Abstract
Background
Preeclampsia (PE) is a serious pregnancy-specific syndrome associated with the inadequate invasion of trophoblast cells and inflammation of the uterus. A previous study found that lncRNA HOXD-AS1 promotes PE. However, its regulatory network requires additional exploration.
Methods
HOXD-AS1-targeted miRNAs and genes were predicted by different databases in a bioinformatics analysis. The expression HOXD-AS1 and its potential m6A methylase (METTL3) were detected in placentas from healthy female patients with PE. The targeting relationship and role of the HOXD-AS1/miR-135a/β-TRCP axis in trophoblast cells were demonstrated by overexpression/knockdown assays. The levels of β-TRCP downstream pathway proteins IκBα, NF-κB, and p65 were measured. The levels of inflammatory factors in cell supernatants were detected by ELISA. To verify the molecular mechanism of β-TRCP in PE, IκBα was co-overexpressed in β-TRCP in trophoblast cells.
Results
The levels of METTL3, HOXD-AS1, and β-TRCP were elevated in PE placental tissues, while miR-135a levels were reduced. MiR-135a was found to be targeted by HOXD-AS1, and HOXD-AS1 expression was maintained at a high level by METTL3-mediated m6A methylation. Overexpression of METTL3, HOXD-AS1, and β-TRCP, and knockdown of miR-135a in HTR-8/SVneo cells all inhibited cell invasion and migration, and promoted apoptosis and the secretion of inflammatory factors. Knockdown of METTL3, HOXD-AS1, and β-TRCP, and overexpression of miR-135a had the opposite effects. Furthermore, IκBα expression was negatively associated with β-TRCP expression, and the levels of NF-κB, p65, and NLRP3 were positively regulated by β-TRCP. A high level of β-TRCP expression attenuated the effect of HOXD-AS1 knockdown in trophoblast cells.
Conclusion
METTL3 functioned to maintain a high level of HOXD-AS1 expression in PE, which influenced inflammation and the migration and invasion of trophoblast cells via the miR-135a/β-TRCP axis and NF-κB pathway.
Keywords: Preeclampsia, m6A methylation, lncRNA, Ubiquitin, Trophoblast
Highlights
-
•
METTL3, HOXD-AS1 and β-TRCP were up-regulated in preeclampsia placentas tissue.
-
•
Highly expressed METTL3 maintained the stability of HOXD-AS1 RNA in preeclampsia.
-
•
HOXD-AS1 regulated the inflammatory response, migration and invasion of trophoblast cells via miR-135a/β-TRCP axis.
1. Introduction
Preeclampsia (PE) is a multisystem progressive disease unique to pregnancy. It is characterized by the onset of hypertension after the 20th week of gestation, and is accompanied by proteinuria and pathological changes in multiple maternal organs and the placenta-fetus [1]. PE occurs in 2∼8 % of pregnancies worldwide, and causes maternal and neonatal morbidity and mortality [2]. In a normal pregnancy, in order to create a high-flow and low-resistance zone in the placenta for adequate exchange of nutrients and waste products between the mother and fetus, fibrinoid matrix stacks and musculoelastic tissue disappear, and spiral artery remodeling occurs [3]. Studies have found that invasion of the walls by extravillous trophoblast cells is important for spiral artery remodeling. Inadequate invasion by trophoblast cells in the uterus and immoderate trophoblast cell apoptosis have been found in the placentas of preeclamptic pregnancies [4,5]. Impaired invasion leads to hypoxia and oxidative damage, and further causes inflammatory responses, which in turn, damage vascular endothelium [6]. Therefore, the impaired invasion and increased apoptosis of trophoblast cells play a vital role in maintaining placental development. Investigating the mechanisms of this impaired invasion and apoptosis will help to prevent and treat PE.
Long non-coding RNAs (lncRNAs) participate in cellular physiological processes by influencing the expression of various coding genes. LncRNAs regulate gene expression via different mechanisms, including altering chromosome formation, regulating transcription initiation, and influencing post-transcription processes [7]. In addition, when compared to mRNA molecules, lncRNAs have higher tissue-specificity, making them excellent biomarkers for diagnostic and therapeutic applications [8]. Accumulating evidence demonstrates that lncRNAs participate in the impaired invasion and increased apoptosis of trophoblast cells. Chen et al. [9] reported that upregulation of lncRNA SH3PXD2A-AS1 prevented trophoblast cell metastasis, which may promote the PE pathogenesis process. The levels of LncRNA H19 were found to be elevated in PE, and its overexpression induced cell autophagy and invasion by activating the PI3K/AKT/mTOR pathways in trophoblast cells [10]. LncRNA HOXD-AS1 has been identified as an oncogene in many cancers. It promotes cancer cell proliferation, invasion, and migration via different pathways [[11], [12], [13]]. More importantly, a transcriptomics study [14] revealed that lncRNA HOXD-AS1 (HAGLR) was abnormally high expressed in first trimester placentas of women destined to develop preeclampsia (https://www.ncbi.nlm.nih.gov/geoprofiles/57672732) and Jiang et al. found that lncRNA HOXD-AS1 promotes preeclampsia progression [15]. Given that there are similarities between cancer cells and trophoblast cells (e.g., the ability to inhibit inflammation and apoptosis, invade, and migrate) [16], we speculated that HOXD-AS1 might be involved in the insufficient invasion and abnormal apoptosis of trophoblast cells in PE.
Through predictive analysis from the StarBase v2.0 database, β-transducin repeat containing E3 ubiquitin protein ligase (β-TRCP) and was miR-135a identified as a potential target of HOXD-AS1. β-TRCP mediates the ubiquitylation and degradation of numerous proteins via the ubiquitin-proteasome pathway [17]. Hakakeyama et al. [18] found that IκBα was recognized by β-TRCP and rapidly ubiquitinated and degraded, and in turn caused the nuclear translocation of NF-κB. Our previous study found that β-TRCP is regulated by miR-135a in PE [19]. Based on these findings, we speculated that miR-135a/β-TRCP might regulate trophoblast invasion and inflammation in PE via NF-κB/NLRP3. An excessive inflammatory response is an important feature, and also a common cause of PE. Inflammasomes are composed of pattern recognition receptor, apoptosis-associated speck-like proteins containing a caspase recruitment domain, and proinflammatory caspase-1. They play a vital role in the activation of an inflammatory response and the release of inflammatory factors, and further cause inflammation-induced apoptosis [20]. The NF-κB pathway may be an important inflammatory regulator involved in PE, and is activated by the hypoxia and inflammation that occurs in early pregnancy. However, its expression in PE increases 10-fold when compared to that in healthy pregnant women [21]. Under conditions of external stimulation, the activation of NF-κB after dissociation from IκB initiates gene transcription and protein synthesis, and further promotes the development of inflammation, which is involved in regulating the development of PE. Recently, NF-κB has been shown to activate the NLRP3 inflammasome in the occurrence of PE [22,23]. NF-κB also regulates the invasion of trophoblast cells by inducing the autocrine and paracrine secretion of certain inflammatory factors [24]. Hence, it is of great importance to further investigate the mechanism by which the inflammatory signaling pathway in PE becomes activated, in order to elucidate the pathogenesis of PE.
To demonstrate the function of HOXD-AS1 in PE, we predicted that HOXD-AS1 might regulate β-TRCP as a competing endogenous RNA (ceRNA) of miR-135a, and that an abnormal level of HOXD-AS1 may be related to its m6A methylation. Therefore, we hypothesized that METTL3-mediated m6A methylation of HOXD-AS1 might regulate inflammation, as well as trophoblast cell invasion, migration, and apoptosis by competing with miR-135a to regulate β-TRCP expression.
2. Materials and methods
2.1. Specimen collection
Totals of 10 healthy pregnant women and 10 PE patients with early onset PE hospitalized at the Hainan Affiliated Hospital of Hainan Medical University were enrolled in this study. The PE patient inclusion criteria were as follows: (1) patient had been diagnosed as early onset PE (systolic pressure ≥140 mmHg or diastolic pressure ≥90 mmHg on two or more occasions after 20-weeks of gestation, accompanied by proteinuria); (2) patient was >18 years old; (3) no evidence of diabetes, kidney disease, cardiovascular, or blood disease. The inclusion criteria for the control group (healthy pregnant women) were the same, except for the presence of PE. Placental trophoblast samples (located in placental disc center) were gathered after delivery and stored in liquid nitrogen. The study protocol was approved by the Ethics Committee of the Hainan Affiliated Hospital of Hainan Medical University (Med-Eth-Re [2022] 745). Each participant provided their written informed consent for study participation.
