Abstract
Background
Population-based surveillance of pediatric cancer incidence trends is critical to determine high-risk populations, drive hypothesis generation, and uncover etiologic heterogeneity. We provide a comprehensive update to the current understanding of pediatric cancer incidence trends by sex, race and ethnicity, and socioeconomic status (SES).
Methods
The Surveillance, Epidemiology, and End Results 22 data (2000-2019) was used to summarize age-adjusted incidence rates for children and adolescents aged 0-19 years at diagnosis. The annual percentage change (APC) and 95% confidence interval (CI) were estimated to evaluate incidence trends by sex, race and ethnicity, and SES overall and for cancer subtypes. Tests of statistical significance were 2-sided.
Results
Substantial variation was observed overall and for several histologic types in race and ethnicity– and SES–specific rates. Overall, we observed a statistically significant increase in incidence rates (APC = 0.8%, 95% CI = 0.6% to 1.1%). All race and ethnic groups saw an increase in incidence rates, with the largest occurring among non-Hispanic American Indian and Alaska Native children and adolescents (APC = 1.7%, 95% CI = 0.5% to 2.8%) and the smallest increase occurring among non-Hispanic White children and adolescents (APC = 0.7%, 95% CI = 0.5% to 1.0%). The lowest SES quintiles saw statistically significant increasing trends, while the highest quintile remained relatively stable (quintile 1 [Q1] APC = 1.6%, 95% CI = 0.6% to 2.6%; quintile 5 [Q5] APC = 0.3%, 95% CI = –0.1% to 0.7%).
Conclusions
Childhood cancer incidence is increasing overall and among every race and ethnic group. Variation by race and ethnicity and SES may enable hypothesis generation on drivers of disparities observed.
Childhood cancers are rare, heterogeneous diseases, which will have been diagnosed in approximately 10 470 children aged 0-14 years in the United States in 2022 (1). Although cancer mortality rates for children aged 0-14 years and adolescents aged 15-19 years have dropped by 71% and 61% over the last 5 decades, respectively, the overall incidence rate for both groups has increased by 0.8% annually since 1975 (1). Due to their rarity, the etiology of malignant neoplasms in these age groups remain elusive. This highlights the need for continuous population-based surveillance of incidence trends by various factors, which could shed light on the underlying causes of disease.
Although there has been research on pediatric US incidence trends over the last decades, there is large variance in time range and population coverage (2-11). The population of the United States has undergone changes in demographic characteristics and in exposure to certain risk factors, necessitating periodic updates to assess trends overtime. Historically, the effect of socioeconomic status (SES) on US incidence trends has been underreported, with existing studies focusing on adult populations or specific cancer types (12-14). Marcotte et al. (15) hypothesized that racial and ethnic variations in incidence of some tumor types may be attributable to SES. We provide an updated comprehensive report of national incidence trends overall and by sex, race and ethnicity, and SES, using population-based data. Further investigation of this area is important for determining high-risk populations, driving hypothesis generation, and uncovering etiologic heterogeneity.
Methods
Incidence data and population denominators
Cancer incidence data were obtained from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute. The SEER 22 database provides long-term, high-quality, population-based data from 22 cancer registries in the United States for the years 2000-2019, which covers 47.9% of the total US population (16). Cancers were classified as per the International Classification of Childhood Cancer, Third Revision, Oncology codes for tumor morphology and primary site, as reported in SEER (17). Race and ethnicity and sex information were obtained as reported in SEER. Cases were restricted to patients diagnosed with primary malignant neoplasms during the years 2000-2019 and aged 0-19 years at diagnosis. Twelve major histologic groups and 50 subgroups were investigated (Supplementary Table 1, available online).
Frequency counts and age-adjusted rates and by sex, age, race and ethnicity, and year were obtained from the SEER 22 database (16). Frequency counts and age-adjusted rates of cancer cases by SES were obtained from the SEER 18 census tract–level SES and Rurality Database (2006-2018). According to SEER (16), census-tract level Yost US-based SES quintiles were defined using the Decennial Census 2010 census tract boundaries and generated using various sets of American Community Survey 5-year estimates. Matching the American Community Survey year to the tumor diagnosis year, SES quintiles were linked to tumor cases at the census tract level. Tumors diagnosed between 2006 and 2007 were linked with quintiles calculated using American Community Survey 2006-2010 survey data. Tumors diagnosed between 2008 and 2017 were linked to quintiles calculated using American Community Survey data from different survey years spanning that period. Quintiles for tumors diagnosed in 2018 were linked to the index estimated from 2015 to 2019 data. The composite SES scores for census tracts were derived through a factor analysis using 7 SES variables, including median household income, median house value, median rent, percent below 150% of the poverty line, education index, percent working class, and percent unemployed (16). Unknown race and ethnicity cases were excluded.
Statistical analysis
Incidence rates were age adjusted to the 2000 US Census standard population and reported as cases per 100 000. The annual percentage change (APC) for age-adjusted incidence rates and corresponding 95% confidence interval (CI) were reported for each cancer type overall, by sex, and by race and ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, non-Hispanic Asian and Pacific Islander, and non-Hispanic American Indian and Alaska Native) for the years 2000 to 2019. Because of the observation that there is etiologic heterogeneity by acute lymphoblastic leukemia (ALL) subtype (18) and that the distribution of cytogenetic subtype varies by age at diagnosis (19), we hypothesized that there may be differences in ALL incidence trends by age group. Therefore, the APC and 95% CI were also reported for age at diagnosis grouping (0 years, 1-4 years, 5-9 years, 10-14 years, and 15-19 years) in ALL by sex and race and ethnicity for the years 2000-2019. Finally, we reported the APC and 95% CI for each cancer type by SES quintile (quintile 1 [Q1]: lowest through quintile 5 [Q5]: highest) for the years 2006-2018.
APCs were computed using the weighted least squares method. The APC was not calculated when 1 or more years in the range 2000-2019 contained zero cases. Groups were considered evaluable if the APC was calculated. The reported results were restricted to cancers with at least 10 cases reported within each year for each group. Tests of statistical significance were 2-sided, and the threshold for statistical significance was set at an alpha of 0.05. All analyses were done using SEER*Stat 8.4.0.1 and SAS 9.4.
