Abstract
Biochemical investigations of the muconate and chloromuconate cycloisomerases from the chlorophenol-utilizing strain Rhodococcus opacus (erythropolis) 1CP had previously indicated that the chlorocatechol catabolic pathway of this strain may have developed independently from the corresponding pathways of proteobacteria. To test this hypothesis, we cloned the chlorocatechol catabolic gene cluster of strain 1CP by using PCR with primers derived from sequences of N termini and peptides of purified chlorocatechol 1,2-dioxygenase and chloromuconate cycloisomerase. Sequencing of the clones revealed that they comprise different parts of the same gene cluster in which five open reading frames have been identified. The clcB gene for chloromuconate cycloisomerase is transcribed divergently from a gene which codes for a LysR-type regulatory protein, the presumed ClcR. Downstream of clcR but separated from it by 222 bp, we detected the clcA and clcD genes, which could unambiguously be assigned to chlorocatechol 1,2-dioxygenase and dienelactone hydrolase. A gene coding for a maleylacetate reductase could not be detected. Instead, the product encoded by the fifth open reading frame turned out to be homologous to transposition-related proteins of IS1031 and Tn4811. Sequence comparisons of ClcA and ClcB to other 1,2-dioxygenases and cycloisomerases, respectively, clearly showed that the chlorocatechol catabolic enzymes of R. opacus 1CP represent different branches in the dendrograms than their proteobacterial counterparts. Thus, while the sequences diverged, the functional adaptation to efficient chlorocatechol metabolization occurred independently in proteobacteria and gram-positive bacteria, that is, by functionally convergent evolution.
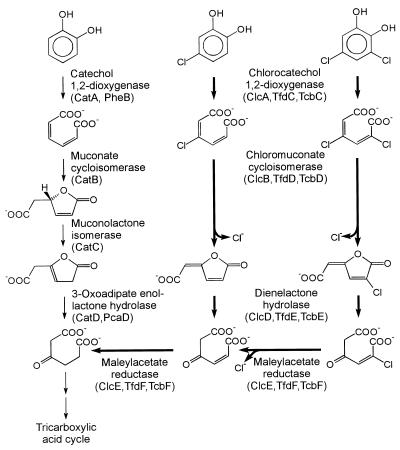
Although various chloroaromatic compounds are known to be degraded slowly or incompletely under certain conditions (23), numerous bacteria are able to utilize some representatives of this class of chemicals as sole sources of carbon and energy (26, 54). In many of the cases where growth occurs, the chloroaromatic substrates are initially converted to chlorocatechols. These can be cleaved in ortho position, i.e., between the hydroxyl groups, to chlorosubstituted cis,cis-muconates. Cycloisomerization of the latter and concomitant chloride elimination will yield dienelactones (4-carboxymethylenebut-2-en-4-olides) which may still carry the remaining chlorine substituents (Fig. 1). Subsequent hydrolysis of the lactone and reduction of the resulting maleylacetate, in most cases, generate 3-oxoadipate, an intermediate also of the catechol and the protocatechuate branch of the ubiquitous 3-oxoadipate pathway (Fig. 1).
FIG. 1.
Degradative pathways for catechol, 4-chlorocatechol, and 3,5-dichlorocatechol. Designations as gene products, as previously assigned for gram-negative or gram-positive bacteria, are given in parentheses. Enzymes with a specific function in chlorocatechol catabolism are set off by heavy arrows.
As initially shown especially for the 3-chlorobenzoate-utilizing Pseudomonas sp. strain B13 and for the 2,4-dichlorophenoxyacetate-degrading strain Ralstonia eutropha (Alcaligenes eutrophus) JMP134(pJP4), different sets of enzymes are induced for catechol catabolism on the one hand and for chlorocatechol catabolism on the other (13, 39, 53, 59, 60). Thus, chlorocatechol 1,2-dioxygenase (EC 1.13.11.-), chloromuconate cycloisomerase (EC 5.5.1.7), and dienelactone hydrolase (EC 3.1.1.45) catalyze reactions analogous to those of catechol 1,2-dioxygenase (EC 1.13.11.1), muconate cycloisomerase (EC 5.5.1.1), and 3-oxoadipate enol-lactone hydrolase (EC 3.1.1.24). They differ from the latter, however, in having higher relative turnover numbers and/or lower Km values for chlorosubstituted substrates or the metabolites generated from them by chloride elimination. The fourth enzyme characteristic for chlorocatechol catabolism, maleylacetate reductase (EC 1.3.1.32), has no equivalent among catechol catabolic enzymes and compensates for the different oxidation states of the metabolites of the two pathways.
Genetic studies, which have so far focused on gram-negative bacteria, have shown that the enzymes of chlorocatechol pathways are often encoded by catabolic plasmids such as pJP4 of R. eutropha JMP134 (11), pAC27 of the 3-chlorobenzoate-utilizing Pseudomonas putida AC866 (6), pP51 of the 1,2,4-trichlorobenzene-mineralizing Pseudomonas sp. strain P51 (68), and pEST4011 isolated from the 2,4-dichlorophenoxyacetate-degrading P. putida EST4021 (42). Several years ago, sequencing of chlorocatechol gene clusters from the former three plasmids (20, 52, 69) showed the chlorocatechol 1,2-dioxygenases and chloromuconate cycloisomerases to be homologous to catechol 1,2-dioxygenases and muconate cycloisomerases, respectively. More interestingly, the chlorocatechol 1,2-dioxygenases of these plasmids were derived from a common ancestor not shared by the catechol 1,2-dioxygenases, as the chloromuconate cycloisomerases diverged from an ancestral enzyme not in the line of descent of known muconate cycloisomerases (58). The dienelactone hydrolases are evolutionarily so remote from the 3-oxoadipate enol-lactone hydrolases that their common evolutionary background could be identified only by their three-dimensional structure or sequence signatures related to it (29, 50, 58). These observations, as well as conserved structures of the gene clusters, suggested that the proteobacterial chlorocatechol pathways, as represented by those on pAC27, pJP4, and pP51, diverged from the same ancestral pathway (58). The catabolic genes from many other strains were shown by hybridization to be related to those of the plasmids mentioned above (4, 21, 36, 42, 65). In other cases, no hybridization was found under the conditions used (4, 21, 34, 36).
The gram-positive bacterium Rhodococcus opacus (erythropolis) 1CP has previously been isolated with 2,4-dichlorophenol as the carbon source (25). Purification and characterization of its chlorocatechol 1,2-dioxygenase, chloromuconate cycloisomerase, and dienelactone hydrolase revealed unusual properties (43, 44, 63). Especially interesting was the observation that with respect to the conversion of 2-chloro-cis,cis-muconate and the chloromuconolactones derived from it, the muconate, and the chloromuconate cycloisomerase of R. opacus 1CP were more similar to each other than to proteobacterial muconate or chloromuconate cycloisomerases characterized thus far (63). If these phenotypic similarities of the Rhodococcus enzymes resulted from a closer evolutionary relationship, then this would mean that the adaptation to chlorosubstituted substrates occurred independently among the proteobacteria and the gram-positive bacteria. Thus, on the basis of a sequence divergence, a functional convergence (12) would have to be assumed. Proof of this hypothesis would require sequence information from both the enzymes involved in catechol catabolism and those involved in chlorocatechol catabolism of Rhodococcus. We recently reported on the characterization of the catechol gene cluster (17), and in the accompanying report (18) we present sequences of enzymes for protocatechuate degradation, some of which also contribute to the catechol branch of the 3-oxoadipate pathway. In this communication, we show that the chlorocatechol enzymes of R. opacus 1CP do indeed represent different branches than the previously characterized enzymes encoded on plasmids pAC27, pJP4, and pP51.
(Some of the results reported here have recently been presented in a preliminary communication [16].)
MATERIALS AND METHODS
Strains, plasmids, and cultivation conditions.
R. opacus 1CP was isolated with 2,4-dichlorophenol as the carbon source (25, 66). To obtain biomass for the enzyme purifications, the strain was cultivated in a mineral medium with 4-chlorophenol as described previously (63). Escherichia coli DH5α (5), obtained from Gibco BRL, was grown aerobically at 37°C with constant shaking in LB (Luria-Bertani) medium (56) supplied with ampicillin (100 μg/ml), if appropriate. The plasmids used in this study are described in Table 1.
TABLE 1.
Plasmids used in this study
| Plasmid | Relevant characteristicsa | Source (reference) |
|---|---|---|
| pBluescript II KS (+) | Phagemid derived from pUC19; Plac lacZ′ Apr; f1(+) origin; ColE1 origin | Stratagene (2) |
| pRERD1 | Apr; ca. 220-bp PCR product obtained with dioxygenase-derived primers in EcoRV site of pBluescript II KS (+) | This study |
| pRERC1 | Apr; ca. 60-bp PCR product obtained with cycloisomerase-derived primers in EcoRV site of pBluescript II KS (+) | This study |
| pRER5 | Apr; ca. 2.9-kb PstI fragment of R. opacus 1CP DNA in pBluescript II KS (+) | This study |
| pRER6 | Apr; ca. 10-kb HindIII fragment of R. opacus 1CP DNA in pBluescript II KS (+) | This study |
| pRER61 | Apr; ca. 2.4-kb SacII fragment of pRER6 in pBluescript II KS (+) | This study |
| pRER7 | Apr; ca. 3.7-kb EcoRI fragment of R. opacus 1CP DNA in pBluescript II KS (+) | This study |
Apr, plasmid carries information for ampicillin resistance.
Enzyme assays and estimation of protein concentration.
