Summary
Background
The biological aging process can be modified through lifestyle interventions to prevent age-related diseases and extend healthspan. However, evidence from population-based studies on whether tea consumption could delay the biological aging process in humans remains limited.
Methods
This study included 7931 participants aged 30–79 years from the China Multi-Ethnic Cohort (CMEC) Study and 5998 participants aged 37–73 years from the UK Biobank (UKB) who participated in both the baseline and first follow-up surveys. Tea consumption information was collected through questionnaires. Biological age (BA) acceleration was calculated using clinical biomarkers and anthropometric measurements based on the Klemera Doubal method (KDM). Change-to-change analyses were performed to estimate the associations between changes in tea consumption status and changes in BA acceleration using multiple linear models. Follow-up adjusted for baseline analyses were further conducted to examine the prospective exposure-response relationship between tea consumption and BA acceleration among individuals with constant tea consumption status.
Findings
During a median follow-up of 1.98 (1.78, 2.16) years in the CMEC and 4.50 (3.92, 5.00) years in the UKB, tea consumption was consistently associated with attenuated BA acceleration in both cohorts. Transitioning from nondrinking to tea-drinking was associated with decreased BA acceleration (CMEC: β = −0.319, 95% CI: −0.620 to −0.017 years; UKB: β = −0.267, 95% CI: −0.831 to 0.297 years) compared to consistent nondrinking. Even stronger associations were found in consistent tea drinkers. The exposure-response relationship suggested that consuming around 3 cups of tea or 6–8 g of tea leaves per day may offer the most evident anti-aging benefits.
Interpretation
Tea consumption was associated with attenuated BA acceleration measured by KDM, especially for consistent tea drinkers with moderate consumption. Our findings highlight the potential role of tea in developing nutrition-oriented anti-aging interventions and guiding healthy aging policies.
Funding
National Natural Science Foundation of China (Grant No. 82273740).
Keywords: Biological aging, Tea consumption, Change-to-change analysis, Follow-up adjusted for baseline analysis, Exposure-response relationship
Research in context.
Evidence before this study
We searched PubMed from database inception to February 1, 2023, for studies that had investigated the effects of tea or tea components on aging-related health outcomes, using the search terms “tea” and (“aging” or “biological aging” or “biological age” or “telomere length” or “epigenetic age” or “phenotypic age” or “Klemera Doubal method” or “homeostatic dysregulation” or “frailty” or “age-related” or “cardiovascular” or “diabetes” or “cancer” or “dementia” or “lifespan” or “healthspan”). Animal studies have suggested that tea polyphenols may extend life expectancy in worms, flies, and mice. Epidemiological studies have also indicated that tea consumption may protect against age-related diseases, such as cardiovascular diseases, diabetes mellitus, dementia, and cancer. Therefore, based on the existing evidence, we hypothesized that tea consumption might delay the biological aging process in humans. However, only a few studies from Asian population have investigated the associations between tea consumption and biological aging measured by telomere length or frailty index.
Added value of this study
To the best of our knowledge, this is the first longitudinal study to investigate the association between tea consumption and biological aging measured by clinical biomarkers, using a change-to-change design. Based on the longitudinal data from the China Multi-Ethnic Cohort (CMEC) and the UK Biobank (UKB), we found that both the transition from nondrinking to drinking tea and consistent tea drinking were associated with attenuated biological age acceleration. Moreover, moderate tea consumption exhibited the strongest anti-aging benefits among consistent tea drinkers.
Implications of all the available evidence
Our findings, along with previous animal and epidemiological studies, provided further support for the hypothesis that tea drinking may delay the biological aging process in humans. As a globally consumed beverage, tea could play an important role in developing nutrition-oriented anti-aging interventions, guiding public health policy, and promoting healthy aging.
Introduction
From 2020 to 2050, the number of people aged 60 years and older is expected to double, reaching 2.1 billion, or 22% of the world's population.1 Since population aging has become one of the most significant global challenges, determining how to extend human health and longevity has emerged as a critical research issue. Despite the fact that everyone ages chronologically at the same rate, the biological aging process can be modified through genetic, pharmacological, and dietary interventions to delay or prevent the onset and progression of age-related diseases and multimorbidity, enhance overall health, and extend lifespan and healthspan.2,3
Tea is one of the most popular beverages consumed globally with a long history. Tea contains a variety of bioactive substances, especially polyphenols. It has been found that polyphenols can exert anti-oxidant, anti-inflammatory, and apoptotic effects,4 as well as modulate epigenetic changes,5 thereby may delay the aging process.6 Meanwhile, animal studies have suggested that flavonoids, a kind of polyphenol that is rich in tea, may extend life expectancy in worms, flies, and even mice.7 Moreover, epidemiological studies are accumulating that tea consumption may protect against age-related diseases, such as cardiovascular diseases,8 diabetes mellitus,9 dementia,10,11 and some types of cancer,12 and that tea consumption was associated with lower mortality risk.13,14 Given the existing evidence, it is plausible to hypothesize that drinking tea may delay the biological aging process in humans.
However, evidence linking tea consumption to biological aging from population-based studies remains limited. To date, there is no gold standard to quantify the biological aging process. Given that age-related changes accumulate at hierarchical levels in organisms,15 several aging measures have been proposed, such as epigenetic clocks,16,17 telomere length,18 biological age (BA) assessed by composite biomarkers (e.g., PhenoAge),19, 20, 21 and functional age (e.g., frailty index, FI).22, 23, 24 Previous studies have suggested that different types of aging measures may reflect distinct aspects of aging.25,26 Though several Asian studies have reported inverse associations between tea consumption and telomere shortening,27,28 or FI,29, 30, 31 little is known about tea consumption and biological aging assessed by composite biomarkers, which incorporate clinical biomarkers from multiple organs and systems and have demonstrated good performance in predicting age-related health outcomes.32, 33, 34 Compared to other measures of biological aging process, composite biomarker BA is cost-effective while ensuring measurement accuracy, making it feasible for large population-based studies. To the best of our knowledge, there is only one relevant cross-sectional study from Singaporean population.35 Thus, longitudinal studies from more representative populations are still required to provide credible evidence. Moreover, most longitudinal studies have mainly used baseline tea consumption as exposure,9,11 with little consideration of changes in tea consumption status (e.g., transition from nondrinking to drinking). This may lead to misclassification of exposure and thus bias in the estimates. Accordingly, it is necessary to assess the association of changes in tea consumption status with changes in BA acceleration (i.e., the deviation of biological age from chronological age). Analyzing changes can provide a more intuitive understanding of how changes in exposure might lead to changes in outcomes, and may offer unique perspectives for developing interventions and guiding policy.
Therefore, based on the longitudinal data from the China Multi-Ethnic Cohort (CMEC) Study and the UK Biobank (UKB), and using a well-performed BA estimated by composite biomarkers and the Klemera Doubal method19 (KDM), we proposed to investigate the association between change in tea consumption status and concurrent change in BA acceleration. For those who consume tea consistently over time, we further investigated the prospective exposure-response relationship between tea consumption and BA acceleration.
Methods
Study population
The CMEC study is an ongoing prospective cohort study based on community populations in five provinces (Sichuan, Chongqing, Yunnan, Guizhou, and Tibet) in Southwest China.36 The baseline survey was initiated in May 2018 and recruited a representative sample of 99,556 participants aged 30–79 years, given a full consideration of ethnic characteristics, socioeconomic status, population size and disease patterns (Supplementary Methods). The first follow-up survey was conducted between August 2020 and July 2021 and involved approximately 10% of the baseline participants, with the remaining 90% followed up by telephone. In both surveys, participants were required to complete a tablet-administered electronic questionnaire via face-to-face interviews, medical examinations, and clinical laboratory tests. Each participant provided written informed consent before data collection by the interviewer. The CMEC study received approval from the Sichuan University Medical Ethical Review Board and local ethics committees at each participating site. For the present study, Tibetan participants were excluded due to their distinct tea-drinking habits, which are characterized by favoring sweet tea and buttered tea that are high in calories and salt.
The UK Biobank is a large-scale, population-based prospective study that enrolled over 500,000 participants aged 37–73 years across 22 assessment centers in England, Wales, and Scotland.37 During the baseline period (2006–2010), detailed information about participants was collected at these assessment centers, including sociodemographic, lifestyle, and health-related information through touch-screen questionnaires, physical measures, and biological samples. This assessment was repeated in 2012–2013 at the Cheadle assessment center within a subgroup of 20,343 participants (referred to as the first follow-up survey hereafter). All participants provided electronic informed consent. The UKB study received approval from the National Information Governance Board for Health and Social Care and the National Health Service North West Multi-Centre Research Ethics Committee.
