Abstract
RpoS, the stationary-phase sigma factor of Escherichia coli, is responsible for increased transcription of an array of genes when cells enter stationary phase and under certain stress conditions. RpoS is rapidly degraded during exponential phase and much more slowly during stationary phase; the resulting changes in RpoS accumulation play an important role in providing differential expression of RpoS-dependent gene expression. It has previously been shown that rapid degradation of RpoS during exponential growth depends on RssB (also called SprE and MviA), a protein with homology to the family of response regulators, and on the ClpXP protease. We find that RssB regulation of proteolysis does not extend to another ClpXP substrate, bacteriophage lambda O protein, suggesting that RssB acts on the specific substrate RpoS rather than on the protease. In addition, the activity of RpoS is down-regulated by RssB when degradation is blocked. In cells blocked for RpoS degradation by a mutation in clpP, cells devoid of RssB show a four- to fivefold-higher activity of an RpoS-dependent reporter fusion than cells overproducing RssB. Therefore, RssB allows specific environmental regulation of RpoS accumulation and may also modulate activity. The regulation of degradation provides an irreversible switch, while the regulation of activity may provide a second, presumably reversible level of control.
Proteolysis of specific proteins under particular conditions provides an important mechanism for regulation of gene expression in all organisms (7, 19). An important component of understanding the role of proteolysis in regulatory cascades is understanding how particular substrates are selected, and in particular, how they are selected under certain environmental conditions and not others. In principle, regulated degradation could reflect regulation of protease synthesis or activity or regulation of substrate availability/susceptibility. For instance, the caspases (or ICE proteases) implicated in eukaryotic cell death appear to be activated (by a protein cleavage) at an early step in the commitment to cell death (6). In prokaryotes, Bacillus subtilis proteases involved in activation of developmental sigma factors are made as part of a developmental cycle and therefore appear and become active only under appropriate conditions (15). A number of bacteriophages inactivate cellular proteases during infection. Bacteriophage T4 synthesizes an inhibitor of the Lon protease, PinA (13, 30, 31), and lambda RexB stabilizes lambda O protein, protecting it from degradation, probably by a general inactivation of Clp proteases (5, 27). Lambda cIII appears to inhibit the FtsH (HflB) protease, leading to stabilization of both lambda cII and the heat shock sigma factor RpoH (11). The most general case for selective modification of substrates for degradation is the use, in eukaryotic cells, of ubiquitination to mark proteins destined for rapid turnover (12, 14). For instance, degradation of cyclins at given points in the cell cycle is due to regulated ubiquitination; presumably, cell cycle signals are fed through the ubiquitination machinery in ways that are still unclear (24). No posttranslational tagging mechanism for degradation equivalent to ubiquitination in prokaryotes has been described; the only known tagging system is a cotranslational mechanism for degrading certain protein fragments (17).
One of the most striking examples of regulated proteolysis in Escherichia coli is degradation of the stationary-phase sigma factor RpoS. RpoS is responsible for the transcription of a variety of genes expressed after cells enter stationary phase and during some sorts of starvation and stress (10). The promoter recognition for the holoenzyme containing RpoS is similar to that for the holoenzyme containing the major sigma factor of E. coli, RpoD, and some promoters can be transcribed by both holoenzymes (34). Unlike RpoD-dependent promoters, however, the activity of RpoS-dependent promoters appears to be regulated in large part by changes in the accumulation of the RpoS protein, a result of changes in rpoS transcription, translation, and, most dramatically, degradation (18, 33). Under exponential growth conditions at 37°C, RpoS is quite unstable, with a half-life of less than 2 min. However, when cells enter stationary phase or under some stress conditions, RpoS becomes stable, with a half-life of greater than 30 min. The protease responsible for the rapid in vivo degradation of RpoS has been shown to be the cytoplasmic ATP-dependent ClpXP protease (29).
