Abstract
The catechol and protocatechuate branches of the 3-oxoadipate pathway, which are important for the bacterial degradation of aromatic compounds, converge at the common intermediate 3-oxoadipate enol-lactone. A 3-oxoadipate enol-lactone-hydrolyzing enzyme, purified from benzoate-grown cells of Rhodococcus opacus (erythropolis) 1CP, was found to have a larger molecular mass under denaturing conditions than the corresponding enzymes previously purified from γ-proteobacteria. Sequencing of the N terminus and of tryptic peptides allowed cloning of the gene coding for the 3-oxoadipate enol-lactone hydrolase by using PCR with degenerate primers. Sequencing showed that the gene belongs to a protocatechuate catabolic gene cluster. Most interestingly, the hydrolase gene, usually termed pcaD, was fused to a second gene, usually termed pcaC, which encodes the enzyme catalyzing the preceding reaction, i.e., 4-carboxymuconolactone decarboxylase. The two enzymatic activities could not be separated chromatographically. At least six genes of protocatechuate catabolism appear to be transcribed in the same direction and in the following order: pcaH and pcaG, coding for the subunits of protocatechuate 3,4-dioxygenase, as shown by N-terminal sequencing of the subunits of the purified protein; a gene termed pcaB due to the homology of its gene product to 3-carboxy-cis,cis-muconate cycloisomerases; pcaL, the fused gene coding for PcaD and PcaC activities; pcaR, presumably coding for a regulator of the IclR-family; and a gene designated pcaF because its product resembles 3-oxoadipyl coenzyme A (3-oxoadipyl-CoA) thiolases. The presumed pcaI, coding for a subunit of succinyl-CoA:3-oxoadipate CoA-transferase, was found to be transcribed divergently from pcaH.
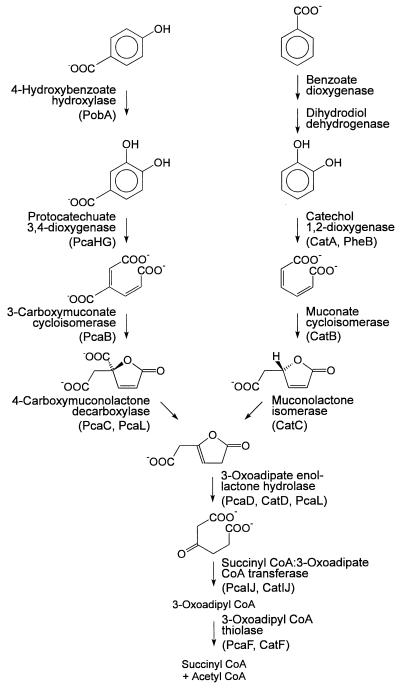
Many aromatic compounds under aerobic conditions are degraded by bacteria via the catechol or protocatechuate branch of the 3-oxoadipate pathway (26, 66). Following ortho cleavage of protocatechuate by protocatechuate 3,4-dioxygenase (EC 1.13.11.3), five additional enzymes, i.e., 3-carboxy-cis,cis-muconate cycloisomerase (EC 5.5.1.2), 4-carboxymuconolactone decarboxylase (EC 4.1.1.44), 3-oxoadipate enol-lactone hydrolase (EC 3.1.1.24), succinyl coenzyme A (succinyl-CoA):3-oxoadipate CoA-transferase (EC 2.8.3.6), and 3-oxoadipyl-CoA thiolase, convert the ring cleavage product to intermediates of the tricarboxylic acid cycle (Fig. 1). In Acinetobacter calcoaceticus, the corresponding genes are clustered into one pca operon which is governed by PcaU (14, 21, 36), while in Pseudomonas putida several operons with pca structural genes (50, 57) and in Agrobacterium tumefaciens several operons and several regulatory proteins appear to be involved (51, 52). ortho cleavage of catechol yields 3-oxoadipate enol-lactone, the first common intermediate of the catechol and the protocatechuate branch, in three reactions catalyzed by catechol 1,2-dioxygenase (EC 1.13.11.1), muconate cycloisomerase (EC 5.5.1.1), and muconolactone isomerase (EC 5.3.3.4) (Fig. 1). While in P. putida the cat gene cluster comprises only the genes for these three enzymes and for a LysR-type regulator (30), A. calcoaceticus expresses isoenzymes for the reactions common to the catechol and protocatechuate branches of the 3-oxoadipate pathway (25, 63). Ralstonia eutropha (Alcaligenes eutrophus) is intermediate between these species in that it induces different enol-lactone hydrolases but not different CoA-transferases and thiolases (35).
FIG. 1.
The protocatechuate and catechol branches of the 3-oxoadipate pathway. Designations as gene products are given in parentheses. For the reactions common to both branches, some bacteria possess only a single set of genes, while others harbor separate pca and cat genes for one or more of the steps.
Rhodococcus opacus (erythropolis) 1CP has previously been isolated by virtue of its ability to grow with 4-chlorophenol and 2,4-dichlorophenol (23). The characterization of its chlorocatechol catabolic enzymes revealed unusual properties (37, 38, 64). Interestingly, the muconate and the chloromuconate cycloisomerase of strain 1CP in their product formation from 2-chloro-cis,cis-muconate and in their inability to convert (+)-2-chloromuconolactone were more similar to each other than to muconate and chloromuconate cycloisomerases of proteobacteria (64). If this phenotypic similarity of the Rhodococcus enzymes indicates a higher degree of relatedness compared to proteobacterial enzymes, then the improved turnover of 3-chloro- and 2,4-dichloro-cis,cis-muconate by the chloromuconate cycloisomerases should have evolved independently among gram-positive bacteria and proteobacteria by functionally convergent evolution. Despite the structural similarity of 3-oxoadipate enol-lactone and dienelactones (4-carboxymethylenebut-2-en-4-olides), the enol-lactone hydrolase was apparently not adapted to convert dienelactones during evolution of the proteobacterial chlorocatechol pathway (60). Rather, some other lactone-hydrolyzing enzyme was recruited from an unknown pathway. An independent evolution of the chlorocatechol pathway among the gram-positive bacteria would imply that a different solution to the chemical problem of dienelactone hydrolysis may have been found. And especially since the dienelactone hydrolase of R. opacus 1CP has an unusual substrate specificity (38), we considered it appropriate to include the lactone hydrolases in sequence comparisons of enzymes for catechol and chlorocatechol catabolism. We have previously reported on the sequences of the catechol 1,2-dioxygenase, the muconate cycloisomerase, and the muconolactone isomerase (16). In the accompanying report (18), we characterize a gene cluster for chlorocatechol degradation of R. opacus 1CP. Here we describe the cloning and sequence analysis of a DNA fragment comprising the gene for the 3-oxoadipate enol-lactone-hydrolyzing enzyme of strain 1CP. We found the gene to be part of a protocatechuate catabolic gene cluster and to be merged to the pcaC gene, which in proteobacteria codes for a separate 4-carboxymuconolactone decarboxylase.
(Some of the results reported in this study have previously been mentioned in a preliminary communication [17].)
MATERIALS AND METHODS
Strains, plasmids, and cultivation conditions.
Strain 1CP was previously isolated with 2,4-dichlorophenol as the carbon source and, based on phenotypic properties, assigned to the species Rhodococcus erythropolis (23). Very recently, 16S rDNA and fatty acid analyses (68) called for a reclassification of the strain to the species R. opacus, which also belongs to the Rhodococcus rDNA group IV (55).
For the purpose of this study, R. opacus 1CP was cultivated in a 10-liter glass fermentor at 30°C in a mineral medium (13) with 50 mM phosphate buffer (pH 7) and with 25 mM sodium benzoate (fed in 5-mM portions) as the only carbon and energy source. To provide biomass for the purification of protocatechuate dioxygenase, sodium 4-hydroxybenzoate (5 portions of 5 mM) was used as the only carbon and energy source. Cells were harvested by centrifugation, frozen in liquid nitrogen, and stored at −20°C until used.
