A 33-year-old man with a history of ulcerative colitis (UC) treated with tofacitinib (a janus kinase (JAK) inhibitor) presented to the hospital with fatigue and bloody diarrhea. The fatigue was progressive over the previous eight months, during which time he had developed increasingly frequent diarrhea. Over the preceding four months he noted fevers (maximum temp 39.2°C) occurring in the late afternoon and evening, associated with drenching night sweats and an unintentional 50 pound weight loss. In the two weeks prior to presentation, he reported 6 to 9 liquid stools per day associated with urgency, passage of blood and nocturnal awakenings. He reported no cough, dysuria, joint pain or skin rashes.
His fevers and night sweats are concerning for infection, malignancy, or inflammation related to his known UC or another inflammatory disorder. The persistent and worsening bloody diarrhea may reflect a flare of UC despite tofacitinib or a superimposed enteric infection. Clostridiodes difficile and cytomegalovirus infections are known complications of UC and should be excluded as should serious infections associated with tofacitinib use such as tuberculosis. Volume depletion or iron deficiency anemia from persistent bloody diarrhea may contribute to fatigue. The unintentional weight loss may be explained by chronic infection, malignancy, or another disorder such as hyperthyroidism.
The patient had a history of left-sided UC diagnosed seven years before presentation and initially managed with oral and rectal mesalamine resulting in clinical remission. Five years prior to presentation, the patient had a flare of his UC at which point he was transitioned to combination therapy with subcutaneous adalimumab and oral azathioprine with an improvement in his symptoms. Prior to initiation of adalimumab, the patient had negative testing for latent tuberculosis by interferon-gamma release assay and negative testing for hepatitis B surface antigen and core antibody.
The patient worked as a grain elevator operator in the upper Midwest and continued work until symptoms worsened two weeks prior to presentation. He had completed 2 tours of active duty in Afghanistan, 11 and 13 years prior to presentation. He was not taking glucocorticoids or other medications at the time of presentation and he had quit smoking tobacco 15 years prior.
Two months prior to presentation, the patient underwent flexible sigmoidoscopy that demonstrated severe (Mayo endoscopic subscore 2) colitis in the examined segments from the rectum to the descending colon. Biopsies demonstrated chronic active colitis without dysplasia, granulomas, or viral inclusions. At the time, he was taking adalimumab with azathioprine and his trough adalimumab level was 15 micrograms per milliliter (reference range > 0.6). Tofacitinib was started and adalimumab was stopped two weeks prior to hospital presentation.
Anti-TNF therapies such as adalimumab are effective in many patents with UC, but are associated with an increased risk of infectious complications, including tuberculosis and other mycobacterial infections, listeriosis and invasive fungal infections. Azathioprine use has been associated with Epstein-Barr virus related lymphomas and in rare cases with hepatosplenic T-cell lymphoma. Tofacitinib also increases risk for infections, in particular herpes zoster, and malignancy, but notably his symptoms antedated his starting this.
On physical examination, the patient’s temperature was 37°C, his heart rate was 117 beats per minute and regular, and blood pressure was 109/77 mm Hg. He appeared comfortable in a supine position and in no acute distress. Oral examination did not demonstrate ulcerations. Lung auscultation was normal without wheezing, rales or rhonchi. The abdominal exam was soft and non-tender in all four quadrants; normal bowel sounds were present and there was no hepatomegaly or splenomegaly. There was no appreciable lymphadenopathy.
The white-cell count was 1,100 per cubic millimeter with 66% neutrophils, 13% lymphocytes, 18% monocytes, and 2% eosinophils. The hemoglobin level was 4.7 grams per deciliter and the platelet count 61,000 per microliter. The mean corpuscular volume was 100 femtoliters (normal range, 82 to 96). The iron level was 20 micrograms per deciliter (normal range, 40 to 160), total iron binding capacity was 170 micrograms per deciliter (normal range, 230–430) and ferritin was 1863 nanograms per milliliter (normal range, 20 to 300). The sodium level was 125 millimoles per liter and potassium 3.7 millimoles per liter. Serum creatinine and thyroid stimulating hormone were normal. Total protein was 5.4 grams per deciliter (normal range, 6 to 8.3) and albumin 2.6 grams per deciliter (normal range, 3.5 to 5). The C-reactive protein was 67 milligrams per liter (normal range, < 5). Transfusion was given of 3 units of packed red blood cells.
