Abstract
Pediatric anxiety and depressive disorders are common, can be highly impairing, and can persist despite the best available treatments. Here, we review research into novel treatments for childhood anxiety and depressive disorders designed to target underlying cognitive, emotional, and neural circuit mechanisms. We highlight three novel treatments lying along a continuum relating to clinical impact of the disorder and the intensity of clinical management required. We review cognitive training, which involves the lowest risk and may be applicable for problems with mild to moderate impact; psychotherapy, which includes a higher level of clinical involvement and may be sufficient for problems with moderate impact; and brain stimulation, which has the highest potential risks and is therefore most appropriate for problems with high impact. For each treatment, we review the specific underlying cognitive, emotional, and brain circuit mechanisms that are being targeted, whether treatments modify those underlying mechanisms, and efficacy in reducing symptoms. We conclude by highlighting future directions, including the importance of work that leverages developmental windows of high brain plasticity to time interventions to the specific epochs in childhood that have the largest and most enduring life-long impact.
Subject terms: Translational research, Attention
Introduction
Psychiatric disorders affect up to 30% of youth [1, 2], can be highly impairing [3–5], and can persist despite the best available treatments [6]. Among individuals who develop a psychiatric disorder, the median age of onset of their first disorder is during childhood [1, 7], and having a childhood psychiatric disorder increases risk for additional disorders later in life [8]. Given data relating early-life brain features to risk [9–11], one hypothesis attributes many psychiatric illnesses across the lifespan to brain development, reflecting effects of accumulating atypicality [12]. Thus, new treatments are needed for childhood psychiatric illnesses, not only to reduce impairments but to potentially interrupt cascades of atypical brain development.
Ideas for new treatments have arisen over the past decade. While most treatments for psychiatric disorders have been discovered through serendipity, some new treatments attempt to alter underlying mechanisms. These treatments target cognitive-, emotional- and brain-based differences between healthy children and children with psychiatric disorders. These treatments support a long-term vision in which clinicians first measure a range of specific psychological and biological targets, as well as how these targets interact with environmental and socio-demographic influences, and then prescribe treatments designed to specifically correct the measured alterations [13]. With this approach, treatment success can be gauged by monitoring these cognitive, emotional, and brain-based targets in addition to symptoms.
The current review focuses on new treatments that target underlying cognitive, emotional, interpersonal, and neural circuit mechanisms disrupted in pediatric psychiatric disorders. Because an exhaustive description of all emerging treatments is not possible in a single review article, we focus primarily on treatments designed to correct underlying mechanisms of anxiety and depressive disorders, while also providing a few specific examples of treatments for other disorders. Current gold-standard treatment of pediatric anxiety and depressive disorders includes use of selective serotonin reuptake inhibitors (SSRIs) and cognitive behavioral therapy (CBT) [14, 15]. While both treatments show medium effect sizes in pediatric anxiety and depression, with some evidence of higher effect sizes for treating anxiety [16], up to 50% of children remain symptomatic following treatment [16–18]. In addition, many children relapse after an initial response to SSRIs or CBT [6, 19, 20]. Thus, new mechanism-based treatments are clearly needed.
Because children are a unique and vulnerable population, research into novel treatments must consider the vulnerable state of the child in a way not required with adults [21–26]. Risks for a vulnerable child must be weighed against the impact of a disorder on the child and the efficacy of the treatment. Relatively invasive treatments or treatments that involve high levels of clinical care can be justified in children with relatively severe or persistent disorders and/or if these treatments are highly effective. Research into novel pediatric treatments can thus be considered along a continuum relating to clinical impact of the disorder in the context of level of risk and clinical involvement required for the treatment.
The current review highlights three novel treatments lying along this continuum: cognitive training, which involves low risk but has a modest effect size, such that stand-alone treatment may be most applicable for problems with no more than moderate impact; psychotherapy, which includes a higher level of clinical involvement and has a medium-to-large effect size, justifiable for problems with at least moderate impact; and brain stimulation, which may have the highest potential risks and unclear efficacy, thus only appropriate for problems with high impact. The three sections below describe three sets of underlying illness mechanisms that can be targeted by novel treatments. Sections review features of novel treatments designed to reduce symptoms by targeting a mechanism. Moreover, the last of the three sections, on treatments lying on the high-risk end of the spectrum, delineates factors that justify such risk despite children’s vulnerable state. The review concludes by highlighting promising approaches for leveraging the lessons learned from these recent efforts. Such future efforts might seek mechanism-based treatments with potential for reducing the burden of childhood psychiatric disorders and their impact on development.
Cognitive training
Cognitive training programs as mechanism-based treatments for psychiatric illnesses
Cognitive training aims to reduce symptoms by targeting cognitive and neural mechanisms underlying mental illnesses. The intervention can be considered low risk, based on available evidence, coupled with the treatment’s non-invasive nature and similarity to activities such as videogames forming everyday components of children’s routines. The general strategy first identifies a specific cognitive alteration that correlates with symptoms of a mental illness. Next, investigators create training tasks that reverse or compensate for the alteration. Although regimens vary, training is generally performed for 15–30 minutes per day, many times per week for one to two months. When successful, the cognitive/emotional adaptations that manifest on multiple tasks administered under controlled circumstances (‘near transfer’) generalize to real-world interactions (‘far transfer’) [27], alleviating processes that generate symptoms.
In the domain of cognitive training, attention bias modification for the treatment of anxiety disorders represents one of the best studied therapies among children [28]. This cognitive training program is designed to reduce symptoms by targeting a patient’s tendency to attend to task-irrelevant threat stimuli. Based on cognitive theories of anxiety disorders developed by Beck and others [29–31], MacLeod et al. were among the first to empirically demonstrate that adults with high anxiety preferentially attend to mildly threatening as compared to neutral stimuli [32, 33]. While initial studies relied on words to convey threats, other paradigms soon adapted procedures to include mildly threatening pictures, in the form of angry faces, or other forms of mildly threatening stimuli. This work provided support for the hypothesis that ‘attention bias to threat’ contributes to the onset or maintenance of anxiety disorders [34].
The first experimental evidence to suggest a causal role for the biases in anxiety again appeared from MacLeod and colleagues [35]. This group showed that experimentally inducing the type of bias found in anxiety disorders increases anxiety among healthy individuals exposed to stressful experiences. This, in turn, led to other paradigms that attempted to reduce anxiety in affected people by repeatedly pairing threatening and neutral stimuli very briefly (500 ms) before then presenting a ‘probe’ at the location previously occupied by the neutral stimulus. The paradigms required the subjects to respond to an aspect of the probe (e.g., its location). Because the probe always appeared at the location of the neutral stimulus, over many hundreds of trials, attention was implicitly trained to the location of the neutral object. Indeed, while debate persists on the robustness of clinical effects, in both this initial report and in subsequent studies, attention bias modification (ABM) reduced bias to threat and symptoms in adults with anxiety disorders [28, 36–38]. Preliminary work indicates that ABM may reduce attention bias towards threat by affecting brain activity involved in control of attention [39–41] and in the amygdala [42]. Figure 1 illustrates how ABM reduces anxiety by reducing attention bias towards threat.
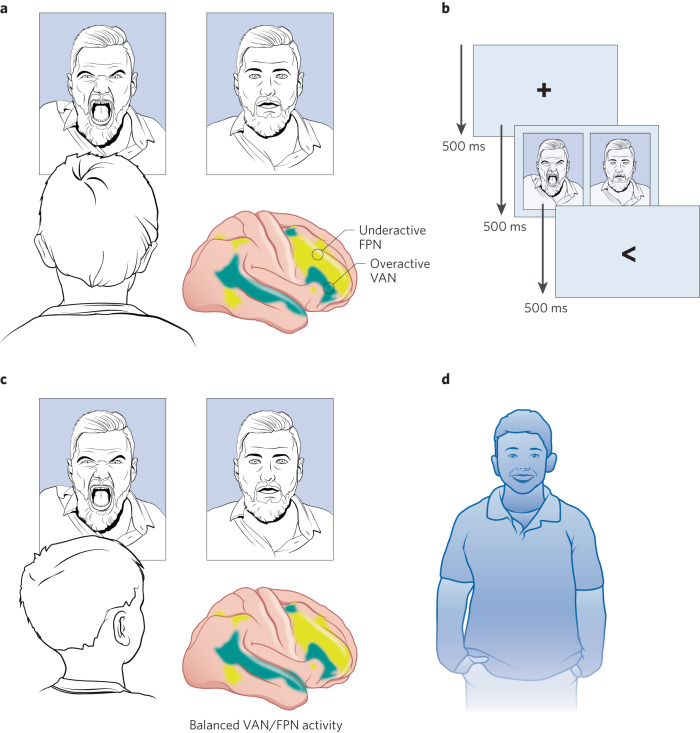
Fig. 1. Attention bias modification (ABM) as a mechanism-based treatment for anxiety disorders.
a Children with anxiety may attend towards threatening rather than neutral stimuli, reinforcing their anxiety. This attention bias towards threat may be associated with an imbalance in brain systems that orient attention, including increased influence by brain systems involved in the involuntary capture of attention by salient stimuli and decreased influence by brain systems involved in top-down, executive control. b ABM is a low-risk cognitive training program in which individuals with anxiety disorders are implicitly trained over 100 s of trials to attend to neutral rather than threatening stimuli. c ABM has been linked to decreases in attention bias to threat and may be associated with a rebalancing of brain systems that orient attention. d Following ABM, anxiety symptoms are reduced. Shaded yellow areas on the brain indicate the fronto-parietal network, FPN, involved in top-down executive control. Shaded teal areas indicate the ventral attention network, VAN, involved in the involuntary capture of attention by salient stimuli.
Cognitive training programs for children with psychiatric illnesses
Cognitive training programs are a good fit for children because they require minimal clinician involvement and are considered low risk (see limitations section below for specific risks). Training tasks can be highly engaging when made enjoyable or embedded in the contexts of a video game. A major benefit of these therapeutic tools is that they can be administered by staff with relatively minimal training and no clinician involvement. The programs can be performed as standalone treatments or as adjuvants to other treatments such as psychotherapy [43–46]. Because they require minimal expert involvement, cognitive training can be implemented as part of a stepped care program for children with mild to moderate symptoms prior to engagement with more time-intensive or higher risk treatments [47].
While many cognitive training programs have been devised and tested for childhood psychiatric illnesses, here we focus on attention-related cognitive training for anxiety disorders. We review research in this area because it leverages studies of normative development to inform the creation of novel, mechanism-based treatments. We point interested readers to other reviews that provide a broader coverage [48, 49].
Developmental trajectories of attention bias towards threat linked to pediatric anxiety
Attempts to reduce anxiety by altering attention or attention bias to threat benefit from knowledge on the normative and atypical developmental trajectories of these processes. Current models hypothesize relatively early maturation of bottom-up, involuntary, automatic attention processes and associated neural systems, in contrast with later maturing top-down, voluntary, sustained attention processes [50, 51]. Although still an active area of investigation, some evidence supports a ‘moderation model’, in which most infants and young children manifest an attention bias towards threat. As children age, the model suggests that the normative threat bias attenuates in children with low anxiety but is maintained in children with high anxiety. Other data supports an ‘acquisition’ model, in which children with anxiety disorders acquire an attention bias towards threat as they get older [52].
