Abstract
The intestinal tight junction (TJ) barrier is a crucial defense mechanism that prevents the passage of intestinal content into the intestinal wall, tissue, and systemic circulation. A compromised intestinal TJ barrier has been identified as a significant factor in inflammatory bowel disease (IBD), necrotizing enterocolitis, and other gut-related inflammatory conditions. Recent studies have revealed the importance of the probiotic bacterial strains of Bifidobacterium in protecting against intestinal inflammation and IBD pathogenesis via the regulation of intestinal TJ barrier function. Numerous species and strains of Bifidobacterium have been found to regulate TJ proteins and the signaling pathways responsible for maintaining intestinal barrier integrity and permeability. In this review, we provide a summary of recent studies that highlight the regulatory role of Bifidobacterium species and the strain effect on the intestinal TJ barrier. We also discuss the intracellular mechanisms involved in Bifidobacterium modulation of the intestinal barrier and the potential therapeutic efficacy of targeting the barrier function to regulate intestinal inflammation.
Keywords: intestinal tight junction barrier, gastrointestinal inflammation, Bifidobacterium, cytokines, gut microbiota
Introduction
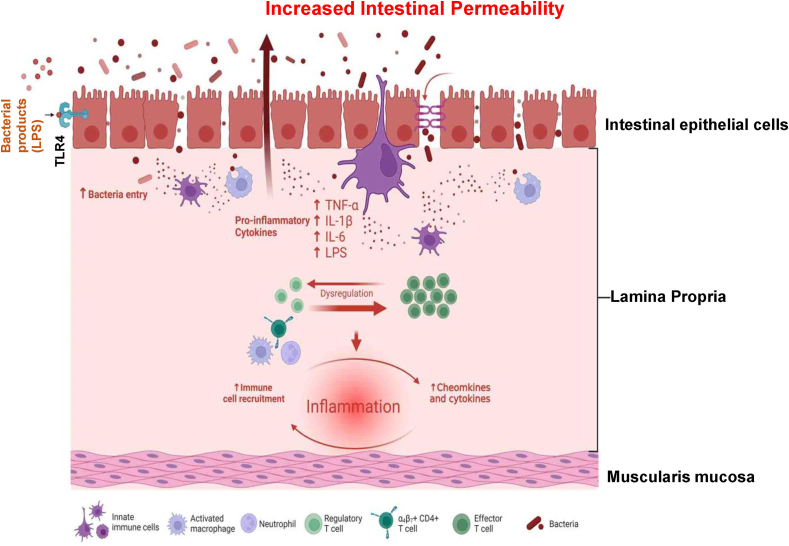
The intestinal epithelium cells (IECs) act as a critical barrier, separating luminal contents from internal tissues. These cells maintain selective permeability by regulating the transport of molecules through tight junctions (TJs), which serve as structural and functional barriers against the passage of luminal substances. Cell junctions in IECs have a crucial role in facilitating selective paracellular permeability. This selective permeability allows for the absorption of essential nutrients, regulates the transportation of ions and macromolecules, and provides protection to IECs against various toxins (Figure 1). The TJ complex is composed of occludin and claudin proteins, adhesion junctions (integral membrane-bound cadherins and cytoplasmic accessory proteins, i.e., α- and β-catenin), junctional adhesion molecule A (JAM-A), and accessory proteins (zonula occludens [ZO]-1, ZO-2, ZO-3) [[1], [2], [3], [4]]. These TJs play a crucial role in preserving epithelial polarity and ensuring the proper functioning of the paracellular barrier as a physical barrier against luminal content including microorganisms, antigens, and xenobiotics [1,[5], [6], [7]]. Figure 1 illustrates the development and critical impact of an impared intestinal epithelial barrier. Accumulating evidence indicates that TJ opening is triggered by the phosphorylation of myosin light chain, which predominantly depends on myosin light-chain kinase (MLCK) activation. This makes MLCK a key regulator of intestinal TJ barrier function [8,9]. The defective intestinal TJ barrier, also known as “leaky gut,” significantly contributes to the development of several chronic inflammatory conditions [6,7]. These conditions include celiac disease, inflammatory bowel disease (IBD) [10,11], necrotizing enterocolitis (NEC) [[12], [13], [14]], alcoholic liver disease [[15], [16], [17]], fatty liver disease [18,19], and rheumatoid arthritis (RA) [[20], [21], [22], [23]].
FIGURE 1.
The impaired tight junction barrier plays a crucial role in the pathology of inflammatory bowel disease. When this barrier is compromised within the intestinal tract, it facilitates the passage of antigens and large molecules such as bacteria and their byproducts into the lamina propria. This breach in intestinal integrity subsequently leads to increased intestinal permeability and triggers an uncontrolled and dysregulated inflammatory response. Abbreviation: TLR, toll-like receptor. (Created with BioRender).
Therapeutic tightening of the intestinal TJ barrier could be an important treatment option for IBD and other inflammatory gut conditions [24]. Recent studies have emphasized the vital role of probiotics in cellular functions, such as regulating TJs and adaptor proteins, as well as maintaining the intestinal barrier. Moreover, an expanding body of research highlights the advantages of targeting the gut microbiota in the treatment of IBD [25,26]. Based on systematic review and meta-analysis studies, probiotics have shown substantial evidence of enhancing gut barrier function, reducing inflammation, and modulating the gut microbiota structure. These benefits are achieved primarily through the enrichment and colonization of Lactobacillus and Bifidobacterium species [27]. Additional studies have demonstrated that the administration of different probiotic strains, mainly Bifidobacteria, can directly influence and regulate both the TJ barrier function and the host immune response by modulating different signaling pathways in IECs (Table 1) [[28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]].
TABLE 1.
Bifidobacteria species and strains have the potential to regulate the TJ barrier in vitro or in a mouse model of colitis, with a specific focus on targeting the function of the TJ barrier
| Probiotic bacteria | Cell and/or animal model | Treatment or disease condition | Effect on TJ proteins and barrier function | Mode of mechanism and effect on animal model | References |
|---|---|---|---|---|---|
| Bifidobacterium (B.) | Caco-2; Rat NEC | LPS damage | Restore occludin, claudin-3, ZO-1 | Normalize intestinal permeability, TNF-α, IL-6. | [28] |
| Bifidobacterium (BIF) | Caco-2 | TNF-α induced inflammation | Increase TER | Suppress TNF-α-induced overexpression of IL-6, IL-8, and autophagy pathway. | [29] |
| B. adolescentis CGMCC15058 | Rat | D-galactosamine induced liver injury | Enhance ZO-1 expression | Decrease the proinflammatory cytokine TNF-α and IL-6 and enhance mucin-4 expression. | [30] |
| B. adolescentis IM38 | Caco-2; Mouse | HFD, LPS damage, colitis | Enhance ZO-1, occludin and claudin-1 expression | Inhibit NF-κB activation; increase HFD suppressed expression of IL-10). | [31] |
| B. animalis ssp. lactis CNCM-I2494 | Mouse model of low-grade inflammation | Chronic DNBS-induced low-grade inflammation | Restore intestinal permeability and normalize claudin-4 | Decrease the proinflammatory cytokines IL-12, IL-2, IL-4 and IFN-γ. | [32] |
| B. bifidium | Rat | NEC | Normalize occludin and claudin-3 expression and localization the ileum | Normalize levels of IL-6, mucin-3, and Tff3 in the ileum. | [33] |
| B. bifidium (BB1) | Caco-2; Mouse | DSS-induced colitis | Increase TER, decrease intestinal permeability | Activation of TLR2, p38 kinase activity, and restore intestinal permeability. | [34] |
| B. bifidium (BB1) | Caco-2 | — | Increase TER, decrease inulin flux | Enhances intestinal epithelial TJ barrier in a strain-specific and TLR2/TOLLIP dependent manner, independent of MYD88 pathway. | [35] |
| B. bifidium (BB1) | Caco-2; Mouse | IL-1β induced inflammation | Increase TER, decrease inulin; decrease intestinal permeability | Prevent the IL-1β induced increase in intestinal permeability by TLR2 dependent activation of PPAR-γ and inhibition of NF-κB and MLCK signaling pathway. | [36] |
| B. bifidium E3 and B. longum subsp. infantis E4 | Mouse | LPS-induced intestinal injury | Increase ZO-1, occludin, claudin-1 | Ameliorate LPS-induced injury by enhancing intestinal TJ barrier and intestinal mucosal immunity, reducing intestinal inflammation (IL-1β, IL-6, and TNF-α) by inhibiting TLR4/NF-κB MAPK signaling pathways. | [37] |
| B. bifidium FL-228.1 | Caco-2; Mouse | DSS-induced colitis | Increase TER, decrease permeability, and increase claudin-4 | Protects against DSS-induced intestinal barrier damage by increasing ratio of IL-10/IL-12, increase mucin-2, inhibits NLRP3, and activates PPAR-γ. | [38] |
| B. bifidium FL-228.1 and B. bifidium FL-276.1 | Caco-2; Mouse | DSS-induced colitis | Increase TER, decrease permeability, and increase ZO-1, occludin, and claudin-4 | Protects against DSS-induced intestinal barrier damage by increasing production of indole-3-lactic acid by activating AHR/NRF2/NLRP3 inflammasome pathways. | [39] |
| B. breve CCFM1078 | Rat | collagen-induced arthritis | Normalize ZO-1, claudin-1, claudin-3, occludin, and JAM-B | Alleviates collagen-induced arthritis, repairs damage to the intestinal barrier, and modulates the gut microbiota through the inhibition of TLR4/MYD88-dependent pathways. | [40] |
| B. dentium N8, isolated from the feces of breastfed infants | Caco-2 | LPS | Increases ZO-1, occludin, claudin-1 mRNA expression/downregulated mRNA expression level of TLR4 and proinflammatory cytokines (TNF-α, IL1B, IL6). Also increases TER and decreases paracellular (FITC) permeability of LPS-stimulated Caco-2 | Anti-inflammation (downregulate mRNA expression level of TLR4 and proinflammatory cytokines) (TNF-α, IL-1β, IL-6). | [41] |
| B. infantis | Neonatal mouse | NEC | Preserve intestinal permeability and normalize claudin-4 and occludin | — | [42] |
| B. infantis 35624 | Mouse | — | Increase colonic TER | Inhibition of colonic ion transport. | [43] |
| B. infantis conditioned medium | Caco-2, H4 cells | IL-1β induced damage | Increase TER and occludin, decrease claudin-1 | Inhibit NF-κB p65 activation. | [44] |
| B. infantis conditioned media | T84 human epithelial cells; IL-10-deficient mouse | TNF-α and IFN-γ damage | Increase TER; increase ZO-1 and occludin expression and decrease claudin-2 expression | Attenuate inflammation, normalize colonic permeability, and decrease colonic and splenic IFN-γ secretion. | [45] |
| B. lactis 420 | Caco-2 | — | Increase TER | — | [46] |
| B. longum CCM 7952 | HEK293; Mouse | DSS-induced colitis | Decrease colon permeability; restore ZO-1 and occludin expression | Activation of TLR2 and NOD2 pathways. | [47] |
| B. longum HB55020 | Mouse | Trichinella spiralis infection induced IBS | No effect on TER; restore ZO-1 and claudin-1 expression | Change gut microbiota. | [48] |
| B. longum LC67 | Mouse | TNBS-induced colitis | Prevent TNBS-suppressed expression of ZO-1, occludin and claudin-1 | Prevent TNBS-induced NF-κB activation and restore Th17/Treg balance. | [49] |