While active UC can present with an iron deficiency anemia, the high ferritin and low total iron binding capacity are more consistent with an anemia of chronic disease. Azathioprine can cause cytopenias and macrocytosis. Infectious processes that could cause bone marrow suppression include mycobacterial infection, fungal infections and herpesviruses such as cytomegalovirus and Epstein-Barr virus.
A chest radiograph demonstrated diffuse reticulonodular opacities (Figure 1). A computed tomography (CT) scan of the abdomen demonstrated circumferential wall thickening of the rectum and descending colon. Stool PCR for Clostridiodes difficile infection and serum cytomegalovirus PCR were negative. A stool multiplex PCR panel was negative for common viral, bacterial and protozoal causes of diarrhea including norovirus, Salmonella, Cryptosporidium and Giardia lamblia. Serologic testing for Epstein-Barr virus demonstrated a positive immunoglobulin G to the viral capsid and negative immunoglobulin M testing. Human immunodeficiency virus antibody was non-reactive. Blood cultures were obtained for bacterial, fungal and mycobacterial infection and serum testing was performed for (1→3)-β-D-glucan (Fungitell, Associates of Cape Cod, Inc. East Falmouth, MA).
Figure 1: Chest Radiograph.
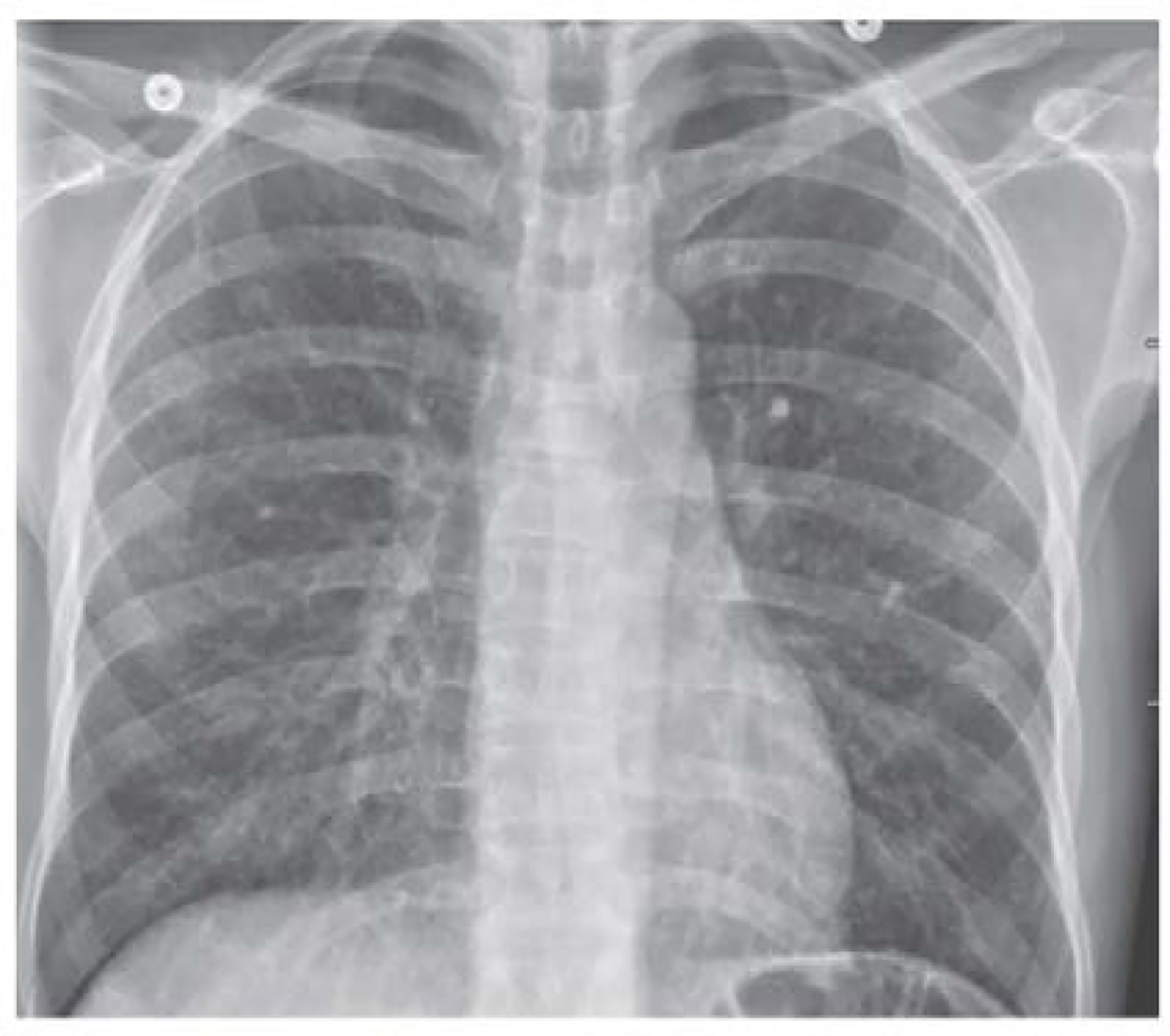
An anteroposterior chest radiograph shows diffuse reticulonodular opacities.
The negative cytomegalovirus PCR testing argues against systemic infection, but exclusion of cytomegalovirus colitis requires colonic biopsies. Results of Epstein-Barr virus testing are inconsistent with acute infection. The reticulonodular opacities on the chest radiograph raise suspicion for an atypical mycobacterial or fungal infection. Testing for (1→3)-β-D-glucan can serve as an adjunctive test for invasive fungal infections from fungi expressing (1→3)-β-D-glucan in their cell wall--including Candida, Aspergilli, Pneumocystis jirovecii, Histoplasma capsulatum and Coccidioides species -- and has a high negative predictive value for infection with these organisms. In contrast, Cryptococcus and Blastomyces produce very low levels of (1→3)-β-D-glucan and are therefore not readily detected by serum testing against the cell wall antigen.