Escherichia coli DH5α (4), obtained from GIBCO BRL, was cultivated aerobically with constant shaking at 37°C in Luria-Bertani (LB) medium (58) supplied, if appropriate, with 100 μg of ampicillin per ml. For induction of E. coli DH5α harboring pARO523 (51), 5 ml of overnight precultures was used to inoculate 200 ml of LB medium (incubation at 37°C). After induction with 1 mM IPTG (isopropyl-β-d-thiogalactoside) at an optical density at 600 nm of 0.6, incubation was continued for 3 h at 30°C.
Properties of plasmids used in this study are summarized in Table 1.
TABLE 1.
Plasmids used in this study
| Plasmid | Relevant characteristicsa | Source (reference) |
|---|---|---|
| pBluescript II KS (+) | Derived from pUC19; Plac lacZ′ Apr; f1(+) origin; ColE1 origin | Stratagene (1) |
| pARO523 | Apr; pcaHG and pcaB from A. tumefaciens A348 on mobilizable pUC19 derivative pARO190 | D. Parke (51) |
| pRERE1 | Apr; 0.17-kb PCR product in EcoRV site of pBluescript II KS (+) | This study |
| pRERE2 | Apr; 0.44-kb PCR product in EcoRV site of pBluescript II KS (+) | This study |
| pRER3 | Apr; 3.6-kb EcoRI fragment of R. opacus 1CP DNA in pBluescript II KS (+) | This study |
| pRER4 | Apr; 2.5-kb PstI fragment of R. opacus 1CP DNA in pBluescript II KS (+) | This study |
Apr, plasmid carries information for ampicillin resistance. Fragment sizes are as determined by agarose gel electrophoresis.
Preparation of cell extracts.
Extracts of R. opacus 1CP were prepared by resuspension in 50 mM Tris-HCl (pH 7.5), disintegration by grinding with glass beads, and clarification of the preparation as described previously (16).
E. coli cells were harvested by centrifugation, washed with 0.25 volume 50 mM Tris-HCl (pH 7), and resuspended in 0.01 volume of the same buffer. The cells were disintegrated by passage through a precooled Aminco French pressure cell (115 MPa), and cell debris was removed by centrifugation as described above.
Enzyme assays and estimation of protein concentration.
The activity of 3-oxoadipate enol-lactone hydrolase was measured spectrophotometrically at 230 nm as described previously (61). The assay mixtures contained 0.67 mM racemic muconolactone (prepared by the method of Elvidge et al. [15]) plus partially purified preparations of muconolactone isomerase (47, 48). The activity of 4-carboxymuconolactone decarboxylase was also measured at 230 nm (47). The assay mixture (1 ml) initially contained 50 mM Tris-Cl (pH 7.5), 0.167 mM protocatechuate, and 6 μl of cell extract from IPTG-induced E. coli DH5α(pARO523) (corresponding to 0.21 mg of protein) with the protocatechuate 3,4-dioxygenase and the 3-carboxymuconate cycloisomerase from A. tumefaciens. The mixture was incubated for 40 min at room temperature to generate 4-carboxymuconolactone before addition of preparations of partially purified 3-oxoadipate enol-lactone hydrolase and of the fraction that was to be measured. The activities of protocatechuate 3,4-dioxygenase were measured at 290 nm as described previously (65). The following extinction coefficients were used: for the conversion of 3-oxoadipate enol-lactone to 3-oxoadipate, 1,430 M−1 · cm−1 (48); for the conversion of 4-carboxymuconolactone to 3-oxoadipate, 4,200 M−1 · cm−1 (47); and for the conversion of protocatechuate to 3-carboxy-cis,cis-muconate, 2,300 M−1 · cm−1 (65). Protein concentrations were measured by the method of Bradford (6), with bovine serum albumin as the standard.
Protein purifications.
For the initial purification of 3-oxoadipate enol-lactone hydrolase (for sequencing purposes), extract from benzoate-grown cells (volume, 160 ml; protein, 370 mg; total activity, 157 U; specific activity, 0.42 U/mg) was chromatographed on a Pharmacia Q Sepharose High Performance column (HR 16/10; bed volume, 20 ml) with 50 mM Tris-HCl (pH 7.5)–0.1 mM dithiothreitol and, for elution, an NaCl gradient (0 to 0.15 M over 60 ml and 0.15 to 0.5 M over 350 ml). The most active fractions (eluting at ca. 0.4 M NaCl) were pooled (volume, 25 ml; protein, 17.9 mg; total activity, 135 U; specific activity, 7.5 U/mg). After addition of (NH4)2SO4 to 1 M (final concentration), the preparation was filtered and subjected to Pharmacia Phenyl Superose HR 10/10 chromatography. Elution was achieved with a decreasing (NH4)2SO4 gradient of 1 to 0.9 M over 5 ml, 0.9 to 0.4 M over 120 ml, and 0.4 to 0 M over 10 ml. The fractions with the highest activities, eluting at about 0.8 to 0.75 M (NH4)2SO4 (volume, 4.5 ml; protein, 0.42 mg; total activity, 38 U; specific activity, 90 U/mg), were pooled and desalted by using Pharmacia PD-10 columns. The pool was then subjected to chromatography on Pharmacia MonoQ (HR 5/5) by using an NaCl gradient (0 to 0.2 M over 5 ml and 0.2 to 0.5 M over 70 ml) for elution, which resulted in a preparation (volume, 2 ml; protein, 0.14 mg; total activity, 21.9 U) with a specific activity of 153 U/mg, equivalent to a 364-fold purification. The details for a second enrichment of 3-oxoadipate enol-lactone hydrolase, performed to track the presumed 4-carboxymuconolactone decarboxylase activity of the enzyme, are given in Table 2.
TABLE 2.
Copurification of the 3-oxoadipate enol-lactone-hydrolyzing and 4-carboxymuconolactone-decarboxylating activities of R. opacus 1CP
| Prepna | Vol (ml) | Protein (mg) | 3-Oxoadipate enol-lactonehydrolyzing enzyme
|
4-Carboxymuconolactonedecarboxylating enzyme
|
||||
|---|---|---|---|---|---|---|---|---|
| Total activity (U) | Sp act (U/mg) | Purification (fold) | Total activity (U) | Sp act (U/mg) | Purification (fold) | |||
| Cell extract | 142 | 256 | 157 | 0.61 | 1.0 | 145 | 0.57 | 1.0 |
| Q-Sepharose HP eluateb | 20 | 8.3 | 102 | 12.4 | 20.3 | 74 | 8.8 | 15.4 |
| Phenyl Superose eluatec | 4 | 0.15 | 9.1 | 61 | 100 | 8.0 | 53 | 93 |
The purification was performed basically as the first one but with different loading, with the buffer set to pH 7 and without dithiothreitol, and with changes in the chromatographic conditions as specified below.
The NaCl gradient for the Q-Sepharose High Performance (HP) step was 0 to 0.15 M over 10 ml and 0.15 to 0.5 M over 400 ml.
A phenyl Superose HR5/5 column was used with an (NH4)2SO4 gradient of 1 to 0.9 M over 3 ml and 0.9 to 0.5 M over 70 ml.
Protocatechuate 3,4-dioxygenase was purified by subjecting an extract from 4-hydroxybenzoate-grown cells (volume, 120 ml; protein, 492 mg; total activity, 153 U, specific activity, 0.32 U/mg) to Q Sepharose High Performance (HR 16/10) chromatography with 50 mM Tris-HCl (pH 7.6) and an increasing NaCl gradient (0 to 1 M over 400 ml) for elution. Dioxygenase-containing fractions (eluting at ca. 0.35 M NaCl) were pooled (volume, 10 ml; protein, 29.5 mg; total activity, 77 U; specific activity, 2.6 U/mg), mixed with (NH4)2SO4 to 1.5 M (final concentration), and centrifuged. The preparation was then chromatographed on a Phenyl Superose HR 10/10 column with a gradient decreasing from 1.5 to 0 M (NH4)2SO4 in buffer over 120 ml [activity peak at ca. 0.56 M (NH4)2SO4]. The combined fractions (volume, 3 ml; protein, 0.6 mg; total activity, 29.5 U; specific activity, 49 U/mg) were then subjected to gel filtration (Pharmacia Superdex 200 prep-grade HiLoad 16/60; bed length, 60 ml). Elution with 50 mM Tris-HCl (pH 7.6)–0.1 M NaCl resulted in a preparation (volume, 1 ml; protein, 62 μg; total activity, 3.3 U) with a specific activity of 53 U/mg, equivalent to a 166-fold purification.
