Abstract
Honey bees are essential pollinators for several economically important crops. In temperate countries, honey bee colonies face multiple threats during the overwintering period, such as food availability, diseases, and confinement. Beekeepers commonly use chemicals to improve colony health during winter, but these products can have a negative impact on bee health and pathogens can develop resistance to them. Thus, there is a need for further development of alternative treatments. The aim of this study was to evaluate the impact of one endogenic bacterium (Bombella apis) and 2 commercial probiotic formulas (Bactocell and Levucell) on colony survival, spring development, and Vairimorpha (formerly Nosema) spp. spore count. Probiotic treatments were given in 1: 1 sugar syrup in October 2017 and April 2018, once a week for 2 wk. One experimental group was given Fumagilin-B, the only product approved in Canada to prevent nosemosis, once in October. The administration of 2 commercial probiotics, Bactocell (Pediococcus acidilactici) and Levucell (Saccharomyces cerevisiae boulardii), led to a significant increase in the number of sealed brood cells in spring. None of the probiotic treatments impacted the honey bee gut load of Vairimorpha spp. spores. The results suggest that beneficial microorganisms can improve spring development and performance of honey bee colonies.
Keywords: Vairimorpha, beekeeping, yeast, Lactobacillaceae, overwintering
Introduction
The honey bee (Apis mellifera, Linnaeus, 1758) provides essential pollination services all around the globe. Unfortunately, the beekeeping industry is threatened by increasing overwintering mortality rates across Canada (CAPA 2022) and other countries (Neumann and Carreck 2010, Potts et al. 2010, Bruckner et al. 2023). During the winter of 2021–2022, beekeepers from Québec, Canada reported a total colony loss of 48.4%, mainly caused by ineffective varroa treatment and weather, which is the highest rate observed since 2007 (CAPA 2022).
Overwintering in Canada corresponds to a period of sharp climate shift that results in the confinement of bees in the hive and lack of defecation, which significantly affects colony health (Döke et al. 2015). During this stressful period, the prevalence of the parasitic microsporidia Vairimorpha sp., formerly Nosema sp. (Tokarev et al. 2020), the pathogen causing nosemosis, increases in colonies (Fries 2009), which reduces worker lifespan and, ultimately triggers colony collapse (Higes et al. 2008). Importantly, this stressful period is associated with a reduction of the gut microbiota diversity and the increase in abundance of several bacterial strains associated with dysbiosis (Bleau et al. 2020). Increased abundance of noncore bacterial strains belonging to the Enterobacteriaceae family is positively correlated with gut dysbiosis (Kwong and Moran 2016) and unhealthy colonies (Budge et al. 2016), but is negatively correlated with core microbiota members belonging to Lactobacillaceae, Orbaceae, and Neisseriaceae, 3 bacterial families known to contribute to the innate immune system of the honey bee (Kwong et al. 2017).
Beekeepers frequently treat their colonies with chemicals in fall to reduce the prevalence of parasites such as Vairimorpha sp.(microsporidian) and Varroa destructor (acarian). These parasites are known to negatively impact both the health of individual bees and colony survival (Fries 2009, Rosenkranz 2010). However, there is an increasing evidence suggesting that these molecules can affect negatively the honey bee health, and that most pathogens can develop a resistance to these treatments. For example, fumagilin, a common medication for nosemosis, alters honey bee midgut tissues at concentrations that do not eliminate Vairimorpha sp. (Huang et al. 2013). In some experiments, the quantity of Vairimorpha sp. spores was not reduced using fumagillin, but the survival of the bees improved (El-Khoury et al. 2018, Prouty et al. 2023). Moreover, fumagillin, the active compound of Fumagilin-B, is restricted in many European countries due to its toxicity (van den Heever et al. 2014). Consequently, there is a need to develop safe and effective alternative treatments to ensure honey bee colony winter survival and spring development.
Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host (Hill et al. 2014). For example, under laboratory conditions, several probiotic strains of Lactobacillaceae and Bifidobacteriaceae can inhibit the growth of pathogenic bacteria, Paenibacillus larvae and Melissococcus plutonius, the causative agents of American and European foulbrood, respectively (Sabate et al. 2009, Forsgren et al. 2010, Killer et al. 2014, Janashia et al. 2016). In addition, trials conducted with caged honey bees showed that some probiotic bacterial strains provoke a reduction of the number of spores present in the gut of infected bees (Baffoni et al. 2016, Arredondo et al. 2018, Peghaire et al. 2020), but is it not always the case (Andrearcyzk et al. 2014, El-Khoury et al. 2018). Probiotic treatments can also mitigate the deleterious impacts of by improving honey bee survival, likely enhancing tolerance to the parasite (El-Khoury et al. 2018).
In field trials, colonies supplemented with lactic acid bacterium raised more brood (De Piano et al. 2017, Alberoni et al. 2018, Lyubimov et al. 2021), had a larger population (Audisio and Benítez-Ahrendts 2011, Daisley et al. 2023), and tended to produce more honey compared to nontreated colonies (Sabate et al. 2009, Audisio and Benítez-Ahrendts 2011, Patruica and Hutu 2013, Alberoni et al. 2018). The administration of Lactobacillus strains also reduces the Paenibacillus larvae pathogen loads in supplemented honey bee colonies, even in the presence of oxytetracycline, the medication used to treat American foulbrood (Daisley et al. 2021). Considering the benefits probiotics can have on honey bee health and colony performance, this alternative treatment has the potential to reduce the threat the overwintering period represents in northern regions, allowing colonies to thrive in spring.
Our goal was to assess the impacts of 3 probiotic formulas added to the fall feeding on the winter survival, spring development and Vairimorpha sp. spore load of honey bee colonies. One endogenous bacterium, Bombella apis (previously called Parasaccharibacter apium, hereafter B. apis) (Smith et al. 2021), and 2 commercial probiotic formulas (Bactocell and LevucellSB, Lallemand Inc.) were selected for this research.
Bombella apis is an endogenous bacterium that is mainly found in the gut of the queen bee and larvae, as well as in colony pollen bread and honey (Kwong and Moran 2016). Laboratory trials showed that this bacterium improves honey bee larval survival (Corby-Harris et al. 2014) and resistance to V. Ceranae (El Khoury et al. 2018). It also slightly increased colony winter survival (Corby-Harris et al. 2016).
