Abstract
Older adults with Type II Diabetes Mellitus (DM) experience mild cognitive impairment, specifically in the domain of recall/working memory. No consistent causative structural cortical deficits have been identified in persons with DM (PwDM). Memory deficits may be exacerbated in older adult females, who are at the highest risk of cardiovascular decline due to DM. The focus of the current study was to evaluate functional cortical hemodynamic activity during memory tasks in postmenopausal PwDM. Functional Near Infrared Spectroscopy (fNIRS) was used to monitor oxyhemoglobin (HbO) and deoxyhemoglobin (HbR) during memory-based tasks in a cross-sectional sample of postmenopausal women with DM. Twenty-one community-dwelling DM females (age = 65 ± 6 years) and twenty-one age- and sex-matched healthy controls (age = 66 ± 6 years) were evaluated. Working memory performance (via N-back) was evaluated while study participants donned cortical fNIRS. Health state, metabolic data, and menopausal status data were also collected. Deficits in working memory accuracy were found in the DM group as compared to controls. Differences in HbO responses emerged in the DM group. The DM group exhibited altered PFC activity magnitudes and increased functional cortical activity across ROIs compared to controls. HbO and HbR responses were not associated with worsened health state measures. These data indicate a shift in cortical activity patterns with memory deficits in postmenopausal PwDM. This DM-specific shift of HbO is a novel finding that is unlikely to be detected by fMRI. This underscores the value of using non-MRI-based neuroimaging techniques to evaluate cortical hemodynamic function to detect early mild cognitive impairment.
Keywords: fNIRS, Cortical oxygenation, Cognition, Brain, Neuroimaging, Dementia
Introduction
Over 14.3 million individuals in the United States aged 60 + are living with either diagnosed or undiagnosed Type II Diabetes Mellitus (DM) (Centers for Disease Control and Prevention 2020). DM and cardiovascular disease are hypothesized to share an underlying constellation of causes, referred to as the 'common soil' hypothesis (Lebovitz 2006). With advanced age, persons with DM (PwDM) exhibit losses in several functional abilities; including development of mild cognitive impairment (MCI), amnesiac mild cognitive impairment (aMCI), and sensorimotor dysfunction (van den Berg et al. 2008; Christman et al. 2009; Janoutová et al. 2015; Gorniak et al. 2019a, b). Patients are not self-aware of these losses (Gorniak et al. 2014) which are typical precursors to development of dementias such as Alzheimer’s disease or vascular dementia.
Emerging evidence reveals differences in presentation of DM and its complications between the sexes with advanced age (Seghieri et al. 2017; Campesi et al. 2017b; Centers for Disease Control and Prevention 2020). Older adult women are significantly more negatively impacted by risks, complications, and comorbidities associated with DM, including development of dementia (Campesi et al. 2017a).
Female participants have been largely excluded from biomedical science for decades, as estrus has been perceived to render females more physiologically variable than males (Beery and Zucker 2011; Prendergast et al. 2014; Vitale et al. 2017). However, sex-based differences in DM presentation and its complications, including cognitive impairment, is an emerging area of interest. Sex-based differences in DM in animal models have not translated well to humans (Campesi et al. 2017b), which has significantly complicated attempts to understand DM-related complications.
Cognitive impairment in memory adversely affects the ability to manage complex daily DM self-management tasks such as meal preparation, taking medications, and exercise (Christman et al. 2009; Vance et al. 2011; Gold 2012). Many of these self-care tasks involve the use of one or both upper extremities in tasks which may require more cognitive resources for successful task completion. Our prior work has found that inclusion of a motor task while performing memory-based cognitive tasks (known as dual-tasking) may lead to reduced accuracy in both cognitive and motor tasks (Gorniak et al. 2019a, b); however, the cortical roots of these deficits are underexplored.
Traditional neuroimaging approaches (i.e., magnetic resonance imaging (MRI)) have been used to search for structural cortical roots of DM-related complications such as MCI and sensorimotor dysfunction (Manschot et al. 2006; Harten et al. 2006; van Harten et al. 2007; Christman et al. 2010; Brundel et al. 2012; Biessels and Reijmer 2014). Inconclusive structural evidence of cortical damage via MRI in individuals with DM has led to investigation of cortical activation differences using functional MRI (fMRI). This is in line with the assumption that altered hemodynamic responses due to micro- and macro-vascular changes are the most likely source of global behavioral changes in individuals with DM (Zochodne 2007). However, inconsistent fMRI evidence of cortical dysfunction in individuals with DM has been reported (Manschot et al. 2006; Harten et al. 2006; van Harten et al. 2007; Christman et al. 2010; Brundel et al. 2012). The blood oxygenation level dependent (known as BOLD) response of fMRI is based on measured changes in one aspect of the hemodynamic response—deoxygenated hemoglobin (HbR) (Buxton 2013). fMRI is only sensitive to HbR (due to its strong paramagnetization (Huettel et al. 2014)), whereas the oxygenated hemoglobin (HbO) aspect of the hemodynamic response is diamagnetic and undetected by fMRI approaches. Acknowledging this shortcoming of fMRI, alternative functional cortical investigations using technologies such as functional near infrared spectroscopy (fNIRS) has revealed altered HbO concurrent with sensorimotor dysfunction in postmenopausal women with DM (Gorniak et al. 2020).
Our overarching hypothesis is that altered hemodynamic function of the cortex leads to DM-complications including cognitive and sensorimotor impairments. In particular, postmenopausal women likely experience significant deterioration of both hemodynamic function and overt behaviors (e.g., cognitive function) given their disproportionate risk of cardiovascular complications as compared to men with DM and individuals without DM (Kautzky-Willer et al. 2016; Raparelli et al. 2017). The focus of this study was to evaluate changes in cortical oxygenation indices of postmenopausal women both with and without DM during memory-based cognitive single- and dual-tasks via fNIRS.
In line with our previous work (Gorniak et al. 2019a, b), we expected to see between-group differences in cognitive function, with impaired memory/recall in the DM group (Hypothesis #1). Concurrent with impaired cognitive function, we expected between-group differences in cortical oxygenation indices of oxygenated hemoglobin (HbO) and deoxygenated/reduced hemoglobin (HbR) (Hypothesis #2) across regions of the cortex involving memory and sensorimotor function during tasks involving cognitive components. No specific hypotheses regarding changes in cortical hemodynamic function with disease state were developed a priori, as multiple mechanistic pathways have been suggested with different levels of support in the evidence base (e.g., high A1c, hypertension, etc.). To examine our two hypotheses, cortical hemodynamic activity was measured via fNIRS during performance of cognitive tasks. The goal of the study was to evaluate the relationship between cortical hemodynamic activity and cognitive function in persons with DM versus controls.
Materials and methods
Participants
Twenty-one postmenopausal women with DM and twenty-one age- and sex-matched healthy controls volunteered to participate in this case control study, see Table 1 for demographics. Handedness was assessed by the Edinburgh Inventory (Oldfield 1971), ranging from a laterality quotient (LQ) of −100 (strong left-handedness) to +100 (strong right-handedness). Participants had an LQ average of +88 and had no previous history of trauma to the upper limbs. Both the DM and control groups included women from self-identified underrepresented racial and ethnic minority groups (n = 24/42 (57%)). Study participants were excluded if they reported a history of neurological and/or musculoskeletal disorders (Parkinson disease, Huntington’s disease, polio, multiple sclerosis, stroke, traumatic brain injury, carpal tunnel syndrome, rheumatoid arthritis, Monoclonal Gammopathy of Undetermined Significance (MGUS), Paraproteinaemic Demyelinating Neuropathy (PDN), Myasthenia Gravis), a history of amputation, a history of major surgical intervention to the upper extremity, or hereditary or compression neuropathies. In accordance with the Declaration of Helsinki, participants provided informed consent according to the regulations established by the Institutional Review Board at the University of Houston (protocol #15615–01). Data collection processes failed on five participants (e.g., a reliable fNIRS signal was not detected (control participants #2, #7, and #10; DM participants #9 and #19)). Data from those participants have been excluded from fNIRS analyses but not behavioral data for completeness of reporting.
