Abstract
Objectives
Loneliness may influence aging biomarkers related to cognitive functioning, for example, through accelerated DNA methylation (DNAm) aging.
Methods
In the present study, we tested whether six common DNAm age acceleration measures mediated the effects of baseline loneliness and five different longitudinal loneliness trajectories on general cognitive ability, immediate memory recall, delayed memory recall, and processing speed in 1,814 older adults in the Health and Retirement Study.
Results
We found that baseline loneliness and individuals who belong to the highest loneliness trajectories had poorer general cognitive ability and memory scores. Only DNAm age acceleration measures that index physiological comorbidities, unhealthy lifestyle factors (e.g., smoking), and mortality risk-mediated effects of baseline loneliness on general cognitive ability and memory functioning but not processing speed. These same DNAm measures mediated effects of the moderate-but-declining loneliness trajectory on cognitive functioning. Additionally, immediate and delayed memory scores were mediated by GrimAge Accel in the lowest and two highest loneliness trajectory groups. Total and mediated effects of loneliness on cognitive functioning outcomes were mainly accounted for by demographic, social, psychological, and physiological covariates, most notably self-rated health, depressive symptomatology, objective social isolation, and body mass index.
Discussion
Current findings suggest that DNAm biomarkers of aging, particularly GrimAge Accel, have promise for explaining the prospective association between loneliness and cognitive functioning outcomes.
Keywords: Epigenetic age, Cognition, DNA methylation, Loneliness, Longitudinal
The biological mechanisms underlying the association between loneliness and cognitive functioning, including dementia, have been hypothesized but rarely tested (Boss et al., 2015). A major effort in tackling that question is testing whether physiological dysregulation is one such mechanism. The present study adds to the literature on the physiological dysregulation hypothesis of loneliness by testing associations between markers of physiological functioning (i.e., epigenetic aging measures) with loneliness, and a cognitive functioning measure that has been used to discriminate dementia risk (Crimmins et al., 2011).
Loneliness and Cognitive Functioning
Loneliness is conceptualized as the negative feelings that result from a perceived lack of sufficient social connection, either in quantity or quality, regardless of one’s objective social connections (Perlman & Peplau, 1981). Loneliness is distinguished conceptually from, and weakly correlated with, objective social isolation (Coyle et al., 2012). The experience of loneliness is not uniform across age—some age groups, on average, experience chronic or increasing loneliness, whereas others report low levels of loneliness (Hawkley & Cacioppo, 2013). For example, 15%–30% of the population report chronic levels of loneliness. Across the life span, loneliness follows a U-shaped trend, with highest levels during younger and older adulthood (Beam & Kim, 2020; Victor & Yang, 2012).
In older adults, loneliness correlates with poorer cognitive performance, declines in global cognition (Boss et al., 2015; Tilvis et al., 2004), and greater risk for Alzheimer’s disease (Lara et al., 2019; Penninkilampi et al., 2018). With respect to cognitive functioning, loneliness is associated with general cognitive ability, processing speed, immediate recall, and delayed memory recall (Boss et al., 2015; Harrington et al., 2023). Although correlation coefficients between loneliness and each domain tend to be small, they are reliable for cognitive functioning outcomes as well as dementia risk (Lara et al., 2019). Among specific cognitive domains, loneliness shows the strongest associations with memory outcomes (Boss et al., 2015). Processing speed was also correlated with loneliness in a minority of studies. These studies mostly consist of cross-sectional associations, leaving open the question of whether different loneliness trajectories predict cognitive functioning outcomes differently.
Loneliness trajectories in older adulthood show high variability. For example, although the prevalence of loneliness in 25 European countries increased across middle to late adulthood, only a minority of older adults (i.e., <20%) reported high or moderate levels of loneliness (Yang & Victor, 2011) with most reporting low levels of loneliness. In three population-representative samples—two from the United States and one from Sweden—age trajectories of loneliness differed in older cohorts (Beam & Kim, 2020). In the Health and Retirement Study (HRS), loneliness scores tended to remain stable in older cohorts, whereas in the National Social Life, Health, and Aging Project and the Swedish Twin Registry, loneliness levels were lower and more stable for younger cohorts than older cohorts. In a diurnal study of 55 older adults, intraindividual loneliness scores were heterogeneous over a 5-week period (Awad et al., 2023). Taken together, this expands on our question of whether group differences in older adults’ loneliness trajectories differentially predict cognitive functioning across time. Older adults with persistently high or increasing levels of loneliness would be expected to perform more poorly on cognitive ability assessments compared to older adults with relatively low levels of loneliness.
Given the heterogeneity of loneliness across individuals and the life span, it is important to identify common life span trajectories of loneliness to better understand who among lonely people are at risk of worse cognitive outcomes. One prior study of loneliness and cognitive impairment defined four loneliness trajectories (i.e., no loneliness, persistently high, increasing levels, and decreasing levels; Akhter-Khan et al., 2021), in which it was shown that people with persistently high loneliness have greater cognitive impairment. We did not make a priori hypotheses regarding the number of trajectory groups that we would identify in the current study. We did, however, reason that increasing and decreasing levels of loneliness may have different effects on cognitive ability depending on the average starting level of loneliness (i.e., whether individuals start with high levels of loneliness and decline rapidly or slowly). Thus, we empirically explored different trajectories of loneliness in older adulthood prior to testing whether different loneliness trajectories differentially correlated with cognitive functioning.
DNA Methylation Age Acceleration and Physiological Dysregulation
Although research suggests at least three different models by which loneliness can influence morbidity—health risk behaviors, psychological distress, and physiological dysregulation (Hawkley & Cacioppo, 2010)—here we focus specifically on physiological dysregulation as the potential mechanism by which loneliness affects cognitive functioning. Epigenetic alterations (e.g., DNA methylation [DNAm]) affect gene expression without changing the genome, which in turn alters phenotypes (Fraga, 2009). Epigenome alterations result from a plethora of factors, for example, prenatal health, early-life adversities, and lifetime stress (Heijmans et al., 2008; Sumner et al., 2019; Zannas, 2019), which also affect the aging process, for better or worse. One way to quantify the accumulation of epigenetic alterations is through associating DNAm at specific sites on the genome—cytosine phosphate guanine (CpG) markers—with aging processes, and then aggregating the methylation into a score. In this way, the DNAm score reflects the toll of aging and includes effects from lifetime experiences and environmental exposures.
DNA methylation markers can be used to quantify whether people are aging faster or slower relative to their chronological age. Often referred to as DNA methylation age acceleration (DNAm AgeAccel) in the literature, numerous measures have been developed based on different tissue types, CpG markers, demographic characteristics, and other biomarkers of physiological function (e.g., C-reactive protein, CRP). Initial DNAm AgeAccel measures that were trained primarily on chronological age were developed as better predictors of longevity and cancer risk (Hannum et al., 2013; Horvath, 2013) and are collectively referred to as “first generation” DNAm AgeAccel measures. Among these, Horvath’s (2013), Hannum et al.’s (2013), and Horvath’s skin-based measure (Horvath et al., 2018) have been found to correlate with cognitive ability (Levine et al., 2015; Marioni et al., 2015). Additional DNAm AgeAccel measures, considered “second generation” measures, were constructed based on immune and inflammatory biomarker profiles (Levine et al., 2018), lifestyle factors (e.g., smoking behavior), mortality risk (Lu et al., 2019), and change in biomarker profiles used to quantify “pace of aging” over time (Belsky et al., 2022). Second-generation measures have been shown to be more robust predictors of cognitive ability, cognitive impairment, and neuropathological markers associated with cognitive impairment (Belsky et al., 2022; Levine et al., 2018; Lu et al., 2019). In the current study, we used three first-generation measures of DNAm AgeAccel and three second-generation measures to evaluate the utility of various DNAm AgeAccel measures as mediating mechanisms in the association between loneliness and cognitive functioning, as in prior studies (e.g., see Beydoun et al., 2020). We hypothesized that second-generation DNAm AgeAccel measures would outperform first-generation measures (McCrory et al., 2021; Reed et al., 2022; Vaccarino et al., 2021).
There are several reasons why DNAm-based estimators of age have promise for clarifying whether physiological dysfunction mediates the effects of loneliness on cognitive functioning. First, individuals with persistently high loneliness may be at risk for altered gene expression (Cole et al., 2015). Second, loneliness triggers stress reactivity, resulting in increased circulating levels of cortisol (Adam et al., 2006) that may cause neuronal damage associated with altered cognition (Epel, 2009). In lonely individuals, genes involved in immune activation, transcription control, and cell proliferation are found to be upregulated, whereas genes supporting mature B lymphocyte function and type I interferon response are downregulated (Cole et al., 2007). Together, these epigenetic changes result in reduced anti-inflammatory responses, which may explain why loneliness is associated with inflammation-mediated morbidity despite increases in cortisol. Thus, transcriptional processes and genetic expression appear to be part of the process by which loneliness correlates with physiological functioning.
The effect of different loneliness trajectories on epigenetic processes has not been explored. Effects of long-term psychosocial stress on DNAm AgeAccel have focused mostly on early life stressors, with discordant evidence for lifetime exposures to psychosocial stress. DNAm AgeAccel measures correlate with early-life socioeconomic status (SES; Austin et al., 2018) and early-life adversities (Sumner et al., 2019) but not adult SES. Cumulative lifetime stress also correlates with DNAm AgeAccel (Zannas, 2019). Taken together, loneliness may differentially alter the epigenome that may, in turn, differentially affect cognitive functioning. We, thus, investigate whether differences in loneliness trajectories predict individual differences in various measures of DNAm AgeAccel. To the extent that DNAm AgeAccel depends on loneliness trajectories, we propose that the epigenome may explain, at least in part, the association between loneliness and cognitive functioning.
DNA Methylation Age Acceleration and Cognitive Functioning
Changes in the epigenome affect physiological processes implicated in cognitive impairment (Chouliaras et al., 2010). First, methylation levels in the apolipoprotein E (APOE) gene promoter region relate to Alzheimer’s disease and related diseases over and above the genetic risk associated with APOE variation alone (Karlsson et al., 2018). Epigenetic mechanisms also correlate with biomarkers of neuropathology of dementia (e.g., amyloid load) and poorer global cognitive functioning (Levine et al., 2015). In some studies, higher DNAm AgeAccel scores are associated with lower cognition (Hillary et al., 2021; Wu et al., 2021), but in others the effect is not statistically significant (Sibbett et al., 2020).