Results
During the period 2000-2019, there were 148, 888 malignant neoplasms reported in SEER 22 among children and adolescents aged 0-19 years at diagnosis. There was evidence of a positive trend overall (APC = 0.8%, 95% CI = 0.6% to 1.1%) and in a similar magnitude for both sexes. All race and ethnic groups saw an increase in incidence rates, with the largest occurring among non-Hispanic American Indians and Alaska Native persons (APC = 1.7%, 95% CI = 0.5% to 2.8%) and the smallest increase occurring among non-Hispanic White persons (APC = 0.7%, 95% CI = 0.5% to 1.0%). Trends within SES quintiles decreased in magnitude as SES increased. The first 3 SES quintiles, representing the groups with lowest SES, showed similar increasing rates of approximately 1.5% per year, after which there was a moderate but noteworthy decrease that saw the incidence trend in the highest SES quintile remain relatively stable (APC = 0.3%, 95% CI = –0.1% to 0.7%).
Incidence rates and trends for all histologies across all years reported by race and ethnicity, sex, and SES quintile are available in Supplementary Tables 2-4 (available online). Incidence rates and trends for ALL by pediatric age group and stratified by sex and race and ethnicity are available in Supplementary Tables 5 and 6 (available online). APCs were not calculated by tumor type overall for 80 cases (unspecified malignant renal, n = 23; unspecified malignant hepatic, n = 18; Kaposi sarcoma, n = 39) due to insufficient case counts reported within each year. A total of 1,609 cases were excluded from the race and ethnicity analysis because of an unknown race and ethnicity classification.
Overall
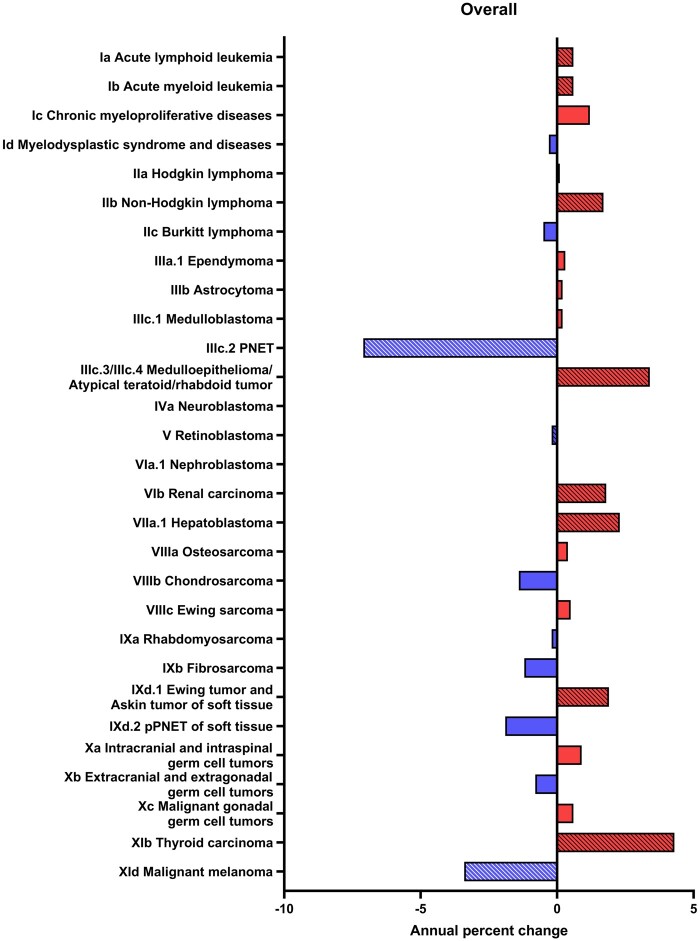
Both ALL and acute myeloid leukemia (AML) saw a statistically significant increase of 0.6% per year. Hodgkin lymphoma remained stable (APC = 0.1%, 95% CI = –0.2% to 0.4%). Non-Hodgkin lymphoma, renal carcinoma, hepatoblastoma, and Ewing tumor and Askin tumor of soft tissue had moderate statistically significant positive trends (Figure 1). The largest increasing trends among specified cancers were observed for medulloepithelioma/atypical teratoid/rhabdoid tumor (APC = 3.4%, 95% CI = 1.4% to 5.6%) and thyroid carcinomas (APC = 4.3%, 95% CI = 3.6% to 5.0%). We observed statistically significant decreases for primitive neuroectodermal tumors and malignant melanoma.
Figure 1.
Annual percentage change by malignant tumor histology type among children and adolescents aged 0-19 years at diagnosis stratified by tumor type, Surveillance, Epidemiology, and End Results 22 (2000-2019). PNET = primitive neuroectodermal tumors; pPNET = peripheral primitive neuroectodermal tumors.