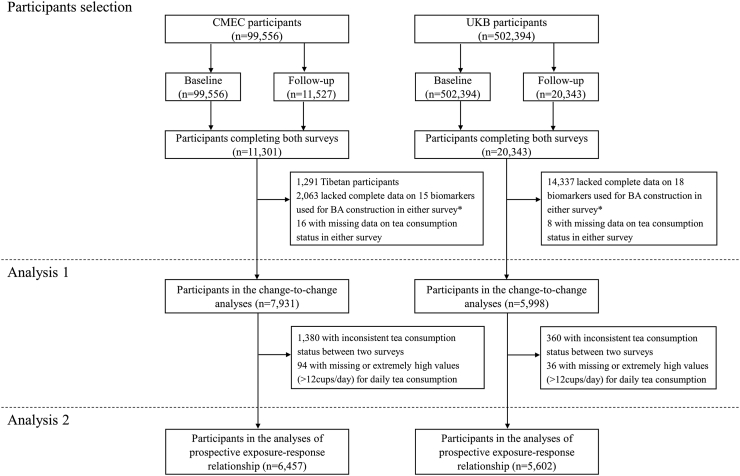
For both cohorts, to examine the association of change in tea consumption status with concurrent change in BA acceleration between the baseline and the first follow-up surveys (Analysis 1), we only included individuals who had available data for both tea consumption and all biomarkers used for BA construction in both surveys. Overall, 7931 participants in CEMC and 5998 participants in UKB were included in the present study (Fig. 1). For further analysis of the exposure-response relationship (Analysis 2), we restricted the study to participants who maintained a consistent tea consumption status throughout both surveys. Individuals with missing data or exceptionally high values (>12 cups/day) for daily tea consumption were excluded (Fig. 1).
Fig. 1.
Flowchart of the study. ∗See the Supplementary Material (Supplementary Tables S1–S4) for more information on the availability of biomarker data.
Assessment of tea consumption
Information on tea consumption were assessed in both cohorts using questionnaires. In CMEC, participants were asked about their tea consumption status (yes or no, including green tea, scented tea, dark tea, sweet tea, black tea, oolong tea, yellow tea and white tea), current frequency of tea consumption (don’t drink currently, 1–2 day(s)/week, 3–5 days/week, or almost every day/daily), and the numbers of cups of tea consumed (with a standard cup size of 200 ml) on a typical drinking day during the past 12 months. Additionally, data were collected on the quantities of loose tea leaves (in grams) consumed on a drinking day since tea is usually served loose instead of tea bags in China. Standard cups and containers were provided to each participant to measure the amount of tea and tea leaves. We calculated the average daily tea consumption (in cups/day and grams/day) by multiplying the weekly frequency of tea consumption (in days/week) by the amount of tea consumed on a drinking day (in cups and grams) and dividing by 7 (days of the week). In UKB, participants were asked the question, “How many cups of tea do you drink a day? (Including black and green tea)” (UKB Data-field ID: 1488). We further defined tea consumption status as “yes” (daily consumed cups > 0) and “no” (daily consumed cups = 0). In both cohorts, participants were divided into four categories based on change in their tea consumption status between the baseline and first follow-up surveys: consistent nondrinking, nondrinking to drinking, drinking to nondrinking, and consistent drinking.
Assessment of covariates
Covariate information was mainly obtained through questionnaires. To properly determine the potential confounders, we constructed a directed acyclic graph (DAG) according to the protocol of “Evidence Synthesis for Constructing Directed Acyclic Graphs” (ESC-DAGs)38 (Supplementary Figure S1). Based on the proposed DAG and backdoor criteria,39 we adjusted statistical models for: age, sex, race and ethnicity, education, Townsend Deprivation Index (TDI, UKB only), urbanicity (CEMC only), occupation, marital status (CEMC only), menopausal status for women, smoking status, alcohol consumption, beverage consumption, dietary score, total energy intake (kcal/day, CMEC only), non-sedentary physical activity in hours of metabolic equivalent tasks per day (METs-h/week), insomnia symptom, depressive symptom, anxiety symptom, body mass index (BMI, continuous) and self-reported physician-diagnosed chronic diseases, including cancer, cardiovascular diseases (CVD), diabetes and chronic obstructive pulmonary disease (COPD). More details of variables can be found in the Supplementary Methods.
Construction of KDM-BA and KDM-BA acceleration
In this study, we used the clinical biomarkers and anthropometric measurements to construct BA based on KDM, which has been validated in both Chinese and UK populations and shown good performance in predicting age-related health outcomes.32, 33, 34 The biomarkers were selected by considering their function in the aging process, their utilization in previous research, their availability in the data sets, and the statistical significance and strength of their correlations with chronological age (CA).33,34 For each available biomarker, a Box–Cox transformation was performed to achieve normal distribution. We first excluded biomarkers with a high missing rate in both waves, leaving 43 biomarkers for CMEC and 74 biomarkers for UKB. Next, we only retained biomarkers that are significantly correlated with CA with a correlation coefficient of |r| > 0.1. We further excluded redundant biomarkers that might reflect the same aspects of aging based on the existing knowledge and correlation among those biomarkers. Finally, we selected 15 biomarkers for CMEC, which includes systolic blood pressure (SBP), waist-to-hip ratio (WHR), peak expiratory flow, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, glycated hemoglobin (HBA1C), triglyceride (TG), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), albumin (ALB), alkaline phosphatase (ALP), creatinine, urea, mean corpuscular volume (MCV), and platelet count. Following the same procedure, a set of 18 biomarkers were selected for UKB: SBP, WHR, body fat percentage, forced expiratory volume in 1 s, MCV, ALP, AST, C-reactive protein, Cystatin C, GGT, heel quantitative ultrasound index, HBA1C, insulin-like growth factor 1, TG, urate, urea, ALB, and vitamin D.
The KDM-BA is derived from information obtained from m regression lines that regress CA on m selected biomarkers in a reference population.19 The formula is as follows:
where is the estimated biological age. is the measured value of the jth biomarker. , and represent the slope, intercept and root mean squared error obtained from the regression of the jth biomarker on chronological age in the reference population, respectively. CA is chronological age. is the estimated variance in CA explained by the selected biomarker set in the reference population. The regression lines were trained separately by sex using the baseline data. BA alone cannot capture the differences in biological aging between individuals because it increases with age. To account for the impact of age, we calculated KDM-BA acceleration as the difference between KDM-BA and CA. A positive KDM-BA acceleration may indicate that the individual is biologically older in terms of physiological function compared to what is expected in the reference sample, and vice versa. The calculation of KDM-BA was performed using the “BioAge” R package.40 More details of biomarker selection and BA construction were shown in the Supplementary Material (Supplementary Methods, Supplementary Tables S1–S4, and Supplementary Figures S2–S5).
Validation analysis
To evaluate that the newly constructed KDM-BAs in both CMEC and UKB were well-performed aging measures, we conducted validation analysis by utilizing KDM-BAs to predict age-related outcomes. For CMEC, due to its relatively short time of establishment and follow-up, we were unable to estimate the associations of KDM-BA acceleration with mortality. Instead, we examined the associations of KDM-BA acceleration with self-reported age-related diseases such as cancer, CVD, diabetes, and COPD, using the baseline data and logistic regression models. Additionally, we constructed an alternative aging measure—FI—which has been suggested as a good predictor of mortality in the Chinese population,41 and estimated the associations of KDM-BA acceleration and FI. FI consisted of 25 health deficit items and was calculated by dividing the total number of deficits included by the number of deficits present in a person (Supplementary Table S5). For UKB, we estimated the associations between the baseline KDM-BA acceleration and all-cause mortality using Cox proportional hazard regression models. Initially, KDM-BA acceleration was fitted as a continuous variable in the model, followed by categorical divisions: |BA acceleration| ≤ 1, BA acceleration < −1, and BA acceleration > 1, with |BA acceleration| ≤ 1 serving as the reference group. Full models were adjusted for age, race and ethnicity, education, occupation, TDI, menopause status in women, smoking status, alcohol consumption, beverage consumption, healthy diet, insomnia, depressive symptom, anxiety symptom, physical activity, body mass index, and chronic diseases.
Statistical analysis
We described the baseline characteristics of CMEC and UKB participants based on their change in tea consumption status between the baseline and first follow-up surveys. Continuous variables were presented as median (25th, 75th percentile), while categorical variables were presented as count (percentage). In addition, we described the changes in time-varying characteristics between the baseline and first follow-up surveys. To assess the representativeness of the study population, we compared the baseline characteristics of participants who were available for the follow-up with those who were not.