SDS-polyacrylamide gel electrophoresis and Western blotting.
For purity checks of enzyme preparations, discontinuous sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was carried out and gels were stained as described previously (16). For N-terminal sequencing of the protocatechuate 3,4-dioxygenase subunits, ca. 7 μg of the purified enzyme was loaded onto an SDS-polyacrylamide gel for separation of the α and β subunits. Electrotransfer of the subunits onto an Immobilon-P membrane (Millipore) was performed for 90 min at 80 V and 4°C in a Bio-Rad Trans-Blot cell as described by Moos (43). After staining with Coomassie brilliant blue R-250 (42), the protein bands were cut out, rinsed, air dried, and subjected to N-terminal sequencing.
Protein cleavage, isolation of peptides, and sequencing of peptides and N termini.
Trypsin digestion of 3-oxoadipate enol-lactone hydrolase and subsequent separation of tryptic peptides by reversed-phase high-performance liquid chromatography (HPLC) were performed by the procedure of Stone et al. (67) as described previously (16). The amount of enol-lactone hydrolase after trichloroacetate precipitation, washing, and drying was 40 μg.
Purified 3-oxoadipate enol-lactone hydrolase prior to sequencing was partially desalted by changing the buffer to 5 mM sodium phosphate buffer (pH 7) by ultrafiltration and repeated dilution, while selected peptide-containing fractions from reversed-phase HPLC as well as the blotted subunits of the protocatechuate 3,4-dioxygenase were sequenced directly in automated sequencers (Applied Biosystems model 473A or model 491, respectively).
Purification and general in vitro manipulations of DNA.
Genomic DNA of R. opacus 1CP was obtained as described previously (16). Plasmid DNA from E. coli DH5α was isolated with Pharmacia Flexiprep kits. Vector DNA digested with only one enzyme was dephosphorylated prior to ligation. Insert DNA for ligation or digoxigenin labeling was isolated from gels by use of a Bio 101 Gene Clean II or a Biozym Easy Pure kit. Transformation of E. coli DH5α was achieved by the method of Chung et al. (9) or Inoue et al. (33). Loss of LacZ complementation by insertion of DNA into vectors was detected by using LB plates with 0.13 mM IPTG and 32 μg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactoside) per ml.
Amplification of segments of the 3-oxoadipate enol-lactone hydrolase gene.
Oligonucleotides were custom synthesized according to the sequences of the N terminus and of two internal peptides (Table 3). The codon usage was assumed to correspond to that of catA, catB, and catC of strain 1CP (16). PCR mixtures (50 μl) for the amplification of genomic DNA contained 50 pmol of each primer, 0.1 to 0.2 μg of genomic template DNA, 0.1 mM each deoxynucleotide triphosphate, Goldstar polymerase reaction buffer [75 mM Tris-HCl (pH 9.0), 20 mM (NH4)2SO4, 0.01% (wt/vol) Tween 20], 1.5 mM MgCl2, and 0.5 U of Eurogentech Goldstar DNA polymerase. As a denaturing agent (70), formamide was used in concentrations between 5 and 7% (vol/vol). The PCR was performed with a touchdown thermocycle program (12): initial denaturation (94°C; 3 min) before addition of the polymerase; 6 cycles with decreasing annealing temperature (66 to 56°C; 1 min), polymerization (72°C; initially 1 min, plus 1 additional s for each cycle), and denaturation (94°C; 30 s); 28 more cycles with 54°C as the annealing temperature; an additional 14 min of polymerization during the last cycle. Of the two products initially obtained, the larger one (ca. 440 bp as determined by agarose gel electrophoresis) required reamplification by the same program to increase the amounts of DNA for cloning.
TABLE 3.
Sequences of N termini, tryptic peptides, and deduced primers
| Protein or peptide | Amino acid sequencea | Deduced primer sequence |
|---|---|---|
| 3-Oxoadipate enol-lactone hydrolase | ||
| N terminus | XXVALA(H)EISGPRNGAAXAPVVVLLGXL | GGYCCSCGNAACGGYGCSGC |
| ELH52.80 | GHGESPAPDGPYSVVR | GTASGGRCCGTCSGGSGCSGG |
| ELH70.65 | WFSEGFAAK | CTTSGCSGCGAARCCYTCSG |
| ELH71.92 | SMWDPQIAALSDE | |
| Protocatechuate 3,4-dioxygenase | ||
| N-terminus α-subunit | MIDTQNP | |
| N-terminus β-subunit | MLHLPAH |
Segments used for the design of oligonucleotides for cloning are underlined. X, amino acid that could not be identified.
Cloning strategy and hybridization procedures.
The PCR products were ligated into a T-tailed vector (39) prepared from pBluescript II KS (+), yielding pRERE1 and pRERE2, respectively. The ca. 170-bp insert of pRERE1 was digoxigenin labeled by using a Boehringer Mannheim DIG DNA Labeling and Detection Kit Nonradioactive. A Southern blot of 3 μg of EcoRI-digested R. opacus 1CP DNA (run on a 1% agarose gel with TAE buffer [58]) was then hybridized with the digoxigenin-labeled probe to detect the corresponding fragment (procedure as described in the Boehringer manual). An area that corresponded in size to the hybridization signal (about 2.5 to 4 kb) was excised from a second gel, and DNA eluted from the gel slice was ligated into pBluescript II KS (+). This ligation mixture was used to transform E. coli DH5α. By colony hybridization with the labeled insert of pRERE1 (performed in a manner analogous to the procedure described in reference 16), a clone carrying pRER3, which contains a ca. 3.6-kb insert of R. opacus DNA, was identified. After sequencing had shown that the gene coding for 3-oxoadipate enol-lactone hydrolase was not complete on the insert, a clone with an overlapping, ca. 2.5-kb PstI insert (pRER4) was isolated in an analogous way by using a digoxigenin-labeled, ca. 650-bp BamHI fragment of pRER3 as a probe.
DNA sequence analysis.
The nucleotide sequence of the overlapping inserts of pRER3 and pRER4 was determined by sequencing subclones in pBluescript II KS (+), by sequencing of nested deletions generated with a double-stranded nested deletion kit (Pharmacia), and by sequencing with custom-synthesized oligonucleotides as primers. For sequencing reactions, an Applied Biosystems Prizm kit was used, with subsequent electrophoresis and analysis in an Applied Biosystems A373 sequencer. Computer-based sequence analyses were performed mainly with the PC/GENE program package (Intelligenetics Inc., Mountain View, Calif.). The PC/GENE version of FASTA (53) as well as the BLAST programs (2, 22) were used to screen the EBI and NCBI databases. Multiple sequence alignments were performed by the PC/GENE version of CLUSTAL (28), with open and unit gap costs set to 10.
Nucleotide sequence accession number.