2.2. Detection of m6A levels
The total m6A methylation level was detected using an EpiQuik m6A RNA methylation quantification kit (EpigenTek, Farmingdale, NY, USA). The m6A methylation levels of HOXD-AS1 were analyzed by MeRIP-qRT-PCR. First, MeRIP was performed with a MeRIP m6A Transcriptome profiling Kit (RIBOBIO, China) to obtain m6A-modified RNA. Next, the level of m6A-modified HOXD-AS1 was determined by qRT-PCR.
2.3. Quantitative reverse transcription -PCR
The total RNA in samples was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). After qualitative and quantitative detection, the RNA was reverse transcribed into cDNA by using a PrimeScript™ RT Kit (Takara, Japan). qPCR was performed by using SYBR green mix (Takara) on an ABI 7500 Real-Time System (Applied Biosystems, Beverly Hill, CA, USA). Primers were synthesized by RiboBio (Guangzhou, China). The primer sequences were as follows: GAPDH: (F: 5-CCAGGTGGTCTCCTCTGA-3 and R: 5-GCTGTAGCCAAATCGTTGT-3); METTL3 (F: 5-TTGTCTCCAACCTTCCGTAGT and R: 5-CCAGATCAGAGAGGTGGTGTAG-3); HOXD-AS1 (F: 5—ACCTCAACAGATGGAGAGCC-3 and R: 5-ACTAGCAGCCTTTGTCCCTT-3); miR-135a (F: 5-ACACTCCAGCTGGGTATGGCTTTTTATTCCTA-3 and R: 5-CTCAACTGGTGTCGTGGA-3); β-TRCP (F: 5- ACCAACATGGGCACATAAACTC-3 and R: 5-TGGCATCCAGGTATGACAGAAT-3); U6 (F: 5-CTCGCTTCGGCAGCACA-3 and R: 5-AACGCTTCACGAATTTGCGT-3). Relative gene expression was calculated using the 2−ΔΔCt method. GAPDH and U6 served as internal controls.
2.4. Western blotting
Total protein was extracted from placental tissues or cell lines by using ice cold RIPA lysis buffer (Sigma, St. Louis, MO, USA) containing a protease inhibitor (Sigma). After centrifugation, the supernatants were collected and the protein concentration in each supernatant was detected using a BCA Protein Assay Kit (Beyotime, Shanghai, China). Next, an equal amount of total protein from each sample was separated by dodecyl sulfate polyacrylamide electrophoresis, and the protein bands were transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA), which were subsequently blocked with 5 % bovine serum albumin (BSA) for 1 h. The membranes were then incubated with primary antibodies (anti-METTL3, β-TRCP, IκBα, NF-κB p65 [Abcam, Cambridge, UK]) for 1 h, and subsequently incubated with a secondary antibody (Abcam) for 1 h at room temperature. Immunostaining was detected with an ECL kit (Yeasen, Shanghai, China).
2.5. Immunohistochemistry
Immunohistochemistry assays were conducted to detect the levels of METTL3 in tissues. Samples of tissue were fixed, dehydrated, paraffined embedded, and then cut into 4-μm-thick sections. After dewaxing and re-hydration, the sections were blocked with 5 % BSA and then incubated with anti-METTL3 (Abcam) for 2 h, followed by incubation with a secondary antibody for 1 h. After being stained with diaminobenzidine (ZSGB-BIO, Beijing, China), and hematoxylin, the sections were mounted in neural resin and visualized under a microscope (Olympus, Japan). Brownish yellow granules in the cytoplasm indicated METTL3 expression.
2.6. Cell culture and transfection
HTR-8/Svneo cells were obtained from American Type Culture Collection (Manassas, VA, USA). The cells were incubated in RPMI 1640 medium (GIBCO, Waltham, MA, USA) containing 10 % fetal bovine serum (GIBCO), plus 100 μg/mL streptomycin and penicillin in a humidified incubator with a 5 % CO2 atmosphere at 37 °C.
A pcDNA4.0 vector containing the coding sequence of METTL3/HOXD-AS1/β-TRCP/IκBα was chemically synthesized by IGE Biotech (Guangzhou, China) for use in overexpression studies, and a pcDNA4.0 vector containing short-hairpin RNA (shRNA) sequences targeting METTL3/HOXD-AS1/β-TRCP was chemically synthesized by IGE Biotech for use in knockdown studies. For miR-135a overexpression and inhibition studies, the corresponding sequences of the mimics and inhibitors were chemically synthesized by IGE Biotech. The plasmids or segments were respectively transfected using Lipofectamine 3000 (Thermo Fisher).
2.7. Cell activity detection
Cell activity was analyzed using the cell counting kit 8 (CCK-8) assay. Cells were added to the wells of 96-well culture plates. The next day, the corresponding treatments were performed on cells in the different groups. Subsequently, the cells were incubated with CCK-8 solution (10 μL/well, Beyotime) for 4 h. Finally, the optical density (OD) of each well at 450 nm was measured with a microplate reader (BIO-RAD, Hercules, CA, USA).
2.8. Transwell assay
To assess cell invasiveness, cells in serum-free medium were added to the upper chambers of Transwell inserts (Corning, Corning, New York, USA) that had been coated with Matrigel. Serum containing culture medium was added each lower chamber. After 24 h of culture, the media and cells in upper chambers were removed, and the invaded cells attached to the lower side of each partition were fixed and stained. Finally, the numbers of cells in 6 randomly selected image fields were counted under a light microscope (Olympus).
2.9. Cell apoptosis detection
Cell apoptosis was quantified by flow cytometry. Treated cells were collected and incubated with reagents in an Annexin V-FITC Apoptosis Detection Kit (Keygen Biotech, Jiangsu, China) in the dark for 10 min. Next, the cells with positive signals were counted with a flow cytometer (Beckman Coulter, San Jose, CA, USA), and the data were analyzed using FlowJo 10.5.3 software.
2.10. Wound healing assay
Cell metastasis was detected by the wound healing assay. In brief, cells were added to 6-well culture plates. After group processing, a pipette tip was used to make a scratch across the center of each well. After washing with PBS, images of each wound were obtained with an inverted microscope (Olympus). Next, the cells were cultured in medium for another 24 h, and the wounds were examined again. The width of each wound was determined using Image J software, and metastasis was quantified by the formula: (W0 h − W24 h)/W0 h × 100 %).
2.11. Inflammatory factor detection
The levels of IL-18 and IL-1β in cell supernatants were measured using ELISA kits (Solarbio, Beijing, China). Briefly, aliquots of supernatants and standards diluted at different multiples were added into the wells of a 96-well culture plate that had been pre-coated with anti-IL-18/IL-1β antibodies. After 2 h of incubation at 37 °C, the supernatants were discarded and the wells were washed with buffer. Next, 100 μL of Detection Reagent A was added, followed by incubation for 1 h, 100 μL of Detection Reagent B was added, followed by incubation for 0.5 h, and 90 μL of Substrate Solution was added, followed by incubation for 15 min. All incubations were conducted at 37 °C. Finally, 50 μL of Stop Solution was added to each well, and the OD value of each well was measured at a suitable wavelength with a plate reader (Thermo Fisher).
2.12. Co-immunoprecipitation (Co-IP)
For detection of protein-protein binding, a recombinant plasmid expressing FLAG tagged β-TRCP or GFP tagged IκBα was transfected into HTR-8/SVneo cells. After 3 days of transfection, the cells were washed and lysed. The two cell lysates were incubated overnight at 4 °C, and then subsequently incubated with anti-FLAG or anti-GFP Magarose beads (Smart-Lifescience, Changzhou, China). Twelve hours later, the beads were washed and the immunoprecipitation complexes were separated and detected by western blotting.
2.13. RNA pull-down
A Pierce™ Magnetic RNA-Protein Pull-Down Kit (Thermo Fisher) was used to perform RNA pull-down experiments to verify the combination of METTL3 and HOXD-AS1. A probe sequence imitating HOXD-AS1 was synthesized by Guangzhou IGE Biotech (China). The probe was labeled with biotinylated cytidine bisphosphate, and then used to capture streptavidin magnetic beads, which were then incubated with HTR-8/SVneo cell lysates. Finally, the captured proteins were washed and subsequently analyzed by western blotting.