To examine the association between change in tea consumption status with the concurrent change in KDM-BA acceleration, we conducted change-to-change analysis using multiple linear regression models, with never drinking as the reference group (Analysis 1). Change-to-change analysis is essentially a self-controlled method using only within-individual information and has been shown to produce valid findings comparable to those obtained from randomized controlled trials by reducing unobserved time-invariant confounding.42,43 In the final model, we adjusted for demographics and self-reported disease at baseline, as well as the baseline and concurrent changes of time-variant variables, including age, lifestyle factors and other covariates identified by the DAG mentioned above (Supplementary Methods). To further examine the prospective exposure-response relationship between tea consumption (cups/day and grams/day) and KDM-BA acceleration, we conducted a follow-up adjusted for baseline analysis using multiple linear models among individuals with constant tea consumption status (Analysis 2). The follow-up KDM-BA acceleration was regressed on the baseline tea consumption and adjusted for the baseline KDM-BA acceleration and covariates identified by the DAG mentioned above. Follow-up adjusted for baseline analysis mitigates reverse causation and, to some extent, reduces unmeasured confounding by adjusting for the baseline outcome.44 Tea consumption was first fitted in the model as a restricted cubic spline to explore the potential non-linear relationship with KDM-BA acceleration, and then as a categorical variable to better inform dietary-based anti-aging therapy. We divided it into four categories: 0 (never drinking), ≤1 cup/day (low), 2–3 cups/day (moderate), and ≥4 cups/day (high), via a comprehensive consideration of the sample size and the magnitude of association in each group. In CMEC, where data on daily tea leaf consumption were available, we replicated the analyses of the exposure-response relationship using the amount of tea leaves consumed. In both of the two main analyses above, to capture the non-linear effects of age on biological aging,45 the restricted cubic spline was used to adjust for the baseline age. Missing values of covariates were imputed using multiple imputation (with 5 imputations) by the chained equations method, and the estimates from imputed data sets were combined using Rubin’s rules.46 The detailed missing information on covariates was shown in Supplementary Table S6.
To explore the effect heterogeneity, we performed stratification analysis among predefined subgroups according to their baseline characteristics, including sex, age, BA acceleration, race and ethnicity, urbanicity (CMEC only), TDI (UKB only), smoking status, alcohol consumption and social participation. Social participation was assessed based on the frequency of engaging in social activities or social visits. It was categorized as “less than once a month” (occasional) and “at least once a month” (frequent) in CMEC, while “less than or equal to once a week” (occasional) and “more than once a week” (frequent) in UKB. To assess the robustness of the findings, we conducted several sensitivity analyses. First, we repeated the analyses by taking an alternative widely-used definition of KDM-BA acceleration: the residual of KDM-BA regressed on CA using linear regression. Second, we restricted the analyses to relatively healthy populations by excluding individuals with self-reported cancer, CVD, diabetes, and COPD. Third, to evaluate whether our findings were consistent within a traditional longitudinal study framework, we performed survival analyses using Cox proportional hazard models. We dichotomized KDM-BA acceleration (i.e., KDM-BA acceleration > 0, occurrence of accelerated biological aging) and examined the associations between baseline tea consumption and incident accelerated biological aging among individuals with constant tea consumptions status. Participants who had experienced accelerated biological aging at baseline were excluded from this analysis. Fourth, we repeated the analyses using complete case samples (n = 7303 for CMEC, n = 3960 for UKB) instead of the multiple imputation approach. Last, we calculated the E-values to examine the potential impact of unmeasured confounding.47
In all of the analyses described above, we did not apply any correction for multiple hypothesis testing. Two-sided P < 0.05 was considered to be statistically significant. All analyses were performed with R project for Statistical Computing version 4.1.0. Data analysis was conducted between October 2022 and February 2023.
Role of the funding source
This study was funded by National Natural Foundation of China (Grant No. 82273740). The study sponsor played no role in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. YX, XX, and XZ had access to all data and had final responsibility for the decision to submit it for publication.
Results
Characteristics of study population
Baseline characteristics of study participants from CMEC and UKB according to change in tea consumption status are presented in Table 1. Among 7931 participants from CMEC, the median age was 50.98 (44.30, 59.74) years, 4880 (61.5%) participants were women, and 5141 (64.8%) were Han Chinese. During a median follow-up of 1.98 (1.78, 2.16) years, 9.5% of participants transitioned from nondrinking to drinking tea, while 18.4% maintained their tea-drinking habit. Among 5998 participants from UKB, the median age was 58.83 (52.08, 63.17) years, 2851 (47.5%) participants were women, and 5847 (97.5%) were White. During a median follow-up of 4.50 (3.92, 5.00) years, only 3.2% of participants transitioned from nondrinking to drinking tea, while 82.9% maintained their tea-drinking habit. Compared with consistent nondrinkers, participants who transitioned from nondrinking to drinking and participants with a consistent drinking habit exhibited a lower increase in KDM-BA acceleration (Supplementary Table S7). Additionally, these individuals were more likely to be men, consume alcohol, and maintain a healthier diet. Furthermore, they were less likely to experience insomnia, depressive symptoms, and anxiety symptoms, especially in CMEC. Compared with consistent drinkers, participants who transitioned from drinking to nondrinking showed a higher increase in KDM-BA acceleration. More detailed information on the characteristics of study populations is presented in Supplementary Tables S7–S9. In CMEC, participants with available data for follow-up were more likely to come from urban cities. In UKB, participants who attended the follow-up tended to be healthier and had lower prevalence of self-reported chronic diseases (Supplementary Table S10).
Table 1.
Baseline characteristics of participants from China Multi-Ethnic Cohort (CMEC) and UK Biobank (UKB) according to change in tea consumption status.a
| Characteristic | CMEC |
UKB |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Consistent nondrinking | Nondrinking to drinking | Drinking to nondrinking | Consistent drinking | Overall | Consistent nondrinking | Nondrinking to drinking | Drinking to nondrinking | Consistent drinking | |
| No. of participants | 7931 | 5088 | 751 | 629 | 1463 | 5998 | 667 | 191 | 169 | 4971 |
| KDM-BA (years) | 51.27 (42.43, 60.26) | 51.00 (41.72, 60.13) | 50.99 (41.90, 60.00) | 51.68 (43.27, 61.73) | 51.89 (44.49, 60.18) | 56.92 (50.06, 62.64) | 57.02 (49.17, 62.63) | 56.41 (50.41, 61.96) | 56.38 (49.35, 62.70) | 56.93 (50.20, 62.65) |
| KDM-BA acceleration (years) | −0.45 (−3.43, 2.62) | −0.41 (−3.65, 2.71) | −0.24 (−2.99, 2.99) | −0.46 (−3.57, 2.58) | −0.68 (−3.04, 2.06) | −1.62 (−5.29, 2.25) | −0.93 (−4.66, 3.07) | −1.82 (−5.44, 3.05) | −0.57 (−4.89, 3.86) | −1.70 (−5.37, 2.06) |
| Age (years) | 50.98 (44.30, 59.74) | 50.64 (43.85, 59.68) | 50.49 (43.74, 58.00) | 51.94 (45.42, 61.11) | 51.98 (45.65, 60.18) | 58.83 (52.08, 63.17) | 58.00 (50.71, 62.67) | 58.17 (51.21, 62.17) | 57.92 (49.83, 62.92) | 59.00 (52.42, 63.25) |
| Female | 4880 (61.5) | 3767 (74.0) | 399 (53.1) | 340 (54.1) | 374 (25.6) | 2851 (47.5) | 327 (49.0) | 99 (51.8) | 75 (44.4) | 2350 (47.3) |