Recently, a protein necessary for this rapid degradation of RpoS and an excellent candidate to mediate environmental signalling has been identified, named by various groups RssB or SprE (in E. coli), and MviA (in Salmonella typhimurium) (2, 23, 25). Null mutations in the rssB gene lead to RpoS stabilization; overproduction of the protein leads to rapid degradation of RpoS even in stationary phase (23, 25). The RssB N terminus has homology to the family of response regulators, and therefore its activity would be predicted to be subject to phosphorylation, as these response regulators generally are. Regulation by RssB of RpoS accumulation is dependent upon ClpXP; RpoS accumulates in clpX and clpP mutants even when RssB is overproduced (25). We began the work described here to ask if RssB-mediated regulation of RpoS degradation reflected regulation of the protease or of the substrate. We find that RssB acts in a substrate-specific fashion and modulates RpoS activity as well as its degradation.
MATERIALS AND METHODS
Bacterial strains.
For the protein turnover experiments, an isogenic set of derivatives of MG1655 (1) was constructed by P1 transduction, carrying clpP::Cat (20) or rssB::Tet (23) insertion mutations. Strains were lysogenized with λcI857 and, in some cases, transformed with pUM-E, which overproduces RssB (3) (received from T. Silhavy and L. Pratt). Note that pUM-E also encodes a number of other proteins, including RssA (unknown function, gene in operon with the rssB gene) and Tgs (transient glycine starvation) (3, 23). Strains for assaying RpoS activity were derived by P1 transduction or transformation with the pUM-E plasmid from DDS1340, and all contain the dsrB::lacZ fusion (32).
Protein turnover experiments.
Cells carrying the heat-inducible λcI857 prophage were grown in Luria-Bertani (LB) broth with 50 μg of ampicillin per ml at 30°C to an optical density at 600 nm (OD600) of 0.3 to 0.5 for logarithmic growth and to an OD600 of 2.0 for stationary-phase samples, transferred to 42°C for 8 min, and then transferred to 37°C and treated with 100 μg of spectinomycin per ml. Samples were removed at appropriate intervals and precipitated with 5% trichloroacetic acid in the cold. Precipitated pellets were washed with 80% acetone and resuspended in sodium dodecyl sulfate gel-loading buffer (Novex). Samples were normalized by optical density, electrophoresed on 12% sodium dodecyl sulfate gels, blotted to 0.2-μm-pore-size nitrocellulose, and probed with either anti-lambda O antibody (gift from R. McMacken) or anti-RpoS monoclonal antibody (gift from R. Burgess), and Western blots were developed with the ECL system (Amersham). Films were scanned with an Eagle Eye II scanner and normalized to the density of the band after 8 min of induction, and the half-life was estimated.
Quantitative Western blots.
To estimate the amounts of RpoS in strains, cell extracts were analyzed by Western blots, using anti-RpoS monoclonal antibody. Extracts were normalized for cell OD, and serial dilutions were used for gel electrophoresis as described above. Estimates of RpoS amounts were extrapolated from a series of sample dilutions that showed a linear response on the film after scanning with the Eagle Eye II scanner.
β-Galactosidase assays.
Strains were grown in M63 salts (21) with 0.2% glucose and vitamin B1 (0.0001%) at 32°C to an OD600 of approximately 0.45. One-tenth-milliliter samples of cells were permeabilized with 0.05 ml of permeabilization buffer (100 mM Tris [pH 7.8], 32 mM NaPO4, 8 mM dithiothreitol, 8 mM trans-1,2-diaminocyclohexane-N,N,N′,N′,tetraacetic acid, 4% Triton X-100, with 0.2 mg of polymyxin B per ml [28]) in microtiter plate wells for at least 10 min and assayed by adding 0.05 ml of o-nitrophenyl-β-d-galactopyranoside (ONPG) solution (4 mg of ONPG per ml in M63, 2 mM Na citrate) and measuring absorption at 420 nM as a function of time in a SpectraMax 250 spectrophotometer. Specific activity was calculated by dividing the slope of the line over time by the OD600 for the sample. In other experiments, we find that units of activity calculated in this manner are about 25-fold lower than Miller units.
RESULTS
RssB regulation of RpoS degradation is substrate specific.
Schweder and coworkers have shown that the protease ClpXP is present both in exponential phase and stationary phase, suggesting that regulation of the availability of the protease is unlikely (29). Therefore, to distinguish between protease-specific and substrate-specific effects of RssB, we investigated the effect of the switch from exponential to stationary phase and the effect of rssB mutants and RssB overproduction on the stability of another ClpXP substrate, lambda O protein. We have previously shown that lambda O protein is degraded with a half-life of 1 to 2 min in wild-type cells; the half-life increases to more than 40 min in cells carrying mutations in either clpX or clpP (8). In vitro, ClpXP is also able to degrade lambda O protein (35).