Table 1.
Demographic and clinical characteristics of DM participants
| Participant # | Age (years) | Menopausal age (years) |
BMI (kg/m2) | DM duration (months) |
A1c (%) | Total cholesterol (mg/dL) |
Systole (mmHg) | Diastole (mmHg) |
|---|---|---|---|---|---|---|---|---|
| 1 | 63 | 50 | 27.4 | 60 | 6.7 | – | 151 | 81 |
| 2 | 79 | 45 | 28.3 | 144 | 7.9 | – | 155 | 75 |
| 3* | 65 | 50 | 40.7 | 120 | 7.1 | – | 145 | 97 |
| 4‡ | 66 | 50 | 29.3 | 186 | 8.7 | 199 | 111 | 62 |
| 5* | 64 | 40 | 44.1 | 60 | 6.2 | 109 | 180 | 91 |
| 6 | 60 | 50 | 37.5 | 387 | 10.4 | 224 | 161 | 78 |
| 7* | 60 | 55 | 33.7 | 245 | 8 | 143 | 130 | 70 |
| 8 | 57 | 49 | 36.9 | 41 | 8.6 | 176 | 130 | 88 |
| 9^ | 73 | 60 | 25.3 | 201 | 6.8 | 125 | 167 | 78 |
| 10 | 68 | 23 | 31.8 | 168 | 5.7 | 219 | 164 | 89 |
| 11 | 70 | 45 | 26.9 | 200 | 6.1 | 266 | 130 | 71 |
| 12 | 62 | 38 | 32.4 | 36 | 6.2 | 189 | 124 | 70 |
| 13 | 67 | 45 | 30.2 | 1 | 8 | 144 | 158 | 97 |
| 14*‡ | 66 | 45 | 31.4 | 262 | 6.3 | 175 | 142 | 75 |
| 15* | 69 | 55 | 42.3 | 298 | 8.4 | 185 | 139 | 63 |
| 16 | 58 | 51 | 32.8 | 95 | 7.4 | 143 | 153 | 89 |
| 17* | 55 | 27 | 38.6 | 385 | 7.4 | 126 | 133 | 68 |
| 18 | 67 | 25 | 30.5 | 1 | 7.7 | 183 | 148 | 73 |
| 19*^ | 71 | 52 | 42.9 | 196 | 8.5 | 173 | 105 | 60 |
| 20 | 69 | 27 | 36.3 | 149 | 8.7 | 187 | 202 | 100 |
| 21 | 60 | 37 | 30.1 | 1 | 6.7 | 183 | 179 | 111 |
| Mean | 65 | 43 | 33.8 | 154 | 7.5 | 175 | 148 | 80 |
| SD | 6 | 11 | 5.6 | 117 | 1.2 | 39 | 23 | 14 |
| Controls | 67 ± 6 | 50 ± 7 | 24.1 ± 4.5 | N/A | 5.3 ± 0.3 | 200 ± 43 | 147 ± 21 | 86 ± 14 |
Indicates a clinical diagnosis of diabetic peripheral neuropathy
Indicates a history of Prempro Rx (in addition to 3 control participants); – Indicates lipid data collection failure; SD standard deviation
indicates omitted fNIRS data due to lack of reliable signal
Health status data
Blood pressure, cholesterol, and glycated hemoglobin (A1c) values were assessed for all study participants onsite at the onset of each session. Cholesterol and A1c values were assessed using a commercially available point of care evaluation kit (Cardiocheck + and A1c Now + kits, PTS Diagnostics, Indianapolis, IN, USA). Blood pressure was measured using a commercially available device (Omron Intellisense 10 series Blood Pressure Monitor, Model BP785, Bannockburn, IL, USA). The presence of peripheral neuropathy (PN status) was determined by abnormalities on either clinical examination or EMG/NCV testing (per physician). A brief menopause questionnaire was also administered regarding several aspects of menopausal characteristics (e.g., age at onset of menopause, hormone replacement therapy history, etc.). All study participants declared themselves to be postmenopausal; with 11 participants claiming a history of hormone replacement therapy (5 with a history of Prempro use). Of the 11 participants with a history of hormone replacement therapy, 4 were in the control group and 7 were in the DM group.
Baseline cognitive evaluation
Montreal cognitive assessment (MoCA)
Cognitive function of each participant was screened using the Montreal Cognitive Assessment (MoCA) (Nasreddine et al. 2005). This is a brief examination of the cognitive domains: attention and concentration, executive functions, working memory/recall, language, visuo-constructional skills, conceptual thinking, calculations, and orientation. The number of years of patient education is accounted for within the MoCA scoring structure. This evaluation was performed prior to placement of the fNIRS cap.
Experimental tasks
Working memory (N-back) evaluation (single-task)
Working memory of each participant was probed using the working memory (N-back) evaluation while wearing the fNIRS device. Working memory was assessed while participants were seated in a quiet location. This test required participants to repeat the “Nth” word back in a list of random words presented as auditory stimuli, consistent with our prior work (Gorniak et al. 2019a, b). The difficulty level is controlled by requiring participants to remember words further back in the series. Three conditions of the N-back task were assigned to each subject (easiest to most difficult: 0-, 1-, and 2-back conditions) in a block randomized manner. Participants wore a headset with headphone and microphone capabilities (Plantronics Inc., Santa Cruz, California), through which they heard a randomized sequence of words via audio provided by E-prime 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA). The software program generated randomized words through the headphones at an interval of 2 s per word. Participants were instructed to verbally repeat the words into the headset in the correct sequence for a task duration of 30 s. The rate of correct responses and verbal reaction time were recorded by the E-prime software and extracted to evaluate performance. Three trials were collected in each of the N-back conditions. N-back conditions were block randomized across all participants.
Working memory (N-back) + motor task evaluation (dual-task)
Working memory function was probed at a baseline (single-task) as well as during motor function evaluations (dual-task). All single-tasks occurred prior to dual-tasks to avoid subject confusion. Each subject was asked to perform a series of working memory + motor task (dual-task) interleaved by 30 s periods of rest, see Fig. 1 for details. Presentation of visual stimuli, timing, and synchronization TTL signals were controlled via E-prime 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA). Three trials were collected in each of the N-back conditions for dual-task evaluation. N-back conditions were block randomized across all participants in dual-task conditions.
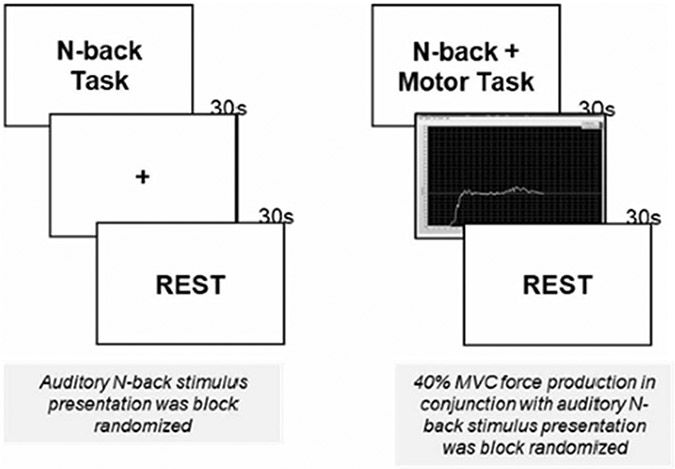
Fig. 1.
