Summary
Background
Hong Kong contained COVID-19 for two years but experienced a large epidemic of Omicron BA.2 in early 2022 and endemic transmission of Omicron subvariants thereafter. We reflected on pandemic preparedness and responses by assessing COVID-19 transmission and associated disease burden in the context of implementation of various public health and social measures (PHSMs).
Methods
We examined the use and impact of pandemic controls in Hong Kong by analysing data on more than 1.7 million confirmed COVID-19 cases and characterizing the temporal changes non-pharmaceutical and pharmaceutical interventions implemented from January 2020 through to 30 December 2022. We estimated the daily effective reproductive number (Rt) to track changes in transmissibility and effectiveness of community-based measures against infection over time. We examined the temporal changes of pharmaceutical interventions, mortality rate and case-fatality risks (CFRs), particularly among older adults.
Findings
Hong Kong experienced four local epidemic waves predominated by the ancestral strain in 2020 and early 2021 and prevented multiple SARS-CoV-2 variants from spreading in the community before 2022. Strict travel-related, case-based, and community-based measures were increasingly tightened in Hong Kong over the first two years of the pandemic. However, even very stringent measures were unable to contain the spread of Omicron BA.2 in Hong Kong. Despite high overall vaccination uptake (>70% with at least two doses), high mortality was observed during the Omicron BA.2 wave due to lower vaccine coverage (42%) among adults ≥65 years of age. Increases in antiviral usage and vaccination uptake over time through 2022 was associated with decreased case fatality risks.
Interpretation
Integrated strict measures were able to reduce importation risks and interrupt local transmission to contain COVID-19 transmission and disease burden while awaiting vaccine development and rollout. Increasing coverage of pharmaceutical interventions among high-risk groups reduced infection-related mortality and mitigated the adverse health impact of the pandemic.
Funding
Health and Medical Research Fund.
Keywords: COVID-19, SARS-CoV-2, Pandemic preparedness, Pandemic responses, Public health, Control, Interventions, Impact, Disease burden
Research in context.
Evidence before this study
Coronavirus disease (COVID-19) has rapidly spread all over the world, with over 17 million deaths recorded globally since late 2019. While many locations intended to apply non-pharmaceutical interventions (NPIs) to control surges in SARS-CoV-2 infections, Hong Kong is one of the few locations that used containment measures to keep infections at a low level for two years. However, Hong Kong experienced catastrophic epidemic waves caused by Omicron subvariants in early 2022 whereas with largely similar control measures implemented. The comprehensive data collected over the pandemic provided a unique opportunity to reflect on pandemic responses and preparation by analyzing the transmission and/or burden of COVID-19 in the context of various public health measures, including NPIs, COVID-19 vaccination and antiviral treatment. We searched PubMed on June 7, 2023, for peer-reviewed publications from studies analyzing the transmission and/or burden of COVID-19 and implementation of NPIs and pharmaceutical interventions during the COVID-19 pandemic by limiting the earliest publication time to January 2020 and using keywords related to COVID-19, SARS-CoV-2, pandemic responses, NPIs (e.g., mitigation, containment, elimination, social distancing), vaccine, antiviral (Paxlovid, nirmatrelvir/ritonavir, molnupiravir), transmission, disease burden, and severity. By excluding studies using mathematical models to make predictions or evaluations based on hypothetical scenarios, we found that most of the remaining studies estimated the impact of interventions on COVID-19 control either through a narrative assessment of pandemic responses during a certain period, or specifically focusing on one or a few control measures.
We found one Lancet Commission report that illustrated pandemic responses, transmission, and COVID-19 disease burden globally without including detailed local data. One study on the national response to COVID-19 in New Zealand provided a descriptive analysis of identified cases and interventions implemented up to mid-May 2020, and another study compared the effects of vaccinations and NPIs on reducing community transmission in eight countries up to August 2021. However, we did not find any ecological studies examining potential impact of pharmaceutical interventions on mortality and case-fatality of COVID-19 from perspectives of pandemic control.
Added value of this study
This study is the first to utilize a comprehensive database covering multiple epidemic waves over 3 years to systematically analyze COVID-19 progression in the context of locally adopted NPIs and pharmaceutical measures implemented over the pandemic, to discuss the potential impact of these interventions on reducing transmission and disease burden of COVID-19 in order to inform pandemic responses and preparedness. Our findings suggested that stringent NPIs targeting importations and community transmissions, along with high population adherence, could minimize local spread of infections with SARS-CoV-2 variants with low-to-moderate transmissibility (e.g., ancestral strain), and therefore reduce disease burden from severe infections even when effective pharmaceutical interventions were unavailable. COVID-19 vaccines and antivirals could substantially decrease mortality impact of the pandemic through reducing case-fatality risks in high-risk individuals, indicating that timely assessment on severity of infection together with the pharmaceutical interventions can be included as critical components of control measures in mitigating the public health impact of the pandemic particularly from more transmissible virus variants. Our findings demonstrated the critical role of parameters in informing transmissibility and severity of infections during the pandemic, highlighting the importance of real-time assessment on population immunity (through infection or vaccination) and the availability of pharmaceutical agents in designing sustainable pandemic response measures in the future.
Implications of all the available evidence
Hong Kong's unique experiences in response to multiple epidemic waves of COVID-19 from 2020 to 2022 provided valuable insights into pandemic preparedness and responses. By assessing COVID-19 transmission dynamics and associated disease burden in the context of implementation of various control measures, we identified critical factors and challenges that arose over the course of the pandemic.
Introduction
The pandemic of coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 led to billions of infections, and more than 17 million deaths had been recorded globally from late 2019.1 Compared to the most recent influenza pandemic in 2009–2011, significant improvements have been made in pandemic responses including surveillance, diagnostics, and innovation and rapid development of vaccines and antivirals.2 The subsequent availability of effective pharmaceuticals for SARS-CoV-2 particularly increased vaccination coverage enabled many locations to relax stringent non-pharmaceutical interventions (NPIs), given the substantial social and economic costs of sustaining these measures. Rapid evolution of SARS-CoV-2 resulted in the emergence of new variants, including Omicron that has dominated the largest waves of global transmission.3,4 The timing and impact of pandemic waves have varied substantially around the world.5, 6, 7 Identifying the optimal combination of pharmaceutical and non-pharmaceutical measures at different points in time remains a priority8 not only for reflecting on the response to the COVID-19 pandemic but also for preparing for the next global outbreak.
As a unique case, Hong Kong adopted a stringent containment strategy since 2020, with successful control of four local epidemic waves in 2020 and the first half of 2021, and no major outbreaks between April and December 2021.3,9 However, a large community epidemic of Omicron beginning in January 2022 caused a large number of fatalities within three months, despite the availability of COVID-19 vaccines for over a year by that time, and more stringent NPIs being implemented to attempt to contain transmission.3,10 Here, we systematically examine the progression and control of COVID-19 pandemic waves in Hong Kong between January 2020 and December 2022, focusing on locally adopted control measures and their impact on transmission and burden of COVID-19, to identify critical factors in response and preparedness over the course of the pandemic.
