Abstract
Cancer-associated cachexia occurs in 50% to 80% of cancer patients and is responsible for 20% to 30% of cancer-related deaths. Cachexia limits survival and treatment outcomes, and is a major contributor to morbidity and mortality during cancer. Ovarian cancer is one of the leading causes of cancer-related deaths in women, and recent studies have begun to highlight the prevalence and clinical impact of cachexia in this population. Here, we review the existing understanding of cachexia pathophysiology and summarize relevant studies assessing ovarian cancer–associated cachexia in clinical and preclinical studies. In clinical studies, there is increased evidence that reduced skeletal muscle mass and quality associate with worse outcomes in subjects with ovarian cancer. Mouse models of ovarian cancer display cachexia, often characterized by muscle and fat wasting alongside inflammation, although they remain underexplored relative to other cachexia-associated cancer types. Certain soluble factors have been identified and successfully targeted in these models, providing novel therapeutic targets for mitigating cachexia during ovarian cancer. However, given the relatively low number of studies, the translational relevance of these findings is yet to be determined and requires more research. Overall, our current understanding of ovarian cancer–associated cachexia is insufficient and this review highlights the need for future research specifically aimed at exploring mechanisms of ovarian cancer–associated cachexia by using unbiased approaches and animal models representative of the clinical landscape of ovarian cancer.
Keywords: ovarian cancer, cachexia, skeletal muscle, animal models, survival
Cachexia, a multifactorial and multiorgan comorbidity of cancer, negatively impacts quality of life, reduces treatment tolerance and drives higher morbidity and mortality rates (1-5). A major component of cachexia is the wasting of skeletal muscle tissue (often referred to as sarcopenia), which also correlates with poorer survival. Cachexia is generally underexplored, especially in a setting of ovarian cancer (OC), which is the deadliest of gynecologic malignancies, thereby representing a major gap in the understanding of this deadly syndrome. Despite difficulties in diagnosing changes in muscle mass due to ascites formation and bloating, sarcopenia plays a critical role as a prognostic factor for survival in patients with OC (6-8). Here we review the current understanding of OC-associated cachexia by summarizing the current clinical and preclinical literature and by highlighting pathophysiologic mechanisms and relevant signaling pathways involved in promoting the onset of this deadly condition.
Cachexia Epidemiology and Clinical Relevance
Definition, Diagnosis, and Treatment of Cancer-Associated Cachexia
Cachexia is a wasting condition associated with a variety of diseases such as cancer, chronic obstructive pulmonary disease, AIDS, and cystic kidney disease. In cancer, cachexia was originally defined as “a multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment” (9). Due to its multifactorial nature, cachexia can be difficult to diagnose, especially in individuals with cancer. In an attempt to fill this gap, a framework was established for diagnosing cachexia in subjects affected with cancer and was based on 3 criteria: (1) weight loss >5% over 6 months; (2) body mass index <20 and any degree of weight loss >2%; or (3) presence of sarcopenia (males <7.26 kg/m2, females <5.45 kg/m2) (9). Given the elevated average age of patients with cachexia and co-occurrence of obesity and/or large tumors that can hide changes in overall body weight and composition, use of measures such as skeletal muscle radiation attenuation, which also take into account muscle quality, are increasingly being applied as tools to interrogate aspects of skeletal muscle biology (10, 11) and contributed to establishing diagnostic criteria that could determine presence or absence of cachexia in patients, often retrospectively. The same measures could be used to assign a stage to the condition of cachexia, thereby leading to the distinguishing of stages such as precachexia, cachexia, and refractory cachexia (9). It is worth noting that, given the absence of reliable biomarkers for early diagnosis of cachexia, these stages are currently defined essentially on the basis of a patient's clinical history and prognosis. Research is underway to determine other metrics clinically available and feasible that may be informative for cachexia diagnosis and survival outcomes.
Despite recent progress, to date there are no approved therapeutic interventions for the treatment of cachexia, with the only exception of Japan, which approved the ghrelin receptor agonist anamorelin for use in certain cancers in 2020 (12). Recently, the American Society for Clinical Oncology provided evidence-based guidance for adults with advanced cancer, suggesting, in certain contexts, dietary counseling and progesterone or corticosteroid use to improve appetite or weight gain (13). However, given a lack of robust preclinical and clinical data, pharmacologic agents directly targeting molecular mechanisms of the condition are not recommended.
Prevalence, Mortality, Morbidity
It is estimated that cachexia affects over 80% of patients diagnosed with cancer worldwide, and as many as 20% to 30% of cancer-related deaths are directly attributed to the development of this syndrome (14). Based on traditional diagnosis, cachexia is more prevalent in certain cancer patient populations, including patients with pancreatic, lung, and colon cancer (15). Regardless of specific disease or cancer type, cachexia associates strongly with poor prognosis and reduced quality of life. In patients with cancer, cachexia also predicts worse response to chemotherapy, compounded by the fact that certain anticancer therapies independently exacerbate symptoms and mechanisms underlying the wasting condition (16-18). Despite recent progress in diagnosis and treatment, cancer-associated cachexia remains a major unmet medical need worldwide.
Phenotype and Mechanisms of Cachexia
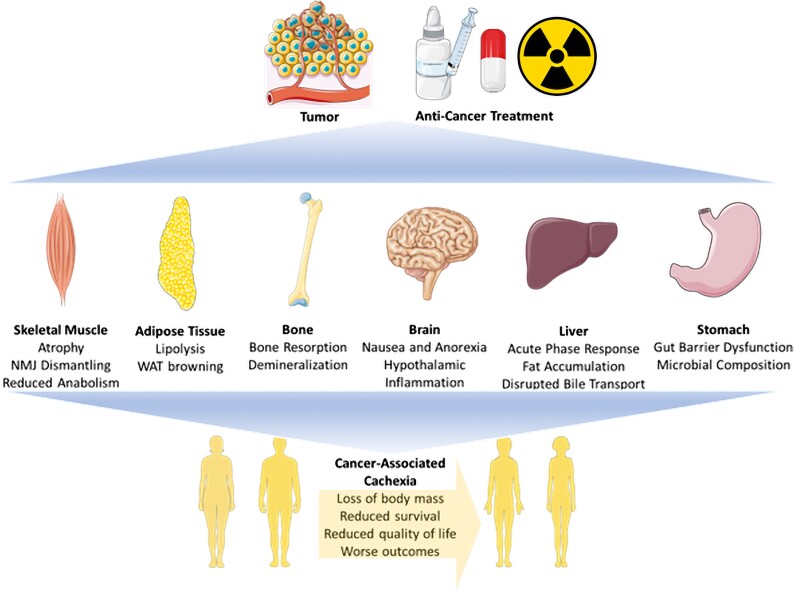
Traditionally, cachexia was characterized as a wasting syndrome mainly resulting from muscle and fat depletion. There is now general agreement on the idea that cachexia is rather a multifactorial and multiorgan syndrome that promotes systemic effects and impacts the entire body (Fig. 1). Here we summarize the phenotype of cachexia and highlight the underlying pathophysiologic mechanisms.
Figure 1.
A summary of the cachexia phenotype. Cachexia is induced during cancer by tumor burden and anticancer therapy, impacting a variety of tissues including skeletal muscle, adipose tissue, bone, brain, liver, and stomach. In these tissues, various structures and functions are deregulated, generating the cachectic phenotype. This figure was created using BioRender.com
Skeletal Muscle
Skeletal muscle remains a primary focus of cancer-associated cachexia research since muscle mass, quality, and function can all determine outcomes such as response to therapy and even overall survival. Indeed, cachexia leads to several derangements in skeletal muscle, including increased inflammation, disrupted metabolism, dismantling of neuromuscular junctions, fibrosis, intramuscular fat accumulation, and skeletal muscle atrophy which precipitate dysfunction. Gene expression analysis of skeletal muscle during cachexia reveals increased expression of a variety of genes involved in protein catabolism including the ubiquitin-mediated proteasome (UPS) and autophagy–lysosome system, consequently termed atrogenes (19).
Historically, UPS has been recognized as the main driver of muscle protein degradation and involves ubiquitin-activating enzyme, ubiquitin-conjugating enzyme, ubiquitin ligase, protease, and their substrates. Muscle-specific ubiquitin ligase ligases Atrogin-1 and MuRF1 have been shown to be strongly involved in skeletal muscle atrophy in patients and preclinical cancer-associated cachexia models. These atrogenes are promoted by several signaling pathways including signal transducer and activator of transcription 3 (STAT3), nuclear factor kappa-light-chain-enhancer of activated B cells, fork head box O (FOXO), mothers against decapentaplegic 2/3 (SMAD2/3), and mitogen-activated protein kinase cellular signaling (14, 20-24). These pathways are stimulated by a variety of factors including interleukin (IL)-1) (25-27), IL-6 (28-35), IL-8 (21, 36-39), and tumor necrosis factor alpha (TNFα) (40, 41), which may be released by the tumor itself or by the host in response to the tumor.
Autophagy is another mechanism utilized for removal of damaged organelles and dysfunctional proteins, making it critical for normal tissue homeostasis. During cancer-associated cachexia, this system is often overactive in skeletal muscle and along with several major signaling pathways including FOXO, mammalian target of rapamycin (mTOR), p38, SMAD1/5/8, and mitogen-activated protein kinase (42). FOXO3 is suggested to be a critical regulator of autophagy during cancer-associated cachexia given its inhibitory effects on phosphoinositide 3-kinase/AKT (Protein Kinase B/PKB) signaling and well-documented overexpression in skeletal muscle during muscle wasting (43).
