Abstract
Background
The emergence of human papillomavirus (HPV)–positive oropharyngeal cancer and evolving tobacco use patterns have changed the landscape of head and neck cancer epidemiology internationally. We investigated updated trends in oropharyngeal cancer incidence worldwide.
Methods
We analyzed cancer incidence data between 1993 and 2012 from 42 countries using the Cancer Incidence in Five Continents database volumes V through XI. Trends in oropharyngeal cancer incidence were compared with oral cavity cancers and lung squamous cell carcinomas using log-linear regression and age period–cohort modeling.
Results
In total, 156 567 oropharyngeal cancer, 146 693 oral cavity cancer, and 621 947 lung squamous cell carcinoma patients were included. Oropharyngeal cancer incidence increased (P < .05) in 19 and 23 countries in men and women, respectively. In countries with increasing male oropharyngeal cancer incidence, all but 1 had statistically significant decreases in lung squamous cell carcinoma incidence, and all but 2 had decreasing or nonsignificant net drifts for oral cavity cancer. Increased oropharyngeal cancer incidence was observed both in middle-aged (40-59 years) and older (≥60 years) male cohorts, with strong nonlinear birth cohort effects. In 20 countries where oropharyngeal cancer incidence increased for women and age period–cohort analysis was possible, 13 had negative or nonsignificant lung squamous cell carcinoma net drifts, including 4 countries with higher oropharyngeal cancer net drifts vs both lung squamous cell carcinoma and oral cavity cancer (P < .05 for all comparisons).
Conclusions
Increasing oropharyngeal cancer incidence is seen among an expanding array of countries worldwide. In men, increased oropharyngeal cancer is extending to older age groups, likely driven by human papillomavirus–related birth cohort effects. In women, more diverse patterns were observed, suggesting a complex interplay of risks factors varying by country, including several countries where female oropharyngeal cancer increases may be driven by HPV.
The epidemiologic landscape of oropharyngeal cancer has evolved in recent decades (1-4). For much of the past century, the primary risk factors for developing oropharyngeal cancer were tobacco and alcohol use (5,6). More recently, changes in sexual behaviors and hypothesized increases in the prevalence of oral human papillomavirus (HPV) infections have produced a wave of HPV-related oropharyngeal cancers in many countries at a time when tobacco-related malignancies in the same populations are declining (7-9).
Previously, our group reported worldwide trends in oropharyngeal cancer incidence across 23 countries between 1983 and 2002 (1). That study showed that among men, oropharyngeal cancer incidence was primarily increasing among younger persons (<60 years of age) in economically developed countries, accompanied by concomitant declines in the incidence of other smoking-associated cancers, underscoring a key role for HPV in this population. In women, increasing oropharyngeal cancer incidence was limited to a group of European countries with parallel increases in lung cancer and oral cavity cancer, strongly suggesting that tobacco was the primary driver of these changes in women. The epidemiologic data from our previous study are now more than 2 decades old, however, and in many countries, HPV-related oropharyngeal cancer incidence has continued to surge. Moreover, rates of HPV-related oropharyngeal cancer are substantially increasing among older patients in some countries (10,11). Finally, more cancer registry data are now available, especially from parts of the world that were relatively underrepresented in our prior study, including Asia, eastern Europe, Africa, and the Middle East.
Here, we update international trends in oropharyngeal cancer incidence from 1993 to 2012 in 42 countries across 6 continents. We compare these changes with trends in oral cavity cancer and lung squamous cell carcinoma to enable interpretation of the potential effects of HPV vis-à-vis smoking (and alcohol use for oral cavity cancers) (1).
Methods
Data sources
Cancer incidence data were obtained from the Cancer Incidence in Five Continents, volumes V through XI, a compendium of data from high-quality population-based cancer registries maintained by the International Agency for Research on Cancer. Each quinquennial volume contains cancer incidence data stratified by sex, year of diagnosis, and 5-year age group. We assembled data from 85 registries encompassing 42 countries across 6 continents (excluding Antarctica) (Supplementary Methods, available online).
Anatomic site and histology codes
Cancers were grouped according to International Statistical Classification of Diseases codes. For oropharyngeal cancer, all histologies were included, given that more than 95% are squamous cell carcinoma. We also analyzed incidence trends in lung squamous cell carcinoma, representing a cancer driven primarily by smoking, and oral cavity cancers, representing a cancer driven by both tobacco and alcohol use. Full codes used are in the Supplementary Methods (available online).
Statistical analysis
Data were aggregated from all available registries within each country. Cancer-specific, age-standardized incidence rates were estimated for each calendar year (1983-2012). Trends in oropharyngeal cancer incidence were estimated using weighted least squares log-linear regression. Log-liner regression was used as the initial analysis methodology because it could be applied to all countries, including those with sparser data. To quantify the direction and magnitude of the trends, the estimated annual percentage change was calculated by taking the antilog of the regression coefficient of (calendar year – 1) × 100.
