Abstract
Background & Aim
Causes of hepatocellular carcinoma (HCC) may change as treatments become available for some liver diseases. We examined the distribution of HCC cause and survival of a nationwide cohort of insured patients.
Methods
Optum’s de-identified Clinformatics® Data Mart Database (CDM), 2003–2021.
Results
A total of 34707 patients with HCC were included: mean age: 68.3±11.6 years, 61% male, 62% Caucasian, 74% cirrhosis. Non-alcoholic fatty liver disease (NAFLD) was the most common etiology (38.9%), then hepatitis C virus (HCV) (25.3%), cryptogenic (18.0%), alcohol-associated liver disease (9.4%), other liver diseases (5.8%) and hepatitis B virus (HBV) at 2.6%. NAFLD patients were the oldest (mean age 71.1±11.2) and had the highest Charlson Comorbidity Index (CCI) (mean 10.5±3.9), while HCV were the youngest (mean age 64.2±9.2 years) and HBV had the lowest CCI (mean 7.2±4.4) (both P<0.0001). The overall 5-year survival was 18.8% (95% CI 18.2–19.3) but was lower in the recent 2014–2021 period vs 2003–2013 (18.1% vs 19.5%, P=0.003). The 2014–2021 cohort (inclusive of HCV treatment advances) was significantly older, with more females, fewer Caucasians, more African Americans, more Hispanics, fewer Asians, more cirrhosis, more NAFLD, and higher CCI (all P<0.001). On multivariable analysis, males (aHR: 1.13), Caucasians (aHR: 1.46), African Americans (aHR: 1.53) and Hispanics (aHR: 1.28) vs Asians, 2014–2021 (vs 2003–2013) cohort (aHR: 1.12), NAFLD (aHR: 1.14) or cryptogenic liver disease (aHR: 1.45) were associated with increased mortality (all P<0.001).
Conclusion
HCC patients in more recent time 2014–2021 were more likely to be older, more likely to have nonviral etiology, and had worse survival compared to those from 2003 to 2013.
Keywords: ethnic disparities, NAFLD, nonviral, epidemiology
Introduction
Worldwide, hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer-related death.1 HCC is also among the few cancers with both rising incidence and mortality in the United States.2
HCC develops most commonly in patients with chronic liver disease, particularly those chronically infected with hepatitis C virus (HCV), hepatitis B virus (HBV), those with alcohol-associated liver disease (ALD), and non-alcoholic fatty liver disease (NAFLD).2 Globally, HBV is the most common etiology of HCC, while previous studies have found HCV to be the main cause of HCC in the United States.2,3 However, in the past decade, several major changes in the causes of liver disease have occurred which may have affected the trends of HCC.4–6
Specifically, with the increasing burden of obesity and diabetes globally, NAFLD may now be a major and increasing cause of HCC.6,7 In fact, studies on the prevalence of NAFLD have shown that NAFLD is increasing around the world,8–11 while the prevalence of HBV is decreasing and the availability of interferon-free direct-acting antivirals for CHC occurred with the expectation that the number of CHC-related HCC would decrease.12–14
Existing studies either examined trends in the mortality of decedents with HCC using death certificate data rather than examining the distribution of the causes of HCC or survival rates among patients with HCC who are currently alive. Most prior studies also primarily included data prior to the wide availability of direct-acting antiviral for hepatitis C. Thus, updated data to evaluate more contemporary impact of antiviral therapy as well as of the rising obesity epidemic in the causes and survival of patients with HCC are needed.
Given the implications for public health planning, the goal of this study was to examine the distribution of causes of HCC, their changes over time, and the long-term survival of patients with HCC in a large national sample of patients. Using nationwide data inclusive of community and academic centers to minimize selection bias, this study provides updated and etiologic-specific survival rates for patients with HCC to help identify higher risk patients and to inform future prevention research and intervention. We also provide detailed population-level subgroup data by sex, race/ethnicity, and etiology to inform future modeling studies and healthcare/public health planning.
Methods
Study Design and Population
We conducted a retrospective study using a nationwide sample of patients with hepatocellular carcinoma from Optum’s de-identified Clinformatics® Data Mart Database (CDM), which contains de-identified administrative health claims for patients enrolled in commercial and Medicare Advantage health plans, with medical and prescription drug claims data and enrollment information for approximately 61 million people, as wells as laboratory test results in a subset of about 31 million people.15
Adult (≥18 years) patients were included if they had HCC (defined by ICD codes 155.0, C22.0, C22.7, C22.8, C22.9 as consistent with previous studies)16 and ≥6 months of insurance coverage before the earliest HCC diagnosis (index date), and no prior liver transplant. Patients were identified as having cirrhosis if they had diagnoses of cirrhosis or complications of cirrhosis such as hepatic encephalopathy and/or ascites in the presence of a chronic liver disease as previously described.17 Information about additional variables are included in the Supplemental Methods.
Etiology of Liver Disease
Because liver disease can have more than one cause, we used the following rules to create mutually exclusive categories of underlying liver disease:
Patients were first categorized into the HCV or HBV groups regardless of the presence of any additional etiologies, such as alcohol use. (Supplemental Table 1)
Patients without HCV or HBV were then classified into the “other liver diseases” group if they had autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, hemochromatosis, alpha-1 antitrypsin deficiency, or Wilson’s disease.
