Neuronal, glial, and endothelial brain tissue express myriad sensors for mechanical force, osmotic pressure, and shear stress. Focused ultrasound (fUS) can create pressure fields on deep brain regions to engage these endogenous sensory mechanisms. Numerous studies have demonstrated that fUS can alter resting membrane potential, local molecular content, or the frequency of spiking events [1]. However, electromechanical properties and mechanosensory gene expression profiles are highly heterogeneous across the brain, and the fUS responsiveness of sub-cortical therapeutically tractable targets is largely unknown.
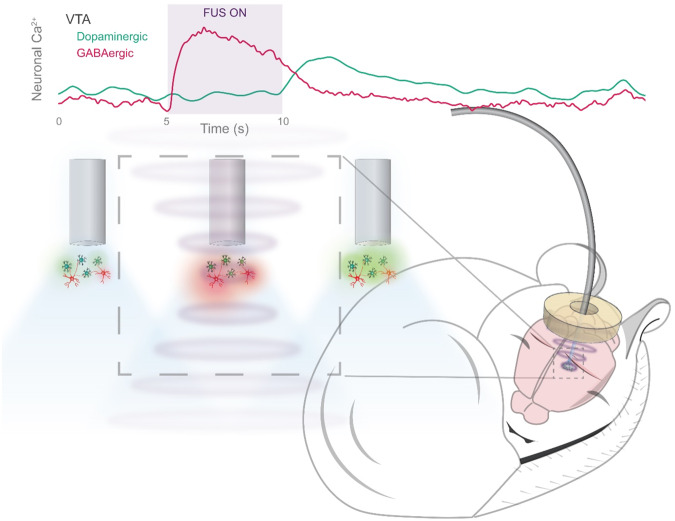
We recently developed Photometry Coupled UltraSound (PhoCUS), an ultrasound transducer integrated with an optical fiber brain implant, allowing us to monitor deep-brain neuronal responses to fUS in freely behaving animals [2]. In monitoring numerous cell types across the brain, we observed distinct fUS response profiles (Fig. 1). In the mouse model, hippocampal pyramidal neurons and their neighboring inhibitory basket cells exhibit a bidirectional response to rapidly pulsed fUS, where inhibitory cells were activated, and excitatory neurons were suppressed which effectively suppressed epileptic activity [2]. The same protocol elicited an asynchronous response between ventral tegmental area GABAergic (VTAGABA) and dopaminergic (VTADA) cells; VTAGABA neurons were powerfully excited during the stimulus, whereas dopaminergic activity rose sharply following the stimulus offset, concurrent with the decline of VTAGABA activity (Fig. 1). This temporal differentiation suggests that both cell types are sensitive to fUS, and that local GABA release temporarily overrides the response of the dopaminergic population. VTADA neurons drive goal-oriented wakefulness, and can result in mania-like behavior if left unregulated [3]. This regulation is a key function of VTAGABA neurons, which act to prevent reward-seeking hyperarousal and allow for sleep induction. Thus, the development of a protocol that optimizes for sustained VTAGABA activity and reduces post-fUS dopaminergic activity in animal models could have potential therapeutic implications. Specifically, it may treat sleep comorbidities in psychosis conditions where VTA activity is aberrantly high, such as in bipolar and substance use disorder. PhoCUS complements optogenetic and designer drug research by providing a translatable arm for circuit-based alleviation of symptoms in model systems.
Fig. 1. PhoCUS on Ultrasound for neuromodulation.
An illustration of photometry coupled ultrasound used to record asynchronous dopaminergic and GABAergic neuronal response to focused ultrasound.
In addition to fUS suppression of wake circuitry, other strategies may have potential as sleep therapeutics. For instance, chronic low-intensity fUS stimulation of the mouse prefrontal cortex increased REM sleep duration and improved memory consolidation [4]. Both ultrasound-based entrainment of hippocampal oscillations in phase with slow activity and fUS modulation of theta waves affect sleep architecture [5]. Targeting the centromedial thalamus (CMT) may also be a viable strategy for enhancing slow wave sleep since glutamatergic CMT neurons are powerfully activated by fUS [2]. The reticular nucleus of the thalamus (RTN) contains GABAergic inputs to the CMT, but is spatially segregated to a shell structure outside of the larger thalamus. Furthermore, ultrasonic neuromodulation of the preoptic area in mice lowers core body temperature suggesting that torpor-like states may also be used to alter vigilance states in non-hibernating animals [6]. These collective findings suggest that optimizing fUS for cell-type specific neuromodulation is a critical step towards future studies attempting to translate FUS in clinical studies for treatment of neuropsychiatric conditions and sleep disorders.
Competing interests
This manuscript was funded by a grant from NIDA (DA05505601A1) to LdL. KM and LdL are co-inventors on a patent application (US2022/0378360A1) assigned to Stanford University containing disclosures related to the technology described in this article.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blackmore DG, Razansky D, Götz J. Ultrasound as a versatile tool for short- and long-term improvement and monitoring of brain function. Neuron. 2023;111:1174–90. [DOI] [PubMed]
- 2.Murphy KR, Farrell JS, Gomez JL, Stedman QG, Li N, Leung SA, et al. A tool for monitoring cell type–specific focused ultrasound neuromodulation and control of chronic epilepsy. Proc Natl Acad Sci USA. 2022;119:e2206828119. [DOI] [PMC free article] [PubMed]
- 3.Yu X, Ba W, Zhao G, Ma Y, Harding EC, Yin L, et al. Dysfunction of ventral tegmental area GABA neurons causes mania-like behavior. Mol Psychiatry. 2021;26:5213–28. [DOI] [PMC free article] [PubMed]
- 4.Jo Y, Lee SM, Jung T, Park G, Lee C, Im GH, et al. General-Purpose Ultrasound Neuromodulation System for Chronic, Closed-Loop Preclinical Studies in Freely Behaving Rodents. Adv Sci (Weinh). 2022;9:e2202345. [DOI] [PMC free article] [PubMed]
- 5.Dong S, Xie Z, Yuan Y. Transcranial ultrasound stimulation modulates neural activities during NREM and REM depending on the stimulation phase of slow oscillations and theta waves in the hippocampus. Cereb Cortex. 2023:bhad174. 10.1093/cercor/bhad174. Epub ahead of print. [DOI] [PubMed]
- 6.Yang Y, Yuan J, Field RL, Ye D, Hu Z, Xu K, et al. Induction of a torpor-like hypothermic and hypometabolic state in rodents by ultrasound. Nat Metab. 2023;5:789–803. [DOI] [PMC free article] [PubMed]