Abstract
This study investigated the impacts of Wooden Breast (WB) abnormality on in vitro protein digestibility and cytotoxicity of cooked chicken breast meat. Chicken breasts without (non-WB, n = 6) or with severe WB condition (WB, n = 6) were cooked and subjected to static in vitro protein digestion. The results showed no significant differences in free-NH2, degree of hydrolysis and distribution of peptide molecular weight between non-WB and WB samples at late intestinal digestion (P5), suggesting no adverse effects of WB on protein digestibility. Based on peptidomic analysis, P5 fraction of WB showed greater content of peptides with oxidative modification than that of non-WB. Untargeted metabolomics did not find any metabolites with potential toxicity either in non-WB and WB. Hydrolyzed non-WB and WB (1.56–100 µg/mL) did not affect viability of Caco-2 and Vero cells but addition of WB samples reduced Caco-2 cell viability compared with non-WB.
Key words: in vitro protein digestion, peptidomics, metabolomics, cell cytotoxicity, Wooden Breast
INTRODUCTION
An occurrence of growth-related myopathies, including White Striping (WS), Wooden Breast (WB), and Spaghetti Meat (SM), has raised a wide concern among broiler industry in the past decade. Among the three myopathies, WB, characterized by hardened ridges extending from the cranial to the caudal regions of the breast, exerts severe deleterious effects on chicken breast quality. The abnormality is occasionally covered by viscous fluid with surface hemorrhage (Sihvo et al., 2014). Despite of an unknown actual causes, WB myopathy has been associated with an artificial selection for fast growth rate and enlarged muscle mass (Malila et al., 2018; Sihvo et al., 2018; Lake et al., 2021). Circulatory and respiratory systems of the fast-growing chickens are shown to be impaired (Lake et al., 2020). The vascular deficiency, leading to limited oxygenation, have been considered the main condition giving rise to the biological cascades, particularly oxidative stress and metabolic changes, responsible for WB development (Abasht et al., 2016; Lake et al., 2021; Malila et al., 2021). Increased reactive oxygen species (ROS) was found in WB muscle compared with non-WB ones, supporting the increased oxidative condition within the abnormal muscles (Pan et al., 2021).
A majority of previous studies has extensively reported impact of WB abnormality on chemical composition and technological properties of the affected chicken breasts. As shown, the WB meat consistently exhibited decreased proportion of protein, increased fat, reduced water holding capacity along with increased hardness in texture (Soglia et al., 2016; Tasoniero et al., 2016; Cai et al., 2018; Malila et al., 2018; Thanatsang et al., 2020). However, no adverse effects of WB consumption on human health were reported.
In our recent study (Thanatsang et al., 2020), increased protein carbonyl content, an indicator of protein oxidation in meat system, together with reduced proportion of essential amino acids were observed in WB samples compared with that of non-WB group. Later, similar findings have been reported (Li et al., 2022). Protein carbonylation generally results in polymerization and aggregation among the oxidized proteins themselves or between the derivatives and other polypeptide chains which might impact protein digestibility. On the other hand, in the severe environment, the carbonyls can further attack the peptide backbone, leading to breakdown of the polypeptides and increased carbonyl-containing peptides (Domínguez et al., 2022). In the study of Soglia et al. (2019), an increase in concentration of free amino acids was observed in raw WB meat, suggesting the breakdown of muscular protein due to myogeneration within the abnormal muscle. The released amino acids could be lost with dripping fluids and during cooking. More importantly, ROS may influence modification of proteins, resulting in the conversion of essential amino acids to oxidative derivatives (Stadtman and Levine, 2003). Oxidized form of essential amino acids, particularly sulfur-containing amino acids, could limit their nutritional availability (Rutherfurd and Moughan, 2012). Once consumed, oxidized proteins could induce in vivo oxidative stress and pathological conditions in the lumen (Estévez and Xiong, 2019; Keller et al., 2020). The chronic oxidative stress is not only responsible for the virulence and severity of the disease but also the oxidative DNA damage of the epithelial cells (Hardbower et al., 2013).
Together, we hypothesize that oxidative stress occurred within the WB samples might reduce protein digestibility as well as its nutritive value within chicken breasts. In addition, ones may assume adverse health effects due to the consumption of WB chicken breast. However, such investigation has not been reported. Generally, when detected, chicken breasts affected with WB condition was removed from the poultry processing line (Caldas-Cueva and Owens, 2020). However, the detection in several plants depends on laborious visual evaluation; hence, some abnormal meat may not be graded. In addition, in some regions that the issues of growth-related myopathies are not widely recognized, the abnormal meat can be consumed.
An application of in vitro protein digestion has become more common in food science research to investigate the quality of dietary protein (Butts et al., 2012). This is because concentration of components in foods alone is not a complete determinant reflecting nutritional quality of the foods. In addition, the biological utilization of a protein primarily depends its digestibility and bioavailability which can be altered by structural changes of the protein (Swaisgood and Catignani, 1991). The in vitro method offers such investigation through a series of enzymatic reactions under gastrointestinal conditions in the human system including oral, gastric, and duodenal digestion (Trithavisup et al., 2022). Although the in vitro method may not completely mimic physiological complexity of the digestive tract, it does not entail any ethical aspect in comparison to the in vivo study. Indeed, an agreement between these in vitro results and in vivo rat true fecal nitrogen digestibility was documented (Butts et al., 2012).
The objective of this study was to investigate the influences of WB abnormality on in vitro protein digestibility, oxidative modification of proteins and peptides of cooked chicken meat. In addition, metabolic profiles and cell cytotoxicity were compared between non-WB and WB cooked meat.
MATERIALS AND METHODS
Samples
Chicken breast samples of Ross 308 broilers (49 d of age) were collected from one processing batch at the end of a manufacturing line of a local slaughterhouse (Nakhon Ratchasrima, Thailand). The meat was classified as “non-WB” or “WB” based on criteria of Sihvo et al. (2017). In brief, the meat with hardened consistency, which was consistent with the breasts graded as “WB1hard” in the study of Sihvo et al. (2017). On the other hand, “non-WB” showed no abnormal characteristics. A total of 12 breasts (n = 6 for non-WB and n = 6 for WB) was used in this study. Each meat sample was packed in a plastic zip-lock bag and kept at −30°C until further analyses. Upon the analysis, the breasts were thawed overnight at 4°C. Two pieces of meat were dissected from the cranial region as shown in Figure 1. The sample of location 1 was used for determining myofibril fragmentation index (MFI), protein carbonyl content, untargeted metabolomics and cell cytotoxicity. On the other hand, the meat dissected from location 2 was cooked and used for in vitro protein digestion.
Figure 1.
Chicken breasts with or without Wooden Breast (WB) abnormality. The dashed boxes indicate sampling locations. The samples from location 1 were used for determining myofibril fragmentation index, protein carbonylation, untargeted metabolomics and cell cytotoxicity. The samples from location 2 were cooked and used for in vitro protein digestion. The scale was in inch unit.
In Vitro Protein Digestion
Chicken breast samples, non-WB and WB, were prepared similarly described in Trithavisup et al. (2022). The samples were thawed at 4°C overnight. The samples (40 g) were individually vacuum-packed in a plastic bag and cooked at 95°C in water bath until temperature of the sample reached 75°C. The samples were then cooled in an iced water bath until the temperature reduced to 15°C and were left to rest at 4°C for at least 1 h. Moisture of the cooked non-WB and WB were determined using a halogen moisture analyzer (HX204, Mettler-Toledo, Switzerland). Crude protein of the cooked meat was examined following Kjeldahl method. A conversion factor of 6.25 was used in a calculation for crude protein.