On the morning of the third hospital day, the patient had an acute change in his clinical presentation with the development of diffuse abdominal pain, distension and guarding. Heart rate was elevated to 119 beats per minute and blood pressure was 109/78 mm Hg. The white-cell count was 2,300 per cubic millimeter and the hemoglobin level was 8.7 grams per deciliter. Serum sodium was 128 millimoles per liter, potassium was 3.8 millimoles per liter and C-reactive protein was 109 milligrams per liter. Serum lactic acid was 2.4 millimoles per liter (normal range, 0.5–2). Serum (1→3)-β-D-glucan returned at 67 picograms per milliliter (normal < 60).
The acute change in clinical status and his abdominal examination is concerning for perforation. The elevated serum lactate is concerning for focal ischemia or intestinal perforation. Moreover, the persistent fevers, pancytopenia, and chest radiograph findings are concerning for an invasive infection. While false positive serum (1→3)-β-D-glucan results may occur in individuals with advanced liver disease and impairments in intestinal permeability, including UC, the patient’s history of immune suppression with an anti-TNF therapy and his residence in the upper Midwest (including work as a grain operator), combined with other findings, suggests that infection due to Histoplasma capsulatum is most likely.
A supine and standing flat plate radiograph of the abdomen demonstrated a focally dilated loop of colon in the mid-abdomen measuring 6.9 cm without the presence of free air (Figure 2). A CT exam of the abdomen and pelvis showed diffuse rectosigmoid wall thickening, a punctate area of pneumoperitoneum and oral contrast extravasation adjacent to the sigmoid colon with contrast layering in the right lower quadrant consistent with an acute perforation (Figure 3). The patient was taken to the operating room, where he was found to have pancolitis with a large perforation in the anterior rectum requiring a total proctocolectomy with an end ileostomy. Additionally, a small bowel perforation was noted 20 cm distal to the ligament of Treitz arising from a small bowel stricture in the jejunum, which required resection and stapled primary anastomosis.
Figure 2: Abdominal Radiograph.