Bactocell contains Pediococcus acidilacti, a bacterium that produces lactic acid, which regulates gut pH, and pediocin, an antibacterial compound (Di Giancamillo et al. 2008). This product is known to improve weight gain of the colonies and reduce pathogen load in honey bees (Castex 2009, Angelakis 2017). The bacterium P. acidilacti is naturally found in pollen collected by bees (Belhadj et al. 2010).
LevucellSB consists of Saccharomyces cerevisiae boulardii, a yeast used to reduce pathogen load in poultry (Rychen et al. 2017) and humans (Czerucka et al. 2007). In the beekeeping industry, S. cerevisiae is often used to enrich pollen substitutes (Kast & Roetschi, 2017).
Finally, Fumagilin-B antibiotic, which is the only medication authorized against Vairimorpha sp. in Canada (McCallum et al. 2020), was used in this study to compare both its effectiveness in mitigating nosemosis and its potential adverse effects on Spring brood production to that of the 3 probiotic formulas tested.
Materials and Methods
No animal health care permits were required for this research. The study took place at the Centre de Recherche en Sciences Animales de Deschambault (CRSAD, Deschambault, Québec, Canada; 46 40030.000 N, 71 54052.300 O) between September 2017 and June 2018. In mid-June 2017, 45 honey bee colonies were prepared with young sister queens, then placed in 2 honey-producing apiaries located on farmland near the research facility. At the beginning of September, honey supers were removed, and colonies were reduced to one brood chamber. Fall feeding started in mid-September and all colonies were given 24 liters of 2:1 sucrose solution using a top box feeder (Wooden Miller feeder # FE-1100 from Propolis-etc., Beloeil, QC, Canada). Colonies received a Thymovar anti-varroa treatment starting on September 12, followed by an oxalic acid treatment on November 5 (drip method: 35 g/L in a sucrose 1:1 solution, 5 ml between every frame of the hive body crowded with honey bees, to a maximum of 50 ml per colony). Colonies were wintered indoors in an environmentally controlled room (4–5 °C, 50–60% RH) from 22 November 2017, to 20 April 2018, and then moved to 2 spring apiaries until the end of June 2018.
Experimental Design
Prior to the experiment, the strength of the colonies was measured by visually estimating frame area covered with brood (Giovenazzo and Dubreuil 2011). Then, experimental groups were formed to have similar mean strength. The groups of 9 colonies were as follows: the first group (CTRL) was the control and received plain sucrose solution 1:1 (w/w); the second group (FMG) was treated with Fumagilin-B; the third group (PB1) was treated with the endogenous bacteria B. apis; the fourth (PB2) and fifth (PB3) groups were administered Bactocell and Levucell respectively.
Treatment Solutions and Their Administration
The B. apis strain was isolated from the gut of healthy workers in Québec, Canada, and characterized as described by El Khoury et al. (2018).The 16S RNA gene sequence of the B. apis strain used is ACAGTCAGATGTGAAATCCCCGGGCTTAACCTGGGAACTGCATTTGATACGTGCAGACTAGAGTCCGAGAGAGGGTTGTGGAATTCCCAGTGTAGAGGTGAAATTCGTAGATATTGGGAAGAACACCGGTTGCGAAGGCGGCAACCTGGCTCGGAACTGACGCTGAGGCGCGAAAGCGTGGGGAGCGAACAGGATTAGATACCCTGGTAGTCCACGCTGTAAACGATGTGTGCTGGATGTTGGGTGATTTTATCATTCAGTGTCGGAGCTAACGCGTTAAGCACACCGCCTGGGGAGTACGGCCGCAAGGTTGAAACTCAAAGGAATTGACGGGGGCCCGCACAAGCGGTGGAGCATGTGGTTTAATTCGAAGCAACGCGCAGAACCTTACCAGGGCTTGCATGGGGAGGCTGTATTCAGAGATGGATATTTCTTCGGACCTCCCGCACAGGTGCTGCATGGCTGTCGTCAGCTCGTGTCGTGAGATGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTTGTCTTTAGTTGCCATCACGTCTGGGTGGGCACTCTAGAGAGACTGCCGGTGACAAGCCGGAGGAAGGTGGGGATGACGTCAAGTCCTCATGGCCCTTATGTCCTGGGCTACACACGTGCTACAATGGCGGTGACAGAGGGATGCTACATGGTACATGGTGCTGATCTCAAAAAACCGTCTCAGTTCGGATTGTACTCTGCAACTCGAGTGCATGAAGGTGGAATCGCT. The sequence can be accessed through GeneBank, and the accession number is OR540530.
To initiate bacterial growth, an inoculum of the glycerol stock stored at −80 ℃ was streaked on Sabouraud Dextrose Agar (SDA) plates under sterile conditions and incubated aerobically at 37 °C for 48 h. Then, 3–4 colony-forming units (CFU) were transferred into 10 ml of liquid SDA medium and incubated under the same conditions as above. After 48 h, the broth was added to 1 liter of fresh SDA medium and incubated again at 37 °C on a rotary agitator. Every 24 h, the optical density of the broth was measured with a spectrophotometer, and the bacterial concentration was calculated with the standard curve. When the desired concentration was obtained, the bacterial broth was centrifuged at 4,000 RCF for 20 min and the supernatant was removed. The remaining bacterial pellet was divided and dissolved in nine 1 liter bottles of 1:1 sucrose solution the day prior to administration to ensure survival of the bacterias (El-Khoury et al. 2018).
The Bactocell and Levucell treatments were prepared dissolving the respective probiotic formula into 1:1 sucrose solution. The concentration of the 3 probiotic treatments was 109 CFU/L. probiotic solutions were prepared the day before administration and stored at 4 °C. Probiotic treatments PB1, PB2, and PB3 were given 4 times during the project: twice in fall (12 October and 18 October 2017) and twice in spring (27 April and 4 May 2018). For each treatment, colonies were given 1 liter of the prepared solution using a top box feeder (Wooden Miller feeder # FE-1100 from Propolis-etc., Beloeil, QC, Canada).