In vitro protein digestion was conducted according to the method of Bordoni et al. (2014) with a slight modification. All steps of the enzymatic digestion were performed in a 50-mL centrifuge tube, stirred in a trayster digital (IKA TRAYSTER digital, IKA Works, Inc., Staufen, Germany) at 10 rpm in an incubator set at 37°C. To simulate oral digestion, 2 g (dry basis) of non-WB or WB samples were mixed with 4 mL of buffer solution (120 mM NaCl, 5 mM KCl, and 6 mM CaCl2, pH 6.9) containing 75 U/mL α-amylase. After amylase digestion for 5 min, 8 mL of the buffer was added and pH of the mixture was then decreased to pH 2.0 by adding 6N HCl. Pepsin was then added to a final concentration of 2,000 U/mL to simulate gastric digestion. The reaction was carried out for 60 min. Subsequently, 8 mL of the buffer solution was added and pH of the solution was then increased to pH 5.0 using 1.5M NaHCO3 to stop pepsin digestion. Pancreatin (final concentration of 100 U/mL based on trypsin) and bile salt (10 mM final concentration) were added to simulate intestinal digestion. The pH of the reaction was then adjusted to pH 6.0 with 1.5 M NaHCO3, and the digestion was conducted for 300 min. During the digestion, 2 mL of the mixture was collected at the beginning of gastric digestion (P1, after decreasing pH to 2.0, and before adding pepsin), at the end of the gastric digestion (P2), at the beginning of the intestinal phase (P3, after the increase of pH to 5.0), after 180 min of intestinal digestion (P4), and after 300 min of intestinal digestion (P5). To stop enzymatic reaction, the samples collected at P1 and P2 were adjusted to pH 8.0 with 35% NaOH. While the samples collected at P3, P4, and P5 were acidified to pH 2.0 with 6N HCl to stop pancreatic hydrolysis. All samples were centrifuged at 3,000 × g for 30 min. Supernatant was filtered through a 0.2-mm membrane. The filtered supernatants of all samples were stored at −20°C until further analyses, including bicinchoninic acid (BCA) assay and determination of free NH2 content based on trinitrobenzenesulfonic (TNBS) acid method. The samples with final protein concentration of 7 mg/mL were lyophilized to obtain sample powder and used for peptidomic analysis using a liquid chromatography-mass spectrometry/ mass spectrometry (LC-MS/MS).
Bicinchoninic Acid Assay
Protein concentration was determined using a Pierce BCA protein assay kit (Thermo Fishes Scientific, Rockford, IL) according to the manufacturer's instruction. Bovine serum albumin (BSA) was used as a protein standard. All reactions containing either supernatant from in vitro digestion or standards were performed in duplicates. Ten microliters of the sample or standard solutions were mixed with 200 µL of BCA working reagent and incubated at 37°C for 30 min. The absorbance at 562 nm was read in a microplate reader (SpectraMax Plus 384, Molecular Devices, LLC, San Jose, CA).
Trinitrobezenesulfonic Acid Method
Trinitrobezenesulfonic (TNBS) method was performed to determine free NH2 groups and degree of protein hydrolysis (%DH) using a method previously described (Kristoffersen et al., 2020). Briefly, supernatants from in vitro digestion were diluted 1:200 with 1% sodium dodecylsulfate (SDS) solution. A series of leucine solutions (0, 0.075, 0.15, 0.3, 0.6, 0.9, 1.2, and 1.5 mM) diluted using 1% SDS was prepared for standard curve construction. The assay was performed (in duplicate) in 96-well plates where 15 µL of samples or standard solutions were mixed with 45 µL of 0.21 M sodium phosphate buffer (pH 8.2) and 45 µL TNBS solution (0.05% w/v in water). The plate was wrapped using aluminum foil and incubated at 50°C for 1 h. To stop the reaction, 90 µL of 0.1N HCl was added. The absorbance at 340 nm was read in a microplate reader (SpectraMax Plus 384, Molecular Devices, LLC, San Jose, CA). Free NH2 content was expressed in the unit of micromoles per gram protein, as leucine amino equivalents, after subtraction of a blank. Degree of protein hydrolysis (%DH) was calculated using equations as follows.
where α = 1.00, β = 0.40 (Adler-Nissen, 1986), htot = 7.6 mmoL/g protein (Nielsen et al., 2001).
Myofibril Fragmentation Index
The values of myofibril fragmentation index (MFI) of non-WB and WB were determined following the method described by Hopkins et al. (2000). Chopped raw meat (0.5 g) was homogenized, on ice, at 13,500 rpm in 30 mL of ice-cold MFI buffer using an ULTRA-TURRAX T25 Basic homogenizer (IKA Werke, Staufen, Germany) equipped with an S25KV-18G dispersing probe (IKA Werke). Composition of MFI buffer includes 25 mM potassium phosphate buffer (pH 7.0), 0.1 M KCl, 1 mM EDTA and 1 mM sodium azide. Homogenization was carried out for 2 min (30 s on followed by 30 s rest, 2 cycles). The homogenate was filtered through two layers of gauzes. The filtrate was centrifuged at 1,000 × g for 10 min. The resulting pellet was resuspended in 20 mL of cold MFI buffer and subjected to another round of extraction process. The final pellet was resuspended in 10 mL of cold MFI buffer. The protein concentration of the suspensions was determined using a Pierce BCA Protein Assay Kit. An aliquot of suspension was diluted with cold MFI buffer to protein concentration of 0.5 mg/mL. The absorbance of a 2 mL-diluted myofibril suspension was measured at 540 nm using MFI buffer as blank. MFI was calculated by multiplying the average absorbance with 150 (Olson and Stromer, 1976). The experiment was performed in triplicates.
Protein Carbonyl Assay
Protein carbonyls in raw and cooked samples were determined following the method described by Thanatsang et al. (2020.) In brief, chopped meat (1 g) was homogenized with 10 mL of 0.15 M KCl for 30 s. Protein in the homogenate (0.1 mL) was precipitated with 1 mL of ice-cold acetone and centrifuged at 3,500 × g for 2 min. The pellet was collected and resuspended in 0.4 mL of 5% SDS at 100°C with a constant shaking at 1,000 rpm for 10 min. Protein carbonyls in the protein solution (0.2 mL) were then examined using a protein carbonyl assay kit (Sigma-Aldrich, St. Louis, MO) according to the company's recommendation. Protein carbonyl content (nmol/mg protein) was calculated using a millimolar extinction coefficient at 375 nm of 22 mM−1 cm−1.
Peptidomic Analysis Between P5-Fraction of Non-WB and WB
Lyophilized peptide powder from P5 fraction in both non-WB and WB groups was solubilized in 0.1% formic acid in water (200 ng/mL). The characteristics of peptidome was analyzed using Orbitrap HF mass spectroscopy (Thermo Scientific, Waltham, MA) with an electrospray ionization (ESI) ion source operated at 3.5 kV. Four microliters of the samples were loaded on a C18 column (Hypersil Gold C18-reverse phase, 100 mm × 2.00 mm, 2.5 μm pore size). The peptides were separated for 35 min with the application of multistep gradient. Two LC-MS grade solvents were used in gradient steps to separate the analytes. Mobile phase A contained water in 0.1% formic acid and mobile phase B was 100% acetonitrile in 0.1% formic acid. The gradient was run as follows: 1 min, 1% B; 20 min, 45% B; 4 min, 55% B; 2 min 95% B; 8 min 1% B with a flow rate of 0.2 mL/min. Mass spectrum were acquired in positive mode under established parameters of auxiliary gas: 8; sheath gas: 25; sweep gas: 3; spray voltage: 3 kV; and capillary temperature: 300°C. MS spectral data were acquired using a Top15 method dynamically choosing the most abundant precursor ions with following parameters. MS resolution: 120,000; automatic gain control (AGC): 3×106; maximum IT time: 25; MS2 resolution: 30,000; AGC: 1×105; maximum IT: 60. MS scan range: 300 to 1,600 m/z with charge states (+1 to +5) for high-energy collision dissociation fragmentation = 27. A blank sample (0.1% formic acid/water) was administered after every injection. Raw data were acquired from the Xcalibur MS software (Thermo Electron Corp., Waltham, MA).