The activity of chloromuconate cycloisomerase was measured spectrophotometrically at 260 nm as described by Schlömann et al. (59), using 0.1 mM 2-chloro-cis,cis-muconate as the substrate (extinction coefficient, 17,100 M−1 · cm−1 [13]). The latter was available from a previous preparation (72). Dienelactone hydrolase was assayed at 280 nm, using a modification of the procedure of Schmidt and Knackmuss (60) in the presence of 50 mM Tris-HCl (pH 7.5) and 0.05 mM cis-dienelactone (cis-4-carboxymethylenebut-2-en-4-olide) (extinction coefficient, 17,000 M−1 · cm−1 [59]; obtained from S. R. Kaschabek and W. Reineke, Wuppertal, Germany).
Protein purifications.
Chlorocatechol 1,2-dioxygenase was purified basically as described previously (43).
For the purification of chloromuconate cycloisomerase, a cell extract was prepared in 50 mM BisTris-HCl (bis-[2-hydroxyethyl]-imino-tris[hydroxymethyl]methane-HCl; pH 6.5) plus 1 mM dithiothreitol (DTT) by grinding the cells with glass beads and clarifying the crude extract as described previously (17). The extract (volume, 250 ml; protein, 800 mg; total activity, 14.6 U; specific activity, 0.018 U/mg) was subjected to a purification scheme which differed from that of Solyanikova et al. (63) especially in the first step and in the additional reversed-phase chromatography. From a Pharmacia Q Sepharose High Performance HR 16/10 column (bed volume, 20 ml), used with 50 mM BisTris-HCl (pH 6.5), 1 mM DTT, and a linear NaCl gradient (0 to 0.5 M NaCl over 300 ml), the most active fractions eluted at ca. 0.35 M NaCl. (NH4)2SO4 was added to 1.6 M (final concentration), and the preparation was chromatographed on a Pharmacia Phenyl Superose HR 10/10 column. Elution with a linear gradient of (NH4)2SO4 (1.6 to 0 M over 104 ml) in 50 mM Tris-HCl (pH 7.5), 2 mM MnSO4 yielded cycloisomerase-containing fractions at about 0.35 to 0.4 M (NH4)2SO4. The preparation was subjected to gel filtration on a Pharmacia Superdex 200 prep-grade HiLoad 16/60 column (bed length, 60 cm), using 50 mM Tris-HCl (pH 7.5)–2 mM MnSO4–0.2 M NaCl for elution. The resulting preparation (volume, 9 ml; protein, 12.3 mg; total activity, 8.25 U) had a specific activity of 0.67 U/mg, equivalent to a 37-fold purification. The purified enzyme was prepared for tryptic digestion by reversed-phase chromatography. A ca. 0.2-mg aliquot of the purified enzyme was loaded onto a Pharmacia ProRPC HR 5/10 column with 0.1% (vol/vol) trifluoroacetic acid in H2O and eluted with a linear gradient (over 18 ml) to 0.1% (vol/vol) trifluoroacetic acid in acetonitrile. The absorption peak at ca. 65% (vol/vol) acetonitrile was collected and concentrated in a vacuum centrifuge.
For purification of dienelactone hydrolase, an extract (volume, 65 ml; protein, 124 mg; total activity, 51.9 U; specific activity, 0.42 U/mg) was prepared in 50 mM Tris-HCl (pH 7.2) with 0.1 mM DTT. Compared to a previous purification (44), more advanced chromatographic media were used. Chromatography with Q Sepharose High Performance HR16/10 and a gradient from 0 to 0.75 M NaCl over 400 ml was followed by Phenyl Sepharose Fast Flow HR16/10 chromatography using a gradient from 2 to 0 M (NH4)2SO4 over 280 ml. The final Superdex 75 HR10/30 (bed length, 30 cm) step yielded a preparation (volume, 1.5 ml; protein, 0.28 mg; total activity, 8.3 U) with a specific activity of 29.7 U/mg, equivalent to a 71-fold purification.
The purity of enzyme preparations was investigated by discontinuous sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis as described previously (17). The gels were stained with a Pharmacia silver-staining kit.
Protein cleavage, isolation of peptides, and sequencing of peptides and N termini.
Trypsin digestion of chlorocatechol dioxygenase, chloromuconate cycloisomerase, and dienelactone hydrolase as well as subsequent separation of tryptic peptides by reversed-phase high-pressure liquid chromatography were performed by the procedure of Stone et al. (64), with details as described by Eulberg et al. (17). The amounts of chlorocatechol dioxygenase, chloromuconate cycloisomerase, and dienelactone hydrolase after precipitation, washing, and drying were 66, 90, and 30 μg, respectively.
Purification and general in vitro manipulations of DNA.
Genomic DNA of R. opacus 1CP was obtained as described previously (17). Plasmid DNA from E. coli DH5α was isolated with Pharmacia Flexiprep kits. For other general methods, see the accompanying report (18).
Amplification of segments of the chlorocatechol 1,2-dioxygenase gene and of the chloromuconate cycloisomerase gene.
Oligonucleotides were custom synthesized according to the sequences of an N terminus and of several internal peptides (Table 2). To reduce the degeneracy of the oligonucleotides, a codon usage table for the previously sequenced genes catA, catB, and catC of strain 1CP (17) was used. The 50-μl PCR mixtures contained 50 pmol of each primer, ca. 0.25 μg of genomic template DNA, 0.1 mM each deoxynucleotide triphosphate, Goldstar DNA polymerase buffer [75 mM Tris-HCl (pH 9), 20 mM (NH4)2SO4, 0.01% (wt/vol) Tween 20], 1.5 mM MgCl2, and 0.5 U of Goldstar DNA polymerase (Eurogentech). A denaturing agent (dimethyl sulfoxide or formamide) (71) was added to the reaction mixtures to enhance the specificity of hybridization (2 to 8% [vol/vol] dimethyl sulfoxide or formamide for amplification of a fragment of the dioxygenase gene; 3 and 6% [vol/vol] dimethyl sulfoxide for the amplification of a cycloisomerase gene fragment). The thermocycling parameters for amplification of the chlorocatechol 1,2-dioxygenase gene fragment were as follows: 30 cycles of denaturing (95°C, 30 s), annealing (48°C, 2 min), and polymerization (72°C, 45 s), with an additional 2.5 min of denaturing before addition of the polymerase during the first cycle (10) and an additional 4.25 min of polymerization during the last cycle. For amplification of the chloromuconate cycloisomerase fragment, a thermocycling program without a separate polymerization step was used as appropriate for the small size of the product expected: 30 cycles of denaturing (95°C, 30 s) and annealing (48°C, 1 min), with an additional 2.5 min of denaturing before addition of the polymerase during the first cycle and 20 s of polymerization at 72°C after the last cycle.
TABLE 2.
Sequences of N termini, tryptic peptides, and deduced primers
| Protein or peptide | Amino acid sequencea | Deduced primer sequence |
|---|---|---|
| Chlorocatechol 1,2-dioxygenase | ||
| CCD67.8 | VVPAEDG(V)(I)(P)F(H)SIR | |
| CCD72.0 | GNWT(D)(L)AIQGPF | AASGGBCCYTGKATNGC |
| CCD82.6 | VIELFDEF(T)DL(R) | GTSATCGAGYTNTTYGAYGA |
| Chloromuconate cycloisomerase | ||
| N terminusb | PNLTVSGVRTTIVDLPIL(R)P | ACSACSATCGTSGAYYTNCC |
| CMCI65.74 | ELEAIGLSAVPVEEQR | |
| CMCI76.33 | QIIDEYLAPALIGSDVEDLAGTR | |
| CMCI79.76 | VEVVT(D)AGFVGLGEGV | |
| CMI85.33 | FANHSIDAQTYLLVEVVTDAGFVGL | TCKATBSWGTGGTTBGCRAA |
| Dienelactone hydrolase | ||
| DLH49.82 | SXAPPTPAHISIQQNR | |
| DLH71.46 | XYDNAGHSFLAGLTEPNHPSTAAAH | |
| DLH84.65 | TPGTDPVPYLHASSDTG |
Segments used for the design of oligonucleotides for cloning are underlined. Letters in parentheses indicate uncertain amino acids.
From reference 63. The published N-terminal sequence had an aspartate in the second position instead of the asparagine shown here.
Cloning strategy and hybridization procedures.
