Abstract
Produced by the liver, corticosteroid-binding globulin (CBG) regulates the plasma distribution and actions of glucocorticoids. A sex difference in pituitary growth hormone secretion patterns established during puberty in rats results in increased hepatic CBG production and 2-fold higher plasma corticosterone levels in females. Glucocorticoids control hepatic development and metabolic activities, and we have therefore examined how disrupting the SerpinA6 gene encoding CBG influences plasma corticosterone dynamics, as well as liver gene expression in male and female rats before and after puberty. Comparisons of corticosterone plasma clearance and hepatic uptake in adult rats, with or without CBG, indicated that CBG limits corticosterone clearance by reducing its hepatic uptake. Hepatic transcriptomic profiling revealed minor sex differences (207 differentially expressed genes) and minimal effect of CBG deficiency in 30-day-old rats before puberty. While liver transcriptomes in 60-day-old males lacking CBG remained essentially unchanged, 2710 genes were differentially expressed in wild-type female vs male livers at this age. Importantly, ∼10% of these genes lost their sexually dimorphic expression in adult females lacking CBG, including those related to cholesterol biosynthesis, inflammation, and lipid and amino acid catabolism. Another 203 genes were altered by the loss of CBG specifically in adult females, including those related to xenobiotic metabolism, circadian rhythm, and gluconeogenesis. Our findings reveal that CBG consolidates the sexual dimorphism of the rat liver initiated by sex differences in growth hormone secretion patterns and provide insight into how CBG deficiencies are linked to glucocorticoid-dependent diseases.
Keywords: sex differences, development, hepatic transcriptome, corticosterone
As a metabolic hub, the liver is critical for organ development, integrating and responding to cues from various tissues by altering the metabolism, uptake, and secretion of nutrients and hormones, as well as the production of hormone transport proteins that influence their biological activities (1). One such plasma hormone-binding protein, corticosteroid-binding globulin (CBG), increases during puberty in female but not male rodents (2, 3). Plasma CBG plays an important role in regulating the plasma distribution of glucocorticoid hormones (cortisol in humans, corticosterone in rodents), as well as their output from the adrenal (1, 3). Governed by the hypothalamic-pituitary-adrenal axis, glucocorticoids are essential for survival, allowing the energetic demands of stress to be accommodated, and controlling homeostatic processes, including metabolic, circadian, and immune responses (4). Many of these systemic actions are influenced by liver function, as glucocorticoids modulate major metabolic, energy storage, and immune-related pathways in this organ (5).
In the blood, glucocorticoids circulate largely bound to CBG and albumin, with a small unbound or “free” fraction that is available to target cells (6). They act via the glucocorticoid and mineralocorticoid receptors to regulate gene expression differentially at multiple levels in a tissue-specific manner (7-9). Prior to binding these receptors, the concentrations of glucocorticoids within cells are controlled at 2 key levels: by binding CBG as the primary regulator of plasma glucocorticoid levels and their access to cells (10), and through metabolic conversions mediated by intracellular enzymes (11). Glucocorticoids act in a negative feedback loop on the activity of the hypothalamic-pituitary-adrenal axis, and we have shown that adrenal gland development is choreographed by changes in plasma CBG using CRISPR/cas9 to disrupt CBG (SerpinA6) gene expression. In brief, we found that differences in adrenal growth, morphology, and gene expression in male and female rats emerge in concert with a sex difference in plasma CBG levels that is established during puberty, and that these sex differences in the adrenal are lost in CBG-deficient animals (3).
The 2-fold higher plasma CBG levels developed during puberty in female rats (2, 3) has been attributed to postnatal androgen imprinting on the pituitary secretion of growth hormone that emerges during puberty (12-14), which not only alters hepatic CBG production but has a profound effect on the sexually dimorphic expression of a wide variety of genes in the liver in adult life (15-18). Accordingly, we sought to determine if and how the sex difference in plasma CBG levels postpubertally in rats might influence the metabolic clearance of corticosterone, as well as liver maturation and function. Our findings indicate that sex differences in plasma CBG consolidate the sexually dimorphic expression of genes in the liver initiated by sex differences in the pattern of pituitary growth hormone secretion, and provide insight into why CBG deficiencies in rats (19, 20), mice (21), and pigs (22, 23), are associated with susceptibility to stress (23, 24), inflammation (25), and behavioral abnormalities (26-28), as well as chronic fatigue, central obesity, and hepatic steatosis in CBG-deficient humans (29-31).
Materials and Methods
Animals
A CRISPR/cas9 mutagenesis strategy was employed (SAGE Labs, acquired by Envigo, Indianapolis, IN) to generate Charles River Sprague Dawley rats lacking CBG, as previously described (3). Rats were maintained on a 12-hour lights on, 12-hour lights off cycle (7 Am–7 Pm) with controlled temperature (21-22 °C) and ad libitum access to standard laboratory chow (LabDiet; PicoLab Rodent Diet 20, #5053) and water. All rats used for these studies were bred and maintained at the Center for Disease Modeling (University of British Columbia). Heterozygous SerpinA6+/− females were used to generate male and female SerpinA6+/+ and SerpinA6−/− rats to avoid any differential effects of maternal genotype on progeny, and to control for litter effects. Unless otherwise noted, adult (∼120 days) male (∼600 g) and female (∼300 g) rats were used. All procedures were approved by the University of British Columbia Animal Care Committee.
Plasma Corticosterone Half-Life
Kinetic parameters of interest were determined in wild-type and CBG-deficient rats after injection of a trace quantity of [3H]-corticosterone (PerkinElmer; 95 Ci/mmol; 1.3 nCi/g BW in 0.9% saline) via the right lateral tail vein under isoflurane anesthesia. Sequential blood samples (200 μL) were taken from 4 minutes to 40 minutes using a catheter placed in the left lateral tail vein, and replacement volumes of saline were delivered via the same route. Blood samples were centrifuged (2500g) for 20 minutes at 4 °C to obtain plasma, and the radioactive counts per minute (CPM) were determined in a scintillation counter. The plasma terminal half-life (t1/2) was generated based on logarithmic transformations of radioactivity as a function of time, as previously described (32). To further assess the contribution of CBG, within-sex profiles obtained in CBG-deficient rats were subtracted from wild-type animals.
Hepatic Corticosterone Metabolism
Based on previously published methods (33-35), fresh liver minces were first washed in ice-cold phosphate-buffered saline (PBS) to remove blood-born contamination (confirmed by the absence of CBG binding in subsequent extracts). Supernatants obtained from livers homogenized in PBS (1 gram tissue per 10 mL buffer) were warmed to 37 °C before adding NADPH (1mM) and [3H]-corticosterone (American Radiolabeled Chemicals Inc.; 40 Ci/mmol; 4nM). Diethyl ether was added to samples of the reaction mixture at 0 minutes and 30 minutes, and this layer was evaporated under nitrogen gas and then resuspended in PBS for subsequent steroid detection using anti-corticosterone antibodies (RRID:AB_90543, 1:5000). Following a 1-hour incubation (4 °C), dextran-coated charcoal (DCC) was added to adsorb nonspecific and/or unbound steroid. The DCC was pelleted by centrifugation, and the radioactivity in the supernatant representing specific [3H]-corticosterone was used as an index of metabolism by dividing the counts obtained at 30 minutes from those at the start of the reaction (0 minutes). No-liver controls did not show changes in [3H]-corticosterone, indicating that degradation of the tracer did not occur over this 30-minute interval.
Hepatic Corticosterone Metabolism and Uptake
To assess the contribution of CBG to steroid uptake and metabolism, livers obtained from CBG-knockout animals were incubated with NADPH (1mM) and [3H]-corticosterone (American Radiolabeled Chemicals Inc.; 40 Ci/mmol; 4nM), matched against same-sex plasma derived from male or female rats with or without CBG (pretreated with DCC to remove endogenous steroids). At 30 minutes of incubation, the total radioactivity and that attributed to corticosterone specifically were determined in both incubation media and tissue extracts. Incubation media was separated by light centrifugation, whereas isolated liver minces were repeatedly washed in ice-cold water before inducing cell lysis by repeated freeze-thawing. Lysate and incubation media were further processed by denaturing CBG in citric acid (pH 3) at 60 °C, followed by neutralization in Tris buffer (pH 9). Samples were then split for separate determinations of total and corticosterone-specific radioactivity and processed using DCC and the corticosterone antibody described above. Buffer-only incubates were also employed as controls.
