic/vagal activation is estimated | PNS | Schultz et al.,[25] Takada et al.,[26] Bozkurt et al.,[27] Nahshoni et al.,[28] Royster et al.,[29] Suzuki et al.[30] |
| LF power | ms2 | Low-frequency power (between 0.04 and 0.15 Hz) extracted from RR interval time series power spectrum | SNS | Takada et al.,[26] Bozkurt et al.,[27] Nahshoni et al.,[28] Royster et al.[29] | |
| VLF power | ms2 | Very low-frequency power (between 0 and 0.04 Hz) extracted from RR interval time series power spectrum | Undefined | NR | |
| LF/HF | LF/HF power ratio | SNS vs. PNS | Takada et al.,[26] Bozkurt et al.,[27] Royster et al.,[29] Nahshoni et al.[31] | ||
| HF | n.u. | HF power in normalized units (n.u.) representing the relative power in proportion to total power (TP) minus VLF power: HF [n.u.] = HF power/(TP – VLF power) | PNS vs. SNS | Ebert et al.,[9] Nahshoni et al.[31] | |
| LF | n.u. | LF power in normalized units (n.u.) representing the relative power in proportion to total power (TP) minus VLF power: LF [n.u.] = LF power/(TP – VLF power) | SNS vs. PNS | Ebert et al.,[9] Nahshoni et al.[31] | |
| Time-domain parameters | SDNN | ms | The standard deviation of all normal RR intervals (normal-to-normal intervals, NN), demonstrating overall variability | PNS ↑, SNS | Ebert et al.,[9] Bozkurt et al.,[27] Karpyak et al.[32] |
| RMSDD | ms | Root mean square of successive differences between RR intervals, demonstrating beat-to-beat variation | PNS ↑ | Bozkurt et al.,[27] Royster et al.[29] | |
| pNN50 | % | The percentage of adjacent NN intervals that differ from each other by more than 50 ms | PNS ↑ | Bozkurt et al.,[27] Karpyak et al.[32] |
NR=not reported. RR intervals, interbeat intervals between all successive heartbeats; NN intervals, interbeat intervals between successive heartbeats with artifacts removed
Associations between the ECT, HRV, and autonomic nervous system
The associations between ECT, HRV, and the autonomic nervous system are summarized in Table 4. Schultz et al.[25] observed a decrease in the amplitude of respiratory sinus arrhythmia, in other words, a decrease in parasympathetic activity, in depressed patients who were treated with ECT. While Ebert et al.[9] showed that response to ECT treatment was associated with increased parasympathetic activity. They found that after six sessions of ECT, baseline autonomic modulation did not change significantly. In another study by Nahshoni et al.,[31] vagal modulation of the heart was shown to increase significantly after ECT, also in another study, Nahshoni et al.[28] found that elderly patients with MDD who responded to ECT showed a degree of vagal modulation that increased after ECT. Also, Royster et al.[29] found that there was no significant difference between patients who responded to ECT and those who did not in terms of the HRV parameters of RMSDD and LF/HF. The changes in RSMDD were not significant, but this measure (which represents the vagus nerve's influence on autonomic control of the heart) decreased in the responders and increased in the nonresponders from pre-ECT-1 to pre-ECT-6.
Table 4.
