Abstract
The lack of site-specific chemotherapeutic agents after osteosarcoma surgeries often induce severe side effects. We propose the utilization of curcumin as an alternative natural chemo-preventive drug for tumor-specific delivery systems with 3D printed tricalcium phosphate (TCP) based artificial bone grafts. The poor bioavailability and hydrophobic nature of curcumin restrict its clinical use. We have used polydopamine (PDA) coating with Zn2+ functionalization to enhance the curcumin release in the biological medium. The obtained PDA- Zn2+ complex is characterized by X-ray photoelectron spectroscopy (XPS). The presence of PDA- Zn2+ coating leads to ~ 2 times enhancement in curcumin release. We have computationally predicted and validated the optimized surface composition by a novel multi-objective optimization method. The experimental validation of the predicted compositions indicates that the PDA-Zn2+ coated curcumin immobilized delivery system leads to a ~ 12 folds decrease in osteosarcoma viability on day 11 as compared to only TCP. The osteoblast viability shows ~ 1.4 folds enhancement. The designed surface shows the highest ~ 90% antibacterial efficacy against gram-positive and gram-negative bacteria. This unique strategy of curcumin delivery with PDA-Zn2+ coating is expected to find application in low-load bearing critical-sized tumor-resection sites.
Keywords: Additive Manufacturing, Drug Delivery, Curcumin, Polydopamine coating, Calcium Phosphate, Antibacterial surface
Graphical Abstract

The graphical abstract summarizes our work, starting from substrate fabrication with 3D printing, PDA-Zn2+ coating, curcumin loading, and computational optimization, followed by the assessment of resultant biological properties such as antibacterial properties, enhanced osteoblast viability, and osteosarcoma inhibition.
1. Introduction
Osteosarcoma is the third most common form of cancer for pediatric patients. The life span of more than 70% of patients with recurring osteosarcoma is under 5 years [1,2]. Sometimes, a critical-sized, patient-specific bone graft is required to support new bone growth after tumor removal [3]. This treatment approach for osteosarcoma has five major drawbacks (a) complex surgical procedure (b) requirement of a defect-specific bone graft (c) lack of localized chemopreventive agents (d) possibility of post-surgical infection, and (e) implant loosening due to insufficient bone growth [4–6].
Several other musculoskeletal disorders due to birth defects, trauma, war injuries, and the active lifestyle of younger patients impose a total of $ 136.8 billion in annual burdens on the US economy [7]. The major scientific question posed in this study is, can we optimize the design of a patient-specific and defect-specific artificial bone graft substitute for localized drug delivery that can simultaneously inhibit osteosarcoma and promote bone-forming osteoblast cells, with ensuring antibacterial efficacy? To achieve this goal, our strategy is to utilize calcium phosphate-based 3D printed bone grafts as a substrate, curcumin from turmeric as a natural chemo-preventive agent, and polydopamine-Zn2+ complex as a multifunctional osteoblast promoting and antibacterial coating.
The ancient Indian medical text Ayurveda documents the utilization of curcumin, the active polyphenol compound of turmeric (Curcuma longa) as an immunity booster, and anti-inflammatory herbal medicine [8,9]. Modern research and preclinical studies show that curcumin induces cytotoxicity toward various tumor cells [10]. Currently, drug-resistant cancer cells are a serious concern that needs to be addressed by utilizing alternative chemopreventive agents [11]. In this regard, curcumin has an added advantage over available chemotherapeutic drugs. However, its poor bioavailability, low solubility, and slower absorption in a biological medium hinder clinical applications [12]. Polydopamine (PDA) is a unique polymer, known for its versatile coating ability [13,14]. PDA has excellent biocompatibility, metal-chelating ability, free radical scavenging potential, wound-repairing properties, antibacterial potential, and antioxidating potential [15–17]. However, a detailed study about the application of PDA coating for a 3D printed TCP-curcumin-based localized drug delivery system has not been performed yet. This work aims to answer the parenting question regarding the applicability of this coating when investigated from a bone tissue engineering point of view. The evaluation of PDA coating’s cytotoxicity is still an open debate and there is no available report to predict its cytotoxicity by computational experimentations.
Antimicrobial surfaces and an implant with inherent antibacterial properties are expected to be beneficial in osteomyelitis, which accounts for a majority of the costlier and more complex revision surgeries [18]. Zinc is an essential element with antibacterial properties that also help in new bone formation, DNA synthesis, and protein synthesis [19]. The possibility of utilizing PDA-Zn2+ complex coatings for curcumin delivery is yet to be explored. To maximize the outcome of this multi-component system in terms of antibacterial properties, osteosarcoma inhibition, and osteoblast proliferation, it is essential to optimize the PDA coating time, Zn2+ functionalization level, and the amount of curcumin. The general approach to optimization for such a multicomponent system is the time-consuming trial-and-error method. Multi-objective optimization strategies through computational methods not only address the limitations of trial-and-error methods but also expediates the optimization process and fosters new material development [20]. Our work aims to develop a novel computational optimization strategy to achieve the maximum possible output in terms of in vitro biological properties for this multifunctional system with PDA- Zn2+ and curcumin.
Additive manufacturing or 3D printing is the only manufacturing method that can produce defect-specific and patient-specific artificial bone grafts with interconnected porosity and complex designs [7,21]. The presence of porosity in 3D-printed grafts helps in nutrient supply and new bone formation after implantation [22–24]. Parametric optimization during 3D printing is important to ensure the production of a functional and porous graft that can help new bone regeneration by providing a suitable microenvironment [25]. Binder jetting-based additive manufacturing of ceramics allows the fabrication of porous Tricalcium phosphate [TCP, Ca10(PO4)6] bone grafts for low load-bearing skeletal reconstruction [26,27]. However, the possibility of utilizing these 3D printed PDA- Zn2+ coated grafts for curcumin delivery with multifunctionality has not been studied before.
The objectives of our work are two folds (a) to investigate the applicability of PDA- Zn2+ coating on 3D printed porous TCP substrates, from the perspective of curcumin delivery for bone tissue engineering applications and (b) to computationally optimize the PDA coating time, PDA- Zn2+ molar ratio, and curcumin amount, a combination of which can maximize the biological outcomes. The novelty of this work lies in (a) the computational optimization of this multifunctional system from a bone-tissue engineering perspective and (b) the demonstration of enhanced biological properties in the presence of these components. The experimental and computational results indicate that 3D-printed porous TCP grafts with PDA-Zn2+ coatings show enhanced curcumin delivery. The novel multi-objective optimization strategy regarding the PDA coating time, PDA: Zn2+ molar ratio, and curcumin level is successfully validated by physical experiments. Curcumin immobilization or incorporation in the fabricated 3D printed grafts with PDA-Zn2+ coating leads to enhanced biological properties.
2. Experimental
2.1. Substrate preparation
The polydopamine was coated on calcium phosphate substrates fabricated by binder jet-based 3D printing. One previously reported method by our group was followed to prepare pure β-TCP powder by solid-state synthesis [28]. In summary, a 2:1 molar ratio of dicalcium phosphate (calcium phosphate dibasic, CaHPO4, ≥98.0%) and calcium carbonate (CaCO3, ≥99.0%) (Sigma-Aldrich, St. Louis, MO) were ball milled for 2 h at speed 70 using zirconia milling media (2:1 weight ratio of the ball: powder), followed by calcination at 1050 °C for 24 h [29]. In the next step, wet milling of the calcined powder was carried out with 1.5x w/v of ethanol in the presence of 2x w/w milling media to powder ratio for 2 h (at 300 rpm). The solvent ethanol was then evaporated, and the dried powder was washed with deionized water for the removal of impurities. After this, the powders were further dried at 250 °C to remove chemisorbed moisture. With the dried powders, TCP scaffolds of 400 μm designed porosity, 11 mm height, and 7- or 3.5-mm diameter were fabricated using a binder jet 3D printer (ExOne LLC, Irwin, PA, USA) [24]. Porous corkscrew structure and cylindrical structure with 400 μm designed porosity, and ridge structures without any designed porosity were fabricated. Careful optimization of the printing parameters was done to ensure crack-free workable parts. The green scaffolds were cured at 175 °C for 1.5 h, followed by the removal of the loosely adherent powders using compressed air. After this depowderization step, the green scaffolds were sintered at 1250 °C for 2 h [30]. The in vitro cell and bacterial studies were carried out using 3D printed circular scaffolds with 5 mm diameter and 3 mm height.
2.2. Coating with polydopamine and Zn2+ functionalized polydopamine
The polydopamine coating was carried out as per the previously reported works [13]. 2 mg mL−1 dopamine hydrochloride [β-(3,4-dihydroxy phenyl)- ethylamine hydrochloride 98%), Millipore Sigma] was dissolved in 0.05 M Tris buffer solution at a pH of 8.5. Each substrate was kept inside a glass vial and submerged in 5 mL of this solution for different periods (0.5 h, 3 h, 10 h, 16 h, 24 h, and 48 h) under constant shaking at 37 °C in dark. To make the polydopamine-zinc complex coating, different concentrations of ZnCl2 were dissolved in 250 mL DI water and ultrasonicated, followed by the addition of Tris buffer and dopamine hydrochloride. Different molar ratios of PDA: Zn2+ (1:0.05, 1:0.2, 1:0.8, 1:1, 1:3, and 1:4) were used for the optimization and initial biological properties assessment studies, and the substrates were coated in a method like that of PDA coating.
2.3. X-ray diffraction (XRD) study
Phase identification of pure TCP and PDA-Zn2+ coated TCP was performed by using the X-Ray diffraction (Siemens D500 diffractometer) in a 2θ range of 20° to 60° (step size of 0.05°). The data were collected at 40 kV, and 30 mA, using Cu Kα radiation.
