Abstract
Recent advancements in digital-light-processing (DLP)-based bioprinting and hydrogel engineering have enabled novel developments in organs-on-chips. In this work, we designed and developed a multi-material, DLP-based bioprinter for rapid, one-step prototyping of hydrogel-based microfluidic chips. A composite hydrogel bioink based on poly-ethylene-glycol-diacrylate (PEGDA) and gelatin methacryloyl (GelMA) was optimized through varying the bioprinting parameters such as light exposure time, bioink composition, and layer thickness. We showed a wide range of mechanical properties of the microfluidic chips for various ratios of GelMA:PEGDA. Microfluidic features of hydrogel-based chips were then tested using dynamic flow experiments. Human-derived tumor cells were encapsulated in the 3D bioprinted structures to demonstrate their bioactivity and cell-friendly environment. Cell seeding experiments then validated the efficacy of the selected bioinks for vascularized micro-tissues. Our biofabrication approach offers a useful tool for the rapid integration of micro-tissue models into organs-on-chips and high-throughput drug screening platforms.
Keywords: Digital-light-processing, organ-on-a-chip, microfluidics, hydrogel models
1. Introduction
Organ-on-a-chip (OoC) platforms include extracellular matrix (ECM)-like micro-tissues [1] that regulate the biological behavior of embedded cells [2] and microfluidic assemblies. The biomimicry of these micro-tissues makes them useful for drug development and personalized medicine, while the microfluidic assemblies help deliver biological agents to the embedded cells [1, 3–5]. Recent OoC research has focused on our ability to control the microstructure, vascular complexity, mechanical properties, and intrinsic heterogeneity of the micro-tissues [5, 6]. Conventional biofabrication methods, such as micro-molding and photolithography, have limitations in controlling the composition of micro-tissues [7], as well as material selection. Three-dimensional (3D) bioprinting, when equipped with multi-material platforms, can be used to make heterogenous micro-tissues [11]. By the synergistic use of multi-material 3D bioprinting and hydrogel engineering, we can create cell-laden OoCs integrated with vascular networks embedded in and/or beside micro-tissues [8–10].
The performance of OoCs depends on the capacity of the microfluidic assemblies to control the continuous flow of cell culture medium, exogenous cues, drugs, and other chemicals [1]. The screening assays may be conducted by way of a microfluidic chip, which is by flowing one or more components of a biochemical system through reaction channels within the chip. The micro-tissue channels may mimic the target tissue depending on the desired application [11]. Perfusion-based microfluidic chips can be applied in optimizing drugs, screening, and toxicology testing [12]. Conventional microfluidic devices are made by curing polydimethylsiloxane (PDMS) or plastic molding. PDMS platforms provide gas permeability for biochemical processes, optical transparency for real-time imaging, ease of manufacturability, and surface functionality after oxygen plasma treatments [13–15]. These PDMS models benefit from high stability under biochemical processes and low costs of ingredients [16]. The PDMS or plastic platforms have difficulties in encasing micro-tissues, and PDMS-based OoCs suffer from lengthy preparation processes and weak functionality in mimicking the physiological environments of target tissues [4]. Sequential integrations of multiple PDMS layers, which are required to model complex architecture, are very time-consuming, labor-intensive, and challenging to automate for the continuous production of OoCs [17].
The use of natural or synthetic hydrogels as the foundation of microfluidic chips has enabled the creation of cell-laden OoCs [10]. The combination of hydrogels with tissue-specific cells can be used to replicate the target tissue in OoCs [18]. Hydrogels are hydrophilic polymer networks containing high water content, which can be used to mimic ECM molecules [19]. They provide a microenvironment for cell interaction [20], and diffusion of biological molecules [21, 22], leading to the transportation of nutrients to cells embedded in the hydrogel. Such permeation-based microfluidic chips may be used based on the convective-diffusion mechanism for liquid and solute transportation. This property can be used to transport nutrients and media, and it can also be used to study the chemotactic response of biological cells to a specific molecule [23].
Among common hydrogels [6], gelatin-based systems benefit from a conducive biochemical environment, photocrosslinking ability, excellent biocompatibility, and ease of preparation [24, 25], and they additionally provide a cell-friendly encasement for various tissues [1, 26, 27]. Previous research revealed tunable stiffness of photocrosslinkable gelatin methacryloyl (GelMA) hydrogels as a function of the mass concentration, chemical substitution, and degree of photocrosslinking [20]. The limited structural fidelity and fast (bio-) degradation of GelMA make it challenging to use as the sole backbone. The microfluidic chips require an additional level of support, which can be provided by adding a highly printable synthetic hydrogel: poly-(ethylene-glycol)-diacrylate-(PEGDA). PEGDA is biocompatible, photocrosslinkable, and tunable with a wide range of mechanical properties [28]. To induce cell-ECM adhesion, other agents such as fibronectin can be added to the hydrogel formulation. Optimizing hydrogel composition requires comparing the structural and mechanical properties of micro-tissues with those of target tissues [29].
Other key elements in creating functional OoCs are fabrication speed and precision [30]. Integration of additive manufacturing and hydrogel engineering can allow the rapid fabrication of OoCs [31]. Automation in 3D bioprinting is beneficial for manufacturing hydrogel-based micro-tissues [32, 33]. 3D bioprinting of cell-laden hydrogels potentially allows for the scale-up and rapid creation of micro-tissues in microfluidic chips [34]. Direct fabrication of microchannels seems challenging due to the collapse of the upper layers into the channels, and bioprinting approaches are commonly combined with support materials [35, 36]. Selective removal of the additional sacrificial material leads to the creation of microchannels [37]. For example, sacrificial Pluronic-F127 printing was used within a GelMA chip for creating a vascular thrombosis-on-a-chip [37]. The removal of the sacrificial material can be challenging in high-resolution channels [36].
Current fabrication approaches are limited to single-material micro-tissue models [38], and few approaches that allow multi-material models are time-consuming and cost ineffective [39]. They also have limited control over microscale heterogeneity of biological components such as cells and growth factors [40]. In addition, existing multi-material bioprinters are unable to continuously fabricate cell-laden constructs with clinically relevant dimensions and multicomponent structures with high precisions. There is still a need for multi-material bioprinters capable of using multiple bioinks for the rapid manufacture of hydrogel-based OoCs [17], as well as high-throughput screening microfluidic chips. Multi-material extrusion bioprinting was also used to create human umbilical vein endothelial cells (HUVECs)-seeded micro-channels using Pluronic F-127 filaments, in which the chip was embedded by human mesenchymal stem cells-laden gelatin/fibrin filaments and neonatal dermal fibroblasts [9]. Culture media containing growth factors were used for osteogenic differentiation to create dense osteogenic tissue. Similarly, perfusable 3D human renal proximal tubules were formed for a human kidney-on-achip and kept viable for up to eight weeks [41]. These works maintained a fabrication timing in the range of a few hours, which illustrated how extrusion 3D bioprinting suffers from low speed and modest resolution of microchannel fabrication [42].
Light-matter interactions solidify a hydrogel construct through conventional stereolithography (SLA), which can improve the speed and resolution of the process [43]. While SLA cannot be easily applied to cell-laden constructs [44], digital light processing (DLP)-based bioprinting was proposed to pattern 2D shapes onto the bioink solutions [10]. To integrate these parameters, a manual exchange of GelMA as the parenchymal tissue combined with GelMA-glycidal hyaluronic acid as the vasculature was used to fabricate the liver-on-a-chip model. Human-induced pluripotent cell-derived hepatic progenitor cells and HUVECs were used in the liver-on-a-chip, and their interactions were studied [45]. Zhu et al. applied a similar DLP bioprinting for making pre-vascularized tissue models of ~50 μm channels seeded by HUVECs [46]. This study highlighted the versatility and accuracy of DLP bioprinting and emphasized the high speed of bioprinting associated with photocrosslinking since all tissue models were bioprinted in less than one minute. A more recent effort demonstrates the development of a multi-material DLP-based bioprinter to fabricate high-resolution micro-tissues [8]. The bioprinter was able to alternate different bioink solutions in a short time, leading to the rapid fabrication of micro-tissues. These studies indicate the capacity of DLP-based bioprinting in creating micro-tissues [6, 17].