2.14. RNA and protein stability assays
For RNA stability detection, cells after treatment were treated with a transcription inhibitor, Actinomycin D (Macklin, Shanghai, China), for periods of 0, 2, 6, 12, and 18 h, respectively; after which, HOXD-AS1 expression was measured by qRT-PCR. For protein stability detection, cells were exposed to 10 μg/mL cycloheximide (CHX, Solarbio) for periods of 0, 2, 6, 12, 18 and 24 h, respectively, and then harvested for analysis by western blotting.
2.15. Dual-luciferase reporter assay
HTR-8/SVneo cells were seeded into the wells of a 96-well plate. A psiCHECK-2 basic vector containing the wild-type or mutant HOXD-AS1 and β-TRCP 3′UTR sequence was co-transfected along with a negative control or miR-135a mimics into HTR-8/SVneo cells by use of Lipofectamine 3000 (Invitrogen). Luciferase activity was measured 48 h later.
2.16. Statistical analysis
All data were analyzed using GraphPad Prism 8 software (La Jolla, CA, USA), and results are presented as a mean value ± standard deviation. Comparisons between 2 groups were performed using the t-test. Comparisons among 3 or more groups were performed using one-way analysis of variance. A P-value <0.05 was considered to be statistically significant.
3. Results
3.1. METTL3 and HOXD-AS1 were dysregulated in PE
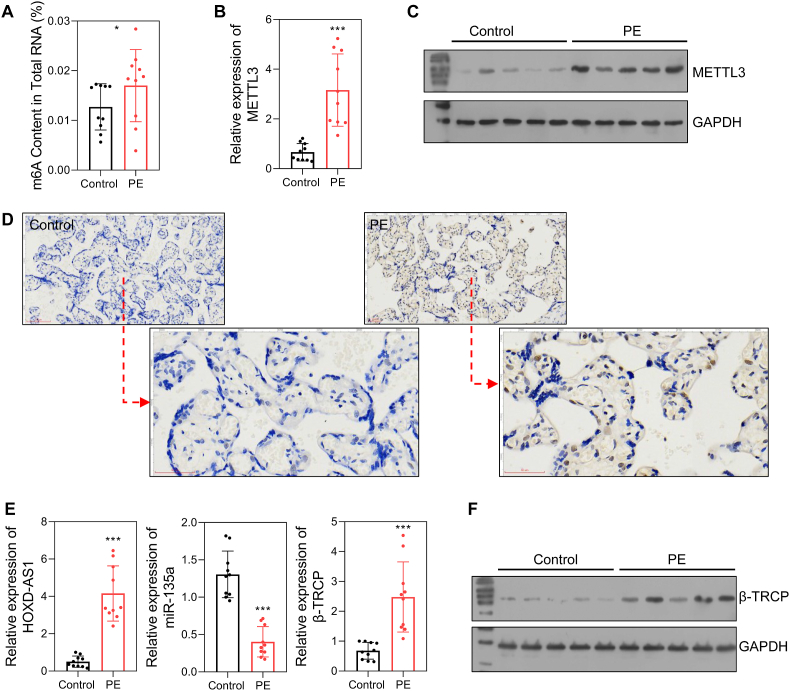
We first detected the levels of m6A methylation and METTL3 expression in placental tissues. Our results showed that the levels of m6A-modified RNA were higher in the PE group than in the control group (Fig. 1A). Moreover, the levels of both METTL3 mRNA and protein were significantly up-regulated in the PE patients (Fig. 1B–D). The levels of HOXD-AS1, miR-135a, and β-TRCP were also detected by qRT-PCR and/or western blotting. Those results showed that HOXD-AS1 and β-TRCP expression were elevated, and miR-135a expression was reduced in the PE patients (Fig. 1E and F).
Fig. 1.
The expression of the METTL3/HOXD-AS1/miR-135a/β-TRCP in PE placentas and health placentas.
A m6A content in total RNA. B The mRNA level of METTL3 detected by qRT-PCR. C The protein level of METTL3 detected by Western blot. D The protein level of METTL3 detected by immunohistochemistry. E The RNA level of HOXD-AS1, miR-135a, and β-TRCP. F The protein level of β-TRCP. Control, placentas from health pregnant women; PE, placentas from PE patients. ns, not significant; *p < 0.05; ***p < 0.001.
3.2. METTL3 regulated the inflammatory response and cellular behavior in trophoblast cells
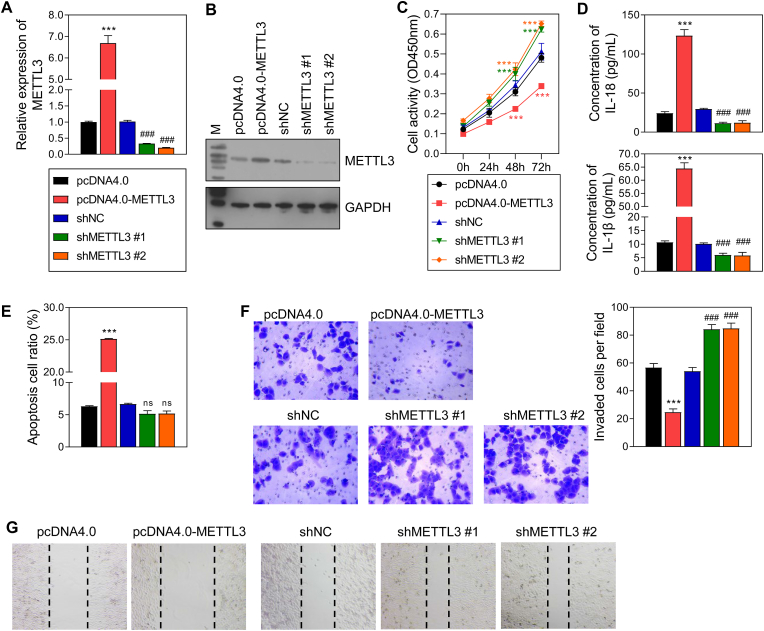
Based on predictions made by the MethyTranscriptome DataBase and our protein and mRNA expression data gathered from PE placentas and healthy placentas, METTL3 was regarded as a potential writer for the m6A methylation of HOXD-AS1. To verify the relationship between METTL3 and HOXD-AS1, METTL3 overexpression and knockdown studies were performed with HTR-8/SVneo cells. First, qRT-PCR and Western blot assays revealed that METTL3 levels were significantly increased in the overexpression group, and significantly decreased in the shMETTL3#1/2 group (Fig. 2A and B). Results from CCK-8, Transwell, and wound healing assays, respectively, revealed that cell activity, invasion, and migration were all inhibited by METTL3 overexpression, and promoted by METTL3 knockdown (Fig. 2C, F, 2G). Meanwhile, flow cytometry data revealed that cell apoptosis had the opposite trends (Fig. 2E). Furthermore, the levels of IL-18 and IL-1β,were both up-regulated in the pcDNA4.0-METTL3 group when compared with those in the pcDNA4.0 group, and were significantly downregulated in the shMETTL3#1/2 group when compared with those in the shNC group (Fig. 2D and E).
Fig. 2.
The effect of METTL3 overexpression and knockdown on HTR-8/SVneo cells.
A, B The efficacy of METTL3 overexpression and knockdown in HTR-8/SVneo cells detected by qRT-PCR and Western blot. C The cell activity detected by CCK-8 assay. D The level of 2 inflammatory factors in the supernatant of HTR-8/SVneo cells detected by ELISA. E Cell apoptosis detected by flow cytometry. F Cell invasion detected by transwell assay. G Cell migration detected by wound healing assay. pcDNA4.0, the control of METTL3 overexpression group; pcDNA4.0-METTL3, METTL3 overexpression group; shNC, the control of METTL3 knockdown group; shMETTL3 #1/2, METTL3 knockdown via 2 different fragments. ***p < 0.001, vs. pcDNA4.0 group; ###p < 0.001, vs. shNC group.