| B. longum LC67 | Caco-2, Mouse | Ethanol and LPS damage, alcohol steatosis | Increase the ethanol/LPS-suppressed occludin and claudin-1 | Reduce ethanol-induced ALT, AST, TG, and TC levels in the blood and liver and inhibit NF-κB activation. | [50] |
| B. longum subsp. longum 2-21-4 | CCD841 CoN | LPS damage | Restore ZO-1, claudin-1, and occludin | Prevent LPS-induced intestinal barrier injury, enhance cell proliferation, reduce apoptosis, and active Wnt/β-catenin and PI3K/Akt/mTOR signaling pathway. | [51] |
| B. longum subsp. longum K5 and K15 | HT-29, Caco-2 RAW264.7 | LPS damage | Increase TER, increase ZO-1, occludin, and claudin-1 expression | Prevent LPS-induced intestinal barrier injury and downregulate TLR4, proinflammatory cytokines (TNF-α, IL-1β, and IL-6). | [52] |
| B. longum subsp. longum YS108R | Mouse | DSS colitis | Maintain ZO-1, claudin-1 and mucin-2 expression | Decrease the proinflammatory cytokine IL-6 and IL-17A levels after DSS challenge. | [53] |
| Bifidobacterium pseudocatenulatum MY40C and CCFM68 | Mouse | DSS-induced colitis | Increase the localization and expression of β-catenin, claudin-3, and ZO-1 | Alleviate DSS-induced colitis and downregulate TNF-α and IL-6, upregulate IL-10 and PPAR-γ, and inhibit TLR4/NF-κB pathway. | [54] |
| Bifidobacterium pseudolongum Bp7 and Bp8 | Mouse | DSS colitis | Elevates transcription levels of occludin, claudin-1, and ZO-1 relative to DSS group | Regulates the PPAR-γ/STAT3 pathway (elevate MYD88); anti-inflammatory (reduces colonic TNF-α and IL-1β levels and elevates colonic IL-10 levels). | [55] |
| Fermentation products B. Bb12 | Caco-2 | — | Increase TER | Protect against deoxycholic acid-disruption of barrier. | [56] |
| Purified galactooligosaccharide, derived from a mixture produced by the enzymic activity of B. bifidum | HT-29 16E; mouse model | S. typhimurium infection | Preserve formation of brush borders and tight junctions | Prevent the adherence or invasion of S. typhimurium to enterocytes. | [57] |
| Two strains of B. isolated from humans | Germ-free mouse model | Killed E. coli, Klebsiella pneumoniae, Yersinia pseudotuberculosis, S. aureus, and S. typhimurium | — | — | [58] |
Abbreviations: AHR, aryl hydrocarbon receptor; ALT, alanine transaminase; AST, aspartate transaminase; DNBS, dinitrobenzene sulfonic acid; DSS, dextran sodium sulfate; FITC, fluorescein isothiocyanate; HFD, high-fat diet; IBS, irritable bowel syndrome; IFN, interferon; JAM, junctional adhesion molecule; MLCK, myosin light-chain kinase; mTOR, mammalian target of rapamycin; MYD88, myeloid differentiation primary response protein 88; NCRP, native C-reactive protein; NEC, necrotizing enterocolitis; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; NOD2, nucleotide-binding oligomerization domain-containing protein 2; NRF, nuclear respiratory factor; PI3K, phosphatidylinositol 3-kinase; PPAR, peroxisome proliferator-activated receptor; TC, total cholesterol; TER, transepithelial resistance; Tff3, trefoil factor 3; TG, triglyceride; TJ, tight junction; TLR, toll-like receptor; TNBS, 2,4,6-trinitrobenzene sulfonic acid; TOLLIP, toll-interacting protein; ZO, zonula occludens.
Bifidobacterium Species and Strains
Among the various probiotic bacteria reported to date, Bifidobacterium spp. are one of the most widely studied and utilized probiotic bacteria. The genus Bifidobacterium belongs to the phylum Actinobacteria and currently comprises 80 (sub) species, which are distributed across different ecological niches, including the gastrointestinal (GI) tract and oral cavity of humans [59,60]. They are gram-positive, nonmotile, nonsporulating anaerobic bacilli. Bifidobacterium spp. are the first to colonize the human intestine, a phenomenon driven by the bifidogenic activities of certain mother milk-derived oligosaccharides [61]. In gram-positive bacteria, the cell wall has unique features; it includes a thick peptidoglycan sacculus with added proteins, lipoteichoic acids, exopolysaccharides, polysaccharides, and cell surface β-glucan/galactan (CSGG). Some species also have an outer layer of proteins arranged in a special pattern called sortease-dependent pili, tight adherence (Tad) pili, or other specific surface proteins (shown in Figure 2). These structures give each bacterium its own distinct properties, which can vary by species and strain. Consequently, they account for 40% to 80% of microorganisms in the intestinal tract of breastfed infants [62,63]. The absence of colonization by Bifidobacteria has been found to be associated with the development of allergic disorders in childhood [64]. B. breve, B. bifidum, B. longum, and B. infantis are the most commonly detected bacteria at the infant stage, with B. bifidum being the most prominent species, followed by B. breve, B. longum, and B. infantis [26,59]. As age progresses, the overall concentration of Bifidobacterium decreases but remains relatively stable (2%–14%) throughout adulthood and decreases again in old age [65]. The most commonly identified species in the adult gut include B. adolescentis and B. catenulatum, followed by B. longum and B. bifidum [66,67]. However, there is no absolute infant compared with adult division of bifidobacterial species. Healthy adults generally have a prevalence of Bifidobacterium exceeding 90%, with only a small number of species observed per individual [68]. Additionally, this decline in Bifidobacteria population has been linked to a decrease in their ability to adhere to the intestinal mucosa. However, it is still uncertain whether this reduced adhesion is caused by changes in the microbiota composition, alterations in the structure of the mucus, or the alteration of TJ barrier permeability [69,70].
FIGURE 2.
Schematic overview of the bifidobacterial surface structure. The bacterial surface molecules responsible for bifidobacterial-host cross-talk encompass a range of molecules, including exopolysaccharides, cell wall polysaccharides, lipoteichoic acid, surface protein, peptidoglycan and proteins such as CSGG. Abbreviation: CSGG, cell surface β-glucan/galactan. (Created with BioRender).
One of the crucial functions of Bifidobacterium is its ability to metabolize undigested food residues, such as poly- and oligosaccharides, and utilize host mucins or hyaluronic acid [71]. The carbohydrate metabolism of Bifidobacterium is based on a unique pathway called the “bifid shunt” or fructose-6-phosphate shunt [72]. This pathway involves the conversion of fructose-6-phosphate from glucose to erythrose-4-phosphate by the enzyme fructose-6-phosphate phosphoketolase [73,74]. Furthermore, knockouts of the bifid shunt pathway demonstrate that this pathway plays a crucial role in the survival and proliferation of Bifidobacteria [75]. As a result, acetate and lactate, which are classified as short-chain fatty acids (SCFAs), are formed (Figure 3) [76]. Bifidobacteria exhibit various mechanisms of probiotic action, often with strain-specific characteristics and the secretion of specific metabolites. To provide an overview of the complexities and interactions involved, refer to Figure 3. In addition to SCFAs, Bifidobacteria can produce antioxidants, conjugated linoleic acids, polyphenols, vitamins B and K, γ-aminobutyric acid, and a specific type of bacteriocin known as lantibiotics as secondary products [74,[77], [78], [79], [80]], as shown in Figure 3.
FIGURE 3.
Metabolic capacities of Bifidobacterium microbial metabolites are relevant to the microbe-host interaction network. These capacities do not necessarily imply species-specificity or universality among all strains; instead, they suggest a more complex interaction and mechanism involving the metabolites [75,77,79]. Abbreviation: TJ, tight junction. (Created with Setworks v1).
Bifidobacteria have been commercially exploited as probiotic agents due to their well-established health benefits and “Generally Recognized As Safe” status [81]. The claimed homeostatic and health-promoting activities exerted by Bifidobacteria are numerous. These activities include the establishment of a healthy microbiota in preterm infants [53], protection against pathogens [82], enhancement of the intestinal gut barrier [83,42], promotion of an anti-inflammatory environment through modulation of the host immune response [84], production of vitamins and SCFAs, digestion of plant oligo- and polysaccharides, and suppression of the production of potentially toxic and carcinogenic metabolites [58] (Figure 3). As an innate member of the human gut, Bifidobacterium has proven to be essential for maintaining intestinal epithelial barrier integrity [56]. Furthermore, in a randomized intervention trial conducted on extremely premature infants, a probiotic mixture consisting of 4 strains of Bifidobacterium species and one strain of Lactobacillus was administered [46]. The study utilized 16S and internal transcribed spacer rRNA sequencing, as well as metabolomics and cytokine levels in stool, to analyze the effects. The results demonstrated that Bifidobacterium strains, but not Lactobacillus rhamnosus, were able to effectively establish colonization in the gut of premature infants. Moreover, the maturation of the gut microbiome driven by Bifidobacterium strains was associated with a reduction in intestinal inflammation and the promotion of an anti-inflammatory immune environment [46]. Indeed, a recent study cataloging the microbiota of patients with ulcerative colitis (UC) by 16S rRNA microbial profiling revealed a substantial decrease of Bifidobacteria, notably B. bifidum, suggesting that this taxon plays a biological role in the etiology of UC and also highlighting the importance of B. bifidum as a microbial biomarker for UC [67]. Notably, numerous studies spotlight Bifidobacteria, with many of them being referenced within this comprehensive review. These studies specifically target strains that are prevalent in both infants and adults, as well as those that exhibit dysregulation in disease states.