An explanation of the changes made in the HRV parameter, the test used, the results, and conclusions. The upper arrow (↑) indicates the increase of the HRV parameter after the treatment compared to before the treatment and the down arrow (↓) shows the opposite
| Study, year | Change in HRV parameter (before and after treatment) | Test used | Main results | Conclusions | |
|---|---|---|---|---|---|
| Schultz et al.,[25] 1997 | HF ↓ | Two-tailed Wilcoxon tests and Spearman correlation coefficients | RR interval tended to decrease after ECT and ECT markedly decreased the amplitude of respiratory sinus arrhythmia. A positive correlation was observed between the reduction of HF variability and the improvement of depressive symptoms. | The parasympathetic activity was reduced in patients treated with ECT. | |
| Nahshoni et al,.[31] 2001 | LFnu ↓, HFnu ↑, and LF/HF↓ | Two-tailed paired t-tests | LF norms and the LF/HF ratio decreased significantly. The HF norm also increased significantly. | Increased cardiac vagal modulation was observed in patients treated with ECT. | |
| Nahshoni et al.,[28] 2004 | LF ↓, HF↑, and LF/HF ↓ | Two-tailed paired t-test or Mann –Whitney test were performed as appropriate. | Following the ECT course, no significant changes were observed in the LF and HF bands. | ECT may result in increased vagal modulation in elderly patients with MDD. Nonlinear HRV measures may shed light on the increased risk for cardiac mortality associated with depression since they are reduced by aging, similar to cholinergic deficits. | |
| Karpyak et al.,[32]2004 | SDNN ↑, pNN50 (Uncertain change) | The difference between the mean before and after ECT within the group was evaluated using a one-tailed paired t-test. Differences between groups of patients with different responses to treatment were evaluated using two-sample t-tests. | A significant increase in SDNN was observed in eight of the patients, indicating that the HRV will improve with the positive response to ECT. As a result of ECT complications and a comorbid somatic condition, SDNNs changed in the opposite direction in two patients. | Response to ECT treatment was associated with increased SDNN. According to the results of this study, physiologic differences may be able to predict the outcome of depression treatment with ECT. | |
| Takada et al.,[26] 2005 | LF ↓, HF ↓ | To compare HR and HRV, between time points, was used repeated measures of one-way ANOVA. | A study of HRV demonstrated that sympathetic activity increased during shock and that both parasympathetic and sympathetic activity decreased following shock. | Electroconvulsive therapy was found to cause transitory increases in HR and blood pressure in middle-aged and elderly patients without systemic disease, but not to result in serious adverse clinical outcomes. | |
| Ebert et al.,[9]2010 | SDNN ↑(NS), LFnu ↑(NS), HFnu ↓(NS) | repeated measures MANOVA univariate ANOVAs | After six sessions of ECT, baseline autonomic modulation did not change significantly. Response to ECT treatment was associated with increased parasympathetic activity. | Higher parasympathetic modulation before treatment can be a useful criterion for deciding to use ECT in a particular patient. | |
| Royster et al.,[29] 2012 | Patients who have responded to ECT (before ECT and after 6 ECT) | RMSSD ↓, LF ↓, HF ↓ and LF/HF ↑ | Two-sample t-test | Between the patients who responded to ECT and those who did not, there were no significant differences in the HRV indices of SD1/SD2, RMSDD, and LF/HF. | Short-term analyses indicate that HRV does not significantly improve among patients treated with ECT who respond to the treatment compared to those who do not. |
| Patients who have not responded to ECT (before ECT and after 6 ECT) | RMSSD ↑, LF ↓, HF ↑, and LF/HF ↑ | ||||
| Bozkurt et al.,[27] 2013 | Patients who have responded to ECT (before ECT and after 6 weeks) | RMSSD↓, LF↓, HF↓and LF/HF ↑ | Friedman and Wilcoxon signed-rank tests were used to analyze the continuous variables. evaluated by Spearman correlation coefficients. | ECT was effective for seven patients. Over the course of six weeks, there was a change in the 2-h resting HF, the RMSSD, and the pNN50 scores. After the first and third weeks, this change was not significant. There was no significant difference between the HRV values of those who responded to ECT and those who did not respond to ECT except for the value of resting HF value between week 0 and week 6 for responders and the value of 24-hour | The HRV of patients with MDD did not change consistently in response to ECT. In nine male patients with MDD who are resistant to treatment, ECT does not lead to a significant change in cardiac autonomic function when HRV is accepted as a promising surrogate marker. |
| Patients who have not responded to ECT (before ECT and after 6 weeks) | RMSSD↓, LF↓, HF↓and LF/HF ↑ | HF for non-responders between week 1 and week 6. | |||
| Suzuki et al.,[30] 2015 | HF ↑ | Paired t-test y repeated-measure ANOVA | Between 30 and 80 s after stimulation, LF/HF power increased significantly, while between 80 and 130 s after stimulation, HF power increased significantly, indicating sympathetic activity in the second phase and parasympathetic activity It is in the third stage. | After the onset of ECT stimulus, in patients with depression observed a triphasic change from parasympathetic to sympathetic to parasympathetic in cardiac autonomic activity. | |
HRV=heart rate variability, HR=heart rate, ECT=electroconvulsive therapy, LF=peak frequency of the low-frequency band, LFnu=LF power in normalized units, HF=Peak frequency of the high-frequency band, HFnu=HF power in normalized units, LF/HF=ratio of LF-to-HF power, pNN50=the percentage of adjacent NN intervals that differ from each other by more than 50 ms, RMSSD=root mean square of successive differences between RR intervals, demonstrating beat-to-beat variation, SDNN=standard deviation of NN intervals, SD1=Poincaré plot standard deviation perpendicular the line of identity, SD2=Poincaré plot standard deviation along the line of identity, SD1/SD2=Ratio of SD1-to-SD2
Takada et al.,[26] based on HRV, found that during shock, sympathetic activity increased with a decrease in both sympathetic and parasympathetic drive afterward. In addition, using HR and HRV analysis from four minutes before and four minutes after ECT stimulus onset, Suzuki et al.[30] demonstrated a triphasic change from parasympathetic to sympathetic to parasympathetic cardiac autonomic activity. The time-domain variables SDNN, SDANN, RMSSD, and pNN50 were used in another study by Bozkurt et al.[27] as well as the frequency-domain variables, HF and LF. It has been shown that ECT does not affect HRV and does not affect cardiac autonomic function immediately after treatment, as well as three weeks later. In another study, using SDNN as an indicator of ECT response, Karpyak et al.[32] found that SDNN increases are associated with ECT's positive effects. Moreover, SDNN may be able to predict the outcome of ECT when candidates are selected, as well as monitor its effectiveness and minimize its side effects. Physical activity, talking, and related changes in breathing patterns are known to affect pNN50 as well as the frequency-domain measures.[35,36] Due to the authors did not restrict patients’ activity during HRV monitoring, they found no clear pattern of ECT-related changes in pNN50 in any group of subjects.
