Abstract
Background
The low implementation rate of guideline-directed medical therapy for heart failure (HF) remains a problem worldwide. To address this issue, we hypothesized that a smartphone application (app) based on behavioral economics that nudges physicians and patients towards optimal medical therapy would be a scalable approach.
Methods and Results
The app prototype was developed, and its usability was tested with 5 HF patients in the outpatient setting. Adherence to the app was outstanding, with a high usability rating from the patients.
Conclusions
It appears feasible to further study our app in a larger cohort to evaluate its efficacy.
Key Words: Clinical inertia, Digital therapeutics, Heart failure
Heart failure (HF) is a disease with a poor prognosis and a high rate of rehospitalization.1 Despite substantial evidence that guideline-directed medical therapy (GDMT) improves prognosis, the low implementation rate of GDMT is a problem worldwide.2
Why don’t physicians prescribe and patients accept GDMT? Addressing such “clinical inertia” is a significant challenge. The real-world gap in medical practice can be explained from the perspective of behavioral economics, which is a field of research that seeks to explain the process of human decision-making, whereby people are not always capable of making rational decisions due to various cognitive biases.
From this perspective, one of the potential cause of clinical inertia is the “status quo bias”,3 which is the tendency to avoid changes from the current state, even if a change from it would be more desirable. GDMT for HF requires frequent drug induction/escalation and laboratory testing in the clinic. As most of these drugs are not for alleviating symptoms but to improve long-term prognosis (so patients cannot immediately perceive the benefits), changing the medication from the current prescription would overemphasize short-term negative aspects such as concerns about side effects and cost, consequently leading physicians and patients to stick to the status quo.
Given that smartphone applications (apps) have gained attention in recent years as a tool to support physicians and patients, we hypothesized that an app aimed at improving GDMT adherence using behavioral economics insights could be a scalable and effective tool to overcome clinical inertia in the management of HF. The aim of this study was to develop a novel HF app designed to improve GDMT adherence and to test its usability for future trials.
Methods
Features of the App
The prototype app was developed by the study team in Oita University. The app was coded using Flutter Software (version3.7.10). To overcome cognitive biases occurring in the clinic, nudge theory was implemented in the app. Thaler and Sunstein defined nudges as any aspect of the choice architecture that alters people’s behavior in a predictable way without forbidding any options or significantly changing their economic incentives.4 The app design was based on this theory and several features were implemented.
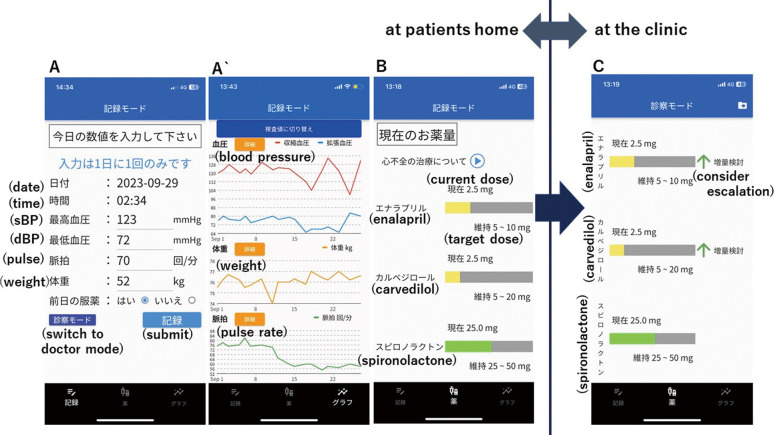
The first feature of the app is the ability to record and collect daily vital data (health records) in the patient’s home setting (Figure A,A`). Patients are able to measure their daily blood pressure, pulse, and weight themselves and manually enter the data into the app. Laboratory values, especially potassium and creatinine, can also be entered into the app by the physician during clinic visits. All data are visualized as charts, making it easy to discuss the data during clinic visits.
Figure.