3.3. METTL3 maintained the stability of HOXD-AS1 RNA
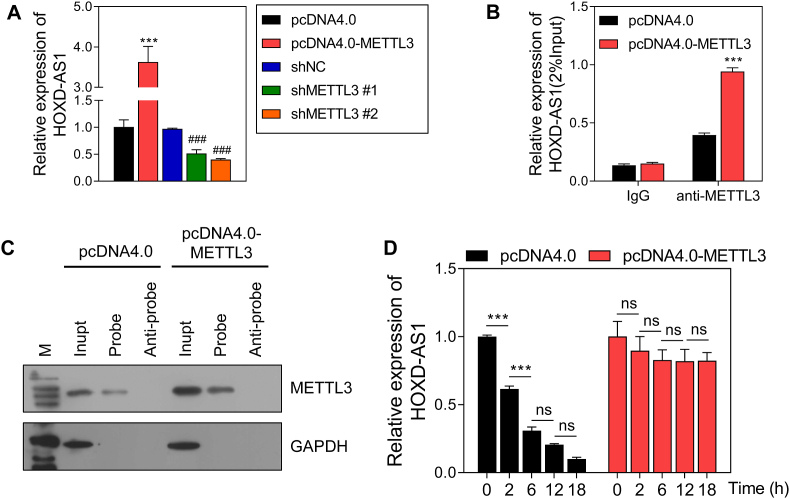
After overexpression or knockdown of METTL3, the levels of HOXD-AS1 were detected by qRT-PCR, and it found that METTL3 overexpression promoted HOXD-AS1 expression and METTL3 knockdown reduced HOXD-AS1 expression (Fig. 3A). A RIP-QPCR assay found that the level of HOXD-AS1 binding with METTL3 was increased in the pcDNA4.0-METTL3 group when compared to that in the pcDNA4.0 group (Fig. 3B). RNA pull-down assays also proved the binding between HOXD-AS1 and METTL3 (Fig. 3C). In addition, METTL3 overexpression prolonged the half-life of HOXD-AS1 (Fig. 3D) in Actinomycin D-treated trophoblast cells. These results indicated the binding between HOXD-AS1 and METTL3, and the promotion of HOXD-AS1 stability by METTL3.
Fig. 3.
HOXD-AS1 is regulated by METTL3.
A The expression of HOXD-AS1 detected by qRT-PCR. B The level of HOXD-AS1 binding on METTL3 showed by RIP- QPCR assay. C The combination between HOXD-AS1 and METTL3 verified by RNA pull-down. D The half-life of HOXD-AS1. pcDNA4.0, the control of METTL3 overexpression group; pcDNA4.0-METTL3, METTL3 overexpression group; shNC, the negative control of METTL3 knockdown group; shMETTL3 #1/2, METTL3 knockdown via 2 different fragments. ns, not significant; ***p < 0.001, vs. pcDNA4.0 group; ###p < 0.001, vs. shNC group.
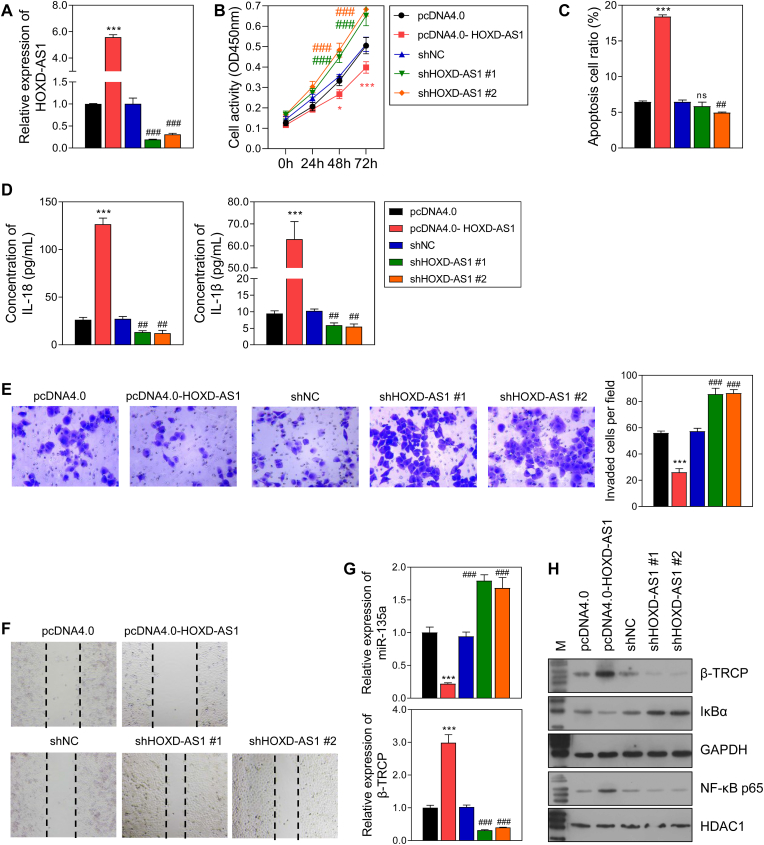
3.4. HOXD-AS1 regulated the inflammatory response and cellular behavior in PE
To verify the function of HOXD-AS1 in trophoblast cells, HOXD-AS1 was overexpressed or knocked-down in HTR-8/SVneo cells (Fig. 4A). Cell activity, invasion, and migration were promoted, and apoptosis was inhibited by HOXD-AS1 overexpression, while HOXD-AS1 knockdown had the opposite effects (Fig. 4B, 4D-4F). Furthermore, the levels of IL-18, IL-1β, β-TRCP, and nuclear NF-κB p65 were up-regulated by HOXD-AS1 overexpression, and downregulated by HOXD-AS1 knockdown, while the levels of miR-135a and IκBα showed the reverse trends in expression (Fig. 4C, G, 4H).
Fig. 4.
The effect of HOXD-AS1 overexpression and knockdown on HTR-8/SVneo cells.
A The efficacy of HOXD-AS1 overexpression and knockdown in HTR-8/SVneo cells. B Cell activity detected by CCK-8 assay. C The level of 2 inflammatory factors in the supernatant of HTR-8/SVneo cells detected by ELISA. D Cell apoptosis detected by flow cytometry. E Cell invasion. F Cell migration. G The mRNA level of miR-135a and β-TRCP. H The protein level of β-TRCP and NF-κB pathway. pcDNA4.0, the control of HOXD-AS1 overexpression group; pcDNA4.0-HOXD-AS1, HOXD-AS1 overexpression group; shNC, the control of HOXD-AS1 knockdown group; shHOXD-AS1 #1/2, HOXD-AS1 knockdown via 2 different fragments. ns, not significant; ***p < 0.001, vs. pcDNA4.0 group; ###p < 0.001, vs. shNC group.
3.5. MiR-135a/β-TRCP affected the behavior of trophoblast cells via NK-κB/NLRP3
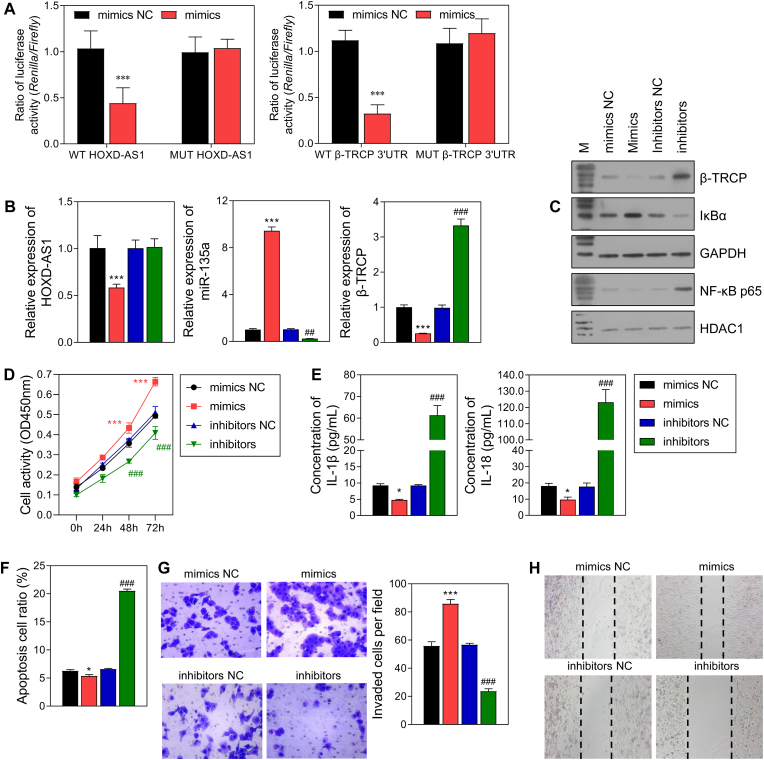
Predictions made by the TargetScanHuman 7.0 and StarBase v2.0 database, and our data obtained from the dual luciferase reporter gene assay indicated that HOXD-AS1 serves as a sponge for miR-135a, and miR-135a can bind to the β-TRCP 3′UTR (Fig. 5A). Furthermore, when miR-135a mimics and inhibitors were used to transfect HTR-8/SVneo cells. miR-135a expression was up-regulated by the miR-135a mimics and down-regulated by the miR-135a inhibitors. Meanwhile, the expression levels of HOXD-AS1, β-TRCP, IL-18, IL-1β, and NF-κB p65 showed the reverse trend, and IκBα had a trend similar to that of miR-135a expression (Fig. 5B, C, 5E). In addition, miR-135a mimics promoted cell proliferation, invasion, and migration, and suppressed cell apoptosis, while the miR-135a inhibitors had the opposite effects (Fig. 5D, 5F–5H).
