Abstract
The acquisition of iron is an essential component in the pathogenesis of Yersinia pestis, the agent of bubonic and pneumonic plague. A cosmid library derived from the genomic DNA of Y. pestis KIM6+ was used for transduction of an Escherichia coli mutant (SAB11) defective in the biosynthesis of the siderophore enterobactin. Recombinant plasmids which had a common 13-kb BamHI fragment were isolated from SAB11 transductants in which growth but not enterobactin synthesis was restored on media containing the iron chelator EDDA [ethylenediamine-di(o-hydroxyphenyl acetic acid)]. Subcloning and transposon mutagenesis revealed a 5.6-kb region, designated yfe, essential for SAB11 growth stimulation. In vitro transcription-translation analysis identified polypeptides of 18, 29.5, 32, and 33 kDa encoded by the yfe locus. Sequence analysis shows this locus to be comprised of five genes in two separate operons which have potential Fur-binding sequences in both promoters. A putative polycistronic operon, yfeABCD, is Fur regulated and responds to iron and manganese. A functional Fur protein is required for the observed manganese repression of this operon. This operon encodes polypeptides which have strong similarity to the ATP-binding cassette (ABC) family of transporters and include a periplasmic binding protein (YfeA), an ATP-binding protein (YfeB), and two integral membrane proteins (YfeC and -D), which likely function in the acquisition of inorganic iron and possibly other ions. The ∼21-kDa protein encoded by the separately transcribed yfeE gene may be located in the cell envelope, since a yfeE::TnphoA fusion is PhoA+. Mutations in this gene abrogate growth of SAB11 on iron-chelated media.
Yersinia pestis is the facultative intracellular, gram-negative bacterium that causes bubonic and pneumonic plague. In nature, the organism exists as a zoonotic disease of rodents and their associated fleas and has been responsible for the widespread loss of human life during several catastrophic pandemics (59). Colonization and growth of bacterial pathogens, including Y. pestis, are dependent on the ability of the invading organism to acquire iron. Since most of the iron in a mammalian host is complexed with metalloproteins or sequestered by the iron storage protein ferritin, bacteria have evolved elaborate strategies to overcome this iron-deficient environment in order to obtain iron for growth (37, 54). Many utilize a variety of extracellular, low-molecular-weight ferric iron chelators termed siderophores (15, 36, 37). Still others obtain iron directly from hemoproteins or the high-affinity iron-binding glycoproteins transferrin and lactoferrin through specific receptors on the bacterial surface (22, 35, 51, 54).
The relationship between iron availability and virulence was clearly demonstrated in spontaneous nonpigmented (Pgm−) mutants of Y. pestis, in which virulence in mice was restored when the strains were inoculated via peripheral routes and supplemented with hemin or inorganic iron (49). Iron-independent virulence in mice and formation of dark brown, or pigmented, colonies at 26°C from adsorption of exogenous hemin were the original defining characteristics of the chromosomally encoded pigmentation (Pgm+) phenotype of Y. pestis (48, 49). Pgm− mutants often arise via spontaneous deletion of the 102-kb pigmentation (pgm) locus, which is known to encode an ∼7-kb hemin storage (hms) locus required for the Hms+ phenotype (pigmented colony formation) and the yersiniabactin-iron transport system (Ybt) (59).
The Ybt transport system is a siderophore-dependent uptake mechanism that is common to the three pathogenic species of Yersinia: Y. pestis, Yersinia pseudotuberculosis, and Yersinia enterocolitica (18, 32, 43, 59, 83). An ∼23-kb region possesses at least four genes likely encoding enzymes for the biosynthesis of the Ybt siderophore (9, 38) and for the receptor (Psn/FyuA) for Ybt and the bacteriocin pesticin (30, 31, 43, 61). Expression of Psn and the Ybt biosynthetic genes is negatively regulated by the Fur repressor but is also activated by YbtA (an AraC-type transcriptional activator located immediately upstream of the Ybt biosynthetic operon) and by the Ybt siderophore (29, 30, 43, 72, 74). Mutations in the Ybt transport system cause a drastic, or possibly complete, loss of virulence in mice infected subcutaneously (9).
However, Y. pestis cells possess additional independent hemoprotein and iron transport systems. A hemin utilization system (Hmu) allows the use of hemin and hemoproteins (46, 58, 69, 73). In addition to the Ybt system, inorganic iron transport is mediated by at least two other separate mechanisms. First, studies which monitored the growth of Pgm− strains in the presence of iron chelators suggest that Y. pestis cells possess an iron transport system that is functional at 26°C but not at 37°C (52). Second, Ybt− mutants of Y. pestis retain the ability to grow at 37°C under iron-deficient but not iron-chelated conditions (9, 30, 58, 68, 69).
In this study, we have used cloning and transposon mutagenesis to identify a pgm-independent transport system (Yfe) encoded in two distinct operons (yfeABCD and yfeE) and located on the Y. pestis chromosome. The data presented here suggest that the yfe locus of Y. pestis is an ATP-binding cassette (ABC) transport system involved in the acquisition of inorganic iron.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The relevant characteristics of all plasmids and bacterial strains are given in Table 1. All bacterial strains were maintained at −20°C in phosphate-buffered glycerol. Yersinia strains were grown routinely in heart infusion broth or on tryptose blood agar base (Difco Laboratories) at either 26 or 37°C. For iron-deficient growth, Y. pestis strains were grown in the chemically defined medium PMH, which had been deferrated prior to use by extraction with Chelex 100 (Bio-Rad Laboratories); this results in a residual iron content of ∼0.3 μM (73). Iron-deficient growth of Escherichia coli strains was accomplished by using Tris-glucose-thymidine medium (TG) prepared as described previously (46). Y. pestis KIM6+ and its Δpgm derivative KIM6 were grown on Congo red agar (78) to determine their hemin storage (Hms) phenotypes (31, 60). E. coli strains were cultured in Luria broth (LB) or Terrific broth (79) or on LB solidified with 1.4% (wt/vol) Bacto agar (Difco Laboratories). For growth studies requiring iron-chelated media, E. coli SAB11 cells containing various recombinant plasmids were grown on either LB agar containing 750 μM EDDA [ethylenediamine-di(o-hydroxyphenyl acetic acid)] (LA-EDDA) or TG medium solidified with 1.2% (wt/vol) agarose and supplemented with 75 μM EDDA (TGE). Where appropriate, media included antibiotics at the following concentrations: ampicillin, 100 μg/ml; tetracycline, 12.5 μg/ml; kanamycin, 25, 50, or 200 μg/ml; and chloramphenicol, 30 μg/ml.
TABLE 1.