RNA Sequencing and Bioinformatics
Rats (n = 4 per group) were sacrificed during the light phase of the circadian cycle (between 10 Am and 11 Am). Total RNA was extracted from liver using TRIzol reagent (Thermo Fisher Scientific) and Ultra Turrax T-25 Basic (IKA), as per the manufacturer's instructions. A Bioanalyzer (Agilent) was used to assess RNA concentration and integrity number (all RIN scores ≥9). Samples were then prepared following the standard protocol for NEBnext Ultra ii Stranded mRNA (New England Biolabs) with polyA selection. Libraries, sequencing, and de-multiplexing were performed by the Biomedical Research Centre Sequencing Core (University of British Columbia) using Illumina NextSeq 500 with a sequencing depth of 20 million paired end reads (43 bp × 43 bp). The data were aligned to the Rattus Norvegicus (Rnor_5.0) reference sequence using Illumina suite programs: STAR and cufflinks. In R, individual spreadsheets with gene count data were loaded, compiled, organized and genes with low reads (<5 in all groups) were removed. Tidyverse (36) and dplyr (37) were used to rearrange data tables where necessary. Base R heatmap, lattice functions, base principal component analysis (PCA), and base R plots were used to identify outliers, of which there were none. A Linear model (limma and voom) and variancePartition (38) were used to assess main effects of sex, genotype, and age, and their interactions (39). Individual contrasts were corrected using the Benjamini-Hochberg method, and a false discovery rate cutoff of 0.05 was applied. Venn diagrams, P value distributions, violin plots, and heatmaps (Complex Heatmap and circlize) were used to visualize the data (40, 41). PANTHER 18.0 was used to categorize genes and perform gene enrichment analysis. Original RNA-seq data and analyses discussed in this publication have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (42).
Statistical Analyses
Plasma corticosterone clearance was compared using 2-way ANOVAs (genotype and time as between subject variables). Steroid kinetic analysis, including corticosterone half-life, was compared using unpaired t tests. Liver steroid metabolism and uptake were compared using 2-way ANOVAs (genotype and sex as between subject variables), and post hoc analyses using Sidak's multiple comparisons test.
Results
CBG Reduces the Plasma Clearance of Corticosterone
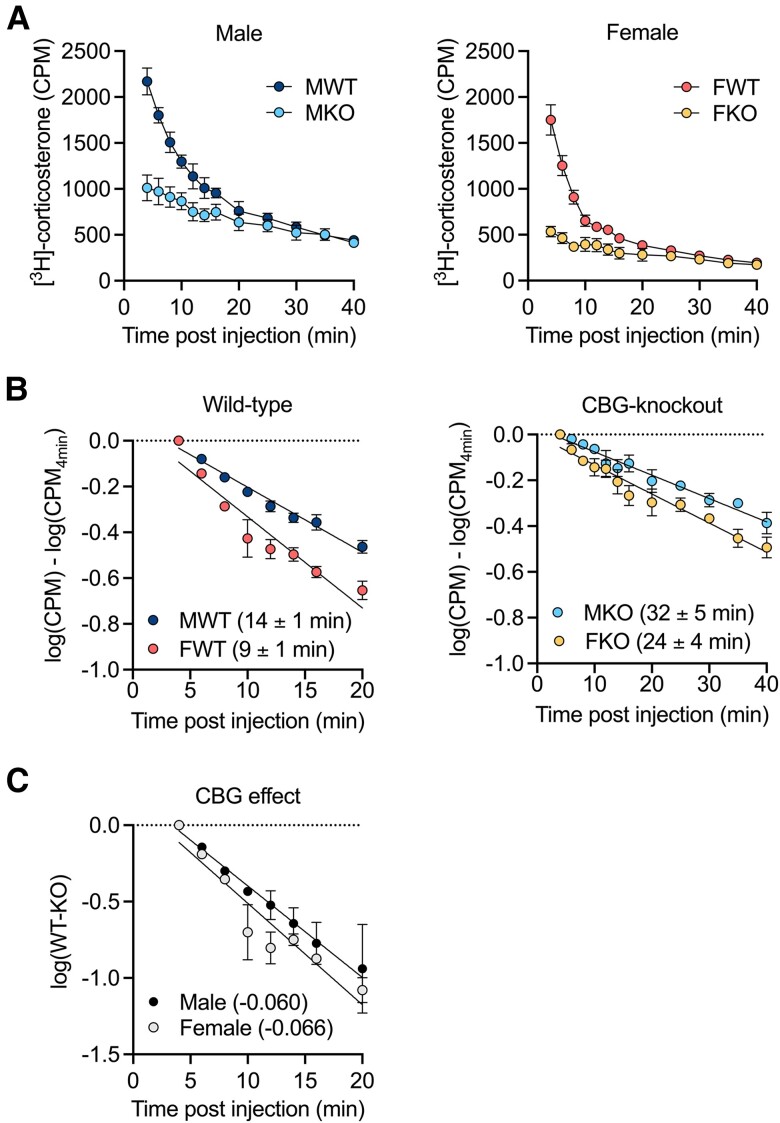
The [3H]-corticosterone elimination profile in wild-type rats showed 2 distinct phases, reflecting the presence of CBG and albumin (Fig. 1A). By contrast, rats without CBG showed only one elimination phase, attributed primarily to albumin binding. The levels of plasma radioactivity in wild-type rats were greater than in rats lacking CBG between 4 minutes and 20 minutes post injection, suggesting that CBG normally retains corticosterone in the blood and limits its clearance over this interval. As expected, the volume of distribution (determined by the dose injected divided by the plasma concentration at 2 minutes) was greater in rats lacking CBG (2-fold in males; 4-fold in females), consistent with observations that postnatal increases in CBG accompany decreases in the apparent volume of distribution of the steroid (43).
Figure 1.
CBG reduces the plasma clearance of corticosterone. A, Mean ± SEM plasma [3H]-corticosterone counts per minute (CPM) in male and female rats with and without CBG, after intravenous injection of [3H]-corticosterone. In both sexes, 2-way ANOVA revealed main effects of time (F's ≤ 124.3; P's < .0001) and genotype (F's ≤ 26.55; P's < .0391) and an interaction between time and genotype (F's ≤ 30.16 P's < .0001). B, Slope profiles revealed a faster clearance rate in female compared to male wild-type (P = .007), and the clearance rate tended to be faster in female compared to male CBG-knockout rats (P = .1). C, Average CPM values from CBG-knockout rats were subtracted from individual wild-type CPM values to isolate the effect of CBG. The slopes were not different between males and females (P = .6). N = 3 per group. M = male, F = female, WT = wild-type, KO = CBG-knockout.
To calculate the plasma half-life of corticosterone, [3H]-corticosterone amounts (CPM) were log-transformed and normalized to the starting value (Fig. 1B). Given the biphasic nature of the wild-type clearance profiles, the analysis was restricted to the first phase (4-20 minutes) wherein the effect of CBG could be assessed. The half-life of [3H]-corticosterone in wild-type rats during this period was 14 ± 1 minute in males and 9 ± 1 minute in females (Fig. 1B), reflecting a faster clearance rate in females. Rats lacking CBG showed half-life values of 32 ± 5 minutes in males and 24 ± 4 minutes in females, suggesting that the sex difference in corticosterone clearance is independent of CBG. The shorter corticosterone half-life in wild-type rats compared to CBG-knockout rats may appear counterintuitive but reflects the half-life calculation based on the rate of decline (slope) that occurs 4 minutes after the intravenous injection of [3H]-corticosterone, that is, after the initial tissue redistribution phase was complete. However, plasma [3H]-corticosterone levels were consistently higher in rats with CBG, and this implies that [3H]-corticosterone is lost much more rapidly during the initial tissue redistribution phase in rats without CBG.
To isolate the effect of CBG, [3H]-corticosterone levels in plasma of CBG-knockout rats were subtracted from wild-type CPM values, and the results were log-transformed to linear plots for slope analysis (Fig. 1C). This revealed a similar influence of CBG to prevent plasma corticosterone clearance between wild-type male and female rats and indicates that CBG does not explain the apparent sex difference in corticosterone clearance, which could be accounted for by an underlying sex difference in the metabolic clearance rate.
Intrinsic Hepatic Corticosterone Metabolism Between Sexes Does Not Vary as a Function of CBG
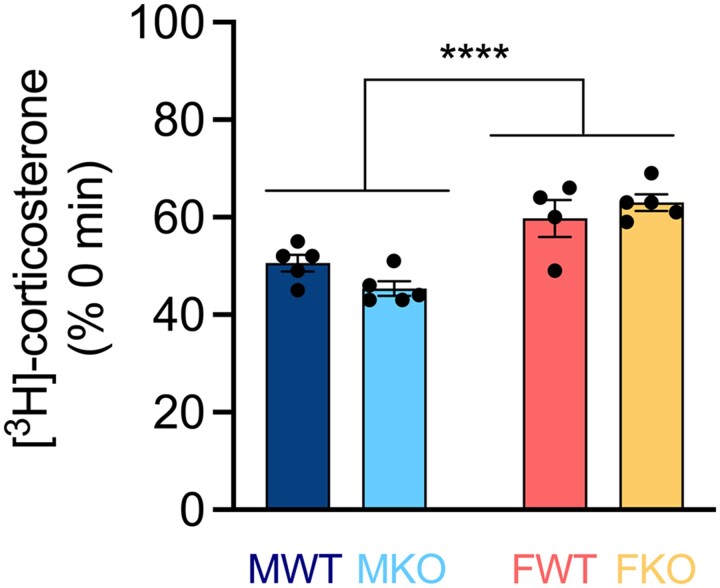
Since the liver is a major site of corticosterone metabolism, we determined whether underlying sex- and/or genotype-dependent differences in hepatic corticosterone metabolism could explain differences in plasma corticosterone half-life. The hepatic metabolism of corticosterone was overall higher in males compared to females (Fig. 2), suggesting that sex differences in plasma corticosterone clearance (females faster than males) cannot be explained by hepatic corticosterone metabolism. Within sex, there was no difference in hepatic corticosterone metabolism between rats with and without CBG, indicating that genotypic differences in corticosterone half-life are not due to intrinsic differences in corticosterone metabolism in the liver.