While protein catabolism is stimulated in muscle during cancer-associated cachexia, anabolism is often inhibited through several distinct mechanisms. One of the main pathways involved in anabolism is mTOR and adenosine monophosphate–activated protein kinase signaling. mTOR contains 2 distinct complex mTORC1 and mTORC2. mTORC1 regulates protein anabolism and is critical for muscle autophagy (44). mTORC1 also stimulates mitochondrial biogenesis by activating eukaryotic translation initiation factor 4E binding protein 1 and increasing peroxisome proliferator-activated receptor gamma coactivator 1-alpha expression (45, 46). Inhibition or deletion of mTORC1 can lead to skeletal muscle wasting, although it appears dispensable for maintenance of skeletal muscle in adult animals (47, 48). In early stages of cancer-associated cachexia, protein synthesis is impaired, and this is correlated with reduced insulin-like growth factor (IGF1)–mTOR signaling (49). During late stages of cancer-associated cachexia, mTORC1 can be inhibited by IL-6 due to adenosine monophosphate–activated protein kinase activation (50). Despite this, activity of mTORC1 may contribute to sarcopenia since mTORC1 signaling is elevated in muscle during advanced age in both mice and humans (51). Further, overexpression of mTORC1 in skeletal muscle causes a sarcopenic molecular signature, suggesting it may contribute to muscle wasting that occurs with age (48). Therefore, mTORC1 could be a potential therapeutic target for treatment of age and/or cancer related muscle wasting.
During cancer-associated cachexia, IGF1 levels are often lower and skeletal muscle can become insulin resistant (52-55). IGF1 and insulin are factors that bind to the IGF1 receptor and activate phosphoinositide 3-kinases stimulating protein synthesis via AKT–mTOR. Further, activation in these pathways can diminish FOXO activity, reducing protein degradation (56-58). IGF1 is primarily produced by the liver, whereas insulin is primarily produced by the pancreas. Decreased insulin abundance is linked to reduced protein synthesis in cancer patients likely due to decreased glucose tolerance and insulin sensitivity (54).
The transforming growth factor β (TGFβ) superfamily of ligands is known to have both beneficial and detrimental effects on skeletal muscle size (59). For instance, compared with healthy controls bone morphogenic protein (BMP) was found to be reduced in patients with cancer and linked to reduced muscle mass. Factors that become elevated during cancer such as IL-6 and activin A can stimulate expression of BMP inhibitor Noggin in skeletal muscle (60). The reduction in BMP signaling in muscle and motor nerves was found to cause disruptions at the neuromuscular junction, denervation, and eventually muscle wasting, which we reported in models of cancer- and chemotherapy-driven cachexia (61). BMP7 also has similar functions where its overexpression contributes to AKT–mTOR activation and inhibition can stimulate muscle wasting (62, 63). Both BMP and BMP7 activate Smad1/5/8 signaling. Conversely, myostatin and Activin A are also TGFβ family members and are negative regulators of muscle growth (63-66). Deletion of myostatin causes muscle growth and overexpression leads to skeletal muscle atrophy (63, 65). Both myostatin and activin A stimulate Smad2/3 signaling by engaging with activin receptor type 2A. Therefore, this family of proteins exerts both hypertrophic and atrophic signaling and regulating TGFβ family signaling has shown promise as a countermeasure to muscle wasting during cancer.
Adipose Tissue
Adipose tissue wasting and browning of white adipose tissue (WAT) occur alongside muscle wasting during cancer-induced cachexia (67). Adipose tissue can be divided into 2 distinct types, WAT and brown adipose tissues. WAT browning promotes lipolysis and alters the secretome of adipose depots to induce protein catabolism (68). During WAT browning, uncoupling protein 1 is upregulated which increases thermogenesis leading to elevated energy expenditure (69, 70). Lipolysis usually occurs prior to WAT browning in cancer-associated cachexia and both processes are enhanced by inflammatory factors (eg, IL-6) (34, 35, 71).
Bone
The musculoskeletal system is compromised during cancer, and new research highlights the role of bone remodeling in cancer-associated cachexia where it contributes to muscle wasting, frailty, and risk of injury (72-74). In fact, given the close proximity of muscle and bone, numerous myokines and osteokines have been identified that contribute to crosstalk between bone and muscle (75). Cancers that metastasize to the bone induce muscle weakness due to bone-derived factors (76). Interestingly, cancers without bone metastasis can cause bone weakness and remodeling (5, 77, 78). Several bone-derived molecules, termed osteokines, have been shown to exert catabolic and anabolic impact on skeletal muscle. For instance, bone-derived Ihh and osteocalcin have been shown to have positive impacts on skeletal muscle size and function whereas TGFβ, activins, FGF23, and FGF12 drive muscle dysfunction. Alternatively, muscle-derived factors, termed myokines, have similar impacts. For instance, IGF1 and FGF2 produced by the muscle can stimulate bone formation (75). Certain factors, including receptor activator of nuclear factor kappa beta ligand (RANKL), from the tumor can also impact both muscle and bone further dysregulating their relationship whose contributions to muscle and bone dysfunctions in cancer will be discussed later in this review (79).
Gut Dysfunction
The gastrointestinal system and microbial communities that inhabit it are strongly affected during cancer and can contribute to the onset of cachexia. This is proposed to occur through several mechanisms including gut barrier dysfunction and alterations to microbiome composition (80, 81). Indeed, gut barrier dysfunction has been detailed in several preclinical cancer-associated cachexia models likely due to disruptions of tight junction, increased gut permeability, and local immunosuppression (67, 82-84). Consequently, gut microbiota is likely a source for Inflammation during cancer due to dislocation of pathogen associated molecular patterns. Interestingly, lipopolysaccharide can influence muscle cell catabolism via Toll Like Receptor 4. Finally, the microbiome composition can change during cancer leading to outgrowth of opportunistic bacteria such as Klebsiella oxytoca (85). Microbial composition has been reported to be altered in several models of cancer-associated cachexia and there is some evidence in mouse models that prebiotics and probiotics may be useful at preventing muscle and fat wasting while lengthening cancer survival and this was attributed to modulation of the gut microbiota composition (86).
Brain and Anorexia
The brain is a critical mediator of systemic health and exerts many functions that regulate systemic metabolism. Hypothalamic inflammation has been shown to drive lost appetite or nausea that results in lower food intake or anorexia (87). Anorexia is a major comorbidity of cachexia in cancer and contributes to the systemic energy deficit that stimulates wasting (88). The hypothalamus controls appetite by integrating signals from various organs in the body with hypothalamic centers. There are 2 different neuronal populations that mediate appetite including the anorexigenic pro-opiomelanocortin and cocaine and amphetamine-regulated transcript expressing neurons, as well as orexigenic neuropepetide Y/agouti-related peptide neurons (89). For instance, ghrelin, a gut-derived hormone that has been widely investigated as a therapeutic target, can increase appetite by suppressing pro-opiomelanocortin and stimulating neuropepetide Y/agouti-related peptide neurons (90). During cancer-associated cachexia the orexigenic axis is dysfunctional and anorexigenic axis is overstimulated leading to appetite suppression (91). Although decreased food intake is an important feature of cachexia, clinical trials have demonstrated that nutritional interventions to address underlying anorexia in cancer patients was insufficient to reverse the wasting (13). The hypothalamus also has major roles in regulation of energy expenditure and a large portion of cancer patients have elevated resting energy expenditure likely due to inflammatory factors such as TNFα that contribute to muscle and fat wasting (92). The hypothalamic pituitary is also disrupted during cancer by proinflammatory factors leading to an increase in glucocorticoid production by the adrenal gland. Glucocorticoids are known to be elevated during cancer and directly cause skeletal muscle wasting (92-94).
Liver
The liver is a major metabolic organ that is impacted by tumor burden and can contribute to cancer-associated cachexia through several mechanisms. Many proinflammatory molecules upregulated due to tumor burden (eg, serum amyloid A, C-reactive protein, fibrinogen) and involved in activation of acute phase response (APR) (95) are known to influence inflammatory signaling in the liver leading to lost insulin and glucose homeostasis (96, 97), circadian homeostasis (98), fat accumulation (99), and bile transport (100), further disrupting the tissues function. The liver is also responsible for acute phase response to tissue injury and inflammation. Muscle breakdown to free amino acids to facilitate APR activity results in lower muscle mass and this is thought to be mediated by IL-6-STAT3 (95). Activation in APR therefore results in increased resting energy expenditure. Ketogenesis has also been reported to be aberrant in livers from cancer-associated cachexia models (101, 102). Ketones are produced by the liver and can be used for energy generation in a variety of tissues including skeletal muscle. Low levels of ketones have been reported in cancer models and are thought to contribute to the systemic energy imbalance and elevated glucocorticoid signaling, both of which worsen muscle atrophy by stimulating catabolic signaling and inhibiting anabolic signaling (103).
Sex Organs and Hormones
Differences between male and females including body composition, fat distribution, metabolism, and hormones have been reported in both humans and animals. It was reported that muscle depletion is more prevalent in male vs female cancer patients (104). These male patients also demonstrate higher fatigue and greater reduction in force than female patients with cancer. This is potentially due to estrogen levels in females. Estrogen has been shown to regulate muscle function and anabolism (105). Estrogen can also impact the hypothalamus resulting in altered physical activity and resting energy expenditure (106). Hormone replacement therapy can actually mitigate wasting in postmenopausal women (107). In contrast, testosterone is an anabolic agent, although testosterone can improve lean body mass but not impact overall survival in patients (108, 109). Also, certain treatments are only effective in male or female mice with cancer, and this suggests that sex may be a critical consideration for the treatment of cachexia (110). Notably, most preclinical cachexia data is produced in male mice, thereby prompting for future studies investigating the role of sex and hormone activity in cancer-associated cachexia.