In this work, DLP-based bioprinting was used to create functional hydrogel-based microfluidic chips as potential OoCs. We customized a multi-material, DLP-based bioprinter to fabricate OoCs to highlight the limitation of conventional systems by recapitulating the multicellular architecture of the target micro-tissue [47]. To provide structural stability and long-term considerations, poly-ethylene glycol-diacrylate (PEGDA) was used as the backbone of the hydrogel-based chips. The proposed bioink may provide readily tunable features for hydrogel-based microfluidic chips. We assessed the effectiveness of the biofabrication approach in creating multi-material, hydrogel-based microfluidic chips with potential outlooks in OoCs. Finally, the biological response of the proposed bioink for cell encapsulation and seeding was evaluated using standard assays.
2. Materials and methods
2.1. 3D Bioprinter
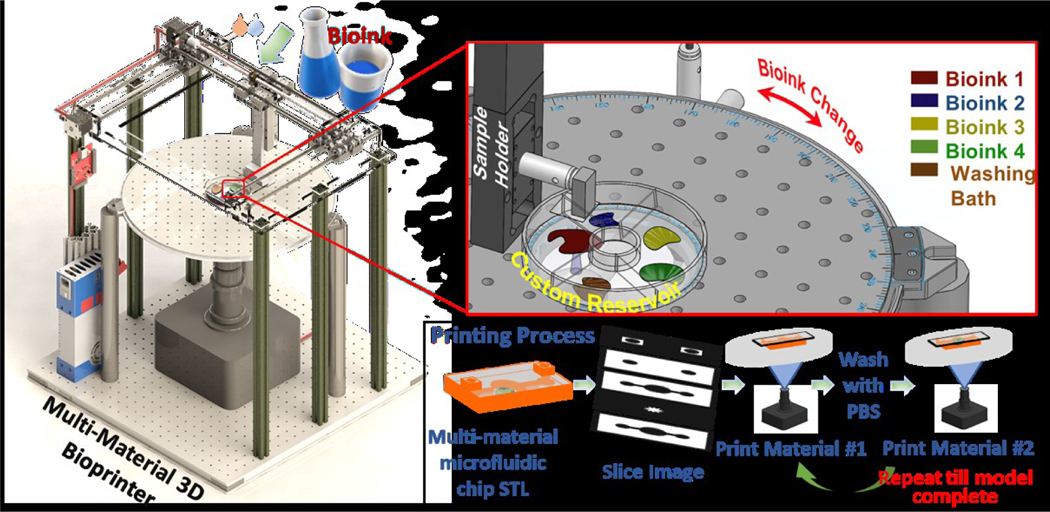
We designed and assembled a DLP-based bioprinter that consists of a digital micromirror device (DMD; Texas Instruments, Dallas, TX) and a UV light system (Visitech; Wetzlar, Germany) at a wavelength of ~ 380 nm and light intensity of ~ 0.7 W.cm−2 (at the focal plane which is located at ~ 2 cm above the lens). The bioprinter is capable of bi-axial movements supported by the stepper motors and z-axis movement by a high precision linear stage (Newport Corp., Irvine, CA). A LabVIEW program was written to control the individual movements of the mechanism, optical tools, and various other parts. The fabrication of hydrogel samples has multiple steps. First, a pre-defined computer-aided design (CAD) model is sliced into a layer-by-layer structure (~50–200 μm layer thickness). While the bioink is kept within the UV-Grade petri-dish (Soda Lime, Corning, NY), the sample holder is lowered by the linear z-stage (see Fig. 1). To enhance the adhesion of PEGDA- and GelMA-based bioinks on the build platform, the glass substrate was coated with 3-(trimethoxysilyl)-propyl methacrylate [17] (Sigma-Aldrich, St. Louis, MO). In our bottom-up bioprinting approach, the bottom surface should be detachable when each layer is crosslinked. A very thin layer of PDMS by using spin coating (at a speed of ~ 1000 rpm) was applied onto our glass substrate [48]. The program then turns on a uniform UV light source, and the reflection from the DMD panel reflects below the stage for about ~ 0.1−0.2 s. When the first layer is formed by photocrosslinking, the stage is raised to the second layer position. The bioink from the surrounding flows inside the first cured layer, and the second layer is cured. The process can be repeated up to the last layer.
Figure 1.
Schematics of the custom-built, multi-material 3D bioprinter and the fabrication process proposed for a hydrogel-based microfluidic chip while highlighting the key steps: bioink preparation, photo-curing, washing, and exchanging bioinks.
The rotation of the upper table (Thorlabs, Newton, NJ) allows bioink exchange (Fig. 1) for multi-material bioprinting. The first bioink cures, and the software retracts back the build platform to the initial position. The upper table is then rotated (90°) for washing the bioink residual. Next, the platform is retracted back to the previous position and the upper table adjusts to the next bioink. The printhead is lowered for curing the next layer, and the process is repeated until our 3D model is completed.
In case of interposed multi-material models, for instance a double-material model (e.g., Gel A and Gel B), the program can be modified to select the bioink that covers smaller spaces or dispersed patterns (Gel A). After washing the bioink residual (Gel A), the second pattern is created (Gel B). The return of the printhead to the same height this process very challenging; hence, it should be positioned at a reasonable tolerance (e.g., ~ 5% of layer thickness) and be approached at a very low speed (e.g., 10 μm/s). Another common issue is the cross-contamination among bioinks induced by high viscosity (i.e., high mass concentration of hydrogels), in which the washing process is hampered by surface tension and capillary forces. The residual bioink requires more washing steps that can be applied to improve bioink exchange. One practical way is to apply mechanical agitation to improve the washing process (not performed in the current work). In addition to these concerns, buffer solution may induce physical variations (e.g., swelling) in bioprinted chips made of soft hydrogels. The mass concentrations of GelMA and PEGDA were consistent in the current work as the buffer washing step was applied for a short time. This had negligible influence on the mass concentration of crosslinked bioinks as the time scale for any swelling or shrinking response of the hydrogel was found to be at a much higher time scale (see Refs. [49] and [50]).
To control UV light penetration into the bioink, gel-based commercial orange food dye (AmeriColor, Placentia, CA) was used to limit the UV penetration within the region required (~ 1% w/v). The total time for fabricating centimeter-sized microfluidic chips and 100 μm-sized layers is less than ~ 2 min (Supplementary Materials: Video S.1). The bioprinter has a custom-built UV source such that the print area at the focal plane is 19.3 × 12.1 mm. The position of the build platform can be moved by using the x-y axis to achieve the area equivalent to the size of the build platform. After the required location is achieved, the build platform is lowered down, and the next layer is cured. During the fabrication of large models, the x-y stage can be used to cure the other parts in each layer.
2.2. Bioink preparation
The proposed bioink formulation was a mixture of GelMA and PEGDA (Mn = 700; Sigma-Aldrich, St. Louis, MO) precursors. The GelMA precursor was synthesized following an established protocol [20], based on porcine skin gelatin (Sigma-Aldrich, St. Louis, MO) and methacrylic anhydride (MA; Sigma-Aldrich, St. Louis, MO). Gelatin solution (10% w/v) was prepared in phosphate-buffered saline (PBS; Corning, AZ). Methacryloyl was induced by adding MA to the gelatin solution at a rate of 8% v/v. The solution was dialyzed for one week and lyophilized to obtain the GelMA powder necessary for the next steps. The bioink solution was prepared at three concentrations of PEGDA (20, 30, and 40% v/v), three concentrations of GelMA (0, 3, and 5% w/v), and two mass concentrations of lithium phenyl-2,4,6-trimethyl-benzoyl-phosphinate (LAP; Allevi, PA) as the photo-initiator (PI; 0.05 and 0.07% w/v). The solution was pre-warmed (> 37° C) for better cleaning of solutions in bioprinting [8]. In case of cell-laden bioink, human-derived tumor cells (HT-1080, ATCC, DC) were cultured in DMEM (Corning, NY) and mixed with the bioinks at a density of ~ 1 M cells/mL.
