Abstract
Coral species in the genus Acropora are key ecological components of coral reefs worldwide and represent the most diverse genus of scleractinian corals. While key species of Indo-Pacific Acropora have annotated genomes, no annotated genome has been published for either of the two species of Caribbean Acropora. Here we present the first fully annotated genome of the endangered Caribbean staghorn coral, Acropora cervicornis. We assembled and annotated this genome using high-fidelity nanopore long-read sequencing with gene annotations validated with mRNA sequencing. The assembled genome size is 318 Mb, with 28,059 validated genes. Comparative genomic analyses with other Acropora revealed unique features in A. cervicornis, including contractions in immune pathways and expansions in signaling pathways. Phylogenetic analysis confirms previous findings showing that A. cervicornis diverged from Indo-Pacific relatives around 41 million years ago, with the closure of the western Tethys Sea, prior to the primary radiation of Indo-Pacific Acropora. This new A. cervicornis genome enriches our understanding of the speciose Acropora and addresses evolutionary inquiries concerning speciation and hybridization in this diverse clade.
Keywords: Acropora cervicornis, acroporidae, scleractinia, de novo assembly, comparative genomics, time-calibrated phylogeny
Introduction
The Acropora are one of the most speciose and important genera of reef-building scleractinian corals globally (Wallace 1999). The genus Acropora are divided into multiple speciose Indo-Pacific clades and a single depauperate Caribbean clade (Wallace 1999). The two sister species of Caribbean Acropora—the Staghorn coral A. cervicornis and Elkhorn coral A. palmata—and their hybrid—called A. prolifera (Vollmer and Palumbi 2002) are thought to have diverged from the Indo-Pacific Acropora during the late Eocene after the closure of the western Tethys Sea prior to the rapid diversification in the Indo-Pacific Acropora (Wallace 1999; Wallace and Portell 2022). To date, all 16 published de novo assembled and annotated Acropora genomes are of Indo-Pacific species (Shinzato et al. 2011, Ying et al. 2019; Fuller et al. 2020; Shinzato et al. 2021; López-Nandam et al. 2023).
Acropora, like all corals, are severely threatened by anthropogenic climate change leading to elevated water temperatures that can cause acute bleaching and subsequently death (Hughes et al. 2018). The Caribbean Acropora are also experiencing a secondary range-wide pressure in the form of White Band Disease, which has resulted in ∼95% population losses of both species Caribbean wide and is the direct cause of their listing on the Endangered Species List (Aronson and Precht 2001; National Marine Fisheries Service 2006). Because these two species are such important foundational species in the Caribbean reef ecosystem, these losses have likely had tremendous unknown effects on higher order taxa which depend on Acropora dominant reefs for survival.
Here we present the first fully annotated genome for the endangered Caribbean staghorn coral Acropora cervicornis, importantly representing the first Caribbean species of this diverse clade. This genome was assembled using a combination of long-read nanopore and short-read shotgun sequences and annotated and validated using mRNA sequencing. This reference genome will accelerate genomic research on this endangered coral and address fundamental evolutionary questions about speciation and hybridization in the speciose Acroporids.
Materials and methods
Sample collection and sequencing
High molecular weight genomic DNA was extracted in June 2021 from adult tissue of the K2 genotype maintained in the Coral Restoration Foundation (CRF) Key Largo, Florida nursery. Three libraries were prepared using Oxford Nanopore Technologies (ONT) kit SQK-LSK112. Two libraries were not size selected while the third included 20+kb PippenPrep size-selection. All ONT prepared libraries were sequenced separately on three Minion flow cells (FLO-MIN112). High-quality base-calling was performed using Guppy v6.1.7 (ONT).
Four additional Illumina PCR-free shotgun libraries were constructed using the Discovar protocol to produce libraries with fragments between 400 and 600 bp (Love et al. 2016). KAPA PCR-free library kits were leveraged with the addition of a second round of 0.7× Agencourt AmPure XP SPRI bead cleanup post-adapter ligation. Libraries were multiplexed and sequenced on a single rapid-run HiSeq 2500 flowcell with 250 bp paired-end sequencing. Additionally, a library of paired 150 bp reads was prepared using the Illumina DNA Prep kit and sequenced as part of a NovaSeq S4 run.
To acquire transcriptome data, mRNA sequence data was obtained for 48 individuals (including the K2 genotype) using NEBs unidirectional mRNA library preparations sequenced on an Illumina NEXTSEQ 550 platform and combined with previously published RNA sequencing data from 38 additional A. cervicornis (PRJNA222758: Libro and Vollmer 2016; PRJNA423227: Parkinson et al. 2018).
Sequence quality control
Nanopore long-reads (DNA) were quality controlled using Porechop (v 0.2.3_seqan2.1.1, https://github.com/rrwick/Porechop) to remove adapter sequences and then quality trimmed into longer assembly reads (minimum average quality 3, minimum length 1,000 bp) and shorter polishing reads (minimum average quality 5, minimum length 500 bp) using NanoFilt (De Coster et al. 2018). Illumina sequenced short-reads (DNA and RNA) were quality controlled initially using Fastp (Chen et al. 2018) to remove adapters and barcodes, filter low quality sequences (PHRED < 30), trim sequences shorter than 140 bp, and PCR artifacts. Contaminants were removed with Fastq_screen (Wingett and Andrews 2018) by mapping reads against a suite of potential contaminant genomes (e.g. human, viral, and bacterial) as well the 13 available genomes of Symbiodiniaceae (Supplementary Table 1) and removing reads which had hits to any potential contaminant genome.
Genome assembly
We estimated the genome size using a k-mer counting approach implemented in Jellyfish based on the quality-controlled Illumina short reads (Marçais and Kingsford 2011). An initial genome assembly of the ONT sequenced reads was built using Flye (Kolmogorov et al. 2019) with the nano-hq parameter setting to fit with the chemistry and base-calling method of the sequencing. After initial assembly of the raw nanopore reads, duplicated sequences were removed using purge_dups (Guan et al. 2020; Guiglielmoni et al. 2021). We polished the genome using two rounds of long-read polishing with Racon (Vaser et al. 2017) followed by a round of polishing with Medaka v1.7.2 (ONT) and then two rounds of polishing with paired-end Illumina sequences using Pilon (Walker et al. 2014). To ensure a final genome assembly of the highest quality and contiguity, we corrected misassembly errors with short reads using Mec (Wu et al. 2020) and remove the mitochondrial genome and other potential genomic contaminants using Blobtools (Laetsch and Blaxter 2017). This was followed by misassembly correction using the long-read data (Coombe et al. 2021), scaffolding (Coombe et al. 2021), gap closing (Xu et al. 2020), and a final round of long-read polishing with Racon and short-read polishing with Pilon (Walker et al. 2014; Vaser et al. 2017). Finally, all contigs shorter than 1,000 bp and/or with no gene annotations (see below) were removed as they did not contain any BUSCOs.