No studies have investigated whether measures of DNAm AgeAccel mediate effects of loneliness on cognitive functioning. Indeed, few studies have tested whether dysregulation of physiological functions mediates effects of baseline measures of loneliness on cognitive functioning (Spithoven et al., 2019). In a study of U.S. older adults spanning 10 years, physiological dysregulation at 5-year follow-up did not mediate baseline effects of loneliness on cognitive ability at 10-year follow-up, whereas functional ability, self-rated health, depressive symptomatology, and social participation did (Kim et al., 2020). In a study of older adults in the HRS, only HbA1C, a measure of metabolic functioning, mediated effects of loneliness on subsequent measures of cognitive ability but not after adjusting for covariates (Yu & Ng, 2022). Although these studies did not support a role for physiological processes in the association between loneliness and cognitive functioning over time, the primary motivation for studying whether measures of DNAm AgeAccel might be more robust mediators is that DNAm-based measures of age have been found to outperform all other measures of cumulative physiological functioning (Horvath & Raj, 2018).
Present Study
In a large sample of nationally representative older adults from the HRS, we tested the general hypothesis that DNAm AgeAccel mediates effects of loneliness on cognitive functioning. As the current study is the first to examine whether DNAm AgeAccel mediates effects of loneliness on cognitive functioning, we tested whether DNAm AgeAccel (measured in 2016) mediated effects of baseline measures of loneliness (measured in 2008) on general cognitive ability, immediate memory recall (IMR), delayed memory recall, and processing speed (measured in 2016). We hypothesized that DNAm AgeAccel measures would mediate, at least partially, the effect of baseline loneliness on general cognitive ability, processing speed, and memory functioning. In a multicategorical mediation analysis, we tested whether DNAm AgeAccel mediated effects of the highest loneliness trajectory groups on all measures of cognitive functioning. Finally, based on the literature review above, we hypothesized that second-generation DNAm AgeAccel measures would primarily mediate associations between loneliness and cognitive functioning outcomes.
Method
Sample
Data come from three waves of the HRS. The HRS is sponsored by the National Institute on Aging (NIA U01AG009740) and is conducted by the University of Michigan. The sample in the present study includes individuals in the HRS who completed the UCLA Loneliness Scale in 2008, 2012, or 2016 (i.e., provided at least one measurement) through the Core survey, provided blood samples for DNAm analysis in 2016 through the Venous Blood Study (HRS, 2021), and completed the Core HRS cognitive assessment in 2016. The base sample in the current study was the 4,104 HRS individuals who participated in the Venous Blood Study. Eighty-six individuals were excluded because their DNAm samples did not pass quality control. Out of the 4,018 remaining, 2,204 were excluded because they did not provide a single measure of loneliness in 2008, 2012, and 2016. The analytic sample consisted of 1,814 individuals: 61.69% provided data in 2008, 82.25% in 2012, and 83.13% in 2016. More than half (59.10%) of the sample was female. The sample was 77.29% White, 15.55% Black, and 7.17% “Other” race. The average age of the sample was 69.76 (SD = 9.77). Further sample-level descriptive statistics are provided in Table 1. The study was approved by the University of Southern California Institutional Review Board in the Office for the Protection of Research Subjects (IRB #: UP-17-00067).
Table 1.
Descriptive Statistics of the Analytic Sample
| N | M | SD | Range | |
|---|---|---|---|---|
| UCLA 2008 | 1,119 | 16.50 | 4.70 | 11.00–31.00 |
| UCLA 2012 | 1,492 | 16.59 | 4.77 | 11.00–33.00 |
| UCLA 2016 | 1,508 | 16.92 | 4.81 | 11.00–33.00 |
| Age (2016) | 1,814 | 69.76 | 9.77 | 50.00–98.00 |
| Horvath Accel | 1,814 | −0.03 | 6.40 | −31.35–48.38 |
| Hannum Accel | 1,814 | −0.01 | 5.34 | −25.64–39.84 |
| HorvathSkin Accel | 1,814 | −0.02 | 4.30 | −24.40–18.96 |
| DNAm PhenoAge Accel | 1,814 | −0.01 | 6.80 | −28.11–30.75 |
| GrimAge Accel | 1,814 | −0.04 | 8.79 | 42.67–97.05 |
| Dunedin PoAm38 Accel | 1,814 | 0.00 | 0.09 | −0.34–0.37 |
| TICS (2016) | 1,814 | 14.80 | 4.22 | 0.00–26.00 |
| Processing speed | 1,814 | 1.86 | 0.51 | 0.00–2.00 |
| Immediate recall | 1,814 | 5.24 | 1.57 | 0.00–10.00 |
| Long delay recall | 1,814 | 4.20 | 1.87 | 0.00–10.00 |
| BMI | 1,775 | 34.50 | 8.26 | 14.65–78.11 |
| Depression (CES-D) | 1,037 | 1.69 | 2.07 | 0.00–10.00 |
| Self-rated health | 1,813 | 2.90 | 1.03 | 1.00–5.00 |
| Social isolation | 1,016 | 2.83 | 1.01 | 0.00–4.00 |
| APOEe4 | 1,814 | 1.70 | 0.73 | 0.00–2.00 |
| N | % | |||
|---|---|---|---|---|
| Sex | ||||
| Female | 1,072 | 59.10 | ||
| Male | 742 | 40.90 | ||
| Race | ||||
| White | 1,402 | 77.29 | ||
| Black | 282 | 15.55 | ||
| Other | 130 | 7.17 | ||
| Education | ||||
| No college | 287 | 15.82 | ||
| GED | 86 | 4.74 | ||
| High school diploma | 894 | 49.28 | ||
| 2-Year college degree | 111 | 6.12 | ||
| 4-Year college degree | 253 | 13.95 | ||
| Master degree | 132 | 7.28 | ||
| Professional degree | 38 | 2.10 | ||
| Degree unknown | 13 | 0.72 | ||
| Current smoking | ||||
| No | 798 | 43.99 | ||
| Yes | 201 | 11.08 | ||
| Cohort 1 (1890–1923) | 109 | 6.01 | ||
| Cohort 2 (1924–1930) | 518 | 28.56 | ||
| Cohort 3 (1931–1941) | 277 | 15.27 | ||
| Cohort 4 (1942–1947) | 384 | 21.17 | ||
| Cohort 5 (1948–1953) | 445 | 24.53 | ||
| Cohort 6 (1954–1959) | 64 | 3.53 |
Measures
Loneliness
An 11-item modified version of the 20-item UCLA Loneliness Scale was administered to all participants to measure social loneliness (Lee & Cagle, 2017). The 11-item measure included the following items, all beginning with the stem “How much of the time do you feel …”: “you lack companionship?”; “left out?”; “isolated from others?”; “that you are ‘in tune’ with the people around you?”; “alone?”; “that there are people you can talk to?”; “that there are people you can turn to?”; “that there are people who really understand you?”; “that there are people you feel close to?”; “part of a group of friends?”; and “that you have a lot in common with the people around you?” All items were rated on a 3-point Likert scale (1 = “often”; 2 = “some of the time”; 3 = “hardly ever or never”). Items were scored so that higher values indicated higher loneliness and then summed (range: 11–33) at each time point. Scale reliability was substantial in 2008 (ω = 0.88), 2012 (ω = 0.88), and 2016 (ω = 0.87).
Cognitive functioning
General cognitive ability was measured using a composite measure of cognitive functioning developed from the modified Telephone Interview for Cognitive Status (TICSm; Weir et al., 2011). The 27-point index includes scores from (1) immediate and delayed 10-noun free recall test to measure memory (0 to 20 points); (2) a serial sevens subtraction test to measure working memory (0 to 5 points); and (3) a counting backwards test to measure speed of mental processing (0 to 2 points; Langa et al., 2020). Immediate memoryrecall (IMR) consisted of the sum score of the free recall word list learning task. Participants were read 10 nouns and asked to repeat as many as they could remember upon the psychometrist finishing the list. Long delayed recall (LDR) consisted of the sum score of the free recall of the same word list in the immediate recall task approximately 5 min later. Processing speed was conceptualized as speed of mental processing and was measured using the backward counting test in which participants were asked to count backwards from 20 as quickly as possible for 10 continuous numbers. Participants were given two tries. Scores of 2 indicate two successful attempts, scores of 1 indicate one successful attempt, and scores of 0 indicate neither attempt was successful.
DNA methylation age acceleration
DNAm age measures were estimated by the HRS (Crimmins et al., 2020). First-generation DNAm age measures included Horvath’s (2013) measure that is defined by DNAm at 353 CpG sites; Hannum et al.’s (2013) measure defined by methylation at 71 CpG and biological sex; and Horvath’s skin-based measure that was defined by methylation of 391 CpG sites based on fibroblasts and other skin cell tissue types.
We used three second-generation DNAm age measures: DNAm PhenoAge (Levine et al., 2018) was trained using measures of age-related morbidity (albumin, creatine, glucose (serum), CRP (log), lymphocyte percent, mean red cell volume, red cell distribution width, alkaline phosphatase, and white blood cell count) and chronological age to define phenotypic age that was characterized by 513 CpG sites. DNAm GrimAge or “GrimAge” (Lu et al., 2019) was developed in two steps. First, surrogate DNAm biomarkers of physiological risk and plasma proteins (adrenomedullin, CRP, plasminogen activation inhibitor 1, and growth differentiation factor 15) and smoking pack-years were identified. Second, time-to-death was then regressed on seven DNAm surrogates and scaled in years to estimate DNAm age. DunedinPoAm38 is based on a pace of aging measures previously estimated (Belsky et al., 2015) that defines how quickly people age biologically based on 18 biomarkers that measure cardiovascular, metabolic, renal, hepatic, pulmonary, periodontal, and immune systems. Elastic-net regression was used to estimate methylation pace of aging.