Methods
COVID-19 cases and pandemic waves
We obtained demographic, clinical, and epidemiological data (situation as of 29 January 2023) on all laboratory-confirmed and self-reported cases between January 2020 and December 2022 from the Hong Kong Department of Health and Hospital Authority of Hong Kong. Laboratory-confirmed COVID-19 cases were defined by positive results of reverse transcription polymerase chain reaction (RT-PCR) tests throughout the pandemic as well as identified based on self-reported positive rapid antigen tests (RAT) since 26 February 2022. Between January 2020 and February 2022, all confirmed cases, including asymptomatic cases, were required to be isolated in hospital, during which the clinical outcomes were recorded.9 Confirmed COVID-19 cases were classified into mild/moderate, serious, critical, and fatal according to clinical outcomes, and linked to epidemiological information collected through contact tracing, e.g., local vs imported infections, contact history, etc. (Appendix section 1).
We classified the COVID-19 pandemic in Hong Kong into six waves by confirmation date of cases. Each wave was divided into pre-peak (16–50 days before peak), peak (15 days before or after peak), and post-peak periods (16–50 days after peak) to reflect the trajectory, where peak was defined as the day recording the largest number of confirmed cases. Waves 5 and 6 were further divided into two periods to reflect changes in healthcare services (suspension of contact tracing on 6 Feb 2022) and dominant virus variant (from Omicron BA.4 to BA.5) over the epidemic, namely 5a (31 Dec 2021–6 Feb 2022), 5b (7 Feb–22 May 2022), 6a (23 May–30 Sep 2022) and 6b (1 Oct–31 Dec 2022). Although the Hong Kong SAR Government classified the resurgence of infections in June 2022 with Omicron BA.4/5 as a continuation of the fifth wave, because daily cases never declined to zero prior to the resurgence, here we refer to this as the sixth wave for clarity and consistency.
Characterization of non-pharmaceutical interventions
Information on NPIs implemented in Hong Kong was collected through the government press releases and classified into travel-related, community-wide, and case-based measures (Appendix pages 4–8 and section 5). Travel-related measures include entrance restrictions, inbound traveller testing, quarantine, and exemptions. Community-wide measures include school closures, work-from-home policies, mask wearing, restrictions on group gatherings and measures to reduce crowding and mixing in the population. Case-based interventions refer to targeted measures related to case identification, timely isolation, and tracing and quarantine of contacts.
We used contact tracing data to assess the temporal changes in local case clustering patterns. A cluster was defined as at least two RT-PCR confirmed cases with contact history and initiated by index local cases (Appendix section 1).11 We characterized the monthly distribution and density distribution of cluster size, stratified by waves.
Case finding and contact tracing
We obtained data on COVID-19 RT-PCR tests performed in Hong Kong through various testing schemes (Appendix section 2) up to wave 5a (7 February 2022),12 including daily numbers of specimens tested and test positives. The testing schemes focused on different risk populations, including inbound travellers, cases and their close contacts, patients in clinical settings, and persons in the general community. We describe temporal changes in testing capacity and case detection proportions and compare the two-week moving average of numbers of specimens collected from close contacts to that from the confirmed cases. We report the distribution of the delays between illness onset and case confirmation for symptomatic cases as indicators of the potential impact on transmissibility of case finding and isolation across waves, stratified by type of case, wave and trajectory.
Population behavioural responses to community-based measures
To assess population behavioural responses during the COVID-19 pandemic, we conducted 107 rounds of cross-sectional random digit dialling telephone surveys from January 2020 to December 2022 (Appendix section 3).9,13 In each survey, we randomly sampled Hong Kong adults ≥18 years using random-digit dialling during working and non-working hours, with a 1:1 ratio of landline and mobile numbers. Participants who provided consent reported on face mask usage, personal hygiene, and social distancing behaviours in the week prior to each survey. We calculated the rim-weighted13 proportion of respondents who reported specific behaviours in each survey, with binomial confidence intervals (CIs).
We compared population mobility in the community during the pandemic to the data recorded at the pre-pandemic phase (1 January 2020) using transport transactions made through the Octopus card which is the most widely used, age-stratified online and offline payment system in Hong Kong.14 Overall mobility based on all card types was calculated and weighted by population age-structure. Changes in community mobility were analysed, stratified by time to the peak of each epidemic wave and age group. We also obtained data from the Immigration Department of Hong Kong to assess changes in inbound travellers during the study period.
COVID-19 transmission and impact of non-pharmaceutical interventions
Over epidemic waves 1–3, boarder and more stringent control measures appeared not to be able to completely block importations of COVID-19 while presumably the transmissibility of imported cases had to some extent been reduced. Therefore, we extended the approach described by Cori et al.15 to estimate the time-varying effective reproductive numbers (Rt) for transmissions of COVID-19 induced by imported and local cases separately (Appendix section 4).16 During wave 4, no locally infected cases were identified with a link to imported cases given the further enhanced border control measures, and epidemic waves 5–6 were dominated by local transmissions of the Omicron variants. Therefore, we used the Cori et al. approach to estimate Rt for waves 4–6, allowing for time-varying infectiousness from symptom onset.17 We inferred the epidemic curve by infection date for Rt estimation by applying the deconvolution approach,18 using the incubation period (mean 5.2 (SD 3.9) days for waves 1–4 and mean 3.5 (SD 2.6) days for waves 5–6),19,20 and infection-to-report delay from empirical data.9
Since various NPIs were often implemented collectively over the pandemic, we examined the impact of the combined NPIs (“NPI package”) adopted in Hong Kong on reducing community transmission from locally infected cases by monitoring the Rt over time. The inclusion of specific NPIs into different NPI packages over the epidemic waves was informed by the clustering patterns of NPIs shown in the hierarchical clustering model and the variance of transmission explained in the log-linear regression on the estimated daily Rt and individual NPIs (Appendix section 5). Vaccination policies had been incorporated into social distancing measures for COVID-19 controls in Hong Kong aiming to minimise community transmission of SARS-CoV-2 (“Vaccine Bubble” in 2021) and improve protection for high-risk population against severe infections (in 2022). Therefore, delineating the impact of vaccine policy on COVID-19 transmission from the impact of population mobility caused by other factors were challenging. Nevertheless, the vaccination campaigns were launched after the fourth wave and therefore had no effect on the first four waves of COVID-19. The high infection attack rate in the fifth wave despite high vaccine coverage (>70% of the population with 2 doses) indicates that vaccination only provided limited protection against Omicron BA.2 infections. Therefore, our study highlighted the importance of non-pharmaceutical interventions (NPIs). Log-linear regression models were used with daily Rt as an outcome and interventions as time-varying covariates across waves 1 to 5a, allowing for different initial Rt and effect of work-from-home of each wave. Of note, the wave-specific initial transmissibility (i.e., initial Rt) was estimated to reflect the basic reproduction number of the dominant strain given the population susceptibility and behaviours at the beginning of each wave. We repeated the above analyses by dropping one wave at each time to test potential changes in NPIs effects across waves. Rt was estimated for defined NPI packages representing different intensities of interventions and the initial condition as reflected by Rt, i.e., the initial transmissibility in each wave, in comparison with the scenarios of no NPIs and applying all available NPIs. 95% CIs were estimated using 1000 bootstraps of coefficient estimates following a multivariate normal distribution.