For the Fumagilin-B treatment (Group FMG), 9.08 g of the antibiotic was added to 1 liter of 1: 1 sucrose solution and given to each colony, as recommended by the manufacturer. This solution was prepared the day of administration, on 12 October 2017.
Colony Performance
To assess the impact of treatments on colony performance, we measured winter survival, brood area, weight variation, and bee population (Table 1).
Table 1.
Timeline of the manipulations and data collection during the experiment
| 2017 | ||||
|---|---|---|---|---|
| Mid-June | September 12 | October 12 | October 18 | November 5 |
| 45 colonies equally formed with sister queens | Colonies reduced to one brood chamber | Probiotic treatment | Probiotic treatment | Colonies moved to indoor wintering facility |
| 24L 2: 1 sucrose feeding | Fumagilin treatment | Varroa sampling | ||
| Thymovar (Varroa treatment) | Varroa sampling | Vairispora sp. sampling | ||
| Varroa sampling | Hive weight | Hive weight | ||
| Vairispora sp. sampling | Bee cluster size | |||
| Broad area | ||||
| Hive weight | ||||
| Overwintering period (November, 2017 to April, 2018) | ||||
| 2018 | ||||
| April 20 | April 27 | May 4 | May 11 | May 31 |
| Colonies moved to spring apiary | Probiotic treatment | Probiotic treatment | Brood area | Brood area |
| Colony survival | Vairispora sp. sampling | Hive weight | Hive weight | |
| Hive weight | Varroa sampling | Varroa sampling | Varroa sampling | |
| Bee cluster size | ||||
Winter survival:
Colony survival was noted in April when we moved the hives from the overwintering chamber to their spring apiary. A colony without brood or with less than 2 frames covered in bees was considered dead.
Brood area:
The area occupied by immature worker honey bees (eggs + larva + sealed brood) in colonies was evaluated by measuring width and length of the brood area on each side of every brood frame. The rectangular area obtained was multiplied by 0.8 to compensate for the elliptic form of the brood pattern (Giovenazzo and Dubreuil 2011). These values were added to calculate the total brood area in each colony. A factor of 25 worker cells per 6.25 cm2 (i.e., a square inch) was used to calculate the number of immature worker honey bees from the area. This calculation was first performed in September 2017 to create equal experimental groups, then twice in May 2018 (11 May and 1 June).
Weight:
Hives were weighed monthly from September to November 2017, and from April to June 2018, using a numeric platform scale (total capacity of 500 kg, minimum weight sensitivity of 0.1 kg).
Bee population:
The size of the bee cluster was measured in November and April, before and after the overwintering period. The number of frames covered by the cluster was noted, both from above and below, and the mean value was calculated for each colony.
Vairimorpha spp. Infection Level
Honey bees were sampled 3 times between September 2017 and April 2018: before the treatments (T0, 6 September 2017); after the fall treatments and before entering the wintering room (T1, 2 November 2017); and after removal from the wintering room (T2, 27 April 2018). Older worker honey bees were sampled to ensure the highest Vairimorpha sp infestation potential (Jack et al. 2016). These bees were sampled on side frames without brood and each sampling consisted of approximately 100 workers. Samples were immediately stored at −86 °C (Thermofisher −86 °C FORMA 908, Waltham, MA, USA).
Sixty worker bees per sample were pooled and the average spore load per bee was determined as described in (Cantwell 1970). Briefly, bees sampled were homogenized in 60 ml of 70% ethanol (1 ml/bee) in a Stomacher for 60 s at normal speed. One thousand microliters of the homogenate was transferred into a small tube for further microscopic examination using a hemocytometer under 400× magnification with a volume of 6 µL aliquot from the tube previously prepared. The Vairimorpha spores were identified and counted without segregating the species V. ceranae from V. apis.
V. destructor Infestation
Once a month, from September to November 2017 and from April to June 2018, the V. destructor mite population was monitored by placing sticky boards on the bottom board of each hive, covering the entire area. They were left in place for 7 consecutive days, and after removal, the daily mite drop was calculated. This variable was included in statistical analyses, as it is known to influence colony performance (Rosenkranz et al. 2010).
Statistical Analyses
Statistical analyses were conducted using R (v 3.3.1, Vienna, Austria), and P values < 0.05 were considered significant. To determine the impact of treatments on brood area and bee population, a mixed effect linear model was carried out. In each model, the fixed effects were the treatment and the varroa load, and the random effect was the apiary. The effects of the treatments on colony weight were determined using a linear model for repeated measures. The fixed effects were the time and the treatments, and the random effects were the colonies and the apiary. Finally, the impact of the treatments on Vairimorpha spp. spore load was assessed using a Negative Binomial mixed model for repeated measures that considered time and treatment as fixed effects, and the colonies as a random effect. For all measured variables, we compared treated groups to the control group with a Dunnett test.
Results
Colony Performance
During our study, one colony died during the overwintering period (FMG) and 2 died in spring (PB1 and PB3). These colonies were excluded from the analyses.