Meta-analyses and studies that pool across many hundreds of participants indicate that, as with adults, children with anxiety disorders have increased attention bias towards threat relative to children without anxiety disorders [34, 53, 54]. This relation between threat bias and anxiety appears evident for children over the age of 6, with some evidence indicating that this relation is unchanged with age [53] and other data suggesting that the difference in attention bias between anxious and non-anxious children increases with age [54, 55]. Threat bias associated with anxiety in children appears to follow a ‘vigilance-avoidance’ pattern, such that attention is rapidly oriented towards threatening stimuli when they first appear, followed by avoidance at longer intervals [56]. Notably, the time course of attention bias to threat may depend on the effector: support for the vigilance-avoidance hypothesis is largely derived from studies measuring reaction times of button presses; studies that use eye-tracking generally report reduced dwell time on threatening faces (avoidance) with less evidence for the early vigilance phase [57].
One hypothesis is that threat bias in anxiety is associated with increased functioning of bottom-up attention mechanisms coupled with poorer top-down voluntary attentional control [31, 58]. This hypothesis is supported by several findings. First, in terms of task performance chronometry, the timing of the early vigilance and later avoidance of attention bias towards threat mirrors the timing of the capture of salient stimuli in involuntary, automatic attention systems [59]. Second, in terms of children’s maturation, the developmental timing of the normative decrease in attention bias to threat may parallel the maturation of voluntary attention systems [50, 51], overriding the involuntary capture of attention by threatening stimuli. Third, inter-individual differences in attention bias to threat correlate with inter-individual differences in functioning of automatic attention capture by non-emotional salient stimuli [60]. Fourth, infants with relatively poor sustained attention at 9 months, expressed as distractibility by salient targets while watching Sesame Street, show relatively high temperamental risk for anxiety at 4 and 7 years [61]. Finally, children with anxiety disorders have increased involuntary capture of attention by generic, non-emotional stimuli [62], suggesting anxiety may be linked to a generalized imbalance in bottom-up and top-down attention mechanisms.
Brain circuits associated with altered attention in pediatric anxiety disorders
Explicating the neural circuitry associated with altered attention in pediatric anxiety disorders is an important goal. Such work informs creation and application of new treatments, based on their capacity to correct dysfunction in brain circuitry. Some evidence in children suggests that neural activity as measured with fMRI is more reliable than behavioral measures of attention to threat [63]. Thus, biological targets may have clinical utility even when behavioral measures are unreliable. In the future, precision medicine approaches may be able to measure target engagement (e.g., changes in functioning of neural circuitry as measured with fMRI) after a few sessions of ABM to determine whether the treatment is likely to be successful, prior to the patient investing more time and energy into completing the full 4-week protocol [64, 65].
Attention to threat increases activity in many brain regions among children with anxiety disorders [63, 66]. Many studies show greater activity in the right ventrolateral prefrontal cortex (VLPFC) in children with high anxiety relative to children with low anxiety [62, 67–71]. Such increased activity has been seen during passive viewing [67–69] as well as during the capture of attention by aversive images [69–71]. Additional work indicates that functional connections (measured using fMRI) [72–74] and structural connections (measured using diffusion tensor imaging) [75] between the VLPFC and the amygdala are disrupted in pediatric anxiety disorders.
The VLPFC is an anatomically defined brain region that includes many closely juxtaposed functional brain areas from various functional networks [76, 77]. Different portions of the VLPFC, therefore, are involved in different cognitive and emotional processes. Recent work by our group indicates that pediatric anxiety disorders are associated with increased activity in the portion of the VLPFC lying within the ventral attention network (VAN) [62]. In this work, elevated activity is elicited in VAN circuitry following the involuntary capture of attention by generic, non-emotional salient stimuli. Further work in the same dataset isolates the clinical importance of functional connectivity between the VAN and the cingulo-opercular network (CON). This pattern of connectivity relates to individual differences in both clinician-rated anxiety and involuntary attention capture by salient stimuli [78]. Together, this prior work is consistent with the model that children with anxiety disorders have increased involuntary capture of attention by all salient stimuli (threatening and non-threatening) that is linked to an overactive VAN [58]. Notably, however, other groups have posited that increased VLPFC activity associated with pediatric anxiety reflects an adaptive, compensatory process [69]; and so further work is needed in this area.
Studies of cognitive training based on modifying attention in pediatric anxiety disorders
Two recent meta-analysis find statistical evidence of benefits from attention bias modification for pediatric anxiety disorders [79, 80]. Notably, however, neither report includes as many as 15 studies, and both document small effect sizes. In addition, the smaller of the two meta-analyses included two studies without an active control [80]; the larger meta-analysis was only significant after one outlier study was removed [79]; and significant effects were noted only for clinician- but neither parent- nor child- reported anxiety. Thus, while attention bias modification may provide some benefit, further studies are needed to clarify the treatment’s robustness. Individual studies support the use of attention bias modification as a stand-alone treatment [81–83], as an adjuvant with psychotherapy [43–46], or as the first step in a stepped care program [47].
Consistent with a small overall effect size across studies, several clinical trials find that ABM fails to outperform active controls in reducing symptom of anxiety in pediatric samples [45, 84]. In these trials, equal reductions in anxiety are noted in the two arms. ABM and the active control arm generally involve equal amounts of cognitive training using the same basic task, with differences in the treatment arms relating to whether the target always appears at the location of the non-threatening stimulus (ABM) or could appear at the location of either the threatening or non-threatening stimulus (active control). In some cases, the active control outperforms ABM [84]. Note that while this control may not modify attention bias to threat, it includes an equal amount of time performing a task that requires attentional control and, in some versions, exposure to threatening stimuli. Therefore, the active control could generate clinical benefits over more simple control treatments.
One possibility is that ABM improves symptoms through several mechanisms including reducing attention bias to threat (hence outperforming active control arms in some studies) but also training aspects of attention independent of associations with threat (thus explaining the high response rates in active control arms). For example, in addition to reducing attention bias to threat, training could also decrease the functioning of automatic, bottom-up attention systems and increase in the functioning of top-down, voluntary control attention systems. Such an explanation would account for comparable clinical effects in many studies for active and control training. Moreover, this possibility receives support from data reviewed above linking pediatric anxiety disorders to imbalances between overactive involuntary automatic attention and underactive top-down voluntary attention. Consistent with this account, some studies find that improvements associated with training in children are linked to improvements in attentional control rather than changes in attention bias to threat [45, 84].
Interestingly, the specific mechanisms by which ABM works may vary as a function of age, such that reducing attention bias to threat may be more relevant at older ages, while non-threat-related changes in attention may be more relevant at younger ages. Consistent with this hypothesis, one study suggested that ABM was better than a control for older adolescents but not younger children [85]. Another study reported that learning was higher in adults for ABM versus control; but in children learning was the same across both versions [86].
Few studies have examined the impact of ABM on the functioning of attention-related neural circuitry in children. Liu et al. [82] reported that ABM in children was associated with activity changes in the left VLPFC and right amygdala, and White et al. [87] indicated that features of insula-amygdala functional connectivity predicted which children would respond to ABM. Additional work indicates that ABM decreases the error-related negativity (ERN) as measured by EEG in children [88, 89]. Finally, a visual search version of attention bias modification reduced neural activity as measured with fMRI to all emotional faces [90]; thus, ABM may work through a variety of cognitive and neural mechanisms.
Other cognitive training for pediatric psychiatric disorders
Many other cognitive training protocols have been devised for pediatric anxiety and depression that aim at other targets, including threat-cue safety discrimination, memory bias, bias in stimulus appraisal, or error monitoring [48, 56]. The evidence for these other treatments in decreasing symptoms of anxiety is less well established than ABM. Cognitive training regimens have also been devised for other childhood psychiatric disorders, though the evidence base for reducing symptoms is less well established compared to anxiety disorders. These other disorders include ADHD, autism, depression, and disruptive mood dysregulation disorder (DMDD) [91–93]. While these treatments aim to alter many targets, one of the most well studied pertains to training different executive functions in ADHD. One such treatment is FDA approved for targeting attention in children with ADHD [94]. However, this FDA approved therapy only outperformed a control treatment on a laboratory-based cognitive measure; it failed to reduce symptoms beyond the control treatment. This pattern is consistent with recent meta-analyses and reviews suggesting that cognitive training for ADHD improves laboratory-based measures of cognition but not symptoms or other behaviors outside of the lab [95–97].
Limitations of cognitive training
Given the small effect sizes generally associated with attention training, this treatment is likely to serve best for children with mild symptoms or as the first stage in a stepped-care program. Benefits should be weighed against the potential risk of investing substantial time that could be better devoted towards other treatments with more established efficacy, such as CBT. The main established risks associated with cognitive training reflect the adverse effects that follow from engagement in any repetitive cognitive task for 15–30 minutes, including frustration and headache [94]. There also exists the theoretical possibility for ABM to worsen symptoms. For example, training individuals to avoid threats could be harmful for people without an attention bias to threat prior to treatment. Similarly, repeated exposure to threatening stimuli also could be harmful. Concerns with the potential for worsening symptoms receive some limited, indirect support from findings among individuals exposed to trauma. In these populations, training individuals to attend towards as opposed to away from threat can be therapeutic [98, 99]. Thus, inducing a form of attention bias typically associated with clinical anxiety might be therapeutic in some contexts. Nevertheless, the bulk of findings from randomized controlled trials refute most concerns about such adverse effects. In general, clinical trials in hundreds of children provide no evidence that cognitive training leads to worsening among affected patients [79, 80, 100].
Psychotherapy
Psychotherapy aims to reduce symptoms by targeting cognitive, emotional, and interpersonal factors underlying mental illnesses. Many forms of psychotherapy originally developed and tested in adults have been adapted for adolescent and school age children with anxiety and depression. These interventions include adaptations of cognitive behavioral therapies (CBT) [101], interpersonal psychotherapies (ITP) [102], behavioral activation therapy (BA) and more transdiagnostic models such as the FIRST program [103]. While much empirical work supports the efficacy of these interventions, overall treatments for depression in childhood and adolescents have had only moderate effect sizes, leaving a substantial proportion of patients as non-responders. Some evidence suggests that effect sizes might be increased by tailoring psychotherapy to target age-specific mechanisms. One study, for example, suggested children ages 7–12 years with anxiety disorders undergoing CBT had improved outcomes with a greater number of sessions containing exposures to fear-inducing situations, while children ages 13–17 years did not show this dependence [104].
A newer wave of psychotherapies aims to have larger effect sizes by building on this theme of targeting age-specific mechanisms, delivered earlier in development. This effort seeks to identify affective disorders earlier in life in the preschool period to capture periods of greater developmental change, where treatments can leverage higher associated neuroplasticity. The following section describes preschool therapies following this path that have been empirically tested for affective disorders.
Targeting the caregiver child relationship
A rich and extensive developmental literature has documented the vital role that a nurturing, supportive, and reliable primary caregiver plays in the healthy social and emotional development of the young child [105, 106]. Many studies examine children living in contexts that limit the availability of such critical support in the first years of life, as can occur in institutional care, when children are neglected, or when caregivers face high adversity, substance abuse, or impairing mental disorders. In these contexts, risk for developmental delays and psychopathology is markedly increased. Thus, suboptimal caregiver support is a mechanism of increased risk for psychopathology in young children and thus can serve as a target for novel interventions. Based on this framework, strengthening the caregiver-child relationship, by teaching caregivers to be more sensitive, supportive, attuned to the child’s needs, and emotionally available in early childhood, is a critical prevention and early intervention strategy. Such a strategy has little risk and reaps abundant benefits for most young children as well as caregivers. In fact, treatments in early childhood that do not address the caregiver miss a very important opportunity to enhance change.