COVID-19 fatality and pharmaceutical interventions
To characterize the changes in disease burden and severity over wave and trajectory, we used the weekly mortality rate and case-fatality risk (CFR) for the overall population, older adults ≥65 years, and unvaccinated individuals, respectively. Within each group and study period, the weekly mortality rate was calculated as the weekly number of COVID-19 deaths over person-times observed, while the CFR was defined as the number of deaths over the total number of confirmed COVID-19 cases (Appendix section 6). Data from 1 October 2022 onwards were excluded from our analysis given the fact that more COVID-19 cases were confirmed based on self-reported positive RATs during that time period, suggesting a possible declining case finding from the government through RT-qPCR confirmation.
Two vaccines including the inactivated vaccine CoronaVac (Sinovac) and the mRNA vaccine BNT162b2 (BioNTech/Fosun Pharma/Pfizer) were provided for free in Hong Kong starting from February 2021, with third doses available for adults since November 2021. We collected age-specific population and daily numbers of vaccine doses administered by age from the territory-wide vaccine registry to calculate the proportion fully vaccinated (≥2 doses) among the overall population and older adults ≥65 years of age, stratified by wave and trajectory.
Two COVID-specific oral antiviral drugs, molnupiravir (Merck) and nirmatrelvir/ritonavir (Pfizer) have been authorized for use in Hong Kong since late February 2022 and mid-March 2022 respectively. Drug prescriptions for individual patients were used to calculate the proportion of patients who were confirmed by either RT-PCR or RAT and received the antivirals in all ages and unvaccinated older adults ≥65 years, stratified by wave and trajectory.
All analyses were conducted in R version 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria). Our project was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Non-pharmaceutical interventions and COVID-19 transmission
Hong Kong experienced six epidemic waves from January 2020 through December 2022 (Fig. 1, Figure S1 and Table S1), with over 2.6 million laboratory- and RAT-confirmed COVID-19 cases (Table 1). Notwithstanding differential ascertainment by wave, only 12,631 (0.5%) cases were confirmed during the first four epidemic waves predominated by the SARS-CoV-2 ancestral strain, corresponding to 1.6 cases per 1000 population. In the second half of 2021, in total there were 841 (98%) imported cases but only five sporadic/index local cases reported (Table 1, Table S2). A superspreading event associated with a case of Omicron BA.2.2 who acquired the infection in a quarantine hotel initiated a large fifth wave, with a cumulative incidence of 162 cases per 1000 persons during January–May 2022 (wave 5), and the incidence rates dropped by 90% afterwards (Fig. 1).
Fig. 1.
Confirmed cases (A), transmission dynamics (B), non-pharmaceutical interventions (NPIs, C), testing schemes (D), self-reported population behaviours (E), and population mobility (F) across the six epidemic waves of COVID-19 in Hong Kong. (A) COVID-19 cases. Coloured bars indicated cases laboratory-confirmed by RT-PCR or self-reported rapid antigen test (RAT) positives, which were only available after February 25, 2022. Laboratory-cases were classified into sporadic/index imported (dark blue), linked-to-imported (light blue), sporadic/index local (brown) and contacts of local COVID-19 cases (orange). Self-reported RAT positive cases were classified into imported (green) or local (pink) cases. Pie charts shows the share of case type for the first four waves. (B) Estimated effective reproduction numbers (Rt) for local transmission based on identified local cases. Shaded areas in dark grey indicated the estimated 95% credible intervals of Rt. (C) NPIs taken to suppress and contain the Covid-19 transmission in Hong Kong by time. NPIs were classified into three target groups: inbound travellers (blue), community (yellow) and case and contact tracing (red). Darker shading represents more stringent measures, with details in Table S4. (D) COVID-19 testing schemes by target groups and settings. Items with asterisks indicate testing for individuals with respiratory illness. (E) Population behaviours related to physical distancing and personal hygiene measured among the general adult population across 107 cross-sectional telephone surveys, January 2020 to December 2022. Point estimates (points) and 95% confidence intervals (vertical segments) were estimated by each survey. (F) Percentage changes in transport transactions using Octopus cards relative to 1 January 2020, weighted by age-structure of Hong Kong population in 2021.
Table 1.
Characteristics of COVID-19 cases confirmed in Hong Kong by epidemic wave.
| Wave 1&2 |
Wave 3 |
Wave 4 |
Post wave 4 |
Wave 5a |
Wave 5b |
Wave 6a |
Wave 6b |
pa | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1/1/20–30/4/20) | (1/5/20–30/9/20) | (1/10/20–30/4/21) | (1/5/21–30/12/21) | (31/12/21–6/2/22) | (7/2/22–22/5/22) |
(23/5/22–30/9/22) |
(1/10/22–31/12/22) |
|||||
| PCR | RAT | PCR | RAT | PCR | RAT | |||||||
| N | 1038 | 4050 | 6682 | 861 | 2804 | 745,787 | 449,173 | 189,298 | 365,711 | 222,148 | 638,080 | |
| Age group (n, %) | <0.001 | |||||||||||
| 0–18 | 108 (10.4) | 374 (9.2) | 591 (8.8) | 87 (10.1) | 437 (15.6) | 62,994 (8.4) | 55,915 (12.4) | 18,932 (10.0) | 65,650 (18.0) | 14,726 (6.6) | 107,658 (16.9) | |