Age period–cohort modeling was used in countries with statistically significant increases in oropharyngeal cancer incidence, as assesed by log-linear regression, to study the concomitant effects of age, calendar year, and birth year on cancer incidence (12). Five-year age groups (25-29, 30-34, etc), 5-year calendar periods (1993-1997, 1998-2002, etc), and 10-year partially overlapping birth cohorts (1918, 1923, … , 1983) were used. Given sparse data in certain age groups for some countries, the specific age range varied by country (Supplementary Figure 1, available online), in contrast to the log-linear regression analysis that used all cases. Because years of available data varied by country, a cubic interpolation from 5-year to 1-year age groups was performed. Some countries had insufficient case numbers to generate robust age period–cohort modeling and thus were excluded from these analyses, despite increasing oropharyngeal cancer incidence (men: Iceland; women: Brazil, China, and Colombia).
Net drifts were estimated from age period–cohort modeling. The net drift was interpreted as the estimated annual percentage change of the age-standardized rate over time. Net drifts were used as the primary analytic tool for comparing various cancer incidence trends because they adjust for both nonlinear period and birth cohort effects. Net drifts for oropharyngeal cancer vs lung squamous cell carcinoma and oral cavity cancer were compared using a 1-df Wald test. Local drifts, which are the age group–specific estimated annual percentage changes, were estimated and compared with the overall net drift for a given country using a 2(A ‒ 1)–df Wald test for heterogeneity, where A is the number of age groups analyzed. To explore cohort effects in countries with significant increases in oropharyngeal cancer incidence, we constructed lexis diagrams for rates, as described by Carstensen, using 5-year intervals of cohort and age (13). We estimated the Pearson correlation of oropharyngeal cancer net drift with the 2000 Human Development Index (Supplementary Methods, available online).
All statistical analyses were performed using R, version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). All statistical comparisons were based on 2-sided tests, with P less than .05 considered significant.
Results
Age-standardized incidence rates for oropharyngeal cancer
In total, 156 567 oropharyngeal cancer cases, 146 693 oral cavity cancer cases, and 621 947 lung squamous cell carcinoma cases diagnosed between 1993 and 2012 from 42 countries were included. Wide variations in the age-standardized incidence rates of oropharyngeal cancer were observed across countries for patients diagnosed between 2008 and 2012, the most recent period available (Supplementary Figure 2, available online). For example, in men there was an approximately 28-fold difference between oropharyngeal cancer incidence in the highest-incidence (France: 19.7 cases/100 000 people) and lowest-incidence countries (Kuwait: 0.7 cases/100 000 people). Substantial variation was also observed for oropharyngeal cancer incidence in women, with a 52-fold difference in the highest-incidence (Germany: 5.2 cases/100 000 people) vs lowest-incidence countries (Bahrain: 0.1 cases/100 000 people). Oropharyngeal cancer incidence was approximately 3 to 4 times higher among men than women in most countries (Supplementary Figure 3, available online). Notably, this sex ratio was skewed more heavily toward men in certain eastern and central European countries, including Lithuania (ratio of male to female incidence, 8.1), Croatia (7.1), Slovakia (6.9), Estonia (6.3), Bulgaria (6.2), and Slovenia (4.5).
Oropharyngeal cancer estimated annual percentage change
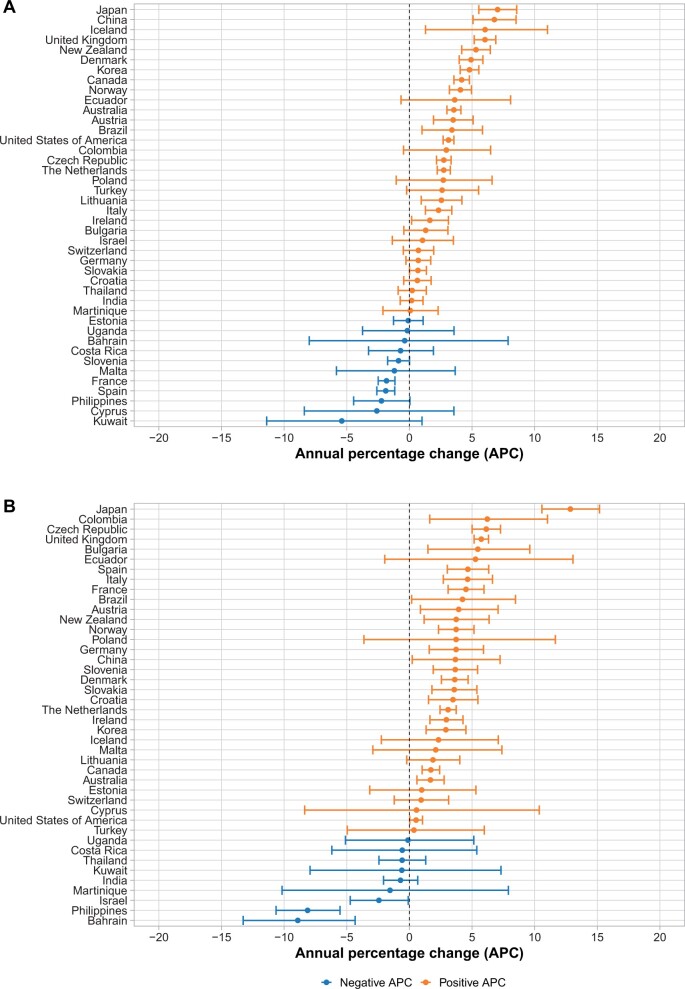
There was a statistically significant increase or a positive trend in oropharyngeal cancer incidence in most countries between 1993 and 2012 in both men and women as assessed by log-linear regression (Figure 1). In men, 19 countries had statistically significant increases in oropharyngeal cancer incidence (Supplementary Figure 4, available online). Thirteen of these countries exhibited marked increases, with annual percentage increases of more than 3% per year, largely in economically developed countries in Asia (Japan, China, Republic of Korea), North America (United States, Canada), South America (Brazil), Oceania (Australia, New Zealand), and northern Europe (United Kingdom, Denmark, Austria, Iceland, Norway). Oropharyngeal cancer incidence showed statistically significant decreases over time for men in only 2 countries: France (‒1.8%/year, P < .001) and Spain (‒1.9%/year, P < .001).