A set of isogenic strains was constructed, all carrying a λcI857 lysogen and a wild-type allele of rpoS, and varying in the presence or absence of mutations in rssB or clpP or of a plasmid overexpressing RssB. For each of these strains, cells were grown in LB broth at 30°C, the temperature was raised to 42°C for 8 min to induce lambda lytic growth and O protein synthesis and then lowered to 37°C, and spectinomycin was added to the culture to inhibit further protein synthesis. Culture samples were removed before induction, at the start of the spectinomycin treatment, and at various times after spectinomycin addition; extracts were compared on Western blots for the presence of RpoS and lambda O protein.
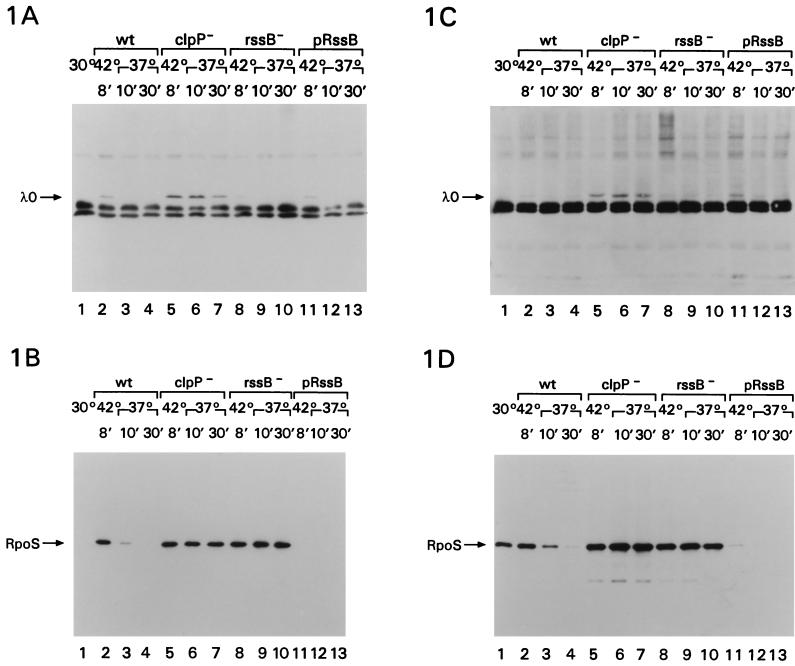
Figure 1A and B show the results of such an experiment, with induction of the cI857 prophage when cells are at an OD600 of 0.3 (during exponential growth). Figure 1C and D show a similar experiment, with induction of the cells at an OD600 of around 2.0 (during stationary phase). The half-lives for lambda O protein and RpoS under each of these conditions are summarized in Table 1. The band of lambda O protein is visible after induction of lambda in wild-type cells during logarithmic growth and disappears rapidly, as expected, in the presence of spectinomycin (Fig. 1A, lanes 1 to 4). Neither a mutation in rssB nor overproduction of RssB perturbed lambda O protein turnover in exponentially growing cells (Fig. 1A, lanes 8 to 10 and 11 to 13). However, as previously seen, lambda O protein was quite stable in cells with a mutation in clpP (Fig. 1A, lanes 5 to 7) (8). In the same induced cultures, RpoS showed the stability pattern previously seen (25); it was unstable during exponential phase (Fig. 1B, lanes 2 to 4) and stable in clpP (lanes 5 to 7) or rssB (lanes 8 to 10) mutants. We could not detect RpoS in cells overproducing RssB (Fig. 1B, lanes 11 to 13). Overproduction of RssB in cells with a mutation in clpP gave an easily detectable, stable band of RpoS (Fig. 2, lanes 10 to 12; see below), consistent with the observations of Pratt and Silhavy that clpP is epistatic to rssB (25) and supporting the idea that the absence of an RpoS band in cells overproducing RssB reflects accelerated RpoS degradation. We also noted an induction of RpoS after the 8-min heat shock, compared to growth at 30°C (Fig. 1B, compare lanes 1 and 2). This heat shock induction of RpoS has been reported previously and attributed to decreased turnover (16, 22). Our results would suggest that this decreased turnover is blocked by RssB overproduction (Fig. 1B, lanes 11 to 13).