| 19–29 | 308 (29.7) | 611 (15.1) | 931 (13.9) | 219 (25.4) | 464 (16.5) | 85,369 (11.4) | 61,363 (13.7) | 21,395 (11.3) | 48,326 (13.2) | 24,416 (11.0) | 76,807 (12.0) | |
| 30–44 | 272 (26.2) | 936 (23.1) | 1917 (28.7) | 315 (36.6) | 767 (27.4) | 169,209 (22.7) | 126,669 (28.2) | 40,886 (21.6) | 101,816 (27.8) | 52,147 (23.5) | 171,390 (26.9) | |
| 45–64 | 269 (25.9) | 1376 (34.0) | 2132 (31.9) | 203 (23.6) | 836 (29.8) | 263,295 (35.3) | 131,810 (29.3) | 63,887 (33.7) | 105,726 (28.9) | 78,735 (35.4) | 194,644 (30.5) | |
| ≥65 | 81 (7.8) | 753 (18.6) | 1111 (16.6) | 37 (4.3) | 296 (10.6) | 157,587 (21.1) | 73,035 (16.3) | 43,719 (23.1) | 43,959 (12.0) | 51,794 (23.3) | 87,069 (13.6) | |
| Missing | – | – | – | – | 4 (0.1) | 7333 (1.0) | 381 (0.1) | 479 (0.3) | 234 (0.1) | 330 (0.1) | 512 (0.1) | |
| Sex (n, %) | <0.001 | |||||||||||
| Male | 559 (53.9) | 1960 (48.4) | 3134 (46.9) | 452 (52.5) | 1316 (46.9) | 366,375 (49.1) | 193,498 (43.1) | 91,853 (48.5) | 164,372 (44.9) | 105,579 (47.5) | 278,859 (43.7) | |
| Female | 479 (46.1) | 2090 (51.6) | 3548 (53.1) | 409 (47.5) | 1484 (52.9) | 372,618 (50.0) | 255,512 (56.9) | 97,021 (51.3) | 201,173 (55.0) | 116,310 (52.4) | 358,752 (56.2) | |
| Missing | – | – | – | – | 4 (0.1) | 6794 (0.9) | 163 (0.0) | 424 (0.2) | 166 (0.0) | 259 (0.1) | 469 (0.1) | |
| Case classifications (n, %) | ||||||||||||
| Sporadic/index imported | 616 (59.3) | 664 (16.4) | 1107 (16.6) | 841 (97.7) | 610 (21.8) | 1156 (0.2) | 221 (0.05) | 18,886 (10.0) | 2985 (0.8) | 45,286 (20.4) | 6628 (1.0) | |
| Linked-to-imported | 88 (8.5) | 5 (0.1) | 11 (0.2) | 10 (1.2) | 848 (30.2) | 56 (0.01) | 4 (0.001) | 7 (0.0) | 30 (0.01) | 0 (0.0) | 0 (0.0) | |
| Local | 169 (16.3) | 1293 (31.9) | 1795 (26.9) | 5 (0.6) | 485 (17.3) | 740,658 (99.3) | 448,822 (99.9) | 170,186 (89.9) | 362,227 (99.0) | 176,862 (79.6) | 631,452 (99.0) | |
| Contact of local | 165 (15.9) | 2088 (51.6) | 3769 (56.4) | 5 (0.6) | 861 (30.7) | 3917 (0.5) | 126 (0.03) | 219 (0.04) | 469 (0.1) | 0 (0.0) | 0 (0.0) | |
| Severity status (n, %) | <0.001 | |||||||||||
| Severe | 57 (5.5) | 495 (12.2) | 924 (13.8) | 27 (3.1) | 69 (2.5) | 11,978 (1.6) | 1511 (0.3) | 3635 (1.9) | 1199 (0.3) | 5753 (2.6) | 2275 (0.4) | |
| Critical | 50 (4.8) | 144 (3.6) | 211 (3.2) | 3 (0.3) | 3 (0.1) | 1508 (0.2) | 143 (0.0) | 435 (0.2) | 110 (0.0) | 643 (0.3) | 248 (0.0) | |
| Fatal | 5 (0.5) | 105 (2.6) | 102 (1.5) | 1 (0.1) | 3 (0.1) | 8969 (1.2) | 704 (0.2) | 682 (0.4) | 174 (0.0) | 1661 (0.7) | 728 (0.1) | |
| Delay distribution, days (mean, sd) | ||||||||||||
| Onset to hospitalizationb | 5.8 (5.4) | 4.7 (4.2) | 3.2 (3.0) | 2.5 (4.6) | 2.6 (2.3) | 5.0 (7.3) | 11.6 (10.4) | 4.5 (11.4) | 6.7 (16.3) | 3.7 (10.1) | 8.0 (12.3) | <0.001 |
| Onset to confirmation | 6.5 (5.4) | 5.2 (3.8) | 4.3 (3.0) | 3.7 (4.4) | 2.8 (1.7) | 5.5 (5.4) | 2.3 (4.9) | 4.6 (41.0) | 4.2 (5.5) | 2.7 (12.9) | 4.2 (35.2) | <0.001 |
| Onset to discharge | 29.1 (13.2) | 18.6 (14.2) | 17.3 (15.2) | 20.9 (9.9) | 14.4 (4.6) | 17.5 (20.3) | 21.5 (17.1) | 11.3 (14.2) | 13.0 (18.5) | 10.1 (11.6) | 13.1 (13.5) | <0.001 |
| Onset to death | 36.4 (54.2) | 36 (79.3) | 29.9 (28.6) | –c | 31.0 (–)d | 11.9 (9.1) | 17.7 (11.4) | 14.2 (7.6) | 14.4 (8.7) | 16.1 (29.5) | 15.5 (8.6) | <0.001 |
| Confirmation to last positive sample | 18.0 (12.5) | 10.7 (25.7) | 9.5 (10.2) | 10.4 (6.8) | 8.9 (4.6) | 8.0 (19.1) | 5.8 (16.6) | 6.9 (9.0) | 6.1 (8.3) | 6.5 (6.5) | 6.1 (5.9) | <0.001 |
Fisher's exact test was used for categorical variables and Kruskal–Wallis rank sum test was used for all continuous variables.
5215 (10%) cases were excluded from the analysis due to the recorded date of onset later than the date of hospital admission (possibly nosocomial infection).
The onset-to-death delay distribution could not be estimated because the onset date of the only fatal case confirmed during post-Wave 4 was missing.
The standard deviation (sd) could not be estimated as the onset date is only available for one of the three fatal cases confirmed during Wave 5a.
The estimated Rt for local infections over the epidemic waves (Fig. 1B and Figures S2–S6) indicated that transmissibility of SARS-CoV-2 decreased following (re-)implementation of strict NPIs and rose after relaxation of the measures during the first four waves. In the Omicron BA.2.2 wave (wave 5), Rt remained above 1 even after increasingly stringent control measures were implemented during pre-peak and peak periods (wave 5), and mostly fluctuated at around 1 during the Omicron BA.4/5 wave (wave 6).
Hong Kong adopted stringent border controls to prevent importation of cases, including suspending 12 out of 15 boundary control points, barring entry of non-local residents if they have been to overseas places, and mandating on-arrival quarantine with RT-PCR testing in designated facilities for up to 21 days (Fig. 1C and D and Tables S3 and S4). We observed a significant decrease in daily passenger arrivals from over 150,000 in January to below 3000 since April 2020 (a 98% decrease from January 2020) and an increase in daily COVID-19 testing for inbound travellers from 1000 in March 2020 to 6000 between July and November 2021 (Figures S7 and S8). As a result, the Rt for imported cases was maintained well below 1 since mid-February 2020 (Figure S9) and eventually became unquantifiable due to too few sporadic transmission events during the following waves (Table 1).