Figure 1.
Annual percentage change in incidence of oropharyngeal cancers in men and women from 1993 to 2012.
In women, 23 countries had statistically significant increases in oropharyngeal cancer incidence (Supplementary Figure 5, available online). Similar to men, the magnitude of increased oropharyngeal cancer incidence observed was substantial, with annual percentage changes greater than 3% per year in 19 countries. Notably, the geographic distribution of countries with large relative oropharyngeal cancer increases (>3%/year) was different in women than in men, with prominent increases in eastern and central Europe (Austria, Bulgaria, Croatia, Czech Republic, Slovakia, Slovenia), southern Europe (Italy, Spain), western Europe (France, Germany, Denmark), and South America (Brazil, Columbia), among other countries. The only countries with statistically significant decreases in oropharyngeal cancer among women were Bahrain (‒8.9%/year, P = .03), Israel (‒2.4%/year, P = .04) and the Philippines (‒8.1%/year, P = .001).
Age period–cohort modeling
We evaluated potential factors underlying these epidemiologic changes using age period–cohort modeling in countries with increasing oropharyngeal cancer incidence. Of note, only 18 of 19 countries for men (excluding Iceland) and 20 of 23 countries for women (excluding Colombia, China, and Brazil) had sufficient data for robust age period–cohort analysis.
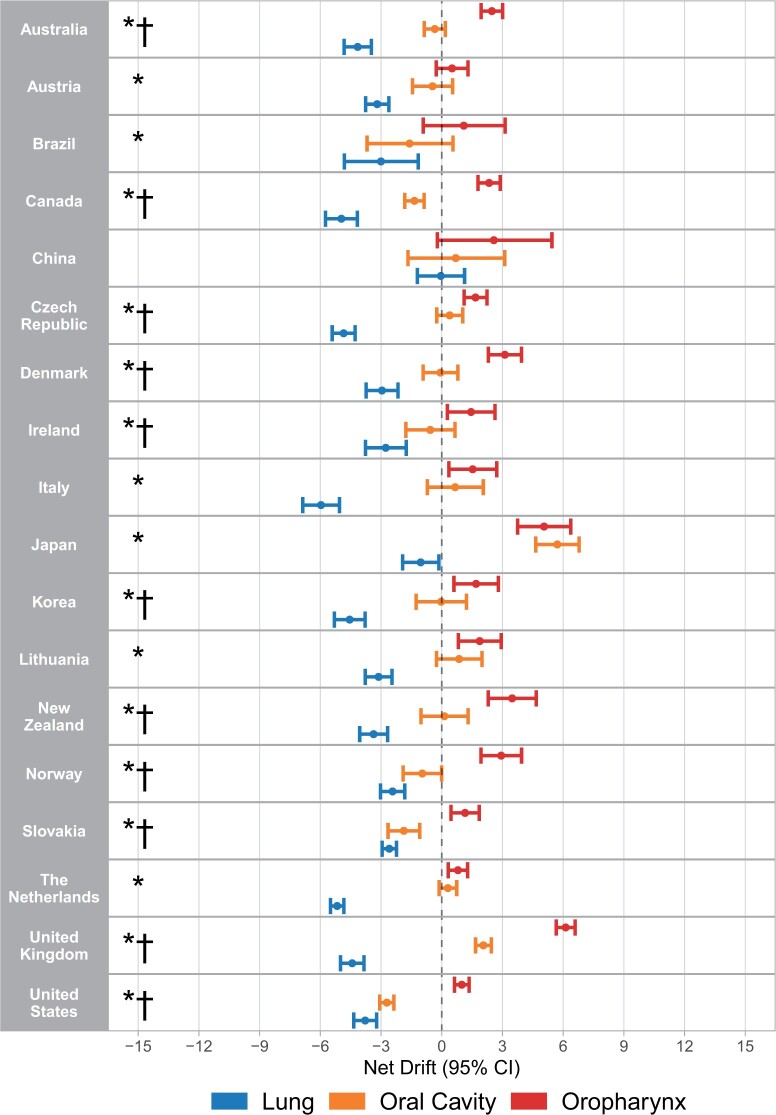
Despite similar increases in oropharyngeal cancer incidence for men and women from 1993 to 2012, different epidemiologic signatures were observed according to sex. In men, 17 of 18 analyzable countries with increasing oropharyngeal cancer incidence had significant decreases in lung squamous cell carcinoma incidence, with the exception of China, where the net drift was zero (Figure 2). The net drift for oropharyngeal cancer incidence was significantly higher than for lung squamous cell carcinoma (P < .05) in all these countries, except China. Incidence changes for oral cavity cancer in men tended to fall between those of oropharyngeal cancer and lung squamous cell carcinoma for most countries. Sixteen of 18 countries had either negative or nonsignificant oral cavity cancer net drifts, with significantly higher oropharyngeal cancer vs oral cavity cancer net drifts in 11 of 18 countries (Supplementary Table 1, available online).
