Abstract
This review updates three key concepts of autonomic neuroscience—stress, the autonomic nervous system (ANS), and homeostasis. Hans Selye popularized stress as a scientific idea. He defined stress variously as a stereotyped response pattern, a state that evokes this pattern, or a stimulus that evokes the state. According to the “homeostat” theory stress is a condition where a comparator senses a discrepancy between sensed afferent input and a response algorithm, the integrated error signal eliciting specific patterns of altered effector outflows. Scientific advances since Langley’s definition of the ANS have incited the proposal here of an “extended autonomic system,” or EAS, for three reasons. (1) Several neuroendocrine systems are bound inextricably to Langley’s ANS. The first to be described, by Cannon in the early 1900s, involves the hormone adrenaline, the main effector chemical of the sympathetic adrenergic system. Other neuroendocrine systems are the hypothalamic-pituitary-adrenocortical system, the arginine vasopressin system, and the renin-angiotensin-aldosterone system. (2) An evolving body of research links the ANS complexly with inflammatory/immune systems, including vagal anti-inflammatory and catecholamine-related inflammasomal components. (3) A hierarchical network of brain centers (the central autonomic network, CAN) regulates ANS outflows. Embedded within the CAN is the central stress system conceptualized by Chrousos and Gold. According to the allostasis concept, homeostatic input-output curves can be altered in an anticipatory, feed-forward manner; and prolonged or inappropriate allostatic adjustments increase wear-and-tear (allostatic load), resulting in chronic, stress-related, multi-system disorders. This review concludes with sections on clinical and therapeutic implications of the updated concepts offered here.
Keywords: Autonomic, Stress, Homeostasis, Allostasis, Dyshomeostasis
INTRODUCTION
This review updates three classic concepts in autonomic neuroscience—stress, the autonomic nervous system (ANS), and homeostasis. These have figured prominently in thinking about numerous acute and chronic disorders. Each can be viewed simply or complexly, as evidenced by myriad internet-based media reports and published peer-reviewed original research articles. A concept-driven approach to stress, the ANS, and homeostasis may be beneficial, especially given that multi-disciplinary, multi-system disorders of regulation are prevalent and likely will become increasingly so in the future.
STRESS AS A SCIENTIFIC IDEA
Stress is engrained in folklore and the mass media. Previous publications have traced the evolution of stress as a scientific idea (Goldstein, 1995b; Goldstein et al., 2007) and in particular the contributions of Hans Selye (Jackson, 2014). This section extracts some of the main points.
Selye and stress
Hans Selye introduced and popularized the stress concept. He defined stress variously as a stereotyped response pattern (the General Adaptation Syndrome, with three sequential stages of alarm, adaptation, and exhaustion), a condition or state that evokes this response pattern, or a stimulus that evokes the state (“stressor”) (Selye, 1956). This definitional ambiguity weakened his theory. Ffrangcon Roberts famously remarked that according to Selye, “…stress, in addition to being itself and the result of itself, is also the cause of itself” (Jackson, 2014).
Kagan has questioned the usefulness of Selye’s stress concept, arguing that “stress should be limited to select events that pose a serious threat to an organism’s well-being or discarded as too ambiguous to be theoretically useful” (Kagan, 2016)
According to the “homeostat” theory (Goldstein, 1995a), discussed in more detail below, stress is a condition or state in which a comparator homeostat senses a discrepancy between perceived or anticipated afferent input to the brain and a set-point or other algorithm for responding, the integrated error signal resulting in generation of relatively specific patterns of altered effector outflows (Goldstein, 2019; Goldstein et al., 2017).
Relatively late in Selye’s career he defined distress as a form of stress that is unpleasant or harmful, in contrast with eustress, which he defined as pleasant and unharmful (Selye, 1974). These definitions were circular and ambiguous. Subsequently the distress/eustress dichotomy was considered temporally, with acute, adaptive “eustress” associated with immunological enhancement and chronic, maladaptive “distress” associated with immunosuppression (Dhabhar et al., 1997).
A non-circular definition of distress holds that distress is consciously experienced, associated with a perceived inability to cope, aversive (i.e., motivates escape or avoidance), produces instinctively communicated signs, and is associated with adrenocortical and adrenomedullary activation and homeostatic resetting (Goldstein, 2006). According to this definition, emotional excitement that is joyful is not distress, although “happy heart syndrome” can have acute adverse consequences (Ghadri et al., 2016).
Selye emphasized the role of glucocorticoids in the stress response. This was expanded by his colleagues and students to the pituitary gland and then to the hypothalamic-pituitary-adrenocortical (HPA) axis. According to the General Adaptation Syndrome idea, catecholaminergic systems would be involved in the first, alarm, stage.
Selye’s doctrine of non-specificity (the “General Adaptation Syndrome”)
Selye’s doctrine of non-specificity states that stress is the non-specific response of the body to any demand imposed upon it (Selye, 1974). About a half century went by before the doctrine of non-specificity underwent experimental evaluation. The testing was based on stressors eliciting HPA, sympathetic noradrenergic system (SNS), and sympathetic adrenergic system (SAS) responses. The doctrine of non-specificity predicts that at different stress intensities ratios of increments in neuroendocrine responses should be the same; however, when arterial plasma corticotropin (ACTH), norepinephrine (NE), and epinephrine (EPI) were measured simultaneously in conscious rats exposed to cold, intravenous insulin, hemorrhage, or immobilization, all of which increased ACTH levels, cold evoked large NE responses, insulin large EPI responses, and hemorrhage small NE and EPI responses, while immobilization elicited large increases in levels of both NE and EPI. These results were inconsistent with Selye’s doctrine of non-specificity and the existence of a unitary “stress syndrome” and were more consistent with the concept that each stressor has a particular HPA, SNS, and SAS “signature” (Pacak et al., 1998b). A variety of other studies have supported the notion of stressor specificity (Kvetnansky et al., 1998; Pacak et al., 1998a; Pacak et al., 1998c).
HPA responses are especially pronounced in distressing situations that are novel. With repeated exposure, the magnitude of the response decreases (Dobrakovova et al., 1993). Habituation is a characteristic of even primitive animals, although whether HPA adaptation to repeated stressors follows “rules of habituation” has been questioned (Rabasa et al., 2015).
The term, “dishabituation,” is used to refer to a return to the initial magnitude of response after habituation has taken place. A related phenomenon is exaggerated responsiveness of adapted organisms to a novel (“heterotypic”) stressor. Individuals who have habituated to one form of stress exhibit exaggerated SNS, SAS, and HPA responses to heterotypic stressors (Kvetnansky, 2004; Kvetnansky et al., 2009; Oka, 2018; Uschold-Schmidt et al., 2012). The doctrine of non-specificity does not account for this phenomenon. Clinical laboratory studies have led to the conclusion that specific emotions are associated with patterned ANS responses, with bi-directional influences of emotions on diseases and of diseases on emotional experiences (Levenson, 2019).
Dishabituation is observable even in primitive animals such as Aplysia and Hirudo and involves the neurotransmitter serotonin (Traina, 2020). Exaggerated responsiveness to a heterotypic stressor requires an ability to recognize that the stressor is novel. Most research on this topic has focused on the involved neurochemical systems including catecholaminergic systems rather than on the central neuroanatomical pathways (Dronjak et al., 2004).
Cannon’s doctrine of non-specificity (the “Fight or Flight” response)
Walter B. Cannon was one of the first critics of Selye’s theory. Cannon’s critique centered on the assumption that a stereotyped response pattern would be adaptive regardless of the character of the stressor. Since a non-specific stress response would not have provided a natural selective advantage, a stereotyped stress response would not have evolved.
This criticism was ironic, because Cannon himself taught that the sympathetic nervous system and adrenal gland act together act as a functional unit, the “sympathico-adrenal” system. The brain was taken to respond to all emergencies the same way, by evoking increased secretion of adrenaline. How could the same response help maintain homeostasis in very different situations? Surely some effects would work against rather than toward homeostasis for at least some body functions at least some of the time. Cannon’s answer was that the body’s response to emergencies, with adrenaline dominating that response, enhance long-term survival, even if in the short-term aspects of the response moved some of the levels for key variables from the ideal values.
This view is still widely held, although by now there is abundant evidence that the neuronal component, the SNS, and the hormonal component, the SAS, are constitutively active, responsive to activities of daily life, (Lake et al., 1976), and can be activated separately (Goldstein et al., 2001; Goldstein et al., 2008).
Cannon introduced the notion of “fight or flight” (Cannon, 1919). By this term he meant that a rather stereotyped pattern of activation prepares the organism for extreme exertion in emergencies posed by antagonistic encounters. The state of knowledge at the time did not allow for the possibility of different patterns of physiological and biochemical responses that would be coupled to the behavioral and emotional experiences. Experimentally, active avoidance behavior is associated with greater noradrenergic activation and passive freezing behavior with greater adrenergic activation (De Boer et al., 1990). Central neural mechanisms underlying differential responses of the SNS and AHS are poorly understood.
Aggression in rodents has a circadian rhythm. In male mice a daily rhythm in aggression propensity is gated by GABAergic subparaventricular zone neurons, the main post-synaptic targets of the central circadian clock in the suprachiasmatic nucleus (SCN) (Todd et al., 2018). A relay from the SCN to the ventromedial hypothalamus might drive attack behavior and to the dorsomedial hypothalamus fight or flight responses. It has been proposed that abnormal circadian control of aggression may explain “sundowning” in elderly hospitalized patients and those with Alzheimer’s disease, but how this would happen has not been explored.
The central stress system
In the 1990s George Chrousos and Philip Gold at the NIH proposed the existence of a central stress system, activation of which would elicit a “stress syndrome,” in line with Selye’s conceptualization (Chrousos et al., 1992; Sternberg et al., 1992). Key elements of the stress system are the paraventricular nucleus (PVN) of the hypothalamus, from which CRH is derived; and the locus ceruleus (LC) of the pons, from which NE in most of the brain is derived (Figure 1, left panel). CRH drives pituitary release of ACTH in the HPA axis. Arginine vasopressin (AVP) is another neuroendocrine factor derived from the PVN.
Figure 1: Early and current conceptualizations of the central stress system.
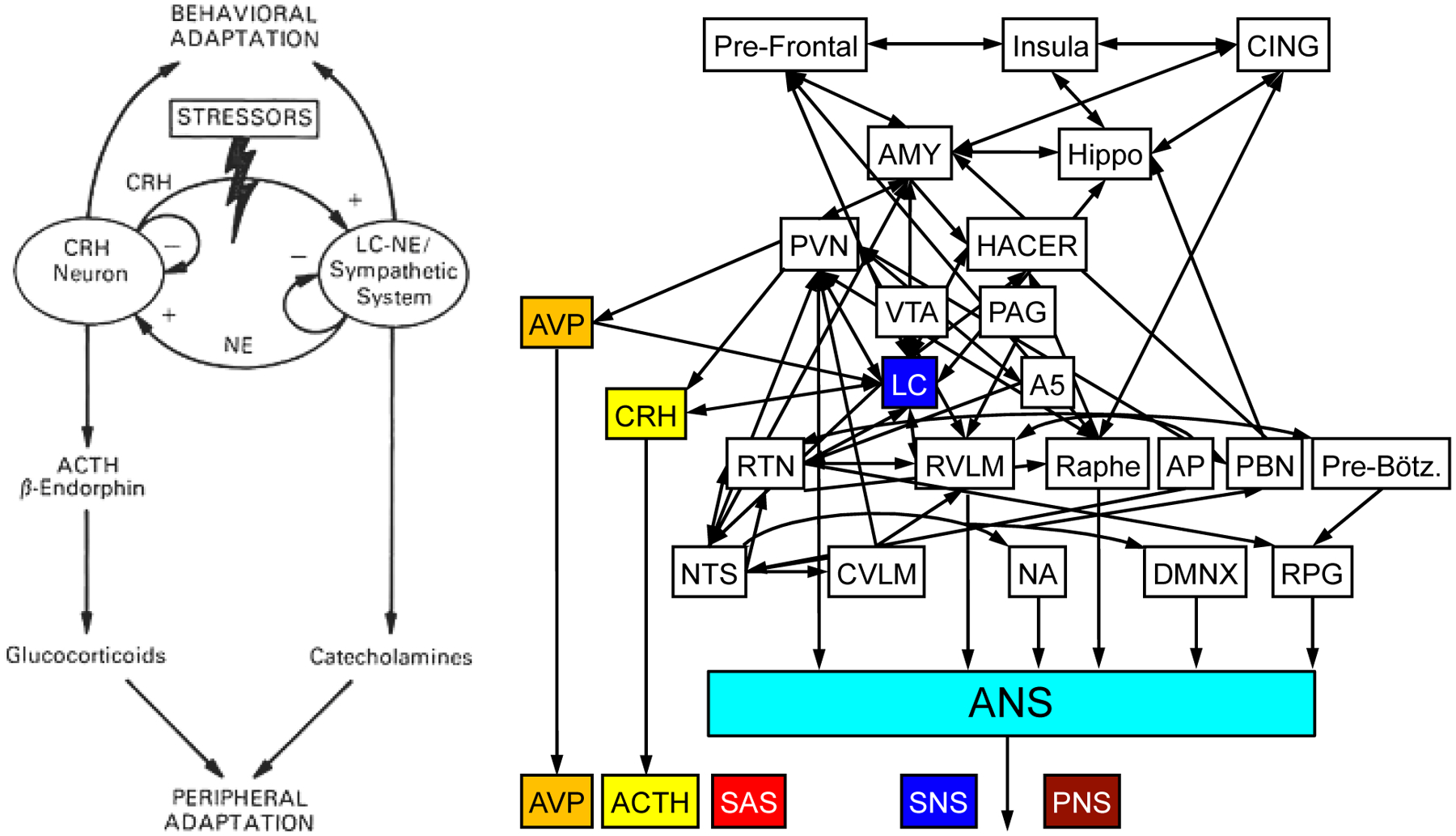
The diagram on the left depicts the original conception, in which corticotropin-releasing hormone (CRH) in the PVN and norepinephrine (NE) in the locus ceruleus (LC) are the major effector chemicals (reproduced from (Sternberg et al., 1992) with permission of the American College of Physicians). The diagram on the right shows the central system stress embedded in the central autonomic network. Arginine vasopressin (AVP) emanates from the PVN. Adrenocorticotropin (ACTH) is the anterior pituitary hormone released by CRH. Across stressors, SAS responses are closely tied to ACTH and AVP responses.
According to the concept of the extended autonomic system (EAS), discussed in the next section, the central stress system is embedded within the CAN (Benarroch, 1993) (Figures 2 and 3).
Figure 2: Overview of the Extended Autonomic System (EAS).
