Abstract
Objective:
We investigate how risk of sexually acquiring or transmitting HIV in adolescent girls and young women (AGYW) changed following the real-world implementation of DREAMS (Determined, Resilient, Empowered, AIDS free, Mentored and Safe) HIV prevention programme.
Design:
A representative population-based prospective cohort study of AGYW living in rural KwaZulu-Natal.
Methods:
Between 2017–2019 we interviewed a random sample of AGYW aged 13–22 annually. We measured exposure to DREAMS as self-reported receipt of an invitation to participate and/or participation in DREAMS activities that were provided by DREAMS implementing organizations. HIV and Herpes Simplex Virus type 2 (HSV-2) statuses were ascertained through blood tests on Dried Blood Spot (DBS). We used multivariable regression analysis to assess the association between exposure to DREAMS and risk of acquiring HIV: measured as incident HSV-2 (a proxy of sexual risk) and incident HIV; and the risk of sexually transmitting HIV: measured as being HIV positive with a detectable HIV viral load (>=50 copies per millitre (mL)) on the last available DBS. We adjusted for socio-demographic, sexual relationship, and migration.
Results:
2184 (86.4%) of those eligible agreed to participate and 2016 (92.3%) provided data for at least one follow-up time-point. 1030 (54%) were exposed to DREAMS; HIV and HSV-2 incidence were 2.2/100 person-years (95% Confidence Interval [CI]: 1.66–2.86) and 17.3/100 person-years (95%CI: 15.5–19.4) respectively. There was no evidence that HSV-2 and HIV incidence were lower in those exposed to DREAMS: adjusted rate ratio (aRR) 0.96 (95%CI: 0.76–1.23 and 0.83 (95%CI: 0.46–1.52) respectively. HIV viral load was detectable for 169 (8.9%) respondents; there was no evidence this was lower in those exposed to DREAMS with an adjusted risk difference, compared to those not exposed to DREAMS, of 0.99% [95%CI: −1.52–3.82]. Participants who lived in peri-urban/urban setting were more likely to have incident HIV and transmissible HIV. Both HSV-2 incidence and the transmissible HIV were associated with older age and ever having sex. Findings did not differ substantively by respondent age group.
Conclusions:
DREAMS exposure was not associated with measurable reductions in risk of sexually acquiring or transmitting HIV amongst a representative cohort of AGYW in rural South Africa
Keywords: combination HIV prevention, HIV, Adolescent Girls and Young Women, HSV-2, HIV viral load
Introduction:
South Africa (SA) has an estimated 7.7 million people living with HIV – the highest number of any country globally; HIV remains the leading cause of death. Despite highly efficacious and cost-effective HIV prevention tools, HIV incidence has remained stubbornly high, especially in KwaZulu-Natal (KZN) where we have shown an annual incidence of 8% amongst females aged 20–24[1, 2]. There is an urgent need to reduce the impact of the HIV epidemic in adolescent girls and young women (AGYW)[3].
There have long been calls to scale-up evidence-based combination structural, behavioural and biomedical HIV prevention interventions[4–8]. This has been reinvigorated by evidence that ‘layering’, i.e. providing multiple interventions together, can accelerate progress towards the Sustainable Development Goals in adolescents[9]. In response, the US Presidents’ Emergency Plan for AIDS Relief with others, supported the ‘DREAMS (Determined, Resilient, Empowered, AIDS free, Mentored and Safe) Partnership’, a multi-sectoral package of interventions to reduce HIV incidence amongst AGYW, hereafter referred to as DREAMS[10, 11]. The aim of DREAMS was to reduce HIV incidence through strengthening existing HIV testing, prevention and linkage to care interventions and the introduction of evidence based interventions for gender-based violence, family and caregiving, social asset building, and cash transfers for AGYW[10, 12, 13].
DREAMS in South Africa was implemented with high-level oversight by government and funders, through local implementing partners who were resourced to deliver defined and target-focused packages of interventions to AGYW in selected geographic areas [14, 15]. Two of the pathways through which we hypothesised DREAMS would reduce HIV amongst AGYW was through reducing sexual risk and reducing the prevalence of transmissible HIV amongst AGYW and their male partners[12, 16].
Between 2016 and 2018 we evaluated DREAMS roll-out in a poor rural district in northern KZN, South Africa, with a high burden of HIV[16]. We present the prespecified analysis of the impact of the real-world implementation of the DREAMS combination prevention intervention on the incidence of Herpes Simplex Virus type 2 (HSV-2, as a measure of sexual risk), HIV incidence and detectable HIV viral load (as a measure of sexually transmissible HIV) in AGYW.
Methods
Study design:
As part of a multicounty DREAMS impact evaluation, we conducted a cohort study to evaluate the impact of exposure to DREAMS on risk of sexually acquiring or transmitting HIV amongst a representative sample of ~2000 AGYW in a DREAMS district of rural South Africa. In 2017 a random sample of AGYW, stratified by age and geographical area, were enrolled from the Africa Health Research Institute (AHRI) demographic surveillance area[17] and followed up annually for two years.
Setting and population.
The AHRI demographic Surveillance System is situated in the uMkhanyakude district in rural northern KZN which is mostly rural and poor with high levels of HIV and youth unemployment (over 85% of those aged 18–24 are unemployed)[17]. DREAMS was rolled-out in 2016 and delivered until the end of 2018 In uMkhanyakude[13, 14].
In 2017 the AHRI demographic surveillance was used as a sampling frame to identify and invite a random sample of 3,013 AGYW, stratified by age (13–17 and 18–22) and area. This longitudinal cohort was followed prospectively at three specific time points over a two-year study period: baseline, 12 months, and 24 months, to study the influence of exposure to DREAMS on HIV outcomes and sexual risk[16]. Up to 6 contact attempts (at home and by phone) were made at each study time point by a team of experienced researchers.
Data collection
Following informed consent researchers collected data in the local language (isiZulu) using a structured quantitative questionnaire programmed in REDCap onto a tablet computer [16]. They used interviewer-administered and self-administered tablet-assisted interviews. The interview included questions on socio-demographics, general health, sexual relationships, awareness and uptake of DREAMS, migration, and gender norms. Interviewers took a Dried Blood Spot (DBS) at baseline and follow-up. They were consented separately for HSV-2 testing on DBS and storage of DBS for future testing, that included for sexually transmitted infections. At the end-line survey, informed consent was obtained separately for DBS, HSV2 testing, HIV antibody and viral load testing, and retrospective HIV antibody testing on stored DBS. All participants were also offered point-of-care HIV testing and linkage to services. Those who were not found at end-line survey but had provided consent for their DBS to be stored and tested for future testing that included sexually transmitted infections, as approved by a research ethics committee, were included in the retrospective HIV antibody testing and viral load testing. For sexual behaviour questions, violence and other sensitive questions participants were given the tablet to complete a self-interview; the research assistants were available to provide support and referral as required.
Laboratory:
We used the HerpeSelect®2 ELISA IgG assay (FOCUS Diagnostics, Cypress, California, USA) for the qualitative detection of human IgG class antibodies to HSV-2 on DBS samples collected on Whatman 903 filter cards[18]. A 6mm diameter punch of a DBS spot was incubated overnight in 150ul Assay Diluent and the assay was performed with 50ul of the eluent in accordance with the manufacturer’s instructions. Following optimisation studies comparing DBS with plasma samples, we multiplied the mean cut-off calibrator absorbance values by a factor of 1.5 before determining the index value for each sample[19, 20].