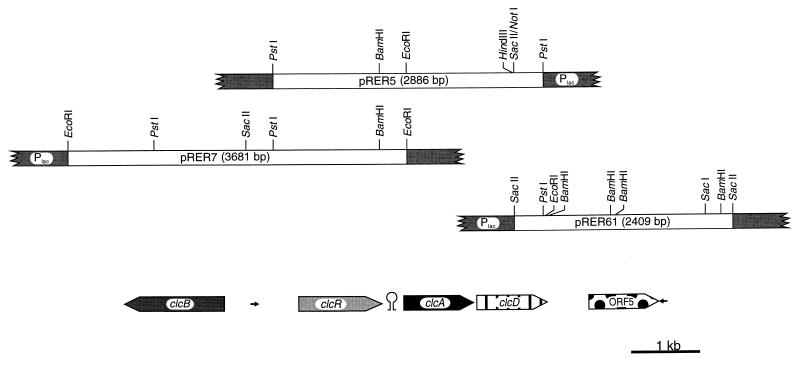
PCR products were ligated into a T-tailed vector (45). The plasmids containing the ca. 220-bp product obtained with the chlorocatechol 1,2-dioxygenase-derived primers and the ca. 60-bp product obtained with the chloromuconate cycloisomerase-derived primers were designated pRERD1 and pRERC1, respectively. The insert of pRERD1 was digoxigenin labeled by using a DIG DNA Labeling and Detection Kit Nonradioactive (Boehringer), while the insert of pRERC1 was labeled, after denaturation (100°C, 10 min) and immediate chilling on ice, by tailing with digoxigenin-dUTP using a DIG Oligonucleotide Tailing Kit (Boehringer). On Southern blots of genomic DNA of strain 1CP digested with PstI or EcoRI, we could identify a ca. 2.9-kb PstI fragment by hybridization with the labeled insert of pRERD1 and a ca. 3.7-kb EcoRI fragment by hybridization with the labeled insert of pRERC1 (procedure as described in the Boehringer manual). Identical digests were loaded onto second gels, and slices that corresponded in size to the positions of the respective hybridization signals were excised. The DNA was eluted from the gel slices and ligated into the dephosphorylated PstI or EcoRI site of pBluescript II KS (+). The resulting gene banks were transformed into cells of E. coli DH5α. The clones which carried the DNA of interest were then identified with the corresponding probe (performed by a procedure analogous to that described in reference 17). The plasmid hybridizing to the labeled insert of pRERD1 was designated pRER5; the one which hybridized with pRERC1 was designated pRER7. An adjacent clone, pRER6, which completed the open reading frame for dienelactone hydrolase on pRER5, was obtained by digoxigenin labeling a ca. 300-bp NotI-PstI fragment of the open reading frame and by using that to identify a ca. 10-kb HindIII fragment. A 2,409-bp SacII subclone of pRER6 which overlaps with pRER5 was also identified by hybridization with the labeled ca. 300-bp NotI-PstI fragment and was designated pRER61.
DNA sequence analysis.
The nucleotide sequences of the overlapping inserts of pRER5, pRER7, and a part of pRER6 were determined partly by sequencing nested deletions or subclones and partly by sequencing with custom-synthesized primers. For the sequencing reactions, an Applied Biosystems Prizm kit was used, with subsequent electrophoresis and analysis in an Applied Biosystems A373 sequencer. Computer-based sequence analyses were done with the PC/GENE program package (Intelligenetics Inc., Mountain View, Calif.). Database searches were performed by the programs BLASTX (3, 24) and FASTA (51). Multiple sequence alignments were performed by the PC/GENE version of CLUSTAL (31), with open and unit gap costs set to 10. Dendrograms based on the Fitch-Margoliash algorithm were obtained by using the programs PROTDIST, FITCH, and DRAWTREE, and bootstrap data were obtained by additionally using the programs SEQBOOT and CONSENSE, all from the PHYLIP program package (19).
Nucleotide sequence accession number.
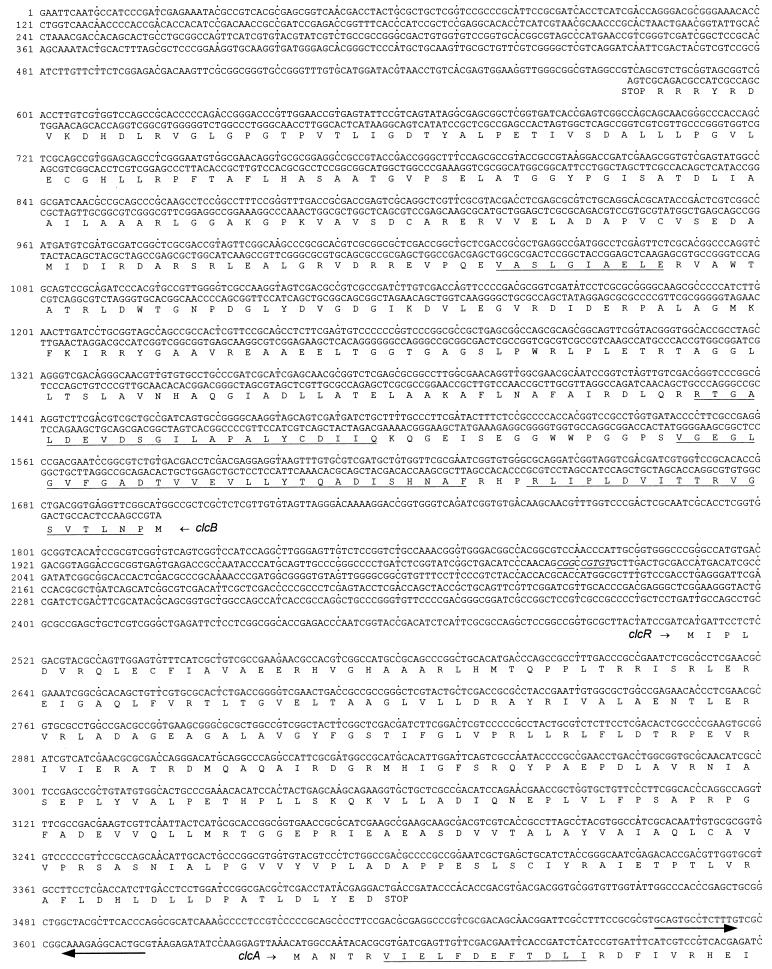
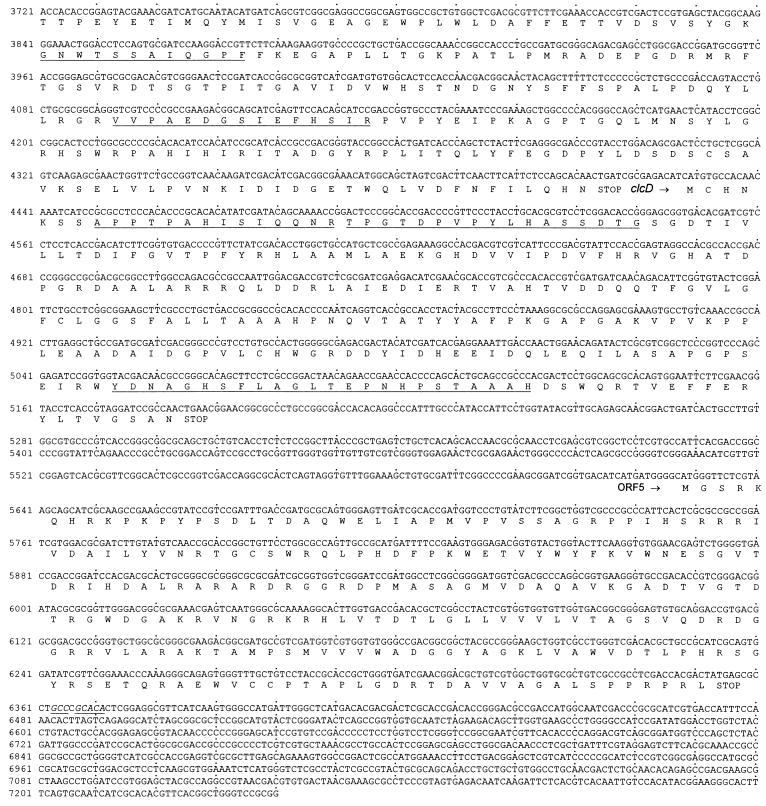
The 7,240-bp sequence of the overlapping inserts of pRER7, pRER5, and pRER61 shown in Fig. 3 will appear in the EMBL, GenBank, and DDBJ nucleotide sequence databases under accession no. AF003948.
FIG. 3.
Sequence of the 7,240 bp of R. opacus 1CP DNA cloned in pRER5, pRER7, and pRER61. The predicted amino acid sequences of the open reading frames are shown below the DNA sequence. Amino acid sequences obtained by direct sequencing of proteins or tryptic peptides are underlined. For clcB, which is divergently transcribed from all other open reading frames, the complementary (in this case, coding) strand is also given. A segment between clcR and clcA, which forms a hairpin structure and possibly represents a transcription terminator, is indicated by two arrows of opposite orientation. An imperfect inverted repeat is represented by underlined and italicized bases between clcB and clcR as well as downstream of ORF5.
RESULTS
Cloning of the clc gene cluster from R. opacus 1CP.
As a basis for the cloning of the clc gene cluster, the chlorocatechol 1,2-dioxygenase, the chloromuconate cycloisomerase, and the dienelactone hydrolase from strain 1CP, which previously had been purified and characterized biochemically (43, 44, 63), were purified again from 4-chlorophenol-grown R. opacus 1CP cells. Aliquots of the purified proteins were subjected to trypsin digestion, and selected isolated peptides were sequenced (Table 2). Some of these peptide sequences, as well as the previously sequenced N terminus of the chloromuconate cycloisomerase (63), served for the design of degenerate primers for PCR experiments (Table 2). PCR products were obtained independently for the chlorocatechol 1,2-dioxygenase gene (a ca. 220-bp fragment) and for the chloromuconate cycloisomerase gene (a ca. 60-bp fragment). The plasmids that resulted from the ligation of the PCR products into pBluescript II KS (+), designated pRERD1 and pRERC1, respectively, were checked by sequencing to contain the fragment of interest. The inserts of pRERD1 and pRERC1 were labeled with digoxigenin and were used to identify a ca. 2.9-kb PstI clone and a ca. 3.7-kb EcoRI clone (designated pRER5 and pRER7, respectively). By DNA sequence analysis, it was shown that the inserts of the two plasmids overlap, which means that the chlorocatechol 1,2-dioxygenase gene and the chloromuconate cycloisomerase gene are part of the same cluster of chlorocatechol catabolic genes. A major clone with an insert overlapping that of pRER5, pRER6, and its subclone, pRER61, were constructed to allow the complete sequencing of this gene cluster.