Figure 2.
Net drifts calculated using age period–cohort analysis for oropharyngeal cancer, oral cavity cancer, and squamous cell carcinoma of the lung in men for countries with increasing incidence of oropharyngeal cancer in log-linear regression. * = net drift for oropharyngeal cancer greater than squamous cell carcinoma of the lung (P < .05), † = net drift for oropharyngeal cancer greater than oral cavity cancer (P < .05). CI = confidence interval.
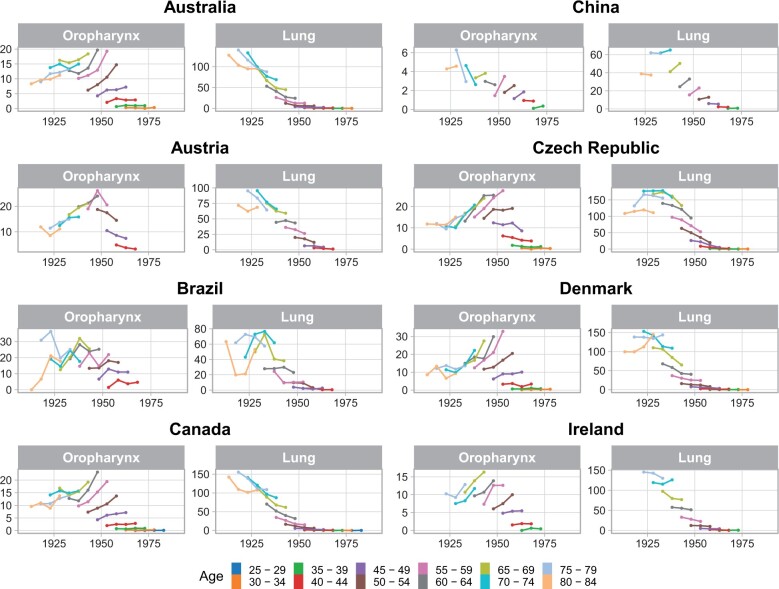
Strong birth cohort effects were observed among countries with increasing oropharyngeal cancer incidence among men, with significant heterogeneity in birth cohort rate ratios observed in 17 of 18 countries (Supplementary Figure 6, available online). In these countries, an increasing incidence of oropharyngeal cancer was typically observed initially for birth cohorts born around 1940. Incidence generally continued to rise for subsequent birth cohorts, although the confidence intervals were wide for more contemporary birth cohorts because of the low absolute incidence of oropharyngeal cancer in younger patients. When analyzing birth cohorts stratified by age, it was observed that generally in most countries, oropharyngeal cancer incidence increased strongly for each subsequent birth cohort within each age group for patients 45 years of age and older, including the subset of patients older than 60 years. Decreases in lung squamous cell carcinoma incidence were seen for these age groups across birth cohorts (Figure 3).
Figure 3.
Birth cohort effects for oropharyngeal cancer and lung squamous cell carcinoma incidence in men, stratified by age for countries with statistically significant increases in oropharyngeal cancer incidence.
Figure 3.
Continued.
We assessed age-specific incidence changes (local drifts) in oropharyngeal cancer for each of these countries with increased oropharyngeal cancer incidence (Supplementary Figure 7, available online). Generally, oropharyngeal cancer local drifts increased for patients older than 50 years of age, peaking in middle age (∼55-65 years) but remaining positive even for those 70 years of age and older in most countries (P < .05 for heterogeneity of local drifts for the United States, Canada, the Czech Republic, New Zealand, Australia, the Netherlands, the United Kingdom, Slovakia, Italy, Lithuania, and China). The Republic of Korea and China were exceptions in which patients 70 years of age and older had negative oropharyngeal cancer local drifts.