Horvath, Hannum, Horvath (skin), DNAm PhenoAge, and GrimAge measures were scaled in years. DunedinPoAm38 was scaled in years per chronological year. DNAm AgeAccel was calculated by using residualized scores from a bivariate ordinary least squares regression of individuals’ DNAm age score regressed on their chronological age. Sample means of all DNAm AgeAccel measures are zero with positive values indicating DNAm age scores older than would be predicted from chronological age and negative values indicating scores younger than would be predicted from chronological age.
Demographics
Sex was coded based on individuals’ sex at birth, male or female. Education was coded based on the highest grade of school completed or year of college completed. Birth cohort was dummy coded according to delineations summarized by the RAND Center for the Population of Aging (2023). Race was based on self-identification, and coded into the categories: White/Caucasian, Black/African American, and Other.
Covariates
Additional covariates were included because they have been found to confound the association between loneliness and cognitive functioning or the association between DNAm AgeAccel and cognitive functioning. For example, depressive symptomatology, self-rated health, and social participation have been found to mediate the association between loneliness and cognitive functioning (Kim et al., 2020) or confound the association (Kuiper et al., 2016). Direct and proxy measures of health behaviors, like body mass index (BMI) and smoking, and genetic risk factors of severe cognitive impairment (i.e., APOE ε4) were adjusted in our final models because they may confound effects of DNAm AgeAccel and cognitive functioning. Body mass index was calculated using English measurements: . Self-rated health was assessed using a single item “Would you say your health is excellent, very good, good, fair, or poor?” Higher scores indicate poorer self-rated health. Current smoking status was assessed using a single item “Do you smoke cigarettes now? (not including pipes, cigars, or e-cigarettes)” and coded as 1 = Yes, 2 = No. Depressive symptomatology was measured using 10 items from the Center for Epidemiologic Studies Depression scale (CES-D; Radloff, 1977). One item measuring feelings of loneliness was excluded from the composite score. Higher scores indicate higher depressive symptomatology. Social isolation was coded as the amount of contact with four types of relationships (spouse, children, friends, other family) each month on a scale of 0 (social integration) to 4 (social isolation). Sum scores were created so that higher scores indicate greater social isolation (Sutin et al., 2020). APOE ε4 status was coded as the number of ε4 alleles (0, 1, or 2). All covariates were collected in the 2016 interview.
Data Analysis
First, we present results from basic descriptive analyses, including means, standard deviations, and correlations for key variables. We then identified subgroups of longitudinal trajectories of loneliness using Covariance Pattern Mixture Modeling (CPMM; McNeish & Harring, 2020), an approach that is an extension of Growth Mixture Modeling (GMM; Ram & Grimm, 2009; Supplementary Figure 1). Unstructured, Toeplitz, and compound symmetric covariance structures were tested. Bayesian Information Criterion (BIC), Akaike Information Criterion (AIC), entropy values, and substantive interpretation of the intercept and slope parameters were used to identify the most plausible number of different latent loneliness trajectory classes (LTCs).
We then performed two sets of mediation analyses. We first present results in which we tested whether the six DNAm AgeAccel measures mediated effects of baseline loneliness scores measured in 2008 on all cognitive functioning measures assessed in 2016. The general expressions used to test the baseline model are:
| (1) |
| (2) |
Equation 1 demonstrates effects of baseline loneliness, a, on measures of DNAm AgeAccel for individual i. The parameter iDNAmAgeAccel is the intercept for measures of DNAm AgeAccel, and eDNAmAgeAccel is the residual variance. Equation 2 specifies the adjusted direct effect of baseline loneliness (c’), DNAm Age Accel (b), and all covariates, W2016 (d), on cognitive functioning (CF). The parameters iCF and eCF represent the intercept and residual variance of cognitive functioning, respectively.
Relative indirect and total effects were calculated as:
| (3) |
| (4) |
The primary parameters of interest are the indirect effects (Equation 3) with statistically significant values supporting our hypothesis that DNAm AgeAccel measures mediate effects of baseline loneliness on cognitive functioning outcomes.
We then present results from a multicategorical mediation analysis to test whether the six DNAm AgeAccel measures mediate effects of LTC on cognitive functioning. We included k–1 indicator codes, using the largest group as the reference group (Hayes & Preacher, 2013). Next, DNAm AgeAccel measures and cognitive functioning were regressed onto the indicator codes (Figure 1), and cognitive functioning was regressed onto the DNAm AgeAccel measures. As in the mediation models for baseline loneliness above, the primary hypotheses tested were the indirect effect (aj*b) of each loneliness trajectory class on cognitive functioning via each DNAm AgeAccel measure.
Figure 1.
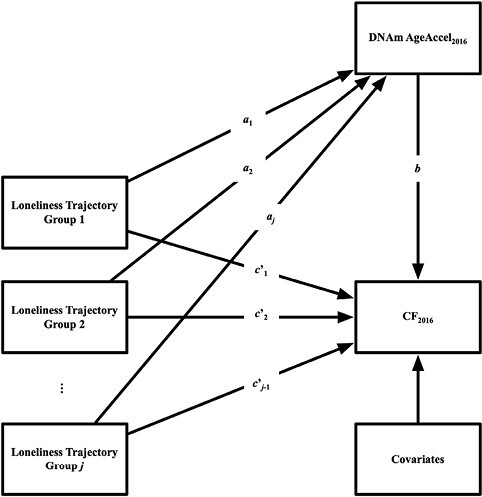
Multicategorical Mediation Model. Loneliness trajectory groups 1, 2, … , j refer to the different loneliness trajectory classes found in the Growth Mixture Models. DNAm AgeAccel2016 refers to Horvath Age Acceleration, Hannum Age Acceleration, Horvath (skin) Age Acceleration, DNAm PhenoAge Acceleration, GrimAge Acceleration, and Dunedin PoAm38 Acceleration. CF2016 refers to TICSm, immediate memory recall, delayed memory recall, and processing speed. Covariates include cohort, sex, race/ethnicity, APOE ε4 status, social isolation, current smoking status, depressive symptomatology, self-rated health, and BMI. The parameters a1, a2, … , aj−1, refer to the effect of each loneliness trajectory group on DNAm AgeAccel for j–1 groups (the largest latent class group was selected as the reference group). The parameter b refers to the direct effect of DNAm AgeAccel measure on the cognitive functioning outcome. The parameters c’1, c’2, … , c’j−1, refer to the adjusted direct effect of each loneliness trajectory group for j–1 groups on the cognitive outcome.
In both mediation analyses, we tested a Baseline Model in which all covariates were excluded against a demographic covariate-only model (Demographic Model) and a full model in which all covariates were included (Full Model). Covariances among all predictor variables were included. All analyses were conducted in Mplus 8.6 (Muthén & Muthén, 1998-2017) using full information maximum likelihood. The α cutoff for all null-hypothesis significance tests was set to .05. Likelihood ratio tests, AIC, and BIC, were used to compare Baseline, Demographic, and Full models. Standard errors and 95% confidence intervals were bootstrapped in Mplus using the BOOTSTRAP function.
Missing data on the UCLA Loneliness Scale was analyzed to determine whether data were missing completely at random. There were no differences between individuals who provided no measure of loneliness (n = 2,204) and those who provided at least one measurement (n = 1,814).
Across the entire study sample, 330 individuals provided a single measure of loneliness, 663 provided two measures, and 821 provided three measures. For those who provided data, there were no statistically significant differences between individuals who provided three loneliness scores and those who provided one or two scores for any DNAm AgeAccel measure or any cognitive functioning outcome except for processing speed (t(1,812) = 3.82, p < .001, d = 0.18). Overall, the missing at random assumption was met.
Results
Descriptive Statistics
Across the entire sample, loneliness scores increased over the 8-year measurement window, albeit slightly (Table 1). The zero-order correlations between DNAm AgeAccel measures and TICSm were: .00 for Horvath, −.03 for Hannum, −.04 for Horvath (skin), −.09 for DNAm PhenoAge, −.19 for GrimAge, and −.11 for DunedinPoAm38. The correlations for immediate recall were: −.02 for Horvath, −.05 for Hannum, −.06 for Horvath (skin), −.08 for DNAm PhenoAge, −.19 for GrimAge, and −.12 for DunedinPoAm38. The correlations for delayed memory recall were: −.01 for Horvath, −.02 for Hannum, −.04 for Horvath (skin), −.08 for DNAm PhenoAge, −.19 for GrimAge, and −.12 for DunedinPoAm38. For processing speed, correlations were: .04 for Horvath, .04 for Hannum, .03 for Horvath (skin), .01 for DNAm PhenoAge, −.01 for GrimAge, and −.03 for DunedinPoAm38. Intercorrelations between key variables and covariates are provided in Supplementary Table 1.
Covariance Pattern Mixture Modeling and Growth Mixture Modeling
Model fit statistics for CPMMs are provided in Supplementary Tables 2 and 3. A compound symmetric error structure fit the data in a one-class solution better than an unstructured error model or a Block Toeplitz structure (Supplementary Table 2). A five-class solution was selected as the best model based on the BIC values and interpretability of the LTCs (Supplementary Table 3). Table 2 shows the intercept and slope parameters for each trajectory group. Group 1 was defined as having “Low” loneliness; group 2 (the largest group) as “Low-But-Increasing” loneliness; group 3 as “Moderate” loneliness; group 4 as “Moderate-But-Declining” loneliness; and group 5 as “High-But-Declining” loneliness. As slope values are in units per decade, groups 2, 4, and 5, tend to change slowly over time.
Table 2.
Model Estimated Intercepts and Slopes of the Five Loneliness Trajectory Classes
| Group | Description | N | Intercept | Slope |
|---|---|---|---|---|
| 1 | Low | 489 | 14.44 | 0.11 |
| 2 | Low-but-increasing | 539 | 12.83 | 0.21 |
| 3 | Moderate | 286 | 17.91 | −0.16 |
| 4 | Moderate-but-declining | 345 | 21.39 | −0.45 |
| 5 | High-but-declining | 155 | 25.42 | −0.50 |
Note: Bolded values are statistically significant at the .05 level. Slope estimates are in units per decade.