Figure 2.
Hepatic metabolism of corticosterone. Liver extract from the indicated source was incubated with [3H]-corticosterone and NADPH, and the amount of [3H]-corticosterone remaining after 30 minutes was determined (Mean ± SEM [3H]-corticosterone % 0 minutes). Two-way ANOVA revealed a main effect of sex (F [1, 15] = 38.13, P < .0001), wherein males showed greater metabolism than females, with no genotype effect (F [1, 15] = .2026, P < .6590). N = 4-5 per group. M = male, F = female, WT = wild-type, KO = CBG-knockout.
Plasma CBG Limits Hepatic Corticosterone Uptake
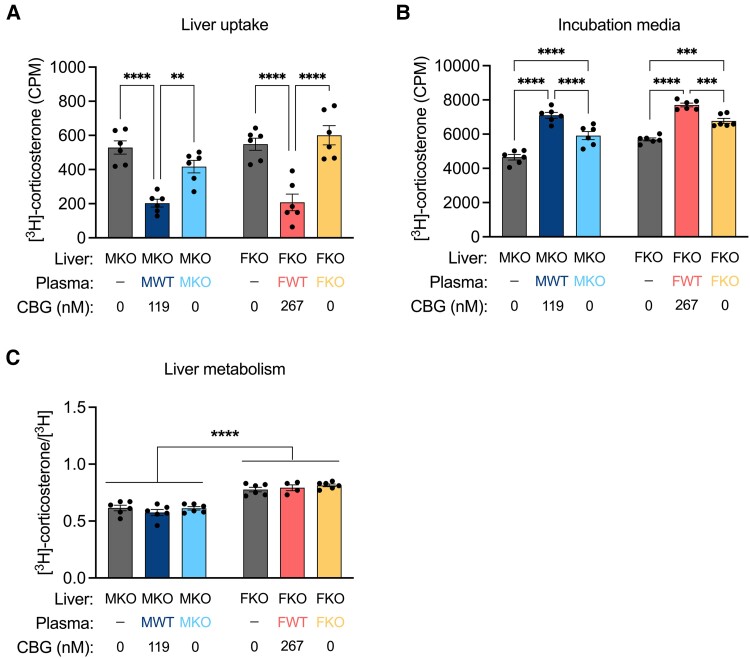
We therefore sought to assess whether the effect of CBG to limit corticosterone clearance could be explained by changes in corticosterone bioavailability to the liver. Analysis of hepatic corticosterone uptake ex vivo against a background of varying levels of CBG revealed that CBG reduced hepatic corticosterone uptake, which was mirrored by higher levels of steroid in the incubation media for samples containing CBG (Fig. 3A). The ratio of [3H]-corticosterone to total [3H] (corticosterone + metabolites) within the liver was then determined to assess whether CBG in the incubation media affected the intrinsic rate of hepatic corticosterone metabolism (Fig. 3B). Males showed more intracellular hepatic corticosterone metabolism than females overall, reflecting our previous findings (Fig. 2), but CBG in the incubation media did not affect the degree of intracellular corticosterone metabolism. Taken together, the data show that CBG reduces the hepatic metabolism of corticosterone by limiting its hepatic uptake.
Figure 3.
CBG limits hepatic corticosterone uptake. Liver minces were incubated with [3H]-corticosterone in PBS or the indicated plasma source for 30 minutes. A, [3H]-corticosterone (Mean ± SEM CPM) in the liver were lower in samples containing CBG in the incubation media, indicated by a main effect of experimental condition (F [2, 30] = 40.06, P < .0001). B, In the incubation media, [3H]-corticosterone levels were higher in samples containing CBG (F [2, 30] = 100.8, P < .0001). There was also a main effect of sex (F [1, 30] = 43.24, P < .0001) whereby females retained more [3H]-corticosterone in the incubation media compared to males. C, The ratio of [3H]-corticosterone to total [3H] was used to assess the extent of [3H]-corticosterone metabolism. In the liver, there was a main effect of sex (F [1, 28] = 126.1, P < .0001) whereby males showed greater metabolism than females. In the incubation media, there was lower metabolism in samples containing CBG (F [2, 30] = 44.8, P < .0001). There was also a main effect of sex (F [1, 30] = 26.39, P < .0001), wherein females showed lower [3H]-corticosterone metabolism than males. N = 6 per group. M = male, F = female, WT = wild-type, KO = CBG-knockout. CPM = counts per minute.
Liver Transcriptome
To determine how sex differences in CBG during the pubertal to adult transition influence liver maturation and function, hepatic transcriptomic profiles were obtained in wild-type and CBG-knockout males and females, before (postnatal day [PD] 30) and after (PD60) puberty, during which the sex difference in plasma CBG levels is established.
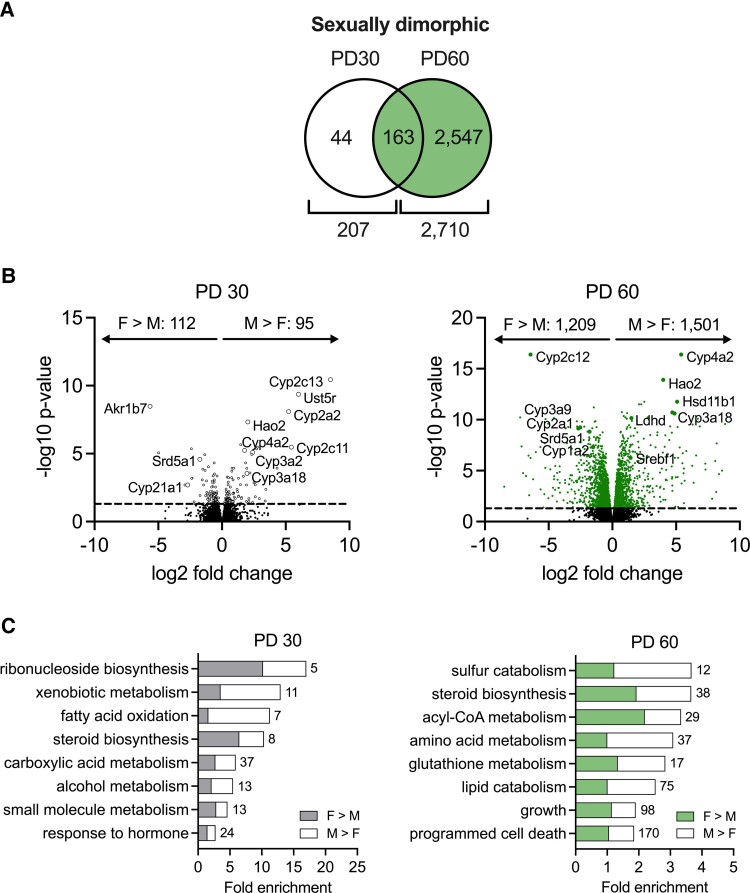
Normal Sexual Dimorphism
Sexual dimorphism of the liver transcriptome was determined by comparing wild-type male and female rats. At PD30, there were 207 differentially expressed genes (DEGs) between sexes, while 2710 DEGs were identified at PD60 (Fig. 4A). These sexually dimorphic genes were plotted according to the fold difference in expression between males and females (Fig. 4B). Many Cyp genes showed notable differences in expression level between sexes, at both PD30 and PD60. To identify the biological processes to which these sexually dimorphic genes belonged, gene ontology analysis was performed at each age (Fig. 4C). This showed that xenobiotic metabolism, fatty acid oxidation, and carboxylic acid metabolism are sexually dimorphic processes at PD30, while acyl-CoA metabolism, amino acid metabolism, lipid catabolism, growth, and cell death are sexually dimorphic at PD60. Our metabolic activity assays (Figs. 2 and 3) indicated a faster rate of hepatic glucocorticoid metabolism in wild-type male rats compared to female rats, and this may be explained by increased expression of hydroxysteroid dehydrogenases in males (Hsd11b2, Akr1c1, Akr1c2, Akr1c3). However, the male rats also showed lower expression of the glucocorticoid-metabolizing enzyme Srd5a1 than female rats, and higher expression of the regenerating enzyme Hsd11b1, which contradict this. Since over 60 hydroxysteroid dehydrogenases, cytochrome P450s, sulfotransferases, glutathione transferases, and glucuronosyltransferases were found to be sexually dimorphic in expression, the net effect of this on glucocorticoid metabolism remains unknown.
Figure 4.