| Race and ethnicity, majorityb | 5141 (64.8) | 3017 (59.3) | 514 (68.4) | 453 (72.0) | 1157 (79.1) | 5847 (97.5) | 653 (97.9) | 181 (94.8) | 162 (95.9) | 4851 (97.6) |
| Educationc | ||||||||||
| Less than high school | 5959 (75.1) | 3927 (77.2) | 552 (73.5) | 472 (75.0) | 1008 (68.9) | 531 (8.9) | 52 (7.8) | 12 (6.3) | 9 (5.3) | 458 (9.2) |
| High school or equivalent | 1040 (13.1) | 618 (12.1) | 104 (13.8) | 79 (12.6) | 239 (16.3) | 2898 (48.3) | 342 (51.3) | 98 (51.3) | 81 (47.9) | 2377 (47.8) |
| College or above | 931 (11.7) | 543 (10.7) | 95 (12.6) | 78 (12.4) | 215 (14.7) | 2537 (42.3) | 273 (40.9) | 79 (41.4) | 79 (46.7) | 2106 (42.4) |
| TDId | – | – | – | – | – | −2.75 (−4.02, −0.81) | −2.62 (−3.84, −0.63) | −2.06 (−3.65, 0.58) | −2.27 (−3.86, −0.63) | −2.79 (−4.06, −0.90) |
| Urban residence | 3020 (38.1) | 1974 (38.8) | 241 (32.1) | 254 (40.4) | 551 (37.7) | – | – | – | – | – |
| Occupation | ||||||||||
| Employed | 6772 (85.4) | 4397 (86.4) | 651 (86.7) | 539 (85.7) | 1185 (81.0) | 3592 (59.9) | 417 (62.5) | 121 (63.4) | 107 (63.3) | 2947 (59.3) |
| Unemployed | 330 (4.2) | 208 (4.1) | 30 (4.0) | 19 (3.0) | 73 (5.0) | 57 (1.0) | 5 (0.7) | 0 (0.0) | 4 (2.4) | 48 (1.0) |
| Retired | 819 (10.3) | 478 (9.4) | 69 (9.2) | 70 (11.1) | 202 (13.8) | 2307 (38.5) | 240 (36.0) | 68 (35.6) | 58 (34.3) | 1941 (39.0) |
| Married | 7130 (89.9) | 4504 (88.5) | 697 (92.8) | 575 (91.4) | 1354 (92.5) | – | – | – | – | – |
| Post-menopause in women | 2343 (48.0) | 1812 (48.1) | 200 (50.1) | 161 (47.4) | 170 (45.5) | 2120 (74.4) | 227 (69.4) | 70 (70.7) | 52 (69.3) | 1771 (75.4) |
| Current smoking | 1524 (19.2) | 509 (10.0) | 178 (23.7) | 145 (23.1) | 692 (47.3) | 373 (6.2) | 50 (7.5) | 12 (6.3) | 14 (8.3) | 297 (6.0) |
| Current drinking | 3458 (43.6) | 1875 (36.9) | 350 (46.6) | 320 (50.9) | 913 (62.4) | 5662 (94.4) | 602 (90.3) | 183 (95.8) | 160 (94.7) | 4717 (94.9) |
| Current beverage consumptione | 239 (3.0) | 126 (2.5) | 27 (3.6) | 32 (5.1) | 54 (3.7) | 4783 (79.7) | 561 (84.1) | 164 (85.9) | 150 (88.8) | 3908 (78.6) |
| Healthy dietf | 2736 (34.5) | 1665 (32.7) | 277 (36.9) | 240 (38.2) | 554 (37.9) | 2002 (33.4) | 196 (29.4) | 67 (35.1) | 61 (36.1) | 1678 (33.8) |
| Total energy intake (kcal/day) | 1756.18 (1395.21, 2196.15) | 1685.52 (1342.61, 2112.83) | 1851.70 (1436.68, 2239.91) | 1809.22 (1413.62, 2215.45) | 1968.06 (1575.29, 2446.48) | – | – | – | – | – |
| Physical activity (MET-hours/week) | 158.08 (88.20, 264.26) | 162.71 (89.71, 266.70) | 159.92 (91.20, 277.37) | 171.32 (95.95, 265.62) | 142.46 (81.20, 244.25) | 29.55 (13.50, 57.73) | 27.61 (12.33, 54.69) | 34.01 (13.11, 58.71) | 27.60 (13.82, 65.40) | 29.83 (13.62, 57.80) |
| Insomnia symptom | 3533 (44.5) | 2397 (47.1) | 302 (40.2) | 286 (45.5) | 548 (37.5) | 1538 (25.6) | 170 (25.5) | 59 (30.9) | 47 (27.8) | 1262 (25.4) |
| Depressive symptom | 416 (5.2) | 283 (5.6) | 39 (5.2) | 35 (5.6) | 59 (4.0) | 186 (3.1) | 20 (3.0) | 13 (6.8) | 4 (2.4) | 149 (3.0) |
| Anxiety symptom | 490 (6.2) | 354 (7.0) | 50 (6.7) | 36 (5.7) | 50 (3.4) | 199 (3.3) | 25 (3.7) | 14 (7.3) | 6 (3.6) | 154 (3.1) |
| BMI (kg/m2) | 24.06 (21.87, 26.58) | 23.89 (21.76, 26.26) | 24.69 (22.04, 27.43) | 24.38 (22.35, 26.97) | 24.33 (22.15, 27.03) | 26.13 (23.77, 28.93) | 26.99 (24.17, 30.38) | 26.29 (23.99, 30.05) | 26.63 (24.33, 29.83) | 25.99 (23.67, 28.73) |
| Self-reported diseases | ||||||||||
| Cancer | 67 (0.8) | 49 (1.0) | 7 (0.9) | 6 (1.0) | 5 (0.3) | 357 (6.0) | 42 (6.3) | 6 (3.1) | 6 (3.6) | 303 (6.1) |
| CVD | 1524 (19.2) | 979 (19.2) | 143 (19.0) | 118 (18.8) | 284 (19.4) | 1547 (25.8) | 162 (24.3) | 48 (25.1) | 39 (23.1) | 1298 (26.1) |
| Diabetes | 405 (5.1) | 234 (4.6) | 41 (5.5) | 39 (6.2) | 91 (6.2) | 221 (3.7) | 34 (5.1) | 8 (4.2) | 6 (3.6) | 173 (3.5) |
| COPD | 485 (6.1) | 304 (6.0) | 47 (6.3) | 33 (5.2) | 101 (6.9) | 34 (0.6) | 5 (0.7) | 0 (0.0) | 1 (0.6) | 28 (0.6) |
Abbreviations: TDI = Townsend deprivation index; BMI = body mass index; CVD = cardiovascular disease; COPD = chronic obstructive pulmonary disease.
Data are presented as median (25th, 75th percentile) for continuous variables and count (percentage) for categorical variables. The numbers of missing covariates in CMEC/UKB were as follows: race and ethnicity (0/10), education (1/32), TDI (3 in UKB), occupation (10/42), marital status (1 in CMEC), menopause status (3/110), smoking (0/8), alcohol consumption (1/4), beverage consumption (0/5), healthy diet (25/98), total energy intake (25 in CMEC), physical activity (40/998), insomnia symptom (27/1), depressive symptom (27/285), anxiety symptom (27/158), BMI (11/3), cancer (0/12), CVD (0/6), COPD (0/3).
Majority denoted Han Chinese in CMEC and White in UKB.
In UKB, education level was defined according to education qualifications: college or above (college or university degree); high school or equivalent (A levels, AS levels, or equivalent; O levels, GCSEs, or equivalent; CSEs or equivalent; NVQ, HND, HNC, or equivalent; other professional qualifications); less than high school (none of the above).
TDI was an area level variable of socioeconomic status, A higher numbers of TDI denotes lower area level SES.
In CMEC, beverage included sweeten beverages, coffee and caffeine beverages, and others. In UKB, beverage referred to coffee.
Healthy diet denoted the top two fifths of healthy diet score in the two cohorts. In CMEC, a Dietary Approaches to Stop Hypertension (DASH) score was used. In UKB, healthy diet score was calculated based on consumption of 7 dietary components (fruits, vegetables, fish, processed meats, unprocessed red meats, whole grains and refined grains).
Validation analysis
Table 2 presents the estimated associations between KDM-BA acceleration and all-cause mortality in UKB. In the multivariable-adjusted model, one-year increase of KDM-BA acceleration elevated mortality risk by 56% (HR = 1.56, 95% CI: 1.52–1.60). Additionally, compared to participants with |BA acceleration| ≤ 1, the HRs for participants who had BA acceleration < −1 and BA acceleration > 1 were 0.85 (0.81, 0.90) and 1.34 (1.27, 1.41), respectively. For CMEC, KDM-BA acceleration demonstrated good performance in predicting most age-related diseases, particularly CVD and diabetes (Supplementary Table S11). Moreover, compared to participants with |BA acceleration| ≤ 1, the risk of frailty decreased by 63% (OR = 0.37, 95% CI: 0.33–0.41) for BA acceleration < −1 and increased by 230% (OR = 3.30, 95% CI: 2.97–3.67) for BA acceleration > 1 (Supplementary Table S12). The distribution of frailty in CMEC was presented in Supplementary Table S13.
Table 2.
Associations of KDM-BA acceleration with All-Cause Mortality in UKB.
| KDM-BA acceleration | Total |
Female |
Male |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Participants | Deaths | HR (95% CI) | Participants | Deaths | HR (95% CI) | Participants | Deaths | HR (95% CI) | |
| Model 1 | |||||||||
| BA acceleration, continuous | 330,150 | 22,358 | 1.76 (1.73, 1.79) | 176,834 | 8980 | 1.66 (1.62, 1.71) | 153,316 | 13,378 | 1.79 (1.75, 1.82) |
| BA acceleration, categorical | |||||||||
| |BA acceleration| ≤ 1 | 43,676 | 2652 | 1.00 | 23,068 | 1085 | 1.00 | 20,608 | 1567 | 1.00 |
| BA acceleration < −1 | 149,076 | 7097 | 0.79 (0.76, 0.83) | 80,063 | 2996 | 0.82 (0.76, 0.88) | 69,013 | 4101 | 0.77 (0.73, 0.82) |
| BA acceleration > 1 | 137,398 | 12,609 | 1.57 (1.50, 1.63) | 73,703 | 4899 | 1.49 (1.39, 1.59) | 63,695 | 7710 | 1.62 (1.53, 1.71) |
| Model 2 | |||||||||
| BA acceleration, continuous | 236,186 | 14,636 | 1.56 (1.52, 1.60) | 120,884 | 5369 | 1.46 (1.40, 1.53) | 115,342 | 9267 | 1.60 (1.55, 1.66) |
| BA acceleration, categorical | |||||||||
| |BA acceleration| ≤ 1 | 31,634 | 1808 | 1.00 | 15,911 | 695 | 1.00 | 15,723 | 1113 | 1.00 |
| BA acceleration < −1 | 112,888 | 5017 | 0.85 (0.81, 0.90) | 58,112 | 1941 | 0.84 (0.77, 0.92) | 54,776 | 3076 | 0.86 (0.80, 0.92) |
| BA acceleration > 1 | 91,664 | 7811 | 1.34 (1.27, 1.41) | 46,821 | 2733 | 1.22 (1.12, 1.34) | 44,843 | 5078 | 1.41 (1.32, 1.50) |
Participants with baseline KDM-BA data (n = 330,150) were included for this validation analysis. During the median follow-up of 13.82 (13.11, 14.53) years, 22,358 deaths occurred.