Sequence analysis of pRER5, pRER7, and pRER61.
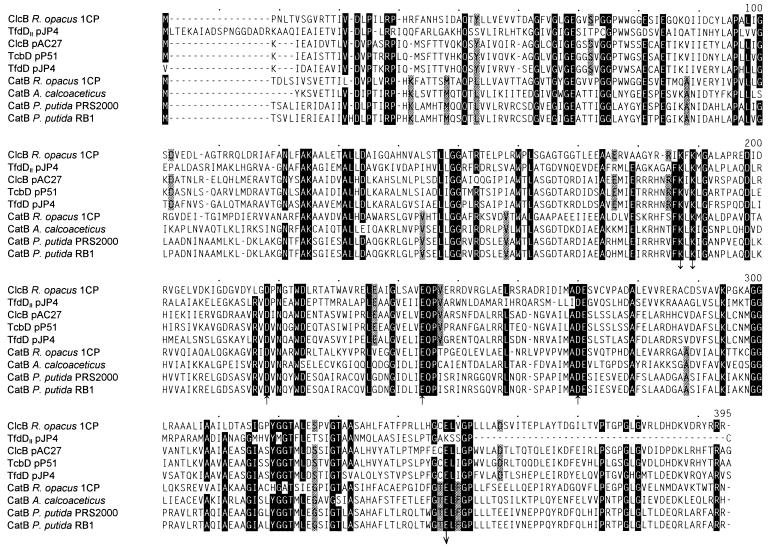
The sequenced 7,240 bp of the overlapping inserts of pRER5, pRER7, and pRER61 comprised five complete open reading frames (Fig. 2 and 3). The first open reading frame (position 1,700 to 579) is transcribed in the opposite direction of the other open reading frames. It was identified as clcB, the gene of the chloromuconate cycloisomerase, by showing that the sequence of its predicted gene product contains all determined peptide sequences as well as the reported N-terminal sequence (63) in which one position turned out to be wrong (Table 2; Fig. 3). In addition, the predicted subunit molecular mass of the protein encoded by this open reading frame (39,532 Da) fits well with the 40 to 42 kDa determined by SDS-polyacrylamide gel electrophoresis of purified chloromuconate cycloisomerase (63). The product of the clcB gene shows high sequence similarities to known bacterial muconate and chloromuconate cycloisomerases: 41.8% identical positions with R. opacus CatB (in the alignment of Fig. 4), 36.2 to 38.3% with muconate cycloisomerases of γ-proteobacteria, and 36.5 to 40.3% identical positions with chloromuconate cycloisomerases of proteobacterial plasmids (excluding TfdDII, for which no biochemical data have been reported [33.9%]). The residues which are involved in manganese coordination, as well as those which are directly involved in the enzyme mechanism (22, 30, 33), are conserved throughout the known muconate and chloromuconate cycloisomerases, including ClcB from Rhodococcus (but excepting TfdDII) (Fig. 4).
FIG. 2.
Restriction map of the inserts of pRER5, pRER7, and pRER61 carrying 7,240 bp of R. opacus 1CP DNA in the multiple cloning site of pBluescript II KS (+). The lengths and orientations of open reading frames are indicated by large arrows. Two small arrows indicate the locations of the imperfect inverted repeats. Between the presumed clcR and clcA was found a hairpin structure that possibly represents a transcription terminator.
FIG. 4.
Sequence alignment of muconate cycloisomerases (CatB), chloromuconate cycloisomerases (TfdD, ClcB, and TcbD), and a cycloisomerase of unknown biochemistry (TfdDII) as calculated by the CLUSTAL program. Numbering above the sequences refers to positions in the alignment, not in single sequences. Amino acids in positions in which at least eight of the nine aligned sequences are identical are highlighted. Amino acids that are identical in all muconate but different in all chloromuconate cycloisomerases (or vice versa) are shaded. (TfdDII was not included because of the lack of biochemical data for this hypothetical protein.) Aspartate and glutamate residues involved in manganese coordination (arrows pointing upward), as well as lysine and glutamate residues directly involved in the enzyme mechanism (arrows pointing downward), are indicated (22, 30, 33). Accession numbers and references for the published sequences: TfdDII, U16782 (67); ClcB of pAC27, M16964 (20); TcbD, M57629 (69); TfdD, M35097 (52); CatB R. opacus 1CP, X99622 (17); CatB A. (Acinetobacter) calcoaceticus, M76991 (62); CatB P. putida PRS2000, U12557 (35) with two corrections in positions 158 and 263 of the alignment (57); CatB P. putida RB1, M19460 (1). The N-terminal amino acid of TfdD has been determined to be methionine, although the associated codon is GTG (27).
Divergently transcribed from clcB, a second open reading frame, coding for a protein with a molecular mass of 33,261 Da, was found to extend from positions 2509 to 3420. It is similar to transcriptional activators of the LysR type, especially to those regulating chlorocatechol catabolic gene clusters on proteobacterial plasmids (36.8 to 39.0% identical positions in the alignment of Fig. 5) and to γ-proteobacterial regulators of catechol catabolism (29.8 and 26.7% identical positions, respectively, with CatR and CatM). Thus, this open reading frame was considered to represent the clcR gene, coding for a regulatory protein.
FIG. 5.
Sequence alignment of the presumed ClcR of R. opacus to known LysR-type regulatory proteins that govern operons for catechol catabolism (CatM and CatR) or chlorocatechol catabolism (ClcR, TfdR, TcbR, and TfdT). Numbers above the sequences refer to positions in the alignment, not in single sequences. Amino acids in positions in which at least seven of the eight sequences are identical are highlighted. Accession numbers and references for the published sequences: TfdR of pJP4, M98445 (46); ClcR of pAC27, L06464 (8); TcbR, M57629 (70); TfdT, U16782 (40); CatM, M76991 (55); CatR, U12557 (35). The amino acid sequence of TfdR from pEST4011 was obtained by translating the DNA sequence after generating a frameshift by insertion of one nucleotide behind position 726 of the submitted sequence (U32188 [38]).
The product predicted from the third open reading frame, positions 3643 to 4416, contains all of tryptic peptides that were sequenced of chlorocatechol 1,2-dioxygenase (Table 2; Fig. 3). The gene was thus designated clcA, although the deduced N-terminal amino acid sequence differed in 10 of 26 positions from the previously published, obviously incorrect N-terminal sequence of the purified protein (43). ClcA shows high sequence similarities to bacterial catechol and chlorocatechol 1,2-dioxygenases: 38.9 to 40.5% identical positions with Rhodococcus or Arthrobacter CatA (in the alignment of Fig. 6), 29.2 to 33.3% with catechol 1,2-dioxygenases of γ-proteobacteria, and 34.1 to 39.5% identical positions with chlorocatechol dioxygenases of proteobacterial plasmids. The amino acids that have been reported to be involved in iron binding (28, 41) are also conserved in the sequence predicted for chlorocatechol 1,2-dioxygenase of strain 1CP. The calculated molecular mass of ClcA is 28,952 Da, which is consistent with calculated molecular masses of other chlorocatechol dioxygenases (27.6 to 29.0 kDa). The rhodococcal ClcA thus does not seem to be significantly shorter than its counterparts from gram-negative strains as suggested by Maltseva et al. (43), who reported to have determined a molecular mass of 26.5 kDa for the purified enzyme by SDS-polyacrylamide gel electrophoresis.
FIG. 6.
Sequence alignment of catechol 1,2-dioxygenases (CatA and PheB) and chlorocatechol 1,2-dioxygenases (ClcA, TcbC, TfdC, and TfdCII) as calculated by the CLUSTAL program. The numbering above the sequences refers to positions in the alignment, not in single sequences. Amino acids in which at least 12 of the 13 sequences are identical are highlighted. Amino acids that are identical in all catechol but different in all chlorocatechol 1,2-dioxygenases (or vice versa) are shaded. Histidine and tyrosine residues that were previously reported to be involved in iron binding (28, 41) are indicated by arrows. Accession numbers and references for the published sequences: TfdCII, U16782 (67); TfdC of pEST4011, U32188 (38); ClcA of pAC27, M16964 (20); TcbC, M57629 (69); TfdC of pJP4, M35097 (52); CatA R. opacus 1CP, X99622 (17); CatA R. erythropolis AN-13, D83237 (47); CatA Arthrobacter sp. mA3, M94318 (14); CatA A. calcoaceticus ADP1, M76991 (49); CatA for phenol degradation by A. calcoaceticus NCIB8250, Z36909 (15); CatA P. putida PRS2000, U12557 (35); PheB Pseudomonas sp. EST1001, M57500 (37). Two sequences of catechol 1,2-dioxygenases from Pseudomonas strains (48) which are very similar to the sequence of CatA from P. putida PRS2000 have been omitted for clarity.
