Abstract
The French National Immunization Program was updated in 2013 for vaccination against diphtheria, tetanus, pertussis, and poliomyelitis. Our previous findings on the evolution of age-specific booster vaccination coverage rates (VCRs) up to 2017 suggested suboptimal vaccination coverages due to the pre-2013 recommendation-residual vaccination practices. In the current analysis, we evaluated all age-specific booster VCR and distribution of age at vaccination visits in 2018. In this retrospective observational cohort study, the cumulative booster VCRs were updated at all vaccination visits up to 2018 among the people who were eligible for a booster vaccination, using a 1/97th random sample of French national healthcare reimbursement databases. The cumulative booster VCR for individuals from all age groups increased from 2017 to 2018, except for 85-years-old vaccination visit. Majority of the individuals from all age groups were vaccinated (boosted) with a vaccine containing the pertussis valence. In 2018, sharp peaks corresponding to the recommended ages for booster vaccination visits were observed for individuals aged 6, 11 to 13, 25, 45, and 65 years. Our study reiterates suboptimal coverages in France and implies the need for booster vaccination throughout life for the protection of the population.
Keywords: Booster vaccine, DTaP-IPV, France, Pertussis, National Immunisation Programme, Vaccination coverage rate
Graphical abstract
1. Introduction
The French National Immunization Program (NIP) was updated in 2013 to ensure optimal protection against diphtheria, tetanus, pertussis, and poliomyelitis at all ages [1]. The new calendar included the recommendation of a booster dose of diphtheria, tetanus, pertussis, and inactivated poliomyelitis (DTaP-IPV) vaccine for children aged 6 years; a booster dose of the vaccine containing reduced antigen content for diphtheria toxoid and pertussis antigens (Tdap-IPV) for individuals aged 11 to 13 years and 25 years; and a booster dose of the vaccine containing tetanus, inactivated poliomyelitis, and reduced content of diphtheria toxoid (Td-IPV) for adults aged 45 and 65 years, followed by a booster dose once every 10 years. In the pre-2013 vaccination schedule, pertussis vaccination was not recommended at 6 and 25 years of age, and for adults aged >35 years, a booster dose was recommended every 10 years with no fixed age for vaccination visits [1,2].
We recently reported suboptimal booster vaccination coverage in France up to 2017 after the change in the NIP [2]. A rise in pertussis VCR from 26.7% in 2013 to 35.9% in 2017 was reported for the vaccination visit at 25 years of age. However, the overall VCR remained below the 95% target recommended by the 2004 Public Health Law. Additionally, gap between the recommended age and vaccination age along with a strong decrease in cumulative rates with increasing age were observed [2]. These findings were consistent with data from the United States [3] and Australia [4], whereby the pertussis VCR decreased with increasing age.
Implementation of changes in national recommendations is a long-term process. Based on the previous observations, some vaccinations were followed per the pre-2013 recommendations. Focusing on the long-term vision of implementing the 2013 vaccination schedule, the study was extended to 2018. The current study evaluated the booster VCR and distribution of age at vaccination visits in 2018.
2. Methods
2.1. Study design
This retrospective observational cohort study was conducted using a 1/97th random representative sample of the French national healthcare reimbursement databases (the General Sample of Beneficiaries [Échantillon Généraliste des Bénéficiaires {EGB}]) [5]. The reference system of the EGB population is updated on a quarterly basis from the health insurance administrative databases. The findings of the data analyzed from January 01, 2013 to December 31, 2017 have been recently published [2]. These results were updated with data up to December 31, 2018. The study was performed according to the Good Epidemiology Practices [6].
2.2. Study population
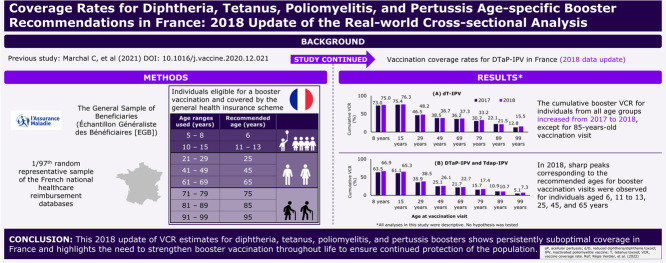
Individuals from the general French population who were eligible for a booster vaccination and were covered by the general health insurance scheme were included in the study. However, there is a frequent delay between the recommended vaccination age and the actual age at the time of vaccination in clinical practice. Hence, broader age ranges of 5 to 8 years, 10 to 15 years, 21 to 29 years, 41 to 49 years, 61 to 69 years, 71 to 79 years, 81 to 89 years, and 91 to 99 years corresponding to the recommended age of 6, 11 to 13, 25, 45, 65, 75, 85, and 95 years (common practice approach) were used. The detailed inclusion and exclusion criteria have been described previously by Marchal et al. [2].
