Abstract
Digital light processing (DLP) bioprinting has been widely introduced as a fast and robust biofabrication method in tissue engineering. The technique holds a great promise for creating tissue models since it can replicate the resolution and complexity of natural tissues and constructs. A DLP system projects 2D images onto layers of bioink using a digital photomask. The resolution of DLP bioprinting strongly depends on the characteristics of the projected light and the photocrosslinking response of the bioink micro-environment. In this review, we present a summary of DLP fundamentals with a focus on bioink properties, photo-initiator selection, and light characteristics in resolution of bioprinted constructs. A simple guideline is provided for bioengineers interested in using DLP platforms and customizing technical specifications for its design. The literature review reveals the promising future of DLP bioprinting for disease modeling and biofabrication.
Keywords: Digital Light Processing, Tissue Engineering, Bioprinter Design, Vascularized Models
1. Introduction
Advances in microfabrication have led to a rapid growth in the development of three-dimensional (3D) cell culture models of various pathologies for preclinical studies and therapeutic screening applications. Conventional two-dimensional (2D) in vitro models lack physiological cues for mimicking tissue microenvironment, and they cannot accurately predict cell response1. Animal models, the traditional gold standard are time-consuming, expensive, and ineffective for replicating human diseases, such as solid tumors2. Extracellular matrix (ECM) cues are required to model the target tissue3,4. The use of biomaterial-based scaffolds provides ECM molecules for cellular behavior while in vivo-like vasculature have been used for creating biomimetic disease models5,6. The micro-physiological parts that recapitulate the native tissue and the relevant pathological conditions are called micro-tissue models, which have been the key part of preclinical screening platforms7. The current challenges in the fabrication of micro-tissue models are maintaining metabolic levels of living cells, controlling ECM-cell interactions, and creating proper vascularization at a high fidelity7,8.
Recent efforts have been made on the use of advanced bioprinting technologies for creating highly-organized, multi-component micro-tissue models with a controlled ECM vasculature complex. These technologies can be classified as contact-based and contactless methods. The common contact-based methods consist of fused deposition modeling (FDM), extrusion, and inkjet bioprinting. FDM is an affordable method of 3D printing based on extruding thermoplastics around melt temperature without live cells. Extrusion bioprinting has the versatility and affordability in making small to large scale tissue scaffolds9. It is a low-cost technology that benefits from a relatively high fabrication speed10. This allows a high density of biological agents10 and the control over the mechanical properties of bioinks11. However, extrusion bioprinting has much worse resolutions, though, typically ~ 100–1200 μm compared to 10–50 μm in inkjet bioprinting12. Inkjet bioprinters deposit liquid-binding bioinks, benefiting from multiple reservoirs to be used for direct writing10. The low speed and high shear forces13, along with the possibility of needle clogging, limit the use of this technology.
Contactless methods consist of unstructured illumination and structured fabrication by stereo-lithography (SLA) and digital light processing (DLP) bioprinting (see Figure 1)14,15. SLA is a solid freeform additive manufacturing16,17, which uses visible or ultraviolet (UV) light to fuse the bioink to fabricate structures, and it has a practical resolution of 40–150 μm18. DLP19 benefits from a higher speed and easy control over the mechanical properties of bioinks11, and a superior practical resolution down to 10 μm compared to other methods20. In DLP systems, the light is projected onto a planar surface by a digital photomask. The mask can be a liquid crystal display (LCD) or a digital micro-mirror device (DMD). In LCD, light passes through the display while a DMD reflects light to a surface. The LCD based projectors have a sharper image and superior picture quality at lower intensities, while the DMD based projectors are lighter, portable, and more reliable for the delivery of high intensity light patterns. The x-y resolution can approach to around 10 μm (and 1 μm for non-biocompatible regular inks), and the speed of fabrication is typically between 0.5 and 15 mm/s20. Ultraviolet and infrared wavelengths are promisingly used for rapid fabrication of tissue constructs by DLP method21,22 (Figure 1-b). The application of other wavelengths such as green light is less studied in the literature due to limitations in accessing to cell friendly photo-initiator systems, lower printing speeds, and lower resolutions. Holographic, or volumetric patterning of light by special lasers have attracted attentions in recent years (Figure 1-b), but the implementation of this approach with biomaterials for bioprinting is still limited. In case of volumetric 3D printers based on multi-photon polymerization, the accessibility to the costly femtosecond lasers is an important limitation. However, the speed of printing and the size of structures which can be produced by multi-photon polymerization are lower compared to DMD/LCD-based DLP systems20,22.
Figure 1.
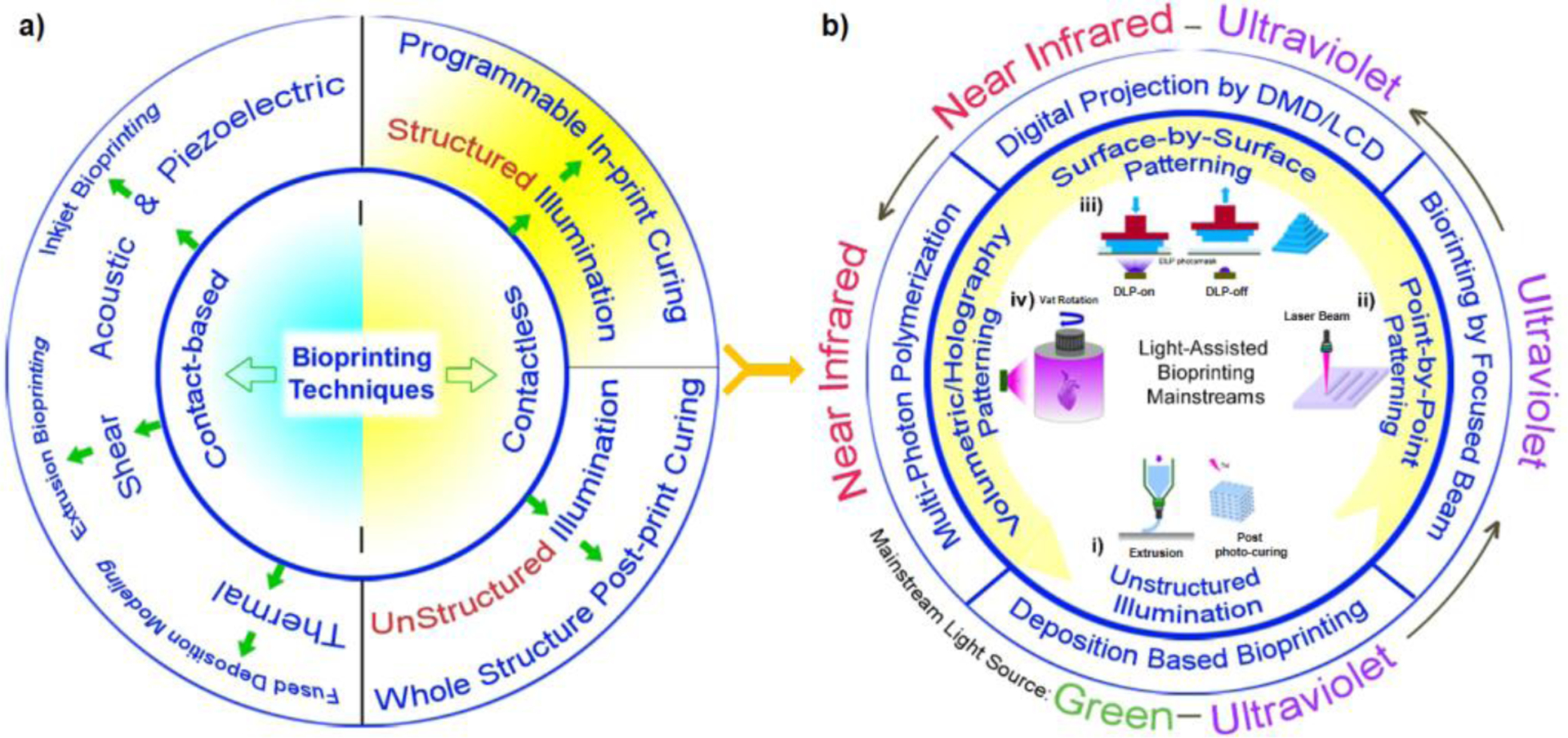
a) Common bioprinting methods used for creating micro-tissue models and scaffolds, and b) light-assisted bioprinting methods showing: i) unstructured illumination of a printed construct followed by a post-print photocuring, ii) point-by-point bioprinting using a focused laser beam, iii) surface-by-surface light patterning using DLP devices composed of digital micro-mirror devices or LCDs as a light mask, iv) volumetric light patterning in multi-photon polymerization techniques using a near infrared beam. The type of light which has found widespread applications in each category is shown.
In this manuscript, we review the governing parameters in the DLP bioprinting for creating micro-tissue models and other structures with an emphasis on technical and practical aspects of the photo-crosslinking process. A list of existing photo-initiators (PIs) are summarized for distinct applications, and the technical specifications of the current bioprinting platforms are discussed for various micro tissue applications. The typical applications include micro-tissue models for disease modeling and drug screening systems. We finally review recent advances in micro-tissue modeling using DLP bioprinting and envision the potential outlook for biomaterials (or bioinks).
2. A Practical Guideline for DLP Bioprinting
2.1. Fundamentals of Light-Assisted Polymerization
The development of DLP systems requires optimizing light characteristics for desired criteria such as resolution, sample size, timing, and toxicity level. A short description of the governing equations on the polymerization process is presented in this subsection (taken from Jakubiak et al.23). The distribution of light intensity follows an empirical equation showing the relation between the cure depth Cd, the energy dosage per area Emax, the critical energy dosage Ec for initiating polymerization, and the depth of light penetration Dp, as:
| (1) |
According to this equation, there exists a critical energy (Ec) for the liquid resin to reach gelation point. As Emax approaches Ec, the layer is cured and the bioink is solidified. It is further developed by considering the kinetics of the polymerization reaction24. Cure depth depends on the light intensity and the concentration of photoinitiator. The cure depth can reach to a maximum zc defined by:
| (2) |
where, ϵ is the molar extinction coefficient of photoinitiator, [PI] is the molar concentration of photoinitiator, and Emax represents the maximum energy exposure per unit area. The variable α2 contains the photochemical parameters such as:
| (3) |
pc, the critical threshold for gelation, kt is the kinetic rate constant for termination, kp is the kinetic rate constant for propagation, and ϕ is the quantum yield of the photoinitiator. β2 incorporates the light processing parameters such as:
| (4) |
where, c is light speed, h is Planck’s constant, Nav is Avogadro’s number, λ is the wavelength, PL is the power, and W0 is the Gaussian half-width of the light. These relations clearly show the role of main parameters which govern DLP printing. The photocrosslinking behavior strongly depends on the capacity of the hydrogel system to absorb and scatter the emitted light. The presence of high concentrations of polymer molecules decreases Ec, which occurs by increased absorption of the emitted light and rapid crosslinking. This commonly leads to an improved resolution. Adding low-to-high concentrations of polymers and PIs of higher molar extinction coefficient (in the hydrogel system) may decrease Ec and regulate zc. The light absorbing dyes can play a major role for improvement of the resolution by limiting light scattering.