Fig. 5.
The effect of miR-135a mimics and inhibitors on HTR-8/SVneo cells.
A The banding between HOXD-AS1 and miR-135a, miR-135a and β-TRCP 3′UTR was evaluated by dual luciferase reporter assay. B The effect of miR-135a mimics and inhibitors on the RNA level of HOXD-AS1, miR-135a and β-TRCP in HTR-8/SVneo cells. C The protein level of β-TRCP and NF-κB pathway. D Cell activity. E The level of 2 inflammatory factors in the supernatant of HTR-8/SVneo cells. F Cell apoptosis. G Cell invasion. H Cell migration. NC: nagtive control. *p < 0.05, ***p < 0.001, vs. mimics NC group; ##p < 0.01, ###p < 0.001, vs. inhibitor NC group.
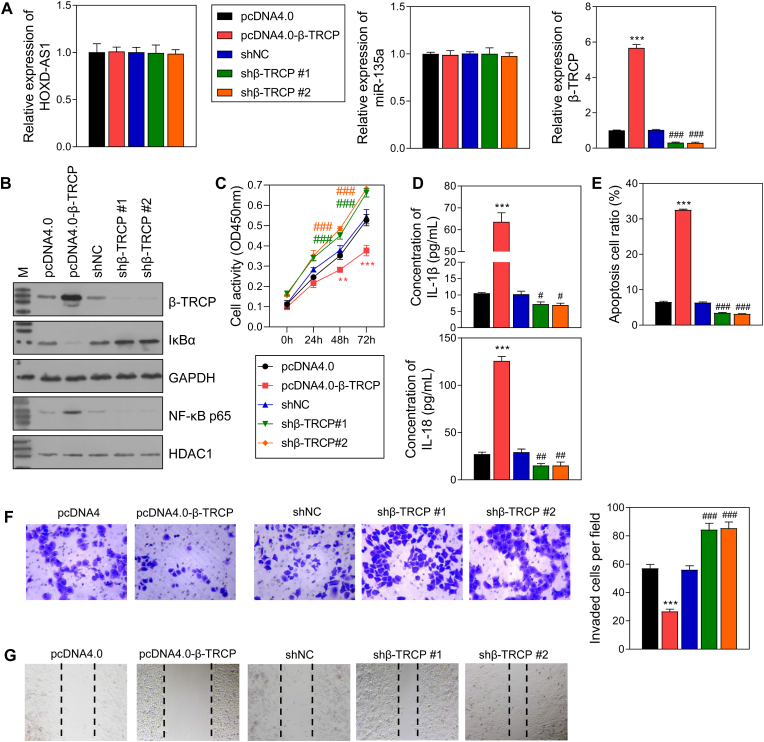
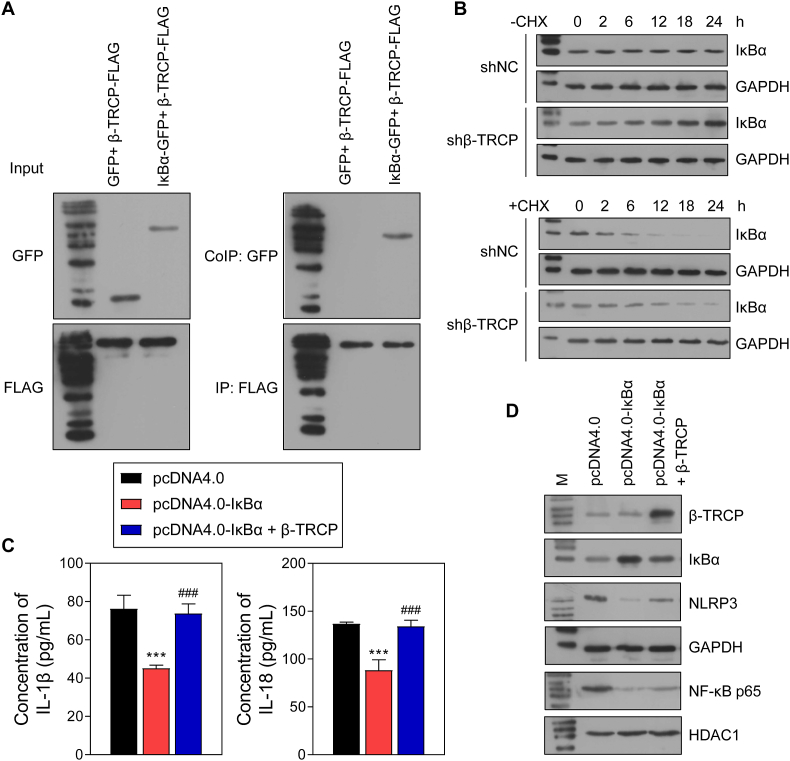
β-TRCP overexpression and knockdown experiments were also performed with HTR-8/SVneo cells, and the successful overexpression and knockdown were verified (Fig. 6A and B). However, the levels of HOXD-AS1 and miR-135a were not affected by either β-TRCP overexpression or knockdown (Fig. 6A). In addition, IκBα expression was lower in the pcDNA4.0-β-TRCP group when compared that in the pcDNA4.0 group, and the levels of nuclear NF-κB p65, IL-18, and IL-1β expression showed the reverse changes (Fig. 6 B, 6D). Of note, cell activity, invasion, and migration were inhibited by β-TRCP overexpression and increased by β-TRCP knockdown (Fig. 6C, F, 6G), while β-TRCP overexpression produced the opposite effects on cell apoptosis (Fig. 6E). Moreover, Co-IP assays verified the binding between IκBα and β-TRCP (Fig. 7A). In cells treated with CHX, the reduced levels of IκBα protein in the sh-β-TRCP group were lower than those in the shNC group (Fig. 7B). In cells treated with LPS, IκBα overexpression downregulated the levels of NLRP3, nuclear NK-κB p65, IL-18, and IL-1β. However, β-TRCP overexpression neutralized the effects of IκBα overexpression on the levels of NLRP3, nuclear NK-κB p65, IL-18, and IL-1β (Fig. 7C and D).
Fig. 6.
The effect of β-TRCP overexpression and knockdown on HTR-8/SVneo cells.
A The RNA level of HOXD-AS1, miR-135a and β-TRCP in HTR-8/SVneo cells. B The protein level of β-TRCP and NF-κB pathway. C Cell activity. D The level of 2 inflammatory factors in the supernatant of HTR-8/SVneo cells. E Cell apoptosis. F Cell invasion. G Cell migration. pcDNA4.0, the control of β-TRCP overexpression group; pcDNA4.0-β-TRCP, β-TRCP overexpression group; shNC, the control of β-TRCP knockdown group; shβ-TRCP #1/2, β-TRCP knockdown via 2 different fragments. **p < 0.01, ***p < 0.001, vs.pcDNA4.0 group; #p < 0.05, ##p < 0.01, ###p < 0.001, vs. shNC group.
Fig. 7.
Interaction between β-TRCP and IκBα activates NF-κB pathway.
A Interaction between β-TRCP and IκBα was verified by bidirectional Co-IP assay. B The HTR-8/SVneo cells were treated with Cycloheximide (CHX) after β-TRCP knockdown and the protein of IκBα was measured by WB. C Co-overexpression of β-TRCP and IκBα was performed in HTR-8/SVneo cells and the cells was subsequently stimulated with lipopolysaccharide (LPS). The level of IL-18 and IL-1β in the supernatant was detected by ELISA assay. D The protein level of β-TRCP, IκBα, NF-κB p65 and NLRP3. ***p < 0.001, vs. pcDNA4.0 group; ###p < 0.001, vs. pcDNA4.0- IκBα group.