2.4. Drug loading and release
For in vitro optimization studies, ethanolic solution of different amounts of curcumin (100 μg, 200 μg, 400 μg, 600 μg, 1000 μg, and 1500 μg) was loaded on top of TCP substrates by drop casting method [31]. Another set of samples was prepared by incorporating curcumin in the dopamine hydrochloride-Tris-ZnCl2 solution during the polymerization step and the grafts were coated with this solution, as followed for PDA coating. In vitro curcumin release was quantified in phosphate buffer solution (PBS) at pH 7.4 and acetate buffer solution at pH 5.0 for 30 days. The pH of 7.4 was used to mimic the physiological pH and acidic microenvironments after surgery was replicated by using pH 5.0 [32].
The drug-containing samples were submerged in 4 mL of the buffer, in a glass vial and kept in the dark at 37 °C inside a shaker (150 rpm). At each time point, the buffer solution was collected and replaced with fresh ones. The curcumin release was quantified by measuring the optical density of 100 μL of the collected buffer solution using a Biotek (VT, USA) Synergy 2 SLFPTAD microplate reader at 427 nm. A standard calibration curve of curcumin was computed with different concentrations of the drug and a cumulative release (%), based on the standard curve is reported.
2.5. Analysis of the drug release kinetics
The release kinetics data were fitted as per the power-law model [33]. The corresponding equation is provided below:
| (1) |
In the above equations, , is the cumulative drug release (%) and t is the time in days. is the order of reaction and is the constant.
2.6. Nuclear magnetic resonance (NMR) study
The chemical structures of curcumin, PDA, and PDA-Zn2+ powders were analyzed using 1H NMR. The powders were suspended in dimethyl sulfoxide (DMSO) and the spectra were collected using a Bruker DRX 400 MHz spectrometer.
2.7. X-ray photoelectron spectroscopy (XPS)
The XPS study of dried PDA and PDA-zinc powders was carried out in Kratos AXIS 165 multi-technique electron spectrometer using an MgKα source. The high-resolution spectra were taken with 40 eV pass energy and 120 μm spot size. The obtained data and deconvoluted peaks of high-resolution spectra were analyzed after normalizing the peaks for carbon.
2.8. In vitro cell-material interaction: osteoblast cell viability
2.8.1. Cell seeding on the sample surface
The potential of these samples for osteoblast viability was assessed up to day 11 by using human fetal osteoblast cells (hFOB, obtained from ATCC, Manassas, VA), as reported in our previous works [26]. The cell media was made using a 1:1 mixture of Ham’s F12 medium and Dulbecco’s Modified Eagle’s Medium (DMEM/F12, Sigma, St. Louis, MO). To prepare the basal medium, a 2.5mM sterilized L-glutamine (without phenol red) solution was utilized. This media was further mixed with fetal bovine serum (10% FBS, ATCC, Manassas, VA) and 0.3mg/mL G418 (Sigma, St. Louis, MO) to make the cell culture media [34]. In the next step, sterilized samples were placed on a 24-well plate inside the culture hood. 40 ×103 cells were seeded on top of each sample surface and 1 mL growth media was added followed by incubation at 37 °C under a 5 % CO2 atmosphere.
2.8.2. Cell viability assessment by MTT assay
The cell viability was quantified with MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay at each timepoint of cell-material interaction [35]. On each day, the samples were transferred to a new well plate and 100 μL of MTT (Sigma, St. Louis, MO) solution was added to each sample top followed by the addition of 900 μL cell media. The well plates were then incubated at 37 °C for 2 h. Next, the mixed solution was replaced with 600 μL of MTT solubilizer solution (a mixture of 10% Triton X-100, 0.1M HCl, and isopropanol) on each sample. The optical density of 100 μL of this obtained solution was measured at 570 nm with a UV–Vis microplate reader (BioTek). Triplicate methods were followed for each test, ensuring the repeatability of the results.
2.8.3. Cell differentiation assessment with Alkaline Phosphatase assay
The osteoblast differentiation was assessed by alkaline phosphatase (ALP) assay (SensoLyte pNPP ALP Assay Kit, AnaSpec, CA, USA). First, the sample washing was done with 1X assay buffer, followed by the addition of 20 μL Triton X-100 in 10 mL of the 1X assay buffer. Adhered cells on the sample top were scraped off after adding this solution to each sample and the resultant suspension was put in an incubator for 10 min at 4 °C with constant shaking. In the next step, centrifugation was done at 2,500 rpm for 10 min at 4 °C. After centrifugation, the resultant suspension was poured into a 96-well plate followed by the addition of 50 μL pNPP solution. The plate was shaken for 30 s to mix the reagents, followed by 45 min incubation at room temperature. Next, 50 μL of the stop solution was poured into each well and the absorbance was measured at 405 nm after shaking the plate for 1 min. The obtained data is plotted as ALP/cell ratio after normalizing the optical densities, obtained in ALP and MTT (OD405/0D570).
2.8.4. Cellular morphology assessment
The cellular morphology and attachment were assessed with a field-emission scanning electron microscope (FESEM) (FEI Inc., Hillsboro, OR, USA). The sample fixation was done using 2% paraformaldehyde and 2% glutaraldehyde in 0.1 M phosphate buffer, followed by overnight refrigeration at 4 °C. In the next step, each sample was rinsed thrice with 0.1 M phosphate buffer solution, followed by post-fixation with 2% osmium tetroxide (OsO4). Dehydration of the samples was done once with an ethanolic series of 30%, 50%, 70%, and 90% and thrice with 100% ethanol. After the dehydration, hexamethyldisilane (HMDS) was added on top of each sample, and the well plates were dried overnight inside a desiccator. Gold coating of these samples was done before FESEM [32].
2.9. Assessment of osteosarcoma cell viability
The potential of the prepared samples for inhibiting osteosarcoma was assessed with the human osteosarcoma cell line (MG-63, ATCC, USA). The cell culture was carried out in Eagles Minimum Essential Medium (EMEM, ATCC, USA). 35,000 – 40,000 cells were seeded on top of each sample and a similar method to that of osteoblast was followed for MTT assay and cellular morphology, and attachment assessment using FESEM.
2.10. Anti-bacterial efficacy assessment
2.10.1. The modified ISO 22196: 2011 Standard
The antibacterial efficacy of the fabricated samples was measured against S. aureus and P. aeruginosa according to the modified ISO 22196: 2011 Standard [36]. First, the optical density of activated bacterial cells (Carolina Biological, USA) was measured at 625 nm using a UV-Vis microplate reader (BioTek) and quantified based on the McFarland standard. In the next step, 106 CFU of bacteria was loaded on top of each sample, after keeping those in a sterile 24-well plate, followed by 1 mL media addition. After this step, incubation of the well plates was done at 37 ° C and a humidity of 90 % for 24, 48, and 72 h. At each time point, samples were moved to glass vials with 1 mL PBS and 10 μL of the diluted bacterial solution (after vertexing for 15 sec) was poured on each specific agar plate followed by 24 h incubation at 37 ° C. After 24 h, bacterial colonies were counted from the photographed agar plates. The bacterial cell viability (%) was computed using the following equation. The antibacterial efficacy (%) of the treatments is calculated as 100 – bacterial cell viability (%).
| (2) |
2.10.2. Live-dead staining, confocal microscopy, and investigation of bacterial morphology
To carry out the live-dead staining, 800 μL of 1: 1 mixture of calcein M (Biolegend, CA, USA) and propidium iodide (Invitrogen, MA, USA) solution (in PBS) was poured on each sample top. The stained samples were analyzed by confocal microscopy with the TCS SP5 confocal laser microscope. The calcein M was detected using a laser in the wavelength range 485 nm - 535 nm and the propidium iodide was detected using a 530 nm - 620 nm laser. In the confocal microscopic images, the live cells were detected with green colors, whereas the red colors detected the dead cells. The bacterial attachment on the sample surfaces was analyzed with FESEM and a similar method was followed like that for osteoblast cells.
2.11. Computational optimization and validation
Polydopamine coating time, PDA-Zn2+ ratio, and the curcumin dosing utilized for long-term biological properties assessment were optimized based on a multi-objective optimization strategy, using MATLAB 2021b. In this regard, the initial osteoblast viability (5 d), osteosarcoma viability (5 d), and antibacterial properties (24 h) were used as input variables for a univariate multi-objective optimization model to choose the best-predicted composition. For each optimization, five random experiments with multiple statistical replicates were performed based on the domain knowledge. This is followed by several computational experiments (n = 50–100) to generate the objective function, which predicted the optimized individual compositions. The predicted results were validated with a 7-day osteoblast cell culture study combining the compositions around the boundary conditions and the mean of each parameter. To carry out this validation, three composite compositions were selected i.e., composition 1: PDA coating time of 10 h, PDA: Zn2+ ratio of 1:0.8, and curcumin level of 400 μg; composition 2: PDA coating time of 12 h, PDA: Zn2+ ratio of 1:1.5, and curcumin levels of 500 μg, and; composition 3: PDA coating time of 16 h, PDA: Zn2+ ratio of 1:1.8, and curcumin levels of 600 μg. Based on the experimental outcome, composition 2 was utilized for the detailed biological properties assessment.
Throughout the manuscript, the control TCP grafts are denoted as TCP, PDA-coated TCP grafts are denoted as TCP-PDA, TCP-PZ is used to denote the samples coated with PDA-Zn2+ (1:1.5 molar ratio), curcumin incorporated samples are denoted as TCP-Cur-PZ, and TCP-PZ-Cur is used to denote the samples with 500 μg curcumin immobilization. Only curcumin-loaded TCP samples are noted as TCP-Cur. The samples denoted as TCP-PZ-Cur 1 represent composition 1 and TCP-PZ-Cur 2 represent composition 2 respectively, utilized in the validation studies, mentioned above.