Bifidobacterium Regulation of Intestinal TJ Barrier
Effects of Bifidobacterium and TJ barrier in IBD
Previous studies have demonstrated that pretreatment of human intestinal epithelial cell lines (Caco-2, HT-29, and T-84) with Bifidobacteria species confers protective effects against TJ barrier impairment induced by various factors [28,43,85]. These protective effects are mediated through upregulation of TJ protein expression (notably occludin and ZO-1). Furthermore, Bifidobacteria species also modulate various protein kinase signaling pathways, leading to the phosphorylation of TJ proteins. This phosphorylation can either promote TJ formation or redistribution and complex stabilization [32,33,83]. The beneficial effects of Bifidobacteria in treating various GI disorders has also been reported in various mouse models [47]. Bergmann et al. [56] reported the protective effect of B. infantis in a mouse model of NEC. Mouse pups administered B. infantis exhibited an attenuated increase in intestinal permeability compared to dam-fed controls. The pups also showed preserved occludin and claudin-4 localization at TJs and a decreased incidence of NEC. Additional studies in Caco-2 cells demonstrated a dose-dependent enhancement of the TJ barrier by B. lactis 420 supernatant [43]. Another species, B. bifidum, has been shown to improve intestinal integrity in a rat model of NEC [41]. To the contrary, B. animalis subspecies lactis was shown to efficiently restore the gut barrier permeability in a DNBS (dinitrobenzene sulfonic acid)-induced low-grade inflammation model in mice. B. animalis subspecies lactis protected the intestinal barrier by normalizing the levels of several TJ proteins, particularly claudin-4, and also by restoring the Th1/Th2 ratio balance in colonic goblet cell population [54]. Srutkova et al. [52] showed that Bifidobacterium can ameliorate acute dextran sodium sulfate (DSS)-induced colitis in mice. Prophylactic administration of B. longum CCM 7952 was capable of preventing the damage by DSS-induced colitis is a strictly strain-specific manner. A recent study by Zhao et al. [38] showed that B. dentium N8 offered protective effects [39] in lipopolysaccharide (LPS)-treated Caco-2 cells by increasing the expression of TJ genes ZO-1, occludin, and claudin-1. Additional studies in DSS-induced colitis in mice demonstrated that supplementation with B. pseudocatenulatum MY40C and CCFM680 strains alleviated DSS-induced colitis by protecting the intestinal barrier, modulating the gut microbiota, suppressing proinflammatory cytokines, and inhibiting the toll-like receptor 4 (TLR4)/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway [39]. These treatments led to increased levels of TJ proteins (ZO-1, β-catenin, and claudin-3) and mucin-2, as well as decreased levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6 while IL-10 and peroxisome proliferator-activated receptor (PPAR)-γ levels were increased [39]. Additionally, they examined how B. pseudocatenulatum MY40C and CCFM680 strains altered the gut microbiome landscape, finding that both strains increased Allobaculum and reduced Sutterella, Bacteroides, and Oscillospira in the gut microbiota [39]. Hsieh et al. [33] demonstrated B. bifidum W1U2 strains can protect the intestinal epithelial barrier from TNF-α-induced disruption and facilitate the maintenance of epithelial TJ integrity. Moreover, the upregulation of SCFA production, particularly acetate and formate, has been identified as a mechanism that contributes to the restoration of the epithelial TJ barrier [33] (Figure 3 depicts a potential metabolite secreted by Bifidobacterium). Zhao et al. [34] showed that B. longum subsp. K5 strain, but not the K15 strain, has the ability to alleviate the inflammatory response and provide protection against LPS-induced intestinal barrier injury. This effect is achieved by upregulating the mRNA expression levels of ZO-1, occludin, and claudin-1, which are important TJ proteins involved in maintaining barrier integrity. Additionally, the K5 strain downregulated the expression of TLR4, a receptor involved in LPS recognition, as well as the production of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6. In a related study with B. bifidum FL-228.1, it was found that this strain protects against DSS-induced intestinal damage in mice by significantly increasing the ratio of IL-10/IL-12, as well as enhancing levels of mucin-2 production and claudin-4 in the colon [86]. A recent investigation involving B. bifidum FL-228.1 illustrated that administration of B. bifidum FL-228.1 and B. bifidum FL-276.1 for 2 wk before DSS induction resulted in more effective alleviation of colitis symptoms compared to intervention after DSS induction began [87]. This protection was attributed to the increased production of indole-3-lactic acid (ILA) by B. bifidum FL-228.1 and B. bifidum FL-276.1, which regulates the aryl hydrocarbon receptor (AHR)/nuclear respiratory factor 2 (NRF2)/NOD-like receptor family pyrin domain-containing 3 (NLRP3) pathway [87]. Additional studies conducted on Caco-2 cells demonstrated that ILA enhances epithelial barrier function by upregulating ZO-1, occluding, and claudin-4 through AHR activation via the AHR/NRF2/NLRP3 pathway [87]. In a more recent study by Al-Sadi et al. [88], it was demonstrated that B. bifidum significantly increased intestinal TJ barrier function in cell culture and live mice in a strain-specific manner. These studies also revealed that B. bifidum protected against DSS-induced colitis by targeting the intestinal TJ barrier in mice. In addition to live B. bifidum providing barrier enhancement, a novel study demonstrated that both B. bifidum (BIA-7) and its extracellular vesicles (EVs) can activate distinct signaling pathways [89]. B. bifidum was found to activate the Notch-1/Hes-1 pathway, possibly through its interaction with Notch-1 receptors on the cell’s surface and by increasing AHR gene expression. However, it was observed that B. bifidum alone did not activate the AHR pathway, as evidenced by the absence of increased cytochrome P450 family 1 subfamily A member 1 (CYP1A1) gene expression, which indicates AHR pathway activation [89]. Intriguingly, only the B. bifidum EVs were able to penetrate the cells and activate the AHR pathway, leading to significant upregulation of CYP1A1, occludin, and ZO-1 gene expressions. These findings indicate that B. bifidum and its derived EVs can activate different signaling pathways, with EVs specifically modulating the gene expression of TJ proteins [89].
Effect of Bifidobacterium and TJ barrier on early-life development and longevity
Kiu et al. [90] aimed to investigate the potential impact of Bifidobacterium on the maintenance of IEC homeostasis during the crucial developmental phase of early life. The administration of B. breve UCC2003 resulted in significant modifications in the transcriptome of neonatal murine IECs. Approximately 4000 genes were found to be significantly upregulated while around 450 genes were downregulated [90]. The upregulated genes include important ones associated with the functionality of the epithelial barrier, such as ZO-1, ZO-2, ZO-3, occludin, and claudin-12 [90]. Furthermore, a substantial increase in the expression levels of JAM-B and claudin-34c1 was observed [90]. These findings provide further confirmation regarding the critical role of Bifidobacterium, as a member of the early-life microbiota, in the development of the intestinal epithelium.
Extended studies conducted in aged mice have provided further evidence supporting the efficacy of administering L. casei LC122 and B. longum BL986 in exhibiting promising antiaging effects [50]. This treatment regimen has been observed to elicit enhancements in cognitive performance and muscular function while concurrently reducing inflammation and oxidative stress in peripheral tissues. Moreover, it has demonstrated efficacy in ameliorating age-related deterioration of gut barrier function by upregulating the expression of claudin-1, ZO-1, and JAM-A mRNA in both the jejunum and colon [50]. Notably, treatment with B. longum BL986 showed a substantial increase in JAM-A expression and a more pronounced anti-inflammatory response compared to the treatment with L. casei LC122 [50].
Effects of Bifidobacterium and TJ barrier on liver disease
Numerous studies involving animal models and human patients with alcoholic liver disease and metabolic disorders have shown that prolonged consumption of alcohol and a high-fat diet (HFD) causes an imbalance in gut microbiota and microbial metabolites, leading to defects in the intestinal epithelial barrier [13,50,89,90]. It is hypothesized that this increased gut permeability leads to a higher concentration of LPS in portal blood circulation, where LPS then binds to TLR4 and activates NF-κB, which in turn stimulates the expression of proinflammatory cytokines [39,91]. Bifidobacterium has been reported to improve the paracellular permeability in Caco-2 monolayers treated with LPS by significantly decreasing the production of proinflammatory cytokines (such as IL-6 and TNF-α) and upregulating TJ protein (occludin, claudin-3, and ZO-1) expression and localization [32]. Li et al. [40] showed that B. adolescentis CGMCC 15058 improved liver injury, enhanced the intestinal barrier, and restored the gut microbiota from dysbiosis in D-galactosamine-treated rats. Prior treatment with B. adolescentis led to a significant reduction in elevated levels of alanine transaminase and total bile acid while enhancing the expression of mucin-4 and ZO-1. Additional studies in HFD utilizing oral administration of B. adolescentis IM38 to mice showed potent suppression of inflammatory markers such as inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2), TNF-α, and IL-17, which are typically elevated in HFD inflammation. IM38 also reversed the HFD-induced suppression of IL-10 levels in mice. Furthermore, in both HFD-induced obese mice and LPS-stimulated Caco-2 cells, IM38 increased the levels of TJ proteins (ZO-1, occludin, and claudin-1) in the colon [92].
Effects of Bifidobacterium and TJ barrier on RA
It has been observed that patients with RA often have an altered gut microbiota [93]. RA is a chronic inflammatory condition characterized by joint inflammation, swelling, and stiffness [94]. These disturbances in the gut microbiota can potentially lead to damage to the integrity of the intestinal TJ barrier in patients with RA. Li et al. [95] examined the impact of B. breve CCFM1078 on the intestinal barrier and systemic inflammation in rats with collagen-induced arthritis (CIA). The results revealed that B. breve CCFM1078 has the ability to restore the intestinal barrier (via ZO-1, claudin-1, claudin-3, occludin, and JAM-B), decrease the translocation of LPS, regulate the composition of gut microbiota (B. breve and B. pseudolongum subsp. pseudolongum), and increase the levels of SCFAs (acetic acid, propionic acid, butyric acid, and valeric acid) in the intestine. Consequently, CCFM1078 reduced the release of proinflammatory cytokines, regulated immune dysfunction, and inhibited the TLR4/MYD88-dependent pathways and downstream inflammatory pathways, thereby alleviating joint inflammation in CIA rats. These findings indicate that B. breve CCFM1078 has the potential to alleviate joint inflammation by modulating the gut microbiota profile and enhancing the intestinal TJ barrier [95].
Mechanisms of Bifidobacteria-Induced Modulation of the Intestinal TJ Barrier
Bifidobacterium adhesion and metabolism in the gut epithelium
Probiotics exhibit strain-specific and even subspecies-specific effects on the intestinal epithelial TJ barrier. Recent research has made significant progress in unraveling the molecular mechanisms underlying the beneficial effects of certain Bifidobacteria strains. Comparative genomics has begun to reveal differences in genes associated with host adhesion between various strains and subspecies of Bifidobacteria [96]. In a recent comparison of B. dentium N8 and E7 (isolated from the feces of breastfed infants), N8 offered significant protective effects against DSS in mouse models and attenuated the LPS-induced increase in paracellular permeability in Caco-2 monolayers, while E7 had no significant effects in either model [38]. Comparative genomics of these strains revealed the presence of genes specific to adhesion ability and immune system regulation in N8 that were absent in the E7 genome [38]. Genes coding for extracellular structures, the main types present in Bifidobacteria being Tad pili and sortase-dependent pili (the structure of the Tad pili is depicted in Figure 2), have been identified in several Bifidobacterium species, including B. longum DSM 20219 and B. bifidum JCM 1254 [96]. Additionally, the DSM 20219 genome revealed 3 sortase A genes and fimbrial isopeptide formation D2 domain-containing protein, which are also associated with adherence and interaction with host proteins [96]. A recent comparative genomic analysis of commercial multispecies probiotic product VSL#3 found gene clusters coding for Tad pili (shown in Figure 2) in B. breve BB02, B. animalis subsp. lactis BL03 and BI04, structures which are known to maintain intestinal barrier integrity and encourage host–bacteria interaction [97]. Adhesion to the intestinal mucosa is a critical factor for successful colonization and functioning of Bifidobacteria. Zhang et al. [35] investigated the impact of different carbon sources, including 2′-fucosyllactose (2′-FL), galactooligosaccharides, and glucose, on the growth and adhesion properties of B. bifidum DNG6 to Caco-2 cells. Notably, the utilization of 2′-FL as a carbon source significantly enhanced the adhesion ability of B. bifidum DNG6. Additionally, the expression levels of adhesion-associated genes, including transaldolase (TAL), enolase, GroEL, and elongation factor Tu, were markedly higher in B. bifidum DNG6 grown in 2′-FL compared to galactooligosaccharides and glucose following incubation with Caco-2 cells [35]. These findings support the potential utilization of 2′-FL as a prebiotic in infant nutritional supplements and underscore the importance of its role as a carbon source, emphasizing its beneficial effects on the adhesion of Bifidobacteria and barrier enhancement. Recently, Zhang et al. [98] demonstrated that dietary supplementation with fructooligosaccharide (FOS) in weanling pigs led to notable increases in the mRNA expression levels of important TJ proteins (claudin-1, claudin-2, claudin-4, and occludin) in the ileal mucosa. Additionally, significant upregulation was observed in the mRNA expression of ZO-1, claudin-1, occludin, and pBD-1 within the colonic mucosa upon FOS supplementation [98]. FOS supplementation improved the expression of TJ proteins in weanling pigs, possibly linked to increased concentrations of lactic acid and acetic acid, which decrease pH in the ileal digesta, thereby promoting a healthier intestinal microenvironment [98].
