Abstract
Background:
Implantable cardioverter-defibrillators (ICDs) have been established for primary and secondary prevention of fatal arrhythmias and effectively reduce the rate of sudden cardiac death (SCD). This study aims to evaluate the indications and effectiveness of ICD for primary and secondary prevention of SCD.
Materials and Methods:
This retrospective study was conducted on 229 patients (136 for primary and 93 for secondary prevention) with ICD implantations in Imam Khomeini Hospital, Ahvaz, between 2017 and 2020. The incidence of arrhythmic events after implantation of ICDs was saved in electrograms, and the performed treatments (antitachycardia pacing (ATP)/shock) were recorded from the device memory.
Results:
The indications for ICD implantation in primary and secondary prevention were different (P < 0.0001). The most common cause of ICD implantation for primary prevention was ischemic cardiomyopathy (ICMP, 90.4%) and for secondary prevention was ICMP (58.1%) followed by dilated cardiomyopathy (31.2%). During ICD implantation, 54 patients (39.7%) with ICD implantation for primary prevention and 50 patients (53.8%) for secondary prevention had arrhythmia (P = 0.043). The rate of appropriate therapies in patients with secondary prevention was higher than the primary prevention (57.9% vs. 42.1%), while the rate of inappropriate treatments in patients with primary prevention indication was more than the secondary prevention (63% vs. 37%) (P = 0.060).
Conclusions:
ICMP was the main cause of ICD implantation for the prevention of SCD in both groups. At follow-up, the high prevalence of appropriate ICD therapy was observed in both groups, and this risk was slightly higher in the secondary prevention group.
Keywords: Arrhythmia, ICD, primary prevention, secondary prevention, sudden cardiac death
INTRODUCTION
Cardiovascular diseases are the leading cause of death worldwide. Sudden cardiac death (SCD) occurs annually in 40 out of every 100,000 people in every Asian country.[1,2] One of the treatment measures in patients with high-risk heart disease, especially heart failure, is the installation of an intra-cardiac defibrillator (ICD) to prevent SCD. It reduces mortality and increases survival.[1,2,3,4]
Today, ICD is known as the preferred choice, compared to antiarrhythmic drugs for the primary or secondary prevention of SCD.[5,6] Primary prevention is the treatment of ICD in patients with risk factors for sudden death without a history of cardiac arrest or persistent ventricular tachyarrhythmia (VTa) or syncope. Secondary prevention also includes ICD implantation after resuscitated cardiac arrest or life-threatening ventricular arrhythmia (VA).[5,7] Between 1980 and 1991, the most indications for ICD implantation (76%) were secondary prevention.[8] However, in the last two decades, this trend has expanded toward early prevention of SCD.[5,7,9]
Indications for ICD treatment for primary and secondary prevention have been reviewed in various studies; however, due to low follow-up and ambiguous definitions for treated arrhythmias, conflicting results have been reported.[10] There are also little data on the recurrence of VTa and its characteristics during the follow-up period in patients at risk of or rescued from SCD due to ventricular fibrillation (VF). According to a report, in 70% of patients with ICD treatment, there were more than five cases of VTa after 4.5 years.[4] In addition, as the indications for ICD implantation, whether for primary or secondary prevention of SCD, vary depending on the pathology of the heart,[5] this study was conducted to provide information on the indications, risks, and benefits of ICD implantation to improve ICD results and to investigate the indications and outcomes of ICD implantation for primary and secondary prevention.
MATERIALS AND METHODS
This retrospective cohort study was performed on patients who underwent ICD treatment for various clinical reasons for primary or secondary prevention of life-threatening VTa in Imam Khomeini Hospital in Ahvaz from 2017 to 2020. This study was conducted after approval by the Research Ethics Committee of Ahwaz Jundishapur University of Medical Sciences (Code: IR.AJUMS.HGOLESTAN.REC.1400.011). It is approximated that at least 212 patients who underwent ICD treatment with indications for primary and secondary prevention of the disease would be needed to find a difference between desired groups, with probability of type I error (Alpha, α=0.05) and the accuracy of 0.08. The following formula was used to find out sample size from most similar studies[2,11]: 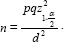 Sampling was performed consecutively from the available samples and continued until reaching the final sample size.