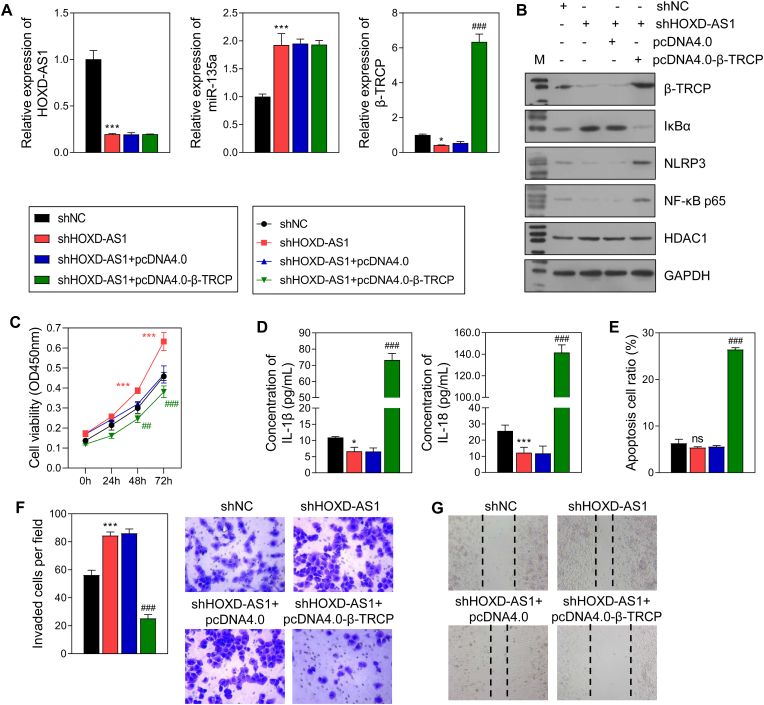
3.6. Overexpression of β-TRCP attenuated the effect of HOXD-AS1 knockdown in trophoblast cells
When β-TRCP was overexpressed in HTR-8/SVneo cells transfected with shHOXD-AS1, we found that the levels of HOXD-AS1 and miR-135a expression remained unchanged. However, the decreases/increases in β-TRCP, IκBα, NLRP3, NK-κB p65, IL-18, and IL-1β levels caused by shHOXD-AS1 were reversed by β-TRCP overexpression (Fig. 8A, B, 8D). Furthermore, the changes in cell activity, invasion, migration, and apoptosis induced by shHOXD-AS1 were also reversed by β-TRCP overexpression (Fig. 8C, 8E-8G).
Fig. 8.
The effect of β-TRCP overexpression in HTR-8/SVneo cells with shHOXD-AS1.
β-TRCP overexpression and HOXD-AS1 knockdown was performed in HTR-8/SVneo cells. A The RNA level of HOXD-AS1, miR-135a and β-TRCP in HTR-8/SVneo cells. B The protein level of β-TRCP, IκBα, NF-κB p65, and NLRP3. C Cell activity deteced by CCK8 assay. D The level of IL-18 and IL-1β in the supernatant of HTR-8/SVneo cells. E Cell apoptosis measured by flow cytometry. F Cell invasion. G Cell migration. ns, not significant; *p < 0.05, ***p < 0.001, vs. shNC group; ###p < 0.001, vs. sh HOXD-AS1 + pcDNA4.0 group.
4. Discussion
Insufficient invasion of trophoblast cells during pregnancy is a major cause of PE. HOXD-AS1 has been reported to inhibit the invasion of various tumor cells. In this study, we demonstrated that methylation of HOXD-AS1 was abnormal in PE, and may regulate the inflammation, invasion, migration, and apoptosis of trophoblast cells by influencing miR-135a/β-TRCP.
m6A modification is dysregulated and plays a vital role in the placentas of PE patients, and might be related to endoplasmic reticulum stress (ERS). Chen et al. [2] and Gu et al. [25] revealed that the levels of m6A-modified RNA and METTL3 (a writer of m6A methylation), are elevated in PE placentas, which is consistent with our results. Furthermore, m6A modification is also related to trophoblast invasion. The dysregulation of Wilms' tumor 1-associating protein (WTAP), another writer of m6A methylation, has been found to inhibit trophoblast cell invasion in early-onset PE [26]. Moreover, m6A modification affects cell apoptosis. Silencing of alpha-ketoglutarate-dependent homolog 5 (ALKBH5) inhibits cell apoptosis and oxidative stress, and increases cell proliferation and invasion in HTR-8/SVneo cells [27]. In the current study, we found that the levels of METTL3 and m6A modification were up-regulated in placentas from PE patients and METTL3 inhibits cell invasion and migration, increases cell apoptosis, and induces the inflammatory reactions of trophoblast cells via m6A methylation of HOXD-AS1. However, Zhao et al. [28]. showed that METTL3 expression was downregulated in PE placentas when compared with normal placentas, and the downregulation induced trophoblast cell invasion. After comparing those findings with the aforementioned report by Bian et al. [26], we speculated that the levels of m6A and METTL3 expression may be associated with the period of PE.
HOXD-AS1 is significantly up-regulated in bladder cancer, cervical cancer, colorectal cancer, gastric cancer, glioma, hepatocellular carcinoma, melanoma, osteosarcoma, ovarian cancer, and prostate cancer. A previous study reported that HOXD-AS1 serves as a ceRNA for miRNAs to regulate gene expression, and then further maintains the proliferation and migration of bladder cancer cells, hepatocellular carcinoma cells, and osteosarcoma cells [29]. In addition, HOXD-AS1 promotes an inflammatory response and apoptosis by activating the NLRP3 inflammasome in lipopolysaccharide-treated SH-SY5Y cells (human neuron blastoma cell line) [30]. Cancer cells and trophoblast cells have similar behavioral characteristics with regards to migration and invasion. HOXD-AS1 was also up-regulated in PE placentas when compared to normal placentas. Knockdown of HOXD-AS1 was found to increase cell proliferation via the MAPK pathway in trophoblast cells [15]. Consistent with these results, our study found that HOXD-AS1 is up-regulated was PE placentas. HOXD-AS1 downregulation suppressed cell apoptosis and promoted cell migration and invasion, and the secretion of inflammatory factors. Of note, our results suggest that HOXD-AS1 affects trophoblast cell behaviors via the miR-135a/β-TRCP axis, and HOXD-AS1 expression is regulated by m6A methylation with METTL3. Therefore, HOXD-AS1 appears to regulate cell behavior in different ways in cancer cells vs. trophoblast cells.
MiR-135a, a member of miR-135 family, is dysregulated in various diseases, and especially in cancers. It acts either as a tumor suppressor or an oncogene to regulate tumor cell behavior via different pathways. Most reports indicate that miR-135a inhibits tumor cell proliferation and cancer progression. In thyroid carcinoma cells, miR-135a promotes G0/G1 arrest and suppresses the malignant characteristics of tumor cells by targeting Veersican. A similar inhibitory effect of miR-135a was revealed in pancreatic cancer, gallbladder cancer, and ovarian cancer by conducting an analysis of different downstream pathways. However, miR-135a acts as a tumor promoter by up-regulating oncogene expression, downregulating cancer suppresser gene expression, or serving as a target of oncoproteins in other cancers [31]. MiR-135-5p expression has also been shown to be up-regulated in cardio-cerebral ischemic disease, and thereby inhibit ischemia-reperfusion-related injuries. In the context of unexplained spontaneous abortion, miR-135a-5p is up-regulated and inhibits trophoblast proliferation, migration, invasion, and angiogenic activity [32]. Petracco et al. [33] found that endometrial miR-135a expression is up-regulated in endometriosis patients, and miR-135a suppresses the expression of homeo box A 10, which is an implantation-related gene. In addition, Zhao et al. [30] revealed that miR-135 overexpression increases cell invasiveness and restricts NLRP3 inflammasome activity, thereby relieving inflammation in placenta tissues caused by PE. Our previous study showed that miR-135a-5p induces the invasiveness of trophoblast cells by directly controlling β-TRCP expression [19]. In in the present study, we also found that miR-135a-5p inhibits trophoblast cell apoptosis, IL-18 and IL-1β secretion, and more notably, miR-135a-5p is modulated by its interaction with HOXD-AS1.