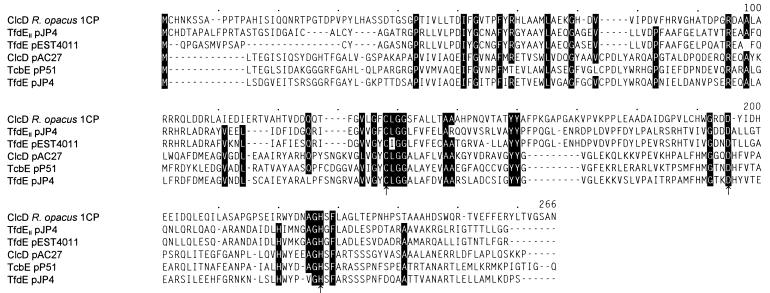
The fourth open reading frame, between positions 4429 and 5187, represents clcD, the gene of the dienelactone hydrolase. All tryptic peptides obtained from the purified enzyme occur within the predicted sequence (Table 2; Fig. 3). The calculated molecular mass of the clcD gene product (27,304 Da) is somewhat lower than the one (30 kDa) determined for the purified protein (44). With the dienelactone hydrolases TfdE of pJP4, TcbE of pP51, and ClcD of pAC27, ClcD from Rhodococcus shares between 17.9 and 22.2% identical positions in the alignment of Fig. 7. This is considerably less than the respective scores obtained for comparisons of chlorocatechol 1,2-dioxygenases or chloromuconate cycloisomerases from the same sources. The protein predicted from clcD was found to be most similar to the dienelactone hydrolases TfdE of pEST4011 and TfdEII of module 2 on pJP4, with which it shares 30.0 and 31.1% identical positions, respectively. Thus, while the rhodococcal dienelactone hydrolase in the dendrogram of Fig. 8 resides on the same branch as two proteobacterial enzymes, TfdE of pEST4011 and TfdEII of module 2 on pJP4, the sequence similarity to them is still significantly less than the similarity between the rhodococcal chloromuconate cycloisomerase or chlorocatechol 1,2-dioxygenase and their proteobacterial counterparts. The catalytic triad residues identified for ClcD of pAC27 (50) are also contained in ClcD from Rhodococcus (Fig. 7).
FIG. 7.
Sequence alignment of dienelactone hydrolases as calculated by the CLUSTAL program. Numbers above the sequences refer to positions in the alignment, not in individual sequences. Amino acids in those positions in which at least five of the six sequences are identical are highlighted. Presumed catalytic triad residues (50) are indicated by arrows. Accession numbers and references for the published sequences: TfdEII, U16782 (67); TfdE of pEST4011, U32188 (38); ClcD of pAC27, M16964 (20); TcbE, M57629 (69); TfdE of pJP4, M35097 (52).
FIG. 8.
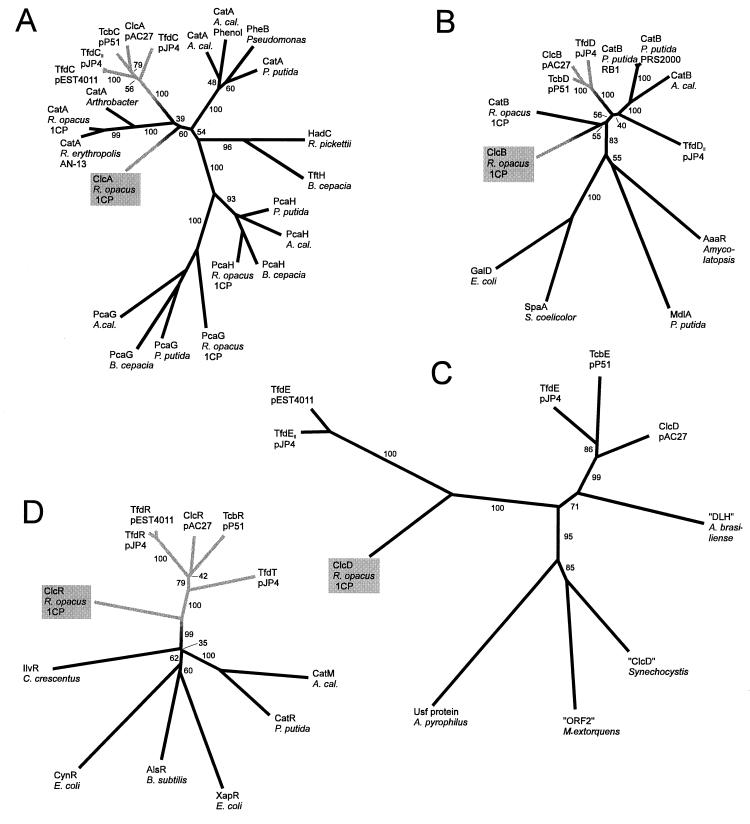
Dendrograms illustrating sequence similarities between proteins involved in catechol and chlorocatechol catabolism. (A) Catechol and chlorocatechol 1,2-dioxygenases; (B) muconate and chloromuconate cycloisomerases; (C) dienelactone hydrolases; (D) LysR-type regulatory proteins. The dendrograms were calculated by the PHYLIP program package (19) based on CLUSTAL alignments of the sequences in Fig. 4 to 7 and the respective outgroup sequences. For the sequences used in Fig. 4 to 7, the resulting alignments were very similar but, close to some gaps, not quite identical to those shown. Numbers on the branches indicate the frequency (percentage) with which the corresponding cluster occurred during bootstrap analyses. They show that the affiliation of a certain protein to one of the major groups could be deduced with great confidence. The exact branching pattern of these major groups, in contrast, as well as the branching within the groups is not certain, as indicated by low bootstrap numbers. The dendrograms for cycloisomerases and dienelactone hydrolases were calculated from the complete alignments (with the exception that the amino acids in positions 3 to 38 of “ORF2” of Methylobacterium extorquens were omitted). For the dioxygenases, only positions 140 to 258 of the alignment in Fig. 6, and for the regulators, only positions 1 to 217 of the alignment in Fig. 5, were considered. All dendrograms are drawn to the same scale with respect to evolutionary distance (in centimeters per unit branch length). Gray branches represent enzymes which are specific for chlorocatechol catabolism; the position of the transition from black to gray was chosen arbitrarily. Functions and accessions numbers of the proteins used as outgroups: PcaG and PcaH, α and β subunits of protocatechuate 3,4-dioxygenases, of A. calcoaceticus (A. cal.) (M33798), Burkholderia cepacia (M30791), P. putida (L14836) and R. opacus (AF003947) TftH, hydroxyquinol 1,2-dioxygenase, of B. cepacia (U19883); HadC, hydroxyquinol 1,2-dioxygenase, of Ralstonia pickettii (D86544); GalD, galactonate dehydratase, of E. coli (L10328 and U19577); SpaA, protein possibly involved in the metabolism of signalling lactones, of Streptomyces coelicolor (X94190); MdlA, mandelate racemase, of P. putida (J05293); AaaR, N-acyl amino acid racemase, of Amycolatopsis sp. (D30738); IlvR, regulator of isoleucine and valine synthesis, of Caulobacter crescentus (L24392); CynR, regulator for cyanate detoxification, of E. coli (M93053); AlsR, acetoin synthesis regulator, of Bacillus subtilis (L04470); XapR, xanthosine catabolic regulator, of E. coli (X73828); Usf protein, hypothetical protein, of Aquifex pyrophilus (U17575); “ORF2,” hypothetical protein, of M. extorquens (U72662); “ClcD,” hypothetical protein homologous to dienelactone hydrolases, of Synechocystis sp. PCC6803 (D90904); “DLH,” hypothetical protein homologous to dienelactone hydrolases, of Azospirillum brasiliense (X67216).
A fifth open reading frame (ORF5; bases 5628 to 6356) was detected 441 bp downstream of clcD. Its predicted gene product shows significant sequence similarity to putative transposition-related proteins, especially to those of Tn4811 from Streptomyces lividans 66 (7) (42.2% identical positions) and of IS1031C from Acetobacter xylinum (9) (38.0% identical positions). Comparison of the sequences of the inverted repeats of IS1031C to the sequence in Fig. 3 indicated that the left inverted repeat is most similar (10 out of 21 bases identical) to the segment directly following the stop codon of ORF5 (positions 6357 to 6377). This corresponds to the arrangement in IS1031C. The right inverted repeat is most similar (8 out of 21 bases identical) to a segment of the intergenic region between clcB and clcR (positions 2002 to 2022). Comparison of the R. opacus 1CP sequences of the two regions to each other revealed that they form an imperfect inverted repeat as indicated in Fig. 3.
A hairpin structure could be detected between catR and catA, suggesting that the presumed clcR gene might be transcribed independently from the clcAD genes (Fig. 3).
DISCUSSION
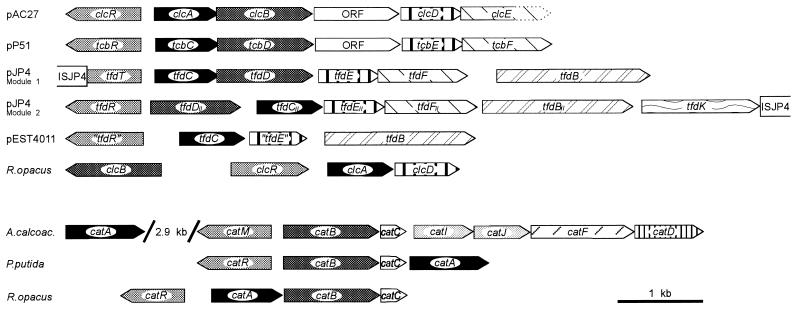
The chlorocatechol degradative pathways which are represented by the clcR,ABDE gene cluster of plasmid pAC27 (8, 20), by the tfdT,CDEF cluster (module 1) of pJP4 (40, 52), and by the tcbR,CDEF cluster of pP51 (69, 70) diverged from a common ancestral pathway already adapted for chlorocatechol catabolism (58). This conclusion could be drawn basically from two sorts of evidence. (i) The proteins encoded by the genes mentioned above are more similar to each other than to their counterparts in catechol degradation or to enzymes of other pathways. Correspondingly, they belong to the same branches in all four dendrograms depicted in Fig. 8. (ii) The three gene clusters referred to above show a marked similarity (Fig. 9). All three consist of a LysR-type regulatory gene divergently transcribed from an operon comprising, in that order, genes for a chlorocatechol 1,2-dioxygenase, a chloromuconate cycloisomerase, a dienelactone hydrolase, and a maleylacetate reductase, with an additional ORF between the cycloisomerase and the hydrolase genes of pAC27 and pP51. Since such a similar genetic structure is unlikely to have evolved two or more times independently, it should have occurred prior to the divergence of the lines giving rise to the gene clusters now present on pAC27, pJP4, and pP51.