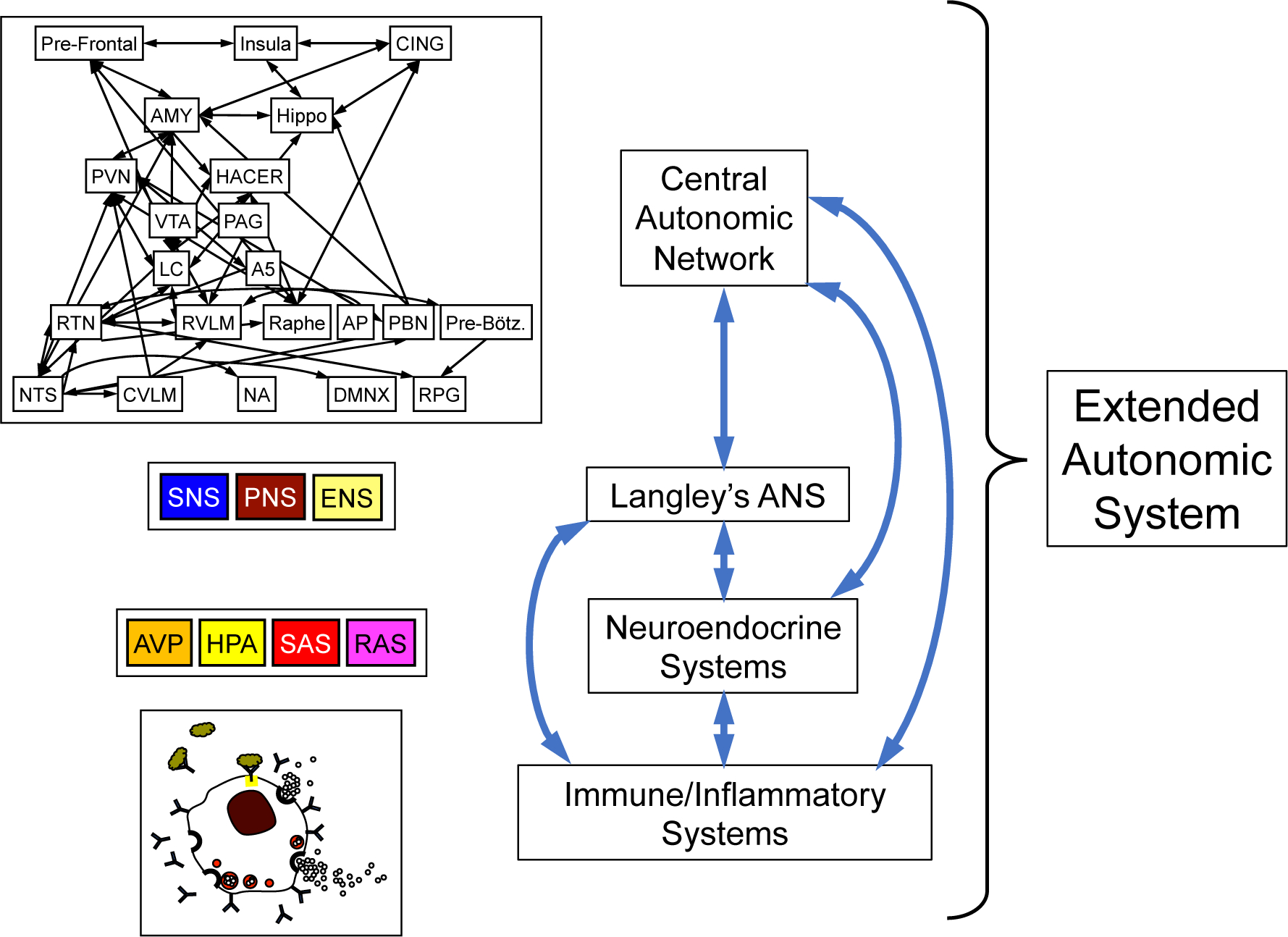
The EAS is conceptualized to consist of the central autonomic network (CAN); Langley’s autonomic nervous system (ANS), with its three component sub-systems the sympathetic nervous system (SNS), parasympathetic nervous system (PNS), and enteric nervous system (ENS); neuroendocrine systems including the arginine vasopressin (AVP) system, hypothalamic-pituitary-adrenocortical (HPA) system, sympathetic adrenergic system (SAS), and renin-angiotensin-aldosterone system (RAS); and immune/inflammatory systems, represented by a stylized mast cell. Not shown here, Langley’s SNS involves three chemical messengers, norepinephrine (sympathetic noradrenergic system, abbreviated as SNS in this review), acetylcholine (sympathetic cholinergic system), and epinephrine (SAS). The 4 components of the EAS are complexly inter-related (blue bi-directional arrows).
Figure 3: The Central Autonomic Network (CAN).
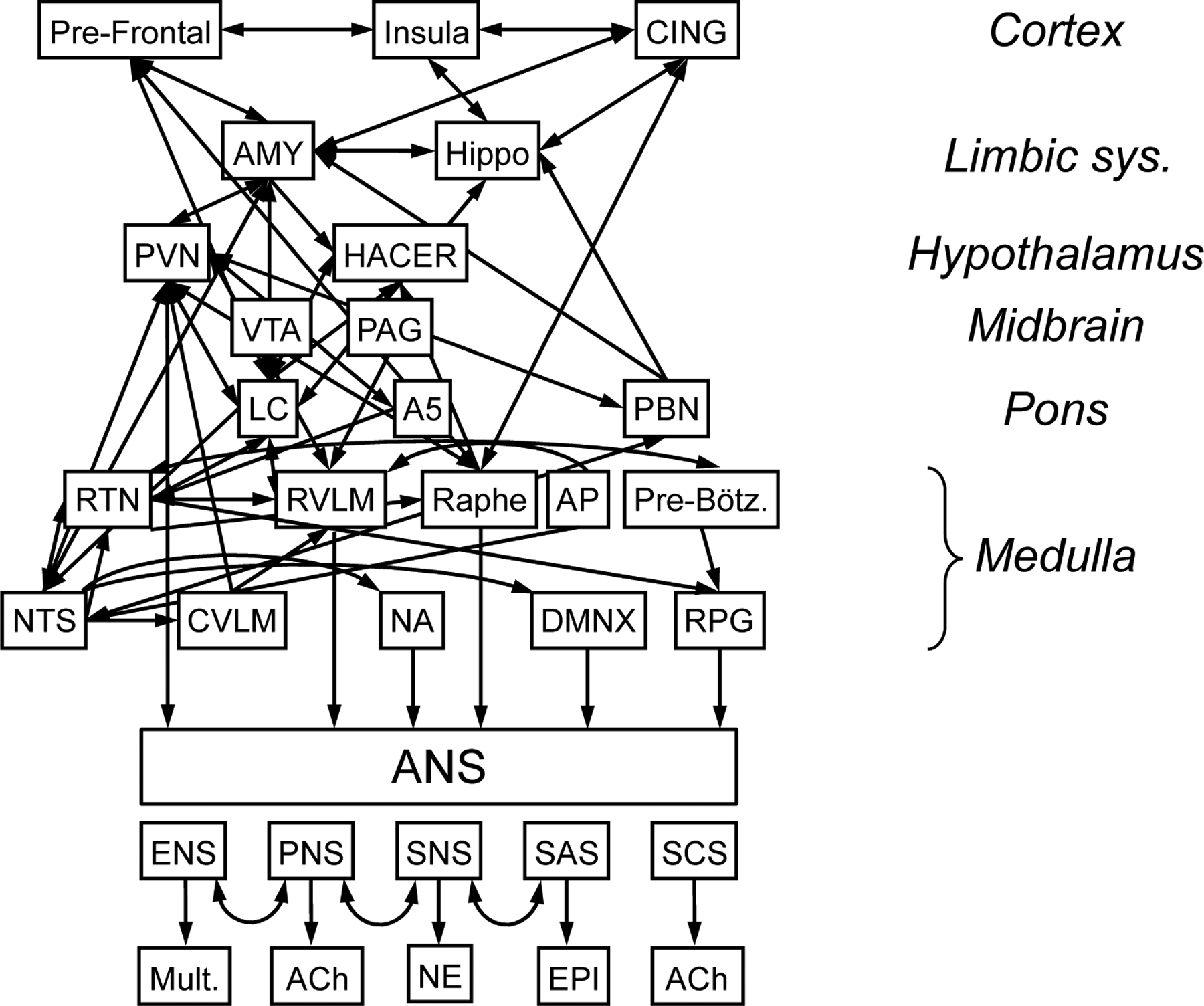
The CAN involves all levels of the central neuraxis. At the cortical level the CAN includes the medial prefrontal cortex (PFC), anterior cingulate (CING), and insula (INS). Limbic system sites include the amygdala (AMY) and hippocampus (Hippo). Hypothalamic regions include the paraventricular nucleus (PVN) and the hypothalamic area controlling emotional responses (HACER). Midbrain components include the peri-aquaductal gray (PAG) and ventral tegmental area (VTA). Pontine components are the locus ceruleus (LC), parabrachial nucleus (PBN), and A5 noradrenergic cell groups. Among several medullary nuclei in the CAN there are the raphe serotonergic cell groups (Raphe), C1 noradrenergic neurons and respiration-related neurons (respiratory pattern generator (RPG), pre-Bötzinger (Pre-Bötz) complex, retrotrapezoid nucleus (RTN) in the rostral ventrolateral medulla (RVLM), A1 noradrenergic neurons in the caudal ventrolateral medulla (CVLM), the nucleus ambiguus (NA) the dorsal motor nucleus of the vagus nerve (DMNX), and the nucleus of the solitary tract (NTS), which is the main site of initial synapse formation in the baroreceptor and chemoreceptor reflexes. Other regions associated with the CAN include the circumventricular regions with an imperfect blood-brain barrier (e.g., the area postrema (AP) in the dorsal medulla). Five components of the autonomic nervous system are the enteric nervous system (ENS), which involves multiple neurotransmitter (Mult.), the parasympathetic nervous system (PNS) and sympathetic cholinergic system (SCS), which use acetylcholine (ACh) as the neurotransmitter, the sympathetic noradrenergic system (SNS), with norepinephrine (NE) the neurotransmitter, and the sympathetic adrenergic system (SAS), with epinephrine (EPI) the hormone. Neuronal clusters in regions of the CAN interact complexly, often in a bi-directional manner.
A recent recapitulation continues these concepts (Tsigos et al., 2020), with key components of the stress system being the HPA axis and autonomic nervous system (ANS), which interact with other vital centers in the brain and periphery to mobilize a successful adaptive response against imposed stressors. The stress system concept continues the tradition begun by Selye, that dysregulation of the stress system (hyper- or hypo-activation) disrupts homeostasis leading to a state of “cacostasis” or allostasis (Chrousos, 2009), resulting in a variety of chronic clinical disorders.
Unlike other autonomic effectors, the parasympathetic nervous system (PNS) is active in situations that are not distressing and tend to build up rather than use up energy (Goldstein, 2006). When the central stress system is activated, PNS outflows generally decrease. Manifestations of PNS inhibition include tachycardia, decreased gastrointestinal motility, decreased production of saliva and tears, and decreased urinary bladder tone.
THE “EXTENDED” AUTONOMIC SYSTEM
The ANS as originally conceptualized by Langley about 120 years ago had three components—the sympathetic nervous system, the PNS, and the enteric nervous system (ENS) (Langley, 1903). In the more than a century that has gone by since then, many discoveries have come to light that justify extending on Langley’s ANS. Three such extensions are neuroendocrinology, inflammation/immunity, and the central autonomic network (CAN) (Figure 2).
Neuroendocrine Extension
The Sympathetic Adrenergic System (SAS)
Cannon added a neuroendocrine component to the ANS when he discovered that adrenaline (synonymous with epinephrine, EPI) is the chemical messenger secreted into the bloodstream during emotional stress (Cannon et al., 1911). One may reasonably claim that EPI was the first identified hormone (Rao, 2019). Cannon viewed the sympathetic nervous system and the adrenal gland to function as a unit, the “sympathico-adrenal system,” which is crucial for maintaining homeostasis (a term he coined) in emergencies.
EPI is a remarkably potent hormone. Probably because of this potency, circulating concentrations of EPI in resting humans are extremely low—in the range of about 100 pM (Kagedal et al., 1988).
EPI exerts numerous bodily effects. Via beta-2 adrenoceptors on vascular smooth muscle cells there is a fall in skeletal muscle vascular resistance, which tends to shift the cardiac output toward skeletal muscle. Vasoconstriction in the splanchnic bed and kidneys decreases gastrointestinal and renal perfusion. Cutaneous vasoconstriction likely results in the pallor that characterizes people during distress. Blood flows to vital organs (the heart, brain, and lungs) generally are preserved. EPI increases cardiac rate and contractility (Mezzacappa et al., 1999), increasing cardiac output. EPI increases glucose levels (Darbar et al., 1996) by multiple mechanisms, including anti-insulin effects, breakdown of hepatic glycogen, stimulation of hepatic gluconeogenesis (Sherwin et al., 1984), and stimulation of pancreatic secretion of glucagon (Hamilton et al., 2018). By stimulation of beta-2 adrenoceptors on bronchiolar smooth muscle cells, EPI produces a well known bronchodilator effect. EPI increases hepatic breakdown of lipids to free fatty acids, generating heat, and also evokes thermogenesis via uncoupling protein-1 in brown adipose tissue (Thomas et al., 1997). EPI activates platelets (Mills et al., 1967), via occupation of alpha-2 adrenoceptors (Larsson et al., 1992a; Larsson et al., 1992b). EPI increases sweating (Ogawa, 1976). The combination of cutaneous vasoconstriction, causing skin temperature to decrease, and adrenergic sweating explains the “cold sweat” characterizing people in shock. Finally, EPI intensifies the negative emotional experience of fear (Schachter et al., 1962), probably via afferent information to the brain from physiological changes exerted by occupation of beta-adrenoceptors, although altered vagal afferent traffic (Chen et al., 2012) and delivery to brain regions with a deficient blood-brain barrier (Weil-Malherbe et al., 1959) are other possibilities.
Unlike the SAS, in which plasma EPI levels are infinitesimally low in healthy people during supine rest, the sympathetic noradrenergic system (abbreviated as SNS in this review) is tonically active (Lake et al., 1976). The SNS plays key roles in patterned alterations in the distribution of the cardiac output among the vascular beds during activities of daily life such as standing up (Lake et al., 1976), eating a meal, mild exercise (Seals et al., 1988), the Valsalva maneuver, and adjustments to altered environmental temperatures. In addition to the cardiovascular system, SNS outflows to the irises, sweat glands, gastrointestinal tract, pancreas, and kidneys play important “housekeeping” roles. Such activities are unconscious, automatic, involuntary processes that are tonically active and phasically modulated.
It is important to keep in mind that NE is a neurotransmitter, not a hormone. Increases in renal sympathetic outflow promote sodium reabsorption by proximal tubular cells (Gill, 1979). Both NE and EPI increase cellular uptake of potassium and therefore tend to decrease serum potassium levels (Clausen, 1983).
EPI might evoke psychological effects via afferent regulation of the locus coeruleus (Aston-Jones et al., 1991). If so, regulation via EPI as a hormone is indirect because of the efficient blood-brain barrier for circulating epinephrine (Weil-Malherbe et al., 1959). EPI is synthesized within the brain, such as in C1 neurons of the RVLM that express phenylethanolamine-N-methyltransferase; however, whether EPI actually is a neurotransmitter in the brain has not been demonstrated (Sved et al., 1985).
The Hypothalamic-Pituitary-Adrenocortical (HPA) System
Concepts about functions of the HPA system have evolved over centuries from the discovery of the pituitary gland by Vesalius and of the adrenal gland by Eustachio in the 1600s. Miller has reviewed the history of the field (Miller, 2018). The writings of Selye (Selye, 1950; Selye, 1956), McEwen (McEwen et al., 2002), and Sapolsky (Sapolsky, 1998) on mechanisms and effects of chronic stress have emphasized the HPA axis.
Stress-related adrenocortical hormones produce numerous bodily effects. Intravenous administration of glucocorticoids can evoke abdominal pain, nausea, and vomiting, disturbed sleep, and neurobehavioral changes such as confusion, irritability, and restlessness (Jongen et al., 2016). Chronically increased glucocorticoid levels, as in Cushing’s syndrome from adrenocortical hyperplasia or a pituitary tumor (Cushing’s disease), produce truncal obesity, easy bruising, abdominal striae, hyperglycemia, hypokalemia, hypertension, osteoporosis, depressed mood, and susceptibility to non-bacterial infections and viral reactivation.
Corticotropin-releasing hormone (CRH) is expressed in cell bodies of limbic regions of the brain including the bed nucleus of the stria terminalis (BNST) and the central nucleus of the amygdala, as well as in the hypothalamic PVN. CRH derived from the PVN drives corticotropin (ACTH) release from the anterior pituitary gland. CRH, urocortins (UCNs), and CRH receptors (in particular CRFR1) have been viewed as the major effector system for responses to aversive stimuli (Deussing et al., 2018; Yuan et al., 2019). CRH projections to the ventral tegmental area (VTA), the source of the mesolimbocortical dopaminergic system, are derived from the basal nucleus of the stria terminalis (BNST), central nucleus of the amygdala, and PVN (Rodaros et al., 2007).