De Novo Peptide Sequencing and Oxidized Sites Identification
For peptide sequence and post-translational modifications (PTM) characterization, 3 replicate analytical LC-MS runs (.raw files) were achieved by integrating database searching (Uniprot database: Gallus gallus; accessed on 04 May 2022) and de novo sequencing algorithms using Peak StudioX software (Bioinformatics Solutions Inc., Waterloo, Canada) (Zhang et al., 2012). The peptide mass tolerance was set to 20 ppm and 0.1 Da for MS/MS. Variable modifications were as follows previous report with minor modification (Bouclon et al., 2017). Briefly, oxidation (+15.99 Da) of methionine, histidine (H), and tryptophan (W); Kynurenin (+3.99 Da), oxolactone (+13.98 Da), and hydroxykynurenine (+19.99 Da) of W; pyroglutamic (+13.98 Da) and pyrrolidinone (−30.01 Da) of proline (P); and aminotyrosine (+15.01 Da) of tyrosine (Y). To obtain high-confidence peptide identification, false discover rate of 1% was used for identified peptide filtering. Quality control of non-WB and WB samples was conducted for confirming the intra- and inter-reproducibility data, we used both chromatographic analysis and MS-acquisition analysis of 12 LC-MS runs.
Untargeted Metabolome Analysis
Chicken samples were cooked in a microtube at 95°C for 15 min. The cooked samples were homogenized using a tissue lyzer to retrieve a powder form. The meat powder was mixed with pepsin (ratio of pepsin to meat powder = 1:20 by weight). Hydrolysis was carried out at 37°C for 4 h. The pepsin-treated hydrolysate was then divided into 2 portions for cell cytotoxicity and metabolomics.
To extract the metabolites, the hydrolysate (20 mg) was mixed with 980 μL of 90% methanol and incubated at −20°C for 24 h. The samples were centrifuged for 30 min at 16,000 × g at 4°C to remove precipitated protein and the debris. Supernatants were carefully transferred to new tubes and cleaned-up using solid phase extraction (SPE) with C18-reverse phase based. SPE was preconditioned using 15 mL of acetonitrile and equilibrated with 30 mL of water. The supernatants were loaded on the equilibrated SPE and eluted by 99% acetonitrile/water. The elutes were then evaporated under a vacuum using rotatory evaporation. The samples were reconstituted in 500 μL methanol and diluted with 1,000 μL of 0.2% formic acid/water before being subjected to LC-MS/MS analysis.
The metabolome profiles were analyzed using an electrospray ionization source conjoined to an Orbitrap HF mass spectroscopy. The metabolites were separated on a Dionex UltiMate-3000 (Sunnyvale, CA) by injecting a 5 μL sample on an analytical column Hypersil Gold C18-reverse phase (100 mm × 2.00 mm, 2.5 μm). The untargeted metabolome method, adapted from Yingchutrakul et al. (2021), separated for 28 min with the application of multistep gradient. Two LC-MS grade solvents were used in gradient steps to separate the analytes. Mobile phase A contained 5% methanol in 0.1% formic acid and mobile phase B was 100% acetonitrile in 0.1% formic acid. The gradient was run as follows: 0 min, 0% B; 5 min, 20% B; 13 min, 55% B; 15.5 min, 95% B; 19 min, 0% B; 25 min, 0% B with a flow rate of 0.3 mL/min. Mass spectrum were acquired in positive mode under established parameters of auxiliary gas: 8; sheath gas: 25; sweep gas: 3; spray voltage: 3.00 kV; and capillary temperature: 300°C. The data-dependent acquisition parameters were set as follows: MS resolution: 120,000; AGC: 3×106; maximum IT time: 75; MS2 resolution: 30,000; AGC: 1×105; maximum IT: 50. MS scan range: 90 to 900 mass-to-charge ratio (m/z). Raw data were acquired from the Xcalibur MS software. Quality control of the samples was conducted for confirming the reproducibility data, we used the LC-MS/MS approach to determine the total ion intensity of all identified compounds from 6 independent extraction batches and 3 technical injections.
Data Processing for Identification and Quantification of Metabolites
The raw spectral files were analyzed using Compound Discoverer 3.1 (Thermo Fisher Scientific). The retention time (RT) and m/z of different LC runs were conducted according to the RT deviation of <0.5 min and the mass deviation of <5 ppm. Signal/noise ratio threshold was set at 1.5. The 9 ion types ([2M+ACN+H]+1, [2M+H]+1, [M+2H]+2, [M+ACN+H]+1, [M+H]+1, [M+H+MeOH]+1, [M+H+NH4]+2, [M+H-H2O]+1, [M+H-NH3]+1 were used to compound detections. The target m/z ions were then integrated to predict the molecular formula, which was compared to mzCloud (https://www.mzcloud.org; accessed date: 04/12/2021) and ChemSpider (http://www.chemspider.com; accessed date: 04/12/2021) online databases, including Chemical Entities of Biological Interest (ChEBI), ChemBank, and Kyoto Encyclopedia of Genes and Genomes, KEGG) for the identification and confirmation of the compounds. The FISh coverage score was calculated, and fragments on the MS/MS spectrum were auto-annotated with structure, molecular weight, and elemental composition. Among candidate metabolites obtained from mzCloud and ChemSpider with FISh, the highest MS/MS coverage scores were selected for annotation. Heat maps were generated using Euclidean clustering algorithm. Pathway enrichment analysis was conducted using MetaboAnalyst 5.0 software (database: Gallus gallus; accessed date: 27/02/2022).
Cell Cytotoxicity
The pepsin-treated hydrolysate, the same sample used for untargeted metabolomic study, used in this study. The hydrolysate was centrifuged at 14,000 × g for 30 min. The supernatant was collected for determining cell cytotoxicity. Epithelial cells isolated from colon tissue (Caco-2) and kidney cell (Vero; CCL-81) were used to determine cell cytotoxicity by 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay (Riss et al., 2016). The cells were cultured in the complete Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and 100 U/mL antibiotic at 37°C in a 5% CO2 overnight before the MTT experiment. To assess the effects of WB abnormality on cell cytotoxicity, the cells were treated with 7 different concentrations of the extracts (100, 50, 25, 12.5, 6.25, 3.125, 1.56 µg/mL) for 24 h. Control was the cells without the pepsin-treated hydrolysate. After 24 h, the culture medium was removed and 10 μL of 0.5 μg/μL of MTT solution was added to each well. After 3-h incubation at 37°C, 50 μL of dimethyl sulfoxide (DMSO) was added to each well. The optical absorbance (OD) at 570 nm of each well was then measured using a microplate reader and transformed the results into the cell survival rate percentage using following equation.
The cell cytotoxicity experiments were conducted in triplicates (n = 3). The resulting graph was presented in mean ± standard deviation (SD) of cell viability compared with the control.
Statistical Analysis
Differences in in vitro protein digestibility at each digestion point between non-WB and WB were assessed using Student's t-test. Significant level was set at P < 0.05. For metabolomics analysis, metabolites showing adjusted P < 0.05 in the comparison between non-WB and WB samples were considered differentially abundant.