β-TRCP has various substrates that regulate inflammation (IκBs), cell differentiation-related genes (β-catenin, Snail), DNA damage responders (Cdc25A, Claspin), and the cell cycle (Emi2, Wee1) [34]. β-TRCP is necessary for NF-κB activation. A previous study reported that β-TRCP recognizes the phosphorylated IκB degron, and thereby couples phosphorylation to ubiquitination, resulting in IκB ubiquitination and degradation within a few minutes, while ablation of β-TRCP leads to increases the levels of IκBs and induces NF-κB inhibition [34]. Some factors have been found to regulate the NF-κB pathway via β-TRCP. Human rotaviruses induce β-TRCP degradation by interacting with β-TRCP, and thus inhibit NF-κB activation [35]. Moreover, histidine triad nucleotide binding protein 1 stabilizes IκBα by targeting β-TRCP [36]. Our results suggest that HOXD-AS1 knockdown increases IκBα expression to inhibit the NF-κB pathway, and β-TRCP overexpression recovers that pathway. Therefore, the NF-κB pathway is regulated by HOXD-AS1 via β-TRCP, and HOXD-AS1 acts as a sponge that competitively binds with miR-135a to regulate β-TRCP RNA translation in trophoblast cells. In addition, our previous study [37] found that β-TRCP restricts trophoblast cell invasiveness by decreasing Snail in PE. These studies indicate that PE is regulated by a complex regulatory system.
The NLRP3 inflammasome is an important mediator of sterile inflammation; it participates in the pathogenesis of placental inflammation, and especially in PE. The levels of NLRP3 are significantly higher in placental tissues and blood mono-nuclear cells from PE patients when compared samples from healthy pregnant women [38]. The NLRP3 inflammasome exacerbates PE by inducing hypertension. NLRP3 deficiency relieves the development of Angiotensin II-induced hypertension [39]. DAMPs, including cholesterol, free fatty acids, uric acid crystals, and high-mobility group box 1 released from various cells under stress, all induce inflammation via the NLRP3 inflammasome pathway in placentas, and the inflammation can result in PE [40]. These findings indicate that hyperactivation of the NLRP3 inflammasome contributes to PE. The NLRP3 inflammasome is also associated with preterm birth [41] and gestational diabetes mellitus [42]. Our present study showed that NLRP3 levels were up-regulated when NF-κB was activated by the METTL3/HOXD-AS1/miR-135a/β-TRCP axis. NLRP3 has been also been reported to be directly regulated by METTL3. In trophoblast cells, lnc-HZ14 induces pyroptosis by up-regulating METTL3 to increase m6A-methylated NLRP3 mRNA levels and its stability [43]. Therefore, the inflammatory response of trophoblast cells regulates METTL3 not only via the HOXD-AS1/miR-135a/β-TRCP axis, but also by direct methylation of NLRP3. However, the trophoblast cell line HTR-8/Svneo cannot completely represent the cellular model of PE, which may be a limitation of the study. Furthermore, the demethylation and methylation recognition of HOXD-AS1 still needs more experimental exploration. To further validate the function of HOXD-AS1 in PE, a intervention based on animal models and a single cell sequencing with tissue samples may be needed.
5. Conclusion
We found that HOXD-AS1 expression was elevated in PE, and the elevation was perhaps caused by METTL3-mediated m6A methylation. The METTL3/HOXD-AS1/miR-135a/β-TRCP axis was found to regulate the invasion, migration, apoptosis, and inflammation of trophoblast cells via the NF-κB signaling pathway. Therefore, that axis may play an important role in the pathogenesis of PE, and could serve as a potential target for PE therapy.
Funding
This research is granted by Hainan Province Science and Technology Special Fund (ZDYF2022SHFZ120) and Hainan Health Scientific Research Project (No.21A200248).
Disclosure
All authors declare that there are no competing interests.
CRediT authorship contribution statement
Ling Wang: Data curation, Formal analysis, Investigation, Methodology. Li Shi: Data curation, Formal analysis, Investigation, Supervision, Validation. Bo Zhou: Resources, Software, Validation, Visualization. Lan Hong: Project administration, Resources, Software, Supervision, Validation, Visualization. Humin Gong: Conceptualization, Project administration, Supervision, Writing – original draft. Dongcai Wu: Conceptualization, Funding acquisition, Project administration, Validation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
None of the authors are members of the editorial board/editor-in-chief of Non-coding RNA Research.
All authors declare that there are no competing interests.
References
- 1.Ives C.W., Sinkey R., Rajapreyar I., Tita A.T.N., Oparil S. Preeclampsia-pathophysiology and clinical presentations: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;76:1690–1702. doi: 10.1016/j.jacc.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y., Liu X., Li L., He X., Zheng F., Zhang Y., Gao H., Jin Z., Wu D., Wang Q., Tao H., Zhao Y., Liu W., Zou L. Methyltransferase-like 3 aggravates endoplasmic reticulum stress in preeclampsia by targeting TMBIM6 in YTHDF2-dependent manner. Mol. Med. 2023;29:19. doi: 10.1186/s10020-023-00604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitley G.S., Dash P.R., Ayling L.J., Prefumo F., Thilaganathan B., Cartwright J.E. Increased apoptosis in first trimester extravillous trophoblasts from pregnancies at higher risk of developing preeclampsia. Am. J. Pathol. 2007;170:1903–1909. doi: 10.2353/ajpath.2007.070006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H., Meng T., Liu X., Sun M., Tong C., Liu J., Wang H., Du J. Long non-coding RNA MALAT-1 is downregulated in preeclampsia and regulates proliferation, apoptosis, migration and invasion of JEG-3 trophoblast cells. Int. J. Clin. Exp. Pathol. 2015;8:12718–12727. [PMC free article] [PubMed] [Google Scholar]
- 5.Redman C.W., Sargent I.L. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y., Li B., Zhao Y. Inflammation in preeclampsia: genetic biomarkers, mechanisms, and therapeutic strategies. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.883404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monteiro L.J., Peñailillo R., Sánchez M., Acuña-Gallardo S., Mönckeberg M., Ong J., Choolani M., Illanes S.E., Nardocci G. The role of long non-coding RNAs in trophoblast regulation in preeclampsia and intrauterine growth restriction. Genes. 2021;12 doi: 10.3390/genes12070970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou G., Fichorova R.N., Holzman C., Chen B., Chang C., Kasten E.P., Hoffmann H.M. Placental circadian lincRNAs and spontaneous preterm birth. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.1051396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q., Jiang S., Liu H., Gao Y., Yang X., Ren Z., Gao Y., Xiao L., Hu H., Yu Y., Yang X., Zhong M. Association of lncRNA SH3PXD2A-AS1 with preeclampsia and its function in invasion and migration of placental trophoblast cells. Cell Death Dis. 2020;11:583. doi: 10.1038/s41419-020-02796-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J., Xia Y., Zhang H., Guo H., Feng K., Zhang C. Overexpression of long non-coding RNA H19 promotes invasion and autophagy via the PI3K/AKT/mTOR pathways in trophoblast cells. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2018;101:691–697. doi: 10.1016/j.biopha.2018.02.134. [DOI] [PubMed] [Google Scholar]
- 11.Ji W., Wang Q., Yang J. LncRNA HOXD-AS1 promotes the metastasis of human hepatocellular carcinoma via modulating miR-326/SLC27A4. Cancer Cell Int. 2020;20:161. doi: 10.1186/s12935-020-01217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng L., Chen J., Zhou Z., He Z. Knockdown of long non-coding RNA HOXD-AS1 inhibits gastric cancer cell growth via inactivating the JAK2/STAT3 pathway. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2017;39 doi: 10.1177/1010428317705335. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y., Dun Y., Zhou S., Huang X.H. LncRNA HOXD-AS1 promotes epithelial ovarian cancer cells proliferation and invasion by targeting miR-133a-3p and activating Wnt/β-catenin signaling pathway. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2017;96:1216–1221. doi: 10.1016/j.biopha.2017.11.096. [DOI] [PubMed] [Google Scholar]