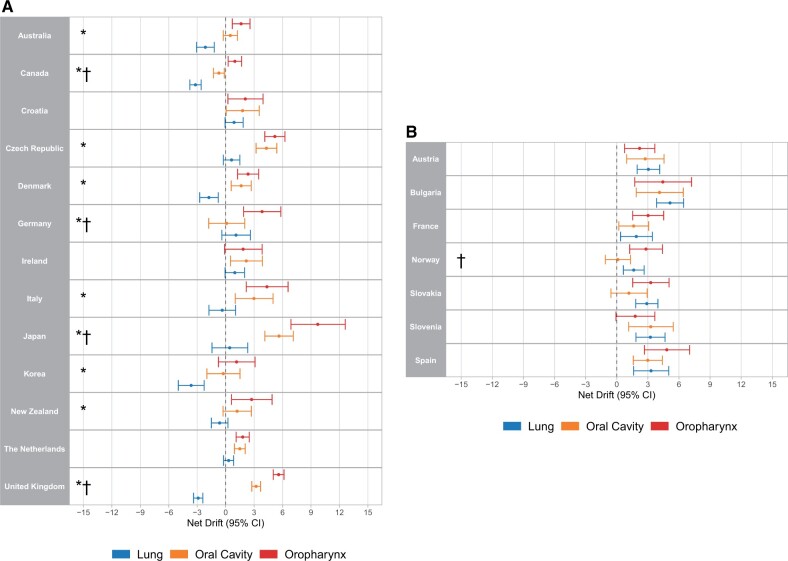
In contrast, more diverse epidemiologic patterns were observed in women (Figure 4). Of 20 analyzed countries with statistically significant positive oropharyngeal cancer annual percentage changes (Supplementary Table 2, available online), 13 had either decreasing or nonsignificant lung squamous cell carcinoma net drifts (Australia, Canada, Croatia, the Czech Republic, Denmark, Germany, Ireland, Italy, Japan, the Republic of Korea, the Netherlands, New Zealand, and the United Kingdom). Of these, 10 had statistically significant differences between oropharyngeal cancer and lung squamous cell carcinoma incidence trends (P < .05), whereas Croatia, Italy, and Ireland did not. In the 10 countries in which changes in oropharyngeal cancer incidence were statistically significantly higher than lung squamous cell carcinoma, 2 different patterns emerged. In 5 countries, oral cavity cancer and oropharyngeal cancer tracked fairly closely together (the Czech Republic, Denmark, the Republic of Korea, the Netherlands, New Zealand). In 4 other countries (the United Kingdom, Canada, Germany, and Japan), the increase in oropharyngeal cancer incidence was much greater than oral cavity cancer incidence changes. In the 7 countries with statistically significant increases in both oropharyngeal cancer and lung squamous cell carcinoma incidence in women (Austria, Bulgaria, France, Norway, Slovakia, Slovenia, Spain), no statistical differences were observed between the magnitude of the net drifts for oropharyngeal cancer vs lung squamous cell carcinoma or oral cavity cancer, with the exception of higher oropharyngeal cancer vs oral cavity cancer net drifts in Norway. Strong birth cohort effects were observed in women (Supplementary Figures 8 and 9, available online), with significant heterogeneity in birth cohort rate ratios observed in 16 of 20 countries. No significant age variations in oropharyngeal cancer incidence changes were observed in women, except in Australia and Germany (Supplementary Figure 10, available online). Notably, relatively wide confidence intervals were present for age-specific oropharyngeal cancer incidence change estimates in women because of smaller absolute case numbers for women than for men.
Figure 4.
Net drifts calculated using age period–cohort analysis for oropharyngeal cancer, oral cavity cancer, and squamous cell carcinoma of the lung for women in countries with increasing incidence of oropharyngeal cancer in log-linear regression and A) without or B) with significantly positive lung squamous cell carcinoma net drifts. * = net drift for oropharyngeal cancer greater than lung squamous cell carcinoma (P < .05), † = net drift for oropharyngeal cancer greater than oral cavity cancer (P < .05). CI = confidence interval.
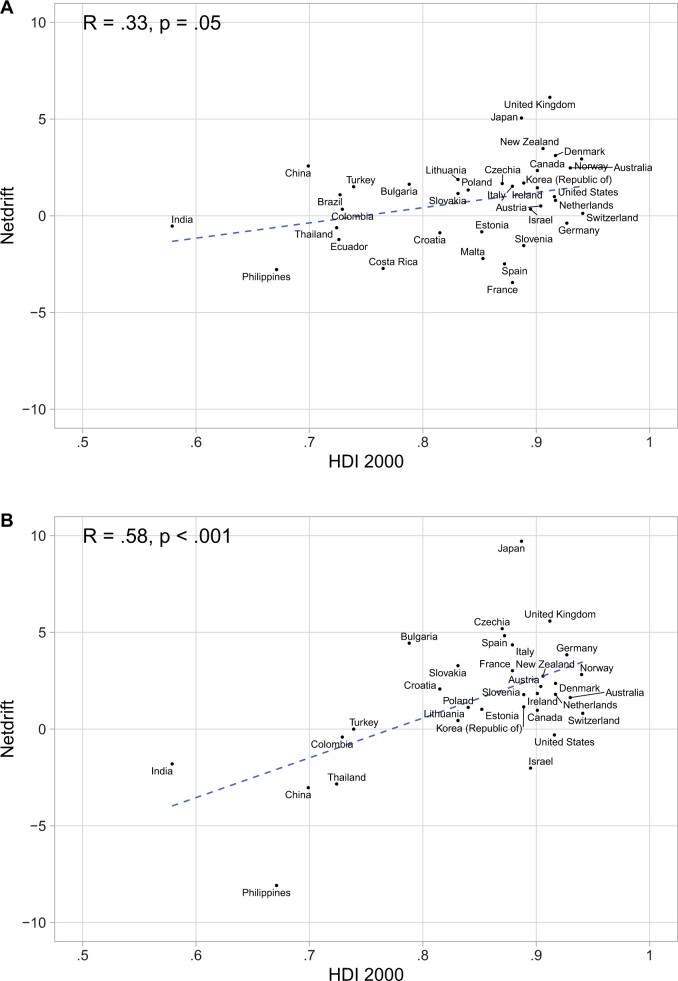
Correlation with the Human Development Index
Because both smoking and incidence of oral HPV infection can vary according to socioeconomic factors, we compared the oropharyngeal cancer net drift to the Human Development Index scores for men and women (Figure 5). For men, there was a mild correlation between Human Development Index scores and oropharyngeal cancer net drift (R = 0.33, P = .05), whereas in women a stronger correlation was observed (R = 0.58, P < .001).