2.3. Mechanical Testing
Compression testing was used to determine the mechanical stiffness of 3D bioprinted samples. Cylindrical samples with diameters in 10 mm (and heights in 5 mm) were made and placed in a universal testing machine (Shimadzu EZ-SX, Columbia, MD). Displacement-controlled tests were performed at a strain rate of 10% per min. In addition to the proposed bioink variation, the UV exposure time was changed between 0.1 and 0.2 s (for each layer sized around 100 μm). Three tests were performed for each group to attain statistical significance. The linear slope of stress-strain curves was used as the elastic modulus. In addition to this, the swelling ratio was measured for the 3D bioprinted samples (10 mm in diameter and 3 mm in height). Selected samples were submerged in PBS at 37°C for 24 hr, and swelling weight (Ws) was measured. The hydrogels were freeze-dried at −50°C to obtain the dry weight (Wd), and the swelling ratio was then calculated as (Ws −Wd)/Wd.
2.4. Burst Pressure
Burst pressure shows the resistance of micro-channels before any sign of failure or bulging. The hydrogel chips with straight, 500-μm-diameter channels were fabricated and assembled by placing metallic connectors into the gels (PEGDA was indented to tighten the tubing). The PBS solution was pumped through the tubing and micro-channel, and the flow-induced forces were recorded using a 5-mL syringe (BD syringe Leuer-Lock tip) and 22-gauge needle as proposed in a previous study [51]. The syringe was kept in direct contact with the load cell, the fluid was circulated, and a solid needle plug was connected to the end of the tubing to make a closed loop. Pressure built up inside the micro-channel led to an eventual rupture of the internal walls. The syringe wall and plunger exert the frictional force, which was measured and subtracted from the recorded data [51]. The pressure data were summarized and reported for the selected groups.
2.5. Structural Imaging
Scanning electron microscopy (SEM, FEI Nova Nano 650) was used to obtain the images of channels on the microfluidic chip. Samples were initially washed using PBS and fixed using formaldehyde (Sigma-Aldrich, St. Louis, MO) for 24 hr. The samples were then rinsed by a buffer solution and dehydrated by a series of gradually increasing ethanol concentrations in DI water from 50–100% v/v (at 10% interval) for 56 Sparta Ave, Newton, NJ 0786010–15 min at each concentration. Furthermore, the samples were chemically dried using hexamethyldisilane (Sigma-Aldrich, St. Louis, MO) and left to dry in a fume hood overnight. Prior to SEM imaging, the samples were then sputter-coated using gold-palladium using an EMS 550X Sputter Coater.
2.6. Biocompatibility
GelMA and PEGDA hydrogels were used to mimic the targeted tissue [52]. The biocompatibility of the bioink was tested at 0 hr and 24 hr post-printing with a selected PI concentration (0.07%) and exposed to high UV exposure (i.e., 0.2 s). The HT-1080 cells were encapsulated in the bioinks with a density of 1 M/mL. For 24 hr test, the samples were submerged in media and incubated at 37°C for 24 hr and tested by a standard live/dead staining kit (PromoKine Live/Dead Staining kit II, Heidelberg Germany). The samples were imaged using a Nikon fluorescent microscope, and the images were processed using ImageJ (NIH, Bethesda, MD). The test results would demonstrate the range of the bioink formulation that can be used to encapsulate cells. The functionality of the proposed bioinks was further tested by seeding fibroblasts (mouse cell line, 3T3, ATCC) onto the surface of 3D bioprinted samples (using fibronectin solution as an adhesive agent), and a similar protocol was used for imaging viable cells. Alamar Blue (Sigma-Aldrich) was also used to assess the bioactivity of one selected hydrogel for different PI concentrations and UV exposure times.
2.7. Endothelial Cell Seeding
To create a vascular model, HUVECs (CC-2519, Lonza Group AG, Walkersville, MD) and the growth medium (Lonza Group AG) were purchased. First, the channel was created in our (3% w/v GelMA/ 40% v/v PEGDA) chip and was coated by fibronectin solution (100 μg/mL, Sigma-Aldrich) and kept at a temperature of 37 °C for ~ 2 hr. Then HUVECs with a density of 10 × 106 cells/mL were injected into the channel (using a 27-gauge needle) and incubated for ~ 1 hr. Then, a dynamic flow of cell culture medium was pumped into the channel (at a rate of 1 μL/min for ~ 1 hr every 6 hr) up to 10 days.
2.8. Immunostaining
The cell morphology was captured by the immunofluorescent staining of actin filaments in the cytoskeleton by using F-actin and nuclei staining (phalloidin/DAPI), while the angiogenic response of HUVECs was measured by CD31 staining (ab182981, Abcam, Cambridge, MA). The chip was fixed with 4% paraformaldehyde (Thermo Fisher) and was stained following standard protocols (REF). Briefly, it was permeabilized with 0.2% Triton X-100 solution (Sigma-Aldrich) for 30 min, blocked with 1% bovine serum albumin (Sigma-Aldrich) for 1 hr, and then incubated with the staining for 2 hr. Samples were then washed and mounted on slides with DAPI (Sigma-Aldrich) for nuclear staining. Images were captured with a laser scanning confocal microscope (Nikon, Melville, NY).
3. Results
3.1. Multi-Material Bioprinting
The performance of the proposed custom-built bioprinter was evaluated by established testing procedures. The resolution (i.e., the threshold of precision for hydrogels) of the bioprinter was characterized by using a high concentration of PEGDA (60% v/v) and creating straight lines. Following SEM imaging, the smallest size achieved by this approach is considered the benchmark, and it depends on the stage precision and optical properties of the bioink as well as light characteristics. The lowest dimension was about ~ 15 μm in the x-y plane, and it was shown by the edge of the triangular shape (Fig. 2A). The high-resolution feature in the bioprinter allows fabricating micro-tissues and organoids with complex architectures.
Figure 2. Printability data using 40% v/v PEGDA:
A) (i) Light-microscopy images of bar patterns for resolution analysis, ii) Star pattern printed, iii) Triangle printed to demonstrate the x-y resolution; B) SEM images of (i) mixing channels for fluid flows, (ii) a zigzag channel, (iii) pyramid 3D structure; C) Multi-material samples using (i) cylindrical channel in a block, (ii) Yin-Yang pattern with the height reference for multi-material samples, and (iii) Rowan University Logo using a three-material system.
The capacity of the bioprinter in creating composite structures was then assessed via the fabrication of different patterns such as channel distribution, channel mixing model, and pyramid (Fig. 2B). The pyramid structure highlights the z-axis resolution of the construct, which was found to be ~ 50 μm. We then tested the multi-material capability of the bioprinter throughout a cylindrical channel, yin yang pattern, and Rowan University Logo (40% v/v PEGDA, see Fig. 2C). In these cases, a key step is the washing process via a buffer solution to minimize any possible cross-contamination, which may result in an unclear interpretation of cell behavior. The present results indicate the ability of the bioprinter in creating multi-material constructs for future generations of micro-tissue and organoid models in OoCs. The fabricated structures showed proper stability in a wide temperature range of 4–37°C.
In the bioink formulation, one constituent (i.e., PEGDA) has a significantly higher stiffness than the other constituents (as shown by our mechanical characterization); thus, it dominates the mechanical properties of our chips. However, the interface bonding between this constituent and others may affect the stability and stiffness of multi-material constructs. The bonding strength depends on the chemistry of the molecular chains formed, joint topology, and surface adhesion [53]. The bonding chemistry can be regulated by extending the UV initiated photopolymerization, or adding molecular chains and adhesive agents to the functional groups for chemical coupling [54]. Another example is the creation of oppositely-charged hydrogels, resulting in a stronger bonding among bioinks [55]. Some common hydrogels are negatively charged, and other hydrogels such as PEGDA can be modified [56] to control the bonding stability.
3.2. Mechanical Stiffness
A key biomimicry feature in OoCs is the bulk stiffness of micro-tissues. We used uniaxial compression testing to determine the stiffness of 3D bioprinted constructs and the range of physical properties controlled by the bioprinter parameters and bioink formulation. The governing parameters include the PEGDA/GelMA composition, PI mass concentration, UV light exposure time, and degree of photocrosslinking. The results indicate highly tunable stiffness values for the proposed bioink formulation: PEGDA/GelMA/PI (see the results in Table 1). As the concentration of GelMA increases from 0% to 3% w/v, there is an increase in the stiffness of PEGDA/GelMA samples, but a rise in GelMA concentration, from 3% to 5% w/v, has a combined increasing and decreasing effect on the stiffness of PEGDA/GelMA samples. The minimum average elastic modulus recorded was 24 ± 5 kPa for 3% w/v GelMA and 20% v/v PEGDA with 0.05% w/v PI at 0.1 s UV exposure for each layer, and the maximum modulus 1180 ± 9 kPa for 3% w/v GelMA and 40% v/v PEGDA concentration with 0.05% PI and 0.2 s UV exposure. As expected, the increase in the PI concentration led to increased stiffness of the construct (Table 1) while maintaining other parameters. The stiffness increases when the UV exposure time increases from 0.1 to 0.2 s with other similar conditions.