Bifidobacterium-mediated effects on the TLR2 signaling pathway
Previous studies of probiotics known to exert beneficial effects on the intestinal barrier, including Lactobacillus acidophilus [99], Akkermansia muciniphila [74], and Bacteroides fragilis [75], have identified TLR2 as a crucial pathway in mediating this host–bacterial interaction. Khailova et al. [41] found that oral administration of B. bifidum to rat pups activates TLR2 signaling, increases COX-2 expression, leads to elevated production of prostaglandin E2 in the ileum, and provides protection against intestinal apoptosis associated with NEC. Similarly, a recent study identified a specific strain of B. bifidium (BB1) that caused an enhancement of the intestinal epithelial TJ barrier both in vivo and in vitro and found that attachment to TLR2 at the apical membrane of enterocytes was the main mechanism of action [88]. Additionally, this binding was found to be mediated by the heterodimerization of TLR2 with TLR6 but not TLR1 [88]. The nature of the host–bacteria interaction in 2 subspecies of B. longum (BI 371 and BI 7952) was also found to involve signaling through TLR2, although only BI 7952 showed a protective effect on the intestinal epithelial barrier [52]. Interestingly, both of these strains were found to also contain ligands for nucleotide-binding oligomerization domain-containing protein 2 (NOD2), although the nature of this interaction and downstream pathways have yet to be investigated [52]. Further studies by Abdulqadir et al. [44] demonstrated that B. bifidum strain BB1, but not BB4, is responsible for strengthening the intestinal epithelial TJ barrier. This enhancement is achieved through an increase in toll-interacting protein (TOLLIP) mediated by TLR2 in a manner independent of MYD88 (myeloid differentiation primary response 88) [44,49]. TOLLIP is a powerful inhibitory cytosolic adaptor protein that suppresses MYD88 activity. The observed enhancement in the TJ barrier caused by BB1 was abolished when TLR2 and TOLLIP were specifically silenced through targeted knockdown [44]. The specific components of B. bifidum that are responsible for enhancing the TJ barrier and their interactions with host cells have not been determined conclusively. In a study by Verma et al. [29], it was found that CSGG (the structure of CSGG is depicted in Figure 2) derived from B. bifidum plays a crucial role in suppressing intestinal inflammation. These CSGG molecules induce the generation of regulatory T cells (Foxp3+ T cells), which help regulate the immune response. Furthermore, CSGG stimulates the production of regulatory dendritic cells (DCs) through a mechanism that involves TLR2. This suggests that CSGG-mediated induction of Treg cells occurs through a TLR2-dependent process involving regulatory DCs [29]. In a subsequent study, cell surface polysaccharides were isolated from B. bifidum strain PRI1 [37]. The results demonstrated that this bacterium produces a complex mixture of polysaccharides that can be classified into 2 main groups: phospho-glycero-β-galactofuranan (PGβG) and a mixture of 4 neutral polysaccharides known as CSGG. The CSGG fraction is composed of β-(1 → 6)-glucan, β-(1 → 4)-galactan, β-(1 → 6)-galactan, β-galactofuranan, and starch. These 2 fractions exhibited distinct immune responses when tested on DCs. PGβG stimulated proinflammatory immune responses by increasing interferon-γ levels, whereas CSGG induced immunosuppressive regulatory T cells and IL-10 production [37]. An overview of Bifidobacteria’s effect on TLR2 and these related pathways is shown in Figure 4.
FIGURE 4.
Schematic representation of Bifidobacteria targeting TJ barrier function via TLRs signaling pathways. Abbreviations: IκB, inhibitor of nuclear factor kappa B; MLCK, myosin light-chain kinase; MYD88, myeloid differentiation primary response protein 88; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; PGE2, prostaglandin E2, PI3K, phosphatidylinositol 3-kinase; P-MLC, phosphorylated myosin light chain; PPAR-γ, peroxisome proliferator-activated receptor gamma; TGF, transforming growth factor; TLR, toll-like receptor, TNFR, tumor necrosis factor receptor; TOLLIP, toll-interacting protein. (Created with BioRender).
Bifidobacterium’s role in modulating the NF-κB and MAP kinase pathway
The 2 known downstream pathways primarily involved in regulating the TJ barrier include NF-κB and MAP kinase [88]. Al-Sadi et al. [88] found that the protective effect of BB1 was mediated by the activation of p38 kinase and was independent of the NF-κB signaling pathway. Consistent with these data, Guo et al. [51] showed that B. infantis conditioned media inhibited the IL-1β induced NF-κB p65 activation in Caco-2 cells by normalizing the protein expression of occludin and claudin-1. Additionally, Jang et al. [100] showed that the strain-specific B. longum LC67 prevented NF-κB activation, transforming growth factor-β-activated kinase 1, and IκBα (inhibitor of nuclear factor kappa B, alpha) phosphorylation, as well as COX-2 and iNOS expression in a 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis mouse model and restored expression of TJ proteins(occludin, ZO-1, and claudins). Furthermore, treatment with Bifidobacterium has a protective effect on the inflammatory response induced by TNF-α in Caco-2 cells. This effect is achieved through the inactivation of NF-κB and p38 kinase pathways [55]. Inhibition of NF-κB activation is a commonly observed mechanism of action of Bifidobacteria. Yue et al. [101] investigated the effects of combining B. bifidum E3 with B. longum subsp. infantis E4 (BLBB) in mice treated with LPS. They found that this combination inhibited the TLR4/NF-κB and mitogen activated protein kinase (MAPK) signaling pathways. Additionally, there was an increase in the number of IgA+ plasma cells, CD4+/CD8+ T cells, and DCs in the BLBB group [101]. The levels of diamine oxidase and D-lactic acid in the serum were reduced in the BLBB group after LPS injection compared to the LPS alone group. Furthermore, the BLBB treated group also showed increased expression levels of TJ proteins (ZO-1, occludin, and claudin-1), mucin-2 mRNA, and decreased expression levels of inflammatory cytokines (IL-1β, IL-6, and TNF-α) [101]. Guan et al. [102] demonstrated that B. longum K2-21-4 exhibits a protective effect against LPS-induced injury. B. longum K2-21-4 enhanced cellular proliferation, boosted overall cellular protein levels, accelerated the cell cycle, diminished apoptosis, and heightened the activity of ATPases, as well as aspartate aminotransferase and alanine aminotransferase, in LPS-induced injury. Additionally, this protective effect was achieved through the activation of 2 signaling pathways, namely the Wnt/β-Catenin pathway and the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway. This study showed that activated B. longum K2-21-4 enhances the TJ barrier of ZO-1, occludin, and claudin-1, further contributing to its protective properties against LPS-induced injury in CCD841 CoN cells [102]. In similar studies, Shang et al. [103] demonstrated the effective alleviation of DSS-induced colitis pathogenesis in mice and the mitigation of LPS-induced disruption in CCD-841 CoN cells using B. bifidum H3-R2. This protective effect was attributed to the treatment with recombinant GroEL and TAL proteins of B. bifidum H3-R2, which facilitated the upregulation of TJ proteins (ZO-1, occludin, and claudin-1) in the context of LPS-induced inflammation [103]. Furthermore, in-depth analyses indicated that these proteins may exert their beneficial effects through the inhibition of NF-κB, MLCK, RhoA/Rho-associated protein kinase, and MAPK pathways [103].
Bifidobacterium’s role in modulating the PPAR-γ/Signal transducer and activator of transcription 3 (STAT3) pathway
Another important pathway for enhancing and repairing the intestinal barrier is the PPAR-γ/STAT3 pathway. In a study by Guo et al. [104], B. pseudolongum strains Bp7 and Bp8 were found to ameliorate intestinal barrier function in DSS colitis mice via increased expression and activation of the PPAR-γ/STAT3 pathway. Previous studies have also shown that probiotics influence bacteria–host interactions by modulating PPAR-γ [36,[105], [106], [107]]. Nepelska et al. [107] examined conditioned media from 57 commensal gut bacterial strains, including 23 species of Bifidobacteria, to modulate PPAR-γ activities. They linked transcriptional activity of PPAR-γ to the presence of butyrate and propionate, 2 of the main metabolites of intestinal bacteria. Only 2 species of probiotics (A. parvulum and P. copri) were found to induce the expression of PPAR-γ genes, including adipose differentiation-related protein and angiopoietin-like protein 4 in HT-29 cells. Another study showed a protective effect of live B. bifidum against IL-1β induced increase in intestinal permeability in vitro and in vivo. The strain’s specific protective effect of live B. bifidum against the IL-1β induced increase in intestinal TJ permeability was mediated by TLR2/PPAR-γ activation, dependent inhibition of NF-κB, and MLCK gene activation [108]. Interestingly, Abdulqadir et al. [49] showed that in administration of 5 live strains of B. bifidum to Caco-2 cells, only 1 strain (BB1) activated PPAR-γ, leading to a cyto-to-nuclear translocation. Additionally, pretreatment with BB1 inhibited TNF-α-induced increase in transepithelial electrical resistance (TER) in Caco-2 cells and prevented the TNF-α-induced activation of NF-κB in a PPAR-γ dependent manner [49,109]. Probiotics are known to activate STAT3 by activating anti-inflammatory factors, maintaining gut integrity, and regenerating the epithelium in the intestinal crypts [[110], [111], [112], [113]]. Sonicated Lactobacillus spp., Bifidobacterium spp., and a mixed cocktail showed significant anti-inflammatory effects on HT-29 cells [110]. This was achieved by downregulating certain genes in the NF-κB pathway, including Janus kinase, Toll-interleukin-1 receptor domain-containing adaptor protein, interleukin-1 receptor-associated kinase 4, NF-κB essential modulator, and ribosomeinactivating protein [110]. Additionally, Bifidobacterium spp. treatment enhanced STAT3 signaling and led to reduced production of IL-6 and IL-1β, further supporting the anti-inflammatory response [110].
Based on the presented research, it is likely that different species and strains of Bifidobacteria will have varying effects on the enhancement of the intestinal TJ barrier (as shown in Figure 4) and the degree of intestinal anti-inflammatory response. Therefore, it is important to acknowledge Bifidobacteria’s role in the intestinal TJ barrier as a fundamental target for therapy in treating IBD, NEC, and other GI-related disease.
The Role of Bifidobacteria in Therapeutically Targeting the Intestinal TJ Barrier and Microbiome in Disease
Results obtained from various human clinical trials also agree on the beneficial effects of Bifidobacterium in maintaining and treating intestinal epithelial barrier. For example, the administration of B. longum subsp. infantis CECT 7210 and B. breve K-110 inhibited rotavirus-induced sporadic diarrhea in infants [114]. A recent study by Patole et al. [115] reported that routine administration of B. breve M-16V lowered the incidence of NEC in neonates born before 34 wk gestation. Another double-blind study showed a significant reduction in antibiotic-associated diarrhea in infants treated with a commercial probiotic formula containing B. bifidum and Streptococcus thermophiles [116]. B. animalis subsp. lactis BB12 helps maintain fecal acetate levels in subjects receiving antibiotics, which is necessary to prevent postantibiotic dysbiosis [48]. Additionally, in a study involving 113 preterm infants who were exclusively breastfed, it was observed that there was a significant increase in B. breve strains. These strains are characterized by their exceptional ability to metabolize carbohydrates, which is essential for the development and maturation of the intestinal barrier. This process is important in preventing increased intestinal permeability and NEC [117].