Sampling was performed consecutively from the available samples and continued until reaching the final sample size.
At first, written consent was obtained from the participating patients after the purpose of the study had been fully explained to them. Furthermore, in all stages of this study, the ethical statement in the Declaration of Helsinki study and the principles of patient information confidentiality were observed. Patients with ICD implantation during the period of 2017–2020, who had surgery at least 3 months before the study, were included in the study if desired. Patients were excluded from the study if the indication for ICD implantation was not clear and there was no documented evidence. Finally, 229 patients with ICD implantation, including 136 patients with primary indication and 93 patients with secondary prevention indication, entered the study. Patients were divided into primary and secondary prevention of SCD, based on the indications for ICD implantation. Primary prevention is the use of ICD in individuals at risk for episodes of stable ventricular tachycardia (VT), VF, or resuscitated cardiac arrest. Secondary prevention is the prevention of SCD in patients, rescued from resuscitated cardiac arrest or with previous stable VT.[5,7]
Basic patient profiles including demographic and clinical information including age, sex, and family history of SCD in the first-degree relatives were extracted from patients’ records. Clinical information included duration of ICD implantation, patient left ventricular ejection fraction (LVEF) within ICD implantation, ischemic heart disease, history of percutaneous coronary intervention (PCI), and concurrent use of antiarrhythmic drugs during ICD implantation. The presence of underlying diseases leading to ICD implantation including ischemic cardiomyopathy (ICMP), right ventricular arrhythmogenic cardiomyopathy, dilated cardiomyopathy (DCM), and hypertrophic cardiomyopathy was evaluated and recorded. Diagnosis of ICMP was based on a previous history of myocardial infarction and/or a history of PCI and/or coronary stenosis on angiography ≥50%. In follow-up visits after ICD implantation, patients underwent ICD check by an electrophysiologist. Arrhythmic events that occurred during the installation of the device stored in the electrogram (EGMs) from the device memory were reviewed and the treatments performed were recorded. VTa is defined as events with a sudden increase in rate along with a change in the morphology of the near-field and far-field ventricular EGM, relative to baseline rhythm. If an atrial EGM is present, ventricular atrial rate (ventricular rate > atrial rate) is used to diagnose VTa. ICD appropriate treatment was defined as ATP or shock for VTa (VT/VF).[4] Finally, data about the occurrence of arrhythmia during ICD implantation as well as arrhythmia treatment method (ATP/shock) were reviewed and recorded.
Statistical analysis
SPSS software (SPSS Inc., Chicago, IL, USA.) version 24 was used for statistical analysis. Mean, standard deviation, frequency, and percentage were used to describe the data. The normality of the data was determined by Kolmogorov–Smirnov test. Independent t-test and Chi-square (or Fisher's exact test) test were used to compare quantitative and qualitative variables, respectively. Significance level was considered 0.05 in the tests.
RESULTS
In this study, 229 patients including 148 males (64.6%) and 81 females (35.4%) with a mean age of 64.48 ± 10.12 years (range of 34–86 years) and a mean follow-up period of 11.5 ± 21.92 months participated. A total of 136 patients (59.4%) with primary indication and 93 patients (40.6%) with secondary indication for prevention were treated with ICD implantation. The basic characteristics of the patients in both the groups of ICD implantation with indications of primary and secondary prevention are presented in Table 1. The mean age of the patients with ICD implantation for primary prevention was significantly higher than the patients with ICD implantation for secondary prevention (P = 0.023). The family history of SCD was higher in patients with ICD implantation for secondary prevention (P = 0.001). Furthermore, the frequency of antiarrhythmic drug use during ICD implantation in the group of patients with ICD implantation for primary prevention was significantly higher than the group of patients with secondary prevention indication (P < 0.0001). ICD implantation indications in primary and secondary prevention were different (P < 0.0001).
