Abstract
Inhibiting a key metabolic enzyme, ACLY, in cancer cells impacts T cell function in immunotherapy-resistant tumors and may offer a target for therapeutic treatment.
To enhance the effectiveness of immunotherapies, an increased understanding is needed of how cancer cells, whose métier is to adapt their metabolism at all costs to support growth, impair T cell function. In this issue of Science Advances, Xiang et al. (1) show that ATP-citrate lyase (ACLY) inhibition and immune checkpoint molecule (ICM) blockade act synergistically to support increased T cell numbers, enhanced T cell effector function, and tumor control in difficult-to-treat tumors. Leveraging their mechanistic insights into reprogramming events following ACLY inhibition, the authors also provide a novel strategy of dietary intervention with ICM blockade to reinvigorate T cell antitumor function.
ACLY plays a central role in cancer cell metabolism, growth, and metastasis (2). Functionally, ACLY metabolizes citrate to acetyl-CoA and oxaloacetate (OAA). ACLY-derived acetyl-CoA provides an indispensable precursor for de novo fatty acid as well as cholesterol synthesis in the cytoplasm, and a substrate for chromatin-modifying enzymes in the nucleus (3). Foreshadowing its importance in biology, the introduction of null mutations in Acly at the level of the murine germline is embryonic lethal (4). ACLY is highly expressed in several forms of cancer including glioblastoma, colorectal, breast, and hepatocellular carcinomas (HCCs) (5, 6), and its important role in proliferation makes it a promising target for drug development. This enzymatic driver sits at the nexus of interventional strategies for lung and prostate cancer, as well as hypercholesterolemia and obesity. Together, these aspects position ACLY at the helm of metabolic fate in proliferating cells and a promising target in cancer cells.
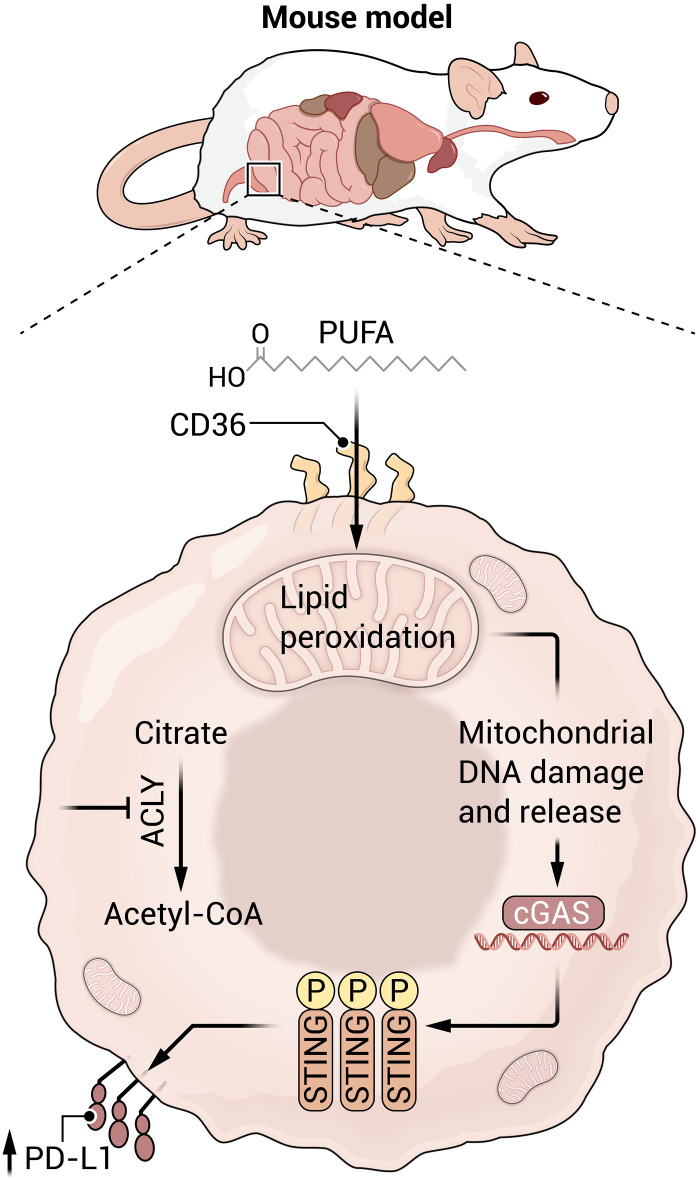
The study by Xiang et al. adds to the growing body of knowledge that ACLY inhibition in cancer cells enforces the uptake of polyunsaturated fatty acids (PUFAs) from the local milieu. ACLY knockdown promotes an up-regulation of CD36, the fatty acid (FA) translocase that facilitates FA uptake (Fig. 1). In a metabolomics screen, they show that Acly-deficient Pan02 cells opt for arachidonic acid, α-linoleic acid, and eicosapentaenoic acid to fuel their growth. The study data support a model in which cancer cells respond to ACLY inhibition via intracellular PUFA accumulation and metabolism. In general, PUFA can elicit a kaleidoscopic range of effects from the formation of eicosanoid signaling molecules, support of phospholipid bilayer formation, and the regulation of gene expression via nuclear receptor signaling.