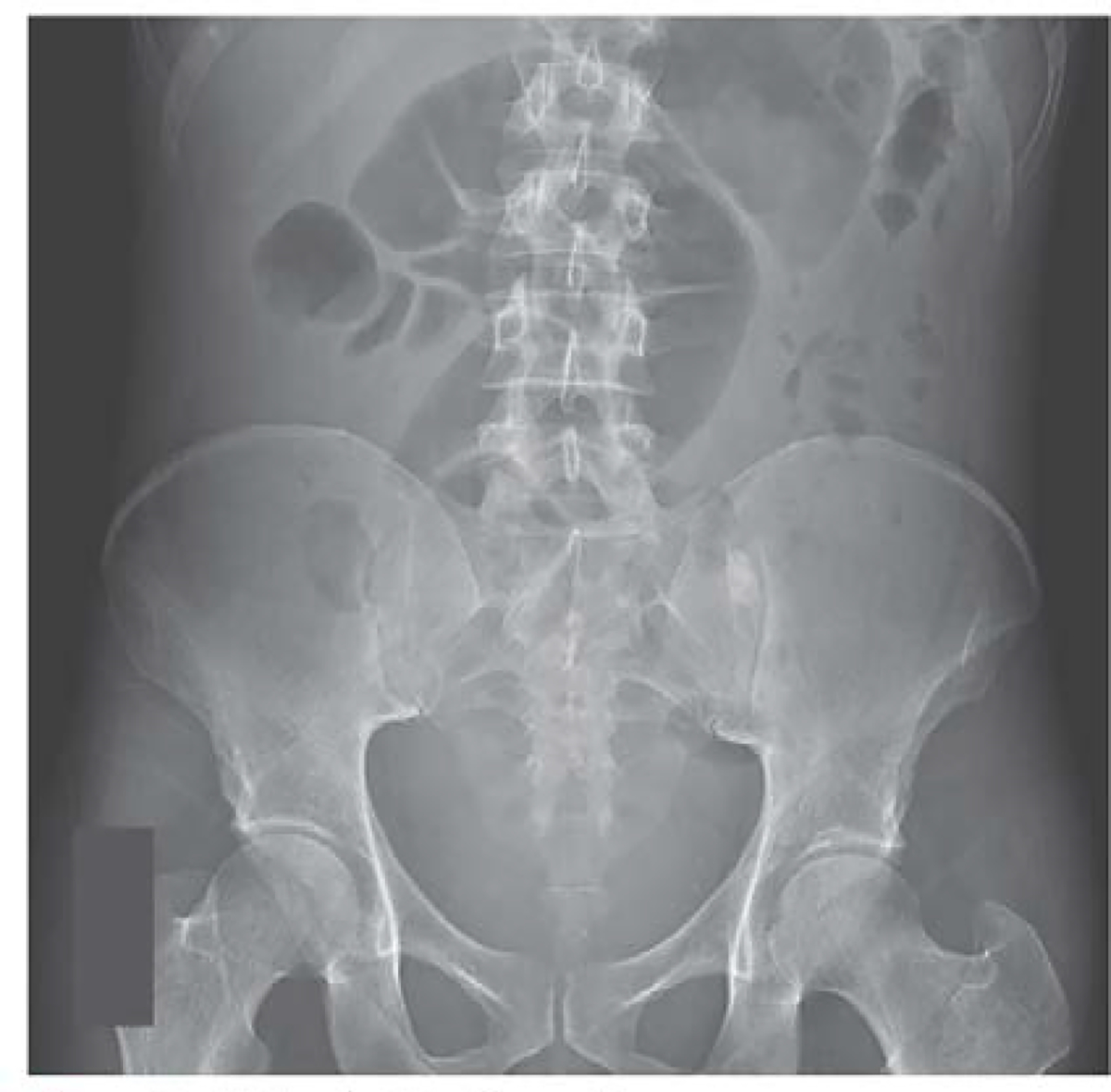
An anteroposterior abdominal radiograph obtained with patient in the supine position shows a focally dilated loop of colon.
Figure 3: CT of the Abdomen and Pelvis.