In infancy and early childhood, the child is inherently dependent upon the caregiver for survival needs and reciprocal, validating social interactions. In the area of emotional and social development, caregiver response to a child’s emotional expression, also known as ‘socialization of emotion’, plays a pivotal role in establishing the child’s emotion response style [107]. When caregivers signal that intense emotional expression is unacceptable or intolerable, children are taught to suppress emotional expression. When caregivers validate and help regulate intense emotions as they arise, children learn how to adaptively express and regulate emotion. This adaptive regulation reflects healthy development given the inevitability of intense emotions as a part of the human experience. Therefore, basic elements of parenting can be used as targets for reducing risk of psychopathology, including how parents respond to the child’s emotional expression and being emotionally available, nurturing, and sensitive to the needs of the child. Importantly, targeting these aspects of parenting behavior do not require deep psychological change on the part of the caregiver, but rather only changes in parents’ education and parenting style. Such targets are highly feasible and have been shown to be rapidly modifiable to change especially when addressed in a dyadic context (e.g. [108]).
Psychotherapy targeting the caregiver-child relationship benefits child affective disorders
Based on targeting parent-child interactions, one advantageous methodology used in the treatment of affective disorders in early childhood involves work with the child and caregiver “dyad” together. This method is developmentally appropriate and particularly effective in early childhood between the ages of 3–7 years when the caregiver plays this integral role in the daily functioning of the child and when the child can actively engage in treatment. Treatments using this method have targeted parenting behavior and aimed to strengthen the caregiver-child relationship. These approaches teach the caregiver to intervene more effectively with the child during this critical developmental phase with skills they may continue to utilize for many years. Such approaches have been used effectively to treat anxiety disorders and appear more effective than targeting the child alone [109]. Using this method in some parent-child interaction therapies, the parent becomes “the arm of the therapist” to effectively deliver a stronger and more enduring “dose” of guidance and corrective emotional experience. There is increasing evidence that such an approach achieves more enduring behavioral change when compared to models that directly work only with the parent or child alone [110, 111].
A central mechanism targeted by such dyadic therapies for affective disorders is the parental response to the child’s expression of intense emotion, especially negative emotions. In the domain of early childhood depression treatment, one novel parent-child therapy adapts the well validated Parent Child Interaction Therapy (PCIT): PCIT- Emotion Development (ED) [112]. This approach teaches parents to become more effective external emotion coaches and regulators for their child by helping them to tolerate and validate negative emotions and to encourage appropriate expression of a wide array of emotions. These elements are illustrated in Fig. 2. While the therapy has many components, one key element helps parents tolerate the child’s expression of emotions as opposed to invalidate the emotion (e.g., “there is nothing to cry about”). Parents are encouraged to validate those emotions and then engage with the child to help teach them to regulate emotions using strategies like cognitive control, reappraisal, and relaxation. In this way, PCIT-ED focuses on changing a parent’s socialization of the child’s emotion. Another unexpected benefit of this approach can be the improvement of a parent’s mood. This is an important benefit as parental depression increases risk of child depression, likely by numerous familial mechanisms including the suboptimal parenting practices noted above [112, 113].
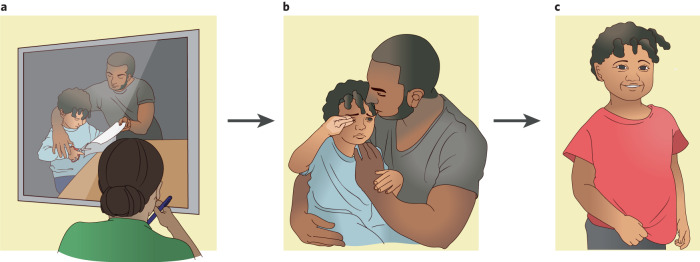
Fig. 2. Parent Child Interaction Therapy: Emotion Development (PCIT: ED) as a mechanism-based treatment for preschool onset depression.
Nurturing, empathic caregiving with high emotional competence is associated with decreasing depression in preschool children. PCIT:ED was developed to increase empathic and emotionally responsive caregiving and tested for its ability to reduce preschool depression. a In PCIT:ED, the parent is taught by the therapist how to play with the child like a play therapist: following the child’s lead, showing interest in the child, and being non-intrusive. b In a second phase, the therapist coaches the caregiver on emotion socialization, emphasizing validation and tolerance for intense emotions as well as teaching the parent to model adaptive emotion regulation. c Following PCIT:ED, empirical evidence indicates that the child develops enhanced emotional competence, and depressive symptoms are significantly reduced.
PCIT-ED was tested in a large-scale clinical trial showing large effect sizes in reducing symptoms of depression and yielding high rates of depression remission. Improvements in depression were maintained at 3 months post treatment [114]. In addition, caregivers had significant decreases in depression [112]. Follow-up work revealed the specific underlying mechanisms by which PCIT-ED may have led to clinical improvement. Consistent with a role for suboptimal caregiver-child relations, compared to the control arm, PCIT-ED improved both self-report and observed parenting practices [115]. More specifically, PCIT-ED decreased observed negative emotions and increased observed positive emotions in caregivers. In addition, parents reported using more positive parenting practices and fewer punitive practices following treatment. The child’s internal representation of parenting also changed, as evidenced by observational measures of narrative completion; children expected different parenting responses to conflictual circumstances and intense emotions [116]. Finally, PCIT-ED modulates targets other than caregiver-child interactions. Prior work had shown that reduced neural response to reward is a key component of early childhood depression [117]. Neural responses to reward, as measured by event-related potentials (ERPs) with EEG, were increased more in the group that got PCIT-ED compared to the control group [118].
Compared to traditional PCIT, PCIT-ED adds an additional emotional development (ED) module. This module targets the child’s ability to recognize emotions in themselves and others and teaches the parent to serve as an external emotion regulator and coach for the child. In this way, it seeks to improve childhood emotional development and well-being by teaching the parent to be directly and actively involved in this developmental process. An analysis showed that changes in parental response to the child’s expression of emotion occurred in the ED module specifically. This contrasts with positive changes in the parent-child relationship more generally, which also occurred in the Child Directed Interaction (CDI) module, a module designed to help the parent validate and accept the child’s interests [113]. These findings suggest that the novel ED module directly targets parental socialization of emotion and begins to connect specific therapeutic elements to different mechanisms underlying childhood depression.
Sensitive periods as windows of opportunity for treatment
Data on neuroplasticity further justify the targeting of child-caregiver dyads between the ages of three and seven years. Different brain areas express different ‘sensitive periods’, or developmental epochs in which their growth and lifelong function is especially sensitive to external influences, such as interactions with caregivers [119–121]. Thus, caregiver-child psychotherapies may have highest impact specifically during these sensitive periods. Consistent with this model, evidence is emerging in several domains for more powerful effects of psychosocial interventions on behavior in early childhood compared to older ages as cited above (e.g., autism, disruptive behavioral disorders, depressive disorders) [111, 122]. Whether this is a true sensitive period effect, an effect of engaging the caregiver as a central target of treatment or both, remains unclear. However, these findings taken together suggest that the earliest identification of affective disorders and risk for these disorders is critical to capture a closing window of opportunity for greater treatment efficacy.
Limitations of psychotherapy
Potential limitations for psychotherapies such as PCIT-ED are the investment in time and energy required of families, the need for an engaged child and parent, and the availability of trained therapists. Currently, there is a major shortage of child mental health providers, and access to care is difficult even for those families with sufficient resources. Based on this shortage, there are a number of efforts to deliver treatment outside of mental health clinics, in primary care or school settings where the vast majority of children receive services. Several of the caregiver-child therapies that have been developed have more abbreviated forms that can be delivered by video conference in a limited number of sessions. Further, children in need of these services may be easily screened and accessed in primary care and school settings prior to the onset of serious clinical psychopathology, when interventions are more difficult. These brief manualized therapies, many of which specifically target certain areas of function such as disruptive behavior (e.g. Incredible Years [123], PCIT [124]), emotion development (PCIT-ED) [112], or anxiety (Taming Sneaky Fears) [125], can be learned by master’s level clinicians which make them more cost effective. There is a new movement to make these approaches even more feasible and to increase the mental health workforce to access more children in need, by training bachelor’s level personnel to deliver these interventions. It should also be noted that PCIT-ED for depressed preschoolers has yet to be compared to an active treatment condition. This is an important next step before the efficacy of the intervention for more widespread use can be determined.
Transcranial magnetic stimulation
The current section elucidates key factors shaping research on novel therapies that have the potential for inducing more serious adverse effects than induced by cognitive training or psychotherapy. The section begins by describing considerations broadly influencing research with many pediatric mental illnesses while highlighting work specifically on treatment refractory adolescent major depressive disorder (MDD). The section then describes studies examining the treatment of MDD in adults using transcranial magnetic stimulation (TMS). Finally, the section summarizes research on TMS in youth, including a randomized controlled trial (RCT) which yielded disappointing results. This final summary highlights novel approaches for future RCTs of TMS that might yield more encouraging results.
Ethical considerations of research in youth
Studying treatments with possible adverse effects in children requires complex considerations. On the one hand, researchers have an ethical obligation to extend the benefits of research from adults to children, to ensure that well studied treatments are available for debilitating illnesses affecting youth. On the other hand, children represent a vulnerable population, being unable to provide informed consent and thus having to rely on adults to weigh the risks and benefits of participating in research. Guidelines and expert opinions are widely available that balance these and other competing influences on regulations for conducting research in children and adolescents [21–26]. These guidelines generally agree that studying the impact of new treatments in children and adolescents requires a consideration of an underlying condition’s severity and thus the potential benefit of having that condition improved. In this context, higher risk associated with a novel treatment is viewed as more acceptable in children and adolescents with relatively severe conditions. Moreover, as part of balancing risks and benefits for individual children and adolescents, guidelines recommend weighing the potential to generate new information that may be useful for society at large [23], the likelihood of benefit of the studied treatment, and the known or potential risks. Thus, studying a novel treatment for a given condition requires careful consideration of complex ethical and scientific issues. Severe neurodevelopmental disorders and treatment refractory MDD provide two examples of relatively severe disorders where treatments with greater risk might be considered. These examples illustrate the range of factors that can justify research with novel therapies.
Severe neurodevelopmental disorders arise early in life, profoundly impact development, and can unfold with a progressive course. These severe neurodevelopmental disorders include some subtypes of autism spectrum disorder (ASD) that can arise from specific genetic anomalies [126]. With advances in genetics research, novel treatments may be discovered based on the treatments’ capacity to alter genomic function. While such treatments may have the potential for serious adverse effects, studying the impact of these treatments on children with severe forms of ASD may be justified given the severity of the underlying condition (and thus the potential for large benefit), the potential for the knowledge gained to eventually benefit other children with different genetic syndromes, and when weighed against known potential risks.
Research into treatments for pediatric MDD similarly requires a consideration of the potential benefits and risks. MDD represents one of the most impairing medical conditions in the world, based on the high cost to individuals in terms of Disability Adjusted Life Years (DALYs) [127]. This impact relates to high prevalence, early onset, and chronic nature of the condition. Somewhere between 10 and 20% of young people will manifest an episode of MDD prior to age 25, and most episodes of MDD in adulthood are preceded by episodes of MDD during adolescence [128–131]. As a result, it is vital to effectively treat MDD in adolescence, so that a cycle of recurrent episodes might be interrupted before the condition becomes chronic [129, 132]. Given these longitudinal data, treatments for adolescents can be discovered by adapting treatments efficacious in adults.