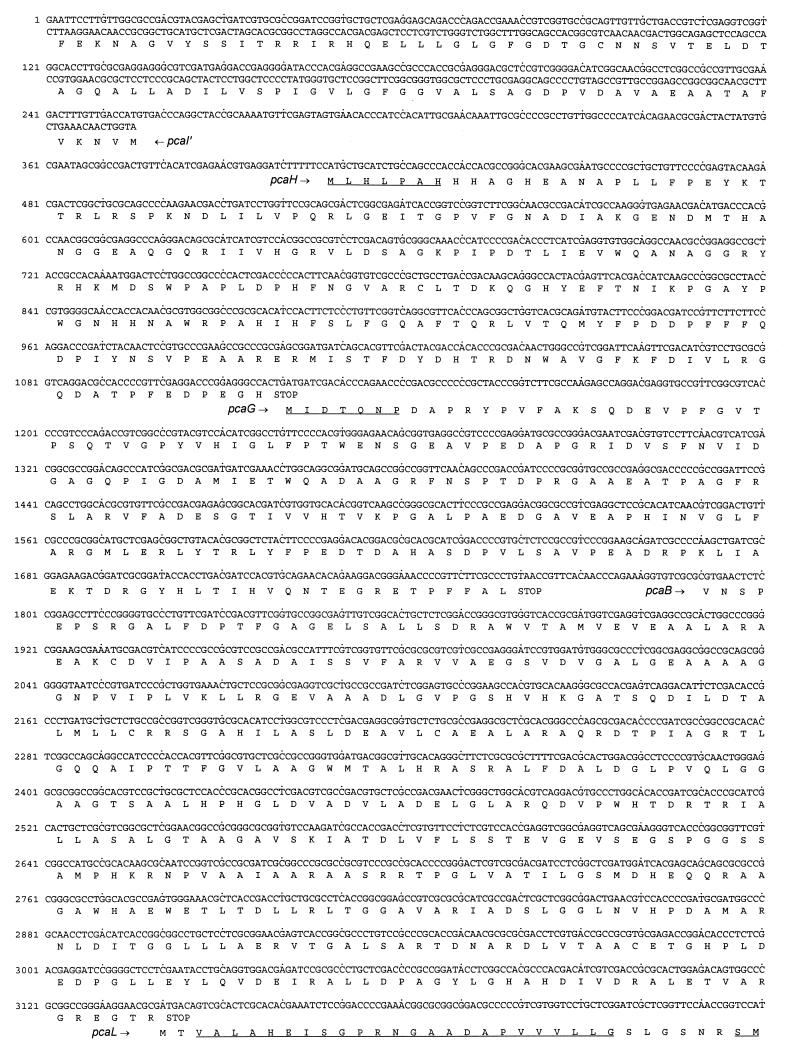
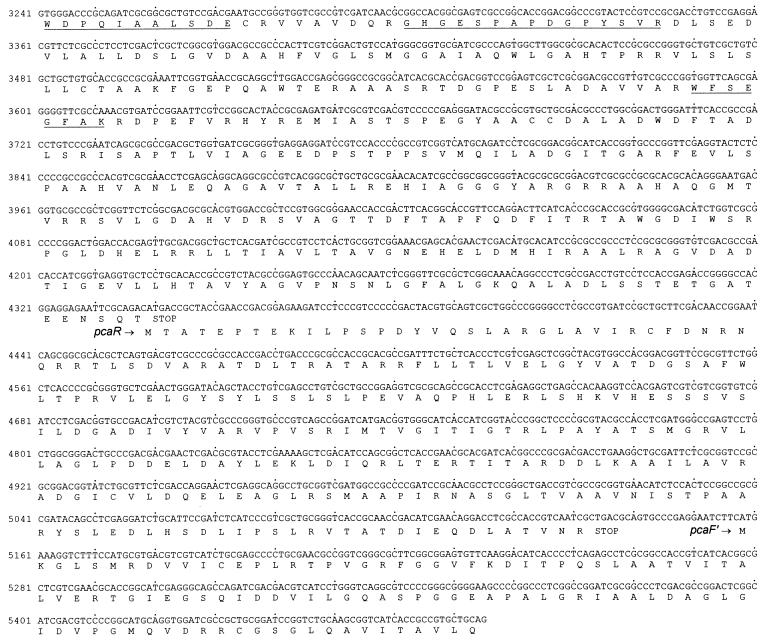
The 5,475-bp sequence of the overlapping inserts of pRER3 and pRER4 shown in Fig. 4 will appear in the EMBL, GenBank, and DDBJ nucleotide sequence databases under accession no. AF003947.
FIG. 4.
Sequence of the 5,475 bp carried on pRER3 and pRER4. The predicted amino acid sequences are shown below the DNA sequence. Amino acid sequences derived from direct sequencing of N termini or tryptic fragments are underlined. For pcal′, presumably transcribed in the opposite orientation, the complementary (here, coding) strand is also shown.
RESULTS
Cloning of pca genes based on sequences from the purified 3-oxoadipate enol-lactone-hydrolyzing enzyme.
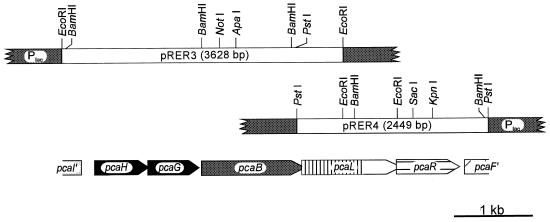
As a basis for cloning of the corresponding gene, the 3-oxoadipate enol-lactone-hydrolyzing enzyme was purified to homogeneity from an extract of benzoate-grown cells of R. opacus 1CP. Surprisingly, the subunit molecular mass of the enzyme turned out to be ca. 42 kDa (Fig. 2), considerably greater than reported for the 3-oxoadipate enol-lactone hydrolases of P. putida and A. calcoaceticus. On SDS-gels, the latter enzymes appeared to have masses of between 30 and 33 kDa (40, 72); for the Acinetobacter catD and pcaD gene products, sizes of 29.3 and 29.1 kDa, respectively, were calculated (25, 63). Tryptic peptides were prepared from the Rhodococcus 3-oxoadipate enol-lactone hydrolase, and several of them as well as the N terminus were sequenced (Table 3). Degenerate oligonucleotides derived from these sequences allowed the amplification of ca. 170- and ca. 440-bp fragments with genomic DNA of strain 1CP as the template. After labeling with digoxigenin, the ca. 170-bp fragment first served to identify hybridizing bands on a Southern blot of EcoRI-digested Rhodococcus DNA and later to screen colonies of transformed E. coli DH5α for the presence of plasmids with the desired insert. A clone containing plasmid pRER3 with a 3,628-bp EcoRI insert was isolated (Fig. 3). A labeled ca. 650-bp BamHI fragment of pRER3 served to clone the overlapping 2,449-bp PstI insert present on pRER4.
FIG. 2.
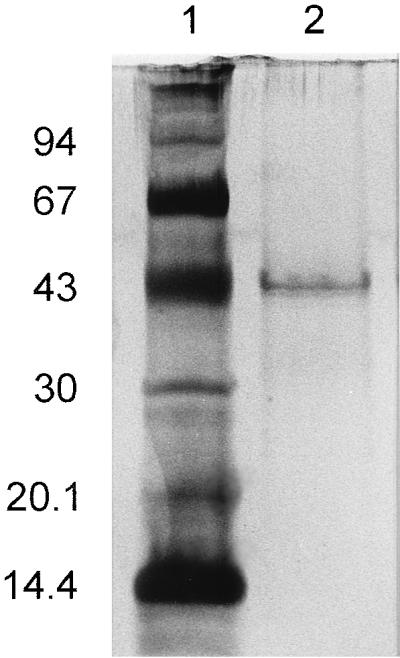
Silver-stained SDS-polyacrylamide gel showing purified 4-carboxymuconolactone decarboxylase:3-oxoadipate enol-lactone hydrolase from R. opacus 1CP in lane 2. Lane 1, Pharmacia low-molecular-weight markers: bovine α-lactalbumin (14.4 kDa), soybean trypsin inhibitor (20.1 kDa), bovine carbonic anhydrase (30 kDa), chicken egg white ovalbumin (43 kDa), bovine serum albumin (67 kDa), and rabbit phosphorylase b (94 kDa).
FIG. 3.
Restriction map of the inserts of pRER3 and pRER4, together carrying 5,475 bp of R. opacus 1CP DNA in the multiple cloning site of pBluescript II KS (+). Arrows indicate the lengths and orientations of open reading frames. Overlapping open reading frames (between pcaH and pcaG, 1 bp; between pcaB, pcaL, and pcaR, 4 bp) are indicated by blunt arrows.
Characterization of the inserts of pRER3 and pRER4.