Cox proportional hazard regression models were used. Model 1 adjusted for baseline age. For male, model 2 adjusted for age, race and ethnicity, education, occupation, TDI, smoking status, alcohol consumption, beverage consumption, healthy diet, insomnia, depressive symptom, anxiety symptom, physical activity, body mass index, and chronic diseases. For female, model 2 additionally adjusted for menopause status.
Associations of change in tea consumption status with change in KDM-BA acceleration
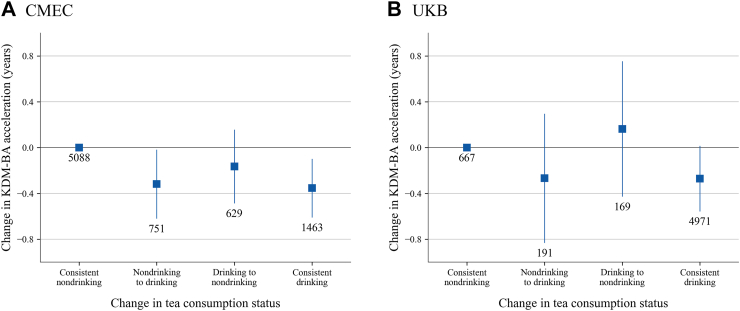
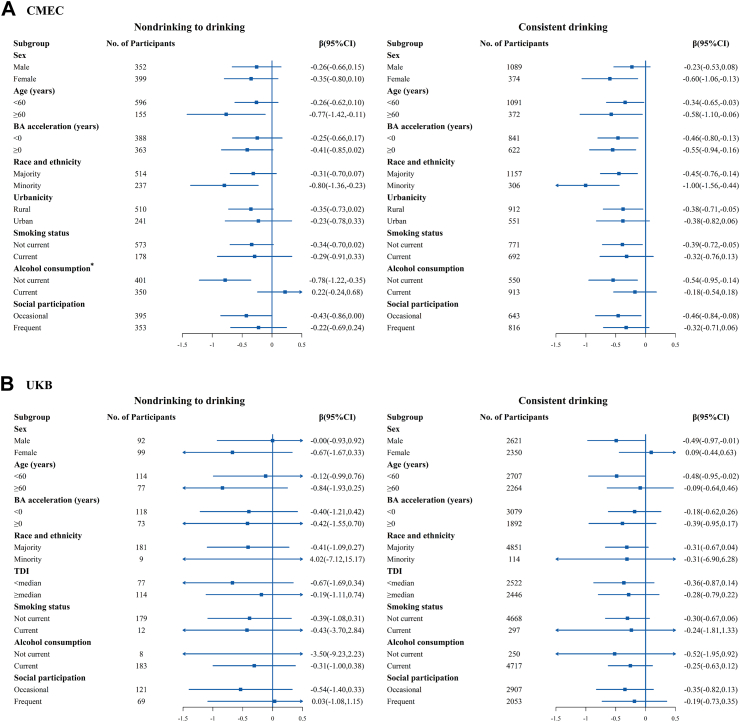
Fig. 2 shows the estimated associations of change in tea consumption status with change in KDM-BA acceleration after adjusting for potential confounders. Compared with consistent nondrinkers, participants who transitioned from nondrinking to drinking showed a decrease in KDM-BA acceleration in CMEC (β = −0.319, 95% CI: −0.620 to −0.017 years), with a slightly stronger association observed for consistent drinkers (β = −0.354, 95% CI: −0.610 to −0.098 years). In UKB, similar trends were found for the transition from nondrinking to drinking (β = −0.267, 95% CI: −0.831 to 0.297 years) and consistent drinking (β = −0.354, 95% CI: −0.610 to −0.098 years). While these two associations were not statistically significant in UKB, possibly due to the relatively small sample sizes of the transition group (n = 191) and nondrinkers (n = 667), the direction and magnitude of associations remained largely consistent across both cohorts. For those who stopped consuming tea (transition from drinking to nondrinking), the estimates showed opposite directions in CMEC (β = −0.165, 95% CI: −0.487 to 0.158 years) and UKB (β = 0.164, 95% CI: −0.428 to 0.756 years), although neither was statistically significant. In the stratified analysis, the results consistently showed the same direction across most subgroups in both cohorts (Fig. 3). Notably, the protective benefits of tea were more pronounced in participants who did not consume alcohol compared to those who did, especially for the transition from non-tea drinking to drinking, suggesting that alcohol consumption might attenuate the anti-aging effects of tea.
Fig. 2.
Estimated associations between change in tea consumption status and change in BA acceleration. Results were adjusted for demographics and self-reported diseases at baseline: sex, race and ethnicity, education, TDI (UKB only), urbanicity (CMEC only); and the baseline and concurrent changes of time-variant variables: age, occupation, marital status (CMEC only), menopause status in women, smoking status, alcohol consumption, beverage consumption, healthy diet, total energy intake (CMEC only), insomnia, depressive symptom, anxiety symptom, physical activity, and body mass index. The boxes represent point estimations and error bars represent 95% CI. The numbers below error bars are numbers of participants in each group.
Fig. 3.
Stratified analysis of estimated associations between change in tea consumption status and change in BA acceleration according to predefined characteristics. All models were adjusted for demographics and self-reported diseases at baseline: sex, race and ethnicity, education, TDI (UKB only), urbanicity (CMEC only); and the baseline and concurrent changes of time-variant variables: age, occupation, marital status (CMEC only), menopause status in women, smoking status, alcohol consumption, beverage consumption, healthy diet, total energy intake (CMEC only), insomnia, depressive symptom, anxiety symptom, physical activity, and body mass index, with exclusion of the stratified variable as appropriate. The boxes represent point estimations. Horizontal lines represent 95% CI. ∗Heterogeneity test: P = 0.002.
Prospective exposure-response relationship between tea consumption and BA acceleration
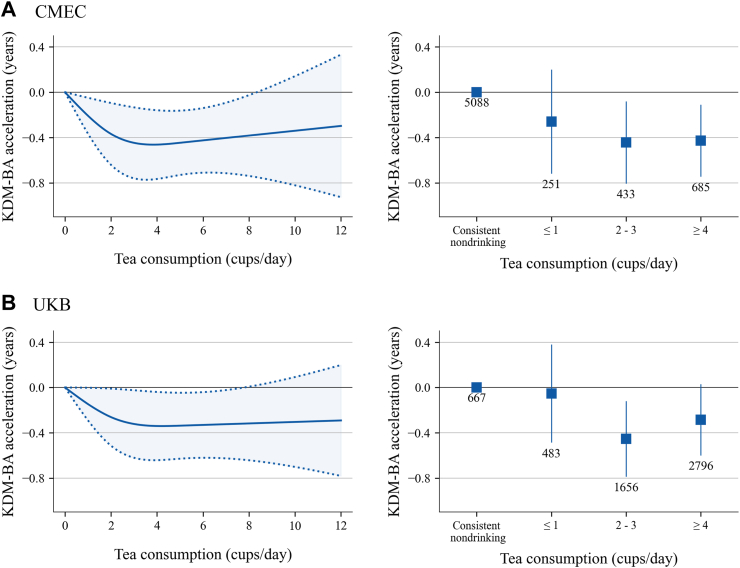
The prospective associations between tea consumption and KDM-BA acceleration among individuals with a constant tea consumption status are presented in Fig. 4. A non-linear exposure-response association was found in restricted cubic splines in both CMEC and UKB, with the curve declining up to approximately 3 cups/day and then plateauing. These splines were estimated using one of the five imputed data sets in CMEC and UKB respectively, and the results estimated by the remaining data sets can be found in Supplementary Figure S6. The results were robust when we categorized tea consumption into four groups. Compared to consistent nondrinkers, consistent drinkers who consumed 2–3 cups/day had the lowest KDM-BA acceleration in CMEC (β = −0.453, 95% CI: −0.815 to −0.090 years) and UKB (β = −0.454, 95% CI: −0.788 to −0.121 years) (Supplementary Table S14). The analyses using tea leaf amount as the exposure in CMEC suggested that a daily consumption of 6–8 g of tea leaves exhibited the strongest association (Supplementary Figure S7). In the stratified analysis, the directions and magnitudes of associations were roughly consistent across various subgroups in both cohorts, with only a few exceptions where estimates were unstable due to extremely small sample sizes (Supplementary Figure S8).
Fig. 4.
Estimated prospective associations between tea consumption and BA acceleration. Results were adjusted for the baseline BA acceleration and baseline covariates: age, sex, race and ethnicity, education, occupation, marital status (CMEC only), menopause status in women, TDI (UKB only), urbanicity (CMEC only), smoking status, alcohol consumption, beverage consumption, healthy diet, total energy intake (CMEC only), insomnia, depressive symptom, anxiety symptom, physical activity, body mass index, and self-reported diseases. The left panel of the figure displays the non-linear relationships between tea consumption and BA acceleration fitted using restricted cubic spline. The right panel displays the estimated associations of tea consumption fitted in models as a categorical variable with BA acceleration. The boxes represent point estimations and error bars represent 95% CI. The numbers below error bars are numbers of participants in each group.