DISCUSSION
There is considerable diversity in the combination of HRV values used in the literature relating HRV to ECT treatment. Our systematic review of the available literature manifested that various factors might influence HRV components such as the duration of the recording and the various changes of physiological and physical agents that could occur during the recording.
Short recording times (2-to-40-min ECG recordings) provide information on cardiac autonomic status, reflecting vagal activity. The short recording durations can create physiologically fixed or stationary states without much difficulty. However, in long-term recordings, it is more difficult to maintain strictly standardized conditions. In this systematic review, four studies either used short-term recording or did not the report duration of the recording. Four other studies used the long-term recording of HRV and generated inconsistent results with some HRV parameters. For instance, Bozkurt et al.[27] observed a significant increase in the HF component, whereas Royster et al.[29] found, although insignificant, a decrease in the HF component. This contradiction may be due in part to the patients who do not remain supine during the recording period of 24 h and changes in their physical activity over this period lead to changes in HRV. Nevertheless, the overall effect of activity and environmental factors on the heart and the autonomic nervous system is best evaluated over a longer 24-h recording period.
Another result evidenced by the review was that there might not be a significant difference in some of the HRV parameters between the subjects who responded to ECT and those who did not. However, insignificant changes in HRV parameters were different between the responders and the non-responders. For example, Schultz et al.[25] and Royster et al.[29] reported that the LF/HF ratio, which represented the sympathovagal balance at the level of the sinus node, decreased in the non-responders and increased in the responders. In addition, Royster et al.[29] observed that RSMDD, which described the vagus nerve-mediated cardiac autonomic control, decreased from pre-ECT-1 to pre-ECT-6 in the non-responders and increased in the responders. Indeed, we should also pay attention to the different responses of subjects in each group of responders and non-responders in these studies. Four subjects had a decrease, three had an increase, and four had a slight change among the responders. This indicates that the alterations of some HRV parameters cannot be used as possible predictors of the response.
Nevertheless, parameters like SDNN, which reflected joint sympathetic and parasympathetic modulation of heart rate, were significantly higher in value at baseline in the group of subjects that sustained response compared with the group with relapse within three weeks after ECT in the study conducted by Karpyak et al[32]. However, Bozkurt et al.[27] found that the SDNN was not different at baseline in comparison to the groups of responders or non-responders.
The changes in the HF parameter, modulated by the parasympathetic nervous system, after ECT were different in the studies. Increased HF (in normalized units), indicating increased vagal modulation, was addressed by Karpyak et al.,[32] but Ebert et al.[9] observed no change in this component after ECT treatment. These discordant results may be attributed, at least in part, to the effects of co-administered medications upon HRV. The effect of electrical charge in ECT upon the ANS during an acute administration could also be the other factor resulting in these conflicting results.
According to the reviews, HRV measures after ECT appeared to be influenced by various other factors that differed among studies. The age of the study population could be an effective factor. A rather elderly population may affect therapeutic response by influencing the means of autonomic measures. Three studies in this review included patients of an elderly population and other studies included patients of both the youth and the elderly. Another factor may be psychotropic medications taken by patients, which seem to interact with autonomic modulation and their effect on HRV has been revealed in the studies. Short recording studies demonstrated that tricyclic antidepressants were associated with a decline in most measures of HRV. For medications that could potentially alter HRV, a large sample size could have allowed adjustment in statistical models; however, the sample size of the included study in this review was small. Other influencing factors may be the treatment schedule and at what point in the treatment course the change of each HRV parameter is assessed. Treatments were given twice or three times per week in the studies. However, some studies only reported the overall number of ECT sessions and did not describe a treatment schedule. In addition, recordings related to HRV were performed in the third or sixth session and even after the stimulus onset. Therefore, the recording times were not similar in the studies for each HRV parameter. All of these considerable differences between the studies included in this review prevented a meta-analysis.