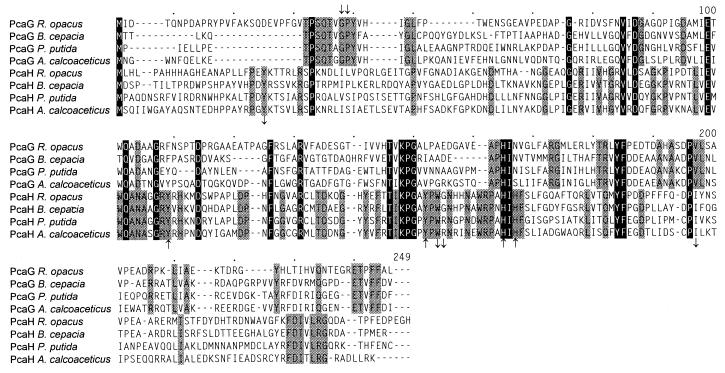
The sequence of the overlapping inserts of pRER3 and pRER4 comprises five complete and two incomplete open reading frames (Fig. 3 and 4). The first two complete open reading frames start at positions 408 and 1121, respectively. They are transcribed in the same direction and overlap by 1 bp. Their predicted N-terminal sequences exactly match those determined for the two subunits of purified protocatechuate 3,4-dioxygenase (Table 3). The predicted sequences of the gene products were found to be homologous to PcaH and PcaG of A. calcoaceticus, Burkholderia cepacia, and P. putida (Fig. 5). Thus, the two open reading frames, coding for polypeptides with molecular masses of 26,837 and 22,936 Da, were designated pcaH and pcaG.
FIG. 5.
Sequence alignment of the subunits of protocatechuate 3,4-dioxygenases. Positions identical in both α and β subunits of all four enzymes are highlighted by black boxes; those identical in only one subunit of all four enzymes are shaded. Numbers above the sequences refer to positions in the alignment, not in a single sequence. Amino acid residues which for the enzyme of P. putida ATCC 23975 have been found to be involved in the active site are indicated by downward-pointing arrows; iron ligands by upward-pointing arrows (above the alignment for the α subunit and below the alignment for the β subunit) (45). Accession numbers and references for the published sequences: M30791 (74), M33798 (24), and L14836 (19).
A third complete open reading frame extends from positions 1791 to 3143 (Fig. 4). The predicted gene product is identical to the sequences predicted for 3-carboxymuconate cycloisomerases of A. calcoaceticus, Bradyrhizobium japonicum, and P. putida (L05770 [36], Y10223 [3], and L17082 [71]) in 36.9, 42.0, and 40.1%, respectively, of the positions. Thus, the third complete open reading frame was considered to represent the pcaB gene coding for 3-carboxymuconate cycloisomerase.
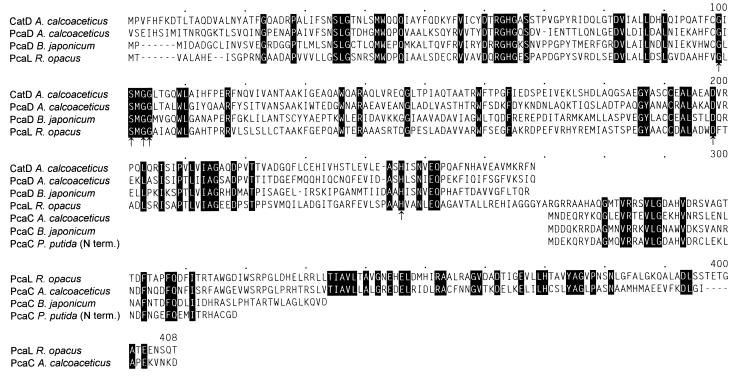
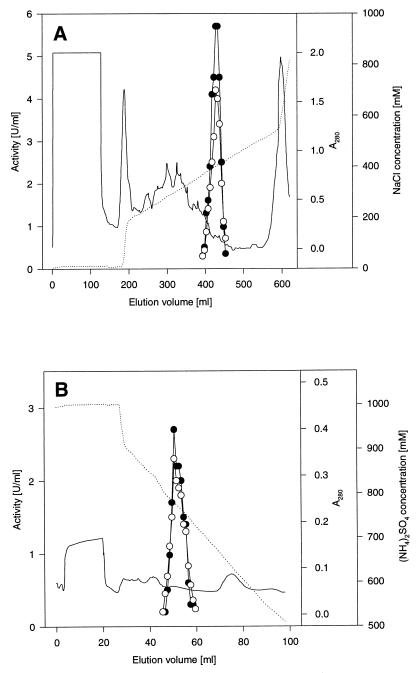
Overlapping pcaB by 4 bp, a fourth complete open reading frame, extending from positions 3140 to 4342, was detected (Fig. 4). All sequences of tryptic peptides and of the N terminus of the 3-oxoadipate enol-lactone-hydrolyzing enzyme occur in the protein sequence predicted from the open reading frame (Table 3; Fig. 4). In addition, the calculated molecular mass of the gene product (42,230 Da) corresponds to that of the purified protein (Fig. 2). Most interestingly, comparisons of the sequence to databases revealed similarities to both 3-oxoadipate enol-lactone hydrolases and 4-carboxymuconolactone decarboxylases. Further analyses showed that the N-terminal two-thirds of the protein are homologous to the hydrolases whereas the C-terminal third is homologous to the decarboxylases (Fig. 6). These results suggested that the protein encoded by the fourth open reading frame has 4-carboxymuconolactone-decarboxylating activity in addition to 3-oxoadipate enol-lactone-hydrolyzing activity. To test this hypothesis, we attempted to purify the enzymes responsible for these activities from an extract of benzoate-grown cells of R. opacus 1CP. As shown in Fig. 7 and Table 2, the 4-carboxymuconolactone decarboxylase and 3-oxoadipate enol-lactone hydrolase activities could be separated from each other by neither anion-exchange chromatography nor hydrophobic interaction chromatography. The fused gene coding for both PcaC and PcaD activities was designated pcaL, to differentiate it from the two precursor genes.
FIG. 6.
Sequence alignment of the 4-carboxymuconolactone decarboxylase:3-oxoadipate enol-lactone hydrolase PcaL of R. opacus 1CP to the 4-carboxymuconolactone decarboxylases PcaC of A. calcoaceticus (M33798 [24]) and B. japonicum (Y10223 [3]), to the N terminus (term.) of PcaC from P. putida (73), and to the 3-oxoadipate enol-lactone hydrolases PcaD of B. japonicum (Y10223 [3]) and CatD and PcaD of A. calcoaceticus (L05770 [25] and M76991 [63]). Positions identical in all compared sequences are highlighted. Possible catalytic triad residues (25, 46) of the hydrolases as well as glycine residues allowing formation of the “nucleophile elbow” (46) are indicated by arrows. Numbers above the sequences refer to positions in the alignment, not in a single sequence.
FIG. 7.
Elution profiles of 3-oxoadipate enol-lactone-hydrolyzing and 4-carboxymuconolactone-decarboxylating activities during chromatography on Q-Sepharose High Performance (A) and Phenyl Superose (B). Experimental details are given in Table 3. Closed and open circles, 3-oxoadipate enol-lactone hydrolase and 4-carboxymuconolactone decarboxylase activities, respectively; dotted lines, NaCl or (NH4)2SO4 concentration; solid lines, absorption at 280 nm.
The fifth complete open reading frame, between positions 4339 and 5136, overlaps pcaL by 4 bp. The predicted gene product shares 43% identical positions with PcaR of P. putida (L33795 [57]), 34.7% with PcaU of A. calcoaceticus (U04359 [20]), and 37.4% with PobR of A. calcoaceticus (L13114 [11]). All three proteins belong to the IclR family of regulators and are activators of pca and pob genes, respectively, in the two species. Thus, the fifth open reading frame was assumed to represent the regulatory gene pcaR.
Downstream of the presumed pcaR (start at position 5158) and transcribed in the same direction, an incomplete open reading frame was detected. The predicted gene product appeared to be homologous to 3-oxoadipyl-CoA thiolases, as shown by 38.8% identical positions to PcaF of A. calcoaceticus (L05770 [36]) and P. putida (U10895 [27]). Therefore, this incomplete open reading frame was designated pcaF′.