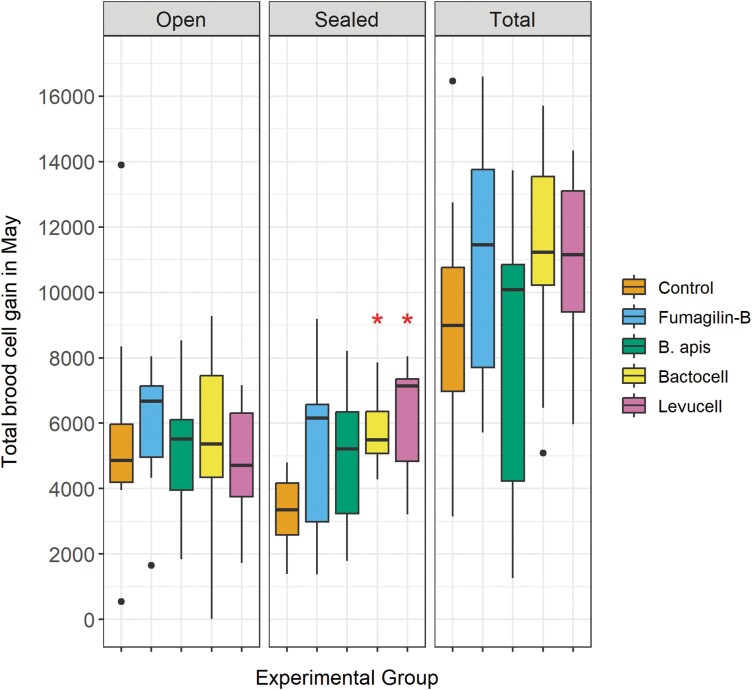
Sealed brood gain in spring was influenced by the experimental treatments given to the colonies (Fig. 1). The amount of sealed brood increased significantly in colonies treated with Bactocell (t = 2.50, P = 0.017) and Levucell (t = 2.72, P = 0.010), compared to control. Between May 11 and 31, colonies treated with Bactocell and Levucell gained respectively an average of 5,682 and 6,106 sealed brood cells, compared to 3,208 for the control colonies. The treatments did not impact the open and total brood gain in spring (Fig. 1).
Fig. 1.
Open, sealed and total brood cell gain between May 11 and 31, 2018 for each experimental group. Each group is compared to the control group. N = 9 colonies per group. * : P ≤ 0.05.
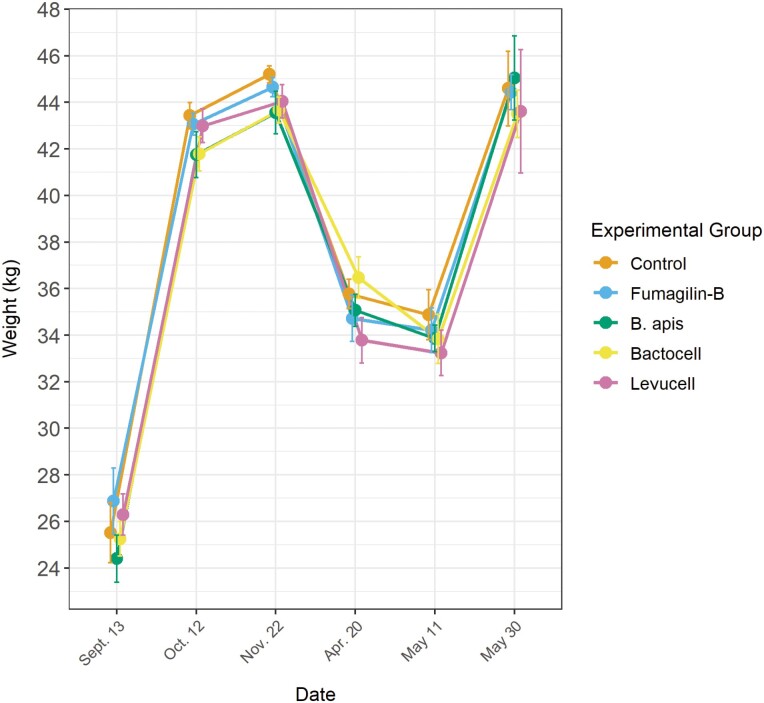
From September 2017 to June 2018, the weight of the colonies from all treated groups was neither influenced by the antibiotic nor by the probiotic treatments (Fig. 2). Hives lost weight similarly during the overwintering period (from 22 November 2017 to 20 April 2018).
Fig. 2.
Average weight of the colonies during the experiment. Weight was measured once a month, except during the overwintering period (December to March). Error bars indicate standard error. N = 9 colonies per group. No significant differences between treatments were detected.
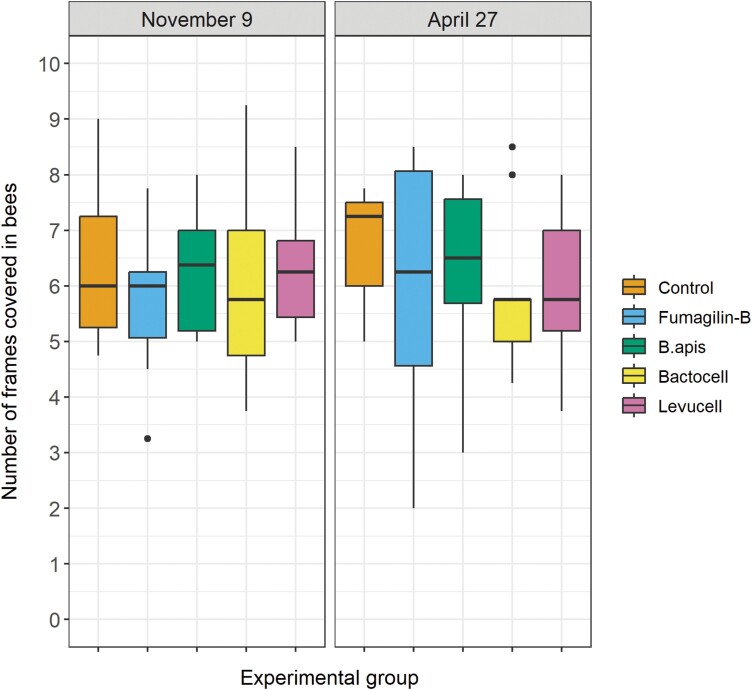
There was no significant difference in the number of frames covered in bees between the treated groups and the control group either in November, before the overwintering period, or in April, when they were moved from the indoor facility to the apiary (Fig. 3).
Fig. 3.
Number of frames covered in bees for each experimental group, in fall (November 9, 2017) and spring (April 9, 2018). N = 9 colonies per group. No significant differences between treatments were detected.