FIG. 9.
Structures of gene clusters for catechol and chlorocatechol catabolism. Homologous genes are shaded in the same way. Functions of the proteins encoded by the genes are evident from Fig. 1. For tfdR of pEST4011, see the legend to Fig. 5. The information for this figure was compiled from references given in a previous review (58) as well as from references 17, 35, 38, 40, 55, and 67.
In concordance with our hypothesis derived from biochemical evidence (63), the sequences of all four rhodococcal proteins involved in chlorocatechol degradation differed considerably from those of their protobacterial counterparts, but still proved to be homologous to the respective proteins of catechol and other chlorocatechol pathways (Fig. 4 to 8). In fact, in the dendrograms which illustrate the relatedness of the respective gene products, the Rhodococcus proteins represent branches separate from those of the corresponding proteins from proteobacteria (Fig. 8). In accordance with the sequence differences, the structure of the chlorocatechol gene cluster of strain 1CP also differs from those of pAC27, pP51, and module 1 of pJP4 (Fig. 9). The cycloisomerase gene clcB is transcribed divergently from clcR,AD, and a maleylacetate reductase gene could not be detected in this gene cluster and was cloned on a separate fragment (61). Thus, the organization of the genes does not provide any indication that the Rhodococcus pathway may have evolved from the same origin as the chlorocatechol pathways of the plasmids pAC27, pP51, and pJP4 (module 1).
The deep branching of the enzymes and regulatory proteins of chlorocatechol pathways in Fig. 8 challenges the general opinion that catechol catabolism evolved first and then gave rise to chlorocatechol catabolism. However, the two different known branches of catechol degradative pathways, from gram-positive bacteria on the one hand and from γ-proteobacteria on the other, have one feature in common: catC directly follows catB (Fig. 9). This feature is also conserved in R. eutropha, a β-proteobacterium (32). Since the catC product, muconolactone isomerase, is an enzyme characteristic of catechol catabolism (Fig. 1), this conserved structure suggests that the catechol pathway has indeed evolved first. This conclusion is consistent with aromatic compounds, which give rise to catechol during their degradation, being more common in nature than chloroaromatic compounds that are degraded via chlorocatechols. Thus, according to present knowledge, chlorocatechol degradation did in fact evolve from catechol degradation. And obviously, it did so independently in gram-positive bacteria and proteobacteria, that is, by functionally convergent evolution.
The sequences of proteobacterial dienelactone hydrolases are known to be so dissimilar to those of their counterparts in catechol pathways, the 3-oxoadipate enol-lactone hydrolases, that recruitment of a dienelactone hydrolase from a preexisting pathway other than for catechol or protocatechuate turnover had to be postulated (58). Given that the Rhodococcus chlorocatechol pathway evolved independently from that of pAC27, pP51, and pJP4 (module 1), it was considered possible that Rhodococcus found a completely different solution for lactone hydrolysis. It was therefore interesting to find that ClcD of strain 1CP is, in fact, related to other dienelactone hydrolases and not, for example, to 3-oxoadipate enol-lactone hydrolases. However, in contrast to the chlorocatechol 1,2-dioxygenase and chloromuconate cycloisomerase of R. opacus 1CP, its dienelactone hydrolase resides on the same branch of the dendrogram as two putative isofunctional enzymes of proteobacterial origin (Fig. 8C). These putative dienelactone hydrolases are encoded by plasmid pEST4011 and by a second chlorocatechol module on pJP4, respectively (38, 67 [U16782]). The respective catabolic genes have only very recently been characterized, and no biochemical evidence has yet been reported for the function of either of the putative dienelactone hydrolases. But even if one accepts that these gene products are dienelactone-hydrolyzing enzymes, their relatively close evolutionary relationship to the dienelactone hydrolase of R. opacus 1CP does not call into question the conclusion that the chlorocatechol pathway of this strain evolved independently from that of proteobacterial plasmids. Considering that the absolute evolutionary distance between Rhodococcus ClcD and TfdE of pEST4011 or TfdEII of pJP4 is even greater than that between the major groups of dioxygenases and cycloisomerases, a plausible interpretation of the similarity pattern of the dienelactone hydrolases would be that similar enzymes and their genes were independently recruited into the gene clusters of pEST4011 and module 2 of pJP4 on the one hand and R. opacus 1CP on the other.
It is interesting that while the putative dienelactone hydrolases of pEST4011 and module 2 of pJP4 are relatively similar to the Rhodococcus enzyme, the respective chlorocatechol 1,2-dioxygenases are more closely related to the other proteobacterial dioxygenases (Fig. 8A). The latter is also true for the LysR-type regulator TfdR of pJP4 (46, 74) and for a presumed analogous regulator which may be deduced from the reported sequence of pEST4011 when allowing for one frameshift (Fig. 8D). As argued above, the ancestral chlorocatechol gene cluster from which those of pAC27, pP51, and module 1 of pJP4 diverged must be assumed to have included all necessary genes, among them those coding for chloromuconate cycloisomerase and dienelactone hydrolase. Since the dioxygenases of pEST4011 and of module 2 of pJP4 diverged from that same origin (Fig. 8A), they should, at some time, have been associated with cycloisomerases and hydrolases closely related to those of pAC27, pP51, and module 1 of pJP4. By some sort of gene shuffling, the hydrolase genes have apparently been replaced by those more closely related to the corresponding Rhodococcus gene. A cycloisomerase gene has so far not been found in the gene cluster of pEST4011. The presumed chloromuconate cycloisomerase of module 2 of pJP4, TfdDII, might even represent a new branch of cycloisomerizing enzymes (Fig. 8B). This, however, must be considered speculation because of the absence of biochemical data on TfdDII.
Not only with respect to the evolution of whole pathways but also for the constituting groups of enzymes, the sequence information from R. opacus 1CP bears potentially significant new information. Comparison of the dioxygenase sequences revealed that even though they represent different evolutionary groups, the chlorocatechol 1,2-dioxygenases of R. opacus and of proteobacterial plasmids are considerably shorter at the N termini than are catechol 1,2-dioxygenases. It would be interesting to see whether this feature influences the catalytic properties. Especially informative should be those positions in the dioxygenases and cycloisomerases in which the chlorocatechol enzymes of Rhodococcus and proteobacteria carry the same amino acids but different ones than the respective catechol enzymes (Fig. 4 and 6). These positions either may have been conserved or may have been gained independently because of their importance for the turnover of chlorinated substrates. Based on structural data of muconate and chloromuconate cycloisomerases (30, 33), we have previously identified some of the amino acids relevant for substrate specificity by using site-directed mutagenesis (73). The occurrence of functionally convergent evolution should allow us to obtain new insight into the adaptations which are necessary at the molecular level to yield an enzyme with altered catalytic properties.
ACKNOWLEDGMENTS
We are indebted to H.-J. Knackmuss for valuable discussions and for making it possible for us to do this work. We thank H. Weber and R. Getzlaff for protein and peptide sequencing, R. Schmid for the opportunity to use an automated DNA sequencer, and S. Bürger as well as S. Lakner for performing the DNA sequencing. We are grateful to W. Reineke and S. R. Kaschabek for a gift of cis-dienelactone.