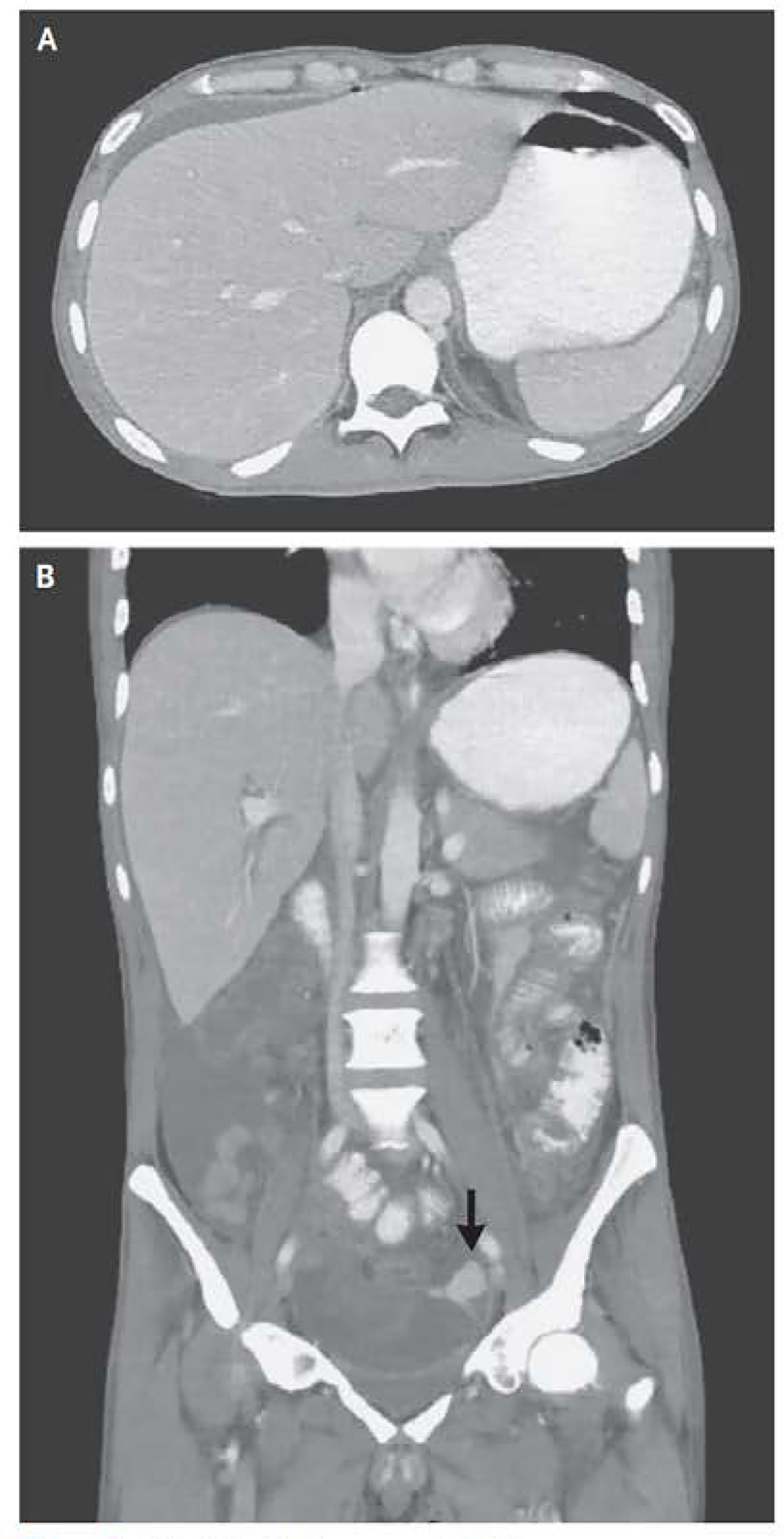
CT of the abdomen and pelvis was performed after the adminstraion of oral contrast material. An axial iamge of the abdomen (Panel A) shows a punctate focus of pneumoperitoneum anterior to the liver, with small volume perihepatic ascites. A coral image of the abdomen and pelvis (Panel B) shows diffuse wall thickening in the rectosigmoid colon and extravasation of extraluminal contrast material (arrow) into the area adjucent to the sigmoid colon, with layering of the contrast material, findings that are thought to indicate a perforation.