Although it is established that CRH/CRHR1 signaling mediates aversive responses, including anxiety and depression-like behaviors, recent studies have revealed anxiolytic and appetitive properties of specific CRH/CRHR1 circuits (Dedic et al., 2018). The detailed pathways and molecular mechanisms by which the CRH/urocortin-system translates negative or positive stimuli into integrated biological responses are incompletely understood.
The Renin-Angiotensin-Aldosterone System (RAS)
The renin-angiotensin-aldosterone system (RAS) plays a dominant role in the maintenance of sodium balance, blood volume, and vascular tone.
Dietary sodium restriction potently stimulates RAS activity. Specialized tubule cells of the macula densa monitor the concentration of sodium in the glomerular filtrate. When the amount of sodium falls below a certain level, macula densa cells send a message to nearby juxtaglomerular cells in the walls of the afferent arterioles. The juxtaglomerular cells release into the bloodstream the first effector chemical of the RAS, renin. The juxtaglomerular cells act as sensors themselves. They detect stretch, and therefore the distending pressure, in the blood vessels to the kidneys. A fall in the distending pressure also leads to release of renin.
Renin accelerates the conversion of a protein, angiotensinogen, to a peptide, angiotensin I. Angiotensin I has no known physiological action; however, angiotensin-converting enzyme (ACE) converts angiotensin I to angiotensin II (AII), which is a potent vasoconstrictor. AII stimulates the adrenal cortex to release aldosterone. Aldosterone, the main sodium-retaining hormone of the body, increases reabsorption of sodium from renal tubular cells.
One can conceptualize the RAS as an effector in regulation of both blood pressure and blood volume. Activation of the RAS tends to increase the blood pressure, via the vasoconstrictor effect of AII, and the blood volume, via the sodium-retaining effect of aldosterone.
Circulating AII can reach the brain at sites where the blood-brain barrier is deficient, such as the area postrema (AP) in the dorsal medulla. In addition, there is a brain RAS in which AII is generated via ACE, ACE2 is expressed, and occupation of AT1 receptors within the CAN increases HPA and SNS outflows (Mohammed et al., 2020).
Catecholamine systems of the body interact with the RAS in several ways. First, stimulation of both the SNS and SAS increases renin secretion (Gordon et al., 1967). Second, AII acts in the brain to increase SNS outflows. Third, there are abundant AII receptors in the adrenal medulla, and AII can evoke release of adrenaline directly (Zimlichman et al., 1987). Meanwhile, EPI increases renin secretion. Fourth, dopamine (DA) attenuates the amount of aldosterone secretion from the adrenal cortex in response to AII. Angiotensin II is a potent vasoconstrictor and in concert with EPI would be expected to augment splanchnic and renal vascular resistance.
Aldosterone, the body’s main mineralocorticoid, promotes sodium reabsorption and renal potassium loss. EPI decreases serum potassium and magnesium levels (Darbar et al., 1996; Nayyar et al., 2017), via augmentation of Na/K ATPases that mediate transmembrane cation influx (Baron et al., 1985). The fall in serum potassium may help to explain a dissociation of stimulated plasma renin activity and less clear effects on plasma aldosterone (Kruse et al., 1994).
The Arginine Vasopressin/Anti-Diuretic Hormone (AVP/ADH) System
Arginine vasopressin (AVP), acting as the anti-diuretic hormone (ADH), promotes renal retention of water. In acute illnesses this can manifest with decreased serum osmolality and hyponatremia (Friedman et al., 2013). AVP acting as a pressor contributes to vasoconstriction and blood pressure; however, the effects may be masked by other determinants of systemic vascular resistance. In addition, in the brain AVP shifts the arterial baroreflex to lower blood pressures, reducing the maximum amount of sympathetic activation for a given decrease in blood pressure (Hasser et al., 1997).
PACAP
Pituitary adenylate cyclase-activating polypeptide (PACAP) was first identified in hypothalamus, based on its ability to increase cyclic AMP generation in the anterior pituitary. PACAP also has been proposed to be a “master stress hormone,” especially with regard to SAS activation (Eiden et al., 2018). Acetylcholine and PACAP acting as neurotransmitters at adrenomedullary chromaffin cells release EPI for given rates of splanchnic sympathetic nerve firing. PACAP is also expressed at several brain sites in the CAN such as the PVN, amygdala, and prefrontal cortex.
Modern neuroendocrinology refers rather specifically to peptides secreted by hypothalamic neurons into the circulation. Even in this restricted sense the neuroendocrine and autonomic systems clearly interact. For instance, thyroidectomy, which increases thyrotropin secretion, also increases plasma levels of the sympathetic neurotransmitter norepinephrine (NE) (Fukuhara et al., 1996). Infusion of the beta-adrenoceptor agonist isoproterenol into humans decreases plasma levels of ACTH and EPI (Eisenhofer et al., 1987). Hypopituitarism and adrenocortical failure decrease plasma EPI and increase plasma NE (Rudman et al., 1981; Sverrisdottir et al., 1998). AVP inhibits sympathetic noradrenergic responses to hemorrhage (Hasser et al., 1997).
Inflammatory/Immune Extension
The ANS and inflammatory/immune system affect each other. Immune cells express neurotransmitter receptors, and neurons express cytokine receptors. Additionally, immune cells can synthesize and release neurotransmitters such as catecholamines themselves, probably via autocrine-paracrine mechanisms. Natural killer cells are innate lymphocytes that are important for early and effective immune reactions. Their activities are regulated by all the major effector chemicals of the EAS, including glucocorticoids, EPI, serotonin, and DA (Capellino et al., 2020).
Whereas the roles of afferents (e.g., via vagal and somatic sensory nerves) and ANS and HPA efferents in inflammatory reflexes have been studied intensively, central pathways mediating inflammatory reflexes remain poorly understood.
Catecholamines and immunity
It has been proposed that overactivity of myocardial beta-adrenoceptors by SNS activation exerts pro-inflammatory effects and thereby contributes to the pathogenesis of atherosclerotic cardiovascular disease (Karakas et al., 2018). This concept does not take into account that there are three types of peripheral catecholamine system—the SNS, where NE is the locally acting neurotransmitter, the SAS, where EPI is the hormone, and the DOPA-DA autocrine-paracrine system (Goldstein et al., 1995), in which DA is produced in, released from, and acts locally on parenchymal cells that take up DOPA from the circulation and decarboxylate the amino acid to form the catecholamine. The latter mechanism is by definition an activity of “APUD” cells (APUD standing for amine precursor uptake and decarboxylation) that release polypeptides hormones in a diffuse neuroendocrine system (Modlin et al., 2006).
In primary human monocytes, alpha-1 adrenoceptor stimulation by phenylephrine has been reported to suppress the NLRP3 inflammasome (Horstmann et al., 2016); however, the same drug has been reported to induce cardiac dysfunction and inflammation in vivo as evidenced by increased expression of IL-6 and NLRP3 (Xin et al., 2020).
Across a variety of stressful situations increases in EPI levels are associated with elevations of the pro-inflammatory cytokine IL-6 (Danobeitia et al., 2012; Hashizaki et al., 2018; Jan et al., 2009; Koelsch et al., 2016; Kulp et al., 2010; Papanicolaou et al., 1996; Piira et al., 2013); however, bases for this relationship have not been systemically studied.
It has been proposed that during restraint stress, activation of catecholaminergic and glutamatergic C1 neurons in the RVLM stimulates a splenic anti-inflammatory pathway, which may protect tissues against ischemic injury (Stornetta et al., 2018).
A program consisting of meditation, cyclic hyperventilation followed by breath retention, and cold exposure has been reported to increase plasma EPI levels substantially; and during subsequent endotoxin administration, the intervention group had more rapid increases in plasma levels of the anti-inflammatory cytokine IL-10, lower levels of pro-inflammatory cytokine TNFα, IL-6, and IL-8, and milder flu-like symptoms, suggesting that voluntary activation of the SAS suppresses in vivo innate immune responses in humans (Kox et al., 2014)..
Although most research on effects of catecholamines on immunity has focused on adrenoceptors on immune cells, vasoconstriction from SNS activation decreases the exit of B and T lymphocytes and leukocytes (Devi et al., 2021). This may explain elevated neutrophil counts in critical illness.
C1 neurons of the rostral ventrolateral medulla (RVLM) may be a key way-station in catecholamine-immune interactions. Experimental neural manipulations of C1 neurons mediate restraint stress-induced anti-inflammation (Abe et al., 2018).
HPA system and immunity
In interpreting the literature about the HPA system and immunity one must separate reports about effects of exogenously administered glucocorticoid from effects of alterations in endogenous glucocorticoid release resulting from increased HPA system activity. One might presume that in the “alarm phase” of stress (see the section on Stress as a Scientific Idea) high circulating levels of cortisol blunt inflammatory/immune functions. Instead, acute stress, via HPA activation, seems to if anything augment immune responses. Studies by Dhabhar, McEwen, and colleagues have suggested that acute stress exposure redistributes leukocytes from the blood to organs such as the skin and lymph nodes (Dhabhar et al., 1997). Based on pharmacological blockade experiments, the redistribution depends on actions at type II (glucocorticoid) adrenocortical hormone receptors (Dhabhar et al., 1996). Subsequently the investigators reported that rats subjected to immobilization have rapid mobilization of neutrophils, lymphocytes, helper T cells, cytolytic T cells, and B cells into the blood, followed by a decreased trafficking of all cell types out of the blood, with the exception of neutrophils, numbers of which continue to increase (Dhabhar et al., 2012). A limitation in the latter experiments was that trunk blood samples were assayed, and even in the first sample plasma levels of NE and EPI were drastically increased. This means the immediate mobilization and subsequent decline could have reflected direct effects of high circulating catecholamine levels or indirect effects of local catecholamine-induced circulatory changes, especially in the skin. In general, over the course of 2 hours, acute restraint decreases lymphocyte and increases neutrophil counts (Dhabhar et al., 1996).
After infection, HPA system activation augments production and release of glucocorticoids; however, the multiplicity of effects of the hormones on functions of immune cells makes it difficult to delineate specific roles in vivo. Quatrini et al. (Quatrini et al., 2018) found that the regulation of natural killer cell function by the glucocorticoid receptor is required for surviving mouse cytomegalovirus infection. Endogenous glucocorticoids produced shortly after infection induce selective, tissue-specific expression of the checkpoint molecule PD-1 on natural killer cells. The glucocorticoid–PD-1 pathway limits the production of the cytokine interferon-γ by splenic natural killer cells, which in turn prevents lethal immunopathology—without compromising viral clearance. HPA axis activation in response to viral infection therefore preserves tissue integrity without impairing pathogen elimination.
The cytokine IL-6 not only activates the HPA axis but also directly stimulates production of aldosterone, cortisol, and androgenic steroids (Path et al., 1997). In conscious, unrestrained rats, TNFα administration increases plasma levels of glucagon, corticosterone, ACTH, NE, and 3,4-dihydroxyphenylglycol (DHPG, the main neuronal metabolite of NE) (Darling et al., 1989).
Pro-inflammatory cytokines increase expression of CRH and AVP in the hypothalamus (Chikanza et al., 2000), probably via vagal afferents (Gaykema et al., 1995). In critically ill patients, non-survivors have been reported to have higher ACTH levels in response to exogenously administered CRH and longer release of cortisol, associated with higher levels of IL-6 and IL-8 (Dimopoulou et al., 2007). In patients with sepsis or ARDS, high dose corticosteroid administration does not improve survival; however, low doses of corticosteroids alleviate inflammation and improve survival (Chadda et al., 2002).
A key example of neuroimmune-autonomic interactions is co-regulation by CRH of the HPA axis and the adrenal medulla. Corticosteroid synthesis by cultured adrenocortical cells is increased 10-fold by co-culture with adrenomedullary chromaffin cells (Haidan et al., 1998). Across a variety of stressors plasma EPI responses are more closely tied to ACTH responses than to NE responses (Goldstein et al., 2008); however, effects on immune cell populations of stressors that specifically stimulate SNS outflow (cold exposure) or SAS outflow (glucoprivation) do not seem to have been studied.
The vagus/inflammasome system
An example of autonomic-immune interactions is regulation of cytokines and the “inflammasome” by the vagus nerve (Koopman et al., 2016; Woody et al., 2017). In the cholinergic anti-inflammatory pathway (Tracey, 2007), efferent nerve traffic from the dorsal motor nucleus of the vagus nerve increases delivery of acetylcholine to α7 nicotinic receptor subunits on celiac-superior mesenteric post-ganglionic neurons that terminate in the spleen and act on splenic immune cells to decrease TNFα generation (Koopman et al., 2016; Lerman et al., 2016; Rosas-Ballina et al., 2008; Yi et al., 2016) A randomized, blinded, healthy control pilot trial of non-invasive transcutaneous vagal stimulation reported down-regulation of inflammatory cytokine release (Lerman et al., 2016).
The inflammasome concept is based on the NOD-, LRR- and pyrin domain-containing protein 3, or NLRP3 (Swanson et al., 2019). NLRP3 is conceptualized to be an intracellular sensor that can detect a wide variety of microbes, including RNA viruses. NLRP3 inflammasome formation leads to release of the pro-inflammatory cytokines IL-1beta and IL-18 and to cell death by pyroptosis. Pyroptosis is a form of programmed cell death that may remove intracellular viral replication niches in the tissue. The sulfonylurea oral hypoglycemic drug glyburide is a NLRP3 inhibitor (Voet et al., 2019); however, effective doses may be high enough to produce cardiovascular side effects.
Dopamine
DA, via the type-1 DA receptor-1 (DRD1) and cyclic AMP signaling inhibits the NLRP3 inflammasome (Yan et al., 2015). This introduces the possibility of modulating cytokine “storm” by a DRD1 agonist. Fenoldopam is a catechol-containing DRD1 partial agonist that does not cross the blood-brain barrier. The drug also exerts vasodilator effects in coronary, renal, mesenteric, and peripheral arteries and is used intravenously clinically as an anti-hypertensive agent.
The sources of endogenous DA outside the brain are relatively poorly understood. In the kidneys DA is formed from uptake and decarboxylation of circulating 3,4-dihydroxyphenylalanine (DOPA) (Wolfovitz et al., 1993) by proximal tubular cells and acts as an autocrine-paracrine substance (Goldstein et al., 1995) that promotes natriuresis (Baines, 1990). In patients with decompensated congestive heart failure, levodopa treatment increases urinary sodium excretion (Grossman et al., 1999). Whether intravenously administered DOPA or DA affects the NLRP3 inflammasome is unknown.
The Central Autonomic Network
A hierarchy of brain regions collectively called the central autonomic network (CAN) (Benarroch, 1993) determines ANS outflows (Figure 3). The CAN involves all levels of the neuraxis. At the cortical level the CAN includes the medial prefrontal cortex, anterior cingulate, and insula. In addition, trans-neuronal tract tracing with pseudorabies virus has revealed that the motor cortex is connected multi-synaptically to the adrenal gland (Strack et al., 1989).
At the level of the limbic system there are the amygdala and hippocampus. Hypothalamic regions include the paraventricular nucleus (PVN), lateral hypothalamus, the dorsomedial hypothalamus (involved with stress-induced hypothermia (Oka, 2018)), and the hypothalamic area controlling emotional responses (HACER), which is also called the peri-fornical area (Smith et al., 1984). Midbrain components include the peri-aquaductal gray and ventral tegmental area (VTA), and pontine components the locus ceruleus (LC, the main source of norepinephrine in the brain), the parabrachial nucleus (PBN), and A5 noradrenergic cell groups.