Illustration of experimental stimuli during the N-back single task and N-back + motor performance (dual-task) during fNIRS testing. Subjects viewed a fixation cross during N-back single task blocks; they viewed real-time feedback on their force production during N-back + motor performance (dual-task) blocks. The order of N-back presentation was block randomized within each testing type. N-back single task tasks always occurred prior to N-back + motor performance dual-tasks
During the working memory + motor task, participants used a precision pinch grip to exert an isometric force against a set of force transducers. Participants were instructed to match their pinch force to the target force line as accurately as possible. Two different force levels were tested for the dominant (right) hand (15% MVC and 40% MVC). Three trials of 30 s each, were performed with at 30 s of rest/washout periods between each block. Force level order (15% or 40% MVC) was block randomized.
The motor task involved using digits 1 and 2 in a precision pinch grip to produce a constant level of pinch force, with feedback from a computer screen. All forces and moments of force produced were recorded simultaneously using 2 identical 6-component force-moment transducers (Nano-25 transducers; ATI Industrial Automation, Garner, NC, USA). Instrument details have been published previously (Gorniak et al. 2014; Ochoa and Gorniak 2014).
Cortical hemodynamics measurements
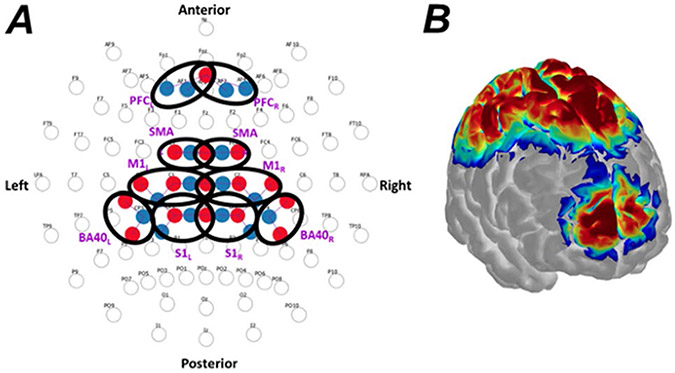
Cortical hemodynamics were measured with a continuous-wave functional near infrared spectroscopy instrument (NIRScout, NIRx Technologies, Glen Head, NY, USA) via 16 optical emitters and 16 optical detectors. Each emitter consisted in a dual-wavelength LED (central wavelengths: 760 nm and 850 nm) directly coupled to the scalp, while each detector was a silicon photodiode collecting backscattered light from the scalp via an optical fiber. The geometrical layout of optical emitters and detectors (collectively referred to as optodes) is shown in Fig. 2A, alongside the corresponding sensitivity map of the optical probing on the cerebral cortex (Fig. 2B) estimated with Monte Carlo-based simulation of photon migration in AtlasViewer (Aasted et al. 2015). We ensured reproducibility of placement to the best of our ability by fitting the standard 10–10 headset (EasyCap, Germany) with reference to anatomical landmarks (nasion Nz, inion Iz, vertex Cz, and preauricular points LPA and RPA), to achieve an optode landing according to the layout depicted in Fig. 2A. We also digitized the spatial location of all optodes and registered such position to a scalp-brain atlas (Colin 27) to ensure placement accuracy within reasonable range (10 mm from standard EEG labels). Regarding the association between optode placements and cortical regions, we inferred cortical areas interrogated by each group of optical channels (ROIs) from the sensitivity map projected onto a Colin 27 model computed with photon migration simulations using AtlasViewer. Although Fig. 2B shows the sensitivity map of the entire probe, we displayed the projections of each ROIs separately and denoted cortical regions accordingly. This configuration resulted in 28 optical channels (i.e., emitter-detector pairings) that interrogated the prefrontal, motor, and somatosensory cortices bilaterally. The geometrical distance between optode pairings ranged from 26 to 37 mm, ensuring the interrogation of the cerebral cortex in all optical channels (Strangman et al. 2013). Proper scalp-optode coupling was ensured by using the PHOEBE toolbox (Pollonini et al. 2016).
Fig. 2.
Cortical fNIRS layout and sensitivity map. A Geometrical layout of sources (red) and detectors (blue) with respect to the international 10–10 EEG system (Oostenveld and Praamstra 2001). Bold black ovals denote the regions of interest (ROIs), which are subsequently labeled nearby in purple boldface. ROIs included: prefrontal cortex (PFC), supplementary motor area (SMA), primary motor cortex (M1), primary sensory cortex (S1), and Broadmann Area 40 (B40). Hemisphere side as well as anterior and posterior of the cranium are noted. B Correspondent sensitivity map overlaid onto the Colin27 brain model. Sensitivity computed and displayed with AtlasViewer (Aasted et al. 2015)
Raw optical signals were collected continuously throughout the N-back portions of the experiment at the frequency of 3.91 Hz from all channels at both wavelengths, and were subsequently converted to optical density (i.e., logarithm of the raw intensity) and then to concentration changes of oxygenated (HbO) and deoxygenated hemoglobin (HbR) compared to a zeroed baseline according to the modified Beer-Lambert Law (Cope and Delpy 1988; Delpy et al. 1988). For each channel, HbO and HbR measurements were analyzed separately with a general linear model approach that estimated the scalar weight coefficient (a.k.a., beta weight (Barker et al. 2013)) of the canonical hemodynamic response that best fitted the measured hemodynamic response. The general linear model approach is described in detail in (Santosa et al. 2018). We did not apply particular preprocessing steps to fNIRS data, since autoregressive pre-whitening approach using iteratively reweighted least-squares (AR-IRLS) can deal with data outliers produced by motion artifacts and extracerebral and physiological responses (Santosa et al. 2018). For each subject, we considered channels as hemodynamically active if their weight coefficient was statistically different from zero at the significance level of 5%.
At the group level, we used a mixed linear model to estimate the weighting coefficient of all channels to determine which of them were hemodynamically active at a statistically significant level. We considered the interaction between the experimental condition (N-back condition) and the group (DM vs. control) as the fixed effect contributing to the weight coefficient, while the magnitude of the coefficient of individual subjects were considered as a random effect.
We grouped optical channels into ten bilateral (right and left) regions of interest (ROIs), namely the prefrontal cortex (PFC), supplementary motor area (SMA), primary motor cortex (M1), primary sensory cortex (S1), and Brodmann Area 40 (B40) as depicted in Fig. 2A. We computed individual-level ROI-level statistics (weight coefficient, t-value, p-value). Positive HbO values and negative HbR values each indicate cortical activity, respectively. Some ROIs did not produce significant t-scores in HbO or HbR. Those data are shown as zeroes in mean and standard error (SE) values in figures within the results section.