2.2. Bioprinting Resolution
The resolution based on DMDs is limited by the width of light reflected from a single micromirror. A set of lenses can demagnify the projected images down to a diffraction limit, i.e. Abbe limit. The demagnification hampers the printing speed for (very) large constructs. Proper exposure time and head positions are thus required to gain resolution in a DLP platform. Doping a small amount of absorbing agents25–27 in the bioink is another useful strategy which can help to gain control over light penetration and minimize the light scattering. A photo-absorber such as Ponceau 4R (1 % w/v) was used for 400–450 nm wavelength light, and it reduced the light penetration depth, which allows better control over the bioprinting resolution and prevents unwanted over-curing of the bio-resin28. In another approach, flash exposure29 reduces the light scattering. Adding a non-dye additive such as TEMPO was also used to reduce the diffusion and enhance the resolution22,25. Adjusting the viscosity of biomaterial was suggested to enhance the density and reduce the light diffusion in the environment30. Proper design modification and adjustment of the optical parts are solutions that can improve the resolution of the image pixels. With one-third magnification in light projection when the initial pixel size is 7.5 μm, the theoretical resolution can reach 2.5 μm31.
2.3. PI Selection
A free radical initiator should be relatively stable during preparation but should decompose rapidly enough upon exposure to a specific range of wavelengths to ensure a practical reaction rate. The solubility of the photoinitiator in water is desired when encapsulating biological components32. The type of PI can affect the resolution as the absorbance behaviour of two well-used PIs for bioprinting purposes is tested by the authors in Figure 2. Figure 2-a shows absorbance of LAP for the polymerization of water-soluble bioinks. LAP is well suited for absorption at UV and blue wavelengths (350–400 nm), and the blue range allows photocrosslinking under more cell friendly conditions compared to other photoinitiators such as Irgacure. It also has a fast polymerization kinetics which enables cell encapsulation at a rapid rate. Figure 2-b represents a non-nitrile halogen-free azo based PI (VA-086) having a high decomposition temperature. It is highly soluble in water and absorbs lights λ = 350–400 nm. Comparison of absorbance in the near blue wavelength (395–405 nm) is highlighted in Figure 2-c. It is evident that LAP releases almost zero-free radicals by exposure to the visible light at high concentrations while the visible light absorbance (λ = 400–700 nm) for VA086 increases proportional to the photoinitiator concentration. Since full implementation of the DLP bioprinting in the dark is challenging, the release of free radicals at the visible wavelengths can negatively affect the printing resolution. Crosslinking due to exposure to light coming from the environment is a potential source of scattering, unwanted photopolymerization, and thus resolution reduction. Although the presented data here is reported for high concentration of the photoinitiator which is rarely used in cell studies, comparable challenges may arise when high intensity light sources are employed and the light scatters or refracts in presence of the cells since a lower concentration of photoinitiator is adequate for DLP bioprinting at higher light intensities. Therefore, proper selection of the photoinitiator and its absorbance behaviour both need to be carefully considered in a DLP bioprinter.
Figure 2.

Variations of the absorption by wavelength for different concentrations of PIs including a) LAP, b) VA086, in water solution obtained by plate reader.
The molar absorptivity or extinction coefficient (ε) of the photoinitiator is another deterministic characteristic of a photoinitiator, which was introduced in Eq. 2 and Eq. 3. For instance, the set of Ruthenium (Ru): sodium persulfate (SPS) photoinitiators show a high molar absorptivity (ε = 14600 M−1cm−1 at 450 nm) approximately 300-fold higher than that of LAP (ε = 50 M−1cm−1 at 405 nm)33. The higher molar absorptivity leads to higher photo-reactivity, lower exposure time, lower scattering, and thus promotes a higher resolution. In addition to aforementioned parameters, the cytotoxicity of the selected photoinitiator is an important factor that must be considered for DLP bioprinting. While the dosage of some of PIs such as LAP must be kept lower than a certain level34 (ca. 0.1 wt%) due to the PI’s cytotoxicity potential, the amount of PI in some other systems such as Ru:SPS can be increased without compromising the cell viability after DLP bioprinting35. Guidelines for using different PI systems in DLP platforms as well as their cytotoxicity information are summarized in Table 1.
Table 1-.
Guidelines for PI selection in DLP bioprinting platforms36
| Initiator | Molecular Structure | Cyto-toxicity | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Irgacure 819 |

|
High |
|
|
37 |
| LAP |

|
Medium |
|
|
22,29,31,38–46 |
| Ru:SPS |


|
Low |
|
|
33,35 |
| Ivocerin |

|
Low |
|
|
[tested by the authors] |
| Eosin Y |

|
Medium |
|
|
47,48 |
| UCNP@ LAP |

|
Low |
|
|
21 |
2.4. Light Considerations
In early stages of the technology invention only ultraviolet (UV) light was used for prototyping applications17. Today UV (λ = 320 to 380 nm) and blue light have found many applications in bioprinting22,49. Due to high energy exposure, the UV-assisted bioprinting has shown limitations in penetrating the tissue and suffers from weak cell viability after printing which is an important issue in many clinical applications. Recently, lights with other wavelengths such as green light (500–580 nm)50 and near-infrared light (900–1000 nm)21 are considered as promising alternatives to extend the applications of DLP and resolve the limitations of UV assisted bioprinting. By changing the light wavelength, a corresponding PI should be selected for preparing the bioink. Some PIs not only drastically decrease the cell viability, but also devastate the printing resolution as discussed in the previous section. In addition, by increasing the light wavelength, the low energy characteristic of the light and inadequate light absorption by the initiators can challenge the photo-polymerization needed to bioprint a robust structure. Therefore, proper manipulation of the light intensity, polarization, collimation, and alignment are required to achieve a balanced setting in the design of a DLP bioprinter. Among them, proper selection of the light intensity is of utmost importance.
In DLP bioprinters, precise selection of the lower limit for light intensity is important to achieve printability. Studies reveal that there exists a minimum light intensity requirement to achieve gelation for each bioink system depending on the PI concentration. For instance, a recent study investigated the unstructured illumination (Figure 1) of extrusion printed scaffolds by postcuring using 3, 30 and 50 mW/cm2 light intensities. It revealed that at least 30 mW/cm2 light intensity is required to achieve whole structure gelation after 15 minutes of exposure using a visible light source (400–450 nm) and a relevant photoinitiator system (Ru:SPS)51. In a DLP bioprinting platform, the same group showed that a light source of 7.5 mW/cm2 intensity together with the same photoinitiator system (0.2 mM:2 mM Ru:SPS) can be used to achieve 25–50 μm thickness layers within 10 seconds of exposure33. Another study showed that increasing the amount of photoinitiator by 10-folds (2 mM:20 mM Ru:SPS) and increasing the exposure up to 30–60 seconds per layer, it is possible to successfully print structures with a DLP even using very low light intensities as low as 0.23 mW/cm2 accessible through commercial mini-projectors35.
The upper limit for the used light intensity is also important as increasing the light intensity imposes negative influences on the cell viability, which leads to over-crosslinking, resolution deterioration, and the increase of the cost of suitable optical components. The technical specifications including the used light intensities in several successful DLP platforms are summarized in Table 2 as a guideline for comparing DLP bioprinters.
Table 2.
Technical specifications of emerging DLP bioprinting platforms
| Bioink Composition | Photo Initiator | Light Characteristics | Output of the DLP platform | ||||
|---|---|---|---|---|---|---|---|
| λ [nm] | W [mW/cm2] | Time [s] | Expected Cell-viability | Resolution [μm] | Ref. | ||
| GelMA (15% w/v) | UCNP@ LAP | 980 | - | 15 | High | >100 | 21 |
| PVA-MA (10% w/v), PEGDA (40 %v/v) | Ru:SPS | 400–700 | 0.23–7.25 | 10–60 | High | 20–50 | 33,35 |
| GelMA (10% w/v), PEGDA (10% v/v) | Eosin Y | 400–700 | - | 240 | High | 50 | 47,48 |
| PEGDA (25 %v/v)-GelMA (5 %w/v), PEGDA (20 %w/v) | LAP | 405 | 2.8–17 | 5–120 | High | 10–50 | 31,56 |
| HAMA, GelMA (20 %w/v) | LAP | 395 | - | 30 | High | 50 | 38,46 |
| BPADA-GMA-BA (69:23:8) | Irgacure 819 | 380 | 500 | 20 | NA | 20–50 | 37 |
| GelMA-PEGDA | LAP | 380 | 700 | 0.2 | Low | 15–50 | 34 |
| PEGDA (20 to 50 % v/v), GelMA (5% w/v) | LAP | 365 | 2.7–11 | 3–45 | Medium | ~20 | 29,44,45 |
| GelMA-PEGDA (<10 %w/v)-Cells, GelMA (2.5% w/v)-HA (1 %w/v)-Cells, GelMA (<10% w/v)-GMHA (1% w/v)-Cells | LAP | 365 | 88–100 | 1–30 | Medium | 50 | 22,40–43 |
| PETA (95 %w/v) | DCPI | 520 & 375 | 215 | <1 | NA | <10 | 57 |
NA: not applicable; “-”: not enough data reported.
2.5. Physical and Rheological Properties of Bioinks
Approaching low viscosities allows the bioink to flow under gravity between consecutive polymerization steps and facilitates the draining of non-polymerized bioink from the bioprinted construct. A dynamic viscosity of 5 Pa·s is the upper limit52, and there is no reported lower limit for the viscosity of the bioink. Lower viscosity makes a faster flow of monomer solution and faster relaxation process; thus, it provides a better quality of DLP bioprinting. Viscosity depends on the molecular weight and concentration of monomers if solvent is used in bioink formulation. Low molecular weights require a high conversion of functional groups to reach the gelation point under light exposure. A high molecular weight monomer or multifunctional macro-monomer can be crosslinked at a much lower conversion due to light exposure. When live cells are incorporated in DLP printing, additional physical properties for the bioink need to be considered. Bioink must be sufficiently viscous to prevent cell settling or precipitation during the printing process, to avoid inhomogeneous cell distribution in the printed construct particularly in top-down fabrication53. The cell settling is dependent on the buoyant density of the cells compared to that of the bioink material. It is shown that in PEG based bioinks the addition of 37.5% v/v of Percoll can match the buoyant density of the live cells with that of the bioink resin, and thus prevent cell settling32. In many other hydrogel systems, a homogeneous distribution of cells is achievable without the need for adding any buoyancy-modifying component22,28.
2.6. Bioink Types and Compositions
Several types of bioink and their compositions, which are widely used to characterize a DLP bioprinter, are summarized in Table 2. Among them, PEGDA and GelMA are considered model materials which show the potentials of the DLP platform for bioprinting micro-tissue models. PEGDA (400 <Mn <700) is mostly used as a model material to evaluate the resolution in printing structures with a DLP platform. Compared to PEGDA, printing of GelMA with a same platform typically leads to a lower printing resolution due to lower concentration of polymer content, higher molecular weight of the polymer, and thus lower density of acrylate functional groups in the bioink solution. However, owing to improved cell attachment to gelatin macromers, GelMA is considered as a model material to evaluate the cytotoxicity of the platform and the potential of the DLP bioprinter for printing constructs including live cells. In addition, mixing certain amount of PEGDA with GelMA is another strategy to achieve a proper printing resolution as well as cell attachment in the micro-tissues obtained from a DLP platform. Depending on the application and target micro-tissue model, some studies use other hydrogels such as HAMA, HA, or GMHA in bioink formulation22,40–43.