Transcriptome assembly
A preliminary transcriptome was assembled using a genome-guided assembly in Trinity (Grabherr et al. 2011). mRNA sequencing reads were splice-aware mapped to the genome using Gsnap (Wu et al. 2016). Trinity was used to perform a genome guided assembly of the transcriptome with an assumed max intron length of 100,000 bp.
Genome annotation
Genome annotation was performed using MAKER (Cantarel et al. 2008; Holt and Yandell 2011). Repetitive elements were identified and masked using RepeatModeler (Flynn et al. 2020) and RepeatMasker (Smit et al. 2015). Evidence-based gene annotation was performed using the assembled transcriptome and proteins identified in either all Acroporids in the UniProt database (The UniProt Consortium 2023) or the closest reference proteomes in UniRef from Stylophora pistillata (Voolstra et al. 2017), Pocillopora damicornis (Cunning et al. 2018), Actinia tenebrosa (Surm et al. 2019), and Nematostella vectensis (Putnam et al. 2007). This initial round of annotation was used to train the ab initio gene identification models of Augustus (Stanke et al. 2006), Snap (Korf 2004), and Genemark-ES (Lomsadze et al. 2005). After the initial round of annotation based solely on protein and RNA evidence, we performed four subsequent rounds of annotation with the results of the previous round being used to train the ab initio gene predictors run in the subsequent round of annotation. Genes were functionally annotated using EnTAP (Hart et al. 2020) and Interproscan (Zdobnov and Apweiler 2001), and formatted the annotations for NCBI using GAG and Annie (Tate et al. 2014; Geib et al. 2018).
Mitochondrial assembly
The mitochondrial genome was assembled using the quality-controlled Illumina short-reads using MitoZ (Meng et al. 2019). Briefly, this was done by first assembling a subset of reads into initial contigs which are then identified as mitogenome sequences using a profile Hidden Markov Model (Wheeler and Eddy 2013; Xie et al. 2014; Nurk et al. 2017). Contigs were then annotated to find the 13 protein-coding mitochondrial genes along with tRNAs and rRNAs with any contigs not containing any annotations removed (Birney et al. 2004; Gertz et al. 2006; Li and Durbin 2009; Jühling et al. 2012; Nawrocki and Eddy 2013). Finally, retained contigs were assembled and circularized into the complete mitochondrial genome and visualized (Gertz et al. 2006; Krzywinski et al. 2009; Meng et al. 2019).
Phylogenetic analysis
Sixteen published Acropora genomes with structural genome annotations along with two Montipora species, Montipora capitata and Montipora efflorescens; two Pocilloporids, P. damicornis and S. pistillata; and three anemones, N. vectensis, A. tenebrosa, and Exaiptasia diaphana were downloaded from NCBI (Table 1). Protein sequences for all annotated genes were extracted and clustered into orthogroups derived from a single gene in the last common ancestor of the group using OrthoFinder (Emms and Kelly 2019). Orthogroups were used to infer rooted gene and species trees to develop a phylogenetic hypothesis for the group (Emms and Kelly 2017, 2018). Specifically, we used the STAG (Emms and Kelly 2018) algorithm to infer the species tree from 7,110 multicopy gene trees which had all species present, each created using DendroBLAST (Kelly and Maini 2013), this species tree was then rooted using the STRIDE algorithm (Emms and Kelly 2017). To infer divergence times, we time-calibrated the species tree using least-squares dating and 1,000 bootstraps to estimate divergence time confidence intervals (To et al. 2016). Ancestral dates were gathered from the Fossilworks database (Supplementary Table 2; Behrensmeyer and Turner 2013).
Table 1.
National Center for Biotechnology Information accession numbers and citations for coral genomes with structural gene annotations used to build phylogeny and for comparative genomic analysis.
| Species | Genome size (Mb) | Number of genes | Accession number | Citation |
|---|---|---|---|---|
| Acropora acuminata | 394.7 | 26,151 | GCA_014633975 | Shinzato et al. (2021) |
| Acropora awi | 428.8 | 26,801 | GCA_014634005 | Shinzato et al. (2021) |
| Acropora cervicornis | 308 | 28,059 | GCA_032359415 | (This Study) |
| Acropora cytherea | 426.3 | 27,327 | GCA_014634045 | Shinzato et al. (2021) |
| Acropora digitifera | 415.8 | 25,278 | GCA_014634065 | Shinzato et al. (2021) |
| Acropora echinata | 401.5 | 26,170 | GCA_014634105 | Shinzato et al. (2021) |
| Acropora florida | 442.8 | 27,573 | GCA_014634605 | Shinzato et al. (2021) |
| Acropora gemmifera | 401 | 26,269 | GCA_014634125 | Shinzato et al. (2021) |
| Acropora hyacinthus | 447.2 | 27,215 | GCA_014634145 | Shinzato et al. (2021) |
| Acropora intermedia | 416.9 | 26,982 | GCA_014634585 | Shinzato et al. (2021) |
| Acropora microphthalma | 383.9 | 26,384 | GCA_014634165 | Shinzato et al. (2021) |
| Acropora millepora | 475.4 | 41,860 | GCA_013753865 | Fuller et al. (2020) |
| Acropora muricata | 420.7 | 27,409 | GCA_014634545 | Shinzato et al. (2021) |
| Acropora nasuta | 416.4 | 27,379 | GCA_014634205 | Shinzato et al. (2021) |
| Acropora selago | 392.9 | 27,036 | GCA_014634525 | Shinzato et al. (2021) |
| Acropora tenuis | 403.1 | 27,236 | GCA_014633955 | Shinzato et al. (2021) |
| Acropora yongei | 438 | 27,452 | GCA_014634225 | Shinzato et al. (2021) |
| Montipora cactus | 652.7 | 29,158 | GCA_014634245 | Shinzato et al. (2021) |
| Montipora efflorescens | 643.3 | 29,424 | GCA_014634505 | Shinzato et al. (2021) |
| Nematostella vectensis | 356.6 | 34,311 | GCF_000209225 | Putnam et al. (2007) |
| Pocillopora damicornis | 234.3 | 25,183 | GCF_003704095 | Cunning et al. (2018) |
| Stylophora pistillata | 397.6 | 33,252 | GCF_002571385 | Voolstra et al. (2017) |
| Actinia tenebrosa | 238.2 | 27,037 | GCF_009602425 | Surm et al. (2019) |
| Exaiptasia diaphana | 256.1 | 27,753 | GCF_001417965 | Baumgarten et al. (2015) |
Comparative genomics
Structural gene annotations for all species included in the phylogenetic hypothesis were functionally annotated using BLAST (Altschul et al. 1990; Camacho et al. 2009) against the Swiss-Prot curated portion of the UniProt database (The UniProt Consortium 2023). To identify KEGG orthologs for all orthogroups, we matched KEGG gene annotations to orthogroups across species and found the consensus KEGG ortholog across all species in which the orthogroup was found. KEGG ortholog membership within KEGG pathways was identified using KEGGREST (Tenenbaum and Volkening 2023).