Vairimorpha spp. Infection Level
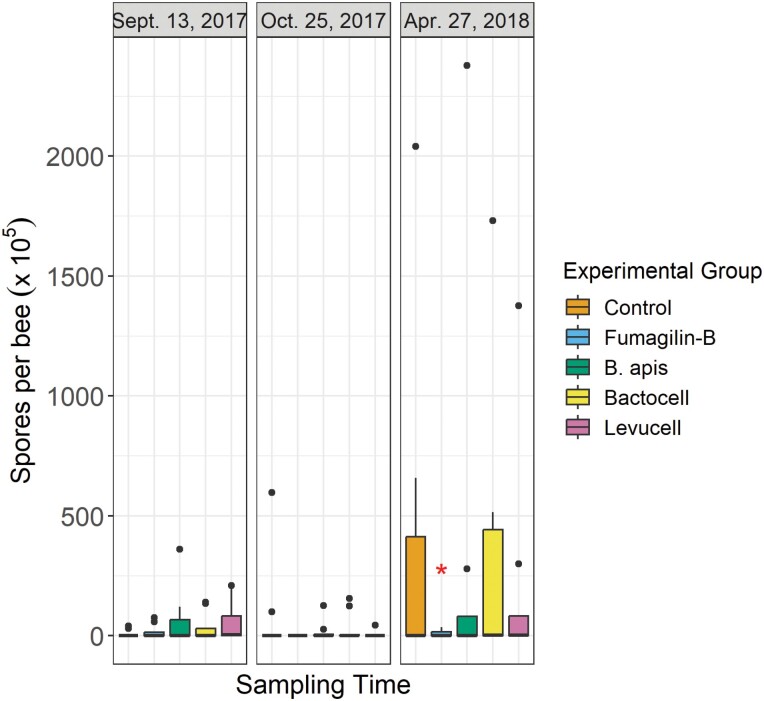
At the beginning of the experiment, the Vairimorpha spp. spore load was low for all groups and there was no difference between them (Fig. 4). The only treatment that significantly reduced the spore load in spring was Fumagilin-B (Z = −2.22, P = 0.027). The groups treated with probiotics showed similar spore loads to the control group throughout the entire experiment.
Fig. 4.
Number of Vairimorpha spp. spores per bee at three sampling times. Each group is compared to the control group. * : P ≤ 0.05.
Discussion and Conclusion
The goal of this study was to assess the effects of 3 probiotic treatments on honey bee colony winter survival, spring performance, and Vairimorpha spp. spore load. Colonies treated with the commercial probiotic formulas Bactocell and Levucell gained respectively 77% and 90% more sealed brood in spring than the control group colonies. Some authors suggest that colonies treated with probiotics produce more brood because the treatment stimulates egg laying (Audisio and Benítez-Ahrendts 2011, Sabate et al. 2012). However, this hypothesis is not supported by any mechanism identified to date.
In a healthy colony, 85% of laid eggs will develop into adults, compared to 64% in a weak colony (Fukuda and Sakagami 1968). The increase in sealed brood observed in our experiment suggests that larval survival rate was higher in colonies supplemented with probiotics. A first factor that may have improved larval survival was increased food availability and quality. In a trial conducted in bumble bee (Bombus terrestris) microcolonies and using Lactobacillus kunkeei as a probiotic (now reclassified as Apilactobacillus kunkeei (Zheng et al. 2020)), the bee population increased in treated colonies when available food was of low quality (Billiet et al. 2017). The authors hypothesized that A. kunkeei increased food digestion and assimilation, thus allowing these colonies to thrive and raise more larvae. Poorly fed colonies were observed to reduce larvae production instead of raising poor quality offspring (Torres et al. 2015). Since food sources are scarce in fall and spring in Québec, it is plausible that colonies supplemented with Bactocell and Levucell were able to maximize nutrients from the foraged pollen and nectar, consequently increasing brood rearing and reducing larval mortality. It would be relevant to assess further the impact of both probiotic strains on nutrition in controlled caged trials, in order to measure accurately survival and body fat percentage, for instance.
Secondly, many researchers have shown that endogenic and commercial strains of Lactobacillus sp. enhance colony performance by increasing the worker population and honey production (Audisio and Benítez-Ahrendts 2011, Sabate et al. 2012, Patruica and Hutu 2013, Audisio et al. 2015, Alberoni et al. 2018, Khaled et al. 2018). In our study, no impact on hive weight was observed between September and June. However, since honey is mainly harvested from June to September in Québec, it is possible that treated colonies with more sealed brood would have a larger population later in summer, harvest more honey and be better prepared to survive the following winter. A long-term study of the impact of Bactocell and Levucell as a preventive treatment in summer would test this hypothesis.
To reduce winter mortality and promote colony growth, many beekeepers prevent nosemosis using fumagillin. Our results confirm the efficacy of this treatment in reducing Vairimorpha spp. cells in treated colonies. Despite its efficacy, Vairimorpha spp. could develop resistance to fumagillin in the coming years, as has been observed in several microorganisms (Tyers and Wright 2019). Furthermore, it was shown that fumagillin must be used with caution: when exposed to a low concentration of the product, Vairimorpha spp. spore production increases, while the honey bee gut epithelial barrier is disrupted (Huang et al. 2013). It is therefore essential to explore the potential of probiotics as an effective and safe alternative to conventional treatments.
In our experiment, although no significant impact of our probiotic treatments on Vairimorpha spp. spore load was detected, B. apis and Levucell showed a trend in reducing spore per bee (Fig. 4). During caged trials, it was shown that bees supplemented with B. apis (called P. apium) have a lower Vairimorpha spp. spore load than nontreated bees (Corby-Harris et al. 2016). El Khoury et al. (2018) also noted that this endogenic bacterium, Bactocell and Levucell improved honey bee survival when infected with Vairimorpha spp. In their experiment, treated bees were found to have the same spore load as nontreated bees, but their survival rate was significantly higher. This tolerance to the presence of Vairimorpha spp. could be explained by the fact that certain probiotics can protect the gut epithelium (Oelschlaeger 2010) and stimulate the immune response of the bee (Janashia et al. 2016). Interestingly, in the present in situ trial, 2 out of 3 probiotic strains (B. apis and Levucell) showed a trend to reduce spore loads in bees but it was not significant. Therefore, administration of higher probiotic concentrations, adding treatments during the overwintering period or alternate ways of delivering the probiotics (Daisley et al. 2023) should be undertaken to validate their potential efficiency in reducing spore loads. Here, we settled on a concentration of 109 CFU/L based on previous in situ experiments (Audisio et al. 2015, Alberoni et al. 2018). However, considering that the hive environment may decrease probiotic survival, it could be interesting to increase either treatment concentration, frequency of delivery mechanism to ensure its effectiveness.