The surgical specimens revealed a jejunal stricture containing granulomas and evidence of perforation as well as granulomas in the colon and perforation in the rectum. Grocott-Gomori’s methenamine silver (GMS) and Periodic acid–Schiff (PAS) stains showed numerous yeast forms consistent with Histoplasma capsulatum (Figure 4). Ziehl-Neelsen staining for acid-fast bacilli was negative and an immunostain for cytomegalovirus was negative for viral inclusions. Urinary Histoplasma antigen returned at 16.7 nanograms per milliliter (normal range 0.2–25) three days after surgery. Serum antibody titers by complement fixation against the Histoplasma antigen were 1:128 and against yeast antigen 1:32 (>1:32 associated with active disease). Post-operatively, the patient had intermittent episodes of delirium and a lumbar puncture was performed to exclude cerebrospinal fluid (CSF) infection, which was negative for the presence of Histoplasma based on fungal culture and antibody testing.
Figure 4: Resection Specimens.
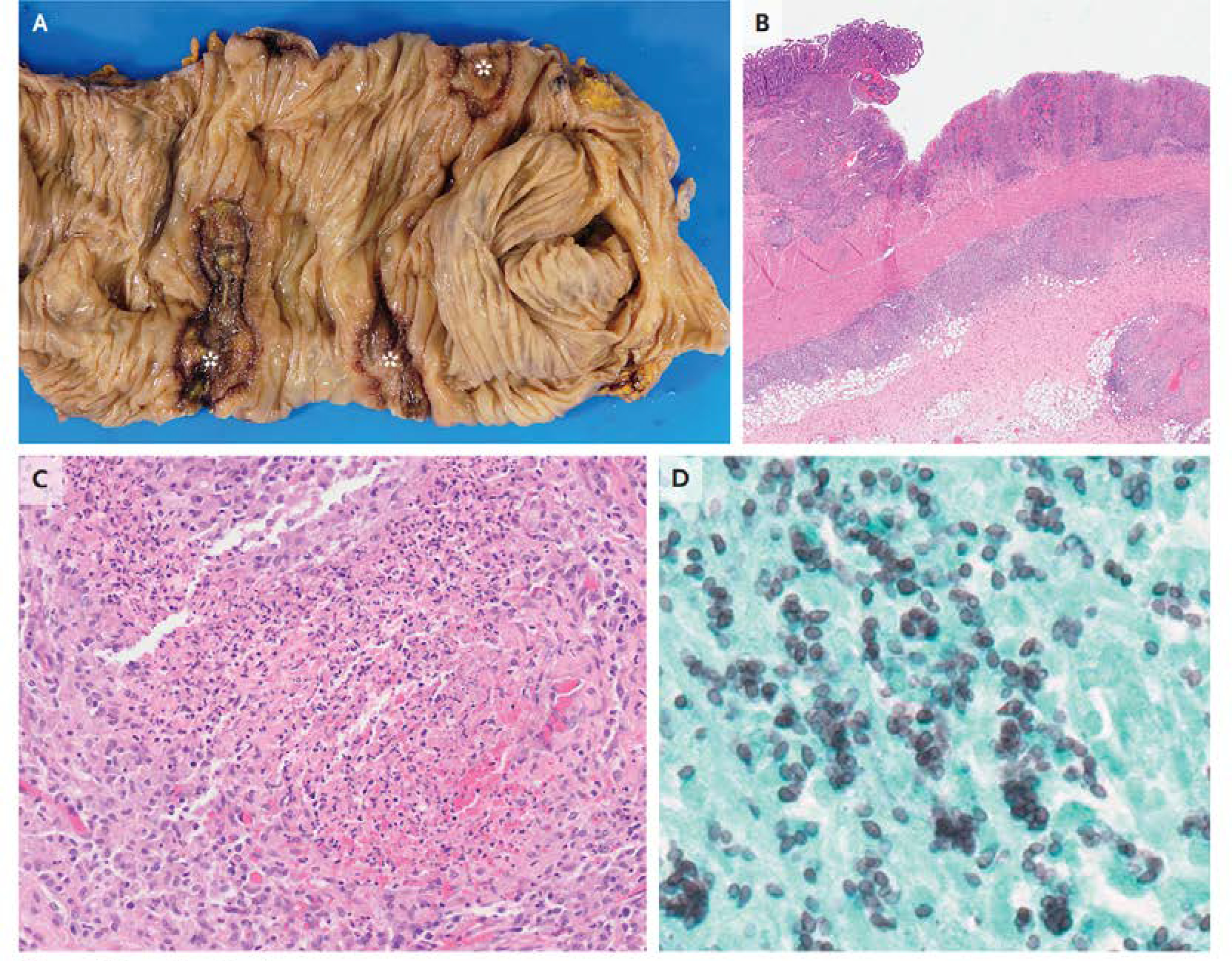
Gross examination of resection specimen of the colon (Panel A) reveals ulceration (asterisks). Hematoxylin and eosin staining (Panel B) shows ulceration with transmural granuloma. At higher power (Panel C), necrotizing granulomas are seen. Grocott-Gomori methenamine silver staining (Panel D) highlights clusters of small, ovoid yeast forms that are consistent with Histoplasma capsulatum.