There are several medullary nuclei in the CAN, including the raphe serotonergic cell groups, C1 adrenergic neurons and respiration-related neurons (respiratory pattern generator (RPG), pre-Botzinger complex, retrotrapezoid nucleus) in the rostral ventrolateral medulla (RVLM), A1 noradrenergic neurons in the caudal ventrolateral medulla (CVLM), the ventromedial medulla (VMM), the nucleus ambiguus (NA) the dorsal motor nucleus of the vagus nerve (DMNX), and the nucleus of the solitary tract (NTS), which is the main site of initial synapse formation in the baroreceptor and chemoreceptor reflexes.
Other regions associated with the CAN include the circumventricular regions with an imperfect blood-brain barrier (e.g., the area postrema (AP) of the dorsal medulla, subfornical organ, organum vasculosum of the lamina terminalis (OVLT), and median eminence.
Signals from interoceptors related to blood volume and pressure connect with many brain sites in the CAN. Structures along the lamina terminalis include the subfornical organ (SFO), median preoptic nucleus (MnPO), organum vasculosum of the lamina terminalis (OVLT), and PVN. The SFO is the primary forebrain target for angiotensin II (AII), while cells in the OVLT function as osmoreceptors. The MnPO, which lies inside the blood–brain barrier, receives input from both the SFO and the OVLT and probably processes information about the status of intracellular and extracellular fluid compartments and blood pressure.
SFO, MnPO and OVLT provide input to the PVN, which directly and indirectly (e.g., via the RVLM) connects to preganglionic sympathetic neurons. The area postrema (AP), caudal ventrolateral medulla (CVLM), nucleus of the solitary (NTS), and parabrachial nucleus (PBN) all also directly or indirectly influence activity in the RVLM. A conceptualization of the “stress system” has adopted more of a mind-body approach (Charmandari et al., 2005). According to this view, the stress response is subserved by components located both in the brain and periphery. The principal effectors are CRH, AVP, the proopiomelanocortin-derived peptides alpha-melanocyte-stimulating hormone and beta-endorphin, glucocorticoids, and the catecholamines NE and EPI.
Ding et al. recently used resting-state brain functional magnetic resonance imaging to assess CAN functional connectivity and baroreflex measures at rest (Ding et al., 2020). Functional connectivity between the left amygdala and left medial frontal gyrus, bilateral post-central gyri, and bilateral paracentral lobules was found to be associated with baroreflex-cardiovagal gain and with low-frequency power of heart rate variability, the latter thought to be a measure of the ability to modulate cardiac autonomic outflows via baroreflexes (Goldstein et al., 2011).
The definition of structures subsumed under the heading of CAN is somewhat arbitrary. For instance, the motor cortex has not been considered to be a component of the CAN, despite multi-synaptic anatomic links to the adrenal gland (Strack et al., 1989) (Recall the etymology of the term, “emotion,” from the French émouvoir, “to move emotionally,” and the antecedent Latin emovere, “to move.”) Neither has the mesolimbocortical system, an intensively studied catecholaminergic system involved with reward-related motor function learning; and the nigrostriatal dopaminergic system, which is crucial for locomotion. It should be noted that anatomic organization and neurotransmitter pathways in the brain are far from congruent.
In conclusion, the EAS concept provides a framework for the complex, integrative, interacting, dynamic nature of the ANS, neuroendocrine systems, and inflammatory/immune systems in daily life, numerous stressful situations, and several distress-related clinical conditions. The reciprocal inter-relationships of the SAS, HPA axis, and other neuroendocrine systems with inflammatory/immune systems is especially complex and ripe for future applications of systems biologic approaches.
HOMEOSTASIS AND THE NOTION OF PURPOSE
Homeostasis is a founding principle of integrative physiology. In systems biology, however, homeostasis is almost invisible. In integrative physiology homeostasis is a key goal that drives body processes. In systems biology homeostasis is a result that emerges from continual adjustments in the operations of complex networks (Goldstein, 2019).
In the 1800s the French physiologist Claude Bernard introduced the idea of the internal environment, the milieu intérieur. In the face of the vicissitudes of the external environment, organisms maintain a constant fluid environment that bathes cells of the body. Even more meaningful, he proposed a purpose for body processes: “The constancy of the internal environment is the condition for free and independent life…All the vital mechanisms, however varied they might be, always have one purpose, that of maintaining the integrity of the conditions of life within the internal environment” (Bernard, 1974).
In the 1900s Walter B. Cannon took up Bernard’s theme. Cannon wrote, “My first article of belief is based on the observation, almost universally confirmed in present knowledge, that what happens in our bodies is directed toward a useful end.” (Cannon, 1945) His Bodily Changes in Pain, Hunger, Fear and Rage, includes the statement, “It has long been recognized that the most characteristic feature of reflexes is their “purposive” nature, or their utility either in preserving the welfare of the organism or in safeguarding it against injury (Cannon, 1919).
The common denominator in Bernard’s and Cannon’s concepts is the notion of purpose. Bernard’s assertion about the purpose of body processes and Cannon’s about the “useful end” are teleological. Teleology refers to a purpose or goal as the reason for something. To a systems biologist, teleological explanations are unnecessary. Physiological phenomena do not occur because they have the purpose of maintaining homeostasis. Instead, homeostasis is an emergent consequence.
Different writers refer to homeostasis of the entire organism, in line with Cannon, or the maintenance of levels of particular physiological variables or biochemical compounds within bounds, while levels of others can vary—e.g., blood pressure homeostasis, sodium homeostasis, glucose homeostasis, and temperature homeostasis.
The evolutionary biologist and philosopher Ernst Mayr proposed the existence of “teleonomic” forms of goal-directed processes that depend on the operation of a program, “coded or prearranged information that controls a process (or behavior) leading it toward a goal. The program contains not only the blueprint of the goal but also the instructions of how to use the information of the blueprint. A program is not a description of a given situation but a set of instructions persisting toward an end point under varying conditions, where the end state of the process is determined by its properties at the beginning” (Mayr, 1992). Mayr emphasizes that “the goal of a teleonomic activity does not lie in the future, but is coded in the program” (108).
McEwen and Wingfield (McEwen et al., 2010) and McEwen and Lasley (McEwen et al., 2002) have offered examples of what seem to be teleonomic processes.
…a cow that begins lactation undergoes morphological, physiological and behavioral changes so it can raise a calf. None of this is essential for the maintenance of homeostasis of the cow, although homeostatic set points will have changed… Another example is the migration of a songbird from Mexico to Alaska in spring and back again in autumn. Here again, there are major changes in morphology, physiology and behavior that allow this animal to complete a journey of almost 5000 km in less than a month. But none of this is essential for maintenance of homeostasis. In both examples, the process of preparing for lactation or migration involves regulation of gene expression
McEwen and Lasley especially emphasize the role of cortisol in one of the more spectacular examples of a teleonomic process—that determining the migration, spawning, and death of salmon.
The whole reason for the migration is to produce the next generation of salmon, so in the cortisol-soaked salmon, unlike other chronically stressed animals, reproduction is not a luxury system to be put on hold. Far from it; the sex glands continue to mature and the sex hormones skyrocket. In fact, biologists consider death by cortisol to be programmed into the salmon
Homeostats
Hierarchical networks involving input-output relationships continuously orchestrate and learn adaptive patterns of observable behaviors, cognition, memory, mood, and autonomic systems. Taken together, these networks determine levels of internal variables and act as if there were homeostatic comparators–i.e., “homeostats” (Goldstein, 2019).
Homeostats are unnecessary, simplistic, and teleological, so what good are they?
First, lumping the complex networks that make up the metaphorical regulators and conceptualizing purposes in controlling the regulated variables enable definitions of otherwise difficult ideas—such as stress. According to the homeostatic theory (Goldstein, 1995a), in stress a homeostat senses a discrepancy between afferent information about the regulated variable and the setpoint for arousing a response. Stress is then the condition, and the error signal is the measure of the extent of stress.
The integrated error signal could correspond to a kind of “memory” that would more be more efficient than the instantaneous error signal in returning the regulated variable to the setpoint value (Goldstein, 2008). Reference to the analogy of a modern home heating system conveys the idea about where memory may fit in here (Goldstein, 2013). With “proportionate control” the response of the furnace to a decrease in room temperature is proportionate to the magnitude of the error signal. The level of the monitored variable reaches a steady state, but unless the system has infinite gain the steady-state level never completely reaches the thermostatic setting. With “integrated control” added to proportionate control, not only does the magnitude of the error signal itself drive the effector but so does the integral of the error signal. That is, the thermostat responds not only to the error signal but also to how the error signal has accumulated over time.
Second, the homeostat theory lends itself straightforwardly to computer models that define homeostatic resetting, compensatory activation of alternative effectors, effector sharing, allostatic load, and induction of pathophysiologic positive feedback loops (Goldstein, 2006; Goldstein, 2008; Goldstein, 2013).
Third, the homeostat idea helps explain clinical phenomena in autonomic medicine. For instance, a patient had “complex” sleep apnea, meaning that his condition worsened rather than improved with continuous positive airway pressure. He brought with him and used during his hospitalization a modified continuous positive airway pressure device that administered CO2 via the mask. Inhaling CO2 produces a metabolic acidosis. The patient’s “chemostat” sensed the hypercarbia and metabolic acidosis, and this released the sympathetic noradrenergic and adrenergic systems from restraint by the “barostat,” resulting in extreme hypertension and high plasma catecholamine levels (Goldstein, 2019).
Homeostats are metaphorical comparators that sense discrepancies between afferent information a set-point for responding, implying a goal optimal state. Hu et al. recently reported by studying a neural network model of the pre-frontal cortex that the optimal performance of working memory co-occurs with the critical dynamics at the network level and the excitatory/inhibitory balance at the level of individual neurons, suggesting the existence of a unified, multi-scale, optimal state modulated by DA (Hu et al., 2019).
Allostasis and Allostatic Load
Sterling and Eyre introduced the concept of allostasis (Sterling et al., 1988), from the Greek allos (“other”) and stasis (“standing, stoppage”). Whereas homeostasis refers to processes that maintains physiological and biochemical regulated variables within narrow operating ranges, in allostasis values for these variables are altered.
Schulkin and Sterling have emphasized that allostatic changes importantly reflect anticipatory, “feed-forward” regulation as opposed to reactive, negative feedback regulation (Schulkin et al., 2019; Sterling, 2020). Even in echinoderms, which lack a brain and spinal cord, evidence has been reported for immune responses not only in reaction to but also in anticipation of stressors. Remarkably, such animals produce cortisol, and cortisol levels increase in the hydrovascular fluid of the Polian vesicle in response to the scent of a predator (Hamel et al., 2021).
Cannon’s classic monograph, “Organization for Physiological Homeostasis” (Cannon, 1929), contained only one figure, which depicted opposing roles of the “sympathico-adrenal” system in raising and the “insular or vago-insular” system in lowering blood glucose. The actions of these opposing effectors would keep blood glucose within bounds (Figure 1A). Allostasis can be conceptualized in terms of homeostatic resetting manifested by shifts in input-output curves (Goldstein et al., 2002). The low-grade fever attending a flu-like illness is an example of allostasis. This seems to correspond to an acute alteration in the optimal state.
Allostatic adjustments use up more energy than do homeostatic adjustments, and the input-output curves generally become flatter, implying decreased homeostatic efficiencies. Allostatic states that are temporary are thought to be beneficial (McEwen et al., 2002). Once the individual recovers, the input-output curves revert to those before the acute illness, with no apparent harm done. Allostatic states, however, also increase wear and tear—allostatic load, defined as the cumulative biological burden on the body as a whole due to attempts to adapt to life’s demands (Seeman et al., 2001).
Quantitative indexes of allostatic load have been based on prediction of mortality and declines in cognitive and physical functioning (Seeman et al., 2001). Recently, the allostatic burden among adults in the United States was assessed across race/ethnicity, gender, and age groups over a 30-year time period, based on data from the National Health and Nutrition Examination Survey from 1988 through 2018. The allostatic load score was calculated from the sum total of abnormal measures for serum albumin, body mass index, serum C-reactive protein, serum creatinine, systolic and diastolic blood pressure, glycated hemoglobin, total cholesterol, and serum triglycerides. Among adults aged 18 or older, the prevalence of high allostatic load increased from 34% in 1988–1991 to 49% in 2015–2018. Adults aged 40 years old and older had over 2-fold higher risks of elevated allostatic load than did adults 18–29 years old.
Estimating the severity of allostatic load based on combining factors that are known to be associated with increased risk seems to entail an element of circularity.
CLINICAL ASPECTS OF STRESS AND THE EAS
Dyshomeostasis in the Elderly
That resilience declines with aging is part of humankind’s evolutionary heritage. In his The Wisdom of the Body Cannon devoted an entire chapter to this phenomenon. Cannon summarized already substantial literature that with aging the abilities to maintain body temperature, glucose, blood pH, and circulatory-respiratory delivery of oxygenated blood under baseline conditions are preserved, but for each of these vital functions there are decreased abilities to keep appropriate levels during stress—e.g., heat or cold exposure, glucose ingestion, and exercise.
During cooling by intravenous infusion of cold saline, older people 55–72 years old have larger decreases in core temperature and smaller increments in plasma NE levels, systemic vascular resistance, and heat generation than younger people 18–23 years old (Frank et al., 2000).
After taking a high carbohydrate diet, in young adults postprandial plasma EPI levels follow a biphasic diurnal pattern that is inversely related to glucose and insulin levels. Aging is associated with a dysregulation of this response (Penev et al., 2005). Insulin sensitivity declines with aging; body mass index and mean arterial blood pressure are independent predictors, although age and plasma NE levels are not (Supiano et al., 1993).
Plasma NE levels, NE responses to stress, skeletal muscle sympathetic outflow, and cardiac NE spillover all increase with aging (Davy et al., 1998; Esler et al., 1995; Palmer et al., 1978), probably from a combination of decreased neuronal reuptake of NE and increased sympathetic nerve traffic. For a given amount of reflexively increased sympathetic outflow there is a blunted vasoconstrictor response (Davy et al., 1998). A potential explanation for this is an aging-related decline in releasable NE stores in sympathetic noradrenergic nerves.
Responses of cardiac NE spillover to exercise are similar in elderly vs. young men, but this is due to an aging-related decline in neuronal reuptake of released NE, meaning that the increment in cardiac sympathetic outflow probably is blunted (Esler et al., 1995).
As people age the efficiency of immune responsiveness also generally declines. Depending on genetics, epigenetics, and life experiences “immune age” can be estimated and is correlated with all-cause mortality (Alpert et al., 2019).
The ability of catecholamines to break down triglycerides to free fatty acids decreases with aging. This may increase susceptibilities to exercise intolerance, decreased ability to maintain core body temperature during cold exposure, and reduced ability to survive starvation, as well as increase visceral adiposity and indolence. Unbiased whole-transcriptome analyses of adipose macrophages has revealed that aging upregulates the gene encoding monoamine oxidase-A (MAO-A) in an NLRP3 inflammasome-dependent manner, and MAO-A inhibition restores NE-induced lipolysis (Camell et al., 2017). It should be noted, however, that MAO-A plays only a small role in sympathetic neuroeffector function, and beneficial effects of MAO-A inhibition might reflect decreased formation of hydrogen peroxide and of the autotoxic catecholaldehydes 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde (Goldstein et al., 2014; Kang et al., 2020; Panneton et al., 2010).
Acute Illness
Virtually all acute, severe illnesses are associated with several physiological and biochemical changes that can be traced to alterations in activities of components of the EAS. These include hyperglycemia (Brealey et al., 2009; Ding et al., 2019; Gearhart et al., 2006; Halter et al., 1984; Ritsinger et al., 2019; Shahid et al., 2020), hypokalemia (Darbar et al., 1996; Nayyar et al., 2017), hyponatremia (Friedman et al., 2013; Riegger et al., 1982; Szatalowicz et al., 1981), hyperthermia (Oka, 2018)), hypertension, arrhythmias, baroreflex inhibition, platelet activation or intravascular thrombosis (Bentur et al., 2018; Golaszewska et al., 2021), and stress-related heart failure (e.g., takotsubo cardiomyopathy (Akashi et al., 2008)). Acute immunity-related changes include IL-6 generation (Herold et al., 2020; Qing et al., 2020). A concept for relating the EAS to pathophysiological mechanisms in the pandemic viral illness COVID-19 is presented in Figure 4.