We retrieved samples from participants who had consented to be tested for HIV and tested them using the Genscreen™ ULTRA HIV Ag-Ab ELISA immunoassay (BioRad, Marnes-la-Coquette, France). A 4.7mm punch spot of DBS was incubated overnight the eluate was assayed as per the manufacturer’s instructions. Optical density measurements were read using an ELx800 Universal microplate reader (BioTek, Vermont, USA) and calculations were performed using the Gen5 v3.03 (BioTek, Vermont, USA).
HIV viral loads were measured on all serology positive samples. Nucleic acid extraction was performed using the automated EasyMag magnetic bead-based extraction protocol on the Nuclisens® easyMAG® instrument (bioMerieux, Marcy l’EtoileFrance). Two x 50mm DBS spots were incubated in the NucliSens Lysis Buffer (2ml) for 1 hour with rotation. The supernatant was transferred to the onboard consumables containing magnetic silica beads and an internal control. The eluted nucleic acids were aliquoted for testing using the Generic HIV Charge Viral assay (Biocentric, Bandol, France). The quantitative qPCR assay was performed using the CFX-96 Touch instrument and analysed using the CFX Manager Software v3.0. Standard curves were calculated per run while baselines were set manually.
All laboratory tests underwent internal and external quality control. An incident HSV-2 or HIV individual was defined as having been negative at baseline and positive at follow-up. Those who were equivocal at follow-up were not considered seroconversions.
Measures
Exposure definitions:
Exposure to DREAMS intervention was defined as self-reported receipt of an invitation to participate in DREAMS activities and/or participation in DREAMS activities that were provided by known DREAMS implementing organizations in the baseline (2017) and/or 2018 interview. Eleven organisations were receiving DREAMS funding to deliver 28 different interventions, grouped into categories: HIV testing services; condom promotion and provision; expanding contraception mix; post violence care; PrEP for young women who sell sex; social asset building; social protection; parenting/caregiver programmes; community mobilisation and norms change; and targeting male partners of AGYW[13, 14]. Of the AGYW who were invited to participate in DREAMS activities (2017 and/or 2018), 88.2% received 3 or more interventions and 96.3% received 2 or more interventions[13].
Outcome definitions:
For HIV and HSV-2 incidence analysis, we included participants who had at least 2 or more test results with the first test being negative. The sero-conversion dates were estimated at the midpoint between the date of the last negative and first positive test result. All participants who remained negative throughout the study were censored at their last negative test date. Transmissible HIV was defined as being HIV positive with a detectable HIV viral load (>=50 copies per millitre (mL) on their last available DBS. Those who only provided a DBS at baseline were excluded.
Explanatory variables included age and other socio-demographic variables: level of education (in school or completed school), geographic area (urbanicity); household wealth index calculated using principal component analysis based on household asset ownership and access to safe drinking water and sanitation; food insecurity defined as any report of reducing the size of food portions or skipping meals by any member of a household because there was not enough money to buy food in the past 12 months; and migration status (defined as ever having moved outside or within the surveillance area since the age of 13). A composite categorical variable with three levels (coded as 0 if Never had sex, 1 if Ever had sex but never pregnant and 2 if Ever pregnant) was generated to measure sexual and pregnancy history. All explanatory variables were measured at baseline in 2017.
Statistical analysis
We calculated the proportion of AGYW who were enrolled and consented to either HSV-2 or HIV testing at baseline and follow-up. HIV, HSV-2 and transmissible viral load prevalence were calculated at baseline and at follow-up among participants who have at least 1 follow-up HIV or HSV-2 test results. A directed acyclic graph (DAG) was constructed to identify a set of variables to adjusted for to control for confounding when estimating the association between DREAMS exposure and the outcome[21]. In the DAG, we included individual and household characteristics, DREAMS exposure and the outcome variable to show the hypothesized causal links between these variables. We conducted multivariable regression analysis (adjusted for confounders identified in the DAG) to measure the effect of DREAMS exposure on HIV incidence, HSV-2 incidence, and transmissible HIV. We calculated HIV and HSV-2 incidence per 100 person-years and used a multivariable Poisson regression model, adjusting for potential confounders identified in the DAG, to estimate the rate ratio of the outcome comparing AGYW with exposure to DREAMS compared with those without exposure. Follow-up time was split up according to an AGYW’s current age, distinguishing the age groups 13–14, 15–17, 18–19 and 20–24, when controlling for age group in multivariate analysis.
For transmissible HIV, which was measured cross-sectionally, we first performed a classic logistic regression to explore the association of the explanatory variables that were identified in the DAG with prevalence of transmissible HIV. We then used logistic regression to predict the percentage of AGYW with the outcome in two counterfactual scenarios, that all AGYW were invited to DREAMS vs no AGYW were invited to DREAMS. We first estimated the “propensity to be invited to DREAMS” by fitting a logistic regression model with “exposure to DREAMS’ as the outcome and explanatory variables that were identified in the DAG as potential confounding variables for the association between DREAMS and the outcome. We then fitted two separate logistic regression models, one among AGYW who were invited to DREAMS and one among AGYW who were not invited to DREAMS; the outcome variable was transmissible HIV and the explanatory variables were age group and the propensity score. After fitting these two models, we used the first to predict the probability of the outcome (transmissible HIV) for all AGYW under the scenario that all were invited to DREAMS, and the second to predict the probability of the outcome for all AGYW under the scenario that none were invited to DREAMS. We calculated the average of these probabilities for each of the two alternative scenarios, and from that estimated the difference between them, with 95% confidence intervals estimated using bootstrapping. We checked the robustness of the “propensity-score regression adjustment” estimates by comparing them with predictions from a multivariable logistic regression model of the outcome on explanatory variables, with estimates from stratification on the propensity score, and with “inverse probability of treatment” weighting” (IPTW) based on the propensity score. Item-specific missing data was uncommon; we used analysis-specific complete case analysis.
Ethics approval
Approval of the DREAMS Partnership impact evaluation protocol was obtained from the University of KwaZulu-Natal Biomedical Research Ethics Committee (BFC339/19), the AHRI Somkhele Community Advisory Board, and the London School of Hygiene & Tropical Medicine Research Ethics Committee (REF11835). Additional ethical approval for secondary data analysis was attained from University College London (18321/001). Written consent was provided from participants aged 18 years or older and, for participants below 18 years of age, written parental consent and participant assent was obtained.
Results
Participants
Figure 1 shows that 2184 (86.4%) of those eligible agreed to participate in the cohort. n=1853 (84.8%) and 1712 (78.4%) were retained at year one and year two follow-up respectively; n= 2016 (92.3%) had at least one follow-up survey. Consent to HSV2 and HIV testing was high (92–95%) in all rounds.