2.7. Oxygen-Penetrating Substrate
A key part of a DLP printer is the monomer bath and a gas permeable substrate located on the bottom of the vat that directly interfaces with the bioink monomers. Polydimethylsiloxane (PDMS) substrate is known as a highly permeable and porous nature which induces oxygen inhibition of free radical polymerization and leads to a thin uncrosslinked prepolymer layer between the PDMS and the polymerized construct. A fluorinated ethylene-propylene (FEP) polymer or Teflon film can also be used as an alternative to PDMS to play the role of the oxygen inhibition layer for continuous liquid interface production. However, PDMS is more affordable due to its lower price. Moreover, Teflon film is more flexible compared to PDMS and can cause convex/concave deformations during the printing which may shift the printing layer from the designed focal length and compromise the printing quality. Moreover, it is worth to note that a PDMS film54 represents oxygen diffusivity around 3.25 × 10−5 cm2/s while a Teflon FEP film55 represents oxygen diffusivity around 0.028 × 10−5 cm2/s. In other words, a PDMS membrane shows much more oxygen permeability compared to a Teflon membrane in similar conditions. PDMS can easily be coated or glued onto any designed geometry, which allows better integration. The ease of fabrication of PDMS membrane in a lab with tailorable thickness and stiffness is another advantage for using PDMS in DLP platforms.
2.8. Optical Engineering
In DLP printers, proper design of the optics is of utmost importance. The optics used in different bioprinters show a high variety depending on the type of used light source, source power, required light intensity at the focal length, the method of light collimation, homogenization, and polarization. Not all applications require a sophisticated optical design, but those with high resolution and versatility requirements need a carefully designed integrated optics. One of the design parameters of the optical system is called numerical aperture (N) which is defined by the following equation:
| (5) |
where, n is the refraction index of the light transfer medium (1 for air and 1.33 for pure water), and θ is the maximal angle of the cone of light that can be formed by the lens. Proper design of the numerical aperture is important since it denotes to the resolving power of the system. Theoretically, the resolution (the size of the finest detail that can be resolved by the bioprinter) is proportional to the wavelength λ and numerical aperture (N) by following equation:
| (6) |
This means that a set of lenses with larger aperture can visualize finer details compared to a set of lenses with smaller aperture. Lenses with larger numerical apertures collect more light and generally provide a brighter picture. However, larger numerical apertures return a shallower depth of field. In modern optics, the numerical aperture can reach around ~ 1.5; hence, the diffraction limit (Abbe limit) can be estimated by d = λ/3. This means that in a well-established DLP platform designed for 365 nm UV light (see Table 2) without changing the lens hardware by sole replacing the light source to green light (550 nm) and then to infra-red light (980 nm), the maximum achievable resolution can shift from 50 μm to 75 μm and then 135 μm, respectively. Unfortunately, most of publications do not clearly disclose the information about their used optics for DLP bioprinting including the numerical aperture data. Here, we could compare the only reported parameters shown in Table 2 for different platforms. In following, fabricated DLP bioprinting platforms are classified based on the complexity in customization of their optical comportments. A selected number of the fabricated micro-tissue models with these DLP platforms is also summarized in Table 2 .
3. Development of DLP Bioprinters
3.1. Commercial DLPs
The advances in the design of DLP bioprinters with commercial light projectors (using built-in optics) are described here. A simple DLP platform makes use of a simple projector to pattern two dimensional digital photomasks on hydrogel bioinks (Figure 3-a). Wang et al.47 used a built-in high intensity white light (400–700 nm) as a cell friendly light source and Eosin Y (peak absorption at 550 nm) as the photoinitiator. Without any light filtration, they reported an undesirable infra-red radiation from the light source which could increase the temperature of vat from 25°C to beyond 40°C within 15 min47. To avoid heating of the vat, a bath of water was used between the projector and the photosensitive bioink container to filter infrared radiations (Figure 3-a). Around 50 μm resolution and 60–85% cell viability was reported for this DLP platform, beside a low printing speed around 16 minutes to print 8 layers of 100 μm of the hydrogel. To enhance the speed of DLP printing, Wang et al.47 suggested replacing the light source of 2500 lumen with a higher intensity lamp. However recent studies showed that the sole enhancing the light intensity of the projector is not an effective strategy39, since despite simplicity, this design is not optimized for long term usage. Further studies showed that by replacing the visible light with near-UV light and replacing Eosin Y with Irgacure-2959, the printing speed can increase by a factor of 10 times at the expense of significantly lower cell viability due to exposure of cells to UV light58. Moreover, the proposed design was suffering from capabilities to bioprint multi component systems to replicate micro-tissues and real biological constructs.
Figure 3-. DLP Bioprinters Based on Commercial DLP:
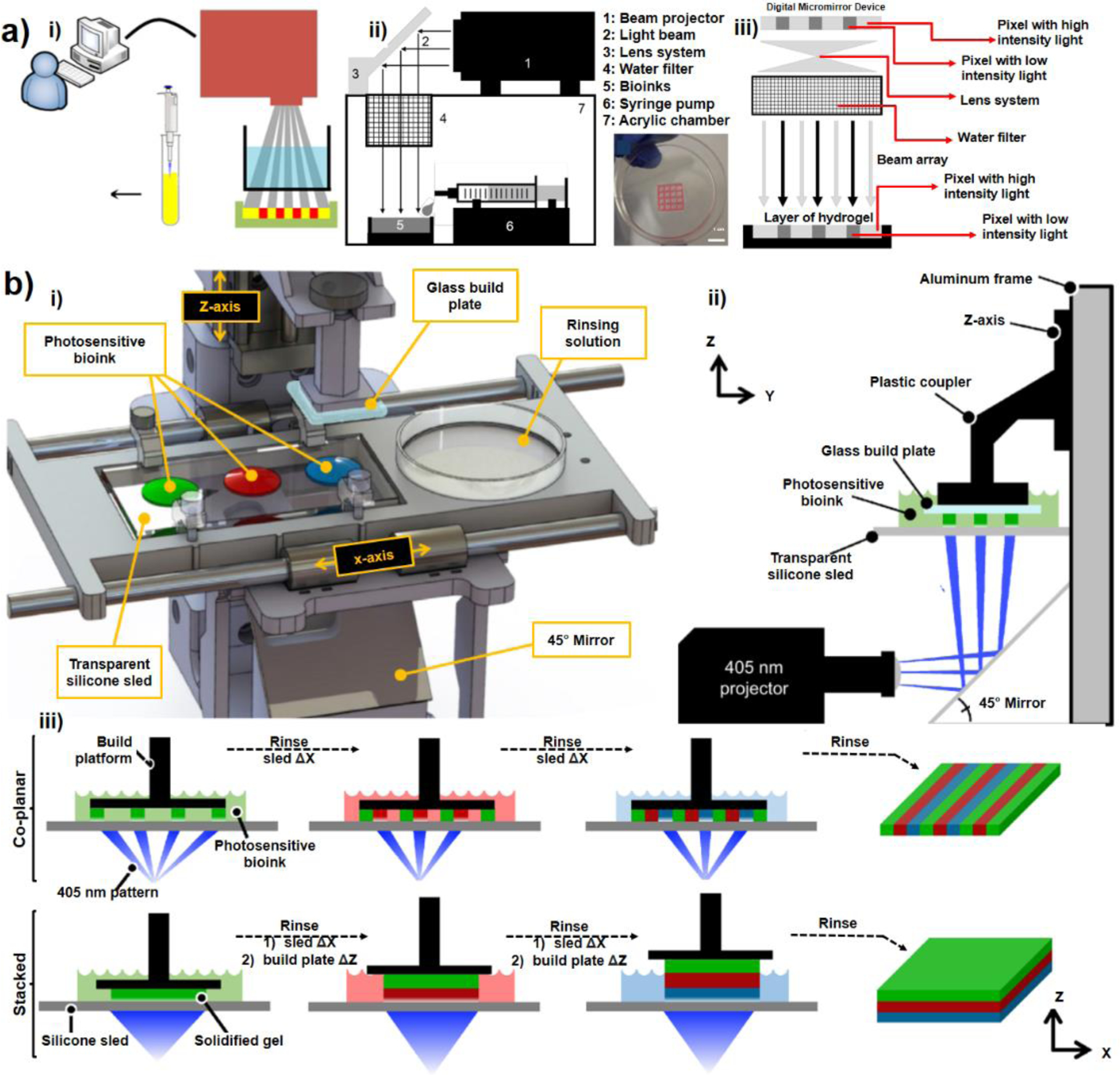
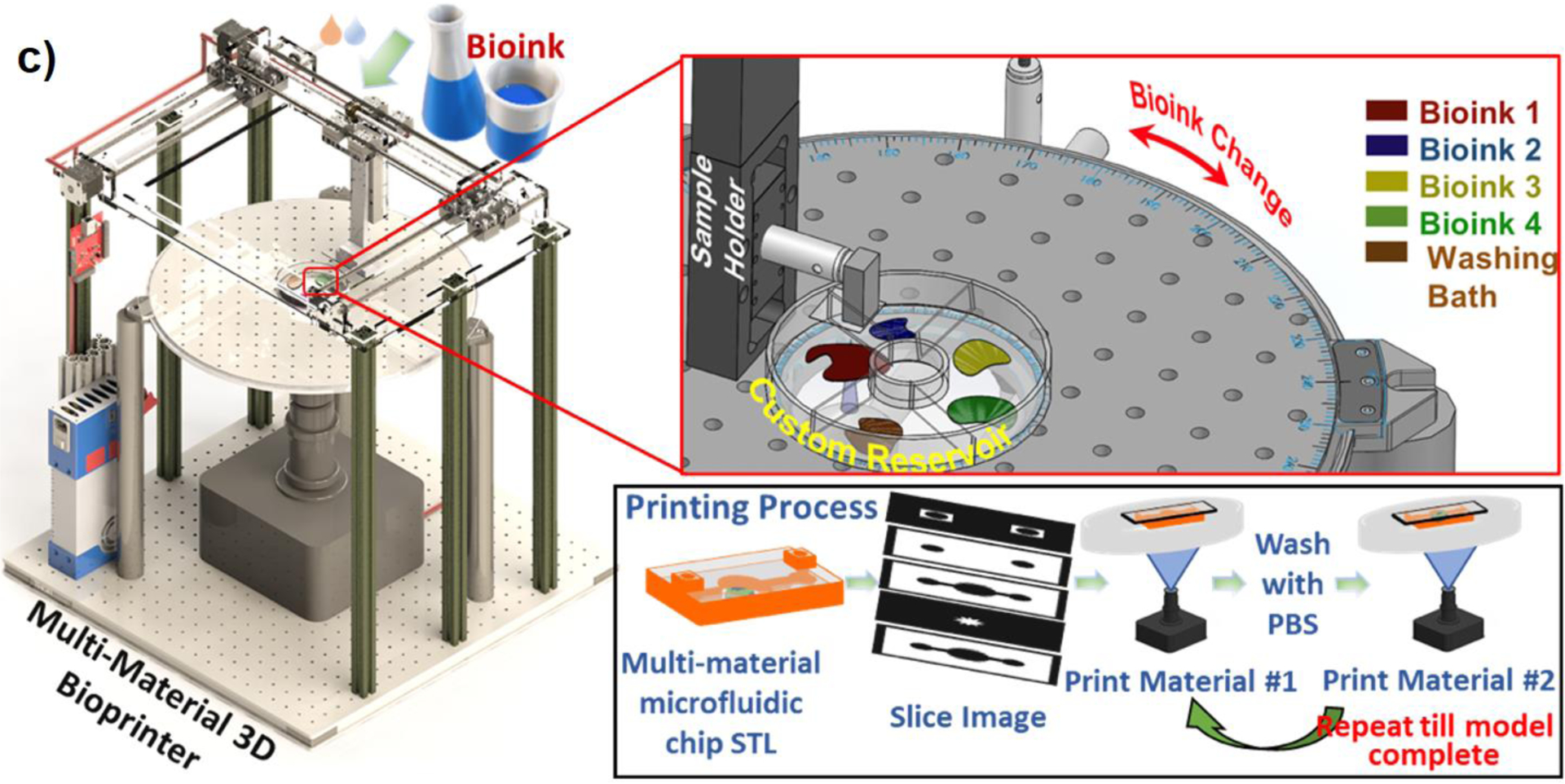
a) A simple stereolithography-based 3D bioprinting system developed for crosslinking bioinks with high intensity white light. i) the binary patterns were directly transferred to the bioinks (red areas in the picture) layer by layer 47,48 , ii) a mirror (lens system) is used to transfer the patterned light, iii) demonstration of DMD capabilities for grayscale patterning to change the light intensity on each pixel; (Reproduced with permission from ref. 47 Copyright 2015 IOP Publishing), b) Design of a multi-material stereolithography bioprinter: i) Perspective view rendering of bioprinter components, where a motorized silicone sled translates laterally in the X-axis to automate bioink selection and rinsing, ii) Side view schematic showing the glass build plate lowered into a bioink droplet, creating a thin first layer (50 μm) for photocrosslinking, iii) Print workflow for fabricating hydrogels. The motorized sled allows nascent structures to interface with separate bioinks of variable chemical or cellular composition, providing heterogeneity within individuals layer (co-planar; XY) and across sequential layers (stacked; Z), (Reprinted with permission from ref 39. Copyright 2021 Springer Nature), c) Design of a customized bioprinter using a commercial DLP box and multi-material module for creating hydrogel constructs: left picture shows the whole apparatus with x-y-z stage for large constructs and right pictures show the rotation model used to exchange materials on a UV-glass substrate (Reprinted with permission from ref 34. Copyright 2021 IOP Publishing).