Table 1.
Basic characteristics of patients with implantable cardioverter-defibrillators implantation for primary and secondary prevention
| Variable | Group | Secondary prevention (n=93), n (%) | Primary prevention (n=136), n (%) | P* |
|---|---|---|---|---|
| Age (years) | 66.80±8.76 (47–86) | 62.54±11.61 (34–86) | 0.023 | |
| Gender | Male | 64 (68.8) | 84 (61.8) | 0.325 |
| Female | 29 (31.2) | 52 (38.2) | ||
| ICD installation period (months) | 22.11±11.29 (4–48) | 21.95±11.58 (3–48) | 0.918 | |
| Family history of sudden cardiac death | 21 (22.6) | 9 (6.6) | 0.001 | |
| LVEF (%) | 26.94±10.65 (10–55) | 26.07±7.13 (10–45) | 0.492 | |
| Antiarrhythmic drug at the time of ICD implantation | 50 (53.8) | 106 (77.9) | <0.0001 | |
| ICD embedding indication | ICMP | 54 (58.1) | 123 (90.4) | <0.0001 |
| DCM | 29 (31.2) | 12 (8.8) | ||
| HCM | 8 (8.6) | 0 (0) | ||
| ARVD | 2 (2.2) | 1 (0.7) |
*P<0.05 is significant. Data are presented as mean±SD (minimum–maximum) or frequency (%). ICD: Implantable cardioverter-defibrillator, LVEF: Left ventricular ejection fraction, ICMP: Ischemic cardiomyopathy, DCM: Dilated cardiomyopathy, HCM: Hypertrophic cardiomyopathy, ARVD/ARVC: Arrhythmogenic right ventricular dysplasia/cardiomyopathy, SD: Standard deviation
In the present study, 54 patients (39.7%) with ICD implantation for primary prevention and 50 patients (53.8%) for secondary prevention had arrhythmia during ICD implantation (P = 0.043). The frequency of arrhythmias in both the groups of patients with ICD implantation for primary and secondary prevention was not significantly different (P = 0.703), and the results are presented in Table 2. In the present study, the need for treatment (shock/ATP) in the primary prevention group was 24.26% while in the secondary prevention group was 34.4%. The frequency of arrhythmia therapy during ICD implantation for primary and secondary prevention is presented in Table 3.
Table 2.
Type of arrhythmia during implantable cardioverter-defibrillator implantation for primary and secondary prevention
| Type of arrhythmia | Secondary prevention (n=50), n (%) | Primary prevention (n=54), n (%) | P* |
|---|---|---|---|
| Sinus tach | 2 (4.0) | 5 (9.3) | 0.703 |
| V-tach | 18 (36.0) | 11 (20.4) | |
| NSVT | 12 (24.0) | 14 (25.9) | |
| AF | 4 (8.0) | 7 (13.0) | |
| AFL | 3 (6.0) | 2 (3.7) | |
| SVT | 5 (10.0) | 7 (13.0) | |
| AT | 2 (4.0) | 3 (5.6) | |
| VF | 4 (8.0) | 5 (9.3) |
*P<0.05 is significant. Data are presented as frequency (%). Sinus tach: Sinus tachycardia, V-tach: Ventricular tachycardia, NSVT: Nonsustained ventricular tachycardia, AF: Atrial fibrillation, AFL: Atrial flutter, SVT: Supraventricular tachycardia, AT: Atrial tachycardia, VF: Ventricular fibrillation
Table 3.