2.12. Statistical data analysis
The experimental data are plotted as mean ± standard deviation. To carry out the statistical analysis, a one-way ANOVA model, using JMP®, Version 14 (SAS Institute Inc., Cary, NC) is used. The Tukey-Kramer model is utilized for multiple comparison correction [37]. In the presented data, a p-value of <0.05 concludes significant results, and a p-value of <0.0001 denotes extremely statistically significant results.
3. Results
Fig. 1a shows the schematic of different CAD designs and the binder jetting method, used in this study. Different 3D printed substrates after PDA coating are shown in Fig. 1b. The chemical structure of dopamine is shown in Fig. 1c. Summary of the multifunctionality of these fabricated coated grafts are shown in Fig. 1d. The detailed process parameters for binder jetting are reported in Table 1. The polymerization steps and formation of PDA from dopamine are schematically represented in Fig. S1.
Fig. 1:
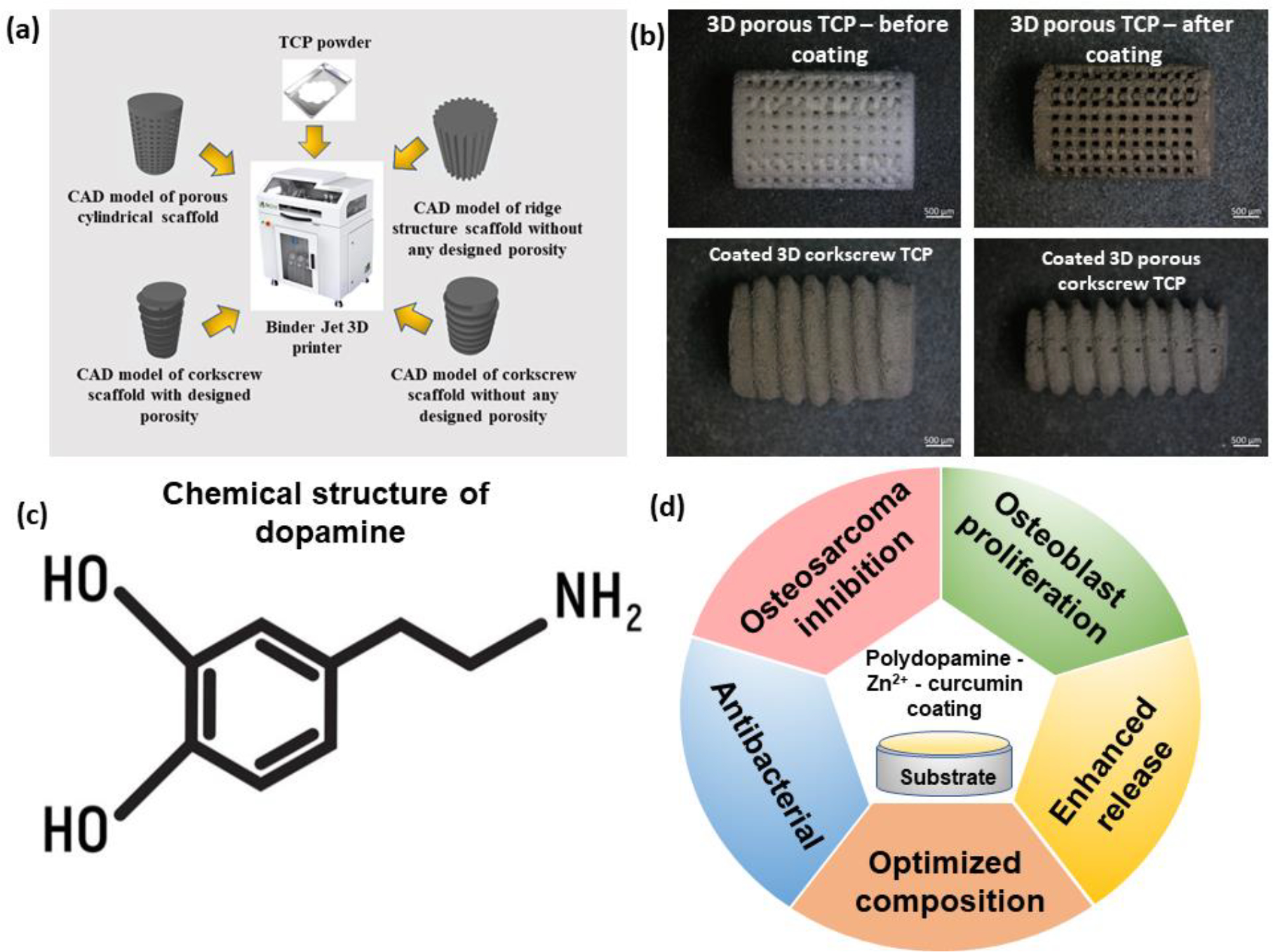
Fabrication of 3D printed substrates with different designs, optical microscopic images of the substrates after PDA coating, the chemical structure of dopamine, and resultant properties at a glance after PDA-zinc-curcumin coating; (a) Schematic of the binder jetting-based 3D printing process and CAD designs of complex structures, printed with TCP powder in this machine (b) optical microscopic images of different 3D printed substrates after PDA coating (c) the chemical structure of dopamine, the monomer of PDA (d) resultant properties of the coated surface at a glance
Table 1:
Process parameters for substrate preparation by binder jet 3D printing of TCP
| (a) Process | Sample | Layer thickness (μm) | Y Print Speed (mm/s) | Binder Set time (s) | Binder drying time (s) | Binder saturation (%) |
|---|---|---|---|---|---|---|
|
| ||||||
| Binder jet Based 3D printing | TCP grafts with complex designs | 50 | 150 | 2 s | 10 s | 85 |
The results obtained from the multi-objective optimization-based experimental design are shown in Figs. 2a–c. This design is constructed using PDA coating time, PDA: Zn2+ molar ratio, and curcumin loading amount as inputs. The osteoblast viability (5 d), osteosarcoma viability (5 d), and antibacterial efficacy (24 h) are considered as the output in this experimental design. The results of these initial experiments are summarized in Table 2. Multiple computational experiments are carried out in MATLAB 2021b with these input parameters to predict the optimum output in terms of osteoblast viability, osteosarcoma viability, and antibacterial efficacy. The optimum range in the predicted plot is shown in Figs. 2a–c. Fig. 2a shows the results obtained as a normalized output after five physical experiments followed by many computational experiments with different PDA coating times as input variables. The predicted results indicate that the optimum PDA coating time is between 10 h to 15 h. Fig. 2b shows the optimization results with different PDA: Zn2+ molar ratios as input variables. The predicted results indicate that a molar ratio between 1:1.2 and 1:1.8 is expected to show the optimum output values. In the next step, similar optimization was carried out with different curcumin loading amounts in the range of 100 μg to 1,500 μg. The curcumin was loaded on bare TCP without any PDA or Zn2+ to independently assess its effects. Fig. 2c shows the obtained predicted compositions. These indicate that a curcumin level between 400 μg to 600 μg is optimum for the best output. Fig. 2d shows the MTT results, utilized for the validation of the predicted compositions after a 7-day osteoblast cell culture experiment. Corresponding representative FESEM images are shown in Fig. S2. It is evident from the MTT results that TCP-PZ-Cur 2 shows a decrease in cellular viability as compared to the control. In contrast, TCP-PZ-Cur 1 and TCP-PZ-Cur both show a statistically significant enhancement in cellular viability than the control. However, no significant difference in cell viability is observed when these two compositions are compared individually. Based on these validation results, TCP-PZ-Cur (PDA: Zn2+ molar ratio of 1:1.5, a coating time of 12 h, and a curcumin amount of 500 μg) is selected for detailed biological properties assessment.
Fig. 2:

Multi-objective optimization strategy with different PDA coating time, PDA: Zn2+ molar ratio, and the amount of curcumin as an input and osteoblast viability (5 d), antibacterial efficacy (24 h), and osteosarcoma viability (5 d) as an output; (a) PDA coating time of 10 h-15 h shows the optimum output (b) the optimization results predict a ratio of PDA: Zn2+ between 1.2–1.8 as the optimum, (c) the optimum output for biological properties are predicted for a curcumin amount in the range of 400–600 μg (d) validation of the predicted experimental design with day 7 osteoblast study of different compositions, such as TCP-PZ-Cur 1, TCP-PZ-Cur, and TCP-PZ-Cur 2; ** indicates a p-value of <0.0001.