Figure 4: From stress system activation to dyshomeostasis to death due to a viral illness (in particular, COVID-19).
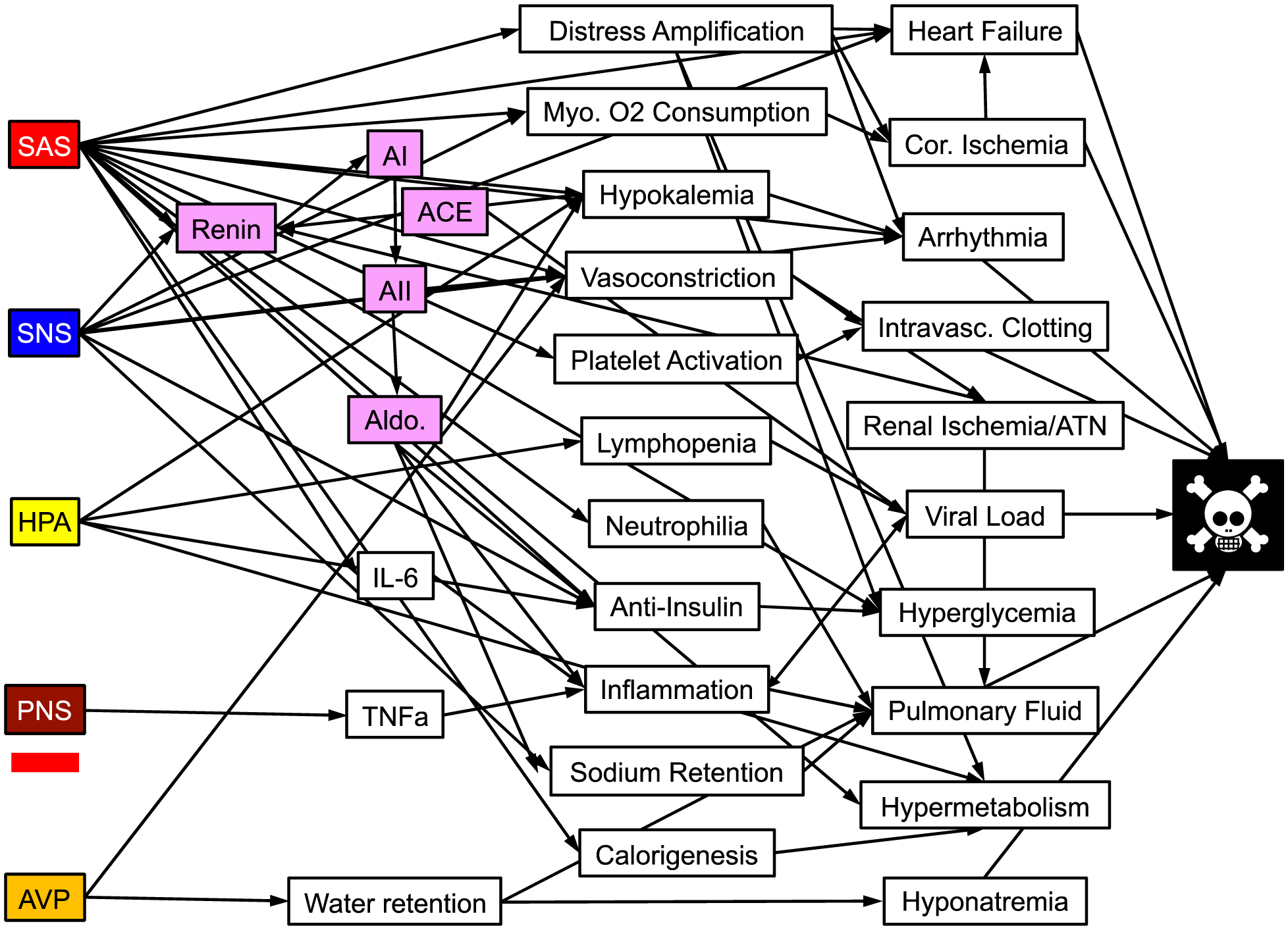
Five effectors of the central stress system are on the left. Intervening variables are in the center. Factors contributing the critical illness or death are on the right. The red bar under PNS indicates PNS inhibition. Other abbreviations besides those defined previously: AI = angiotensin I; ACE = angiotensin-converting enzyme; AII = angiotensin II; Aldo = aldosterone; Myo. = myocardial; Cor. = coronary; IL-6 = interleukein 6; TNFa = tumor necrosis factor alpha; ATN = acute tubular necrosis.
The neuroendocrine pattern accompanying fainting reactions illustrates an acute allostatic patterned response. In a distressing situation where an individual cannot fight and cannot flee, the brain can evoke a particular response characterized by SAS activation (Benditt et al., 2003; Kikushima et al., 1999; Takase et al., 2001) and SNS inhibition (Jardine et al., 2002; Mosqueda-Garcia et al., 1997) or attenuated NE release (Evans et al., 2001; Fritsch-Yelle et al., 1996; Vaddadi et al., 2011). We call this “sympathoadrenal imbalance,” or SAI (Goldstein et al., 2003). In this situation, EPI-induced skeletal muscle vasodilation that is unopposed by NE-induced vasoconstriction shunts the cardiac output toward the limbs and away from the brain. Concurrently, there are substantial increases in circulating AVP (Jardine et al., 1997; Meck et al., 2004; Nilsson et al., 2016), which may be relevant to the mechanism of SNS inhibition in that AVP augments baroreflexive restraint of SNS outflows, probably at the level of the area postrema (Bishop et al., 1987; Hasser et al., 1997).
Chronic Disorders of Regulation
Decreased efficiency of regulation of monitored variables of the body’s inner world threatens homeostasis. The homeostasis theory encompasses integration of autonomic nervous with behavioral, endocrine, autocrine/paracrine, and cytokine effectors. The general proposal is that all these systems mediate automatic adjustments that maintain health, and disintegration of these systems causes disorders of regulation in disease (Figure 5). Some of these are post-traumatic stress disorder (Preston et al., 2021), cardiovascular (Esler, 2017; Grassi et al., 2016; Silva et al., 2019; Templin et al., 2019), metabolic (Deussing et al., 2018; Wulsin et al., 2018), and gastrointestinal (Kano et al., 2017; Levinthal et al., 2020) disorders, and neurodegeneration (Foffani et al., 2018; Fornai et al., 2021; Ren et al., 2021; Wu et al., 2016).
Figure 5: Homeostasis, stress, allostasis, allostatic load, dyshomeostasis, and death.
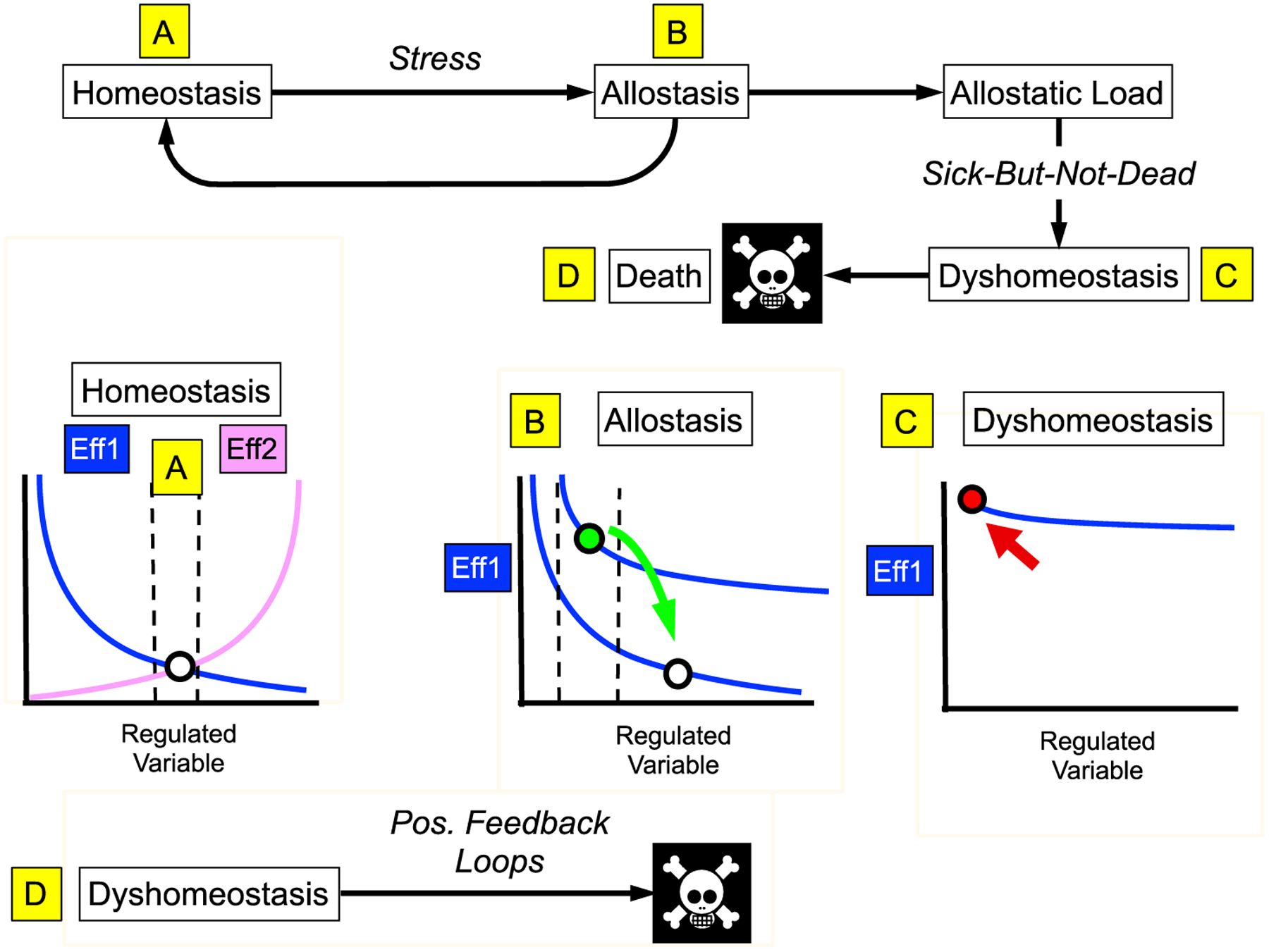
(A) In homeostasis effectors (Eff1, Eff2) with opposite effects on a Regulated Variable keep the level of the variable (circle) within bounds (vertical dashed lines). This panel is adapted from that drawn by Cannon (Cannon, 1929), where the Regulated Variable was blood glucose and the effectors the sympathico-adrenal system (activated when blood glucose falls) and the vago-insular system (activated when blood glucose is increased). (B) Allostatic processes result in a shift in the input-output curve relating the level of the Regulated Variable to the extent of activation of the effector (here only one effector is depicted). The level of the Regulated Variable is kept within bounds, but the acceptable bounds are different. The low grade fever attending a viral infection is an example of allostasis. Temporary allostatic adjustments are beneficial, and afterward the level of the Regulated Variable reverts to baseline (green arrow). (C) Prolonged, exaggerated, or inappropriate allostatic changes result in wear-and-tear (allostatic load), which decreases efficiencies of allostatic processes and the ability of the organism to adapt (dyshomeostasis). Dysfunctions may be sufficient to produce symptoms or signs of illness, without overt loss of the effectors (“sick-but-not-dead” phenomenon). (D) Dyshomeostatic states decrease thresholds for induction of destabilizing positive feedback loops that can be lethal.
Hypertension provides an example of a chronic disorder of regulation. A role for increased SNS outflow in hypertension has long been suspected (Goldstein, 1981). Elevated SNS outflow seems especially prominent in relatively young patients, consistent with a contribution to pathogenesis (Goldstein et al., 1983). Many studies have addressed brain sites and mechanisms underlying increased SNS outflows. The sites are within the CAN, such as the rostral ventrolateral medulla (RVLM) (Guyenet et al., 2018) and PVN (Dampney et al., 2018).
The hypertensive response to pressor stimuli can become sensitized to particular stimuli. This seems to be a form of classical conditioning. Hypertensive response sensitization is mediated by neuroplasticity (Johnson et al., 2018). The brain circuitry involved, in the CAN, controls SNS outflows. Central regions thought to be anatomic loci for hypertensive response sensitization include the PVN and the lamina terminalis (Xue et al., 2019). The latter includes the OVLT, a circumventricular hypothalamic area that lacks an efficient blood-brain barrier. Hypertensive response sensitization involves interplays among the sympathetic noradrenergic system, RAS, and brain and peripheral immune systems (Xiao et al., 2020; Xue et al., 2020) and probably other sites in the CAN.
Baroreflex sensitivity, and therefore the ability to keep blood pressure within bounds, declines with age in a manner associated with hypertension (Bristow et al., 1969). The rate of pulse-synchronous bursts of skeletal muscle sympathetic nerve traffic increases with aging (Sundlof et al., 1978); however, arterial baroreflex control of muscle sympathetic nerve traffic (Matsukawa et al., 1998) and of cardiovagal outflow are decreased in the elderly (Bristow et al., 1969).
All forms of distress increase blood pressure and inhibit baroreflex functions (Goldstein, 1983). In baboons trained by operant conditioning to raise their diastolic blood pressure, baroreflex-cardiovagal gain decreases when the blood pressure increases (Goldstein et al., 1977). When psychological factors are attenuated by treatment with a benzodiazepine, the baroreflex function is less decreased. For instance, in the hyperdynamic circulatory state syndrome, sedation with intravenous diazepam acutely decreases plasma NE levels, increases baroreflex-cardiovagal gain, and mitigates yohimbine-evoked systolic hypertension (Goldstein et al., 1985a). A major source of descending inhibition of baroreflex function seems to be the PVN, since PVN stimulation interferes with phenylephrine-induced increased neuronal firing in the NTS (Duan et al., 1999).
Decreased baroreflexive function is generally associated with poor outcome. This is well documented for myocardial infarction and the risk of sudden death from ventricular fibrillation (La Rovere et al., 1998). Conversely, baroreflex activation therapy improves survival in advanced heart failure (Borisenko et al., 2018).
TREATMENT
The multiple reciprocal inter-relationships among components of the EAS provide a conceptual framework for inducing therapeutic options that transcend unidirectional cause-effect relationships. One can classify such options in terms of education, non-drug treatments, and drug treatments.
The first line in management of a clinical autonomic disorder is education. Given the efficacy of allostatic adjustments in anticipation of a stressor, mutual information exchange between patient and clinician should improve quality of life in dealing with a chronic dysautonomia. A patient can learn about situations that exacerbate or alleviate symptoms, drug interactions, diurnal patterns, dietary constituents, herbal remedies, and danger signs that could mitigate threats to homeostasis by anticipating them. Meanwhile, the clinician learns from the experience gained from what in essence are N=1 experiments.
A variety of non-drug treatments may be considered that exploit the EAS concept. For instance, a patient with orthostatic hypotension or orthostatic intolerance might benefit from swimming or supine bicycle exercise training focusing on anti-gravity muscles (Fu et al., 2018) or, applying the allostasis idea, from drinking 16 ounces of water before the exercise session (Lu et al., 2003; Savard et al., 1995).
Regarding drug treatments, in a patient with post-traumatic stress disorder, electrical carotid sinus stimulation might exert a calming influence (Dworkin et al., 1979). A patient with hypertension and elevated sympathetic noradrenergic outflows might benefit from a central sympatholytic agent like clonidine (Goldstein et al., 1985b), while in a patient with a tendency to faint related to sympathoadrenal imbalance a benzodiazepine and a non-selective beta-adrenoceptor blocker might decrease the frequency of presyncope (Breier et al., 1992; Dendi et al., 2002).