FIG. 1.
Cells were grown to an OD600 of 0.3 to 0.5 for logarithmic growth (A and B) and to 2.0 for stationary-phase samples (C and D) in LB broth with 50 μg of ampicillin per ml at 30°C, transferred to 42°C for 8 min, and then transferred to 37°C and treated with 100 μg of spectinomycin per ml. Samples were removed before the heat treatment (lanes 1) for the wild-type strain, after 8 min at 42°C (lanes 2, 5, 8, and 11), after 10 min of chase with spectinomycin (lanes 3, 6, 9, and 12), and after 30 min of chase with spectinomycin (lanes 4, 7, 10, and 13) and treated as described in Materials and Methods. (A and C) Probed with anti-lambda O antibody; (B and D) probed with anti-RpoS monoclonal antibody. All strains were derivatives of MG1655; all carried a λcI857 prophage. In addition, they carried the following: YN186, wild-type (lanes 1 to 4); YN187 clpP1::Cat (lanes 5 to 7); YN188 rssB::Tet (23) (lanes 8 to 10); YN189, plasmid pUM-E, overproducing RssB (3) (lanes 11 to 13).
TABLE 1.
Effect of rssB on RpoS and lambda O protein degradation
| Strain | Genotype | Growth phase | Half-life (min)a
|
|
|---|---|---|---|---|
| λ O | RpoS | |||
| YN186 | clp+ rssB+ | Exponential | <2 | <2 |
| YN186 | clp+ rssB+ | Stationary | 3 | 7 |
| YN187 | clpP::Cat | Exponential | >30 | >30 |
| YN188 | rssB::Tet | Exponential | <2 | >30 |
| YN189 | prssB+/clp+ | Exponential | <2 | Not detectable |
Gels obtained from experiments shown in Fig. 1 were scanned with an Eagle Eye II scanner and normalized for protein after 8 min of induction, and the half-lives were estimated. While these are results of a single set of experiments, the general pattern was the same for other experiments. In another strain background (MC4100 [4]), we saw similar results for lambda O protein turnover, although RpoS was somewhat more stable during logarithmic growth in our MC4100 derivative.
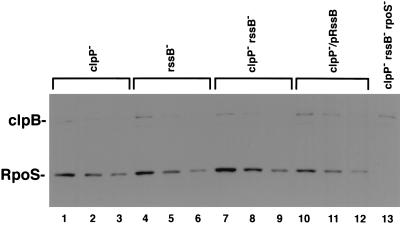
FIG. 2.
RpoS levels in the absence of ClpXP. Strains grown for assays (see Table 2) were sampled for RpoS levels. An equal amount of cell extract was loaded in the first lane for each strain (lanes 1, 4, 7, 10, and 13). The amounts of RpoS in the second and third lanes for each strain are twofold dilutions of the previous lane. Gels were processed as described in Materials and Methods.
During stationary phase, RpoS was somewhat more stable, as expected (Fig. 1D, lanes 2 to 4; Table 1). Overproduction of RssB again led to barely detectable RpoS even during stationary phase (Fig. 1D, lanes 11 to 13). In stationary-phase cells, it was more difficult to detect lambda O protein by Western blot unless the cells carried a clpP mutation (Fig. 1C). This is consistent with our previous observations that accumulation of lambda O protein after induction of a prophage is primarily regulated by the half-life of the protein (8) and with the observations that rssB mutations do not interfere with rapid lambda O protein degradation in exponentially growing cells. Presumably, the 8-min heat induction of the lambda prophage leads to less lambda protein synthesis in cells in stationary phase. To confirm that lambda O protein turnover was not perturbed by rssB during stationary phase, we carried out a parallel series of experiments with cells carrying a multicopy plasmid (pRLM71) (26) expressing lambda O protein from the pL promoter. In those cells, lambda O protein was degraded in stationary-phase cells with a half-life of 3 min, and turnover was not significantly changed in an rssB mutant or when RssB was overproduced (data not shown). Therefore, these results demonstrate that RssB and stationary phase change RpoS stability without perturbing degradation of another ClpXP substrate, suggesting that RssB acts on RpoS, not on the protease.