Patients not classified into any of the above groups were then categorized into the ALD group using a definition adapted from previous work: patients were classified as having ALD if they were diagnosed with alcohol use disorders, including alcohol abuse, dependence, alcohol withdrawal, alcohol-induced delirium, alcohol-induced mental disorders, acute alcohol intoxication, alcohol polyneuropathy, alcohol cardiomyopathy, alcohol pancreatitis, acute alcoholic hepatitis, and alcoholic liver damage in the absence of a known specific liver disease.7
Patients not classified into any of the above groups were evaluated for inclusion into the NAFLD group. As NAFLD-HCC does not have pathognomonic serological, radiological, or histological features, we adapted a clinical definition of NAFLD based on previous work that reflects the diagnostic process used in clinical practice, ie, NAFLD is suspected in the presence of risk factors, obesity and diabetes, after the exclusion of other etiologies.7
Statistical Analysis
We reported descriptive statistics as counts and percentages for categorical variables and for continuous variables, we reported mean and standard deviation (SD) for normally distributed variables; otherwise, we reported median and interquartile range (IQR). Comparisons between subgroups were done using the Student’s t-test of variance or Wilcoxon rank-sum test for continuous variables and the Pearson chi-squared test for categorical variables. For comparison between three or more groups, we used the one-way ANOVA test or the Kruskal–Wallis H-test. Overall survival rates were evaluated via the Kaplan–Meier methods and comparisons among the subgroups were performed using the Log rank test. Predictors for overall survival were performed using univariable and multivariable Cox proportional hazard regression in a stepwise backward fashion for the latter. Variables with potential association with survival by prior knowledge or variables with univariable hazard ratio (HR) with P-value of 0.10 or less were included in multivariable model to estimate adjusted HR (aHR). All analyses were conducted using Stata 17.0 (College Station, TX), and P-values <0.05 were considered significant.
Results
Study Population and Characteristics of HCC Patients
Overall
In total, there were 34,707 patients with HCC who met our inclusion criteria (Supplemental Figure 1). The overall cohort’s mean age was 68.3±11.6 years, 61% were male, 62% were Caucasian, 74% had cirrhosis, the average CCI was 9.0± 4.2, 4.4% received a liver transplant and 35% received hospice/palliative care (Table 1).
Table 1.
Baseline Characteristics of the Total HCC Cohort by Liver Disease Etiology, 2003–2021
| Characteristic | Total N=34,707 |
HCV N=8,765 25.3% | HBV N=905 2.6% | ALD N=3,265 9.4% |
NAFLD N=13,48,338.9% | Cryptogenic N=6,261 18.0% |
Other* N=2,028 5.8% | P-value |
|---|---|---|---|---|---|---|---|---|
| Mean age (years±SD) | 68.3±11.6 | 64.2±9.2 | 65.2±12.6 | 68.5±10.3 | 71.1±11.2 | 68.8±14.0 | 67.8±12.2 | <0.0001 |
| Sex (%) | <0.001 | |||||||
| Male | 61.4 | 74.7 | 72.8 | 80.8 | 54.0 | 48.0 | 57.6 | |
| Female | 38.6 | 25.3 | 27.2 | 19.2 | 46.0 | 52.1 | 42.4 | |
| Race/ethnicity (%) | <0.001 | |||||||
| Caucasian | 62.1 | 54.6 | 31.9 | 65.6 | 63.0 | 71.0 | 68.9 | |
| African American | 11.7 | 16.3 | 9.7 | 8.5 | 11.5 | 9.1 | 7.3 | |
| Hispanic | 13.7 | 15.6 | 9.1 | 17.3 | 15.1 | 7.5 | 12.1 | |
| Asian | 4.7 | 5.8 | 41.1 | 1.9 | 3.0 | 3.1 | 3.7 | |
| Unknown | 7.9 | 7.7 | 8.2 | 6.8 | 7.5 | 9.3 | 8.0 | |
| Cirrhosis (%) | 73.7 | 93.3 | 75.7 | 83.0 | 65.8 | 51.6 | 93.3 | <0.001 |
| Advance care directive (%) | 8.0 | 6.7 | 8.1 | 9.1 | 9.7 | 6.0 | 6.7 | <0.001 |
| Hospice/palliative care (%) | 35.2 | 30.2 | 25.5 | 39.2 | 38.4 | 34.1 | 36.7 | <0.001 |
| DCCI (±SD) | 9.0±4.2 | 7.4±4.3 | 7.2±4.4 | 8.8±4.3 | 10.5±3.9 | 8.7±3.2 | 8.5±4.0 | <0.0001 |
| Antiviral therapy (%) | – | – | – | – | – | – | ||
| HCV DAA** | 29.8 | |||||||
| HBV | 40.8 | |||||||
| Liver transplant (%) | 4.4 | 11.0 | 6.5 | 4.0 | 1.2 | 0.4 | 8.2 | <0.001 |
| Follow-up (person-years) | 46,670 | 15,782 | 1794 | 3807 | 15,620 | 6660 | 3006 | – |
Notes: *Other liver diseases: autoimmune hepatitis, primary sclerosing cholangitis, primary biliary cholangitis, hemochromatosis, alpha-1 antitrypsin deficiency, and Wilson’s disease. **Includes only patients from 2014 and after.
Abbreviations: HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HBV, hepatitis B virus; ALD, alcohol-associated liver disease; NAFLD, non-alcoholic fatty liver disease; DCCI: Deyo Charlson Comorbidity Index.