Baseline Loneliness Mediation Models
Total effect of baseline loneliness (UCLA Loneliness score in 2008) on TICSm was significant in the baseline models across all DNAm AgeAccel measures (Table 3). Estimates slightly varied based on the DNAm AgeAccel variable. Indirect effects were statistically significant only for GrimAge Accel and Dunedin PoAm38 Accel in the baseline model. Total effects of loneliness remained significant after adjusting for demographic variables (Supplementary Table 4), but indirect effects for GrimAge Accel and Dunedin PoAm38 Accel were no longer significant. All other effects of loneliness on TICSm were no longer statistically significant after adjusting for all covariates. Self-rated health, depressive symptomatology, and BMI were the most likely confounds (Supplementary Table 4).
Table 3.
Baseline Mediation Parameter Estimates, Indirect and Total Effects
| Parameter | Horvath Accel | HorvathSkin Accel | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Full | Baseline | Full | |||||
| Est. | .95 CI | Est. | .95 CI | Est. | .95 CI | Est. | .95 CI | |
| Total effect of loneliness | ||||||||
| UCLA2008 → TICS2016 | −0.154 | [−0.215, −0.088] | −0.040 | [−0.092, 0.010] | −0.154 | [−0.216, −0.090] | −0.040 | [−0.093, 0.011] |
| Indirect effect | ||||||||
| UCLA2008 → DNAm AgeAccel2016 → TICS2016 | 0.000 | [−0.001, 0.002] | 0.000 | [−0.002, 0.001] | 0.000 | [−0.003, 0.002] | 0.000 | [−0.001, 0.001] |
| Adjusted direct effect of loneliness | ||||||||
| UCLA2008 → TICS2016 | −0.154 | [−0.215, −0.088] | −0.040 | [−0.093, 0.010] | −0.154 | [−0.216, −0.089] | −0.040 | [−0.092, 0.011] |
| Effect of DNAm AgeAccel2016 on TICS2016 | ||||||||
| DNAm AgeAccel2016 → TICS2016 | 0.001 | [−0.029, 0.029] | 0.011 | [−0.014, 0.035] | −0.036 | [−0.082, 0.008] | 0.002 | [−0.036, 0.041] |
| Effects of loneliness on DNAm AgeAccel2016 | ||||||||
| UCLA2008 → DNAm AgeAccel2016 | −0.011 | [−0.090, 0.069] | −0.005 | [−0.084, 0.072] | 0.010 | [−0.045, 0.064] | 0.014 | [−0.042, 0.068] |
| Parameter | Hannum Accel | DNA PhenoAge Accel | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Full | Baseline | Full | |||||
| Est. | .95 CI | Est. | .95 CI | Est. | .95 CI | Est. | .95 CI | |
| Total effect of loneliness | ||||||||
| UCLA2008 → TICS2016 | −0.153 | [−0.215, −0.088] | −0.040 | [−0.093, 0.010] | −0.151 | [−0.212, −0.085] | −0.039 | [−0.093, 0.011] |
| Indirect effect | ||||||||
| UCLA2008 → DNAm AgeAccel2016 → TICS2016 | −0.001 | [−0.003, 0.001] | 0.000 | [−0.002, 0.001] | 0.001 | [−0.004, 0.006] | 0.000 | [−0.001, 0.002] |
| Adjusted direct effect of loneliness | ||||||||
| UCLA2008 → TICS2016 | −0.153 | [−0.214, −0.087] | −0.040 | [−0.092, 0.010] | −0.151 | [−0.213, −0.087] | −0.039 | [−0.093, 0.011] |
| Effect of DNAm AgeAccel2016 on TICS2016 | ||||||||
| DNAm AgeAccel2016 → TICS2016 | −0.020 | [−0.059, 0.015] | −0.007 | [−0.040, 0.025] | −0.057 | [−0.085, −0.028] | −0.014 | [−0.040, 0.010] |
| Effects of loneliness on DNAm AgeAccel2016 | ||||||||
| UCLA2008 → DNAm AgeAccel2016 | 0.031 | [−0.035, 0.093] | 0.036 | [−0.029, 0.099] | −0.011 | [−0.092, 0.070] | −0.013 | [−0.094, 0.068] |
| Parameter | GrimAge Accel | DunedinPoAm38 Accel | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Full | Baseline | Full | |||||
| Est. | .95 CI | Est. | .95 CI | Est. | .95 CI | Est. | .95 CI | |
| Total effect of loneliness | ||||||||
| UCLA2008 → TICS2016 | −0.144 | [−0.206, −0.078] | −0.045 | [−0.098, 0.006] | −0.148 | [−0.211, −0.082] | −0.041 | [−0.095, 0.009] |
| Indirect effect | ||||||||
| UCLA2008 → DNAm AgeAccel2008 → TICS2016 | −0.012 | [−0.021, −0.002] | −0.004 | [−0.009, 0.000] | −0.007 | [−0.013, −0.001] | −0.001 | [−0.005, 0.002] |
| Adjusted direct effect of loneliness | ||||||||
| UCLA2008 → TICS2016 | −0.132 | [−0.193, −0.066] | −0.041 | [−0.095, 0.009] | −0.141 | [−0.203, −0.075] | −0.040 | [−0.094, 0.010] |
| Effect of DNAm AgeAccel2016 on TICS2016 | ||||||||
| DNAm AgeAccel2016 → TICS2016 | −0.155 | [−0.195, −0.118] | −0.051 | [−0.100, −0.006] | −4.625 | [−6.754, −2.614] | −0.745 | [−2.981, 1.398] |
| Effects of loneliness on DNAm AgeAccel2016 | ||||||||
| UCLA2008 → DNAm AgeAccel2016 | 0.075 | [0.012, 0.137] | 0.071 | [0.009, 0.133] | 0.002 | [0.000, 0.003] | 0.001 | [0.000, 0.003] |
Note: Estimates are unstandardized. Bolded values are statistically significant at the .05 level.
Total effects of baseline loneliness on IMR were statistically significant, suggesting that greater levels of loneliness in 2008 predicted slightly worse scores in 2016 (Supplementary Table 5). Only GrimAge Accel significantly mediated effects of loneliness on IMR in the baseline model but were no longer statistically significant in the Demographic Model. Total effects of baseline loneliness on IMR were no longer statistically significant in the Full Model, which, like the TICSm, suggests that other factors including objective social isolation, self-rated, health, and BMI confound the association between loneliness and IMR.
The total effect of baseline loneliness on LDR was significant in the Baseline models across all DNAm AgeAccel measures, indicating that higher levels of loneliness predicted worse word retrieval scores (Supplementary Table 6). Only the indirect effect of baseline loneliness on LDR via GrimAge Accel was statistically significant in the baseline model but was no longer statistically significant in the Demographic Model. Total and adjusted effects of baseline loneliness on LDR were no longer statistically significant in the Full Model.
Total, adjusted, and indirect effects of baseline loneliness on processing speed were not statistically significant in the baseline, demographic, or full models (Supplementary Table 7). The only significant predictors of processing speed across all models were sex and education.
Loneliness Trajectory Class Mediation Models
Compared to the Low-But-Increasing group, all other LTC groups were predicted to have lower TICSm scores across all DNAmAge Accel measures (Table 4). Adjusted direct effects for the High-But-Declining group showed the most negative effect on TICSm scores followed by the Moderate-But-Declining and Moderate groups. Only the second-generation DNAmAge Accel measures—DNA PhenoAge, GrimAge Accel, and Dunedin PoAm38 Accel—significantly mediated effects on TICSm for the two highest loneliness trajectories. All second-generation measures mediated the association between the Moderate-But-Declining group, whereas only GrimAge Accel mediated the association between the High-But-Declining group. We note, too, that GrimAge Accel mediated effects of the Low group on TICSm. Only GrimAge Accel continued to mediate effects of the Moderate-But-Declining group in the Demographic (Supplementary Table 8) and Full Models (Table 4). Adjusted direct effects of the loneliness trajectory groups on TICSm were no longer statistically significant in the Full Model, which means that GrimAge Accel fully mediated effects of the Moderate-But-Declining group on TICSm. Self-rated health, depressive symptomatology, and BMI likely confounded effects of loneliness trajectories on TICSm scores.
Table 4.