RESULTS AND DISCUSSION
Influences of WB Abnormality on Protein Carbonyls and In Vitro Protein Digestion of Cooked Chicken Breast Meat
Protein carbonylation, an irreversible nonenzymatic modification of proteins, has been used as a biomarker for protein oxidation in meat system (Estévez et al., 2009). The main route for protein carbonylation in the meat system has been associated with the direct oxidation of susceptible amino acid side chains (Estévez et al., 2009). In this study, protein carbonyls in raw chicken breasts with and without WB abnormality were investigated. The current results showed that protein carbonyl of the WB samples was at a greater extent compared with that of non-WB ones (Figure 2A). The findings, indicating the greater protein oxidation in WB meat, were well agreed with previous reports (Soglia et al., 2019; Thanatsang et al., 2020; Li et al., 2022).
Figure 2.
Effects of Wooden Breast (WB) abnormality on protein carbonylation and in vitro protein digestion in cooked chicken breast. (A) Protein carbonyls, (B) protein concentration, (C) free NH2-groups, (D) degree of protein hydrolysis (%) of cooked non-WB and WB samples and collected at end of oral phase (P1), end of gastric phase (P2) and before (P3), in the middle (P4) and the end of intestinal phase (P5). Bars and error bars represent means and standard deviations, respectively. (E) box-plot analysis with turkey test of peptide mass distribution according to molecular weight in P5 of cooked non-WB and WB samples. (F) myofibril fragment index of raw non-WB and WB samples. Statistical significance was analyzed using Student's t-test for comparing the mean values of the effects of different chicken types within each check point and marked as “*” for indicating statistically significant difference (P < 0.05).
To determine whether development of WB abnormality exert adverse effects on chicken protein quality, in terms of digestibility, cooked meat was subjected to in vitro digestibility, mimicking enzymatic reaction in gastrointestinal (GI) track (Bordoni et al., 2014; Trithavisup et al., 2022). Protein concentration of cooked non-WB and cooked WB samples was determined using BCA assay (Figure 2B). The resulted showed that no significant difference in protein concentration was observed between cooked non-WB and cooked WB samples at every digestion step (P > 0.05), except for P3. Considering free NH2 groups (Figure 2C) and %DH (Figure 2D), cooked non-WB showed significantly lower free NH2 groups and %DH when compared to cooked WB at P1, P2 and P4 (P < 0.05). However, no significant difference in free NH2 groups and %DH between cooked non-WB and cooked WB samples was found at P5 (P > 0.05).
The analysis by PEAKS studioX software revealed peptide sequence and PTM profiling. A total of 11,630 and 11,135 peptide groups were identified from the non-WB and WB groups, respectively. Molecular weight of the peptide sequenced from the non-WB and WB followed a gradient distribution (Figure 2E) with an average molecular weight of 1,118.73 ± 297.57 and 1,112.05 ± 290.91 Da and a length of 9.51 ± 2.68 and 9.45 ± 2.65 residues for non-WB and WB, respectively. The size distribution of non-WB and WB was shown in Figure S1A and S1B, respectively. Peptide length and molecular weight from P5 fraction were not significantly different across the global peptide profile for each non-WB and WB (Figure 2E). The peptidomic results of the P5 fraction, mimicking the intestinal digestion phase, agreed well with the detection of free-NH2 group. The findings suggested no differences in in vitro protein digestion between non-WB and WB chicken breasts.
Initially, we anticipated a greater free-NH2 at P2 to P5 and greater small peptides at P5 in non-WB compared to those of WB based on our hypothesis that the oxidized proteins in the WB would inhibit in vitro protein digestion. However, this was not the case. To further elucidate what could underline the current findings, we compared MFI between non-WB and WB. MFI is a useful indicator of the proteolytic extent indicating both rupture of the I-band and breakage of intermyofibrillar linkages of the muscle (Hopkins et al., 2000). In this study, MFI value of WB was higher than that of non-WB (Figure 2F). The higher MFI values suggested that myofibril structure of the WB meat would undergo fragmentation at a greater extent compared to non-WB. The results aligned well with the event of myogeneration due to WB myopathy (Soglia et al., 2019). The breakdown of the myofibril might allow gastric and pancreatic enzymes to access the action sites in the WB samples. This might be the reason why free NH2 groups and %DH of WB meat were higher than those of non-WB meat at P1, P2 and P4.
Oxidative Modification Sites
To better elucidate the potential oxidative changes associated with WB development in chicken breasts, the profiles of oxidized amino acids in P5 fraction were determined. As shown in Table 1, oxidative products, particularly oxolactone, 2-aminotyrosine, hydrozyl-kynurenin, and pyrrolidinone, were more abundant in WB samples. Oxolactone and hydroxyl-kynurenin, the oxidation products of tryptophan, were identified in actin and troponin I of murine rate exposed to acute oxidative stress (Fedorova et al., 2010). In the case of hydroxyl-kynurenin, even though its detrimental effects are still controversial, previous studies suggested its pro-oxidative role at certain physiological conditions (Mor et al., 2021). Methionine and tyrosine residues are the important targets of oxidant OH radicals (Bergès et al., 2011).
Table 1.
Number of oxidized peptides identified in nonwooden breast (WB) and WB meat.
| Oxidized modifications | Number of identified peptides | |
|---|---|---|
| Non-WB | WB | |
| Oxidation at histidine and tryptophan | 48 | 53 |
| Oxidation at methionine | 56 | 60 |
| Tryptophan oxidation to oxolactone | 12 | 22 |
| Tyrosine oxidation to 2-aminotyrosine | 12 | 29 |
| Tryptophan oxidation to hydroxyl-kynurenin | 14 | 18 |
| Tryptophan oxidation to kynurenin | 20 | 10 |
| Proline oxidation to pyroglutamic acid | 198 | 191 |
| Proline oxidation to pyrrolidinone | 207 | 219 |
Metabolome Profiling and Differential Metabolites Quantification
Untargeted metabolomic analysis was conducted to explore the difference of metabolite compositions of pepsin-hydrolyzed cooked non-WB and WB samples. This analysis served as a high-throughput screening method, primarily targeting biomolecules in cooked WB samples that might exert adverse health effects.
To explore the variance in the metabolic profiles between non-WB and WB samples, principal component analysis (PCA) was performed by comparing the 2 sample groups in the 36 LC-runs and evaluating intra- and intervariations (Figure 3A). The PCA analysis showed a complete separation between cooked non-WB and WB samples, indicating differences in the metabolic profiles between the 2 groups. In total, 1,155 metabolites were successfully identified in the current study (Table S1). Relative abundance of metabolites with adjusted P values of less than 0.05 are considered significantly difference in abundance (Figure 3B). Hence, a total of 322 differentially expressed metabolites was identified between non-WB and WB samples. Among them, 151 metabolites were downregulated while 171 metabolites were upregulated in WB samples (Figure 3C). Regarding pathway analysis, only 27 metabolites were recognized by KEGG pathway. Metabolites related with taurine and hypotaurine metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, and D-glutamine and D-glutamate metabolism were affected the most due to WB abnormality (Figure 3D, Table S2). Such changes in amino acid metabolisms in breast muscle with WB abnormality have been consistently reported (Abasht et al., 2016, 2019; Kuttappan et al., 2017; Cônsolo et al., 2020).
Figure 3.