Overall, our results support current knowledge concerning the benefits of using probiotics to enhance performance of honey bee colonies. In this experiment, 3 single strains of beneficial bacteria were tested on honey bee colonies during the overwintering period. Colonies treated with Bactocell and Levucell showed a significant increase in sealed brood in spring. Our results show that probiotic treatments can play an important role in the beekeeping industry due to their beneficial impact on honey bee health. Additional experiments are needed to determine the optimal concentration, administration frequency, and delivery method for probiotic formulas to become a highly effective treatment option for the beekeeping industry.
Contributor Information
N Bleau, Biology Department, Laval University, Québec, Canada; Centre de Recherche en Sciences Animales de Deschambault (CRSAD), Deschambault, Québec, Canada; Institut de Biologie Intégrative et des Systèmes (IBIS), Laval University, Québec, Canada.
N Derome, Biology Department, Laval University, Québec, Canada; Institut de Biologie Intégrative et des Systèmes (IBIS), Laval University, Québec, Canada.
P Giovenazzo, Biology Department, Laval University, Québec, Canada; Centre de Recherche en Sciences Animales de Deschambault (CRSAD), Deschambault, Québec, Canada.
Funding
This research was funded by Project Apis m., grant number MOU-021816.
Author Contributions
Naomie Bleau (Conceptualization [Equal], Data curation [Equal], Formal analysis [Equal], Investigation [Equal], Methodology [Equal], Software [Equal], Visualization [Equal], Writing – original draft [Equal], Writing – review & editing [Equal]), Nicolas Derome (Conceptualization [Equal], Methodology [Equal], Project administration [Equal], Resources [Equal], Supervision [Equal], Validation [Equal], Writing – review & editing [Equal]), and pierre giovenazzo (Conceptualization [Equal], Funding acquisition [Equal], Methodology [Equal], Project administration [Equal], Resources [Equal], Supervision [Equal], Validation [Equal], Writing – review & editing [Equal])
References
- Alberoni D, Baffoni L, Gaggia F, Ryan PM, Murphy K, Ross PR, Stanton C, Di Gioia D.. Impact of beneficial bacteria supplementation on the gut microbiota, colony development and productivity of Apis mellifera L. Benef Microbes. 2018:9(2):269–278. 10.3920/BM2017.0061 [DOI] [PubMed] [Google Scholar]
- Andrearcyzk S, Khadim MJ, Knaga S.. Influence of a probiotic on the mortality, sugar syrup ingestion and infection of honeybees with Nosema spp under laboratory assessment. Med Weter. 2014:70(12):762–765. [Google Scholar]
- Angelakis E. Weight gain by gut microbiota manipulation in productive animals. Microb Pathog. 2017:106:162–170. 10.1016/j.micpath.2016.11.002 [DOI] [PubMed] [Google Scholar]
- Arredondo D, Castelli L, Porrini MP, Garrido PM, Eguaras MJ, Zunino P, Antúnez K.. Lactobacillus kunkeei strains decreased the infection by honey bee pathogens Paenibacillus larvae and Nosema ceranae. Benef Microbes. 2018:9(2):279–290. 10.3920/BM2017.0075 [DOI] [PubMed] [Google Scholar]
- Audisio MC, Benítez-Ahrendts MR.. Lactobacillus johnsonii CRL1647, isolated from Apis mellifera L bee-gut, exhibited a beneficial effect on honey bee colonies. Benef Microbes. 2011:2(1):29–34. 10.3920/BM2010.0024 [DOI] [PubMed] [Google Scholar]
- Audisio MC, Sabate DC, Benitez-Ahrendts MR.. Effect of Lactobacillus johnsonii CRL1647 on different parameters of honey bee colonies and bacterial populations of the bee gut. Benef Microbes. 2015:6(5):687–695. 10.3920/BM2014.0155 [DOI] [PubMed] [Google Scholar]
- Baffoni L, Gaggia F, Alberoni D, Cabbri R, Nanetti A, Biavati B, Di Gioia D.. Effect of dietary supplementation of Bifidobacterium and Lactobacillus strains in Apis mellifera L. against Nosema ceranae. Benef Microbes. 2016:7(1):45–51. 10.3920/BM2015.0085 [DOI] [PubMed] [Google Scholar]
- Belhadj H, Harzallah D, Khennouf S, Dahamna S, Bouharati S, Baghiani A.. Isolation, identification and antimicrobial activity of lactic acid bacteria from Algerian honey bee collected pollen. Xiii Int Conf Med Aromatic Plants. 2010:854:51–58. [Google Scholar]
- Billiet A, Meeus I, Cnockaert M, Vandamme P, Van Oystaeyen A, Wackers F, Smagghe G.. Effect of oral administration of lactic acid bacteria on colony performance and gut microbiota in indoor-reared bumblebees (Bombus terrestris). Apidologie. 2017:48(1):41–50. 10.1007/s13592-016-0447-5 [DOI] [Google Scholar]
- Bleau N, Bouslama S, Giovenazzo P, Derome N.. Dynamics of the honey bee (Apis mellifera) gut microbiota throughout the overwintering period in Canada. Microorganisms. 2020:8(8):1146. 10.3390/microorganisms8081146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner S, Wilson M, Aurell D, Rennich K, vanEngelsdorp D, Steinhauer N, Williams GR.. A national survey of managed honey bee colony losses in the USA: results from the Bee Informed Partnership for 2017–18, 2018–19, and 2019–20. J Api Res. 2023:62(3):429–443. 10.1080/00218839.2022.2158586 [DOI] [Google Scholar]
- Budge GE, Adams I, Thwaites R, Pietravalle S, Drew GC, Hurst GDD, Tomkies V, Boonham N, Brown M. Identifying bacterial predictors of honey bee health. J Invertebr Pathol. 2016:141:41–44. 10.1016/j.jip.2016.11.003 [DOI] [PubMed] [Google Scholar]