To add multi-material capabilities, several amendments are implemented to the conventional bioprinters. One straight forward strategy has been manual lifting of the cross-linked biopolymer after printing the initial layers and switching between different materials which inevitably compromises cellular and biochemical purity due to the mixing of the bioinks. To avoid mixing and contamination, washing the structure with water or saline after lifting the structure out of the liquid polymer solution has been proposed either manually59,60 or through automatic approaches such as using an automated fluid-exchanging microfluidics22, using rotating monomer baths61, or with automated proprietary bioprinters38,46. As a semi-automated approach Grigoryan et al.39 developed a method based on transferring biomaterial droplets using a set of syringes. They introduced an open-source multi-material DLP bioprinter as shown in Figure 3-b. In this design, the glass build-plate is lowered on top of one of the 4 positions designed to reserve bioink droplets of 100–1000 μl. This way, a 50 μm thin layer will form between the build plate and the underlying transparent silicone sled (PDMS coated glass). A horizontally installed projector and a mirror are then used to pattern 2D grayscale digital photomasks at the thin bioink layer from the bottom of the transparent silicon sled (Figure 3-b). Two projectors were tested in a same setup including a classroom type projector of white light (3000 lumen of 40 mW/cm2 intensity at 540 nm) and an industrial projector of near-UV light (16.5 mW/cm2 at 400 nm). It was found that compared to the classroom projector, the industrial projector provided more homogeneity and narrower Gaussian distribution. After printing each layer, the build plate raised up by a user specified layer height (50–300 um) to draw more liquid under the build plate to be photocrosslinked by the next photomask. Next, the silicone sled is moved horizontally to put the active layer into a petri dish for manual cleaning while pumping 2–5 ml of phosphate buffered saline (PBS) and removing the residual PBS with an autoclaved cellulose wiper then changing the bioink for next layer printing. Here, rinsing the structures with PBS could prevent proper adhesion of the subsequent layer of the biopolymer. To fix this problem, the formulation of the rinsing solution was modified by the addition of a dilute photopolymer (2.5–10 % w/v) to PBS. This semi-automated DLP platform had limitations with respect to the resolution, material selection, and extensive manual washing with specific formulations. Another strategy for removing the non-crosslinked residual polymer solution between printing layers in a DLP platform can be an automated cleaning of the monomer residue with compressed air62. However, using compressed air is not an effective strategy for biofabrication of cell laden hydrogels which must be well hydrated to support the cell viability during and after print. Kuang et al. [48] demonstrated a single-vat greyscale g-DMD printing method that allows to fabricate functionally graded materials with tunable mechanical properties with 50 μm resolution. They used a hybrid ink combining bisphenol A ethoxylate diacrylate (BPADA), glycidyl methacrylate (GMA), n-butyl acrylate (BA) (weight ratio of 69:23:8), a diamine cross-linker [poly (propylene glycol) bis(2-aminopropyl ether); D230], a photoinitiator (Irgacure 819), and a photo-absorber (Sudan I). First, they crosslinked ink by grayscale light patterns to form a structure with location-specific properties followed by a second-stage thermal curing that allows elimination of the residual monomers to enhance the property gradients. They also demonstrated the fabrication of the shape memory effect of the g-DLP printed graded material using the printed active composite. Despite interesting 4D printing and folding behaviors, the particular choice of resins used in this method leads to many limitations for using this technique for biofabrication.
Recently, Bhusal et al.34 presented a DLP rotational optical plate (Thorlabs, Newton, NJ) that allows bioink exchange during bioprinting process. It allows bioprinting different materials thus different cell types, which is crucial for a successful disease modelling through DLP printing. For the multi-material bioprinting, the first structural material is cured then the software rotates the motorized upper table and brings the printhead lower for the washing process to remove excess material, where the first layer is submerged in the buffer solution (Figure 3-c). Thus, a desired cell-laden hydrogel layer can be printed by this technique. The approach can be adopted to model vascularized complex disease modelling allowing to the use of different hydrogels and cell types (Figure 5-d).
Figure 5. Various Bioprinted Tissue Constructs:

a) Bioprinted 3D triculture construct. i) Bioprinted hepatic construct on a piece of coverslip held by a pair of forceps. (Scale bar, 5 mm.) ii) 3D reconstruction of the construct showing the patterns of hepatic cells (green) and supporting cells (red). (Scale bar, 500 μm) (Reprinted with permission from ref 83. Copyright 2020 Elsevier); b) i) A tendon-to-bone insertion model showing the schematic of the tendon-to-bone insertion site; the mask for printing; bright-field optical image showing a bioprinted dye-laden GelMA structure; bioprinted structure of GelMA containing patterned osteoblasts (blue), MSCs (red), and fibroblasts (green); the inset shows a magnified image of the region hosting fibroblasts, where the cells were stained for f-actin (green) and nuclei (blue). ii) A tumor angiogenesis model showing schematic showing the tumor angiogenesis model; the mask for printing; bioprinted microvasculature in PEGDA; bioprinted MCF7 cell (blue)-laden microvascular bed of GelMA further seeded with HUVECs (green) in the channels (Reprinted with permission from ref 22. Copyright 2018 Wiley-VCH), c) Engraftment of functional hepatic hydrogel carriers and vascularized alveolar models i) Prevascularized hepatic hydrogel carriers created by seeding endothelial cells (HUVECs) in the vascular network post-bioprinting. ii) Confocal microscopy observations show that hydrogel anchors physically entrap fibrin gel containing the hepatocyte aggregates (Hep) (scale bar, 1 mm). iii) Tidal ventilation and oxygenation in hydrogels with vascularized alveolar model topologies (Reprinted with permission from ref 27. Copyright 2019 Springer Nature). d) Bioprinted hydrogel-based microfluidic chips: i) A ready-to-use microfluidic chip made of PEGDA and GelMA. ii) Vascular modelling in bioprinted microfluidic chip: fluorescence images showing HUVECs stained for F-actin (green) and nuclei (blue) at Day 10 (left); and fluorescence images showing HUVECs stained for CD31 (red) and nuclei (blue) at Day 10 (right) (Adapted with permission from ref 34. Copyright 2021 IOP Publishing). e) Micropatterning of cardiac tissue and effects on displacement. i) 3D schematic of full tissue-measuring scaffold and confocal 3D reconstruction of stained cardiac tissue. ii) Masks of various complex patterns and DIC images showing each patterned tissue at Day 10 (scale bar is 500 μm). iii) Fluorescent images of printed cardiac tissue stained for α-actinin for each scaffold type showing alignment of sarcomeres (scale bar is 25 μm) and summary of max displacement for various patterned tissues at 1, 2, and 4 Hz (Reprinted with permission from ref 44. Copyright 2020 Elsevier).
3.2. Custom-Built Optical Components
Advances in designing DLP bioprinters with major optical customization are summarized here. Ma et al.41 used a pioneer DLP bioprinter with customized optics. As illustrated in Figure 4-a, they used a set of projection lenses to adjust the numerical aperture and collimation of UV light (88 mW/cm2 at 365 nm) on the vat which allowed printing each layer in several seconds (Table 2). Hexagonal shape GelMA scaffolds were made without live cells on a stationary substrate, and the scaffolds were seeded by cells afterwards. The printer brought about an in-plane resolution as low as 30 μm. Same apparatus was used to bioprint a liver-on-chip model representing a part of a liver lobule structure. Two bioinks including live cells were used including GelMA (5% w/v) to form the parenchymal tissue and GelMA (25% w/v)- glycidal methacrylate hyaluronic acid (1% w/v) to shape the vasculature. Before printing with the device, the bioinks were manually mixed with live cells. A lower resolution around 50 μm was achieved due to incorporation of the cells and enhanced light scattering in the microenvironment40,41,63. Zhu et al.31 modified the DLP platform by inclusion of a motorized stage to allow continuous lift of the bioprinted construct from the vat surface and enabled a robust fabrication of nerve guide conduits (NGC) using less harmful light at 405 nm wavelength (Figure 4-b). Another modification to the DLP platform was introduced by Chen et al.21 where an interesting non-invasive portable in vivo DLP bioprinter was developed for patterning near infra-red (NIR) light by a DMD chip and exposing the pattern to skin tissues (Figure 4-c). A new photoinitiator system called up-conversion nanoparticle coated with LAP photoinitiator (UCNP@LAP) was introduced which could be activated by infra-red light of high penetration depth. Despite many advantages, the approach still suffers from lack of capabilities for multi-material fabrication.