Sensitivity analysis
First, the results remained robust in both cohorts regardless of the definition of KDM-BA acceleration (Supplementary Figures S9 and S10). Second, the association persisted in the same direction in the healthy populations, but with a modest attenuation in CMEC and an increase in UKB (Supplementary Figures S11 and S12). Third, results from Cox proportional hazard regression models also exhibited a non-linear relationship between tea consumption and incident accelerated biological aging, with around 3 cups/day showing the lowest hazard ratio (Supplementary Figures S13 and S14). Fourth, the results were similar when using complete case samples, except for slightly increased magnitudes of associations (Supplementary Figures S15 and S16). Last, the E-value for the estimated associations between change in tea consumption status and change in KDM-BA acceleration by comparing consistent drinkers and consistent nondrinkers was 2.1 in CMEC and 1.9 in UKB (Supplementary Table S15 and S16), which indicated that the observed estimates could be explained away by an unmeasured confounder that was associated with both the exposure and the outcome by a risk ratio of 2.1 (or 1.9)-fold each, above and beyond the measured confounders, but weaker confounding could not do so.
Discussion
Summary of main results
In the two independent cohorts of CMEC and UKB, tea consumption was found to be consistently associated with attenuated biological aging acceleration measured by KDM-BA. Both the transition from nondrinking to drinking and consistent drinking were inversely associated with BA acceleration compared to consistent non-drinking. These associations were roughly consistent in various subgroups. Furthermore, the exposure-response relationship suggested that consuming 3 cups of tea or 6–8 g of tea leaves per day may offer the most evident anti-aging benefits.
Comparison with previous studies
Our findings are in line with previous evidence showing that tea consumption may delay biological aging in humans.27, 28, 29, 30, 31,35 Most previous studies used telomere length or FI as indicators of biological aging,27, 28, 29, 30, 31 while only one study used composite biomarker BA.35 Previous studies indicated that different types of aging measures correlate weakly with each other and may reflect different aspects of the aging process.25,26 Molecular measures, such as epigenetic clocks, telomere length, and omics age have been proposed based on age-related alterations at cellular and molecular levels.16, 17, 18 For example, telomeres are repetitive DNA sequences that protect chromosome ends, shortening with each cell division. When telomeres shorten enough to become deprotected, cellular senescence may be accelerated.48 Functional age, such as FI, however, reflects overt age-related functional decline and health deficits such as, symptoms, diseases, and disabilities.22, 23, 24 In contrast, composite biomarker BA captures age-related changes in physiological integrity from multiple organs and systems.19,21,34,49 In fact, aging is a multidimensional deterioration in biological homeostasis and integrity that begins at cellular levels, then progresses to multiple organs and systems, and ultimately affects whole-body functions.15 Our findings, along with previous studies, further confirm the hypothesis that tea drinking may delay human aging from different perspectives.
In the only one cross-sectional study that explored tea consumption and composite biomarker BA, including 2844 Singaporean participants aged 55–94 years, the consumption of at least 1 cup of tea per day was negatively associated with KDM-BA acceleration.35 Though KDM was also used in our study to estimate BA for participants from CMEC and UKB, the selected biomarkers were not exactly the same among different cohorts due to data availability and distinct aging phenotypes. As a result, the BA estimates and association sizes were not directly comparable across populations. However, the diverse populations and aging biomarkers enhanced the consistency of our findings. Besides, epidemiological studies focusing on the composite biomarker BA, not just the association between tea consumption and composite biomarker BA, have mostly used cross-sectional data.35,50,51 One possible reason could be the scarcity of data resources for large cohort studies with multiple measurements of biomarkers, which limited the implementation of longitudinal studies on BA as well as the development of novel aging predictors.
In our change-to-change analysis, we found that both the transition from nondrinking to drinking and consistent tea consumption were associated with lower BA acceleration, with slightly stronger associations for consistent tea consumption in both CMEC and UKB. Rare epidemiological studies have investigated the behavioral changes from nondrinking to drinking tea. Even in most longitudinal studies with multiple assessments of tea consumption, the transition from nondrinking to drinking was usually classified as inconsistent tea-drinking, along with the transition from drinking to nondrinking.30,52 One possible explanation for this is that most longitudinal studies mainly focused on morbidity or mortality outcomes, which are unsuitable for change analysis. Additionally, sample sizes of participants who transitioned from nondrinking to drinking were generally small, resulting in low statistical power. Importantly, randomized clinical trials have the potential to study behavioral changes. However, the conclusions of clinical trials on the benefits of tea or tea extracts have remained controversial,53, 54, 55, 56 possibly due to their short-term nature that precludes the observation of potential protective effects of tea. Our results suggested that the transition from nondrinking to drinking within at least a 2-year follow-up has been associated with attenuated BA acceleration, although we had no information on when the change started. Furthermore, our study highlighted the importance of consistent tea consumption, which has also been reported to provide more evident beneficial effects in many prospective cohort studies.30,52,57
Our study may shed light on future clinical trials, not just on intervention durations but also on intervention doses. We found a non-linear relationship between tea consumption and BA acceleration, suggesting that 3 cups or 6–8 g of tea leaves per day might provide the most evident anti-aging benefits. A growing number of population-based studies also reported nonlinear relationships between tea consumption and age-related diseases.8,10,11 For example, a large cohort study suggested that around 3 cups of tea per day provided the best prevention against dementia.11 Similar findings have also been observed between coffee consumption and cardiovascular risks.58 Possible mechanisms might involve the hormesis of bioactive substances in tea, which is characterized by low-dose stimulation and high-dose inhibition.59 In a Caenorhabditis elegans (C. elegans) model, the effect of EGCG (a green tea polyphenol) on C. elegans lifespan showed an inverted U-shaped dose-response relationship.60 In addition, tea polyphenol (EGCG) is believed to have hepatotoxicity, and green tea-associated acute liver injury has been widely discussed.61, 62, 63 Excessive tea consumption may also increase caffeine intake, which has been associated with headache, hypertension, anxiety and restlessness.64
Potential anti-aging mechanisms of tea
Tea contains various bioactive compounds, such as polyphenols, purine alkaloids, theanine, tea polysaccharide and caffeine, which may be related to its potential anti-aging effects. Polyphenols are the main bioactive substances in tea and have been extensively studied for their functions in oxidative stress, inflammation response, epigenetic alterations, mitochondrial activation, and autophagy.5,6,65,66 Moreover, polyphenols have been reported to modulate gut microbiota,6,67 which might have an important effect on regulating age-related changes in immunity, metabolism, and cognitive function.68,69 Tea polyphenols in fresh leaves can transfer into other derivatives during the manufacturing process of different types of tea, such as theaflavins in black tea. Thus, the contents of the main bioactive compounds vary greatly in different tea types, which might result in the heterogeneity of their beneficial effects.67 While we did not perform subgroup analyses stratified by tea types within each cohort, it can be mentioned that we did not observe substantial differences in the association sizes between the CMEC and UKB cohorts, where green tea and black tea are the predominant types, respectively. Given the potential anti-aging properties of tea and its components, they have been considered as anti-aging candidates. Further research is needed to clarify the precise anti-aging mechanisms of tea and its components, and to evaluate their efficacy, safety, and bioavailability in vivo.
Strengths and limitations
To our knowledge, this is by far the first longitudinal study examining the association between changes in tea consumption status and changes in BA acceleration. We constructed a validated aging indicator that is modifiable and conducted change-to-change analyses, which produced evidence comparable to randomized clinical trials and offers unique insights for guiding nutrition-oriented anti-aging interventions. This type of study has been rarely performed before due to the lack of repeated measurements of clinical biomarkers that were required to construct BA. Besides, we provided more accurate estimates of the prospective exposure-response relationship between tea consumption and BA acceleration by exploring it among individuals with constant tea consumption status. Furthermore, our findings were cross-validated by incorporating two independent cohorts from Southwest China and the UK, and the findings were generally consistent within both cohorts regardless of huge differences in genetics, demographics, behaviors and lifestyles.