Subgroup Analysis
Demographic and Clinical Characteristics by Individual Liver Disease Etiology
By individual liver disease etiology (Table 1), NAFLD was the most prevalent cause of HCC (38.9%, n = 13,483) followed by HCV (25.3%, n = 8,765), then cryptogenic liver disease (18.0%, n = 6,261), ALD (9.4%, n = 3,265), “other” liver disease (5.8%, n = 2,028) and lastly HBV at 2.6% (n = 905). Those with NAFLD were the oldest (mean age 71.1± 11.2 years) while those with HCV were the youngest (mean age 64.2±9.2 years, P<0.0001). Those with ALD were more frequently male (80.8%) while those with cryptogenic were more frequently female (52%, P<0.0001). There were significant differences in the distribution of liver disease etiology among the racial/ethnic groups (P<0.0001). Those with HBV were more frequently Asian (41%) compared to all the other etiologies who were more frequently Caucasian (54.6%–71.0%). Those with other liver diseases and HCV more frequently had cirrhosis (93%) followed by ALD (83%), HBV (76%), NAFLD (66%) and cryptogenic (52%) (P<0.0001). Those with NAFLD had the highest CCI (mean 10.5±3.9) while those with HBV had the lowest CCI (mean 7.2±4.4, P<0.0001).
Laboratory Characteristics by Individual Liver Disease Etiology
Overall, the majority of patients fell into ALBI category 1 (57%). However, there were several significant and clinically relevant laboratory differences among patients with HCC and the different disease etiologies. (Supplemental Table 2) Specifically, those with HCV had the lowest platelet count and highest AST and ALT values (all P<0.0001). Those with “other” liver disease also had higher AST and ALT values compared to the other liver disease etiologies except for HCV but had the highest ALP overall and the largest percent of patients with an ALBI score of 2 (56%) (P<0.0001). On the other hand, those with HBV, NAFLD and cryptogenic disease were less likely to have abnormal laboratory values and more frequently had the majority of patients classified as an ALBI 1 compared to the other liver disease etiologies (all P<0.001).
By Viral versus Nonviral Etiology
In comparing viral versus nonviral etiology (Supplemental Table 3), those with nonviral etiology of HCC were older (69.9±12.0 vs 64.3±9.5), more frequently female (44% vs 26%), Caucasian (66% vs 53%), more frequently had a higher CCI (9.7±3.9 vs 7.3±4.3), more frequently received hospice/palliative care (37% vs 30%), less frequently had cirrhosis (67% vs 92%), and less frequently received a liver transplant (2% vs 11%) (all P<0.001).
By Sex
Females were slightly older than males overall (68.9±12.5 vs 68.0±11.0, P<0.0001). Males were more frequently Caucasian, Hispanic, and Asian (P<0.0001), while females were more frequently African American (P<0.0001). (Supplemental Table 4) Males more frequently had cirrhosis (78% vs 67%), an ALBI grade of 2 (45% vs 38%), received HBV treatment (44% vs 33%) or underwent liver transplantation (5% vs 3%) (all P<0.01). Females were slightly more likely to have an advanced directive (9% vs 8%), received hospice/palliative care (37% vs 34%), and had a higher CCI (9.3±4.0 vs 8.9± 4.2) (all P<0.001).
By Study Time Period
There were notable differences between the study time points of 2003–2013 (time period before HCV DAA treatment) and 2014–2021 (time period inclusive of HCV DAA treatment). (Table 2A) The study sample was significantly older in the 2014–2021 cohort compared to the 2003–2013 cohort (69.9±10.8 vs 65.1±12.6, P<0.0001). Other notable shifts in the 2014–2021 cohort included there were more females, fewer Caucasians, more African Americans, more Hispanics and fewer Asians and “others” compared to the 2003–2013 cohort (all P<0.001). In addition, the 2014–2021 cohort more frequently had cirrhosis, an advanced directive, received hospice/palliative care, had a higher DCCI (all P<0.001) but less frequently received a liver transplantation (P=0.006).
Table 2.
Baseline Characteristics in HCC Subgroup by Study Time Period (A) and Race and Ethnicity (B)
| Characteristic | (A) By Time Period | (B) By Race/Ethnicity | ||||||
|---|---|---|---|---|---|---|---|---|
| 2003–2013 N=11,581 |
2014–2021 N=23,125 |
P-value | Caucasian N=21,550 |
African American N=4051 |
Hispanic/Latino N=4764 |
Asian N=1619 |
P-value | |
| Mean age (years±SD) | 65.1±12.6 | 69.9±10.8 | <0.0001 | 68.5±11.7 | 68.1±11.0 | 68.9±11.5 | 67.2±12.9 | <0.0001 |
| Sex (%) | <0.001 | <0.001 | ||||||
| Male | 62.7 | 60.8 | 61.6 | 57.6 | 62.9 | 65.4 | ||
| Female | 37.3 | 39.3 | 38.4 | 42.4 | 37.1 | 34.6 | ||
| Race/ethnicity | <0.001 | – | – | – | – | – | ||
| Caucasian | 62.4 | 61.9 | ||||||
| African American | 10.3 | 12.4 | ||||||
| Hispanic | 11.7 | 14.7 | ||||||
| Asian | 5.4 | 4.3 | ||||||
| Unknown | 10.2 | 6.7 | ||||||
| Cirrhosis (%) | 71.9 | 74.6 | <0.001 | 72.6 | 73.1 | 79.6 | 73.6 | <0.001 |
| Advance care directive (%) | 0.9 | 11.6 | <0.001 | 7.5 | 9.8 | 8.9 | 8.2 | <0.001 |
| Hospice/palliative care (%) | 19.5 | 43.0 | <0.001 | 35.7 | 38.8 | 35.1 | 25.0 | <0.001 |
| DCCI (±SD) | 8.3±4.0 | 9.4±4.2 | <0.0001 | 9.1±4.1 | 9.6±4.3 | 8.9±4.2 | 7.7±4.3 | <0.0001 |
| ALBI | 0.21 | <0.001 | ||||||
| 1 | 56.3 | 57.4 | 58.1 | 58.3 | 51.4 | 63.8 | ||
| 2 | 43.7 | 42.6 | 41.9 | 41.7 | 48.6 | 36.2 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Antiviral treatment (%) | ||||||||
| HCV DAA* | – | 29.8 | – | 31.6 | 31.5 | 25.6 | 21.5 | <0.001 |
| HBV | 39.1 | 41.8 | 0.41 | 24.6 | 44.3 | 24.4 | 56.2 | <0.001 |
| Liver transplant (%) | 4.8 | 4.1 | 0.006 | 4.2 | 3.7 | 5.8 | 5.6 | <0.001 |
| Follow-up (person-years) | 22,103 | 24,567 | – | 28,779 | 5053 | 6779 | 3518 | – |
Notes: *Includes only patients from 2014 and after.