2.3. Study outcomes
The cumulative booster VCRs against diphtheria, tetanus, and poliomyelitis (DTaP-IPV, Tdap-IPV, or Td-IPV), and pertussis (DTaP-IPV or Tdap-IPV) from 2013 to 2017 have been reported previously [2]. The primary objective of the current analysis was to estimate the proportion of people vaccinated in 2018 (cumulative vaccination coverage in common practice) according to the strict application of the vaccination schedule established in 2013. Additionally, the distribution of age at vaccination visits in 2018 were evaluated.
2.4. Statistical analysis
All analyses in this study were descriptive. No hypothesis was tested; hence, sample size calculation was not performed. The processing and statistical analysis of the data were performed using SAS Enterprise Guide software version 7.13 HF1 (Raleigh).
3. Results
3.1. Study population
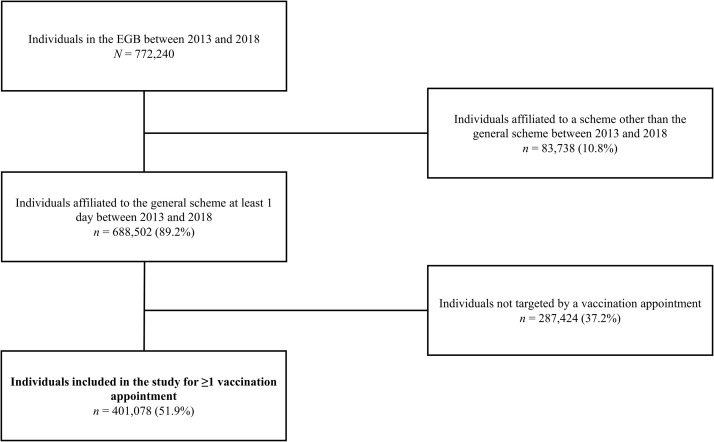
Out of the 772,240 individuals recorded in the EGB database between 2013 and 2018, 401,078 individuals affiliated with the general health insurance scheme and eligible for a booster vaccination in common practice were included in the study (Fig. 1).
Fig. 1.
Flowchart of study population.
3.2. Booster vaccination coverage rates
3.2.1. Diphtheria-tetanus-poliomyelitis
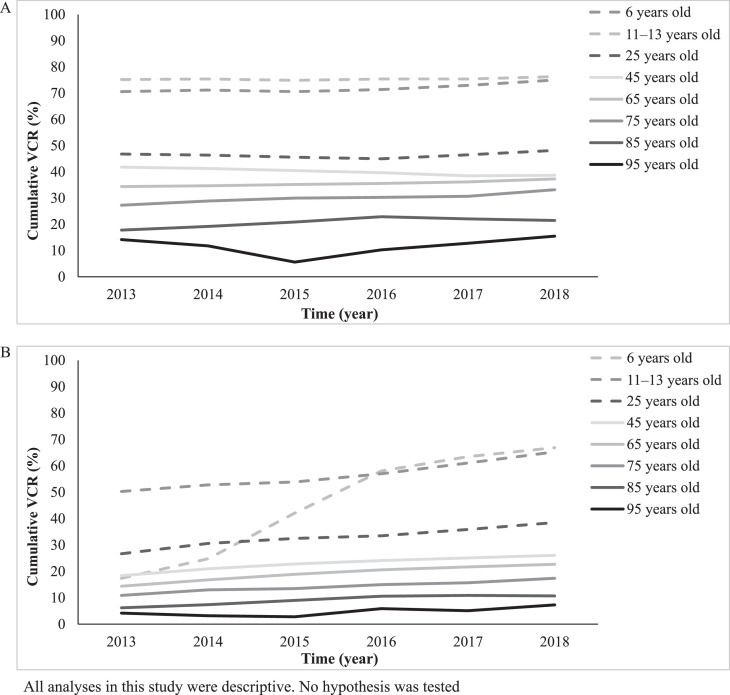
Consistent with our previous findings [2], the cumulative booster VCR for diphtheria–tetanus–poliomyelitis was the highest for vaccination visits at 11 to 13 years of age throughout the study period and remained the highest with a cumulative booster VCR of 76.3% in 2018. Starting from the vaccination visit at 25 years of age and above, the cumulative VCR decreased with age (Fig. 2A).