Bioelectronic medicine
The myriad neurotransmission pathways to, from, and within the brain offer fertile ground for treatment via “bioelectronic medicine.” (Cracchiolo et al., 2021) Bioelectronic medicine is an emerging approach for neuromodulation therapies in disorders that until recently were treated medicinally.
This concept has attracted media attention that so far is beyond what the data justify (Blase et al., 2021). The use of vagus nerve stimulation has been advocated for myocardial reperfusion injury (Statz et al., 2020) and stress-related psychiatric disorders (Bremner et al., 2020; Noble et al., 2019). Appropriately controlled studies designed to address placebo effects and observer bias are needed.
Left cardiac sympathetic denervation is well established for decreasing the risk of ventricular fibrillation after myocardial infarction (La Rovere et al., 2020).
Bioelectronic approaches such as cardiac sympathetic denervation, renal denervation, vagal stimulation, and ganglionated plexi ablation are expanding in treating arrhythmias in clinical cardiology (Manolis et al., 2020).
Acknowledgement:
Dr. Goldstein’s research is supported (in part) by the Division of Intramural Research, NINDS, NIH.
Abbreviations:
- ACTH
adrenocorticotropin (corticotropin)
- ADH
anti-diuretic hormone
- AII
angiotensin II
- ANS
autonomic nervous system
- APUD
amine precursor uptake and decarboxylation
- AVP
arginine vasopressin
- CRH
corticotropin-releasing hormone
- DA
dopamine
- DOPA
3,4-dihydroxyphenylalanine
- DRD1
type 1 dopamine receptor
- ENS
enteric nervous system
- EPI
epinephrine (synonymous with adrenaline)
- NLRP3
NOD-, LRR- and pyrin domain-containing protein 3
- HPA
hypothalamic-pituitary-adrenocortical
- IL-6
interleukin-6
- LC
locus ceruleus
- MnPO
median preoptic nucleus
- NE
norepinephrine
- OVLT
organum vasculosum of the lamina terminalis
- PNS
parasympathetic nervous system
- PVN
paraventricular nucleus of the hypothalamus
- RAS
renin-angiotensin-aldosterone system
- SAS
sympathetic adrenergic system
- SCN
suprachiasmatic nucleus
- SNS
sympathetic noradrenergic system (SNS is also used as an abbreviation for Langley’s sympathetic nervous system)
- SFO
Subfornical organ
- TNFα
tumor necrosis factor alpha
- VTA
ventral tegmental area
REFERENCES
- Abe C, Inoue T 2018. Role of C1 neurons in anti-inflammatory reflex: Mediation between afferents and efferents. Neurosci Res 136, 6–12. [DOI] [PubMed] [Google Scholar]
- Akashi YJ, Goldstein DS, Barbaro G, Ueyama T 2008. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 118, 2754–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert A, Pickman Y, Leipold M, Rosenberg-Hasson Y, Ji X, Gaujoux R, Rabani H, Starosvetsky E, Kveler K, Schaffert S, Furman D, Caspi O, Rosenschein U, Khatri P, Dekker CL, Maecker HT, Davis MM, Shen-Orr SS 2019. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med 25, 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, Van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B 1991. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog.Brain Res 88, 47–75. [DOI] [PubMed] [Google Scholar]
- Baines AD 1990. Functional effects of proximal tubular dopamine production. Am. J. Hypertension 3, 68S–71S. [PubMed] [Google Scholar]
- Baron DN, Green RJ, Khan FA 1985. Adrenaline and ion flux in isolated human leucocytes. Clin. Sci 68, 517–521. [DOI] [PubMed] [Google Scholar]
- Benarroch EE 1993. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin. Proc 68, 988–1001. [DOI] [PubMed] [Google Scholar]
- Benditt DG, Ermis C, Padanilam B, Samniah N, Sakaguchi S 2003. Catecholamine response during haemodynamically stable upright posture in individuals with and without tilt-table induced vasovagal syncope. Europace 5, 65–70. [DOI] [PubMed] [Google Scholar]
- Bentur OS, Sarig G, Brenner B, Jacob G 2018. Effects of Acute Stress on Thrombosis. Semin Thromb Hemost 44, 662–668. [DOI] [PubMed] [Google Scholar]
- Bernard C 1974. Lectures on the Phenomena of Life Common to Animals and Vegetables. Charles C Thomas, Springfield, IL. [Google Scholar]
- Bishop VS, Hasser EM 1987. Vasopressin and sympathetic nerve activity: involvement of the area postrema. In: Buckley JP, Ferrario CM, (Eds.), Brain Peptides and Catecholamines in Cardiovascular Regulation. Raven, New York. pp. 373–382. [Google Scholar]
- Blase K, Vermetten E, Lehrer P, Gevirtz R 2021. Neurophysiological Approach by Self-Control of Your Stress-Related Autonomic Nervous System with Depression, Stress and Anxiety Patients. Int J Environ Res Public Health 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisenko O, Muller-Ehmsen J, Lindenfeld J, Rafflenbeul E, Hamm C 2018. An early analysis of cost-utility of baroreflex activation therapy in advanced chronic heart failure in Germany. BMC Cardiovasc Disord 18, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brealey D, Singer M 2009. Hyperglycemia in critical illness: a review. J Diabetes Sci Technol 3, 1250–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier A, Davis O, Buchanan R, Listwak SJ, Holmes C, Pickar D, Goldstein DS 1992. Effects of alprazolam on pituitary-adrenal and catecholaminergic responses to metabolic stress in humans. Biol. Psychiatry 32, 880–890. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Gurel NZ, Wittbrodt MT, Shandhi MH, Rapaport MH, Nye JA, Pearce BD, Vaccarino V, Shah AJ, Park J, Bikson M, Inan OT 2020. Application of Noninvasive Vagal Nerve Stimulation to Stress-Related Psychiatric Disorders. J Pers Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow JD, Gribbin B, Honour AJ, Pickering TG, Sleight P 1969. Diminished baroreflex sensitivity in high blood pressure and ageing man. J. Physiol 202, 45P–46P. [PubMed] [Google Scholar]
- Camell CD, Sander J, Spadaro O, Lee A, Nguyen KY, Wing A, Goldberg EL, Youm YH, Brown CW, Elsworth J, Rodeheffer MS, Schultze JL, Dixit VD 2017. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550, 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB 1919. Bodily Changes in Pain, Hunger, Fear and Rage D. Appleton & Co., New York. [Google Scholar]
- Cannon WB 1929. Organization for physiological homeostasis. Physiol. Rev 9, 399–431. [Google Scholar]
- Cannon WB 1945. The Way of an Investigator W. W. Norton, New York. [Google Scholar]
- Cannon WB, de la Paz D 1911. Emotional stimulation of adrenal gland secretion. Am. J. Physiol 28, 64–70. [Google Scholar]
- Capellino S, Claus M, Watzl C 2020. Regulation of natural killer cell activity by glucocorticoids, serotonin, dopamine, and epinephrine. Cell Mol Immunol 17, 705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadda K, Annane D 2002. The use of corticosteroids in severe sepsis and acute respiratory distress syndrome. Ann Med 34, 582–589. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G 2005. Endocrinology of the stress response. Annu Rev Physiol 67, 259–284. [DOI] [PubMed] [Google Scholar]
- Chen CC, Williams CL 2012. Interactions between epinephrine, ascending vagal fibers, and central noradrenergic systems in modulating memory for emotionally arousing events. Front. Behav. Neurosci 6, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikanza IC, Petrou P, Chrousos G 2000. Perturbations of arginine vasopressin secretion during inflammatory stress. Pathophysiologic implications. Ann. N.Y. Acad. Sci 917, 825–834. [DOI] [PubMed] [Google Scholar]
- Chrousos GP 2009. Stress and disorders of the stress system. Nat. Rev. Endocrinol 5, 374–381. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW 1992. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. J. Am. Med. Assoc 267, 1244–1252. [PubMed] [Google Scholar]
- Clausen T 1983. Adrenergic control of Na+-K+-homoeostasis. Acta Med. Scand. Suppl 672, 111–115. [DOI] [PubMed] [Google Scholar]
- Cracchiolo M, Ottaviani MM, Panarese A, Strauss I, Vallone F, Mazzoni A, Micera S 2021. Bioelectronic medicine for the autonomic nervous system: clinical applications and perspectives. J Neural Eng 18. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Michelini LC, Li DP, Pan HL 2018. Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. Am. J. Physiol. Heart Circ. Physiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danobeitia JS, Sperger JM, Hanson MS, Park EE, Chlebeck PJ, Roenneburg DA, Sears ML, Connor JX, Schwarznau A, Fernandez LA 2012. Early activation of the inflammatory response in the liver of brain-dead non-human primates. J Surg Res 176, 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbar D, Smith M, Morike K, Roden DM 1996. Epinephrine-induced changes in serum potassium and cardiac repolarization and effects of pretreatment with propranolol and diltiazem. Am. J. Cardiol 77, 1351–1355. [DOI] [PubMed] [Google Scholar]
- Darling G, Goldstein DS, Stull R, Gorschboth CM, Norton JA 1989. Tumor necrosis factor: immune endocrine interaction. Surgery 106, 1155–1160. [PubMed] [Google Scholar]
- Davy KP, Seals DR, Tanaka H 1998. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension 32, 298–304. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Slangen JL, Van der Gugten J 1990. Plasma catecholamine and corticosterone levels during active and passive shock-prod avoidance behavior in rats: effects of chlordiazepoxide. Physiol Behav 47, 1089–1098. [DOI] [PubMed] [Google Scholar]
- Dedic N, Chen A, Deussing JM 2018. The CRF Family of Neuropeptides and their Receptors - Mediators of the Central Stress Response. Curr Mol Pharmacol 11, 4–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendi R, Goldstein DS 2002. Meta-analysis of nonselective versus beta-1 adrenoceptor-selective blockade in prevention of tilt-induced neurocardiogenic syncope. Am. J. Cardiol 89, 1319–1321. [DOI] [PubMed] [Google Scholar]
- Deussing JM, Chen A 2018. The Corticotropin-Releasing Factor Family: Physiology of the Stress Response. Physiol Rev 98, 2225–2286. [DOI] [PubMed] [Google Scholar]
- Devi S, Alexandre YO, Loi JK, Gillis R, Ghazanfari N, Creed SJ, Holz LE, Shackleford D, Mackay LK, Heath WR, Sloan EK, Mueller SN 2021. Adrenergic regulation of the vasculature impairs leukocyte interstitial migration and suppresses immune responses. Immunity 54, 1219–1230. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Malarkey WB, Neri E, McEwen BS 2012. Stress-induced redistribution of immune cells--from barracks to boulevards to battlefields: a tale of three hormones--Curt Richter Award winner. Psychoneuroendocrinology 37, 1345–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS 1997. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun 11, 286–306. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL 1996. Stress-induced changes in blood leukocyte distribution. Role of adrenal steroid hormones. J Immunol 157, 1638–1644. [PubMed] [Google Scholar]
- Dimopoulou I, Alevizopoulou P, Dafni U, Orfanos S, Livaditi O, Tzanela M, Kotanidou A, Souvatzoglou E, Kopterides P, Mavrou I, Thalassinos N, Roussos C, Armaganidis A, Tsagarakis S 2007. Pituitary-adrenal responses to human corticotropin-releasing hormone in critically ill patients. Intensive Care Med 33, 454–459. [DOI] [PubMed] [Google Scholar]
- Ding K, Tarumi T, Wang C, Vernino S, Zhang R, Zhu DC 2020. Central autonomic network functional connectivity: correlation with baroreflex function and cardiovascular variability in older adults. Brain Struct Funct 225, 1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XS, Wu SS, Chen H, Zhao XQ, Li HW 2019. High admission glucose levels predict worse short-term clinical outcome in non-diabetic patients with acute myocardial infraction: a retrospective observational study. BMC Cardiovasc Disord 19, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrakovova M, Kvetnansky R, Oprsalova Z, Jezova D 1993. Specificity of the effect of repeated handling on sympathetic-adrenomedullary and pituitary-adrenocortical activity in rats. Psychoneuroendocrinology 18, 163–174. [DOI] [PubMed] [Google Scholar]
- Dronjak S, Jezova D, Kvetnansky R 2004. Different effects of novel stressors on sympathoadrenal system activation in rats exposed to long-term immobilization. Ann N Y Acad Sci 1018, 113–123. [DOI] [PubMed] [Google Scholar]
- Duan YF, Kopin IJ, Goldstein DS 1999. Stimulation of the paraventricular nucleus modulates firing of neurons in the nucleus of the solitary tract. Am. J. Physiol 277, R403–R411. [DOI] [PubMed] [Google Scholar]
- Dworkin BR, Filewich RJ, Miller NE, Craigmyle N, Pickering TG 1979. Baroreceptor activation reduces reactivity to noxious stimulation: Implications for hypertension. Science 205, 1299–1301. [DOI] [PubMed] [Google Scholar]
- Eiden LE, Emery AC, Zhang L, Smith CB 2018. PACAP signaling in stress: insights from the chromaffin cell. Pflugers Arch 470, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Goldstein DS, Stull RW, Gold PW, Keiser HR, Kopin IJ 1987. Dissociation between corticotrophin and catecholamine responses to isoprenaline in humans. Clin. Exp. Pharmacol. Physiol 14, 337–341. [DOI] [PubMed] [Google Scholar]
- Esler M 2017. Mental stress and human cardiovascular disease. Neurosci Biobehav Rev 74, 269–276. [DOI] [PubMed] [Google Scholar]
- Esler MD, Thompson JM, Kaye DM, Turner AG, Jennings GL, Cox HS, Lambert GW, Seals DR 1995. Effects of aging on the responsiveness of the human cardiac sympathetic nerves to stressors. Circulation 91, 351–358. [DOI] [PubMed] [Google Scholar]
- Evans JM, Leonelli FM, Ziegler MG, McIntosh CM, Patwardhan AR, Ertl AC, Kim CS, Knapp CF 2001. Epinephrine, vasodilation and hemoconcentration in syncopal, healthy men and women. Auton. Neurosci 93, 79–90. [DOI] [PubMed] [Google Scholar]
- Foffani G, Obeso JA 2018. A Cortical Pathogenic Theory of Parkinson’s Disease. Neuron 99, 1116–1128. [DOI] [PubMed] [Google Scholar]
- Fornai F, Puglisi-Allegra S 2021. Autophagy status as a gateway for stress-induced catecholamine interplay in neurodegeneration. Neurosci. Biobehav. Rev 123, 238–256. [DOI] [PubMed] [Google Scholar]
- Frank SM, Raja SN, Bulcao C, Goldstein DS 2000. Age-related thermoregulatory differences during core cooling in humans. Am. J. Physiol 279, R349–354. [DOI] [PubMed] [Google Scholar]
- Friedman B, Cirulli J 2013. Hyponatremia in critical care patients: frequency, outcome, characteristics, and treatment with the vasopressin V2-receptor antagonist tolvaptan. J. Crit. Care 28, 219 e211–212. [DOI] [PubMed] [Google Scholar]
- Fritsch-Yelle JM, Whitson PA, Bondar RL, Brown TE 1996. Subnormal norepinephrine release relates to presyncope in astronauts after spaceflight. J Appl Physiol 81, 2134–2141. [DOI] [PubMed] [Google Scholar]
- Fu Q, Levine BD 2018. Exercise and non-pharmacological treatment of POTS. Auton Neurosci 215, 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara K, Kvetnansky R, Cizza G, Pacak K, Ohara H, Goldstein DS, Kopin IJ 1996. Interrelations between sympathoadrenal system and hypothalamo-pituitary-adrenocortical/thyroid systems in rats exposed to cold stress. J. Neuroendocrinol 8, 533–541. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Dijkstra I, Tilders FJ 1995. Subdiaphragmatic vagotomy suppresses endotoxin-induced activation of hypothalamic corticotropin-releasing hormone neurons and ACTH secretion. Endocrinology 136, 4717–4720. [DOI] [PubMed] [Google Scholar]
- Gearhart MM, Parbhoo SK 2006. Hyperglycemia in the critically ill patient. AACN Clin. Issues 17, 50–55. [DOI] [PubMed] [Google Scholar]