Type of arrhythmia treatment during implantable cardioverter-defibrillator implantation for primary and secondary prevention
| Type of arrhythmia treatment | Secondary prevention (n=93), n (%) | Primary prevention (n=136), n (%) | P* |
|---|---|---|---|
| Untreated | 61 (65.6) | 103 (75.7) | 0.109 |
| ATP/shock | 3 (3.2) | 7 (5.1) | |
| Shock | 18 (19.4) | 12 (8.8) | |
| ATP | 11 (11.8) | 14 (10.3) | |
| Appropriate | 22 (57.9) | 16 (42.1) | 0.060 |
| Inappropriate | 10 (37.0) | 17 (63.0) |
*P<0.05 is significant. Data are presented as frequency (%). ATP: Antitachycardia pacing
Although most of the appropriate treatments were in patients with secondary prevention group (57.9%) and most of the inappropriate treatments were in patients with primary prevention indications (63.0%), there was no significant difference between the two groups in terms of inappropriate and appropriate arrhythmia treatments (P = 0.060). The frequency of different arrhythmia treatment methods during ICD implantation, based on the type of arrhythmia, is presented in Table 4, and as it can be seen, there was a difference between the treatment methods for different arrhythmias (P < 0.0001).
Table 4.
Types of arrhythmia treatment based on type of arrhythmia during implantable cardioverter-defibrillator implantation
| Type of arrhythmia | Untreated | ATP/shock | Shock | ATP | P* |
|---|---|---|---|---|---|
| Sinus tach | 3 (28.6) | 2 (28.6) | 1 (14.3) | 2 (28.6) | <0.0001 |
| V-tach | 0 | 8 (27.6) | 15 (51.7) | 6 (20.7) | |
| NSVT | 20 (76.9) | 0 | 2 (6.7) | 4 (16.0) | |
| AF | 5 (76.9) | 0 | 2 (18.2) | 4 (36.4) | |
| AFL | 4 (80.0) | 0 | 0 | 1 (20.0) | |
| SVT | 5 (41.7) | 0 | 1 (8.3) | 6 (50.6) | |
| AT | 4 (80.0) | 0 | 0 | 1 (20.1) | |
| VF | 0 | 0 | 9 (100) | 0 |
*P<0.05 is significant. Data are presented as frequency (%). Sinus tach: Sinus tachycardia, V-tach: Ventricular tachycardia, NSVT: Nonsustained ventricular tachycardia, AF: Atrial fibrillation, AFL: Atrial flutter, SVT: Supraventricular tachycardia, AT: Atrial tachycardia, VF: Ventricular fibrillation
In patients with primary prevention indication, there was no significant relationship between patient characteristics (age, sex, duration of ICD implantation, LVEF ICD implantation time, family history of SCD, and concurrent antiarrhythmic drug use), and arrhythmia incidence (P < 0.05). However, in patients with secondary prevention indication, the duration of ICD implantation in patients with arrhythmia was significantly longer than in patients without arrhythmia (22.44 ± 11.17 months vs. 19.11 ± 10.94 months, P = 0.031). In addition, LVEF in time of ICD implantation was significantly higher in patients with arrhythmia, compared to the patients without arrhythmia (29.10% ±11.00% vs. 24.42% ±9.77%, P = 0.034). No dramatic relationship was reported between other patients’ characteristics and the presence of arrhythmia (P > 0.05).
DISCUSSION
The results of the present study showed that the indications for ICD implantation in primary and secondary prevention were different so that the most common cause of ICD implantation for primary prevention was ICMP (90.4%) while the most common causes of ICD implantation for secondary prevention were ICMP (58.1%) and DCM (31.2%). In the study of Nagahara et al.,[12] ischemic heart disease (34%) and nonischemic heart disease (66%) were the causes of ICD for secondary prevention. However, in a study by Boulé et al.[6] on patients with ICD implantation for secondary prevention of SCD, most patients (72%) had ICMP. In a study by Schaer et al. in Switzerland[10] on the indications and predictors of ICD treatment for secondary prevention, 83% of cases were ICMP. These results suggest that ICD is used in patients at risk for SCD with different clinical indications. Indications for ICD implantation for the primary and secondary prevention of SCD are various in different regions.