Fig. 2.
Cumulative booster vaccination coverage rate for diphtheria–tetanus–poliomyelitis vaccines (A) and pertussis vaccines (B) for each vaccination visit.
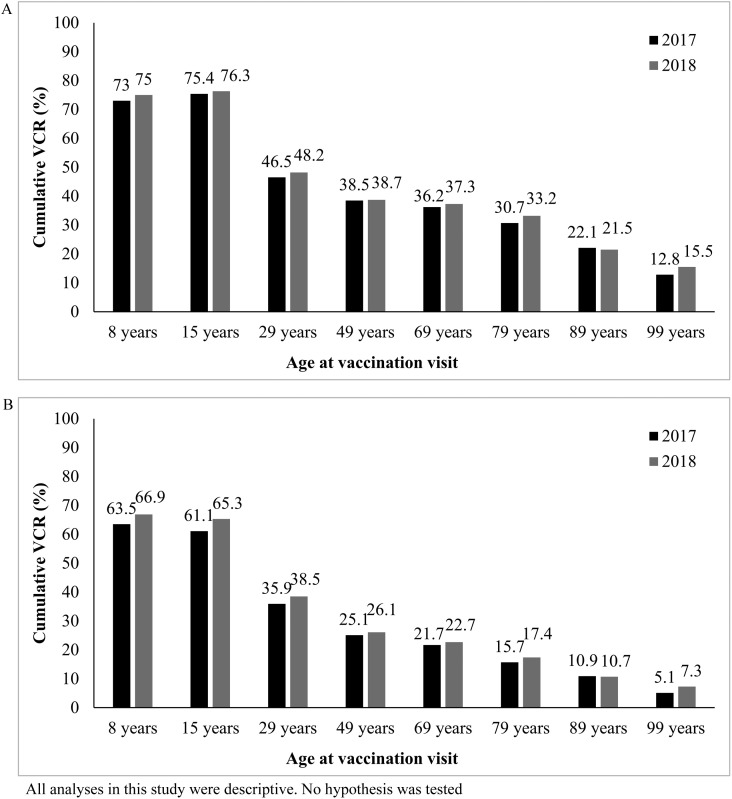
The cumulative booster VCRs for all age groups increased from 2017 to 2018, except for 85-year-old age group, whereby the cumulative booster VCR slightly decreased (from 22.1% in 2017 to 21.5% in 2018 [Fig. 3A]). The VCR for the vaccination visit at 45 years of age that had previously declined from 41.8% in 2013 to 38.5% in 2017 [2] remained constant at 38.7% in 2018.
Fig. 3.
Cumulative booster vaccination coverage rate for diphtheria–tetanus–poliomyelitis vaccines (A) and pertussis vaccines (B) for each vaccination visit in 2017 and 2018.
3.2.2. Pertussis
Pertussis booster VCR for vaccination visit at 6 years of age sharply increased from 17.4% in 2013 to 63.5% in 2017 and further increased to 66.9% in 2018. Pertussis booster VCR for vaccination visit at 11 to 13 years was >50% throughout the study duration and reached 65.3% in 2018 (Fig. 2B).
All age groups demonstrated a rise in pertussis booster VCRs from 2017 to 2018, except among the 85-year-old age group, whereby it remained constant (from 10.9% in 2017 to 10.7% in 2018 [Fig. 3B]).
Similar to 2017, a majority of the individuals from all age groups were vaccinated with a vaccine containing the pertussis valence in 2018. However, for the vaccination visit at 6 and 11 to 13 years of age, approximately 10% of children did not receive a pertussis antigen-containing vaccine.