Table 2:
Design of experiments and obtained outputs, used for computational prediction. Three inputs were selected: PDA coating time, PDA-Zn2+ molar ratio, and curcumin level.
| Input parameter | Levels | Antibacterial efficacy (%) | Osteoblast Viability (OD) | Osteosarcoma Viability (OD) |
|---|---|---|---|---|
|
| ||||
| PDA coating time (h) | 0.5 | 2.2 ± 1 | 0.14 ± 0.001 | 0.23 ± 0.002 |
| 3 | 12 ± 2 | 0.14 ± 0.002 | 0.23 ± 0.001 | |
| 10 | 23 ± 2 | 0.16 ± 0.001 | 0.24 ± 0.003 | |
| 24 | 32 ± 1 | 0.14 ± 0.001 | 0.23 ± 0.002 | |
| 48 | 44 ± 1 | 0.07 ± 0.002 | 0.23 ± 0.001 | |
|
| ||||
| PDA: Zn2+ molar ratio | 1:0.05 | 27 ± 1 | 0.16 ± 0.002 | 0.23 ± 0.003 |
| 1:0.2 | 33 ± 2 | 0.16 ± 0.001 | 0.23 ± 0.002 | |
| 1:1 | 43 ± 3 | 0.17 ± 0.003 | 0.23 ± 0.001 | |
| 1:3 | 52 ± 2 | 0.13 ± 0.002 | 0.24 ± 0.001 | |
| 1:4 | 67 ± 3 | 0.06 ± 0.004 | 0.23 ± 0.004 | |
|
| ||||
| Curcumin amount (μg) | 100 | 2 ± 1 | 0.14 ± 0.003 | 0.19 ± 0.002 |
| 200 | 22 ± 2 | 0.14 ± 0.001 | 0.11 ± 0.001 | |
| 400 | 43 ± 2 | 0.15 ± 0.002 | 0.08 ± 0.001 | |
| 1000 | 81 ± 3 | 0.13 ± 0.004 | 0.07 ± 0.001 | |
| 1500 | 96 ± 3 | 0.08 ± 0.003 | 0.06 ± 0.001 | |
The XRD results (Fig. S3) of TCP, TCP-PDA, and TCP-PZ indicate that the coating leads to no significant phase change of TCP or impurity generation [26]. The 1H NMR results of curcumin and PDA are shown in Figs. 3a–b. The sharp singlet ~ 9.7 ppm (Fig. 3a) originates due to the hydroxyl group of curcumin. The signature peak of the methoxy group, present in curcumin is visible at ~ 3.8 ppm (Fig. 3a). This observation is well supported by available literature reports [38]. The 1H NMR spectra of PDA are shown in Fig. 3b. The indole peaks are visible at ~ 8.3 ppm and the resonance of protons on pyrocatechol is visible at ~ 6 ppm. Previous works report that because of complexation with Zn2+, these pyrocatechol peaks shifts ~ 0.01 ppm [15]. The solvent peaks in the 1H NMR spectra of PDA are separately shown in Fig. S4. The chemical structure of the resultant PDA-Zn2+ complex is shown in Fig. 3c. The chemical structures of the curcumin and PDA are shown in Figs. 3d and 3e respectively.
Fig. 3:

The 1H NMR and XPS results; (a) 1H NMR of free curcumin; the prominent hydroxyl and methoxy peaks are marked with Δ symbol; (b) the prominent peaks in the 1H NMR of PDA are marked with * symbol; (c) the chemical structure of the resultant PDA-Zn complex (d) chemical structure of curcumin (e) chemical structure of PDA after polymerization from dopamine (f) the full scan XPS spectra of PDA and PDA-zinc complex shows characteristics peaks such as C1s, N1s, and O1s. The presence of Zn2P peaks is noticed in the PDA-zinc complex (g) the high-resolution spectra of Zn2P indicate peaks at ~ 1046.06 eV and ~ 1022.8 eV (h) the deconvoluted O1s spectra of the complex shows indication of zinc-oxygen interaction at ~ 531.2 eV.
The full scan XPS spectra of the PDA and PDA-zinc complex is shown in Fig. 3f. The characteristic peaks of PDA such as C1s, N1s, and O1s are observed in the spectra [39]. The presence of Zn2P peaks is noticed in the PDA-zinc complex (Fig. 3f). The deconvoluted high-resolution Zn2P peaks (Fig. 3g) at ~ 1022.8 eV and ~ 1046.06 eV indicate the Zn-O bonding instead of free Zn, as reported in previous works [39]. Deconvolution of O1s spectra of the PDA-zinc complex (Fig. 3h) indicates the presence of zinc-oxygen interaction peak at ~ 531 eV, whereas the peak at ~ 534 eV indicates oxygen, as a characteristic of polydopamine [39,40].
The cumulative release profiles of curcumin from TCP-Cur, TCP-Cur-PZ, and TCP-PZ-Cur samples at pH 7.4 and pH 5.0 after 30 days of release are shown in Figs. 4a–b. At pH 7.4, curcumin immobilization on PDA-Zn2+ coated surface leads to the highest release of ~ 40 ± 2.3 % curcumin for 30 days (Fig. 4a). In contrast, the free curcumin shows a release of only ~ 23±2 %, and the incorporation of curcumin in the PDA-Zn2+ coating leads to a release of 33±1.5 %. The cumulative release profile at pH 5.0 (Fig. 4b) indicates that TCP-PZ-Cur leads to the highest release of ~ 51 ± 1 % curcumin. In contrast, the free curcumin shows a release of only ~ 30±2 %, and the incorporation of curcumin leads to a release of 42±3 %. The first 24 h release data at pH 7.4 shows the best fit with a power law (Fig. 4c). The obtained parameters after the power law fit are reported in Table S1.
Fig. 4:

The cumulative release profiles at (a) pH 7.4 and (b) pH 5.0 of free curcumin, immobilized curcumin on the PDA-Zn2+ coating, and curcumin incorporated within the PDA-Zn2+ matrix; the obtained results show that at pH 7.4, curcumin immobilization on PDA-Zn2+ coated surface leads to the highest release of 40 ± 2.3 % curcumin for 30 days. In contrast, the free curcumin shows a release of only 23±2 %, and the incorporation of curcumin leads to a higher release of 33±1.5 %; curcumin immobilization leads to the highest release of 51 ± 1 % curcumin at pH 5.0. In contrast, the free curcumin shows a release of only 30±2 %, and the incorporation of curcumin leads to a higher release of 42±3 % (c) power law fit at pH 7.4 for the first 24 h data (d) FESEM images after drug release at pH 7.4 show no significant degradation of the grafts (e) similar observation is noticed at pH 5.0. However, the generation of porosity is noticed and marked with arrows.
The microstructure of the 3D-printed grafts after drug release is shown in Figs. 4d–e. At pH 7.4, no significant degradation of the scaffolds is noticed during the whole period of study (Fig. 4d). At pH 5.0, the coating remains intact but the generation of microporosity is noticed on the samples, as marked with the arrows (Fig. 4e).
The MTT assay results in Fig. 5a show osteoblast cell viability of control TCP, TCP-PDA, TCP-PZ, TCP-Cur-PZ, and TCP-PZ-Cur respectively. No compositions show cytotoxicity as per the ISO 10993 standard [41]. On day 7, the only PDA coating shows a slight enhancement in cell viability compared to that of the control. The complexation of Zn2+ with PDA coating leads to further enhancement of cell viability and TCP-PZ composition shows ~ 1.2 times enhanced cell viability compared to that of control. The incorporation of curcumin with the PDA-Zn2+ coating does not show any cytotoxicity and leads to enhanced cell viability compared to that of control or TCP-PDA. However, no significant change in cell viability is noticed for this composition (TCP-Cur-PZ) as compared to TCP-PZ. Immobilization of curcumin on the PDA-Zn2+ coated surface shows the highest cell viability among all tested compositions on day 7 and day 11. This composition (TCP-PZ-Cur) shows the highest enhancement in cell viability up to ~ 1.4 times. The statistical analysis, performed by the ANOVA and the Tukey-Kramer method shows statistically significant differences in cell viability as compared to control and all treatments on day 7 and day 11. Interestingly, on both the later time points, Zn2+ functionalization with PDA and curcumin incorporation or immobilization leads to significant enhancement in cell viability compared to that of only PDA. Additionally, the TCP-PZ-Cur shows significantly higher cell viability on day 7 and day 11 than that of the TCP-Cur-PZ.
Fig. 5:

The osteoblast-3D printed graft substitutes interactions (a) MTT assay results indicate cytocompatibility of all the tested compositions; on day 3, all compositions show similar cell viability; on day 7 and day 11, the cell viability of the treatment samples enhances significantly compared to that of control. * denotes a p-value of <0.05 and the ** indicates a p-value of <0.0001 (b) The ALP assay result shows that TCP-PZ-Cur results in ~ 2 times higher cell differentiation than the control; (c) The FESEM images show good cellular attachment on each sample surface. Extend filopodia is noticed in the TCP-PZ-Cur sample. The dotted circles show cellular attachments and filopodia is marked with the arrows in the images.
The ALP assay results on day 11 (Fig. 5b) indicate that TCP-PZ-Cur composition shows ~ 2 times increase in the osteoblast differentiation as compared to the control. The FESEM images in Fig. 5c corroborate well with the MTT and ALP data and further confirm the biocompatibility of the tested compositions. Healthy, mature osteoblast cellular attachments with extended filopodia are visible on the sample surface.
The antibacterial assay results for 24 h, 48 h, and 72 h against S. aureus are shown in Fig. 6. The agar plate images (Fig. 6a) show that both TCP-PZ and TCP-PZ-Cur result in a significantly lesser number of bacterial colonies compared to the control. Fig. 6b denotes that the TCP-PZ composition has up to ~ 52 % antibacterial efficacy and the TCP-PZ-Cur shows up to ~ 90 % antibacterial efficacy. The antibacterial efficacy of all treatment compositions is retained at later time points of 48 h and 72 h. The obtained FESEM images (Fig. 6c) corroborate well with the agar plate results. Lesser number of bacterial colonies on the treatment sample is visible in the FESEM images at each time point than that of the control. The high magnification FESEM image (Fig. 6d) shows that the TCP-PZ surface leads to bacterial cell membrane rupture and the formation of debris due to bacterial cell death. The punctured cell membranes are marked with arrows in the image. The proposed antibacterial mechanism due to the presence of PDA, Zn2+, and curcumin is shown in Fig. 6e.
Fig. 6:

The antibacterial assay results for 24 h, 48 h, and 72 h against S.aureus (a) The agar plate images show that both TCP-PZ and TCP-PZ-Cur lead to a significantly lesser number of bacterial colonies compared to that of control (b) The TCP-PZ composition shows up to ~ 52 % antibacterial efficacy and the TCP-PZ-Cur shows up to ~ 90 % antibacterial efficacy. The ** indicates a p-value of <0.0001 (c) The FESEM results show a lesser number of bacterial colonies in the treatment samples. The bacterial colonies are marked with a dotted circle and the debris are marked with arrows (d) The punctured cell membranes and bacterial debris are marked with arrows in the image. (e) the proposed antibacterial mechanism.