Table 1.
Stiffness (elastic modulus) for selected PI concentrations and UV exposure times in each layer
|
|
|
||||
|---|---|---|---|---|---|
| 0.05% PI & 0.1 s UV exposure in each layer | 0.07% PI & 0.1 s UV exposure in each layer | ||||
|
|
|
||||
| GelMA (%) | PEGDA (%) | Modulus (kPa) | GelMA (%) | PEGDA (%) | Modulus (kPa) |
|
|
|
||||
| 0 | 26.0 ± 0.6 | 0 | N/A | ||
| 3 | 20 | 24.0 ± 5.0 | 3 | 20 | 75.0 ± 7.0 |
| 5 | N/A | 5 | 56.0 ± 6.0 | ||
|
|
|
||||
| 0 | 47.0 ± 3.0 | 0 | 97.0 ± 10 | ||
| 3 | 30 | 49.0 ± 6.0 | 3 | 30 | 106 ± 20 |
| 5 | N/A | 5 | 109 ± 10 | ||
|
|
|
||||
| 0 | 420 ± 6.0 | 0 | 920 ± 60 | ||
| 3 | 40 | 660 ± 30 | 3 | 40 | 530 ± 70 |
| 5 | N/A | 5 | 650 ± 40 | ||
|
|
|
||||
|
|
|
||||
|---|---|---|---|---|---|
| 0.05% PI & 0.2 s UV exposure in each layer | 0.07% PI & 0.2 s UV exposure in each layer | ||||
|
|
|
||||
| GelMA (%) | PEGDA (%) | Modulus (kPa) | GelMA (%) | PEGDA (%) | Modulus (kPa) |
|
|
|
||||
| 0 | 78.0 ± 3.0 | 0 | 51.0 ± 1.0 | ||
| 3 | 20 | 41.0 ± 2.0 | 3 | 20 | 120 ± 7.0 |
| 5 | 52.0 ± 5.0 | 5 | 100 ± 9.0 | ||
|
|
|
||||
| 0 | 97.0 ± 20 | 0 | 110 ± 30 | ||
| 3 | 30 | 140 ± 60 | 3 | 30 | 180 ± 30 |
| 5 | 100 ± 1.0 | 5 | 130 ± 10 | ||
|
|
|
||||
| 0 | 880 ± 30 | 0 | 110 ± 30 | ||
| 3 | 40 | 1180 ± 90 | 3 | 40 | 880 ± 60 |
| 5 | 750 ± 10 | 5 | 980 ± 50 | ||
|
|
|
||||
The mechanical properties of human tissues can be mimicked by the bioink composition and light parameters. Lower stiffness values range from 20 kPa to 30 kPa, similar to those of soft connective tissues [57]. For example, the modulus for brain tumor tissue, such as meningiomas, low-grade gliomas, high-grade gliomas, and metastasis, falls within the range of 10 – 40 kPa [58]. Encapsulation of primary cells and cell lines into hydrogel chips may provide a functional OoC. This will allow for a case of kidney tissue, with stiffness ranging from 10 kPa to 50 kPa [59], vocal cord tissue with 50 – 200 kPa stiffness [60], spinal cords with 1,000 – 1,500 kPa stiffness [61], and other soft tissues. The range of mechanical properties indicates the potential of this approach in creating biomimetic OoCs for future applications.
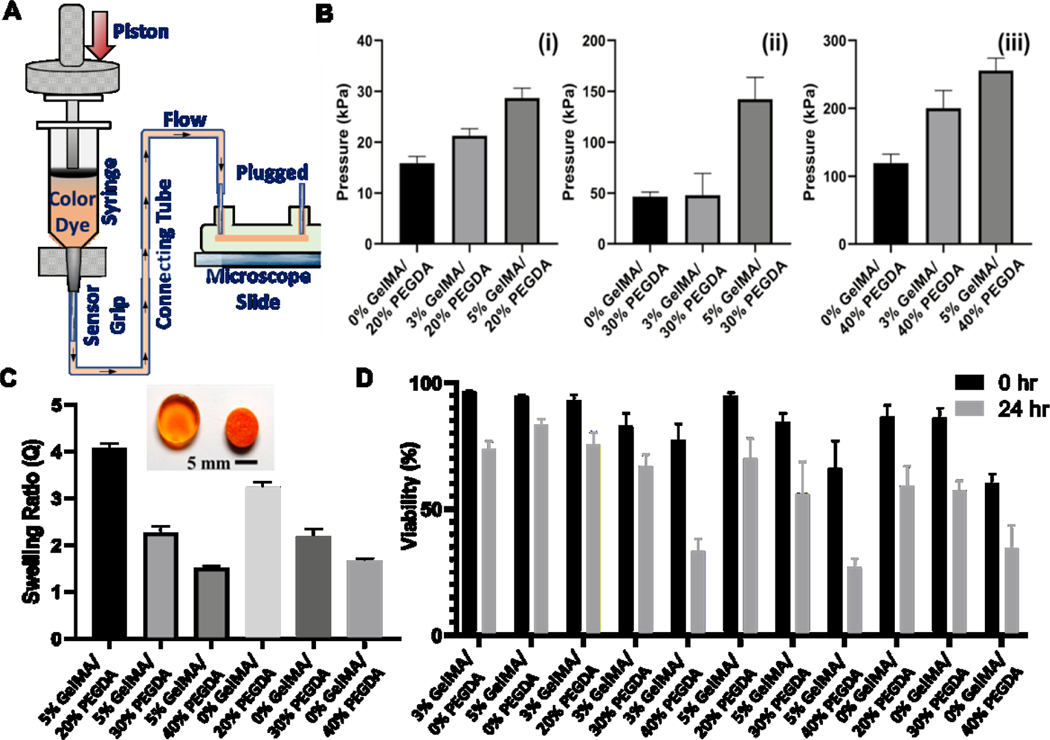
Potential swelling behavior of hydrogel chips may result in increased volumes and weights over time [62]; thus, the impact of swelling on the performance of the chip and potential blockage of micro-channels should be studied. GelMA-based hydrogels swell over time and the equilibrium can be reached a time scale of 6–12 hr [63]. This was observed in our preliminary experiments (Supplementary Materials: Video S.2). The samples were submerged in the buffer solution for 24 hr to allow full swelling and removing any residual stresses. The microfluidic chips were subjected to the buffer for this test, and we selected six different combinations of GelMA and PEGDA (Fig. 3C). For the case of GelMA 0% w/v, the swelling ratio was decreased from 3.2 ± 0.1 to 1.7 ± 0.1 when PEGDA concentration changed from 20% to 40% v/v. Similarly, for the case of GelMA 5% w/v, the swelling ratio was decreased from 4.0 ± 0.1 to 1.5 ± 0.0, with PEGDA concentration increased from 20% to 40% v/v. This observation can be associated with the degree of crosslinking induced by the semi-synthetic PEGDA network. The inherent swelling of hydrogels also affects the mechanical performance and deformation of the 3D bioprinted structures post-incubation. Less-swelling hydrogels seem to be helpful to preserve the strength and channel morphology when incubated. Such hydrogel chips could be applicable for cell-related applications, performing comparably to conventional PDMS microfluidic chips.