Chemotherapy
While cancer itself can cause cachexia, certain anticancer treatments also drive muscle and fat wasting, acute sickness behavior, and elevate levels of inflammatory factors. Chemotherapies, in particular, are known for their dose-limiting off-target effects that complicate their clinical utility including nausea, nephrotoxicity, fatigue, and weakness. Studies have identified the mechanism responsible for chemotherapy-induced muscle, fat, and/or bone wasting. Interestingly, many mechanisms responsible for these processes in cancer seem to also contribute to chemotherapy-induced cachexia. In such regard, we and others have shown that routinely administered chemotherapeutics can drive occurrence and sustainment of a cachexia-like state, along with loss of muscle mass and function (16, 111), and can overall exacerbate cachexia in cancer hosts (17). Weight instability is also a major component of patient assessment, and failure to maintain weight often results in less treatment options and reduced treatment capacity (112). This further highlights the necessity for generating treatments that can protect against cancer and cancer-therapy induced wasting to improve overall survival and quality of life for people with cancer.
Cachexia in Ovarian Cancer
Ovarian Cancer Epidemiology and Treatment
OC comprises a group of diseases that can originate in the ovaries, within the fallopian tubes, or the peritoneum. OC is the fifth leading cause of cancer-related deaths in women and the leading cause of cancer-related deaths for all gynecologic malignancies. In the United States, it is estimated that 19 710 new cases of OC will be diagnosed, whereas 13 270 women will die from the disease in 2023 (113). OC most often affects postmenopausal women, and the majority of OCs are diagnosed at advanced stages, therefore leading to disease recurrence, chemoresistance, and poor outcomes (114). The overall 5-year survival rate for OC from 2013 to 2019 is 50.8%, though it strongly depends on the stage of disease at diagnosis and is dramatically influenced by its progression (113). While women affected with localized (∼18% of cases) or regional disease (∼20% of new diagnoses) present with 92.4% and 72.9% survival rates, respectively, in the case of distant, metastatic disease (making up to 55% of new cases, often consequential to late diagnosis and to the onset of chemoresistance) survival rates are drastically lower at 31.5% (113). Epithelial OC represents about 90% of new diagnoses and the majority are considered high-grade serous carcinoma (115), being characterized by near ubiquitous TP53 mutations, DNA repair deficiencies, high propensity for chromosomal copy numbers, and a worse prognosis (116, 117). The standard of care for OC regardless of stage involves the administration of platinum-based (eg, carboplatin, cisplatin) and taxane-based (eg, paclitaxel, docetaxel) drugs (118, 119). These chemotherapies are often given as a combination therapy and can be delivered by intravenous or intraperitoneal injection. Intraperitoneal injection can improve outcomes but also exacerbates treatment related toxicity and is not recommended unless tumors are optimally debulked (120, 121).
Clinical Assessment of Cachexia in Ovarian Cancer
Diagnosis of cachexia in OC has long been neglected and/or understudied due to the typical presentation of the disease, associated with ascites formation and bloating, often masking the underlying weight loss and muscle condition. However, recent data from clinical and preclinical research is now highlighting the burden of cachexia also in patients with OC (6-8). Despite limitations associated with the low number of studies assessing cachexia and/or sarcopenia, based on meta-analysis of clinical studies there is now general consensus on the idea that loss of skeletal muscle mass is a frequent feature accompanying the onset of OC (122-128) (Table 1).
Table 1.
Association of sarcopenia with survival in patients with ovarian cancer
| Reference info | Study inclusion | Sarcopenia diagnosis | Outcomes | |||||
|---|---|---|---|---|---|---|---|---|
| Author, year | OC stage | N | Age | Muscle mass assessed by CT scan at | Sarcopenic cut-off | % Sarcopenia | Survival | Notes |
| Ataseven et al, 2018 | III-IV | 323 | NR | L3 | SMI < 41 cm2/m2 | 29.40% | HR 1.14, P = .730 (OS) | |
| SMD < 32 HU | 21.1% | HR 1.32, P = .003 (OS) | ||||||
| Aust et al, 2015 | III-IV | 140 | 60 | L3 | SMA < 39 HU | 35% | HR 2.25; P = .028 (OS) | Eotaxin and IL-10 correlate with low SMA |
| SMI < 41 cm2/m2 | 28.90% | HR 1.23; P = .565 (OS) | ||||||
| Bronger et al, 2017 | III-IV | 105 | 63 | L3 | SMI 38.5 cm2/m2 | 11.40% | HR 3.17; P = .012 (OS) | Change in muscle mass over time not associated with survival |
| HR 2.64; P = .012 (PFS) | ||||||||
| Bruno, 2021 | I-IV | 239 | NR | L3 | SMI < 38.9 cm2/m2 | 35.10% | HR 1.2, P > .05 | |
| SMD < 21.24 HU | 50% | HR 2.66, P < .05 | ||||||
| Chae et al, 2021 | I-II | 82 | 52 | L3 | SMI ≤ 38.7 cm2/m2 | 20.7 | HR 58.4; P = .0008 (OS) | BMI not associated with survival |
| Huang et al, 2020 | III | 139 | 54.4 | L3 | SMI 39.0 cm2/m2 | 34.10% | HR 1.08; P = .002 (OS) | SMI loss associates with worse OS and PFS |
| HR 1.03; P = .04 (PFS) | ||||||||
| Grande et al, 2021 | III-IV | 69 | 63.6 | L3 | SMI < 41.0 cm2/m2 | 29% | HR .95; P > .05 (OS) | SMA was associated with early discontinuation of chemotherapy |
| Kim et al, 2020 | III-IV | 179 | 57.5 | L3 | SMI < 39.0 cm2/m2 | 42.50% | HR .87; P = .636 (OS) | High fat to muscle ratio showed significantly worse OS |
| HR 1.29; P = .157 (PFS) | ||||||||
| Kumar et al, 2016 | III-IV | 296 | 64.6 | L3 | SMI < 39 cm2/m2 | 44.60% | HR .99, P = .97 (PFS) | |
| SMD < 36.4 HU | NR | HR 1.26, P = .0009 (PFS) | ||||||
| Matsubara et al, 2019 | III-IV | 92 | 55.3 | L3 | SMA < 92.92 cm2 | 50% | HR 2.186; P = .030 (OS) | Psoas major volume was superior to SMA for prognosis prediction |
| HR 1.272; P = .402 (PFS) | ||||||||
| Nakayama et al, 2019 | III-IV | 94 | 61.8 | L3 | SMI 30.88 cm2/m2 | 77% | P = .337 (OS) | |
| Rutten et al, 2017 | II-IV | 216 | 63.1 | L3 | SMI 38.73 cm2/m2 | 32.40% | HR 1.36; P = .076 | Low SMI shows trend towards association with poor OS |
| Rutten et al, 2016 | III-IV | 123 | 66.5 | L3 | SMI 41.5 cm2/m2 | 50.40% | HR .89; P = .613 | Loss of SM during chemotherapy associates with worse OS |
| Staley et al, 2020 | III-IV | 201 | 63.6 | L3 | SMI ≤ 41cm2/m2 | 64% | P > .05 (OS) | Trend toward worse neutropenia was noted in the sarcopenic group |
| P > .05 (PFS) | ||||||||
| Ubachs et al, 2020 | III | 212 | 60.9 | L3 | SMI > 2%/100 days | 58% | HR 1.41; P > .05 | Patients with SMI loss demonstrate more perioperative adverse events |
| Wood et al, 2023 | II-IV | 174 | 64.1 | L4 | SMI 38.0 cm2/m2 | 55.70% | P = .95 (OS) | > 2% decrease in VAT per 100 days was significantly associated with a decreased OS |
| P = .68 (PFS) | ||||||||
| Yoshikawa et al, 2021 | I-IV | 72 | 62 | L5 | PMI < 5.4 cm2/m2 | NR | HR 3.87; P = .0098 (OS) | |
| Yoshino et al, 2020 | I-IV | 60 | 63.5 | L3 | SMI 39.0 cm2/m2 | 60% (pre), 68% (post) | HR 3.17; P = .022 (OS) | Post-IC neutrophil count correlates with SMI |
Clinical retrospective and prospective studies were collected that looked at associations of OS or PFS with sarcopenia defined by SMI, SMD, SMA, or PMI. Multivariate statistics were reported when available.
Abbreviations: IC, immune complex; NR, not recorded; OS, overall survival; PFS, progression-free survival; PMI, psoas muscle index; SMA, skeletal muscle attenuation; SMD, skeletal muscle density; SMI, skeletal muscle index.
Symptoms associated with cachexia such as physical frailty, weakness, and early satiety are frequently reported by patients with OC. Further, a major component of cachexia is sarcopenia, which is muscle wasting and dysfunction that can occur independent of age and is easily assessed using computed tomography (CT)-based metrics, as we recently showed in a cohort of women affected with OC (79). The impact of sarcopenia on survival and treatment outcomes in OC is still partially controversial, with recent meta-analyses reaching different conclusions (122, 123, 128, 129), whereas other studies suggest that skeletal muscle wasting correlates with overall survival and progression free survival especially in patients with advanced disease (130-138). Some discrepancies in defining inclusion criteria and skeletal muscle condition may explain misalignment in conclusions, as in the case of recent studies, which failed to see similar associations between sarcopenia and survival outcomes (139-146). The data is further complicated by the varied definitions used to clinically define sarcopenia. Indeed, different CT-based metrics have been utilized such as skeletal muscle index (SMI), skeletal muscle density (SMD), psoas muscle index (PMI), and skeletal muscle attenuation (SMA), thus contributing to generating confusion. It is also important to note that body mass index, which is often used for the diagnosis of cachexia, does not always correlate well with markers of skeletal muscle loss and should be utilized with caution (131, 133). Current evidence also suggests a negative impact of obesity on cancer progression, though this is still being debated in the case of OC, where body composition, and in particularly the presence of high visceral adiposity (with or without sarcopenia) was found to associate with worse survival (147). The staging of OC may also be important to look at association of sarcopenia with outcomes, since pooling of higher stage studies (III-IV) was found to identify higher risk of mortality linked to low SMI (128).