Such downward extensions have led to some effective treatments for depression in adolescents, yet many youth remain symptomatic even after receiving the best available current treatments [132]. Moreover, most downward extensions simply transfer the treatment used in adults to children and adolescents. Research is needed that adapts treatments efficacious in adults to unique features of MDD in adolescents. Finally, compared to children with syndromic ASD, outcomes in adolescents with MDD are better, where a significant minority respond to early treatment and exhibit lasting remissions. Hence, justification is weaker for studying novel treatments with serious adverse effects in this group.
The study of novel treatments might be justified for adolescents who fail to respond to the two available first-line treatments. One effective treatment is serotonin reuptake inhibitor (SSRI) medications, where both fluoxetine (approved for children 8 and older) and escitalopram (approved for children 12 and older) carry Food and Drug Administration (FDA) indications for childhood MDD (escitalopram and duloxetine are FDA approved for generalized anxiety disorder in children). Nevertheless, Cohen’s d effect sizes for SSRIs compared to placebo in the treatment of childhood MDD are not large, falling generally in the 0.3–0.5 range [16, 17]. Psychological therapy is the other first-line treatment for childhood MDD; among these, the best-established treatment applies principles of cognitive behavioral therapy (CBT). However, effect sizes for the comparison of CBT versus control psychotherapy in treating childhood MDD are generally smaller than for medication comparisons with placebo, falling in the 0.2–0.4 range [133–136]. Moreover, in the largest head-to-head trial to compare placebo, medication, and CBT for adolescent MDD, medication but not CBT was superior to placebo [137]. Findings of modest efficacy for first line treatments are supported by recent meta-analyses [16, 17, 134, 138]. Clearly, there is a major need for new treatments [132, 139].
Because neither first-line treatment is highly effective for all youth, testing novel therapies with potential severe adverse effects could be justified for the many patients who fail to respond to these treatments. Glutamate antagonists (ketamine, esketamine) and psilocybin, for example, show promise in treating adult depression [140]. Limited evidence also supports the use of ketamine in adolescents with depression [141], while studies relating recreational use of psilocybin to mental health outcomes in youth have yielded mixed results [142, 143]. Thus, further work may be justified to further test the efficacy and safety of these treatments in youth. In addition to this approach that tests whether medications that show efficacy in adults also are useful in youth, it may be justified to test novel treatments that are tailored to the developmental stage of adolescent patients and their potentially unique underlying problems in MDD-linked physiology. TMS, for example, provides an avenue for a novel treatment that can be targeted to specific brain circuit-level anomalies identified in youth with depression.
Transcranial magnetic stimulation (TMS) in adults
In adults, forms of TMS designed to increase activity in the left dorsolateral prefrontal cortex (dlPFC) represent accepted treatments for MDD [144]. Targeting reduced functioning of the left dlPFC extends a wealth of data in both adolescents and adults. Initial findings involved lesion studies in adults, which suggested that damage to the left dlPFC increased risk for MDD. This was followed by neuropsychological, electrophysiological, and brain imaging studies in adults providing additional evidence of reduced left hemisphere function in MDD [145, 146]. Finally, attempts to extend such work to adolescents also found links between symptoms of MDD and lateralized alterations in brain function [147, 148].
Most findings in adults and adolescents connect reduced left hemisphere functioning with an increased risk for depression. However, some evidence further implicates imbalanced functioning between the right and left hemisphere, which can involve increased engagement of right-hemisphere processes in tandem with reduced left-hemisphere functioning [147, 148]. Thus, while many TMS studies attempt to increase functioning of the left dlPFC, others attempt to reduce functioning of the right dlPFC. Finally, MDD relates to asymmetric function in posterior brain areas. While such findings justify clinical trials of TMS applied to extra-frontal regions, most current research targets the frontal cortex. This reflects the success of initial TMS trials, coupled with extensive evidence of frontal dysfunction in MDD at all ages.
In the treatment of adult MDD, one conventional form and a second alternative form of TMS have been cleared by the Food and Drug Administration (FDA), both of which target the left dlPFC. Published meta-analyses suggest that the conventional form of TMS, which applies 10-Hz stimulation, is an effective treatment for MDD in adults [149–151]. Typically, this form of TMS is used to increase activity in the left dlPFC, though other successful studies apply TMS to the right frontal area in an attempt to reduce activity in the right dlPFC. A second, alternative form of TMS uses a different mode of stimulation—intermittent theta burst stimulation (iTBS), which is thought to increase brain function over the region that is stimulated. Recent reviews from the first seven clinical trials suggest that iTBS is also effective for the treatment of MDD in adults [152–154].
While many novel treatments in adults inform research with adolescents, TMS provides a unique, mechanism-based path for novel therapeutic research. This path is considered mechanism-based because recent studies in adults attempt to correct underlying pathophysiology by stimulating brain regions localized with resting state functional magnetic resonance imaging (rsfMRI). Figure 3 illustrates the path for using rsfMRI to extend TMS in both adolescents and adults. Prior work supports the hypothesis that TMS of the left dlPFC in adults with depression works, in part, by indirectly altering activity in the subgenual anterior cingulate cortex (sgACC) [155]. This hypothesis is supported by work identifying alterations of the sgACC in the individuals with MDD [156], indicating that correcting these alterations is an avenue for novel treatment development. While the sgACC is a midline brain structure not readily accessible by TMS, it is functionally connected to portions of the dlPFC, i.e., the sgACC and parts of the dlPFC show correlated fMRI activity at rest [157]. Because TMS can indirectly alter brain activity in regions functionally connected to the stimulation site [158], the sgACC may be indirectly targeted by stimulating the dlPFC. By conducting systematic research on dlPFC-sgACC circuitry in adolescents and adults, future work could seek tailor findings generated in adults to adolescents with treatment refractory MDD.
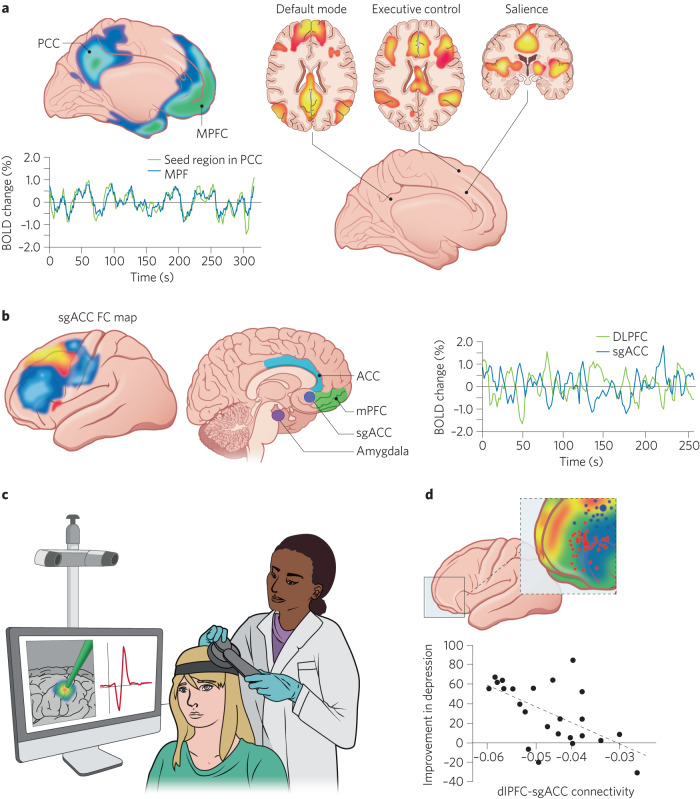
Fig. 3. Key findings in research on the interface among resting state functional magnetic resonance imaging (rsfMRI), major depressive disorder (MDD), and transcranial magnetic stimulation (TMS).
a Functional brain networks demonstrate correlated blood-oxygen level-dependent (BOLD) activity over time, measured using resting rsfMRI. The default mode (DMN), salience (SN), and executive control networks (ECN) have all been implicated in the pathophysiology of anxiety and depressive disorders. b The left panel illustrates positive (orange) and negative (blue) correlations in BOLD activity of the dorsolateral prefrontal cortex (DLPFC) with the subgenual anterior cingulate cortex (sgACC), which is depicted in the middle panel. The right panel shows BOLD timeseries from the sgACC and portions of the DLPFC illustrated in blue, showing the negative correlation between these timeseries. The blue portions of the DLPFC largely reside within the ECN, whereas the sgACC largely resides within the DMN; activity from these two networks is known to be negatively correlated at rest, potentially representing top-down control of the DLPFC over the sgACC. c Machinery that allows a clinician to place a TMS coil over a region of the scalp related to the DLPFC. d Some evidence suggests that TMS is most effective for depression when targeted at the portion of the DLPFC that is most negatively correlated with the sgACC, potentially because this strengthens the top-down control of the ECN over the DMN. Panel d illustrates data based on adults that could be studied for extension to adolescents. The top panel colors the lateral cortex based on correlations in BOLD activity with the sgACC and the overlaid dots indicate TMS targets that were successful (red) or unsuccessful (blue). Note that successful applications target the portion of the portion of the DLPFC that is negatively correlated with the sgACC. The bottom panel is a plot that shows theoretical findings that might be expected across adolescents if they replicate findings from adults. In these theoretical findings, TMS targets at parts of the DLFPC with the most negative connectivity with the sgACC (x-axis) would be expected to result in greater levels of improvement in symptoms of MDD following TMS (y-axis).
Transcranial magnetic stimulation (TMS) in adolescents
Compared to adults, far fewer studies of TMS examine adolescents with MDD. Initial work provided some evidence of TMS being helpful [159], but only one reasonably powered RCT compared active and control TMS in adolescent MDD. In this two-group, sham-controlled trial, Croarkin and colleagues studied 103 patients with treatment-resistant depression and found similar response rates, approximately 40%, in both groups [160].
Findings from Croarkin and colleagues suggest two avenues for identifying subgroups of adolescents who might be most likely to respond to TMS. One relevant feature of the study concerned the definition of treatment refractoriness, where patients only had to fail one trial of an antidepressant medication. This relatively low threshold for defining treatment resistance could explain the relatively high response rate to both active and sham controlled TMS. Consistent with this possibility, additional analyses stratified patients based on refractoriness. Results suggested that stronger differential effects of the active relative to sham treatment manifested among the more severe patients. Future RCTs might identify patients with more treatment refractory forms of MDD, based on insufficient response to multiple treatments. Not only might this select samples uniquely responsive to active TMS, but this could also strengthen the ethical justification for delivering a treatment with potentially serious adverse effects.
The other relevant feature of the study from Croarkin and colleagues concerned the method used to target the dlPFC, which did not utilize imaging to consider individualized aspects of brain circuitry. Individuals vary in the exact dlPFC location that is functionally connected to the sgACC [161, 162]; thus, tailoring stimulation sites to an individual’s functional neuroanatomy may yield better outcomes than a one-size-fits-all approach. Following methods developed in adults, studies in adolescents could target sections of the dlPFC where particularly robust negative rsfMRI correlations manifest with the sgACC. Data in adults suggest that TMS is most successful in the treatment of MDD when it targets such dlPFC sections [155]. Additional research could usefully guide these attempts to tailor TMS to MDD in adolescents by studying healthy adolescents and adolescents with varying levels of MDD. Such work could attempt to relate levels of rsfMRI connectivity among the dlPFC, sgACC, and other regions not implicated in adult MDD to levels of MDD symptoms in adolescents. Not only could such research inform novel therapeutics, but it also could provide a path for integrating studies of treatment, pathophysiology, and neurodevelopment.