Untargeted metabolomic profiles of cooked chicken breasts with or without Wooden Breast (WB) abnormality. (A) Principal component analysis (PCA) of the metabolomic data. (B) Volcano plot of pepsin-hydrolyzed cooked chicken breasts. Each dot represents a metabolite and those in the green and red area indicated downregulated and upregulated metabolites, respectively. (C) Heat map of 322 differentially expressed metabolites identified between pepsin-hydrolyzed cooked chicken breasts from non-WB and WB samples. (D) Pathway analysis revealed that in the cooked samples (a) taurine and hypotaurine metabolism, (b) phenylalanine, tyrosine and tryptophan biosynthesis, and (c) D-Glutamine and D-glutamate metabolism were affected the most due to WB abnormality. The circle with the darker the color indicates a greater significance of the pathway. Names of other pathways were listed in Table S2.
According to the current results, there was no apparent compounds that could exert acute adverse health effects found in both hydrolyzed samples (Table S1). Several identified metabolites were amino acids along with short peptides (e.g., L-glutamyl-L-leucyl-L-lysine, log2 fold change, FC = 3.7, L-alanyl-L-tyrosine, log2FC = −3.8) as shown in Table 2. Carboxamides, known as organic amides (RC(=O)NR'R”), were also identified in the samples (Table S1), well corresponding with pepsin hydrolysis. Flavor compounds formed during cooking such as derivatives of butanone (e.g., 1-[4-(4-methoxyphenyl)-2-methyl-1-piperazinyl]-4-[5-(4-morpholinylmethyl)-1H-tetrazol-1-yl]-1-butanone, log2FC = 2.9) were identified. An increased abundance of derivatives of butanone is of interest. The group of butanone has been reported as volatile components formed during prolonged cooking of chicken meat under oxidation-favoring condition (Pippen et al., 1960). The greater value of butanone in the WB samples could be due to an increased proportion of fat in WB meat. In addition, an increase in derivatives of benzamide (e.g., 4-(dimethylamino)-N-[6-(hydroxyamino)-6-oxohexyl]benzamide, log2FC = 3.2) was found in WB samples. Such compounds were naturally found in some foods and associated with bitter flavor (Wiener et al., 2012).
Table 2.
Amino acids and peptides with different abundance between pepsin-hydrolyzed cooked nonwooden breast (WB) and WB meat identified based on untargeted metabolomics.
| Name | Formula | MW | Log2 fold change | Adj. P value |
|---|---|---|---|---|
| Increased abundance in WB | ||||
| Amino acids | ||||
| Methionine | C5 H11 N O2 S | 149.0 | 1.1 | 0.015 |
| Short peptides | ||||
| L-Glutamyl-L- leucyl -L-lysine | C17 H32 N4 O6 | 776.5 | 3.7 | 0.007 |
| L-Methionyl-L-histidyl-L-arginyl-L-seryl-L-leucyl-L-leucylglycyl-L-arginyl-L-methionyl-L-lysylglycyl-L-alanine | C56 H101 N21 O14 S2 | 1355.7 | 2.6 | 0.015 |
| L-Alanyl-L-prolyl-L-histidine | C14 H21 N5 O4 | 323.2 | 2.3 | 0.044 |
| L-Phenylalanyl-L-glutamyl-L-histidine | C20 H26 N6 O5 | 860.4 | 1.6 | 0.030 |
| L-Isoleucyl-L-prolyl-L-histidine | C17 H27 N5 O4 | 347.2 | 1.5 | 0.013 |
| L-Arginyl-L-leucine | C12 H25 N3 O3 | 259.2 | 1.3 | 0.034 |
| L-Asparaginyl-L-arginyl-L-arginyl-L-asparaginylglycyl-L-glutaminyl-L-methionyl-L-arginyl-L-arginine | C44 H82 N24 O13 S | 1186.6 | 1.0 | 0.048 |
| L-Isolecyl-L-methionyl-L-lysine | C17 H34 N4 O4 S | 390.2 | 1.0 | 0.040 |
| L-Phenylalanyl-L-methionyl-L-lysine | C16 H30 N4 O4 S | 712.4 | 1.0 | 0.038 |
| N∼2∼-[Amino(oxo)acetyl]-L-arginyl-L-valyl-N-methyl-L-tyrosyl-L-isoleucyl-L-histidyl-L-prolyl-L-phenylalanine | C49 H69 N13 O11 | 1015.5 | 0.5 | 0.036 |
| Decreased abundance in WB | ||||
| Amino acids | ||||
| Alanine | C3 H7 N O2 | 89.0 | −1.4 | 0.026 |
| Short peptides | ||||
| L-Histidyl-L-phenylalanyl-L-lysine | C17 H28 N6 O4 | 362.2 | −1.1 | 0.009 |
| L-Alanyl-L-phenylalanyl-L-lysine | C14 H26 N4 O4 | 712.4 | −0.5 | 0.034 |
| L-Alanyl-L-tyrosine | C12 H16 N2 O4 | 252.1 | −3.8 | 0.026 |
| L-lysyl-L-leucine | C12 H25 N3 O3 | 259.2 | −3.3 | 0.014 |
| L-Tryptophanyl-L-lysyl-L-lysine | C23 H36 N6 O4 | 230.1 | −3.0 | 0.032 |
| L-Alanyl-L-tyrosine | C12 H16 N2 O4 | 252.1 | −2.0 | 0.048 |
| L-leucinyl-L-asparaginyl-L-lysine | C16 H31 N5 O5 | 355.2 | −1.7 | 0.039 |
| L-Ariginyl-L-valine | C11 H23 N3 O3 | 245.2 | −1.4 | 0.031 |
Furthermore, the changes of several compounds agreed with previous studies. Abasht et al. (2016) reported decreased abundances of adenosine monophosphate (AMP) in association with perturbed AMP/ATP ratio and glycolysis signaling. Lake et al. (2022) recently highlighted a reduced carnitine in pectoralis major muscle but increased plasma carnitine and L-acetyl carnitine in WB-affected broilers relative with unaffected birds, suggesting an increased uptake of fatty acids from plasma lipoproteins in the breast of affected chickens. Here, we found decreased abundances of AMP (log2FC = −1.2), L-carnitine (log2FC = −1.4), and acetyl-L-carnitine (log2FC = −2.2) although the meat samples were cooked and hydrolyzed by pepsin (Table S1). While Abasht et al. (2016) and Greene et al. (2020) reported an increased hypoxanthine in the WB-affected muscle, the current study found a decreased hypoxanthine (log2FC = −1.4) in cooked WB samples (Table S1). Cônsolo et al. (2020) addressed no significant difference in hypoxanthine between normal and WB muscle samples.
To further elucidate the obtained metabolomic results, those compounds were grouped based on their relevant biological functions. As shown in Figure 4A and B, the majority of identified metabolic compounds were amino acids, short peptides and phosphonates/phosphinates which are the breakdown products of proteins and carbon-phosphorous containing molecules (e.g., glycoproteins and phospholipids), respectively. In addition, abundances of metabolites related with fatty acid metabolisms, particularly choline metabolism, as well as purine metabolisms were affected due to WB myopathy. The impact of WB abnormality on those pathways was recently highlighted by Greene et al. (2020) and Lake et al. (2022). However, it remains to be further investigated whether the change in purine metabolisms was associated with increased activities of pentose phosphate pathway or decreased nucleotide utilization (Abasht et al., 2016; Lake et al., 2022). Metabolites related with oxidative stress responses, cytochrome P450 and [Ca2+] regulation were significantly increased in WB relative to those of non-WB samples (Figure 4B). The results aligned well with the condition of oxidative stress and altered [Ca2+] in WB-affected pectoralis major muscle (Mutryn et al., 2015; Abasht et al., 2016; Malila et al., 2019; Cônsolo et al., 2020; Thanatsang et al., 2020). Overall, the current findings showed that metabolomic changes between non-WB and WB that were previously identified at physiological stage were carried over in the cooked meat. However, our study did not find any biomolecules with potential toxicity in cooked chicken breast with WB abnormality.