Figure 5.
Correlation of the oropharyngeal cancer net drift with the 2000 Human Development Index (HDI) for each country in A) men and B) women.
Discussion
The impact of oropharyngeal cancer is expanding, becoming an increasingly important public health problem in both men and women. In this comprehensive analysis of international cancer registry data from 42 countries spanning 6 continents from 1993 to 2012, strong increases in oropharyngeal cancer incidence were observed across a broad spectrum of countries involving both sexes. Statistically significant increases in oropharyngeal cancer incidence were observed in 19 and 23 countries for men and women, respectively, compared with only 2 and 3 countries with statistically significant decreases. The magnitude of the increase in oropharyngeal cancer incidence worldwide is substantial, with annual percentage change for oropharyngeal cancer of more than 3% per year in the majority of countries with rising oropharyngeal cancer incidence. Although the relative change in countries with oropharyngeal cancer increases in women were similar to those observed in men, the absolute increases in oropharyngeal cancer remains substantially higher in men than in women, with male:female incidence ratios of approximately 3:1 to 4:1 in most countries. Despite generally rising rates worldwide, marked variations in the absolute oropharyngeal cancer rates remain, with 28-fold and 52-fold differences from the highest and lowest incidence countries in men and women, respectively.
Oropharyngeal cancer has 3 major extrinsic risk factors: tobacco, alcohol, and oral HPV infection. To better characterize factors driving increases in the rate of oropharyngeal cancer, we compared changes in oropharyngeal cancer incidence using age period–cohort modeling with 2 other cancers: lung squamous cell carcinoma, which is strongly associated with tobacco use but has no direct association with alcohol or HPV, and oral cavity cancer, which is associated with both tobacco and alcohol use but has minimal association with HPV. Using this methodology, we saw strong evidence that internationally, oropharyngeal cancer increases in men are almost universally caused by HPV. In 18 countries with increasing oropharyngeal cancer incidence in men that were analyzable with age-period-cohort modeling, 17 had statistically significant declines in lung squamous cell carcinoma incidence, consistent with reduced rates of smoking. Moreover, in 16 of 18 countries, oral cavity cancer had negative or nonsignificant incidence changes, with oropharyngeal cancer incidence increases statistically higher than oral cavity cancer in 11 countries. There was an expansion of the international patterns we previously described, with oropharyngeal cancer increases among men observed in numerous countries not previously identified (Austria, China, the Czech Republic, Iceland, Ireland, Italy, the Republic of Korea, Lithuania, New Zealand, and Norway) in addition to continued increases in all countries identified in our prior analysis (1). Others changes in the epidemiology of oropharyngeal cancer in men were apparent as well. For example, we previously reported that increased rates of oropharyngeal cancer were predominantly confined to men 60 years of age and younger. As those birth cohorts have aged, however, they have carried their increase in oropharyngeal cancer risk into older age groups. Indeed, increasing oropharyngeal cancer incidence was observed in patients older than 60 years of age, including the subset aged 70 and older, in the majority of countries where oropharyngeal cancer was rising in men, similar to what has been reported in the United States (10,11).
Our study also revealed a substantial evolution in the epidemiology of oropharyngeal cancer among women. Previously, we had shown that from 1983 to 2002, oropharyngeal cancer only increased in women in certain European countries, always in parallel with tobacco-associated cancers such as lung squamous cell carcinoma and oral cavity cancer, strongly implicating increased tobacco use as the underlying cause. With updated international data, the geographic pattern of increasing oropharyngeal cancer incidence in women has dramatically expanded and diversified. In addition to involving a wider swath of European countries, increased oropharyngeal cancer incidence among women was observed in North America (Canada), Oceania (Australia, New Zealand), Asia (China, Japan, the Republic of Korea), and South America (Brazil, Colombia) in this study. In the 20 countries with increasing oropharyngeal cancer incidence in women that were analyzable with age period–cohort techniques, 7 had increasing lung squamous cell carcinoma incidence and 10 had no significant difference in oropharyngeal cancer vs lung squamous cell carcinoma net drifts. This suggests that tobacco remains a major factor driving increases in oropharyngeal cancer incidence among women in certain places. In contrast, 4 countries (the United Kingdom, Canada, Germany, Japan), all with top-15 gross domestic product economies, had oropharyngeal cancer incidence increases that were significantly greater than both lung squamous cell carcinoma and oral cavity cancer and negative or nonsignificant lung squamous cell carcinoma trends. This constellation of epidemiologic findings is evidence that oral HPV may be contributing to increased oropharyngeal cancer incidence among women in these highly economically developed countries. Patterns are less straightforward than in men, however. Evidence for HPV was strongest in Canada, where lung squamous cell carcinoma and oral cavity cancer net drifts were both strongly negative. Supporting HPV as an emerging important risk factor for oropharyngeal cancer in women, the proportion of oropharyngeal cancers associated with HPV has dramatically increased over time (14), currently accounting for approximately 80% of oropharyngeal cancers at some tertiary care centers in the United States (15). In contrast, the United Kingdom and Japan had highly positive oral cavity cancer net drifts, albeit substantially lower than was what seen in oropharyngeal cancer. It is possible that non-HPV–related risk factors shared by oral cavity cancer and oropharyngeal cancer (eg, alcohol) may be contributing to the changes in these countries. Nevertheless, in the coming decades, as smoking continues to decrease in women, we hypothesize that more countries will exhibit stronger epidemiologic influences from HPV-associated oropharyngeal cancer among women.