While hospitalized, the patient completed two weeks of induction therapy with intravenous liposomal amphotericin B (3 mg/kg daily) and was started on oral itraconazole (200 mg three times per day for three days, followed by 200 mg twice daily). The performance of a colectomy eliminated the need for further immunosuppressive therapy for his UC. 12 months after initiation of therapy, a follow-up chest CT scan demonstrated improvement in the diffuse bilateral lung nodularity, and itraconazole therapy was discontinued. At that point the patient had regained 27 pounds, fevers and pancytopenia had resolved, and he remained well, with a functioning ileostomy.
Commentary:
Our patient presented with bloody diarrhea suggestive of severe UC. Evaluation for infection initially was unrevealing, but ultimately invasive histoplasmosis was identified on pathological specimens from the urgent colectomy performed after the patient experienced a bowel perforation.
Histoplasma capsulatum is an endemic fungal organism with a worldwide distribution. It is most commonly found in the central and eastern parts of the United States, with a high prevalence in the Ohio and Mississippi River valley regions. Histoplasma conidia inhabit soil with high nitrogen content often arising from bird or bat droppings and result in infection via inhalation when the soil is disturbed (1). In the lungs, the conidia transform from a mold state into a yeast state (dimorphic fungus) after which they are phagocytized by the alveolar macrophages. Most patients with histoplasmosis are asymptomatic or develop mild symptoms with a self-limited disease course. However, patients with a high inoculation burden or those who are immunosuppressed are at risk for severe or disseminated disease (2).
The gold standard for diagnosis of histoplasmosis is fungal culture or histologic diagnosis. However, the diagnosis is strongly suggested by a positive enzyme immunoassay (EIA) targeting the galactomannan antigen contained within the Histoplasma polysaccharide cell wall. Antigen testing can be performed on urine and most other clinical specimens; sensitivity for detection is increased when antigen testing is combined with serum antibody testing against Histoplasma (3). History taking in endemic areas should assess for activities that result in high exposure risk, such as digging in soil where there are bird or bat droppings, cleaning chicken coops, exploring caves, or remodeling or demolishing old buildings. It is possible that the patient’s history of employment as a grain elevator operator exposed him to aerosolized conidia. A high index of clinical suspicion is important, as symptoms can be nonspecific, including fever, weight loss, and malaise, as in this case.
Histoplasmosis can affect numerous organ systems, including the gastrointestinal tract, but most commonly causes pulmonary disease. Chest imaging characteristically shows pulmonary small nodular pulmonary opacities with a diffuse distribution. Gastrointestinal findings can include polypoid masses and/or ulcerations in the small bowel and colon, which can mimic IBD (4). In a large case series of individuals presenting with disseminated disease, approximately 70% of patients were found to have gastrointestinal involvement at autopsy, but only 3–12% of cases were known to have clinical gastrointestinal manifestations (5). Perforation is a rare complication of disseminated histoplasmosis. When it occurs, the most common sites are the terminal ileum or proximal colon, which has been attributed to the high density of lymph nodes that can harbor Histoplasma in these regions (6).