Bacterial strains and plasmidsa
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Y. pestis | ||
| KIM6+ | Pgm+ Ybt+ Yfe+ Lcr− | 68 |
| KIM6 | Pgm− (Δpgm) Ybt− Yfe+ Lcr− | 32, 68 |
| KIM6-2030 | Pgm− Fur− (fur::kan-9) Ybt− Yfe+ Lcr− Kmr | 73 |
| Y. enterocolitica WA | Serotype O:8, Ybt+ Yfu+ Psts Lcr+ | 32, 82 |
| Y. pseudotuberculosis PB1/+ | Serotype 1, Psts Lcr+ | 32, 82 |
| E. coli | ||
| CC118 | Strain for detecting PhoA+ fusion proteins, PhoA− | 53 |
| DH5α | Cloning strain, Yfe− PhoA− | 5 |
| HB101 | K-12 × B hybrid strain, Ent+ Yfe− Smr | 5 |
| LE392 | Strain for propagating λTnphoA | 5 |
| SAB11 | Ent− derivative of HB101 | 6 |
| Plasmids | ||
| pACYC184 | 4.2-kb cloning vector, Cmr Tcr | 5 |
| pBluescript II KS+ | 3.0-kb cloning vector, Apr | Stratageneb |
| pBR322 | 4.4-kb cloning vector, Apr Tcr | 5 |
| pUC19 | 2.7-kb cloning vector, Apr | 65 |
| pEUPP1 | 15.3-kb pEU730-derived reporter plasmid containing the psn promoter region upstream of a promoterless lacZ gene, Spr | 29 |
| pSC27.1 | Reporter plasmid containing an iron- and Fur-regulated lacZ gene | 17 |
| pYHE3 | pHC79-derived cosmid clone containing an ∼23-kb fragment from Y. pestis KIM6+ genomic DNA, Apr | This study |
| pYFE1 | 12.8-kb BamHI fragment from pYHE3 cloned into pUC19, Yfe+ Apr | This study |
| pYFE1.1 | 12.8-kb BamHI fragment from pYHE3 cloned into pACYC184, Yfe+ Cmr | This study |
| pYFE1.2 | pYFE1.1 with BamHI fragment in the opposite orientation, Yfe+ Cmr | This study |
| pYFE2 | 5.2-kb ClaI/BamHI fragment of pYFE1 inserted into pBR322, Yfe− Apr | This study |
| pYFE3 | 7.7 kb BamHI/ClaI fragment of pYFE1 cloned into pBR322, Yfe+ Apr | This study |
| pYFE3.1 | 7.7-kb BamHI/ClaI fragment of pYFE3 cloned into pBluescript, Yfe+ Apr | This study |
| pYFE4 | 5.3-kb BamHI/HindIII fragment of pYFE1 inserted in pBR322, Yfe− Apr | This study |
| pYFE6 | 3.6-kb PvuI/PstI fragment of pYFE1 cloned into pBR322, Yfe− Tcr | This study |
| pYFE8 | 9.7-kb NcoI fragment of pYFE1 cloned into pACYC184, Yfe+ Tcr | This study |
| pYFE9 | 6.1-kb PstI fragment of pYFE1 inserted into pBR322, Yfe− Tcr | This study |
| pYFE10 | pYFE1.1 lacking internal 5.3-kb SphI fragment, Yfe+ Cmr | This study |
| pYFE11 | pYFE3 with a cat cassette inserted at the HindIII site, Yfe+/− Apr Cmr | This study |
| pYFE12 | pYFE10 with a kan cassette inserted at the PstI site, Yfe− Kmr Cmr | This study |
| pYFE13 | pYFE10 with a kan cassette inserted at the XhoI site, Yfe− Kmr Cmr | This study |
| pYFE15 | pYFE1 with 4.5-kb XhoI fragment deletion, Yfe− Apr | This study |
| pYFE21 | 4.2-kb EcoRI/PstI fragment of pYFE1 cloned into pBluescript, Yfe− (ΔyfeAB) Apr | This study |
| pYFE30 | ∼1.8-kb EcoRI fragment deletion of pYFE3.1, Yfe− (ΔyfeAB) Apr | This study |
| pYFE34 to -46 | TnphoA insertion mutants of pYFE1.1 (Fig. 1B), Cmr Kmr | This study |
| pYFE47 | 14.2-kb SalI fragment deletion of pYFE34, Yfe−, PhoA+ reporter for Y. pestis KIM6-2030, Cmr Kmr | This study |
Y. pestis KIM6+ possesses an intact 102-kb pgm locus containing the genes for hemin storage (hms), psn, ybtA, and the Ybt biosynthetic genes irp1, irp2, ybtT, and ybtE. Y. pestis KIM6 is an isolate of KIM6+ with the 102-kb region deleted. Yersinia strains producing the siderophore yersiniabactin have been designated Ybt+, while those defective in yersiniabactin secretion are Ybt−. The Yfe+ designation indicates the presence of operons yfeABCD and yfeE. Mutations in either yfe operon are depicted as Yfe−. Lcr+ and Lcr− refer to the presence or absence, respectively, of the low-calcium response phenotype conferred by the 70- to 75-kb virulence plasmid. Ent+ and Ent− indicate production or lack of the siderophore enterobactin, respectively. E. coli strains which fail to express alkaline phosphatase are designated PhoA−. Apr, Cmr, Kmr, Smr, Spr, and Tcr, resistance to ampicillin, chloramphenicol, kanamycin, streptomycin, spectinomycin, and tetracycline, respectively.
Stratagene Cloning Systems, La Jolla, Calif.
Recombinant DNA methodology.
Plasmids were introduced into E. coli strains by standard CaCl2 transformation (65). Isolation of plasmid and cosmid DNAs was achieved by alkaline lysis (12), and the DNAs were further purified when necessary by polyethylene glycol precipitation (47). DNA probes used in Southern hybridizations (5) were radiolabeled with [α-32P]dCTP (ICN Biomedicals, Inc.) by nick translation with a commercially available kit (Life Technologies, Inc.). Hybridization to confirm the presence of the yfe locus in various recombinant cosmids was carried out as described by Fetherston et al. (32). Radiolabeled blots were exposed to Kodak XAR-5 film at −80°C with intensifying screens. Subclones derived from pYFE1.1 (Table 1; Fig. 1) were constructed by using restriction endonucleases and DNA-modifying enzymes according to the manufacturers’ instructions. The Yfe insertion mutants depicted in Fig. 1 were generated by introduction of antibiotic resistance cassettes (Pharmacia, Inc.) into functional pYFE subclones (Table 1).
FIG. 1.
(A) Restriction map of pYFE1.1 and various subclones used to test for restoration of growth of E. coli SAB11. +, plasmids which promote SAB11 growth; −, no growth stimulation. Arrows indicate the directions of transcription of genes included in the yfe locus. Dashed lines and open triangles represent deletions and sites of antibiotic cassette insertion, respectively. (B) Map of relevant TnphoA insertions within the yfe locus. Filled triangles represent PhoA+ translational fusions. + and − signs above each insertion site indicate iron-deficient growth and no growth, respectively, of SAB11 carrying that insertion in pYFE1.1. Numbers above each triangle represent plasmids pYFE34 to -46 (pYFE1.1::TnphoA34 to -46), respectively (Table 1). Shaded boxes upstream of yfeA and yfeE represent potential Fur boxes.
DNA sequencing was performed by the dideoxynucleotide chain termination method (66) with Sequenase version 2.0 and 35S-dATP (Amersham Corp.). Resolution of sequencing artifacts and compressions was achieved by using 7-deaza-dGTP (Boehringer Mannheim Biochemicals) and by the inclusion of dimethyl sulfoxide (Sigma Chemical Co.) to 10% (vol/vol) in primer-template annealing reactions. Sequencing reaction products were resolved on 6% polyacrylamide gels containing 8.3 M urea (Sigma Chemical Co.) and cast in Tris-borate-EDTA buffer (65). Dried gels were exposed to Kodak BioMax MR film at room temperature. Oligonucleotide primers (Integrated DNA Technologies, Inc.) were used to extend the sequence in the yfe region and to determine the sequence of the opposite strand within yfe coding regions. DNA sequence analysis was performed with programs from the Intelligenetics software suite.
Isolation of yfe clones.
Construction of a cosmid library derived from Y. pestis KIM6+ and its transduction into E. coli SAB11 (Table 1) have been described previously (46). Transduced cells were plated onto LA-EDDA plates supplemented with 5 μM hemin and ampicillin at 100 μg/ml. Isolated colonies were subsequently plated onto LA-EDDA containing either 0 or 10 μM hemin. Transductants requiring hemin for growth have been described elsewhere (46). Isolates exhibiting hemin-independent iron-chelated growth were designated yfe clones and are the subject of this study.
TnphoA mutagenesis of pYFE1.1.
For transposon mutagenesis, E. coli CC118 cells bearing plasmid pYFE1.1 (Table 1; Fig. 1) were transduced with λTnphoA (39) at a multiplicity of infection of 5.0 according to the method of de Bruijn and Lupski (23) with minor modifications. Briefly, transduced cells were spread onto LB agar containing chloramphenicol (30 μg/ml), kanamycin (200 μg/ml), and 5-bromo-4-chloro-3-indolyl phosphate (XP) (40 μg/ml) (Sigma Chemical Co.) and incubated at 30°C for 48 h. Kanamycin-resistant colonies were harvested, and plasmid DNAs from this pool of transformants were isolated by alkaline lysis (12). Pooled plasmid DNA was subsequently used to transform E. coli DH5α (PhoA−) cells for the selection of individual blue (cells expressing alkaline phosphatase) and white colonies (63). Transposon insertion sites of individual pYFE1.1::TnphoA recombinant plasmids were estimated by restriction endonuclease digestion. TnphoA insertion sites of mutant plasmids which abrogated growth stimulation of E. coli SAB11 cells on TGE medium were verified by DNA sequence analysis with the primer phoA.1 (5′-GTGCAGTAATATCGCCCTGAGC-3′) derived from the sequence of the E. coli phoA gene (19).
Alkaline phosphatase and β-galactosidase assays.
Cell lysates were prepared from cultures of Y. pestis grown in PMH or in PMH supplemented with either 1.0 μM MnCl2, 1.0 μM FeCl3, or 10 μM FeCl3. Cells were passaged twice for a total of approximately six to eight generations as described previously (72). Alkaline phosphatase and β-galactosidase activities were determined spectrophotometrically by monitoring cleavage, respectively, of the substrates p-nitrophenyl phosphate and 4-nitrophenyl-β-d-galactopyranoside (Sigma Chemical Co.). Units of alkaline phosphatase activity were calculated according to the formula described by Brickman and Beckwith (16), and β-galactosidase activity is expressed in Miller units (55).
Protein analyses.
In vitro transcription-translation of plasmid-encoded proteins was performed with an E. coli S30 cell extract system (Promega Corp.). Proteins were radiolabeled with 35S-labeled amino acids (DuPont NEN Research Products) according to the manufacturer’s recommendations, and equal amounts of trichloroacetic acid-precipitable counts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Dried gels were exposed to Kodak BioMax MR film at room temperature. Homology searches of protein databases were performed with BLAST (3). Pairwise and multiple sequence alignments were accomplished with the programs Gap (University of Wisconsin Genetics Computer Group software package version 8.1) and CLUSTALW (80), respectively. Determination of the transmembrane-spanning domains in the predicted amino acid sequences of YfeC and YfeD was performed with the program TopPred II (21).
PCR analyses.