We used a logistic regression model to test if there are differences in the percentage of orthogroups with KEGG annotations across taxa. Post-hoc tests were used to compare the rate of annotation in A. cervicornis to other Acropora sp. and make pairwise comparisons between genera. To identify systematic differences in the distribution of genes in pathways within the Acropora, we used χ2 tests followed by a post-hoc analysis with FDR correction to identify the species and KEGG pathways with significantly more or less gene copies (i.e. orthogroups) than would be expected compared to the other Acropora (Beasley and Schumacker 1995; Benjamini and Hochberg 1995).
The time-calibrated phylogeny along with the number of genes found within orthogroups for each species was used to estimate the rate of gene family evolution across the phylogeny using Cafe5 (Hahn et al. 2005; Mendes et al. 2020) and identify KEGG orthologs which exhibit significant expansions/contractions at each node of the phylogeny. In the Cafe5 analysis we assumed a single rate of evolution across all gene families using the error rate estimating model and Poisson prior distribution (De Bie et al. 2006; Han et al. 2013). We then performed an overrepresentation analysis using Fisher's exact test to determine if any pathways in A. cervicornis showed significant expansions/contractions. All statistical analyses were performed using R v4.2.1 (R Core Team 2022).
Results and discussion
Genome/transcriptome assembly and annotation
Nanopore sequencing resulted in ∼6.6 million reads containing 15.5 Gb with an N50 of 5,072 bp with 3.3 million high-quality reads after filtering used in the initial long-read assembly containing 13.3 Gb of DNA with an N50 of 6,078 bp and a polishing set of 4.5 million reads containing 13.3 Gb of DNA with an N50 of 5,269 bp. 92 million paired-end Illumina reads (totally 40 Gb) were retained after filtering and decontamination for x polishing. mRNA sequencing from 86 corals totaled 1.4 billion single-end reads and 174 Gb of mRNA sequencing data for gene annotation.
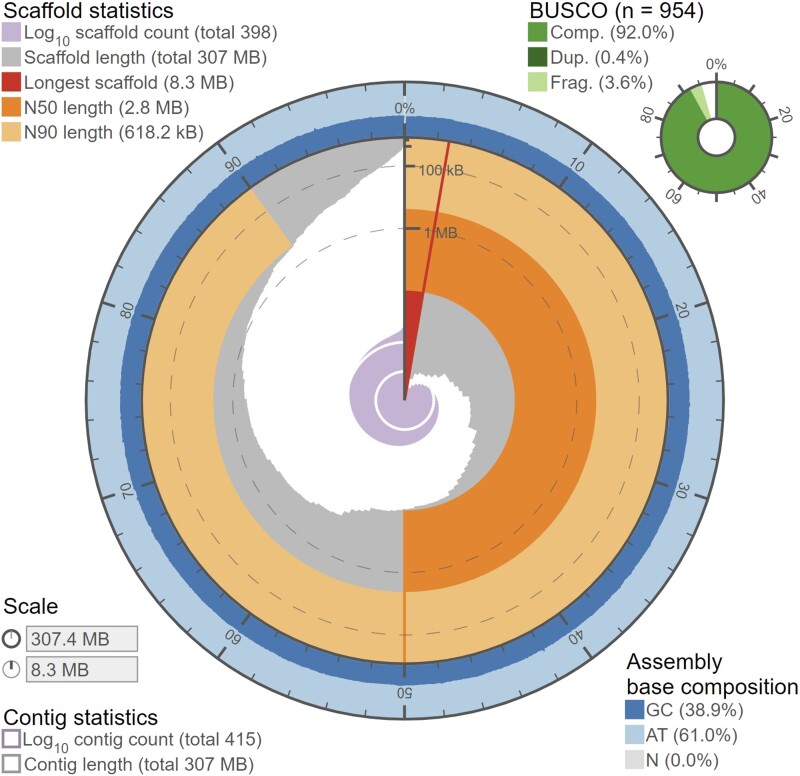
Using the filtered paired-end short-read sequences, we estimated the genome size of A. cervicornis to be ∼318 Mb, somewhat smaller than the Pacific Acroporids (384–475 Mb; Shinzato et al. 2011, 2021; Ying et al. 2019; Fuller et al. 2020), and obtained ∼42× genomic coverage of the long-read nanopore sequences for the genomic assembly. The initial genome assembly measured 328 Mb with 3,014 contigs (N50 = 0.94 Mb; L50 = 99) with the longest contig being 4.9 Mb and a BUSCO completeness of 92.8% (1.2% duplicated, 3.7% fragmented). After genomic post-processing, polishing, and scaffolding, the final genome assembly measured 307 Mb (96.5% the estimated genome size, Table 2) with 398 scaffolded contigs (N50 = 2.8 Mb; L50 = 35) with the longest scaffold measuring 8.3 Mb and a BUSCO completeness of 92.4% (0.4% duplicated, 3.6% fragmented, Fig. 1). The pooled transcriptome contained 374,749 transcripts with a total N50 of 3,659 and longest isoform N50 of 1,483 with a BUSCO completeness of 93.4% (78% duplicated, 3.5% fragmented). The high degree of transcriptome duplication in the BUSCO value is likely due to the pooling of individuals to create the transcriptome. Similar to other Acropora genomes (Shinzato et al. 2021), A. cervicornis contained 39% interspersed repeats (Supplementary Table 3) with the most common identifiable class of repeat being short interspersed nuclear elements, though the plurality (17%) of repeats in the genome were unable to be classified and may be taxon specific (Shinzato et al. 2021). A. cervicornis had 28,059 validated genes (Table 3), intermediate among other Acroporids (25,278–41,860; Shinzato et al. 2011; Ying et al. 2019; Fuller et al. 2020; Shinzato et al. 2021), with 53% having a Swiss-Prot annotation at an e-value < 10−6 and a BUSCO completeness of 81.6% (2.8% duplicated, 10% fragmented).
Table 2.
A. cervicornis assembly statistics.
| Cumulative scaffold length (bp) | 307.4 |
| Number of scaffolds | 398 |
| Number of contigs | 415 |
| G + C content (%) | 38.95 |
| Number of Ns/100 kbp | 37.23 |
| Largest scaffold (Mb) | 8.337 |
| Scaffold N50 (Mb) | 2.8 |
| Scaffold L50 | 35 |
| Largest contig (Mb) | 8.337 |
| Contig N50 (Mb) | 2.7 |
| Contig L50 (Mb) | 36 |
| BUSCO complete single-copy | 878 |
| BUSCO complete multicopy | 4 |
| BUSCO fragmented | 34 |
| BUSCO missing | 38 |
Fig. 1.
Genome snail plot showing genome contiguity and completeness statistics. For an interactive version of the figure see: https://jdselwyn.github.io/assembly-stats/. Created using Challis (2017).
Table 3.