Of note, several studies also highlighted considerable heterogeneity in treatment outcomes related to the extent of sarcopenia (8, 130, 134, 138, 141, 148, 149), mainly as a consequence of the fact that the loss of skeletal muscle was assessed at different stages of the disease relative to the start of anticancer therapy (eg, prior, after, or during surgery and/or chemotherapy). Nonetheless, the currently available data suggests that the loss of skeletal muscle during surgery and chemotherapy is an important indicator of outcomes in OC (134, 150). In particular, patients with OC with sarcopenia were found to be more likely to receive neoadjuvant chemotherapy (ie, chemotherapy prior to cytoreductive surgery) with cytoreduction instead of primary cytoreductive surgery alone, and this has been shown to reduce morbidity and mortality following treatment (151, 152). Similarly, a meta-analysis of 35 articles involving 6894 patients also showed that sarcopenia was present in ∼39% of patients with OC prior to initiation of chemotherapy treatments, and it was significantly and independently associated with postoperative complications, chemotherapy-induced toxicity and poor survival (153).
Lastly, systemic inflammation, despite being well known as a major driver of muscle wasting during cancer, was assessed in relation with sarcopenia in only a modest number of OC studies, though a recent one identified a clear association between malignant ascites and muscle loss after primary debulking surgery and adjuvant chemotherapy in advanced-stage OC (79, 131, 154). In a recent study, we showed that RANKL (Tnfsf11), a TNF superfamily member and master regulator of bone remodeling normally responsible for osteoclast differentiation and osteoclastic bone resorption (155), was elevated in the blood of 31 women with OC compared with women affected with benign gynecologic conditions, consistently with increases in circulating levels of C-telopeptide of type I collagen, a marker of bone turnover. Interestingly, RANKL expression is aberrantly expressed in many solid tumors, including breast, prostate, liver, gastric and ovarian (156-159), and was described to correlate with poorer clinical outcomes in patients with OC (159). Consistently, in our report we observed increased expression of RANKL in different OC histological types and showed that patients with OC with low RANKL–expressing tumors correlated with improved overall survival.
Cachexia in Preclinical Models of Ovarian Cancer
Preclinical and basic cancer-associated cachexia research has historically relied on certain tumor models that recapitulate the wasting phenotype in mice. Much of our molecular understanding of cancer-associated cachexia is gleaned from these models, which have predominately looked at colorectal, lung, and pancreatic cancer models, usually in male mice only. Because of this, research related to the OC field remains inadequate, particularly due to the limited availability of optimal preclinical models that are characterized in terms of muscle and bone phenotype or function. Indeed, until recent, only 1 OC xenograft, the mouse bearing the TOV-21G clear cell carcinoma cell line, a distinct pathologic subtype of epithelial OC only accounting for less than 5% of all ovarian malignancies, had been described and partially characterized for cancer-associated effects on skeletal muscle (160).
To date, new OC mouse models have emerged that can be utilized to shed light on mechanisms and potential targets for therapy (Table 2). We were the first to show that implantation of ES-2 OC cells caused ascites accumulation in the peritoneal cavity, accompanied by severe cachexia, as suggested by marked body weight loss, as well as by concurrent muscle and bone wasting (5, 79). It is important to note that although the ES-2 cells were originally classified as ovarian clear cell carcinoma (161), their genomic profiling followed by comparison with the most extensively studied high grade serous OC (HGS-OC) histotypes (eg, SK-OV-3, OVCAR-3, CAOV-3) recently led to reclassification of the ES-2 cells as a suitable model for the study of HGS-OC (162, 163). Our model also demonstrated that cachexia-associated factors such as IL-6 were elevated in plasma and, at a greater extent, in ascites (5, 79). Interestingly, ascites fluid accumulation has been suggested to be a contributing factor to cachexia in OC, potentially by causing early satiety and driving elevated levels of inflammatory factors (23). In a recent study, patient ascites was used to treat C2C12 myotubes in vitro. Myotubes demonstrated reduced protein synthesis in response to patient ascites, and this reduction correlated with SMI assessment in patients with OC. These data also suggest that ascites itself can promote the appearance of sarcopenia potentially by reducing protein synthesis directly in skeletal muscle (164).
Table 2.
Summary of preclinical ovarian cancer cachexia studies
| Cell culture | Animals | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | OC cell lines | Phenotype | Biomarkers | Outcomes | N | Mouse strain | Tumor burden (days) | cell # | Biomarkers | Muscle wasting | Muscle pathways (RNA) | Muscle pathways (WB) | Main finding |
| Kim-Muller et al, 2023 | TOV-21G | NA | NA | NA | 12-15 | BALB/C | 48 | 5 × 106 SC | ↑ GDF15 | ↓ Lean and Hindlimb mass, ↓ TA and Gast CSA | ↑ Atrogin-1, MuRF-1 | NA | GDF15 Neutralization attenuates muscle wasting and dysfunction |
| Pin et al, 2019 | ES2 | ↓ Myotube diameter | ↑ IL-6 | ↑ pSTAT3/STAT3 | 6-10 | NSG | 15 | 1 × 107 IP | ↑ IL-6 | ↓ TA, Gast, Quad, Heart mass; ↓ TA CSA | ↑ Atrogin-1, MuRF-1, Fbxo31 | ↑ p-P38, pSTAT3 | ES2 caused bone and fat wasting, IL-6 higher in ascites than plasma |
| ↓ OPA1, PGC1a, Cytochrome C | ↓ AKT1/2, OPA1, Mitofusin-2, Cytochrome-C | ||||||||||||
| Pin et al, 2021 | ES2 | ↓ Myotube Diameter | ↑ IL-6 and RANKL | 5-7 | NSG | 15 | 1 × 107 IP | ↑ IL-6 and RANKL | ↓ TA, Gast, Quad, Heart mass; ↓ TA CSA | ↑ Atrogin-1, MuRF-1 | NA | RANKL neutralization lessens bone and muscle wasting during ES2 tumor burden | |
| Straughn et al, 2020 | A2780 | NA | NA | NA | 10 | NSG | 28 | 8 × 105 IP | NA | ↓ Forelimb and Total grip strength, | ↓ MyoD, Ern1 (Quad) | ↓ P62 (Quad) | WFA attenuates muscle mass loss and activation in ALS and UPS signaling |
| ↓ TA mass and CSA, GA mass, QF Mass | ↑ RelA, IKKB, Pax7, EIF2a, Atf4, Ddit3, Ppplr15a, Hspa5, Hsp90b1, Tnfrsf10b; Traf6, Fbx030, Fbx032, Trim63, Sqstm1, Map1lc3b, and Becn1 | ↑ pP65, pERK, IRE1a, Ubiquitin LC3, Beclin1 (Quad) | |||||||||||
| Ubarchs et al, 2021 | ↓ Protein synthesis | ↑ IL-6 and IL-8 (ascites) | ↑ NF-kB | 15 | NA | NA | NA | ↑ IL-6 and IL-8 (ascites) | NA | ↑ NF-kB (C2C12) | NA | 6 sarcopenic patients showed significant, lower SMI which correlated to the loss of protein synthesis in the in vitro model. | |
| Yi Luan et al, 2022 | NA | NA | NA | NA | 6 | Pik3ca | 65 and 83 | NA | ↑ Activin A, GDF15, IL-6, IL-1B, TNFα | ↓ Lean mass, TA CSA, TA, Gast, and Quad mass | ↑ Murf1, Atrogin1, Lc3, Myogenin | ↑ FOXO3 and P38 phosphorylation | |
| ↓ Pax7 | |||||||||||||
| Zhou et al, 2010 | TOV-21G | NA | ↑ Activin A | NA | 12 | BALB/C | NA | 5 × 106 SC | ↑ Activin A | ↓ Lean and Gast mass | NA | NA | ActRIIB Antagonisms attenuates muscle wasting and improves survival |
Preclinical studies were compiled and summarized from various mouse models of cancer cachexia. Disrupted muscle phenotype and signaling pathways as well as tumor- and host-derived biomarkers were recorded.
Abbreviations: ALS, autophagy-lysosomal system; FOXO, fork head box O; IL, interleukin; IP, intraperitoneally; NA, not available; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cell; OC, ovarian cancer; PGC1, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; SC, subcutaneously; SMI, skeletal muscle index; STAT, signal transducer and activator of transcription; TNF, tumor necrosis factor; UPS, ubiquitin-mediated proteasome; WFA, withaferin A.
In a recent study employing the same model, we also showed that ES-2 OCs express high RANKL and that OC hosts present high circulating level of this factor, whereas its neutralization by means of anti-RANKL monoclonal antibodies was sufficient to mitigate muscle and bone decline in ES-2 tumor bearing mice (79). Altogether, these observations contributed to identifying RANKL as a novel therapeutic target for the treatment of musculoskeletal complications associated with nonbone metastatic OCs.
In a work by Luan et al genetically engineered mice presenting constitutive activation of phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (Pik3ca) were shown to develop OC accompanied by ascites formation and occurrence of cancer-associated cachexia identified by muscle and fat wasting. Interestingly, in this model elevated activin A and growth differentiation factor 15 (GDF15), known procachexiogenic factors (64, 165) were also observed in serum (166). This is not the first evidence that links OC growth with the onset of activin A- or GDF15-driven cachexia. Indeed, Zhou et al previously reported that growth of TOV-21G promotes progressive body and muscle weight loss, whereas administration of ACVR2B/Fc, an inhibitor of the activin receptor 2B signaling known to preserve muscle mass and prolong survival in tumor hosts and to increase muscle and bone mass following chemotherapy treatment (167), was shown to restore muscle mass in this model (160). The therapeutic potential deriving from targeting the activin-dependent signaling was further supported by evidence from Pettersen et al, showing a functional link between the activin A and IL-6 signaling pathways in causing cachexia in OC (168). In the same model, recent observations by Kim-Muller and colleagues (169) showed that treatment with anti-GDF15 antibody mAB2 promotes body weight gain with near-complete restoration of muscle mass and markedly improves muscle function and physical performance.