Statistical analysis
The data are presented as means ± SE. For HbO and HbR, statistically significant individual-level ROI t-scores were compared between Groups using mixed model analyses of covariance (ANCOVAs) via SPSS 25 (IBM Corporation, Armonk, NY, USA). Between-subject primary factors were Group (two levels: DM vs. controls). Within-subject factors included Hemisphere (two levels for the cortex: left and right) and ROI (five levels: 1 = PFC, 2 = SMA, 3 = M1, 4 = S1, and 5 = B40). For N-back data, main factors included: Group, Task Type (two levels: one level each for single- and dual-tasks), and Condition (three levels: 0-back, 1-back, and 2-back). Evaluation of health state covariates was done to control for health state variability both within and across the two sample groups. Covariates were selected via Automatic Linear Modeling (ALM) using forward stepwise selection functions in SPSS. ALM was utilized to reduce the potential for expectation biases that may occur when hand-selecting potential statistical models. In the event of significant covariates determined via ALM and ANCOVA, follow-up correlation analyses were performed between the health state or performance covariate and the measured behavior. ANCOVAs included health state covariates of: A1c, systolic and diastolic blood pressures, total cholesterol, high-density lipoprotein (HDL) cholesterol, disease duration, menopausal age, body mass index (BMI), PN status (via indicator variable), history of hormone replacement therapy (via indicator variable), history of treatment with Prempro (conjugated estrogens/medroxyprogesterone acetate; via indicator variable), and working memory performance variables of response time and accuracy (in HbO and HbR analyses). Specific attention to use of Prempro in our work is warranted as long-term use of Prempro is associated with development of cardiovascular disease and potential cognitive complications (Wells and Herrington 1999; Grady et al. 2002; Cagnacci and Venier 2019; Manson et al. 2020). Prempro use was largely abandoned in the early 2000’s; however, patients with a history of Prempro use are still alive. In multiple comparison situations, Bonferroni corrected posthocs were used. Significant differences are denoted by the following in figures: * at p < 0.05, ** at p < 0.001, *** at p < 0.005, and **** at p < 0.001.
Results
Cognitive evaluation
Montreal cognitive assessment (MoCA)
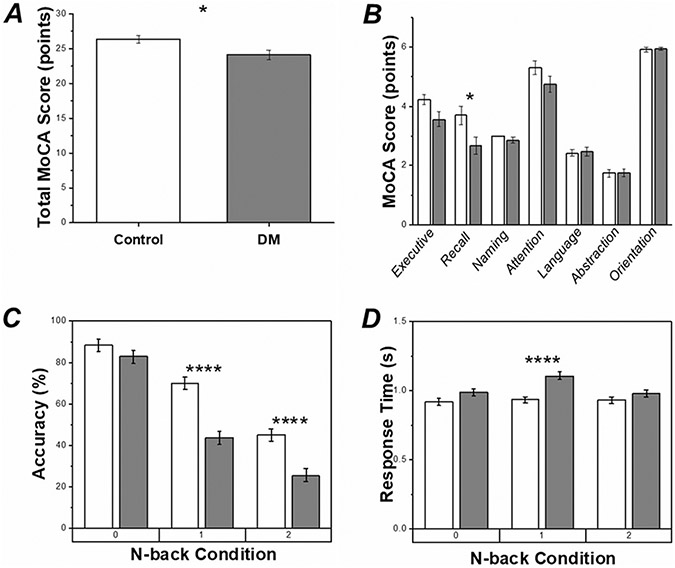
Via ALM, the MoCA data show a significant Group difference (F1,42 = 6.45, p < 0.05) in which the average total MoCA scores were lower in the DM group as compared to controls, Fig. 3A. Further analyses of the individual MoCA domains indicated Group differences in working memory/recall (F1,19 = 7.27, p < 0.05), such that working memory/recall scores in the DM group were lower as compared to controls. MoCA data scores can be found in Fig. 3B.
Fig. 3.
Group mean and standard error (SE) for MoCA and working memory data. White bars indicate data from the control group, gray bars indicate data from the DM group. Significant differences between Groups at p < 0.05 (*) and p < 0.001 (****) are shown. A Total MoCA scores. B Domain specific MoCA scores. C Correct response rates (accuracy) in N-back evaluations. D Response times in N-back evaluations
Working memory (N-back) evaluations: accuracy
Differences between single- and dual-task accuracy rates were not found via ALM; subsequent analyses of N-back data were performed collapsed across both single- and dual-task conditions. Significant Group differences in N-back accuracy were found (F1, 230 = 46.73, p < 0.001); such that the DM group was less accurate than controls (Fig. 3C). Condition (F2, 230 = 142.61, p < 0.001) and Group x Condition (F2, 230 = 6.29, p < 0.005) effects were found such that accuracy declined as the Condition became more difficult; however, the decline in accuracy was more dramatic in the DM group (Fig. 3C). When health state covariates were included in statistical analyses, the Condition (F2,123 = 170.39, p < 0.001) effect remained significant. However, health state covariates of Total Cholesterol (F1,123 = 9.95, p < 0.005), Menopausal Age (F1,123 = 14.47, p < 0.001), and Prempro Use (F1,123 = 13.86, p < 0.001) replaced the Group effect. These health state covariates were positively correlated with accuracy (Total Cholesterol: r264 = 0.277, p < 0.001; Menopausal Age: r252 = 0.219, p < 0.001; Prempro Use: r252 = 0.137, p < 0.05).
Working memory (N-back) evaluations: response time
Differences between single- and dual-task response times were not found via ALM; subsequent analyses of N-back data were performed collapsed across both single- and dual-task conditions. Group differences in N-back response times were found (F1, 217 = 21.20, p < 0.001); such that the DM group had longer response times than controls (Fig. 3D). Significant Condition (F2, 217 = 4.72, p < 0.05) and Group x Condition (F2, 217 = 3.44, p < 0.05) effects were found such that response times were generally flat in the control Group but were significantly higher in the 1-back condition for DM group as compared to all other Conditions (Fig. 3D). When health state covariates were included in statistical analyses, the main effects of Group and Condition disappeared. Instead, Total Cholesterol (F1,90 = 16.92, p < 0.001) dominated the model and was negatively correlated with response time (r240 = −0.257, p < 0.001).
Cortical hemodynamic responses
Cortical hemodynamic responses during working memory (N-back) evaluation
ALM analyses indicated significant differences in Task in the HbO data, but not the HbR data. In the following paragraphs, we present the HbO data first with results presented in the single-task separate from the dual-task. Afterwards, we present the HbR data collapsed across Task, as Task was not found to be a significant factor for HbR.