A. cervicornis annotation statistics.
| Number of genes | 28,059 |
| Cumulative length of CDSs (bp) | 36,143,406 |
| Median gene length (bp) | 4,072 |
| Median CDS length (bp) | 119 |
| Median exon length (bp) | 124 |
| Median intron length (bp) | 569 |
| Number of intronless genes | 4,591 |
| Median number of exons per gene | 4 |
| Median number of exons per multiexon gene | 4 |
| BUSCO complete single-copy | 752 |
| BUSCO complete multicopy | 27 |
| BUSCO fragmented | 95 |
| BUSCO missing | 80 |
Mitochondrial genome
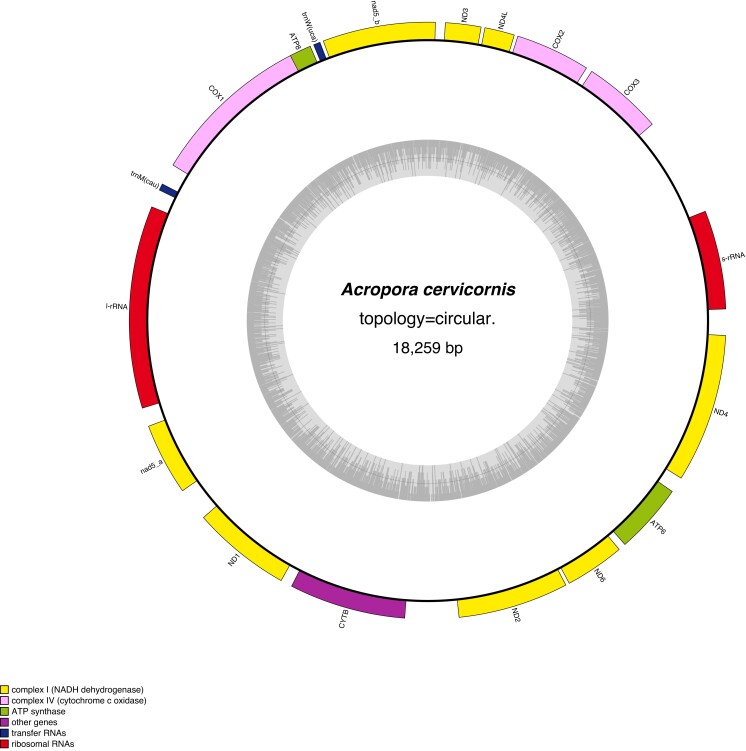
The A. cervicornis mitochondrial genome was assembled into a single circular structure containing 18,259 bp, 13 protein-coding genes, two tRNA genes (tRNA-Met and tRNA-Trp), and two rRNA genes (16 and 12 s; Fig. 2, GenBank accession number: OQ772303). Like all Acropora, the A. cervicornis mitochondrial genome has a particular dearth of tRNA coding sequences when compared to other metazoans, likely due to this diversification occurring after the split between cnidarians and metazoans (van Oppen et al. 1999; van Oppen, Catmull, et al. 2002; Liu et al. 2015; Tian and Niu 2017; Colin et al. 2021). The gene order found in A. cervicornis is the same as that found in other Acroporids, including ND5 being split into two portions with a large intron containing all genes except the two tRNA coding genes, the large ribosomal subunit rRNA, ATP8, and COI (Figure Y; van Oppen, Catmull, et al. 2002; Liu et al. 2015; Zhang et al. 2016; Colin et al. 2021).
Fig. 2.
Mitochondrial genome plot showing the relative locations and orientations of genes on the mitochondria with gene groups color coded by type. Plot made using Lohse et al. 2013).
Comparative genomics
The 28,059 genes found in A. cervicornis belong to 15,191 distinct orthogroups of which 54.4% had a KEGG annotation. The percent of orthogroups with KEGG annotations varied significantly by species (χ2(23) = 595.8, P < 0.0001) due primarily to differences between genera (Acropora vs Montipora, Z = 12.9, P < 0.0001) and higher-order taxonomic comparisons outside of the Acropora (Acropora vs outgroups, Z = −17.6, P < 0.0001) rather than within Acropora which all show a similar degree of annotation (A. cervicornis vs other Acropora, Z = −0.87, P = 0.38). The number of orthogroups per KEGG pathway were similar for all Acropora sp. (χ2(4,976) = 478.1, P = 1) indicating that no Acropora genomes had broad gains or losses of genes. Amongst five major KEGG categories, 25.7% of the orthogroups were found in pathways involved in organismal systems and 24.8% were involved in environmental information processing. The remaining orthogroups were split between cellular processes (18.8%), metabolism-related processes (16.7%), and genetic information processing (14.1%). A. cervicornis possessed 135 unique orthogroups, more than the other Acropora species (17–70), possibly as a result of the separate evolutionary history in the Caribbean. An alternative hypothesis for this discrepancy could be that the ab initio gene models for most other Acropora were trained on A. digitifera rather than being trained for each species independently (Shinzato et al. 2021).
Six gene pathways were significantly overrepresented in A. cervicornis among the significantly expanded and contracted KEGG orthologs (Supplementary Table 4). Two immune system pathways—NOD-like receptor signaling (map04621, OR = 19.7, P < 0.001, padj < 0.001) and Neutrophil extracellular trap formation (map04613, OR = 16.9, P < 0.001, padj = 0.018)—and the related cellular growth and death pathway Necroptosis (map04217, OR = 17.6, P < 0.001, padj = 0.018) were significantly overrepresented due to one expansion and three contractions in NACHT, LRR, and PYD domain-containing proteins, three contractions in histone proteins (H2A, H3, and H4), and one expansion in a cation channel protein (TRPM7). In corals, NOD-like receptor signaling pathway has been found to be an integral part of the innate immune system (Hamada et al. 2013) and histone phosphorylation has been shown to be a key response to nutrient stress and regulating the dinoflagellate symbionts (Rodriguez-Casariego et al. 2018).
The second major group of overrepresented pathways are signaling pathways, particularly the calcium signaling pathway (map04020, OR = 9.93, P < 0.001, padj = 0.025) and the neuroactive ligand–receptor interaction (map04080, OR = 39.8, P < 0.001, padj < 0.001). While the immune-related pathways were predominantly gene contractions in these pathways, all KEGG orthologs showed expansions (Supplementary Table 4). The calcium signaling and neuroactive ligand–receptor pathways have been found to be important in regulation reproduction (Hilton et al. 2012; Rosenberg et al. 2017), nematocyst regulation (Russell and Watson 1995), and biomineralization (Reyes-Bermudez et al. 2009). Further, these pathways appear to be differentially expressed between the growth tips and bases in the Caribbean Acropora (Hemond et al. 2014).