Multicategorical Mediation Parameter Estimates, Indirect and Total Effects
| Parameters | Horvath Accel | HorvathSkin Accel | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Full | Baseline | Full | |||||
| Est. | .95 CI | Est. | .95 CI | Est. | .95 CI | Est. | .95 CI | |
| Total effects of loneliness trajectories | ||||||||
| Low → TICS2016 | −0.655 | [−1.186, −0.148] | −0.062 | [−0.514, 0.367] | −0.655 | [−1.186, −0.147] | −0.062 | [−0.515, 0.366] |
| Moderate → TICS2016 | −1.119 | [−1.717, −0.486] | −0.447 | [−1.014, 0.121] | −1.119 | [−1.717, −0.485] | −0.448 | [−1.012, 0.121] |
| Moderate-But-Declining → TICS2016 | −1.341 | [−1.880, −0.794] | −0.209 | [−0.733, 0.318] | −1.341 | [−1.879, −0.794] | −0.211 | [−0.734, 0.317] |
| High-But-Declining → TICS2016 | −1.643 | [−2.345, −0.956] | −0.403 | [−1.106, 0.287] | −1.643 | [−2.344, −0.955] | −0.409 | [−1.112, 0.282] |
| Indirect effects | ||||||||
| Low → DNAm AgeAccel2016 → TICS2016 | 0.002 | [−0.017, 0.024] | 0.006 | [−0.008, 0.030] | −0.008 | [−0.035, 0.011] | 0.001 | [−0.012, 0.020] |
| Moderate → DNAm AgeAccel2016 → TICS2016 | 0.000 | [−0.015, 0.014] | −0.001 | [−0.018, 0.015] | −0.003 | [−0.031, 0.021] | 0.000 | [−0.013, 0.015] |
| Moderate-But-Declining → DNAm AgeAccel2016 → TICS2016 | 0.002 | [−0.023, 0.030] | 0.008 | [−0.010, 0.036] | −0.025 | [−0.070, 0.012] | 0.003 | [−0.029, 0.040] |
| High-But-Declining → DNAm AgeAccel2016 → TICS2016 | 0.001 | [−0.021, 0.028] | 0.004 | [−0.016, 0.035] | −0.019 | [−0.074, 0.014] | 0.002 | [−0.026, 0.039] |
| Adjusted direct effects of loneliness trajectories | ||||||||
| Low → TICS2016 | −0.657 | [−1.191, −0.146] | −0.067 | [−0.519, 0.359] | −0.647 | [−1.183, −0.142] | −0.063 | [−0.516, 0.367] |
| Moderate → TICS2016 | −1.119 | [−1.718, −0.493] | −0.445 | [−1.014, 0.120] | −1.117 | [−1.719, −0.482] | −0.449 | [−1.007, 0.114] |
| Moderate-But-Declining → TICS2016 | −1.343 | [−1.881, −0.793] | −0.217 | [−0.745, 0.309] | −1.317 | [−1.842, −0.776] | −0.214 | [−0.744, 0.305] |
| High-But-Declining → TICS2016 | −1.644 | [−2.350, −0.954] | −0.408 | [−0.745, 0.309] | −1.624 | [−2.325, −0.939] | −0.412 | [−1.116, 0.271] |
| Effect of DNAm AgeAccel2016 on TICS2016 | ||||||||
| DNAm AgeAccel2016 → TICS2016 | 0.003 | [−0.026, 0.032] | 0.012 | [−0.013, 0.036] | −0.031 | [−0.077, 0.013] | 0.004 | [−0.034, 0.042] |
| Effects of Loneliness on DNAm AgeAccel2016 | ||||||||
| Low → DNAm AgeAccel2016 | 0.483 | [−0.301, 1.296] | 0.483 | [−0.301, 1.296] | 0.257 | [−0.272, 0.799] | 0.257 | [−0.272, 0.799] |
| Moderate → DNAm AgeAccel2016 | −0.121 | [−0.970, 0.783] | −0.121 | [−0.970, 0.783] | 0.086 | [−0.527, 0.684] | 0.086 | [−0.527, 0.684] |
| Moderate-But-Declining → DNAm AgeAccel2016 | 0.636 | [−0.271, 1.591] | 0.636 | [−0.271, 1.591] | 0.800 | [0.235, 1.374] | 0.800 | [0.235, 1.374] |
| High-But-Declining → DNAm AgeAccel2016 | 0.342 | [−0.915, 1.529] | 0.342 | [−0.915, 1.529] | 0.616 | [−0.238, 1.511] | 0.616 | [−0.238, 1.511] |
| Parameters | Hannum Accel | DNAm PhenoAge Accel | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Full | Baseline | Full | |||||
| Est. | .95 CI | Est. | .95 CI | Est. | .95 CI | Est. | .95 CI | |
| Total effects of loneliness trajectories | ||||||||
| Low → TICS2016 | −0.655 | [−1.186, −0.147] | −0.067 | [−0.519, 0.366] | −0.655 | [−1.186, −0.147] | −0.068 | [−0.520, 0.361] |
| Moderate → TICS2016 | −1.120 | [−1.717, −0.487] | −0.453 | [−1.012, 0.125] | −1.120 | [−1.718, −0.486] | −0.455 | [−1.014, 0.116] |
| Moderate-But-Declining → TICS2016 | −1.342 | [−1.880, −0.794] | −0.215 | [−0.744, 0.312] | −1.341 | [−1.881, −0.793] | −0.222 | [−0.750, 0.308] |
| High-But-Declining → TICS2016 | −1.644 | [−2.345, −0.956] | −0.415 | [−1.115, 0.279] | −1.643 | [−2.389, −0.897] | −0.418 | [−1.124, 0.266] |
| Indirect effects | ||||||||
| Low → DNAm AgeAccel2016 → TICS2016 | 0.003 | [−0.016, 0.025] | 0.001 | [−0.012, 0.016] | −0.043 | [−0.102, 0.002] | −0.010 | [−0.031, 0.011] |
| Moderate → DNAm AgeAccel2016 → TICS2016 | 0.008 | [−0.013, 0.038] | 0.002 | [−0.014, 0.024] | −0.023 | [−0.080, 0.025] | −0.005 | [−0.031, 0.011] |
| Moderate-But-Declining → DNAm AgeAccel2016 → TICS2016 | −0.013 | [−0.053, 0.010] | −0.004 | [−0.034, 0.019] | −0.065 | [−0.133, −0.013] | −0.016 | [−0.055, 0.015] |
| High-But-Declining → DNAm AgeAccel2016 → TICS2016 | −0.016 | [−0.070, 0.014] | −0.005 | [−0.042, 0.024] | −0.066 | [−0.154, 0.004] | −0.016 | [−0.066, 0.016] |
| Adjusted direct effects of loneliness trajectories | ||||||||
| Low → TICS2016 | −0.658 | [−1.190, −0.151] | −0.068 | [−0.524, 0.362] | −0.612 | [−1.140, −0.116] | −0.058 | [−0.511, 0.366] |
| Moderate → TICS2016 | −1.127 | [−1.719, −0.498] | −0.455 | [−1.013, 0.119] | −1.097 | [−1.699, −0.457] | −0.450 | [−1.011, 0.112] |
| Moderate-But-Declining → TICS2016 | −1.329 | [−1.866, −0.781] | −0.211 | [−0.735, 0.311] | −1.276 | [−1.809, −0.727] | −0.206 | [−0.739, 0.325] |
| High-But-Declining → TICS2016 | −1.628 | [−2.329, −0.942] | −0.410 | [−1.114, 0.268] | −1.577 | [−2.281, −0.886] | −0.402 | [−1.108, 0.277] |
| Effect of DNAm AgeAccel2016 on TICS2016 | ||||||||
| DNAm AgeAccel2016 → TICS2016 | −0.020 | [−0.058, 0.014] | −0.006 | [−0.039, 0.025] | −0.051 | [−0.079, −0.023] | −0.012 | [−0.038, 0.012] |
| Effects of loneliness on DNAm AgeAccel2016 | ||||||||
| Low → DNAm AgeAccel2016 | −0.148 | [−0.814, 0.520] | −0.148 | [−0.814, 0.520] | 0.849 | [−0.041, 1.689] | 0.849 | [−0.041, 1.689] |
| Moderate → DNAm AgeAccel2016 | −0.392 | [−1.138, 0.340] | −0.392 | [−1.138, 0.340] | 0.444 | [−0.521, 1.382] | 0.444 | [−0.521, 1.382] |
| Moderate-But-Declining → DNAm AgeAccel2016 | 0.617 | [−0.091, 1.331] | 0.617 | [−0.091, 1.331] | 1.278 | [0.333, 2.197] | 1.278 | [0.333, 2.197] |
| High-But-Declining → DNAm AgeAccel2016 | 0.791 | [−0.210, 1.782] | 0.791 | [−0.210, 1.782] | 1.293 | [−0.097, 2.594] | 1.293 | [−0.097, 2.594] |
| Parameters | GrimAge Accel | Dunedin PoAm 38 Accel | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Full | Baseline | Full | |||||
| Est. | .95 CI | Est. | .95 CI | Est. | .95 CI | Est. | .95 CI | |
| Total effects of loneliness trajectories | ||||||||
| Low → TICS2016 | −0.655 | [−1.186, −0.149] | −0.096 | [−0.552, 0.340] | −0.655 | [−1.186, −0.144] | −0.076 | [−0.531, 0.360] |
| Moderate → TICS2016 | −1.122 | [−1.721, −0.486] | −0.486 | [−1.050, 0.090] | −1.120 | [−1.719, −0.485] | −0.458 | [−1.018, 0.114] |
| Moderate-But-Declining → TICS2016 | −1.341 | [−1.879, −0.794] | −0.269 | [−0.800, 0.269] | −1.340 | [−1.880, −0.790] | −0.229 | [−0.757, 0.299] |
| High-But-Declining → TICS2016 | −1.643 | [−2.346, −0.950] | −0.477 | [−1.180, 0.229] | −1.641 | [−2.345, −0.950] | −0.435 | [−1.144, 0.256] |
| Indirect effects | ||||||||
| Low → DNAm AgeAccel2016 → TICS2016 | −0.099 | [−0.187, −0.011] | −0.032 | [−0.084, 0.000] | −0.025 | [−0.082, 0.029] | −0.004 | [−0.028, 0.013] |
| Moderate → DNAm AgeAccel2016 → TICS2016 | −0.070 | [−0.178, 0.033] | −0.023 | [−0.072, 0.011] | −0.016 | [−0.086, 0.052] | −0.003 | [−0.027, 0.017] |
| Moderate-But-Declining → DNAm AgeAccel2016 → TICS2016 | −0.174 | [−0.289, −0.073] | −0.057 | [−0.134, −0.005] | −0.101 | [−0.183, −0.037] | −0.017 | [−0.073, 0.027] |
| High-But-Declining → DNAm AgeAccel2016 → TICS2016 | −0.175 | [−0.344, −0.019] | −0.057 | [−0.147, 0.000] | −0.022 | [−0.117, 0.070] | −0.004 | [−0.037, 0.021] |
| Adjusted direct effects of loneliness trajectories | ||||||||
| Low → TICS2016 | −0.557 | [−1.084, −0.061] | −0.064 | [−0.520, 0.367] | −0.630 | [−1.157, −0.119] | −0.071 | [−0.527, 0.359] |
| Moderate → TICS2016 | −1.052 | [−1.640, −0.436] | −0.463 | [−1.033, 0.103] | −1.103 | [−1.698, −0.477] | −0.455 | [−1.018, 0.113] |
| Moderate-But-Declining → TICS2016 | −1.166 | [−1.668, −0.623] | −0.212 | [−0.734, 0.320] | −1.239 | [−1.772, −0.698] | −0.212 | [−0.737, 0.311] |
| High-But-Declining → TICS2016 | −1.467 | [−2.165, −0.770] | −0.420 | [−1.135, 0.265] | −1.619 | [−2.327, −0.943] | −0.432 | [−1.143, 0.252] |
| Effect of DNAm AgeAccel2016 on TICS2016 | ||||||||
| DNAm AgeAccel2016 → TICS2016 | −0.156 | [−0.196, −0.119] | −0.051 | [−0.099, −0.006] | −4.891 | [−6.979, −2.918] | −0.839 | [−3.082, 1.353] |
| Effects of loneliness on DNAm AgeAccel2016 | ||||||||
| Low → DNAm AgeAccel2016 | 0.633 | [0.071, 1.179] | 0.633 | [0.071, 1.179] | 0.005 | [−0.007, 0.016] | 0.005 | [−0.007, 0.016] |
| Moderate → DNAm AgeAccel2016 | 0.447 | [−0.215, 1.102] | 0.447 | [−0.215, 1.102] | 0.003 | [−0.011, 0.017] | 0.003 | [−0.011, 0.017] |
| Moderate-But-Declining → DNAm AgeAccel2016 | 1.116 | [0.473, 1.707] | 1.116 | [0.473, 1.707] | 0.021 | [0.008, 0.033] | 0.021 | [0.008, 0.033] |
| High-But-Declining → DNAm AgeAccel2016 | 1.124 | [0.111, 2.115] | 1.124 | [0.111, 2.115] | 0.005 | [−0.014, 0.022] | 0.005 | [−0.014, 0.022] |
Note: Estimates are unstandardized. Bolded values are statistically significant at the .05 level.