Moreover, a study carried out in patients with UC in Japan strongly demonstrated that administration of B. longum BB536, isolated from the feces of a healthy infant, was well tolerated and effective in preventing remission [118]. Previous studies have demonstrated that the viable strain of B. bifidum MIMBb75 significantly alleviates symptoms of irritable bowel syndrome (IBS) [119,120]. However, it remains uncertain whether the nonviable form of B. bifidum MIMBb75 exerts a significant impact on IBS. In a recent randomized, double-blind, placebo-controlled clinical trial involving 443 participants (221 receiving the heat-inactivated bacterial strain of B. bifidum HI-MIMBb75 and 222 receiving placebo), significant alleviation of IBS and its symptoms was observed in the group administered [121]. Additionally, certain Bifidobacteria probiotics have the potential to decrease the accumulation of fat in the host. A study conducted using heat-killed B. lactis CECT8145 showed a notable increase in lean mass and an improvement in metabolic syndrome among cafeteria-fed obese rats [122]. This finding was further supported by similar studies conducted on abdominally obese individuals using the same probiotic. These studies demonstrated that both live and heat-treated forms of B. lactis CECT8145 can effectively reduce anthropometric adiposity biomarkers associated with alterations in the regulation of the host immune system while also promoting the enrichment of the Akkermansia genus in the gut of individuals with abdominal obesity [123]. Upon conducting sex-specific analyses, statistical significance was observed exclusively among females [123]. Furthermore, a randomized, double-blind, placebo-controlled trial demonstrated that the administration of the Lab4P consortium of probiotics, which consists of 2 strains of Lactobacilli and 2 strains of Bifidobacteria, resulted in significant reductions in body weight, BMI, and waist-to-hip ratio (W/H ratio) circumference. The trial also revealed decreases in small dense LDL cholesterol levels overall and improvements in overall well-being [45]. In contrast, the efficacy of one strain of B. longum APC1472 in addressing obesity only partially translates from mice studies to humans. In HFD-fed mice, supplementation of B. longum APC1472 was linked to reduced body weight, decreased accumulation of fat deposits, and improved glucose tolerance [124]. However, in overweight/obese adults, the supplementation of B. longum APC1472 strain did not yield significant changes in the primary outcomes of BMI or W/H ratio. Nevertheless, a positive impact was observed in secondary outcomes of reduced fasting blood glucose levels, cortisol awakening responses and increased active ghrelin in healthy obese adults [124]. Moreover, Niu et al. [57] demonstrated that a higher dosage of B. animalis subsp. lactis (1 × 1010 CFU/kg b.w.) provided significant protection to HFD-induced mice. This protection extended to mitigating body-weight gain, reducing elevated fat percentage, and alleviating dyslipidemia. Additionally, the higher dosage of B.animals subsp. lactis exhibited remarkable efficacy in safeguarding against increased levels of LPS or endotoxemia [57]. It is important to note that this study has not been replicated in humans.
However, there is evidence that show the effect of Bifidobacterium is strain-specific and hence, the interaction of different Bifidobacterium species with host cells may have distinct effects [125]. For instance, supplementation of B. longum showed a decrease in the expression of genes encoding proinflammatory cytokines [42], while ingestion of B. animalis subsp. lactis caused an increase in the anti-inflammatory cytokine IFN-α and phagocytic activity [82]. These studies caution that the beneficial effects of one probiotic strain cannot be applied to other species or even subspecies of the same genus.
A major concern regarding the use of probiotics in treating infections is the potential side effects caused by the ingestion of large bacterial loads in sick or immunocompromised patients. One approach to address this concern is to use isolated probiotic-derived bioactive factors that retain the functions of live bacteria. Some studies have suggested that secreted products from specific probiotics alleviate disruptions in intestinal barrier function associated with intestinal disease. Multiple in vitro studies found that bacteria-free conditioned media from B. infantis demonstrated several beneficial effects [126]. It increased the TER, reduced the levels of claudin-2, and increased the expression of ZO-1 and occludin. These effects were accompanied by increased levels of phosphorylated extracellular-signal regulated kinase and decreased levels of phosphorylated p38 in T-84 cells [126]. Furthermore, bacteria-free conditioned media of B. infantis was found to protect against NEC by suppressing the activation of NF-κB via preserved IκB expression. The conditioned media also suppressed the production of the proinflammatory cytokine TNF-α, a downstream target of the NF-κB-pathway [127] and prevented the intestinal permeability damage caused by IL-1β [51]. Purified galactooligosaccharide, derived by the galactosyltransferase activity of B. bifidum (using lactose as the substrate) was shown to reduce the adhesion and invasion of Salmonella enterica serovar and S. typhimurium both in vitro and in vivo [128]. Furthermore, it was found that the fermentation products of B.Bb12 protect against deoxycholic acid-induced disruption of the barrier and enhance the intestinal barrier, as measured by TER in Caco-2 cells [28].
The majority of studies involving Bifidobacteria-derived bioactives have only been demonstrated in laboratory models. More studies are ongoing to progress to the translational stage and ultimately to clinical trials. Another promising method is the use of engineered Bifidobacteria that have been modified to perform a therapeutic function. Numerous clinical trials are currently underway, investigating the potential of engineered probiotics in the treatment of IBD, exemplified by studies like E. coli Nissle 1917 (NCT04969679) and B. breve Bif195 (NCT04842149) [129,130]. In addition, ActoGenix has pioneered the development of 2 engineered L. lactis-based products designed to address IBD, both of which have advanced to the clinical trial phase. The first, AG011, features an engineered L. lactis strain capable of secreting human IL-10 and has demonstrated safety and excellent tolerance in a cohort of patients with UC (NCT00729872) [25,130,131]. Meanwhile, AG014, built upon the same L. lactis platform as AG011, has been engineered to produce an anti-TNF-α antibody fragment similar to certolizumab [132]. Although AG014 underwent evaluation in a phase I clinical trial, it has not progressed further [130,132,133]. Ongoing in vitro studies are conducting similar investigations on the use of Bifidobacteria [134], and future research endeavors will delve into the potential role of Bifidobacteria in the treatment of IBD without the side effect of ingesting large bacterial loads in sick or immunocompromised patients.
Conclusion and Future Perspectives
Although a plethora of studies have demonstrated the health-promoting activities of Bifidobacteria species, particularly their role in maintaining the intestinal epithelial TJ barrier, the underlying molecular mechanisms still remain largely unknown. Major challenges behind this include: 1) the strain-specific activity of Bifidobacterium, which makes it difficult to define a specific pathway of action; 2) the complex interaction of Bifidobacteria with human host cells and other members of the gut; and 3) the notoriously recalcitrant nature of Bifidobacteria to genetic modification. The development of effective molecular tools and more focused studies is expected to unravel molecular mechanisms that explain how Bifidobacteria interact with human host cells and exert their beneficial effect.
Author contributions
The authors’ responsibilities were as follows—RA, RA-S: conceptualized and designed the review; RA, J.E: wrote the draft manuscript; RA: prepared figures; RA, JE, RA-S: revised the first draft; and all authors: read and approved the final manuscript.
Funding
This research was funded by Penn State Department of Medicine.
Conflict of interest
The authors report no conflict of interest.
References
- 1.Ma T.Y., Nighot P., Al-Sadi R. In: Physiology of the Gastrointestinal Tract. 6th ed. Said H.M., editor. Elsevier; 2018. Tight junctions and the intestinal barrier; pp. 587–639. Available from: [DOI] [Google Scholar]
- 2.Cordenonsi M., D’Atri F., Hammar E., Parry D.A.D., Kendrick-Jones J., Shore D., et al. Cingulin contains globular and coiled-coil domains and interacts with Zo-1, Zo-2, Zo-3, and myosin. J. Cell Biol. 1999;147(7):1569–1582. doi: 10.1083/jcb.147.7.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odenwald M.A., Choi W., Kuo W.T., Singh G., Sailer A., Wang Y., et al. The scaffolding protein ZO-1 coordinates actomyosin and epithelial apical specializations in vitro and in vivo. J. Biol. Chem. 2018;293(45):17317–17335. doi: 10.1074/jbc.RA118.003908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buschmann M.M., Shen L., Rajapakse H., Raleigh D.R., Wang Y., Wang Y., et al. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol. Biol. Cell. 2013;24(19):3056–3068. doi: 10.1091/mbc.E12-09-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner J.R. ‘Putting the squeeze’ on the tight junction: understanding cytoskeletal regulation, Semin. Cell Dev. Biol. 2000;11(4):301–308. doi: 10.1006/scdb.2000.0180. [DOI] [PubMed] [Google Scholar]
- 6.Turner J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 7.Buckley A., Turner J.R. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb. Perspect. Biol. 2018;10(1):a029314. doi: 10.1101/cshperspect.a029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma T.Y., Boivin M.A., Ye D., Pedram A., Said H.M. Mechanism of TNF-α modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288(3):G422–G430. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- 9.Al-Sadi R., Guo S., Ye D., Ma T.Y. TNF-α modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am. J. Pathol. 2013;183(6):1871–1884. doi: 10.1016/j.ajpath.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michielan A., D’Incà R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. 2015;2015:628157. doi: 10.1155/2015/628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad R., Sorrell M.F., Batra S.K., Dhawan P., Singh A.B. Gut permeability and mucosal inflammation: bad, good or context dependent. Mucosal Immunol. 2017;10(2):307–317. doi: 10.1038/mi.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore S.A., Nighot P., Reyes C., Rawat M., McKee J., Lemon D., et al. Intestinal barrier dysfunction in human necrotizing enterocolitis. J. Pediatr. Surg. 2016;51(12):1907–1913. doi: 10.1016/j.jpedsurg.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weaver L.T., Laker M.F., Nelson R. Intestinal permeability in the newborn. Arch. Dis. Child. 1984;59(3):236–241. doi: 10.1136/adc.59.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Elburg R.M., Fetter W.P., Bunkers C.M., Heymans H.S. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch. Dis. Child. Fetal Neonatal Ed. 2003;88(1):F52–F55. doi: 10.1136/fn.88.1.f52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer N., Vaishnava S. Alcohol lowers your (intestinal) inhibitions. Cell Host Microbe. 