Using another model, Straughn et al reported that inoculation of ovarian surface epithelium cancer cell line A2780 into female mice lead to development of cachexia (170, 171), whereas treatment of A2780 tumor-bearing mice with Withaferin A, a steroidal lactone purified from the Withania somnifera plant known for its anti-inflammatory properties and inhibitory effects on cell proliferation and invasion of OC (172), was sufficient to ameliorate cachexia via nuclear factor kappa-light-chain-enhancer of activated B cells suppression (170). Interestingly, in a follow up study, Withaferin A was then demonstrated to cause muscle hypertrophy likely due to its regulation of myogenic progenitors and proteolytic systems in skeletal muscle (171).
Conclusions
Cancer-associated cachexia remains a substantially neglected area of research and represents a significant challenge for patients affected with cancer and for their caregivers, mainly because of poorer response to anticancer therapies, worsened quality of life and increased morbidity and mortality rates. While the mechanisms responsible for the onset of cachexia are generally well studied in pancreatic, lung, and colorectal cancers, less is known about the effects of cachexia in OC, which represents a leading causes of gynecologic cancer-related deaths worldwide.
As we reported herein, this is primarily a consequence of difficulties in diagnosing changes in muscle mass in subjects affected with OC, and mainly results from ascites formation and bloating, which ultimately contribute to masking the progressive decreases in skeletal muscle mass, strength and function. Due to recent advancements, CT scan imaging is becoming widely utilized to assess changes in skeletal muscle mass and has contributed to validating sarcopenia as a powerful indicator of poor clinical characteristics, chemotherapy response and adverse prognosis also in patients with OC.
Our knowledge of initiators and regulators of cachexia, as well as the availability of reliable cachexia biomarkers are currently insufficient and have led to very limited translatability, mainly because of the lack of optimal preclinical animal models. This is true especially for OC. Until recently, most of the available data related to OC cachexia were generated in mice bearing the TOV-21G, neglecting the fact that this cell line is representative of clear cell carcinoma, hence barely accounting for less than 5% of all OC histotypes (160), and possesses few copy number alterations and a “hypermutated” genome, which sets it apart from most OC cell lines and in particular from HGS-OC lines (162). Recent attempts by our group and others have led to developing more clinically relevant and representative animal models of OC and contributed to the identification of novel mediators of musculoskeletal wasting, including RANKL (5, 79).
Nonetheless, while cachexia researchers remain focused on the longer-term objective of developing effective and novel treatments for cachexia, further research is necessary to assess the true extent of OC cachexia and associated symptoms, in both preclinical and clinical settings.
Abbreviations
- APR
acute phase response
- BMP
bone morphogenic protein
- CT
computed tomography
- FOXO
fork head box O
- GDF
growth differentiation factor
- HGS
high grade serous
- IGF
insulin-like growth factor
- IL
interleukin
- mTOR
mammalian target of rapamycin
- OC
ovarian cancer
- PMI
psoas muscle index
- RANKL
receptor activator of nuclear factor kappa beta ligand
- SMA
skeletal muscle attenuation
- SMAD
mothers against decapentaplegic
- SMD
skeletal muscle density
- SMI
skeletal muscle index
- STAT
signal transducer and activator of transcription
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- UPS
ubiquitin-mediated proteasome
- WAT
white adipose tissue
Contributor Information
Chandler S Callaway, Department of Pathology, University of Colorado Anschutz Medical Campus, Aurora, CO 80045, USA.
Lila M Mouchantat, Department of Pathology, University of Colorado Anschutz Medical Campus, Aurora, CO 80045, USA.
Benjamin G Bitler, Department of Obstetrics & Gynecology, University of Colorado Anschutz Medical Campus, Aurora, CO 80045, USA; Comprehensive Cancer Center, University of Colorado Anschutz Medical Campus, Aurora, CO 80045, USA.
Andrea Bonetto, Department of Pathology, University of Colorado Anschutz Medical Campus, Aurora, CO 80045, USA; Comprehensive Cancer Center, University of Colorado Anschutz Medical Campus, Aurora, CO 80045, USA.
Funding
This work was supported by Department of Pathology and Comprehensive Cancer Center at University of Colorado Anschutz Medical Campus, and by grants from National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR079379, R01AR080051) and American Cancer Society (132013-RSG-18-010-01-CCG) to A.B., as well as National Cancer Institute (R37CA261987) and Department of Defense (W81XWH-21-1-0382, W81XWH-21-1-0233) to B.G.B. C.S.C. was supported by a T32 Institutional Training Grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR080630).
Disclosures
The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Waning DL, Mohammad KS, Reiken S, et al. Excess TGF-beta mediates muscle weakness associated with bone metastases in mice. Nat Med. 2015;21(11):1262‐1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pin F, Bonetto A, Bonewald LF, Klein GL. Molecular mechanisms responsible for the rescue effects of pamidronate on muscle atrophy in pediatric burn patients. Front Endocrinol (Lausanne). 2019;10:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huot JR, Novinger LJ, Pin F, et al. Formation of colorectal liver metastases induces musculoskeletal and metabolic abnormalities consistent with exacerbated cachexia. JCI Insight. 2020;5(9):e136687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Essex AL, Pin F, Huot JR, Bonewald LF, Plotkin LI, Bonetto A. Bisphosphonate treatment ameliorates chemotherapy-induced bone and muscle abnormalities in young mice. Front Endocrinol (Lausanne). 2019;10:809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pin F, Barreto R, Kitase Y, et al. Growth of ovarian cancer xenografts causes loss of muscle and bone mass: a new model for the study of cancer cachexia. J Cachexia Sarcopenia Muscle. 2018;9(4):685‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Torres ML, Hartmann LC, Cliby WA, et al. Nutritional status, CT body composition measures and survival in ovarian cancer. Gynecologic Oncol. 2013;129(3):548‐553. [DOI] [PubMed] [Google Scholar]
- 7. Bronger H, Hederich P, Hapfelmeier A, et al. Sarcopenia in advanced serous ovarian cancer. Int J Gynecol Cancer. 2017;27(2):223‐232. [DOI] [PubMed] [Google Scholar]
- 8. Rutten IJ, Ubachs J, Kruitwagen RF, et al. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur J Surg Oncol. 2017;43(4):717‐724. [DOI] [PubMed] [Google Scholar]
- 9. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489‐495. [DOI] [PubMed] [Google Scholar]
- 10. Aubrey J, Esfandiari N, Baracos VE, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf). 2014;210(3):489‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MacDonald AJ, Greig CA, Baracos V. The advantages and limitations of cross-sectional body composition analysis. Curr Opin Support Palliat Care. 2011;5(4):342‐349. [DOI] [PubMed] [Google Scholar]
- 12. Wakabayashi H, Arai H, Inui A. The regulatory approval of anamorelin for treatment of cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: facts and numbers. J Cachexia Sarcopenia Muscle. 2021;12(1):14‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roeland EJ, Bohlke K, Baracos VE, et al. Management of cancer cachexia: ASCO guideline. J Clin Oncol. 2020;38(21):2438‐2453. [DOI] [PubMed] [Google Scholar]
- 14. Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14(11):754‐762. [DOI] [PubMed] [Google Scholar]
- 15. Anker MS, Holcomb R, Muscaritoli M, et al. Orphan disease status of cancer cachexia in the USA and in the European Union: a systematic review. J Cachexia Sarcopenia Muscle. 2019;10(1):22‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barreto R, Waning DL, Gao H, Liu Y, Zimmers TA, Bonetto A. Chemotherapy-related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs. Oncotarget. 2016;7(28):43442‐43460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pin F, Barreto R, Couch ME, Bonetto A, O’Connell TM. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J Cachexia Sarcopenia Muscle. 2019;10(1):140‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jordan KR, Sikora MJ, Slansky JE, et al. The capacity of the ovarian cancer tumor microenvironment to integrate inflammation signaling conveys a shorter disease-free interval. Clin Cancer Res. 2020;26(23):6362‐6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taillandier D, Polge C. Skeletal muscle atrogenes: from rodent models to human pathologies. Biochimie. 2019;166:251‐269. [DOI] [PubMed] [Google Scholar]
- 20. Zhou J, Liu B, Liang C, Li Y, Song YH. Cytokine signaling in skeletal muscle wasting. Trends Endocrinol Metab. 2016;27(5):335‐347. [DOI] [PubMed] [Google Scholar]
- 21. Webster JM, Kempen LJAP, Hardy RS, Langen RCJ. Inflammation and skeletal muscle wasting during cachexia. Front Physiol. 2020;11:597675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Argilés JM, Moore-Carrasco R, Fuster G, Busquets S, López-Soriano FJ. Cancer cachexia: the molecular mechanisms. Int J Biochem Cell Biol. 2003;35(4):405‐409. [DOI] [PubMed] [Google Scholar]
- 23. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89(2):381‐410. [DOI] [PubMed] [Google Scholar]
- 24. Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16(2):153‐166. [DOI] [PubMed] [Google Scholar]
- 25. Fong Y, Moldawer LL, Marano M, et al. Cachectin/TNF or IL-1 alpha induces cachexia with redistribution of body proteins. Am J Physiol. 1989;256(3):R659‐R665. [DOI] [PubMed] [Google Scholar]