Race and ethnicity
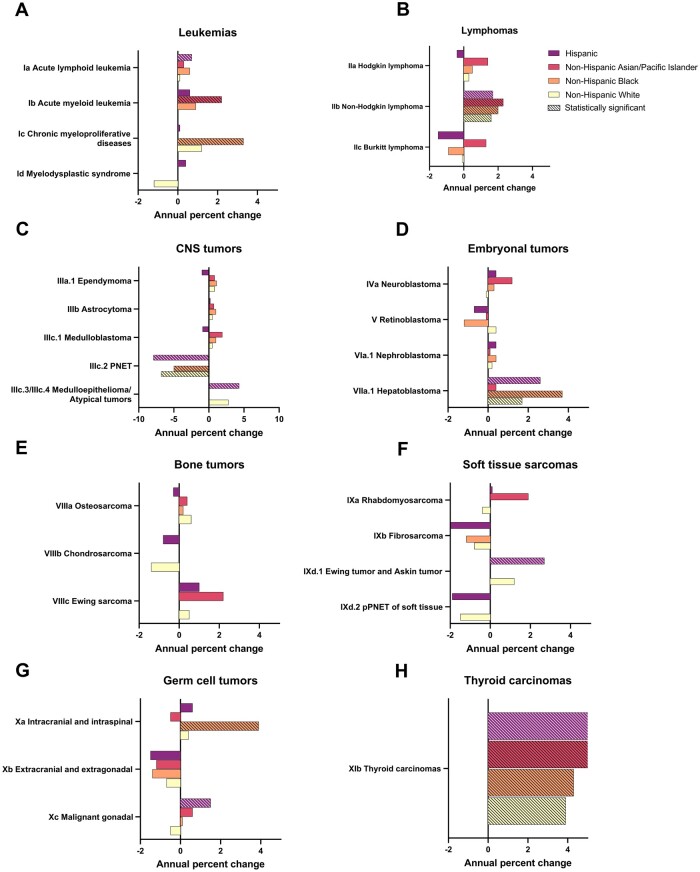
For ALL, the only statistically significant increase was observed among the Hispanic group (Figure 2). Non-Hispanic Asian and Pacific Islander individuals saw a statistically significant positive trend for AML, while a large statistically significant increase for chronic myeloproliferative diseases was observed among the non-Hispanic Black group (APC = 3.3%, 95% CI = 0.9% to 5.8%). Although none reached significance, all evaluable groups saw an increase in Hodgkin lymphoma except the Hispanic group, which saw a decrease (Figure 2). Non-Hodgkin lymphoma increased significantly for all evaluable groups, with the largest annual increase observed among non-Hispanic Asian and Pacific Islander persons (APC = 2.3%, 95% CI = 0.2% to 4.4%). Burkitt lymphoma decreased among non-Hispanic Black and Hispanic groups, remained stable among non-Hispanic White individuals, and increased among non-Hispanic Asian and Pacific Islander persons, although no trends were significant.
Figure 2.
Annual percentage change by malignant tumor histology type and race and ethnicity among children and adolescents aged 0-19 years at diagnosis stratified by tumor type, Surveillance, Epidemiology, and End Results 22 (2000-2019). CNS = central nervous system; PNET = primitive neuroectodermal tumors; pPNET = peripheral primitive neuroectodermal tumors.
For hepatoblastoma, there was a statistically significant increase among non-Hispanic White, Hispanic, and, with the largest increase, non-Hispanic Black individuals (APC = 3.7%, 95% CI = 0.3% to 7.2%). An increasing incidence for Ewing tumor and Askin tumor was seen only among the Hispanic group (APC = 2.7%, 95% CI = 0.4% to 5.1%). We observed a large increase for intracranial and intraspinal germ cell tumors only among non-Hispanic Black persons (APC = 3.9%, 95% CI = 1.0% to 6.9%) and a moderate increase for malignant gonadal germ cell tumors only among Hispanic persons. Thyroid carcinomas saw statistically significant positive trends across all evaluable groups, the largest among the non-Hispanic Asian and Pacific Islander group and the smallest among the non-Hispanic White group (Figure 2).
Sex
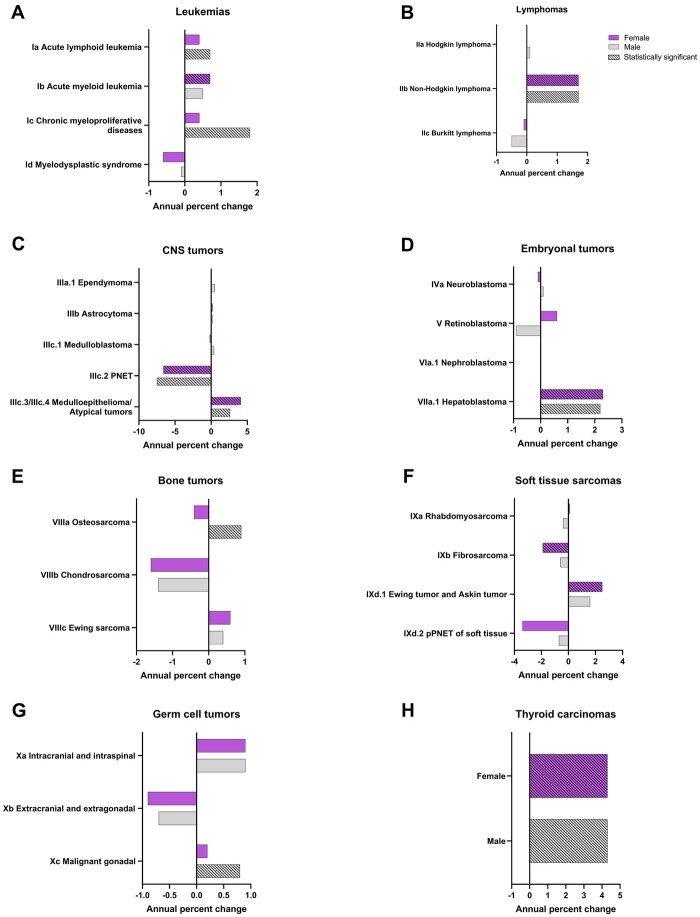
A statistically significant increase was observed only among males for ALL and chronic myeloproliferative diseases. AML increased statistically significantly only among females. For retinoblastoma, we observed an increase among males and a decrease among females, although neither were statistically significant (Figure 3). Osteosarcoma increased statistically significantly only among males (APC = 0.9%, 95% CI = 0.4% to 1.4%). A statistically significant increase was observed for Ewing tumor and Askin tumor of soft tissue in females, while a statistically significant decrease was observed for fibrosarcoma in females (Figure 3). For most histologies, trends were not different by sex.
Figure 3.
Annual percentage change by malignant tumor histology type and sex among children and adolescents aged 0-19 years at diagnosis stratified by tumor type, Surveillance, Epidemiology, and End Results 22 (2000-2019). PNET = primitive neuroectodermal tumors; pPNET = peripheral primitive neuroectodermal tumors.