2016;19(2):131–133. doi: 10.1016/j.chom.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann P., Seebauer C.T., Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin. Exp. Res. 2015;39(5):763–775. doi: 10.1111/acer.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicoletti A., Ponziani F.R., Biolato M., Valenza V., Marrone G., Sganga G., et al. Intestinal permeability in the pathogenesis of liver damage: from non-alcoholic fatty liver disease to liver transplantation. World J. Gastroenterol. 2019;25(33):4814–4834. doi: 10.3748/wjg.v25.i33.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luther J., Garber J.J., Khalili H., Dave M., Bale S.S., Jindal R., et al. Hepatic injury in nonalcoholic steatohepatitis contributes to altered intestinal permeability. Cell. Mol. Gastroenterol. Hepatol. 2015;1(2):222–232. doi: 10.1016/j.jcmgh.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Munck T.J.I., Xu P., Verwijs H.J.A., Masclee A.A.M., Jonkers D., Verbeek J., Koek G.H. Intestinal permeability in human nonalcoholic fatty liver disease: a systematic review and meta-analysis. Liver Int. 2020;40(12):2906–2916. doi: 10.1111/liv.14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flak M.B., Colas R.A., Muñoz-Atienza E., Curtis M.A., Dalli J., Pitzalis C. Inflammatory arthritis disrupts gut resolution mechanisms, promoting barrier breakdown by Porphyromonas gingivalis. JCI Insight. 2019;4(13) doi: 10.1172/jci.insight.125191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tajik N., Frech M., Schulz O., Schälter F., Lucas S., Azizov V., et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-15831-7. 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taneja V. Arthritis susceptibility and the gut microbiome. FEBS Lett. 2014;588(22):4244–4249. doi: 10.1016/j.febslet.2014.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandl C., Bucci L., Schett G., Zaiss M.M. Crossing the barriers: revisiting the gut feeling in rheumatoid arthritis. Eur. J. Immunol. 2021;51(4):798–810. doi: 10.1002/eji.202048876. [DOI] [PubMed] [Google Scholar]
- 24.Fortea M., Albert-Bayo M., Abril-Gil M., Ganda Mall J.P., Serra-Ruiz X., Henao-Paez A., et al. Present and future therapeutic approaches to barrier dysfunction. Front. Nutr. 2021;8:718093. doi: 10.3389/fnut.2021.718093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pesce M., Seguella L., Del Re A., Lu J., Palenca I., Corpetti C., et al. Next-generation probiotics for inflammatory bowel disease. Int. J. Mol. Sci. 2022;23(10):5466. doi: 10.3390/ijms23105466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolacek S., Grgurić J. [Long-term effect of nutrition in early infancy] Lijec. Vjesn. 1996;118(3–4):80–83. [PubMed] [Google Scholar]
- 27.Zheng Y., Zhang Z., Tang P., Wu Y., Zhang A., Li D., et al. Probiotics fortify intestinal barrier function: a systematic review and meta-analysis of randomized trials. Front. Immunol. 2023;14:1143548. doi: 10.3389/fimmu.2023.1143548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Commane D.M., Shortt C.T., Silvi S., Cresci A., Hughes R.M., Rowland I.R. Effects of fermentation products of pro- and prebiotics on trans-epithelial electrical resistance in an in vitro model of the colon. Nutr. Cancer. 2005;51(1):102–109. doi: 10.1207/s15327914nc5101_14. [DOI] [PubMed] [Google Scholar]
- 29.Verma R., Lee C., Jeun E.J., Yi J., Kim K.S., Ghosh A., et al. Cell surface polysaccharides of Bifidobacterium bifidum induce the generation of Foxp3+ regulatory T cells. Sci. Immunol. 2018;3(28) doi: 10.1126/sciimmunol.aat6975. [DOI] [PubMed] [Google Scholar]
- 30.Pendyala S., Walker J.M., Holt P.R. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012;142(5):1100–1101.e2. doi: 10.1053/j.gastro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boroni Moreira A.P., Salles Texeira T.F., Barbosa Ferreira A., Do Carmo Gouveia Peluzio M., De Cássia Gonçalves Alfenas R. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012;108(5):801–809. doi: 10.1017/S0007114512001213. [DOI] [PubMed] [Google Scholar]
- 32.Ling X., Linglong P., Weixia D., Hong W. Protective effects of Bifidobacterium on intestinal barrier function in LPS-induced enterocyte barrier injury of Caco-2 monolayers and in a rat NEC model. PLOS ONE. 2016;11(8) doi: 10.1371/journal.pone.0161635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh C.Y., Osaka T., Moriyama E., Date Y., Kikuchi J., Tsuneda S. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol. Rep. 2015;3(3) doi: 10.14814/phy2.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao L., Xie Q., Evivie S.E., Yue Y., Yang H., Lv X., et al. Bifidobacterium longum subsp. longum K5 alleviates inflammatory response and prevents intestinal barrier injury induced by LPS in vitro based on comparative genomics. J. Funct. Foods. 2022;92:105030. doi: 10.1016/j.jff.2022.105030. [DOI] [Google Scholar]
- 35.Zhang G., Zhao J., Wen R., Zhu X., Liu L., Li C. 2′-Fucosyllactose promotes Bifidobacterium bifidum DNG6 adhesion to Caco-2 cells. J. Dairy Sci. 2020;103(11):9825–9834. doi: 10.3168/jds.2020-18773. [DOI] [PubMed] [Google Scholar]
- 36.Oh H.Y.P., Visvalingam V., Wahli W. The PPAR–microbiota–metabolic organ trilogy to fine-tune physiology. FASEB J. 2019;33(9):9706–9730. doi: 10.1096/fj.201802681RR. [DOI] [PubMed] [Google Scholar]
- 37.Speciale I., Verma R., Di Lorenzo F., Molinaro A., Im S.H., De Castro C. Bifidobacterium bifidum presents on the cell surface a complex mixture of glucans and galactans with different immunological properties, Carbohydr. Polym. 2019;218:269–278. doi: 10.1016/j.carbpol.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Zhao L., Xie Q., Etareri Evivie S., Liu D., Dong J., Ping L., et al. Bifidobacterium dentium N8 with potential probiotic characteristics prevents LPS-induced intestinal barrier injury by alleviating the inflammatory response and regulating the tight junction in Caco-2 cell monolayers. Food Funct. 2021;12(16):7171–7184. doi: 10.1039/d1fo01164b. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y., Yang B., Stanton C., Ross R.P., Zhao J., Zhang H., et al. Bifidobacterium pseudocatenulatum ameliorates DSS-induced colitis by maintaining intestinal mechanical barrier, blocking proinflammatory cytokines, inhibiting TLR4/NF-κB signaling, and altering gut microbiota. J. Agric. Food Chem. 2021;69(5):1496–1512. doi: 10.1021/acs.jafc.0c06329. [DOI] [PubMed] [Google Scholar]
- 40.Li Y., Lv L., Ye J., Fang D., Shi D., Wu W., et al. Bifidobacterium adolescentis CGMCC 15058 alleviates liver injury, enhances the intestinal barrier and modifies the gut microbiota in d-galactosamine-treated rats. Appl. Microbiol. Biotechnol. 2019;103(1):375–393. doi: 10.1007/s00253-018-9454-y. [DOI] [PubMed] [Google Scholar]
- 41.Khailova L., Dvorak K., Arganbright K.M., Halpern M.D., Kinouchi T., Yajima M., et al. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297(5):G940–G949. doi: 10.1152/ajpgi.00141.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan S., Yang B., Ross R.P., Stanton C., Zhang H., Zhao J., et al. Bifidobacterium longum subsp. longum YS108R fermented milk alleviates DSS induced colitis via anti-inflammation, mucosal barrier maintenance and gut microbiota modulation. J. Funct. Foods. 2020;73:104153. doi: 10.1016/j.jff.2020.104153. [DOI] [PubMed] [Google Scholar]
- 43.Mokkala K., Laitinen K., Röytiö H. Bifidobacterium lactis 420 and fish oil enhance intestinal epithelial integrity in Caco-2 cells. Nutr. Res. 2016;36(3):246–252. doi: 10.1016/j.nutres.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Abdulqadir R., Al-Sadi R., Ma T. Bifidobacterium bifidum causes an enhancement of the intestinal epithelial tight junction barrier is mediated by TLR-2 dependent increases of Toll-interacting protein (TOLLIP) expression in a MYD88 independent manner. Physiology. 2023;38(S1):5734013. doi: 10.1152/physiol.2023.38.S1.5734013. [DOI] [Google Scholar]
- 45.Michael D.R., Jack A.A., Masetti G., Davies T.S., Loxley K.E., Kerry-Smith J., et al. A randomised controlled study shows supplementation of overweight and obese adults with lactobacilli and bifidobacteria reduces bodyweight and improves well-being. Sci. Rep. 2020;10(1):4183. doi: 10.1038/s41598-020-60991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samara J., Moossavi S., Alshaikh B., Ortega V.A., Pettersen V.K., Ferdous T., et al. Supplementation with a probiotic mixture accelerates gut microbiome maturation and reduces intestinal inflammation in extremely preterm infants. Cell Host Microbe. 2022;30(5):696–711.e5. doi: 10.1016/j.chom.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Lomasney K.W., Houston A., Shanahan F., Dinan T.G., Cryan J.F., Hyland N.P. Selective influence of host microbiota on cAMP-mediated ion transport in mouse colon. Neurogastroenterol. Motil. 2014;26(6):887–890. doi: 10.1111/nmo.12328. [DOI] [PubMed] [Google Scholar]
- 48.Merenstein D., Fraser C.M., Roberts R.F., Liu T., Grant-Beurmann S., Tan T.P., et al. Bifidobacterium animalis subsp. lactis BB-12 protects against antibiotic-induced functional and compositional changes in human fecal microbiome. Nutrients. 2021;13(8):2814. doi: 10.3390/nu13082814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdulqadir R.F., Al-Sadi R., Ma T.Y. Tu1113: Bifidobacterium bifidum causes an enhancement of intestinal epithelial tight junction barrier by a novel mechanism involving peroxisome proliferation-activated receptor gamma (PPAR-γ) Gastroenterology. 2022;162(7) doi: 10.1016/S0016-5085(22)62101-2. S-887. [DOI] [Google Scholar]
- 50.Ni Y., Yang X., Zheng L., Wang Z., Wu L., Jiang J., et al. Lactobacillus and Bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol. Nutr. Food Res. 2019;63(22) doi: 10.1002/mnfr.201900603. [DOI] [PubMed] [Google Scholar]
- 51.Guo S., Gillingham T., Guo Y., Meng D., Zhu W., Walker W.A., et al. Secretions of Bifidobacterium infantis and Lactobacillus acidophilus protect intestinal epithelial barrier function. J. Pediatr. Gastroenterol. Nutr. 2017;64(3):404–412. doi: 10.1097/MPG.0000000000001310. [DOI] [PubMed] [Google Scholar]
- 52.Srutkova D., Schwarzer M., Hudcovic T., Zakostelska Z., Drab V., Spanova A., et al. Bifidobacterium longum CCM 7952 promotes epithelial barrier function and prevents acute DSS-induced colitis in strictly strain-specific manner. PLOS ONE. 2015;10(7) doi: 10.1371/journal.pone.0134050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C., Shoji H., Sato H., Nagata S., Ohtsuka Y., Shimizu T., Yamashiro Y. Effects of oral administration of Bifidobacterium breve on fecal lactic acid and short-chain fatty acids in low birth weight infants. J. Pediatr. Gastroenterol. Nutr. 2007;44(2):252–257. doi: 10.1097/01.mpg.0000252184.89922.5f. [DOI] [PubMed] [Google Scholar]
- 54.Martín R., Laval L., Chain F., Miquel S., Natividad J., Cherbuy C., et al. Bifidobacterium animalis ssp. lactis CNCM-I2494 restores gut barrier permeability in chronically low-grade inflamed mice. Front. Microbiol. 2016;7:608. doi: 10.3389/fmicb.2016.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nie N., Bai C., Song S., Zhang Y., Wang B., Li Z. Bifidobacterium plays a protective role in TNF-α-induced inflammatory response in Caco-2 cell through NF-κB and p38MAPK pathways. Mol. Cell Biochem. 2020;464(1–2):83–91. doi: 10.1007/s11010-019-03651-3. [DOI] [PubMed] [Google Scholar]