- 26. Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med (Berl). 2008;86(10):1113‐1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li W, Moylan JS, Chambers MA, Smith J, Reid MB. Interleukin-1 stimulates catabolism in C2C12 myotubes. Am J Physiol Cell Physiol. 2009;297(3):C706‐C714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fujita J, Tsujinaka T, Yano M, et al. Anti-interleukin-6 receptor antibody prevents muscle atrophy in colon-26 adenocarcinoma-bearing mice with modulation of lysosomal and ATP-ubiquitin-dependent proteolytic pathways. Int J Cancer. 1996;68(5):637‐643. [DOI] [PubMed] [Google Scholar]
- 29. Barton BE, Murphy TF. Cancer cachexia is mediated in part by the induction of IL-6-like cytokines from the spleen. Cytokine. 2001;16(6):251‐257. [DOI] [PubMed] [Google Scholar]
- 30. Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol (1985). 2005;98(3):911‐917. [DOI] [PubMed] [Google Scholar]
- 31. Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol. 2008;294:R393‐R401. [DOI] [PubMed] [Google Scholar]
- 32. Bonetto A, Aydogdu T, Jin X, et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol. 2012;303:E410‐E421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pelosi M, De Rossi M, Barberi L, Musarò A. IL-6 impairs myogenic differentiation by downmodulation of p90RSK/eEF2 and mTOR/p70S6K axes, without affecting AKT activity. Biomed Res Int. 2014;2014:206026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Han J, Meng Q, Shen L, Wu G. Interleukin-6 induces fat loss in cancer cachexia by promoting white adipose tissue lipolysis and browning. Lipids Health Dis. 2018;17(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rupert JE, Narasimhan A, Jengelley DHA, et al. Tumor-derived IL-6 and trans-signaling among tumor, fat, and muscle mediate pancreatic cancer cachexia. J Exp Med. 2021;218(6):e20190450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matsushima K, Oppenheim JJ. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989;1(1):2‐13. [DOI] [PubMed] [Google Scholar]
- 37. Hou YC, Wang CJ, Chao YJ, et al. Elevated serum interleukin-8 level correlates with cancer-related cachexia and sarcopenia: an indicator for pancreatic cancer outcomes. J Clin Med. 2018;7(12):502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cury SS, de Moraes D, Freire PP, et al. Tumor transcriptome reveals high expression of IL-8 in non-small cell lung cancer patients with low Pectoralis muscle area and reduced survival. Cancers (Basel). 2019;11(9):1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Callaway CS, Delitto AE, Patel R, et al. IL-8 Released from human pancreatic cancer and tumor-associated stromal cells signals through a CXCR2-ERK1/2 axis to induce muscle atrophy. Cancers (Basel). 2019;11(12):1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goodman MN. Tumor necrosis factor induces skeletal muscle protein breakdown in rats. Am J Physiol. 1991;260(5):E727‐E730. [DOI] [PubMed] [Google Scholar]
- 41. Patel HJ, Patel BM. TNF-α and cancer cachexia: molecular insights and clinical implications. Life Sci. 2017;170:56‐63. [DOI] [PubMed] [Google Scholar]
- 42. Franco-Romero A, Sandri M. Role of autophagy in muscle disease. Mol Aspects Med. 2021;82:101041. [DOI] [PubMed] [Google Scholar]
- 43. Mammucari C, Milan G, Romanello V, et al. Foxo3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6(6):458‐471. [DOI] [PubMed] [Google Scholar]
- 44. Tang H, Inoki K, Brooks SV, et al. mTORC1 underlies age-related muscle fiber damage and loss by inducing oxidative stress and catabolism. Aging Cell. 2019;18(3):e12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tsai S, Sitzmann JM, Dastidar SG, et al. Muscle-specific 4E-BP1 signaling activation improves metabolic parameters during aging and obesity. J Clin Invest. 2015;125(8):2952‐2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bentzinger CF, Romanino K, Cloëtta D, et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8(5):411‐424. [DOI] [PubMed] [Google Scholar]
- 47. Risson V, Mazelin L, Roceri M, et al. Muscle inactivation of mTOR causes metabolic and dystrophin defects leading to severe myopathy. J Cell Biol. 2009;187(6):859‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ham AS, Chojnowska K, Tintignac LA, et al. mTORC1 signalling is not essential for the maintenance of muscle mass and function in adult sedentary mice. J Cachexia Sarcopenia Muscle. 2020;11(1):259‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. White JP, Baynes JW, Welle SL, et al. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PLoS One. 2011;6(9):e24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. White JP, Puppa MJ, Gao S, Sato S, Welle SL, Carson JA. Muscle mTORC1 suppression by IL-6 during cancer cachexia: a role for AMPK. Am J Physiol Endocrinol Metab. 2013;304(10):E1042‐E1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Markofski MM, Dickinson JM, Drummond MJ, et al. Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women. Exp Gerontol. 2015;65:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Asp ML, Tian M, Wendel AA, Belury MA. Evidence for the contribution of insulin resistance to the development of cachexia in tumor-bearing mice. Int J Cancer. 2010;126(3):756‐763. [DOI] [PubMed] [Google Scholar]
- 53. Costelli P, Muscaritoli M, Bossola M, et al. IGF-1 is downregulated in experimental cancer cachexia. Am J Physiol Regul Integr Comp Physiol. 2006;291(3):R674‐R683. [DOI] [PubMed] [Google Scholar]
- 54. Dev R, Bruera E, Dalal S. Insulin resistance and body composition in cancer patients. Ann Oncol. 2018;29:ii18‐ii26. [DOI] [PubMed] [Google Scholar]
- 55. Honors MA, Kinzig KP. The role of insulin resistance in the development of muscle wasting during cancer cachexia. J Cachexia Sarcopenia Muscle. 2012;3(1):5‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hornsveld M, Dansen TB, Derksen PW, Burgering BMT. Re-evaluating the role of FOXOs in cancer. Semin Cancer Biol. 2018;50:90‐100. [DOI] [PubMed] [Google Scholar]
- 57. Salminen A, Kaarniranta K. Insulin/IGF-1 paradox of aging: regulation via AKT/IKK/NF-kappaB signaling. Cell Signal. 2010;22(4):573‐577. [DOI] [PubMed] [Google Scholar]
- 58. Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle. 2011;1(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen JL, Walton KL, Hagg A, et al. Specific targeting of TGF-beta family ligands demonstrates distinct roles in the regulation of muscle mass in health and disease. Proc Natl Acad Sci U S A. 2017;114(26):E5266‐E5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sartori R, Hagg A, Zampieri S, et al. Perturbed BMP signaling and denervation promote muscle wasting in cancer cachexia. Sci Transl Med. 2021;13(605):eaay9592. [DOI] [PubMed] [Google Scholar]
- 61. Huot JR, Pin F, Bonetto A. Muscle weakness caused by cancer and chemotherapy is associated with loss of motor unit connectivity. Am J Cancer Res. 2021;11:2990‐3001. [PMC free article] [PubMed] [Google Scholar]
- 62. Schiaffino S, Reggiani C, Akimoto T, Blaauw B. Molecular mechanisms of skeletal muscle hypertrophy. J Neuromuscul Dis. 2021;8(2):169‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen MM, Zhao YP, Zhao Y, Deng SL, Yu K. Regulation of myostatin on the growth and development of skeletal muscle. Front Cell Dev Biol. 2021;9:785712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen JL, Walton KL, Winbanks CE, et al. Elevated expression of activins promotes muscle wasting and cachexia. FASEB J. 2014;28(4):1711‐1723. [DOI] [PubMed] [Google Scholar]
- 65. Loumaye A, de Barsy M, Nachit M, et al. Role of activin A and myostatin in human cancer cachexia. J Clin Endocrinol Metab. 2015;100(5):2030‐2038. [DOI] [PubMed] [Google Scholar]
- 66. Zimmers TA, Davies MV, Koniaris LG, et al. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296(5572):1486‐1488. [DOI] [PubMed] [Google Scholar]
- 67. Argilés JM, Stemmler B, López-Soriano FJ, Busquets S. Inter-tissue communication in cancer cachexia. Nat Rev Endocrinol. 2018;15(1):9‐20. [DOI] [PubMed] [Google Scholar]
- 68. Daas SI, Rizeq BR, Nasrallah GK. Adipose tissue dysfunction in cancer cachexia. J Cell Physiol. 2018;234(1):13‐22. [DOI] [PubMed] [Google Scholar]
- 69. Machado SA, Pasquarelli-do-Nascimento G, da Silva DS, et al. Browning of the white adipose tissue regulation: new insights into nutritional and metabolic relevance in health and diseases. Nutr Metab (Lond). 2022;19(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Guilherme A, Yenilmez B, Bedard AH, et al. Control of adipocyte thermogenesis and lipogenesis through β3-adrenergic and thyroid hormone signal integration. Cell Rep. 2020;31(5):107598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kliewer KL, Ke JY, Tian M, Cole RM, Andridge RR, Belury MA. Adipose tissue lipolysis and energy metabolism in early cancer cachexia in mice. Cancer Biol Ther. 2015;16(6):886‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bonetto A, Kays JK, Parker VA, et al. Differential bone loss in mouse models of colon cancer cachexia. Front Physiol. 2016;7:679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Greco EA, Pietschmann P, Migliaccio S. Osteoporosis and sarcopenia increase frailty syndrome in the elderly. Front Endocrinol (Lausanne). 2019;10:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Castañeda S, Casas A, González-Del-Alba A, et al. Bone loss induced by cancer treatments in breast and prostate cancer patients. Clin Transl Oncol. 2022;24(11):2090‐2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pin F, Bonewald LF, Bonetto A. Role of myokines and osteokines in cancer cachexia. Exp Biol Med (Maywood). 