Abbreviations: HCC, hepatocellular carcinoma; DCCI, Deyo Charlson Comorbidity Index; HCV, hepatitis C virus; DAA, direct acting antiviral; HBV, hepatitis B virus; ALBI, albumin-bilirubin grade for HCC.
Additionally, there has also been a significant shift in the distribution of HCC cause between the two time periods (P<0.001). Compared to the 2003–2013 period, the proportions of HCV (29.5% to 23.1%) and HBV (3.0% to 2.4%) in the 2014–2021 have decreased. Meanwhile, the proportions of ALD (7.0% to 10.6%) and NAFLD (31.0% to 42.8%) have increased. The proportions of cryptogenic and “other” liver disease were 21.3% and 8.2% in 2003–2013 and 16.4% and 4.7% in 2014–2021, respectively.
Among those with HCV in 2014–2021 cohort, 30% were treated with an HCV DAA regimen. For HBV, the antiviral treatment rate did not change significantly between the 2003–2013 cohort and the 2014–2021 cohort (39.1% vs 41.8%, P=0.41).
By Race/Ethnicity
Hispanics were more frequently older (68.9±11.5), had cirrhosis (80%) and more frequently received a liver transplant (5.8%) compared to the other ethnicities (all P<0.001). (Table 2B)
Asians were the youngest (67.2±12.9), more frequently male (65%), less frequently received hospice/palliative care (25%), more frequently received HBV treatment (56%), less frequently received treatment for HCV (22%) and had the lowest CCI score (7.7±4.3) compared to the other ethnicities (all P<0.001). African Americans were less likely to be male (58%), more frequently had an advanced directive (10%) and to receive hospice/palliative care (39%), had the highest CCI (9.6%±4.3), and less frequently received a liver transplant (3.7%) compared to the other ethnicities (all P<0.001).
Characteristics of NAFLD-Related HCC with and without Cirrhosis
Among the patients with NAFLD, there was no difference in age among those with and without cirrhosis (Table 3). However, those with cirrhosis were more frequently Caucasian and Hispanic while African Americans were more likely to have HCC without cirrhosis (P<0.001). Those with cirrhosis were more frequently seen in 2014–2021 (75% vs 70%), more frequently had diabetes (79% vs 76%), an advanced directive (11% vs 7%), received hospice/palliative care (44% vs 28%), received a liver transplant (2% vs 0.3%), and had an ALBI score of 2 (45% vs 24%) (all P<0.0001).
Table 3.
Comparison Between NAFLD-Related HCC Patients with and without Cirrhosis
| Characteristic | No cirrhosis N=4614 |
Cirrhosis N=8869 |
P-value |
|---|---|---|---|
| Mean age (years±SD) | 71.1±12.1 | 71.1±10.7 | 0.96 |
| Sex | <0.001 | ||
| Male | 50.6 | 55.8 | |
| Female | 49.4 | 44.2 | |
| Race/ethnicity (%) | <0.001 | ||
| Caucasian | 62.8 | 63.1 | |
| African American | 13.5 | 10.4 | |
| Hispanic | 13.1 | 16.1 | |
| Asian | 3.0 | 3.0 | |
| Unknown | 7.6 | 7.4 | |
| Time period | <0.001 | ||
| 2003–2013 | 30.5 | 24.6 | |
| 2014–2021 | 69.5 | 75.4 | |
| Diabetes mellitus (%) | 75.6 | 79.2 | <0.001 |
| Advance care directive (%) | 7.2 | 11.0 | <0.001 |
| Hospice/palliative care | 27.9 | 43.8 | <0.001 |
| DCCI (±SD) | 10.5±3.8 | 10.5±3.9 | 0.97 |
| ALBI grade | <0.001 | ||
| 1 | 76.0 | 55.1 | |
| 2 | 24.0 | 44.9 | |
| 3 | 0 | 0 | |
| Liver transplant (%) | 0.3 | 1.7 | <0.001 |
| Follow-up (person-years) | 6135 | 9486 | – |
Abbreviations: NAFLD, non-alcoholic fatty liver disease; HCC, hepatocellular carcinoma; DCCI, Deyo Charlson Comorbidity Index; HCC, hepatocellular carcinoma; ALBI, albumin-bilirubin grade for HCC.