Hong Kong implemented proactive case finding and contact tracing measures, with substantially expanded laboratory testing capacity over time, leading to gradually shortened onset-to-report intervals (e.g., for close contacts of local cases from a median of 5 to 3 days between wave 3 and 5a) (Figures S10–S12, Table S5). Quarantine of close contacts were enforced in designated quarantine facilities since January 2020 and home quarantine was introduced since February 28, 2022, and continued until December 28, 2022. Numbers of specimens collected from close contacts for testing peaked before the fifth wave, but the ratio to confirmed case numbers decreased substantially compared to previous waves (Figure S10), consistent with widespread community transmission. In clinical settings, testing was required for all inpatients and outpatients with respiratory illness since January 2020, and expanded to all hospital admissions in September 2020 (Fig. 1 and Figure S10). In the community, some predefined groups of individuals who were at a higher risk of transmission or providing essential services (Table S5) had also received mandatory testing on a regular basis or by random sampling since May 2020, leading to an increasing contribution to the total number of tested specimens from 40% in the third wave to 88% in wave 5a.
The government closed all schools and recommended civil servants to work remotely in late January 2020 (Fig. 1A and Table S2–S4). The work-from-home recommendation was removed and re-introduced in subsequent waves, while schools re-opened with restrictions when local cases were low (Fig. 1). During the second wave, the government instituted infection control requirements for group gatherings, restaurant capacity and operating hours and other non-essential business sectors in response to local case clusters associated with restaurants and bars. These measures were relaxed and retightened through the following waves. A face mask mandate in all indoor and outdoor public areas was issued during the third wave in mid-July 2020 and remained in place throughout the remaining study period and has only ultimately been relaxed from March 2023 onwards.
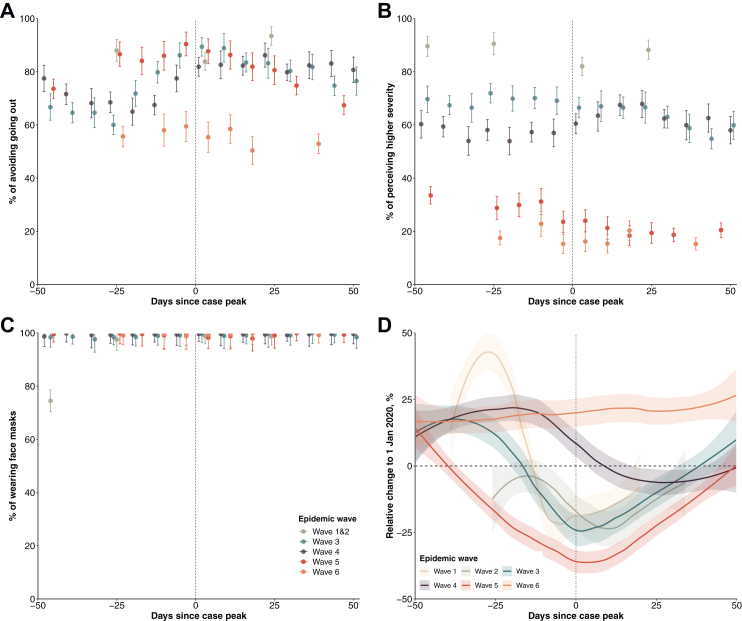
Telephone surveys conducted from late January 2020 and December 2022 (total n = 82,562) revealed notable behavioural changes in population response to the implemented NPIs. Physical distancing behaviours were observed at high proportions during waves 1–6 especially when local transmission was high (67%–90%; Fig. 2 and Figure S8). Face mask use increased to a high level in January 2020 and remained very high (over 98%) throughout the study period. Reduced behaviour changes and risk perceptions were observed during the period between wave 4 and 5, and during wave 6 (Fig. 2 and Figures S13 and S14). There were notable reductions in public transport transactions, with the greatest reductions in wave 5 (largest reduction at 37%, 95% CI, 33%–40%), compared to previous waves (Fig. 2 and Figures S15 and S16). Clusters with more than 10 cases involved were less frequently observed after strict community-based measurements were implemented in the third wave, with a median size of 10 (IQR: 5–19) (Figure S17).
Fig. 2.
Changes in population behaviours and mobility relative to pre-pandemic levels, stratified by epidemic wave in Hong Kong. The case peak was determined as the day with the largest number of cases in each wave. Point estimates (dots) and 95% confidence intervals (vertical line segments) were estimated for each survey. (A) Avoid going out as much as possible. (B) Higher perceived severity of COVID-19. (C) Wear face masks when going out. (D) Percentage changes in transport transactions using Octopus cards relative to 1 January 2020, weighted by age-structure of Hong Kong population in 2021.
We included a combination of NPIs that parsimoniously reflected changes in controlling local transmission in the multivariate analysis (Appendix, Figure S18; adjusted R2 67%), to estimate the effects of different NPI packages. The NPI packages adopted in wave 4 appeared to be the strictest among all the waves (Fig. 3A). The initial Rt for waves 1&2, 3, 4 and 5a were estimated to be 1.2 (95% CI: 1.0–1.5), 2.6 (95% CI: 1.9–3.5), 3.0 (95% CI: 2.3–3.9) and 9.3 (95% CI: 6.5–13.4), respectively (Fig. 3B and Table S6). Applying all locally implemented NPIs at the most intensive level was estimated to bring Rt down to below 1 (e.g., 0.6, 95% CI: 0.4–0.9 for wave 3), except for wave 5a (3.1, 95% CI: 1.5–5.9), while the analysis on the strictest wave-specific NPI packages showed that only the NPI package used in wave 4 seemed sufficient to control epidemics in waves 1–3, but was only marginally effective during wave 4. However, none of the NPI packages from earlier waves could have sufficiently suppressed Omicron transmission in wave 5 (Fig. 3C).
Fig. 3.
Prediction of effective reproduction numbers (Rt) of COVID-19 associated with non-pharmaceutical interventions (NPIs) taken across epidemic waves. Wave-specific intercepts were used as the baseline level for each epidemic wave. The initial transmissibility is interpreted as the reproduction number of the circulating strain in the context of pre-existing population immunity (derived from vaccination and/or infections) and contact patterns determined by population behaviours. (A) NPIs packages used for model prediction: the most stringent measures used in each epidemic wave, no measure, and all measures. (B) Wave-specific Rt (row) when no (i.e., initial transmissibility) or all measures (column) were implemented. (C) Wave-specific Rt (row) under the most stringent NPIs packages (column) of each epidemic wave.
Pharmaceutical interventions against COVID-19
In total 13,134 COVID-19 deaths were reported throughout the six waves, among which 74% (n = 9676) were reported between January and May 2023 (wave 5) with a peak in the overall mortality rate of 2.36 (95% CI, 2.31–2.41) deaths per 10,000 person-weeks (Fig. 4, Fig. 5). Among all fatal cases recorded, 92% (n = 12,073) were amongst older adults ≥65 years of age.
Fig. 4.