Limitations of TMS
The main limitation of TMS in youth at this time is that there are no large studies clearly demonstrating efficacy over sham, and more research is needed to determine whether TMS is effective in youth depression. Side effects that are more common in youth with active versus sham TMS include headache, eye pain, nausea, and facial twitching [160]. While the absolute risk is low, there is also the potential for seizures given TMS directly induces brain activity. An additional limitation in both adults and adolescents is that the optimal brain stimulation site for treating depression remains an active area of investigation. The most consistent fMRI findings in TMS research link treatment response to pre-treatment levels of dlPFC-sgACC circuitry function. Adolescent depression has been linked to alterations in many brain regions beyond the dlPFC and sgACC [163–165], however, and it is not known whether stimulating these other brain regions is effective in treating youth depression. Moreover, the relation between dlPFC-sgACC functional connectivity and depression is not well established, further calling into question whether this is the optimal target in youth [166, 167]. Future work is needed to clarify the role of dlPFC-sgACC connectivity in youth, symptoms of depression, and the optimal brain stimulation site for adolescent MDD.
Future research directions
This article reviews new and emerging treatments for pediatric psychiatric disorders designed to target underlying mechanisms contributing to the disorders. We focused on specific examples of cognitive training, psychotherapy, and TMS for treatment of childhood anxiety and depression to exemplify the range of techniques available for targeting illness mechanisms. Although beyond the scope of the current review, we note that there are many other mechanism-based treatments emerging for youth anxiety and depression, such as glutamate antagonists [141]. Considering this range also elucidates the complex ethical hurdles confronting studies in children [21–26]. This requires a tighter matching than in adults between levels of risk for a treatment and clinical impact of a disorder. To strengthen this bench-to-bedside approach, more work is needed into underlying environmental, social, economic, behavioral, emotional, and brain-based mechanisms of childhood psychiatric disorders. Given well established concerns with replicability and reproducibility [168], it is especially important to pre-register specific hypotheses and measures that might serve as markers or treatment targets prior to study initiation, to increase likelihood that putative mechanisms will extend to new groups such as clinical samples. In parallel, more work is needed on ascertaining the reliability of behavioral, emotional, and brain-based measures that might serve as treatment targets [64, 65, 169]. While low to moderate reliability of measures may be reasonable in an exploratory study with a large sample size, high reliability is required for measures to have clinical utility.
To further devise appropriately targeted treatments, we also need to better incorporate developmental models of underlying mechanisms, both in typical and atypical development. In the section on cognitive training above, we highlight how attention and attention bias to threat emerge through a complex developmental cascade, and deviations from normal need to be characterized within the context of this normative development. These developmental models highlight how deviations in very early developing sensory, cognitive, and emotional brain systems can have cascading, non-linear impacts on neurodevelopment and subsequently observable behavior and symptoms. Additionally, many brain circuits have high plasticity during specific developmental epochs, such that the development of the system is highly influenced by natural or experimental manipulations (e.g., sounds specific to native vs foreign languages; the presence or absence of expected caregiver interactions) [119–121]. Influences during these periods of high plasticity (sensitive periods) can have enduring, even lifelong impact on the functioning of the brain system. An important goal of future work is to characterize when periods of high plasticity occur for specific brain systems, as interventions that are directed at specific brain systems (and their cognitive/social/emotional correlates) during these epochs may have much higher impact than targeting treatments at other times. Moreover, intervening early in life may be the only way to prevent an altered neurodevelopmental cascade associated with later risk [12].
We note that not all approaches to devising novel treatments for pediatric psychiatric disorders necessarily require engaging a specific, pre-defined target; though we have focused on such targeted treatments in this review. Other approaches that may have high impact, especially early in development, are general treatments that promote overall brain health, growth, and plasticity. Such approaches may include interventions that focus on diet or sleep, or medicines that have a brain-wide effect of reopening windows of plasticity. Another approach that may ultimately have high utility is using machine learning or artificial intelligence to select among potential treatment options but analyzing very high dimensional brain imaging data [170]. Such an approach could be valuable and inform precision medicine even if it is not known ‘why’ the algorithm selects a particular treatment for a particular patient.
The past decade has seen a flurry of new mechanism-based treatments for pediatric anxiety disorders. Given the morbidity of child psychiatric disorders, and that treating mental illnesses in childhood may prevent additional illnesses from emerging later in life, these new treatments are highly needed. Biological studies of psychiatric disorders are leading to increased awareness that the cascading neurodevelopment associated with many illnesses starts early in life, and thus interventions early in development, when brain circuitry is highly plastic, may ultimately have the highest impact. This review highlights how researchers are targeting known mechanisms of childhood psychiatric disorders to devise new treatments.
Author contributions
All three authors contributed to the conception and interpretations offered in this review. The authors worked together to draft the work, make revisions, and approval the final version. All authors agree that they are accountable for all aspects of the work.
Funding
Funding is through the National Institute of Mental Health (R01MH122389 and R01MH131584 to CMS; R01MH098454 to JLL) and the Taylor Family Foundation (to CMS); NIMH Intramural Research Program ZIA-MH002781.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Merikangas KR, He J-P, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–9. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langley AK, Bergman RL, McCracken J, Piacentini JC. Impairment in childhood anxiety disorders: preliminary examination of the child anxiety impact scale-parent version. J Child Adolesc Psychopharmacol. 2004;14:105–14. doi: 10.1089/104454604773840544. [DOI] [PubMed] [Google Scholar]
- 4.La Greca AM, Lopez N. Social anxiety among adolescents: linkages with peer relations and friendships. J Abnorm Child Psychol. 1998;26:83–94. doi: 10.1023/A:1022684520514. [DOI] [PubMed] [Google Scholar]
- 5.Franz L, Angold A, Copeland W, Costello EJ, Towe-Goodman N, Egger H. Preschool anxiety disorders in pediatric primary care: prevalence and comorbidity. J Am Acad Child Adolesc Psychiatry. 2013;52:1294–303.e1. doi: 10.1016/j.jaac.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piacentini J, Bennett S, Compton SN, Kendall PC, Birmaher B, Albano AM, et al. 24- and 36-Week Outcomes for the Child/Adolescent Anxiety Multimodal Study (CAMS) J Am Acad Child Adolesc Psychiatry. 2014;53:297–310. doi: 10.1016/j.jaac.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kessler RC, Avenevoli S, Costello EJ, Georgiades K, Green JG, Gruber MJ, et al. Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry. 2012;69:372–80. doi: 10.1001/archgenpsychiatry.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kessler RC, Ormel J, Petukhova M, McLaughlin KA, Green JG, Russo LJ, et al. Development of lifetime comorbidity in the World Health Organization world mental health surveys. Arch Gen Psychiatry. 2011;68:90–100. doi: 10.1001/archgenpsychiatry.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sylvester CM, Smyser CD, Smyser T, Kenley J, Ackerman JJ, Jr., Shimony JS, et al. Cortical Functional Connectivity Evident After Birth and Behavioral Inhibition at Age 2. Am J Psychiatry. 2018;175:180–87.. doi: 10.1176/appi.ajp.2017.17010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sylvester CM, Myers MJ, Perino MT, Kaplan S, Kenley JK, Smyser TA, et al. Neonatal Brain Response to Deviant Auditory Stimuli and Relation to Maternal Trait Anxiety. Am J Psychiatry. 2021. 10.1176/appi.ajp.2020.20050672. [DOI] [PMC free article] [PubMed]
- 11.Thomason ME. Development of Brain Networks In Utero: Relevance for Common Neural Disorders. Biol Psychiatry. 2020;88:40–50. doi: 10.1016/j.biopsych.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry Allied Discip. 2007;48:631–48. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- 13.Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biological Psychiatry. 2014;76:350–3. [DOI] [PubMed]
- 14.Walter HJ, Bukstein OG, Abright AR, Keable H, Ramtekkar U, Ripperger-Suhler J, et al. Clinical Practice Guideline for the Assessment and Treatment of Children and Adolescents With Anxiety Disorders. J Am Acad Child Adolesc Psychiatry. 2020;59:1107–24.. doi: 10.1016/j.jaac.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Walter HJ, Abright AR, Bukstein OG, Diamond J, Keable H, Ripperger-Suhler J, et al. Clinical Practice Guideline for the Assessment and Treatment of Children and Adolescents With Major and Persistent Depressive Disorders. J Am Acad Child Adolesc Psychiatry. 2023;62:479–502. doi: 10.1016/j.jaac.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Locher C, Koechlin H, Zion SR, Werner C, Pine DS, Kirsch I, et al. Efficacy and Safety of Selective Serotonin Reuptake Inhibitors, Serotonin-Norepinephrine Reuptake Inhibitors, and Placebo for Common Psychiatric Disorders Among Children and Adolescents: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2017;74:1011–20. doi: 10.1001/jamapsychiatry.2017.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cipriani A, Zhou X, Del Giovane C, Hetrick SE, Qin B, Whittington C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388:881–90. doi: 10.1016/S0140-6736(16)30385-3. [DOI] [PubMed] [Google Scholar]
- 18.Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N. Engl J Med. 2008;359:2753–66. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emslie GJ, Kennard BD, Mayes TL, Nightingale-Teresi J, Carmody T, Hughes CW, et al. Fluoxetine versus placebo in preventing relapse of major depression in children and adolescents. Am J Psychiatry. 2008;165:459–67. doi: 10.1176/appi.ajp.2007.07091453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vostanis P, Feehan C, Grattan E, Bickerton WL. A randomised controlled out-patient trial of cognitive-behavioural treatment for children and adolescents with depression: 9-month follow-up. J Affect Disord. 1996;40:105–16. doi: 10.1016/0165-0327(96)00054-7. [DOI] [PubMed] [Google Scholar]
- 21.Wendler D, Belsky L, Thompson KM, Emanuel EJ. Quantifying the federal minimal risk standard: implications for pediatric research without a prospect of direct benefit. JAMA. 2005;294:826–32. doi: 10.1001/jama.294.7.826. [DOI] [PubMed] [Google Scholar]
- 22.Rid A, Abdoler E, Roberson-Nay R, Pine DS, Wendler D. Evaluating the risks of clinical research: direct comparative analysis. J Child Adolesc Psychopharmacol. 2014;24:390–8. doi: 10.1089/cap.2014.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schupmann W, Li X, Wendler D. Acceptable Risks in Pediatric Research: Views of the US Public. Pediatrics. 2022;149:e2021052687. [DOI] [PMC free article] [PubMed]
- 24.Wherrett DK, Chiang JL, Delamater AM, DiMeglio LA, Gitelman SE, Gottlieb PA, et al. Defining pathways for development of disease-modifying therapies in children with type 1 diabetes: a consensus report. Diabetes Care. 2015;38:1975–85. doi: 10.2337/dc15-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lepage S, Conway A, Goodson N, Wicks P, Flight L, Devane D. Online randomised trials with children: A scoping review. PLoS One. 2023;18:e0280965. doi: 10.1371/journal.pone.0280965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandina G, Busner J, Horrigan JP, McSherry C, Bateman-House A, Pani L, et al. Implications of Pediatric Initiatives on CNS Drug Development for All Ages-2020 and Beyond: Second of Three Sets of Expanded Proceedings from the 2020 ISCTM Autumn Conference on Pediatric Drug Development. Innov Clin Neurosci. 2023;20:18–24. [PMC free article] [PubMed] [Google Scholar]
- 27.Schmiedek F, Lövdén M, Lindenberger U. Hundred Days of Cognitive Training Enhance Broad Cognitive Abilities in Adulthood: Findings from the COGITO Study. Front Aging Neurosci. 2010;2:27. [DOI] [PMC free article] [PubMed]
- 28.Bar-Haim Y. Research review: Attention bias modification (ABM): a novel treatment for anxiety disorders. J Child Psychol Psychiatry, Allied Discip. 2010;51:859–70. doi: 10.1111/j.1469-7610.2010.02251.x. [DOI] [PubMed] [Google Scholar]
- 29.Barlow DH. Anxiety and its disorders: The nature and treatment of anxiety and panic. Second ed. New York, NY: Guilford Press; 2002.