The antibacterial efficacy assessment after the interaction of P. aeruginosa with the fabricated scaffolds is shown in Fig. 7. The agar plate results after 48 h and 72 h of bacterial interaction are shown in Fig. 7a and Fig. 7b respectively. The control TCP shows denser bacterial colony formation at both time points. In contrast, a significantly lesser number of colonies are visible in the TCP-PZ-Cur composition. The confocal microscopic images (Fig. 7c) show the presence of live dense bacterial colonies (green zone) in the control sample, whereas dead bacterial colonies (red zone) are noticed in the treatment. The FESEM images in Figs. 7d and 7e corroborate well with the observed agar plate results. The denser bacterial colony is noticed in the control TCP at both time points. The treatment samples show ruptured bacterial cell walls, marked with the arrows in the images. Fig. 7e shows that TCP-PZ-Cur leads to bacterial cell wall rupture and the ruptured zones are marked with arrows. The agar plate quantification results (Fig. 7f) show that the TCP-PZ-Cur composition leads to the highest ~ 85% lesser bacterial cell viability as compared to the control TCP. Fig. 7g schematically shows the P. aeruginosa bacterial cell wall rupture in the presence of polydopamine-Zn2+ complex and curcumin and possible applications of these antibacterial surfaces for surgical reconstruction.
Fig. 7:

Interaction of P. aeruginosa with the fabricated scaffolds. (a) The agar plate results at 48 h and, (b) 72 h indicate that TCP-PZ-Cur shows a significant reduction in bacterial colonies than the control, (c) the confocal microscopic images at 72 h indicate a significant number of dead bacteria colonies in the treatment as opposed to living colonies in the control, (d) the FESEM images at 48h, and (e) 72 h respectively show lesser bacterial densities in the TCP-PZ-Cur surface. Ruptured bacterial cell membranes are noticed for this composition and marked with white arrows in the image (f) quantification results by agar plate colony count indicate that the TCP-PZ-Cur sample shows the highest ~ 85% antibacterial efficacy; ** indicates a p-value of <0.0001 (g) schematic of the bacterial cell membrane rupture. It is expected to reduce the chances of post-surgical infections.
The MTT assay results after a day-11 osteosarcoma study are shown in Fig. 8a. It indicates significantly reduced cell viability at each time point, due to the presence of curcumin. The TCP-Cur-PZ shows up to ~ 10 times reduction in osteosarcoma viability on day 11, compared to the control. The TCP-PZ-Cur shows ~ 4 times reduction in cellular viability on day 7, which further increases to ~ 12 folds reduction on day 11, compared to the control. The TC-PPZ and TCP-PDA do not show any significant reduction in cellular viability than that of the control. FESEM results (Fig. 8b) show healthy osteosarcoma attachments on control TCP at each time point. The filopodial extension of the cell is shown in the day 11 image of this composition. In contrast, the curcumin-containing composition shows a significantly lesser number of cells.
Fig. 8.

(a) MTT assay results after osteosarcoma cell culture indicate significantly reduced cell viability at each time point, in the presence of curcumin. The TCP-PZ-Cur shows ~ 4 times reduction in cellular viability on day 7, which further increases to ~ 12 folds reduction on day 11, compared to the control. The ** indicates a p-value of <0.0001 (b) FESEM results show healthy osteosarcoma attachments on control TCP at each time point. In contrast, the curcumin-containing composition does not show a significant number of cells at each time point.
Discussions
The uniqueness of PDA coating and its limitations
Some of the existing methods to prepare functional surfaces require complex instrumentations, multiple steps, and a defined size or shape of the substrates [13,42]. In contrast, PDA can be coated on various substrates by a simple dip-coating method [17]. We have tested the feasibility of PDA coating in various 3D-printed ceramic substrates. PDA coating is an easy approach for introducing surface functionalities in almost all kinds of inorganic and organic substrates [43,44]. The presence of catechol and amines in polydopamine structure mimics the adhesive proteins of mussels and allows good adhesion to various wet substrates. The major drawbacks of PDA coatings are (a) lower stability of the coating in an extremely acidic or basic environment, and (b) formation of oligomeric aggregates on the coated substrates [45,46]. Previous works have reported laser annealing, the addition of cross-linkers, and oxidizing agents to improve the physical and mechanical properties of PDA coating [47].
There are limited studies to develop an alternate strategy for the enhancement of PDA coating’s biological properties, specific to bone tissue engineering applications. To bridge this gap, we have constructed a novel strategy by functionalizing PDA with Zn2+ and utilizing curcumin as a chemo-preventive natural drug with that formulation. The complex formation between PDA and Zn2+ is confirmed by the XPS results (Figs. 3f–h). Previous works report that the Zn2p peaks at ~ 1046 and ~ 1022 eV results due to bonding between zinc and oxygen as opposed to free zinc [40,48]. The structure of the PDA-Zn2+ complex is shown in Fig. 3c [15]. Our results indicate the entrapment of Zn2+ within the PDA matrix. Previous works report that the non-covalent interactions and strong electrostatic repulsions among oligomers lead to PDA coating degradation in acidic conditions [49]. However, the presence of Zn2+ with PDA acts as a crosslinker and Zn2+ binds with the catechol groups of PDA (Fig. 3c), which enhances the coating stability.
Enhancing curcumin release in biological medium
Localized drug delivery vehicles are gaining importance in the bone tissue engineering field due to their site-specificity and lesser side effects on the patient’s health [3]. Curcumin, the “golden specie” has chemo-preventive potential, but its clinical applications are limited due to its lower release in the biological medium. We have used a novel approach to enhance curcumin release by constructing a drug formulation with PDA- Zn2+. The presence of this polymer-metal complex enhances the curcumin release both at pH 5.0 and pH 7.4. Further, the curcumin immobilized PDA- Zn2+ coated surface shows higher drug release than the curcumin incorporated substrate. This difference can be attributed to the electrostatic interaction of immobilized curcumin with the PDA- Zn2+ complex, as opposed to the direct entrapment of curcumin within the matrix in the case of incorporation [50]. Previous works report that PDA improves the hydrophilicity of the coated surface without any additional functionalization [13,51]. Due to this reason, the interaction of hydrophobic curcumin with PDA-coated surfaces leads to enhanced curcumin release in the biological medium, as evident in our results (Figs. 4a–b). The generation of microporosity in the tested grafts at pH 5.0 can be attributed to the higher drug release at this pH than at pH 7.4 (Figs. 4d–e). The drug release kinetics from a calcium phosphate matrix is complex and generally explained by different factors such as (a) matrix degradation (b) diffusion (c) electrostatic interactions within the system (d) interaction between the drug molecule and environment in terms of hydrophobicity and hydrophilicity [31]. Our obtained power-law fitting results corroborate well with previously reported works on drug release from calcium phosphate and calcium phosphate-polymer composite matrix [3,52].
Innovative computational optimization strategies and validation
To ensure the optimum output of an artificial bone graft, it is important to create a proper balance of all the resulting biological properties [15]. For example, the complexation of Zn2+ with PDA leads to enhanced antibacterial efficacy, but we need to confirm that this enhancement is not happening at the cost of cytotoxicity. We have to ensure that our composite system with PDA- Zn2+-curcumin is showing the optimum output in terms of antibacterial efficacy, osteoblast viability, and osteosarcoma inhibition. In this regard, the conventional trial-and-error optimization method has several drawbacks such as (a) a large number of experiments (b) higher chances of human error (c) higher cost depending on the number of experiments (d) difficulty in replication [53]. In this study, we have proposed an alternate strategy by utilizing multi-objective optimization [20]. We have carried out a few physical experiments (Table 2) followed by a numerical interpolation to ascertain the best combinations of the inputs required for the most satisfactory output. The cubic spline interpolation has been used to generate this piecewise polynomial as shown in equation 3.
| (3) |
Each coefficient of this polynomial represents four individual segments of the obtained curve. The x-axis input is represented by and ‘’ is the th segment of the curve. If we take an example of PDA: Zn 2+ molar ratio optimization (Fig. 2b), is the 1st breakpoint at an input level of 0.05, is the second breakpoint at level 0.2, and so on. The first segment of the curve is the region between the x-axis in the range of 0 to 0.05; the second segment is between 0.05 and 0.2 and so on. Hence each input property with five different levels generates four segments in the curve. The coefficient values for each curve are shown in Table S2. This method is followed for other input properties such as PDA coating time and curcumin level. Utilization of this method represents the data with the least noise and is expected to result in higher accuracy in the predicted optimization results.
The predicted optimization results are validated with a 7-day osteoblast study. The obtained results (Fig. 2d) with two composite boundary condition regions and one mean of the boundary region indicate that the composition around the upper boundary condition (PDA-Zn-Cur 2) leads to a decrease in osteoblast viability as compared to the control. Due to this reason, this composition is excluded from further analysis. Both other two compositions i.e., PDA-Zn-Cur 1 and PDA-Zn-Cur show a significant enhancement in cellular viability than that of the control. Between these two, the PDA-Zn-Cur composition is selected for further studies. A relatively higher amount of Zn2+ and curcumin in this composition than the PDA-Zn-Cur 1 is expected to show better antibacterial efficacy and osteosarcoma inhibition respectively.