Fig. 1. Inhibiting ACLY, a key metabolic enzyme, reprograms tumor cells to consume exogenous polyunsaturated fatty acids (PUFAs).
Fatty acid oxidation can promote mitochondrial DNA damage and release which then triggers the cGAS STING pathway to induce the protein PD-L1 on the surface of tumor cells. In immunotherapy-resistant tumors, combining ACLY inhibition (or a dietary PUFA intervention) with immune checkpoint molecule (ICM) blockade can reinvigorate tumor-targeting T cells. Illustration credit: Austin Fisher/Science Advances.
Viewed through a metabolic lens, PUFAs are carbon-rich molecules whose oxidation can trigger free radical production and mitochondrial damage. Xiang et al. show that damaged mitochondria release their DNA which activates the cGAS/STING innate immune pathway. This induces PD-L1 expression thereby inhibiting T cell antitumor function. Informed by these data, the authors developed an innovative strategy that combines ACLY inhibition with immune checkpoint blockade (anti–PD-L1) to curb tumor growth in mouse models of Pan02 pancreatic cancer and B16 melanoma. ACLY inhibition also enhances ICM blockade in vivo and recovers T cell antitumor function in a Hepa1-6–CD38 liver cancer model. This breadth of therapeutic benefit will appeal widely to the immunotherapy community.
The research reveals a promising combinatorial strategy to reinvigorate T cell cytolytic function in immunotherapy-resistant tumors. An interesting finding of the study is that the mitochondrial-targeting antioxidant, mitoTEMPO, reduces STING activation and PD-L1 induction suggesting an important link between oxidative stress, STING signaling, and ICM induction in cancer cells. It will be interesting to see if N-acetylcysteine, which is a dietary supplement, can buffer reactive oxygen species (ROS) sufficiently to limit mitochondrial damage and reverse ICM induction in tumor cells.
Xiang et al. shed light on an important yet correlative observation in the field: Low ACLY expression in tumor cells is associated with an increased abundance of exhausted T cells in the tumor microenvironment. This manifests as poor clinical outcomes and patient responses in human HCC and pancreatic ductal adenocarcinoma (PDAC). These effects are independent of changes in immunogenicity/antigen presentation as MHC-1 expression is unaltered by ACLY inhibition. The study’s insights into mechanisms of immunosuppression following ACLY inhibition are supported by a mouse model of pancreatic cancer when Acly is posttranscriptionally suppressed with short hairpin–mediated RNA interference (shAcly): shAcly tumors (Pan02) are infiltrated by exhausted CD8+ T cells characterized phenotypically by high PD-1 expression and functionally by limited cytolytic ability. The authors provide mechanistic insights into the nature of immunosuppression: ACLY inhibition promotes PD-L1 expression through the cytoplasmic DNA sensing signaling pathway cGAS STING. They also provide genetic and pharmacologic evidence that Acly knockdown promotes the phosphorylation of STING Tbk1 and Irf3 in Pan02, B16, and Hepa1-6 cells. Consistent with the study model, low ACLY expression correlated with cGAS-STING activation in several human cancers including HCC, PDAC, and melanoma.
The data point to ACLY in tumor cells as the critical target underlying the phenotype. However, stromal cells that rely on metabolic reprogramming for their antitumor function may be affected by compounds targeting ACLY. Undoubtedly, this will fuel a series of compelling studies in this area. For example, macrophages govern the immune landscape of several cancers and are key regulators of tumor growth (7). Macrophages can also direct antitumor functions. These opposing roles are related to their phenotypic polarity, differentiating into a proinflammatory (M1) versus immunosuppressive (M2) fate. A previous study characterized a “hybrid” M1/M2 metabolic state in response to the TLR9 agonist, CpG, that enabled antitumor activity that depended on CPT1A and ACLY (8). As central carbon metabolism is an important determinant of macrophage antitumor activity, future research is needed to define how ACLY inhibition or PUFA dietary intervention affects macrophage fate, in the context of ICM blockade.