For IMR (Supplementary Table 9), the Moderate, Moderate-But-Declining, and High-But-Declining groups showed significantly lower scores. The Low and Low-But-Increasing groups did not differ. Only the second-generation clocks mediated associations between loneliness trajectory groups and IMR. All second-generation DNAm acceleration measures mediated the association between the Moderate-But-Declining group whereas only GrimAge Accel mediated the association between the High-But-Declining group and the Low Group. GrimAge Accel continued to mediate effects of these loneliness trajectory groups in the Demographic Model as well as the Full Model. Notably, GrimAge Accel mediated effects of the Moderate-But-Declining and High-But-Declining groups on IMR more strongly than the Low Group. Objective social isolation, self-rated health, depressive symptomatology, and BMI likely confounded the association between all acceleration measures, but to a lesser degree for GrimAge Accel.
Results for LDR (Supplementary Table 10) were similar to IMR. Compared to the Low-But-Increasing group, all other loneliness trajectory groups showed significantly lower LDR scores with those in the High-But-Declining and Moderate-But-Declining groups predicted to score the lowest. Only the second-generation clocks again mediated associations between latent trajectory groups and LDR. All second-generation DNAm acceleration measures mediated the association between the Moderate-But-Declining group, whereas only GrimAge Accel mediated effects of the High-But-Declining and Low groups. As with IMR, GrimAge Accel mediated effects of these groups on LDR in the Demographic and Full Models. GrimAge Accel mediated effects of the Moderate-But-Declining and High-But-Declining groups on LDR more strongly than the Low Group. As the adjusted effects in this model were no longer statistically significant, GrimAge Accel fully mediated effects of the Low, Moderate-But-Declining, and High-But-Declining groups, holding constant all covariates.
As in the baseline loneliness model, the total, adjusted, and indirect associations between any loneliness trajectory group and processing speed were not statistically significant (Supplementary Table 11). The only significant predictors of processing speed across all models were sex and education.
Discussion
The current study showed that one second-generation DNAm AgeAccel measure, GrimAge Accel, consistently explained the association between loneliness and general cognitive ability, immediate recall, and delayed memory recall in older adulthood. GrimAge Accel fully mediated effects of the Moderate-But-Declining loneliness trajectory on general cognitive ability and both measures of episodic memory after adjusting for all covariates. In addition, GrimAge Accel fully mediated effects of the High-But-Declining and Low Group loneliness trajectories on both memory measures. No other DNAm AgeAccel measure mediated any effect of loneliness on any other cognitive functioning outcome. For the majority of DNAm AgeAccel measures, demographic, social, physiological, and psychosocial variables explained both direct and indirect associations.
As the first study to examine whether DNAm AgeAccel measures explain the association between loneliness and cognitive functioning, this study is among the strongest tests of the physiological mechanisms underlying loneliness and cognitive functioning given that DNAm-based measures of aging constitute the most robust estimators of cumulative physiological dysregulation (Horvath & Raj, 2018). These results suggest a complicated role that physiological dysfunction might have in the association between loneliness and cognitive functioning, as demographic, social, psychological, and lifestyle factors confounded the mediating role that most DNAm AgeAccel measures had on all cognitive ability outcomes.
GrimAge Accel was the DNAm AgeAccel measure most strongly correlated with loneliness and cognitive functioning, excluding processing speed. Although not exclusively, individuals primarily in the highest loneliness trajectories—the High-But-Declining and the Moderate-But-Declining groups—were found to have lower general cognitive ability and episodic memory scores after adjusting for all covariates. This result is consistent with findings that GrimAge Accel has predictive utility for clinical and clinically related outcomes, including cognitive and functional ability measures (McCrory et al., 2021). GrimAge Accel takes into account proxy measures of adverse lifestyle factors like BMI and smoking pack-years, so it may reflect that as people’s time horizons shorten, higher levels of chronic loneliness have stronger effects on cognitive functioning.
The finding that GrimAge Accel fully mediated effects of the Moderate-But-Declining loneliness trajectory on general cognitive ability and both memory measures partially supported the hypothesis that second-generation DNAm AgeAccel measures would mediate effects of loneliness on cognitive ability in older adulthood. In addition, GrimAge Accel fully mediated effects of the High-But-Declining trajectory on both memory measures but not general cognitive ability. Given the slow rate of decline in the High and Moderate-But Declining loneliness trajectories, the loneliness levels of individuals in both of these groups probably represent greater stability than change over time. Persistent loneliness, be it moderate to high, may put older adults at greater risk of physiological dysregulation and lower cognitive functioning, especially memory functioning.
Unexpectedly, GrimAge Accel mediated effects of the group with the lowest loneliness scores across the three measures, the Low Group, on both memory measures. Yet the indirect effects were slight for both immediate and delayed recall scores, with differences between the Low Group and the Low-But-Increasing group no greater than a .05 units on either memory measure. A judgment about which group characterizes “normal aging” cannot be made here, as the majority of older adults report feeling little to no loneliness (Victor & Yang, 2012). Still, these results suggest that “normal aging” might include small increases in loneliness across older adulthood, as reported elsewhere (Beam & Kim, 2020). People with Low-But-Increasing loneliness, thus, would be expected to perform in the normal range of cognitive functioning compared to same-aged peers. For any number of reasons, including response bias, endogeneity, and sampling error, people whose loneliness trajectories deviate from population norms also may show differences in their cognitive functioning.
A strength of the current study includes employing GMM to identify different loneliness trajectory groups. These latent classes represent both loneliness level and change over an 8-year time span, thus the classes characterize differences in the stability and change of loneliness over time. In the baseline loneliness analyses, no DNAm AgeAccel measure mediated effects of loneliness on any cognitive outcome once demographic, social, psychological, and physiological covariates were included in the model. Yet, when disentangling the different trajectory groups, GrimAge Accel consistently mediated differences between the largest group—the Low-But-Increasing group—and the Moderate-But-Declining, High-But-Declining, and Low groups on general cognitive ability and both memory measures. As a result of using participants’ longitudinal data, larger between-group differences were found. One possibility for this difference in rejecting the null hypothesis is that a single measurement of loneliness precludes identifying groups of individuals with chronic levels of loneliness across the 8-year measurement window. For example, someone who may have been high in loneliness in 2008 yet low in 2012 and 2016 may have been classified in one of the lower LTC groups and performed well cognitively whereas in a cross-sectional analysis this person would have been identified as high in loneliness in 2008 yet also as someone who performed well cognitively compared to someone who was persistently high in loneliness across all three measurements.
Another significant contribution of the current study is that only second-generation DNAm AgeAccel measures mediated effects of loneliness on at least one cognitive functioning outcome in baseline models. Although previous studies have found that Horvath’s (2013) measures correlated with cognitive ability (Levine et al., 2015), loneliness and cognitive functioning are complex traits on which first-generation measures were not necessarily intended to predict; they were designed to predict longevity and disease risk (Crimmins et al., 2020). Second-generation measures include additional biomarkers in their development, most notably metabolic, cardiovascular, and immune markers, that have been shown to correlate with cognitive decline (Reed et al., 2022; Vaccarino et al., 2021). Thus, DNAm AgeAccel measures that explain the association between loneliness and cognitive functioning need to index age-related processes other than methylation alone, like mortality risk, lifestyle factors, and other physiological biomarkers.
We also replicated previous studies, including HRS studies (Sutin et al., 2020), in finding that baseline levels of loneliness predicted differences in cognitive functioning in older adults (Boss et al., 2015; Harrington et al., 2023). We extended these results by showing that loneliness trajectories are also associated with cognitive functioning in the hypothesized direction. Our longitudinal findings are consistent with Akhter-Khan et al.’s (2021) findings that high, persistent levels of loneliness conferred poorer cognitive functioning.
By testing direct and indirect effects of loneliness on specific cognitive domains, we were able to identify which cognitive faculties may have contributed to the association between loneliness and general cognitive ability. In the current study, both memory measures correlated with baseline loneliness as well as the loneliness trajectory groups. Unexpectedly, and in contrast to previous research (Boss et al., 2015), loneliness was uncorrelated with processing speed. One possibility is that the measure used, counting backwards, may be a better assessment of attention or working memory, as the time score was not used in score construction. Alternatively, loneliness and processing speed may not be correlated temporally unlike in cross-sectional studies (Boss et al., 2015). Finally, missingness also may explain the lack of association, as processing speed was the only measure on which missing loneliness scores was correlated.
Depressive symptomatology, BMI, self-rated health, cohort, and education were significantly associated with general cognitive ability and both memory measures. For example, correlations between depressive symptomatology and loneliness scores were moderate (rs = .28 to .31; Supplementary Table 1). Different cohorts, for example, may reflect not only age but generational trends associated with cognitive impairment (Walters et al., 2016). Taken together, our results suggest that hypotheses such as the health risk behavior and psychological distress hypothesis may be more relevant; lifestyle factors that affect physical health directly such as diet and exercise may be critical to the association between loneliness and cognitive functioning.