HbO data, single-task
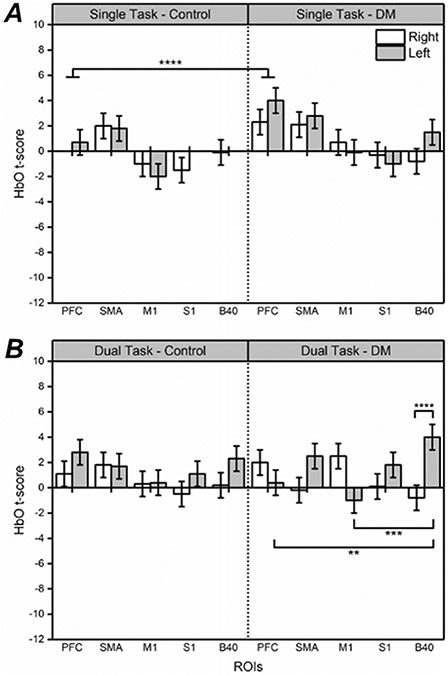
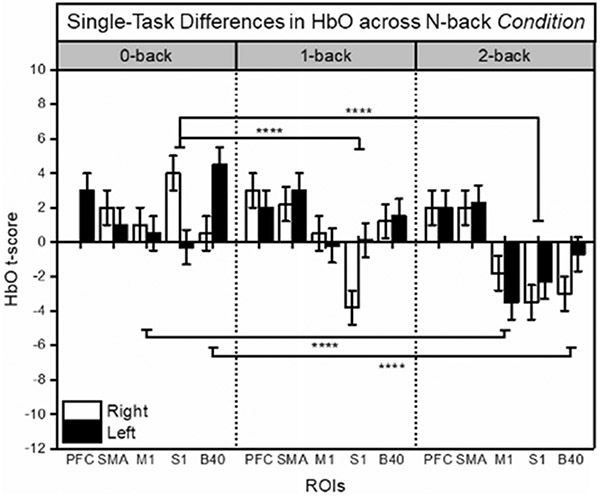
During the single-task working memory evaluation, significant effects of Group (F1,76 = 4.07, p < 0.05), ROI (F4,76 = 5.40, p < 0.001), and Condition (F2,76 = 5.77, p < 0.001) were found in HbO t-scores via ALM. Overall, the data show significantly larger average HbO t-scores in the DM Group as compared to controls; this is particularly noticeable in PFC (between Group differences are denoted in Fig. 4A). As the N-back Condition became more difficult (0-back vs. 2-back), HbO t-scores decreased significantly on average across ROIs except for PFC and SMA, denoted in Fig. 5. HbO t-scores in PFC were significantly different from S1 and M1 as N-back Condition difficulty increased (shown in Fig. 5), supported by a near significant interaction in Condition x ROI (F8,76 = 1.77, p = 0.096). No health state covariates were found impact to HbO t-scores in the single-task condition.
Fig. 4.
fNIRS t-scores for HbO during single-task and dual-task evaluations for each Group, depicted by ROI and Hemisphere. Mean and standard error (SE) values are shown. Significant at p < 0.01 (**), p < 0.005 (***), p < 0.001 (****) are shown. White bars indicate right hemisphere, gray bars indicated left hemisphere
Fig. 5.
fNIRS t-scores for HbO during N-back single-task evaluations (0-, 1-, and 2-back Conditions), depicted by ROI and Hemisphere. Data are averaged across Group. Mean and standard error (SE) values are shown. Significant differences between N-back Conditions at p < 0.001 (****) are shown. White bars indicate right hemisphere, black bars indicate left hemisphere
HbO data, dual-task
During the dual-task working memory evaluation, a significant interaction effect in HbO of Group x Side x ROI (F13,170 = 1.971, p < 0.05), shown in Fig. 4B, was found when response time and accuracy were included as covariates within the statistical model via ALM. Posthoc analysis of this data show significantly higher HbO t-scores by the DM Group in the left hemisphere in the dual-task (most notably in B40 as compared to PFC and M1), denoted in Fig. 4B. No other health state covariates were found impact to HbO t-scores in the dual-task condition.
HbR data, collapsed across task
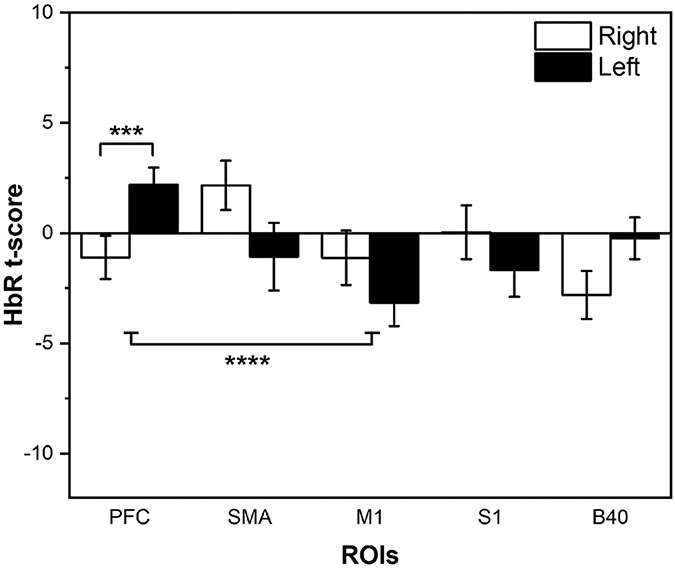
With respect to HbR, a significant effect of ROI (F4,187 = 2.60, p < 0.05) was found along with a significant Side x ROI interaction (F4,187 = 3.93, p < 0.005) via ALM, as indicated in Fig. 6. HbR t-scores showed significant asymmetry in the PFC region, as well as significant differences between PFC and M1 activation in both hemispheres (supported by posthoc testing). No health state covariates were found impact HbR t-scores.
Fig. 6.
fNIRS t-scores for HbR collapsed across all Tasks and Conditions, depicted by ROI and Hemisphere. Mean and standard error (SE) values are shown. Significant differences at p < 0.005 (***) and p < 0.001 (****) are shown. White bars indicate right hemisphere, black bars indicated left hemisphere
Discussion
The purpose of the current study was to evaluate changes in cortical oxygenation indices of postmenopausal women both with and without DM during cognitive tasks. The data support each of our hypotheses. In support of Hypothesis #1, cognitive impairment in memory/recall was observed in postmenopausal women with DM as compared to controls. Impaired memory function appeared as reduced accuracy and did not differ if the task was performed alone or coupled with a simultaneous motor task. In support of Hypothesis #2, HbO values differed between groups during memory/recall tasks; in some ROIs, differences in HbO were magnified in the DM group, suggesting changes in memory activation patterns with increased functional activity of non-PFC regions in PwDM. With respect to our exploratory arm of the study, there is an influence of poor health state and earlier menopausal age on poor memory function; however, no influence of health state was found to impact HbO or HbR. In the following paragraphs, we discuss the results of this study regarding cortical oxygenation, functional neuroimaging, the impact of health state markers, and menopause in assessment of both behavior and cortical hemodynamic function.
DM-changes in hemodynamic response and use of fNIRS
The data indicate a significant difference in the use of HbO concurrent with impaired memory function, such that the DM group exhibited differences in PFC HbO activity during dual-tasks and dedifferentiation of functional brain activity across remaining ROIs as compared to controls. Functional activity changes concurrent with deficits in working memory in the DM group indicate a functional root for memory deficits in persons with DM that is linked to HbO. This is consistent with our recent finding of altered cortical HbO use in PwDM in sensorimotor tasks (Gorniak et al. 2020). Together, these data indicate that it is a problem with the hemodynamic response that leads to behavioral deficits in DM. This supports use of behavioral monitoring along with fNIRS to detect early MCI development since techniques such as fMRI rely on the paramagnetism of HbR, thereby not fully measuring cortical hemodynamic activity which involves both HbO and HbR. Increased HbO use during dual-tasks is notable in the DM group, as HbO use is not indicated by other functional imaging techniques—including fMRI. By its nature, HbO is diamagnetic and not attracted to any magnetic field. Use of (f)MRI also limits the possible sample for study participants, as implanted devices (e.g., stents, pacemakers, etc.) commonly used to treat cardiovascular comorbidities of DM are an exclusion criterion for (f)MRI (Manschot et al. 2006; Harten et al. 2006; van Harten et al. 2007; Christman et al. 2010; Brundel et al. 2012). Techniques such as fNIRS offer better insight into cortical activity using a more inclusive approach that may better reflect early markers of MCI during realistic tasks similar to activities of daily living in populations at high risk of developing dementia (Pinti et al. 2020). Aberrations in cortical activity may be a potential biomarker for tracking changes in cognitive decline in DM using wearable technology such as fNIRS ahead of development of dementias such as Alzheimer’s disease. Detection of cortical activity differences via fNIRS provides an inclusive approach and expands monitoring eligibility for persons with implanted devices (e.g., stents, pacemakers, etc.). This is consistent with other work done in fNIRS supporting its use in investigating cognitive function with respect to both advanced age and disease (Sato et al. 2013; Bonetti et al. 2019; Beishon et al. 2021; Koo et al. 2022; St George et al. 2022; Hou et al. 2002).