- Ghadri JR, Sarcon A, Diekmann J, Bataiosu DR, Cammann VL, Jurisic S, Napp LC, Jaguszewski M, Scherff F, Brugger P, Jancke L, Seifert B, Bax JJ, Ruschitzka F, Luscher TF, Templin C, Inter, T.A.K.C.-i. 2016. Happy heart syndrome: role of positive emotional stress in takotsubo syndrome. Eur Heart J 37, 2823–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JR Jr. 1979. Neural control of renal tubular sodium reabsorption. Nephron 23, 116–118. [DOI] [PubMed] [Google Scholar]
- Golaszewska A, Misztal T, Marcinczyk N, Chabielska E, Rusak T 2021. Adrenaline May Contribute to Prothrombotic Condition via Augmentation of Platelet Procoagulant Response, Enhancement of Fibrin Formation, and Attenuation of Fibrinolysis. Front Physiol 12, 657881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS 1981. Plasma norepinephrine in essential hypertension. A study of the studies. Hypertension 3, 48–52. [DOI] [PubMed] [Google Scholar]
- Goldstein DS 1983. Arterial baroreflex sensitivity, plasma catecholamines, and pressor responsiveness in essential hypertension. Circulation 68, 234–240. [DOI] [PubMed] [Google Scholar]
- Goldstein DS 1995a. Stress as a scientific idea: A homeostatic theory of stress and distress. Homeostasis 4, 177–215. [Google Scholar]
- Goldstein DS 1995b. Stress, Catecholamines, and Cardiovascular Disease. Oxford University Press, New York. [Google Scholar]
- Goldstein DS 2006. Adrenaline and the Inner World: An Introduction to Scientific Integrative Medicine The Johns Hopkins University Press, Baltimore, MD. [Google Scholar]
- Goldstein DS 2008. Computer models of stress, allostasis, and acute and chronic disease. Ann. N.Y. Acad. Sci 1148, 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS 2013. Concepts of scientific integrative medicine applied to the physiology and pathophysiology of catecholamine systems. Compr. Physiol 3, 1569–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS 2019. How does homeostasis happen? Integrative physiological, systems biological, and evolutionary perspectives. Am. J. Physiol. Regul. Integr. Comp. Physiol 316, R301–R317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Bentho O, Park MY, Sharabi Y 2011. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp. Physiol 96, 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Frank SM 2001. The wisdom of the body revisited: The adrenomedullary response to mild core hypothermia in humans. Endocrine Regul. 35, 3–7. [PubMed] [Google Scholar]
- Goldstein DS, Harris AH, Brady JV 1977. Baroreflex sensitivity during operant blood pressure conditioning. Biofeedback Self-Regul. 2, 127–138. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Holmes C, Frank SM, Naqibuddin M, Dendi R, Snader S, Calkins H 2003. Sympathoadrenal imbalance before neurocardiogenic syncope. Am. J. Cardiol 91, 53–58. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Keiser HR 1985a. Neural circulatory control in the hyperdynamic circulatory state syndrome. American heart journal 109, 387–390. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ 2007. Evolution of concepts of stress. Stress 10, 109–120. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ 2008. Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: A meta-analysis. Endo. Regul 42, 111–119. [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ 2017. Homeostatic systems, biocybernetics, and autonomic neuroscience. Auton. Neurosci 208, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ, Sharabi Y 2014. Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders. Pharmacol Ther 144, 268–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Lake CR, Chernow B, Ziegler MG, Coleman MD, Taylor AA, Mitchell JR, Kopin IJ, Keiser HR 1983. Age-dependence of hypertensive-normotensive differences in plasma norepinephrine. Hypertension 5, 100–104. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Levinson PD, Zimlichman R, Pitterman A, Stull R, Keiser HR 1985b. Clonidine suppression testing in essential hypertension. Ann. Intern. Med 102, 42–49. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, McEwen B 2002. Allostasis, homeostats, and the nature of stress. Stress 5, 55–58. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Mezey E, Yamamoto T, Aneman A, Friberg P, Eisenhofer G 1995. Is there a third peripheral catecholaminergic system? Endogenous dopamine as an autocrine/paracrine substance derived from plasma DOPA and inactivated by conjugation. Hypertens. Res. 18 Suppl 1, S93–S99. [DOI] [PubMed] [Google Scholar]
- Gordon RD, Kuchel O, Liddle GW, Island DP 1967. Role of the sympathetic nervous system in regulating renin and aldosterone production in man. J Clin Invest 46, 599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Ram VS 2016. Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens 10, 457–466. [DOI] [PubMed] [Google Scholar]
- Grossman E, Shenkar A, Peleg E, Thaler M, Goldstein DS 1999. Renal effects of L-DOPA in heart failure. J. Cardiovasc. Pharmacol 33, 922–928. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Holloway BB, Souza G, Abbott SBG 2018. Rostral Ventrolateral Medulla and Hypertension. Hypertension 72, 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidan A, Bornstein SR, Glasow A, Uhlmann K, Lubke C, Ehrhart-Bornstein M 1998. Basal steroidogenic activity of adrenocortical cells is increased 10-fold by coculture with chromaffin cells. Endocrinology 139, 772–780. [DOI] [PubMed] [Google Scholar]
- Halter JB, Beard JC, Porte D Jr. 1984. Islet function and stress hyperglycemia: plasma glucose and epinephrine interaction. Am. J. Physiol 247, E47–52. [DOI] [PubMed] [Google Scholar]
- Hamel JF, Jobson S, Caulier G, Mercier A 2021. Evidence of anticipatory immune and hormonal responses to predation risk in an echinoderm. Sci Rep 11, 10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A, Zhang Q, Salehi A, Willems M, Knudsen JG, Ringgaard AK, Chapman CE, Gonzalez-Alvarez A, Surdo NC, Zaccolo M, Basco D, Johnson PRV, Ramracheya R, Rutter GA, Galione A, Rorsman P, Tarasov AI 2018. Adrenaline Stimulates Glucagon Secretion by Tpc2-Dependent Ca(2+) Mobilization From Acidic Stores in Pancreatic alpha-Cells. Diabetes 67, 1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizaki T, Nishimura Y, Teramura K, Umemoto Y, Shibasaki M, Leicht CA, Kouda K, Tajima F 2018. Differences in serum IL-6 response after 1 degrees C rise in core body temperature in individuals with spinal cord injury and cervical spinal cord injury during local heat stress. Int J Hyperthermia 35, 541–547. [DOI] [PubMed] [Google Scholar]
- Hasser EM, Bishop VS, Hay M 1997. Interactions between vasopressin and baroreflex control of the sympathetic nervous system. Clin. Exp. Pharmacol. Physiol 24, 102–108. [DOI] [PubMed] [Google Scholar]
- Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, Bergwelt-Baildon MV, Klein M, Weinberger T 2020. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann JP, Marzi I, Relja B 2016. Adrenergic stimulation alters the expression of inflammasome components and interleukins in primary human monocytes. Exp. Ther. Med 11, 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Huang X, Jiang T, Yu S 2019. Multi-Scale Expressions of One Optimal State Regulated by Dopamine in the Prefrontal Cortex. Front Physiol 10, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M 2014. Evaluating the Role of Hans Selye in the Modern History of Stress. In: Cantor D, Ramsden E, (Eds.), Stress, Shock, and Adaptation in the Twentieth Century, Rochester (NY). [PubMed] [Google Scholar]
- Jan BU, Coyle SM, Oikawa LO, Lu SE, Calvano SE, Lehrer PM, Lowry SF 2009. Influence of acute epinephrine infusion on endotoxin-induced parameters of heart rate variability: a randomized controlled trial. Ann. Surg 249, 750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine DL, Melton IC, Crozier IG, Bennett SI, Donald RA, Ikram H 1997. Neurohormonal response to head-up tilt and its role in vasovagal syncope. Am. J. Cardiol 79, 1302–1306. [DOI] [PubMed] [Google Scholar]
- Jardine DL, Melton IC, Crozier IG, English S, Bennett SI, Frampton CM, Ikram H 2002. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am. J. Physiol. Heart Circ. Physiol 282, H1804–1809. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Xue B 2018. Central nervous system neuroplasticity and the sensitization of hypertension. Nat Rev Nephrol 14, 750–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen PJ, Stavrakaki I, Voet B, Hoogervorst E, van Munster E, Linssen WH, Sinnige LG, Verhagen WI, Visser LH, van der Kruijk R, Verheul F, Boringa J, Heerings M, Gladdines W, Lonnqvist F, Gaillard P 2016. Patient-reported adverse effects of high-dose intravenous methylprednisolone treatment: a prospective web-based multi-center study in multiple sclerosis patients with a relapse. J. Neurol 263, 1641–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J 2016. An Overly Permissive Extension. Perspect Psychol Sci 11, 442–450. [DOI] [PubMed] [Google Scholar]
- Kagedal B, Goldstein DS 1988. Catecholamines and their metabolites. J. Chromatog 429, 177–233. [DOI] [PubMed] [Google Scholar]
- Kang SS, Liu X, Ahn EH, Xiang J, Manfredsson FP, Yang X, Luo HR, Liles LC, Weinshenker D, Ye K 2020. Norepinephrine metabolite DOPEGAL activates AEP and pathological Tau aggregation in locus coeruleus. J Clin Invest 130, 422–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Muratsubaki T, Van Oudenhove L, Morishita J, Yoshizawa M, Kohno K, Yagihashi M, Tanaka Y, Mugikura S, Dupont P, Ly HG, Takase K, Kanazawa M, Fukudo S 2017. Altered brain and gut responses to corticotropin-releasing hormone (CRH) in patients with irritable bowel syndrome. Sci Rep 7, 12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas M, Haase T, Zeller T 2018. Linking the sympathetic nervous system to the inflammasome: towards new therapeutics for atherosclerotic cardiovascular disease. Eur. Heart J 39, 70–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikushima S, Kobayashi Y, Nakagawa H, Katagiri T 1999. Triggering mechanism for neurally mediated syncope induced by head-up tilt test: role of catecholamines and response to propranolol. J. Am. Coll. Cardiol 33, 350–357. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Boehlig A, Hohenadel M, Nitsche I, Bauer K, Sack U 2016. The impact of acute stress on hormones and cytokines, and how their recovery is affected by music-evoked positive mood. Sci Rep 6, 23008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ, Tak PP 2016. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl. Acad. Sci. U S A 119, 8284–8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kox M, van Eijk LT, Zwaag J, van den Wildenberg J, Sweep FC, van der Hoeven JG, Pickkers P 2014. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc. Natl. Acad. Sci. U.S.A 111, 7379–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse HJ, Kreutz R, Staib S, Overlack A, Stumpe KO, Kolloch RE 1994. Dissociation of renin and aldosterone during low-dose epinephrine infusion. Am. J. Hypertens 7, 913–918. [DOI] [PubMed] [Google Scholar]
- Kulp GA, Herndon DN, Lee JO, Suman OE, Jeschke MG 2010. Extent and magnitude of catecholamine surge in pediatric burned patients. Shock 33, 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvetnansky R 2004. Stressor specificity and effect of prior experience on catecholamine biosynthetic enzyme phenylethanolamine N-methyltransferase. Ann. N. Y. Acad. Sci 1032, 117–129. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Pacak K, Sabban EL, Kopin IJ, Goldstein DS 1998. Stressor specificity of peripheral catecholaminergic activation. Adv. Pharmacol 42, 556–560. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Sabban EL, Palkovits M 2009. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev 89, 535–606. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Bigger JT Jr., Marcus FI, Mortara A, Schwartz PJ 1998. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 351, 478–484. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Porta A, Schwartz PJ 2020. Autonomic Control of the Heart and Its Clinical Impact. A Personal Perspective. Front Physiol 11, 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake CR, Ziegler MG, Kopin IJ 1976. Use of plasma norepinephrine for evaluation of sympathetic neuronal function in man. Life Sci. 18, 1315–1325. [DOI] [PubMed] [Google Scholar]
- Langley JN 1903. The autonomic nervous system. Brain 26, 1–26. [Google Scholar]
- Larsson PT, Wallen NH, Egberg N, Hjemdahl P 1992a. Alpha-adrenoceptor blockade by phentolamine inhibits adrenaline-induced platelet activation in vivo without affecting resting measurements. Clin. Sci 82, 369–376. [DOI] [PubMed] [Google Scholar]
- Larsson PT, Wallen NH, Martinsson A, Egberg N, Hjemdahl P 1992b. Significance of platelet beta-adrenoceptors for platelet responses in vivo and in vitro. Thromb. Haemost 68, 687–693. [PubMed] [Google Scholar]
- Lerman I, Hauger R, Sorkin L, Proudfoot J, Davis B, Huang A, Lam K, Simon B, Baker DG 2016. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: A randomized, blinded, healthy control pilot trial. Neuromodulation 19, 283–290. [DOI] [PubMed] [Google Scholar]
- Levenson RW 2019. Stress and Illness: A Role for Specific Emotions. Psychosom Med 81, 720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinthal DJ, Strick PL 2020. Multiple areas of the cerebral cortex influence the stomach. Proc Natl Acad Sci U S A 117, 13078–13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CC, Diedrich A, Tung CS, Paranjape SY, Harris PA, Byrne DW, Jordan J, Robertson D 2003. Water ingestion as prophylaxis against syncope. Circulation 108, 2660–2665. [DOI] [PubMed] [Google Scholar]
- Manolis AA, Manolis TA, Apostolopoulos EJ, Apostolaki NE, Melita H, Manolis AS 2020. The role of the autonomic nervous system in cardiac arrhythmias: The neuro-cardiac axis, more foe than friend? Trends Cardiovasc Med. [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T 1998. Baroreflex control of muscle sympathetic nerve activity is attenuated in the elderly. J. Auton. Nerv. Syst 73, 182–185. [DOI] [PubMed] [Google Scholar]
- Mayr E 1992. The Idea of Teleology. J. Hist. Ideas 53, 117–135. [Google Scholar]
- McEwen BS, Lasley EN 2002. The End of Stress As We Know It The Joesph Henry Press and The Dana Press, Washington, D.C. [Google Scholar]
- McEwen BS, Wingfield JC 2010. What is in a name? Integrating homeostasis, allostasis and stress. Horm Behav 57, 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck JV, Waters WW, Ziegler MG, deBlock HF, Mills PJ, Robertson D, Huang PL 2004. Mechanisms of postspaceflight orthostatic hypotension: low alpha1-adrenergic receptor responses before flight and central autonomic dysregulation postflight. Am J Physiol Heart Circ Physiol 286, H1486–1495. [DOI] [PubMed] [Google Scholar]
- Mezzacappa ES, Kelsey RM, Katkin ES 1999. The effects of epinephrine administration on impedance cardiographic measures of cardiovascular function. Int. J. Psychophysiol 31, 189–196. [DOI] [PubMed] [Google Scholar]
- Miller WL 2018. The Hypothalamic-Pituitary-Adrenal Axis: A Brief History. Horm Res Paediatr 89, 212–223. [DOI] [PubMed] [Google Scholar]
- Mills DCB, Roberts GCK 1967. Effects of adrenaline on human blood platelets. J. Physiol 193, 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin IM, Champaneria MC, Bornschein J, Kidd M 2006. Evolution of the diffuse neuroendocrine system--clear cells and cloudy origins. Neuroendocrinology 84, 69–82. [DOI] [PubMed] [Google Scholar]