- 30.Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry. 2008;165:969–77. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- 31.Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7:336–53. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 32.MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. J Abnorm Psychol. 1986;95:15–20. doi: 10.1037/0021-843X.95.1.15. [DOI] [PubMed] [Google Scholar]
- 33.Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol. 2005;1:167–95. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- 34.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 35.MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. J Abnorm Psychol. 2002;111:107–23. doi: 10.1037/0021-843X.111.1.107. [DOI] [PubMed] [Google Scholar]
- 36.Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, et al. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biol Psychiatry. 2010;68:982–90. [DOI] [PMC free article] [PubMed]
- 37.Lowther H, Newman E. Attention bias modification (ABM) as a treatment for child and adolescent anxiety: a systematic review. J Affect Disord. 2014;168:125–35. doi: 10.1016/j.jad.2014.06.051. [DOI] [PubMed] [Google Scholar]
- 38.Mogg K, Waters AM, Bradley BP. Attention Bias Modification (ABM): Review of Effects of Multisession ABM Training on Anxiety and Threat-Related Attention in High-Anxious Individuals. Clin Psychol Sci. 2017;5:698–717. doi: 10.1177/2167702617696359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Browning M, Holmes EA, Murphy SE, Goodwin GM, Harmer CJ. Lateral Prefrontal Cortex Mediates the Cognitive Modification of Attentional Bias. Biol Psychiatry. 2010;67:919–25. doi: 10.1016/j.biopsych.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke PJF, Browning M, Hammond G, Notebaert L, MacLeod C. The causal role of the dorsolateral prefrontal cortex in the modification of attentional bias: evidence from transcranial direct current stimulation. Biol Psychiatry. 2014;76:946–52. doi: 10.1016/j.biopsych.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Hakamata Y, Mizukami S, Komi S, Sato E, Moriguchi Y, Motomura Y, et al. Attentional bias modification alters intrinsic functional network of attentional control: A randomized controlled trial. J Affect Disord. 2018;238:472–81. doi: 10.1016/j.jad.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 42.Britton JC, Suway JG, Clementi MA, Fox NA, Pine DS, Bar-Haim Y. Neural changes with attention bias modification for anxiety: a randomized trial. Soc Cogn Affect Neurosci. 2015;10:913–20. doi: 10.1093/scan/nsu141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shechner T, Rimon-Chakir A, Britton JC, Lotan D, Apter A, Bliese PD, et al. Attention bias modification treatment augmenting effects on cognitive behavioral therapy in children with anxiety: randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2014;53:61–71. doi: 10.1016/j.jaac.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Britton JC, Bar-Haim Y, Clementi MA, Sankin LS, Chen G, Shechner T, et al. Training-associated changes and stability of attention bias in youth: Implications for Attention Bias Modification Treatment for pediatric anxiety. Dev Cogn Neurosci. 2013;4:52–64. doi: 10.1016/j.dcn.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pettit JW, Bechor M, Rey Y, Vasey MW, Abend R, Pine DS, et al. A Randomized Controlled Trial of Attention Bias Modification Treatment in Youth With Treatment-Resistant Anxiety Disorders. J Am Acad Child Adolesc Psychiatry. 2020;59:157–65. doi: 10.1016/j.jaac.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bechor M, Pettit JW, Silverman WK, Bar-Haim Y, Abend R, Pine DS, et al. Attention Bias Modification Treatment for children with anxiety disorders who do not respond to cognitive behavioral therapy: a case series. J Anxiety Disord. 2014;28:154–9. doi: 10.1016/j.janxdis.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pettit JW, Rey Y, Bechor M, Melendez R, Vaclavik D, Buitron V, et al. Can less be more? Open trial of a stepped care approach for child and adolescent anxiety disorders. J Anxiety Disord. 2017;51:7–13. doi: 10.1016/j.janxdis.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lazarov A, Bar-Haim Y. Emerging Domain-Based Treatments for Pediatric Anxiety Disorders. Biol Psychiatry. 2021;89:716–25. [DOI] [PubMed]
- 49.Edwards EJ, Zec D, Campbell M, Hoorelbeke K, Koster EHW, Derakshan N, et al. Cognitive control training for children with anxiety and depression: A systematic review. J Affect Disord. 2022;300:158–71. doi: 10.1016/j.jad.2021.12.108. [DOI] [PubMed] [Google Scholar]
- 50.Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, et al. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–40. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 51.Posner MI, Rothbart MK, Sheese BE, Voelker P. Developing Attention: Behavioral and Brain Mechanisms. Adv Neurosci. 2014;2014:405094. doi: 10.1155/2014/405094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Field AP, Lester KJ. Is there room for ‘development’ in developmental models of information processing biases to threat in children and adolescents? Clin Child Fam Psychol Rev. 2010;13:315–32. [DOI] [PubMed]
- 53.Abend R, de Voogd L, Salemink E, Wiers RW, Perez-Edgar K, Fitzgerald A, et al. Association between attention bias to threat and anxiety symptoms in children and adolescents. Depress Anxiety. 2018;35:229–38. doi: 10.1002/da.22706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dudeney J, Sharpe L, Hunt C. Attentional bias towards threatening stimuli in children with anxiety: A meta-analysis. Clin Psychol Rev. 2015;40:66–75. doi: 10.1016/j.cpr.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 55.Jenness JL, Lambert HK, Bitran D, Blossom JB, Nook EC, Sasse SF, et al. Developmental Variation in the Associations of Attention Bias to Emotion with Internalizing and Externalizing Psychopathology. Res Child Adolesc Psychopathol. 2021;49:711–26. doi: 10.1007/s10802-020-00751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lau JYF, Waters AM. Annual Research Review: An expanded account of information-processing mechanisms in risk for child and adolescent anxiety and depression. J child Psychol psychiatry, allied Discip. 2017;58:387–407. doi: 10.1111/jcpp.12653. [DOI] [PubMed] [Google Scholar]
- 57.Lisk S, Vaswani A, Linetzky M, Bar-Haim Y, Lau JYF. Systematic Review and Meta-Analysis: Eye-Tracking of Attention to Threat in Child and Adolescent Anxiety. J Am Acad Child Adolesc Psychiatry. 2020;59:88–99.e1. doi: 10.1016/j.jaac.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 58.Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, et al. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35:527–35. [DOI] [PMC free article] [PubMed]
- 59.Klein R. Inhibition of return. Trends Cogn Sci. 2000;4:138–47. doi: 10.1016/S1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- 60.Sylvester CM, Hudziak JJ, Gaffrey MS, Barch DM, Luby JL. Stimulus-Driven Attention, Threat Bias, and Sad Bias in Youth with a History of an Anxiety Disorder or Depression. J Abnorm Child Psychol. 2016;44:219–31. doi: 10.1007/s10802-015-9988-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pérez-Edgar K, Mcdermott JNM, Korelitz K, Degnan KA, Curby TW, Pine DS, et al. Patterns of sustained attention in infancy shape the developmental trajectory of social behavior from toddlerhood through adolescence. Dev Psychol. 2010;46:1723–30. doi: 10.1037/a0021064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perino MT, Yu Q, Myers MJ, Harper JC, Baumel WT, Petersen SE, et al. Attention Alterations in Pediatric Anxiety: Evidence From Behavior and Neuroimaging. Biol Psychiatry. 2021;89:726–34. [DOI] [PMC free article] [PubMed]
- 63.White LK, Britton JC, Sequeira S, Ronkin EG, Chen G, Bar-Haim Y, et al. Behavioral and neural stability of attention bias to threat in healthy adolescents. Neuroimage. 2016;136:84–93. [DOI] [PMC free article] [PubMed]
- 64.Baumel WT, Lu L, Huang X, Drysdale AT, Sweeny JA, Gong Q, et al. Neurocircuitry of treatment in anxiety disorders. Biomark Neuropsychiatry. 2022;6:100052. [DOI] [PMC free article] [PubMed]
- 65.Lenze EJ, Nicol GE, Barbour DL, Kannampallil T, Wong AWK, Piccirillo J, et al. Precision clinical trials: a framework for getting to precision medicine for neurobehavioural disorders. J Psychiatry Neurosci. 2021;46:E97–E110. doi: 10.1503/jpn.200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Price RB, Siegle GJ, Silk JS, Ladouceur CD, Mcfarland A, Dahl RE, et al. Looking under the hood of the dot-probe task: an fMRI study in anxious youth. Depression Anxiety. 2014;31:178–87. doi: 10.1002/da.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJR, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 68.Strawn JR, Bitter SM, Weber WA, Chu W-J, Whitsel RM, Adler C, et al. Neurocircuitry Of Generalized Anxiety Disorder In Adolescents: A Pilot Functional Neuroimaging And Functional Connectivity Study. Depression Anxiety. 2012;29:939–47. doi: 10.1002/da.21961. [DOI] [PubMed] [Google Scholar]
- 69.Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163:1091–7. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 70.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–34. doi: 10.1016/j.biopsych.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Telzer EH, Mogg K, Bradley BP, Mai X, Ernst M, Pine DS, et al. Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biol Psychol. 2008;79:216–22. doi: 10.1016/j.biopsycho.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamm LL, Jacobs RH, Johnson MW, Fitzgerald DA, Fitzgerald KD, Langenecker SA, et al. Aberrant amygdala functional connectivity at rest in pediatric anxiety disorders. Biol Mood Anxiety Disord. 2014;4:15. doi: 10.1186/s13587-014-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, Carlisi C, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:290–99.e2. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HMC, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–76. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tromp DPM, Williams LE, Fox AS, Oler JA, Roseboom PH, Rogers GM, et al. Altered Uncinate Fasciculus Microstructure in Childhood Anxiety Disorders in Boys But Not Girls. Am J Psychiatry. 2019;176:208–16. doi: 10.1176/appi.ajp.2018.18040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Power Jonathan D, Cohen Alexander L, Nelson Steven M, Wig Gagan S, Barnes Kelly A, Church Jessica A, et al. Functional Network Organization of the Human Brain. Neuron. 2011;72:665–78. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perino MT, Myers MJ, Wheelock MD, Yu Q, Harper JC, Manhart MF, et al. Whole-Brain Resting-State Functional Connectivity Patterns Associated With Pediatric Anxiety and Involuntary Attention Capture. Biol Psychiatry: Glob Open Sci. 2021;1:229–38. doi: 10.1016/j.bpsgos.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hang Y, Zhang G, Wang C, Zhang N, Liu G. Attention bias modification for anxiety disorders in children and adolescents: A systematic review and meta-analysis. Psychiatry Res. 2021;300:113896. doi: 10.1016/j.psychres.2021.113896. [DOI] [PubMed] [Google Scholar]
- 80.Grist R, Croker A, Denne M, Stallard P. Technology Delivered Interventions for Depression and Anxiety in Children and Adolescents: A Systematic Review and Meta-analysis. Clin Child Fam Psychol Rev. 2019;22:147–71. doi: 10.1007/s10567-018-0271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang SW, Kuckertz JM, Bose D, Carmona AR, Piacentini J, Amir N. Efficacy of Attention Bias Training for Child Anxiety Disorders: A Randomized Controlled Trial. Child Psychiatry Hum Dev. 2019;50:198–208. doi: 10.1007/s10578-018-0832-6. [DOI] [PubMed] [Google Scholar]