Figure 1:
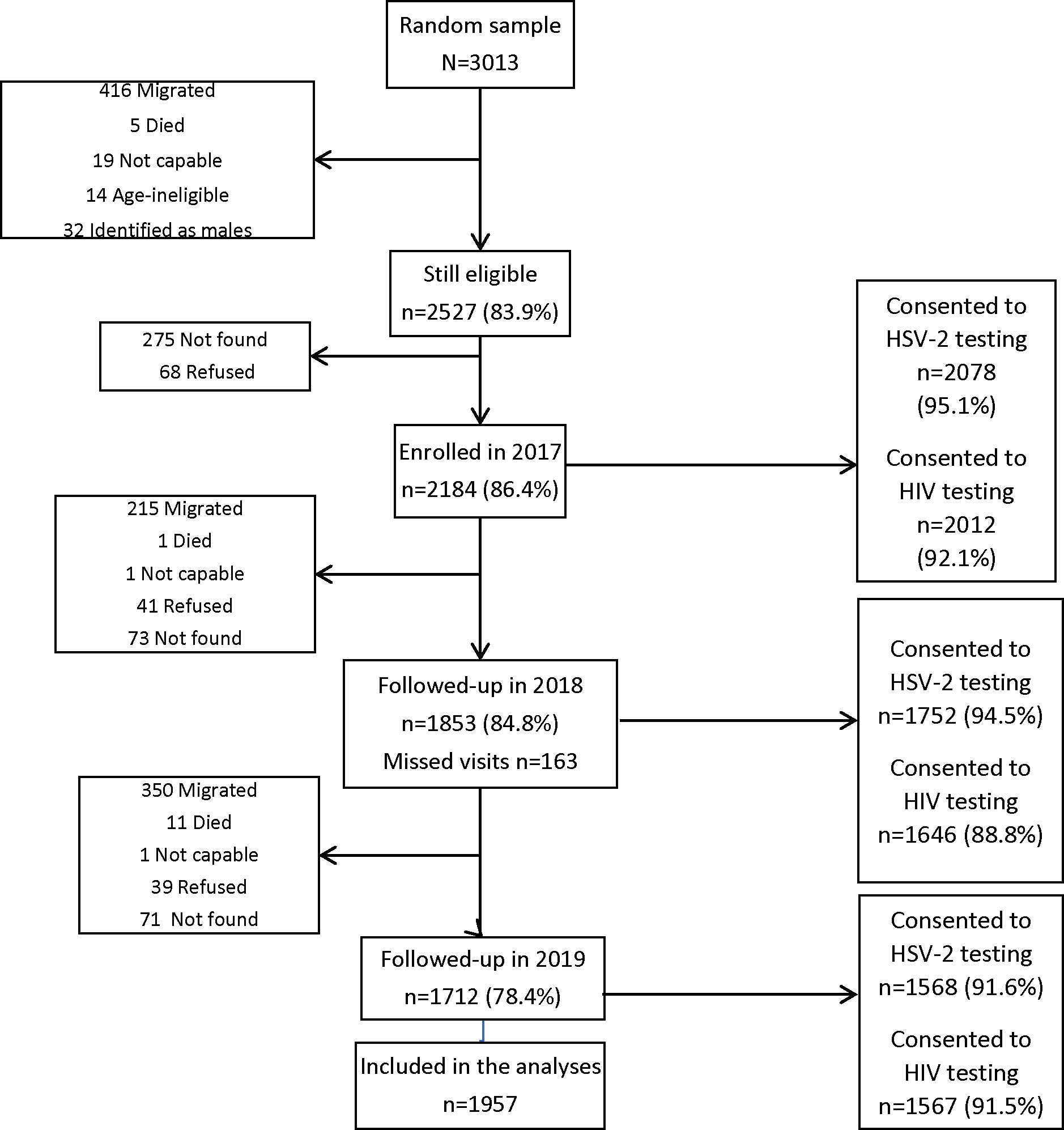
Flow chart of cohort recruitment and follow-up 2017–2019
At baseline (table 1), median age was 16 years, three quarters were still attending school, 31% described food insecurity, 64% lived in rural areas, and 20% had migrated since the age of 13. The majority (59%) had not yet reported sex. Those who had at least one follow-up HSV-2 or HIV test results were younger, more likely to be in school and less likely to have migrated or had sex compared to those not contributing follow-up data (table 1). The majority (54%) of AGYW included in follow-up analysis had been exposed to DREAMS (table 1).
TABLE 1.
Characteristics of AGYW who were enrolled and consented to HSV-2 or HIV testing
| All AGYW | AGYW consent HSV-2/HIV testing baseline (2017) | AGYW consented to HSV-2/HIV testing at follow-up (2018/2019) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| n(%) | n(%) | All n(%) | Invited/received DREAMS by 2018 n(%) | Never Invited/received DREAMS by 2018 n(%) | Chi2 test p-value | |
| Total | 2184 | 2078 (95.1) | 1957 (89.6) | 1056 (54.0) | 901 (46.0) | |
| Age group | <0.001 | |||||
| 13–14 | 460 (21.1) | 445 (20.4) | 435 (22.2) | 261 (24.7) | 174 (19.3) | |
| 15–17 | 688 (31.5) | 667 (30.5) | 638 (32.6) | 430 (40.7) | 208 (23.1) | |
| 18–19 | 475 (21.7) | 442 (20.2) | 413 (21.1) | 200 (18.9) | 213 (23.6) | |
| 20–22 | 561 (25.7) | 524 (24.0) | 471 (24.1) | 165 (15.6) | 306 (34.0) | |
| Currently in school | <0.001 | |||||
| No | 540 (24.7) | 498 (22.8) | 443 (22.6) | 140 (13.3) | 303 (33.6) | |
| Yes | 1644 (75.3) | 1580 (72.3) | 1514 (77.4) | 916 (86.7) | 598 (66.4) | |
| Age and education | <0.001 | |||||
| 13–17 or 18–22 & In school | 1664 (76.3) | 1600 (73.3) | 1529 (78.2) | 924 (87.5) | 605 (67.2) | |
| 18–22 & Not completed secondary | 188 (8.6) | 175 (8.0) | 161 (8.2) | 52 (4.9) | 109 (12.1) | |
| 18–22 & completed secondary | 330 (15.1) | 301 (13.8) | 266 (13.6) | 80 (7.6) | 186 (20.7) | |
| Socio-economic status | 0.009 | |||||
| Low | 727 (35.1) | 700 (32.1) | 674 (35.8) | 398 (38.9) | 276 (32.2) | |
| Middle | 747 (36.0) | 717 (32.8) | 677 (36.0) | 354 (34.6) | 323 (37.6) | |
| High | 600 (28.9) | 558 (25.5) | 530 (28.2) | 271 (26.5) | 259 (30.2) | |
| Food insecurity | 0.077 | |||||
| No | 1497 (68.7) | 1419 (65.0) | 1342 (68.8) | 742 (70.5) | 600 (66.7) | |
| Yes | 682 (31.3) | 656 (30.0) | 610 (31.3) | 311 (29.5) | 299 (33.3) | |
| Geographic area | <0.001 | |||||
| Rural | 1388 (64.1) | 1325 (60.7) | 1252 (64.6) | 724 (69.2) | 528 (59.1) | |