Figure 3. Characterization data:
A) Schematics of burst pressure testing setup; (B) Burst pressure data for different bioink groups at 0.2 s UV exposure and 0.07% PI concentration in each layer; C) Swelling ratio data for the selected bioink groups (0.07% PI and 0.2 s UV exposure in each layer) showing the role of GelMA and PEGDA concentrations; D) Cell viability data for the all the bioink combinations (0.07% PI and 0.2 s UV exposure. (symbols w/v and v/v are factored out in the labels)
3.3. Burst Pressure
Burst pressure represents the resistance of micro-channels to the fluid pressure, which can lead to any local failure in the chip. The burst pressure was measured by a custom-built testing setup shown in Fig. 3A, and the results are presented in Fig. 3B. Single-channel chips with 500-μm channels were fabricated following the present protocol. The inlet and outlet were then connected to the tester machine via standard metal connectors, connecting tubes, and standard syringes. The liquid was passed through the channels at a steady flow rate of 0.1 mL/min. We changed bioink mass concentration as the other fabrication parameters were kept constant (PI: 0.07% w/v and UV exposure time: 0.2 s). At low UV exposure time (i.e., 0.1 s), the microfluidic chip was unstable for some GelMA/PEGDA combinations. The present results indicate an increase in the burst pressure when the GelMA concentration was raised (Fig. 3B). The burst pressure increased from 16 ± 1 kPa in GelMA 0% w/v samples to 29 ± 2 kPa in GelMA 5% w/v samples (both for 20% PEGDA). A similar trend was observed when changing from 0% GelMA to 5% GelMA and PEGDA concentrations of 30% (47 ± 5 kPa to 140 ± 20 kPa) and 40% (120 ± 20 kPa to 256 ± 19 kPa), as demonstrated in Fig. 3B. The burst pressure of the microfluidic chip was found to be 200 ± 26 kPa at 3% GelMA and 40% PEGDA, which is less than 5% GelMA/ 40% PEGDA. Hence, the rate of increase in the burst pressure can be linked to the rate of increase in the stiffness.
In some cases, the data related to fluid leakage in the connecting regions between the chips and plastic tubing were excluded from the report (Fig. 4A). The average pressure in arterial circulation can be as high as 13–33 kPa [64]; thus, a resistance above the physiological pressure can ensure the use of hydrogel-based OoCs for vascularized tissue models. The interest here is to design the tunable cell-laden constructs capable of mimicking tissue for OoCs. The microfluidic chip intends to replicate this architecture for perfusion-based screening.
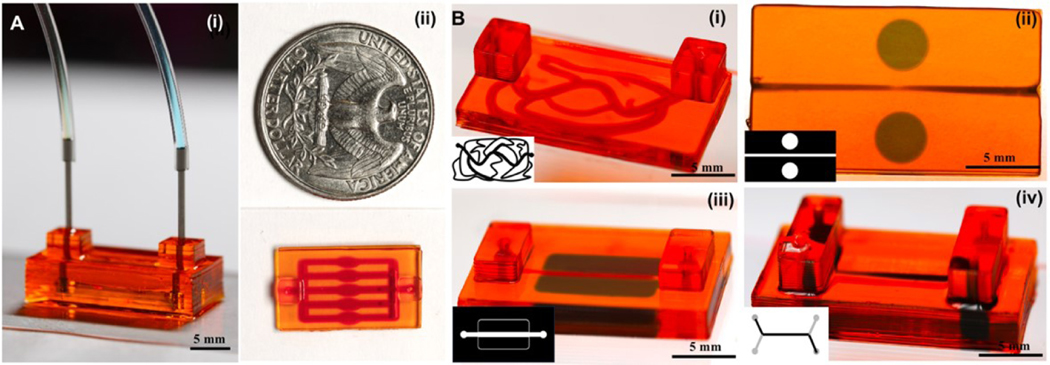
Figure 4. 3D Bioprinted hydrogel-based microfluidic chips:
(A) A ready-to-use microfluidic chip made of 40% PEGDA with the connections (i) and size scale (ii); B) Selected geometries for multi-material chips: 40% PEGDA filled by 3% GelMA (i), 5% GelMA filled disks embedded in 40% PEGDA chip with a straight channel (ii), 5% GelMA filled strips embedded in 40% PEGDA chips (iii), and double-layer stacked channels embedded in 40% PEGDA (iv). (PI: 0.07% w/v and UV exposure time: 0.2 s)
3.4. Microfluidic Chips
The capacity of the proposed fabrication process in creating microfluidic chips is shown by a typical sample having tubing connections and different patterns in Fig. 4. The resolution of the process is depicted in Fig. 4A, where the height of the sample is around 5 mm, and the channel sizes are around ~250 μm. The use of optical bioprinting allows creating complex and diameter-varying tumor vasculature as depicted in Fig. 4A–i, while a second material was bioprinted along the channels. The second material can be used to study cell-drug interactions inside the hydrogel chip. The connectors were press fitted at the inlet and outlet of the microfluidic chip manually. At higher concentrations of PEGDA, the structure showed high fidelity, making it ideal for no-leak attachments to the connectors. The flow did not diffuse outside the metallic connector, which was press-fitted to the channels (by using a higher concentration of PEGDA, such as 40% v/v). The diffusion from the flowing liquid kept the crosslinked hydrogel hydrated and prevented the dehydration of the chip.
Bioink selection depends on several criteria such as functionality, biocompatibility, mechanical properties, and degradation. For microfluidic platforms, we selected PEGDA to create support and channel connections, because it benefits from superior fabrication fidelity and stability. The stiffness of the construct has a direct relation to the printing fidelity. The threshold is dictated by the metallic connectors and required pressure to keep tubing. We observed in our burst pressure experiments that hydrogel stiffness values higher than 100 kPa can offer the desired stability for hydrogel-tubing connections. The stiffness and burst pressure values for PEGDA at 20–40% v/v concentrations showed a good fit to the metallic connectors. The level of stability is very challenging in GelMA hydrogels or other soft hydrogels. GelMA does not provide a high printing fidelity though it is suited for cell encapsulation and growth. Cell-friendly hydrogels can be included within the chip structure while PEGDA or similar hydrogels should be used to encase the micro-tissue. Proper sealing between the tubing and chip can be added by a press fit (Fig. 4A).
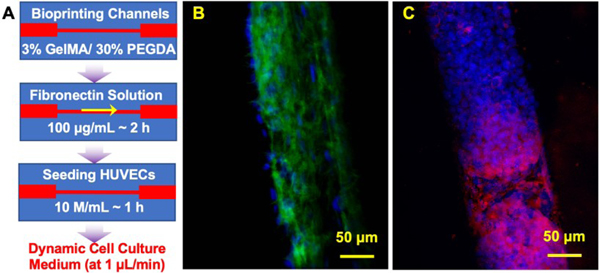
The bioink aims to provide an ECM for cell growth. In addition to encapsulating cells, they can support seeded cells, such as fibroblasts. We showed the application of our bioink for cell seeding in Fig. S4, where fibroblasts were seeded onto the surface of thin layers. The fibroblast showed attachments to the surface of the samples after one day (data taken at Day 3). The chip was then used to grow endothelial cells (HUVECs) to form vascular channels. Bioprinted vasculature allows transportation of any nutrients, solutes, and chemotactic responses. The stiffness of artery and vein ranges from 10 to 1000 kPa. The use of low concentrations of GelMA provides better mimicry for softer tissues such as breast tissue, spinal cord, muscle, and the use of high concentrations of PEGDA yields artery and vein tissues [65]. GelMA/PEGDA mixture seems promising to provide required stiffness while providing better surface attachment and adhesion for HUVECs. It has been shown that osteoblast can adhere and proliferate on the surface of the GelMA/PEGDA hydrogel that indicates the mixture is biocompatible for surface attachment rather than cell encapsulation [66, 67]. We tested the surface properties in our hydrogel-based chip by using HUVECs in micro-channels (100 μm). The channel was bioprinted by a composition of 3% GelMA/30% PEGDA and was subjected to flow for 10 days. The process is shown in Fig. 5A. The long-term dynamic flow-induced physiological shear stresses to form a lumen structure (Fig. 5B) and generate angiogenesis biomarkers (such as CD31, Fig. 5C) after 10 days. The physiological morphology of HUVECs and the spatial distribution of CD31 show bioactivity of HUVECs in response to a dynamic flow.
Figure 5. Vascular modeling in 3D bioprinted microfluidic chip:
(A) The procedure to seed HUVECs and put dynamic flow; B) fluorescence images showing HUVECs stained for F-actin (green) and nuclei (blue) at Day 10; and C) fluorescence images showing HUVECs stained for CD31 (red) and nuclei (blue) at Day 10.