- Mohammed M, Berdasco C, Lazartigues E 2020. Brain angiotensin converting enzyme-2 in central cardiovascular regulation. Clin Sci (Lond) 134, 2535–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosqueda-Garcia R, Furlan R, Fernandez-Violante R, Desai T, Snell M, Jarai Z, Ananthram V, Robertson RM, Robertson D 1997. Sympathetic and baroreceptor reflex function in neurally mediated syncope evoked by tilt. J Clin Invest 99, 2736–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayyar M, Yusuf J, Khan MU, Weber KT 2017. K(+) and Mg(2+) dyshomeostasis in acute hyperadrenergic stressor states. Am. J. Med. Sci 353, 422–424. [DOI] [PubMed] [Google Scholar]
- Nilsson D, Sutton R, Melander O, Fedorowski A 2016. Spontaneous vs nitroglycerin-induced vasovagal reflex on head-up tilt: Are there neuroendocrine differences? Heart Rhythm 13, 1674–1678. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Souza RR, McIntyre CK 2019. Vagus nerve stimulation as a tool for enhancing extinction in exposure-based therapies. Psychopharmacology (Berl) 236, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T 1976. Effects of subcutaneously administered adrenaline on human eccrine sweating, with special reference to the physiological significance of the adrenergic sweating mechanism. Jpn. J. Physiol 26, 517–528. [DOI] [PubMed] [Google Scholar]
- Oka T 2018. Stress-induced hyperthermia and hypothermia. Handb Clin Neurol 157, 599–621. [DOI] [PubMed] [Google Scholar]
- Pacak K, Baffi JS, Kvetnansky R, Goldstein DS, Palkovits M 1998a. Stressor-specific activation of catecholaminergic systems: implications for stress-related hypothalamic-pituitary-adrenocortical responses. Adv. Pharmacol 42, 561–564. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS 1998b. Heterogeneous neurochemical responses to different stressors: a test of Selye’s doctrine of nonspecificity. Am J Physiol 275, R1247–1255. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS 1998c. Heterogeneous neurochemical responses to different stressors: A test of Selye’s doctrine of nonspecificity. Am. J. Physiol 275, R1247–R1255. [DOI] [PubMed] [Google Scholar]
- Palmer GJ, Ziegler MG, Lake CR 1978. Response of norepinephrine and blood pressure to stress increases with age. J. Gerontol 33, 482–487. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Kumar VB, Gan Q, Burke WJ, Galvin JE 2010. The neurotoxicity of DOPAL: behavioral and stereological evidence for its role in Parkinson disease pathogenesis. PLoS One 5, e15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou DA, Petrides JS, Tsigos C, Bina S, Kalogeras KT, Wilder R, Gold PW, Deuster PA, Chrousos GP 1996. Exercise stimulates interleukin-6 secretion: inhibition by glucocorticoids and correlation with catecholamines. Am J Physiol 271, E601–605. [DOI] [PubMed] [Google Scholar]
- Path G, Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA 1997. Interleukin-6 and the interleukin-6 receptor in the human adrenal gland: expression and effects on steroidogenesis. J. Clin. Endocrinol. Metab 82, 2343–2349. [DOI] [PubMed] [Google Scholar]
- Penev P, Spiegel K, Marcinkowski T, Van Cauter E 2005. Impact of carbohydrate-rich meals on plasma epinephrine levels: dysregulation with aging. J Clin Endocrinol Metab 90, 6198–6206. [DOI] [PubMed] [Google Scholar]
- Piira OP, Miettinen JA, Hautala AJ, Huikuri HV, Tulppo MP 2013. Physiological responses to emotional excitement in healthy subjects and patients with coronary artery disease. Auton Neurosci 177, 280–285. [DOI] [PubMed] [Google Scholar]
- Preston G, Emmerzaal T, Radenkovic S, Lanza IR, Oglesbee D, Morava E, Kozicz T 2021. Cerebellar and multi-system metabolic reprogramming associated with trauma exposure and post-traumatic stress disorder (PTSD)-like behavior in mice. Neurobiol Stress 14, 100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing H, Desrouleaux R, Israni-Winger K, Mineur YS, Fogelman N, Zhang C, Rashed S, Palm NW, Sinha R, Picciotto MR, Perry RJ, Wang A 2020. Origin and Function of Stress-Induced IL-6 in Murine Models. Cell. [DOI] [PubMed] [Google Scholar]
- Quatrini L, Wieduwild E, Escaliere B, Filtjens J, Chasson L, Laprie C, Vivier E, Ugolini S 2018. Endogenous glucocorticoids control host resistance to viral infection through the tissue-specific regulation of PD-1 expression on NK cells. Nat Immunol 19, 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabasa C, Gagliano H, Pastor-Ciurana J, Fuentes S, Belda X, Nadal R, Armario A 2015. Adaptation of the hypothalamus-pituitary-adrenal axis to daily repeated stress does not follow the rules of habituation: A new perspective. Neurosci Biobehav Rev 56, 35–49. [DOI] [PubMed] [Google Scholar]
- Rao Y 2019. The First Hormone: Adrenaline. Trends Endocrinol Metab 30, 331–334. [DOI] [PubMed] [Google Scholar]
- Ren C, Li LX, Dong AQ, Zhang YT, Hu H, Mao CJ, Wang F, Liu CF 2021. Depression Induced by Chronic Unpredictable Mild Stress Increases Susceptibility to Parkinson’s Disease in Mice via Neuroinflammation Mediated by P2X7 Receptor. ACS Chem Neurosci 12, 1262–1272. [DOI] [PubMed] [Google Scholar]
- Riegger GA, Liebau G, Kochsiek K 1982. Antidiuretic hormone in congestive heart failure. Am. J. Med 72, 49–52. [DOI] [PubMed] [Google Scholar]
- Ritsinger V, Jensen J, Ohm D, Omerovic E, Koul S, Frobert O, Erlinge D, James S, Lagerqvist B, Norhammar A 2019. Elevated admission glucose is common and associated with high short-term complication burden after acute myocardial infarction: Insights from the VALIDATE-SWEDEHEART study. Diab. Vasc. Dis. Res 16, 582–584. [DOI] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J 2007. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience 150, 8–13. [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ 2008. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. U.S.A 105, 11008–11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudman D, Moffitt SD, Fernhoff PM, Blackston RD, Faraj BA 1981. Epinephrine deficiency in hypocorticotropic hypopituitary children. J. Clin. Endocrinol. Metab 53, 722–729. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM 1998. Why Zebras Don’t Get Ulcers W. H. Freeman and Company, New York. [Google Scholar]
- Savard GK, Stonehouse MA 1995. Cardiovascular response to orthostatic stress: effects of exercise training modality. Can J Appl Physiol 20, 240–254. [DOI] [PubMed] [Google Scholar]
- Schachter S, Singer J 1962. Cognitive, social, and physiological determinants of emotional state. Psychol. Rev 69, 379–399. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Sterling P 2019. Allostasis: A Brain-Centered, Predictive Mode of Physiological Regulation. Trends Neurosci 42, 740–752. [DOI] [PubMed] [Google Scholar]
- Seals DR, Victor RG, Mark AL 1988. Plasma norepinephrine and muscle sympathetic discharge during rhythmic exercise in humans. J. Appl. Physiol 65, 940–944. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH 2001. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A 98, 4770–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H 1950. The Physiology and Pathology of Exposure to Stress. A Treatise Based on the Concepts of the General-Adaptation Syndrome and the Diseses of Adaptation. Acta, Inc., Montreal, Canada. [Google Scholar]
- Selye H 1956. The Stress of Life McGraw-Hill, New York. [Google Scholar]
- Selye H 1974. Stress without Distress New American Library, New York. [Google Scholar]
- Shahid M, Zarif HMA, Farid MS, Abid MS, Akhtar B, Khan MR 2020. Prognostic Value of Hyperglycemia on Admission on In-hospital Outcomes in Patients Presenting with ST-elevation Myocardial Infarction. Cureus 12, e7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin RS, Sacca L 1984. Effect of epinephrine on glucose metabolism in humans: contribution of the liver. Am. J. Physiol 247, E157–165. [DOI] [PubMed] [Google Scholar]
- Silva AR, Magalhaes R, Arantes C, Moreira PS, Rodrigues M, Marques P, Marques J, Sousa N, Pereira VH 2019. Brain functional connectivity is altered in patients with Takotsubo Syndrome. Sci Rep 9, 4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith OA, DeVito JL, Astley CA 1984. Organization of central nervous system pathways influencing blood pressure responses during emotional behavior. Clin Exp Hypertens A 6, 185–204. [DOI] [PubMed] [Google Scholar]
- Statz GM, Olshansky B 2020. Editorial commentary: Vagal nerve stimulation for myocardial ischemia-reperfusion injury: Hope or Hype? Trends Cardiovasc Med 30, 489–490. [DOI] [PubMed] [Google Scholar]
- Sterling P 2020. What is Health? Allostasis and the Evolution of Human Design MIT Press, Cambridge, MA. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J 1988. Allostasis: a new paradigm to explain arousal pathology. In: Fisher J, Reason J, (Eds.), Handbook of Life Stress, Cognition, and Health. Johns Wiley & Sons Inc., New York. pp. 629–649. [Google Scholar]
- Sternberg EM, Chrousos GP, Wilder RL, Gold PW 1992. The stress response and the regulation of inflammatory disease. Ann. Intern. Med 117, 854–866. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Guyenet PG 2018. C1 neurons: a nodal point for stress? Exp Physiol 103, 332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack AM, Sawyer WB, Platt KB, Loewy AD 1989. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res. 491, 274–296. [DOI] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG 1978. Human muscle nerve sympathetic activity at rest: Relationship to blood pressure and age. J. Physiol 274, 621–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supiano MA, Hogikyan RV, Morrow LA, Ortiz-Alonso FJ, Herman WH, Galecki AT, Halter JB 1993. Aging and insulin sensitivity: role of blood pressure and sympathetic nervous system activity. J Gerontol 48, M237–243. [DOI] [PubMed] [Google Scholar]
- Sved AF, Imaizumi T, Talman WT, Reis DJ 1985. Vasopressin contributes to hypertension caused by nucleus tractus solitarius lesions. Hypertension 7, 262–267. [DOI] [PubMed] [Google Scholar]
- Sverrisdottir YB, Elam M, Herlitz H, Bengtsson BA, Johannsson G 1998. Intense sympathetic nerve activity in adults with hypopituitarism and untreated growth hormone deficiency. J. Clin. Endocrinol. Metab 83, 1881–1885. [DOI] [PubMed] [Google Scholar]
- Swanson KV, Deng M, Ting JP 2019. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol 19, 477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatalowicz VL, Arnold PE, Chaimovitz C, Bichet D, Berl T, Schrier RW 1981. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N. Engl. J. Med 305, 263–266. [DOI] [PubMed] [Google Scholar]
- Takase B, Kastushika S, Hamabe A, Uehata A, Isojima K, Satomura K, Nishioka T, Ohsuzu F, Kurita A 2001. Significance of circulatory epinephrine levels in exercise-induced neurally mediated syncope. Clin. Cardiol 24, 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templin C, Hanggi J, Klein C, Topka MS, Hiestand T, Levinson RA, Jurisic S, Luscher TF, Ghadri JR, Jancke L 2019. Altered limbic and autonomic processing supports brain-heart axis in Takotsubo syndrome. Eur Heart J 40, 1183–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD 1997. Thermoregulatory and metabolic phenotypes of mice lacking noradrenaline and adrenaline. Nature 387, 94–97. [DOI] [PubMed] [Google Scholar]
- Todd WD, Fenselau H, Wang JL, Zhang R, Machado NL, Venner A, Broadhurst RY, Kaur S, Lynagh T, Olson DP, Lowell BB, Fuller PM, Saper CB 2018. A hypothalamic circuit for the circadian control of aggression. Nat Neurosci 21, 717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ 2007. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 117, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traina G 2020. Learning processes in elementary nervous systems section sign. J Integr Neurosci 19, 673–678. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Kyrou I, Kassi E, Chrousos GP 2020. Stress: Endocrine Physiology and Pathophysiology. In: Feingold KR, Anawalt B, Boyce A, al., e., (Eds.), Endotext [Internet]. MDText.com, Inc., South Dartmouth, MA. [Google Scholar]
- Uschold-Schmidt N, Nyuyki KD, Fuchsl AM, Neumann ID, Reber SO 2012. Chronic psychosocial stress results in sensitization of the HPA axis to acute heterotypic stressors despite a reduction of adrenal in vitro ACTH responsiveness. Psychoneuroendocrinology 37, 1676–1687. [DOI] [PubMed] [Google Scholar]
- Vaddadi G, Guo L, Esler M, Socratous F, Schlaich M, Chopra R, Eikelis N, Lambert G, Trauer T, Lambert E 2011. Recurrent postural vasovagal syncope: sympathetic nervous system phenotypes. Circ. Arrhythm. Electrophysiol 4, 711–718. [DOI] [PubMed] [Google Scholar]
- Voet S, Srinivasan S, Lamkanfi M, van Loo G 2019. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil-Malherbe H, Axelrod J, Tomchick R 1959. Blood-brain barrier for adrenaline. Science 129, 1226–1227. [DOI] [PubMed] [Google Scholar]
- Wolfovitz E, Grossman E, Folio CJ, Keiser HR, Kopin IJ, Goldstein DS 1993. Derivation of urinary dopamine from plasma dihydroxyphenylalanine in humans. Clin. Sci 84, 549–557. [DOI] [PubMed] [Google Scholar]
- Woody A, Figueroa WS, Benencia F, Zoccola PM 2017. Stress-induced parasympathetic control and Its association with inflammatory reactivity. Psychosom. Med 79, 306–310. [DOI] [PubMed] [Google Scholar]
- Wu Q, Yang X, Zhang Y, Zhang L, Feng L 2016. Chronic mild stress accelerates the progression of Parkinson’s disease in A53T alpha-synuclein transgenic mice. Exp. Neurol 285, 61–71. [DOI] [PubMed] [Google Scholar]
- Wulsin L, Herman J, Thayer JF 2018. Stress, autonomic imbalance, and the prediction of metabolic risk: A model and a proposal for research. Neurosci Biobehav Rev 86, 12–20. [DOI] [PubMed] [Google Scholar]
- Xiao L, do Carmo LS, Foss JD, Chen W, Harrison DG 2020. Sympathetic Enhancement of Memory T-Cell Homing and Hypertension Sensitization. Circ Res 126, 708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin JZ, Wu JM, Hu GM, Gu HJ, Feng YN, Wang SX, Cong WW, Li MZ, Xu WL, Song Y, Xiao H, Zhang YY, Wang L 2020. alpha1-AR overactivation induces cardiac inflammation through NLRP3 inflammasome activation. Acta Pharmacol. Sin 41, 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Yu Y, Wei SG, Beltz TG, Guo F, Felder RB, Johnson AK 2019. Stress-Induced Sensitization of Angiotensin II Hypertension Is Reversed by Blockade of Angiotensin-Converting Enzyme or Tumor Necrosis Factor-alpha. Am J Hypertens 32, 909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Zhang Y, Johnson AK 2020. Interactions of the Brain Renin-Angiotensin-System (RAS) and Inflammation in the Sensitization of Hypertension. Front Neurosci 14, 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R 2015. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160, 62–73. [DOI] [PubMed] [Google Scholar]
- Yi C, Zhang C, Hu X, Li Y, Jiang H, Xu W, Lu J, Liao Y, Ma R, Li X, Wang J 2016. Vagus nerve stimulation attenuates myocardial ischemia/reperfusion injury by inhibiting the expression of interleukin-17A. Exp Ther Med 11, 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Wu W, Chen M, Cai F, Fan C, Shen W, Sun W, Hu J 2019. Reward Inhibits Paraventricular CRH Neurons to Relieve Stress. Curr Biol 29, 1243–1251 e1244. [DOI] [PubMed] [Google Scholar]
- Zimlichman R, Goldstein DS, Zimlichman S, Stull R, Keiser HR 1987. Angiotensin II increases cytosolic calcium and stimulates catecholamine release in cultured bovine adrenomedullary cells. Cell Calcium 8, 315–325. [DOI] [PubMed] [Google Scholar]