Significantly different use of HbO in the cortex in DM may indicate reduced bioavailability of oxygen in DM; consistent with evidence of behavioral impairment in DM (Gorniak et al. 2020). However, the change in HbO use in the DM group during dual-tasks was not accompanied by improved memory, as accuracy and response time were generally worse in the DM group across all conditions. DM is associated with increased hemoglobin-oxygen affinity, which is responsible for lower oxygen delivery rates to tissue (Pu et al. 2012). DM is also associated with impaired hyperemic response, endothelial dysfunction, and microvascular dysfunction (Meyer et al. 2008; Petrofsky 2011; Barwick et al. 2016; Pollonini et al. 2020). However, the increased use of HbO in the DM group within the current data set indicate that increased hemoglobin-oxygen affinity does not contribute to the observed memory deficits; rather the impairment in vascular function drives memory deficits in DM.
Impaired memory function and cortical activity changes in DM
The DM group exhibited significant bilateral PFC activation via HbO in dual-tasks as compared to controls, despite memory error rates not improving with increased PFC activity. These activity differences co-occurred with activation of non-PFC cortical areas involved in movement, priming for movement, phonological processing, and emotional responses (M1, SMA, and B40 respectively). This DM-specific shift in HbO use is a novel finding that cannot be detected by fMRI. An increase of HbO along with higher HbO values in other measured ROIs suggests distributed cortical HbO activity in DM in an attempt to compensate for memory deficits. This change in HbO was not accompanied by Group differences in HbR use, suggesting that altered HbO use across the cortex is the driver of memory deficits in DM. Changes in HbO in the DM group are supported by evidence of increased HbO use in the primary visual cortex in PwDM during visual stimulation (Aitchison et al. 2018), and may suggest an increased sympathetic drive in the autonomic nervous system in postmenopausal women (Barnes et al. 2014). These differences may also suggest potential advanced aging of the brain via cortical dedifferentiation in PwDM beyond what is to be expected with healthy aging (Koen et al. 2020; Seider et al. 2021; Rabipour et al. 2021).
Changes in PFC activity in HbO use are consistent with reports of hypothalamic–pituitary–adrenal axis (HPA) dysfunction, insulin signaling aberrations, and pathological changes in hippocampal functions all associated with DM (Sullivan and Gratton 2002; Eichenbaum 2017; Soto et al. 2019). The PFC-hippocampus interaction is known to be important for episodic memory (Eichenbaum 2017). Metabolic disruption of PFC-hippocampus via endocrine dysfunction in DM impacts memory and behavior (Sullivan and Gratton 2002; Ho et al. 2013). Aberrations in PFC activity spurred by changes in the HPA axis in DM are consistent with impaired stress coping ability and symptoms of cognitive decline (Sullivan and Gratton 2002; Ho et al. 2013)—in line with our observations of impaired working memory in DM (Gorniak et al. 2019a, b).
Influence of health state variables and menopause
Reports of the link between metabolic syndrome and cognitive impairment abound in the evidence base (Yaffe et al. 2004). This is supported by our findings of some health state markers (e.g., lipidemia) being associated with impaired memory function in DM (Gorniak et al. 2019a, b), such that PwDM on statins for lipidemia exhibit lower total cholesterol scores but impaired memory function as compared to controls with higher total cholesterol scores who may not take statins for lipidemia management. No significant influences of health state variables on cortical activity were found in the current study. Our prior work on sensorimotor function indicated that health state variables clarified functional cortical activity deficits in DM. The lack of similar result in the current data indicate that hemodynamic response of some cortical regions may not be moderated by commonly measured health state variables (e.g., cholesterol). Cortical regions closely linked to the limbic system, such as PFC and B40, may be more significantly impacted by disruptions to the neuroendocrine system instead. Such disruption may impact cortical activity by blunting both neurovascular and hemodynamic responses (Drew 2019).
Consistent with (Grady et al. 2002), working memory data was impacted by menopausal age and use of specific hormone replacement therapies (HRT). Increased menopausal age (resulting in a shorter time between menopause and participation in the current study) and Prempro use were associated with higher working memory accuracy. Menopausal age was significantly different between the DM (43 ± 11 years) and control (50 ± 7 years) groups (t40 = 2.85, p < 0.05); however, DM-related deficits in accuracy persisted once menopausal age was considered in our statistical models. In contrast, no significant influences of menopause or HRT were found on cortical activity. The lack of a specific impact of menopausal age on cortical hemodynamic response during memory tasks is an intriguing outcome, as menopause is associated with impaired hemodynamic responses of the cortex and skeletal muscle during sensorimotor tasks (Pollonini et al. 2020; Gorniak et al. 2020). There is some evidence that HRT improves hemodynamic responses in postmenopausal females (Peterson et al. 2000; Fadel et al. 2004); however, it is unclear if HRT is also protective against deficits induced by a combination of DM and menopause. It is also unclear if and how HRT during a certain time window (e.g., early) during menopause may protect both cardiovascular and cognitive function (Grady et al. 2002; Cagnacci and Venier 2019; Manson et al. 2020). A complex interplay among menopause, menopausal symptoms, sex-hormones, and cognitive decline has been suggested (Maki 2015; Cagnacci and Venier 2019; Manson et al. 2020; Maki and Thurston 2020); however, further work is needed to assess what features of menopause may truly underlie memory decline in women, particularly women with DM.
Conclusion
Deficits in working memory accuracy were found in the DM group as compared to controls. Differences in HbO responses occurred such that the DM group exhibited altered PFC activity magnitudes and evidence of increased of functional cortical activity across remaining ROIs. HbO responses in the DM group were not associated with worsened health state measures (e.g., lipidemia). These data indicate a shift in cortical activity regarding memory use in DM concurrent with poor memory. This DM-specific shift of HbO use is a novel finding that cannot be detected by fMRI and is consistent with HPA dysfunction. This work underscores the value of using wearable non-MRI-based neuroimaging technology to monitor functional deficits to detect mild cognitive impairment using a more inclusive approach.
Acknowledgements
This work was supported by American Heart Association Grant (AHA) #16BGIA27250047 to SLG. SMH and LP acknowledge the support of the National Science Foundation under Grant No. CNS 1650536 and 2137255: I/UCRC for Building Reliable Advances and Innovation in Neurotechnology (BRAIN). LP also acknowledges the U.S. Fulbright Scholar Program and the Fulbright Spain Commission for sponsoring his stay at the Basque Center on Cognition, Brain and Language.
Funding
AHA had no role in the design of the study, collection/analysis/interpretation of data, nor the writing/submission of this article.
Footnotes
Code, data, and materials availability Data analyzed in this project will be available via Zenodo (https://zenodo.org/) upon manuscript publication. As public sharing of protected health information such as date of birth, date of diagnosis, dates of treatment and other private medical history information pertinent to each participant may violate HIPAA and the Texas Medical Privacy Act, these data will not be shared to protect patient identity.
Conflict of interest None of the authors has any conflict of interest to disclose.