- 82.Liu P, Taber-Thomas BC, Fu X, Perez-Edgar KE. Biobehavioral Markers of Attention Bias Modification in Temperamental Risk for Anxiety: A Randomized Control Trial. J Am Acad Child Adolesc Psychiatry. 2018;57:103–10. doi: 10.1016/j.jaac.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eldar S, Apter A, Lotan D, Edgar KP, Naim R, Fox NA, et al. Attention bias modification treatment for pediatric anxiety disorders: a randomized controlled trial. Am J Psychiatry. 2012;169:213–20. doi: 10.1176/appi.ajp.2011.11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pettit JW, Rey Y, Marin CE, Bechor M, Lebowitz ER, Vasey MW, et al. Attention Training as a Low-Intensity Treatment for Concerning Anxiety in Clinic-Referred Youth. Behav Ther. 2023;54:77–90. doi: 10.1016/j.beth.2022.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pergamin-Hight L, Pine DS, Fox NA, Bar-Haim Y. Attention bias modification for youth with social anxiety disorder. J Child Psychol Psychiatry. 2016;57:1317–25. doi: 10.1111/jcpp.12599. [DOI] [PubMed] [Google Scholar]
- 86.Abend R, Naim R, Pergamin-Hight L, Fox NA, Pine DS, Bar-Haim Y. Age Moderates Link Between Training Effects and Treatment Response to Attention Bias Modification Treatment for Social Anxiety Disorder. J Abnorm Child Psychol. 2019;47:881–94. doi: 10.1007/s10802-018-0494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.White LK, Sequeira S, Britton JC, Brotman MA, Gold AL, Berman E, et al. Complementary Features of Attention Bias Modification Therapy and Cognitive-Behavioral Therapy in Pediatric Anxiety Disorders. Am J Psychiatry. 2017;174:775–84. doi: 10.1176/appi.ajp.2017.16070847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tan PZ, Rozenman M, Chang SW, Jurgiel J, Truong HV, Piacentini J, et al. The ERN as a neural index of changes in performance monitoring following attention training in pediatric obsessive-compulsive disorder. Biol Psychol. 2021;166:108206. doi: 10.1016/j.biopsycho.2021.108206. [DOI] [PubMed] [Google Scholar]
- 89.Nelson BD, Jackson F, Amir N, Hajcak G. Attention bias modification reduces neural correlates of response monitoring. Biol Psychol. 2017;129:103–10. doi: 10.1016/j.biopsycho.2017.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Waters AM, Cao Y, Kershaw R, Kerbler GM, Shum DHK, Zimmer-Gembeck MJ, et al. Changes in neural activation underlying attention processing of emotional stimuli following treatment with positive search training in anxious children. J Anxiety Disord. 2018;55:22–30. doi: 10.1016/j.janxdis.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 91.Haller SP, Stoddard J, Botz-Zapp C, Clayton M, MacGillivray C, Perhamus G, et al. A Randomized Controlled Trial of Computerized Interpretation Bias Training for Disruptive Mood Dysregulation Disorder: A Fast-Fail Study. J Am Acad Child Adolesc Psychiatry. 2022;61:37–45. doi: 10.1016/j.jaac.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cortese S, Ferrin M, Brandeis D, Buitelaar J, Daley D, Dittmann RW, et al. Cognitive training for attention-deficit/hyperactivity disorder: meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry. 2015;54:164–74. doi: 10.1016/j.jaac.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Benyakorn S, Calub CA, Riley SJ, Schneider A, Iosif AM, Solomon M, et al. Computerized Cognitive Training in Children With Autism and Intellectual Disabilities: Feasibility and Satisfaction Study. JMIR Ment Health. 2018;5:e40. doi: 10.2196/mental.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kollins SH, DeLoss DJ, Canadas E, Lutz J, Findling RL, Keefe RSE, et al. A novel digital intervention for actively reducing severity of paediatric ADHD (STARS-ADHD): a randomised controlled trial. Lancet Digit Health. 2020;2:e168–e78. doi: 10.1016/S2589-7500(20)30017-0. [DOI] [PubMed] [Google Scholar]
- 95.Lee S, Hill TR, Johnson B, Testa R, Priya V, Spencer-Smith M, et al. Can Neurocognitive Outcomes Assist Measurement-Based Care for Children with Attention-Deficit/Hyperactivity Disorder? A Systematic Review and Meta-Analyses of the Relationships Among the Changes in Neurocognitive Functions and Clinical Outcomes of Attention-Deficit/Hyperactivity Disorder in Pharmacological and Cognitive Training Interventions. J Child Adolesc Psychopharmacol. 2022;32:250–77. doi: 10.1089/cap.2022.0028. [DOI] [PubMed] [Google Scholar]
- 96.Penuelas-Calvo I, Jiang-Lin LK, Girela-Serrano B, Delgado-Gomez D, Navarro-Jimenez R, Baca-Garcia E, et al. Video games for the assessment and treatment of attention-deficit/hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry. 2022;31:5–20. doi: 10.1007/s00787-020-01557-w. [DOI] [PubMed] [Google Scholar]
- 97.Karch D, Albers L, Renner G, Lichtenauer N, von Kries R. The efficacy of cognitive training programs in children and adolescents: a meta-analysis. Dtsch Arztebl Int. 2013;110:643–52. doi: 10.3238/arztebl.2013.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Badura-Brack AS, Naim R, Ryan TJ, Levy O, Abend R, Khanna MM, et al. Effect of Attention Training on Attention Bias Variability and PTSD Symptoms: Randomized Controlled Trials in Israeli and U.S. Combat Veterans. The. Am J Psychiatry. 2015;172:1233–41. doi: 10.1176/appi.ajp.2015.14121578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wald I, Fruchter E, Ginat K, Stolin E, Dagan D, Bliese PD, et al. Selective prevention of combat-related post-traumatic stress disorder using attention bias modification training: a randomized controlled trial. Psychol Med. 2016;46:2627–36. [DOI] [PubMed]
- 100.Price RB, Wallace M, Kuckertz JM, Amir N, Graur S, Cummings L, et al. Pooled patient-level meta-analysis of children and adults completing a computer-based anxiety intervention targeting attentional bias. Clin Psychol Rev. 2016;50:37–49. doi: 10.1016/j.cpr.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oud M, de Winter L, Vermeulen-Smit E, Bodden D, Nauta M, Stone L, et al. Effectiveness of CBT for children and adolescents with depression: A systematic review and meta-regression analysis. Eur Psychiatry. 2019;57:33–45. doi: 10.1016/j.eurpsy.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 102.Mufson L, Sills R. Interpersonal Psychotherapy for depressed adolescents (IPT-A): an overview. Nord J Psychiatry. 2006;60:431–7. doi: 10.1080/08039480601022397. [DOI] [PubMed] [Google Scholar]
- 103.Weisz J, Bearman SK, Santucci LC, Jensen-Doss A. Initial Test of a Principle-Guided Approach to Transdiagnostic Psychotherapy With Children and Adolescents. J Clin Child Adolesc Psychol. 2017;46:44–58. doi: 10.1080/15374416.2016.1163708. [DOI] [PubMed] [Google Scholar]
- 104.Peris TS, Caporino NE, O’Rourke S, Kendall PC, Walkup JT, Albano AM, et al. Therapist-Reported Features of Exposure Tasks That Predict Differential Treatment Outcomes for Youth With Anxiety. J Am Acad Child Adolesc Psychiatry. 2017;56:1043–52. doi: 10.1016/j.jaac.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Richter L. The importance of caregiver-child interactions for survival and healthy development of young children: a review. Geneva: World Health Organization; 2004.
- 106.Britto PR, Lye SJ, Proulx K, Yousafzai AK, Matthews SG, Vaivada T, et al. Nurturing care: promoting early childhood development. Lancet. 2017;389:91–102. doi: 10.1016/S0140-6736(16)31390-3. [DOI] [PubMed] [Google Scholar]
- 107.Lunkenheimer E, Hamby CM, Lobo FM, Cole PM, Olson SL. The role of dynamic, dyadic parent-child processes in parental socialization of emotion. Dev Psychol. 2020;56:566–77. doi: 10.1037/dev0000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Timmer SG, Urquiza AJ, Zebell NM, McGrath JM. Parent-child interaction therapy: application to maltreating parent-child dyads. Child Abus Negl. 2005;29:825–42. doi: 10.1016/j.chiabu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 109.Monga S, Rosenbloom BN, Tanha A, Owens M, Young A. Comparison of child-parent and parent-only cognitive-behavioral therapy programs for anxious children aged 5 to 7 years: short- and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2015;54:138–46. doi: 10.1016/j.jaac.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 110.Dawson G, Zanolli K. Early intervention and brain plasticity in autism. Novartis Found Symp. 2003;251:266-74; discussion 74-80, 81-97. [PubMed]
- 111.Eyberg S, Funderburk B, Hembree-Kigin T, McNeil C, Querido J, Hood K. Parent-child interaction therapy with behavior problem children: One and two year maintenance of treatment effects in the family. Child Fam Behav Ther. 2001;23:1–20. doi: 10.1300/J019v23n04_01. [DOI] [Google Scholar]
- 112.Luby JL, Barch DM, Whalen D, Tillman R, Freedland KE. A Randomized Controlled Trial of Parent-Child Psychotherapy Targeting Emotion Development for Early Childhood Depression. Am J Psychiatry. 2018;175:1102–10. doi: 10.1176/appi.ajp.2018.18030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luby JL, Gilbert K, Whalen D, Tillman R, Barch DM. The Differential Contribution of the Components of Parent-Child Interaction Therapy Emotion Development for Treatment of Preschool Depression. J Am Acad Child Adolesc Psychiatry. 2020;59:868–79. doi: 10.1016/j.jaac.2019.07.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Luby J, Donohue MR, Gilbert K, Tillman R, Barch DM. Sustained remission of child depression despite drift in parent emotion management skills 18 weeks following Parent Child Interaction Therapy: emotion development. Eur Child Adolesc Psychiatry. 2021;30:369–79. doi: 10.1007/s00787-020-01522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Whalen DJ, Gilbert KE, Luby JL. Changes in self-reported and observed parenting following a randomized control trial of parent-child interaction therapy for the treatment of preschool depression. J Child Psychol Psychiatry. 2021;62:86–96. doi: 10.1111/jcpp.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Donohue MR, Yin J, Quinones-Camacho L, Hennefield L, Tillman R, Gilbert K, et al. Children’s Maternal Representations Moderate the Efficacy of Parent-Child Interaction Therapy-Emotion Development (PCIT-ED) Treatment For Preschool Depression. Res Child Adolesc Psychopathol. 2022;50:1233–46. doi: 10.1007/s10802-022-00897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Belden AC, Irvin K, Hajcak G, Kappenman ES, Kelly D, Karlow S, et al. Neural Correlates of Reward Processing in Depressed and Healthy Preschool-Age Children. J Am Acad Child Adolesc Psychiatry. 2016;55:1081–89. doi: 10.1016/j.jaac.2016.09.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barch DM, Whalen D, Gilbert K, Kelly D, Kappenman ES, Hajcak G, et al. Neural Indicators of Anhedonia: Predictors and Mechanisms of Treatment Change in a Randomized Clinical Trial in Early Childhood Depression. Biol Psychiatry. 2020;88:879–87. doi: 10.1016/j.biopsych.2020.06.032. [DOI] [PubMed] [Google Scholar]
- 119.Nelson CA. Neural plasticity and human development: the role of early experience in sculpting memory systems. Dev Sci. 2000;3:115–36. doi: 10.1111/1467-7687.00104. [DOI] [Google Scholar]
- 120.Werker JF, Hensch TK. Critical periods in speech perception: new directions. Annu Rev Psychol. 2015;66:173–96. doi: 10.1146/annurev-psych-010814-015104. [DOI] [PubMed] [Google Scholar]
- 121.Reh RK, Dias BG, Nelson CA, 3rd, Kaufer D, Werker JF, Kolb B, et al. Critical period regulation across multiple timescales. Proc Natl Acad Sci USA. 2020;117:23242–51. doi: 10.1073/pnas.1820836117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev Psychopathol. 2008;20:775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- 123.Pidano AE, Allen AR. The Incredible Year series: A review of the independent research base. J Child Fam Stud. 2015;24:1898–916. doi: 10.1007/s10826-014-9991-7. [DOI] [Google Scholar]
- 124.Funderburk B, Eyberg S. Parent-child interaction therapy. In: Norcross JC, VandenBos GR, Freedheim DK, editors. History of psychotherapy: Continuity and change. 2nd Ed. Washington DC: American Psychological Association; 2011. p. 415-20.