HCC Survival
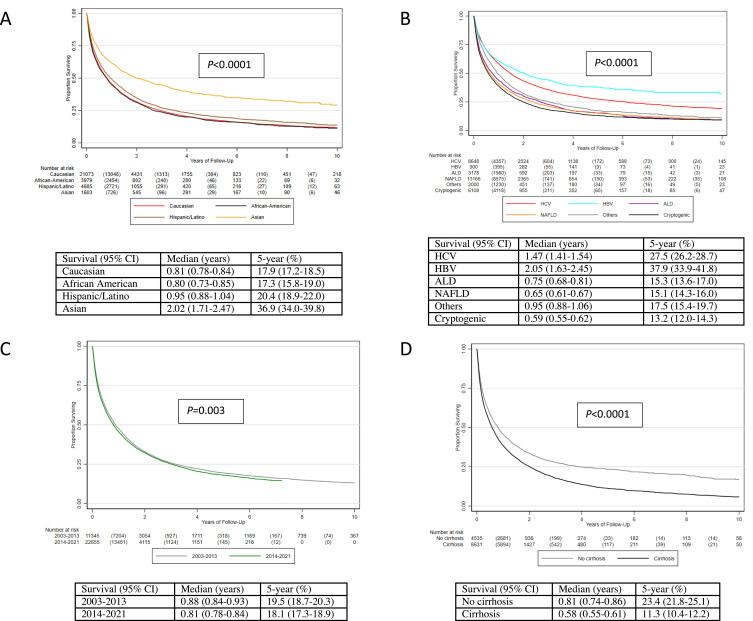
In the Kaplan–Meier assessment, the overall 5-year survival was 18.8% (95% CI 18.2–19.3). In subgroup analysis, males had worse 5-year survival compared to females (17.9% vs 20.2%, P<0.0001). (Supplemental Figure 2) By ethnicity, Asian had by far the best (36.9%) 5-year survival compared to the other ethnicities while African Americans (17.3%) and Caucasians (17.9%) had the worst (P<0.0001). (Figure 1A) By etiology of HCC, those with HBV had the best 5-year survival (37.9%) while those with ALD (15.3%), NAFLD (15.1%) and cryptogenic liver disease (13.2%) had the worse 5-year survivals (P<0.0001). (Figure 1B) Comparing those with viral HCC to nonviral HCC, 5-year survival in those with nonviral HCC was only half the survival rate of viral HCC patients (14.9% vs 28.4%, P<0.0001). (Supplemental Figure 3)
Figure 1.
Survival of HCC patients by race and ethnicity (A), etiology (B), time period (C) and cirrhosis status in the NAFLD subgroup (D).
Abbreviations: HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HBV, hepatitis B virus; ALD, alcohol-associated liver disease; NAFLD, non-alcoholic fatty liver disease.
By time period, those in the 2003–2013 cohort actually had higher 5-year survival (19.5%) compared to the recent 2014–2021 cohort (18.1%) (P=0.003). (Figure 1C) We also investigated survival status by cirrhosis status and among those with NAFLD with and without cirrhosis. As expected, those with cirrhosis had worse 5-year survival (17.0%) compared to those without cirrhosis (24.3%, P<0.0001). (Supplemental Figure 4) This finding was also evident among those with NAFLD cirrhosis whose 5-year survival was significantly lower (11.3%) than those without cirrhosis (23.4%, P<0.0001). (Figure 1D)
Predictors of Survival
Our multivariable analysis adjusting for age, sex, race/ethnicity, time period, individual liver disease etiology, baseline CCI, cirrhosis status, ALBI grade, and HCC treatment, factors associated with increased mortality were older age (aHR 1.02, 95% CI 1.01–1.03, P<0.001); male sex (aHR 1.13 (95% CI: 1.08–1.17, P<0.001); being Caucasian (aHR: 1.46, 95% CI: 1.33–1.60), African American (aHR: 1.53, 95% CI: 1.38–1.70) and Hispanic (aHR: 1.28, 95% CI: 1.16–1.42) compared to Asian (P<0.001); those in the 2014–2021 cohort compared to earlier patients (aHR 1.12, 95% CI: 1.07–1.17, P<0.001); having NAFLD (aHR:1.14, 1.08–1.21, P<0.001) or cryptogenic liver disease (aHR:1.45, 95% CI: 1.36–1.55, P<0.001) compared to untreated HCV; having a higher baseline CCI (aHR: 1.04, 95% CI: 1.03–1.05, P<0.001); having cirrhosis (aHR: 1.28, 95% CI: 1.22–1.34, P<0.001); and having an ALBI grade of 2 (aHR: 1.57, 95% CI: 1.51.-1.64, P<0.001) were all associated with increased mortality (Table 4). Additionally, compared to untreated HCV, DAA-treated HCV and treated HBV were associated with 47% and 31% reduction in mortality, respectively, while there was no significant difference in the mortality risk between untreated HCV and untreated HBV.
Table 4.