Transcribed divergently from pcaH, another incomplete open reading frame was identified (start at position 255). It was termed pcaI′ due to the similarity of the predicted gene product to PcaI, a subunit of succinyl-CoA:3-oxoadipate CoA-transferases, of A. calcoaceticus (L05770 [36]) and P. putida (M88763 [49]) (37.7 and 41.2%, respectively, identical positions).
DISCUSSION
Characterization of the gene coding for the 3-oxoadipate enol-lactone-hydrolyzing enzyme induced during growth of R. opacus 1CP with benzoate showed it to be part of a protocatechuate catabolic gene cluster. This was unexpected, since Rann and Cain (56) as well as Cain (8) had argued that in their Rhodococcus strains, two different 3-oxoadipate enol-lactone hydrolases are induced for the catechol and protocatechuate branches of the 3-oxoadipate pathway. The isoenzymes could be distinguished by their denaturation kinetics at 41°C, by their Km values, and by their gel filtration behavior. Whether the reason for the discrepancy between our results and those of Cain’s group is the investigation of different strains or whether other factors are responsible is not known.
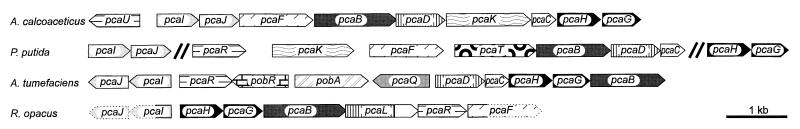
The 3-oxoadipate enol-lactone-hydrolyzing enzyme of R. opacus 1CP did not reveal any unusual similarities to the dienelactone hydrolases of chlorocatechol catabolic pathways. The enzyme was, however, found to be quite unique in being able to catalyze two subsequent reactions, 4-carboxymuconolactone decarboxylation and 3-oxoadipate enol-lactone hydrolysis. This conclusion was inferred from the occurrence of a pcaD-like segment as well as a pcaC-like segment within one open reading frame, from the molecular mass being consistent with such a merged protein, and from the inability to separate the two activities chromatographically. The proteobacterial 4-carboxymuconolactone decarboxylases, in contrast, are separate enzymes (47), i.e., not part of a larger enzyme with additional hydrolytic activity (and vice versa). Since the general trend of protein evolution goes from simple to more complex enzymes and since gene fusions play a major role in this process (5, 34, 62), the enzymatic situation in proteobacteria may be considered the more ancient one. Consequently, during evolution of the protocatechuate pathway found in Rhodococcus, the original pcaD and pcaC genes were fused to code for a protein with both decarboxylase and hydrolase activities. This merger of two proteins catalyzing sequential reactions might be beneficial to the cells for two reasons: (i) it might contribute to minimizing the distance which a metabolic intermediate has to diffuse to the next active site; (ii) alternatively, if the distances are already optimized by the enzymes of the 3-oxoadipate pathway forming a multienzyme complex, as suggested by Meagher et al. (41), this complex might be somewhat stabilized by two of the components being fused into one protein. The fusion could apparently occur without other major rearrangements: in the γ-proteobacterium P. putida as well as in the α-proteobacteria A. tumefaciens and B. japonicum, pcaC is located directly downstream of pcaD within the same operon (Fig. 8) (3, 32, 52). In A. calcoaceticus, this order is also present, but the genes are interrupted by pcaK, a gene for a transporter (36). The order whereby the PcaC segment follows the PcaD segment is retained in the fused protein, which we designated PcaL. The PcaD-homologous region of PcaL extends to position 268 of the alignment, and the PcaC-homologous segment starts at position 271 (Fig. 6). Thus, it was apparently not necessary to introduce any major loops for the new protein to accommodate both domains.
FIG. 8.
Structures of gene clusters for protocatechuate catabolism. Homologous genes are shaded in the same way. Arrows with dotted borderlines represent Rhodococcus genes for which no or only partial sequence information is available. The information was compiled from the following sources: P. putida, references 7, 19, 27, 32, 49, 57, and 71; A. calcoaceticus, references 20 (U04359), 24, 25, and 36; A. tumefaciens, references 51 and 52.
Other features of the protocatechuate gene clusters have also been conserved. Thus, pcaD directly follows pcaB, the gene for 3-carboxymuconate cycloisomerase, also in A. calcoaceticus, B. japonicum, and P. putida (3, 32, 36), whereas in A. tumefaciens a different order occurs (51). Moreover, pcaG, the gene for the β subunit of protocatechuate 3,4-dioxygenase, has been reported to be located downstream of pcaH, the gene for the α subunit, for A. tumefaciens, B. cepacia, A. calcoaceticus, and P. putida, representatives of the α-, β-, and γ-proteobacteria (19, 24, 51, 74).
From the order of the Rhodococcus pca genes, it is clear that pcaI and probably pcaJ, the genes coding for the two sub- units of succinyl-CoA:3-oxoadipate CoA-transferase, are transcribed independently from the other genes for protocatechuate catabolism. This corresponds to the finding of Cain (8) that the transferase activity showed a different behavior in the presence of glucose as a catabolite repressor than the other enzymes of the protocatechuate or catechol branch. It is unclear, however, whether pcaH, -G, -B, -L, -R, and -F are transcribed together as one operon or in separate units which are possibly governed by the same regulator and coinducer. An unusual feature of this organization is that pcaR, the presumed regulatory gene, overlaps pcaL by 4 bp, which suggests that these genes are transcribed together and may even be translationally coupled (44, 69). In contrast, pcaU of A. calcoaceticus and pcaR of A. tumefaciens are transcribed divergently from other pca genes (21, 52), whereas in P. putida pcaR is transcribed in the same direction as the pca structural genes but is separated from them by more than 400 bp (27, 57). Activators of catabolic pathways, like many LysR-type proteins, often repress their own formation at the transcriptional level and thus tend to generate a relatively constant level of the regulator (59). Transcriptional repression of their own gene has also been found for the IclR-type regulators PobR (10), PcaR (29), and PcaU (54). Although genes forming one operon may in addition be transcribed separately, as in the case of P. putida catA (31), the organization of the pca genes of R. opacus 1CP suggests that the presumed pcaR gene may be cotranscribed with the structural genes, presumably resulting in vaying levels of the activator. It would be interesting to study this regulation and the possible influence of other regulatory elements in more detail.
ACKNOWLEDGMENTS
We thank H.-J. Knackmuss for providing the environment in which we could do this work. We are indebted to R. Getzlaff and V. Noedinger for protein sequencing, to R. Schmid for the opportunity to use an automated DNA sequencer, and to S. Bürger for performing the DNA sequencing. Thanks are due to D. Parke for providing pARO523 and for helpful advice on setting up the decarboxylase assay.
The work was supported by a fellowship of the Landesgraduiertenförderung to D.E. and by grants from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Alting-Mees M A, Sorge J A, Short J M. pBluescriptII: multifunctional cloning and mapping vectors. Methods Enzymol. 1992;216:483–495. doi: 10.1016/0076-6879(92)16044-k. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Bedmar, E. J. 1996. Direct submission to the databases.