Nevertheless, several limitations should be noted. First, the information on tea consumption was self-reported, potentially introducing the risk of recall bias and misclassification. Tea consumption status (a binary variable) was less susceptible, and we used information on tea consumption status from both the baseline and repeated surveys and gave consideration to changes in status. However, quantification of tea drinking could still be subject to measurement errors. Notably, in UKB the size of tea cups used by participants was not identified. Second, due to data availability, we were unable to cover all clinical biomarkers associated with aging and thus the constructed BA could only capture specific aspects of the aging process. Third, we failed to investigate the potential effect heterogeneity among different tea types on biological aging. In UKB, data from touchscreen questionnaires used in this study did not provide information on tea types; instead, tea type information was collected through 24-h dietary questionnaires. We did not use 24-h dietary questionnaire data in our analysis because they were not collected concurrently with the biomarker data, which does not align with the requirements of the change-to-change design that exposure and outcome data be measured simultaneously. Despite this, these data have shown that nearly 90% of tea drinkers predominantly drank black tea.14 In CMEC, the majority of participants consumed green tea, with only a few individuals consuming other types. Future studies on different tea types and biological aging are still warranted. Fourth, even in a prospective study design, reverse causality may still exist. For example, tea drinking habits might be influenced by disease status. However, we adjusted for major chronic diseases at baseline in our main analyses and excluded those with major chronic diseases in our sensitivity analyses. Fifth, although the change-to-change design is not susceptible to unmeasured time-invariant confounding, and we have carefully controlled for potential confounders identified by DAG, due to the intrinsic nature of an observational study, residual confounding from unmeasured time-varying factors is still inevitable. Thus, causal conclusions should be interpreted prudently. Finally, although we included two independent populations in the present study, our findings should be generalized with caution. Both the CMEC and UKB studies have limitations in terms of their representativeness. Neither study achieved national coverage, and their age and sex distributions may not perfectly represent the demographics of the entire country's population. The CMEC participants were drawn from Southwest China and the Tibetan population was excluded from this study because of their unique tea drinking habits. The UKB exhibited an extremely low response rate (5.5%) and showed evidence of a potential “healthy volunteer” selection bias.37 Additionally, survival bias was possible because only surviving participants could attend the follow-up survey. Selection bias arising from missing data may also limit the generalizability of our study. In both cohorts, the sample sizes of participants with available BA data at both baseline and follow-up were noticeably small. Furthermore, due to the stability of tea-drinking behavior, the sample sizes of those who changed their habits were small, particularly in UKB, leading to less stable estimates and wide confidence intervals. Therefore, future prospective studies in other populations are required to test the external validity of these findings.
Conclusion
In summary, based on longitudinal data from CMEC and UKB, tea consumption was associated with the attenuation of BA acceleration measured by KDM. Both the transition from nondrinking to drinking and consistent tea consumption might have the potential to modestly mitigate the acceleration of BA, with stronger associations observed for consistent tea drinkers. Moreover, a non-linear relationship between tea consumption and BA acceleration implied the most evident protective association with daily moderate tea consumption of approximately 3 cups or 6–8 g of tea leaves. Given that tea is a worldwide beverage, our findings highlight its potential role in promoting healthy aging within the global aging population, which may have significant implications for guiding public health policies. In addition, our study provides valuable insights for developing and evaluating nutrition-oriented anti-aging interventions in future clinical trials.
Contributors
YX, XX, and XZ conceptualized the present study. XZ was the principal investigator and JY was the co-principal investigator of the CMEC study. YX conducted data analysis, wrote, and revised the manuscript. XX and XH coordinated the data collection, reviewed, and revised the manuscript. HC, DT, ZH, YZ, Zhenghong W, Ziyun W, LY, and MH contributed to the data collection, data management, and data cleaning. JY, XX, and XZ reviewed and commented on the data analysis, all drafts, and the final paper. All authors offered critical comments on all drafts of the report.
Data sharing statement
The CMEC data are accessible by contacting the corresponding author. The UKB data are publicly available on application at www.ukbiobank.ac.uk.
Declaration of interests
The authors declare no conflict of interest.
Acknowledgements
The CMEC study was funded by the National Key R&D Program of China (Grant No. 2017YFC0907300). We thank all the participants and team members involved in the CMEC study for their contributions. We are grateful to Prof. Xiaosong Li at Sichuan University, the former principal investigator of the CMEC study, for his leadership and fundamental contribution to the establishment of the CMEC. Prof. Li passed away in 2019. This research has been conducted using the UK Biobank Resource under Application Number 117185.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100955.
Contributor Information
Xiong Xiao, Email: xiaoxiong.scu@scu.edu.cn.
Xing Zhao, Email: xingzhao@scu.edu.cn.
Appendix A. Supplementary data
References
- 1.WHO . 2022. Ageing and health.https://www.who.int/news-room/fact-sheets/detail/ageing-and-health [Google Scholar]
- 2.Campisi J., Kapahi P., Lithgow G.J., Melov S., Newman J.C., Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571(7764):183–192. doi: 10.1038/s41586-019-1365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barzilai N., Cuervo A.M., Austad S. Aging as a biological target for prevention and therapy. JAMA. 2018;320(13):1321–1322. doi: 10.1001/jama.2018.9562. [DOI] [PubMed] [Google Scholar]
- 4.Alam M., Ali S., Ashraf G.M., Bilgrami A.L., Yadav D.K., Hassan M.I. Epigallocatechin 3-gallate: from green tea to cancer therapeutics. Food Chem. 2022;379 doi: 10.1016/j.foodchem.2022.132135. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal V., Tuli H.S., Tania M., et al. Molecular mechanisms of action of epigallocatechin gallate in cancer: recent trends and advancement. Semin Cancer Biol. 2022;80:256–275. doi: 10.1016/j.semcancer.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Wu M., Luo Q., Nie R., Yang X., Tang Z., Chen H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit Rev Food Sci Nutr. 2021;61(13):2175–2193. doi: 10.1080/10408398.2020.1773390. [DOI] [PubMed] [Google Scholar]
- 7.Pallauf K., Duckstein N., Rimbach G. A literature review of flavonoids and lifespan in model organisms. Proc Nutr Soc. 2017;76(2):145–162. doi: 10.1017/S0029665116000720. [DOI] [PubMed] [Google Scholar]
- 8.Chung M., Zhao N., Wang D., et al. Dose-response relation between tea consumption and risk of cardiovascular disease and all-cause mortality: a systematic review and meta-analysis of population-based studies. Adv Nutr. 2020;11(4):790–814. doi: 10.1093/advances/nmaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nie J., Yu C., Guo Y., et al. Tea consumption and long-term risk of type 2 diabetes and diabetic complications: a cohort study of 0.5 million Chinese adults. Am J Clin Nutr. 2021;114(1):194–202. doi: 10.1093/ajcn/nqab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Yang H., Li S., Li W.D., Wang Y. Consumption of coffee and tea and risk of developing stroke, dementia, and poststroke dementia: a cohort study in the UK Biobank. PLoS Med. 2021;18(11) doi: 10.1371/journal.pmed.1003830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu H.Y., Wu B.S., Ou Y.N., et al. Tea consumption and risk of incident dementia: a prospective cohort study of 377 592 UK Biobank participants. Transl Psychiatry. 2022;12(1):171. doi: 10.1038/s41398-022-01923-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim T.L., Jeong G.H., Yang J.W., et al. Tea consumption and risk of cancer: an umbrella review and meta-analysis of observational studies. Adv Nutr. 2020;11(6):1437–1452. doi: 10.1093/advances/nmaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin S., Lee J.E., Loftfield E., et al. Coffee and tea consumption and mortality from all causes, cardiovascular disease and cancer: a pooled analysis of prospective studies from the Asia Cohort Consortium. Int J Epidemiol. 2022;51(2):626–640. doi: 10.1093/ije/dyab161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue-Choi M., Ramirez Y., Cornelis M.C., Berrington de Gonzalez A., Freedman N.D., Loftfield E. Tea consumption and all-cause and cause-specific mortality in the UK Biobank: a prospective cohort study. Ann Intern Med. 2022;175(9):1201–1211. doi: 10.7326/M22-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrucci L., Levine M.E., Kuo P.L., Simonsick E.M. Time and the metrics of aging. Circ Res. 2018;123(7):740–744. doi: 10.1161/CIRCRESAHA.118.312816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannum G., Guinney J., Zhao L., et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49(2):359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10) doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackburn E.H., Greider C.W., Szostak J.W. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12(10):1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 19.Klemera P., Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127(3):240–248. doi: 10.1016/j.mad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Cohen A.A., Milot E., Yong J., et al. A novel statistical approach shows evidence for multi-system physiological dysregulation during aging. Mech Ageing Dev. 2013;134(3–4):110–117. doi: 10.1016/j.mad.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z., Kuo P.L., Horvath S., Crimmins E., Ferrucci L., Levine M. A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: a cohort study. PLoS Med. 2018;15(12) doi: 10.1371/journal.pmed.1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitnitski A.B., Mogilner A.J., Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rockwood K., Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol Ser A Biol Sci Med Sci. 2007;62(7):722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 24.Searle S.D., Mitnitski A., Gahbauer E.A., Gill T.M., Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belsky D.W., Moffitt T.E., Cohen A.A., et al. Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol. 2018;187(6):1220–1230. doi: 10.1093/aje/kwx346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jylhava J., Pedersen N.L., Hagg S. Biological age predictors. EBioMedicine. 2017;21:29–36. doi: 10.1016/j.ebiom.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan R., Woo J., Suen E., Leung J., Tang N. Chinese tea consumption is associated with longer telomere length in elderly Chinese men. Br J Nutr. 2010;103(1):107–113. doi: 10.1017/S0007114509991383. [DOI] [PubMed] [Google Scholar]