Amplification of a region of the yfeA gene was accomplished by PCR with commercially synthesized oligonucleotides (Integrated DNA Technologies, Inc.) 4151.1 (5′-GAATGGCATGAATCTGGAGCG-3′) and 2021.4 (5′-GGCGGGTTTGTCGGAAATAG-3′), derived from the nucleotide sequence of Y. pestis yfeA. Similarly, synthetic primers yfuA5.2 (5′-CTGGTGAAATCCTGGGTC-3′) and yfuA3.3 (5′-TCCAGATCTTTCAGCGGCAC-3′), derived from the nucleotide sequence of the Y. enterocolitica yfuA gene, were used to amplify a fragment of the yfuA gene, encoding a putative periplasmic binding protein involved in inorganic iron transport (64). All strains were cultured at 37°C in microtiter wells for 4 to 6 h. For PCR analysis, 1.0 μl of cells (104 to 105 CFU) from each strain was used. Amplification reactions with mixtures containing either Taq DNA polymerase (Promega Corp.) or Pfu DNA polymerase (Stratagene), 200 μM deoxynucleoside triphosphates, 1.5 μM MgCl2, and 0.4 μM primers were performed for 30 s at 94°C, 55°C, and 72°C for 30 cycles. All PCRs were carried out with a GeneAmp PCR System 2400 thermal cycler (Perkin-Elmer). Products from these reactions were resolved by agarose gel electrophoresis.
Nucleotide sequence accession numbers.
The sequences of yfeABCD and yfeE have been deposited in the GenBank database and assigned accession numbers U50597 and U50903, respectively.
RESULTS
Isolation and subcloning of the Y. pestis yfe locus.
To identify heme and inorganic iron uptake systems of Y. pestis, a cosmid library derived from Y. pestis KIM6+ was transduced into E. coli SAB11 (Table 1), a mutant defective in the synthesis of the siderophore enterobactin (6). Eleven transductants were isolated from LA-EDDA plates containing 5 μM hemin as described in Materials and Methods and reference 46. Six of the isolates grew on LA-EDDA plates in the absence of added hemin and on TGE medium, precluding the possibility that growth on LA-EDDA was due to contaminating hemin. Southern hybridizations (5) confirmed that these cosmids have a common ∼13-kb BamHI fragment which is present in both Pgm+ and Pgm− strains of Y. pestis and in Y. pseudotuberculosis (data not shown). This fragment was subcloned from the recombinant cosmid pYHE3 into the vector pACYC184 (5) in both orientations and designated pYFE1.1 and pYFE1.2 (Table 1; Fig. 1). Further subcloning as well as insertion and deletion mutagenesis of this yfe-containing recombinant plasmid showed the essential region for SAB11 growth stimulation to be contained within a 7.7-kb BamHI-ClaI fragment represented by pYFE3 (Fig. 1A; Table 1). However, restoration of SAB11 growth mediated by pYFE1.1 and various subclones did not result in concomitant biosynthesis of the siderophore enterobactin. E. coli SAB11 cells carrying either pACYC184 (Table 1) or the nonfunctional pYFE1.1 subclones pYFE4, pYFE6, pYFE9, pYFE12, pYFE13, pYFE15, and pYFE30 (Fig. 1A) could not be cross-fed by adjacently streaked cells harboring either pYFE1.1 or pYFE3 on iron-deficient media. However, HB101, which synthesizes enterobactin, did cross-feed SAB11 in this assay system. Introduction of antibiotic resistance cassettes into the functional subclones pYFE3 and pYFE10 (Table 1) either abolished (pYFE12 and pYFE13) or inhibited (pYFE11) their growth-promoting properties in SAB11. DNA sequence analysis of the yfe locus (see below) places the HindIII site into which the chloramphenicol acetyltransferase (cat) cassette has been ligated in pYFE11 near the carboxy terminus of YfeD. The expression of a truncated YfeD polypeptide which has retained partial function may account for the reduced E. coli SAB11 growth for cells containing this plasmid. While similar growth characteristics might be expected for SAB11 cells transformed with pYFE4, it should be noted that this plasmid construct lacks the yfeE gene locus, which is an essential component in restoring growth to SAB11 on iron-chelated media (Fig. 1A).
Iron-deficient growth of E. coli SAB11.
In addition to scoring individual subclones of pYFE1.1 for their ability to promote the growth of E. coli SAB11 on iron-chelated media (Fig. 1A), we also examined the growth characteristics of this strain in liquid culture. E. coli SAB11 cells containing either pBR322 or the pYFE1.1 subclone pYFE3 (Table 1; Fig. 1A) and HB101(pBR322) were serially transferred twice (six to eight generations) to acclimate cells to medium conditions prior to monitoring their growth in TG medium with or without 10 μM FeCl3 for a period of 9 h. As shown in Fig. 2, the iron-limited growth of E. coli SAB11(pBR322) is enhanced by providing the yfe locus in trans (pYFE3). SAB11(pYFE3) shows a slightly increased initial growth rate over those of SAB11(pBR322) and its enterobactin-producing parent strain HB101(pBR322). The relative ineffectiveness of iron acquisition by the enterobactin siderophore system of HB101 is a curious but highly reproducible result. The increased growth of SAB11(pYFE3) over that of the enterobactin-producing HB101(pBR322) may be due to the copy number of the yfe operons contained within the pBR322-derived pYFE3 plasmid.
FIG. 2.
Growth of E. coli HB101 or SAB11 transformed with either pBR322 or pYFE3. Filled symbols indicate strains grown in TG minimal medium supplemented with 10 μM FeCl3, while open symbols designate growth in TG without supplementation. OD620, optical density at 620 nm.
TnphoA mutagenesis of plasmid pYFE1.1.
To further define the region necessary for restoring growth to E. coli SAB11, we employed a method of random mutagenesis with λTnphoA (39). In mapping transposon insertion sites within pYFE1.1 which abrogated growth of E. coli SAB11 on iron-chelated media, we also identified several TnphoA insertion mutants which exhibited alkaline phosphatase (PhoA+) activity by the phoA gene product, which requires a foreign signal peptide for export of PhoA to the periplasm where it becomes active (39, 53). PhoA+ insertions in three separate open reading frames (ORFs) (Fig. 1B), as determined by DNA sequence analysis, indicate that at least three proteins encoded by the yfe locus contain sequences which target them for export to the cell envelope. The results in Fig. 1B also show that despite multiple insertions within or upstream of ORF33, SAB11 growth was unaffected, indicating that this gene is not essential for promoting the iron-chelated growth of E. coli SAB11. By contrast, insertions in yfeA, yfeD, and yfeE resulted in a loss of the ability of SAB11 to utilize chelated iron. The weak, reduced growth phenotype of the single transposon insertion in yfeB cannot be readily explained but likely is aberrant. This is supported by the negative growth phenotype conferred by pYFE13 and by the genetic organization of yfeABCD (Fig. 1A), which suggests that interruption of the YfeB-coding region should have polar effects on downstream sequences and thus preclude the ability of such a plasmid to promote the growth of SAB11. Transposon insertions within yfeE (Fig. 1B) present apparently conflicting results. DNA sequence analysis of the TnphoA insertion proximal to the 5′ end of yfeE (pYFE45 [Table 1]) shows that the location of the yfeE-phoA fusion occurs just 13 nucleotides downstream of the yfeE translational start site (yfeE::TnphoA45). Despite this disruption of the yfeE coding region, plasmid pYFE45 is able to restore the growth of SAB11 on iron-chelated media. This is in contrast to the case for pYFE44, in which the transposon insertion site follows nucleotide 139 of the yfeE ORF (yfeE::TnphoA44) and does not restore SAB11 growth (Fig. 1B).
DNA sequence analysis of the yfe locus reveals two distinct operons.