- 56.Bergmann K.R., Liu S.X.L., Tian R., Kushnir A., Turner J.R., Li H.L., et al. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am. J. Pathol. 2013;182(5):1595–1606. doi: 10.1016/j.ajpath.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niu X., Zhang N., Li S., Li N., Wang R., Zhang Q., et al. Bifidobacterium animalis subsp. lactis MN-Gup protects mice against gut microbiota-related obesity and endotoxemia induced by a high fat diet. Front. Nutr. 2022;9:992947. doi: 10.3389/fnut.2022.992947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sela D.A., Chapman J., Adeuya A., Kim J.H., Chen F., Whitehead T.R., et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl. Acad. Sci. U. S. A. 2008;105(48):18964–18969. doi: 10.1073/pnas.0809584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bottacini F., Milani C., Turroni F., Sánchez B., Foroni E., Duranti S., et al. Bifidobacterium asteroides PRL2011 genome analysis reveals clues for colonization of the insect gut. PLOS ONE. 2012;7(9) doi: 10.1371/journal.pone.0044229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Dea Lindner J., Canchaya C., Zhang Z., Neviani E., Fitzgerald G.F., Van Sinderen D., et al. Exploiting Bifidobacterium genomes: the molecular basis of stress response. Int. J. Food Microbiol. 2007;120(1–2):13–24. doi: 10.1016/j.ijfoodmicro.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 61.Garrido D., Dallas D.C., Mills D.A. Consumption of human milk glycoconjugates by infant-associated bifidobacteria: mechanisms and implications. Microbiology (Reading) 2013;159(4):649–664. doi: 10.1099/mic.0.064113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soto A., Martín V., Jiménez E., Mader I., Rodríguez J.M., Fernández L. Lactobacilli and Bifidobacteria in human breast milk: influence of antibiotherapy and other host and clinical factors. J. Pediatr. Gastroenterol. Nutr. 2014;59(1):78–88. doi: 10.1097/MPG.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin C., Lin Y., Zhang H., Wang G., Zhao J., Zhang H., et al. Intestinal ‘infant-type’ Bifidobacteria mediate immune system development in the first 1000 days of life. Nutrients. 2022;14(7):1498. doi: 10.3390/nu14071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grönlund M.M., Gueimonde M., Laitinen K., Kociubinski G., Grönroos T., Salminen S., et al. Maternal breast-milk and intestinal bifidobacteria guide the compositional development of the Bifidobacterium microbiota in infants at risk of allergic disease. Clin. Exp. Allergy. 2007;37(12):1764–1772. doi: 10.1111/j.1365-2222.2007.02849.x. [DOI] [PubMed] [Google Scholar]
- 65.Odamaki T., Kato K., Sugahara H., Hashikura N., Takahashi S., Xiao J.Z., et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chaplin A.V., Brzhozovskii A.G., Parfenova T.V., Kafarskaia L.I., Volodin N.N., Shkoporov A.N., et al. [Species diversity of Bifidobacteria in the intestinal microbiota studied using MALDI-TOF mass-spectrometry] Vestn Ross Akad Med Nauk. 2015;70(4):435–440. doi: 10.15690/vramn.v70.i4.1409. [DOI] [PubMed] [Google Scholar]
- 67.Duranti S., Milani C., Lugli G.A., Turroni F., Mancabelli L., Sanchez B., et al. Insights from genomes of representatives of the human gut commensal Bifidobacterium bifidum. Environ. Microbiol. 2015;17(7):2515–2531. doi: 10.1111/1462-2920.12743. [DOI] [PubMed] [Google Scholar]
- 68.Matsuki T., Watanabe K., Tanaka R., Fukuda M., Oyaizu H. Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNA-gene-targeted species-specific primers. Appl. Environ. Microbiol. 1999;65(10):4506–4512. doi: 10.1128/AEM.65.10.4506-4512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He F., Ouwehand A.C., Isolauri E., Hosoda M., Benno Y., Salminen S. Differences in composition and mucosal adhesion of bifidobacteria isolated from healthy adults and healthy seniors. Curr. Microbiol. 2001;43(5):351–354. doi: 10.1007/s002840010315. [DOI] [PubMed] [Google Scholar]
- 70.Hollander D., Tarnawski H. Aging-associated increase in intestinal absorption of macromolecules. Gerontology. 1985;31(3):133–137. doi: 10.1159/000212694. [DOI] [PubMed] [Google Scholar]
- 71.Kelly S.M., Munoz-Munoz J., Van Sinderen D. Plant glycan metabolism by Bifidobacteria. Front. Microbiol. 2021;12:609418. doi: 10.3389/fmicb.2021.609418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gavini F., Van Esbroeck M., Touzel J.P., Fourment A., Goossens H. Detection of fructose-6-phosphate phosphoketolase (F6PPK), a key enzyme of the bifid-shunt. Gardnerella vaginalis. 1996;2(3):191–193. doi: 10.1006/anae.1996.0025. Anaerobe. [DOI] [Google Scholar]
- 73.Pokusaeva K., Fitzgerald G.F., Van Sinderen D. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011;6(3):285–306. doi: 10.1007/s12263-010-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pyclik M., Srutkova D., Schwarzer M., Górska S. Bifidobacteria cell wall-derived exo-polysaccharides, lipoteichoic acids, peptidoglycans, polar lipids and proteins – their chemical structure and biological attributes. Int. J. Biol. Macromol. 2020;147:333–349. doi: 10.1016/j.ijbiomac.2019.12.227. [DOI] [PubMed] [Google Scholar]
- 75.Devika N.T., Raman K. Deciphering the metabolic capabilities of Bifidobacteria using genome-scale metabolic models. Sci. Rep. 2019;9(1):18222. doi: 10.1038/s41598-019-54696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Savi A.O. 2021. Setworks: networks of complex set intersections. [Internet]. PsyArXiv, April. Available from: [DOI] [Google Scholar]
- 77.Rivière A., Selak M., Lantin D., Leroy F., De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hooper L.V., Wong M.H., Thelin A., Hansson L., Falk P.G., Gordon J.I. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291(5505):881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 79.Derrien M., Turroni F., Ventura M., Van Sinderen D. Insights into endogenous Bifidobacterium species in the human gut microbiota during adulthood. Trends Microbiol. 2002;30(10):940–947. doi: 10.1016/j.tim.2022.04.004. [DOI] [PubMed] [Google Scholar]
- 80.Ghosh S., Whitley C.S., Haribabu B., Jala V.R. Regulation of intestinal barrier function by microbial metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021;11(5):1463–1482. doi: 10.1016/j.jcmgh.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Picard C., Fioramonti J., Francois A., Robinson T., Neant F., Matuchansky C. Review article: bifidobacteria as probiotic agents - physiological effects and clinical benefits. Aliment. Pharmacol. Ther. 2005;22(6):495–512. doi: 10.1111/j.1365-2036.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 82.Arunachalam K., Gill H.S., Chandra R.K. Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis (HN019) Eur. J. Clin. Nutr. 2000;54(3):263–267. doi: 10.1038/sj.ejcn.1600938. [DOI] [PubMed] [Google Scholar]
- 83.Chichlowski M., De Lartigue G., German J.B., Raybould H.E., Mills D.A. Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. J. Pediatr. Gastroenterol. Nutr. 2012;55(3):321–327. doi: 10.1097/MPG.0b013e31824fb899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.López P., González-Rodríguez I., Gueimonde M., Margolles A., Suárez A. Immune response to Bifidobacterium bifidum strains support Treg/Th17 plasticity. PLOS ONE. 2011;6(9) doi: 10.1371/journal.pone.0024776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liévin V., Peiffer I., Hudault S., Rochat F., Brassart D., Neeser J.R., Servin A.L. Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut. 2000;47(5):646–652. doi: 10.1136/gut.47.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang R., Kuerman M., Cui Q., Tian X., Zhou Y., Yi H., et al. Protective effects of Bifidobacterium bifidum FL-228.1 on dextran sulfate sodium-induced intestinal damage in mice. Eur. J. Nutr. 2023;62(3):1267–1280. doi: 10.1007/s00394-022-03064-x. [DOI] [PubMed] [Google Scholar]
- 87.Cui Q., Zhang Z., Tian X., Liang X., Lu Y., Shi Y., et al. Bifidobacterium bifidum ameliorates DSS-induced colitis in mice by regulating AHR/NRF2/NLRP3 inflammasome pathways through indole-3-lactic acid production. J. Agric. Food Chem. 2023;71(4):1970–1981. doi: 10.1021/acs.jafc.2c06894. [DOI] [PubMed] [Google Scholar]
- 88.Al-Sadi R., Dharmaprakash V., Nighot P., Guo S., Nighot M., Do T., et al. Bifidobacterium bifidum enhances the intestinal epithelial tight junction barrier and protects against intestinal inflammation by targeting the Toll-like receptor-2 pathway in an NF-κB-independent manner. Int. J. Mol. Sci. 2021;22(15):8070. doi: 10.3390/ijms22158070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hassani S., Sotoodehnejadnematalahi F., Fateh A., Siadat S.D. Evaluation of association between Bifidobacterium bifidum derived extracellular vesicles and intestinal epithelium tight junction proteins through Notch-1 and AhR activation in Caco-2 cell line. Mol. Genet. Microbiol. Virol. 2021;36(S1):S1–S6. doi: 10.3103/S0891416821050086. [DOI] [Google Scholar]
- 90.Kiu R., Treveil A., Harnisch L.C., Caim S., Leclaire C., Van Sinderen D., et al. Bifidobacterium breve UCC2003 induces a distinct global transcriptomic program in neonatal murine intestinal epithelial cells. iScience. 2020;23(7):101336. doi: 10.1016/j.isci.2020.101336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim W.G., Kim H.I., Kwon E.K., Han M.J., Kim D.H. Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 mitigate alcoholic steatosis in mice by inhibiting LPS-mediated NF-κB activation through restoration of the disturbed gut microbiota. Food Funct. 2018;9(8):4255–4265. doi: 10.1039/c8fo00252e. [DOI] [PubMed] [Google Scholar]
- 92.Lim S.M., Kim D.H. Bifidobacterium adolescentis IM38 ameliorates high-fat diet–induced colitis in mice by inhibiting NF-κB activation and lipopolysaccharide production by gut microbiota. Nutr. Res. 2017;41:86–96. doi: 10.1016/j.nutres.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 93.Li M., Wang F. Role of intestinal microbiota on gut homeostasis and rheumatoid arthritis. J. Immunol. Res. 2021;2021:8167283. doi: 10.1155/2021/8167283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Symons R.A., Colella F., Collins F.L., Rafipay A.J., Kania K., McClure J.J., et al. Targeting the IL-6–Yap–Snail signalling axis in synovial fibroblasts ameliorates inflammatory arthritis. Ann. Rheum. Dis. 2022;81(2):214–224. doi: 10.1136/annrheumdis-2021-220875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li B., Ding M., Liu X., Zhao J., Ross R.P., Stanton C., et al. Bifidobacterium breve CCFM1078 alleviates collagen-induced arthritis in rats via modulating the gut microbiota and repairing the intestinal barrier damage. J. Agric. Food Chem. 2022;70(46):14665–14678. doi: 10.1021/acs.jafc.2c04602. [DOI] [PubMed] [Google Scholar]