There are limitations to note in the included studies. First, few studies measured the effects of ECT treatment on each HRV parameter. Second, existing studies have small samples and relatively low numbers of participants. Third, there was no comparison between depressive patients not treated with ECT and healthy individuals.
CONCLUSION
In conclusion, the results of our systematic review provided limited evidence for the influence of ECT on HRV parameters. Although according to the studies, depression might be associated with a decrease in parasympathetic activity and ECT was supposed to result in increased cardiac vagal activity, it seemed that consistent change could not be observed in HRV parameters in response to ECT. However, such a result could be due to contradictory findings and methodological limitations (e.g., small sample sizes, different age groups, etc.). Thus, future studies are needed to examine the association between ECT and HRV parameters.
Financial support and sponsorship
This research was financially supported by the Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Global Health Data Exchange (GHDx) WA, USA: Institute of Health Metrics and Evaluation Seattla; 2021. Available from: http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/d780dffbe8a381b25e1416884959e88b%0A . [Google Scholar]
- 2.Cuijpers P, Karyotaki E, Eckshtain D, Ng MY, Corteselli KA, Noma H, et al. Psychotherapy for depression across different age groups: A systematic review and meta-analysis. JAMA Psychiatry. 2020;77:694–702. doi: 10.1001/jamapsychiatry.2020.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kappelmann N, Rein M, Fietz J, Mayberg HS, Craighead WE, Dunlop BW, et al. Psychotherapy or medication for depression.Using individual symptom meta-analyses to derive a Symptom-Oriented Therapy (SOrT) metric for a personalised psychiatry? BMC Med. 2020;18:170. doi: 10.1186/s12916-020-01623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baghai TC, Möller HJ. Electroconvulsive therapy and its different indications. Dialogues Clin Neurosci. 2008;10:105–17. doi: 10.31887/DCNS.2008.10.1/tcbaghai. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kellner CH, Obbels J, Sienaert P. When to consider electroconvulsive therapy (ECT) Acta Psychiatr Scand. 2020;141:304–15. doi: 10.1111/acps.13134. [DOI] [PubMed] [Google Scholar]
- 6.Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia part 3: Update 2015 management of special circumstances: Depression, suicidality, substance use disorders and pregnancy and lactation. World J Biol Psychiatry. 2015;16:142–70. doi: 10.3109/15622975.2015.1009163. [DOI] [PubMed] [Google Scholar]
- 7.Milev RV, Giacobbe P, Kennedy SH, Blumberger DM, Daskalakis ZJ, Downar J, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 4.Neurostimulation treatments. Can J Psychiatry. 2016;61:561–75. doi: 10.1177/0706743716660033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bär KJ, Ebert A, Boettger MK, Merz S, Kiehntopf M, Jochum T, et al. Is successful electroconvulsive therapy related to stimulation of the vagal system? J Affect Disord. 2010;125:323–9. doi: 10.1016/j.jad.2010.02.110. [DOI] [PubMed] [Google Scholar]
- 9.Ebert A, Jochum T, Ritter J, Boettger MK, Schulz S, Voss A, et al. Does parasympathetic modulation prior to ECT treatment influence therapeutic outcome? Prog Neuro-Psychopharmacol Biol Psychiatry. 2010;34:1174–80. doi: 10.1016/j.pnpbp.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Bassett D. A literature review of heart rate variability in depressive and bipolar disorders. Aust N Z J Psychiatry. 2016;50:511–9. doi: 10.1177/0004867415622689. [DOI] [PubMed] [Google Scholar]
- 11.Rajendra Acharya U, Paul Joseph K, Kannathal N, Lim CM, Suri JS. Heart rate variability: A review. Med Biol Eng Comput. 2006;44:1031–51. doi: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- 12.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: An integrative review of the heart's anatomy and heart rate variability. Front Psychol. 2014;5:1040. doi: 10.3389/fpsyg.2014.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 15.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–2. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 16.Berntson GG, Cacioppo JT, Grossman P. Whither vagal tone. Biol Psychol. 2007;74:295–300. doi: 10.1016/j.biopsycho.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Lehrer PM. Biofeedback training to increase heart rate variability. Princ Pract Stress Manag. 2007;3:227–48. [Google Scholar]