According to the results of the present study, 61.5% of arrhythmias were VT/nonsustained ventricular tachycardia (NSVT)/VF and 11.5% of arrhythmias were supraventricular tachycardia (SVT). In addition, the incidence of arrhythmias in people with ICD for primary prevention was significantly lower than the secondary prevention group (39.7% vs. 53.8%). Previous studies have also shown that patients undergoing ICD treatment for secondary prevention are at high risk for short-term VTs.[13,14] However, the long-term outcome has not been accurately determined. In a study by Theuns et al. in the Netherlands,[4] during the mean follow-up time of 4.5 years after ICD implantation, 690 cases of VF were reported in 91 patients (24%) and 70% of patients had more than five cases of VTa.
In the study by Zhang et al. in China,[11] 86.3% of arrhythmic events were related to VT/VF and 13.7% were related to SVT. In the study of Manuchehry et al.[15] in the United States, monomorphic VT, apart from implantation indication, was the most common cause of ICD treatment in the primary and secondary prevention groups (60.3% and 56.4%, respectively), and there was no significant difference in indication for ICD implantation between the primary and secondary prevention groups. The results of PainFREE Rx II and INTRINSIC RV clinical trials also showed that the frequency distribution of VAs was similar between the two groups of primary and secondary prevention patients.[16,17] However, in both clinical trials, in most patients in the secondary prevention group, VT was as the index arrhythmia. These results are completely consistent with the findings of the present study.
In the present study, the need for treatment (shock/ATP) in the primary prevention group was 24.26% while in the secondary prevention group was 34.4%. The frequency of arrhythmia treatment in both the groups of patients with ICD implantation for primary and secondary prevention was not significantly different. Although inappropriate treatments in patients with primary prevention indication were more than secondary prevention (63% vs. 37%) and appropriate treatment in patients with secondary prevention indication was more than the primary prevention group (57.9% vs. 42.1%), there was no significant difference between the groups in terms of appropriate and inappropriate treatment.
Results of the study by Nagahara et al.[12] in evaluating the results of ICD treatment in patients with structural heart disease (SHD) and the indication for secondary prevention showed that the appropriate therapy in the 5-year follow-up was 54%, while inappropriate treatment was reported 17.6%. In the study by Schaer et al.,[10] it was reported that during 10 years of follow-up, the appropriate treatment rate in patients with ICD implantation for secondary prevention was high (65%), and there was no important predictor for it, especially for life-threatening arrhythmias. These results are inconsistent with the findings of the present study. The reason for the high incidence of arrhythmias in their study may be partly due to long-term follow-up and differences in subjects’ characteristics. The results of a study by Boulé et al.[6] showed that recipients of ICD treatment for secondary prevention in long-term follow-up were at high risk for appropriate device therapy (58.2%). In LOHCAT clinical trial,[13] the rate of appropriate treatments during the average follow-up of 4.5 years was 47%.
Schaer et al.[10] in a cohort study on 357 patients with secondary prevention indication reported a 59% incidence of appropriate treatments over a 6.8-year follow-up period. In a study by Rahmawati et al., during the follow-up period (69.2 months) for secondary prevention, the patients received more appropriate shocks than primary prevention (40.5% vs. 19.2%), and the incidence of inappropriate shocks was high in both primary and secondary prevention groups (25.5% vs. 26%). In a study by Konstantino et al. in the United States,[18] the patients with ICD implantation for primary prevention were significantly at lower risk of appropriate ICD treatment, while the patients with ICD implantation for secondary prevention were at greater risk for ICD treatment, and the time interval between ICD implantation till the first treatment was significantly shorter. In the study of Theuns et al.,[4] during the mean follow-up period of 4.5 years, 24% of patients with ICD implantation for secondary prevention received appropriate treatment (shock or ATP).