RssB regulates RpoS activity.
As seen above (Fig. 1B and D), RpoS accumulates to significant extents in a clpP mutant host, independent of the growth phase of the cells and therefore presumably regardless of the presence or absence of RssB. We confirmed this in a clpP mutant host by overproducing RssB; RpoS still accumulates (Fig. 2, lanes 10 to 12). This allowed us to ask if RssB modifies RpoS activity in the absence of degradation. RpoS activity was monitored with a dsrB::lac fusion that we have previously shown to be fully dependent on RpoS (32). As shown in Table 2 for an isogenic set of clpP::cat hosts, rssB mutant cells have four to fivefold-higher RpoS activities than cells overproducing RssB. Quantitative Western blots demonstrate that the amounts of RpoS protein do not change more than twofold between these strains (Fig. 2). Thus, no new proteolytic system attacks RpoS, but RssB can down-regulate RpoS activity, an effect which is not easy to assess when RpoS is rapidly degraded (in the presence of ClpXP).
TABLE 2.
RpoS activity modulated by RssB
| Straina | Genotype | β-Galactosidase (U)b |
|---|---|---|
| DDS1342 | clpP::Cat | 24 |
| DDS1343 | clpX::Kan | 27 |
| DDS1346 | rssB::Tet | 35 |
| YN236 | clpP::Cat rssB::Ttet | 39 |
| YN238 | clpP::Cat rssB::Tet rpoS::Kan | 1.7 |
| YN218 | clpP::Cat/prssB+ | 7.9 |
| YN216 | clpX::Kan/prssB+ | 7.5 |
Strains all carry the dsrB::lacZ fusion and are derived from DDS1340 (32).
Cells were grown in M63 medium with 0.2% glucose and vitamin B1 at 32°C to an OD600 of approximately 0.45 and assayed for β-galactosidase as described in Materials and Methods. The values (see Materials and Methods for units) remained relatively constant during exponential growth in these strains. In duplicate experiments, the relative values were reproducible. In experiments done under somewhat different growth conditions, wild-type strains have about one-half to one-third the activity of the clpP or clpX mutant host for the dsrB-lacZ fusion.
DISCUSSION
The experiments described here strongly suggest that RssB regulates RpoS degradation by a substrate-specific interaction. This interaction also interferes with RpoS activity when the ClpXP protease is not present. Given the similarity of RssB to response regulators, it seems likely that RssB may be sensitive to environmental signalling via reversible phosphorylation. Because increased RssB (made from the plasmid in our experiments) is sufficient to increase RssB activity even in stationary phase, either phosphorylation is not essential or it can be provided in the absence of the normal signals. If RssB acts directly on RpoS, it could in theory form a complex with RpoS, either modifying RpoS to allow degradation or the complex itself may render either RpoS itself or both RssB and RpoS subject to degradation.
Regions within RpoS necessary for rapid degradation by ClpXP have been identified, primarily by examining the behavior of RpoS-LacZ fusion proteins. Those fusions carrying the N terminus of RpoS up to amino acid 160 did not show evidence of degradation; those carrying up to amino acid 180 were degraded in a manner dependent upon growth phase, ClpXP, and therefore presumably RssB (23, 29). This region is just downstream of the region of sigma known to contact the −10 region of target promoters and differs at relatively few positions from the stable RpoD sigma factor. Whether this region includes recognition sequences for RssB and/or recognition regions for the ClpXP protease, only made accessible in the presence of RssB, remains to be seen. We would predict that the same changes or complex that allows degradation also interferes with RpoS activity. Possibly both reflect a decrease in either core or DNA binding; this region would be predicted to be involved in DNA binding. It is interesting that RpoH, the heat shock sigma factor of E. coli, is also subject to regulated degradation, that this degradation depends on a region of RpoH not far from the one implicated in RpoS degradation, that accessory factors (DnaJ, DnaK, and GrpE) are implicated in accessibility to degradation, and finally, that these factors also participate in regulating activity of RpoH (reviewed in reference 9).