A hydrogel is mainly a fluid-saturated solid network, and the fluid movement within the hydrogel regulates the delivery of nutrients to cells while they can proliferate and migrate within the interconnected space [20]. The interconnected pores within the hydrogel provide the passage of various agents. This characteristic of hydrogel chips was tested in a single-material chip with complex channels in Fig. S2, where the time-dependent diffusion of dye particles was observed over a 120-min time frame. A similar diffusion behavior was observed in a three-material chip with different gel concentrations, as shown in Fig. 6. Thus, microfluidic platforms have facilitated the quantification of diffusivity and how it is affected by both properties of the drug and the ECM setting, with the experimental and theoretical results in agreement with what has been observed in vivo [68].
Figure 6. Diffusion in 3D bioprinted microfluidic chip:
The three-material microfluidic chip pattern (top-left) and time-series of light-microscopy photos taken from the hydrogel chips up to 1.5 hr. The images show gradual diffusion of dyed buffer (red color) into the chip with different rates. The scale bar is 5 mm.
Following the mechanical and physical characterization of hydrogel-based bioinks, we also determined their capacity for cell encapsulation. The viability of cells was examined through biocompatibility assays. The proposed bioink is a combination of GelMA and PEGDA, while a light absorber was used to limit UV-light penetration to the preceding layers. To evaluate the toxicity level of the bioink, the viability of cells was analyzed post-fabrication and after 24 hr. It was observed that the cells in 3% and 5% GelMA (where there is no PEGDA) showed the percentage of viable cells around ~ 74% and 83% at 24 hr while it was around 96% and 94% post-bioprinting (see also Fig. S3) respectively in the presence of the light absorber. Bioprinting GelMA is difficult and has adhesion and breaking issues. The control GelMA with food dye was printed at higher UV exposure of 3 s. Either GelMA or a combination of both gel precursors can be used for OoCs as tested here. For the selected GelMA concentrations (3% and 5% w/v), a 10% v/v step-wise increase in PEGDA was studied in the viability tests (Fig. 3D). The addition of PEGDA led to more toxicity of encapsulated cells, and indeed 30–40% v/v concentrations of PEGDA might be unsuitable for biological components. The bioink used in the fabrication processes is potential candidate for OoCs [6, 69]. The proposed PI concentration and UV exposure were found to be safe for the proposed bioink formulation.
4. Discussions
Natural and synthetic hydrogel-based building blocks can be assembled into large constructs for high-throughput biofabrication of organoids and micro-tissue models [70]. The synergic incorporation of hydrogel building blocks and microfluidic devices may offer novel tools for OoCs and biomimetic models [70, 71]. In this perspective, hydrogel-based microfluidic chips benefit from ease of control in manufacturing, superior structural features at different length scales, and tunability in their biophysical and biochemical properties. They also offer controlled porosity and microenvironment for biological activities of encapsulated cells. Gas and liquid permeability of hydrogels further allows mass transport of small molecules and chemical reagents into the microstructure, which can be used for desired chemical stimuli [70]. In addition, the integration of endothelial cells and perfusable hydrogel chips can lead to biomimetic vascularized constructs. In this work, GelMA was selected as a cell-friendly bioink and PEGDA was used as a highly printable bioink. Their combination provided an optimal choice in generating vascularized micro-tissue models in hydrogel-based microfluidic chips. The light scattering in GelMA makes it challenging for creating small sized channels; thus, PEGDA helped minimizing the light scattering issues.
Recent advances in 3D bioprinting have allowed engineering of hydrogels with physiologically relevant architectures and microchannels with complex geometries. Hydrogels’ architectures and biophysical properties are dictated by the target organ. The current biofabrication method for the hydrogel-based microfluidic chips can address the heterogeneity of the organ or required cell-ECM interactions in the micro-tissue. Existing methods of microfluidic chips are very limited in creating complex environments, and they require long processing times and high costs for creating vasculature. The proposed DLP-based bioprinting makes multi-material chips in a rapid, continuous process (less than 2 min for a typical size). We minimized the contamination between different bioinks for an improved quality of the chip construct. The bioprinter uses multiple combinations of the bioink to fabricate the microfluidic chip with a desired heterogeneity.
The cell-laden part in 3D bioprinted OoCs can mimic the target tissue, and the microfluidic part provides a high level of control over nutrient flow, exogenous cues, integrated sensors, and imaging modules [72–74]. The manipulation of the bioink formulation and bioprinting parameters allows regulating the stiffness of the micro-tissue part and the cell distribution. The microchannels in a microfluidic chip are also tunable in terms of the burst pressure and structural complexity. The pressure ranges from those in arteries to small capillaries. The vascular models, such as double-layer stacked microchannels (Fig. 4Biv), can be used for multi-culture microtissue models [75, 76]. The microchannel design can be simply modified in the CAD model to create diverse geometries and fluid-modulation patterns (e.g., mixing multiples solutions).
Liquid handling equipment, data acquisition, extensive robotic liquid and plate handling are expensive in the drug discovery process. The present biofabrication approach can be employed in creating platforms of drug discovery and therapeutic screening. They offer a small sample size, low reagent consumption, low cost, and ease of handling. They support cell-based screening assays as the cell seeding capability was shown in this work. The microfluidic chip can integrate, multiple biological materials, physiological stiffness, and different biological processes by the application of biological cells with mass transportation. The present observation highlighted the potential use of hydrogel-based microfluidic chips for observing drug mass transport in a high-throughput setting.
5. Conclusion
Most conventional OoCs involve inter-cellular, cell-cell, and cell-ECM interactions, but they cannot mimic the fluid-solid interactions that regulate drug transport into micro-tissues. The lack of control over the microstructural features in micro-tissues has hampered the impact of OoCs for drug testing. By employing a light-assisted biofabrication method and hydrogel engineering, we have developed a biofabrication approach for potential high-throughput screening OoCs. Fluid movements within the hydrogel microstructure regulate the delivery of nutrients to embedded cells while they proliferate and migrate within the interconnected space. The bioprinting process, coupled with hydrogel engineering in cell-laden chips, will reveal how to control realistic biphasic properties of micro-tissues. This approach also allows positioning cells into the structure during the biofabrication process. The range of physical properties shown in this work depicts the flexibility of the proposed biofabrication approach, while the high printing fidelity, tunable properties, and high burst pressure capacity, competitive with traditional PDMSbased OoCs. The outlook of light-assisted bioprinting techniques and microfluidics fits into the pharmaceutical industry.
Supplementary Material
Acknowledgments
A.K.M. acknowledges the receipt of R21-DC18818 from the National Institutes of Health (NIH) and Seed Funding from Rowan University. A.B. and A.K.M. thank all lab members for their valuable feedback and comments on this manuscript. A.K.M. acknowledges Dr. Roya Samanipour (University of British Columbia) for her help in immuno staining. The authors sincerely thank Dr. Berivan Cecen (Rowan University) for her contributions to the cell seeding experiment.
Footnotes
Conflict of Interest
None.