Antibacterial surfaces for the success of an artificial bone graft
The formation of dense bacterial colonies on the implant surface has a high tolerance against conventional antibiotics, and it destroys the host tissue’s natural defense such as phagocytosis [54]. Gram-positive S. aureus is the major cause of osteomyelitis or post-surgical bacterial infection [55]. Additionally, gram-negative P. aeruginosa is another concern due to its antibiotic resistance [56]. Balancing the antibacterial potential and osteogenic properties of an implant or scaffold is challenging. The utilization of alternative antibacterial agents such as transition metals and curcumin is expected to address the issue related to multi-drug resistance bacteria [57,58]. The optimized composition in our study with curcumin, PDA, and Zn2+ shows antibacterial properties against both S. aureus (Fig. 6) and P. aeruginosa (Fig. 7). PDA and Zn2+ both generate ROS, which ruptures the bacterial cell membrane and leads to the death of bacteria [15,59]. The agar plate results are confirmed with the FESEM. The ruptured bacterial cell membrane and debris are visible in the high magnification FESEM images against S. aureus (Fig. 6d). The phenolic groups present in curcumin encounter the bacterial membrane’s lipid bilayer and lead to disruption of the membrane structure, as evident in the microscopic images. This interaction results in bacterial cell death [60]. Previous works suggest that Mn2+ is an essential element to support bacterial nutrition. However, Zn2+ selectively binds with the PsaBCA transporters, present in the bacterial membrane, and resists the Mn2+ uptake by bacteria, which leads to bacterial cell death [61]. The proposed mechanism of PDA-Zn2+-Curcumin’s antibacterial potential against S. aureus due to ROS generation and cell wall rupture is shown in Fig. 6e.
The confocal microscopic (Fig. 7c) and FESEM images (Fig. 7d–e) after the interaction of PDA-Zn2+-Curcumin with P. aeruginosa indicate bacterial cell wall rupture due to the generation of oxidative stress in the presence of PDA, Zn2+, and curcumin. Previous works report that after interaction with the outer bacterial membrane, the benzene ring, present in polydopamine forms active functional groups [62]. These active groups hinder nutrient supply to the bacterial cells and resist their growth on the PDA-coated surface. The denser bacterial colony formation capacity of P. aeruginosa is regulated by quorum sensing (QS), which is a cell-to-cell signaling mechanism [63]. Curcumin and Zn2+ downregulate the virulence factors and associated genes by reducing the QS signals [62,63]. A combination of this mechanism and the generation of active functional groups by PDA leads to P. aeruginosa bacterial cell membrane rupture as evident in Figs. 7d–e. Antibacterial efficacy of the fabricated novel PDA-Zn2+-curcumin composite surfaces against both gram-negative and gram-positive bacteria is beneficial to find an alternative against multi-drug resistance bacteria.
Enhanced osteoblast viability and differentiation
Osteoblast growth and differentiation on the graft surface are crucial for in vivo bone formation [64,65]. In this work, our challenge is to optimize the composition by ensuring that the antibacterial components are not becoming cytotoxic. In this regard, we have considered three factors, all of which may lead to toxicity if utilized at a higher amount (a) PDA coating time (b) PDA: Zn2+ molar ratio (c) curcumin dosing. The prediction through the computational optimization indicates that a PDA coating between 10 h to 15 h is the best for osteoblast viability, compared to the control. The initial experimental results, obtained from a PDA coating time of 24 h do not show any significant enhancement in cell viability, and a coating time of 48 h leads to decreased cell viability due to the formation of PDA aggregates. Similarly, for an 11-day study, we have used, the PDA: Zn2+ molar ratio of 1:1.5, a coating time of 12 h, and a curcumin level of 500 μg, based on the optimization prediction and initial validation (Figs. 2a–d). The MTT assay results (Fig. 5a) indicate that the treatment samples on day 7 show significantly enhanced cell viability than the control. The PDA-coated sample leads to slightly higher cellular viability than the control and Zn2+ complexation further enhanced the cellular viability due to the osteogenic potential of Zn2+. Excess ROS production by PDA or Zn2+ may lead to healthy cell death. However, the complexation of PDA and Zn2+ regulates ROS production by each of them [66]. In our study, the coated layer shows enhanced cell viability, because of the successful optimization of coating time, PDA: Zn2+ molar ratio, and curcumin level. Previous studies report that Zn2+ activates the Mitogen-activated protein (MAP) kinase pathway and promotes osteoblast viability [67]. The presence of curcumin further supports the osteoblast viability at both time points. Available literature reports suggest that curcumin increases the Runx2 and osteocalcin mRNA expressions, which leads to enhanced osteoblast viability [68]. The ALP assay results (Fig. 5b) indicate enhanced osteoblast differentiation in the presence of curcumin. This can be attributed to curcumin’s potential of upregulating the Heme Oxygenase 1 (HO-1) pathway [69]. The MTT results are further supported by the FESEM images (Fig. 5c), which show good cellular attachments on the tested samples with filopodial extension. Cellular adhesion is an important step that ensures the success of a biomaterial [70,71]. In our work, the MTT assay, ALP results, and FESEM altogether confirm the in vitro osteogenic potential of the fabricated compositions.
Curcumin as an alternate chemo-preventive natural molecule for bone tissue engineering
The obtained osteosarcoma cell culture results (Figs. 8a–b) show that curcumin significantly reduces the cell viability on days 7 and day 11, with the highest reduction of ~ 12 folds. The FESEM results further support this observation. No significant number of osteosarcoma cells are visible in the curcumin-containing compositions. Additionally, the presence of curcumin leads to cell wall rupture, as marked by the arrows in the images. In contrast, the control sample shows healthy osteosarcoma attachment with filopodial extension. This observation indicates that curcumin can be a safer alternative for in vitro chemoprevention. Curcumin’s potential for inhibition of the nuclear factor pathway leads to osteosarcoma cell death, as reported in the previous works [3,8]. A summary of our work is presented in the graphical abstract.
Our preliminary results indicate that the novel 3D-printed drug delivery system with PDA-Zn2+-curcumin composite shows enhanced biological properties in terms of osteoblast growth, antibacterial efficacy, and inhibition of osteosarcoma. We have successfully employed and validated a multi-objective optimization strategy to maximize the output of this system.
Conclusions
The obtained results indicate that curcumin-loaded PDA-Zn2+ coated 3D printed TCP significantly enhances osteoblast viability, antibacterial efficacy, and osteosarcoma inhibition. We have successfully utilized a novel multi-objective optimization strategy in terms of PDA coating time, PDA: Zn2+ molar ratio, and curcumin dosing to ensure a proper balance among the resultant biological properties. The predicted optimum composition in terms of the best output is validated with physical experiments. The complexation of PDA with Zn2+ is confirmed by the Zn-O bonding in XPS. The presence of PDA-Zn2+-curcumin coating in the 3D-printed TCP leads to ~ 85 % antibacterial efficacy against gram-negative P. aeruginosa and ~ 90 % antibacterial efficacy against gram-positive S. aureus. In summary, our work redefines the importance of compositional optimization and detailed evaluation of biological properties of curcumin-loaded Zn2+ functionalized PDA-coated surface for drug delivery and bone tissue engineering applications.
Supplementary Material
Highlights.
3D printed ceramic substrates are successfully coated with polydopamine – Zn 2+
Incorporation of curcumin in the coating results in in vitro chemoprevention
The polydopamine – Zn 2+ coating significantly enhances curcumin release
The compositions are computationally predicted for optimized performance
The presence of polydopamine, Zn 2+, and curcumin enhance biological properties
Acknowledgments
The authors would like to acknowledge financial support from the National Institute of Dental and Craniofacial Research (NIDCR) of the NIH grant number R01 DE029204-01 (PI: Bose). The authors would like to thank the Franceschi Microscopy & Imaging Center at WSU.
Footnotes
Conflict of interest
The authors do not have any possible conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability
Data presented in this manuscript will be made available upon reasonable request to the corresponding author.