The yfe locus contains two separate operons (yfeABCD and yfeE) that are essential for iron acquisition in E. coli SAB11. The product of ORF33 (Fig. 1B) is a hypothetical protein, as no evidence for its expression yet exists. The promoter region of each operon has a putative Fur-binding sequence (FBS) (Fig. 3) which has 89% (yfeABCD) and 63% (yfeE) identity to the E. coli FBS consensus sequence (77). Moreover, imperfect inverted repeats can be found downstream of the translational stop codons for yfeD and yfeE and may serve as transcriptional terminators (Fig. 3) YfeE is an ∼21-kDa protein with a pI of 9.3 and significant homology (75.3% similarity) to the product of a 534-bp ORF from E. coli designated f178 (13) (GenBank accession number U00096). The PhoA+ yfeE::TnphoA fusions (Fig. 1B) suggest that YfeE is located in the cell envelope despite the absence of an obvious signal sequence. The genetic organization of yfeABCD suggests that it is a polycistronic operon. Examination of the intergenic nucleotide sequences indicates that expression of YfeABCD is translationally coupled (Fig. 3). BLAST homology searches (3) show that the yfeABCD operon likely encodes components of a periplasmic binding protein-dependent transport system or ABC transporter (27, 44), with the 35.7-kDa YfeA predicted to be a periplasmic binding protein, the 32.2-kDa YfeB predicted to be an ATP-binding protein, and YfeC and YfeD (31.7 and 32.2 kDa, respectively) predicted to be integral membrane permeases. The predicted translation product of yfeA has significant amino acid similarity (82%) to an iron-repressible periplasmic protein of unknown function from Haemophilus influenzae (42). More recently, this polypeptide has been shown to be derived from a complex and as-yet-uncharacterized region of the H. influenzae genome (HI0359 to HI0362) whose genetic organization is identical to that of yfeABCD and whose ORFs have >80% similarity at the amino acid level (33). Additionally, the yfeABCD operon has ∼70% amino acid similarity with an ABC transporter complex for manganese (mntCAB) in the cyanobacterium Synechocystis sp. strain PCC 6803 (7). Moreover, a putative ABC transporter (troABCD products) has recently been identified in the syphilis spirochete Treponema pallidum (41). This operon encodes proteins TroA to -D, which have amino acid similarities of 51, 61, 54, and 60%, respectively, with YfeA to -D. An amino acid sequence alignment between YfeA, -B, and -C and their H. influenzae and Mnt homologs is shown in Fig. 4. For the purpose of clarity, alignments with YfeD were omitted, since the mnt operon lacks this additional ORF (7). The deduced amino acid sequence encoded by yfeB shows strong similarity to ATP-binding proteins in several systems, including those involved in the uptake of inorganic iron (15, 44). The amino acid sequence alignment between YfeB, HI0361, and MntA (Fig. 4) reveals the high degree of conservation surrounding the ATP-binding motifs (Walker A and B) of these proteins (44). These sites are recognized for their ability to form an ATP-binding pocket and are the distinguishing feature among the family of ABC transporters (44). The integral membrane proteins YfeC and YfeD are strongly hydrophobic, with each containing up to seven membrane-spanning domains and a distinctive domain known as the EAA motif (Fig. 5). This signature motif typically occurs in an hydrophilic loop region and contains an invariant glycine residue positioned about 100 amino acids from the C terminus of the protein (119 and 125 residues for YfeC and YfeD, respectively), and it is highly conserved among cytoplasmic membrane permeases (67). In addition to the YfeC sequence homologies depicted in Fig. 4, the products encoded by yfeC and yfeD have homology with a number of hydrophobic integral membrane proteins found in Streptococcus spp. (26, 28, 50, 57). Interestingly, despite similarities in their predicted secondary structures, pairwise alignment of the amino acid sequences for YfeC and YfeD (Fig. 5) shows a lesser degree of similarity (60%) than is indicated for YfeC and HI0360 (83%), YfeC and MntB (72%), or YfeD and HI0359 (80%).
FIG. 3.
Partial DNA sequence representation of the yfeABCD and yfeE operons and ORF33, including single-letter amino acid translations of the 5′ and 3′ ends of each ORF. Only the coding strand of each gene is shown, and omitted nucleotide sequences are depicted as dots. The FBSs in the promoter regions of yfeA and yfeE are overlined and underlined, respectively. Nucleotides in boldface represent potential ribosomal binding sites. Nucleotides overlined with arrows, which follow the yfeD termination codon and the translational stop of yfeE, represent imperfect inverted repeat structures. The translational start sites of the Yfe polypeptides are indicated by arrows. YfeA′ indicates an alternate translational start site for the yfeA coding region. Underlined amino acids in YfeA depict the region of homology with N-terminal amino acid sequence of a 31-kDa iron-repressible periplasmic binding protein from H. influenzae (42). The vertical arrow marks a putative signal sequence cleavage site.
FIG. 4.
CLUSTALW amino acid sequence alignments of Y. pestis proteins YfeA, YfeB, and YfeC with components of a manganese transporter from Synechocystis sp. strain PCC 6803 (MntCAB) and with polypeptides from a putative ABC transporter from H. influenzae (HI0360, HI0361, and HI0362). Amino acids in boldface represent ATP-binding motifs (Walker A and Walker B). The consensus line displayed below the aligned sequences depicts identical amino acids as asterisks, with conserved residues shown as dots.
FIG. 5.
Amino acid sequence alignment of the putative cytoplasmic membrane proteins YfeC and YfeD. Identical amino acids are indicated by vertical lines, while conserved and semiconserved residues are shown by colons and dots, respectively. Overlined (YfeC) and underlined (YfeD) amino acids designate the predicted transmembrane (TM)-spanning domains of these polypeptides. Amino acids in boldface type represent the EAA motif, a conserved hydrophilic loop region common to the integral membrane component of bacterial binding protein-dependent transport systems (67). The invariant glycine residue is marked with an asterisk.
Identification of putative yfe-encoded proteins.
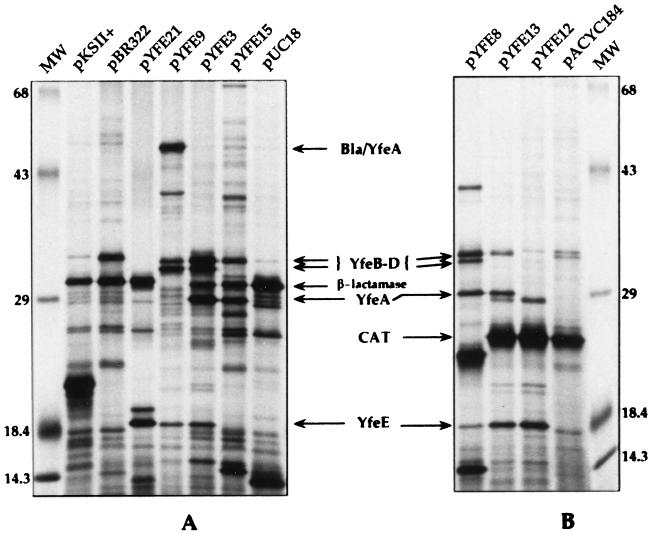
To determine the polypeptides necessary for promoting growth of E. coli SAB11 on iron-chelated media, proteins encoded by the plasmid pYFE3 as well as selected subclones were examined by in vitro transcription-translation (Fig. 6). The similar predicted molecular masses of the proteins encoded by the yfeABCD operon have made specific identification of these gene products problematic due to possible comigration of several polypeptides in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Fig. 6). We have identified at least four polypeptides, with molecular masses of 18, 29.5, 32, and 33 kDa (Fig. 6A), encoded by the 7.7-kb insert of pYFE3, which had previously been designated the essential region for promoting the iron-deficient growth of SAB11. The most readily identifiable gene product, YfeE, migrates at ∼18 kDa, compared to its predicted mass of 21 kDa, and is evident in the protein profiles of pYFE3, pYFE8, pYFE9, pYFE12, pYFE13, and pYFE21 (Fig. 6). YfeE is absent from the profile of pYFE15 (Fig. 6A). In addition to the loss of YfeE expression from pYFE15, it was expected that at least two other proteins (YfeC and YfeD), whose genes are contained within the deleted region of this plasmid (Fig. 1A), would be lost. While the protein band migrating at ∼32 kDa is no longer evident, the persistence of a 33-kDa band may be explained by the presence of an additional 5.1 kb of Y. pestis DNA downstream of the XhoI deletion in this plasmid. Products potentially encoded by this region of pYFE1.1 have not been extensively examined. While yfeA is predicted to encode a 35.7-kDa protein, a polypeptide migrating at this position was not detected (Fig. 6). To determine the relative migration of YfeA, we examined the protein profile of pYFE9 (Fig. 6A). Construction of this plasmid resulted in a fortuitous translational fusion between the pBR322-encoded β-lactamase protein (Bla) and YfeA, resulting in a fusion protein with a predicted molecular mass of ∼53 kDa. The appearance of the Bla-YfeA fusion in pYFE9 and the concomitant loss of the species migrating at 29.5 kDa (Fig. 6A) suggest that this band corresponds to YfeA. The protein bands migrating at 32 and 33 kDa account for the remaining proteins, YfeB to -D. Since there are only two bands present to account for three distinct proteins, two-dimensional gel electrophoresis will be required to resolve individual YfeB-, -C, and -D polypeptides. Finally, since the organization of yfeABCD and supporting DNA sequence data suggest that it is a polycistronic operon, we examined the in vitro transcription-translation protein profiles of plasmids that either lacked the yfeA promoter region or contained an insert which interrupted the yfeA coding region. The profile of plasmid pYFE12 shows that insertion of a kan cassette near the 5′ end of yfeA causes polar effects on downstream sequences, resulting in the apparent loss of polypeptides YfeA to -D (Fig. 6B). Similarly, plasmid pYFE21 (Table 1), which lacks the yfeA promoter region as well as yfeA and a portion of yfeB, fails to express downstream sequences. However, both pYFE12 and pYFE21 maintain expression of the 18-kDa protein, confirming that YfeE is derived from a separate transcriptional unit (Fig. 6).
FIG. 6.
Autoradiograms of plasmid-encoded proteins labeled with 35S-amino acids by in vitro transcription-translation. Lanes MW, molecular mass markers. Other lane designations indicate the plasmids used. Maps of pYFE plasmids are shown in Fig. 1A. Relevant proteins are indicated by arrows. Bla/YfeA, β-lactamase–YfeA translational fusion; CAT, chloramphenicol acetyltransferase. Numbers on the left and right are molecular masses in kilodaltons.