Figure 4.
Bar graphs indicate the numbers of the metabolites, classified into relevant biological function, with (A) decreased or (B) increased abundance in Wooden Breast (WB) samples relative to non-WB.
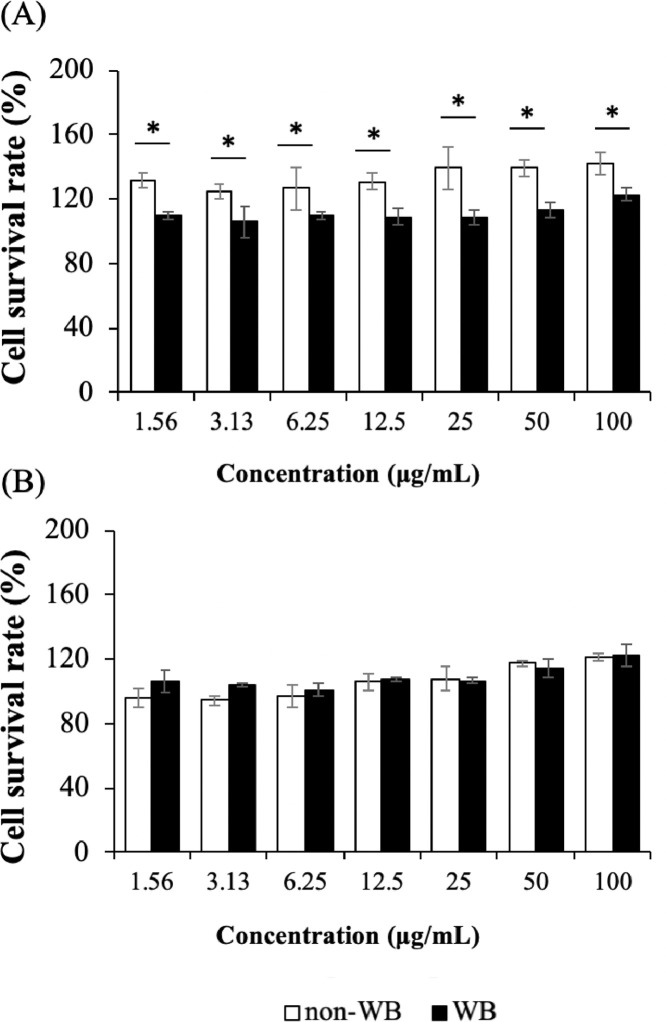
Cell Cytotoxicity
To determine whether cooked WB breast meat exhibited any cytotoxicity on the cells of human GI tract, two cell types (Caco-2 and Vero) were exposed to a varying concentration of the pepsin-hydrolyzed cooked meat extracts for 24 h using the MTT assay. Caco-2 has been widely used as a model of the intestinal epithelial barrier in several food research. The cells exhibited high correlation for studies on the adsorption of the compounds after oral intake in human (Sun et al., 2008). Vero cells which represent kidney cell were used to increase the reliable cell toxicity information.
Viability of the cells is illustrated in Figure 5. The results indicated that supplementation of supernatant collected from pepsin hydrolysis of both cooked non-WB and WB meat showed no effects on cell viability at all tested concentrations. Percentage of cell viability above 100% at all tested concentrations might be because adding the extracts increased nutrients to the cells. Focusing on the effects of WB abnormality, for Caco-2 cells (Figure 5A), the percentage of viable cells in cooked WB meat extracts was significantly lower (P < 0.05) than those of the non-WB samples at all concentrations. The results might be due potentially to an exposure of the cells to oxidized derivatives contained within the cooked WB meat (Vizio et al., 2005; Mor et al., 2021). For instance, while free-radical scavenging kynurenine was detected at higher level in non-WB (Table 1) its derivatives, the hydroxyl kynurenine, which appeared to exert pro-oxidative effects under certain condition (Mor et al., 2021), was at higher level in WB. Oxidative insult might induce DNA damage and apoptotic responses of the Caco-2 cells, leading to cell viability reduction (Vizio et al., 2005; Lezcano et al., 2013). In the study of Lezcano et al. (2013), an exposure of Caco-2 cells to hydrogen peroxide resulted in 50% viability loss. In the current study, on the other hand, the oxidative potential of those biomolecules in the cooked WB meat extracts might not exert as strong effects as hydrogen peroxide. In addition, some peptides contained in the pepsin-hydrolyzed chicken breast extracts might exhibit protective bioactivity and act against stress-induced conditions (Ryan et al., 2011). Hence, the lower cell viability was observed in WB-treated cells compared to those exposed to non-WB samples but no significant cytotoxic effects were detected. In contrast, for Vero cells, no significant differences in cell survival between non-WB and WB treatment were observed. The results might suggest that Vero cells were less susceptible to the WB meat extracts in comparison to the Caco-2 cells. However, such speculations regarding the redox imbalance of both cells along with the long-term exposure requires further in-depth investigation. Overall, the results suggested that the digested cooked WB meat might disturb human colon cells at a greater extent when compared to cooked non-WB samples but no significant cytotoxicity of the WB meat was observed.
Figure 5.
Effect of Wooden Breast (WB) abnormality on cell toxicity of (A) Caco-2 cells and (B) Vero cells. Bars and error bars present means and standard deviation for viable cells after treated with meat extract for 24 h, respectively. Statistical significance was analyzed using Student's t-test for comparing the mean values of the effects of different chicken types within each concentration and marked as “*” for indicating statistically significant difference (P < 0.05).
CONCLUSIONS
The ultimate aim of this study aims was to identify whether WB condition reduced in vitro protein digestibility of cooked chicken meat or exerted any adverse impact in terms of nutritional value to the meat. The results indicated that no significant differences in free-NH2, degree of hydrolysis and distribution of peptide molecular weight between non-WB and WB samples at (P5), suggesting no adverse effects of WB on in vitro protein digestibility at the late phase of intestinal digestion. Untargeted metabolomics found no particular metabolites with potential toxicity either in non-WB and WB. Based on cell cytotoxicity, increased concentration of hydrolyzed non-WB and WB did not affect viability of Caco-2 and Vero cells. The results suggested that WB abnormality did not adversely impact in vitro protein digestibility nor exert cytotoxic effects to the cooked chicken meat. Further investigation regarding long-term exposure of WB meat remains to be elucidated as P5 fraction of WB showed greater content of peptides with oxidative modification and WB samples lowered Caco-2 cell viability in comparison to those of cooked non-WB samples.
ACKNOWLEDGMENTS
This project was funded by National Research Council of Thailand (NRCT) and National Science and Technology Development Agency (NSTDA, Thailand) with project number N42A660312. Postdoctoral fellowships awarded toward T. T. and S. K. by NSTDA (Thailand), and by the Second Century Fund (C2F), Chulalongkorn University (Thailand), respectively.
DISCLOSURES
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Yuwares Malila reports financial support was provided by National Research Council of Thailand (NRCT). Thanatorn Trithavisup reports financial support was provided by National Science and Technology Development Agency.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.103261.