- 28.Sohn I., Shin C., Baik I. Associations of green tea, coffee, and soft drink consumption with longitudinal changes in leukocyte telomere length. Sci Rep. 2023;13(1):492. doi: 10.1038/s41598-022-26186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nanri H., Yoshida T., Watanabe Y., et al. The association between habitual green tea consumption and comprehensive frailty as assessed by Kihon checklist indexes among an older Japanese population. Nutrients. 2021;13(11):4149. doi: 10.3390/nu13114149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao T., Han S., Mo G., Sun Q., Zhang M., Liu H. Long-term tea consumption reduces the risk of frailty in older Chinese people: result from a 6-year longitudinal study. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.916791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S., Cui G., Yin Y., Lv F., Yao Y. Association between tea consumption and frailty among Chinese older adults: a cross-sectional study. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.987911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan M.S., Arnold M., Offer A., et al. A biomarker-based biological age in UK Biobank: composition and prediction of mortality and hospital admissions. J Gerontol Ser A Biol Sci Med Sci. 2021;76(7):1295–1302. doi: 10.1093/gerona/glab069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z. Development and validation of 2 composite aging measures using routine clinical biomarkers in the Chinese population: analyses from 2 prospective cohort studies. J Gerontol Ser A Biol Sci Med Sci. 2021;76(9):1627–1632. doi: 10.1093/gerona/glaa238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine M.E. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol Ser A Biol Sci Med Sci. 2013;68(6):667–674. doi: 10.1093/gerona/gls233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng T.P., Zhong X., Gao Q., Gwee X., Chua D.Q.L., Larbi A. Socio-environmental, lifestyle, behavioural, and psychological determinants of biological ageing: the Singapore longitudinal ageing study. Gerontology. 2020;66(6):603–613. doi: 10.1159/000511211. [DOI] [PubMed] [Google Scholar]
- 36.Zhao X., Hong F., Yin J., et al. Cohort profile: the China multi-ethnic cohort (CMEC) study. Int J Epidemiol. 2021;50(3) doi: 10.1093/ije/dyaa185. 721–721l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fry A., Littlejohns T.J., Sudlow C., et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferguson K.D., McCann M., Katikireddi S.V., et al. Evidence synthesis for constructing directed acyclic graphs (ESC-DAGs): a novel and systematic method for building directed acyclic graphs. Int J Epidemiol. 2020;49(1):322–329. doi: 10.1093/ije/dyz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearl J., Glymour M.M., Jewell N.P. 2016. Causal inference in statistics: a primer. [Google Scholar]
- 40.Kwon D., Belsky D.W. A toolkit for quantification of biological age from blood chemistry and organ function test data: BioAge. GeroScience. 2021;43(6):2795–2808. doi: 10.1007/s11357-021-00480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan J., Yu C., Pang Y., et al. Adherence to healthy lifestyle and attenuation of biological aging in middle-aged and older Chinese adults. J Gerontol Ser A. 2021;76(12):2232–2241. doi: 10.1093/gerona/glab213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gunasekara F.I., Richardson K., Carter K., Blakely T. Fixed effects analysis of repeated measures data. Int J Epidemiol. 2014;43(1):264–269. doi: 10.1093/ije/dyt221. [DOI] [PubMed] [Google Scholar]
- 43.Smith J.D., Hou T., Hu F.B., et al. A comparison of different methods for evaluating diet, physical activity, and long-term weight gain in 3 prospective cohort studies. J Nutr. 2015;145(11):2527–2534. doi: 10.3945/jn.115.214171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tennant P.W.G., Arnold K.F., Ellison G.T.H., Gilthorpe M.S. Analyses of 'change scores' do not estimate causal effects in observational data. Int J Epidemiol. 2022;51(5):1604–1615. doi: 10.1093/ije/dyab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lehallier B., Gate D., Schaum N., et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat Med. 2019;25(12):1843–1850. doi: 10.1038/s41591-019-0673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 47.VanderWeele T.J., Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 48.Blackburn E.H., Epel E.S., Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350(6265):1193–1198. doi: 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- 49.Li Q., Wang S., Milot E., et al. Homeostatic dysregulation proceeds in parallel in multiple physiological systems. Aging Cell. 2015;14(6):1103–1112. doi: 10.1111/acel.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao X., Huang N., Guo X., Huang T. Role of sleep quality in the acceleration of biological aging and its potential for preventive interaction on air pollution insults: findings from the UK Biobank cohort. Aging Cell. 2022;21(5) doi: 10.1111/acel.13610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao X., Ma C., Zheng Z., et al. Contribution of life course circumstances to the acceleration of phenotypic and functional aging: a retrospective study. EClinicalMedicine. 2022;51 doi: 10.1016/j.eclinm.2022.101548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X., Liu F., Li J., et al. Tea consumption and the risk of atherosclerotic cardiovascular disease and all-cause mortality: the China-PAR project. Eur J Prev Cardiol. 2020;27(18):1956–1963. doi: 10.1177/2047487319894685. [DOI] [PubMed] [Google Scholar]
- 53.Nagao T., Hase T., Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity. 2007;15(6):1473–1483. doi: 10.1038/oby.2007.176. [DOI] [PubMed] [Google Scholar]
- 54.Igho-Osagie E., Cara K., Wang D., et al. Short-term tea consumption is not associated with a reduction in blood lipids or pressure: a systematic review and meta-analysis of randomized controlled trials. J Nutr. 2020;150(12):3269–3279. doi: 10.1093/jn/nxaa295. [DOI] [PubMed] [Google Scholar]
- 55.Zhang S., Takano J., Murayama N., et al. Subacute ingestion of caffeine and oolong tea increases fat oxidation without affecting energy expenditure and sleep architecture: a randomized, placebo-controlled, double-blinded cross-over trial. Nutrients. 2020;12(12):3671. doi: 10.3390/nu12123671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hodgson J.M., Croft K.D., Woodman R.J., et al. Black tea lowers the rate of blood pressure variation: a randomized controlled trial. Am J Clin Nutr. 2013;97(5):943–950. doi: 10.3945/ajcn.112.051375. [DOI] [PubMed] [Google Scholar]
- 57.Li X., Yu C., Guo Y., et al. Tea consumption and risk of ischaemic heart disease. Heart. 2017;103(10):783–789. doi: 10.1136/heartjnl-2016-310462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding M., Bhupathiraju S.N., Satija A., van Dam R.M., Hu F.B. Long-term coffee consumption and risk of cardiovascular disease a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation. 2014;129(6):643–659. doi: 10.1161/CIRCULATIONAHA.113.005925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calabrese E.J., Baldwin L.A. Defining hormesis. Hum Exp Toxicol. 2002;21(2):91–97. doi: 10.1191/0960327102ht217oa. [DOI] [PubMed] [Google Scholar]
- 60.Xiong L.G., Chen Y.J., Tong J.W., Gong Y.S., Huang J.A., Liu Z.H. Epigallocatechin-3-gallate promotes healthy lifespan through mitohormesis during early-to-mid adulthood in Caenorhabditis elegans. Redox Biol. 2018;14:305–315. doi: 10.1016/j.redox.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Navarro V.J., Khan I., Björnsson E., Seeff L.B., Serrano J., Hoofnagle J.H. Liver injury from herbal and dietary supplements. Hepatology. 2017;65(1):363–373. doi: 10.1002/hep.28813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoofnagle J.H., Bonkovsky H.L., Phillips E.J., et al. HLA-B∗35:01 and green tea-induced liver injury. Hepatology. 2021;73(6):2484–2493. doi: 10.1002/hep.31538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lambert J.D., Kennett M.J., Sang S., Reuhl K.R., Ju J., Yang C.S. Hepatotoxicity of high oral dose (-)-epigallocatechin-3-gallate in mice. Food Chem Toxicol. 2010;48(1):409–416. doi: 10.1016/j.fct.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gonzalez de Mejia E., Ramirez-Mares M.V. Impact of caffeine and coffee on our health. Trends Endocrinol Metab. 2014;25(10):489–492. doi: 10.1016/j.tem.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 65.Khan N., Mukhtar H. Tea polyphenols in promotion of human health. Nutrients. 2019;11(1):39. doi: 10.3390/nu11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu K., Ouyang J., Huang J., Liu Z. Research progress of black tea thearubigins: a review. Crit Rev Food Sci Nutr. 2021;61(9):1556–1566. doi: 10.1080/10408398.2020.1762161. [DOI] [PubMed] [Google Scholar]
- 67.Zhang L., Ho C.-T., Zhou J., Santos J.S., Armstrong L., Granato D. Chemistry and biological activities of processed Camellia sinensis teas: a comprehensive review. Compr Rev Food Sci Food Saf. 2019;18(5):1474–1495. doi: 10.1111/1541-4337.12479. [DOI] [PubMed] [Google Scholar]
- 68.O'Toole P.W., Jeffery I.B. Gut microbiota and aging. Science. 2015;350(6265):1214–1215. doi: 10.1126/science.aac8469. [DOI] [PubMed] [Google Scholar]
- 69.DeJong E.N., Surette M.G., Bowdish D.M.E. The gut microbiota and unhealthy aging: disentangling cause from consequence. Cell Host Microbe. 2020;28(2):180–189. doi: 10.1016/j.chom.2020.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.