Screenshots of the app. (A) The screen for entering the patient’s daily health data, where blood pressure (BP), pulse, weight, medication adherence can be recorded. (A`) Chart showing the trends in the entered daily health records. (B) The medication screen displays gauges for 3 medications: current prescribed doses of ACEi/ARB/ARNI, β-blocker and MRA relative to the target dose. (C) Recommendation alert to escalate/induce medications. In this screen, the doses of enalapril and carvedilol are recommended for escalation.
The second feature of the app is a “medication” screen, which consists of a gauge to visualize the patient’s current HF medication doses relative to the patient’s target dose (i.e., GDMT) (Figure B). Three categories of drugs, specifically angiotensin-converting enzyme inhibitors (ACEi)/angiotensin-receptor blockers (ARB)/angiotensin-receptor neprilysin inhibitors (ARNI), β-blockers and mineralocorticoid receptor antagonists (MRAs), were adopted in this screen. The target dose for each drug was set according to the doses recommended by the Japan Circulation Society (JCS) guidelines.5 This feature is intended to nudge patients towards GDMT. From a behavioral economics perspective, when the current dosage is the patient’s “reference point”, increasing the drug dosage is perceived as a loss in the short term because these drugs do not relieve symptoms. To correct this cognitive bias in patients, and to accept drug escalation or induction, it is necessary to deliberately shift the patient’s “reference point” to the target dose by visually presenting the patient’s target dose, thereby presenting it as a default to titrate to the maximum dose. The medication screen is designed to make this visually apparent.
The third feature is a recommendation alert that pops up on the app’s medication screen during clinical visits to escalate or initiate each medication (Figure C). The on-screen alert is intended to be seen by the clinician and patient together in the clinic. When the patient brings the app to the clinic, the guideline-based algorithm in the app analyzes health records in the app (the patient’s vital signs at home and laboratory values obtained in the clinic) and gives the physician and patient a recommendation to escalate the medication’s dosage.
Usability Study
After the app was developed, 5 HF patients in the outpatient clinic who were not on the maximum tolerated dose of GDMT were recruited from 3 hospitals (Oita University Hospital, Nagoya City University East Medical Center, and Dokkyo University Nikko Medical Center) in Japan to test the app’s usability. The study was conducted during the patients’ usual clinic visit intervals, which ranged from ≈28 to 56 days. Recruiting 5 patients is common for usability studies as it is typically sufficient to identify usability problems.6
After enrolment, the participants were loaned an iPhone XR (Apple) with the app already downloaded and were given instruction on how to use the app. Participants were encouraged to measure their daily blood pressure, pulse rate, and weight at home and record them in the app until the day of their next clinic visit. At their next visit, participants were instructed to bring the iPhone to the clinic and give it to the physician, who would enter data from laboratory tests, if performed, during the outpatient visit. After entering the results, clinicians showed participants the medication screen, which displayed the drug escalation alert, and discussed the medication dosage adjustments. Although physicians and patients were encouraged to discuss the medication regimen using the app, the physician had the final authority to make decisions about prescription changes. Following the visit, patients returned the iPhone to the investigator and were requested for an interview to identify any usability problems. Additionally, they were asked to complete a System Usability Scale (SUS) questionnaire. The SUS score is used to assess the usability of software, and a score ≥68 is usually considered above average.7 The study complied with the Declaration of Helsinki and was approved by the Clinical Trial Ethics Committee of Oita University Hospital. All participants provided written informed consent. The study was registered at the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000050520).
Endpoints
The primary endpoint was the percentage of days the patient entered their health records in the app from the first day of app use to day 28. This endpoint aimed to evaluate the patients’ willingness and feasibility to consistently use the app in their home settings. As exploratory endpoints, the SUS was used to evaluate the overall usability of the app.
Statistical Analysis
As this was a feasibility study with an exploratory purpose, only descriptive statistics were applied for the analysis. Continuous variables are expressed as median [minimum, maximum].
Results
A total of 5 patients being treated by 4 physicians were recruited from the 3 hospitals between April 2023 and May 2023. One patient was excluded from further analysis due to hospitalization for worsening of pre-existing disease (hepatocellular carcinoma) during the study. Therefore, 4 patients were included in the final analysis.