Appendix. Supplementary materials
REFERENCES
- Abasht B., Mutryn M.F., Michalek R.D., Lee W.R. Oxidative stress and metabolic perturbations in wooden breast disorder in chickens. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abasht B., Zhou N., Lee W.R., Zhuo Z., Peripolli E. The metabolic characteristics of susceptibility to wooden breast disease in chickens with high feed efficiency. Poult. Sci. 2019;98:3246–3256. doi: 10.3382/ps/pez183. [DOI] [PubMed] [Google Scholar]
- Adler-Nissen J. Elsevier Science Pub. Co.; New York, NY: 1986. Enzymic Hydrolysis of Food Proteins. [Google Scholar]
- Bergès J., Trouillas P., Houée-Levin C. Oxidation of protein tyrosine or methionine residues: from the amino acid to the peptide. J. Phys. Conf. Ser. 2011;261 [Google Scholar]
- Bordoni A., Laghi L., Babini E., Di Nunzio M., Picone G., Ciampa A., Valli V., Danesi F., Capozzi F. The foodomics approach for the evaluation of protein bioaccessibility in processed meat upon in vitro digestion. Electrophoresis. 2014;35:1607–1614. doi: 10.1002/elps.201300579. [DOI] [PubMed] [Google Scholar]
- Bouclon J., Le Danvic C., Guettier E., Bray F., Tokarski C., Rolando C., Nagnan-Le Meillour P. Identification of post-translational modifications on odorant-binding protein isoforms from pig olfactory secretome by high-resolution mass spectrometry: o-β-N-acetylglucosaminylation and phosphorylation. Front. Ecol. Evol. 2017;5:142. [Google Scholar]
- Butts C.A., Monro J.A., Moughan P.J. In vitro determination of dietary protein and amino acid digestibility for humans. Br. J. Nutr. Suppl. 2012;2:S282–S287. doi: 10.1017/S0007114512002310. [DOI] [PubMed] [Google Scholar]
- Cai K., Shao W., Chen X., Campbell Y., Nair M., Suman S., Beach C., Guyton M., Schilling M. Meat quality traits and proteome profile of woody broiler breast (pectoralis major) meat. Poult. Sci. 2018;97:337–346. doi: 10.3382/ps/pex284. [DOI] [PubMed] [Google Scholar]
- Caldas-Cueva J.P., Owens C.M. A review on the woody breast condition, detection methods, and product utilization in the contemporary poultry industry. J. Anim. Sci. 2020;98:skaa207. doi: 10.1093/jas/skaa207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cônsolo N.R.B., Samuelsson L.M., Barbosa L.C.G.S., Monaretto T., Moraes T.B., Buarque V.L.M., Higuera-Padilla A.R., Colnago L.A., Silva S.L., Reis M.M., Fonseca A.C., Araújo C.S.d.S., Leite B.G.d.S., Roque F.A., Araújo L.F. Characterization of chicken muscle disorders through metabolomics, pathway analysis, and water relaxometry: a pilot study. Poult. Sci. 2020;99:6247–6257. doi: 10.1016/j.psj.2020.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez R., Pateiro M., Munekata P.E.S., Zhang W., Garcia-Oliveira P., Carpena M., Prieto M.A., Bohrer B., Lorenzo J.M. Protein oxidation in muscle foods: a comprehensive review. Antioxidants (Basel) 2022;11:60. doi: 10.3390/antiox11010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez M., Ollilainen V., Heinonen M. Analysis of protein oxidation markers – α-aminoadipic and γ-glutamic semialdehydes – in food proteins by using LC–ESI-multi-stage tandem MS. J. Agric. Food Chem. 2009;57:3901–3910. doi: 10.1021/jf804017p. [DOI] [PubMed] [Google Scholar]
- Estévez M., Xiong Y. Intake of oxidized proteins and amino acids and causative oxidative stress and disease: recent scientific evidences and hypotheses. J. Food Sci. 2019;84:387–396. doi: 10.1111/1750-3841.14460. [DOI] [PubMed] [Google Scholar]
- Fedorova M., Todorovsky T., Kuleva N., Hoffmann R. Quantitative evaluation of tryptophan oxidation in actin and troponin I from skeletal muscles using a rat model of acute oxidative stress. Proteomics. 2010;10:2692–2700. doi: 10.1002/pmic.201000147. [DOI] [PubMed] [Google Scholar]
- Greene E., Cauble R., Dhamad A.E., Kidd M.T., Kong B., Howard S.M., Castro H.F., Campagna S.R., Bedford M., Dridi S. Muscle metabolome profiles in woody breast-(un)affected broilers: effects of quantum blue phytase-enriched diet. Front. Vet. Sci. 2020;7:458. doi: 10.3389/fvets.2020.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardbower D.M., de Sablet T., Chaturvedi R., Wilson K.T. Chronic inflammation and oxidative stress: the smoking gun for Helicobacter pylori-induced gastric cancer? Gut Microbes. 2013;4:475–481. doi: 10.4161/gmic.25583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins D.L., Littlefield P.J., Thompson J.M. A research note on factors affecting the determination of myofibrillar fragmentation. Meat Sci. 2000;56:19–22. doi: 10.1016/s0309-1740(00)00012-7. [DOI] [PubMed] [Google Scholar]
- Keller J., Chevolleau S., Noguer-Meireles M.H., Pujos-Guillot E., Delosière M., Chantelauze C., Joly C., Blas-Y-Estrada F., Jouanin I., Durand D., Pierre F., Debrauwer L., Theodorou V., Guéraud F. Heme-iron-induced production of 4-hydroxynonenal in intestinal lumen may have extra-intestinal consequences through protein-adduct formation. Antioxidants. 2020;9:1293. doi: 10.3390/antiox9121293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristoffersen K.A., Afseth N.K., Böcker U., Lindberg D., de Vogel-van den Bosch H., Ruud M.L., Wubshet S.G. Average molecular weight, degree of hydrolysis and dry-film FTIR fingerprint of milk protein hydrolysates: Intercorrelation and application in process monitoring. Food Chem. 2020;310 doi: 10.1016/j.foodchem.2019.125800. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Bottje W., Ramnathan R., Hartson S.D., Coon C.N., Kong B.W., Owens C.M., Vazquez-Añon M., Hargis B.M. Proteomic analysis reveals changes in carbohydrate and protein metabolism associated with broiler breast myopathy. Poult. Sci. 2017;96:2992–2999. doi: 10.3382/ps/pex069. [DOI] [PubMed] [Google Scholar]
- Lake J.A., Brannick E.M., Papah M.P., Lousenberg C., Velleman S.G., Abasht B. Blood gas disturbances and disproportionate body weight distribution in broilers with wooden breast. Front. Physiol. 2020;11:304. doi: 10.3389/fphys.2020.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J.A., Dekkers J., Abasht B. Genetic basis and identification of candidate genes for wooden breast and white striping in commercial broiler chickens. Sci. Rep. 2021;11:1–13. doi: 10.1038/s41598-021-86176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J.A., Yan Y., Dekkers J.C.M., Qiu J., Brannick E.M., Abasht B. Identification of circulating metabolites associated with wooden breast and white striping. PLoS One. 2022;17 doi: 10.1371/journal.pone.0274208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezcano V., Gentili C., de Boland A.R. Role of PTHrP in human intestinal Caco-2 cell response to oxidative stress. Biochim. Biophys. Acta. 2013;1833:2834–2843. doi: 10.1016/j.bbamcr.2013.06.029. [DOI] [PubMed] [Google Scholar]
- Li B., Dong X., Puolanne E., Ertbjerg P. Effect of wooden breast degree on lipid and protein oxidation and citrate synthase activity of chicken pectoralis major muscle. LWT. 2022;154 [Google Scholar]