Figure 4-. Custom-Built DLP Bioprinters:
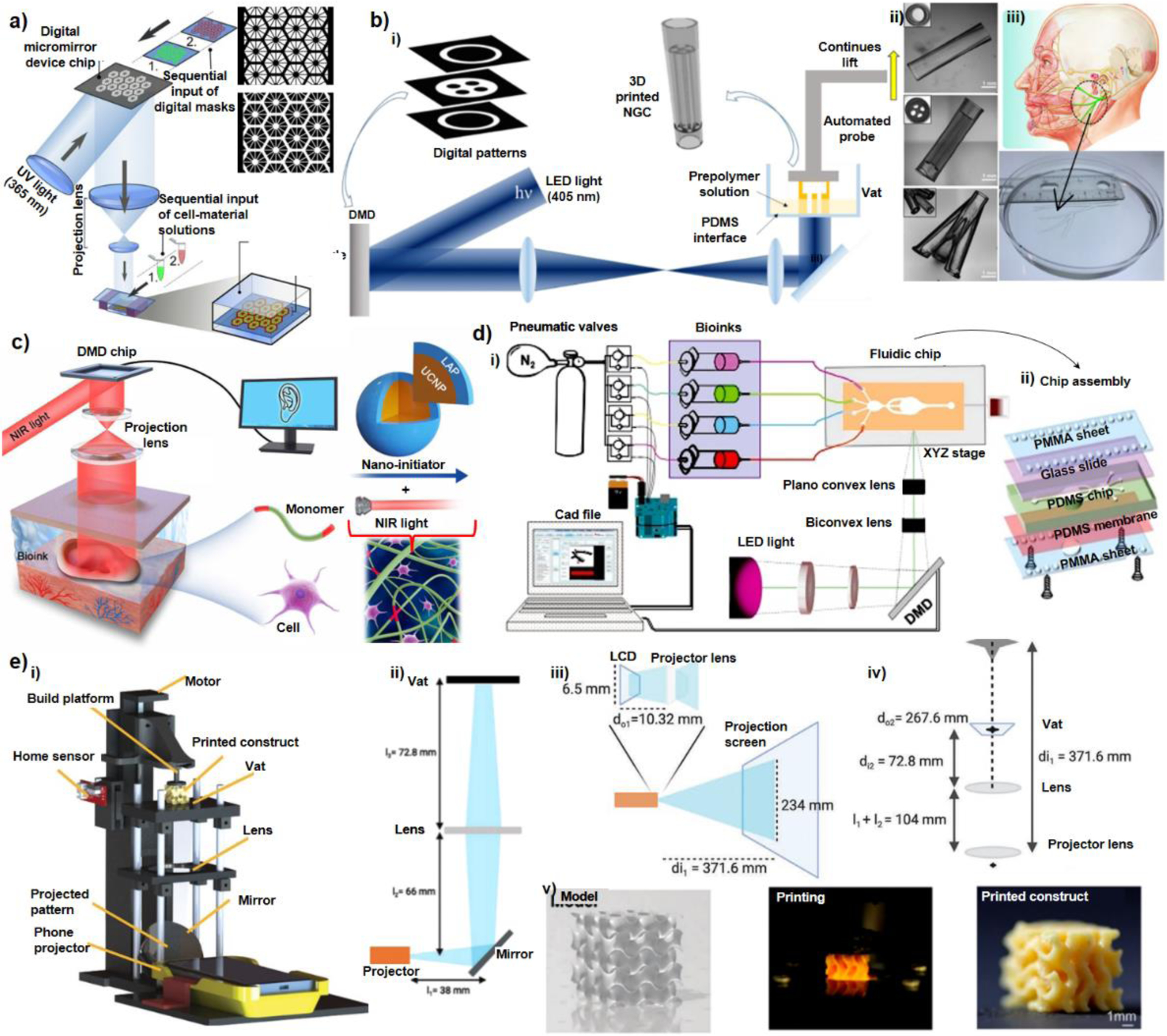
a) Schematic of a two-step bioprinting approach for patterning the first grayscale digital mask (top) for lobule structure followed by the patterning of second grayscale digital mask (down) for vascular structure (Reprinted with permission from ref 41. Copyright 2016 National Academy of Sciences), b) Schematic of the rapid continuous 3D-printer, i) printing customizable nerve guidance conduits (NGCs) with inclusion of a motorized stage, ii) various 3D-printed NGC structures, iii) 3D-printed human life-size NGC based on the facial nerve schematic adapted from Atlas of Human Anatomy (Reprinted with permission from ref 31. Copyright 2018 Elsevier), c) Schematic of digital near-infrared based noninvasive bioprinter: the DMD chip reflects 980 nm light with a pattern across optical lens, onto the bioink, which was injected into the body to noninvasively fabricate a living tissue in vivo. The bioink contains UCNP@LAP nanoinitiators that can convert the NIR light to 365 nm light and thus initiates the pattern–controlled polymerization (Reprinted with permission from ref 21. Copyright 2020 AAAS), d) Planar schematics of the multi-material stereolithographic bioprinter including i) UV lamp, optical lenses, DMD chip, pneumatic module and the microfluidic device, ii) the assembly of the microfluidic chip having four inlets and one common outlet (Reprinted with permission from ref 22. Copyright 2018 Wiley-VCH), e) Smart phone enabled bioprinter, i) schematic diagram of the bioprinter, ii) diagram of the optical relationship between the projector, the mirror, the lens, and the vat; iii) the optical path inside the smartphone-powered projector; iv) the calculation of magnification between the projector lens and the vat, v) an example of a gyroid printing from the 3D model to the printed construct (Reprinted with permission from ref 35. Copyright 2021 Elsevier).
Miri et al.22 designed a microfluidic system to enable multi-material bioprinting in DLP platforms. They used a set of biconvex and planoconvex lenses to pattern 365 nm light (100 mW/cm2) on a microfluidic chip where flow of the bioinks in the chip was controlled by a computer using an Arduino board interface. LAP was used as a photoinitiator. Feature sizes as small as 10 μm and parallel lines with 25 μm resolution could be precisely printed in exposure times between 1–20 seconds depending on the aperture of the light and hydrogel composition. They successfully showed ultrafast 3D multi-material fabrication and co-culture of tissues with an acceptable cell viability in bioprinted constructs.
Recently, Li et al.35 have shown that increasing the concentration of a double-component photoinitiator by 10 folds based on Ru and SPS (i.e. tris(2,2′-bipyridyl)-dichloro-ruthenium(II)-hexahydrate with sodium persulfate), it is possible to crosslink hydrogel systems even at comparably low light intensities without compromising of the cell viability. This founding enabled them to introduce a new portable and modular bioprinter which used a commercial smartphone-powered mini DLP projector. Moreover, they employed a set of lenses to transfer the patterned light on to the Teflon film in vat area. The customization of the optics and lens configurations in the design are illustrated in Figure 4-e. The system was assembled to allow the lenses and the polymerization vat for adjustment across different levels, adding versatility to the printing system. For instance, it allowed lenses with different focal lengths to be switched, resulting in different magnifications and light intensities at the Teflon film. This design has potentials for printing structures with flexibility over multiple length scales. A visible light of intensities as low as 0.23 mW/cm2 (powered by a conventional smartphone) was successfully used to make cell laden hydrogel bioinks with around 50 μm of resolution and 98% cell viability after two weeks.
Among different platforms summarized in Table 2, Li et al.35 introduced a platform with minimum intensities alongside a less harmful light in the visible range. The need to implement high light intensity and high light energy to reach proper crosslink density in bioink formulations has restricted the DLP platforms to exploit costly DMD projectors instead of cost effective LCD displays designed to function at low intensity and low energy lights. The liquid crystal material in LCDs may not be applied to high intensity UV lights. Introduction of effective bioink formulations that work under low light intensities such as the system presented by Li et al.35 paves the way for development of advanced DLP based platforms based on LCDs at much lower cost for the hardware and optics. This makes the DLP bioprinting even more affordable to produce micro-tissue models and complex biological architectures in near future.
4. DLP Bioprinted Micro-Tissue Models
In this part, we discuss how DLP bioprinting has been used to create micro-tissue models and the incentives to apply it for organoids (as summarized in Figure 5). The high fabrication speed (i.e., 100– 1000 cubic millimeters per second as discussed above), faster than other conventional methods used for micro-tissues64, and the efficient photo-polymerization process at a wider range of wavelengths (see Table 1) have made DLP bioprinting ideal for micro-tissue application. The use of DLP bioprinting to create complex structures with micron-sized resolutions would allow fabrication of micro-vascularized tissue models. The control over light-matter interactions would offer modulating scaffold mechanical properties65. Some well-organized tissue constructs have been made based on their complex vascular networks (e.g., ref40,45,66), aligned cardiac scaffolds, and liver microarchitectures (e.g., ref27,41). These examples would demonstrate the adaptability of DLP bioprinting to micro-tissue models.
Soman et al.67 fabricated constructs with various topologies based on cell-laden bioinks, named PEGDA and GelMA (compare Table 2 and Table 3). They showed that the resolution of PEGDA (Mw= 700 Da) can reach nearly 6 × 6 μm in the x-y plane. But, the resolution of 10% w/v cell-laden GelMA (3 × 106 cells/mL) can reach about 17 μm in the sample plane. Researchers have been investigating various different materials that can be used in DLP printing for tissue engineering applications. DLP printing can enable researchers to control over negative and positive poisson’s ratio parameters for tissue engineering68. In this sense, researchers fabricated auxetic tissue structures using PEGDA and GelMA through DLP technique with various poisson’s ratio to investigate neural differentiations of human embryonic stem cells69. The choice of bioinks are growing and include HAMA [37], PVAMA [33], GMHA [39–41], and other new components. Hong et al.70 used silk fibroin for DLP bioprinting in the form of glycidyl-methacrylate (silk-GMA). They evaluated the chondrogenesis properties of chondrocyte-laden silk-GMA through in vitro culture for up to four weeks and then used it in vivo. Silk-GMA hydrogel results showed viability, proliferation, and differentiation to chondrogenesis of encapsulated cells.
Table 3.
Selected DLP bioprinted micro-tissue models
| Bioprinted Model | Bioink/Cell Composition | Patho-Physiological Activity | PI | λ[nm] | Ref. |
|---|---|---|---|---|---|
| Liver | GelMA, HAGM, pluripotent stem cells-derived hepatic progenitor cells and HUVECs | Investigation of multi-cellular hepatic tissue interactions. | LAP | 365 | 41 |
| Bone | GelMA, C2C12, HUVEC, fibroblast, hMSCs, osteoblast | Recapitulating interactions among musculoskeletal junctions | LAP | 385 | 22 |
| Lung | GelMA, PEGDA, HUVEC, Human lung epithelial cells, fibroblast | Recapitulating tidal ventilation and oxygenation in hydrogels with vascularized alveolar model | LAP | 405 | 27 |
| Cardiac muscle | GelMA, mouse ventricular cardiomyocytes. Support and cantilever: HAGM, PEGDA | Capturing the contraction of cardiac micro tissues attached to the printed force gauges and pillars to transmit the force produced by micro tissue. | LAP | 365 | 44 |
| Vessel | PEGDA, GelMA, HUVEC | Addressing the challenge of single operation biorptined vascularized tissue model | LAP | 380 | 34 |
| Brain tumor | GelMA, GMHA, glioma stem cells, astrocytes, neural precursor cells, macrophages | Interrogation of drug sensitivity, cellular crosstalk, invasion, context-specific functional dependencies, immunologic interactions. | LAP | - | 92 |
4.1. Vascularized Models
The vasculature is critical for the delivery of oxygen and nutrients in modeling disease development and growth71. For better tumor microenvironment (TME) mimicry, a viable in vitro disease and tissue model requires complex and hierarchical capillary architectures (~ 10–50 μm)72,73. Lithography and micro-molding based microfluidic devices via sacrificial materials such as hydrogels or sugar crystals were used for microvascular models66,74. These strategies allowed researchers to observe the geometry of capillaries seeded with endothelial cells and how it affects the spatial patterning of diffusive gradients and, as a result, the invasion of endothelial cells during angiogenic sprouting due to the angiogenic growth factors and chemokine mixture66. In this sense, DLP bioprinting can eliminate the need of sacrificial material. Zhu et al.40 developed prevascularized tissues with sophisticated microarchitectures using a continuous DLP technique without any sacrificial material. Endothelial cells spontaneously formed lumen-like structures in vitro through spatially controlled biomaterial characteristics. Similarly, Dai et al.45 used a high-precision DLP to build a platform to fabricate multiscale vascular channels with diameters ranging from > 1100 μm wide down to 17 μm to represent the main trunk channel and relatively small branch channels45. Bhusal et al.34 present a DLP rotational optical plate that allows bioink exchange during the bioprinting process. This way, another type of cell-laden hydrogel layer can be created. This approach was adopted to create vascularized disease models, and this can be extended to different hydrogels with cell types for co-culture systems or high-throughput screening devices (Figure 5-d).