Sexual dimorphism of the liver transcriptome. A, Venn diagrams show the number of differentially expressed genes between wild-type males (M) and females (F) at PD30 and PD60. B, Volcano plots show the sex-dependent genes and their relative expression at PD30 and PD60. C, Gene ontology analysis revealed sexually dimorphic biological processes at each age. Numbers of DEGs belonging to each biological process are indicated. N = 4 per group. PD = postnatal day.
Impact of CBG
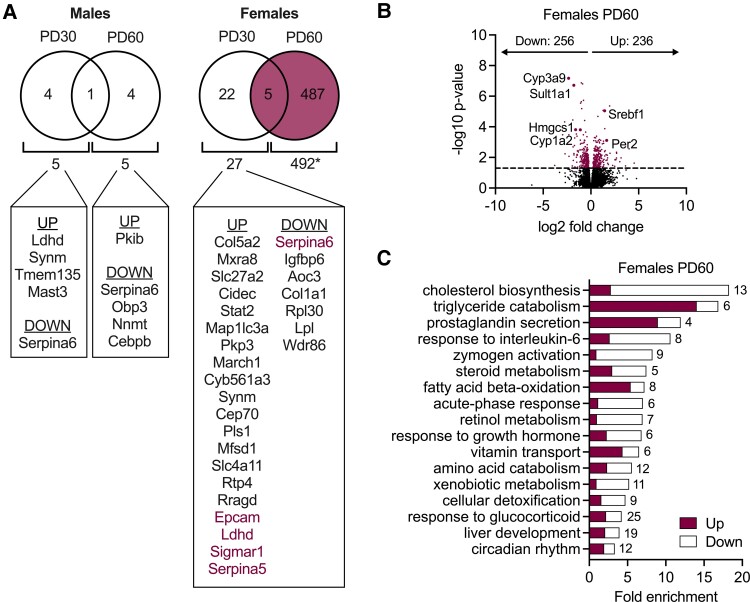
To determine how CBG influences liver function, we then compared the transcriptome of rats with and without CBG. In male rats, there were 5 CBG-dependent genes at each PD30 and PD60, whereas between female rats with and without CBG, there were 27 DEGs at PD30, and 492 DEGs at PD60 (Fig. 5A). Thus, while CBG had minimal effect on the liver transcriptome in males and little effect prepubertally, its influence on the adult female liver was notable. Of the 492 genes identified as CBG-dependent in 60-day-old female rats, the majority of DEGs were identified as metabolite interconversion enzyme (125 genes), protein modifying enzyme (42 genes), or transporter (38 genes). Approximately half of the genes were downregulated, and the remaining were upregulated in the absence of CBG (Fig. 5B). Notable genes downregulated in the absence of CBG included Cyp3a9, Cyp1a2, and Sult1a1, critical for the clearance of endo- and xenobiotics. Also downregulated was Hmgcs1, the rate-limiting enzyme of cholesterol biosynthesis. Among the most upregulated genes were Srebf1, a transcriptional regulator of lipogenesis, and Per2, involved in the regulation of circadian rhythm.
Figure 5.
Sex- and age-selective effect of CBG on the liver transcriptome. A, Venn diagrams show the number of differentially expressed genes between wild-type and CBG-knockout rats in males and females at PD30 and PD60. Genes that were up- and downregulated in CBG-knockout rats compared to wild-type are listed. Genes in purple font indicate that they were differentially expressed at both PD30 and PD60. B, Volcano plot showing CBG-dependent genes in adult females (492 genes). Approximately half of these CBG-dependent genes were downregulated in females lacking CBG, and the remaining were upregulated (adjusted P value < .05). C, Gene ontology analysis revealed CBG-dependent biological processes in adult females. Numbers of DEGs belonging to each biological process are indicated. N = 4 per group. PD = postnatal day.
Gene ontology analysis revealed the most affected biological process to be cholesterol biosynthesis, since almost all cholesterol biosynthetic enzymes were downregulated in the absence of CBG in females (Fig. 5C). Other affected processes included triglyceride catabolism, response to interleukin-6, steroid metabolism, fatty acid beta-oxidation, response to growth hormone, amino acid metabolism, xenobiotic metabolism, response to glucocorticoid, and circadian rhythm. In support of our metabolic activity assays (Figs. 2 and 3), the expression of major glucocorticoid-metabolizing enzymes (Hsd11b1, Srd5a1, Cyp3a4, Akr1c, Hsd3b, Sult2a1, Ugt2b1) was not affected by CBG, except for Hsd11b2, which was increased by the loss of CBG in the female rats. Taken together, analysis of the hepatic transcriptome revealed little effect of CBG deficiency in 30-day-old animals. However, in 60-day-old animals, while the hepatic transcriptome of males lacking CBG was essentially normal, substantial genotype effects were observed in females (492 DEGs).
Contribution of CBG to Hepatic Sexual Dimorphism
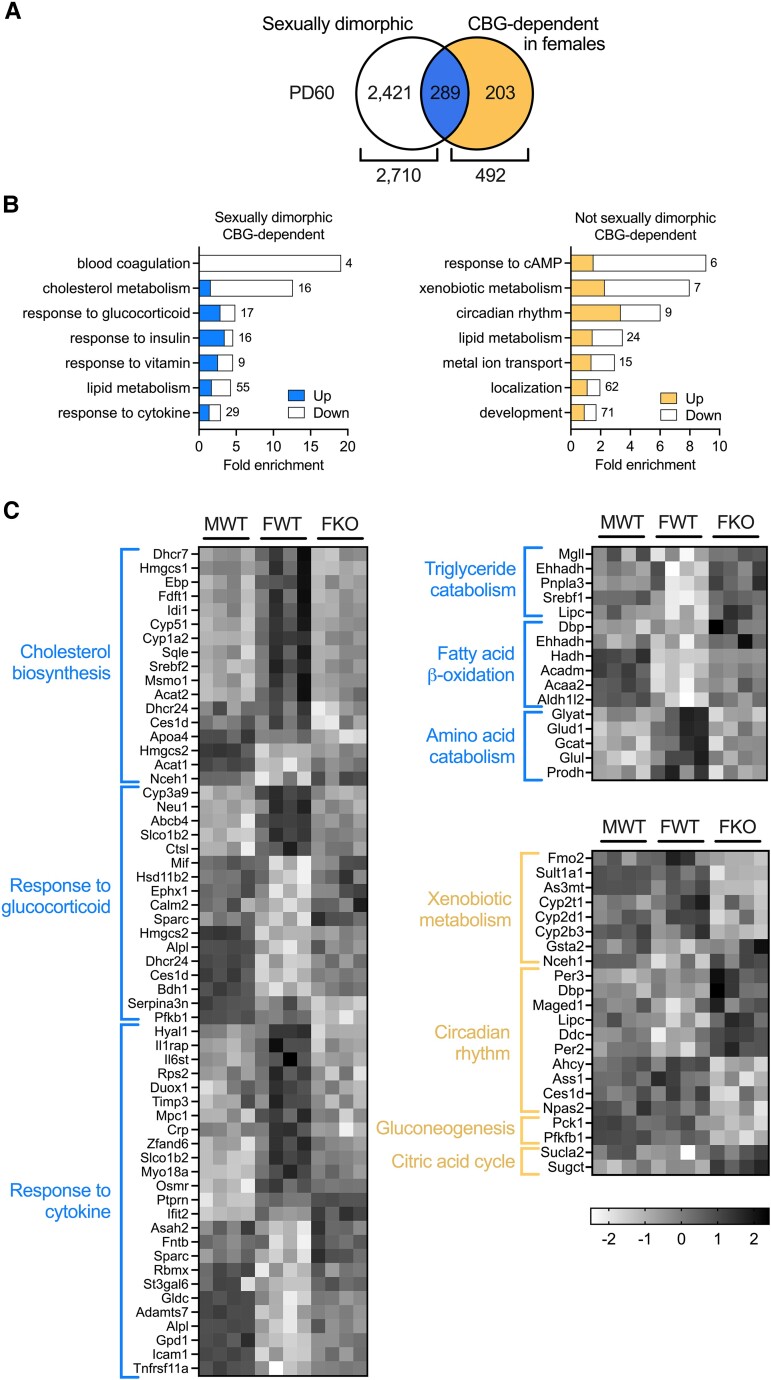
Based on these age- and sex-dependent influences we determined the nature and extent to which the sexual dimorphism of the liver transcriptome is determined by CBG. Of the 492 genes identified as CBG-dependent in adult female rats, approximately 50% were normally sexually dimorphic in expression (Fig. 6A), including those involved in blood coagulation, cholesterol metabolism, triglyceride catabolism, fatty acid beta-oxidation, amino acid metabolism, and response to cytokine and glucocorticoid (Fig. 6B and 6C). The vast majority of these genes lost their sexually dimorphic expression (Fig. 6C). By contrast, female CBG-dependent genes that are not normally sexually dimorphic were related to cAMP signaling, xenobiotic metabolism, circadian rhythm, development, gluconeogenesis, and the citric acid cycle (Fig. 6B and 6C). The data show that CBG consolidates sexually dimorphic gene expression and has little effect on male livers.