SES quintiles
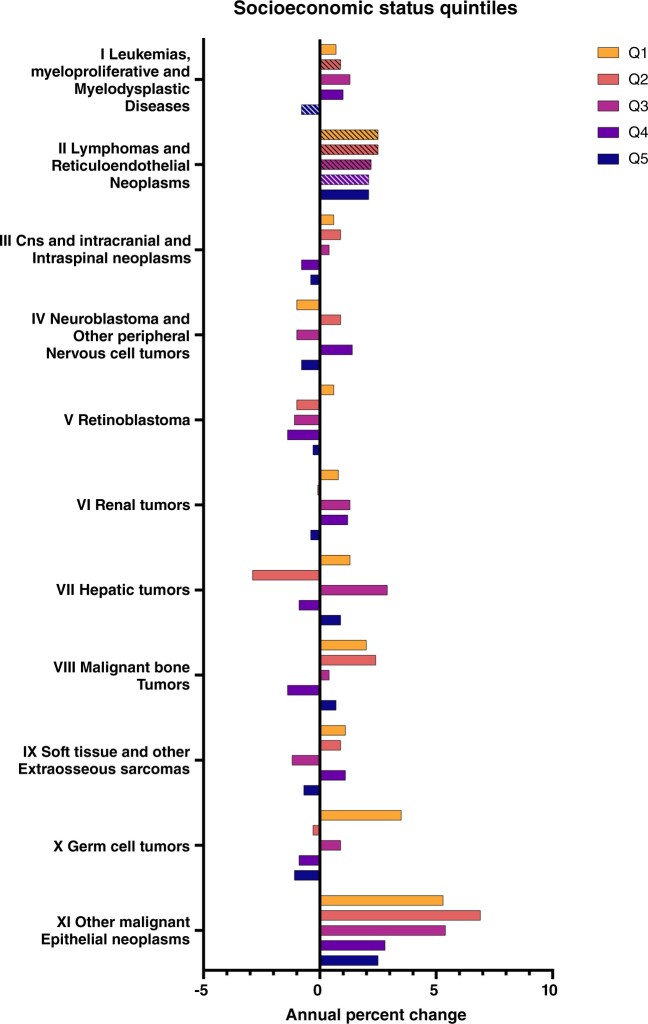
Across leukemias, myeloproliferative and myelodysplastic diseases, there were either suggested or statistically significant increasing trends for the lowest quintiles, whereas the highest quintile saw a statistically significant decrease (Q2 APC = 0.9%, 95% CI = 0.1% to 1.8%; Q5 APC = –0.8%, 95% CI = –1.6% to –0.1%). This trend was reflected for ALL and AML. Lymphomas and reticuloendothelial neoplasms were increasing more rapidly among lower quintiles than higher ones by a small margin (Figure 4). For central nervous system (CNS) and intracranial and intraspinal neoplasms, we observed a suggested increase among the lowest three quintiles as opposed to a suggested decrease in the highest two quintiles. Among germ cell tumors, a suggested positive trend was observed in the lowest quintile (Q1 APC = 3.5%, 95% CI = –0.3% to 7.5%), whereas the highest quintiles pointed toward decreasing trends. For other malignant epithelial neoplasms, although an increasing trend was observed across all quintiles, the effect size was larger in the lowest three quintiles (Q1 APC = 5.3%, 95% CI = 3.7% to 7.0%; Q5 APC = 2.5%, 95% CI = 1.2% to 3.9%).
Figure 4.
Annual percentage change by malignant tumor histology type and socioeconomic quintile among children and adolescents aged 0-19 at diagnosis stratified by tumor type, Surveillance, Epidemiology, and End Results 22 (2000-2019). CNS = central nervous system; Q = quintile.
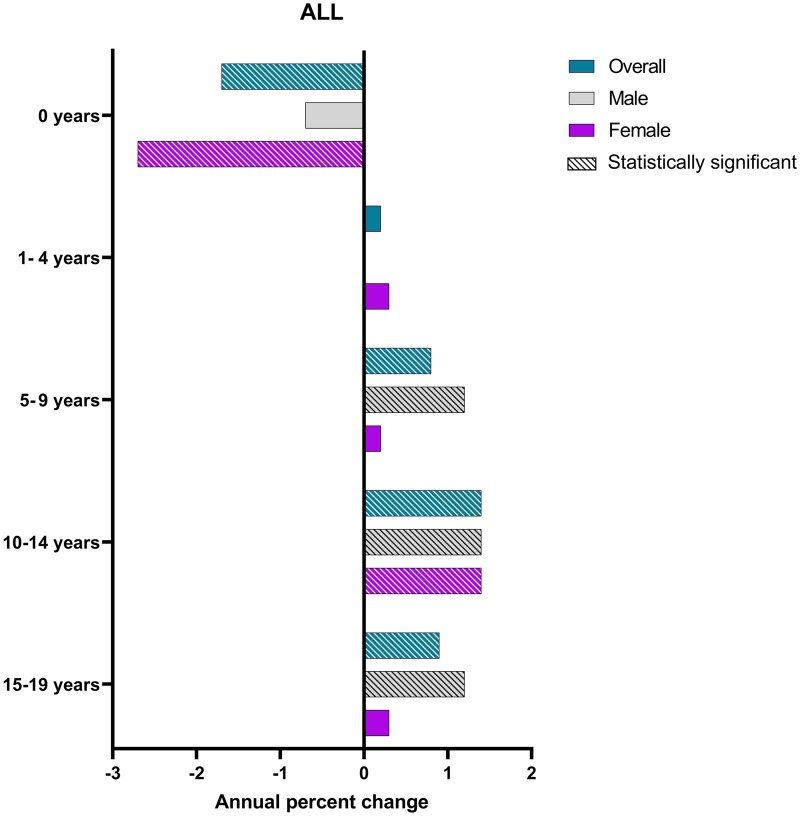
Age at diagnosis (ALL)
For ALL, a statistically significant decrease was observed in infants aged 0 years overall and among females. There was no evidence of change for those aged 1 to 4 years. A similar statistically significant positive trend of near 1% increase in incidence per year was seen in each subsequent age group overall and among males. Only females aged 10-14 years displayed a statistically significant increase, similar in magnitude to males (Figure 5). We observed a decline only among Hispanic infants aged 0 years (APC = –2.1%, 95% CI = –3.9% to –0.2%). Rising trends were noted for non-Hispanic White children aged 5-9 years (APC = 0.7%, 95% CI = 0.1% to 1.4%) and for Hispanic adolescents aged 10-14 years (APC = 1.7%, 95% CI = 0.8% to 2.7%) and 15-19 years (APC = 1.4%, 95% CI = 0.7% to 2.0%).
Figure 5.
Annual percentage change by age at diagnosis grouping and sex among children and adolescents aged 0-19 years at diagnosis stratified by age grouping among children with acute lymphoblastic leukemia (ALL), Surveillance, Epidemiology, and End Results 22 (2000-2019).