Regulation of the yfeA promoter by iron, manganese, and Fur.
We introduced plasmid pYFE34 (yfeA::TnphoA34) into Y. pestis KIM6 and KIM6+ by electroporation to examine expression of the plasmid-encoded YfeA-PhoA fusion protein under repressive or nonrepressive conditions. The alkaline phosphatase activities of these strains grown in the presence or absence of iron or manganese are reported in Table 2. Y. pestis KIM6(pYFE34) cells grown in PMH medium under iron-deficient conditions exhibit a >7-fold increase in enzymatic activity over cells grown in iron-replete medium. Iron-repressible alkaline phosphatase activity was also demonstrated in the Pgm+ derivative KIM6(pYFE34)+, albeit at a slightly lower level. Expression was derepressed ∼5-fold in this strain in the absence of iron, suggesting that Pgm− cells are more iron starved than Pgm+ cells. The lower induction ratio in KIM6(pYFE34)+ is likely due to the presence of the biosynthetic locus for the Y. pestis siderophore, yersiniabactin, and its receptor gene, psn (9, 30, 31). This is in agreement with similar studies measuring β-galactosidase activity, in which Staggs et al. demonstrated slightly higher enzyme activity from an iron- and Fur-regulated promoter construct in Y. pestis KIM6 (Pgm−) cells compared to the Pgm+ KIM6+ strain (72).
TABLE 2.
β-galactosidase and alkaline phosphatase activities of Y. pestis KIM6+ and KIM6 derivatives grown to mid-log phase in defined PMH medium
| Strain | Alkaline phosphatase or β-galactosidase activitya of cells grown in medium with:
|
Induction ratiob
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 μM FeCl3 (37°C) | 1.0 μM FeCl3 (37°C) | 1.0 μM MnCl2 (37°C) | No added FeCl3 or MnCl2
|
−Fe/+10 μM Fe | −Fe/+1.0 μM Fe | −Fe/+Mn | 26°C/37°C | ||
| 37°C | 26°C | ||||||||
| PhoA strains | |||||||||
| KIM6(pYFE34)+c | 229 | 369 | 576 | 1,532 | 5.1 (1.6) | 4.7 (1.4) | 2.6 (0.2) | ||
| KIM6(pYFE34) | 326 | 436 | 647 | 2,071 | 3,295 | 7.2 (2.4) | 5.7 (0.9) | 3.5 (0.2) | 1.8 (0.3) |
| KIM6(pYFE47) | 239 | 317 | 537 | 1,306 | 5.4 (1.4) | 4.8 (2.1) | 2.4 (0.3) | ||
| KIM6-2030(pYFE47) | 1,830 | 1,865 | 1,940 | 2,046 | 1.2 (0.3) | 0.9 (0.4) | 0.9 (0.2) | ||
| LacZ strains | |||||||||
| KIM6(pEUPP1)+ | 196 | 623 | 1,102 | 1,403 | 7.3 (2.7) | 2.3 (0.05) | 1.3 (0.04) | ||
| KIM6(pSC27.1)+ | 1,060 | 1,412 | 2,265 | 2,648 | 2.6 (0.6) | 2.2 (0.2) | 1.2 (0.04) | ||
| KIM6(pSC27.1) | 1,726 | 4,307 | 2.5 (0.1) | ||||||
Units of phosphatase activity were calculated as 1,000 × [(OD420 − 1.75 × OD550)/time × OD620 × volume], where OD420 is optical density at 420 nm (16). β-Galactosidase activity is expressed in Miller units (55). Values are the means for samples taken from at least two independent experiments. In the absence of reporter genes, Y. pestis cells are phenotypically PhoA− and β-galactosidase negative.
Values in parentheses represent standard deviations.
Names followed by a + are those of Y. pestis strains containing an intact 102-kb pigmentation (pgm) locus (59).
Due to the strong homology demonstrated between the yfeABCD gene products and polypeptides encoded by the manganese transporter complex, mntCAB, in the cyanobacterium Synechocystis sp. strain PCC 6803 (7), we examined the influence of manganese on alkaline phosphatase activity from reporter plasmid pYFE34 (Table 1; Fig. 1B) in Y. pestis KIM6 and KIM6+. Enzyme activity was repressed 2.6- and 3.5-fold, respectively, in cells grown in PMH medium supplemented with 1.0 μM MnCl2 compared to cells grown in PMH alone (Table 2). These data suggest that the iron-repressible yfeA promoter is also influenced by the presence of manganese. To test the ability of manganese to repress other iron- and Fur-regulated promoters, we measured the β-galactosidase activity of reporter plasmids pEUPP1 and pSC27.1 in Y. pestis KIM6+ (Table 1). When cells were grown in the presence of 1.0 μM MnCl2, β-galactosidase expression from the Y. pestis psn promoter on pEUPP1 (29) was only slightly repressed (∼1.3-fold). In cells harboring the reporter plasmid pSC27.1, which contains an artificial promoter that requires Fur for the iron-regulated expression of β-galactosidase in Y. pestis (73) and E. coli (17), growth in PMH medium containing 1.0 μM MnCl2 results in a 1.2-fold repression of activity. These results indicate that yfeABCD expression is repressible by both iron and manganese, while expression from two other iron- and Fur-repressible promoters was not significantly affected by the manganese content of the growth medium (Table 2).
To demonstrate Fur regulation of the yfeA promoter, we constructed plasmid pYFE47 (Table 1), a derivative of reporter plasmid pYFE34 (yfeA::TnphoA34). Unlike its parent plasmid, pYFE47 was stable when introduced into the Y. pestis Fur mutant KIM6-2030 (fur::kan-9) (data not shown). The enzyme activity of KIM6-2030(pYFE47) cells was affected only slightly by changes in the growth media, whereas PhoA expression from fur+ Y. pestis KIM6(pYFE47) cells increased ∼5- and 2.4-fold, respectively, over that of cells grown with supplemental iron or manganese (Table 2). These data clearly show that iron and manganese repression of the yfeABCD promoter requires the presence of a functional Fur protein.
Y. pestis possesses a genetically uncharacterized iron transport system that functions at 26°C but not at 37°C (52). Transcriptional repression of this system at 37°C is one possible mechanism for the observed temperature dependence. Consequently, we examined the effects of growth temperature on transcriptional activity of the yfeABCD promoter. In KIM6(pYFE34), transcriptional activity of the yfeABCD promoter was slightly higher at 26°C than at 37°C. However, activity from the artificial Fur-regulated promoter in KIM6(pSC27.1) was similarly enhanced (Table 2). This indicates that transcription from the yfeABCD promoter is significant at 37°C and is not specifically affected by temperature.
PCR amplification of the yfeA and yfuA gene loci in Yersinia spp.
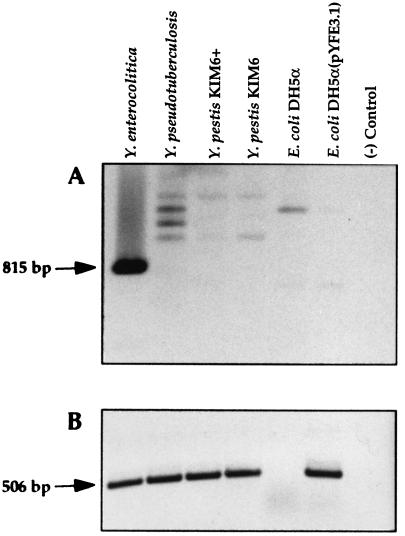
Southern blot analysis indicates that the yfeABCDE locus is present in both Pgm+ and Pgm− Y. pestis strains and in Y. pseudotuberculosis (data not shown). Recently, ABC transporter systems for inorganic iron have been identified in Neisseria gonorrhoeae (fbpABC), H. influenzae (hitABC), Serratia marcescens (sfuABC), and Y. enterocolitica (yfuABC) (1, 2, 20, 64, 85). Although BLAST searches have not identified any significant homologies between Yfe and these systems, primers were designed from a region of Y. enterocolitica yfuA that is conserved in hitA, sfuA, and fbpA (see Materials and Methods) to amplify, by PCR, DNAs from the three pathogenic Yersinia spp. As shown in Fig. 7A, only Y. enterocolitica yielded an abundant PCR product of the expected size of 815 bp, while weakly amplified products of heterologous sizes were observed for Y. pestis, Y. pseudotuberculosis, and E. coli. An analogous experiment using Y. pestis yfeA primers resulted in an abundance of the predicted 506-bp product from all three Yersinia spp. and E. coli DH5α carrying the cloned Y. pestis yfeABCDE genes but not from DH5α alone (Fig. 7B).
FIG. 7.
PCR of DNAs derived from whole cells of Yersinia spp. or E. coli DH5α. (A) Oligonucleotide primers derived from a region of Y. enterocolitica yfuA were used to PCR amplify genomic DNAs from the indicated strains. The predicted product is indicated by the arrow. (B) Oligonucleotide primers were derived from a region of Y. pestis yfeA. The predicted amplicon is designated with an arrow. Reactions were performed with Taq DNA polymerase for 25 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 30 s.