- 96.Abdelhamid A.G., El-Masry S.S., El-Dougdoug N.K. Probiotic Lactobacillus and Bifidobacterium strains possess safety characteristics, antiviral activities and host adherence factors revealed by genome mining. EPMA J. 2019;10(4):337–350. doi: 10.1007/s13167-019-00184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Douillard F.P., Mora D., Eijlander R.T., Wels M., De Vos W.M. Comparative genomic analysis of the multispecies probiotic-marketed product VSL#3. PLOS ONE. 2018;13(2) doi: 10.1371/journal.pone.0192452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Z., Zhang G., Zhang S., Zhao J. Fructooligosaccharide reduces weanling pig diarrhea in conjunction with improving intestinal antioxidase activity and tight junction protein expression. Nutrients. 2022;14(3):512. doi: 10.3390/nu14030512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Al-Sadi R., Nighot P., Nighot M., Haque M., Rawat M., Ma T.Y. Lactobacillus acidophilus induces a strain-specific and Toll-like receptor 2–dependent enhancement of intestinal epithelial tight junction barrier and protection against intestinal inflammation. Am. J. Pathol. 2021;191(5):872–884. doi: 10.1016/j.ajpath.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jang S.E., Jeong J.J., Kim J.K., Han M.J., Kim D.H. Simultaneous amelioratation of colitis and liver injury in mice by Bifidobacterium longum LC67 and Lactobacillus plantarum LC27. Sci. Rep. 2018;8(1):7500. doi: 10.1038/s41598-018-25775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yue Y., Wang Y., Xie Q., Lv X., Zhou L., Smith E.E., et al. Bifidobacterium bifidum E3 combined with Bifidobacterium longum subsp. infantis E4 improves LPS-induced intestinal injury by inhibiting the TLR4/NF-κB and MAPK signaling pathways in vivo. J. Agric. Food Chem. 2023;71(23):8915–8930. doi: 10.1021/acs.jafc.3c00421. [DOI] [PubMed] [Google Scholar]
- 102.Guan J., Liu F., Zhao S., Evivie S.E., Shi J., Li N., et al. Effect of Bifidobacterium longum subsp. longum on the proliferative and tight-junction activities of human fetal colon epithelial cells. J. Funct. Foods. 2021;86:104715. doi: 10.1016/j.jff.2021.104715. [DOI] [Google Scholar]
- 103.Shang J., Yang S., Tang Z., Chen Y., Duan B., Meng X. Bifidobacterium bifidum H3-R2 and its molecular communication within the context of ulcerative colitis. J. Agric. Food Chem. 2022;70(37):11678–11688. doi: 10.1021/acs.jafc.2c02909. [DOI] [PubMed] [Google Scholar]
- 104.Guo W., Mao B., Cui S., Tang X., Zhang Q., Zhao J., et al. Protective effects of a novel probiotic Bifidobacterium pseudolongum on the intestinal barrier of colitis mice via modulating the Pparγ/STAT3 pathway and intestinal microbiota. Foods. 2022;11(11):1551. doi: 10.3390/foods11111551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mencarelli A., Distrutti E., Renga B., D’Amore C., Cipriani S., Palladino G., et al. Probiotics modulate intestinal expression of nuclear receptor and provide counter-regulatory signals to inflammation-driven adipose tissue activation. PLOS ONE. 2011;6(7) doi: 10.1371/journal.pone.0022978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Voltan S., Martines D., Elli M., Brun P., Longo S., Porzionato A., et al. Lactobacillus crispatus M247-derived H2O2 acts as a signal transducing molecule activating peroxisome proliferator activated receptor-γ in the intestinal mucosa. Gastroenterology. 2008;135(4):1216–1227. doi: 10.1053/j.gastro.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 107.Nepelska M., De Wouters T., Jacouton E., Béguet-Crespel F., Lapaque N., Doré J., et al. Commensal gut bacteria modulate phosphorylation-dependent PPARγ transcriptional activity in human intestinal epithelial cells. Sci. Rep. 2017;7:43199. doi: 10.1038/srep43199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abdulqadir R., Al-Sadi R., Ma T. Bifidobacterium bifidum prevents the IL-1B induced increase in intestinal permeability by a novel mechanism: TLR-2 dependent activation of PPAR-gamma and inhibition of NF-kB signaling pathway. Physiology. 2023;38(S1):5732809. doi: 10.1152/physiol.2023.38.S1.5732809. [DOI] [Google Scholar]
- 109.Abdulqadir R.F., Al-Sadi R., Ma T.Y. 768 Bifidobacterium bifidum prevents cytokine induced increase in intestinal tight junction permeability by a novel mechanism: crosstalk between PPAR-g, NF-kB and MLCK. Gastroenterology. 2023;164(6) doi: 10.1016/S0016-5085(23)01352-5. S-163. [DOI] [Google Scholar]
- 110.Aghamohammad S., Sepehr A., Miri S.T., Najafi S., Rohani M., Pourshafiea M.R. The effects of the probiotic cocktail on modulation of the NF-kB and JAK/STAT signaling pathways involved in the inflammatory response in bowel disease model. BMC Immunol. 2022;23(1):8. doi: 10.1186/s12865-022-00484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou Q., Shen B., Huang R., Liu H., Zhang W., Song M., et al. Bacteroides fragilis strain ZY-312 promotes intestinal barrier integrity via upregulating the STAT3 pathway in a radiation-induced intestinal injury mouse model. Front. Nutr. 2022;9:1063699. doi: 10.3389/fnut.2022.1063699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jia D.J.C., Wang Q.W., Hu Y.Y., He J.M., Ge Q.W., Qi Y.D., et al. Lactobacillus johnsonii alleviates colitis by TLR1/2-STAT3 mediated CD206+ macrophagesIL-10 activation. Gut Microbes. 2022;14(1):2145843. doi: 10.1080/19490976.2022.2145843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hou Q., Ye L., Liu H., Huang L., Yang Q., Turner J.R., et al. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018;25(9):1657–1670. doi: 10.1038/s41418-018-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chenoll E., Rivero M., Codoñer F.M., Martinez-Blanch J.F., Ramón D., Genovés S., et al. Complete genome sequence of Bifidobacterium longum subsp. infantis strain CECT 7210, a probiotic strain active against rotavirus infections. Genome Announc. 2015;3(2):e00105–e00115. doi: 10.1128/genomeA.00105-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Patole S.K., Rao S.C., Keil A.D., Nathan E.A., Doherty D.A., Simmer K.N. Benefits of Bifidobacterium breve M-16V supplementation in preterm neonates - a retrospective cohort study. PLOS ONE. 2016;11(3) doi: 10.1371/journal.pone.0150775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Corrêa N.B.O., Péret Filho L.A., Penna F.J., Lima F.M.L.S., Nicoli J.R. A randomized formula controlled trial of Bifidobacterium lactis and Streptococcus thermophilus for prevention of antibiotic-associated diarrhea in infants. J. Clin. Gastroenterol. 2005;39(5):385–389. doi: 10.1097/01.mcg.0000159217.47419.5b. [DOI] [PubMed] [Google Scholar]
- 117.Ma B., Sundararajan S., Nadimpalli G., France M., McComb E., Rutt L., et al. Highly specialized carbohydrate metabolism capability in Bifidobacterium strains associated with intestinal barrier maturation in early preterm infants. mBio. 2022;13(3) doi: 10.1128/mbio.01299-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Takeda Y., Nakase H., Namba K., Inoue S., Ueno S., Uza N., et al. Upregulation of T-bet and tight junction molecules by Bifidobactrium longum improves colonic inflammation of ulcerative colitis, Inflamm. Bowel Dis. 2009;15(11):1617–1618. doi: 10.1002/ibd.20861. [DOI] [PubMed] [Google Scholar]
- 119.Guglielmetti S., Mora D., Gschwender M., Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life -- a double-blind, placebo-controlled study. Aliment. Pharmacol. Ther. 2011;33(10):1123–1132. doi: 10.1111/j.1365-2036.2011.04633.x. [DOI] [PubMed] [Google Scholar]
- 120.Bai T., Zhang L., Wang H., Qian W., Song J., Hou X. Fecal microbiota transplantation is effective in relieving visceral hypersensitivity in a postinfectious model. BioMed Res. Int. 2018;2018:3860743. doi: 10.1155/2018/3860743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Andresen V., Gschossmann J., Layer P. Heat-inactivated Bifidobacterium bifidum MIMBb75 (SYN-HI-001) in the treatment of irritable bowel syndrome: a multicentre, randomised, double-blind, placebo-controlled clinical trial. Lancet Gastroenterol. Hepatol. 2020;5(7):658–666. doi: 10.1016/S2468-1253(20)30056-X. [DOI] [PubMed] [Google Scholar]
- 122.Caimari A., Del Bas J.M., Boqué N., Crescenti A., Puiggròs F., Chenoll E., et al. Heat-killed Bifidobacterium animalis subsp. lactis CECT 8145 increases lean mass and ameliorates metabolic syndrome in cafeteria-fed obese rats. J. Funct. Foods. 2017;38:251–263. doi: 10.1016/j.jff.2017.09.029. [DOI] [Google Scholar]
- 123.Pedret A., Valls R.M., Calderón-Pérez L., Llauradó E., Companys J., Pla-Pagà L., et al. Effects of daily consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: a randomized controlled trial. Int. J. Obes. (Lond.) 2019;43(9):1863–1868. doi: 10.1038/s41366-018-0220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schellekens H., Torres-Fuentes C., Van De Wouw M., Long-Smith C.M., Mitchell A., Strain C., et al. Bifidobacterium longum counters the effects of obesity: partial successful translation from rodent to human. EBioMedicine. 2021;63:103176. doi: 10.1016/j.ebiom.2020.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Braga V.L., Rocha L.P.D.S., Bernardo D.D., de Oliveira Cruz C., Riera R. What do Cochrane systematic reviews say about probiotics as preventive interventions? Sao Paulo Med. J. 2017;135(6):578–586. doi: 10.1590/1516-3180.2017.0310241017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ewaschuk J.B., Diaz H., Meddings L., Diederichs B., Dmytrash A., Backer J., et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295(5):G1025–G1034. doi: 10.1152/ajpgi.90227.2008. [DOI] [PubMed] [Google Scholar]
- 127.Shiou S.R., Yu Y., Guo Y., He S.M., Mziray-Andrew C.H., Hoenig J., et al. Synergistic protection of combined probiotic conditioned media against neonatal necrotizing enterocolitis-like intestinal injury. PLOS ONE. 2013;8(5) doi: 10.1371/journal.pone.0065108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Searle L.E.J., Cooley W.A., Jones G., Nunez A., Crudgington B., Weyer U., et al. Purified galactooligosaccharide, derived from a mixture produced by the enzymic activity of Bifidobacterium bifidum, reduces Salmonella enterica serovar Typhimurium adhesion and invasion in vitro and in vivo. J. Med. Microbiol. 2010;59(12):1428–1439. doi: 10.1099/jmm.0.022780-0. [DOI] [PubMed] [Google Scholar]
- 129.Park S.K., Kang S.B., Kim S., Kim T.O., Cha J.M., Im J.P., et al. Additive effect of probiotics (Mutaflor) on 5-aminosalicylic acid therapy in patients with ulcerative colitis. Korean J. Intern. Med. 2022;37(5):949–957. doi: 10.3904/kjim.2021.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rutter J.W., Dekker L., Owen K.A., Barnes C.P. Microbiome engineering: engineered live biotherapeutic products for treating human disease. Front. Bioeng. Biotechnol. 2022;10:1000873. doi: 10.3389/fbioe.2022.1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martín R., Chain F., Miquel S., Natividad J.M., Sokol H., Verdu E.F., et al. Effects in the use of a genetically engineered strain of Lactococcus lactis delivering in situ IL-10 as a therapy to treat low-grade colon inflammation. Hum. Vaccin. Immunother. 2014;10(6):1611–1621. doi: 10.4161/hv.28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vandenbroucke K., De Haard H., Beirnaert E., Dreier T., Lauwereys M., Huyck L., et al. Orally administered anti-TNFL. lactis secreting an nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol. 2010;3(1):49–56. doi: 10.1038/mi.2009.116. [DOI] [PubMed] [Google Scholar]
- 133.Crowe J.S., Roberts K.J., Carlton T.M., Maggiore L., Cubitt M.F., Clare S., et al. Preclinical development of a novel, orally-administered anti-tumour necrosis factor domain antibody for the treatment of inflammatory bowel disease. Sci. Rep. 2018;8(1):4941. doi: 10.1038/s41598-018-23277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hong N., Ku S., Yuk K., Johnston T.V., Ji G.E., Park M.S. Production of biologically active human interleukin-10 by Bifidobacterium bifidum BGN4. Microb. Cell Fact. 2021;20(1):16. doi: 10.1186/s12934-020-01505-y. [DOI] [PMC free article] [PubMed] [Google Scholar]