Confirmed deaths, vaccine coverage, and antiviral usages across the six epidemic waves of COVID-19 in Hong Kong. (A) Confirmed COVID-19 deaths sorted by date of confirmation. Coloured bars indicated whether the fatal cases were aged 65 years or above (red) or otherwise (blue). (B) Cumulative coverage of one, two, three or four doses of CoronaVac and/or BNT162b2. (C) Antiviral usage among confirmed COVID-19 cases aged 65 and above sorted by date of confirmation.
Fig. 5.
Disease severity and burden of COVID-19 and implementations of pharmaceutical interventions taken across epidemic waves. Estimates were stratified by wave and trajectory (i.e., pre-peak, peak, and post-peak). We also calculated the estimates for all Hong Kong population (overall), individuals aged 65 y and above, and unvaccinated individuals of all ages (unvaccinated). Data used for the plot were provided in Tables S8 and S9. (A) Mortality rate per 1000 persons per week. (B) Case-fatality risk. Cases were all notified cases, including PCR and RAT positives. (C) Coverage of individuals received two or more doses of COVID-19 vaccines. Vaccine coverage among both all Hong Kong (overall; light colours) and individuals aged ≥65 y (dark colours) were calculated. (D) Antiviral usage among confirmed COVID-19 cases aged 65 and above. (E) Case-fatality risk and antiviral usage in the fifth and sixth wave. Estimates were calculated for unvaccinated individuals aged ≥65 y. (F) Case-fatality risk and COVID-19 vaccine coverage in the fifth and sixth wave. We calculated vaccine coverage as individuals aged 65 and above and received two or more doses. Case-fatality risk was calculated for all adults ≥65 y and fully vaccinated adults ≥65 y, respectively. (G) Mortality rate and COVID-19 vaccine coverage in the fifth and sixth wave. We calculated vaccine coverage as individuals aged ≥65 y who had received two or more doses. Mortality rate was calculated for all adults ≥65 y and fully vaccinated adults ≥65 y, respectively.
Uptake of the two COVID-19 vaccines in Hong Kong gradually increased during the first six months after having become available, reaching a plateau between October 2021 and January 2022 (Fig. 4). Before wave 5, approximately 70% of the population and 42% of older adults ≥65 years received ≥2 doses, but fewer than 10% of the whole population and fewer than one-tenth of older adults had received a third dose (Fig. 4, Fig. 5). The coverage of ≥2 doses of vaccine increased to 82% among adults ≥65 years by the end of wave 6.
The two antivirals molnupiravir and nirmatrelvir/ritonavir first became available only during the peak of wave 5 and were provided to 18% of the adult patients ≥65 y within one month (Fig. 4, Fig. 5). The usage of antivirals increased to about 40% among adults ≥65 y.
The CFR in infected adults ≥65 years decreased across epidemic waves from 13% (95% CI, 11% to 16%) in wave 3 to 0.83% (95% CI, 0.76% to 0.89%) in wave 6 (Fig. 5B). Following availability of the antivirals, CFRs among unvaccinated adults ≥65 y decreased from 9.4% (95% CI, 7.5% to 11.6%) pre-peak of wave 5 to 3.0% (95% CI, 2.4% to 3.8%) during the peak of wave 6 (Fig. 5E). We observed reduced differences in CFRs and weekly mortality rate between all and vaccinated adults ≥65 years along with the increasing vaccine uptake in this age group (Fig. 5F and G).
Discussion
Using detailed individual case data and territory-wide population data collected during the COVID-19 pandemic in Hong Kong, we were able to systematically examine the progression of epidemics from 2020 to 2022 in relation to local pandemic responses, including implementation of both NPIs and pharmaceutical interventions over the course of the pandemic.
Stringent NPIs aiming to minimize importation risks and interrupt local transmission chains had been implemented in Hong Kong from early in the pandemic, and successfully controlled multiple epidemic waves in the community caused by the ancestral strain (Fig. 1). These measures reduced COVID-19 mortality in a highly susceptible population in the absence of effective pharmaceutical interventions through effectively suppressing the spread of infection (Fig. 4).21 Nevertheless, the containment strategy relying solely on NPIs may not be sustainable because of the cost and disruption of these measures. Successful suppression of transmission leaves a large population susceptible to infection with newly emerging, immunologically distinct viral strains, and the NPIs would be less effective in preventing pre-symptomatic and superspreading transmissions, and in controlling infections with a relatively higher transmissibility.17,22 In some countries, relaxation of NPIs resulted in increased COVID-19 mortality, accompanied by health and social consequences from an overburdened healthcare system and a slow recovery from the COVID-19 pandemic in a long run.1,23 Achieving high vaccination coverage in the most vulnerable groups, particularly older adults, would minimize COVID-19 mortality after containment measures fail or are relaxed.24
Strict travel measures were able to minimize COVID-19 introductions into the community.25,26 Despite over 2000 infections in arriving travellers, only three independent introductions accounted for 90% of the local cases between the second and fourth waves (Fig. 1 and Table 1).27 The other cornerstone of the approaches to COVID-19 elimination in Hong Kong was the strict isolation of all confirmed cases until viral shedding of the patients reached low levels before discharge, and quarantine of close contacts identified from contact tracing at designated facilities (Fig. 1C). While isolation and quarantine likely reduced transmission of COVID-19, it is well recognized that many infections in the community were never confirmed, and a number of community epidemics occurred despite intense contact tracing and timely quarantine.9 As a consequence, the containment of COVID-19 in Hong Kong cannot be attributed to strict isolation and quarantine alone, while it is clear that moderate social distancing measures were necessary to contain community epidemics with the ancestral strain.
Community-based physical distancing measures were widely adopted in different parts of the world early in the pandemic,5 and individual behaviours might have also changed in response to perceived risk.28 Our analysis showed that the implementation of packages of physical distancing measures, including school closure, working from home and suspension of large gatherings (Fig. 1), etc., correlated with subsequent decline in the effective reproductive number (Fig. 2) during epidemics of the ancestral strain but these declines were not sufficient to achieve containment of the more transmissible Omicron subvariants. It was not possible to estimate the impact from individual measures which were often implemented together, or which were not the only factor shaping the patterns of population mixing or mobility therefore transmission of infection, e.g., adherence to facemask wearing ahead of the mask mandate policies.