Discussion
We observed increasing rates of childhood cancer overall, for both sexes, and for all racial and ethnic groups. When investigating by SES overall, lower quintiles had increasing trends while the highest quintile was stable. For histologic subgroups overall, thyroid carcinomas displayed the highest increase, while the only statistically significant decreases were observed for PNET and malignant melanoma. The Hispanic group experienced the only statistically significant increase for ALL, while the non-Hispanic Asian and Pacific Islander group experienced the only statistically significant increase for AML. Both the leukemias and lymphomas displayed higher increases in the lowest SES quintiles as compared with the highest quintile. Many differences were revealed within each stratum and across most histologies, indicating that etiologic heterogeneity exists across most cancer types and that numerous drivers of disease create different high-risk populations.
Although racial and ethnic disparities in childhood cancer incidence rates have been well investigated in the literature (15), disparities in incidence trends, which are distinct from incidence rates, are not well established for all tumor types and generally do not cover trends during more recent years. Racial and ethnic disparities in pediatric cancer incidence trends have been previously reported for some cancer types in the United States (6-9). There are fewer analyses on incidence trends for all cancer types, but those that are available report increasing rates overall (10,11). Although other publications have investigated the effect of SES on trends (12-14), to our knowledge, this is the first analysis to examine the effect of SES on incidence trends across all major childhood cancer types.
As we investigated those diagnosed with cancer between ages 0 and 19 years from 2000 to 2019, the data set represents birth years from 1981 to 2019. During this near 40-year range in birth years, the prevalence of maternal and perinatal risk factors associated with childhood cancer have changed, such as maternal obesity, parental age, and birth by cesarean section. Maternal obesity is a known teratogen and has been associated with leukemia and hepatoblastoma risk (20-29). From 1988-1994 to 2017-2018, obesity among women of childbearing age rose from 20.7% to 39.7% (30, 31). Advanced parental age, particularly maternal age, is associated with an increased risk for various solid tumors and ALL (32-35). Since 1970, the mean maternal and paternal age have been on the rise, and the proportion of first births has shifted toward advanced maternal age groups (36-38). Birth by cesarean section, particularly when it occurs before the onset of labor, is associated with increased risk of ALL (39-42). In 1980, 16.5% of births were by cesarean section, which rose to account for 32% of births in 2015 (43-45). Although the etiology of childhood cancers are complex, changes in the prevalence of these and other maternal and perinatal risk factors associated with childhood cancer may be contributing to the increasing trends observed in our results.
Changes in environmental exposures are also potential drivers of trends observed in our analysis. Benzene production is increasing over time (46), and both maternal and child exposure are associated with an increased risk for AML and non-Hodgkin lymphoma (47,48). Similarly, pesticide and air pollution exposure are associated with an increased risk for childhood leukemias (49-55). The persistent nature of pesticides coupled with their increased application in food production have put more people at risk of exposure in recent decades (56). Moreover, studies have shown that the impacts of air pollution and pesticides disproportionally affect poor and minority groups (57-60). Although there is insufficient evidence to make direct links to increasing childhood cancer incidence, there is the possibility that the disproportionate exposure of such chemicals to those in lower SES quintiles and minority groups might contribute to the disparities found in this analysis.
Race and ethnicity and SES are intrinsically linked in the United States, with minority groups disproportionally represented in lower SES areas (61). Studies have shown the effect of individual and neighborhood economic status on adverse health outcomes, including cancer incidence and mortality (62-66). Access to resources depend on SES and may further harm socially disadvantaged groups. These groups have elevated behavioral, occupational, and environmental exposures to potential carcinogens, causing cancer-related health disparities (66-68). This underlies the need for monitoring SES-level cancer incidence trends and quantifying temporal shifts in health disparities. We observed overall increasing rates only among the lowest SES groups for all cancer types and higher increasing rates among the lowest SES groups for the leukemias, lymphomas, and CNS tumors.
Attributable to the 2016 change in the World Health Organization diagnostic classification for this tumor type, we observed a large statistically significant overall decrease for PNET, which is no longer recognized as a cancer classification (69). These cases are now classified into CNS embryonal subtypes. Increased sun-protective behaviors and decreased ultraviolet radiation exposure may underlie the observed decrease in malignant melanomas, which has been reported previously (70). Changes in diagnostic procedures may have affected rates for thyroid carcinomas, the largest overall increase in our results. Although changes in exposures to risk factors such as radiation cannot be ruled out, the increased use and sensitivity of advanced diagnostic technologies coincide with global incidence increases for this cancer type (13,71-73).
We used a population-based dataset spanning 20 years, which contains high-quality data following SEER standards and coded using International Classification of Childhood Cancer, Third Revision, Oncology guidelines. The dataset has high case ascertainment and is large enough to examine disease stratifications by race and ethnicity, sex, and SES for many cancers. The SEER registries were chosen to overrepresent non-White individuals making it not completely representative, however, because of the extensive coverage of these registries, the SEER population is comparable to the general US population (16). Although the dataset was large, there were insufficient numbers to provide evaluable stratified rates for rare cancers and minority race and ethnic groups, especially non-Hispanic American Indian and Alaska Native children and adolescents. Insufficient numbers limited SES stratification by race and ethnicity, which could highlight trends not otherwise seen. For the rare cancers, there is a greater possibility of type 1 errors, as some statistically significant findings are close to the cutoff margin. Due to the lack of available data, we did not look at molecular tumor subtypes.
Future studies should aim to analyze risk factors by tumor subtypes and racial and ethnic subpopulations. The findings that hold true across several subgroups should be the subject of future investigations that would help us better understand the forces driving this change.
Supplementary Material
Acknowledgements
The funder did not play a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Contributor Information
Pablo S Monterroso, Division of Epidemiology and Clinical Research, Department of Pediatrics, University of Minnesota, Minneapolis, MN, USA.
Zhaoheng Li, Department of Biostatistics, University of Washington, Seattle, WA, USA.
Allison M Domingues, Division of Epidemiology and Clinical Research, Department of Pediatrics, University of Minnesota, Minneapolis, MN, USA.