4.2. Multi-Culture Tissue Models
Multi-material extrusion bioprinting is widely used to create vascularized tissue and disease models using Pluronic F-127 filaments75. However, the resolutions are far from reaching a resolution of down to 8–10 μm in diameters required for complex and hierarchical capillary architectures. Current biofabrication methods through DLP are commonly based on single-material micro-tissue models68, and few methods offer multi-material biofabrication76. Despite various disease modelling techniques using bioprinting in literature, the lack of co-culturing using different cell types and biomaterials is still challenging. The models indeed consist of only one cell type, resulting in inadequate throughput, especially endothelial-stromal- interactions and tumor invasiveness77–79. To overcome this challenge, we can apply some existing strategies as discussed in the previous sections.
Choi et al.80 developed a soft lithography-based PDMS microfluidic device to mimic the human mammary duct consisting of the co-culture of breast tumor spheroids and human mammary duct epithelial cells mammary fibroblasts. The device had two chambers, each chamber was 1 mm (width) × 3 mm (length) × 200 μm (height), consisting of upper and lower parts separated through an ECM-derived collagen membrane to mimic the basement membrane. It plays a crucial role in cell migration, proliferation, and differentiation81,82. To generate multicellular spheroids, they seeded the cells into a 96-well hanging drop plate, have let them grow for three days. After UV sterilization of assembled device, they seeded the spheroids and epithelial cells in the upper channel, while they formed a fibroblast-laden collagen gel layer attached to the lower part of the ECM membrane to stromal tissue. This work can be combined with DLP bioprinting and adjustable bioink-PI formulations to create advanced models.
The capability of vascular tissue generation through DLP technique has led researchers to focus on more complicated vascularized architectures to mimic healthy and diseased tissues. Miri et al.22 bioprinted structures resembling biological tissues such as tumor angiogenesis, muscle strips, and musculoskeletal junctions through the DLP system. They reached ~ 20–30 μm resolution (x-y plane) using different cell (e.g. muscle cell line C2C12, HUVEC, fibroblast cell line 3T3, hMSCs, human osteoblasts) embedded GelMA structures to recapitulate different biological environments (Figure 5-b). The liver-on-a-chip model was created using a manual interchange of GelMA as the parenchymal tissue and GelMA-glycidal hyaluronic acid as the vasculature (Figure 5-a). In this DLP patterned liver-on-a-chip, they used human induced pluripotent stem cells-derived hepatic progenitor cells and human umbilic vascular endothelial cells (HUVECs), and investigated their interactions41. Similarly, Grigoryan et al.27 created an artificial liver with functional intravascular (Figure 5-c) and a tidal ventilation and oxygenation in hydrogels with vascularized alveolar model (Figure 5-c) topologies using DLP bioprinting. Artificial liver has showed that albumin promoter activity was 60 times higher in the vascularized liver including hepatocyte aggregation than in single hepatocyte tissue. After being implanted in vivo, the DLP bioprinted vascularized liver tissues appeared to have a better compatibility and integration with the host tissue. Researchers recently presented DLP bioprinting of multichannel nerve guidance conduits using GelMA hydrogels that can be potentially used for peripheral nerve regeneration. They printed multichannel with different inner diameters for in vitro co-culturing neural crest stem cells with PC12 cells, which previously demonstrated nerve guidance support for the survival, proliferation, and migration of neural cells along the longitudinal channel. The results showed that neural crest stem cells could be induced to differentiate into neurons on fabricated conduits83.
Liu et al.44 demonstrated how multi-material bioprinted aligned cardiac microtissues with several different orientations could be potentially used for cardiac disease modeling and drug screening. They used DLP bioprinting technique to bioprint GelMA encapsulating mouse ventricular cardio myocytes to obtain contracting micro tissues attached to the printed force gauges and pillars to transmit the force produced by micro tissue. A support and a cantilever were printed using 2% w/v HAGM (hyaluronic acid glycidyl methacrylate), 2% v/v PEGDA. After a washing process, 15% w/v GelMA was used to pattern pillars, and 5% w/v cell-laden GelMA (25 million cells/ml) was then patterned between the pillars. They showed how different alignments and patterns affect contractile force through this bioprinted system (Figure 5-e).
4.3. Tumor Modeling
Early in vitro tumor modelling methods adopted hybrid manufacturing processes that included procedures such as casting on photolithography-based micro-molds, punching, oxygen plasma coating, and cell seeding. These hybrid approaches require a slew of time-consuming operations which are limited in terms of cell density, repeatability, scalability, and capability to control the structure and geometry84. These difficulties have prompted academics to concentrate on modern and more practical manufacturing methods for reducing fabrication time and costs. This section will go through existing methods such as lithography-based, additive manufacturing, bioprinting, and hybrid approaches.
In conventional microfluidics, lithography-based molding and casting procedures are widely used and well-established. Optical transparency for monitoring the progress and response of in vitro disease models is an essential need of microfluidic devices, and this has limited the range of (bio-) materials and manufacturing methods available. Replicate molding, injection molding, and embossing are examples of traditional fabrication procedures. The most common materials used are silicon, glass, and plastic85. Researchers have used characteristics like gas permeability- optical transparency, and biocompatibility of PDMS in conventional microfluidic devices by coating or injecting cell-laden hydrogels into specific regions of devices86,87. This allows researchers to replicate an environment with oxygen, nutrient, and chemical gradient66,88 to monitor cancer progression and migration behaviour86 by promoting cell-cell interactions80 to induce cell signalling89,90, a crucial parameter in tumor thus any type of disease modelling91.
For example, Tang et al. 92used a DLP-based system to bioprint glioblastoma tumor model. They generated a GelMA and glycidyl methacrylate-HA (GMHA) based hydrogel tumor model using glioma stem cell and other stromal cells (astrocytes, neural precursor cells) with/without presence of macrophages to recapitulate tumour microenvironment through DLP. The study showed that using a DLP-based method, they created a spatially separated tumor zone and surrounding non-neoplastic brain tissue with defined cell density, allowing cells to interact in a more realistic manner and giving a highly reproducible platform for the study of cell-cell interactions.
Nashimoto et al.93 developed a tumor-on-a-chip that allows for intraluminal flow analysis of tumor activities in a designed perfusable tumor vascular network. They employed a three-layer PDMS microfluidic system to allow active perfusion of tumor spheroid and vascular network interactions within a fibrin-collagen matrix. The intraluminal flow in an artificial vascular network inside a premade tumor spheroid was found to be critical in evaluating tumor activity in a drug screening platform. This model can also be applicable through a DLP multimaterial bioprinting approach that allows researchers to reduce production time while eliminating many time-consuming processes (e.g., molding, long PDMS curing time, oxygen plasma bonding, sterilization, introducing biologics into cavities) and more precise special control on biologics.
5. Future Directions
Light-assisted bioprinting when combined with suitable chemistry and bioink formulations offers high capabilities for creating tissue models and many biomaterials applications. DLP bioprinting has become a popular modality because of higher fabrication speed (~ 103 mm3/s) and improved resolution (< 20 μm) compared to other modes. This has led to innovative biofabrication approaches for micro-tissue models such as tumor organoids and tumors-on-chips (Table 3). Multi-material DLP bioprinters have been developed to address the heterogeneity of constructs and potential integration to other components. This can be further advanced for multi-organs-on-chips and potential high-throughput screening platforms94. This pathway is still at an early stage with several translational challenges ahead. The transition from single-organ to multi-organ models is the next phase of DLP bioprinted models. Proper culture assays will be required to support the growth and proliferation of multiple cell phenotypes in a single environment.
The optical capacity of DLP systems to create small feature sizes has been used to fabricate 3D capillaries in organs-on-chips and microfluidic systems. More complex vascular models would be desirable for different sectors. To move towards commercialization, DLP bioprinters should be integrated with different platforms while improving the biological response of vascularized micro-tissue models. Within this context, DLP bioprinters have proved a useful tool in creating cell-laden hydrogel constructs with a superior cell function. This direction of research further can be extended to novel microfluidic platforms made of bioprinted components34.
Among many technical challenges in our road, the field has major challenges to address. The optical transparency of bioinks and how they interact with UV light beams has been a bottleneck for creating high-resolution constructs. Further research is required to achieve light-absorbing bioinks with proper transparency for light patterning while being compatible with biological components. The role of light-matter interactions in the biocompatibility of the bioinks has been overlooked by many active researchers and this would be become an issue in translating the technology to clinical applications. Two favourite bioink compositions, GelMA and PEGDA, can be combined with other biomaterials to obtain desirable properties for tissue engineering applications. The next steps involve improving the optical properties of bioink formulations by adding more efficient PIs and maybe photo absorbing molecules.
Acknowledgments
H. G. H. acknowledges the receipt of fellowship funding by the Alexander von Humboldt Foundation during this research. A.K.M. acknowledges the receipt of Start-up Fund from NJIT. Authors would like to thank Ali Yousefinejad, Hoda Fattel, and Ali Abu-Abed for their comments and proofreading.
Footnotes
Conflict of Interest
Authors declare no Conflict of Interest.