Notably, prior studies have described marked geographic variation in the proportion of oropharyngeal cancer associated with HPV (16-19). In an analysis of nearly 4000 head and neck cancer samples collected from 1990 to 2012, more than 50% of oropharyngeal cancer cases were associated with HPV in South America, northern Europe, and central and eastern Europe compared with less than 10% in southern Europe (16). This finding is likely the result of variations in patterns of sexual behaviors and tobacco and alcohol use across countries (20). Most countries that have reported increasing rates of HPV-associated oropharyngeal cancer have generally had relatively high levels of economic development. In this study, we report a statistically significant correlation between a country’s Human Development Index score and the magnitude of oropharyngeal cancer incidence change observed. Interestingly, we found that this correlation was stronger in women. This reason for this finding is unclear. It is possible that the Human Development Index more strongly correlates with smoking in women than smoking in men or oral HPV infection in either sex.
We note key limitations. Most significantly, this study cannot distinguish HPV-related from HPV-unrelated oropharyngeal cancers because no tissue-based data are available. Moreover, data on tobacco or alcohol use among the people included in this study are lacking. Thus, we needed to infer the underlying etiology of the observed epidemiologic changes indirectly by comparing these trends to other tobacco-associated and alcohol-associated cancers. Moreover, the risk factors underlying oral cavity cancers extend beyond tobacco and alcohol (21). For example, genetic conditions such as Fanconi anemia and dyskeratosis congenita have been linked to increased oral cavity cancer risk, as have a variety of acquired genetic abnormalities (22). HPV may be an etiologic factor in 2% to 8% of oral cavity cancers (23,24), and other exogenous risk factors, such as mouthwash use (25), poor dental hygiene (26), immunosuppression (27), and chronic irritation from poorly fitting dentures (28), also have been associated with oral cavity cancer. Changes in any of these factors could influence oral cavity cancer epidemiology. Consequently, the relative roles of HPV, tobacco use, and alcohol use in the epidemiologic patterns observed in this study must be interpreted with caution (1). In addition, we included all histologies of oropharyngeal and oral cavity tumors in our analysis. Although squamous cell carcinomas represent the vast majority of these cases, this approach does overlook some histologic heterogeneity, especially for oral cavity cancers, and we cannot rule out changes in non–squamous cell cancers having some effect on the trends we observed. Our results likely underestimate the total burden of oropharyngeal cancer, given that we had to exclude certain ambiguously coded cancers of the “tongue,” “palate,” and “pharynx.” Finally, although we included data from 42 countries, there are many countries that were not included, and certain geographic regions remain underrepresented. Thus, this study represents an incomplete view of the global burden of oropharyngeal cancer.
In conclusion, we observed a rising incidence of oropharyngeal cancer in both sexes in many countries throughout the world. In men, the increased incidence is likely driven almost entirely by increases in HPV-associated oropharyngeal cancers. In women, more diverse patterns are present. Tobacco and alcohol use appear to be primary drivers in many countries with increasing oropharyngeal cancer incidence in women. However, there are some countries with highly developed economies that show an epidemiologic signature that supports a possible role for increased HPV-associated oropharyngeal cancer in women as well. These data provide a strong rationale for increased HPV vaccination internationally in both boys and girls to help minimize the worldwide burden of this preventable malignancy.
Supplementary Material
Acknowledgements
The funder had no role in the design of the study; the collection, analysis, or interpretation of the data; or the writing of the manuscript and decision to submit it for publication.
Contributor Information
Zachary S Zumsteg, Department of Radiation Oncology, Cedars-Sinai Medical Center, Los Angeles, CA, USA; Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Michael Luu, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA; Department of Biostatistics and Bioinformatics, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Philip S Rosenberg, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Julia K Elrod, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA; Department of Statistics and Data Science, Carnegie Mellon University, Pittsburgh, PA, USA.
Freddie Bray, Cancer Surveillance Branch, International Agency for Research on Cancer, Lyon, France.
Salvatore Vaccarella, Cancer Surveillance Branch, International Agency for Research on Cancer, Lyon, France.
Christopher Gay, Department of Radiation Oncology, University of Arizona, Tucson, AZ, USA.
Diana J Lu, Department of Radiation Oncology, The Queen’s Medical Center, Honolulu, HI, USA.
Michelle M Chen, Department of Surgery, Stanford University, Stanford, CA, USA.
Anil K Chaturvedi, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Marc T Goodman, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA; Division of Cancer Prevention and Control, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Data availability
Data used for this study are available upon request through the International Agency for Research on Cancer (Lyon, France).
Author contributions
Zachary Zumsteg, MD (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Writing—original draft; Writing—review & editing), Michael Luu, MPH (Data curation; Formal analysis; Methodology; Writing—original draft; Writing—review & editing), Philip S. Rosenberg, PhD (Data curation; Formal analysis; Methodology; Software; Writing—original draft; Writing—review & editing), Julia K. Elrod, BA (Formal analysis; Methodology; Software; Writing—review & editing), Freddie Bray, PhD (Conceptualization; Data curation; Writing—review & editing), Salvatore Vaccarella, PhD (Conceptualization; Data curation; Writing—review & editing), Christopher Gay, MD (Data curation; Formal analysis; Writing—review & editing), Diana Lu, MD (Data curation; Formal analysis; Writing—review & editing) Michelle Chen, MD (Writing—original draft; Writing—review & editing), Anil K. Chaturvedi, PhD (Conceptualization; Formal analysis; Methodology; Supervision; Writing—original draft; Writing—review & editing), Marc Goodman, PhD (Conceptualization; Data curation; Investigation; Supervision; Writing—original draft; Writing—review & editing).