Jeannette M Sample, Division of Epidemiology and Clinical Research, Department of Pediatrics, University of Minnesota, Minneapolis, MN, USA.
Erin L Marcotte, Division of Epidemiology and Clinical Research, Department of Pediatrics, University of Minnesota, Minneapolis, MN, USA; Masonic Cancer Center, University of Minnesota, Minneapolis, MN, USA.
Data availability
All data used in this work is publicly available. Cancer incidence data was obtained from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute, specifically the SEER 22 registries database. Instructions for requesting access to SEER data can be found at https://seer.cancer.gov/data/access.html.
Author contributions
Pablo Sebastian Monterroso, BA (Data curation; Writing – original draft; Writing – review & editing), Zhaoheng Li, BA (Data curation; Writing – review & editing), Allison Domingues, MS (Data curation; Writing – review & editing), Jeannette Sample, MPH (Formal analysis; Writing – review & editing), and Erin L Marcotte, PhD (Conceptualization; Methodology; Supervision; Writing – review & editing).
Funding
This work was supported by the National Cancer Institute (R01CA266105 to ELM) and the Children’s Cancer Research Fund.
Conflicts of interest
The authors declare no potential conflicts of interest.
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2. Ward E, DeSantis C, Robbins A, Kohler B, Jemal A.. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83-103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 3. Qian ZJ, Jin MC, Meister KD, Megwalu UC.. Pediatric thyroid cancer incidence and mortality trends in the United States, 1973-2013. JAMA Otolaryngol Head Neck Surg. 2019;145(7):617-623. doi: 10.1001/jamaoto.2019.0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Withrow DR, Berrington de Gonzalez A, Lam C, Warren KE, Shiels MS.. Trends in pediatric central nervous system tumor incidence in the United States, 1998-2013. Cancer Epidemiol Biomarkers Prev. 2019;28(3):522-530. doi: 10.1158/1055-9965.EPI-18-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gittleman HR, Ostrom QT, Rouse CD, et al. Trends in central nervous system tumor incidence relative to other common cancers in adults, adolescents, and children in the United States, 2000 to 2010. Cancer. 2015;121(1):102-112. doi: 10.1002/CNCR.29015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernier MO, Withrow DR, Berrington de Gonzalez A, et al. Trends in pediatric thyroid cancer incidence in the United States, 1998-2013. Cancer. 2019;125(14):2497-2505. doi: 10.1002/cncr.32125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kong Y, Ji X, Han X, Zhang B.. Pediatric neurological cancer incidence and trends in the United States, 2000-2018. Cancer Causes Control. 2022;33(5):687-699. doi: 10.1007/s10552-021-01535-w. [DOI] [PubMed] [Google Scholar]
- 8. Kahla JA, Siegel DA, Dai S, et al. Incidence and 5-year survival of children and adolescents with hepatoblastoma in the United States. Pediatr Blood Cancer. 2022;69(10):e29763. doi: 10.1002/pbc.29763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feng J, Polychronidis G, Heger U, Frongia G, Mehrabi A, Hoffmann K.. Incidence trends and survival prediction of hepatoblastoma in children: a population-based study. Cancer Commun (Lond). 2019;39(1):62. doi: 10.1186/s40880-019-0411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J.. Cancer incidence rates and trends among children and adolescents in the United States, 2001-2009. Pediatrics. 2014;134(4):e945-e955. doi: 10.1542/peds.2013-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Linabery AM, Ross JA.. Trends in childhood cancer incidence in the U.S. (1992-2004). Cancer. 2008;112(2):416-432. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 12. Li N, Du XL, Reitzel LR, Xu L, Sturgis EM.. Impact of enhanced detection on the increase in thyroid cancer incidence in the United States: review of incidence trends by socioeconomic status within the Surveillance, Epidemiology, and End Results registry, 1980-2008. Thyroid. 2013;23(1):103-110. doi: 10.1089/thy.2012.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reitzel LR, Nguyen N, Li N, Xu L, Regan SD, Sturgis EM.. Trends in thyroid cancer incidence in Texas from 1995 to 2008 by socioeconomic status and race/ethnicity. Thyroid. 2014;24(3):556-567. doi: 10.1089/thy.2013.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh GK, Miller BA, Hankey BF, Edwards BK.. Area Socioeconomic Variations in US Cancer Incidence, Mortality, Stage, Treatment, and Survival 1975–1999. Bethesda, MD: National Cancer Institute; 2003. [Google Scholar]
- 15. Marcotte EL, Domingues AM, Sample JM, Richardson MR, Spector LG.. Racial and ethnic disparities in pediatric cancer incidence among children and young adults in the United States by single year of age. Cancer. 2021;127(19):3651-3663. doi: 10.1002/CNCR.33678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute Surveillance, Epidemiology, and End Results SEER 8 Regs Research Data, Nov 2021 Sub (1975-2020). National Cancer Institute, DCCPS, Surveillance Research Program, released April 2023, based on the November 2022 submission. http://www.seer.cancer.gov. Accessed August 15, 2022.