References
- (1).Bhatia SN; Ingber DE Microfluidic Organs-on-Chips. Nat. Biotechnol 2014, 32 (8), 760–772. [DOI] [PubMed] [Google Scholar]
- (2).Dodd RD; Mito JK; Kirsch DG Animal Models of Soft-Tissue Sarcoma. DMM Dis. Model. Mech 2010, 3 (9–10), 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Cox TR; Erler JT Remodeling and Homeostasis of the Extracellular Matrix: Implications for Fibrotic Diseases and Cancer. DMM Dis. Model. Mech 2011, 4 (2), 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Stylianopoulos T; Munn LL; Jain RK Reengineering the Physical Microenvironment of Tumors to Improve Drug Delivery and Efficacy: From Mathematical Modeling to Bench to Bedside. Trends in Cancer 2018, 4 (4), 292–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Kuo C. Te; Chiang CL; Huang RYJ; Lee H; Wo AM Configurable 2D and 3D Spheroid Tissue Cultures on Bioengineered Surfaces with Acquisition of Epithelial-Mesenchymal Transition Characteristics. NPG Asia Mater. 2012, 4 (9), 1–8. [Google Scholar]
- (6).Meng F; Meyer CM; Joung D; Vallera DA; McAlpine MC; Panoskaltsis-Mortari A 3D Bioprinted In Vitro Metastatic Models via Reconstruction of Tumor Microenvironments. Adv. Mater 2019, 31 (10), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Yesil-Celiktas O; Hassan S; Miri AK; Maharjan S; Al-kharboosh R; Quiñones-Hinojosa A; Zhang YS Mimicking Human Pathophysiology in Organ-on-Chip Devices. Adv. Biosyst 2018, 2 (10), 1–25. [Google Scholar]
- (8).Shang M; Soon RH; Lim CT; Khoo BL; Han J Microfluidic Modelling of the Tumor Microenvironment for Anti-Cancer Drug Development. Lab Chip 2019, 19 (3), 369–386. [DOI] [PubMed] [Google Scholar]
- (9).Gu Z; Fu J; Lin H; He Y Development of 3D Bioprinting: From Printing Methods to Biomedical Applications. Asian J. Pharm. Sci 2020, 15 (5), 529–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Mazzocchi A; Soker S; Skardal A 3D Bioprinting for High-Throughput Screening: Drug Screening, Disease Modeling, and Precision Medicine Applications. Appl. Phys. Rev 2019, 6 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Samavedi S; Joy N 3D Printing for the Development of in Vitro Cancer Models. Curr. Opin. Biomed. Eng 2017, 2, 35–42. [Google Scholar]
- (12).Blaeser A; Duarte Campos DF; Fischer H 3D Bioprinting of Cell-Laden Hydrogels for Advanced Tissue Engineering. Curr. Opin. Biomed. Eng 2017, 2, 58–66. [Google Scholar]
- (13).Samavedi S; Joy N 3D Printing for the Development of in Vitro Cancer Models. Curr. Opin. Biomed. Eng 2017, 2, 35–42. [Google Scholar]
- (14).Miri AK; Mostafavi E; Khorsandi D; Huc S-K; Malpica M; Kkhademhosseini A; 12; Khademhosseini. Bioprinters for Organs-on-Chips. Biofabrication, 2019, 0–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Miri AK; Khalilpour A; Cecen B; Maharjan S; Shin SR; Khademhosseini A Multiscale Bioprinting of Vascularized Models. Biomaterials 2019, 198 (May), 204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Melchels FPW; Feijen J; Grijpma DW A Review on Stereolithography and Its Applications in Biomedical Engineering. Biomaterials 2010, 31 (24), 6121–6130. [DOI] [PubMed] [Google Scholar]
- (17).Hull CW; Arcadia C United States Patent (19) Hull (54) (75) (73) 21) 22 (51) 52) (58) (56) APPARATUS FOR PRODUCTION OF THREE-DMENSONAL OBJECTS BY STEREO THOGRAPHY; 1984.
- (18).Blaeser A; Duarte Campos DF; Fischer H 3D Bioprinting of Cell-Laden Hydrogels for Advanced Tissue Engineering. Curr. Opin. Biomed. Eng 2017, 2, 58–66. [Google Scholar]
- (19).Nielsen AV; Beauchamp MJ; Nordin GP; Woolley AT 3D Printed Microfluidics. Annu. Rev. Anal. Chem 2020, 13, 45–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Hwang HH; Zhu W; Victorine G; Lawrence N; Chen S 3D-Printing of Functional Biomedical Microdevices via Light- and Extrusion-Based Approaches. Small Methods 2018, 2 (2), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Chen Y; Zhang J; Liu X; Wang S; Tao J; Huang Y; Wu W; Li Y; Zhou K; Wei X; et al. Noninvasive in Vivo 3D Bioprinting. Sci. Adv 2020, 6 (23), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Miri AK; Nieto D; Iglesias L; Goodarzi Hosseinabadi H; Maharjan S; Ruiz-Esparza GU; Khoshakhlagh P; Manbachi A; Dokmeci MR; Chen S; et al. Microfluidics-Enabled Multimaterial Maskless Stereolithographic Bioprinting. Adv. Mater 2018, 30 (27), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Jakubiak J; Rabek JF Three-Dimensional (3D) Photopolymerization in the Stereolithography. Part II. Technologies of the 3D Photopolymerization. Polimery/Polymers 2001, 46 (3), 164–172. [Google Scholar]
- (24).Lee JH; Prud’homme RK; Aksay IA Cure Depth in Photopolymerization: Experiments and Theory. J. Mater. Res 2001, 16 (12), 3536–3544. [Google Scholar]
- (25).Zhang AP; Qu X; Soman P; Hribar KC; Lee JW; Chen S; He S Rapid Fabrication of Complex 3D Extracellular Microenvironments by Dynamic Optical Projection Stereolithography. Adv. Mater 2012, 24 (31), 4266–4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Janusziewicz R; Tumbleston JR; Quintanilla AL; Mecham SJ; DeSimone JM Layerless Fabrication with Continuous Liquid Interface Production. Proc. Natl. Acad. Sci 2016, 113 (42), 11703–11708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Grigoryan B; Paulsen SJ; Corbett DC; Sazer DW; Fortin CL; Zaita AJ; Greenfield PT; Calafat NJ; Gounley JP; Ta AH; et al. Multivascular Networks and Functional Intravascular Topologies within Biocompatible Hydrogels. Science (80-. ). 2019, 364 (6439), 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Lim KS; Levato R; Costa PF; Castilho MD; Alcala-Orozco CR; Van Dorenmalen KMA; Melchels FPW; Gawlitta D; Hooper GJ; Malda J; et al. Bio-Resin for High Resolution Lithography-Based Biofabrication of Complex Cell-Laden Constructs. Biofabrication 2018, 10 (3). [DOI] [PubMed] [Google Scholar]
- (29).You S; Wang P; Schimelman J; Hwang HH; Chen S High-Fidelity 3D Printing Using Flashing Photopolymerization. Addit. Manuf 2019, 30 (March), 100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Su J Thiol-Mediated Chemoselective Strategies for In Situ Formation of Hydrogels. Gels 2018, Vol. 4, Page 72 2018, 4 (3), 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Zhu W; Tringale KR; Woller SA; You S; Johnson S; Shen H; Schimelman J; Whitney M; Steinauer J; Xu W; et al. Rapid Continuous 3D Printing of Customizable Peripheral Nerve Guidance Conduits. Mater. Today 2018, 21 (9), 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Lin H; Zhang D; Alexander PG; Yang G; Tan J; Cheng AWM; Tuan RS Application of Visible Light-Based Projection Stereolithography for Live Cell-Scaffold Fabrication with Designed Architecture. Biomaterials 2013, 34 (2), 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Lim KS; Levato R; Costa PF; Castilho MD; Alcala-Orozco CR; Dorenmalen K. M. A. van; Melchels FPW; Gawlitta D; Hooper GJ; Malda J; et al. Bio-Resin for High Resolution Lithography-Based Biofabrication of Complex Cell Laden Constructs. Biofabrication 2018, 10 (3). [DOI] [PubMed] [Google Scholar]
- (34).Bhusal A; Dogan E; Nguyen H-A; Labutina O; Nieto D; Miri AK Multi-Material Digital Light Processing Bioprinting of Hydrogel-Based Microfluidic Chips Title: Multi-Material Digital Light Processing Bioprinting of Hydrogel-Based Microfluidic Chips 4. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Li W; Wang M; Mille LS; Robledo Lara JA; Huerta V; Uribe Velázquez T; Cheng F; Li H; Gong J; Ching T; et al. A Smartphone‐Enabled Portable Digital Light Processing 3D Printer. Adv. Mater 2021, 2102153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Mu X; Sahoo JK; Cebe P; Kaplan DL Photo-Crosslinked Silk Fibroin for 3d Printing. Polymers. 2020, pp 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Kuang X; Wu J; Chen K; Zhao Z; Ding Z; Hu F; Fang D; Qi HJ Grayscale Digital Light Processing 3D Printing for Highly Functionally Graded Materials. Sci. Adv 2019, 5 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).L. T; D. T; K. JP; H. S; E. M; T. A; L. R; S. M; K. L Photopolymerizable Gelatin and Hyaluronic Acid for Stereolithographic 3D Bioprinting of Tissue-Engineered Cartilage. J. Biomed. Mater. Res. B. Appl. Biomater 2019, 107 (8), 2649–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Grigoryan B; Sazer DW; Avila A; Albritton JL; Padhye A; Ta AH; Greenfield PT; Gibbons DL; Miller JS Development, Characterization, and Applications of Multi-Material Stereolithography Bioprinting. Sci. Rep 2021, 11 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Zhu W; Qu X; Zhu J; Ma X; Patel S; Liu J; Wang P; Lai CSE; Gou M; Xu Y; et al. Direct 3D Bioprinting of Prevascularized Tissue Constructs with Complex Microarchitecture. Biomaterials 2017, 124, 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Ma X; Qu X; Zhu W; Li Y-SS; Yuan S; Zhang H; Liu J; Wang P; Lai CSE; Zanella F; et al. Deterministically Patterned Biomimetic Human IPSC-Derived Hepatic Model via Rapid 3D Bioprinting. Proc. Natl. Acad. Sci. U. S. A 2016, 113 (8), 2206–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Zhong Z; Deng X; Wang P; Yu C; Kiratitanaporn W; Wu X; Schimelman J; Tang M; Balayan A; Yao E; et al. Rapid Bioprinting of Conjunctival Stem Cell Micro-Constructs for Subconjunctival Ocular Injection. Biomaterials 2021, 267 (January), 120462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Wang P; Li X; Zhu W; Zhong Z; Moran A; Wang W; Zhang K; Chen S 3D Bioprinting of Hydrogels for Retina Cell Culturing. Bioprinting 2018, 12 (September), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Liu J; Miller K; Ma X; Dewan S; Lawrence N; Whang G; Chung P; McCulloch AD; Chen S Direct 3D Bioprinting of Cardiac Micro-Tissues Mimicking Native Myocardium. Biomaterials 2020, 256 (November 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Xue D; Wang Y; Zhang J; Mei D; Wang Y; Chen S Projection-Based 3D Printing of Cell Patterning Scaffolds with Multiscale Channels. ACS Appl. Mater. Interfaces 2018, 10 (23), 19428–19435. [DOI] [PubMed] [Google Scholar]
- (46).Thomas A; Orellano I; Lam T; Noichl B; Geiger MA; Amler AK; Kreuder AE; Palmer C; Duda G; Lauster R; et al. Vascular Bioprinting with Enzymatically Degradable Bioinks via Multi-Material Projection-Based Stereolithography. Acta Biomater 2020, 117, 121–132. [DOI] [PubMed] [Google Scholar]