References
- Aasted CM, Yücel MA, Cooper RJ et al. (2015) Anatomical guidance for functional near-infrared spectroscopy: AtlasViewer tutorial. Neurophotonics 2:020801. 10.1117/1.NPh.2.2.020801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitchison RT, Ward L, Kennedy GJ et al. (2018) Measuring visual cortical oxygenation in diabetes using functional near-infrared spectroscopy. Acta Diabetol 55:1181–1189. 10.1007/s00592-018-1200-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JW, Aarabi A, Huppert TJ (2013) Autoregressive model based algorithm for correcting motion and serially correlated errors in fNIRS. Biomed Opt Express 4:1366–1379. 10.1364/BOE.4.001366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JN, Hart EC, Curry TB et al. (2014) Aging enhances autonomic support of blood pressure in women. Hypertension 63:303–308. 10.1161/HYPERTENSIONAHA.113.02393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barwick AL, Tessier JW, Janse de Jonge X et al. (2016) Peripheral sensory neuropathy is associated with altered postocclusive reactive hyperemia in the diabetic foot. BMJ Open Diab Res Care 4:e000235. 10.1136/bmjdrc-2016-000235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Zucker I (2011) Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35:565–572. 10.1016/j.neubiorev.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beishon L, Panerai RB, Robinson TG, Haunton VJ (2021) Cerebral blood flow response rate to task-activation using a novel method can discriminate cognitive impairment from healthy aging. Physiol Meas. 10.1088/1361-6579/ac1185 [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Reijmer YD (2014) Brain changes underlying cognitive dysfunction in diabetes: what can we learn from MRI? Diabetes 63:2244–2252. 10.2337/db14-0348 [DOI] [PubMed] [Google Scholar]
- Bonetti LV, Hassan SA, Lau S-T et al. (2019) Oxyhemoglobin changes in the prefrontal cortex in response to cognitive tasks: a systematic review. Int J Neurosci 129:195–203. 10.1080/00207454.2018.1518906 [DOI] [PubMed] [Google Scholar]
- Brundel M, van den Berg E, Reijmer YD et al. (2012) Cerebral haemodynamics, cognition and brain volumes in patients with Type 2 Diabetes. J Diabetes Complicat 26:205–209. 10.1016/j.jdiacomp.2012.03.021 [DOI] [PubMed] [Google Scholar]
- Buxton RB (2013) The physics of functional magnetic resonance imaging (fMRI). Rep Prog Phys 76:096601. 10.1088/0034-4885/76/9/096601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnacci A, Venier M (2019) The Controversial History of Hormone Replacement Therapy. Medicina (kaunas) 55:602. 10.3390/medicina55090602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campesi I, Franconi F, Seghieri G, Meloni M (2017a) Sex-gender-related therapeutic approaches for cardiovascular complications associated with diabetes. Pharmacol Res 119:195–207. 10.1016/j.phrs.2017.01.023 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2020) National Diabetes Statistics Report. 32. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf [Google Scholar]
- Christman AL, Vannorsdall TD, Pearlson GD et al. (2009) Cranial volume, mild cognitive deficits, and functional limitations associated with diabetes in a community sample. Arch Clin Neuropsychol 25:49–59. 10.1093/arclin/acp091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman AL, Vannorsdall TD, Pearlson GD et al. (2010) Cranial volume, mild cognitive deficits, and functional limitations associated with diabetes in a community sample. Arch Clin Neuropsychol 25:49–59. 10.1093/arclin/acp091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope M, Delpy DT (1988) System for long-term measurement of cerebral blood and tissue oxygenation on newborn infants by near infra-red transillumination. Med Biol Eng Comput 26:289–294. 10.1007/bf02447083 [DOI] [PubMed] [Google Scholar]
- Delpy DT, Cope M, van der Zee P et al. (1988) Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol 33:1433–1442. 10.1088/0031-9155/33/12/008 [DOI] [PubMed] [Google Scholar]
- Drew PJ (2019) Vascular and neural basis of the BOLD signal. Curr Opin Neurobiol 58:61–69. 10.1016/j.conb.2019.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2017) Prefrontal–hippocampal interactions in episodic memory. Nat Rev Neurosci 18:547–558. 10.1038/nrn.2017.74 [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Wang Z, Watanabe H et al. (2004) Augmented sympathetic vasoconstriction in exercising forearms of postmenopausal women is reversed by oestrogen therapy. J Physiol 561:893–901. 10.1113/jphysiol.2004.073619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold DA (2012) An examination of instrumental activities of daily living assessment in older adults and mild cognitive impairment. J Clin Exp Neuropsychol 34:11–34 [DOI] [PubMed] [Google Scholar]
- Gorniak SL, Khan A, Ochoa N et al. (2014) Detecting subtle fingertip sensory and motor dysfunction in adults with Type II Diabetes. Exp Brain Res 232:1283–1291 [DOI] [PubMed] [Google Scholar]
- Gorniak SL, Lu FY, Lee BC et al. (2019) Cognitive impairment and postural control deficit in adults with Type 2 Diabetes. Diabetes Metab Res Rev 35:e3089. 10.1002/dmrr.3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorniak SL, Ray H, Lee B-C, Wang J (2019) Cognitive-motor impairment in manual tasks in adults with Type 2 Diabetes. OTJR Occup Particip Health 40:113–121. 10.1177/1539449219880536 [DOI] [PubMed] [Google Scholar]
- Gorniak SL, Wagner VE, Vaughn K et al. (2020) Functional neuroimaging of sensorimotor cortices in postmenopausal women with Type II Diabetes. NPh 7:035007. 10.1117/1.NPh.7.3.035007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady D, Yaffe K, Kristof M et al. (2002) Effect of postmenopausal hormone therapy on cognitive function: the Heart and Estrogen/progestin Replacement Study. Am J Med 113:543–548. 10.1016/s0002-9343(02)01270-6 [DOI] [PubMed] [Google Scholar]
- Ho N, Sommers MS, Lucki I (2013) Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci Biobehav Rev 37:1346–1362. 10.1016/j.neubiorev.2013.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Yang J, Xu L et al. (2002) Activation of brain regions associated with working memory and inhibitory control in patients with attention-deficit/hyperactivity disorder in functional near-infrared spectroscopy: a systematic review. Curr Med Imag 18:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G (2014) Functional magnetic resonance imaging, 3 edition. Sinauer associates is an imprint of Oxford University Press, Sunderland, Massachusetts, USA [Google Scholar]
- Janoutová J, Šerý O, Hosák L, Janout V (2015) Is mild cognitive impairment a precursor of Alzheimer’s disease? Short review. Cent Eur J Public Health 23:365–367. 10.21101/cejph.a4414 [DOI] [PubMed] [Google Scholar]
- Kautzky-Willer A, Harreiter J, Pacini G (2016) Sex and gender differences in risk, pathophysiology and complications of Type 2 Diabetes mellitus. Endocr Rev 37:278–316. 10.1210/er.2015-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen JD, Srokova S, Rugg MD (2020) Age-related neural dedifferentiation and cognition. Curr Opin Behav Sci 32:7–14. 10.1016/j.cobeha.2020.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo YW, Neumann DL, Ownsworth T et al. (2022) Understanding the neural basis of prospective memory using functional near-infrared spectroscopy. Front Hum Neurosci. 10.3389/fnhum.2022.905491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz HE (2006) Insulin resistance–a common link between Type 2 Diabetes and cardiovascular disease. Diabetes Obes Metab 8:237–249. 10.1111/j.1463-1326.2005.00521.x [DOI] [PubMed] [Google Scholar]