3.3. Compliance with National Immunization Program
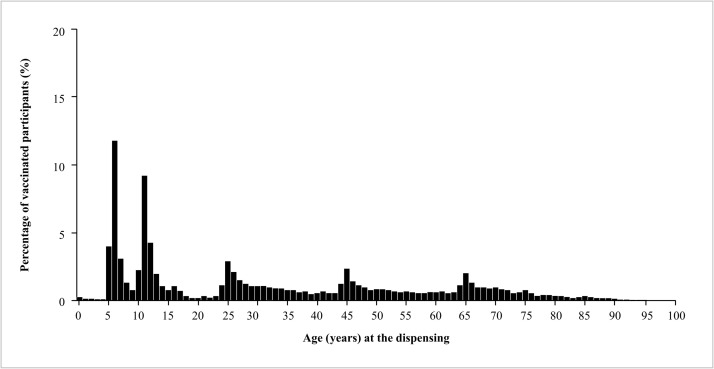
In 2018, sharp peaks corresponding to the recommended ages for booster vaccination visits were observed for individuals aged 6, 11, 25, 45, and 65 years (Fig. 4). A low but continuous dispensing of vaccines was observed at other ages between the peaks.
Fig. 4.
Histogram of the age of individuals who received a booster vaccination in 2018.
4. Discussion
The cumulative diphtheria–tetanus–poliomyelitis VCRs were generally stable from 2013 to 2018, except among the 6-year-old, 65-year-old, and 75-year-old age groups, for whom it increased in 2018. The implementation of the 2013 recommendation on pertussis continued to make progress, as the sharp rise in pertussis VCR demonstrated by 6-year-olds further increased in 2018. This was consistent with a previous study in children <12 months old between 2009 and 2014 [7]. However, the VCR was not optimal for vaccination visits at 6 and 11 to 13 years of age. Furthermore, around 10% did not receive a pertussis booster despite the recommendations being implemented since 2013 for the 6-year-olds and since 1998 for the 11- to 13-year-olds.
For the adult population, previous surveys have reported a decrease in booster VCR with increasing age in older adults (aged >65 years) [8]. Although a booster pertussis vaccination for those aged >25 years is not mentioned in the general population recommendation (except per cocooning strategy), most of the individuals were reported to be vaccinated with a vaccine containing the pertussis valence in 2018.
Our results are consistent with the findings from triennial school-based surveys conducted by Public Health, France (2003–2004 and 2008–2009) [9] and Metropolitan France and Overseas Departments (2016–2017) [10], studies conducted by the Centers for Disease Control and Prevention in the United States using National Immunization Survey–Teen data, and data published by Santé Publique France (French Public Institute for Health, the national public health agency in France), with the difference (higher VCR in these studies) attributed to the difference in the definition of booster VCR and selection methodology [2].
However, despite the increase reported post 2017, the VCR remained non-optimal in 2018 (<95% target recommended by the Public Health Law of 2004 [Law no. 2004-806 of August 09, 2004 on public health policy. Appendix – Report of public health objectives]) [11]. Overall, the VCR decreased with age in adulthood, and the vaccination schedule was not strictly followed for the majority of the studied population throughout the study period. As disease transmission through the adult population remains a significant source of infection for unvaccinated and partially vaccinated infants [12], booster vaccination has an important role in reducing the overall disease burden of pertussis and increase the herd immunity of the populations.
In 2018, continuous dispensing of vaccines outside common practice was observed, similar to that in 2017. Such noncompliance with the vaccination schedule may decrease the population immunity, particularly in 6-year-old children with a significant gap between the last dose at 11 months and a possible late booster at 8 years of age. Vaccinations outside common practice could be due to a continued application of the old vaccination schedule, lack of communication on the updated vaccine schedule, vaccinations made fortuitously, and as a part of the cocooning strategy.
Our study had some limitations. The data collected from EGB provided information about the number of participants who retrieved the vaccine from the pharmacy. However, it did not confirm that the vaccines were administered. This bias could have led to overestimation of VCRs. Conversely, there was possibility of underestimation of VCRs, since health care consumption of participants is not recorded by some health centres.
Analyses of data for 2019 and 2020 are in progress. These analyses will be of interest to assess the impact of coronavirus disease-2019 on the diphtheria, tetanus, poliomyelitis, and pertussis booster VCRs in France.