- (47).Wang Z; Abdulla R; Parker B; Samanipour R; Ghosh S; Kim K A Simple and High-Resolution Stereolithography-Based 3D Bioprinting System Using Visible Light Crosslinkable Bioinks. Biofabrication 2015, 7 (4). [DOI] [PubMed] [Google Scholar]
- (48).Wang Z Development of a Visible Light Stereolithography-Based Bioprinting System for Tissue Engineering, Tianjin University, 2016. [Google Scholar]
- (49).Koffler J; Zhu W; Qu X; Platoshyn O; Dulin JN; Brock J; Graham L; Lu P; Sakamoto J; Marsala M; et al. Biomimetic 3D-Printed Scaffolds for Spinal Cord Injury Repair. Nat. Med 2019, 25 (2), 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Kirillova A; Maxson R; Stoychev G; Gomillion CT; Ionov L 4D Biofabrication Using Shape-Morphing Hydrogels. Adv. Mater 2017, 29 (46), 1–8. [DOI] [PubMed] [Google Scholar]
- (51).Lim KS; Schon BS; Mekhileri NV; Brown GCJ; Chia CM; Prabakar S; Hooper GJ; Woodfield TBF New Visible-Light Photoinitiating System for Improved Print Fidelity in Gelatin-Based Bioinks. ACS Biomater. Sci. Eng 2016, 2 (10), 1752–1762. [DOI] [PubMed] [Google Scholar]
- (52).Fonseca AC; Melchels FPW; Ferreira MJS; Moxon SR; Potjewyd G; Dargaville TR; Kimber SJ; Domingos M Emulating Human Tissues and Organs: A Bioprinting Perspective Toward Personalized Medicine. Chem. Rev 2020, 120 (19), 11128–11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Chan V; Zorlutuna P; Jeong JH; Kong H; Bashir R Three-Dimensional Photopatterning of Hydrogels Using Stereolithography for Long-Term Cell Encapsulation. Lab Chip 2010, 10 (16), 2062–2070. [DOI] [PubMed] [Google Scholar]
- (54).Markov DA; Lillie EM; Garbett SP; McCawley LJ Variation in Diffusion of Gases through PDMS Due to Plasma Surface Treatment and Storage Conditions. Biomed. Microdevices 2014, 16 (1), 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Powers DE; Millman JR; Bonner-Weir S; Rappel MJ; Colton CK Accurate Control of Oxygen Level in Cells during Culture on Silicone Rubber Membranes with Application to Stem Cell Differentiation. Biotechnol. Prog 2010, 26 (3), 805–818. [DOI] [PubMed] [Google Scholar]
- (56).Grigoryan B; Paulsen SJ; Corbett DC; Sazer DW; Fortin CL; Zaita AJ; Greenfield PT; Calafat NJ; Gounley JP; Ta AH; et al. Multivascular Networks and Functional Intravascular Topologies within Biocompatible Hydrogels. [DOI] [PMC free article] [PubMed]
- (57).Regehly M; Garmshausen Y; Reuter M; König NF; Israel E; Kelly DP; Chou CY; Koch K; Asfari B; Hecht S Xolography for Linear Volumetric 3D Printing. Nature 2020, 588 (7839), 620–624. [DOI] [PubMed] [Google Scholar]
- (58).Wang Z; Kumar H; Tian Z; Jin X; Holzman JF; Menard F; Kim K Visible Light Photoinitiation of Cell-Adhesive Gelatin Methacryloyl Hydrogels for Stereolithography 3D Bioprinting. ACS Appl. Mater. Interfaces 2018, 10 (32), 26859–26869. [DOI] [PubMed] [Google Scholar]
- (59).YT K; C. K; B. N; F. A Digital Manufacturing of Selective Porous Barriers in Microchannels Using Multi-Material Stereolithography. Micromachines 2018, 9 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Arcaute K; Mann B; Wicker R Stereolithography of Spatially Controlled Multi-Material Bioactive Poly(Ethylene Glycol) Scaffolds. Acta Biomater. 2010, 6 (3), 1047–1054. [DOI] [PubMed] [Google Scholar]
- (61).Choi JW; Kim HC; Wicker R Multi-Material Stereolithography. J. Mater. Process. Technol 2011, 211 (3), 318–328. [Google Scholar]
- (62).Kowsari K; Akbari S; Wang D; Fang NX; Ge Q High-Efficiency High-Resolution Multimaterial Fabrication for Digital Light Processing-Based Three-Dimensional Printing. 3D Print. Addit. Manuf 2018, 5 (3), 185–193. [Google Scholar]
- (63).Miri AK; Mirzaee I; Hassan S; Mesbah Oskui S; Nieto D; Khademhosseini A; Zhang YS Effective Bioprinting Resolution in Tissue Model Fabrication. Lab Chip 2019, 19 (11), 2019–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Zhu W; Ma X; Gou M; Mei D; Zhang K; Chen S 3D Printing of Functional Biomaterials for Tissue Engineering. Curr. Opin. Biotechnol 2016, 40, 103–112. [DOI] [PubMed] [Google Scholar]
- (65).Ma X; Liu J; Zhu W; Tang M; Lawrence N; Yu C; Gou M; Chen S 3D Bioprinting of Functional Tissue Models for Personalized Drug Screening and in Vitro Disease Modeling. Adv. Drug Deliv. Rev 2018, 132, 235–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Baker BM; Trappmann B; Stapleton SC; Toro E; Chen CS Microfluidics Embedded within Extracellular Matrix to Define Vascular Architectures and Pattern Diffusive Gradients. Lab Chip 2013, 13 (16), 3246–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Soman P; Chung PH; Zhang AP; Chen S Digital Microfabrication of User-Defined 3D Microstructures in Cell-Laden Hydrogels. Biotechnol. Bioeng 2013, 110 (11), 3038–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Dogan E; Bhusal A; Cecen B; Miri AK 3D Printing Metamaterials towards Tissue Engineering. Appl. Mater. Today 2020, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Soman P; Tobe BTD; Lee JW; Winquist AM; Singec I; Vecchio KS; Snyder EY; Chen S Three-Dimensional Scaffolding to Investigate Neuronal Derivatives of Human Embryonic Stem Cells. Biomed Microdevices 2012, 14 (5), 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Hong H; Seo YB; Kim DY; Lee JS; Lee YJ; Lee H; Ajiteru O; Sultan MT; Lee OJ; Kim SH; et al. Digital Light Processing 3D Printed Silk Fibroin Hydrogel for Cartilage Tissue Engineering. Biomaterials 2020, 232. [DOI] [PubMed] [Google Scholar]
- (71).Ellis LM. Tumor Angiogenesis. In Surgical research; Souba WW, Wilmore DW, Eds.; Academic Press: San Diego, 2001; pp 401–413. [Google Scholar]
- (72).KH B; D. S; H. B; H. A; J. A; J. KJ; K. K; K. HJ; M. L; M. R; et al. Engineered in Vitro Disease Models. Annu. Rev. Pathol 2015, 10, 195–262. [DOI] [PubMed] [Google Scholar]
- (73).Bersini S; Moretti M 3D Functional and Perfusable Microvascular Networks for Organotypic Microfluidic Models. Journal of Materials Science: Materials in Medicine. 2015. [DOI] [PubMed] [Google Scholar]
- (74).Wang XY; Jin ZH; Gan BW; Lv SW; Xie M; Huang WH Engineering Interconnected 3D Vascular Networks in Hydrogels Using Molded Sodium Alginate Lattice as the Sacrificial Template. Lab Chip 2014, 14 (15), 2709–2716. [DOI] [PubMed] [Google Scholar]
- (75).Kolesky DB; Homan KA; Skylar-Scott MA; Lewis JA Three-Dimensional Bioprinting of Thick Vascularized Tissues. Proc. Natl. Acad. Sci 2016, 113 (12), 3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Mobaraki M; Ghaffari M; Yazdanpanah A; Luo Y; Mills DK Bioinks and Bioprinting: A Focused Review. Bioprinting. Elsevier B.V. June 1, 2020, p e00080. [Google Scholar]
- (77).Bischel LL; Beebe DJ; Sung KE Microfluidic Model of Ductal Carcinoma in Situ with 3D, Organotypic Structure. BMC Cancer 2015, 15 (1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Hu M; Peluffo G; Chen H; Gelman R; Schnitt S; Polyak K Role of COX-2 in Epithelial-Stromal Cell Interactions and Progression of Ductal Carcinoma in Situ of the Breast. Proc. Natl. Acad. Sci. U. S. A 2009, 106 (9), 3372–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Rothbauer M; Zirath H; Ertl P Recent Advances in Microfluidic Technologies for Cell-to-Cell Interaction Studies. Lab Chip 2018, 18 (2), 249–270. [DOI] [PubMed] [Google Scholar]
- (80).Choi Y; Hyun E; Seo J; Blundell C; Kim HC; Lee E; Lee SH; Moon A; Moon WK; Huh D A Microengineered Pathophysiological Model of Early-Stage Breast Cancer. Lab Chip 2015, 15 (16), 3350–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Miner JH; Nguyen NM EXTRACELLULAR MATRIX. In Encyclopedia of Respiratory Medicine; Laurent GJ, Shapiro SD, Eds.; Academic Press: Oxford, 2006; pp 157–162. [Google Scholar]
- (82).Lowe JS; Anderson PG Support Cells and the Extracellular Matrix. In Stevens & Lowe’s Human Histology; Lowe JS, Anderson PG, Eds.; Mosby: Philadelphia, 2015; pp 55–70. [Google Scholar]
- (83).Ye W; Li H; Yu K; Xie C; Wang P; Zheng Y; Zhang P; Xiu J; Yang Y; Zhang F; et al. 3D Printing of Gelatin Methacrylate-Based Nerve Guidance Conduits with Multiple Channels. Mater. Des 2020, 192, 108757. [Google Scholar]
- (84).Knowlton S; Onal S; Yu CH; Zhao JJ; Tasoglu S Bioprinting for Cancer Research. Trends Biotechnol. 2015, 33 (9), 504–513. [DOI] [PubMed] [Google Scholar]
- (85).Convery N; Gadegaard N 30 Years of Microfluidics. Micro Nano Eng. 2019, 2 (November 2018), 76–91. [Google Scholar]
- (86).Irimia D; Toner M Spontaneous Migration of Cancer Cells under Conditions of Mechanical Confinement. Integr. Biol 2009, 1 (8–9), 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Huang TQ; Qu X; Liu J; Chen S 3D Printing of Biomimetic Microstructures for Cancer Cell Migration. Biomed. Microdevices 2014, 16 (1), 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).SUN W; CHEN YQ; WANG MF; WANG YR; ZHANG M; ZHANG HY; HU P Study on Drug Resistance to Tumor Cell in Oxygen Gradient and Co-Culture Microfluidic Chip. Chinese J. Anal. Chem 2020, 48 (2), 180–186. [Google Scholar]
- (89).Zervantonakis IK; Hughes-Alford SK; Charest JL; Condeelis JS; Gertler FB; Kamm RD Three-Dimensional Microfluidic Model for Tumor Cell Intravasation and Endothelial Barrier Function. Proc. Natl. Acad. Sci. U. S. A 2012, 109 (34), 13515–13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Nguyen DHT; Stapleton SC; Yang MT; Cha SS; Choi CK; Galie PA; Chen CS Biomimetic Model to Reconstitute Angiogenic Sprouting Morphogenesis in Vitro. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (17), 6712–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Kim BJ; Hannanta-anan P; Chau M; Kim YS; Swartz MA; Wu M Cooperative Roles of SDF-1α and EGF Gradients on Tumor Cell Migration Revealed by a Robust 3D Microfluidic Model. PLoS One 2013, 8 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Tang M; Xie Q; Gimple RC; Zhong Z; Tam T; Tian J; Kidwell RL; Wu Q; Prager BC; Qiu Z; et al. Three-Dimensional Bioprinted Glioblastoma Microenvironments Model Cellular Dependencies and Immune Interactions. Cell Research. 2020, pp 833–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Nashimoto Y; Okada R; Hanada S; Arima Y; Nishiyama K; Miura T; Yokokawa R Vascularized Cancer on a Chip: The Effect of Perfusion on Growth and Drug Delivery of Tumor Spheroid. Biomaterials 2020, 229, 119547. [DOI] [PubMed] [Google Scholar]
- (94).Cecen B; Karavasili C; Nazir M; Bhusal A; Dogan E; Shahriyari F; Tamburaci S; Buyukoz M; Kozaci LD; Miri AK Multi-Organs-on-Chips for Testing Small-Molecule Drugs_ Challenges and Perspectives. Pharmaceutics 2021, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]