References:
- [1].Rutkowski P, Antiangiogenic agents combined with systemic chemotherapy in refractory osteosarcoma, Lancet. Oncol. 22 (2021) 1206–1207. [DOI] [PubMed] [Google Scholar]
- [2].Lahr CA, Landgraf M, Wagner F, Cipitria A, Moreno-Jiménez I, Bas O, Schmutz B, Meinert C, Cavalcanti ADS, Mashimo T, Miyasaka Y, Holzapfel BM, Shafiee A, McGovern JA, Hutmacher DW, A humanised rat model of osteosarcoma reveals ultrastructural differences between bone and mineralised tumour tissue, Bone. 158 (2022) 116018. 10.1016/j.bone.2021.116018. [DOI] [PubMed] [Google Scholar]
- [3].Sarkar N, Bose S, Controlled delivery of curcumin and vitamin K2 from hydroxyapatite-coated titanium implant for enhanced in vitro chemoprevention, osteogenesis, and in vivo osseointegration, ACS Appl. Mater. Interfaces. 12 (2020) 13644–13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Damiati LA, Tsimbouri MP, Hernandez V-L, Jayawarna V, Ginty M, Childs P, Xiao Y, Burgess K, Wells J, Sprott MR, Meek RMD, Li P, Oreffo ROC, Nobbs A, Ramage G, Su B, Salmeron-Sanchez M, Dalby MJ, Materials-driven fibronectin assembly on nanoscale topography enhances mesenchymal stem cell adhesion, protecting cells from bacterial virulence factors and preventing biofilm formation, Biomaterials. 280 (2022) 121263. 10.1016/j.biomaterials.2021.121263. [DOI] [PubMed] [Google Scholar]
- [5].Calabrese G, De Luca G, Franco D, Morganti D, Rizzo MG, Bonavita A, Neri G, Fazio E, Neri F, Fazio B, Crea F, Leonardi AA, Lo Faro MJ, Guglielmino S, Conoci S, Structural and antibacterial studies of novel ZnO and ZnxMn(1−x)O nanostructured titanium scaffolds for biomedical applications, Biomater. Adv. 145 (2023) 213193. 10.1016/j.bioadv.2022.213193. [DOI] [PubMed] [Google Scholar]
- [6].Khare D, Majumdar S, Krishnamurthy S, Dubey AK, An in vivo toxicity assessment of piezoelectric sodium potassium niobate [NaxK1-xNbO3 (x = 0.2–0.8)] nanoparticulates towards bone tissue engineering approach, Biomater. Adv. 140 (2022) 213080. 10.1016/j.bioadv.2022.213080. [DOI] [PubMed] [Google Scholar]
- [7].Bhattacharjee A, Bose S, 3D printed hydroxyapatite – Zn2+ functionalized starch composite bone grafts for orthopedic and dental applications, Mater. Des. 221 (2022) 110903. 10.1016/j.matdes.2022.110903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Banerjee S, Ji C, Mayfield JE, Goel A, Xiao J, Dixon JE, Guo X, Ancient drug curcumin impedes 26S proteasome activity by direct inhibition of dual-specificity tyrosine-regulated kinase 2, Proc. Natl. Acad. Sci. 115 (2018) 8155–8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].V Padma T, Ayurveda, Nature. 436 (2005) 486. [DOI] [PubMed] [Google Scholar]
- [10].Bose S, Sarkar N, Banerjee D, Natural medicine delivery from biomedical devices to treat bone disorders: A review, Acta Biomater. 126 (2021) 63–91. 10.1016/j.actbio.2021.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Song Y, Cai L, Tian Z, Wu Y, Chen J, Phytochemical curcumin-coformulated, silver-decorated melanin-like polydopamine/mesoporous silica composites with improved antibacterial and chemotherapeutic effects against drug-resistant cancer cells, ACS Omega. 5 (2020) 15083–15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hassanzadeh K, Buccarello L, Dragotto J, Mohammadi A, Corbo M, Feligioni M, Obstacles against the marketing of curcumin as a drug, Int. J. Mol. Sci. 21 (2020) 6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lee H, Dellatore SM, Miller WM, Messersmith PB, Mussel-inspired surface chemistry for multifunctional coatings, Science (80-. ). 318 (2007) 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pandey N, Soto-Garcia L, Yaman S, Kuriakose A, Rivera AU, Jones V, Liao J, Zimmern P, Nguyen KT, Hong Y, Polydopamine nanoparticles and hyaluronic acid hydrogels for mussel-inspired tissue adhesive nanocomposites, Biomater. Adv. 134 (2022) 112589. 10.1016/j.msec.2021.112589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li J, Tan L, Liu X, Cui Z, Yang X, Yeung KWK, Chu PK, Wu S, Balancing Bacteria–Osteoblast Competition through Selective Physical Puncture and Biofunctionalization of ZnO/Polydopamine/Arginine-Glycine-Aspartic Acid-Cysteine Nanorods, ACS Nano. 11 (2017) 11250–11263. 10.1021/acsnano.7b05620. [DOI] [PubMed] [Google Scholar]
- [16].Chen L, Ma J, Chen Y, Huang C, Zheng Z, Gao Y, Jiang Z, Wei X, Peng Y, Yu S, Yang L, Polydopamine modified acellular dermal matrix sponge scaffold loaded with a-FGF: Promoting wound healing of autologous skin grafts, Biomater. Adv. 136 (2022) 212790. 10.1016/j.bioadv.2022.212790. [DOI] [PubMed] [Google Scholar]
- [17].Song Y, Jiang H, Wang B, Kong Y, Chen J, Silver-incorporated mussel-inspired polydopamine coatings on mesoporous silica as an efficient nanocatalyst and antimicrobial agent, ACS Appl. Mater. Interfaces. 10 (2018) 1792–1801. [DOI] [PubMed] [Google Scholar]
- [18].DeVoe K, Banerjee S, Roy M, Bandyopadhyay A, Bose S, Resorbable Tricalcium Phosphates for Bone Tissue Engineering: Influence of SrO Doping, J. Am. Ceram. Soc. 95 (2012) 3095–3102. 10.1111/j.1551-2916.2012.05356.x. [DOI] [Google Scholar]
- [19].Bose S, Fielding G, Tarafder S, Bandyopadhyay A, Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics, Trends Biotechnol. 31 (2013) 594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lambrinidis G, Tsantili-Kakoulidou A, Multi-objective optimization methods in novel drug design, Expert Opin. Drug Discov. 16 (2021) 647–658. [DOI] [PubMed] [Google Scholar]
- [21].Koons GL, Diba M, Mikos AG, Materials design for bone-tissue engineering, Nat. Rev. Mater. 5 (2020) 584–603. 10.1038/s41578-020-0204-2. [DOI] [Google Scholar]
- [22].Wu R, Li Y, Shen M, Yang X, Zhang L, Ke X, Yang G, Gao C, Gou Z, Xu S, Bone tissue regeneration: The role of finely tuned pore architecture of bioactive scaffolds before clinical translation, Bioact. Mater. 6 (2021) 1242–1254. 10.1016/j.bioactmat.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sahebalzamani M, Ziminska M, McCarthy HO, Levingstone TJ, Dunne NJ, Hamilton AR, Advancing bone tissue engineering one layer at a time: a layer-by-layer assembly approach to 3D bone scaffold materials, Biomater. Sci. 10 (2022) 2734–2758. [DOI] [PubMed] [Google Scholar]
- [24].Ke D, Bose S, Effects of pore distribution and chemistry on physical, mechanical, and biological properties of tricalcium phosphate scaffolds by binder-jet 3D printing, Addit. Manuf. 22 (2018) 111–117. [Google Scholar]
- [25].Pan Q, Gao C, Wang Y, Wang Y, Mao C, Wang Q, Economidou SN, Douroumis D, Wen F, Tan LP, Li H, Investigation of bone reconstruction using an attenuated immunogenicity xenogenic composite scaffold fabricated by 3D printing, Bio-Design Manuf. 3 (2020) 396–409. 10.1007/s42242-020-00086-4. [DOI] [Google Scholar]
- [26].Bose S, Bhattacharjee A, Banerjee D, Boccaccini AR, Bandyopadhyay A , Influence of random and designed porosities on 3D printed tricalcium phosphate-bioactive glass scaffolds, Addit. Manuf. 40 (2021) 101895. 10.1016/j.addma.2021.101895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhou Z, Buchanan F, Mitchell C, Dunne N, Printability of calcium phosphate: Calcium sulfate powders for the application of tissue engineered bone scaffolds using the 3D printing technique, Mater. Sci. Eng. C. 38 (2014) 1–10. 10.1016/j.msec.2014.01.027. [DOI] [PubMed] [Google Scholar]
- [28].Vu AA, Burke DA, Bandyopadhyay A, Bose S, Effects of Surface Area and Topography on Mechanical Properties of 3D Printed Tricalcium Phosphate Scaffolds for Osteoblast Proliferation in Bone Grafting Applications, Addit. Manuf. (2021) 101870. 10.1016/j.addma.2021.101870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Robertson SF, Bose S, Enhanced osteogenesis of 3D printed β-TCP scaffolds with Cissus Quadrangularis extract-loaded polydopamine coatings, J. Mech. Behav. Biomed. Mater. 111 (2020) 103945. 10.1016/j.jmbbm.2020.103945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ke D, Tarafder S, Vahabzadeh S, Bose S, Effects of MgO, ZnO, SrO, and SiO2 in tricalcium phosphate scaffolds on in vitro gene expression and in vivo osteogenesis, Mater. Sci. Eng. C. 96 (2019) 10–19. 10.1016/j.msec.2018.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tarafder S, Banerjee S, Bandyopadhyay A, Bose S, Electrically Polarized Biphasic Calcium Phosphates: Adsorption and Release of Bovine Serum Albumin, Langmuir. 26 (2010) 16625–16629. 10.1021/la101851f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Boanini E, Torricelli P, Gazzano M, Giardino R, Bigi A, Alendronate–hydroxyapatite nanocomposites and their interaction with osteoclasts and osteoblast-like cells, Biomaterials. 29 (2008) 790–796. 10.1016/j.biomaterials.2007.10.040. [DOI] [PubMed] [Google Scholar]
- [33].Dash S, Murthy PN, Nath L, Chowdhury P, Kinetic modeling on drug release from controlled drug delivery systems, Acta Pol Pharm. 67 (2010) 217–223. [PubMed] [Google Scholar]
- [34].Koski C, Vu AA, Bose S, Effects of chitosan-loaded hydroxyapatite on osteoblasts and osteosarcoma for chemopreventative applications, Mater. Sci. Eng. C. 115 (2020) 111041. 10.1016/j.msec.2020.111041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bose S, Sarkar N, Vahabzadeh S, Sustained release of vitamin C from PCL coated TCP induces proliferation and differentiation of osteoblast cells and suppresses osteosarcoma cell growth, Mater. Sci. Eng. C. 105 (2019) 110096. 10.1016/j.msec.2019.110096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang L, Wang M, Li M, Shen Z, Wang Y, Shao Y, Zhu Y, Trace fluorine substituted calcium deficient hydroxyapatite with excellent osteoblastic activity and antibacterial ability, CrystEngComm. 20 (2018) 5744–5753. [Google Scholar]