| Peri-urban/urban | 777 (35.9) | 734 (33.6) | 687 (35.4) | 322 (30.8) | 365 (40.9) | |
| Migrated/moved | <0.001 | |||||
| No | 1781 (81.5) | 1703 (78.0) | 1616 (82.6) | 911 (86.3) | 705 (78.2) | |
| Yes | 403 (18.5) | 375 (17.2) | 341 (17.4) | 145 (13.7) | 196 (21.8) | |
| Ever had sex, ever pregnant | <0.001 | |||||
| Never had sex | 1273 (58.7) | 1209 (55.4) | 1174 (60.4) | 722 (68.6) | 452 (50.7) | |
| Ever sex, never pregnant | 308 (14.2) | 293 (13.4) | 264 (13.6) | 122 (11.6) | 142 (15.9) | |
| Ever pregnant | 588 (27.1) | 563 (25.8) | 507 (26.1) | 209 (19.8) | 298 (33.4) | |
| HIV prevalence | <0.001 | |||||
| Negative | 1776 (81.3) | 1623 (74.3) | 1669 (85.3) | 920 (87.1) | 749 (83.1) | |
| Positive | 236 (10.8) | 270 (12.4) | 288 (14.7) | 136 (12.9) | 152 (16.9) | |
| Did not consent | 172 (7.9) | |||||
| HSV-2 prevalence | <0.001 | |||||
| Negative | 1525 (69.8) | 1116 (51.1) | 1153 (58.9) | 665 (63.0) | 488 (54.2) | |
| Positive | 553 (25.3) | 777 (35.6) | 804 (41.1) | 391 (37.0) | 413 (45.8) | |
| Did not consent | 106 (4.9) | |||||
| Transmissible viral load | 0.158 | |||||
| <50 | 1835 (91.2) | 1733 (79.3) | 1785 (91.2) | 972 (92.0) | 813 (90.2) | |
| >=50 | 139 (6.9) | 160 (7.3) | 172 (8.8) | 84 (8.0) | 88 (9.8) | |
| Insufficient sample (not testable) | 38 (1.9) | |||||
Exposure to DREAMS and HIV and HSV2 outcomes
Table 1 shows n=1030 (54%) were invited to or received DREAMS in 2017 and/ or 2018. n=259 (11.8%) were HIV positive at baseline (either knew their status or tested positive on DBS); 70 (6.1%) and 189 (18.2%) of 13–17 and 18–22 year olds respectively. Overall HIV incidence was 2.2/ 100 py 95% CI (1.66 – 2.86) and HSV2 incidence was 17.3/ 100 py 95% CI (15.5 – 19.4). n=169 (8.9%) had a detectable HIV viral load at last measure.
HIV and HSV2 incidence by DREAM exposure
HIV incidence was 2.75 (1.91–3.96)/ 100 person years in those unexposed to DREAMS, compared with 1.73 (1.15–2.60) / 100 person years in those exposed to DREAMS. After adjusting for potential confounding factors, there was no evidence of an association between DREAMS exposure and HIV incidence: adjusted Rate Ratio (adjRR) 0.83; 95% confidence interval (95%CI) of 0.46–1.52. Findings in the younger age group (aged 13–17) and the older age group (18–22) were similar (figure 2a). Beyond age, the only characteristic (table 2a) for which there was evidence of association with HIV incidence was peri-urban/ urban setting adjRR 1.89: 95%CI (1.05–2.39).
Figure 2:
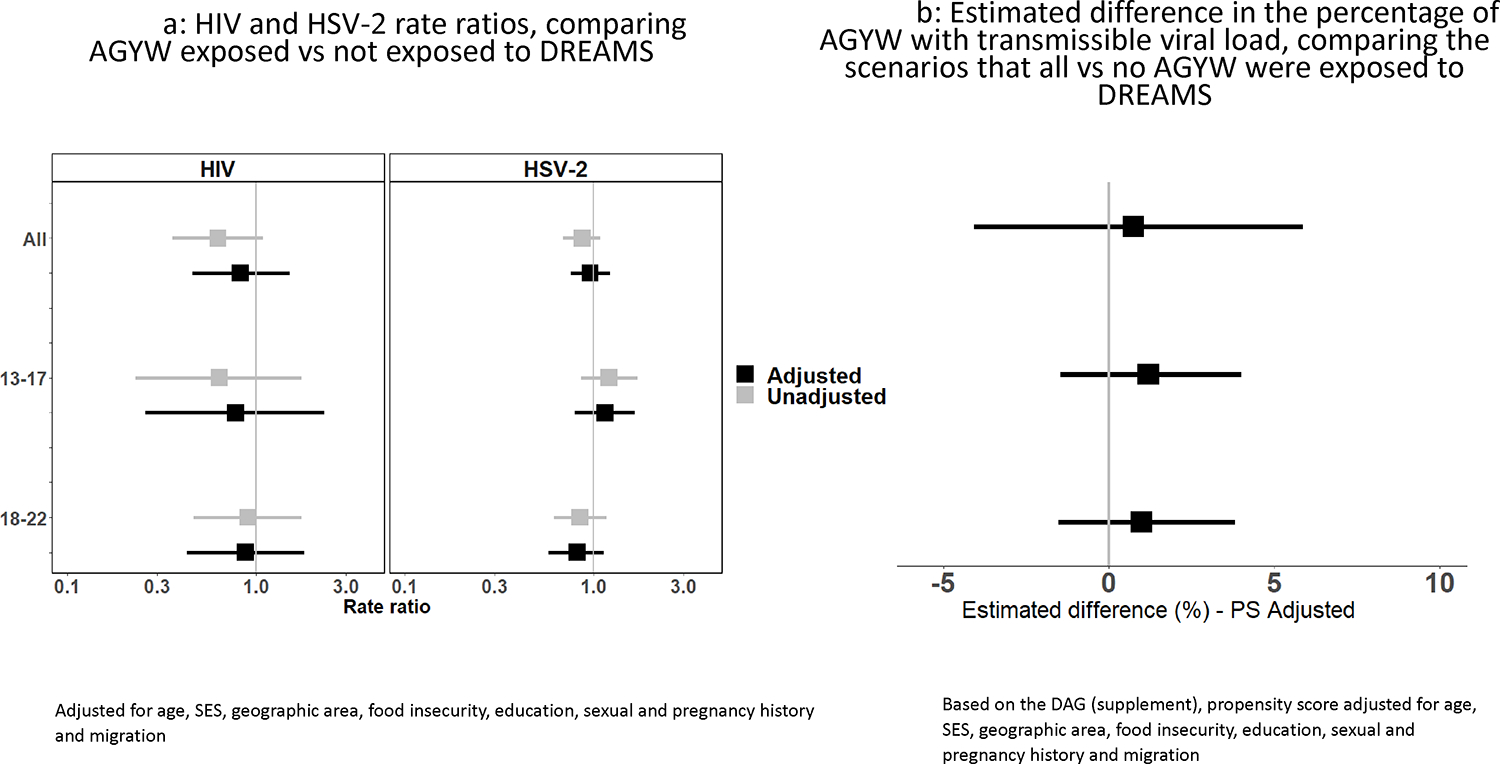
Comparing incident HIV, HSV2 and transmissible HIV between DREAMS exposed and unexposed AGYW
Table 2a.