- Malila Y., U-Chupaj J., Srimarut Y., Chaiwiwattrakul P., Uengwetwanit T., Arayamethakorn S., Punyapornwithaya V., Sansamur C., Kirschke C.P., Huang L., Tepaamorndech S., Petracci M., Rungrassamee W., Visessanguan W. Monitoring of white striping and wooden breast cases and impacts on quality of breast meat collected from commercial broilers (Gallus gallus) Asian-Australas. J. Anim. Sci. 2018;31:1807–1817. doi: 10.5713/ajas.18.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malila Y., Uengwetwanit T., Thanatsang K.V., Arayamethakorn S., Srimarut Y., Petracci M., Soglia F., Rungrassamee W., Visessanguan W. Insights into transcriptome profiles associated with WB myopathy in broilers slaughtered at the age of 6 weeks or 7 weeks. Front. Physiol. 2021;12:996. doi: 10.3389/fphys.2021.691194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malila Y., Thanatsang K., Arayamethakorn S., Uengwetwanit T., Srimarut Y., Petracci M., Strasburg G.M., Rungrassamee W., Visessanguan W. Absolute expressions of hypoxia-inducible factor-1 alpha (HIF1A) transcript and the associated genes in chicken skeletal muscle with white striping and wooden breast myopathies. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0220904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor A., Tankiewicz-Kwedlo A., Krupa A., Pawlak D. Role of kynurenine pathway in oxidative stress during neurodegenerative disorders. Cells. 2021;10:1603. doi: 10.3390/cells10071603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutryn M.F., Brannick E.M., Fu W., Lee W.R., Abasht B. Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using RNA-sequencing. BMC Genomics. 2015;16:399. doi: 10.1186/s12864-015-1623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P., Petersen D., Dambmann C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001;66:642–646. [Google Scholar]
- Olson D., Stromer M. Myofibril fragmentation and shear resistance of three bovine muscles during postmortem storage. J. Food Sci. 1976;41:1036–1041. [Google Scholar]
- Pan X., Zhang L., Xing T., Li J., Gao F. The impaired redox status and activated nuclear factor-erythroid 2-related factor 2/antioxidant response element pathway in wooden breast myopathy in broiler chickens. Anim. Biosci. 2021;34:652–661. doi: 10.5713/ajas.19.0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippen E.L., Eyring E.J., Nonaka M. The occurrence and flavor significance of acetoin in aqueous extracts of chicken. Poult. Sci. 1960;39:922–924. [Google Scholar]
- Riss T. L., R. A. Moravec, A. L. Niles, S. Duellman, H. A. Benink, T. J. Worzella and L. Minor. Cell viability assays. In: S. Markossian, A. Grossman, K. Brimacombe, M. Arkin, D. Auld, C. Austin, J. Baell, T. D. Y. Chung, N. P. Coussens, J. L. Dahlin, V. Devanarayan, T. L. Foley, M. Glicksman, K. Gorshkov, J. V. Haas, M. D. Hall, S. Hoare, J. Inglese, P. W. Iversen, S. C. Kales, M. Lal-Nag, Z. Li, J. McGee, O. McManus, T. Riss, P. Saradjian, G. S. Sittampalam, M. Tarselli, O. J. Trask, Jr., Y. Wang, J. R. Weidner, M. J. Wildey, K. Wilson, M. Xia and X. Xu . Eli Lilly & Company and the National Center for Advancing Translational Sciences; Bethesda, MD: 2016. Assay Guidance Manual [Internet] pp. 1–25. [Google Scholar]
- Rutherfurd S.M., Moughan P.J. Available versus digestible dietary amino acids. Br. J. Nutr. 2012;108:S298–S305. doi: 10.1017/S0007114512002528. [DOI] [PubMed] [Google Scholar]
- Ryan J.T., Ross R.P., Bolton D., Fitzgerald G.F., Stanton C. Bioactive peptides from muscle sources: meat and fish. Nutrients. 2011;3:765–791. doi: 10.3390/nu3090765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvo H.K., Airas N., Lindén J., Puolanne E. Pectoral vessel density and early ultrastructural changes in broiler chicken wooden breast myopathy. J. Comp. Pathol. 2018;161:1–10. doi: 10.1016/j.jcpa.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Sihvo H.K., Immonen K., Puolanne E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014;51:619–623. doi: 10.1177/0300985813497488. [DOI] [PubMed] [Google Scholar]
- Sihvo H.-K., Lindén J., Airas N., Immonen K., Valaja J., Puolanne E. Wooden breast myodegeneration of pectoralis major muscle over the growth period in broilers. Vet. Pathol. 2017;54:119–128. doi: 10.1177/0300985816658099. [DOI] [PubMed] [Google Scholar]
- Soglia F., Mudalal S., Babini E., Di Nunzio M., Mazzoni M., Sirri F., Cavani C., Petracci M. Histology, composition, and quality traits of chicken Pectoralis major muscle affected by wooden breast abnormality. Poult. Sci. 2016;95:651–659. doi: 10.3382/ps/pev353. [DOI] [PubMed] [Google Scholar]
- Soglia F., Silva A., Lião L., Laghi L., Petracci M. Effect of broiler breast abnormality and freezing on meat quality and metabolites assessed by 1 H-NMR spectroscopy. Poult. Sci. 2019;98:7139–7150. doi: 10.3382/ps/pez514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E.R., Levine R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- Sun H., Chow E.C., Liu S., Du Y., Pang K.S. The Caco-2 cell monolayer: usefulness and limitations. Expert Opin. Drug Metab. Toxicol. 2008;4:395–411. doi: 10.1517/17425255.4.4.395. [DOI] [PubMed] [Google Scholar]
- Swaisgood H.E., Catignani G.L. Protein digestibility: in vitro methods of assesments. Pages 185-236 in Adv. Food Nutr. Res. 1991;35:185–236. doi: 10.1016/s1043-4526(08)60065-0. [DOI] [PubMed] [Google Scholar]
- Tasoniero G., Cullere M., Cecchinato M., Puolanne E., Dalle Zotte A. Technological quality, mineral profile, and sensory attributes of broiler chicken breasts affected by white striping and wooden breast myopathies. Poult. Sci. 2016;95:2707–2714. doi: 10.3382/ps/pew215. [DOI] [PubMed] [Google Scholar]
- Thanatsang K.V., Malila Y., Arayamethakorn S., Srimarut Y., Tatiyaborworntham N., Uengwetwanit T., Panya A., Rungrassamee W., Visessanguan W. Nutritional properties and oxidative indices of broiler breast meat affected by wooden breast abnormality. Animals. 2020;10:2272. doi: 10.3390/ani10122272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trithavisup T., Sanpinit P., Sakulwech S., Klamchuen A., Malila Y. In vitro protein digestion of cooked spent commercial laying hen and commercial broilers breast meat. Foods. 2022;11:1853. doi: 10.3390/foods11131853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizio B., Poli G., Chiarpotto E., Biasi F. 4-Hydroxynonenal and TGF-beta1 concur in inducing antiproliferative effects on the Caco-2 human colon adenocarcinoma cell line. Biofactors. 2005;24:237–246. doi: 10.1002/biof.5520240128. [DOI] [PubMed] [Google Scholar]
- Wiener A., Shudler M., Levit A., Niv M.Y. BitterDB: a database of bitter compounds. Nucleic Acids Res. 2012:D413–D419. doi: 10.1093/nar/gkr755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingchutrakul Y., Sittisaree W., Mahatnirunkul T., Chomtong T., Tulyananda T., Krobthong S. Cosmeceutical potentials of grammatophyllum speciosum extracts: anti-inflammations and anti-collagenase activities with phytochemical profile analysis using an untargeted metabolomics approach. Cosmetics. 2021;8:116. [Google Scholar]
- Zhang J., Xin L., Shan B., Chen W., Xie M., Yuen D., Zhang W., Zhang Z., Lajoie G.A., Ma B.PEAKS DB. de novo sequencing assisted database search for sensitive and accurate peptide identification. Mol. Cell Proteomics. 2012;11 doi: 10.1074/mcp.M111.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.