The baseline characteristics for each participant are summarized in Table 1. The median age of the patients was 54.5 [50.0, 66.0] years and all were male. The median left ventricular ejection fraction (LVEF) was 24.5 [23.1, 33.9] %.
Table 1.
Baseline Characteristics of the Participants
| Patient (ID) |
Baseline characteristics | Baseline medication | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) |
LVEF (%) |
Serum potassium (mmol/L) |
Serum creatinine (mg/dL) |
SBP (mmHg) |
DBP (mmHg) |
Resting heart rate (beats/min) |
ACEi/ARB/ ARNI |
β-blocker | MRA | |
| 1 | 66 | 24.0 | 3.9 | 0.78 | 91 | 54 | 85 | En 2.5 mg | C 5 mg | S 50 mg |
| 2 | 51 | 25.0 | 4.8 | 0.83 | 101 | 60 | 73 | S-V 100 mg | C 2.5 mg | Ep 25 mg |
| 3 | 58 | 33.9 | 4.0 | 1.16 | 157 | 91 | 53 | B 3.75 mg | ||
| 4 | 50 | 23.1 | 4.2 | 0.90 | 104 | 77 | 79 | C 10 mg | S 25 mg | |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; ARNI, angiotensin-receptor neprilysin inhibitor; B, bisoprolol; C, carvedilol; DBP, diastolic blood pressure; En, enalapril; Ep, eplerenone; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; SBP, systolic blood pressure; S, spironolactone; S-V, sacubitril-valsartan.
Endpoints for each participant are summarized in Table 2. Regarding the primary endpoint, on average, the patients successfully recorded their health records in the app for 100 [96.4, 100] % of the days during the initial 28-day period. For usability assessments, the median SUS score for the app was 85.0 [77.5, 90.0], which is rated as “good” in the adjective rating.
Table 2.
Outcomes
| Patient (ID) |
Results | |
|---|---|---|
| Percent of days the data was entered during the first 28 days |
System Usability Scale Score |
|
| 1 | 96.4 (27/28) | 77.5 |
| 2 | 100 (28/28) | 82.5 |
| 3 | 100 (28/28) | 87.5 |
| 4 | 100 (28/28) | 90.0 |
The numbers in parentheses indicate the days of data entry over 28 days.
Discussion
A smartphone app developed to nudge patients and clinicians toward GDMT was evaluated for usability, and the results suggested outstanding feasibility for use in the clinical setting. Over the past years, several interventions have been reported to address the issue of low GDMT implementation, with mixed results. For instance, physician and patient reminders to initiate or titrate GDMT have shown limited effectiveness.8,9 On the other hand, an electronically delivered decision aid to nudge patients has been reported to be effective in improving GDMT implementation.10 Remote titration of drugs has also been effective compared with conventional outpatient clinics.11 Additionally, in the era of digital therapy, remote titration using algorithms to remotely analyze physical parameters obtained at home has also been attempted.12 However, many of these interventions face challenges in terms of cost and the burden they place on healthcare professionals, limiting scalability. In contrast, our app offers a very simple and low-cost solution, making it more scalable for wider public use.
The usability test with 4 patients suggested the feasibility of our app with an impressive app adherence of almost 100% for the first 28 days, together with a high SUS score of 85.0. The high SUS score suggests excellent usability, which has motivated us to proceed to larger study assessing the effectiveness of the app.
Study Limitations
Our study has several limitations. First, although the sample size was deemed adequate for assessing the usability and feasibility of the app, it was not powered to evaluate its effectiveness. Future research is warranted to assess the app’s efficacy with a larger number of patients. Second, the study duration was relatively short and longer-term adherence to the app is unknown. Third, the participants in this study may not represent the majority of HF patients because they may have been more motivated to participate in the study.
Conclusions
The encouraging results of a preliminary usability study of a simple, user-friendly smartphone app developed to nudge patients towards GDMT suggest further evaluation of its efficacy in a larger cohort.
Disclosures
N.T. is a member of Circulation Reports’ Editorial Team; K. Hayashi, K. Hachiya, K.Y., N.O., K.F., I.M. are listed as inventors in a patent pending for the smartphone app; other authors declare no conflicts of interest.