- Maki PM (2015) Verbal memory and menopause. Maturitas 82:288–290. 10.1016/j.maturitas.2015.07.023 [DOI] [PubMed] [Google Scholar]
- Maki PM, Thurston RC (2020) Menopause and brain health: hormonal changes are only part of the story. Front Neurol. 10.3389/fneur.2020.562275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschot SM, Brands A, van der Grond J et al. (2006) Brain magnetic resonance imaging correlates of impaired cognition in patients with Type 2 Diabetes. Diabetes 55:1106. [DOI] [PubMed] [Google Scholar]
- Manson JE, Bassuk SS, Kaunitz AM, Pinkerton JV (2020) The Women’s Health Initiative trials of menopausal hormone therapy: lessons learned. Menopause 27:918–928. 10.1097/GME.0000000000001553 [DOI] [PubMed] [Google Scholar]
- Meyer MF, Lieps D, Schatz H, Pfohl M (2008) Impaired flow-mediated vasodilation in Type 2 Diabetes: lack of relation to microvascular dysfunction. Microvasc Res 76:61–65. 10.1016/j.mvr.2008.03.001 [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V et al. (2005) The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Ochoa N, Gorniak SL (2014) Changes in sensory function and force production in adults with Type II Diabetes. Muscle Nerve 50:984–990 [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113 [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Praamstra P (2001) The five percent electrode system for high-resolution EEG and ERP measurements. Clin Neurophysiol 112:713–719. 10.1016/s1388-2457(00)00527-7 [DOI] [PubMed] [Google Scholar]
- Peterson LR, Courtois M, Peterson LF et al. (2000) Estrogen increases hyperemic microvascular blood flow velocity in postmenopausal women. J Gerontol A Biol Sci Med Sci 55:M174–M179. 10.1093/gerona/55.3.M174 [DOI] [PubMed] [Google Scholar]
- Petrofsky JS (2011) The effect of type-2-diabetes-related vascular endothelial dysfunction on skin physiology and activities of daily living. J Diabetes Sci Technol 5:657–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinti P, Tachtsidis I, Hamilton A et al. (2020) The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann N Y Acad Sci 1464:5–29. 10.1111/nyas.13948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollonini L, Bortfeld H, Oghalai JS (2016) PHOEBE: a method for real time mapping of optodes-scalp coupling in functional near-infrared spectroscopy. Biomed Opt Express 7:5104–5119. 10.1364/B0E.7.005104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollonini L, Gulley Cox L, Gorniak SL (2020) Hemodynamic function of forearm muscle in postmenopausal women with Type 2 Diabetes . J Aging Phys Act. 10.1123/japa.2019-0221 [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I (2014) Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev 40:1–5. 10.1016/j.neubiorev.2014.01.001 [DOI] [PubMed] [Google Scholar]
- Pu LJ, Shen Y, Lu L et al. (2012) Increased blood glycohemoglobin A1c levels lead to overestimation of arterial oxygen saturation by pulse oximetry in patients with Type 2 Diabetes. Cardiovasc Diabetol 11:110. 10.1186/1475-2840-11-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabipour S, Rajagopal S, Pasvanis S, Rajah MN (2021) Generalization of memory-related brain function in asymptomatic older women with a family history of late onset Alzheimer’s disease: Results from the PREVENT-AD Cohort. Neurobiol Aging 104:42–56. 10.1016/jj.neurobiolaging.2021.03.009 [DOI] [PubMed] [Google Scholar]
- Raparelli V, Morano S, Franconi F et al. (2017) Sex differences in Type-2 Diabetes: implications for cardiovascular risk management. Curr Pharm Des 23:1471–1476. 10.2174/1381612823666170130153704 [DOI] [PubMed] [Google Scholar]
- Santosa H, Zhai X, Fishburn F, Huppert T (2018) The NIRS Brain AnalyzIR Toolbox. Algorithms 11:73. 10.3390/a11050073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Yahata N, Funane T et al. (2013) A NIRS–fMRI investigation of prefrontal cortex activity during a working memory task. Neuroimage 83:158–173. 10.1016/).neuroimage.2013.06.043 [DOI] [PubMed] [Google Scholar]
- Seghieri G, Policardo L, Anichini R et al. (2017) The effect of sex and gender on diabetic complications. Curr Diabetes Rev 13:148–160. 10.2174/1573399812666160517115756 [DOI] [PubMed] [Google Scholar]
- Seider TR, Porges EC, Woods AJ, Cohen RA (2021) Dedifferentiation of functional brain activation associated with greater visual discrimination accuracy in middle-aged and older adults. Front Aging Neurosci 13:651284. 10.3389/fnagi.2021.651284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto M, Cai W, Konishi M, Kahn CR (2019) Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior. PNAS 116:6379–6384. 10.1073/pnas.1817391116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St George RJ, Jayakody O, Healey R et al. (2022) Cognitive inhibition tasks interfere with dual-task walking and increase prefrontal cortical activity more than working memory tasks in young and older adults. Gait Posture 95:186–191. 10.1016/j.gaitpost.2022.04.021 [DOI] [PubMed] [Google Scholar]
- Strangman GE, Li Z, Zhang Q (2013) Depth Sensitivity and Source-Detector Separations for Near Infrared Spectroscopy Based on the Colin27 Brain Template. PLOS ONE 8:e66319. 10.1371/journal.pone.0066319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A (2002) Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology 27:99–114. 10.1016/s0306-4530(01)00038-5 [DOI] [PubMed] [Google Scholar]
- van den Berg E, Dekker JM, Nijpels G et al. (2008) Cognitive functioning in elderly persons with Type 2 Diabetes and metabolic syndrome: the hoorn study. Dement Geriatr Cogn Disord 26:261–269. 10.1159/000160959 [DOI] [PubMed] [Google Scholar]
- van Harten B, Oosterman JM, Potter van Loon B-J et al. (2007) Brain lesions on MRI in elderly patients with Type 2 Diabetes mellitus. Eur Neurol 57:70–74. 10.1159/000098054 [DOI] [PubMed] [Google Scholar]
- van Harten B, de Leeuw F-E, Weinstein HC et al. (2006) Brain imaging in patients with Diabetes: a systematic review. Diabetes Care 29:2539–2548. 10.2337/dc06-1637 [DOI] [PubMed] [Google Scholar]
- Vance D, Larsen KI, Eagerton G, Wright MA (2011) Comorbidities and cognitive functioning. J Neurosci Nurs 43:215–224. 10.1097/JNN.0b013e3182212a04 [DOI] [PubMed] [Google Scholar]
- Vitale C, Fini M, Spoletini I et al. (2017) Under-representation of elderly and women in clinical trials. Int J Cardiol 232:216–221. 10.1016/j.ijcard.2017.01.018 [DOI] [PubMed] [Google Scholar]
- Wells G, Herrington DM (1999) The Heart and estrogen/progestin replacement study: what have we learned and what questions remain? Drugs Aging 15:419–422. 10.2165/00002512-199915060-00001 [DOI] [PubMed] [Google Scholar]
- Yaffe K, Kanaya A, Lindquist K et al. (2004) The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 292:2237–2242. 10.1001/jama.292.18.2237 [DOI] [PubMed] [Google Scholar]
- Zochodne DW (2007) Diabetes mellitus and the peripheral nervous system: manifestations and mechanisms. Muscle Nerve 36:144–166. 10.1002/mus.20785 [DOI] [PubMed] [Google Scholar]