DISCUSSION
In this study we have used subcloning, transposon mutagenesis, and DNA sequencing to identify a pgm-independent transporter system which restores iron-deficient growth but not siderophore production to an E. coli mutant (SAB11) incapable of synthesizing the siderophore enterobactin (6). The yfe locus of Y. pestis is an ∼5.6-kb region of genomic DNA comprised of five genes arranged in two distinct operons. The larger of these, yfeABCD, encodes polypeptides which are translationally coupled (Fig. 3) and collectively resemble a periplasmic binding protein-dependent transport system (14, 45) belonging to the superfamily of ABC transporters (27, 44). The yfeA gene encodes the putative substrate-binding protein YfeA, which likely functions within the periplasmic space of Y. pestis cells. YfeB has signature ATP-binding motifs (44), while YfeC and YfeD possess characteristics of integral membrane permeases (67). YfeE, encoded by the second operon of the yfe locus, is apparently essential for restoring growth to E. coli SAB11 on iron-chelated media. The mechanism which enables pYFE46 (yfeE::TnphoA46) to restore iron-chelated growth to SAB11 is unknown; however, it is plausible that a spurious promoter generated at the TnphoA insertion site in pYFE46 facilitated some level of ′YfeE (lacking only the first five amino acids) expression. This rationale might also explain the weak growth observed with SAB11(pYFE36), which has a TnphoA insertion in yfeB (Fig. 1B). In most instances insertions of transposon Tn5, and derivatives such as TnphoA, have strongly polar effects on the expression of distal genes. However, other studies have shown that low levels of distal gene expression can occur in some cases with transcription originating from within the end of Tn5 (10). While the function of YfeE is unknown, TnphoA mutagenesis of yfeE suggests that YfeE is transported to the cell envelope (Fig. 1B). Although we have demonstrated, by subcloning and mutagenesis, only that YfeD and YfeE are essential for this system to function in E. coli SAB11, sequence information makes it likely that all five yfe gene products are necessary for transport activity.
Several well-characterized ABC transporters involved in inorganic iron uptake have been described for E. coli. However, these systems are largely defined by their iron-chelating siderophores and associated outer membrane receptors (15). Recently, other ABC transporter systems for inorganic iron that are more readily characterized based on their periplasmic binding proteins rather than an associated siderophore or outer membrane protein receptor have been described. The fbpABC locus of N. gonorrhoeae, hitABC of H. influenzae, and sfuABC of S. marcescens all encode systems which transport iron from the periplasm to the cytosol in a siderophore-independent manner. These transport systems may utilize several different specific outer membrane receptors (1, 2, 4, 20, 34, 85). Amino acid sequence homologies indicate that the yfuABC locus of Y. enterocolitica also belongs to this family of iron transporters and encodes polypeptides which facilitate iron uptake (62, 64). The Yfe system of Y. pestis is functionally and organizationally similar to the Yfu system of Y. enterocolitica. However, except for ATP-binding motifs found in YfeB, it shows no significant amino acid homology to Yfu or to the related ABC iron transport systems of H. influenzae (HitABC), N. gonorrhoeae (FbpABC), and S. marcescens (SfuABC) (1, 2, 4, 20, 34, 85). Moreover, PCR analysis indicates that the yfu locus is restricted to Y. enterocolitica (Fig. 7A), whereas the yfeA gene and presumably yfeBCD were detected in all three pathogenic yersiniae, including Y. pestis KIM6, the Pgm− derivative of KIM6+ (Fig. 7B). Recent database searches and the alignments depicted in Fig. 4 show that the Yfe system has a high degree of similarity with a manganese-specific periplasmic binding protein-dependent transporter of the cyanobacterium Synechocystis sp. strain PCC 6803 (MntCAB) as well as with putative periplasmic permeases found in H. influenzae (HI0359 to HI0362) and T. pallidum (TroA to -D) (7, 33, 41). It has been suggested that the Y. pestis Yfe transport system and the HI0359 to HI0362, TroA to -D, and MntCAB systems are members of a new subfamily of ABC transporters (26). This subfamily also includes a number of potentially metal-binding streptococcal adhesins and two ABC metal permease systems from Streptococcus pneumoniae involved in the transport of zinc (Adc) and manganese (Psa). These two cation transport systems are important for the competence and virulence of this human pathogen (25, 26).
A recent study using the manganese ABC transporter, Mnt, showed that the uptake of 54Mn2+ was uninhibited in the presence of FeCl3 (8). However, the accumulation of manganese by the Mnt system was competitively inhibited by Cd2+, Co2+, and Zn2+ (8). Although it is clear that the yfeABCDE locus functions to provide iron to E. coli SAB11 in our system, the similarities between the Mnt and Yfe ABC transport systems allow for reasonable speculation that the Yfe system is involved not only in the transport of iron but also in that of other metal ions. The promoter regions of both yfe operons (Fig. 3) contain FBSs, and we have used transcriptional reporter gene studies to show that the yfeABCD promoter is iron and Fur regulated. However, the high degree of similarity to the manganese transporter of Synechocystis sp. strain PCC 6803 (7, 8) suggests that the Yfe system may also function in manganese transport.
In E. coli, manganese is transported by a specific, high-affinity system (71). Although the genetic components of this system have not been isolated, studies using E. coli right-side-out membrane vesicles demonstrated functional manganese transport in the absence of ATP, indicating that the E. coli system derives its energy from the chemiosmotic membrane potential of the cell (11). Since the manganese transporter of E. coli is presumed to be encoded by a single gene (70) and there is no evidence to indicate that E. coli SAB11 or its parent strain, HB101, is defective in manganese transport, it is unlikely that the Yfe ABC transporter would restore a specific manganese transport defect in E. coli SAB11. Still, it is interesting that PhoA expression driven by the yfeA promoter was significantly repressed in Y. pestis cells grown in the presence of manganese. In E. coli, binding to Fur-regulated promoters has been demonstrated in DNase I footprinting and gel retardation assays using the Fur repressor complexed with Mn2+ (24, 84). In addition, resistance to high concentrations of Mn2+ was used to select an E. coli Fur− mutant (40). Therefore, we examined the possibility that manganese repression of the yfeABCD promoter is mediated in conjunction with Fur by examining regulation in other Fur-regulated promoters and in a Y. pestis Fur− mutant. We showed that manganese repression of transcription from the yfeABCD promoter was Fur dependent; however, β-galactosidase expression from two separate iron- and Fur-regulated promoters was not significantly repressed by the presence of manganese in the growth medium (Table 2). This indicates that the manganese regulation shown by the yfeABCD promoter is not common to all Fur-regulated promoters. Moreover, it is possible that manganese regulation of yfeABCD occurs through an altered FBS which enhances binding of the Fur-Mn2+ complex. Whether Fur proteins from other bacteria are capable of manganese regulation remains to be determined.
In the Y. pestis and Y. enterocolitica siderophore-mediated iron uptake system, many of the key elements involved in the biosynthesis, uptake, and regulation of the Ybt siderophore have been established (9, 29–31, 43, 59, 61). However, only the outer membrane receptor for the Ybt transport system has been identified (30, 31, 59, 61). In recent cell fractionation studies of Y. pestis, a number of iron-repressible periplasmic and cytoplasmic membrane proteins were detected, several of which were unrelated to the pgm locus (52, 59). The presence of the yfe locus in Y. pestis KIM6+ and KIM6, as determined by PCR (Fig. 7B) and Southern blot analyses (data not shown), suggests that the Yfe system functions independently of the pgm-linked Ybt siderophore-mediated uptake system of Y. pestis (9, 30). Furthermore, the presence of an uptake system which utilizes hemin and hemoproteins (46, 58, 69, 73), an iron transport system that functions at 26°C but not at 37°C, and a Ybt-independent iron transport system functioning at 37°C (30, 52, 69) supports the hypothesis that multiple mechanisms for heme and iron acquisition in Y. pestis are fundamental to the success of plague pathogenesis. The lack of specific temperature-regulated expression from the yfeABCD promoter and the ability of the transport system to function at 37°C in E. coli SAB11 suggest that the yfe locus does not represent the 26°C iron transport system of Y. pestis. However, it may correspond to an iron transport system that, in the absence of a functional Ybt system, allows growth of Y. pestis cells in iron-deficient but not iron-chelated media at 37°C (30, 52, 69).
With at least four independent iron/heme transport systems, Y. pestis is in the company of a number of bacterial pathogens that have evolved several strategies for acquiring iron. Vibrio cholerae, for example, utilizes ferric citrate, heme-containing compounds, and a siderophore-mediated uptake system and produces an iron-regulated hemolysin which may facilitate the acquisition of iron or heme compounds liberated from lysed cells (75, 76). Similarly, Shigella flexneri uses siderophores and heme compounds to obtain iron but is also able to bind lactoferrin (56, 81). Receptors for both lactoferrin and transferrin, as well as heme and hemoprotein transport systems, are expressed by the pathogenic neisseriae and by H. influenzae (51, 54). The multiple systems for obtaining iron possessed by numerous bacterial pathogens emphasize the importance of iron acquisition to the pathogenic process. In addition, it is feasible that different iron/heme transport systems are required for the variety of environmental niches that pathogens inhabit—different organ systems within the host as well as nonhost environments. In the plague bacillus, Y. pestis, the role that the Yfe transport system plays in establishing or maintaining an infection and the conditions under which it is optimally used will likely provide new insights into the relationship between virulence and iron acquisition.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI33481 from the National Institutes of Health.