- 4.Bethesda Research Laboratories. BRL pUC host: E. coli DH5α competent cells. Bethesda Res Lab Focus. 1986;8(2):9. [Google Scholar]
- 5.Bork P. Mobile modules and motifs. Curr Opinion Struct Biol. 1992;2:413–421. [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Buck G W, Houghton J E. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Cloning and sequencing of a dicarboxylic acid transport protein from Pseudomonas putida, abstr. K-123; p. 556. [Google Scholar]
- 8.Cain R B. Regulation of aromatic and hydroaromatic pathways in nocardioform actinomycetes. In: Schaal K P, Pulverer G, editors. Actinomycetes. Stuttgart, Germany: Gustav Fischer Verlag; 1981. pp. 335–354. [Google Scholar]
- 9.Chung C T, Niemela S L, Miller R H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci USA. 1989;86:2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiMarco A A, Ornston L N. Regulation of p-hydroxybenzoate hydroxylase synthesis by PobR bound to an operator in Acinetobacter calcoaceticus. J Bacteriol. 1994;176:4277–4284. doi: 10.1128/jb.176.14.4277-4284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiMarco A A, Averhoff B, Ornston L N. Identification of the transcriptional activator pobR and characterization of its role in the expression of pobA, the structural gene for p-hydroxybenzoate hydroxylase in Acinetobacter calcoaceticus. J Bacteriol. 1993;175:4499–4506. doi: 10.1128/jb.175.14.4499-4506.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Don R H, Cox P T, Wainwright B J, Baker K, Mattick J S. ‘Touchdown’ PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991;19:4008. doi: 10.1093/nar/19.14.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorn E, Hellwig M, Reineke W, Knackmuss H-J. Isolation and characterization of a 3-chlorobenzoate degrading pseudomonad. Arch Microbiol. 1974;99:61–70. doi: 10.1007/BF00696222. [DOI] [PubMed] [Google Scholar]
- 14.Doten R C, Ngai K-L, Mitchell D J, Ornston L N. Cloning and genetic organization of the pca gene cluster from Acinetobacter calcoaceticus. J Bacteriol. 1987;169:3168–3174. doi: 10.1128/jb.169.7.3168-3174.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elvidge J A, Linstead R P, Orkin B A, Sims P, Baer H, Pattison D B. Unsaturated lactones and related substances. Part IV. Lactonic products derived from muconic acid. J Chem Soc. 1950;1950:2228–2235. [Google Scholar]
- 16.Eulberg D, Golovleva L A, Schlömann M. Characterization of catechol catabolic genes from Rhodococcus erythropolis 1CP. J Bacteriol. 1997;179:370–381. doi: 10.1128/jb.179.2.370-381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eulberg D, Hinner I-S, Lohmann M, Schlömann M. Abstracts of the General Meeting of the American Society for Microbiology, 1997. Washington, D.C: American Society for Microbiology; 1997. Characterization of gene clusters involved in catechol catabolism of Rhodococcus erythropolis 1CP and Ralstonia eutropha (Alcaligenes eutrophus) 335, abstr. K-24; p. 346. [Google Scholar]
- 18.Eulberg D, Kourbatova E M, Golovleva L A, Schlömann M. Evolutionary relationship between chlorocatechol catabolic enzymes from Rhodococcus opacus 1CP and their counterparts in proteobacteria: sequence divergence and functional convergence. J Bacteriol. 1998;180:1082–1094. doi: 10.1128/jb.180.5.1082-1094.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frazee R W, Livingston D M, LaPorte D C, Lipscomb J D. Cloning, sequencing, and expression of the Pseudomonas putida protocatechuate 3,4-dioxygenase genes. J Bacteriol. 1993;175:6194–6202. doi: 10.1128/jb.175.19.6194-6202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerischer, U. C. 1993. Direct submission to the databases.
- 21.Gerischer U, Tran S, Ornston L N. Abstracts of the 93rd General Meeting of the American Society for Microbiology 1993. Washington, D.C: American Society for Microbiology; 1993. Physiological effects exerted by pcaU, a newly discovered gene governing synthesis of enzymes for catabolism of protocatechuate in Acinetobacter calcoaceticus, abstr. H-267; p. 238. [Google Scholar]
- 22.Gish W, States D J. Identification of protein coding regions by database similarity search. Nat Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 23.Gorlatov S N, Maltseva O V, Shevchenko V I, Golovleva L A. Degradation of chlorophenols by a culture of Rhodococcus erythropolis. Mikrobiologiya. 1989;58:802–806. . (Microbiology 58:647–651.) [Google Scholar]
- 24.Hartnett C, Neidle E L, Ngai K-L, Ornston L N. DNA sequences of genes encoding Acinetobacter calcoaceticus protocatechuate 3,4-dioxygenase: evidence indicating shuffling of genes and of DNA sequences within genes during their evolutionary divergence. J Bacteriol. 1990;172:956–966. doi: 10.1128/jb.172.2.956-966.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartnett G B, Ornston L N. Acquisition of apparent DNA slippage structures during extensive evolutionary divergence of pcaD and catD genes encoding identical catalytic activities in Acinetobacter calcoaceticus. Gene. 1994;142:23–29. doi: 10.1016/0378-1119(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 26.Harwood C S, Parales R E. The β-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol. 1996;50:553–590. doi: 10.1146/annurev.micro.50.1.553. [DOI] [PubMed] [Google Scholar]
- 27.Harwood C S, Nichols N N, Kim M-K, Ditty J L, Parales R E. Identification of the pcaRKF gene cluster from Pseudomonas putida: involvement in chemotaxis, biodegradation, and transport of 4-hydroxybenzoate. J Bacteriol. 1994;176:6479–6488. doi: 10.1128/jb.176.21.6479-6488.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins D G, Sharp P M. Fast and sensitive multiple sequence alignments on a microcomputer. CABIOS. 1989;5:151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- 29.Houghton, J. E. Personal communication.
- 30.Houghton J E, Brown T M, Appel A J, Hughes E J, Ornston L N. Discontinuities in the evolution of Pseudomonas putida cat genes. J Bacteriol. 1995;177:401–412. doi: 10.1128/jb.177.2.401-412.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houghton J E, Shanley M S. Catabolic potential of pseudomonads: a regulatory perspective. In: Chaudhry G R, editor. Biological degradation and bioremediation of toxic chemicals. Portland, Oreg: Dioscorides Press; 1994. pp. 11–32. [Google Scholar]
- 32.Hughes E J, Shapiro M K, Houghton J E, Ornston L N. Cloning and expression of pca genes from Pseudomonas putida in Escherichia coli. J Gen Microbiol. 1988;134:2877–2887. doi: 10.1099/00221287-134-11-2877. [DOI] [PubMed] [Google Scholar]
- 33.Inoue H, Nojima H, Okayama H. High efficiency transformation of Escherichia coli with plasmids. Gene. 1990;96:23–28. doi: 10.1016/0378-1119(90)90336-p. [DOI] [PubMed] [Google Scholar]
- 34.Jensen R A. Evolution of metabolic pathways in enteric bacteria. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 2649–2662. [Google Scholar]
- 35.Johnson B F, Stanier R Y. Regulation of the β-ketoadipate pathway in Alcaligenes eutrophus. J Bacteriol. 1971;107:476–485. doi: 10.1128/jb.107.2.476-485.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kowalchuk G A, Hartnett G B, Benson A, Houghton J E, Ngai K-L, Ornston L N. Contrasting patterns of evolutionary divergence within the Acinetobacter calcoaceticus pca operon. Gene. 1994;146:23–30. doi: 10.1016/0378-1119(94)90829-x. [DOI] [PubMed] [Google Scholar]
- 37.Maltseva O V, Solyanikova I P, Golovleva L A. Chlorocatechol 1,2-dioxygenase from Rhodococcus erythropolis 1CP. Kinetic and immunochemical comparison with analogous enzymes from Gram-negative strains. Eur J Biochem. 1994;226:1053–1061. doi: 10.1111/j.1432-1033.1994.01053.x. [DOI] [PubMed] [Google Scholar]
- 38.Maltseva O V, Solyanikova I P, Golovleva L A, Schlömann M, Knackmuss H-J. Dienelactone hydrolase from Rhodococcus erythropolis 1CP: purification and properties. Arch Microbiol. 1994;162:368–374. [Google Scholar]