Therapy with anti-TNF agents is a well-recognized risk factor for fungal infections and mycobacterial infections (7–8). Histoplasma replicates within the host macrophages until T-cells become activated. It is hypothesized that TNF-α plays a central role in protection against Histoplasma, potentially through the expansion of T cells (9), and that, correspondingly, anti-TNF therapy predisposes to the development of disseminated histoplasmosis.
Treatment of individuals with moderate-to-severe disseminated histoplasmosis includes liposomal amphotericin B and oral itraconazole. Induction therapy with amphotericin B for a 1–2 week course is recommended, followed by oral itraconazole for a total of at least 12 months with treatment continued until clinical and laboratory findings have returned to normal (10). In a randomized trial comparing induction therapy with liposomal amphotericin B versus the deoxycholate formulation for 2 weeks (in both cases followed by oral itraconazole) in individuals with moderate-to severe histoplasmosis and acquired immunodeficiency syndrome, the regimen containing liposomal amphotericin B resulted in a higher clinical success rate and less nephrotoxicity (11). Alternative azole therapies may be utilized in the setting of itraconazole intolerance or drug interactions, although data are limited to inform their relative benefits and risks (10).
In this case, accurate identification of histoplasmosis as the cause of this patient’s illness led to appropriate treatment and clinical recovery. This case highlights the need to consider alternate causes of colitis in patients with UC with atypical presenting features.
Acknowledgements:
Raghavendra Paknikar wrote the first draft of the manuscript. The authors would like to thank Dr. Robert Clark for assistance in obtaining historical records and Drs. Christopher Lehmann and Carol Semrad for critical review of the manuscript.
Footnotes
Disclosures provided by the authors are available with the full text of this article at NEJM.org.
Support: None
References:
- 1.Salzer HJF, Burchard G, Cornely OA, et al. Diagnosis and management of systemic endemic mycoses causing pulmonary disease. Respiration. 2018;96(3):283–301. [DOI] [PubMed] [Google Scholar]
- 2.Newman SL. Cell-mediated immunity to Histoplasma capsulatum. Sem Respir Inject. 2001;16:102–108. [DOI] [PubMed] [Google Scholar]
- 3.Richer SM, Smedema ML, Durkin MM, et al. Improved Diagnosis of Acute Pulmonary Histoplasmosis by Combining Antigen and Antibody Detection. Clin Infect Dis 2016. Apr 1; 62(7):896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhinder J, Mori A, Cao W, Malieckal A. A case of isolated gastrointestinal histoplasmosis. Cureus. 2018;10(7):e2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodwin RA Jr, Shapiro JL, Thurman GH, et al. Disseminated histoplasmosis: clinical and pathologic correlations. Medicine (Baltimore) 1980;59:1–33. [PubMed] [Google Scholar]
- 6.Psarros G, Kauffman CA. Colonic histoplasmosis: a difficult diagnostic problem. Gastroenterol Hepatol (N Y). 2007;3(6):461–463. [PMC free article] [PubMed] [Google Scholar]
- 7.Winthrop KL, Yamashita S, Beekmann SE, Polgreen PM; Infectious Diseases Society of America Emerging Infections Network Mycobacterial and other serious infections in patients receiving anti-tumor necrosis factor and other newly approved biologic therapies: case finding through the emerging infections network. Clin Infect Dis. 2008;46:1738–1740. [DOI] [PubMed] [Google Scholar]
- 8.Gregory MH, Spec A, Stwalley D, et al. Corticosteroids increase the risk of invasive fungal infections more than tumor necrosis factor-alpha inhibitors in patients with inflammatory bowel disease. Crohns Colitis 360. 2023;5(2):otad010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deepe GS. Modulation of infection with Histoplasma capsulatum by inhibition of tumor necrosis factor-alpha activity. Clin Infect Dis. 2005;41 Suppl 3:S204–207. [DOI] [PubMed] [Google Scholar]
- 10.Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807–825. [DOI] [PubMed] [Google Scholar]
- 11.Johnson PC, Wheat LJ, Cloud GA, et al. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS, Ann Intern Med, 2002, vol. 137 (pg. 105–9) [DOI] [PubMed] [Google Scholar]