The current results should be interpreted in view of several limitations. First, all DNAm AgeAccel measures and cognitive functioning outcomes were measured at the same time as the third loneliness measurement, thus precluding strict conclusions that DNAm AgeAccel measures are a mediating mechanism. Second, cellular composition was not controlled for any of the second-generation DNAm AgeAccel measures, as these estimates (i.e., Houseman estimates; Houseman et al., 2012) were not available from the HRS. Nevertheless, prior research has found negligible differences between estimation of DNAm AgeAccel using blood-based measures of cellular composition from flow cytometry (Crimmins et al., 2021). Third, there was significant age heterogeneity at each wave of measurement that limited our ability to draw conclusions about the number of loneliness trajectories. In our growth mixture models, we addressed this by defining the basis weights of each individual’s slope by each individual’s actual ages of assessment. This analytical decision, however, precluded estimating recommended fit statistics for evaluating the fit of different trajectory groups. Fourth, the majority of the sample reported low levels of loneliness. Still, greater than a quarter of the sample belonged to the two highest loneliness trajectories, which were in the upper half of the range. Despite low levels of loneliness overall, the current sample provides a representation of the general U.S. given that the HRS is a population-based sample of community-dwelling older adults.
Future Directions
Future studies should utilize second-generation DNAm-based aging measures, as they show the greatest promise for understanding the physiological biomarkers and comorbidities (e.g., immunological markers, time-to-death, and cardiovascular disease) underlying association between loneliness and cognitive functioning (Boss et al., 2015; Harrington et al., 2023). Additionally, mediation models in which DNAm AgeAccel is measured temporally between loneliness and cognitive functioning at follow-up would bolster the current study findings. Finally, future studies should determine the temporal direction between loneliness, DNAm age acceleration, and psychosocial factors using repeated-measure designs.
Supplementary Material
Data Availability
For HRS, three categories of data: public, sensitive, and restricted can be accessed through procedures described on the HRS website; genotype data are only available to approved researchers with the HRS. Materials and analysis code for this study are available on the Open Science Framework (https://osf.io/e2p89/). Data were analyzed using Mplus, version 8.6 (Muthén & Muthén, 2017). The study’s design and its analyses were not preregistered.
Contributor Information
Morgan Lynch, Department of Psychology, University of Southern California, Los Angeles, California, USA.
Thalida Em Arpawong, Davis School of Gerontology, University of Southern California, Los Angeles, California, USA.
Christopher R Beam, Department of Psychology, University of Southern California, Los Angeles, California, USA; Davis School of Gerontology, University of Southern California, Los Angeles, California, USA.
Funding
This work was supported by the Alzheimer’s Association (AARF-17505302) and the National Institute on Aging (R01AG063949, T32AG000037, and R01AG068937). The Health and Retirement Study is supported by National Institute on Aging (U01 AG009740).
Conflict of Interest
None declared.
References
- Adam, E. K., Hawkley, L. C., Kudielka, B. M., & Cacioppo, J. T. (2006). Day-to-day dynamics of experience—Cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences of the United States of America, 103(45), 17058–17063. 10.1073/pnas.0605053103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter-Khan, S. C., Tao, Q., Ang, T., Itchapurapu, I. S., Alosco, M. L., Mez, J., Piers, R. J., Steffens, D. C., Au, R., & Qiu, W. Q. (2021). Associations of loneliness with risk of Alzheimer’s disease dementia in the Framingham Heart Study. Alzheimer’s & Dementia, 17(10), 1619–1627. 10.1002/alz.12327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin, M. K., Chen, E., Ross, K. M., McEwen, L. M., Maclsaac, J. L., Kobor, M. S., & Miller, G. E. (2018). Early-life socioeconomic disadvantage, not current, predicts accelerated epigenetic aging of monocytes. Psychoneuroendocrinology, 97, 131–134. 10.1016/j.psyneuen.2018.07.007 [DOI] [PubMed] [Google Scholar]
- Awad, R., Shamay-Tsoory, S., & Palgi, Y. (2023). Fluctuations in loneliness due to changes in frequency of social interactions among older adults: A weekly based diary study. International Psychogeriatrics, 35(6), 293–303. 10.1017/S1041610223000133 [DOI] [PubMed] [Google Scholar]
- Beam, C. R., & Kim, A. J. (2020). Psychological sequelae of social isolation and loneliness might be a larger problem in young adults than older adults. Psychological Trauma, 12(S1), S58–S60. 10.1037/tra0000774 [DOI] [PubMed] [Google Scholar]
- Belsky, D. W., Caspi, A., Corcoran, D. L., Sugden, K., Poulton, R., Arseneault, L., Baccarelli, A., Chamarti, K., Gao, X., Hannon, E., Harrington, H. L., Houts, R., Kothari, M., Kwon, D., Mill, J., Schwartz, J., Vokonas, P., Wang, C., Williams, B. S., & Moffitt, T. E. (2022). DunedinPACE, a DNA methylation biomarker of the pace of aging. eLife, 11, e73420. 10.7554/eLife.73420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky, D. W., Caspi, A., Houts, R., Cohen, H. J., Corcoran, D. L., Danese, A., Harrington, H., Israel, S., Levine, M. E., Schaefer, J. D., Sugden, K., Williams, B., Yashin, A. I., Poulton, R., & Moffitt, T. E. (2015). Quantification of biological aging in young adults. Proceedings of the National Academy of Sciences of the United States of America, 112(30), E4104–E4110. 10.1073/pnas.1506264112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun, M. A., Shaked, D., Tajuddin, S. M., Weiss, J., Evans, M. K., & Zonderman, A. B. (2020). Accelerated epigenetic age and cognitive decline among urban-dwelling adults. Neurology, 94(6), e613–e625. 10.1212/WNL.0000000000008756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss, L., Kang, D. H., & Branson, S. (2015). Loneliness and cognitive function in the older adult: A systematic review. International Psychogeriatrics, 27(4), 541–553. 10.1017/S1041610214002749 [DOI] [PubMed] [Google Scholar]
- Chouliaras, L., Rutten, B. P., Kenis, G., Peerbooms, O., Visser, P. J., Verhey, F., van Os, J., Steinbusch, H. W., & van den Hove, D. L. (2010). Epigenetic regulation in the pathophysiology of Alzheimer’s disease. Progress in Neurobiology, 90(4), 498–510. 10.1016/j.pneurobio.2010.01.002 [DOI] [PubMed] [Google Scholar]
- Cole, S. W., Hawkley, L. C., Arevalo, J. M., Sung, C. Y., Rose, R. M., & Cacioppo, J. T. (2007). Social regulation of gene expression in human leukocytes. Genome Biology, 8(9), R189. 10.1186/gb-2007-8-9-r189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, S. W., Levine, M. E., Arevalo, J. M., Ma, J., Weir, D. R., & Crimmins, E. M. (2015). Loneliness, eudaimonia, and the human conserved transcriptional response to adversity. Psychoneuroendocrinology, 62, 11–17. 10.1016/j.psyneuen.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle, C. E., & Dugan, E. (2012). Social isolation, loneliness and health among older adults. Journal of Aging and Health, 24(8), 1346–1363. 10.1177/0898264312460275 [DOI] [PubMed] [Google Scholar]
- Crimmins, E., Kim, J. K., Fisher, J., & Faul, J. (2020). HRS Epigenetic Clocks—Release 1. Survey Research Center, Institute for Social Research, University of Michigan. [Google Scholar]
- Crimmins, E. M., Kim, J. K., Langa, K. M., & Weir, D. R. (2011). Assessment of cognition using surveys and neuropsychological assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66B(S1), i162–i171. 10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins, E. M., Thyagarajan, B., Levine, M. E., Weir, D. R., & Faul, J. (2021). Associations of age, sex, race/ethnicity, and education with 13 epigenetic clocks in a Nationally Representative U.S. sample: The Health and Retirement Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 76(6), 1117–1123. 10.1093/gerona/glab016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel, E., Daubenmier, J., Moskowitz, J. T., Folkman, S., & Blackburn, E. (2009). Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Annals of the New York Academy of Sciences, 1172, 34–53. 10.1111/j.1749-6632.2009.04414.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga, M. F. (2009). Genetic and epigenetic regulation of aging. Current Opinion in Immunology, 21(4), 446–453. 10.1016/j.coi.2009.04.003 [DOI] [PubMed] [Google Scholar]
- Hannum, G., Guinney, J., Zhao, L., Zhang, L., Hughes, G., Sadda, S., Klotzle, B., Bibikova, M., Fan, J. B., Gao, Y., Deconde, R., Chen, M., Rajapakse, I., Friend, S., Ideker, T., & Zhang, K. (2013). Genome-wide methylation profiles reveal quantitative views of human aging rates. Molecular Cell, 49(2), 359–367. 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington, K. D., Vasan, S., Sliwinski, M. J., & Lim, M. H. (2023). Loneliness and cognitive function in older adults without dementia: A systematic review and meta-analysis. Journal of Alzheimer’s Disease, 91(4), 1243–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley, L. C., & Cacioppo, J. T. (2010). Loneliness matters: A theoretical and empirical review of consequences and mechanisms. Annals of Behavioral Medicine, 40(2), 218–227. 10.1007/s12160-010-9210-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley, L. C., & Cacioppo, J. T. (2013). Social connectedness and health. In Hazan C. & Campa M. I. (Eds.), Human bonding: The science of affectional ties (pp. 343–364). The Guilford Press. [Google Scholar]
- Hayes, A. F., Preacher, K. J. (2013). Statistical mediation analysis with a multicategorical independent variable. British Journal of Mathematical and Statistical Psychology, 67(3):451–470. [DOI] [PubMed] [Google Scholar]
- Health and Retirement Study. (2021). Epigenetic Clock Restricted Use Data set. Produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant number NIA U01AG009740). [Google Scholar]