- Cantwell GE. Standard methods for counting Nosema spores. Am Bee J. 1970:110: 222–223. [Google Scholar]
- CAPA National Survey Committee, Ferland J, Kempers M, Kennedy K, Kozak P, Lafrenière R, Maund C, Menzies C, Mesher C, Muirhead S, et al. Canadian Association of Professional Apiculturists Statement on Honey Bee Wintering Losses in Canada (2022). Canadian Association of Professional Apiculturists; 2022. [accessed 2023 Jan 21]. https://capabees.com/shared/CAPA-Statement-on-Colony-Losses-2021-2022-FV.pdf [Google Scholar]
- Castex, M. Evaluation du probiotique bactérien Pediococcus acidilactici MA18/5M chez la crevette pénéide Litopenaeus stylirostris en Nouvelle-Calédonie. [Thesis]. Paris: École Doctorale ABIES Paris; 2009. [Google Scholar]
- Corby-Harris V, Snyder L, Meador CAD, Naldo R, Mott B, Anderson KE.. Parasaccharibacter apium, gen. nov., sp nov., improves honey bee (Hymenoptera: Apidae) resistance to Nosema. J Econ Entomol. 2016:109(2):537–543. 10.1093/jee/tow012 [DOI] [PubMed] [Google Scholar]
- Corby-Harris V, Snyder LA, Schwan MR, Maes P, McFrederick QS, Anderson KE.. Origin and effect of Alpha 2.2 Acetobacteraceae in honey bee larvae and description of Parasaccharibacter apium gen nov, sp nov. Appl Environ Microbiol. 2014:80(24):7460–7472. 10.1128/AEM.02043-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerucka D, Piche T, Rampal P.. Yeast as probiotics - Saccharomyces boulardii. Aliment. Pharmacol Ther. 2007:26:767–778. [DOI] [PubMed] [Google Scholar]
- Daisley BA, Pitek AP, Chmiel JA, Gibbons S, Chernyshova AM, Al KF, Faragalla KM, Burton JP, Thompson GJ, Reid G.. Lactobacillus spp attenuate antibiotic-induced immune and microbiota dysregulation in honey bees. Commun Biol. 2021:3(1):534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daisley BA, Pitek AP, Torres C, Lowery R, Adair BA, Al KF, Niño B, Burton JP, Allen-Vercoe E, Thompson GJ, et al. Delivery mechanism can enhance probiotic activity against honey bee pathogens. ISME J. 2023:17(9):1382–1395. 10.1038/s41396-023-01422-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Piano FG, Maggi M, Pellegrini MC, Cugnata NM, Szawarski N, Buffa F, Negri P, Fuselli SR, Audisio CM, Ruffinengo SR.. Effects of Lactobacillus johnsonii aj5 metabolites on nutrition, Nosema ceranae development and performance of Apis mellifera L. J Apic Sci. 2017:61:93–104. [Google Scholar]
- Di Giancamillo A, Vitari F, Savoini G, Bontempo V, Bersani C, Dell’Orto V, Domeneghini C.. Effects of orally administered probiotic Pediococcus acidilactici on the small and large intestine of weaning piglets A qualitative and quantitative micro-anatomical study. Histol Histopathol. 2008:23(6):651–664. 10.14670/HH-23.651 [DOI] [PubMed] [Google Scholar]
- Doke SK, Dhawale SC.. Alternatives to animal testing: a review. Saudi Pharm J. 2015:23(3):223–229. 10.1016/j.jsps.2013.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury S, Rousseau A, Lecoeur A, Cheaib B, Bouslama S, Mercier PL, Demey V, Castex M, Giovenazzo P, Derome N.. Deleterious interaction between honey bees (Apis mellifera) and its microsporidian intracellular parasite Nosema ceranae was mitigated by administrating either endogenous or allochthonous gut microbiota strains. Front Ecol Evol. 2018:6:1–15. [Google Scholar]
- Forsgren E, Olofsson TC, Váasquez A, Fries I. Novel lactic acid bacteria inhibiting Paenibacillus larvae in honey bee larvae. Apidologie. 2010:41:99–108. [Google Scholar]
- Fries I. Nosema Ceranae in European honey bees (Apis mellifera). J Invertebr Pathol. 2009:103:S73–S79. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Sakagami SF.. Worker brood survival in Hobeybees. Res. Popul Ecol. 1968:10(1):31–39. 10.1007/bf02514731 [DOI] [Google Scholar]
- Giovenazzo P, Dubreuil P.. Evaluation of spring organic treatments against Varroa destructor (Acari: Varroidae) in honey bee Apis mellifera (Hymenoptera: Apidae) colonies in eastern Canada. Exp Appl Acarol. 2011:55(1):65–76. 10.1007/s10493-011-9447-3 [DOI] [PubMed] [Google Scholar]
- Higes M, Martin-Hernandez R, Botias C, Bailon EG, Gonzalez-Porto AV, Barrios L, del Nozal MJ, Bernal JL, Jimenez JJ, Palencia PG, et al. How natural infection by Nosema ceranae causes honey bee colony collapse. Environ Microbiol. 2008:10(10):2659–2669. 10.1111/j.1462-2920.2008.01687.x [DOI] [PubMed] [Google Scholar]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014:11(8):506–514. 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- Huang WF, Solter LF, Yau PM, Imai BS.. Nosema ceranae escapes fumagillin control in honey bees. PLoS Pathog. 2013:9(3):e1003185. 10.1371/journal.ppat.1003185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janashia I, Choiset Y, Rabesona H, Hwanhlem N, Bakuradze N, Chanishvili N, Haertlé T.. Protection of honey bee Apis mellifera by its endogenous and exogenous lactic flora against bacterial infections. Ann Agrar Sci. 2016:14(3):177–181. 10.1016/j.aasci.2016.07.002 [DOI] [Google Scholar]
- Jack CJ, Lucas HM, Webster TC, Sagili RR. Colony level prevalence and intensity of Nosema ceranae in honey bees (Apis mellifera L.). PLoS One 2016:11(9):e0163522. 10.1371/journal.pone.0163522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast C, Roetschi A.. Evaluation of baker’s yeast in honey using a real-time PCR assay. Food Microbiol. 2017:62:282–288. 10.1016/j.fm.2016.10.025 [DOI] [PubMed] [Google Scholar]