The work was funded by a fellowship of the Landesgraduiertenförderung to D.E. and by grants from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Aldrich T L, Chakrabarty A M. Transcriptional regulation, nucleotide sequence, and localization of the promoter of the catBC operon in Pseudomonas putida. J Bacteriol. 1988;170:1297–1304. doi: 10.1128/jb.170.3.1297-1304.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alting-Mees M A, Sorge J A, Short J M. pBluescriptII: multifunctional cloning and mapping vectors. Methods Enzymol. 1992;216:483–495. doi: 10.1016/0076-6879(92)16044-k. [DOI] [PubMed] [Google Scholar]
- 3.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 4.Amy P S, Schulke J W, Frazier L M, Seidler R J. Characterization of aquatic bacteria and cloning of genes specifying partial degradation of 2,4-dichlorophenoxyacetic acid. Appl Environ Microbiol. 1985;49:1237–1245. doi: 10.1128/aem.49.5.1237-1245.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bethesda Research Laboratories. BRL pUC host: E. coli DH5α competent cells. Bethesda Res Lab Focus. 1986;8(2):9. [Google Scholar]
- 6.Chatterjee D K, Chakrabarty A M. Genetic rearrangements in plasmids specifying total degradation of chlorinated benzoic acids. Mol Gen Genet. 1982;188:279–285. doi: 10.1007/BF00332688. [DOI] [PubMed] [Google Scholar]
- 7.Chen C W, Yu T-W, Chung H-M, Chou C-F. Discovery and characterization of a new transposable element, Tn4811, in Streptomyces lividans 66. J Bacteriol. 1992;174:7762–7769. doi: 10.1128/jb.174.23.7762-7769.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coco W M, Rothmel R K, Henikoff S, Chakrabarty A M. Nucleotide sequence and initial functional characterization of the clcR gene encoding a LysR family activator of the clcABD chlorocatechol operon in Pseudomonas putida. J Bacteriol. 1993;175:417–427. doi: 10.1128/jb.175.2.417-427.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coucheron D H. A family of IS1031 elements in the genome of Acetobacter xylinum: nucleotide sequences and strain distribution. Mol Microbiol. 1993;9:211–218. doi: 10.1111/j.1365-2958.1993.tb01682.x. [DOI] [PubMed] [Google Scholar]
- 10.D’Aquila R T, Bechtel L J, Videler J A, Eron J J, Gorczyca P, Kaplan J C. Maximizing sensitivity and specificity of PCR by preamplification heating. Nucleic Acids Res. 1991;19:3749. doi: 10.1093/nar/19.13.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Don R H, Weightman A J, Knackmuss H-J, Timmis K N. Transposon mutagenesis and cloning analysis of the pathways for degradation of 2,4-dichlorophenoxyacetic acid and 3-chlorobenzoate in Alcaligenes eutrophus JMP134(pJP4) J Bacteriol. 1985;161:85–90. doi: 10.1128/jb.161.1.85-90.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doolittle R F. Convergent evolution: the need to be explicit. Trends Biochem Sci. 1994;19:15–18. doi: 10.1016/0968-0004(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 13.Dorn E, Knackmuss H-J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of catechol. Biochem J. 1978;174:85–94. doi: 10.1042/bj1740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eck R, Belter J. Cloning and characterization of a gene coding for the catechol 1,2-dioxygenase of Arthrobacter sp. mA3. Gene. 1993;123:87–92. doi: 10.1016/0378-1119(93)90544-d. [DOI] [PubMed] [Google Scholar]
- 15.Ehrt S, Schirmer F, Hillen W. Genetic organization, nucleotide sequence and regulation of expression of genes encoding phenol hydroxylase and catechol 1,2-dioxygenase in Acinetobacter calcoaceticus NCIB8250. Mol Microbiol. 1995;18:13–20. doi: 10.1111/j.1365-2958.1995.mmi_18010013.x. [DOI] [PubMed] [Google Scholar]
- 16.Eulberg D, Schlömann M. Convergent evolution of chlorocatechol catabolism in Rhodococcus and proteobacteria. Biospektrum (special issue), abstr. VU016. 1997. p. 35. [Google Scholar]
- 17.Eulberg D, Golovleva L A, Schlömann M. Characterization of catechol catabolic genes from Rhodococcus erythropolis 1CP. J Bacteriol. 1997;179:370–381. doi: 10.1128/jb.179.2.370-381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eulberg D, Lakner S, Golovleva L A, Schlömann M. Characterization of a protocatechuate catabolic gene cluster from Rhodococcus opacus 1CP: evidence for a merged enzyme with 4-carboxymuconolactone-decarboxylating and 3-oxoadipate enol-lactone-hydrolyzing activity. J Bacteriol. 1998;180:1072–1081. doi: 10.1128/jb.180.5.1072-1081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felsenstein J. PHYLIP (Phylogeny Inference Package) version 3.5c. Distributed by the author. Seattle, Wash: Department of Genetics, University of Washington; 1993. [Google Scholar]
- 20.Frantz B, Chakrabarty A M. Organization and nucleotide sequence determination of a gene cluster involved in 3-chlorocatechol degradation. Proc Natl Acad Sci USA. 1987;84:4460–4464. doi: 10.1073/pnas.84.13.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fulthorpe R R, McGowan C, Maltseva O V, Holben W E, Tiedje J M. 2,4-Dichlorophenoxyacetic acid-degrading bacteria contain mosaics of catabolic genes. Appl Environ Microbiol. 1995;61:3274–3281. doi: 10.1128/aem.61.9.3274-3281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerlt J A, Gassman P G. Understanding enzyme-catalyzed proton abstraction from carbon acids: details of stepwise mechanisms for β-elimination reactions. J Am Chem Soc. 1992;114:5928–5934. [Google Scholar]
- 23.Ghisalba O. Chemical wastes and their biodegradation—an overview. Experientia. 1983;39:1247–1257. doi: 10.1007/BF01990362. [DOI] [PubMed] [Google Scholar]
- 24.Gish W, States D J. Identification of protein coding regions by database similarity search. Nat Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 25.Gorlatov S N, Maltseva O V, Shevchenko V I, Golovleva L A. Degradation of chlorophenols by a culture of Rhodococcus erythropolis. Mikrobiologiya. 1989;58:802–806. . (Microbiology 58:647–651.) [Google Scholar]
- 26.Häggblom M M. Microbial breakdown of halogenated aromatic pesticides and related compounds. FEMS Microbiol Rev. 1992;103:29–72. doi: 10.1111/j.1574-6968.1992.tb05823.x. [DOI] [PubMed] [Google Scholar]
- 27.Hammer A, Hildenbrand T, Hoier H, Ngai K-L, Schlömann M, Stezowski J J. Crystallization and preliminary X-ray data of chloromuconate cycloisomerase from Alcaligenes eutrophus JMP134 (pJP4) J Mol Biol. 1993;232:305–307. doi: 10.1006/jmbi.1993.1385. [DOI] [PubMed] [Google Scholar]
- 28.Hartnett C, Neidle E L, Ngai K-L, Ornston L N. DNA sequences of genes encoding Acinetobacter calcoaceticus protocatechuate 3,4-dioxygenase: evidence indicating shuffling of genes and of DNA sequences within genes during their evolutionary divergence. J Bacteriol. 1990;172:956–966. doi: 10.1128/jb.172.2.956-966.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartnett G B, Ornston L N. Acquisition of apparent DNA slippage structures during extensive evolutionary divergence of pcaD and catD genes encoding identical catalytic activities in Acinetobacter calcoaceticus. Gene. 1994;142:23–29. doi: 10.1016/0378-1119(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 30.Helin S, Kahn P C, Guha B L, Mallows D G, Goldman A. The refined X-ray structure of muconate lactonizing enzyme from Pseudomonas putida PRS2000 at 1.85 Å resolution. J Mol Biol. 1995;254:918–941. doi: 10.1006/jmbi.1995.0666. [DOI] [PubMed] [Google Scholar]
- 31.Higgins D G, Sharp P M. Fast and sensitive multiple sequence alignments on a microcomputer. CABIOS. 1989;5:151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- 32.Hinner I-S, Lohmann M, Schlömann M. Two catechol catabolic genes in Ralstonia eutropha 335. Biospektrum (special issue), abstr. PU074. 1997. p. 71. [Google Scholar]
- 33.Hoier H, Schlömann M, Hammer A, Glusker J P, Carrell H L, Goldman A, Stezowski J J, Heinemann U. Crystal structure of chloromuconate cycloisomerase from Alcaligenes eutrophus JMP134 (pJP4) at 3 Å resolution. Acta Crystallogr D. 1994;50:75–84. doi: 10.1107/S090744499300900X. [DOI] [PubMed] [Google Scholar]
- 34.Holben W E, Schroeter B M, Calabrese V G M, Olsen R H, Kukor J K, Biederbeck V O, Smith A E, Tiedje J M. Gene probe analysis of soil microbial populations selected by amendment with 2,4-dichlorophenoxyacetic acid. Appl Environ Microbiol. 1992;58:3941–3948. doi: 10.1128/aem.58.12.3941-3948.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houghton J E, Brown T M, Appel A J, Hughes E J, Ornston L N. Discontinuities in the evolution of Pseudomonas putida cat genes. J Bacteriol. 1995;177:401–412. doi: 10.1128/jb.177.2.401-412.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ka J O, Holben W E, Tiedje J M. Genetic and phenotypic diversity of 2,4-dichlorophenoxyacetic acid (2,4-D)-degrading bacteria isolated from 2,4-D-treated field soils. Appl Environ Microbiol. 1994;60:1106–1115. doi: 10.1128/aem.60.4.1106-1115.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kivisaar M, Kasak L, Nurk A. Sequence of the plasmid-encoded catechol 1,2-dioxygenase-expressing gene, pheB, of phenol-degrading Pseudomonas sp. strain EST1001. Gene. 1991;98:15–20. doi: 10.1016/0378-1119(91)90098-v. [DOI] [PubMed] [Google Scholar]
- 38.Kõiv V, Marits R, Heinaru A. Sequence analysis of the 2,4-dichlorophenol hydroxylase gene tfdB and 3,5-dichlorocatechol 1,2-dioxygenase gene tfdC of 2,4-dichlorophenoxyacetic acid degrading plasmid pEST4011. Gene. 1996;174:293–297. doi: 10.1016/0378-1119(96)00043-1. [DOI] [PubMed] [Google Scholar]