- Heijmans, B. T., Tobi, E. W., Stein, A. D., Putter, H., Blauw, G. J., Susser, E. S., Slagboom, P. E., & Lumey, L. H. (2008). Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proceedings of the National Academy of Sciences of the United States of America, 105(44), 17046–17049. 10.1073/pnas.0806560105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary, R. F., Stevenson, A. J., Cox, S. R., McCartney, D. L., Harris, S. E., Seeboth, A., Higham, J., Sproul, D., Taylor, A. M., Redmond, P., Corley, J., Pattie, A., Hernández, M., Muñoz-Maniega, S., Bastin, M. E., Wardlaw, J. M., Horvath, S., Ritchie, C. W., Spires-Jones, T. L., … Marioni, R. E. (2021). An epigenetic predictor of death captures multi-modal measures of brain health. Molecular Psychiatry, 26(8), 3806–3816. 10.1038/s41380-019-0616-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath, S. (2013). DNA methylation age of human tissues and cell types. Genome Biology, 14(10), R115. 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath, S., Oshima, J., Martin, G. M., Lu, A. T., Quach, A., Cohen, H., & Raj, K. (2018). Epigenetic clock for skin and blood cells applied to Hutchinson Gilford Progeria Syndrome and ex vivo studies. Aging, 10(7), 1758. 10.18632/aging.101508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath, S., & Raj, K. (2018). DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews. Genetics, 19(6), 371–384. 10.1038/s41576-018-0004-3 [DOI] [PubMed] [Google Scholar]
- Houseman, E. A., Accomando, W. P., Koestler, D. C., Christensen, B. C., Marsit, C. J., Nelson, H. H., Wiencke, J. K., & Kelsey, K. T. (2012). DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics, 13, 86. 10.1186/1471-2105-13-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson, I. K., Ploner, A., Wang, Y., Gatz, M., Pedersen, N. L., Hägg, S. (2018). Apolipoprotein E DNA methylation and late-life disease. International Journal of Epidemiology, 47(3), 899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, A. J., Beam, C. R., Greenberg, N. E., & Burke, S. L. (2020). Health factors as potential mediators of the longitudinal effect of loneliness on general cognitive ability. The American Journal of Geriatric Psychiatry, 28(12), 1272–1283. 10.1016/j.jagp.2020.07.017 [DOI] [PubMed] [Google Scholar]
- Kuiper, J. S., Zuidersma, M., Zuidema, S. U., Burgerhof, J. G., Stolk, R. P., Oude Voshaar, R. C., & Smidt, N. (2016). Social relationships and cognitive decline: A systematic review and meta-analysis of longitudinal cohort studies. International Journal of Epidemiology, 45(4), 1169–1206. 10.1093/ije/dyw089 [DOI] [PubMed] [Google Scholar]
- Langa, K.M., Weir, D.R., Kabeto, M., & Sonnega, A. (2020). Langa–Weir Classification of Cognitive Function (1995 Onward). Survey Research Center, Institute for Social Research, University of Michigan. [Google Scholar]
- Lara, E., Martín-María, N., De la Torre-Luque, A., Koyanagi, A., Vancampfort, D., Izquierdo, A., & Miret, M. (2019). Does loneliness contribute to mild cognitive impairment and dementia? A systematic review and meta-analysis of longitudinal studies. Ageing Research Reviews, 52, 7–16. 10.1016/j.arr.2019.03.002 [DOI] [PubMed] [Google Scholar]
- Lee, J., & Cagle, J. G. (2017). Validating the 11-Item Revised University of California Los Angeles Scale to assess loneliness among older adults: An evaluation of factor structure and other measurement properties. The American Journal of Geriatric Psychiatry, 25(11), 1173–1183. 10.1016/j.jagp.2017.06.004 [DOI] [PubMed] [Google Scholar]
- Levine, M. E., Lu, A. T., Bennett, D. A., & Horvath, S. (2015). Epigenetic age of the pre-frontal cortex is associated with neuritic plaques, amyloid load, and Alzheimer’s disease related cognitive functioning. Aging, 7(12), 1198–1211. 10.18632/aging.100864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, M. E., Lu, A. T., Quach, A., Chen, B. H., Assimes, T. L., Bandinelli, S., Hou, L., Baccarelli, A. A., Stewart, J. D., Li, Y., Whitsel, E. A., Wilson, J. G., Reiner, A. P., Aviv, A., Lohman, K., Liu, Y., Ferrucci, L., & Horvath, S. (2018). An epigenetic biomarker of aging for lifespan and healthspan. Aging, 10(4), 573–591. 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, A. T., Quach, A., Wilson, J. G., Reiner, A. P., Aviv, A., Raj, K., Hou, L., Baccarelli, A. A., Li, Y., Stewart, J. D., Whitsel, E. A., Assimes, T. L., Ferrucci, L., & Horvath, S. (2019). DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging, 11(2), 303–327. 10.18632/aging.101684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni, R. E., Shah, S., McRae, A. F., Chen, B. H., Colicino, E., Harris, S. E., Gibson, J., Henders, A. K., Redmond, P., Cox, S. R., Pattie, A., Corley, J., Murphy, L., Martin, N. G., Montgomery, G. W., Feinberg, A. P., Fallin, M. D., Multhaup, M. L., Jaffe, A. E., … Deary, I. J. (2015). DNA methylation age of blood predicts all-cause mortality in later life. Genome Biology, 16(1), 25. 10.1186/s13059-015-0584-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory, C., Fiorito, G., Hernandez, B., Polidoro, S., O’Halloran, A. M., Hever, A., & Kenny, R. A. (2021). GrimAge outperforms other epigenetic clocks in the prediction of age-related clinical phenotypes and all-cause mortality. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 76(5), 741–749. 10.1093/gerona/glaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeish, D., & Harring, J. (2020). Covariance pattern mixture models: Eliminating random effects to improve convergence and performance. Behavior Research Methods, 52, 947–979. 10.3758/s13428-019-01292-4 [DOI] [PubMed] [Google Scholar]
- Muthén, L. K., & Muthén, B. O. (1998-2017). Mplus: Statistical analysis with latent variables: User’s guide (Version 8). Muthén & Muthén. [Google Scholar]
- Penninkilampi, R., Casey, A. N., Singh, M. F., & Brodaty, H. (2018). The association between social engagement, loneliness, and risk of dementia: A systematic review and meta-analysis. Journal of Alzheimer's Disease, 66(4), 1619–1633. 10.3233/JAD-180439 [DOI] [PubMed] [Google Scholar]
- Perlman, D., & Peplau, L. A. (1981). Toward a social psychology of loneliness. In Gilmour R. & Duck S. (Eds.), Personal relationships: 3. Relationships in disorder (pp. 31–56). Academic Press. [Google Scholar]
- Radloff, L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Ram, N., & Grimm, K. J. (2009). Growth mixture modeling: A method for identifying differences in longitudinal change among unobserved groups. International Journal of Behavioral Development, 33(6), 565–576. 10.1177/0165025409343765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND HRS Longitudinal File 2020 (V1). (2023). Produced by the RAND Center for the Study of Aging, with funding from the National Institute on Aging and the Social Security Administration. [Google Scholar]
- Reed, R. G., Carroll, J. E., Marsland, A. L., & Manuck, S. B. (2022). DNA methylation-based measures of biological aging and cognitive decline over 16-years: Preliminary longitudinal findings in midlife. Aging, 14(23), 9423–9444. 10.18632/aging.204376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibbett, R. A., Altschul, D. M., Marioni, R. E., Deary, I. J., Starr, J. M., & Russ, T. C. (2020). DNA methylation-based measures of accelerated biological ageing and the risk of dementia in the oldest-old: A study of the Lothian Birth Cohort 1921. BMC Psychiatry, 20(1), 1–15. 10.1186/s12888-020-2469-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spithoven, A. W. M., Cacioppo, S., Goossens, L., & Cacioppo, J. T. (2019). Genetic contributions to loneliness and their relevance to the evolutionary theory of loneliness. Perspectives on Psychological Science, 14(3), 376–396. 10.1177/1745691618812684 [DOI] [PubMed] [Google Scholar]
- Sumner, J. A., Colich, N. L., Uddin, M., Armstrong, D., & McLaughlin, K. A. (2019). Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biological Psychiatry, 85(3), 268–278. 10.1016/j.biopsych.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin, A. R., Stephan, Y., Luchetti, M., & Terracciano, A. (2020). Loneliness and risk of dementia. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 75(7), 1414–1422. 10.1093/geronb/gby112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilvis, R. S., Kähönen-Väre, M. H., Jolkkonen, J., Valvanne, J., Pitkala, K. H., & Strandberg, T. E. (2004). Predictors of cognitive decline and mortality of aged people over a 10-year period. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 59(3), 268–274. 10.1093/gerona/59.3.m268 [DOI] [PubMed] [Google Scholar]
- Vaccarino, V., Huang, M., Wang, Z., Hui, Q., Shah, A. J., Goldberg, J., Smith, N., Kaseer, B., Murrah, N., Levantsevych, O. M., Shallenberger, L., Driggers, E., Bremner, J. D., & Sun, Y. V. (2021). Epigenetic age acceleration and cognitive decline: A twin study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 76(10), 1854–1863. 10.1093/gerona/glab047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor, C. R., & Yang, K. (2012). The prevalence of loneliness among adults: A case study of the United Kingdom. The Journal of Psychology, 146(1–2), 85–104. 10.1080/00223980.2011.613875 [DOI] [PubMed] [Google Scholar]
- Walters, K., Hardoon, S., Petersen, I., Iliffe, S., Omar, R. Z., Nazareth, I., & Rait, G. (2016). Predicting dementia risk in primary care: development and validation of the Dementia Risk Score using routinely collected data. BMC Medicine, 14, 6. 10.1186/s12916-016-0549-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir, D. R., Faul, J. D., & Langa, K. M. (2011). Proxy interviews and bias in cognition measures due to non-response in longitudinal studies: A comparison of HRS and ELSA. Longitudinal and Life Course Studies, 2(2), 170–184. 10.14301/llcs.v2i2.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. W., Yaqub, A., Ma, Y., Koudstaal, W., Hofman, A., Ikram, M. A., Ghanbari, M., & Goudsmit, J. (2021). Biological age in healthy elderly predicts aging-related diseases including dementia. Scientific Reports, 11(1), 15929. 10.1038/s41598-021-95425-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, K., & Victor, C. (2011). Age and loneliness in 25 European nations. Ageing and Society, 31(8), 1368–1388. 10.1017/s0144686x1000139x [DOI] [Google Scholar]
- Yu, K., & Ng, T. K. S. (2022). Investigating biological pathways underpinning the longitudinal association between loneliness and cognitive impairment. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 78, 1417–1426. 10.1093/gerona/glac213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas, A. S. (2019). Epigenetics as a key link between psychosocial stress and aging: Concepts, evidence, mechanisms. Dialogues in Clinical Neuroscience, 21(4), 389–396. 10.31887/DCNS.2019.21.4/azannas [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