Figure 6.
CBG determines the sexually dimorphic expression of 289 hepatic genes. A, Venn diagram indicating that of the 2710 sexually dimorphic genes in adults, 289 were CBG-dependent in females. B, Gene ontology of sexually dimorphic (left) and non-sexually dimorphic (right), CBG-dependent genes. Numbers of DEGs belonging to each biological process are indicated. C, Z-score analyses describe sexually dimorphic (blue) and non-sexually dimorphic (yellow) genes and pathways impacted by CBG deficiency. N = 4 per group. M = male, F = female, WT = wild-type, KO = CBG-knockout. PD = postnatal day.
Discussion
Our studies of how sex differences in plasma CBG levels influence corticosterone clearance, hepatic glucocorticoid uptake, and metabolism were informative at several levels. As expected, wild-type rats showed much higher initial levels of intravenously administered [3H]-corticosterone in their blood compared to rats without CBG, indicating that CBG retains corticosterone in the blood and limits its clearance, contributing to the lower plasma corticosterone levels in rats lacking CBG (3). After 20 minutes, this effect of CBG was no longer apparent, and the steady state plasma distribution of [3H]-corticosterone reflected the contribution of plasma albumin which was comparable between rats with and without CBG. This allowed us to measure the effect of albumin on the metabolic clearance of corticosterone for the first time, in the absence of CBG. Surprisingly, these experiments revealed that the 2-fold higher CBG levels in female rats (2) did not restrict corticosterone clearance to a greater extent than in males. In fact, wild-type females showed a faster clearance rate of corticosterone than wild-type males, which has been observed by others (32, 44). A faster metabolic clearance rate of corticosterone in female rats may be necessary to balance the higher CBG levels and enhanced corticosterone secretion rate (45) in order to maintain free corticosterone levels, which are comparable between sexes (3).
Our ex vivo experiments verified that genotypic differences in corticosterone clearance were not caused by changes in the glucocorticoid-metabolizing activity of the liver. Interestingly, male rats showed a faster rate of hepatic corticosterone metabolism than females, an opposite pattern to that of corticosterone clearance. This is important because previous reports suggest that the faster clearance in females is due to enhanced hepatic activity of 5α-reductase (32, 33, 44). Since hepatic metabolic activity could not account for the sex difference in plasma corticosterone clearance, the activities of extrahepatic tissues such as intestine, kidney, muscle, heart, adipose, and adrenal, which also contribute to glucocorticoid metabolism (45-49), may explain this. Our experiments also showed that CBG limited the hepatic uptake of corticosterone, without affecting the rate of intracellular corticosterone metabolism. Albumin also prevented the hepatic metabolism of corticosterone to some degree, because incubation media derived from rats without CBG retained greater amounts of [3H]-corticosterone than the PBS control. Though it has been shown that higher levels of CBG prevent corticosterone metabolism to a greater extent (50), we did not observe this dose effect on corticosterone uptake. The relatively low concentration of corticosterone employed may have precluded a dose-dependent effect of CBG that would have otherwise been revealed in a background of high corticosterone. Overall, these experiments demonstrate that CBG limits corticosterone clearance by preventing its hepatic uptake, and that higher CBG levels in females may be important for counteracting the higher metabolic activity of extrahepatic tissues.
To determine how sex differences in CBG during the pubertal to adult transition influence liver maturation and function, we performed RNA sequencing in the liver before and after puberty. Analysis of the hepatic transcriptome revealed a greater number of sexually dimorphic genes at postnatal day (PD) 60 than at PD30 in wild-type rats, reflecting the known effect of growth hormone to induce sexual dimorphism during puberty (51, 52). A comparable number of sexually dimorphic genes have been identified in the mouse liver at PD30 (53). At 60 days of age, GO analysis identified steroid metabolism as the most enriched sexually dimorphic process, due primarily to the higher expression of genes involved in cholesterol metabolism in females compared to males (54). Other sexually dimorphic processes included lipid, fatty acid, and amino acid metabolism, reflecting the fundamental sex difference in the utilization of carbohydrates and lipids as fuel sources: at rest, females tend to synthesize fatty acids and triglycerides for fat storage, while males oxidize circulating fatty acids. During exercise, women utilize more lipids than carbohydrates, while the reverse is true in men (55). In response to fasting, females utilize amino acids to maintain fat storage, while males decrease the activity of anabolic pathways (54). Our transcriptomic data also revealed that cell growth and death are highly sexually dimorphic processes, supported by others who found apoptosis and death receptor signaling to be male-dominant pathways (56).
The effect of CBG on the hepatic transcriptome was centered in postpubertal females with no major effects in males; a sex difference we have also observed in the adrenal gland (3). One of the downregulated genes in males lacking CBG was CCAAT/enhancer-binding protein beta (Cebpb), a transcription factor that facilitates glucocorticoid receptor recruitment to steroid response elements (57) and regulates daily liver metabolic transcriptional programs (58). Despite the widespread influence of this transcription factor on metabolism (59), only 3 other genes were CBG-dependent in adult males, including Obp3, the cAMP-dependent protein kinase inhibitor Pkib, and Nnmt involved in NAD+ metabolism (60). Since Cebpb is induced during the acute-phase response (61), the impact of its downregulation in males lacking CBG may become apparent during stress conditions. In the female liver, CBG maintained the sexual dimorphism of 289 genes related to metabolism and inflammation. Most strikingly, almost all genes involved in cholesterol biosynthesis were downregulated to male-like expression levels, which is consistent with the role of the liver in regulating cholesterol synthesis and transport to the adrenal gland, which is small in female rats lacking CBG (3). Sex differences in the expression of genes related to xenobiotic metabolism, circadian rhythm, and gluconeogenesis appeared only after the loss of CBG, suggesting that the underlying physiology regulating these genes is normally different between sexes (62). In this way, CBG both promotes and constrains the sexual dimorphism of the rat liver.
In the absence of CBG, females showed increased expression of lipolysis genes Lipe and MgII and the major transcription factor Srebf1, which together reflect an increase in lipid catabolism. Glucocorticoids normally stimulate the expression of lipolysis genes to break down triglycerides into fuel, suggesting that glucocorticoid activity is enhanced in CBG-knockout females. Indeed, Addisonian and adrenalectomized individuals show impaired energy mobilization, whereas individuals with Cushing disease show fat accumulation (63). Moreover, glucocorticoid receptor-knockout mice show high levels of hepatic triglycerides due to increased triglyceride storage and decreased catabolism (64). The expression of genes related to fatty acid β-oxidation were also upregulated in the absence of CBG, as was the triacylglycerol lipase Pnpla3, a risk factor for nonalcoholic fatty liver disease (65). Taken together, these changes in the expression of lipid metabolic pathways point to enhanced energy mobilization in the livers of female rats lacking CBG, which may reflect reports of central obesity and hepatic steatosis in CBG-deficient patients (31).
By contrast, changes in the expression of gluconeogenic and amino acid catabolic genes were indicative of reduced energy utilization in the livers of female rats lacking CBG. Amino acids are normally utilized to a greater degree in the female liver compared to male (54), yet many amino acid catabolic genes were downregulated in CBG-knockout females and lost their sexually dimorphic expression (Glyat, Glud1, Gcat, Glul, Prodh). The hepatic expression levels of rate-limiting enzymes in gluconeogenesis (Pck1) and glycolysis (Pfkfb1) were also downregulated in the absence of CBG, pointing to reduced glucocorticoid signaling and energy mobilization (64). Interestingly, fatty acids suppress Pfkfb1 expression, allowing for coordination between lipid and carbohydrate metabolism (66). The data therefore point to a shift from amino acid to lipid catabolism in females lacking CBG, implying that CBG normally maintains this sex difference. Interestingly, these findings are recapitulated to some extent in individuals with chronic fatigue syndrome/myalgic encephalomyelitis (29), which is associated with alterations in cholesterol, lipid, and amino metabolism (67-69), and is common in CBG-deficient individuals (30).
Glucocorticoids strongly repress the inflammatory response, and CBG-deficient mice show increased susceptibility to inflammation because of glucocorticoid insufficiency (25). Many CBG-dependent genes in female rats were annotated as cytokine-responsive, and the vast majority of these lost their sexually dimorphic expression. However, downregulation of transcripts involved in the inflammatory response (Crp, SerpinA3N, Fga, Fgb, Fgg, Hp (70)) reflect a dampened immune response and therefore enhanced glucocorticoid exposure (71). Interestingly, a dampened inflammatory status also predicts a reduction in the expression of xenobiotic enzymes (72), which we observed for Cyp1a2, Cyp2d1, Cyp2d3, Cyp2r1, and Sult1a1; enzymes critical for the biotransformation of common drugs (73-76) and vitamin D metabolism (77-79). How these factors respond to an inflammatory challenge in CBG-deficient rats remains to be seen.