The regulation of RpoS activity as well as degradation by RssB-dependent phosphorylation would not have been detectable without the ability to specifically block degradation independently by mutations in the protease. It is not yet clear whether this modulation of RpoS activity has a physiological role, since normally the protease will be available. However, it seems efficient to couple protein behavior and susceptibility to degradation in this fashion. The additional sensitivity to degradation provides both irreversibility and multiplication of the effect of inactivation on protein activity.
ACKNOWLEDGMENTS
We thank Leslie Pratt and Tom Silhavy for providing sprE mutants and the sprE plasmid and for helpful discussions, G. Storz for providing the rssB::Tet mutation, Nancy Thompson and Richard Burgess for the gift of the anti-sigmaS antibody, and members of the Laboratory of Molecular Biology for comments on the manuscript. We thank Darren Sledjeski for sharing his initial observations suggesting that rssB had effects on RpoS-dependent fusions beyond those attributable to clpP.
REFERENCES
- 1.Bachmann B J. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C: ASM Press; 1996. pp. 2460–2488. [Google Scholar]
- 2.Bearson S M D, Benjamin J W H, Swords W E, Foster J W. Acid shock induction of RpoS is mediated by the mouse virulence gene mviA of Salmonella typhimurium. J Bacteriol. 1996;178:2572–2579. doi: 10.1128/jb.178.9.2572-2579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosl M, Kersten H. Organization and functions of genes in the upstream region of tyrT of Escherichia coli: phenotypes of mutants with partial deletion of a new gene (tgs) J Bacteriol. 1994;176:221–231. doi: 10.1128/jb.176.1.221-231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casadaban M J, Cohen S N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci USA. 1979;76:4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelberg-Kulka, H., M. Reches, S. Narasimhan, R. Schoulaker-Schwarz, Y. Klemes, E. Aizenman, and G. Glaser. Unpublished results. [DOI] [PMC free article] [PubMed]
- 6.Fraser A, Evan G. A license to kill. Cell. 1996;85:781–784. doi: 10.1016/s0092-8674(00)81005-3. [DOI] [PubMed] [Google Scholar]
- 7.Gottesman S. Proteases and their targets in Escherichia coli. Annu Rev Genet. 1996;30:465–506. doi: 10.1146/annurev.genet.30.1.465. [DOI] [PubMed] [Google Scholar]
- 8.Gottesman S, Clark W P, de Crecy-Lagard V, Maurizi M R. ClpX, an alternative subunit for the ATP-dependent Clp protease of Escherichia coli. J Biol Chem. 1993;268:22618–22626. [PubMed] [Google Scholar]
- 9.Gross C. Function and regulation of the heat shock proteins. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 1382–1399. [Google Scholar]
- 10.Hengge-Aronis R. Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell. 1993;72:165–168. doi: 10.1016/0092-8674(93)90655-a. [DOI] [PubMed] [Google Scholar]
- 11.Herman C, Thévenet D, D’Ari R, Bouloc P. Degradation of ς32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc Natl Acad Sci USA. 1995;92:3516–3520. doi: 10.1073/pnas.92.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hershko A, Ciechanover A. The Ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- 13.Hilliard J J, Maurizi M R, Simon L D. Isolation and characterization of the phage T4 PinA protein, an inhibitor of the ATP-dependent Lon protease of Escherichia coli. J Biol Chem. 1998;273:518–523. doi: 10.1074/jbc.273.1.518. [DOI] [PubMed] [Google Scholar]
- 14.Hochstrasser M. Ubiquitin-dependent protein degradation. Annu Rev Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- 15.Hofmeister A E, Londono-Vallejo A, Harry E, Stragier P, Losick R. Extracellular signal protein triggering the proteolytic activation of a developmental transcription factor in B. subtilis. Cell. 1995;83:219–226. doi: 10.1016/0092-8674(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 16.Jishage M, Ishihama A. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of ς70 and ς38. J Bacteriol. 1995;177:6832–6835. doi: 10.1128/jb.177.23.6832-6835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keiler K C, Waller P R H, Sauer R T. Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science. 1996;271:990–993. doi: 10.1126/science.271.5251.990. [DOI] [PubMed] [Google Scholar]