- 14.Founds S.A., P C.Y., F L.-W.j, A J., A H.W., Conrad K.P. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009;30:15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang J., Zhao Z.M. LncRNA HOXD-AS1 promotes preeclampsia progression via MAPK pathway. Eur. Rev. Med. Pharmacol. Sci. 2018;22:8561–8568. doi: 10.26355/eurrev_201812_16618. [DOI] [PubMed] [Google Scholar]
- 16.Ferretti C., Bruni L., Dangles-Marie V., Pecking A.P., Bellet D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum. Reprod. Update. 2007;13:121–141. doi: 10.1093/humupd/dml048. [DOI] [PubMed] [Google Scholar]
- 17.Zheng N., Zhou Q., Wang Z., Wei W. Recent advances in SCF ubiquitin ligase complex: clinical implications. Biochim. Biophys. Acta. 2016;1866:12–22. doi: 10.1016/j.bbcan.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatakeyama S., Kitagawa M., Nakayama K., Shirane M., Matsumoto M., Hattori K., Higashi H., Nakano H., Okumura K., Onoé K., Good R.A., Nakayama K. Ubiquitin-dependent degradation of IkappaBalpha is mediated by a ubiquitin ligase Skp1/Cul 1/F-box protein FWD1. Proc. Natl. Acad. Sci. U.S.A. 1999;96:3859–3863. doi: 10.1073/pnas.96.7.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu D., Shi L., Hong L., Chen X., Cen H. MiR-135a-5p promotes the migration and invasion of trophoblast cells in preeclampsia by targeting β-TrCP. Placenta. 2020;99:63–69. doi: 10.1016/j.placenta.2020.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Michalczyk M., Celewicz A., Celewicz M., Woźniakowska-Gondek P., Rzepka R. The role of inflammation in the pathogenesis of preeclampsia. Mediat. Inflamm. 2020;2020 doi: 10.1155/2020/3864941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S., Lee K.S., Choi S., Kim J., Lee D.K., Park M., Park W., Kim T.H., Hwang J.Y., Won M.H., Lee H., Ryoo S., Ha K.S., Kwon Y.G., Kim Y.M. NF-κB-responsive miRNA-31-5p elicits endothelial dysfunction associated with preeclampsia via down-regulation of endothelial nitric-oxide synthase. J. Biol. Chem. 2018;293:18989–19000. doi: 10.1074/jbc.RA118.005197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matias M.L., Romao-Veiga M., Ribeiro V.R., Nunes P.R., Gomes V.J., Devides A.C., Borges V.T., Romagnoli G.G., Peracoli J.C., Peracoli M.T. Progesterone and vitamin D downregulate the activation of the NLRP1/NLRP3 inflammasomes and TLR4-MyD88-NF-κB pathway in monocytes from pregnant women with preeclampsia. J. Reprod. Immunol. 2021;144 doi: 10.1016/j.jri.2021.103286. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Liu W., Zhong Y., Li Q., Wu M., Yang L., Liu X., Zou L. Metformin corrects glucose metabolism reprogramming and NLRP3 inflammasome-induced pyroptosis via inhibiting the TLR4/NF-κB/PFKFB3 signaling in trophoblasts: implication for a potential therapy of preeclampsia. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/1806344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armistead B., Kadam L., Drewlo S., Kohan-Ghadr H.R. The role of NFκB in healthy and preeclamptic placenta: trophoblasts in the spotlight. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21051775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu Y., Chu X., Morgan J.A., Lewis D.F., Wang Y. Upregulation of METTL3 expression and m6A RNA methylation in placental trophoblasts in preeclampsia. Placenta. 2021;103 doi: 10.1016/j.placenta.2020.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Bian Y., Li J., Shen H., Li Y., Hou Y., Huang L., Song G., Qiao C. WTAP dysregulation-mediated HMGN3-m6A modification inhibited trophoblast invasion in early-onset preeclampsia. Faseb. J. : official publication of the Federation of American Societies for Experimental Biology. 2022;36 doi: 10.1096/fj.202200700RR. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y., Song W., Yang Y. Inhibition of ALKBH5-mediated m(6) A modification of PPARG mRNA alleviates H/R-induced oxidative stress and apoptosis in placenta trophoblast. Environ. Toxicol. 2022;37:910–924. doi: 10.1002/tox.23454. [DOI] [PubMed] [Google Scholar]
- 28.Zhao J., Ding H., Ding J., Shi X., He Y., Zhu H., Yuan H., Zhang T., Zhang J. The m(6)A methyltransferase METTL3 promotes trophoblast cell invasion by regulating MYLK expression. Placenta. 2022;129:1–6. doi: 10.1016/j.placenta.2022.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Li L., Wang Y., Zhang X., Huang Q., Diao Y., Yin H., Liu H. Long non-coding RNA HOXD-AS1 in cancer. Clinica chimica acta. international journal of clinical chemistry. 2018;487:197–201. doi: 10.1016/j.cca.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Zhao X., Zhang X., Wu Z., Mei J., Li L., Wang Y. Up-regulation of microRNA-135 or silencing of PCSK6 attenuates inflammatory response in preeclampsia by restricting NLRP3 inflammasome. Mol. Med. 2021;27:82. doi: 10.1186/s10020-021-00335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao Z., Qiu J., Yang G., Liu Y., Luo W., You L., Zheng L., Zhang T. MiR-135a biogenesis and regulation in malignancy: a new hope for cancer research and therapy. Cancer biology & medicine. 2020;17:569–582. doi: 10.20892/j.issn.2095-3941.2020.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Y., Zhang X., Li X., Deng L., Wei C., Yang D., Tan X., Pan W., Pang L. MiR-135a-5p suppresses trophoblast proliferative, migratory, invasive, and angiogenic activity in the context of unexplained spontaneous abortion. Reprod. Biol. Endocrinol. 2022;20:82. doi: 10.1186/s12958-022-00952-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petracco R., Grechukhina O., Popkhadze S., Massasa E., Zhou Y., Taylor H.S. MicroRNA 135 regulates HOXA10 expression in endometriosis. J. Clin. Endocrinol. Metabol. 2011;96:E1925–E1933. doi: 10.1210/jc.2011-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanarek N., Ben-Neriah Y. Regulation of NF-κB by ubiquitination and degradation of the IκBs. Immunol. Rev. 2012;246:77–94. doi: 10.1111/j.1600-065X.2012.01098.x. [DOI] [PubMed] [Google Scholar]
- 35.Di Fiore I.J., Pane J.A., Holloway G., Coulson B.S. NSP1 of human rotaviruses commonly inhibits NF-κB signalling by inducing β-TrCP degradation. J. Gen. Virol. 2015;96:1768–1776. doi: 10.1099/vir.0.000093. [DOI] [PubMed] [Google Scholar]
- 36.Shi Z., Wu X., Ke Y., Wang L. Hint1 up-regulates IκBα by targeting the β-TrCP subunit of SCF E3 ligase in human hepatocellular carcinoma cells. Dig. Dis. Sci. 2016;61:785–794. doi: 10.1007/s10620-015-3927-y. [DOI] [PubMed] [Google Scholar]
- 37.Wu D., Shi L., Chen X., Cen H., Mao D. β-TrCP suppresses the migration and invasion of trophoblast cells in preeclampsia by down-regulating Snail. Exp. Cell Res. 2020;395 doi: 10.1016/j.yexcr.2020.112230. [DOI] [PubMed] [Google Scholar]
- 38.Weel I.C, Romão-Veiga M., Matias M.L., Fioratti E.G., Peraçoli J.C., Borges V.T., Araujo J.P., Jr., Peraçoli M.T. Increased expression of NLRP3 inflammasome in placentas from pregnant women with severe preeclampsia. J. Reprod. Immunol. 2017;123:40–47. doi: 10.1016/j.jri.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Shirasuna K., Karasawa T., Usui F., Kobayashi M., Komada T., Kimura H., Kawashima A., Ohkuchi A., Taniguchi S., Takahashi M. NLRP3 deficiency improves Angiotensin II-induced hypertension but not fetal growth restriction during pregnancy. Endocrinology. 2015;156:4281–4292. doi: 10.1210/en.2015-1408. [DOI] [PubMed] [Google Scholar]
- 40.Shirasuna K., Karasawa T., Takahashi M. Role of the NLRP3 inflammasome in preeclampsia. Front. Endocrinol. 2020;11:80. doi: 10.3389/fendo.2020.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faro J., Romero R., Schwenkel G., Garcia-Flores V., Arenas-Hernandez M., Leng Y., Xu Y., Miller D., Hassan S.S., Gomez-Lopez N. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasome. Biol. Reprod. 2019;100:1290–1305. doi: 10.1093/biolre/ioy261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lappas M. Activation of inflammasomes in adipose tissue of women with gestational diabetes. Mol. Cell. Endocrinol. 2014;382:74–83. doi: 10.1016/j.mce.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Wang R., Xu X., Yang J., Chen W., Zhao J., Wang M., Zhang Y., Yang Y., Huang W., Zhang H. BPDE exposure promotes trophoblast cell pyroptosis and induces miscarriage by up-regulating lnc-HZ14/ZBP1/NLRP3 axis. J. Hazard Mater. 2023;455 doi: 10.1016/j.jhazmat.2023.131543. [DOI] [PubMed] [Google Scholar]