Although ICD prophylaxis is widely used, there are relatively limited data on the outcome of these patients. In addition, the studies, performed on different populations with different indications and different follow-up durations, have yielded controversial results in this field. It is also substantial to say that the peril of suitable remedies depends on ICD programming.[19] In this research, although ICD programming was standardized at the time of implant placement, subsequent ICD programming was performed with the patient's physician; therefore, changes in ICD programming may have affected the peril of suitable remedies. In addition, despite limited data in this area, it is likely that “modern” tachycardia diagnosis programming (higher threshold for VT detection rate, longer detection duration, etc.) may lead to less appropriate therapies.
What is certain is that ICD is an essential treatment for the secondary prevention of SCD in patients with SHD.[12] Although some studies have shown that the rate of appropriate therapy after ICD implantation in patients with secondary prevention indication was higher than ICD patients for primary prevention,[10,13,20,21,22] it should be noted that clinical trials for ICD treatment have been mostly performed in European countries and the United States, where most of the underlying cases of heart disease are ischemic heart disease, and therefore, these findings may not be applicable for countries with different prevalence of heart diseases.[12] Furthermore, one of the reasons for the different occurrence of appropriate therapy after implantation of ICD for primary and secondary prevention in previous studies is the heterogeneity of different groups of patients with arrhythmia.
In the present study, there was a significant difference between the treatment methods of different arrhythmias so that NSVT, atrial fibrillation (AF), atrial flutter (AFL), and atrial tachycardia (AT) arrhythmias were untreated in most cases (76.9%, 76.9%, 80%, and 80%). SVT arrhythmia was treated with ATP in 50% of cases and untreated in 41.7% of cases. VT arrhythmia was treated by shock method in 51.7% of cases. VF arrhythmia was also treated in all cases (100%) by shock method. In the study of Theuns et al.,[4] the results of VTa after ICD implantation in 378 patients showed that VTa with a duration <300 ms was treated with shock (82%), but most cases (83%) of VTa with a cycle length >300 ms were treated with ATP. No studies were observed on arrhythmia treatment, based on the type of arrhythmia during follow-up within ICD implantation for primary and secondary prevention, so it was impossible to compare this part of the results. In the present study, all VT and VF cases received appropriate treatment. Meanwhile, in a 3-year follow-up study, it was reported that patients with VT as an arrhythmia index were more likely to require appropriate treatment during ICD implantation (75.5% vs. 47.4%), compared to VF index arrhythmias.[23]
In a cohort study by Boulé et al.,[6] which investigated 239 patients with an average follow-up of 7.8 years, the results showed that patients with VF index arrhythmia needed more appropriate treatment than VT arrhythmias.
Finally, as mentioned, various studies have reported conflicting results in terms of indications and outcome of ICD implantation for primary and secondary prevention of SCD, because these studies have been performed on different populations with different underlying heart diseases and different rates of heart failure. The duration of follow-up has also varied in different studies.
The present study also faced limitations including being retrospective and monocenteric. Other limitations include the failure to investigate the relationship between the ICD manufacturer and receiving right or wrong shocks. Further, changes in device programming over time can affect the incidence of appropriate therapies. Therefore, by conducting more multicenter studies with a larger sample size, better results can be achieved.
CONCLUSIONS
The results of the present study showed that the indications for ICD implantation in primary and secondary prevention are different and ICD treatment recipients in both groups, especially patients with secondary prevention indication, were exposed to high incidence of appropriate therapy, which supports the use of existing ICD guidelines for the primary and secondary prevention of SCD. Finally, the results of this study suggest that the patients, treated with ICD, require close monitoring and that the care of patients receiving ICD for primary and secondary prevention may be different.
Financial support and sponsorship
This research was the result of Dr. Maryam Pooyanfar's thesis and was financially supported by Ahvaz Jundishapur University of Medical Sciences under the grant number of CVRC-0001.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are grateful to the staff of the Cardiac Surgery Department of Imam Khomeini Hospital in Ahvaz for their cooperation in conducting research and collecting data.