Cox Proportional Hazards Model for Factors Associated with Mortality for Patients with Hepatocellular Carcinoma
| Predictor | Univariable HR (95% CI) | P-value | Multivariable HR (95% CI) | P-value |
|---|---|---|---|---|
| Age | 1.03 (1.02–1.04) | <0.001 | 1.02 (1.01–1.03) | <0.001 |
| Male | 1.06 (1.03–1.09) | <0.001 | 1.13 (1.08–1.17) | <0.001 |
| Race/ethnicity | ||||
| Asian | Referent | Referent | ||
| Caucasian | 1.69 (1.57–1.81) | <0.001 | 1.46 (1.33–1.60) | <0.001 |
| African American | 1.73 (1.60–1.87) | <0.001 | 1.53 (1.38–1.70) | <0.001 |
| Hispanic | 1.54 (1.43–1.66) | <0.001 | 1.28 (1.16–1.42) | <0.001 |
| Time period | ||||
| 2003–2013 | Referent | Referent | ||
| 2014–2021 | 1.05 (1.02–1.08) | <0.001 | 1.12 (1.07–1.17) | <0.001 |
| Etiology | ||||
| HCV, untreated | Referent | Referent | ||
| HCV, treated | 0.45 (0.42–0.49) | <0.001 | 0.53 (0.48–0.59) | <0.001 |
| HBV, untreated | 0.94 (0.84–1.05) | 0.27 | 0.97 (0.82–1.14) | 0.71 |
| HBV, treated | 0.45 (0.38–0.53) | <0.001 | 0.69 (0.54–0.87) | 0.002 |
| ALD | 1.28 (1.21–1.34) | <0.001 | 1.05 (0.97–1.13) | 0.23 |
| NAFLD | 1.35 (1.31–1.40) | <0.001 | 1.14 (1.08–1.21) | <0.001 |
| Cryptogenic | 1.44 (1.39–1.50) | <0.001 | 1.45 (1.36–1.55) | <0.001 |
| Other* | 1.12 (1.06–1.19) | <0.001 | 0.98 (0.89–1.07) | 0.61 |
| Baseline DCCI | 1.05 (1.04–1.06) | <0.001 | 1.04 (1.03–1.05) | <0.001 |
| Cirrhosis | ||||
| No cirrhosis | Referent | Referent | ||
| Cirrhosis | 1.10 (1.07–1.13) | <0.001 | 1.28 (1.22–1.34) | <0.001 |
| ALBI grade | ||||
| 1 | Referent | Referent | ||
| 2 | 1.55 (1.50–1.61) | <0.001 | 1.57 (1.51–1.64) | <0.001 |
| HCC treatment | ||||
| No transplant | Referent | Referent | ||
| Transplant | 0.17 (0.15–0.19) | <0.001 | 0.22 (0.19–0.25) | <0.001 |
Notes: *Other liver diseases: autoimmune hepatitis, primary sclerosing cholangitis, primary biliary cholangitis, hemochromatosis, alpha-1 antitrypsin deficiency, and Wilson’s disease.
Abbreviations: HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HBV, hepatitis B virus; ALD, alcohol-associated liver disease; NAFLD, non-alcoholic fatty liver disease; ALBI, albumin-bilirubin grade for HCC.
In an alternative model adjusting for the same variables with etiology combined as viral versus nonviral (Supplemental Table 5), the predictors noted in the first model for increased mortality were relatively similar. However, when the etiologies were combined, the nonviral group compared to the viral group had a significantly increased risk of mortality (aHR: 1.33, 95% CI: 1.27–1.40, P<0.001).
Discussion
In this large nationwide cohort of 34,707 privately insured HCC patients with diverse ethnicities and liver disease etiologies, we found the overall 5-year survival to be less than 20% and worse in the recent era (2014–2021, 18.1%) as compared to survival rates about 10–20 years ago (2003–2013, 19.1%, P=0.003). We also noted that nonviral etiology made up 72% of HCC cases overall with the vast majority of nonviral cases being NAFLD and increasing. HCC patients in the latter cohort 2014–2021 (vs 2003–2013) were also older, more likely female, more likely to be Hispanics or African Americans, more likely to have cirrhosis or comorbidities, but not more likely to receive antiviral therapy for HBV (~40%) and less than one-third of HCV patients received DAA treatment. On the other hand, there have been significant improvement in the utility of advance care directives (12%) and hospice/palliative care (43%) in the 2014–2021 cohort (vs 2003–2013, 1% and 20%, respectively), though both services are still very under-utilized.
HCC incidence continues to increase and this is likely in part due to the increasing prevalence of NAFLD, the persistent prevalence of ALD, and the poor linkage to care for patients with HBV or HCV.2,4–6,12,18–20 As the birth cohort of patients with HCV born between 1945 and 1965 ages, this trend will likely continue.20 The initiation of effective HBV vaccine a few decades ago also contributed to an aging cohort of patients with chronic hepatitis B.19 The COVID pandemic with the surge in alcohol disuse likely will further contribute to the rising HCC burden in the coming years.21
In this light, we found that NAFLD-related HCC (38.9%) accounted for the highest percent of HCC among the etiologies as well as having the lowest rate of liver transplantation along with cryptogenic liver disease. We also found that there were significant differences by age, sex, race/ethnicities, etiology, and time period. Patients with HCV were the youngest group while those with NAFLD were the oldest. Patients with HCC overall were more likely to be males, however, among those with NAFLD-related HCC, the gender ratio was almost 50–50 which was the same finding when we split the groups by viral vs nonviral HCC etiology. The 2014–2021 cohort was older with more comorbidities and were less likely to undergo a liver transplant. By ethnicity, African Americans had more comorbidities, the highest percent of females and the lowest liver transplant rate compared to all the other ethnicities. Interestingly, despite the widespread availability of DAA treatments now, only 32% of those with HCV HCC were noted to have undergone treatment while viral suppression treatment was not much better for those with HBV as only 42% of those with HBV HCC received treatment.
Since NAFLD was the most frequent cause of HCC, we further investigated this subgroup and found that those without cirrhosis were just as likely to be male as female while those with cirrhosis were more likely to be male but a significant proportion was still female (44%). Caucasians most frequently had cirrhosis. Perhaps not surprising, the percent of those with diabetes was significantly increased among those with NAFLD-related cirrhosis, as was the use of hospice care and having an ALBI score of 2.