- [37].Sassoubre LM, Yamahara KM, Gardner LD, Block BA, Boehm AB, Quantification of environmental DNA (eDNA) shedding and decay rates for three marine fish, Environ. Sci. Technol. 50 (2016) 10456–10464. [DOI] [PubMed] [Google Scholar]
- [38].Ahmed M, Qadir MA, Shafiq MI, Muddassar M, Hameed A, Arshad MN, Asiri AM, Curcumin: Synthesis optimization and in silico interaction with cyclin dependent kinase, Acta Pharm. 67 (2017) 385–395. [DOI] [PubMed] [Google Scholar]
- [39].Wang P, Zhang Y-L, Fu K-L, Liu Z, Zhang L, Liu C, Deng Y, Xie R, Ju X-J, Wang W, Zinc-coordinated polydopamine surface with a nanostructure and superhydrophilicity for antibiofouling and antibacterial applications, Mater. Adv. 3 (2022) 5476–5487. [Google Scholar]
- [40].Xiang Y, Mao C, Liu X, Cui Z, Jing D, Yang X, Liang Y, Li Z, Zhu S, Zheng Y, Rapid and superior bacteria killing of carbon quantum dots/ZnO decorated injectable folic acid-conjugated PDA hydrogel through dual-light triggered ROS and membrane permeability, Small. 15 (2019) 1900322. [DOI] [PubMed] [Google Scholar]
- [41].Vidal MNP, Granjeiro JM, Cytotoxicity tests for evaluating medical devices: an alert for the development of biotechnology health products, J. Biomed. Sci. Eng. 10 (2017) 431. [Google Scholar]
- [42].Decher G, Fuzzy nanoassemblies: toward layered polymeric multicomposites, Science (80-. ). 277 (1997) 1232–1237. [Google Scholar]
- [43].Ryu JH, Messersmith PB, Lee H, Polydopamine surface chemistry: a decade of discovery, ACS Appl. Mater. Interfaces. 10 (2018) 7523–7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Amin DR, Higginson CJ, Korpusik AB, Gonthier AR, Messersmith PB, Untemplated resveratrol-mediated polydopamine nanocapsule formation, ACS Appl. Mater. Interfaces. 10 (2018) 34792–34801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wu H, Ang JM, Kong J, Zhao C, Du Y, Lu X, One-pot synthesis of polydopamine–Zn complex antifouling coatings on membranes for ultrafiltration under harsh conditions, RSC Adv. 6 (2016) 103390–103398. [Google Scholar]
- [46].Yan J, Yang L, Lin M, Ma J, Lu X, Lee PS, Polydopamine spheres as active templates for convenient synthesis of various nanostructures, Small. 9 (2013) 596–603. [DOI] [PubMed] [Google Scholar]
- [47].Lee K, Park M, Malollari KG, Shin J, Winkler SM, Zheng Y, Park JH, Grigoropoulos CP, Messersmith PB, Laser-induced graphitization of polydopamine leads to enhanced mechanical performance while preserving multifunctionality, Nat. Commun. 11 (2020) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mai NT, Thuy TT, Mott DM, Maenosono S, Chemical synthesis of blue-emitting metallic zinc nano-hexagons, CrystEngComm. 15 (2013) 6606–6610. [Google Scholar]
- [49].Hong S, Na YS, Choi S, Song IT, Kim WY, Lee H, Non-Covalent Self-Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation, Adv. Funct. Mater. 22 (2012) 4711–4717. 10.1002/adfm.201201156. [DOI] [Google Scholar]
- [50].Wulf K, Teske M, Matschegewski C, Arbeiter D, Bajer D, Eickner T, Schmitz K-P, Grabow N, Novel approach for a PTX/VEGF dual drug delivery system in cardiovascular applications—an innovative bulk and surface drug immobilization, Drug Deliv. Transl. Res. 8 (2018) 719–728. [DOI] [PubMed] [Google Scholar]
- [51].Jin A, Wang Y, Lin K, Jiang L, Nanoparticles modified by polydopamine: Working as “drug” carriers, Bioact. Mater. 5 (2020) 522–541. 10.1016/j.bioactmat.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gbureck U, Vorndran E, Müller FA, Barralet JE, Low temperature direct 3D printed bioceramics and biocomposites as drug release matrices, J. Control. Release. 122 (2007) 173–180. 10.1016/j.jconrel.2007.06.022. [DOI] [PubMed] [Google Scholar]
- [53].Qin AK, Huang VL, Suganthan PN, Differential evolution algorithm with strategy adaptation for global numerical optimization, IEEE Trans. Evol. Comput. 13 (2008) 398–417. [Google Scholar]
- [54].Busscher HJ, van der Mei HC, Subbiahdoss G, Jutte PC, van den Dungen JJAM, Zaat SAJ, Schultz MJ, Grainger DW, Biomaterial-associated infection: locating the finish line in the race for the surface, Sci. Transl. Med. 4 (2012) 153rv10–153rv10. [DOI] [PubMed] [Google Scholar]
- [55].Zelmer AR, Nelson R, Richter K, Atkins GJ, Can intracellular Staphylococcus aureus in osteomyelitis be treated using current antibiotics? A systematic review and narrative synthesis, Bone Res. 10 (2022) 53. 10.1038/s41413-022-00227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].An S, Murtagh J, Twomey KB, Gupta MK, O’Sullivan TP, Ingram R, Valvano MA, Tang J, Modulation of antibiotic sensitivity and biofilm formation in Pseudomonas aeruginosa by interspecies signal analogues, Nat. Commun. 10 (2019) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhu X, Wang J, Cai L, Wu Y, Ji M, Jiang H, Chen J, Dissection of the antibacterial mechanism of zinc oxide nanoparticles with manipulable nanoscale morphologies, J. Hazard. Mater. 430 (2022) 128436. [DOI] [PubMed] [Google Scholar]
- [58].Cai L, Zhu X, Ruan H, Yang J, Wei W, Wu Y, Zhou L, Jiang H, Ji M, Chen J, Curcumin-stabilized silver nanoparticles encapsulated in biocompatible electrospun nanofibrous scaffold for sustained eradication of drug-resistant bacteria, J. Hazard. Mater. 452 (2023) 131290. [DOI] [PubMed] [Google Scholar]
- [59].Xie X, Mao C, Liu X, Tan L, Cui Z, Yang X, Zhu S, Li Z, Yuan X, Zheng Y, Yeung KWK, Chu PK, Wu S, Tuning the Bandgap of Photo-Sensitive Polydopamine/Ag3PO4/Graphene Oxide Coating for Rapid, Noninvasive Disinfection of Implants, ACS Cent. Sci. 4 (2018) 724–738. 10.1021/acscentsci.8b00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zheng D, Huang C, Huang H, Zhao Y, Khan MRU, Zhao H, Huang L, Antibacterial Mechanism of Curcumin: A Review, Chem. Biodivers. 17 (2020) e2000171. 10.1002/cbdv.202000171. [DOI] [PubMed] [Google Scholar]
- [61].Couñago RM, Ween MP, Begg SL, Bajaj M, Zuegg J, O’mara ML, Cooper MA, McEwan AG, Paton JC, Kobe B, Imperfect coordination chemistry facilitates metal ion release in the Psa permease, Nat. Chem. Biol. 10 (2014) 35–41. [DOI] [PubMed] [Google Scholar]
- [62].He X, Obeng E, Sun X, Kwon N, Shen J, Yoon J, Polydopamine, harness of the antibacterial potentials-A review, Mater. Today Bio. (2022) 100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Rudrappa T, Bais HP, Curcumin, a known phenolic from Curcuma longa, attenuates the virulence of Pseudomonas aeruginosa PAO1 in whole plant and animal pathogenicity models, J. Agric. Food Chem. 56 (2008) 1955–1962. [DOI] [PubMed] [Google Scholar]
- [64].Zhao X, Lui YS, Choo CKC, Sow WT, Huang CL, Ng KW, Tan LP, Loo JSC, Calcium phosphate coated Keratin–PCL scaffolds for potential bone tissue regeneration, Mater. Sci. Eng. C. 49 (2015) 746–753. 10.1016/j.msec.2015.01.084. [DOI] [PubMed] [Google Scholar]
- [65].Hann SY, Cui H, Esworthy T, Zhou X, Lee S, Plesniak MW, Zhang LG, Dual 3D printing for vascularized bone tissue regeneration, Acta Biomater. 123 (2021) 263–274. 10.1016/j.actbio.2021.01.012. [DOI] [PubMed] [Google Scholar]
- [66].Fu PP, Xia Q, Hwang H-M, Ray PC, Yu H, Mechanisms of nanotoxicity: generation of reactive oxygen species, J. Food Drug Anal. 22 (2014) 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Azriel-Tamir H, Sharir H, Schwartz B, Hershfinkel M, Extracellular Zinc Triggers ERK-dependent Activation of Na+/H+ Exchange in Colonocytes Mediated by the Zinc-sensing Receptor*, J. Biol. Chem. 279 (2004) 51804–51816. 10.1074/jbc.M406581200. [DOI] [PubMed] [Google Scholar]
- [68].Gu Q, Cai Y, Huang C, Shi Q, Yang H, Curcumin increases rat mesenchymal stem cell osteoblast differentiation but inhibits adipocyte differentiation, Pharmacogn. Mag. 8 (2012) 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Yang X, Jiang H, Shi Y, Upregulation of heme oxygenase-1 expression by curcumin conferring protection from hydrogen peroxide-induced apoptosis in H9c2 cardiomyoblasts, Cell Biosci. 7 (2017) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Agheli H, Malmström J, Hanarp P, Sutherland DS, Nanostructured biointerfaces, Mater. Sci. Eng. C. 26 (2006) 911–917. 10.1016/j.msec.2005.09.105. [DOI] [Google Scholar]
- [71].Chatzinikolaidou M, Pontikoglou C, Terzaki K, Kaliva M, Kalyva A, Papadaki E, Vamvakaki M, Farsari M, Recombinant human bone morphogenetic protein 2 (rhBMP-2) immobilized on laser-fabricated 3D scaffolds enhance osteogenesis, Colloids Surfaces B Biointerfaces. 149 (2017) 233–242. 10.1016/j.colsurfb.2016.10.027. [DOI] [PubMed] [Google Scholar]
- [72].Liebscher J, Mrówczyński R, Scheidt HA, Filip C, Hădade ND, Turcu R, Bende A, Beck S, Structure of polydopamine: a never-ending story?, Langmuir. 29 (2013) 10539–10548. [DOI] [PubMed] [Google Scholar]
- [73].Sahoo JK, Nazareth C, VandenBerg MA, Webber MJ, Self-assembly of amphiphilic tripeptides with sequence-dependent nanostructure, Biomater. Sci. 5 (2017) 1526–1530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data presented in this manuscript will be made available upon reasonable request to the corresponding author.