Containment of COVID-19 in Hong Kong allowed vaccination rollout in early 2021 which could provide an opportunity for a transition/exit strategy from the use of NPIs for containment. Despite over 70% of the Hong Kong population being fully vaccinated by the end of 2021, the vaccine uptake among adults ≥65 years was low particularly in adults ≥80 years (approximately 25%) before the Omicron wave (Fig. 3). With a high incidence of infections with Omicron, there was a high mortality rate of 1500 per 1,000,000 persons in 2022, the majority of deaths occurring in unvaccinated older adults (Fig. 4). Conversely, locations such as Singapore that only considered containment as a temporary approach prior to reaching high vaccination coverage among vulnerable groups recorded much lower mortality rates in their Omicron waves.5,7 If all adults ≥65 y were fully vaccinated, a large fraction of deaths in that age group during wave 5 in Hong Kong might have been prevented. The successful control of community transmission for two years in Hong Kong, and the intention to continue with a containment approach regardless of vaccination uptake,29 might have contributed to a lack of urgency in increasing vaccination uptake among vulnerable groups,13,30 further exacerbating the low coverage of vaccine in Hong Kong. Identification of population groups at a higher risk of infection or severe outcomes and factors closely related to vaccine hesitancy may help to increase vaccine uptake in most vulnerable populations along with convenient outreach vaccination programs and risk communication to improve risk perception and to address urgency of vaccine uptake.13,31
Planning for “living with the virus” is a challenge for many different locations with different control strategies during the pandemic. Clear objectives in responses are essential for selecting appropriate strategies over the course of the pandemic, and the ultimate goal is always to minimize severe cases and fatalities, and to protect healthcare systems from being overwhelmed. In late 2021, emerging variants such as Delta showing increased immune evasion caused concerns about reduced vaccine-induced protection against infection.32 However, evidence suggested that COVID-19 vaccines had remained highly effective in preventing severe and fatal outcomes from infections with the variants,10,33 suggesting that vaccination would still likely be an effective way to reduce the severe disease burden even if not preventing infections especially in a population with passive immunity conferred by vaccines.34, 35, 36 While Omicron was associated with milder disease and lower health impact in some locations,4 infections may have a similar intrinsic severity to the ancestral virus in persons who have not been vaccinated or previously infected.37 Real-time risk assessment of emerging variants remains a challenge.
There are several limitations of our study. First, the investigation of the direct health impact of pandemic control measures did not take into consideration the possible effects on indirect health outcomes (e.g., mental health) and social and economic life, and the estimates might vary based on local infrastructure (e.g., personal protection equipment availability) and cultural factors. Second, we estimated the initial transmissibility for each epidemic wave, but could not attribute the estimates to viral features or population immunity. However, the increased transmissibility in the fifth wave was likely due to increased transmissibility and immune invasion of Omicron variant, and relatively low prior immunity acquired through infections in previous epidemic waves or vaccination.34, 35, 36 Third, we only considered the impact of NPIs on COVID-19 transmissions without accounting for population adherence to these NPIs, which might have been affected by factors such as pandemic fatigue and vaccination coverage in the population. Finally, as for any illness, surveillance data we used is not complete in detecting cases. However, previous studies suggested good representativeness of the surveillance data, capturing one fourth of the infections before wave 5.9,36
Experience from Hong Kong has indicated that optimal pandemic control lies in timely and efficient implementation of NPIs, along with a high level of population adherence before pharmaceutical interventions become available. Once vaccines or antivirals can be rolled out, the rationale for continuing to apply disruptive NPIs will gradually weaken. Our findings highlight the value of continuously assessing the level of population immunity against severe disease in the light of viral evolution and the changing availability of pharmaceutical agents. In future pandemics caused by other novel respiratory pathogens, employing NPIs as an initial and temporary strategy can help to contain the spread, providing time to implement more sustainable control measures for high-risk individuals to minimize population mortality and impact on public health.
Contributors
All authors meet the ICMJE criteria for authorship. The study was conceived by BY, GML, BJC and PW. Data analyses were conducted by BY, YL, CL, HG, FH, JZ, RZ, WX and TKT. BY and PW wrote the first draft of the manuscript, and all authors provided critical review and revision of the text and approved the final version.
Data sharing statement
Information of non-pharmaceutical interventions is freely available from the press release of the Government of Hong Kong SAR (https://www.info.gov.hk/gia/general/today.htm). All demographic and epidemiological information of individuals with confirmed COVID-19 and information of COVID-19 vaccinations programme are freely available from the Centre for Health Protection website (https://www.coronavirus.gov.hk/eng/index.html). Access to the hospitalisation, surveillance and antiviral data derived from the electronic medical record system managed by the Hospital Authority and other databases by the Centre for Health Protection in Hong Kong is subject to the approval from the two agencies. Access to daily passenger traffic entering Hong Kong are freely available from the Immigration Department (https://www.immd.gov.hk/eng/facts/passenger-statistics-menu.html). Access to local mobility data are subject to the approval from the Octopus company. Telephone survey data are available from the corresponding author on request.
Declaration of interests
BJC consults for AstraZeneca, Fosun Pharma, GSK, Haleon, Moderna, Novavax, Pfizer, Roche, and Sanofi Pasteur. The authors report no other potential conflicts of interest.
Acknowledgements
We thank the Department of Health and the Health Bureau (former Food and Health Bureau) of the Hong Kong SAR Government for providing the data for the analysis and thank Julie Au and Chloe Chui for technical assistance.
Funding: This project was supported by the Health and Medical Research Fund from the Health Bureau of the Hong Kong SAR Government (grant numbers COVID190118 and 21200212), and the Collaborative Research Scheme (Project No. C7123-20G), the Theme-based Research Scheme (Project No. T11-705/21-N) and the General Research Fund (Project No. 17110221) from the Research Grants Council of the Hong Kong SAR Government. The telephone surveys were supported by the Health and Medical Research Fund (ref: COVID19F04, COVID19F11) from the Health Bureau of the Hong Kong SAR Government. The funding bodies had no role in the design of the study or in the analysis and interpretation of data.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100969.