Association between DREAMS and HIV incidence among AGYW
| Person-years | n HIV-positive | Incidence rate | Unadjusted Rate Ratio (95% CI) | Age Adjusted Rate Ratio (95% CI) | Fully Adjusted Rate Ratio (95% CI) | Likelihood Ratio (LR) p-value | |
|---|---|---|---|---|---|---|---|
| Invited or received DREAMS in 2017/18 | 0.549 | ||||||
| No | 1054 | 29 | 2.8 | 1 | 1 | 1 | |
| Yes | 1329 | 23 | 1.7 | 0.63 (0.36 – 1.09) | 0.78 (0.44 – 1.36) | 0.83 (0.46 – 1.52) | |
| Current age | 0.449 | ||||||
| 13–17 | 1439 | 15 | 1.0 | 1 | 1 | ||
| 18–19 | 475 | 19 | 4.0 | 2.57 (1.23 – 5.39) | 1.74 (0.73 – 4.15) | ||
| 20–24 | 469 | 18 | 3.8 | 3.50 (1.81 – 6.76) | 1.40 (0.51 – 3.84) | ||
| Geographic area | 0.034 | ||||||
| Rural | 1528 | 25 | 1.6 | 1 | 1 | 1 | |
| Peri-urban/urban | 830 | 27 | 3.3 | 1.99 (1.15 – 3.43) | 1.96 (1.14 – 3.38) | 1.89 (1.05 – 3.39) | |
| Socio-economic status | 0.316 | ||||||
| Low | 838 | 23 | 2.7 | 1 | 1 | 1 | |
| Middle | 811 | 16 | 2.0 | 0.72 (0.38 – 1.36) | 0.71 (0.37 – 1.34) | 0.66 (0.34 – 1.28) | |
| High | 649 | 11 | 1.7 | 0.62 (0.30 – 1.27) | 0.63 (0.31 – 1.29) | 0.60 (0.28 – 1.29) | |
| Age and education | 0.358 | ||||||
| 13–17 or 18–22 & In school | 1962 | 34 | 1.7 | 1 | 1 | 1 | |
| 18–22 & Not completed secondary | 148 | 9 | 6.1 | 3.51 (1.68 – 7.32) | 1.96 (0.85 – 4.54) | 1.79 (0.73 – 4.38) | |
| 18–22 & completed secondary | 272 | 9 | 3.3 | 1.91 (0.92 – 3.99) | 1.06 (0.45 – 2.48) | 0.98 (0.39 – 2.46) | |
| Food insecurity | 0.674 | ||||||
| No | 1671 | 31 | 1.9 | 1 | 1 | 1 | |
| Yes | 706 | 21 | 3.0 | 1.60 (0.92 – 2.79) | 1.27 (0.72 – 2.24) | 1.14 (0.62 – 2.11) | |
| Ever had sex, ever pregnant | 0.278 | ||||||
| Never had sex | 1568 | 18 | 1.1 | 1 | 1 | 1 | |
| Ever sex, never pregnant | 266 | 7 | 2.6 | 2.29 (0.96 – 5.48) | 1.68 (0.64 – 4.41) | 1.54 (0.58 – 4.10) | |
| Ever pregnant | 537 | 24 | 4.5 | 3.89 (2.11 – 7.17) | 2.78 (1.26 – 6.14) | 2.02 (0.85 – 4.78) | |
| Migrated/moved | 0.174 | ||||||
| No | 2036 | 36 | 1.8 | 1 | 1 | 1 | |
| Yes | 347 | 16 | 4.6 | 2.61 (1.45 – 4.70) | 1.85 (0.99 – 3.46) | 1.60 (0.81 – 3.16) |
HSV2 incidence was 18.8 (15.9–22.1)/ 100 person years in those unexposed to DREAMS, compared with 16.3 (14.0–18.9) / 100 person years in those exposed to DREAMS. As with HIV incidence, there was no evidence of an association between DREAMS exposure and HSV-2 incidence after adjusting for potential confounding factors: adjRR 0.96: 95% CI (0.76–1.23). Findings in the younger age group (aged 13–17) and the older age group (18–22) were similar (figure 2a). Age and ever having sex were the only factors that remained associated with HSV2 incidence after adjustment (table 2b).
Table 2b.
Association between DREAMS and HSV-2 incidence among AGYW
| Person years | n HSV-2 positive | Incidence rate | Unadjusted Rate Ratio (95% CI) | Age Adjusted Rate Ratio (95% CI) | Fully Adjusted Rate Ratio (95% CI) | LR p-value | |
|---|---|---|---|---|---|---|---|
| Invited or received DREAMS in 2017/18 | 0.759 | ||||||
| No | 741 | 139 | 18.8 | 1 | 1 | 1 | |
| Yes | 1032 | 168 | 16.3 | 0.87 (0.69 –1.09) | 0.99 (0.79 –1.25) | 0.96 (0.76 –1.23) | |
| Current age | 0.002 | ||||||
| 13–14 | 519 | 57 | 11.0 | 1 | 1 | ||
| 15–17 | 656 | 94 | 14.3 | 1.66 (1.09 –2.55) | 1.56 (1.02 –2.40) | ||
| 18–19 | 322 | 85 | 26.4 | 2.68 (1.73 –4.15) | 2.17 (1.36 –3.46) | ||
| 20–24 | 276 | 71 | 25.7 | 3.30 (2.16 –5.05) | 2.55 (1.53 –4.25) | ||
| Geographic area | 0.084 | ||||||
| Rural | 1126 | 212 | 18.8 | 1 | 1 | 1 | |
| Peri-urban/urban | 628 | 94 | 15.0 | 0.79 (0.62 –1.01) | 0.79 (0.62 –1.01) | 0.80 (0.62 –1.03) | |
| Socio-economic status | 0.751 | ||||||
| Low | 639 | 122 | 19.1 | 1 | 1 | 1 | |
| Middle | 584 | 102 | 17.5 | 0.92 (0.70 –1.19) | 0.94 (0.72 –1.22) | 0.95 (0.73 –1.24) | |
| High | 484 | 73 | 15.1 | 0.79 (0.59 –1.06) | 0.82 (0.61 –1.09) | 0.89 (0.66 –1.20) | |
| Age and education | 0.505 | ||||||
| 13–17 or 18–22 & In school | 1515 | 241 | 15.9 | 1 | 1 | 1 | |
| 18–22 & Not completed secondary | 74 | 25 | 33.7 | 2.12 (1.40 –3.20) | 1.24 (0.79 –1.96) | 1.09 (0.67 –1.76) | |
| 18–22 & completed secondary | 184 | 41 | 22.3 | 1.40 (1.01 –1.95) | 0.82 (0.56 –1.20) | 0.83 (0.56 –1.23) | |
| Food insecurity | 0.531 | ||||||
| No | 1256 | 193 | 15.4 | 1 | 1 | 1 | |
| Yes | 510 | 114 | 22.4 | 1.45 (1.15 –1.83) | 1.23 (0.97 –1.56) | 1.08 (0.84 –1.39) | |
| Ever had sex, ever pregnant | 0.028 | ||||||
| Never had sex | 1278 | 171 | 13.4 | 1 | 1 | 1 | |
| Ever sex, never pregnant | 161 | 49 | 30.5 | 2.28 (1.66 –3.13) | 1.68 (1.18 –2.37) | 1.62 (1.13 –2.33) | |
| Ever pregnant | 323 | 85 | 26.3 | 1.97 (1.52 –2.55) | 1.36 (0.98 –1.87) | 1.34 (0.95 –1.89) | |
| Migrated/moved | 0.895 | ||||||
| No | 1546 | 257 | 16.6 | 1 | 1 | 1 | |
| Yes | 227 | 50 | 22.0 | 1.33 (0.98 –1.80) | 0.98 (0.72 –1.35) | 1.02 (0.73 –1.43) |
Transmissible HIV by DREAMS exposure
Prevalence of transmissible HIV was 87/865 (10.1%) in those who had not received DREAMS compared to 82/1030 (8.0%) in those who had received DREAMS, with no evidence of a DREAMS effect after adjusting for potential confounding factors using multivariable logistic regression: adjOR 1.14; 95%CI (0.79–1.64). Those who lived in a peri-urban/urban area, were out of school and had not completed secondary education at baseline, had migrated and who had sex or had been pregnant were more likely to have transmissible HIV (table 3). The propensity-score adjusted analysis, to compare the scenarios that all versus no AGYW were exposed to DREAMS (figure 2b), similarly found no evidence of an effect of DREAMS on transmissible HIV, with an estimated difference in the percentage with a detectable HIV viral load of 0.99%: 95% CI (−1.52,3.82)%. Findings about the association between DREAMS exposure and transmissible HIV were similar in the younger age group (aged 13–17) and the older age group (18–22).