The absence of CBG altered the hepatic expression of core clock genes Per2, Per3, and Dbp (80), suggesting a dysregulation or shift in circadian rhythm (81). Per2-knockout mice show disruptions in the hepatic metabolism of lipids, cholesterol, glucose, and amino acids, the expression of inflammatory genes, and corticosterone rhythm (82), suggesting that the effects of CBG on hepatic metabolism may be secondary to changes in ultradian and/or circadian rhythm. Indeed, diurnal oscillations in hepatic metabolism are coordinated by the glucocorticoid receptor (64), and the expression of a critical transcription factor responsible for integrating circadian and nutritional cues, Srebf1 (83), was altered in the absence of CBG. Rats lacking CBG maintain a diurnal rhythm in total plasma corticosterone levels although low overall (3), but the time at which the diurnal shift occurred has not been investigated.
Genetic ablation of the hepatic glucocorticoid receptor in mice causes a reduction in the number of inflammatory genes that exhibit sexually dimorphic expression (56, 84), similar to the impact of CBG ablation. Glucocorticoid-dependent effects on the sexual dimorphism of the liver may be explained by interactions between the glucocorticoid receptor and sex steroid receptors to alter chromatin accessibility (85). The glucocorticoid and androgen receptors target similar transcription regulatory sequences (86), and this redundancy could provide a candidate mechanism by which males are protected from CBG deficiency. However, the expression of many genes involved in lipid metabolism, an estrogen-sensitive process (87), were altered in female CBG-knockout rats, and glucocorticoid-dependent changes in estrogen receptor activity may therefore contribute to the sex-selectivity of this deficiency.
While free corticosterone levels in the plasma are expected to be similar between sexes and genotypes during our experiments (3), female-specific differences in intracellular ligand availability may occur as a result of an increased expression of Hsd11b2 in female rats lacking CBG compared to their wild-type counterparts. Importantly, hepatocytes synthesize CBG at levels that likely approach those of the intracellular corticosterone levels in this tissue, and the normally higher levels of CBG within female hepatocytes compared to male hepatocytes may affect intracellular ligand availability to a greater extent, such that the loss of CBG affects females disproportionately.
Some of the sex-dependent effects of CBG on the liver may instead be an indirect consequence of changes in adrenal function, which is altered in female CBG-knockout rats but not males (3). For example, reduced cholesterol uptake in the adrenal of female CBG-knockout rats (3) may explain the reduction in cholesterol biosynthetic gene expression in the liver (Fig. 6C), since the liver responds to changes in circulating cholesterol (88). Indeed, circulating cholesterol levels are correlated to adrenal size, steroidogenesis (89, 90) and glucocorticoid levels (91). If this liver-adrenal relationship is true, then Cbg+/− rats showing intermediate adrenal size (3) would be expected to show proportional changes in hepatic cholesterol biosynthesis.
Sex differences in liver function are linked to disparities in the prevalence of diseases such as hepatocellular carcinoma (92), nonalcoholic fatty liver disease (93), liver fibrosis, autoimmune diseases, diabetes, and osteoporosis (56). These sex differences are induced by differential secretory patterns of pituitary growth hormone that emerge during puberty (51, 52, 94), as reflected by widespread changes in the expression of hepatic genes involved in cholesterol homeostasis, lipid metabolism, inflammation, and drug metabolism (17, 95). Our experiments show that CBG, the sexually dimorphic expression of which in rodents is established by sex differences in the pattern of pituitary growth hormone secretion (12-14), represents an additional driver to consolidate the sexual dimorphism of the rat liver postnatally. Although plasma CBG in humans is not normally different between sexes (96), our findings may help understand the various clinical sequelae observed in humans and other mammals that are CBG-deficient (22, 27, 28, 31, 97-104), and provide insight into how changes in CBG levels may modify disease symptoms in response to oral contraceptives (105), pregnancy (106), inflammation (107, 108), and throughout the life cycle (109).
Abbreviations
- CBG
corticosteroid-binding globulin
- CPM
counts per minute
- DCC
dextran-coated charcoal
- PBS
phosphate-buffered saline
- PD
postnatal day
Contributor Information
Julia N C Toews, Department of Cellular and Physiological Sciences, The Life Sciences Institute, University of British Columbia, Vancouver, BC V6T 1Z3, Canada.
Tristan J Philippe, Department of Cellular and Physiological Sciences, The Life Sciences Institute, University of British Columbia, Vancouver, BC V6T 1Z3, Canada.
Matthew Dordevic, Department of Cellular and Physiological Sciences, The Life Sciences Institute, University of British Columbia, Vancouver, BC V6T 1Z3, Canada.
Lesley A Hill, Department of Cellular and Physiological Sciences, The Life Sciences Institute, University of British Columbia, Vancouver, BC V6T 1Z3, Canada.
Geoffrey L Hammond, Department of Cellular and Physiological Sciences, The Life Sciences Institute, University of British Columbia, Vancouver, BC V6T 1Z3, Canada.
Victor Viau, Department of Cellular and Physiological Sciences, The Life Sciences Institute, University of British Columbia, Vancouver, BC V6T 1Z3, Canada.
Funding
This work was supported by the Canadian Institutes of Health Research [136840, 136856], and Natural Sciences and Engineering Research Council of Canada [RGPIN-2014-05714] Grants (V.V.).
Disclosures
The authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repository listed in References.
References
- 1. Toews JNC, Hammond GL, Viau V. Liver at the nexus of rat postnatal HPA axis maturation and sexual dimorphism. J Endocrinol. 2021;248(1):R1‐R17. [DOI] [PubMed] [Google Scholar]
- 2. Gala RR, Westphal U. Corticosteroid-binding globulin in the rat: studies on the sex difference. Endocrinology. 1965;77(5):841‐851. [DOI] [PubMed] [Google Scholar]
- 3. Toews JNC, Philippe TJ, Hill LA, et al. Corticosteroid-binding globulin (SERPINA6) establishes postpubertal sex differences in rat adrenal development. Endocrinology. 2022;163(11):bqac152. [DOI] [PubMed] [Google Scholar]
- 4. Timmermans S, Souffriau J, Libert C. A general introduction to glucocorticoid biology. Front Immunol. 2019;10:1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Præstholm SM, Correia CM, Grøntved L. Multifaceted control of GR signaling and its impact on hepatic transcriptional networks and metabolism. Front Endocrinol. 2020;11:572981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989;10(3):232‐274. [DOI] [PubMed] [Google Scholar]
- 7. de Kloet ER, de Kloet SF, de Kloet CS, de Kloet AD. Top-down and bottom-up control of stress-coping. J Neuroendocrinol. 2019;31(3):e12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reul JM, Collins A, Saliba RS, et al. Glucocorticoids, epigenetic control and stress resilience. Neurobiol Stress. 2015;1:44‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spiga F, Lightman SL. Dynamics of adrenal glucocorticoid steroidogenesis in health and disease. Mol Cell Endocrinol. 2015;408:227‐234. [DOI] [PubMed] [Google Scholar]
- 10. Hammond GL. Plasma steroid-binding proteins: primary gatekeepers of steroid hormone action. J Endocrinol. 2016;230(1):R13‐R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chapman K, Holmes M, Seckl J. 11β-Hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev. 2013;93(3):1139‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chowen JA, Argente J, Gonzalez-Parra S, Garcia-Segura LM. Differential effects of the neonatal and adult sex steroid environments on the organization and activation of hypothalamic growth hormone-releasing hormone and somatostatin neurons. Endocrinology. 1993;133(6):2792‐2802. [DOI] [PubMed] [Google Scholar]
- 13. Gabriel SM, Roncancio JR, Ruiz NS. Growth hormone pulsatility and the endocrine milieu during sexual maturation in male and female rats. Neuroendocrinology. 1992;56(5):619‐625. [DOI] [PubMed] [Google Scholar]
- 14. Jansson JO, Ekberg S, Isaksson O, Mode A, Gustafsson JA. Imprinting of growth hormone secretion, body growth, and hepatic steroid metabolism by neonatal testosterone. Endocrinology. 1985;117(5):1881‐1889. [DOI] [PubMed] [Google Scholar]
- 15. Ahluwalia A, Clodfelter KH, Waxman DJ. Sexual dimorphism of rat liver gene expression: regulatory role of growth hormone revealed by deoxyribonucleic acid microarray analysis. Mol Endocrinol. 2004;18(3):747‐760. [DOI] [PubMed] [Google Scholar]