Appendix A. Supplementary data
References
- 1.World Health Organization . 2020. WHO COVID-19 dashboard.https://covid19.who.int/ [Google Scholar]
- 2.Fineberg H.V. Pandemic preparedness and response--lessons from the H1N1 influenza of 2009. N Engl J Med. 2014;370(14):1335–1342. doi: 10.1056/NEJMra1208802. [DOI] [PubMed] [Google Scholar]
- 3.Mefsin Y.M., Chen D., Bond H.S., et al. Epidemiology of infections with SARS-CoV-2 omicron BA.2 variant, Hong Kong, January-March 2022. Emerg Infect Dis. 2022;28(9):1856–1858. doi: 10.3201/eid2809.220613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolter N., Jassat W., Walaza S., et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399(10323):437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sachs J.D., Karim S.S.A., Aknin L., et al. The lancet commission on lessons for the future from the COVID-19 pandemic. Lancet. 2022;400(10359):1224–1280. doi: 10.1016/S0140-6736(22)01585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonabend R., Whittles L.K., Imai N., et al. Non-pharmaceutical interventions, vaccination, and the SARS-CoV-2 delta variant in England: a mathematical modelling study. Lancet. 2021;398(10313):1825–1835. doi: 10.1016/S0140-6736(21)02276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Foo C., Grépin K.A., Cook A.R., et al. Navigating from SARS-CoV-2 elimination to endemicity in Australia, Hong Kong, New Zealand, and Singapore. Lancet. 2021;398(10311):1547–1551. doi: 10.1016/S0140-6736(21)02186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foo C., Verma M., Tan S.M., et al. COVID-19 public health and social measures: a comprehensive picture of six Asian countries. BMJ Glob Health. 2022;7(11) doi: 10.1136/bmjgh-2022-009863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang B., Tsang T.K., Gao H., et al. Universal community nucleic acid testing for coronavirus disease 2019 (COVID-19) in Hong Kong reveals insights into transmission dynamics: a cross-sectional and modeling study. Clin Infect Dis. 2022;75(1):e216–e223. doi: 10.1093/cid/ciab925. [DOI] [PubMed] [Google Scholar]
- 10.McMenamin M.E., Nealon J., Lin Y., et al. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis. 2022;22(10):1435–1443. doi: 10.1016/S1473-3099(22)00345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adam D.C., Wu P., Wong J.Y., et al. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat Med. 2020;26(11):1714–1719. doi: 10.1038/s41591-020-1092-0. [DOI] [PubMed] [Google Scholar]
- 12.Young B.R., Yang B., Wu P., et al. Residential clustering of COVID-19 cases and efficiency of building-wide compulsory testing notices as a transmission control measure in Hong Kong. J Infect Dis. 2023;228(4):426–430. doi: 10.1093/infdis/jiad107. [DOI] [PubMed] [Google Scholar]
- 13.Xiao J., Cheung J.K., Wu P., Ni M.Y., Cowling B.J., Liao Q. Temporal changes in factors associated with COVID-19 vaccine hesitancy and uptake among adults in Hong Kong: serial cross-sectional surveys. Lancet Reg Health West Pac. 2022;23:100441. doi: 10.1016/j.lanwpc.2022.100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung K., Wu J.T., Leung G.M. Real-time tracking and prediction of COVID-19 infection using digital proxies of population mobility and mixing. Nat Commun. 2021;12(1):1501. doi: 10.1038/s41467-021-21776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cori A., Ferguson N.M., Fraser C., Cauchemez S. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am J Epidemiol. 2013;178(9):1505–1512. doi: 10.1093/aje/kwt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser C. Estimating individual and household reproduction numbers in an emerging epidemic. PLoS One. 2007;2(8):e758. doi: 10.1371/journal.pone.0000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He X., Lau E.H.Y., Wu P., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 18.Becker D.J., Linebarger D.A. Vol. 1. 1991. Constrained iterative deconvolution applied to bearing and amplitude estimation of coherent sources; pp. 579–582. ([1991] Conference record of the twenty-fifth asilomar conference on signals, systems & computers; 1991 4-6 Nov). [Google Scholar]
- 19.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected Pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manica M., De Bellis A., Guzzetta G., et al. Intrinsic generation time of the SARS-CoV-2 Omicron variant: an observational study of household transmission. Lancet Reg Health Eur. 2022;19 doi: 10.1016/j.lanepe.2022.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pei S., Yamana T.K., Kandula S., Galanti M., Shaman J. Burden and characteristics of COVID-19 in the United States during 2020. Nature. 2021;598(7880):338–341. doi: 10.1038/s41586-021-03914-4. [DOI] [PubMed] [Google Scholar]
- 22.Hellewell J., Abbott S., Gimma A., et al. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health. 2020;8(4):e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Campbell H., Kulkarni D., et al. The temporal association of introducing and lifting non-pharmaceutical interventions with the time-varying reproduction number (R) of SARS-CoV-2: a modelling study across 131 countries. Lancet Infect Dis. 2021;21(2):193–202. doi: 10.1016/S1473-3099(20)30785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao J., Cowling B.J., Leung G.M. 2021. Transitioning from covid-19 elimination to sustainable endemicity in East Asia.https://blogs.bmj.com/bmj/2021/08/11/transitioning-from-covid-19-elimination-to-sustainable-endemicity-in-east-asia/ [Google Scholar]
- 25.Yang B., Tsang T.K., Wong J.Y., et al. The differential importation risks of COVID-19 from inbound travellers and the feasibility of targeted travel controls: a case study in Hong Kong. Lancet Reg Health West Pac. 2021;13 doi: 10.1016/j.lanwpc.2021.100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang B., Sullivan S.G., Du Z., Tsang T.K., Cowling B.J. Effectiveness of international travel controls for delaying local outbreaks of COVID-19. Emerg Infect Dis. 2022;28(1):251–253. doi: 10.3201/eid2801.211944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu H., Xie R., Adam D.C., et al. Genomic epidemiology of SARS-CoV-2 under an elimination strategy in Hong Kong. Nat Commun. 2022;13(1):736. doi: 10.1038/s41467-022-28420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Z., Wang L., Shan S., et al. Pandemic fatigue impedes mitigation of COVID-19 in Hong Kong. Proc Natl Acad Sci U S A. 2022;119(48) doi: 10.1073/pnas.2213313119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Economist . 2021. Why Hong Kong's ‘Zero-covid’ strategy could backfire?https://www.economist.com/the-economist-explains/2021/10/06/why-hong-kongs-zero-covid-strategy-could-backfire [Google Scholar]
- 30.Yuan J., Lam W.T.W., Xiao J., Ni Y.M., Cowling B.J., Liao Q. Why do Chinese older adults in Hong Kong delay or refuse COVID-19 vaccination? A qualitative study based on Grounded Theory. J Gerontol B Psychol Sci Soc Sci. 2022;78(4):736–748. doi: 10.1093/geronb/gbac184. [DOI] [PubMed] [Google Scholar]
- 31.Hu S., Xiong C., Li Q., Wang Z., Jiang Y. COVID-19 vaccine hesitancy cannot fully explain disparities in vaccination coverage across the contiguous United States. Vaccine. 2022;40(37):5471–5482. doi: 10.1016/j.vaccine.2022.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrews N., Stowe J., Kirsebom F., et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tseng H.F., Ackerson B.K., Luo Y., et al. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and delta variants. Nat Med. 2022;28(5):1063–1071. doi: 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boon S.S., Wong M.C.S., Ng R.W.Y., et al. Seroprevalence of unidentified SARS-CoV-2 infection in Hong Kong during 3 pandemic waves. JAMA Netw Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.32923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poon R.W., Chan B.P., Chan W.M., et al. SARS-CoV-2 IgG seropositivity after the severe Omicron wave of COVID-19 in Hong Kong. Emerg Microbes Infect. 2022;11(1):2116–2119. doi: 10.1080/22221751.2022.2106899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.To K.K., Cheng V.C., Cai J.P., et al. Seroprevalence of SARS-CoV-2 in Hong Kong and in residents evacuated from Hubei province, China: a multicohort study. Lancet Microbe. 2020;1(3):e111–e118. doi: 10.1016/S2666-5247(20)30053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong J.Y., Cheung J.K., Lin Y., et al. Intrinsic and effective severity of coronavirus disease 2019 cases infected with the ancestral strain and Omicron BA.2 variant in Hong Kong. J Infect Dis. 2023;228(9):1231–1239. doi: 10.1093/infdis/jiad236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.