Grant
This study was supported by a grant from Daiwa Securities Health Foundation and JSPS KAKENHI Grant Number JP23K09552.
IRB Information
Clinical Trial Ethics Committee of Oita University Hospital approved this study (reference number B22-007).
Data Availability
The de-identified participant data will not be shared.
References
- 1. Agarwal MA, Fonarow GC, Ziaeian B.. National trends in heart failure hospitalizations and readmissions from 2010 to 2017. JAMA Cardiol 2021; 6: 952–956, doi:10.1001/jamacardio.2020.7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Savarese G, Kishi T, Vardeny O, Adamsson Eryd S, Bodegård J, Lund LH, et al.. Heart failure drug treatment – inertia, titration, and discontinuation: A multinational observational study (EVOLUTION HF). JACC Heart Fail 2023; 11: 1–14, doi:10.1016/j.jchf.2022.08.009. [DOI] [PubMed] [Google Scholar]
- 3. Suri G, Sheppes G, Schwartz C, Gross JJ.. Patient inertia and the status quo bias: When an inferior option is preferred. Psychol Sci 2013; 24: 1763–1769, doi:10.1177/0956797613479976. [DOI] [PubMed] [Google Scholar]
- 4. Thaler RH, Sunstein CR.. Nudge: Improving decisions about health, wealth, and happiness. New Haven, CT: Yale University Press, 2008.
- 5. Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, et al.. JCS 2017/JHFS 2017 Guideline on diagnosis and treatment of acute and chronic heart failure: Digest version. Circ J 2019; 83: 2084–2184, doi:10.1253/circj.CJ-19-0342. [DOI] [PubMed] [Google Scholar]
- 6. Nielsen J, Landauer TK.. A mathematical model of the finding of usability problems. In: Proceedings of the INTERACT ’93 and CHI ’93 Conference on Human Factors in Computing Systems. Amsterdam, The Netherlands: Association for Computing Machinery; 1993: 206–213. [Google Scholar]
- 7. Hyzy M, Bond R, Mulvenna M, Bai L, Dix A, Leigh S, et al.. System usability scale benchmarking for digital health apps: Meta-analysis. JMIR Mhealth Uhealth 2022; 10: e37290, doi:10.2196/37290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ansari M, Shlipak MG, Heidenreich PA, Van Ostaeyen D, Pohl EC, Browner WS, et al.. Improving guideline adherence: A randomized trial evaluating strategies to increase beta-blocker use in heart failure. Circulation 2003; 107: 2799–2804, doi:10.1161/01.CIR.0000070952.08969.5B. [DOI] [PubMed] [Google Scholar]
- 9. Wadhwa R, Fridsma DB, Saul MI, Penrod LE, Visweswaran S, Cooper GF, et al.. Analysis of a failed clinical decision support system for management of congestive heart failure. AMIA Annu Symp Proc 2008; 2008: 773–777. [PMC free article] [PubMed] [Google Scholar]
- 10. Allen LA, Venechuk G, McIlvennan CK, Page RL, Knoepke CE, Helmkamp LJ, et al.. An electronically delivered patient-activation tool for intensification of medications for chronic heart failure with reduced ejection fraction: The EPIC-HF trial. Circulation 2021; 143: 427–437, doi:10.1161/CIRCULATIONAHA.120.051863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Desai AS, MacLean T, Blood AJ, Bosque-Hamilton J, Dunning J, Fischer C, et al.. Remote optimization of guideline-directed medical therapy in patients with heart failure with reduced ejection fraction. JAMA Cardiol 2020; 5: 1430–1434, doi:10.1001/jamacardio.2020.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong CK, Un KC, Zhou M, Cheng Y, Lau YM, Shea PC, et al.. Daily ambulatory remote monitoring system for drug escalation in chronic heart failure with reduced ejection fraction: Pilot phase of DAVID-HF study. Eur Heart J Digit Health 2022; 3: 284–295, doi:10.1093/ehjdh/ztac024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The de-identified participant data will not be shared.