Table 3.
Logistic regression: Association between DREAMS and transmissible HIV among AGYW aged 13–22 years
| Total | n with viral load>=50 (%) | Unadjusted OR (95% CI) | Age Adjusted OR (95% CI) | Fully Adjusted OR (95% CI) | LR p-value | |
|---|---|---|---|---|---|---|
| Invited or received DREAMS, 2017/18 | ||||||
| No | 865 | 87 (10.1) | 1 | 1 | 1 | |
| Yes | 1030 | 82 (8.0) | 0.77 (0.56 –1.06) | 0.99 (0.71 –1.38) | 1.14 (0.79 –1.64) | 0.477 |
| Age group, 2017 | ||||||
| 13–14 | 433 | 10 (2.3) | 0.13 (0.06 –0.25) | 0.37 (0.15 –0.90) | ||
| 15–17 | 623 | 43 (6.9) | 0.40 (0.27 –0.60) | 1.01 (0.55 –1.84) | ||
| 18–19 | 404 | 48 (11.9) | 0.73 (0.49 –1.08) | 0.94 (0.59 –1.51) | ||
| 20–22 | 435 | 68 (15.6) | 1 | 1 | 0.071 | |
| Geographic area | ||||||
| Rural | 1214 | 86 (7.1) | 1 | 1 | 1 | |
| Peri-urban/urban | 663 | 82 (12.4) | 1.85 (1.35 –2.55) | 1.86 (1.35 –2.57) | 1.91 (1.34 –2.72) | <0.001 |
| Socio-economic status, 2017 | ||||||
| Low | 653 | 64 (9.8) | 1 | 1 | 1 | |
| Middle | 656 | 59 (9.0) | 0.91 (0.63 –1.32) | 0.92 (0.63 –1.34) | 0.86 (0.58 –1.29) | |
| High | 513 | 37 (7.2) | 0.72 (0.47 –1.09) | 0.76 (0.50 –1.17) | 0.71 (0.45 –1.13) | 0.356 |
| Food insecurity, 2017 | ||||||
| No | 1308 | 96 (7.3) | 1 | 1 | 1 | |
| Yes | 582 | 73 (12.5) | 1.81 (1.31 –2.50) | 1.42 (1.02 –1.98) | 1.20 (0.83 –1.73) | 0.329 |
| Age and education, 2017 | ||||||
| 13–17 or 18–22 & In school | 1497 | 99 (6.6) | 1 | 1 | 1 | |
| 18–22 & Not completed secondary | 152 | 42 (27.6) | 5.39 (3.58 –8.12) | 3.13 (1.91 –5.11) | 2.96 (1.72 –5.10) | |
| 18–22 & completed secondary | 245 | 28 (11.4) | 1.82 (1.17 –2.84) | 1.05 (0.62 –1.77) | 1.05 (0.60 –1.84) | <0.001 |
| Migrated/moved, 2017 | ||||||
| No | 1571 | 115 (7.3) | 1 | 1 | 1 | |
| Yes | 324 | 54 (16.7) | 2.53 (1.79 –3.59) | 1.66 (1.15 –2.41) | 1.50 (1.00 –2.25) | 0.05 |
| Ever had sex, ever pregnant composite variable, 2017 | ||||||
| Never had sex | 1158 | 51 (4.4) | 1 | 1 | 1 | |
| Ever sex, never pregnant | 249 | 45 (18.1) | 4.79 (3.12 –7.34) | 3.16 (1.95 –5.13) | 2.55 (1.52 –4.30) | |
| Ever pregnant | 476 | 69 (14.5) | 3.68 (2.52 –5.38) | 2.27 (1.41 –3.65) | 1.79 (1.06 –3.00) | 0.002 |
Discussion
In this representative cohort of women aged 13–22, half of whom were invited to DREAMS (all of whom received at least one of the combination HIV prevention interventions)[13], we found no evidence that exposure to DREAMS was associated with reduction in sexual risk as evidenced by HSV2 incidence. After two years of exposure to DREAMS combination prevention there was no evidence of impact on HIV incidence or transmissible HIV (defined as detectable HIV viral load). Women who lived in peri-urban/urban areas, had recently left school, had a history of migration and were sexually active were at most risk of poor HIV outcomes.
It is plausible that overall declines in HIV incidence, attributable to a reduction in levels of untreated HIV infection among male sexual partners of AGYW may have prevented us from showing small reductions in HIV incidence attributable to DREAMS itself[22, 23]. However, we also found that DREAMS did not impact on sexual risk or prevalence of transmissible HIV, the two pathways through which we hypothesised DREAMS would reduce HIV incidence. This is consistent with other findings from our setting i.e. that DREAMS did not affect any of the behavioural drivers of sexual risk, including condom use, transactional sex or number of sexual partners. It remains to be investigated if DREAMS exposure had an impact on transmissible HIV amongst male partners in our setting.
These disappointing findings may in part be explained by the fact that DREAMS exposure was greater in younger than older AGYW: those still in school and who had not yet reached sexual debut even during the follow-up period. Key outcomes, on the other hand, were more common in older age groups: those who had left school and had a history of migration. It is plausible that over a longer follow-up period, and as this younger cohort age into their sexual debut, we will start to observe an impact of earlier exposure to DREAMS[14, 24].
Our analyse confirms the importance of structural factors in driving HIV risk and poor outcomes[5, 9, 25, 26]. We found that young women who have left the relative protection of school and who had a history of migration were more vulnerable to poor sexual health and HIV outcomes. DREAMS, whilst emphasising some aspects of social asset building, such as cash transfers and school grants, had limited income generation and training activities that appeal to young women transitioning from school into employment[14, 15]. Moreover, our process evaluation suggested that retention in curricular based interventions to change social and gender norms was challenging for young women[14, 15, 27, 28]. Our findings support calls for more radical and fundamental structural interventions to build social capital and create a more enabling environment for young women who are not in education, employment or training [14, 29, 30].