References:
- [1].Yesil-Celiktas O, Hassan S, Miri AK, Maharjan S, Al-kharboosh R, Quiñones-Hinojosa A, Zhang YS, Mimicking Human Pathophysiology in Organ-on-Chip Devices, Advanced Biosystems 0(0) (2018) 1800109. [Google Scholar]
- [2].Huh D, Hamilton GA, Ingber DE, From 3D cell culture to organs-on-chips, Trends Cell Biol 21 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Huh D, Kim HJ, Fraser JP, Shea DE, Khan M, Bahinski A, Hamilton GA, Ingber DE, Microfabrication of human organs-on-chips, Nature protocols 8(11) (2013) 2135. [DOI] [PubMed] [Google Scholar]
- [4].Shen C, Li Y, Wang Y, Meng Q.J.L.o.a.C., Non-swelling hydrogel-based microfluidic chips, 19(23) (2019) 3962–3973. [DOI] [PubMed] [Google Scholar]
- [5].Sun H, Jia Y, Dong H, Dong D, Zheng J.J.C.O.i.C.E., Combining additive manufacturing with microfluidics: an emerging method for developing novel organs-on-chips, 28 (2020) 1–9. [Google Scholar]
- [6].Miri AK, Mirzaee I, Hassan S, Mesbah Oskui S, Nieto D, Khademhosseini A, Zhang YS, Effective bioprinting resolution in tissue model fabrication, Lab Chip 19(11) (2019) 2019–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Billiet T, Vandenhaute M, Schelfhout J, Van Vlierberghe S, Dubruel P, A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering, Biomaterials 33(26) (2012) 6020–6041. [DOI] [PubMed] [Google Scholar]
- [8].Miri AK, Nieto D, Iglesias L, Goodarzi Hosseinabadi H, Maharjan S, Ruiz-Esparza GU, Khoshakhlagh P, Manbachi A, Dokmeci MR, Chen S, Zhang Yu S, Khademhosseini A, Microfluidics-Enabled Multimaterial Maskless Stereolithographic Bioprinting, Advanced Materials (2018) 1800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA, Three-dimensional bioprinting of thick vascularized tissues, Proceedings of the National Academy of Sciences 113(12) (2016) 3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhu W, Qu X, Zhu J, Ma X, Patel S, Liu J, Wang P, Lai CSE, Gou M, Xu Y, Zhang K, Chen S, Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture, Biomaterials 124 (2017) 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nge PN, Rogers CI, Woolley AT, Advances in microfluidic materials, functions, integration, and applications, Chemical reviews 113(4) (2013) 2550–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rothbauer M, Zirath H, Ertl P, Recent advances in microfluidic technologies for cell-to-cell interaction studies, Lab on a Chip 18(2) (2018) 249–270. [DOI] [PubMed] [Google Scholar]
- [13].Damiati S, Kompella UB, Damiati SA, Kodzius R, Microfluidic devices for drug delivery systems and drug screening, Genes 9(2) (2018) 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Torino S, Corrado B, Iodice M, Coppola G, PDMS-based microfluidic devices for cell culture, Inventions 3(3) (2018) 65. [Google Scholar]
- [15].Halldorsson S, Lucumi E, Gómez-Sjöberg R, Fleming RM, Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices, Biosensors and Bioelectronics 63 (2015) 218–231. [DOI] [PubMed] [Google Scholar]
- [16].Guber AE, Heckele M, Herrmann D, Muslija A, Saile V, Eichhorn L, Gietzelt T, Hoffmann W, Hauser PC, Tanyanyiwa J, Gerlach A, Gottschlich N, Knebel G, Microfluidic lab-on-a-chip systems based on polymers—fabrication and application, Chemical Engineering Journal 101(1) (2004) 447–453. [Google Scholar]
- [17].Miri AK, Mostafavi E, Khorsandi D, Hu S-K, Malpica M, Khademhosseini AJB, Bioprinters for organs-on-chips, 11(4) (2019) 042002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Park KM, Lewis D, Gerecht S, Bioinspired hydrogels to engineer cancer microenvironments, Annual review of biomedical engineering 19 (2017) 109–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ratner BD, Hoffman AS, Synthetic hydrogels for biomedical applications, ACS Publications; 1976. [Google Scholar]
- [20].Miri AK, Hosseinabadi HG, Cecen B, Hassan S, Zhang YS, Permeability Mapping of Gelatin Methacryloyl Hydrogels, Acta Biomaterialia (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Daly AC, Riley L, Segura T, Burdick JA, Hydrogel microparticles for biomedical applications, Nature Reviews Materials (2019) 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chung BG, Lee K-H, Khademhosseini A, Lee S-H, Microfluidic fabrication of microengineered hydrogels and their application in tissue engineering, Lab on a Chip 12(1) (2012) 45–59. [DOI] [PubMed] [Google Scholar]
- [23].Cheng S-Y, Heilman S, Wasserman M, Archer S, Shuler ML, Wu M.J.L.o.a.C., A hydrogel-based microfluidic device for the studies of directed cell migration, 7(6) (2007) 763–769. [DOI] [PubMed] [Google Scholar]
- [24].Liu W, Zhong Z, Hu N, Zhou Y, Maggio L, Miri AK, Fragasso A, Jin X, Khademhosseini A, Zhang YS, Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments, Biofabrication 10(2) (2018) 024102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wanjun L, Shrike ZY, H.M. A., Fabio DF, Lin JH, Mahwish BS, Moisés AM, Jingzhou Y, Yi-Chen L, Grissel T.-d.S., M.A. K., Kai Z, Parastoo K, Gyan P, Hao, Xiaofei G, Zhe Z, Jie J, Harry ZG, Xiangyu J, Ryon SS, Remzi DM, Ali K, Rapid Continuous Multimaterial Extrusion Bioprinting, Advanced Materials 29(3) (2017) 1604630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A, Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels, Biomaterials 73 (2015) 254–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Loessner D, Meinert C, Kaemmerer E, Martine LC, Yue K, Levett PA, Klein TJ, Melchels FPW, Khademhosseini A, Hutmacher DW, Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms, Nat. Protocols 11(4) (2016) 727–746. [DOI] [PubMed] [Google Scholar]
- [28].Huang TQ, Qu X, Liu J, Chen S.J.B.m., 3D printing of biomimetic microstructures for cancer cell migration, 16(1) (2014) 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bayly P, Taber L, Kroenke C, Mechanical forces in cerebral cortical folding: a review of measurements and models, Journal of the mechanical behavior of biomedical materials 29 (2014) 568–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sontheimer-Phelps A, Hassell BA, Ingber DE, Modelling cancer in microfluidic human organs-on-chips, Nature Reviews Cancer (2019) 1. [DOI] [PubMed] [Google Scholar]
- [31].Zhang X, Li L, Luo C, Gel integration for microfluidic applications, Lab on a Chip 16(10) (2016) 1757–1776. [DOI] [PubMed] [Google Scholar]
- [32].Memic A, Navaei A, Mirani B, Cordova JAV, Aldhahri M, Dolatshahi-Pirouz A, Akbari M, Nikkhah M.J.B.l., Bioprinting technologies for disease modeling, 39(9) (2017) 1279–1290. [DOI] [PubMed] [Google Scholar]
- [33].Pedde RD, Mirani B, Navaei A, Styan T, Wong S, Mehrali M, Thakur A, Mohtaram NK, Bayati A, Dolatshahi-Pirouz AJAM, Emerging biofabrication strategies for engineering complex tissue constructs, 29(19) (2017). [DOI] [PubMed] [Google Scholar]
- [34].Knowlton S, Yenilmez B, Tasoglu S.J.T.i.b., Towards single-step biofabrication of organs on a chip via 3D printing, 34(9) (2016) 685–688. [DOI] [PubMed] [Google Scholar]
- [35].Gu Z, Fu J, Lin H, He Y, Development of 3D Bioprinting: From Printing Methods to Biomedical Applications, Asian Journal of Pharmaceutical Sciences (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Miri AK, Khalilpour A, Cecen B, Maharjan S, Shin S-R, Khademhosseini A, Multiscale Bioprinting of Vascularized Models, Biomaterials 198 (2019) 204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang YS, Davoudi F, Walch P, Manbachi A, Luo X, Dell’Erba V, Miri AK, Albadawi H, Arneri A, Li X, Wang X, Dokmeci MR, Khademhosseini A, Oklu R, Bioprinted thrombosis-on-a-chip, Lab on a Chip 16(21) (2016) 4097–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dogan E, Bhusal A, Cecen B, Miri A.K.J.A.m.t., 3D Printing metamaterials towards tissue engineering, 20 (2020) 100752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mobaraki M, Ghaffari M, Yazdanpanah A, Luo Y, Mills DJB, Bioinks and bioprinting: A focused review, 18 (2020) e00080. [Google Scholar]
- [40].Richard C, Neild A, Cadarso V.J.J.L.o.a.C., The emerging role of microfluidics in multi-material 3D bioprinting, 20(12) (2020) 2044–2056. [DOI] [PubMed] [Google Scholar]