- 18.Reyes del Paso GA, Langewitz W, Mulder LJM, van Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013;50:477–87. doi: 10.1111/psyp.12027. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein DS, Bentho O, Park MY, Sharabi Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol. 2011;96:1255–61. doi: 10.1113/expphysiol.2010.056259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quintana DS, Elstad M, Kaufmann T, Brandt CL, Haatveit B, Haram M, et al. Resting-state high-frequency heart rate variability is related to respiratory frequency in individuals with severe mental illness but not healthy controls. Sci Rep. 2016;6:37212. doi: 10.1038/srep37212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol. 2007;74:263–85. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Kleiger RE, Stein PK, Bigger JT., Jr Heart rate variability: Measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10:88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baek HJ, Cho CH, Cho J, Woo JM. Reliability of ultra-short-term analysis as a surrogate of standard 5-min analysis of heart rate variability. Telemed e-Health. 2015;21:404–14. doi: 10.1089/tmj.2014.0104. [DOI] [PubMed] [Google Scholar]
- 24.Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: Relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31:593–601. doi: 10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- 25.Schultz SK, Anderson EA, van de Borne P. Heart rate variability before and after treatment with electroconvulsive therapy. J Affect Disord. 1997;44:13–20. doi: 10.1016/s0165-0327(97)01443-2. [DOI] [PubMed] [Google Scholar]
- 26.Takada JY, Solimene MC, da Luz PL, Grupi CJ, Giorgi DMA, Rigonatti SP, et al. Assessment of the cardiovascular effects of electroconvulsive therapy in individuals older than 50 years. Braz J Med Biol Res. 2005;38:1349–57. doi: 10.1590/s0100-879x2005000900009. [DOI] [PubMed] [Google Scholar]
- 27.Bozkurt A, Barcin C, Isintas M, Ak M, Erdem M, Nahit Ozmenler K. Changes in heart rate variability before and after ECT in the treatment of resistant major depressive disorder. Isr J Psychiatry Relat Sci. 2013;50:40–6. [PubMed] [Google Scholar]
- 28.Nahshoni E, Aizenberg D, Sigler M, Strasberg B, Zalsman G, Imbar S, et al. Heart rate variability increases in elderly depressed patients who respond to electroconvulsive therapy. J Psychosom Res. 2004;56:89–94. doi: 10.1016/S0022-3999(03)00037-0. [DOI] [PubMed] [Google Scholar]
- 29.Royster EB, Trimble LM, Cotsonis G, Schmotzer B, Manatunga A, Rushing NN, et al. Changes in heart rate variability of depressed patients after electroconvulsive therapy. Cardiovasc Psychiatry Neurol 2012. 2012:794043. doi: 10.1155/2012/794043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki Y, Miyajima M, Ohta K, Yoshida N, Okumura M, Nakamura M, et al. A triphasic change of cardiac autonomic nervous system during electroconvulsive therapy. J ECT. 2015;31:186–91. doi: 10.1097/YCT.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 31.Nahshoni E, Aizenberg D, Sigler M, Zalsman G, Strasberg B, Imbar S, et al. Heart rate variability in elderly patients before and after electroconvulsive therapy. Am J Geriatr Psychiatry. 2001;9:255–60. [PubMed] [Google Scholar]
- 32.Karpyak VM, Rasmussen KG, Hammill SC, Mrazek DA. Changes in heart rate variability in response to treatment with electroconvulsive therapy. J ECT. 2004;20:81–8. doi: 10.1097/00124509-200406000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders, clinician version (SCID-CV) Struct Clin Interview DSM-IV Axis I Disord. 1996 [Google Scholar]
- 35.Fortrat JO, Formet C, Frutoso J, Gharib C. Even slight movements disturb analysis of cardiovascular dynamics. Am J Physiol Circ Physiol. 1999;277:H261–7. doi: 10.1152/ajpheart.1999.277.1.H261. [DOI] [PubMed] [Google Scholar]
- 36.Bernardi L, Wdowczyk-Szulc J, Valenti C, Castoldi S, Passino C, Spadacini G, et al. Effects of controlled breathing, mental activity and mental stress with or without verbalization on heart rate variability. J Am Coll Cardiol. 2000;35:1462–9. doi: 10.1016/s0735-1097(00)00595-7. [DOI] [PubMed] [Google Scholar]