The final KEGG pathway overrepresented among the rapidly evolving KEGG orthologs is the Taurine and hypotaurine metabolism pathway (map00430, OR = 91.6, P < 0.001, padj = 0.04). This pathway was driven by the loss of a single KEGG orthogroup, cysteine dioxygenase (−2, K00456). Unlike other scleractinian corals, Indo-Pacific Acropora lack the cystathionine beta-synthase gene involved in cysteine biosynthesis (Shinzato et al. 2011) suggesting that Acroporid corals must rely on their algal symbionts to supply this vital amino acid (Shinzato et al. 2014). However, an alternative cysteine biosynthesis pathway has recently been discovered in Acropora loripes suggesting Acropora corals can natively synthesize cysteine through this alternate pathway (Salazar et al. 2022). Similar to the other studied Acropora, A. cervicornis lacks genes coding for cystathionine beta-synthase but does possess the genes required for the alternate cysteine biosynthesis pathway (Salazar et al. 2022). The loss of cysteine dioxygenase in A. cervicornis may suggest that the alternative pathway does not fully compensate the biosynthesis of cysteine resulting in a lack of excess cysteine needing to be metabolized.
Phylogeny
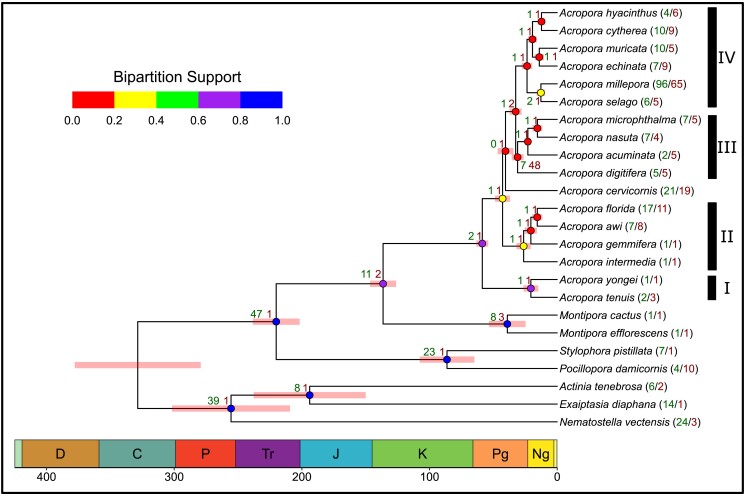
A. cervicornis is estimated to have diverged from the other Acropora 41 million years ago (mya) (35–47, 95% CI) during the Paleogene, approximately coinciding with the initial closure of the Tethys Sea (van Oppen et al. 2001; Wallace and Portell 2022). The addition of A. cervicornis as a Caribbean Acropora to the published Acropora phylogenomic tree (Shinzato et al. 2021) suggests that the Acropora radiation in the Indo-Pacific occurred in at least two stages with an initial split occurring between 58 and 68 mya resulting in clades I and II prior to the divergence of the Caribbean and Indo-Pacific Acropora, followed by a second set of radiations of the Indo-Pacific Acropora 35–47 mya and resulting in the more speciose clades III and IV (Fig. 3). Low bipartition support values within Acropora could result from the relatively rapid radiation within Acropora, incomplete lineage sorting and/or introgressive hybridization (Vollmer and Palumbi 2002; van Oppen, Willis, et al. 2002; Minh et al. 2020). The Acroporids (Montipora and Acropora) likely diverged from the Pocilloporids (S. pistillata and P. damicornis) during the Triassic period 220 mya (188–225, 95% CI) with Acropora and Montipora diverging during the early Cretaceous 136 mya (116–136, 95% CI).
Fig. 3.
Species phylogeny based on identified orthogroups. Node color indicates the proportion of single-locus gene trees supporting each bipartition (i.e. gene concordance factor, a more stringent metric of node support than bootstrap support, Emms and Kelly 2018; Minh et al. 2020). Red bars at each node indicate the 95% confidence interval of the estimated time of divergence marked in geologic periods on the x axis. Green/red node numbers indicate the number of KEGG orthologs which significantly expanded/contracted at each divergence point. Clade groupings from Shizato et al. (2021).
Conclusion
In summary, this study presents the first fully annotated genome of the endangered Caribbean staghorn coral, A. cervicornis. Comparative genomics highlights distinctive genetic traits, including immune pathway contractions and signaling pathway expansions, suggesting further research into the species response to environmental stressors, particularly White Band Disease. Phylogenetic analysis places A. cervicornis within the broader Acropora genus, dating its divergence to around 41 mya, aligning previous morphological findings and supporting the hypothesis of divergence coincident with the closure of the western Tethys Sea. This annotated genome serves as a valuable resource for future research, facilitating conservation and restoration efforts for Caribbean coral reefs, and deepening our understanding of speciation and adaptation within Acropora.
Supplementary Material
Acknowledgment
We would like to thank the staff of the Coral Restoration Foundation for providing samples (permit: CRF-2021-001) and logistical support.
Contributor Information
Jason D Selwyn, Department of Marine and Environmental Sciences, Northeastern University, Nahant, MA 01908, USA.
Steven V Vollmer, Department of Marine and Environmental Sciences, Northeastern University, Nahant, MA 01908, USA.
Data availability
Genome assembly and associated Nanopore and short-read DNA sequencing data can be accessed from NCBI BioProject: PRJNA948411 with the mitochondrial genome assembly accessible at NCBI GenBank: OQ772303. RNA sequencing data used for annotation can be accessed from NCBI BioProject: PRJNA949884. Genome assembly and annotation pipeline code available from GitHub: https://github.com/VollmerLab/Acerv_Genome.
Supplemental material available at G3 online.
Funding
Grant funding was provided to SVV by a National Science Foundation Division of Ocean Sciences grant (NSF OCE-1924145).
Literature cited
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol. 215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Aronson RB, Precht WF. 2001. White-band disease and the changing face of Caribbean coral reefs. In: Porter JW, editors. The Ecology and Etiology of Newly Emerging Marine Diseases. Developments in Hydrobiology. Dordrecht: Springer. p. 25–38. [Google Scholar]
- Baumgarten S, Simakov O, Esherick LY, Liew YJ, Lehnert EM, Michell CT, Li Y, Hambleton EA, Guse A, Oates ME, et al. 2015. The genome of Aiptasia, a sea anemone model for coral symbiosis. Proc Natl Acad Sci U S A. 112(38):11893–11898. doi: 10.1073/pnas.1513318112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley TM, Schumacker RE. 1995. Multiple regression approach to analyzing contingency tables: post hoc and planned comparison procedures. J Exp Educ. 64(1):79–93. doi: 10.1080/00220973.1995.9943797. [DOI] [Google Scholar]
- Behrensmeyer AK, Turner A. Taxonomic occurrences of Suidae recorded in the Paleobiology Database. Fossilworks [Internet]. 2013. Available from: http://fossilworks.org.2013.
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B: Stat Methodol. 57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Birney E, Clamp M, Durbin R. 2004. Genewise and genomewise. Genome Res. 14(5):988–995. doi: 10.1101/gr.1865504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. 2009. BLAST+: architecture and applications. BMC Bioinformatics. 10(1):421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarel BL, Korf I, Robb SMC, Parra G, Ross E, Moore B, Holt C, Sánchez Alvarado A, Yandell M. 2008. MAKER: an easy-to-use annotation pipeline designed for emerging model organism genomes. Genome Res. 18(1):188–196. doi: 10.1101/gr.6743907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis R. 2017rjchallis/assembly-stats 17.02.