5. Conclusions
Our 2018 update on the diphtheria, tetanus, poliomyelitis, and pertussis booster VCR reiterates suboptimal coverage in France. This study implies the need for booster vaccination throughout life for the protection of the population.
Funding
The study was funded by Sanofi, Lyon, France.
Author contributions
All authors contributed to conceptualization and data analysis and/or interpretation. All authors read and approved the final manuscript and are accountable for accuracy and integrity of the data presented therein.
Acknowledgments
Scientific writing support was provided by Saili Dharadhar (Sanofi), and manuscript coordination support was provided by Roopsha Brahma, PhD (Sanofi).
Declaration of competing interest
NG reports personal fees from Sanofi. MB and CM are full time employees of PELyon and have received consulting fees paid by Sanofi to PELyon to perform the study. RC has no conflicts of interest to declare. MLP and RV are full time employees of Sanofi and may hold stocks in the company.
Data available statement
Due to EGB rules, no data sharing is possible as access to data is restricted to habilitated and qualified researchers. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at https://www.vivli.org/.
Ethics statement
An ethical statement is not required as this was not an interventional study.
Informed consent
Informed Consent was not required as this was not an interventional study.
References
- 1.Vaccination schedule and 2013 vaccination recommendations from the Ministry of Social Affairs and Health, according to the opinion of the High Council for Public Health, https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=348; 2013 [Accessed 10 May, 2022].
- 2.Marchal C., Belhassen M., Guiso N., et al. Vaccination coverage rates for diphtheria, tetanus, poliomyelitis and pertussis booster vaccination in France between 2013 and 2017: learnings from an analysis of National Health System Real-World Data. Vaccine. 2021;39:505–511. doi: 10.1016/j.vaccine.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Lu P.J., O’Halloran A., Ding H., et al. National and state-specific Td and Tdap vaccination of adult populations. Am. J. preventive med. 2016;50:616–626. doi: 10.1016/j.amepre.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayliss J., Randhawa R., Oh K.B., et al. Perceptions of vaccine preventable diseases in Australian healthcare: focus on pertussis. Human vaccines & immunotherap. 2021;17:344–350. doi: 10.1080/21645515.2020.1780848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moulis G., Lapeyre-Mestre M., Palmaro A., et al. French health insurance databases: what interest for medical research? La Revue de med. interne. 2015;36:411–417. doi: 10.1016/j.revmed.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Guidelines for Good Pharmacoepidemiology Practices (GPP) by International Society for Pharmacoepidemiology, Available at:https://www.pharmacoepi.org/resources/policies/guidelines-08027/; 2015 [Accessed 10 May, 2022].
- 7.Cohen R., Gaudelus J., Denis F., et al. Pertussis vaccination coverage among French parents of infants after 10years of cocoon strategy. Medecine et maladies infectieuses. 2016;46:188–193. doi: 10.1016/j.medmal.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Report 556: Health and Social Protection Survey 2012. IRDES. Institute for Research in Health Economics, Report 556, Available at:https://www.irdes.fr/recherche/2014/rapport-556-enquete-sur-la-sante-et-la-protection-sociale-2012.html; 2012 [Accessed 10 May, 2022].
- 9.Public Health France; 2011. Diphtheria-Tetanus, Poliomyelitis, Pertussis Vaccination Coverage Data by Age Group.https://www.santepubliquefrance.fr/determinants-de-sante/vaccination/articles/donnees-de-couverture-vaccinale-diphterie-tetanos-poliomyelite-coqueluche-par-groupe-d-age Available at: [Accessed 1 April 2022] [Google Scholar]
- 10.Guignon N. D.M.-C., Fonteneau L. Studies and results, drees; number 1122., Available at:https://drees.solidarites-sante.gouv.fr/sites/default/files/er1122.pdf; 2019 [Accessed 10 May, 2022].
- 11.Health systems in Transition. France, Health System Review, Available at:https://www.euro.who.int/__data/assets/pdf_file/0008/135809/E94856.pdf; 2010 [Accessed 7 March, 2022].
- 12.Choi J.-H., Correia de Sousa J., Fletcher M., et al. Improving vaccination rates in older adults and at-risk groups: focus on pertussis. Aging Clin Exp Res. 2022;34:1–8. doi: 10.1007/s40520-021-02018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]