REFERENCES
- 1.Murakoshi N, Aonuma K. Epidemiology of arrhythmias and sudden cardiac death in Asia. Circ J. 2013;77:2419–31. doi: 10.1253/circj.cj-13-1129. [DOI] [PubMed] [Google Scholar]
- 2.Rahmawati A, Chishaki A, Ohkusa T, Sawatari H, Tsuchihashi-Makaya M, Ohtsuka Y, et al. Influence of primary and secondary prevention indications on anxiety about the implantable cardioverter-defibrillator. J Arrhythm. 2016;32:102–7. doi: 10.1016/j.joa.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haugaa KH, Tilz R, Boveda S, Dobreanu D, Sciaraffia E, Mansourati J, et al. Implantable cardioverter defibrillator use for primary prevention in ischaemic and non-ischaemic heart disease-indications in the post-DANISH trial era: Results of the European Heart Rhythm Association survey. Europace. 2017;19:660–4. doi: 10.1093/europace/eux089. [DOI] [PubMed] [Google Scholar]
- 4.Theuns DA, Bhagwandien RE, Szili-Torok T, Zijlstra F, Yap SC. Evaluation of recurrent ventricular tachyarrhythmias in patients who survived out-of-hospital cardiac arrest due to ventricular fibrillation: Eligibility for subcutaneous implantable defibrillator therapy. J Interv Card Electrophysiol. 2019;55:317–23. doi: 10.1007/s10840-018-0490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pick JM, Batra AS. Implantable cardioverter-defibrillator implantation for primary and secondary prevention: Indications and outcomes. Cardiol Young. 2017;27:S126–31. doi: 10.1017/S1047951116002365. [DOI] [PubMed] [Google Scholar]
- 6.Boulé S, Sémichon M, Guédon-Moreau L, Drumez É, Kouakam C, Marquié C, et al. Long-term outcome of implantable cardioverter-defibrillator implantation in secondary prevention of sudden cardiac death. Arch Cardiovasc Dis. 2016;109:517–26. doi: 10.1016/j.acvd.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and Stroke Statistics-2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silka MJ, Kron J, Dunnigan A, Dick M 2nd. Sudden cardiac death and the use of implantable cardioverter-defibrillators in pediatric patients.The Pediatric Electrophysiology Society. Circulation. 1993;87:800–7. doi: 10.1161/01.cir.87.3.800. [DOI] [PubMed] [Google Scholar]
- 9.Olde Nordkamp LR, Postema PG, Knops RE, van Dijk N, Limpens J, Wilde AA, et al. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: A systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm. 2016;13:443–54. doi: 10.1016/j.hrthm.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Schaer B, Kühne M, Reichlin T, Osswald S, Sticherling C. Incidence of and predictors for appropriate implantable cardioverter-defibrillator therapy in patients with a secondary preventive implantable cardioverter-defibrillator indication. Europace. 2016;18:227–31. doi: 10.1093/europace/euv188. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Ching CK, Huang D, Liu YB, Rodriguez-Guerrero DA, Hussin A, et al. Utilization of implantable cardioverter-defibrillators for the prevention of sudden cardiac death in emerging countries: Improve SCA clinical trial. Heart Rhythm. 2020;17:468–75. doi: 10.1016/j.hrthm.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Nagahara D, Fujito T, Mochizuki A, Shimoshige S, Hashimoto A, Miura T. Predictors of appropriate ICD therapy in Japanese patients with structural heart diseases: A major role of prior sustained ventricular tachycardia in secondary prevention. J Arrhythm. 2018;34:527–35. doi: 10.1002/joa3.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borleffs CJ, van Erven L, Schotman M, Boersma E, Kiès P, van der Burg AE, et al. Recurrence of ventricular arrhythmias in ischaemic secondary prevention implantable cardioverter defibrillator recipients: Long-term follow-up of the Leiden Out-of-Hospital Cardiac ArresT study (LOHCAT) Eur Heart J. 2009;30:1621–6. doi: 10.1093/eurheartj/ehp234. [DOI] [PubMed] [Google Scholar]