Funding
Supported in part by the Intramural Research Program of the National Cancer Institute.
Conflicts of interest
Dr Chaturvedi is supported by the Intramural Research Program of the National Cancer Institute. Dr Zumsteg’s spouse previously performed legal work for Johnson & Johnson, Allergan, Merck, and Boehringer Ingelheim. The other authors have nothing else to disclose.
References
- 1. Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marur S, D'Souza G, Westra WH, et al. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11(8):781-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kreimer AR, Chaturvedi AK, Alemany L, et al. Summary from an international cancer seminar focused on human papillomavirus (HPV)-positive oropharynx cancer, convened by scientists at IARC and NCI. Oral Oncol. 2020;108:104736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lorenzoni V, Chaturvedi AK, Vignat J, et al. The Current Burden of Oropharyngeal Cancer: a global assessment based on GLOBOCAN 2020. Cancer Epidemiol Biomarkers Prev. 2022;31(11):2054-2062. [DOI] [PubMed] [Google Scholar]
- 5. Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99(10):777-789. [DOI] [PubMed] [Google Scholar]
- 6. Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 1988;48(11):3282-3287. [PubMed] [Google Scholar]
- 7. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709-720. [DOI] [PubMed] [Google Scholar]
- 9. D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944-1956. [DOI] [PubMed] [Google Scholar]
- 10. Zumsteg ZS, Cook-Wiens G, Yoshida E, et al. Incidence of oropharyngeal cancer among elderly patients in the United States. JAMA Oncol. 2016;2(12):1617-1623. [DOI] [PubMed] [Google Scholar]
- 11. Tota JE, Best AF, Zumsteg ZS, et al. Evolution of the oropharynx cancer epidemic in the United States: moderation of increasing incidence in younger individuals and shift in the burden to older individuals. J Clin Oncol. 2019;37(18):1538-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenberg PS, Check DP, Anderson WF.. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2296-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carstensen B. Age-period-cohort models for the Lexis diagram. Stat Med. 2007;26(15):3018-3045. [DOI] [PubMed] [Google Scholar]
- 14. D'Souza G, Westra WH, Wang SJ, et al. Differences in the Prevalence of Human Papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncol. 2017;3(2):169-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scott-Wittenborn N, D'Souza G, Tewari S, et al. Prevalence of human papillomavirus in head and neck cancers at tertiary care centers in the United States over time. Cancer. 2022;128(9):1767-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castellsagué X, Alemany L, Quer M, et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. JNCI J. 2016;108(6):djv403. [DOI] [PubMed] [Google Scholar]
- 17. de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehanna H, Franklin N, Compton N, et al. Geographic variation in human papillomavirus-related oropharyngeal cancer: Data from 4 multinational randomized trials. Head Neck. 2016;38 Suppl 1(Suppl 1):E1863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15(12):1319-1331. [DOI] [PubMed] [Google Scholar]
- 20. Heck JE, Berthiller J, Vaccarella S, et al. Sexual behaviours and the risk of head and neck cancers: a pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. Int J Epidemiol. 2010;39(1):166-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chaturvedi AK, Freedman ND, Abnet CC.. The evolving epidemiology of oral cavity and oropharyngeal cancers. Cancer Res. 2022;82(16):2821-2823. [DOI] [PubMed] [Google Scholar]
- 22. Chamoli A, Gosavi AS, Shirwadkar UP, et al. Overview of oral cavity squamous cell carcinoma: risk factors, mechanisms, and diagnostics. Oral Oncol. 2021;121:105451. [DOI] [PubMed] [Google Scholar]
- 23. Hubbers CU, Akgul B.. HPV and cancer of the oral cavity. Virulence. 2015;6(3):244-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gillison ML, Chaturvedi AK, Anderson WF, et al. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3235-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahrens W, Pohlabeln H, Foraita R, et al. Oral health, dental care and mouthwash associated with upper aerodigestive tract cancer risk in Europe: the ARCAGE study. Oral Oncol. 2014;50(6):616-625. [DOI] [PubMed] [Google Scholar]
- 26. Hashim D, Sartori S, Brennan P, et al. The role of oral hygiene in head and neck cancer: Results from International Head and Neck Cancer Epidemiology (INHANCE) consortium. Ann Oncol. 2016;27(8):1619-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tota JE, Engels EA, Madeleine MM, et al. Risk of oral tongue cancer among immunocompromised transplant recipients and human immunodeficiency virus-infected individuals in the United States. Cancer. 2018;124(12):2515-2522. [DOI] [PubMed] [Google Scholar]
- 28. Manoharan S, Nagaraja V, Eslick GD.. Ill-fitting dentures and oral cancer: a meta-analysis. Oral Oncol. 2014;50(11):1058-1061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used for this study are available upon request through the International Agency for Research on Cancer (Lyon, France).