2021;246(19):2118‐2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Waning DL, Guise TA. Molecular mechanisms of bone metastasis and associated muscle weakness. Clin Cancer Res. 2014;20(12):3071‐3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Huot JR, Pin F, Essex AL, Bonetto A. MC38 tumors induce musculoskeletal defects in colorectal cancer. Int J Mol Sci. 2021;22(3):1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pin F, Prideaux M, Huot JR, et al. Non-bone metastatic cancers promote osteocyte-induced bone destruction. Cancer Lett. 2021;520:80‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pin F, Jones AJ, Huot JR, et al. RANKL blockade reduces cachexia and bone loss induced by non-metastatic ovarian cancer in mice. J Bone Miner Res. 2022;37(3):381‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bindels LB, Neyrinck AM, Loumaye A, et al. Increased gut permeability in cancer cachexia: mechanisms and clinical relevance. Oncotarget. 2018;9(26):18224‐18238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Setiawan T, Sari IN, Wijaya YT, et al. Cancer cachexia: molecular mechanisms and treatment strategies. J Hematol Oncol. 2023;16(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Puppa MJ, White JP, Sato S, Cairns M, Baynes JW, Carson JA. Gut barrier dysfunction in the Apc(Min/+) mouse model of colon cancer cachexia. Biochim Biophys Acta. 2011;1812(12):1601‐1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kordes M, Larsson L, Engstrand L, Löhr JM. Pancreatic cancer cachexia: three dimensions of a complex syndrome. Br J Cancer. 2021;124(10):1623‐1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sakakida T, Ishikawa T, Doi T, et al. Water-soluble dietary fiber alleviates cancer-induced muscle wasting through changes in gut microenvironment in mice. Cancer Sci. 2022;113(5):1789‐1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pötgens SA, Brossel H, Sboarina M, et al. Klebsiella oxytoca expands in cancer cachexia and acts as a gut pathobiont contributing to intestinal dysfunction. Sci Rep. 2018;8(1):12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. van Krimpen SJ, Jansen FAC, Ottenheim VL, Belzer C, van der Ende M, van Norren K. The effects of pro-, Pre-, and synbiotics on muscle wasting, a systematic review-gut permeability as potential treatment target. Nutrients. 2021;13(4):1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. van Norren K, Dwarkasing JT, Witkamp RF. The role of hypothalamic inflammation, the hypothalamic-pituitary-adrenal axis and serotonin in the cancer anorexia-cachexia syndrome. Curr Opin Clin Nutr Metab Care. 2017;20(5):396‐401. [DOI] [PubMed] [Google Scholar]
- 88. Yeom E, Yu K. Understanding the molecular basis of anorexia and tissue wasting in cancer cachexia. Exp Mol Med. 2022;54(4):426‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sohn JW. Network of hypothalamic neurons that control appetite. BMB Rep. 2015;48(4):229‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Méquinion M, Foldi CJ, Andrews ZB. The ghrelin-AgRP neuron nexus in anorexia Nervosa: implications for metabolic and behavioral adaptations. Front Nutr. 2019;6:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wyart E, Bindels LB, Mina E, Menga A, Stanga S, Porporato PE. Cachexia, a systemic disease beyond muscle atrophy. Int J Mol Sci. 2020;21(22):8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Braun TP, Zhu X, Szumowski M, et al. Central nervous system inflammation induces muscle atrophy via activation of the hypothalamic-pituitary-adrenal axis. J Exp Med. 2011;208(12):2449‐2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dittrich A, Khouri C, Sackett SD, et al. Glucocorticoids increase interleukin-6-dependent gene induction by interfering with the expression of the suppressor of cytokine signaling 3 feedback inhibitor. Hepatology. 2012;55(1):256‐266. [DOI] [PubMed] [Google Scholar]
- 94. Braun TP, Marks DL. The regulation of muscle mass by endogenous glucocorticoids. Front Physiol. 2015;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bonetto A, Aydogdu T, Kunzevitzky N, et al. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS One. 2011;6(7):e22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Di Gregorio GB, Yao-Borengasser A, Rasouli N, et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54(8):2305‐2313. [DOI] [PubMed] [Google Scholar]
- 97. Kosters A, Karpen SJ. The role of inflammation in cholestasis: clinical and basic aspects. Semin Liver Dis. 2010;30(02):186‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Masri S, Papagiannakopoulos T, Kinouchi K, et al. Lung adenocarcinoma distally rewires hepatic circadian homeostasis. Cell. 2016;165(4):896‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gonçalves DC, Lira FS, Yamashita AS, et al. Liver lipid metabolism disruption in cancer cachexia is aggravated by cla supplementation -induced inflammation. Clin Nutr. 2019;38(5):2219‐2230. [DOI] [PubMed] [Google Scholar]
- 100. Cai SY, Ouyang X, Chen Y, et al. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI Insight. 2017;2(5):e90780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fearon KC, Borland W, Preston T, Tisdale MJ, Shenkin A, Calman KC. Cancer cachexia: influence of systemic ketosis on substrate levels and nitrogen metabolism. Am J Clin Nutr. 1988;47(1):42‐48. [DOI] [PubMed] [Google Scholar]
- 102. Cortez NE, Mackenzie GG. Ketogenic diets in pancreatic cancer and associated cachexia: cellular mechanisms and clinical perspectives. Nutrients. 2021;13(9):3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Flint TR, Janowitz T, Connell CM, et al. Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab. 2016;24(5):672‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Montalvo RN, Counts BR, Carson JA. Understanding sex differences in the regulation of cancer-induced muscle wasting. Curr Opin Support Palliat Care. 2018;12(4):394‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chidi-Ogbolu N, Baar K. Effect of estrogen on musculoskeletal performance and injury risk. Front Physiol. 2018;9:1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tiidus PM. Benefits of estrogen replacement for skeletal muscle mass and function in post-menopausal females: evidence from human and animal studies. Eurasian J Med. 2011;43(2):109‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Falqueto H, Júnior JLR, Silvério MNO, Farias JCH, Schoenfeld BJ, Manfredi LH. Can conditions of skeletal muscle loss be improved by combining exercise with anabolic-androgenic steroids? A systematic review and meta-analysis of testosterone-based interventions. Rev Endocr Metab Disord. 2021;22(2):161‐178. [DOI] [PubMed] [Google Scholar]
- 109. Wright TJ, Dillon EL, Durham WJ, et al. A randomized trial of adjunct testosterone for cancer-related muscle loss in men and women. J Cachexia Sarcopenia Muscle. 2018;9(3):482‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhong X, Narasimhan A, Silverman LM, et al. Sex specificity of pancreatic cancer cachexia phenotypes, mechanisms, and treatment in mice and humans: role of activin. J Cachexia Sarcopenia Muscle. 2022;13(4):2146‐2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hain BA, Xu H, Waning DL. Loss of REDD1 prevents chemotherapy-induced muscle atrophy and weakness in mice. J Cachexia Sarcopenia Muscle. 2021;12(6):1597‐1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bonomi PD, Walsh D, Currow DC, Ballinari G, Skipworth RJE. Cancer cachexia impact on chemotherapy dose reduction, treatment discontinuation, and survival: a qualitative systematic review. J Clin Oncol. 2022;40(16_suppl):e24103. [Google Scholar]
- 113. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: Cancer J Clin. 2023;73(1):17‐48. [DOI] [PubMed] [Google Scholar]
- 114. Arora T, Mullangi S, Lekkala MR. Ovarian Cancer. NCBI; 2023. https://www.ncbi.nlm.nih.gov/books/NBK567760/. [Google Scholar]
- 115. Hayashi T, Konishi I. Molecular histopathology for establishing diagnostic method and clinical therapy for ovarian carcinoma. J Clin Med Res. 2023;15(2):68‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rosen DG, Yang G, Liu G, et al. Ovarian cancer: pathology, biology, and disease models. Front Biosci (Landmark Ed). 2009;14(14):2089‐2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cancer Genome Atlas Research Network . Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Piccart MJ, Bertelsen K, James K, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92(9):699‐708. [DOI] [PubMed] [Google Scholar]
- 119. McGuire WP. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 120. Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34‐43. [DOI] [PubMed] [Google Scholar]
- 121. Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001‐1007. [DOI] [PubMed] [Google Scholar]
- 122. Ubachs J, Ziemons J, Minis-Rutten IJ, et al. Sarcopenia and ovarian cancer survival: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10(6):1165‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rinninella E, Fagotti A, Cintoni M, et al. Skeletal muscle mass as a prognostic indicator of outcomes in ovarian cancer: a systematic review and meta-analysis. Int J Gynecol Cancer. 2020;30(5):654‐663. [DOI] [PubMed] [Google Scholar]
- 124. Allanson ER, Peng Y, Choi A, Hayes S, Janda M, Obermair A. A systematic review and meta-analysis of sarcopenia as a prognostic factor in gynecological malignancy. Int J Gynecol Cancer. 2020;30(11):1791‐1797. [DOI] [PubMed] [Google Scholar]
- 125. Petrelli F, Cortellini A, Indini A, et al. Association of obesity with survival outcomes in patients with cancer: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(3):e213520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sutton EH, Plyta M, Fragkos K, Di Caro S. Pre-treatment sarcopenic assessments as a prognostic factor for gynaecology cancer outcomes: systematic review and meta-analysis. Eur J Clin Nutr. 2022;76(11):1513‐1527. [DOI] [PubMed] [Google Scholar]
- 127. Tranoulis A, Kwong FL, Lakhiani A, Georgiou D, Yap J, Balega J. Prevalence of computed tomography-based sarcopenia and the prognostic value of skeletal muscle index and muscle attenuation amongst women with epithelial ovarian malignancy: a systematic review and meta-analysis. Eur J Surg Oncol. 2022;48(7):1441‐1454. [DOI] [PubMed] [Google Scholar]