- Chen S, Zhou Y, Chen Y, Gu J. 2018. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 34(17):i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin L, Yesson C, Head C, Colin L, Yesson C, Head C, Colin L, Yesson C, Head C, Colin L, et al. 2021. Complete mitochondrial genomes of three reef forming Acropora corals (acroporidae, scleractinia) from chagos archipelago, Indian ocean. Biodivers Data J. 9:e72762. doi: 10.3897/BDJ.9.e72762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombe L, Li JX, Lo T, Wong J, Nikolic V, Warren RL, Birol I. 2021. LongStitch: high-quality genome assembly correction and scaffolding using long reads. BMC Bioinformatics. 22(1):534. doi: 10.1186/s12859-021-04451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunning R, Bay RA, Gillette P, Baker AC, Traylor-Knowles N. 2018. Comparative analysis of the Pocillopora damicornis genome highlights role of immune system in coral evolution. Sci Rep. 8(1):16134. doi: 10.1038/s41598-018-34459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bie T, Cristianini N, Demuth JP, Hahn MW. 2006. CAFE: a computational tool for the study of gene family evolution. Bioinformatics. 22(10):1269–1271. doi: 10.1093/bioinformatics/btl097. [DOI] [PubMed] [Google Scholar]
- De Coster W, D’Hert S, Schultz DT, Cruts M, Van Broeckhoven C. 2018. NanoPack: visualizing and processing long-read sequencing data. Bioinformatics. 34(15):2666–2669. doi: 10.1093/bioinformatics/bty149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emms DM, Kelly S. 2017. STRIDE: species tree root inference from gene duplication events. Mol Biol Evol. 34(12):3267–3278. doi: 10.1093/molbev/msx259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emms DM, Kelly S. 2018. STAG: Species Tree Inference from All Genes. 267914.
- Emms DM, Kelly S. 2019. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20(1):238. doi: 10.1186/s13059-019-1832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JM, Hubley R, Goubert C, Rosen J, Clark AG, Feschotte C, Smit AF. 2020. RepeatModeler2 for automated genomic discovery of transposable element families. Proc Natal Acad Sci U S A. 117(17):9451–9457. doi: 10.1073/pnas.1921046117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller ZL, Mocellin VJL, Morris LA, Cantin N, Shepherd J, Sarre L, Peng J, Liao Y, Pickrell J, Andolfatto P, et al. 2020. Population genetics of the coral Acropora millepora: toward genomic prediction of bleaching. Science. 369(6501):eaba4674. doi: 10.1126/science.aba4674. [DOI] [PubMed] [Google Scholar]
- Geib SM, Hall B, Derego T, Bremer FT, Cannoles K, Sim SB. 2018. Genome annotation generator: a simple tool for generating and correcting WGS annotation tables for NCBI submission. GigaScience. 7(4):giy018. doi: 10.1093/gigascience/giy018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz EM, Yu Y-K, Agarwala R, Schäffer AA, Altschul SF. 2006. Composition-based statistics and translated nucleotide searches: improving the TBLASTN module of BLAST. BMC Biol. 4(1):41. doi: 10.1186/1741-7007-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, et al. 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 29(7):644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan D, McCarthy SA, Wood J, Howe K, Wang Y, Durbin R. 2020. Identifying and removing haplotypic duplication in primary genome assemblies. Bioinformatics. 36(9):2896–2898. doi: 10.1093/bioinformatics/btaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiglielmoni N, Houtain A, Derzelle A, Van Doninck K, Flot J-F. 2021. Overcoming uncollapsed haplotypes in long-read assemblies of non-model organisms. BMC Bioinformatics. 22(1):303. doi: 10.1186/s12859-021-04118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW, De Bie T, Stajich JE, Nguyen C, Cristianini N. 2005. Estimating the tempo and mode of gene family evolution from comparative genomic data. Genome Res. 15(8):1153–1160. doi: 10.1101/gr.3567505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Shoguchi E, Shinzato C, Kawashima T, Miller DJ, Satoh N. 2013. The complex NOD-like receptor repertoire of the coral Acropora digitifera includes novel domain combinations. Mol Biol Evol. 30(1):167–176. doi: 10.1093/molbev/mss213. [DOI] [PubMed] [Google Scholar]
- Han MV, Thomas GWC, Lugo-Martinez J, Hahn MW. 2013. Estimating gene gain and loss rates in the presence of error in genome assembly and annotation using CAFE 3. Mol Biol Evol. 30(8):1987–1997. doi: 10.1093/molbev/mst100. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Ginzburg S, Xu M, Fisher CR, Rahmatpour N, Mitton JB, Paul R, Wegrzyn JL. 2020. EnTAP: bringing faster and smarter functional annotation to non-model eukaryotic transcriptomes. Mol Ecol Resour. 20(2):591–604. doi: 10.1111/1755-0998.13106. [DOI] [PubMed] [Google Scholar]
- Hemond EM, Kaluziak ST, Vollmer SV. 2014. The genetics of colony form and function in Caribbean Acropora corals. BMC Genomics. 15(1):1133. doi: 10.1186/1471-2164-15-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton JD, Brady AK, Spaho SA, Vize PD. 2012. Photoreception and signal transduction in corals: proteomic and behavioral evidence for cytoplasmic calcium as a mediator of light responsivity. Biol Bull. 223(3):291–299. doi: 10.1086/BBLv223n3p291. [DOI] [PubMed] [Google Scholar]
- Holt C, Yandell M. 2011. MAKER2: an annotation pipeline and genome-database management tool for second-generation genome projects. BMC Bioinformatics. 12(1):491. doi: 10.1186/1471-2105-12-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, et al. 2018. Spatial and temporal patterns of mass bleaching of corals in the anthropocene. Science. 359(6371):80–83. doi: 10.1126/science.aan8048. [DOI] [PubMed] [Google Scholar]
- Jühling F, Pütz J, Bernt M, Donath A, Middendorf M, Florentz C, Stadler PF. 2012. Improved systematic tRNA gene annotation allows new insights into the evolution of mitochondrial tRNA structures and into the mechanisms of mitochondrial genome rearrangements. Nucleic Acids Res. 40(7):2833–2845. doi: 10.1093/nar/gkr1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Maini PK. 2013. DendroBLAST: approximate phylogenetic trees in the absence of multiple sequence alignments. PLoS One. 8(3):e58537. doi: 10.1371/journal.pone.0058537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmogorov M, Yuan J, Lin Y, Pevzner PA. 2019. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol. 37(5):540–546. doi: 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- Korf I. 2004. Gene finding in novel genomes. BMC Bioinformatics. 5(1):59. doi: 10.1186/1471-2105-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywinski M, Schein J, Birol İ, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. 2009. Circos: an information aesthetic for comparative genomics. Genome Res. 19(9):1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laetsch DR, Blaxter ML. 2017. BlobTools: Interrogation of genome assemblies. F1000Research. 6. doi: 10.12688/f1000research.12232.1. [DOI] [Google Scholar]
- Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows–wheeler transform. Bioinformatics. 25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libro S, Vollmer SV. 2016. Genetic signature of resistance to white band disease in the Caribbean staghorn coral Acropora cervicornis. PLoS One. 11(1):e0146636. doi: 10.1371/journal.pone.0146636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S-YV, Chan C-LC, Hsieh HJ, Fontana S, Wallace CC. 2015. Massively parallel sequencing (MPS) assays for sequencing mitochondrial genomes: the phylogenomic implications for Acropora staghorn corals (Scleractinia; Acroporidae). Mar Biol. 162(6):1383–1392. doi: 10.1007/s00227-015-2657-1. [DOI] [Google Scholar]
- Lohse M, Drechsel O, Kahlau S, Bock R. 2013. OrganellarGenomeDRAW—a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 41(W1):W575–W581. doi: 10.1093/nar/gkt289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomsadze A, Ter-Hovhannisyan V, Chernoff YO, Borodovsky M. 2005. Gene identification in novel eukaryotic genomes by self-training algorithm. Nucleic Acids Res. 33(20):6494–6506. doi: 10.1093/nar/gki937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Nandam EH, Albright R, Hanson EA, Sheets EA, Palumbi SR. 2023. Mutations in coral soma and sperm imply lifelong stem cell renewal and cell lineage selection. Proc Biol Sci. 290:20221766. doi: 10.1098/rspb.2022.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love RR, Weisenfeld NI, Jaffe DB, Besansky NJ, Neafsey DE. 2016. Evaluation of DISCOVAR de novo using a mosquito sample for cost-effective short-read genome assembly. BMC Genomics. 17(1):187. doi: 10.1186/s12864-016-2531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marçais G, Kingsford C. 2011. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics. 27(6):764–770. doi: 10.1093/bioinformatics/btr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes FK, Vanderpool D, Fulton B, Hahn MW. 2020. CAFE 5 models variation in evolutionary rates among gene families. Bioinformatics. 36(22–23):5516–5518. doi: 10.1093/bioinformatics/btaa1022. [DOI] [PubMed] [Google Scholar]
- Meng G, Li Y, Yang C, Liu S. 2019. MitoZ: a toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 47(11):e63. doi: 10.1093/nar/gkz173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh BQ, Hahn MW, Lanfear R. 2020. New methods to calculate concordance factors for phylogenomic datasets. Mol Biol Evol. 37(9):2727–2733. doi: 10.1093/molbev/msaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Marine Fisheries Service . 2006. Endangered and Threatened Species: Final Listing Determinations for Elkhorn Coral and Staghorn Coral. Silver Spring, MD: National Marine Fisheries Service (NMFS), 71(FR26852). p. 26852–26872. [Google Scholar]
- Nawrocki EP, Eddy SR. 2013. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics. 29(22):2933–2935. doi: 10.1093/bioinformatics/btt509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurk S, Meleshko D, Korobeynikov A, Pevzner PA. 2017. metaSPAdes: a new versatile metagenomic assembler. Genome Res. 27(5):824–834. doi: 10.1101/gr.213959.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JE, Bartels E, Devlin-Durante MK, Lustic C, Nedimyer K, Schopmeyer S, Lirman D, LaJeunesse TC, Baums IB. 2018. Extensive transcriptional variation poses a challenge to thermal stress biomarker development for endangered corals. Mol Ecol. 27(5):1103–1119. doi: 10.1111/mec.14517. [DOI] [PubMed] [Google Scholar]
- Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, Terry A, Shapiro H, Lindquist E, Kapitonov VV, et al. 2007. Sea Anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 317(5834):86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2022. R: A Language and Environment for Statistical Computing.
- Reyes-Bermudez A, DeSalvo MK, Voolstra CR, Sunagawa S, Szmant AM, Iglesias-Prieto R, Medina M. 2009. Gene expression microarray analysis encompassing metamorphosis and the onset of calcification in the scleractinian coral Montastraea faveolata. Mar Genom. 2(3–4):149–159. doi: 10.1016/j.margen.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Casariego JA, Ladd MC, Shantz AA, Lopes C, Cheema MS, Kim B, Roberts SB, Fourqurean JW, Ausio J, Burkepile DE, et al. 2018. Coral epigenetic responses to nutrient stress: histone H2A.X phosphorylation dynamics and DNA methylation in the staghorn coral Acropora cervicornis. Ecol Evol. 8(23):12193–12207. doi: 10.1002/ece3.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg Y, Doniger T, Harii S, Sinniger F, Levy O. 2017. Canonical and cellular pathways timing gamete release in Acropora digitifera, Okinawa, Japan. Mol Ecol. 26(10):2698–2710. doi: 10.1111/mec.14062. [DOI] [PubMed] [Google Scholar]
- Russell TJ, Watson GM. 1995. Evidence for intracellular stores of calcium ions involved in regulating nematocyst discharge. J Exp Zool. 273(3):175–185. doi: 10.1002/jez.1402730302. [DOI] [Google Scholar]
- Salazar OR, Arun PN, Cui G, Bay LK, van Oppen MJH, Webster NS, Aranda M. 2022. The coral Acropora loripes genome reveals an alternative pathway for cysteine biosynthesis in animals. Sci Adv. 8(38):eabq0304. doi: 10.1126/sciadv.abq0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinzato C, Khalturin K, Inoue J, Zayasu Y, Kanda M, Kawamitsu M, Yoshioka Y, Yamashita H, Suzuki G, Satoh N. 2021. Eighteen coral genomes reveal the evolutionary origin of Acropora strategies to accommodate environmental changes. Mol Biol Evol. 38(1):16–30. doi: 10.1093/molbev/msaa216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinzato C, Mungpakdee S, Satoh N, Shoguchi E. 2014. A genomic approach to coral-dinoflagellate symbiosis: studies of Acropora digitifera and Symbiodinium minutum. Front Microbiol. 5:336. doi: 10.3389/fmicb.2014.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinzato C, Shoguchi E, Kawashima T, Hamada M, Hisata K, Tanaka M, Fujie M, Fujiwara M, Koyanagi R, Ikuta T, et al. 2011. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature. 476(7360):320–323. doi: 10.1038/nature10249. [DOI] [PubMed] [Google Scholar]
- Smit AFA, Hubley R, Green P. 2015. RepeatMasker Open-4.0. 2013–2015.
- Stanke M, Schöffmann O, Morgenstern B, Waack S. 2006. Gene prediction in eukaryotes with a generalized hidden Markov model that uses hints from external sources. BMC Bioinformatics. 7(1):62. doi: 10.1186/1471-2105-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surm JM, Stewart ZK, Papanicolaou A, Pavasovic A, Prentis PJ. 2019. The draft genome of Actinia tenebrosa reveals insights into toxin evolution. Ecol Evol. 9(19):11314–11328. doi: 10.1002/ece3.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate R, Hall B, DeRego T, Geib S. 2014 Annie: the ANNotation Information Extractor (Version 1.0). 2014.