DREAMS, whilst ambitious in scope, did not explicitly tackle the well described barriers to AGYW accessing sexual reproductive and HIV treatment services within primary health care settings[27, 28, 31]. Implementing partners delivered community-based HIV testing (which increased testing uptake) but not sexual and reproductive health or HIV care[15]. Work from both our group and others have consistently found that young men and women (aged <30) often do not access HIV care, even after diagnosis[32, 33]. A similar pattern is seen in sexual and reproductive health seeking, and this has led to a high burden of sexually transmitted infections[34] and teenage pregnancy[27]. Despite the growing evidence on the effectiveness of community-based HIV care [35], particularly for adolescents living with HIV [36–38], HIV and sexual reproductive care in DREAMS remained facility based. This may partly account for the limited effect of exposure to DREAMS on HIV viral load amongst the AGYW.
Finally, we looked at the effect of any DREAMS exposure on sexual behaviour and HIV outcomes in AGYW, but not at the effect of different amounts of exposure, different patterns of layering or the fidelity of the intervention content. In work presented elsewhere we have shown that exposure and layering increased with time and that over 80% of those invited received at least three interventions[13]. Our in-depth ethnographic mapping however illustrated some of the challenges that multiple implementing partners faced in scaling up this complex and multifaceted intervention[15, 28] and the competing priorities for out of school women making it difficult for them to engage, either fully or at all, in curriculum based interventions [14, 27]. It is therefore plausible that longer and more sustained DREAMS like combination prevention intervention, led by AGYW that also integrates employability and livelihoods into the curriculum based interventions, would have greater impact [29].
Strengths and Limitations
The strength of our study was our ability to prospectively measure exposure to the DREAMS intervention and biological measures of sexual risk and HIV in a representative sample of AGYW. With over 80% response rate and over 90% contributing to the outcome we are confident that our sample is representative of the experience of DREAMS roll out amongst AGYW in this poor rural community of South Africa. However, our study was observational and we cannot exclude the possibility that those who are exposed to DREAMS are systematically different to those who are not in ways that impact on the outcome but which we did not capture sufficiently in our data collection or account for in our analyses. We attempted to measure key dimensions of sexual risk at baseline, and adjusted for these in our analyses, but we may not have fully accounted for these differences and if so there will be residual confounding. Given that for all outcomes the proportion with a poor outcome was lower among those exposed to DREAMS than among those not exposed, it is possible that systematic channelling bias may have masked a real effect of DREAMS exposure. Another limitation is that we did not track “dose” of exposure and counted any invitation or participation in a DREAMS intervention as an exposure.
Conclusions and implications for the future
In this evaluation of a real-world scale up of a promising combination HIV prevention intervention we did not find a short-term effect (over two years) of DREAMS exposure on sexual risk or HIV outcomes in a representative cohort of AGYW. Sexually active young women who had left school, had a history of migration and were residing in small urban and peri-urban areas had worse sexual risk and HIV outcomes. This suggests a need to improve engagement of older adolescents and young women in DREAMS and DREAMS like interventions with more fundamental structural interventions that build social capital and strengthen health systems for older adolescents and young women.
Supplementary Material
Acknowledgements
The authors acknowledge AHRI research team including the research assistants 9 B. Mbatha, D. Mkhwanazi, K. Ngobese, N. Buthelezi, N. Fakude, N. Mbatha, S. Nsiband, S. Ntshangase, S. Mnyango, Th. Dlamini, Z. Cumbane, Z. Mathenjwa, M.Zikhali, N. Mpanza, S. Xulu, X. Ngwenya, Zakhele Xulu, Z. Mthethwa, S. Hlongwane) and research administrators, especially A. Jalazi and S. Mbili, for their commitment to the study. We also extend our appreciation to our research community including the community advisory boards in uMkhanyakude district.
Funding
This work was supported by the Bill & Melinda Gates Foundation, Grant Number OPP1136774 and OPP1171600 and the National Institutes of Health under award number 5R01MH114560–03. Africa Health Research Institute is supported by a grant from the Wellcome Trust (082384/Z/07/Z). The AHRI population surveillance is partially funded by DSI-MRC South Africa Population Research Network. GH is supported by a fellowship from the Wellcome Trust and Royal Society (210479/Z/18/Z). Funding bodies were not involved in the design of the study, data collection, analysis or interpretation of the data.
Footnotes
Competing interests:
The authors declare that they have no competing interests
Consent for publication: Not applicable
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the AHRI repository and will be made available prior to publication
References
- 1.Chimbindi N, Mthiyane N, Birdthistle I, Floyd S, McGrath N, Pillay D, et al. Persistently high incidence of HIV and poor service uptake in adolescent girls and young women in rural KwaZulu-Natal, South Africa prior to DREAMS. PLoS One 2018; 13(10):e0203193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Human Sciences Research Council (HSRC). The Fifth South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2017: HIV Impact Assessment Summary Report. In. Edited by Press H. Cape Town; 2018. [Google Scholar]
- 3.UNAIDS. HIV prevention among adolescent girls and young women: Putting HIV prevention among adolescent girls and young women on the Fast-Track and engaging men and boys In: UNAIDS 2016. [Google Scholar]
- 4.Chang LW, Serwadda D, Quinn TC, Wawer MJ, Gray RH, Reynolds SJ. Combination implementation for HIV prevention: moving from clinical trial evidence to population-level effects. Lancet Infect Dis 2013; 13(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pettifor A, Stoner M, Pike C, Bekker L-G. Adolescent lives matter: preventing HIV in adolescents. Current opinion in HIV and AIDS 2018; 13(3):265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta GR, Parkhurst JO, Ogden JA, Aggleton P, Mahal A. Structural approaches to HIV prevention. Lancet 2008; 372(9640):764–775. [DOI] [PubMed] [Google Scholar]
- 7.Pronyk PM, Kim JC, Abramsky T, Phetla G, Hargreaves JR, Morison LA, et al. A combined microfinance and training intervention can reduce HIV risk behaviour in young female participants. AIDS 2008; 22(13):1659–1665. [DOI] [PubMed] [Google Scholar]
- 8.Wagman JA, Gray RH, Campbell JC, Thoma M, Ndyanabo A, Ssekasanvu J, et al. Effectiveness of an integrated intimate partner violence and HIV prevention intervention in Rakai, Uganda: analysis of an intervention in an existing cluster randomised cohort. Lancet Glob Health 2015; 3(1):e23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cluver LD, Orkin FM, Campeau L, Toska E, Webb D, Carlqvist A, et al. Improving lives by accelerating progress towards the UN Sustainable Development Goals for adolescents living with HIV: a prospective cohort study. Lancet Child Adolesc Health 2019; 3(4):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DREAMS working together for an AIDS free future for girls and women. Washington D.C.: Office of the U.S. Global AIDS Coordinator; 2018. [Google Scholar]