- 17. Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P.. International Classification of Childhood Cancer, Third Edition. Cancer. 2005;103(7):1457-1467. doi: 10.1002/CNCR.20910 [DOI] [PubMed] [Google Scholar]
- 18. Williams L, Yang JJ, Hirsch BA, Marcotte EL, Spector LG.. Is there etiologic heterogeneity between subtypes of acute lymphoblastic leukemia? A literature review of variation in risk by subtype. Cancer Epidemiol Biomarkers Prev. 2019;28(5):846-856. doi: 10.1158/1055-9965.EPI-18-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marcotte EL, Spector LG, Mendes-de-Almeida DP, Nelson HH.. The prenatal origin of childhood leukemia: potential applications for epidemiology and newborn screening. Front Pediatr. 2021;9:639479. doi: 10.3389/fped.2021.639479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marley AR, Domingues A, Ghosh T, Turcotte LM, Spector LG.. Maternal body mass index, diabetes, and gestational weight gain and risk for pediatric cancer in offspring: a systematic review and meta-analysis. JNCI Cancer Spectr. 2022;6(2):pkac020. doi: 10.1093/jncics/pkac020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brite J, Laughon SK, Troendle J, Mills J.. Maternal overweight and obesity and risk of congenital heart defects in offspring [Erratum in: Int J Obes (Lond). 2014;38(6):886].Int J Obes (Lond). 2014;38(6):878-882. doi: 10.1038/ijo.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Correa A, Marcinkevage J.. Prepregnancy obesity and the risk of birth defects: an update. Nutr Rev. 2013;71(suppl 1):S68-S77. doi: 10.1111/nure.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blanco R, Colombo A, Suazo J.. Maternal obesity is a risk factor for orofacial clefts: a meta-analysis. Br J Oral Maxillofac Surg. 2015;53(8):699-704. doi: 10.1016/j.bjoms.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 24. Mills JL, Troendle J, Conley MR, Carter T, Druschel CM.. Maternal obesity and congenital heart defects: a population-based study. Am J Clin Nutr. 2010;91(6):1543-1549. doi: 10.3945/ajcn.2009.28865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McLaughlin CC, Baptiste MS, Schymura MJ, Nasca PC, Zdeb MS.. Maternal and infant birth characteristics and hepatoblastoma. Am J Epidemiol. 2006;163(9):818-828. doi: 10.1093/aje/kwj104. [DOI] [PubMed] [Google Scholar]
- 26. Spector LG, Johnson KJ, Soler JT, Puumala SE.. Perinatal risk factors for hepatoblastoma. Br J Cancer. 2008;98(9):1570-1573. doi: 10.1038/sj.bjc.6604335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Musselman JR, Georgieff MK, Ross JA, et al. Maternal pregnancy events and exposures and risk of hepatoblastoma: a Children’s Oncology Group (COG) study. Cancer Epidemiol. 2013;37(3):318-320. doi: 10.1016/j.canep.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang X, Hansen J, Lee PC, et al. Maternal diabetes and childhood cancer risks in offspring: two population-based studies. Br J Cancer .2022;127(10):1837-1842. doi: 10.1038/s41416-022-01961-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stacy SL, Buchanich JM, Ma ZQ, et al. Maternal obesity, birth size, and risk of childhood cancer development. Am J Epidemiol. 2019;188(8):1503-1511. doi: 10.1093/aje/kwz118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL.. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. 2018;319(16):1723-1725. doi: 10.1001/jama.2018.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marley AR, Ryder JR, Turcotte LM, Spector LG.. Maternal obesity and acute lymphoblastic leukemia risk in offspring: a summary of trends, epidemiological evidence, and possible biological mechanisms. Leuk Res. 2022;121:106924. doi: 10.1016/j.leukres.2022.106924. [DOI] [PubMed] [Google Scholar]
- 32. Domingues A, Moore KJ, Sample J, Kharoud H, Marcotte EL, Spector LG.. Parental age and childhood lymphoma and solid tumor risk: a literature review and meta-analysis. JNCI Cancer Spectr. 2022;6(3):pkac040. doi: 10.1093/jncics/pkac040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marcotte EL, Druley TE, Johnson KJ, et al. Parental age and risk of infant leukaemia: a pooled analysis. Paediatr Perinat Epidemiol. 2017;31(6):563-572. doi: 10.1111/ppe.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petridou ET, Georgakis MK, Erdmann F, et al. Advanced parental age as risk factor for childhood acute lymphoblastic leukemia: results from studies of the Childhood Leukemia International Consortium. Eur J Epidemiol. 2018;33(10):965-976. doi: 10.1007/s10654-018-0402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Contreras ZA, Hansen J, Ritz B, Olsen J, Yu F, Heck JE.. Parental age and childhood cancer risk: a Danish population-based registry study. Cancer Epidemiol. 2017;49:202-215. doi: 10.1016/j.canep.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mathews TJ, Hamilton BE.. Mean age of mother, 1970-2000. Natl Vital Stat Rep. 2002;51(1):1-13. [PubMed] [Google Scholar]
- 37. Mathews TJ, Hamilton BE.. Mean age of mothers is on the rise: United States, 2000-2014. NCHS Data Brief. 2016;(232):1-8. [PubMed] [Google Scholar]
- 38. Khandwala YS, Zhang CA, Lu Y, Eisenberg ML.. The age of fathers in the USA is rising: an analysis of 168 867 480 births from 1972 to 2015. Hum Reprod. 2017;32(10):2110-2116. doi: 10.1093/humrep/dex267. [DOI] [PubMed] [Google Scholar]
- 39. Williams LA, Richardson M, Spector LG, Marcotte EL.. Cesarean section is associated with an increased risk of acute lymphoblastic leukemia and hepatoblastoma in children from Minnesota. Cancer Epidemiol Biomarkers Prev. 2021;30(4):736-742. doi: 10.1158/1055-9965.EPI-20-1406. [DOI] [PubMed] [Google Scholar]
- 40. Marcotte EL, Thomopoulos TP, Infante-Rivard C, et al. Caesarean delivery and risk of childhood leukaemia: a pooled analysis from the Childhood Leukemia International Consortium (CLIC) [Erratum in: Lancet Haematol. 2016;3(4):e162]. Lancet Haematol. 2016;3(4):e176-e185. doi: 10.1016/S2352-3026(16)00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marcotte EL, Richardson MR, Roesler MA, Spector LG.. Cesarean delivery and risk of infant leukemia: a report from the Children’s Oncology Group. Cancer Epidemiol Biomarkers Prev. 2018;27(4):473-478. doi: 10.1158/1055-9965.EPI-17-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang R, Wiemels JL, Metayer C, et al. Cesarean section and risk of childhood acute lymphoblastic leukemia in a population-based, record-linkage study in California. Am J Epidemiol. 2017;185(2):96-105. doi: 10.1093/aje/kww153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taffel SM, Placek PJ.. Complications in cesarean and non-cesarean deliveries: United States, 1980. Am J Public Health. 1983;73(8):856-860. doi: 10.2105/ajph.73.8.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Taffel SM, Placek PJ, Liss T.. Trends in the United States cesarean section rate and reasons for the 1980-85 rise. Am J Public Health. 1987;77(8):955-959. doi: 10.2105/ajph.77.8.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ.. Births: final data for 2015. Natl Vital Stat Rep. 2017;66(1):1. [PubMed] [Google Scholar]