- 16. Udy GB, Towers RP, Snell RG, et al. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci U S A. 1997;94(14):7239‐7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76(2):215‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waxman DJ, O’Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol. 2006;20(11):2613‐2629. [DOI] [PubMed] [Google Scholar]
- 19. Dhabhar FS, McEwen BS, Spencer RL. Stress response, adrenal steroid receptor levels and corticosteroid-binding globulin levels—a comparison between Sprague-Dawley, Fischer 344 and Lewis rats. Brain Res. 1993;616(1-2):89‐98. [DOI] [PubMed] [Google Scholar]
- 20. Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Differential activation of adrenal steroid receptors in neural and immune tissues of Sprague Dawley, Fischer 344, and Lewis rats. J Neuroimmunol. 1995;56(1):77‐90. [DOI] [PubMed] [Google Scholar]
- 21. Orava M, Zhao XF, Leiter E, Hammond GL. Structure and chromosomal location of the gene encoding mouse corticosteroid-binding globulin: strain differences in coding sequence and steroid-binding activity. Gene. 1994;144(2):259‐264. [DOI] [PubMed] [Google Scholar]
- 22. Guyonnet-Duperat V, Geverink N, Plastow GS, et al. Functional implication of an Arg307Gly substitution in corticosteroid-binding globulin, a candidate gene for a quantitative trait locus associated with cortisol variability and obesity in pig. Genetics. 2006;173(4):2143‐2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ousova O, Guyonnet-Duperat V, Iannuccelli N, et al. Coticosteroid binding globulin: a new target for cortisol-driven obestity. Mol Endocrinol. 2004;18(7):1687‐1696. [DOI] [PubMed] [Google Scholar]
- 24. Tak LM, Cleare AJ, Ormel J, et al. Meta-analysis and meta-regression of hypothalamic-pituitary-adrenal axis activity in functional somatic disorders. Biol Psychol. 2011;87(2):183‐194. [DOI] [PubMed] [Google Scholar]
- 25. Libert C, Wielockx B, Hammond GL, Brouckaert P, Fiers W, Elliott RW. Identification of a locus on distal mouse chromosome 12 that controls resistance to tumor necrosis factor-induced lethal shock. Genomics. 1999;55(3):284‐289. [DOI] [PubMed] [Google Scholar]
- 26. Minni AM, de Medeiros GF, Helbling JC, et al. Role of corticosteroid binding globulin in emotional reactivity sex differences in mice. Psychoneuroendocrinology. 2014;50:252‐263. [DOI] [PubMed] [Google Scholar]
- 27. Minni AM, Dorey R, Piérard C, et al. Critical role of plasma corticosteroid-binding-globulin during stress to promote glucocorticoid delivery to the brain: impact on memory retrieval. Endocrinology. 2012;153(10):4766‐4774. [DOI] [PubMed] [Google Scholar]
- 28. Richard EM, Helbling JC, Tridon C, et al. Plasma transcortin influences endocrine and behavioral stress responses in mice. Endocrinology. 2010;151(2):649‐659. [DOI] [PubMed] [Google Scholar]
- 29. Lim E-J, Ahn Y-C, Jang E-S, Lee S-W, Lee S-H, Son C-G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med. 2020;18(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Torpy DJ, Ho JT. Corticosteroid-binding globulin gene polymorphisms: clinical implications and links to idiopathic chronic fatigue disorders. ClinEndocrinol(Oxf). 2007;67(2):161‐167. [DOI] [PubMed] [Google Scholar]
- 31. Torpy DJ, Bachmann AW, Grice JE, et al. Familial corticosteroid-binding globulin deficiency due to a novel null mutation: association with fatigue and relative hypotension. J Clin Endocrinol Metab. 2001;86(8):3692‐3700. [DOI] [PubMed] [Google Scholar]
- 32. Glenister DW, Yates FE. Sex difference in the rate of disappearance of corticosterone-4-C14 from plasma of intact rats: further evidence for the influence of hepatic Delta4-steroid hydrogenase activity on adrenal cortical function. Endocrinology. 1961;68(5):747‐758. [DOI] [PubMed] [Google Scholar]
- 33. Deckx R, Raus J, Denef C, De Moor P. Sex difference in the metabolism of cortisol by rat liver. Steroids. 1965;6(2):129‐141. [DOI] [PubMed] [Google Scholar]
- 34. Krawczyńska A, Herman AP, Antushevich H, et al. Modifications of western-type diet regarding protein, fat and sucrose levels as modulators of steroid metabolism and activity in liver. J Steroid Biochem Mol Biol. 2017;165(Pt B):331‐341. [DOI] [PubMed] [Google Scholar]
- 35. Woodward CJ, Hervey GR, Oakey RE, Whitaker EM. The effects of fasting on plasma corticosterone kinetics in rats. Br J Nutr. 1991;66(1):117‐127. [DOI] [PubMed] [Google Scholar]
- 36. Wickham H, Averick M, Bryan J, et al. Welcome to the tidyverse. J Open Source Soft. 2019;4(43):1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- 37. Wickham H, François R, Henry L, Müller K. Dplyr: A grammar of data manipulation. R Package Version 0.8.4 (2020). https://CRAN.R-project.org/package=dplyr
- 38. Hoffman GE, Schadt EE. variancePartition: interpreting drivers of variation in complex gene expression studies. BMC Bioinformatics. 2016;17(1):483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32(18):2847‐2849. [DOI] [PubMed] [Google Scholar]
- 41. Gu Z, Gu L, Eils R, Schlesner M, Brors B. circlize Implements and enhances circular visualization in R. Bioinformatics. 2014;30(19):2811‐2812. [DOI] [PubMed] [Google Scholar]
- 42. Toews JNC, Philippe TJ, Dordevic M, Hill LA, Hammond GL, Viau V. Gene expression omnibus repository. National Center for Biotechnology Information. Deposited 3 November 2023. GEO Series accession number GSE245348. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi? acc=GSE245348
- 43. Schroeder RJ, Henning SJ. Roles of plasma clearance and corticosteroid-binding globulin in the developmental increase in circulating corticosterone in infant rats. Endocrinology. 1989;124(5):2612‐2618. [DOI] [PubMed] [Google Scholar]
- 44. Kitay JI. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68(5):818‐824. [DOI] [PubMed] [Google Scholar]
- 45. Saroff J, Wexler BC. Metabolic clearance and production rates of corticosterone in male and female virgin and breeder rats. Acta Endocrinol. 1969;62(3):411‐424. [DOI] [PubMed] [Google Scholar]
- 46. Yates FE, Urquhart J. Control of plasma concentrations of adrenocortical hormones. Physiol Rev. 1962;42(3):359‐433. [DOI] [PubMed] [Google Scholar]
- 47. Bellamy D, Phillips JG, Jones IC, Leonard RA. The uptake of cortisol by rat tissues. Biochem J. 1962;85(3):537‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marandici A, Monder C. The fate of corticosterone and 11-deoxycorticosterone in C57BL/6 and BALB/c strains of mice: distribution and oxidative metabolism. J Steroid Biochem. 1984;21(5):579‐583. [DOI] [PubMed] [Google Scholar]
- 49. Petersen HH, Andreassen TK, Breiderhoff T, et al. Hyporesponsiveness to glucocorticoids in mice genetically deficient for the corticosteroid binding globulin. Mol Cell Biol. 2006;26(19):7236‐7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sandberg AA, Slaunwhite WR Jr. Transcortin: a corticosteroid-binding protein of plasma. V. In vitro inhibition of cortisol metabolism. J Clin Invest. 1963;42(1):51‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol. 2006;20(6):1333‐1351. [DOI] [PubMed] [Google Scholar]
- 52. Wauthier V, Waxman DJ. Sex-specific early growth hormone response genes in rat liver. Mol Endocrinol. 2008;22(8):1962‐1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Conforto TL, Waxman DJ. Sex-specific mouse liver gene expression: genome-wide analysis of developmental changes from pre-pubertal period to young adulthood. Biol Sex Differ. 2012;3(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Della Torre S, Mitro N, Meda C, et al. Short-term fasting reveals amino acid metabolism as a major sex-discriminating factor in the liver. Cell Metab. 2018;28(2):256‐267.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ. 2015;6(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Duma D, Collins JB, Chou JW, Cidlowski JA. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Sci Signal. 2010;3(143):ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Grøntved L, John S, Baek S, et al. C/EBP maintains chromatin accessibility in liver and facilitates glucocorticoid receptor recruitment to steroid response elements. EMBO J. 2013;32(11):1568‐1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Greco CM, Koronowski KB, Smith JG, et al. Integration of feeding behavior by the liver circadian clock reveals network dependency of metabolic rhythms. Sci Adv. 2021;7(39):eabi7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. 2006;86(2):465‐514. [DOI] [PubMed] [Google Scholar]
- 60. Komatsu M, Kanda T, Urai H, et al. NNMT activation can contribute to the development of fatty liver disease by modulating the NAD (+) metabolism. Sci Rep. 2018;8(1):8637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Roos AB, Nord M. The emerging role of C/EBPs in glucocorticoid signaling: lessons from the lung. J Endocrinol. 2012;212(3):291‐305. [DOI] [PubMed] [Google Scholar]
- 62. McCarthy MM, Arnold AP, Ball GF, Blaustein JD, Vries GJD. Sex differences in the brain: the not so inconvenient truth. J Neurosci. 2012;32(7):2241‐2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Rev. 2000;21(1):55‐89. [DOI] [PubMed] [Google Scholar]