- 18.Lange R, Hengge-Aronis R. The cellular concentration of the ςS subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation, and protein stability. Genes Dev. 1994;8:1600–1612. doi: 10.1101/gad.8.13.1600. [DOI] [PubMed] [Google Scholar]
- 19.Maurizi M R. Proteases and protein degradation in Escherichia coli. Experientia. 1992;48:178–201. doi: 10.1007/BF01923511. [DOI] [PubMed] [Google Scholar]
- 20.Maurizi M R, Clark W P, Katayama Y, Rudikoff S, Pumphrey J, Bowers B, Gottesman S. Sequence and structure of ClpP, the proteolytic component of the ATP-dependent Clp protease of Escherichia coli. J Biol Chem. 1990;265:12536–12545. [PubMed] [Google Scholar]
- 21.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 22.Muffler A, Barth M, Marschall C, Hengge-Aronis R. Heat shock regulation of ςS turnover: a role for DnaK and relationship between stress responses mediated by ςS and ς32 in Escherichia coli. J Bacteriol. 1997;179:445–452. doi: 10.1128/jb.179.2.445-452.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muffler A, Fischer D, Altuvia S, Storz G, Hengge-Aronis R. The response regulator RssB controls stability of the ςS subunit of RNA-polymerase in Escherichia coli. EMBO J. 1996;15:1333–1339. [PMC free article] [PubMed] [Google Scholar]
- 24.Murray A. Cyclin ubiquitination: the destructive end of mitosis. Cell. 1995;81:149–152. doi: 10.1016/0092-8674(95)90322-4. [DOI] [PubMed] [Google Scholar]
- 25.Pratt L A, Silhavy T J. The response regulator SprE controls the stability of RpoS. Proc Natl Acad Sci USA. 1996;93:2488–2492. doi: 10.1073/pnas.93.6.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts J D, McMacken R. The bacteriophage lambda O replication protein: isolation and characterization of the amplified initiator. Nucleic Acids Res. 1983;11:7435–7452. [PMC free article] [PubMed] [Google Scholar]
- 27.Schoulaker-Schwarz R, Dekel-Gorodetsky L, Engelberg-Kulka H. An additional function for bacteriophage λ rex: the rexB product prevents degradation of the λ O protein. Proc Natl Acad Sci USA. 1991;88:4996–5000. doi: 10.1073/pnas.88.11.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schupp J M, Trais S E, Price L B, Shand R F, Keim P. Rapid bacterial permeabilization reagent useful for enzyme assays. BioTechniques. 1995;19:18–19. [PubMed] [Google Scholar]
- 29.Schweder T, Lee K-H, Lomovskaya O, Matin A. Regulation of Escherichia coli starvation sigma factor (ςS) by ClpXP protease. J Bacteriol. 1996;178:470–476. doi: 10.1128/jb.178.2.470-476.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon L D, Tomczak K, John A C S. Bacteriophages inhibit degradation of abnormal proteins in E. coli. Nature. 1978;275:424–428. doi: 10.1038/275424a0. [DOI] [PubMed] [Google Scholar]
- 31.Skorupski K, Tomaschewski J, Ruger W, Simon L D. A bacteriophage T4 gene which functions to inhibit Lon protease. J Bacteriol. 1988;170:3016–3024. doi: 10.1128/jb.170.7.3016-3024.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sledjeski D D, Gottesman S. The small RNA, DsrA, is essential for the low temperature expression of RpoS during exponential growth in E. coli. EMBO J. 1996;15:3993–4000. [PMC free article] [PubMed] [Google Scholar]
- 33.Takayanagi Y, Tanaka K, Takahashi H. Structure of the 5′ upstream region and the regulation of the rpoS gene of Escherichia coli. Mol Gen Genet. 1994;243:525–531. doi: 10.1007/BF00284200. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka K, Takayanagi Y, Fujita N, Ishihama A, Takahashi H. Heterogeneity of the principal ς factor in Escherichia coli: the rpoS gene product, ς38, is a second principal ς factor of RNA polymerase in stationary phase Escherichia coli. Proc Natl Acad Sci USA. 1993;90:3511–3515. doi: 10.1073/pnas.90.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wojtkowiak D, Georgopoulos C, Zylicz M. ClpX, a new specificity component of the ATP-dependent Escherichia coli Clp protease, is potentially involved in λ DNA replication. J Biol Chem. 1993;268:22609–22617. [PubMed] [Google Scholar]