- 46. Sekar A, Varghese GK, Ravi Varma MK.. Analysis of benzene air quality standards, monitoring methods and concentrations in indoor and outdoor environment. Heliyon. 2019;5(11):e02918. doi: 10.1016/j.heliyon.2019.e02918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carlos-Wallace FM, Zhang L, Smith MT, Rader G, Steinmaus C.. Parental, in utero, and early-life exposure to benzene and the risk of childhood leukemia: a meta-analysis. Am J Epidemiol. 2016;183(1):1. doi: 10.1093/aje/kwv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Raaschou-Nielsen O, Hvidtfeldt UA, Roswall N, Hertel O, Poulsen AH, Sørensen M.. Ambient benzene at the residence and risk for subtypes of childhood leukaemia, lymphoma and CNS tumour. Int J Cancer. 2018;143(6):1367-1373. doi: 10.1002/ijc.31421. [DOI] [PubMed] [Google Scholar]
- 49. Karalexi MA, Tagkas CF, Markozannes G, et al. Exposure to pesticides and childhood leukemia risk: a systematic review and meta-analysis. Environ Pollut. 2021;285:117376. doi: 10.1016/j.envpol.2021.117376. [DOI] [PubMed] [Google Scholar]
- 50. Kumar A, Vashist M, Rathee R.. Maternal factors and risk of childhood leukemia. Asian Pac J Cancer Prev. 2014;15(2):781-784. doi: 10.7314/apjcp.2014.15.2.781. [DOI] [PubMed] [Google Scholar]
- 51. Wang Y, Gao P, Liang G, et al. Maternal prenatal exposure to environmental factors and risk of childhood acute lymphocytic leukemia: a hospital-based case-control study in China. Cancer Epidemiol. 2019;58:146-152. doi: 10.1016/j.canep.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 52. Wigle DT, Turner MC, Krewski D.. A systematic review and meta-analysis of childhood leukemia and parental occupational pesticide exposure. Environ Health Perspect. 2009;117(10):1505-1513. doi: 10.1289/ehp.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kreis C, Héritier H, Scheinemann K, et al. Childhood cancer and traffic-related air pollution in Switzerland: a nationwide census-based cohort study [published online ahead of print, June 30, 2022]. Environ Int. 2022;166:107380. doi: 10.1016/j.envint.2022.107380. [DOI] [PubMed] [Google Scholar]
- 54. Hvidtfeldt UA, Erdmann F, Urhøj SK, et al. Air pollution exposure at the residence and risk of childhood cancers in Denmark: a nationwide register-based case-control study. EClinicalMedicine. 2020;28:100569. doi: 10.1016/j.eclinm.2020.100569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Reynolds P, Von Behren J, Gunier RB, Goldberg DE, Hertz A, Smith DF.. Childhood cancer incidence rates and hazardous air pollutants in California: an exploratory analysis. Environ Health Perspect. 2003;111(4):663-668. doi: 10.1289/ehp.5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sharma A, Shukla A, Attri K, et al. Global trends in pesticides: a looming threat and viable alternatives. Ecotoxicol Environ Saf. 2020;201:110812. doi: 10.1016/j.ecoenv.2020.110812. [DOI] [PubMed] [Google Scholar]
- 57. Tessum CW, Paolella DA, Chambliss SE, Apte JS, Hill JD, Marshall JD.. PM2.5 polluters disproportionately and systemically affect people of color in the United States. Sci Adv. 2021;7(18):eabf4491. doi: 10.1126/sciadv.abf4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Donley N, Bullard RD, Economos J, et al. Pesticides and environmental injustice in the USA: root causes, current regulatory reinforcement and a path forward. BMC Public Health. 2022;22(1):708. doi: 10.1186/s12889-022-13057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Apelberg BJ, Buckley TJ, White RH.. Socioeconomic and racial disparities in cancer risk from air toxics in Maryland. Environ Health Perspect. 2005;113(6):693-699. doi: 10.1289/ehp.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bell ML, Ebisu K.. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ Health Perspect. 2012;120(12):1699-1704. doi: 10.1289/ehp.1205201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Williams DR, Priest N, Anderson NB.. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35(4):407-411. doi: 10.1037/hea0000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mode NA, Evans MK, Zonderman AB.. Race, neighborhood economic status, income inequality and mortality. PLoS One. 2016;11(5):e0154535. doi: 10.1371/journal.pone.0154535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Singh GK, Jemal A.. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi: 10.1155/2017/2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the Surveillance, Epidemiology, and End Results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20(4):417-435. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boscoe FP, Johnson CJ, Sherman RL, Stinchcomb DG, Lin G, Henry KA.. The relationship between area poverty rate and site-specific cancer incidence in the United States. Cancer. 2014;120(14):2191-2198. doi: 10.1002/cncr.28632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Singh GK, Jemal A.. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi: 10.1155/2017/2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Clarke CA, Moy LM, Swetter SM, Zadnick J, Cockburn MG.. Interaction of area-level socioeconomic status and UV radiation on melanoma occurrence in California. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2727-2733. doi: 10.1158/1055-9965.EPI-10-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Egen O, Beatty K, Blackley DJ, Brown K, Wykoff R.. Health and social conditions of the poorest versus wealthiest counties in the United States. Am J Public Health. 2017;107(1):130-135. doi: 10.2105/AJPH.2016.303515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803-820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 70. Campbell LB, Kreicher KL, Gittleman HR, Strodtbeck K, Barnholtz-Sloan J, Bordeaux JS.. Melanoma incidence in children and adolescents: decreasing trends in the United States. J Pediatr. 2015;166(6):1505-1513. doi: 10.1016/j.jpeds.2015.02.050. [DOI] [PubMed] [Google Scholar]
- 71. Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S.. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid. 2015;25(10):1127-1136. doi: 10.1089/thy.2015.0116. [DOI] [PubMed] [Google Scholar]
- 72. Morris LG, Sikora AG, Tosteson TD, Davies L.. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. 2013;23(7):885-891. doi: 10.1089/thy.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brito JP, Morris JC, Montori VM.. Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. BMJ. 2013;347:f4706. doi: 10.1136/bmj.f4706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this work is publicly available. Cancer incidence data was obtained from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute, specifically the SEER 22 registries database. Instructions for requesting access to SEER data can be found at https://seer.cancer.gov/data/access.html.