- [41].Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, Lewis JA, Bioprinting of 3D convoluted renal proximal tubules on perfusable chips, Scientific reports 6 (2016) 34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yi H-G, Lee H, Cho D-W, 3D printing of organs-on-chips, Bioengineering 4(1) (2017) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Blaeser A, Campos DFD, Fischer H, 3D bioprinting of cell-laden hydrogels for advanced tissue engineering, Current Opinion in Biomedical Engineering 2 (2017) 58–66. [Google Scholar]
- [44].Samavedi S, Joy N, 3D printing for the development of in vitro cancer models, Current Opinion in Biomedical Engineering 2 (2017) 35–42. [Google Scholar]
- [45].Ma X, Qu X, Zhu W, Li Y-S, Yuan S, Zhang H, Liu J, Wang P, Lai CSE, Zanella F, Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting, Proceedings of the National Academy of Sciences 113(8) (2016) 2206–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pirlo RK, Wu P, Liu J, Ringeisen B, PLGA/hydrogel biopapers as a stackable substrate for printing HUVEC networks via BioLP™, Biotechnology and bioengineering 109(1) (2012) 262–273. [DOI] [PubMed] [Google Scholar]
- [47].Melchels FP, Feijen J, Grijpma DW, A review on stereolithography and its applications in biomedical engineering, Biomaterials 31(24) (2010) 6121–6130. [DOI] [PubMed] [Google Scholar]
- [48].Miri AK, Nieto D, Iglesias L, Goodarzi Hosseinabadi H, Maharjan S, Ruiz-Esparza GU, Khoshakhlagh P, Manbachi A, Dokmeci MR, Chen SJAM, Microfluidics-enabled multimaterial maskless stereolithographic bioprinting, 30(27) (2018) 1800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zuo Y, Liu X, Wei D, Sun J, Xiao W, Zhao H, Guo L, Wei Q, Fan H, Zhang X.J.A.a.m., interfaces, Photo-cross-linkable methacrylated gelatin and hydroxyapatite hybrid hydrogel for modularly engineering biomimetic osteon, 7(19) (2015) 10386–10394. [DOI] [PubMed] [Google Scholar]
- [50].Browning M, Cereceres S, Luong P, Cosgriff-Hernandez E.J.J.o.B.M.R.P.A., Determination of the in vivo degradation mechanism of PEGDA hydrogels, 102(12) (2014) 4244–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Schwartz R, Malpica M, Thompson GL, Miri AK, Cell encapsulation in gelatin bioink impairs 3D bioprinting resolution, Journal of the Mechanical Behavior of Biomedical Materials 103 (2020) 103524. [DOI] [PubMed] [Google Scholar]
- [52].Mazzocchi A, Soker S, Skardal A, 3D bioprinting for high-throughput screening: Drug screening, disease modeling, and precision medicine applications, Applied Physics Reviews 6(1) (2019) 011302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bovone G, Dudaryeva OY, Marco-Dufort B, Tibbitt MWJABS, Engineering, Engineering Hydrogel Adhesion for Biomedical Applications via Chemical Design of the Junction, (2021). [DOI] [PubMed] [Google Scholar]
- [54].Yang J, Bai R, Li J, Yang C, Yao X, Liu Q, Vlassak JJ, Mooney DJ, Suo Z.J.A.a.m., interfaces, Design molecular topology for wet–dry adhesion, 11(27) (2019) 24802–24811. [DOI] [PubMed] [Google Scholar]
- [55].Li H, Tan YJ, Liu S, Li L.J.A.a.m., interfaces, Three-dimensional bioprinting of oppositely charged hydrogels with super strong interface bonding, 10(13) (2018) 11164–11174. [DOI] [PubMed] [Google Scholar]
- [56].Tan F, Xu X, Deng T, Yin M, Zhang X, Wang J.J.B.m., Fabrication of positively charged poly (ethylene glycol)-diacrylate hydrogel as a bone tissue engineering scaffold, 7(5) (2012) 055009. [DOI] [PubMed] [Google Scholar]
- [57].Arani A, Murphy MC, Glaser KJ, Manduca A, Lake DS, Kruse SA, Jack CR Jr, Ehman RL, Huston JJN 3rd, Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults, 111 (2015) 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chauvet D, Imbault M, Capelle L, Demene C, Mossad M, Karachi C, Boch A, Gennisson J, Tanter MJUM, In vivo measurement of brain tumor elasticity using intraoperative shear wave elastography, 37(6) (2016) 584–590. [DOI] [PubMed] [Google Scholar]
- [59].Bensamoun SF, Robert L, Leclerc GE, Debernard L, Charleux F.J.C.i., Stiffness imaging of the kidney and adjacent abdominal tissues measured simultaneously using magnetic resonance elastography, 35(4) (2011) 284–287. [DOI] [PubMed] [Google Scholar]
- [60].Miri AK, Mechanical characterization of vocal fold tissue: a review study, Journal of Voice 28(6) (2014) 657–667. [DOI] [PubMed] [Google Scholar]
- [61].Bilston LE, Thibault L.E.J.A.o.b.e., The mechanical properties of the human cervical spinal cordIn Vitro, 24(1) (1995) 67–74. [DOI] [PubMed] [Google Scholar]
- [62].Lee WF, Shieh C.H.J.J.o.a.p.s., pH–thermoreversible hydrogels. II. Synthesis and swelling behaviors of N-isopropylacrylamide-co-acrylic acid-co-sodium acrylate hydrogels, 73(10) (1999) 1955–1967. [Google Scholar]
- [63].Han L, Xu J, Lu X, Gan D, Wang Z, Wang K, Zhang H, Yuan H, Weng J.J.J.o.M.C.B., Biohybrid methacrylated gelatin/polyacrylamide hydrogels for cartilage repair, 5(4) (2017) 731–741. [DOI] [PubMed] [Google Scholar]
- [64].Kumar VA, Brewster LP, Caves JM, Chaikof E.L.J.C.e., technology, Tissue engineering of blood vessels: functional requirements, progress, and future challenges, 2(3) (2011) 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cabot JM, Daikuara LY, Yue Z, Hayes P, Liu X, Wallace GG, Paull B, Electrofluidic control of bioactive molecule delivery into soft tissue models based on gelatin methacryloyl hydrogels using threads and surgical sutures, Scientific Reports 10(1) (2020) 7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Nichol J, Koshy S, Bae H, Hwang C, Yamanlar S, Khademhosseini A, Cell-laden microengineered gelatin methacrylate hydrogels, Biomaterials 31 (2010) 5536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wang Y, Ma M, Wang J, Zhang W, Lu W, Gao Y, Zhang B, Guo Y, Development of a Photo-Crosslinking, Biodegradable GelMA/PEGDA Hydrogel for Guided Bone Regeneration Materials, Materials (Basel) 11(8) (2018) 1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tomasetti L, Breunig M, Preventing obstructions of nanosized drug delivery systems by the extracellular matrix, Advanced Healthcare Materials 7(3) (2018) 1700739. [DOI] [PubMed] [Google Scholar]
- [69].Dogan E, Kisim A, Bati-Ayaz G, Kubicek GJ, Pesen-Okvur D, Miri AK, Cancer Stem Cells in Tumor Modeling: Challenges and Future Directions, Advanced NanoBiomed Research n/a(n/a) 2100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Nie J, Fu J, He Y, Hydrogels: The Next Generation Body Materials for Microfluidic Chips?, Small 16(46) (2020) e2003797. [DOI] [PubMed] [Google Scholar]
- [71].Nie J, Gao Q, Wang Y, Zeng J, Zhao H, Sun Y, Shen J, Ramezani H, Fu Z, Liu Z, Xiang M, Fu J, Zhao P, Chen W, He Y, Vessel-on-a-chip with Hydrogel-based Microfluidics, Small 14(45) (2018) e1802368. [DOI] [PubMed] [Google Scholar]
- [72].Yesil-Celiktas O, Hassan S, Miri AK, Maharjan S, Al-kharboosh R, Quiñones-Hinojosa A, Zhang YS, Mimicking Human Pathophysiology in Organ-on-Chip Devices, Advanced Biosystems 2(10) (2018) 1800109. [Google Scholar]
- [73].Heo YJ, Kang J, Kim MJ, Chung WK, Tuning-free controller to accurately regulate flow rates in a microfluidic network, Scientific reports 6 (2016) 23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Geraili A, Jafari P, Hassani MS, Araghi BH, Mohammadi MH, Ghafari AM, Tamrin SH, Modarres HP, Kolahchi AR, Ahadian S, Controlling Differentiation of Stem Cells for Developing Personalized Organ-on-Chip Platforms, Advanced healthcare materials 7(2) (2018). [DOI] [PubMed] [Google Scholar]
- [75].Choi Y, Hyun E, Seo J, Blundell C, Kim HC, Lee E, Lee SH, Moon A, Moon WK, Huh D.J.L.o.a.C., A microengineered pathophysiological model of early-stage breast cancer, 15(16) (2015) 3350–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sleeboom JJ, Amirabadi HE, Nair P, Sahlgren CM, Den Toonder J.M.J.D.m., mechanisms, Metastasis in context: modeling the tumor microenvironment with cancer-on-a-chip approaches, 11(3) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.