Furthermore, the risk of mortality was increased for these same groups. Specifically, males, being Caucasian or African American, with NAFLD or cryptogenic liver disease, being in the 2014–2021 cohort, with cirrhosis to include NAFLD cirrhosis had decreased survival. In fact, being African American increased the risk of mortality by 1.5 times compared to being Asian. Together, these results show that in addition to the prevalence of NAFLD increasing8–11 so is the burden of NAFLD-related adverse outcomes. In addition, those with cryptogenic liver disease actually had the worst outcomes but there is thought that cryptogenic liver disease is actually a different manifestation of NAFLD.22–24 Which, if this is the case, confirms prior studies that have advocated for the need for increased awareness of this liver disease among both healthcare practitioners and the general public.25–28
Given that this is a cohort of patients with private health insurance, the more likely cause of this lack of HCC treatment is due to late disease presentation rather than lack of access to care although insured patients from rural areas or without sufficient social and family support may struggle with access to care despite having health insurance coverage. A recent study from a safety-net medical center in Arizona found a striking difference in the median survival of their patients who had insurance versus those who did not (32.02 vs 8.35 months).29 Our suggestion of late presentation is aligned with prior studies reports about suboptimal adherence to HCC surveillance in patients with chronic hepatitis B and patients with cirrhosis of any cause.17,19,20 Thus, improved surveillance and adherence strategies are urgently needed as well as methods to reach those in rural areas and African American communities.30
To our knowledge, this is the first real-world nationwide study inclusive of adult HCC patients of all ages in the US that examined the impact of liver disease etiology, the presence of liver cirrhosis, as well as impact of sex and age to inform practice and public health efforts. Our study also provided updated data to 2021 so it can better take into account the effect of the newer direct-acting antivirals for HCV that became available in 2014. The study also provided long-term mortality data since it records vital statistics from national database, rather than from record review at a given institution which would miss patient follow-up at other facilities.
Our study also has several limitations. First, Optum reports race/ethnicity using a proprietary algorithm based on member geography and last name that may not have been well validated. Furthermore, our study suffers inherent limitations of retrospective study design, such as missing data, for example, our study lacks relevant data that many affect HCC survival such as tumor staging, and laboratory data for more comprehensive assessment of liver function were available in only a subset of patients in this database, though laboratory data were still available for the majority of our total patient cohort (60%, 20,662/34,707). However, as we used an administrative claims database, selection bias and referral bias would be minimized in our study unlike studies based on institutional databases which tend to come from more specialized tertiary care centers.
In conclusion, in this large nationwide population-based cohort of insured US patients, 5-year HCC survival remains dismal at 18% and decreased in the more recent time period 2014–2021, likely due to the rise of nonviral etiology, increasing age and comorbidities among this population as well as the persistently low rates of antiviral therapy among those with HCV or HBV. The major causes of HCC in this cohort include NAFLD, HCV and cryptogenic liver disease with Caucasian and African American males with an ALBI grade of 2 having the greatest increased risk for mortality. Culturally sensitive efforts geared towards raising awareness of the main causes of HCC along with linkage to care for treatment and screening are needed to curtail the rising burden of HCC in the US.
Acknowledgments
Data for this project were accessed using the Stanford Center for Population Health Sciences Data Core. The PHS Data Core is supported by the National Institutes of Health National Center for Advancing Translational Science Clinical and Translational Science Award (UL1TR003142) and from Internal Stanford funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding Statement
No external funding to disclose in this study.
Data Sharing Statement
Individual patient data can only be obtained with permission from Optum. Aggregated data we have produced have been submitted in the manuscript.
Statement of Ethics
The study was approved by the Institutional Review Board at Stanford University, Stanford, California, US. Patient consent was not required as this study was based on publicly available data. The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. All patient data accessed complied with relevant data protection and privacy regulations.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
MHN: Research funding: Astra Zeneca, Delfi, Pfizer, Enanta, Gilead, CurveBio, Innogen, Exact Sciences, Helio Health, Glycotest, National Cancer Institute, B.K. Kee Foundation, Vir Biotech; Consulting: Eli Lilly, Exelixis, Gilead, Intercept, GSK, Exact Science, Novartis, Janssen, Bayer. RC: Research funding: Gilead, Siemens Healthineers. DQH: Research support from Singapore Ministry of Health’s National Medical Research Council under its NMRC Research Training Fellowship (MOH-000595-01). Advisory board/consulting: Eisai. Personal fees from Gilead, outside the submitted work. All other authors report no conflicts of interest in this work.