We thank B. R. Byers for providing E. coli SAB11.
REFERENCES
- 1.Adhikari P, Berish S A, Nowalk A J, Veraldi K L, Morse S A, Mietzner T A. The fbpABC locus of Neisseria gonorrhoeae functions in the periplasm-to-cytosol transport of iron. J Bacteriol. 1996;178:2145–2149. doi: 10.1128/jb.178.7.2145-2149.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adhikari P, Kirby S D, Nowalk A J, Veraldi K L, Schryvers A B, Mietzner T A. Biochemical characterization of a Haemophilus influenzae periplasmic iron transport operon. J Biol Chem. 1995;270:25142–25149. doi: 10.1074/jbc.270.42.25142. [DOI] [PubMed] [Google Scholar]
- 3.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 4.Angerer A, Klupp B, Braun V. Iron transport systems of Serratia marcescens. J Bacteriol. 1992;174:1378–1387. doi: 10.1128/jb.174.4.1378-1387.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1987. [Google Scholar]
- 6.Barghouthi S, Payne S M, Arceneaux J E L, Byers B R. Cloning, mutagenesis, and nucleotide sequence of a siderophore biosynthetic gene (amoA) from Aeromonas hydrophila. J Bacteriol. 1991;173:5121–5128. doi: 10.1128/jb.173.16.5121-5128.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartsevich V V, Pakrasi H B. Molecular identification of an ABC transporter complex for manganese: analysis of a cyanobacterial mutant strain impaired in the photosynthetic oxygen evolution process. EMBO J. 1995;14:1845–1853. doi: 10.1002/j.1460-2075.1995.tb07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartsevich V V, Pakrasi H B. Manganese transport in the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1996;271:26057–26061. doi: 10.1074/jbc.271.42.26057. [DOI] [PubMed] [Google Scholar]
- 9.Bearden S W, Fetherston J D, Perry R D. Genetic organization of the yersiniabactin biosynthetic region and construction of avirulent mutants in Yersinia pestis. Infect Immun. 1997;65:1659–1668. doi: 10.1128/iai.65.5.1659-1668.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg D E, Weiss A, Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980;142:439–446. doi: 10.1128/jb.142.2.439-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharyya P. Active transport of manganese in isolated membranes of Escherichia coli. J Bacteriol. 1970;104:1307–1311. doi: 10.1128/jb.104.3.1307-1311.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collada-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 14.Boos W, Lucht J M. Periplasmic binding protein-dependent ABC transporters. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 1175–1209. [Google Scholar]
- 15.Braun V, Hantke K. Genetics of bacterial iron transport. In: Winkelman G, editor. Handbook of microbial iron chelates. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 107–138. [Google Scholar]
- 16.Brickman E, Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and φ80 transducing phages. J Mol Biol. 1975;96:307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- 17.Calderwood S B, Mekalanos J J. Confirmation of the Fur operator site by insertion of a synthetic oligonucleotide into an operon fusion plasmid. J Bacteriol. 1988;170:1015–1017. doi: 10.1128/jb.170.2.1015-1017.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carniel E, Mazigh D, Mollaret H H. Expression of iron-regulated proteins in Yersinia species and their relation to virulence. Infect Immun. 1987;55:277–280. doi: 10.1128/iai.55.1.277-280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang C N, Kuang W-J, Chen E Y. Nucleotide sequence of the alkaline phosphatase gene of Escherichia coli. Gene. 1986;44:121–125. doi: 10.1016/0378-1119(86)90050-8. [DOI] [PubMed] [Google Scholar]
- 20.Chen C-Y, Berish S A, Morse S A, Mietzner T A. The ferric iron-binding protein of pathogenic Neisseria spp. functions as a periplasmic transport protein in iron acquisition from human transferrin. Mol Microbiol. 1993;10:311–318. doi: 10.1111/j.1365-2958.1993.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 21.Claros M G, von Heijne G. TopPred II: an improved software for membrane protein structure predictions. CABIOS. 1994;10:685–686. doi: 10.1093/bioinformatics/10.6.685. [DOI] [PubMed] [Google Scholar]
- 22.Cornelissen C N, Sparling P F. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol Microbiol. 1994;14:843–850. doi: 10.1111/j.1365-2958.1994.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 23.de Bruijn F J, Lupski J R. The use of Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids—a review. Gene. 1984;27:131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]
- 24.De Lorenzo V, Giovannini F, Herrero M, Neilands J B. Metal ion regulation of gene expression. Fur repressor-operator interaction at the promoter region of the aerobactin system of pColV-K30. J Mol Biol. 1988;203:875–884. doi: 10.1016/0022-2836(88)90113-1. [DOI] [PubMed] [Google Scholar]
- 25.Dintilhac A, Alloing G, Granadel C, Claverys J-P. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol. 1997;25:727–939. doi: 10.1046/j.1365-2958.1997.5111879.x. [DOI] [PubMed] [Google Scholar]
- 26.Dintilhac A, Claverys J-P. The adc locus, which affects competence for genetic transformation in Streptococcus pneumoniae, encodes an ABC transporter with a putative lipoprotein homologous to a family of streptococcal adhesins. Res Microbiol. 1997;148:119–131. doi: 10.1016/S0923-2508(97)87643-7. [DOI] [PubMed] [Google Scholar]
- 27.Doige C A, Ames G F-L. ATP-dependent transport systems in bacteria and humans: relevance to cystic fibrosis and multidrug resistance. Annu Rev Microbiol. 1993;47:291–319. doi: 10.1146/annurev.mi.47.100193.001451. [DOI] [PubMed] [Google Scholar]
- 28.Fenno J C, Shaikh A, Spatafora G, Fives-Taylor P. The fimA locus of Streptococcus parasanguis encodes an ATP-binding membrane transport system. Mol Microbiol. 1995;15:849–863. doi: 10.1111/j.1365-2958.1995.tb02355.x. [DOI] [PubMed] [Google Scholar]
- 29.Fetherston J D, Bearden S W, Perry R D. YbtA, an AraC-type regulator of the Yersinia pestis pesticin/yersiniabactin receptor. Mol Microbiol. 1996;22:315–325. doi: 10.1046/j.1365-2958.1996.00118.x. [DOI] [PubMed] [Google Scholar]
- 30.Fetherston J D, Lillard J W, Jr, Perry R D. Analysis of the pesticin receptor from Yersinia pestis: role in iron-deficient growth and possible regulation by its siderophore. J Bacteriol. 1995;177:1824–1833. doi: 10.1128/jb.177.7.1824-1833.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fetherston J D, Perry R D. The pigmentation locus of Yersinia pestis KIM6+ is flanked by an insertion sequence and includes the structural genes for pesticin sensitivity and HMWP2. Mol Microbiol. 1994;13:697–708. doi: 10.1111/j.1365-2958.1994.tb00463.x. [DOI] [PubMed] [Google Scholar]
- 32.Fetherston J D, Schuetze P, Perry R D. Loss of the pigmentation phenotype in Yersinia pestis is due to the spontaneous deletion of 102 kb of chromosomal DNA which is flanked by a repetitive element. Mol Microbiol. 1992;6:2693–2704. doi: 10.1111/j.1365-2958.1992.tb01446.x. [DOI] [PubMed] [Google Scholar]
- 33.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C A, Gocayne J D, Scott J D, Shirley R, Liu L-I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Ventor J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 34.Genco C A, Desai P J. Iron acquisition in the pathogenic Neisseria. Trends Microbiol. 1996;4:179–184. doi: 10.1016/0966-842x(96)10029-9. [DOI] [PubMed] [Google Scholar]
- 35.Gray-Owen S D, Schryvers A B. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996;4:185–191. doi: 10.1016/0966-842x(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 36.Griffiths E. The iron-uptake systems of pathogenic bacteria. In: Bullen J J, Griffiths E, editors. Iron and infection: molecular, physiological, and clinical aspects. New York, N.Y: John Wiley & Sons, Inc.; 1987. pp. 69–137. [Google Scholar]
- 37.Guerinot M L. Microbial iron transport. Annu Rev Microbiol. 1994;48:743–772. doi: 10.1146/annurev.mi.48.100194.003523. [DOI] [PubMed] [Google Scholar]
- 38.Guilvout I, Mercereau-Pujalon O, Bonnefey S, Pugsley A P, Carniel E. High-molecular-weight protein 2 of Yersinia enterocolitica is homologous to AngR of Vibrio anguillarum and belongs to a family of proteins involved in nonribosomal peptide synthesis. J Bacteriol. 1993;175:5488–5504. doi: 10.1128/jb.175.17.5488-5504.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutierrez C, Barondess J, Manoil C, Beckwith J. The use of transposon TnphoA to detect genes for cell envelope proteins subject to a common regulatory stimulus. J Mol Biol. 1987;195:289–297. doi: 10.1016/0022-2836(87)90650-4. [DOI] [PubMed] [Google Scholar]