- Tenenbaum D, Volkening J. 2023. KEGGREST: Client-side REST access to the Kyoto Encyclopedia of Genes and Genomes (KEGG).
- The UniProt Consortium . 2023. Uniprot: the universal protein knowledgebase in 2023. Nucleic Acids Res. 51(D1):D523–D531. doi: 10.1093/nar/gkac1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P, Niu W. 2017. The complete mitochondrial genome of the Acropora pruinosa. Mitochondrial DNA B Resour. 2(2):652–653. doi: 10.1080/23802359.2017.1375882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To T-H, Jung M, Lycett S, Gascuel O. 2016. Fast dating using least-squares criteria and algorithms. Syst Biol. 65(1):82–97. doi: 10.1093/sysbio/syv068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oppen MJH, Catmull J, Mcdonald BJ, Hislop NR, Hagerman PJ, Miller DJ. 2002. The mitochondrial genome of Acropora tenuis (Cnidaria; Scleractinia) contains a large group I intron and a candidate control region. J Mol Evol. 55(1):1–13. doi: 10.1007/s00239-001-0075-0. [DOI] [PubMed] [Google Scholar]
- van Oppen MJ, Hislop NR, Hagerman PJ, Miller DJ. 1999. Gene content and organization in a segment of the mitochondrial genome of the scleractinian coral Acropora tenuis: major differences in gene order within the anthozoan subclass zoantharia. Mol Biol Evol. 16(12):1812–1815. doi: 10.1093/oxfordjournals.molbev.a026094. [DOI] [PubMed] [Google Scholar]
- van Oppen MJH, McDonald BJ, Willis B, Miller DJ. 2001. The evolutionary history of the coral genus acropora (scleractinia, cnidaria) based on a mitochondrial and a nuclear marker: reticulation, incomplete lineage sorting, or morphological convergence? Mol Biol Evol. 18(7):1315–1329. doi: 10.1093/oxfordjournals.molbev.a003916. [DOI] [PubMed] [Google Scholar]
- van Oppen MJH, Willis BL, Van Rheede T, Miller DJ. 2002. Spawning times, reproductive compatibilities and genetic structuring in the Acropora aspera group: evidence for natural hybridization and semi-permeable species boundaries in corals. Mol Ecol. 11(8):1363–1376. doi: 10.1046/j.1365-294X.2002.01527.x. [DOI] [PubMed] [Google Scholar]
- Vaser R, Sović I, Nagarajan N, Šikić M. 2017. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res. 27(5):737–746. doi: 10.1101/gr.214270.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer SV, Palumbi SR. 2002. Hybridization and the evolution of reef coral diversity. Science. 296(5575):2023–2025. doi: 10.1126/science.1069524. [DOI] [PubMed] [Google Scholar]
- Voolstra CR, Li Y, Liew YJ, Baumgarten S, Zoccola D, Flot J-F, Tambutté S, Allemand D, Aranda M. 2017. Comparative analysis of the genomes of Stylophora pistillata and Acropora digitifera provides evidence for extensive differences between species of corals. Sci Rep. 7(1):17583. doi: 10.1038/s41598-017-17484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng Q, Wortman J, Young SK, et al. 2014. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 9(11):e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace CC. 1999. Staghorn Corals of the World: A Revision of the Genus Acropora. Collingwood (VIC): Csiro Publishing. [Google Scholar]
- Wallace CC, Portell RW. 2022. Earliest western Atlantic staghorn corals (Acropora) from the lower Oligocene Suwannee Limestone of Florida, USA, and their significance for modern coral distribution. J Paleontol. 96(6):1390–1399. doi: 10.1017/jpa.2022.47. [DOI] [Google Scholar]
- Wheeler TJ, Eddy SR. 2013. nhmmer: DNA homology search with profile HMMs. Bioinformatics. 29(19):2487–2489. doi: 10.1093/bioinformatics/btt403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingett SW, Andrews S. 2018. FastQ Screen: a tool for multi-genome mapping and quality control. F1000Res. 7:1338. doi: 10.12688/f1000research.15931.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Li M, Liao X, Luo J, Wu F-X, Pan Y, Wang J. 2020. MEC: misassembly error correction in contigs based on distribution of paired-End reads and statistics of GC-contents. IEEE/ACM Transact Comput Biol Bioinform. 17(3):847–857. doi: 10.1109/TCBB.2018.2876855. [DOI] [PubMed] [Google Scholar]
- Wu TD, Reeder J, Lawrence M, Becker G, Brauer MJ. 2016. GMAP And GSNAP for genomic sequence alignment: enhancements to speed, accuracy, and functionality. In: Mathé E, Davis S, editors. Statistical Genomics: Methods and Protocols. Methods in Molecular Biology. New York: Springer. p. 283–334. [DOI] [PubMed] [Google Scholar]
- Xie Y, Wu G, Tang J, Luo R, Patterson J, Liu S, Huang W, He G, Gu S, Li S, et al. 2014. SOAPdenovo-Trans: de novo transcriptome assembly with short RNA-Seq reads. Bioinformatics. 30(12):1660–1666. doi: 10.1093/bioinformatics/btu077. [DOI] [PubMed] [Google Scholar]
- Xu M, Guo L, Gu S, Wang O, Zhang R, Peters BA, Fan G, Liu X, Xu X, Deng L, et al. 2020. TGS-GapCloser: a fast and accurate gap closer for large genomes with low coverage of error-prone long reads. GigaScience. 9(9):giaa094. doi: 10.1093/gigascience/giaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H, Hayward DC, Cooke I, Wang W, Moya A, Siemering KR, Sprungala S, Ball EE, Forêt S, Miller DJ. 2019. The whole-genome sequence of the coral Acropora millepora. Genome Biol Evol. 11(5):1374–1379. doi: 10.1093/gbe/evz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdobnov EM, Apweiler R. 2001. Interproscan – an integration platform for the signature-recognition methods in InterPro. Bioinformatics. 17(9):847–848. doi: 10.1093/bioinformatics/17.9.847. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yu X, Zhou Z, Huang B. 2016. The complete mitochondrial genome of Acropora aculeus (cnidaria, scleractinia, acroporidae). Mitochondrial DNA A DNA Mapp Seq Anal. 27(6):4276–4277. doi: 10.3109/19401736.2015.1082092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genome assembly and associated Nanopore and short-read DNA sequencing data can be accessed from NCBI BioProject: PRJNA948411 with the mitochondrial genome assembly accessible at NCBI GenBank: OQ772303. RNA sequencing data used for annotation can be accessed from NCBI BioProject: PRJNA949884. Genome assembly and annotation pipeline code available from GitHub: https://github.com/VollmerLab/Acerv_Genome.
Supplemental material available at G3 online.