References
- 1.Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77(6):1598–1606. doi: 10.1016/j.jhep.2022.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477–491. doi: 10.1053/j.gastro.2018.08.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi: 10.1038/s41575-019-0186-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol. 2020;18(12):2650–2666. doi: 10.1016/j.cgh.2019.07.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim NG, Nguyen PP, Dang H, et al. Temporal trends in disease presentation and survival of patients with hepatocellular carcinoma: a real-world experience from 1998 to 2015. Cancer. 2018;124(12):2588–2598. doi: 10.1002/cncr.31373 [DOI] [PubMed] [Google Scholar]
- 6.Younossi Z, Stepanova M, Ong JP, et al. Global nonalcoholic steatohepatitis council: nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17(4):748–755.e3. doi: 10.1016/j.cgh.2018.05.057 [DOI] [PubMed] [Google Scholar]
- 7.Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62(6):1723–1730. doi: 10.1002/hep.28123 [DOI] [PubMed] [Google Scholar]
- 8.Le MH, Yeo YH, Li X, et al. 2019 global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;20(12):2809–2817.e28.9. doi: 10.1016/j.cgh.2021.12.002 [DOI] [PubMed] [Google Scholar]
- 9.Ye Q, Zou B, Yeo YH, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(8):739–752. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4(5):389–398. doi: 10.1016/S2468-1253(19)30039-1 [DOI] [PubMed] [Google Scholar]
- 11.Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi: 10.1016/j.jhep.2019.06.021 [DOI] [PubMed] [Google Scholar]
- 12.Mathur K, Mazhar A, Patel M, et al. Changing trends of cirrhotic and noncirrhotic hepatocellular carcinoma in the era of directly-acting antiviral agents. Clin Transl Gastroenterol. 2021;12(11):e00420. doi: 10.14309/ctg.0000000000000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paik JM, Younossi Y, Henry L, Mishra A, Younossi ZM. Recent trends in the global burden of hepatitis B virus: 2007–2017. Gastroenterology. 2021;160(5):1845–1846.e3. doi: 10.1053/j.gastro.2020.11.057 [DOI] [PubMed] [Google Scholar]
- 14.Paik JM, Henry L, De Avila L, Younossi E, Racila A, Younossi ZM. Mortality related to nonalcoholic fatty liver disease is increasing in the United States. Hepatol Commun. 2019;3(11):1459–1471. doi: 10.1002/hep4.1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanford Center for Population Health Sciences. Optum DOD (v 5.0). Redivis Dataset; 2021. Available from: https://redivis.com/StanfordPHS. Accessed 31 August 2022.
- 16.Fernandes G, Campos D, Ballalai A, et al. Epidemiological and clinical patterns of newly diagnosed hepatocellular carcinoma in Brazil: the need for liver disease screening programs based on real-world data. J Gastrointest Cancer. 2021;52(3):952–958. doi: 10.1007/s12029-020-00508-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeo YH, Hwang J, Jeong D, et al. Surveillance of patients with cirrhosis remains suboptimal in the United States. J Hepatol. 2021;75(4):856–864. doi: 10.1016/j.jhep.2021.04.042 [DOI] [PubMed] [Google Scholar]
- 18.Soriano V, Barreiro P, Cachay E, Kottilil S, Fernandez-Montero JV, de Mendoza C. Advances in hepatitis B therapeutics. Ther Adv Infect Dis. 2020;7:2049936120965027. doi: 10.1177/2049936120965027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang DQ, Hoang JK, Nguyen MH. HBV-related hepatocellular carcinoma: a call to improve surveillance and linkage to care. Liver Int. 2021;41(9):2238–2239. doi: 10.1111/liv.15023 [DOI] [PubMed] [Google Scholar]
- 20.Kim NJ, Locke CJ, Park H, Magee C, Bacchetti P, Khalili M. Race and hepatitis C care continuum in an underserved birth cohort. J Gen Intern Med. 2019;34(10):2005–2013. doi: 10.1007/s11606-018-4649-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon AM, Curtis B, Mandrekar P, Singal AK, Verna EC, Fix OK. Alcohol-associated liver disease before and after COVID-19: an overview and call for ongoing investigation. Hepatol Commun. 2021;5(9):1616–1621. doi: 10.1002/hep4.1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jun TW, Yeh ML, Yang JD, et al. More advanced disease and worse survival in cryptogenic compared to viral hepatocellular carcinoma. Liver Int. 2018;38(5):895–902. doi: 10.1111/liv.13613 [DOI] [PubMed] [Google Scholar]
- 23.Younossi Z, Stepanova M, Sanyal AJ, et al. The conundrum of cryptogenic cirrhosis: adverse outcomes without treatment options. J Hepatol. 2018;69(6):1365–1370. doi: 10.1016/j.jhep.2018.08.013 [DOI] [PubMed] [Google Scholar]
- 24.Caldwell SH, Lee VD, Kleiner DE, et al. NASH and cryptogenic cirrhosis: a histological analysis. Ann Hepatol. 2009;8(4):346–352. doi: 10.1016/S1665-2681(19)31748-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le MH, Yeo YH, Cheung R, Wong VW, Nguyen MH. Ethnic influence on nonalcoholic fatty liver disease prevalence and lack of disease awareness in the United States, 2011–2016. J Intern Med. 2020;287(6):711–722. doi: 10.1111/joim.13035 [DOI] [PubMed] [Google Scholar]
- 26.Alqahtani SA, Paik JM, Biswas R, Arshad T, Henry L, Younossi ZM. Poor awareness of liver disease among adults with NAFLD in the United States. Hepatol Commun. 2021;5(11):1833–1847. doi: 10.1002/hep4.1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rai B, Albertian R, Solano L, et al. Lack of liver disease awareness: important contributor to late state hepatocellular carcinoma. Hepatology. 2020;72(S1)644A. [Google Scholar]
- 28.Younossi ZM, Ong JP, Takahashi H, et al. Global nonalcoholic steatohepatitis council: a global survey of physicians knowledge about nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2022;20(6):e1456–e1468. doi: 10.1016/j.cgh.2021.06.048 [DOI] [PubMed] [Google Scholar]
- 29.Turse E, Aboona M, Charley E, et al. Factors associated with survival of hepatocellular carcinoma (HCC) patients at a safety net hospital in Arizona without on-site liver transplant program. J Hepatocell Carcinoma. 2022;9:1–11. doi: 10.2147/JHC.S341690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Estevez J, Yang JD, Leong J, et al. Clinical features associated with survival outcome in African-American patients with hepatocellular carcinoma. Am J Gastroenterol. 2019;114(1):80–88. [DOI] [PubMed] [Google Scholar]