- 128. Ge HP, Song DF, Wu P, Xu HF. Impact of sarcopenia and low muscle attenuation on outcomes of ovarian cancer: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2023;27(10):4544‐4562. [DOI] [PubMed] [Google Scholar]
- 129. McSharry V, Mullee A, McCann L, Rogers AC, McKiernan M, Brennan DJ. The impact of sarcopenia and low muscle attenuation on overall survival in epithelial ovarian cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2020;27(9):3553‐3564. [DOI] [PubMed] [Google Scholar]
- 130. Ataseven B, Luengo TG, du Bois A, et al. Skeletal muscle attenuation (sarcopenia) predicts reduced overall survival in patients with advanced epithelial ovarian cancer undergoing primary debulking surgery. Ann Surg Oncol. 2018;25(11):3372‐3379. [DOI] [PubMed] [Google Scholar]
- 131. Aust S, Knogler T, Pils D, et al. Skeletal muscle depletion and markers for cancer cachexia are strong prognostic factors in epithelial ovarian cancer. PLoS One. 2015;10(10):e0140403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bruno KA, Sobreira da Silva MJ, Chaves GV. Association of body composition with toxicity to first-line chemotherapy and three-year survival in women with ovarian adenocarcinoma. Acta Oncol. 2021;60(12):1611‐1620. [DOI] [PubMed] [Google Scholar]
- 133. Chae SH, Lee C, Yoon SH, et al. Sarcopenia as a predictor of prognosis in early stage ovarian cancer. J Korean Med Sci. 2021;36(1):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Huang CY, Yang YC, Chen TC, et al. Muscle loss during primary debulking surgery and chemotherapy predicts poor survival in advanced-stage ovarian cancer. J Cachexia Sarcopenia Muscle. 2020;11(2):534‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kumar A, Moynagh MR, Multinu F, et al. Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol Oncol. 2016;142(2):311‐316. [DOI] [PubMed] [Google Scholar]
- 136. Matsubara Y, Nakamura K, Matsuoka H, Ogawa C, Masuyama H. Pre-treatment psoas major volume is a predictor of poor prognosis for patients with epithelial ovarian cancer. Mol Clin Oncol. 2019;11(4):376‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Yoshikawa T, Miyamoto M, Aoyama T, et al. Psoas muscle index at the fifth lumbar vertebra as a predictor of survival in epithelial ovarian cancers. Mol Clin Oncol. 2021;15(3):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yoshino Y, Taguchi A, Nakajima Y, et al. Extreme skeletal muscle loss during induction chemotherapy is an independent predictor of poor survival in advanced epithelial ovarian cancer patients. J Obstet Gynaecol Res. 2020;46(12):2662‐2671. [DOI] [PubMed] [Google Scholar]
- 139. Rizzo S, Raia G, Del Grande M, et al. Body composition as a predictor of chemotherapy-related toxicity in ovarian cancer patients: a systematic review. Front Oncol. 2022;12:1057631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kim SI, Kim TM, Lee M, et al. Impact of CT-determined sarcopenia and body composition on survival outcome in patients with advanced-stage high-grade serous ovarian carcinoma. Cancers (Basel). 2020;12(3):559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Rutten IJ, van Dijk DP, Kruitwagen RF, Beets-Tan RG, Olde Damink SW, Van Gorp T. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle. 2016;7(4):458‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Rutten IJ, Ubachs J, Kruitwagen RF, Beets-Tan RG, Olde Damink SW, Van Gorp T. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J Cachexia Sarcopenia Muscle. 2017;8(4):630‐638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nakayama N, Nakayama K, Nakamura K, Razia S, Kyo S. Sarcopenic factors may have No impact on outcomes in ovarian cancer patients. Diagnostics (Basel). 2019;9(4):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Staley SA, Tucker K, Newton M, et al. Sarcopenia as a predictor of survival and chemotoxicity in patients with epithelial ovarian cancer receiving platinum and taxane-based chemotherapy. Gynecol Oncol. 2020;156(3):695‐700. [DOI] [PubMed] [Google Scholar]
- 145. Ubachs J, Koole SN, Lahaye M, et al. No influence of sarcopenia on survival of ovarian cancer patients in a prospective validation study. Gynecol Oncol. 2020;159(3):706‐711. [DOI] [PubMed] [Google Scholar]
- 146. Wood N, Morton M, Shah SN, et al. Association between CT-based body composition assessment and patient outcomes during neoadjuvant chemotherapy for epithelial ovarian cancer. Gynecol Oncol. 2023;169:55‐63. [DOI] [PubMed] [Google Scholar]
- 147. Cuello MA, Gómez F, Wichmann I, et al. Body composition and metabolic dysfunction really matter for the achievement of better outcomes in high-grade serous ovarian cancer. Cancers (Basel). 2023;15(4):1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Can E, Sönmez S, Konal M, Şirinoglu HA, Seyhan NA, AKBAYIR Ö. Does sarcopenia predict perioperative mortality in patients with advanced ovarian cancer? Eur Rev Med Pharmacol Sci. 2022;26(24):9409‐9415. [DOI] [PubMed] [Google Scholar]
- 149. Del Grande M, Rizzo S, Nicolino GM, et al. Computed tomography-based body composition in patients with ovarian cancer: association with chemotoxicity and prognosis. Front Oncol. 2021;11:718815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Hsu WH, Ko AT, Weng CS, et al. Explainable machine learning model for predicting skeletal muscle loss during surgery and adjuvant chemotherapy in ovarian cancer. J Cachexia Sarcopenia Muscle. 2023;14(5):2044‐2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Fadadu PP, Polen-De CL, McGree ME, et al. Patients triaged to neoadjuvant chemotherapy have higher rates of sarcopenia: an opportunity for prehabilitation. Gynecol Oncol. 2021;160(1):40‐44. [DOI] [PubMed] [Google Scholar]
- 152. Patel A, Iyer P, Matsuzaki S, Matsuo K, Sood AK, Fleming ND. Emerging trends in neoadjuvant chemotherapy for ovarian cancer. Cancers (Basel). 2021;13(4):626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Jin Y, Ma X, Yang Z, Zhang N. Low L3 skeletal muscle index associated with the clinicopathological characteristics and prognosis of ovarian cancer: a meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14(2):697‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Weng CS, Huang WC, Chang CL, Jan YT, Chen TC, Lee J. Association of malignant ascites with systemic inflammation and muscle loss after treatment in advanced-stage ovarian cancer. J Cachexia Sarcopenia Muscle. 2023;14(5):2114‐2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Fuller K, Wong B, Fox S, Choi Y, Chambers TJ. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med. 1998;188(5):997‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Jones DH, Nakashima T, Sanchez OH, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440(7084):692‐696. [DOI] [PubMed] [Google Scholar]
- 157. Santini D, Perrone G, Roato I, et al. Expression pattern of receptor activator of NFκB (RANK) in a series of primary solid tumors and related bone metastases. J Cell Physiol. 2011;226(3):780‐784. [DOI] [PubMed] [Google Scholar]
- 158. Zhang X, Song Y, Song N, et al. Rankl expression predicts poor prognosis in gastric cancer patients: results from a retrospective and single-center analysis. Braz J Med Biol Res. 2018;51(3):e6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Wieser V, Sprung S, Tsibulak I, et al. Clinical impact of RANK signalling in ovarian cancer. Cancers (Basel). 2019;11(6):791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zhou X, Wang JL, Lu J, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142(4):531‐543. [DOI] [PubMed] [Google Scholar]
- 161. Lau DH, Lewis AD, Ehsan MN, Sikic BI. Multifactorial mechanisms associated with broad cross-resistance of ovarian carcinoma cells selected by cyanomorpholino doxorubicin. Cancer Res. 1991;51:5181‐5187. [PubMed] [Google Scholar]
- 162. Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4(1):2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev. 2010;10(11):803‐808. [DOI] [PubMed] [Google Scholar]
- 164. Ubachs J, van de Worp WRPH, Vaes RDW, et al. Ovarian cancer ascites induces skeletal muscle wasting in vitro and reflects sarcopenia in patients. J Cachexia Sarcopenia Muscle. 2022;13(1):311‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Lerner L, Tao J, Liu Q, et al. MAP3K11/GDF15 axis is a critical driver of cancer cachexia. J Cachexia Sarcopenia Muscle. 2016;7(4):467‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Luan Y, Zhang Y, Yu SY, et al. Development of ovarian tumour causes significant loss of muscle and adipose tissue: a novel mouse model for cancer cachexia study. J Cachexia Sarcopenia Muscle. 2022;13(2):1289‐1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Barreto R, Kitase Y, Matsumoto T, et al. ACVR2B/Fc counteracts chemotherapy-induced loss of muscle and bone mass. Sci Rep. 2017;7(1):14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Pettersen K, Andersen S, van der Veen A, et al. Autocrine activin A signalling in ovarian cancer cells regulates secretion of interleukin 6, autophagy, and cachexia. J Cachexia Sarcopenia Muscle. 2020;11(1):195‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kim-Muller JY, Song L, LaCarubba Paulhus B, et al. GDF15 neutralization restores muscle function and physical performance in a mouse model of cancer cachexia. Cell Rep. 2023;42(1):111947. [DOI] [PubMed] [Google Scholar]
- 170. Straughn AR, Kakar SS. Withaferin A ameliorates ovarian cancer-induced cachexia and proinflammatory signaling. J Ovarian Res. 2019;12(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Straughn AR, Kelm NQ, Kakar SS. Withaferin A and ovarian cancer antagonistically regulate skeletal muscle mass. Front Cell Dev Biol. 2021;9:636498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Fong MY, Jin S, Rane M, Singh RK, Gupta R, Kakar SS. Withaferin A synergizes the therapeutic effect of doxorubicin through ROS-mediated autophagy in ovarian cancer. PLoS One. 2012;7(7):e42265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.