- 64. Quagliarini F, Mir AA, Balazs K, et al. Cistromic reprogramming of the diurnal glucocorticoid hormone response by high-fat diet. Mol Cell. 2019;76(4):531‐545.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. BasuRay S, Wang Y, Smagris E, Cohen JC, Hobbs HH. Accumulation of PNPLA3 on lipid droplets is the basis of associated hepatic steatosis. Proc Natl Acad Sci U S A. 2019;116(19):9521‐9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jenkins CM, Yang J, Sims HF, Gross RW. Reversible high affinity inhibition of phosphofructokinase-1 by acyl-CoA: a mechanism integrating glycolytic flux with lipid metabolism. J Biol Chem. 2011;286(14):11937‐11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Germain A, Ruppert D, Levine SM, Hanson MR. Metabolic profiling of a myalgic encephalomyelitis/chronic fatigue syndrome discovery cohort reveals disturbances in fatty acid and lipid metabolism. Mol Biosyst. 2017;13(2):371‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hoel F, Hoel A, Pettersen IK, et al. A map of metabolic phenotypes in patients with myalgic encephalomyelitis/chronic fatigue syndrome. JCI Insight. 2021;6(16):e149217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Naviaux RK, Naviaux JC, Li K, et al. Metabolic features of chronic fatigue syndrome. Proc Natl Acad Sci U S A. 2016;113(37):E5472‐E5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Van Zaane B, Nur E, Squizzato A, et al. Hypercoagulable state in Cushing's syndrome: a systematic review. J Clin Endocrinol Metab. 2009;94(8):2743‐2750. [DOI] [PubMed] [Google Scholar]
- 72. Wang X, Rao J, Tan Z, Xun T, Zhao J, Yang X. Inflammatory signaling on cytochrome P450-mediated drug metabolism in hepatocytes. Front Pharmacol. 2022;13:1043836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Klein K, Winter S, Turpeinen M, Schwab M, Zanger UM. Pathway-targeted pharmacogenomics of CYP1A2 in human liver. Front Pharmacol. 2010;1:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sakai N, Saito K, Kim H-S, et al. Importance of CYP2D3 in polymorphism of diazepam p-hydroxylation in rats. Drug Metab Dispos. 2005;33(11):1657‐1660. [DOI] [PubMed] [Google Scholar]
- 75. Teh LK, Bertilsson L. Pharmacogenomics of CYP2D6: molecular genetics, interethnic differences and clinical importance. Drug Metab Pharmacokinet. 2012;27(1):55‐67. [DOI] [PubMed] [Google Scholar]
- 76. Xie Y, Xie W. The role of sulfotransferases in liver diseases. Drug Metab Dispos. 2020;48(9):742‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Manousaki D, Dudding T, Haworth S, et al. Low-frequency synonymous coding variation in CYP2R1 has large effects on vitamin D levels and risk of multiple sclerosis. Am J Hum Genet. 2017;101(2):227‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhu JG, Ochalek JT, Kaufmann M, Jones G, DeLuca HF. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc Natl Acad Sci U S A. 2013;110(39):15650‐15655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Reddy AB, Maywood ES, Karp NA, et al. Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology. 2007;45(6):1478‐1488. [DOI] [PubMed] [Google Scholar]
- 81. Pendergast JS, Niswender KD, Yamazaki S. Tissue-specific function of Period3 in circadian rhythmicity. PLoS One. 2012;7(1):e30254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhen Y, Xi Z, Hu L, et al. Impacts of circadian gene Period2 knockout on intestinal metabolism and hepatic antioxidant and inflammation state in mice. Oxid Cell Med Longev. 2022;2022:7896371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Brewer M, Lange D, Baler R, Anzulovich A. SREBP-1 as a transcriptional integrator of circadian and nutritional cues in the liver. J Biol Rhythms. 2005;20(3):195‐205. [DOI] [PubMed] [Google Scholar]
- 84. Quinn MA, Cidlowski JA. Endogenous hepatic glucocorticoid receptor signaling coordinates sex-biased inflammatory gene expression. FASEB J. 2016;30(2):971‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kroon J, Pereira AM, Meijer OC. Glucocorticoid sexual dimorphism in metabolism: dissecting the role of sex hormones. Trends Endocrinol Metab. 2020;31(5):357‐367. [DOI] [PubMed] [Google Scholar]
- 86. Ruiz D, Padmanabhan V, Sargis RM. Stress, sex, and sugar: glucocorticoids and sex-steroid crosstalk in the sex-specific misprogramming of metabolism. J Endocr Soc. 2020;4(8):bvaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Palmisano BT, Zhu L, Stafford JM. Role of estrogens in the regulation of liver lipid metabolism. Adv Exp Med Biol. 2017;1043:227‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cummins CL, Volle DH, Zhang Y, et al. Liver X receptors regulate adrenal cholesterol balance. J Clin Invest. 2006;116(7):1902‐1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kharb S, Garg MK, Puri P, et al. Assessment of adrenal function in liver diseases. Indian J Endocrinol Metab. 2013;17(3):465‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Marik PE, Gayowski T, Starzl TE. The hepatoadrenal syndrome: a common yet unrecognized clinical condition. Crit Care Med. 2005;33(6):1254‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. van der Geest R, Ouweneel AB, van der Sluis RJ, Groen AK, Van Eck M, Hoekstra M. Endogenous glucocorticoids exacerbate cholestasis-associated liver injury and hypercholesterolemia in mice. Toxicol Appl Pharmacol. 2016;306:1‐7. [DOI] [PubMed] [Google Scholar]
- 92. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264‐1273.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. 2017;34(6):1291‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang Y, Klein K, Sugathan A, et al. Transcriptional profiling of human liver identifies sex-biased genes associated with polygenic dyslipidemia and coronary artery disease. PLoS One. 2011;6(8):e23506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rando G, Wahli W. Sex differences in nuclear receptor-regulated liver metabolic pathways. Biochim Biophys Acta Mol Basis Dis. 2011;1812(8):964‐973. [DOI] [PubMed] [Google Scholar]
- 96. Robinson PA, Langley MS, Hammond GL. A solid-phase radioimmunoassay for human corticosteroid binding globulin. J Endocrinol. 1985;104(2):259‐267. [DOI] [PubMed] [Google Scholar]
- 97. Avvakumov GV, Hammond GL. Substitutions of tryptophan residues in human corticosteroid-binding globulin: impact on steroid binding and glycosylation. J Steroid Biochem Mol Biol. 1994;49(2-3):191‐194. [DOI] [PubMed] [Google Scholar]
- 98. Bolton JL, Hayward C, Direk N, et al. Genome wide association identifies common variants at the SERPINA6/SERPINA1 locus influencing plasma cortisol and corticosteroid binding globulin. PLoS Genet. 2014;10(7):e1004474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Brunner E, Baima J, Vieira TC, Vieira JG, Abucham J. Hereditary corticosteroid-binding globulin deficiency due to a missense mutation (Asp367Asn. CBG Lyon) in a Brazilian kindred. ClinEndocrinol(Oxf). 2003;58(6):756‐762. [DOI] [PubMed] [Google Scholar]
- 100. Cizza G, Bernardi L, Smirne N, et al. Clinical manifestations of highly prevalent corticosteroid-binding globulin mutations in a village in southern Italy. J Clin Endocrinol Metab. 2011;96(10):E1684‐E1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Doe RP, Lohrenz FN, Seal US. Familial decrease in corticosteroid-binding globulin. Metab Clin Exp. 1965;14(8):940‐943. [DOI] [PubMed] [Google Scholar]
- 102. Robinson PA, Hammond GL. Identification and characterization of a human corticosteroid binding globulin variant with a reduced affinity for cortisol. J Endocrinol. 1985;104(2):269‐277. [DOI] [PubMed] [Google Scholar]
- 103. Torpy DJ, Lundgren BA, Ho JT, Lewis JG, Scott HS, Mericq V. CBG Santiago: a novel CBG mutation. J Clin Endocrinol Metab. 2012;97(1):E151‐E155. [DOI] [PubMed] [Google Scholar]
- 104. Van Baelen H, Brepoels R, De Moor P. Transcortin Leuven: a variant of human corticosteroid-binding globulin with decreased cortisol-binding affinity. J Biol Chem. 1982;257(7):3397‐3400. [PubMed] [Google Scholar]
- 105. Verbeeten KC, Ahmet AH. The role of corticosteroid-binding globulin in the evaluation of adrenal insufficiency. J Pediatr Endocrinol Metab. 2018;31(2):107‐115. [DOI] [PubMed] [Google Scholar]
- 106. Meyer EJ, Nenke MA, Rankin W, Lewis JG, Torpy DJ. Corticosteroid-binding globulin: a review of basic and clinical advances. Horm Metab Res. 2016;48(6):359‐371. [DOI] [PubMed] [Google Scholar]
- 107. Bernier J, Jobin N, Emptoz-Bonneton A, Pugeat MM, Garrel DR. Decreased corticosteroid-binding globulin in burn patients: relationship with interleukin-6 and fat in nutritional support. Crit Care Med. 1998;26(3):452‐460. [DOI] [PubMed] [Google Scholar]
- 108. Hill LA, Bodnar TS, Weinberg J, Hammond GL. Corticosteroid-binding globulin is a biomarker of inflammation onset and severity in female rats. J Endocrinol. 2016;230(2):215‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29(1):83‐98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repository listed in References.