- 125.Benoit D, Monga S. Taming sneaky fears. Victoria, British Columbia: Friesen Press; 2018.
- 126.Willsey HR, Willsey AJ, Wang B, State MW. Genomics, convergent neuroscience and progress in understanding autism spectrum disorder. Nat Rev Neurosci. 2022;23:323–41. doi: 10.1038/s41583-022-00576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 128.Copeland W, Shanahan L, Costello EJ, Angold A. Cumulative prevalence of psychiatric disorders by young adulthood: a prospective cohort analysis from the Great Smoky Mountains Study. J Am Acad Child Adolesc Psychiatry. 2011;50:252–61. doi: 10.1016/j.jaac.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Costello EJ, Pine DS, Hammen C, March JS, Plotsky PM, Weissman MM, et al. Development and natural history of mood disorders. Biol Psychiatry. 2002;52:529–42. doi: 10.1016/S0006-3223(02)01372-0. [DOI] [PubMed] [Google Scholar]
- 130.Pine DS, Cohen E, Cohen P, Brook J. Adolescent depressive symptoms as predictors of adult depression: moodiness or mood disorder? Am J Psychiatry. 1999;156:133–5. doi: 10.1176/ajp.156.1.133. [DOI] [PubMed] [Google Scholar]
- 131.Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- 132.Dwyer JB, Stringaris A, Brent DA, Bloch MH. Annual Research Review: Defining and treating pediatric treatment-resistant depression. J Child Psychol Psychiatry. 2020;61:312–32. doi: 10.1111/jcpp.13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cuijpers P, Karyotaki E, Eckshtain D, Ng MY, Corteselli KA, Noma H, et al. Psychotherapy for Depression Across Different Age Groups: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2020;77:694–702. doi: 10.1001/jamapsychiatry.2020.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eckshtain D, Kuppens S, Ugueto A, Ng MY, Vaughn-Coaxum R, Corteselli K, et al. Meta-Analysis: 13-Year Follow-up of Psychotherapy Effects on Youth Depression. J Am Acad Child Adolesc Psychiatry. 2020;59:45–63. doi: 10.1016/j.jaac.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 135.Ng MY, Weisz JR. Annual Research Review: Building a science of personalized intervention for youth mental health. J Child Psychol Psychiatry. 2016;57:216–36. doi: 10.1111/jcpp.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou X, Teng T, Del Giovane C, Furukawa TA, Weisz JR, Cipriani A, et al. Treatment of depression in children and adolescents - Authors’ reply. Lancet Psychiatry. 2021;8:97–98. doi: 10.1016/S2215-0366(20)30537-X. [DOI] [PubMed] [Google Scholar]
- 137.March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA. 2004;292:807–20. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 138.Zhou X, Teng T, Zhang Y, Del Giovane C, Furukawa TA, Weisz JR, et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7:581–601. doi: 10.1016/S2215-0366(20)30137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brent D, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller M, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299:901–13. doi: 10.1001/jama.299.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Marwaha S, Palmer E, Suppes T, Cons E, Young AH, Upthegrove R. Novel and emerging treatments for major depression. Lancet. 2023;401:141–53. doi: 10.1016/S0140-6736(22)02080-3. [DOI] [PubMed] [Google Scholar]
- 141.Dwyer JB, Landeros-Weisenberger A, Johnson JA, Londono Tobon A, Flores JM, Nasir M, et al. Efficacy of intravenous ketamine in adolescent treatment-resistant depression: a randomized midazolam-controlled trial. Focus. 2022;20:241–51. doi: 10.1176/appi.focus.22020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jones G, Arias D, Nock M. Associations between MDMA/ecstasy, classic psychedelics, and suicidal thoughts and behaviors in a sample of U.S. adolescents. Sci Rep. 2022;12:21927. doi: 10.1038/s41598-022-25658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shah K, Trivedi C, Kamrai D, Akbar M, Tankersley W. Association of Psilocybin Use in Adolescents with Major Depressive Episode. European Psychiatry. 2022;65(S1), S329-S329.
- 144.Lapate RC, Samaha J, Rokers B, Hamzah H, Postle BR, Davidson RJ. Inhibition of Lateral Prefrontal Cortex Produces Emotionally Biased First Impressions: A Transcranial Magnetic Stimulation and Electroencephalography Study. Psychol Sci. 2017;28:942–53. doi: 10.1177/0956797617699837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–74. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 146.Padmanabhan JL, Cooke D, Joutsa J, Siddiqi SH, Ferguson M, Darby RR, et al. A Human Depression Circuit Derived From Focal Brain Lesions. Biol Psychiatry. 2019;86:749–58. doi: 10.1016/j.biopsych.2019.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kentgen LM, Tenke CE, Pine DS, Fong R, Klein RG, Bruder GE. Electroencephalographic asymmetries in adolescents with major depression: influence of comorbidity with anxiety disorders. J Abnorm Psychol. 2000;109:797–802. doi: 10.1037/0021-843X.109.4.797. [DOI] [PubMed] [Google Scholar]
- 148.Pine DS, Kentgen LM, Bruder GE, Leite P, Bearman K, Ma Y, et al. Cerebral laterality in adolescent major depression. Psychiatry Res. 2000;93:135–44. doi: 10.1016/S0165-1781(00)00101-3. [DOI] [PubMed] [Google Scholar]
- 149.Kisely S, Li A, Warren N, Siskind D. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35:468–80. doi: 10.1002/da.22746. [DOI] [PubMed] [Google Scholar]
- 150.Chen JJ, Zhao LB, Liu YY, Fan SH, Xie P. Comparative efficacy and acceptability of electroconvulsive therapy versus repetitive transcranial magnetic stimulation for major depression: A systematic review and multiple-treatments meta-analysis. Behav Brain Res. 2017;320:30–36. doi: 10.1016/j.bbr.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 151.Li H, Cui L, Li J, Liu Y, Chen Y. Comparative efficacy and acceptability of neuromodulation procedures in the treatment of treatment-resistant depression: a network meta-analysis of randomized controlled trials. J Affect Disord. 2021;287:115–24. doi: 10.1016/j.jad.2021.03.019. [DOI] [PubMed] [Google Scholar]
- 152.Sonmez AI, Camsari DD, Nandakumar AL, Voort JLV, Kung S, Lewis CP, et al. Accelerated TMS for Depression: A systematic review and meta-analysis. Psychiatry Res. 2019;273:770–81. doi: 10.1016/j.psychres.2018.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ferrarelli F, Phillips MLExamining. and Modulating Neural Circuits in Psychiatric Disorders With Transcranial Magnetic Stimulation and Electroencephalography: Present Practices and Future Developments. Am J Psychiatry. 2021;178:400–13. doi: 10.1176/appi.ajp.2020.20071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Modak A, Fitzgerald PB. Personalising transcranial magnetic stimulation for depression using neuroimaging: A systematic review. World J Biol Psychiatry. 2021;22:647–69. doi: 10.1080/15622975.2021.1907710. [DOI] [PubMed] [Google Scholar]
- 155.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–47. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 158.Wang JX, Rogers LM, Gross EZ, Ryals AJ, Dokucu ME, Brandstatt KL, et al. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345:1054–7. doi: 10.1126/science.1252900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Oberman LM, Hynd M, Nielson DM, Towbin KE, Lisanby SH, Stringaris A. Repetitive Transcranial Magnetic Stimulation for Adolescent Major Depressive Disorder: A Focus on Neurodevelopment. Front Psychiatry. 2021;12:642847. doi: 10.3389/fpsyt.2021.642847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Croarkin PE, Elmaadawi AZ, Aaronson ST, Schrodt GR, Jr., Holbert RC, Verdoliva S, et al. Left prefrontal transcranial magnetic stimulation for treatment-resistant depression in adolescents: a double-blind, randomized, sham-controlled trial. Neuropsychopharmacology. 2021;46:462–69. doi: 10.1038/s41386-020-00829-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gordon EM, Laumann TO, Gilmore AW, Newbold DJ, Greene DJ, Berg JJ, et al. Precision Functional Mapping of Individual Human Brains. Neuron. 2017;95:791–807.e7. doi: 10.1016/j.neuron.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sylvester CM, Yu Q, Srivastava AB, Marek S, Zheng A, Alexopoulos D, et al. Individual-specific functional connectivity of the amygdala: A substrate for precision psychiatry. Proc Natl Acad Sci USA. 2020;117:3808–18. doi: 10.1073/pnas.1910842117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Macedo MA, Sato JR, Bressan RA, Pan PM. Adolescent depression and resting-state fMRI brain networks: a scoping review of longitudinal studies. Braz J Psychiatry. 2022;44:420–33. doi: 10.47626/1516-4446-2021-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Braund TA, Breukelaar IA, Griffiths K, Tillman G, Palmer DM, Bryant R, et al. Intrinsic Functional Connectomes Characterize Neuroticism in Major Depressive Disorder and Predict Antidepressant Treatment Outcomes. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7:276–84. doi: 10.1016/j.bpsc.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 165.Brandl F, Weise B, Mulej Bratec S, Jassim N, Hoffmann Ayala D, Bertram T, et al. Common and specific large-scale brain changes in major depressive disorder, anxiety disorders, and chronic pain: a transdiagnostic multimodal meta-analysis of structural and functional MRI studies. Neuropsychopharmacology. 2022;47:1071–80. doi: 10.1038/s41386-022-01271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lynch CJ, Elbau IG, Ng TH, Wolk D, Zhu S, Ayaz A, et al. Automated optimization of TMS coil placement for personalized functional network engagement. Neuron. 2022;110:3263–77.e4. doi: 10.1016/j.neuron.2022.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Elbau IG, Lynch CJ, Downar J, Vila-Rodriguez F, Power JD, Solomonov N, et al. Functional Connectivity Mapping for rTMS Target Selection in Depression. Am J Psychiatry. 2023;180:230–40. [DOI] [PMC free article] [PubMed]
- 168.Nosek BA, Hardwicke TE, Moshontz H, Allard A, Corker KS, Dreber A, et al. Replicability, Robustness, and Reproducibility in Psychological Science. Annu Rev Psychol. 2022;73:719–48. doi: 10.1146/annurev-psych-020821-114157. [DOI] [PubMed] [Google Scholar]
- 169.Shrout PE. Measurement reliability and agreement in psychiatry. Stat Methods Med Res. 1998;7:301–17. doi: 10.1177/096228029800700306. [DOI] [PubMed] [Google Scholar]
- 170.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]