- 39.Marchuk D, Drumm M, Saulino A, Collins F S. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 1991;19:1154. doi: 10.1093/nar/19.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCorkle G M, Yeh W-K, Fletcher P, Ornston L N. Repetitions in the NH2-terminal amino acid sequence of β-ketoadipate enol-lactone hydrolase from Pseudomonas putida. J Biol Chem. 1980;255:6335–6341. [PubMed] [Google Scholar]
- 41.Meagher R B, McCorkle G M, Ornston M K, Ornston L N. Inducible uptake system for β-carboxy-cis,cis-muconate in a permeability mutant of Pseudomonas putida. J Bacteriol. 1972;111:465–473. doi: 10.1128/jb.111.2.465-473.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Millipore Corp. Immobilon Tech Protocol TP 017. Direct protein microsequencing from the Immobilon-P transfer membrane. Bedford, Mass: Millipore Corp.; 1987. [Google Scholar]
- 43.Moos M., Jr . Isolation of proteins for microsequence analysis. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman G, Smith J A, Struhl K, editors. Current protocols in molecular biology, suppl. 21. New York, N.Y: John Wiley & Sons, Inc.; 1993. pp. 10.19.1–10.19.12. [Google Scholar]
- 44.Normark S, Bergström S, Edlund T, Grundström T, Jaurin B, Lindberg F P, Olsson O. Overlapping genes. Annu Rev Genet. 1983;17:499–525. doi: 10.1146/annurev.ge.17.120183.002435. [DOI] [PubMed] [Google Scholar]
- 45.Ohlendorf D H, Orville A M, Lipscomb J D. Structure of protocatechuate 3,4-dioxygenase from Pseudomonas aeruginosa at 2.15 Å resolution. J Mol Biol. 1994;224:586–608. doi: 10.1006/jmbi.1994.1754. [DOI] [PubMed] [Google Scholar]
- 46.Ollis D L, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken S M, Harel M, Remington S J, Silman I, Schrag J, Sussman J L, Verschueren K H G, Goldman A. The α/β hydrolase fold. Protein Eng. 1992;5:197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- 47.Ornston L N. The conversion of catechol and protocatechuate to β-ketoadipate by Pseudomonas putida. II. Enzymes of the protocatechuate pathway. J Biol Chem. 1966;241:3787–3794. [PubMed] [Google Scholar]
- 48.Ornston L N. The conversion of catechol and protocatechuate to β-ketoadipate by Pseudomonas putida. III. Enzymes of the catechol pathway. J Biol Chem. 1966;241:3795–3799. [PubMed] [Google Scholar]
- 49.Parales R E, Harwood C S. Characterization of the genes encoding β-ketoadipate:succinyl-coenzyme A transferase in Pseudomonas putida. J Bacteriol. 1992;174:4657–4666. doi: 10.1128/jb.174.14.4657-4666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parales R E, Harwood C S. Regulation of the pcaIJ genes for aromatic acid degradation in Pseudomonas putida. J Bacteriol. 1993;175:5829–5838. doi: 10.1128/jb.175.18.5829-5838.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parke D. Supraoperonic clustering of pca genes for catabolism of the phenolic compound protocatechuate in Agrobacterium tumefaciens. J Bacteriol. 1995;177:3808–3817. doi: 10.1128/jb.177.13.3808-3817.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parke D. Acquisition, reorganization, and merger of genes: novel management of the β-ketoadipate pathway in Agrobacterium tumefaciens. FEMS Microbiol Lett. 1997;146:3–12. [Google Scholar]
- 53.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Popp R, Steiner I, Gerischer U. Regulation of the catabolism of aromatic compounds in Acinetobacter calcoaceticus. Biospektrum (special issue), abstr. PF076. 1997. p. 115. [Google Scholar]
- 55.Rainey F A, Burghardt J, Kroppenstedt R M, Klatte S, Stackebrandt E. Phylogenetic analysis of the genera Rhodococcus and Nocardia and evidence for the evolutionary origin of the genus Nocardia from within the radiation of Rhodococcus species. Microbiology. 1995;141:523–528. [Google Scholar]
- 56.Rann D L, Cain R B. Regulation of the enzymes of aromatic ring fission in the genus Nocardia. Biochem Soc Trans. 1973;1:658–661. [Google Scholar]
- 57.Romero-Steiner S, Parales R E, Harwood C S, Houghton J E. Characterization of the pcaR regulatory gene from Pseudomonas putida, which is required for the complete degradation of p-hydroxybenzoate. J Bacteriol. 1994;176:5771–5779. doi: 10.1128/jb.176.18.5771-5779.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning. A laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 59.Schell M A. Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol. 1993;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- 60.Schlömann M. Evolution of chlorocatechol catabolic pathways. Conclusions to be drawn from comparisons of lactone hydrolases. Biodegradation. 1994;5:301–321. doi: 10.1007/BF00696467. [DOI] [PubMed] [Google Scholar]
- 61.Schlömann M, Schmidt E, Knackmuss H-J. Different types of dienelactone hydrolase in 4-fluorobenzoate-utilizing bacteria. J Bacteriol. 1990;172:5112–5118. doi: 10.1128/jb.172.9.5112-5118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schulz G E, Schirmer R H. Priciples of protein structure. New York, N.Y: Springer-Verlag; 1979. [Google Scholar]
- 63.Shanley M S, Harrison A, Parales R E, Kowalchuk G, Mitchell D J, Ornston L N. Unusual G+C content and codon usage in catIJF, a segment of the ben-cat supra-operonic cluster in the Acinetobacter calcoaceticus chromosome. Gene. 1994;138:59–65. doi: 10.1016/0378-1119(94)90783-8. [DOI] [PubMed] [Google Scholar]
- 64.Solyanikova I P, Maltseva O V, Vollmer M D, Golovleva L A, Schlömann M. Characterization of muconate and chloromuconate cycloisomerase from Rhodococcus erythropolis 1CP: indications for functionally convergent evolution among bacterial cycloisomerases. J Bacteriol. 1995;177:2821–2826. doi: 10.1128/jb.177.10.2821-2826.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stanier R Y, Ingraham J L. Protocatechuic acid oxidase. J Biol Chem. 1954;210:799–808. [PubMed] [Google Scholar]
- 66.Stanier R Y, Ornston L N. The β-ketoadipate pathway. Adv Microb Physiol. 1973;9:89–151. [PubMed] [Google Scholar]
- 67.Stone K L, LoPresti M B, Crawford J M, DeAngelis R, Williams K R. Enzymatic digestion of proteins and HPLC peptide isolation. In: Matsudaira P T, editor. A practical guide to protein and peptide purification for microsequencing. San Diego, Calif: Academic Press, Inc.; 1989. pp. 31–47. [Google Scholar]
- 68.Tsitko, T. V., G. M. Zaitsev, A. G. Lobanok, and M. S. Salkinoja-Salonen. Tolerance of Rhodococcus opacus strains to aromatic and aliphatic solvents and the role of cellular fatty acids in the adaptation. Submitted for publication.
- 69.van de Guchte M, Kok J, Venema G. Distance-dependent translational coupling and interference in Lactococcus lactis. Mol Gen Genet. 1991;227:65–71. doi: 10.1007/BF00260708. [DOI] [PubMed] [Google Scholar]
- 70.Varadaraj K, Skinner D M. Denaturants or cosolvents improve the specificity of PCR amplification of a G + C-rich DNA using genetically engineered DNA polymerases. Gene. 1994;140:1–5. doi: 10.1016/0378-1119(94)90723-4. [DOI] [PubMed] [Google Scholar]
- 71.Williams S E, Woolridge E M, Ransom S C, Landro J A, Babbitt P C, Kozarich J W. 3-Carboxy-cis,cis-muconate lactonizing enzyme from Pseudomonas putida is homologous to the class II fumarase family: a new reaction in the evolution of a mechanistic motif. Biochemistry. 1992;31:9768–9776. doi: 10.1021/bi00155a033. [DOI] [PubMed] [Google Scholar]
- 72.Yeh W-K, Ornston L N. p-Chloromercuribenzoate specifically modifies thiols associated with the active sites of β-ketoadipate enol-lactone hydrolase and succinyl CoA: β-ketoadipate CoA transferase. Arch Microbiol. 1984;138:102–105. doi: 10.1007/BF00413008. [DOI] [PubMed] [Google Scholar]
- 73.Yeh W-K, Fletcher P, Ornston L N. Homologies in the NH2-terminal amino acid sequences of γ-carboxymuconolactone decarboxylases and muconolactone isomerases. J Biol Chem. 1980;255:6347–6354. [PubMed] [Google Scholar]
- 74.Zylstra G J, Olsen R H, Ballou D P. Genetic organization and sequence of the Pseudomonas cepacia genes for the alpha and beta subunits of protocatechuate 3,4-dioxygenase. J Bacteriol. 1989;171:5915–5921. doi: 10.1128/jb.171.11.5915-5921.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]