- 39.Kuhm A E, Schlömann M, Knackmuss H-J, Pieper D H. Purification and characterization of dichloromuconate cycloisomerase from Alcaligenes eutrophus JMP 134. Biochem J. 1990;266:877–883. [PMC free article] [PubMed] [Google Scholar]
- 40.Leveau J H J, van der Meer J R. The tfdR gene product can successfully take over the role of the insertion element-inactivated TfdT protein as a transcriptional activator of the tfdCDEF gene cluster, which encodes chlorocatechol degradation in Ralstonia eutropha JMP134(pJP4) J Bacteriol. 1996;178:6824–6832. doi: 10.1128/jb.178.23.6824-6832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipscomb J D, Orville A M. Mechanistic aspects of dihydroxybenzoate dioxygenases. In: Sigel H, Sigel A, editors. Metal ions in biological systems. 28. Degradation of environmental pollutants by microorganisms and their metalloenzymes. New York, N.Y: Marcel Dekker, Inc.; 1992. pp. 243–298. [Google Scholar]
- 42.Mäe A A, Marits R O, Ausmees N R, Kõiv V M, Heinaru A L. Characterization of a new 2,4-dichlorophenoxyacetic acid degrading plasmid pEST4011: physical map and localization of catabolic genes. J Gen Microbiol. 1993;139:3165–3170. [Google Scholar]
- 43.Maltseva O V, Solyanikova I P, Golovleva L A. Chlorocatechol 1,2-dioxygenase from Rhodococcus erythropolis 1CP. Kinetic and immunochemical comparison with analogous enzymes from Gram-negative strains. Eur J Biochem. 1994;226:1053–1061. doi: 10.1111/j.1432-1033.1994.01053.x. [DOI] [PubMed] [Google Scholar]
- 44.Maltseva O V, Solyanikova I P, Golovleva L A, Schlömann M, Knackmuss H-J. Dienelactone hydrolase from Rhodococcus erythropolis 1CP: purification and properties. Arch Microbiol. 1994;162:368–374. [Google Scholar]
- 45.Marchuk D, Drumm M, Saulino A, Collins F S. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 1991;19:1154. doi: 10.1093/nar/19.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matrubutham U, Harker A R. Analysis of duplicated gene sequences associated with tfdR and tfdS in Alcaligenes eutrophus JMP134. J Bacteriol. 1994;176:2348–2353. doi: 10.1128/jb.176.8.2348-2353.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murakami S, Kodama N, Shinke R, Aoki K. Classification of catechol 1,2-dioxygenase family: sequence analysis of a gene for the catechol 1,2-dioxygenase showing high specificity for methylcatechols from Gram+ aniline-assimilating Rhodococcus erythropolis AN-13. Gene. 1997;185:49–54. doi: 10.1016/s0378-1119(96)00629-4. [DOI] [PubMed] [Google Scholar]
- 48.Nakai C, Uyeyama H, Kagamiyama H, Nakazawa T, Inouye S, Kishi F, Nakazawa A, Nozaki M. Cloning, DNA sequencing, and amino acid sequencing of catechol 1,2-dioxygenases (pyrocatechase) from Pseudomonas putida mt-2 and Pseudomonas arvilla C-1. Arch Biochem Biophys. 1995;321:353–362. doi: 10.1006/abbi.1995.1405. [DOI] [PubMed] [Google Scholar]
- 49.Neidle E L, Hartnett C, Bonitz S, Ornston L N. DNA sequence of the Acinetobacter calcoaceticus catechol 1,2-dioxygenase I structural gene catA: evidence for evolutionary divergence of intradiol dioxygenases by acquisition of DNA sequence repetitions. J Bacteriol. 1988;170:4874–4880. doi: 10.1128/jb.170.10.4874-4880.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ollis D L, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken S M, Harel M, Remington S J, Silman I, Schrag J, Sussman J L, Verschueren K H G, Goldman A. The α/β hydrolase fold. Protein Eng. 1992;5:197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- 51.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perkins E J, Gordon M P, Caceres O, Lurquin P F. Organization and sequence analysis of the 2,4-dichlorophenol hydroxylase and dichlorocatechol oxidative operons of plasmid pJP4. J Bacteriol. 1990;172:2351–2359. doi: 10.1128/jb.172.5.2351-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pieper D H, Reineke W, Engesser K-H, Knackmuss H-J. Metabolism of 2,4-dichlorophenoxyacetic acid, 4-chloro-2-methylphenoxyacetic acid and 2-methylphenoxyacetic acid by Alcaligenes eutrophus JMP 134. Arch Microbiol. 1988;150:95–102. [Google Scholar]
- 54.Reineke W. Degradation of chlorinated aromatic compounds by bacteria: strain development. In: Chaudhry G R, editor. Biological degradation and bioremediation of toxic chemicals. Portland, Oreg: Dioscorides Press; 1994. pp. 416–454. [Google Scholar]
- 55.Romero-Arroyo C E, Schell M A, Gaines III G L, Neidle E L. catM encodes a LysR-type transcriptional activator regulating catechol degradation in Acinetobacter calcoaceticus. J Bacteriol. 1995;177:5891–5898. doi: 10.1128/jb.177.20.5891-5898.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 57.Schell, U. Unpublished results.
- 58.Schlömann M. Evolution of chlorocatechol catabolic pathways. Conclusions to be drawn from comparisons of lactone hydrolases. Biodegradation. 1994;5:301–321. doi: 10.1007/BF00696467. [DOI] [PubMed] [Google Scholar]
- 59.Schlömann M, Schmidt E, Knackmuss H-J. Different types of dienelactone hydrolase in 4-fluorobenzoate-utilizing bacteria. J Bacteriol. 1990;172:5112–5118. doi: 10.1128/jb.172.9.5112-5118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt E, Knackmuss H-J. Chemical structure and biodegradability of halogenated aromatic compounds. Conversion of chlorinated muconic acids into maleoylacetic acid. Biochem J. 1980;192:339–347. doi: 10.1042/bj1920339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seibert V, Golovleva L A, Schlömann M. Cloning of maleylacetate reductase genes from Alcaligenes eutrophus JMP289 and Rhodococcus erythropolis 1CP. Biospektrum (special issue), abstr. PA013. 1995. p. 57. [Google Scholar]
- 62.Shanley M S, Harrison A, Parales R E, Kowalchuk G, Mitchell D J, Ornston L N. Unusual G+C content and codon usage in catIJF, a segment of the ben-cat supra-operonic cluster in the Acinetobacter calcoaceticus chromosome. Gene. 1994;138:59–65. doi: 10.1016/0378-1119(94)90783-8. [DOI] [PubMed] [Google Scholar]
- 63.Solyanikova I P, Maltseva O V, Vollmer M D, Golovleva L A, Schlömann M. Characterization of muconate and chloromuconate cycloisomerase from Rhodococcus erythropolis 1CP: indications for functionally convergent evolution among bacterial cycloisomerases. J Bacteriol. 1995;177:2821–2826. doi: 10.1128/jb.177.10.2821-2826.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stone K L, LoPresti M B, Crawford J M, DeAngelis R, Williams K R. Enzymatic digestion of proteins and HPLC peptide isolation. In: Matsudaira P T, editor. A practical guide to protein and peptide purification for microsequencing. San Diego, Calif: Academic Press, Inc.; 1989. pp. 31–47. [Google Scholar]
- 65.Top E M, Holben W E, Forney L J. Characterization of diverse 2,4-dichlorophenoxyacetic acid-degradative plasmids isolated from soil by complementation. Appl Environ Microbiol. 1995;61:1691–1698. doi: 10.1128/aem.61.5.1691-1698.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsitko, T. V., G. M. Zaitsev, A. G. Lobanok, and M. S. Salkinoja-Salonen. Tolerance of Rhodococcus opacus strains to aromatic and aliphatic solvents and the role of cellular fatty acids in the adaptation. Submitted for publication.
- 67.van der Meer, J. R. 1996. Direct submission to the databases.
- 68.van der Meer J R, van Neerven A R W, de Vries E J, de Vos W M, Zehnder A J B. Cloning and characterization of plasmid-encoded genes for the degradation of 1,2-dichloro-, 1,4-dichloro-, and 1,2,4-trichlorobenzene of Pseudomonas sp. strain P51. J Bacteriol. 1991;173:6–15. doi: 10.1128/jb.173.1.6-15.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Meer J R, Eggen R I L, Zehnder A J B, de Vos W M. Sequence analysis of the Pseudomonas sp. strain P51 tcb gene cluster, which encodes metabolism of chlorinated catechols: evidence for specialization of catechol 1,2-dioxygenases for chlorinated substrates. J Bacteriol. 1991;173:2425–2434. doi: 10.1128/jb.173.8.2425-2434.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van der Meer J R, Frijters A C J, Leveau J H J, Eggen R I L, Zehnder A J B, de Vos W M. Characterization of the Pseudomonas sp. strain P51 gene tcbR, a LysR-type transcriptional activator of the tcbCDEF chlorocatechol oxidative operon, and analysis of the regulatory region. J Bacteriol. 1991;173:3700–3708. doi: 10.1128/jb.173.12.3700-3708.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Varadaraj K, Skinner D M. Denaturants or cosolvents improve the specificity of PCR amplification of a G + C-rich DNA using genetically engineered DNA polymerases. Gene. 1994;140:1–5. doi: 10.1016/0378-1119(94)90723-4. [DOI] [PubMed] [Google Scholar]
- 72.Vollmer M D, Fischer P, Knackmuss H-J, Schlömann M. Inability of muconate cycloisomerases to cause dehalogenation during conversion of 2-chloro-cis,cis-muconate. J Bacteriol. 1994;176:4366–4375. doi: 10.1128/jb.176.14.4366-4375.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vollmer M D, Hoier H, Schell U, Gröning J, Pleiss J, Goldman A, Schlömann M. Differences in the catalytic properties of muconate and chloromuconate cycloisomerases analyzed by site-directed mutagenesis. Biospektrum (special issue), abstr. KV002. 1995. p. 24. [Google Scholar]
- 74.You I-S, Ghosal D. Genetic and molecular analysis of a regulatory region of the herbicide 2,4-dichlorophenoxyacetate catabolic plasmid pJP4. Mol Microbiol. 1995;16:321–331. doi: 10.1111/j.1365-2958.1995.tb02304.x. [DOI] [PubMed] [Google Scholar]