T cells also express PD-L1. The puzzle proliferates as the authors provide evidence that Jurkat T cells and cancer cells do not share an identical metabolic response to ACLY inhibition. So far, the phenomenon they describe is exclusive to cancer cells, making it an attractive target for a combinatorial approach involving ICM blockade. Understanding how other stromal cells including primary human T cells respond to ACL inhibition will inform efforts to design next-generation immunotherapies. Of note, acetyl-CoA is replenished principally by glutamine and minimally by exogenous sources of long-chain FAs in primary human T cells (9). This preference for glutamine over exogenous FAs makes the findings of Xiang et al. more impactful. As noted by the authors, an inevitable consequence of FA oxidation is the production of potentially harmful ROS. The inherent aversion of activated T cells (at least in the context of CD28 costimulation) to exogenous long-chain FAs may explain why T cells are spared from the oxidative stress–induced responses induced by ACLY inhibition as shown in this study. Additional studies will be needed to determine how ACLY inhibition would affect T cells expressing chimeric antigen receptors containing built-in 4-1BB costimulatory domains. Direct stimulation via the CAR (as opposed to the endogenous TCR) and costimulation via 4-1BB do redirect metabolism toward exogenous FAs (10).
And here's the kicker: The authors show that a dietary intervention of PUFA combined with PD-L1 blockade replicates the therapeutic benefit of ACLY inhibition and ICM blockade in a Pan02 mouse model of cancer. PUFAs have attracted much interest and controversy for their potential anticancer effects. Deleting cGAS in Pan02 cells abrogates the synergistic benefit of PUFA with anti–PD-L1 treatment. Given their similar mechanisms of action, these findings support the principle that dietary PUFAs can enhance the efficacy of PD-L1 blockade mirroring the impact of ACLY inhibition. Elucidating which subspecies of PUFA culminates in cGAS STING activation will be an important next step for this research. The authors show that α-linoleic acid is less important in the context of Pan02 cells and may be more relevant for melanoma. Conversely, docosahexaenoic acid (DHA), which is an important PUFA, has no obvious effect on STING activity or ICM induction. These findings implicate distinct forms of PUFA in the observed phenotype and warrant further investigation for this exciting field of cancer immunity.
This new study also makes a strong case for revisiting drug development that inhibits ACLY since few clinically relevant compounds exist. While bempedoic acid is an exception, it is a prodrug that requires activation by a liver-specific enzyme. Notably, this enzyme can be expressed, albeit less abundantly, in some cancer cell lines including those used in this study. This activity explains why Xiang et al. emphasize genetic approaches (RNAi) to suppress ACLY. Complementing the genetic approaches to inhibit ACLY with pharmacologic approaches would increase the strength of the study findings. For enhanced, immediate, translational relevance, this research provides strong support for future work in this area of drug development.
REFERENCES AND NOTES
- 1.Xiang W., Lv H., Xing F., Sun X., Ma Y., Wu L., Lv G., Zong Q., Wang L., Wu Z., Feng Q., Yang W., Inhibition of ACLY overcomes cancer immunotherapy resistance via polyunsaturated fatty acids peroxidation and cGAS-STING activation. Sci. Adv. 9, eadi2465 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Icard P., Wu Z., Fournel L., Coquerel A., Lincet H., Alifano M., ATP citrate lyase: A central metabolic enzyme in cancer. Cancer Lett. 471, 125–134 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Wellen K. E., Hatzivassiliou G., Sachdeva U. M., Bui T. V., Cross J. R., Thompson C. B., ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigneux A. P., Kosinski C., Gavino B., Horton J. D., Skarnes W. C., Young S. G., ATP-citrate lyase deficiency in the mouse. J. Biol. Chem. 279, 9557–9564 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaidi N., Swinnen J. V., Smans K., ATP-citrate lyase: a key player in cancer metabolism. Cancer Res. 72, 3709–3714 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Koundouros N., Poulogiannis G., Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 122, 4–22 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantovani A., Allavena P., Marchesi F., Garlanda C., Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 21, 799–820 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu M., O’Connor R. S., Trefely S., Graham K., Snyder N. W., Beatty G. L., Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated 'don't-eat-me' signal. Nat. Immunol. 20, 265–275 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Connor R. S., The CPT1a inhibitor, etomoxir induces severe oxidative stress at commonly used concentrations. Sci. Rep. 8, 6289 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawalekar O. U., O’Connor R. S., Fraietta J. A., Guo L., McGettigan S. E., Posey A. D. Jr., Patel P. R., Guedan S., Scholler J., Keith B., Snyder N. W., Blair I. A., Milone M. C., June C. H., Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44, 380–390 (2016). [DOI] [PubMed] [Google Scholar]