- Khaled JM, Al-Mekhlafi FA, Mothana RA, Alharbi NS, Alzaharni KE, Sharafaddin AH, Kadaikunnan S, Alobaidi AS, Bayaqoob NI, Govindarajan M, et al. Brevibacillus laterosporus isolated from the digestive tract of honey bees has high antimicrobial activity and promotes growth and productivity of honey bee’s colonies. Environ Sci Pollut Res. 2018:25:10447–10455. [DOI] [PubMed] [Google Scholar]
- Killer J, Dubna S, Sedlacek I, Svec P.. Lactobacillus apis sp nov., from the stomach of honey bees (Apis mellifera), having an in vitro inhibitory effect on the causative agents of American and European foulbrood. Int J Syst Evol Microbiol. 2014:64(Pt 1):152–157. 10.1099/ijs.0.053033-0 [DOI] [PubMed] [Google Scholar]
- Kwong WK, Moran NA.. Gut microbial communities of social bees. Nat Rev Microbiol. 2016:14(6):374–384. 10.1038/nrmicro.2016.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubimov AI, Vorobieva SL, Tronina AS, Yudin VM.. Efficiency of probiotic supplements in the dynamics of economically useful indicators of honey-bee colonies. BIO Web Conferences. 2021:36:05014. 10.1051/bioconf/20213605014 [DOI] [Google Scholar]
- McCallum R, Olmstead S, Shaw J, Glasgow K.. Evaluating efficacy of Fumagilin-B against nosemosis and tracking seasonal trends of Nosema spp. in Nova Scotia Honey Bee Colonies. J Apic Sci. 2020:64:277–286. [Google Scholar]
- Neumann P, Carreck NL.. Honey bee colony losses. J Api Res. 2010:49(1):1–6. 10.3896/ibra.1.49.1.01 [DOI] [Google Scholar]
- Oelschlaeger TA. Mechanisms of probiotic actions - a review. Int J Med Microbiol. 2010:300(1):57–62. 10.1016/j.ijmm.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Patruica S, Hutu I.. Economic benefits of using prebiotic and probiotic products as supplements in stimulation feeds administered to bee colonies. Turk. J Vet Anim Sci. 2013:37:259–263. [Google Scholar]
- Peghaire E, Moné A, Delbac F, Debroas D, Chaucheyras-Durand F, El Alaoui H.. A Pediococcus strain to rescue honeybees by decreasing Nosema ceranae- and pesticide-induced adverse effects. Pestic Biochem Physiol. 2020:163:138–146. 10.1016/j.pestbp.2019.11.006 [DOI] [PubMed] [Google Scholar]
- Potts SG, Roberts SPM, Dean R, Marris G, Brown MA, Jones R, Neumann P, Settele J.. Declines of managed honey bees and beekeepers in Europe. J Api Res. 2010:49(1):15–22. 10.3896/ibra.1.49.1.02 [DOI] [Google Scholar]
- Prouty C, Jack C, Sagili R, Ellis JD.. Evaluating the efficacy of common treatments used for Vairimorpha (Nosema) spp. Control Appl Sci. 2023:13(3):1303. [Google Scholar]
- Rosenkranz P, Aumeier P, Ziegelmann B.. Biology and control of Varroa destructor. J Invertebr Pathol. 2010:103(Suppl 1):S96–119. 10.1016/j.jip.2009.07.016 [DOI] [PubMed] [Google Scholar]
- Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos MD, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, et al. Safety and efficacy of Levucell((R)) SB (Saccharomyces cerevisiae CNCM I-1079) as a feed additive for chickens for fattening and minor poultry species. EFSA J. 2017:15:1–9. [Google Scholar]
- Sabate DC, Carrillo L, Audisio MC.. Inhibition of Paenibacillus larvae and Ascosphaera apis by Bacillus subtilis isolated from honey bee gut and honey samples. Res Microbiol. 2009:160(3):193–199. 10.1016/j.resmic.2009.03.002 [DOI] [PubMed] [Google Scholar]
- Sabate DC, Cruz MS, Benitez-Ahrendts MR, Audisio MC.. Beneficial effects of Bacillus subtilis subsp subtilis Mori2, a honey-associated strain, on honey bee colony performance. Probiotics Antimicrob Proteins. 2012:4(1):39–46. 10.1007/s12602-011-9089-0 [DOI] [PubMed] [Google Scholar]
- Smith EA, Anderson KE, Corby-Harris V, McFrederick QS, Parish AJ, Rice DW, Newton ILG.. Reclassification of seven honey bee symbiont strains as Bombella apis. Int J Syst Evol Microbiol. 2021:71(9). 10.1099/ijsem.0.004950 [DOI] [PubMed] [Google Scholar]
- Tokarev YS, Huang W, Solter LF, Malysh JM, Becnel JJ, Vossbrinck CR.. A formal redefinition of the genera Nosema and Vairimorpha (Microsporidia: Nosematidae) and reassignment of species based on molecular phylogenetics. J Invertebr Pathol. 2020:169(10):107279–107279. 10.1016/j.jip.2019.107279 [DOI] [PubMed] [Google Scholar]
- Torres DJ, Ricoy UM, Roybal S.. Modeling honey bee populations. PLoS One. 2015:10(7):e0130966. 10.1371/journal.pone.0130966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyers M, Wright GD.. Drug combinations: a strategy to extend the life of antibiotics in the 21st century. Nat Rev Microbiol. 2019:17(3):141–155. 10.1038/s41579-018-0141-x [DOI] [PubMed] [Google Scholar]
- van den Heever JP, Thompson TS, Otto SJG, et al. Evaluation of Fumagilin-B® and other potential alternative chemotherapies against Nosema ceranae-infected honey bees (Apis mellifera) in cage trial assays. Apidologie. 2014:47:617–630. [Google Scholar]
- Zheng J, Wittouck S, Salvetti E, Franz CMAP, Harris HMB, Mattarelli P, O’Toole PW, Pot B, Vandamme P, Walter J, Watanabe K, et al. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020:70(4):2782–2858. [DOI] [PubMed] [Google Scholar]