- 11.Dreaming of an AIDS-Free Future. Washington D.C, USA: Office of the U.S. Global AIDS Coordinator; 2018. [Google Scholar]
- 12.Saul J, Bachman G, Allen S, Toiv NF, Cooney C, Beamon TA. The DREAMS core package of interventions: A comprehensive approach to preventing HIV among adolescent girls and young women. PLOS ONE 2018; 13(12):e0208167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gourlay A, Birdthistle I, Mthiyane NT, Orindi BO, Muuo S, Kwaro D, et al. Awareness and uptake of layered HIV prevention programming for young women: analysis of population-based surveys in three DREAMS settings in Kenya and South Africa. BMC Public Health 2019; 19(1):1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chimbindi N, Birdthistle I, Floyd S, Harling G, Mthiyane N, Zuma T, et al. Directed and target focused multi-sectoral adolescent HIV prevention: Insights from implementation of the ‘DREAMS Partnership’ in rural South Africa. J Int AIDS Soc 2020; 23 Suppl 5:e25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chimbindi N, Birdthistle I, Shahmanesh M, Osindo J, Mushati P, Ondeng’e K, et al. Translating DREAMS into practice: Early lessons from implementation in six settings. PloS one 2018; 13(12):e0208243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birdthistle I, Schaffnit SB, Kwaro D, Shahmanesh M, Ziraba A, Kabiru CW, et al. Evaluating the impact of the DREAMS partnership to reduce HIV incidence among adolescent girls and young women in four settings: a study protocol. BMC Public Health 2018; 18(1):912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gareta D, Baisley K, Mngomezulu T, Smit T, Khoza T, Nxumalo S, et al. Cohort profile update: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. International Journal of Epidemiology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arvin A, Prober C. Herpes Simplex Viruses. In: Manual of Clinical Microbiology 6th Ed Murray P, Baron E, Pfaller M, Tenover F, Yolkenet R (editors). Washington D.C.: ASM; 1995. pp. 876–883. [Google Scholar]
- 19.Delany-Moretlwe S, Jentsch U, Weiss H, Moyes J, Ashley-Morrow R, Stevens W, et al. Comparison of Focus HerpeSelect ® and Kalon TM HSV-2 gG2 ELISA serological assays to detect herpes simplex virus type 2 (HSV-2) antibodies in a South African population.. Sexually Transmitted Infections, 2009; 86(1):46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashley-Morrow R, Nolkamper J, Robinson N, Bishop N, Smith J. Performance of Focus ELISA test for herpes simplex virus type 1 (HSV-1) and HSV-2 antibodies among women in ten diverse geographical locations.. Clinical Microbiology and Infection 2004; 10(6):530–536. [DOI] [PubMed] [Google Scholar]
- 21.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999; 10(1):37–48. [PubMed] [Google Scholar]
- 22.Shahmanesh M et al. Reaching young men: Evaluating the impact of DREAMS on HIV testing, care and prevention among young men in three diverse settings. In: AIDS 2020. San Francisco; 2020. [Google Scholar]
- 23.Vandormael A, Akullian A, Siedner M, de Oliveira T, Barnighausen T, Tanser F. Declines in HIV incidence among men and women in a South African population-based cohort. Nat Commun 2019; 10(1):5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibbs A, Campbell C, Maimane S. Can local communities ‘sustain’ HIV/AIDS programmes? A South African example. Health Promot Int 2015; 30(1):114–125. [DOI] [PubMed] [Google Scholar]
- 25.Pettifor A, Lippman SA, Gottert A, Suchindran CM, Selin A, Peacock D, et al. Community mobilization to modify harmful gender norms and reduce HIV risk: results from a community cluster randomized trial in South Africa. J Int AIDS Soc 2018; 21(7):e25134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranganathan M, Heise L, Pettifor A, Silverwood RJ, Selin A, MacPhail C, et al. Transactional sex among young women in rural South Africa: prevalence, mediators and association with HIV infection. J Int AIDS Soc 2016; 19(1):20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuma T, Seeley J, Mdluli S, Chimbindi N, McGrath N, Floyd S, et al. Young people’s experiences of sexual and reproductive health interventions in rural KwaZulu-Natal, South Africa. International Journal of Adolescence and Youth 2020; 25(1):1058–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuma T, Seeley J, Sibiya LO, Chimbindi N, Birdthistle I, Sherr L, et al. The Changing Landscape of Diverse HIV Treatment and Prevention Interventions: Experiences and Perceptions of Adolescents and Young Adults in Rural KwaZulu-Natal, South Africa. Front Public Health 2019; 7:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mannell J, Willan S, Shahmanesh M, Seeley J, Sherr L, Gibbs A. Why interventions to prevent intimate partner violence and HIV have failed young women in southern Africa. J Int AIDS Soc 2019; 22(8):e25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell C Social capital, social movements and global public health: Fighting for health-enabling contexts in marginalised settings. Soc Sci Med 2020; 257:112153. [DOI] [PubMed] [Google Scholar]
- 31.Nkosi B, Seeley J, Chimbindi N, Zuma T, Kelley M, Shahmanesh M. Managing ancillary care in resource-constrained settings: Dilemmas faced by frontline HIV prevention researchers in a rural area in South Africa. Int Health 2020; 12(6):543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baisley KJ, Seeley J, Siedner MJ, Koole K, Matthews P, Tanser F, et al. Findings from home-based HIV testing and facilitated linkage after scale-up of test and treat in rural South Africa: young people still missing. HIV Med 2019; 20(10):704–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwuji CC, Orne-Gliemann J, Larmarange J, Balestre E, Thiebaut R, Tanser F, et al. Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomised trial. Lancet HIV 2018; 5(3):e116–e125. [DOI] [PubMed] [Google Scholar]
- 34.Francis SC, Mthiyane TN, Baisley K, McHunu SL, Ferguson JB, Smit T, et al. Prevalence of sexually transmitted infections among young people in South Africa: A nested survey in a health and demographic surveillance site. PLoS Med 2018; 15(2):e1002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dave S, Peter T, Fogarty C, Karatzas N, Belinsky N, Pant Pai N. Which community-based HIV initiatives are effective in achieving UNAIDS 90–90-90 targets? A systematic review and meta-analysis of evidence (2007–2018). PloS one 2019; 14(7):e0219826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mavhu W, Willis N, Mufuka J, Bernays S, Tshuma M, Mangenah C, et al. Effect of a differentiated service delivery model on virological failure in adolescents with HIV in Zimbabwe (Zvandiri): a cluster-randomised controlled trial. Lancet Glob Health 2020; 8(2):e264–e275. [DOI] [PubMed] [Google Scholar]
- 37.Kanters S, Park JJ, Chan K, Ford N, Forrest J, Thorlund K, et al. Use of peers to improve adherence to antiretroviral therapy: a global network meta-analysis. J Int AIDS Soc 2016; 19(1):21141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernays S, Tshuma M, Willis N, Mvududu K, Chikeya A, Mufuka J, et al. Scaling up peer-led community-based differentiated support for adolescents living with HIV: keeping the needs of youth peer supporters in mind to sustain success. J Int AIDS Soc 2020; 23 Suppl 5:e25570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the AHRI repository and will be made available prior to publication