- 14.Freedberg NA, Hill JN, Fogel RI, Prystowsky EN CARE Group. Recurrence of symptomatic ventricular arrhythmias in patients with implantable cardioverter defibrillator after the first device therapy: Implications for antiarrhythmic therapy and driving restrictions.CARE Group. J Am Coll Cardiol. 2001;37:1910–5. doi: 10.1016/s0735-1097(01)01226-8. [DOI] [PubMed] [Google Scholar]
- 15.Manuchehry A, Agusala K, Montevecchi M, Kadish A, Passman R. Ventricular tachyarrhythmias in patients receiving an implantable cardioverter-defibrillator for primary versus secondary prophylaxis indications. Pacing Clin Electrophysiol. 2011;34:571–6. doi: 10.1111/j.1540-8159.2010.03004.x. [DOI] [PubMed] [Google Scholar]
- 16.Sweeney MO, Wathen MS, Volosin K, Abdalla I, DeGroot PJ, Otterness MF, et al. Appropriate and inappropriate ventricular therapies, quality of life, and mortality among primary and secondary prevention implantable cardioverter defibrillator patients: Results from the Pacing Fast VT REduces Shock ThErapies (PainFREE Rx II) trial. Circulation. 2005;111:2898–905. doi: 10.1161/CIRCULATIONAHA.104.526673. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan RM, Russo AM, Berg KC, Stolen KQ, Seth M, Perschbacher D, et al. Arrhythmia rate distribution and tachyarrhythmia therapy in an ICD population: Results from the INTRINSIC RV trial. Heart Rhythm. 2012;9:351–8. doi: 10.1016/j.hrthm.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Konstantino Y, Shafat T, Novack V, Novack L, Amit G. Incidence of implantable cardioverter defibrillator therapy and mortality in primary and secondary prevention of sudden cardiac death. Isr Med Assoc J. 2015;17:760–3. [PubMed] [Google Scholar]
- 19.Wilkoff BL, Fauchier L, Stiles MK, Morillo CA, Al-Khatib SM, Almendral J, et al. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart Rhythm. 2016;13:e50–86. doi: 10.1016/j.hrthm.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Sabbag A, Suleiman M, Laish-Farkash A, Samania N, Kazatsker M, Goldenberg I, et al. Contemporary rates of appropriate shock therapy in patients who receive implantable device therapy in a real-world setting: From the Israeli ICD Registry. Heart Rhythm. 2015;12:2426–33. doi: 10.1016/j.hrthm.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Betts TR, Sadarmin PP, Tomlinson DR, Rajappan K, Wong KC, de Bono JP, et al. Absolute risk reduction in total mortality with implantable cardioverter defibrillators: Analysis of primary and secondary prevention trial data to aid risk/benefit analysis. Europace. 2013;15:813–9. doi: 10.1093/europace/eus427. [DOI] [PubMed] [Google Scholar]
- 22.Satake H, Fukuda K, Sakata Y, Miyata S, Nakano M, Kondo M, et al. Current status of primary prevention of sudden cardiac death with implantable cardioverter defibrillator in patients with chronic heart failure – A report from the CHART-2 Study. Circ J. 2015;79:381–90. doi: 10.1253/circj.CJ-14-0925. [DOI] [PubMed] [Google Scholar]
- 23.Raitt MH, Klein RC, Wyse DG, Wilkoff BL, Beckman K, Epstein AE, et al. Comparison of arrhythmia recurrence in patients presenting with ventricular fibrillation versus ventricular tachycardia in the Antiarrhythmics Versus Implantable Defibrillators (AVID) trial. Am J Cardiol. 2003;91:812–6. doi: 10.1016/s0002-9149(03)00015-8. [DOI] [PubMed] [Google Scholar]