- 40.Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K 12: fur not only affects iron metabolism. Mol Gen Genet. 1987;210:135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- 41.Hardham J M, Stamm L V, Porcella S F, Frye J G, Barnes N Y, Howell J K, Mueller S L, Radolf J D, Weinstock G M, Norris S J. Identification and transcriptional analysis of a Treponema pallidum operon encoding a putative ABC transport system, an iron-activated repressor protein homolog, and a glycolytic pathway enzyme homolog. Gene. 1997;197:47–64. doi: 10.1016/s0378-1119(97)00234-5. [DOI] [PubMed] [Google Scholar]
- 42.Harkness R E, Chong P, Klein M H. Identification of two iron-repressed periplasmic proteins in Haemophilus influenzae. J Bacteriol. 1992;174:2425–2430. doi: 10.1128/jb.174.8.2425-2430.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heesemann J, Hantke K, Vocke T, Saken E, Rakin A, Stojiljkovic I, Berner R. Virulence of Yersinia enterocolitica is closely associated with siderophore production, expression of an iron-repressible outer membrane polypeptide of 65,000 Da and pesticin sensitivity. Mol Microbiol. 1993;8:397–408. doi: 10.1111/j.1365-2958.1993.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 44.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 45.Higgins C F, Hyde S C, Mimmack M M, Gileadi U, Gill D R, Gallagher M P. Binding protein-dependent transport systems. J Bioenerg Biomembr. 1990;22:571–592. doi: 10.1007/BF00762962. [DOI] [PubMed] [Google Scholar]
- 46.Hornung J M, Jones H A, Perry R D. The hmu locus of Yersinia pestis is essential for utilization of free haemin and haem-protein complexes as iron sources. Mol Microbiol. 1996;20:725–739. doi: 10.1111/j.1365-2958.1996.tb02512.x. [DOI] [PubMed] [Google Scholar]
- 47.Humphreys G O, Willshaw G A, Anderson E S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975;383:457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- 48.Jackson S, Burrows T W. The pigmentation of Pasteurella pestis on a defined medium containing haemin. Br J Exp Pathol. 1956;37:570–576. [PMC free article] [PubMed] [Google Scholar]
- 49.Jackson S, Burrows T W. The virulence-enhancing effect of iron on nonpigmented mutants of virulent strains of Pasteurella pestis. Br J Exp Pathol. 1956;37:577–583. [PMC free article] [PubMed] [Google Scholar]
- 50.Kolenbrander P E, Andersen R N, Ganeshkumar N. Nucleotide sequence of the Streptococcus gordonii PK488 coaggregation adhesin gene, scaA, and ATP-binding cassette. Infect Immun. 1994;62:4469–4480. doi: 10.1128/iai.62.10.4469-4480.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee B C. Quelling the red menace: haem capture by bacteria. Mol Microbiol. 1995;18:383–390. doi: 10.1111/j.1365-2958.1995.mmi_18030383.x. [DOI] [PubMed] [Google Scholar]
- 52.Lucier T S, Fetherston J D, Brubaker R R, Perry R D. Iron uptake and iron-repressible polypeptides in Yersinia pestis. Infect Immun. 1996;64:3023–3031. doi: 10.1128/iai.64.8.3023-3031.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manoil C, Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci USA. 1985;82:8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mietzner T A, Morse S A. The role of iron-binding proteins in the survival of pathogenic bacteria. Annu Rev Nutr. 1994;14:471–493. doi: 10.1146/annurev.nu.14.070194.002351. [DOI] [PubMed] [Google Scholar]
- 55.Miller J F. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 56.Payne S M. Iron and virulence in Shigella. Mol Microbiol. 1988;3:1301–1306. doi: 10.1111/j.1365-2958.1989.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 57.Pearce B J, Yin Y B, Masure H R. Genetic identification of exported proteins in Streptococcus pneumoniae. Mol Microbiol. 1993;9:1037–1050. doi: 10.1111/j.1365-2958.1993.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 58.Perry R D, Brubaker R R. Accumulation of iron by yersiniae. J Bacteriol. 1979;137:1290–1298. doi: 10.1128/jb.137.3.1290-1298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perry R D, Fetherston J D. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perry R D, Pendrak M L, Schuetze P. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J Bacteriol. 1990;172:5929–5937. doi: 10.1128/jb.172.10.5929-5937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rakin A, Saken E, Harmsen D, Heesemann J. The pesticin receptor of Yersinia enterocolitica: a novel virulence factor with dual function. Mol Microbiol. 1994;13:253–263. doi: 10.1111/j.1365-2958.1994.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 62.Rakin A V, Saken E, Heesemann J. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Variability of iron acquisition systems in pathogenic Yersinia, abstr. B-377; p. 93. [Google Scholar]
- 63.Rodríguez-Quiñones F, Benedí V J. Escherichia coli strain DH5α is a suitable host for the study of phoA insertions. Focus. 1993;15:110–112. [Google Scholar]
- 64.Saken, E. M., and J. Heesemann. 1995. GenBank accession number Z47200.
- 65.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 66.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saurin W, Köster W, Dassa E. Bacterial binding protein-dependent permeases: characterization of distinctive signatures for functionally related integral cytoplasmic membrane proteins. Mol Microbiol. 1994;12:993–1004. doi: 10.1111/j.1365-2958.1994.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 68.Sikkema D J, Brubaker R R. Resistance to pesticin, storage of iron, and invasion of HeLa cells by yersiniae. Infect Immun. 1987;55:572–578. doi: 10.1128/iai.55.3.572-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sikkema D J, Brubaker R R. Outer membrane peptides of Yersinia pestis mediating siderophore-independent assimilation of iron. Biol Metals. 1989;2:174–184. doi: 10.1007/BF01142557. [DOI] [PubMed] [Google Scholar]
- 70.Silver S. Transport of inorganic cations. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 1091–1102. [Google Scholar]
- 71.Silver S, Lusk J. Magnesium, manganese and zinc transport. In: Rosen B P, Silver S, editors. Ion transport in prokaryotes. New York, N.Y: Academic Press, Inc.; 1987. pp. 165–180. [Google Scholar]
- 72.Staggs T M, Fetherston J D, Perry R D. Pleiotropic effects of a Yersinia pestis fur mutation. J Bacteriol. 1994;176:7614–7624. doi: 10.1128/jb.176.24.7614-7624.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Staggs T M, Perry R D. Identification and cloning of a fur regulatory gene in Yersinia pestis. J Bacteriol. 1991;173:417–425. doi: 10.1128/jb.173.2.417-425.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Staggs T M, Perry R D. Fur regulation in Yersinia species. Mol Microbiol. 1992;6:2507–2516. doi: 10.1111/j.1365-2958.1992.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 75.Stoebner J A, Butterton J R, Calderwood S B, Payne S M. Identification of the vibriobactin receptor of Vibrio cholerae. J Bacteriol. 1992;174:3270–3274. doi: 10.1128/jb.174.10.3270-3274.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stoebner J A, Payne S M. Iron-regulated hemolysin production and utilization of heme and hemoglobin by Vibrio cholerae. Infect Immun. 1988;56:2891–2895. doi: 10.1128/iai.56.11.2891-2895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stojiljkovic I, Bäumler A J, Hantke K. Fur regulon in gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a Fur titration assay. J Mol Biol. 1994;236:531–545. doi: 10.1006/jmbi.1994.1163. [DOI] [PubMed] [Google Scholar]
- 78.Surgalla M J, Beesley E D. Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl Microbiol. 1969;18:834–837. doi: 10.1128/am.18.5.834-837.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tartof K D, Hobbs C A. Improved media for growing plasmid and cosmid clones. Focus. 1987;9:12. [Google Scholar]
- 80.Thompson J D, Higgins D G, Gibson T J. CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tigyi Z, Kishore A R, Mæland J A, Forsgren A, Naidu A S. Lactoferrin-binding proteins in Shigella flexneri. Infect Immun. 1992;60:2619–2626. doi: 10.1128/iai.60.7.2619-2626.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Une T, Brubaker R R. In vivo comparison of avirulent Vwa− and Pgm− or Pstr phenotypes of yersiniae. Infect Immun. 1984;43:895–900. doi: 10.1128/iai.43.3.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wake A, Misawa M, Matsui A. Siderochrome production by Yersinia pestis and its relation to virulence. Infect Immun. 1975;12:1211–1213. doi: 10.1128/iai.12.5.1211-1213.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wee S, Neilands J B, Bittner M L, Hemming B C, Haymore B L, Seetharam R. Expression, isolation and properties of Fur (ferric uptake regulation) protein of Escherichia coli K 12. Biol Metals. 1988;1:62–68. doi: 10.1007/BF01128019. [DOI] [PubMed] [Google Scholar]
- 85.Zimmermann L, Angerer A, Braun V. Mechanistically novel iron(III) transport system in Serratia marcescens. J Bacteriol. 1989;171:238–243. doi: 10.1128/jb.171.1.238-243.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]