Abstract
Introduction:
Neuropathic pain is a common and painful somatosensory nervous system disease, and its treatment remains a medical challenge. Evidence demonstrates that gut microbiota alters in neuropathic pain and, therefore, improvement of the gut flora may affect the disease. The present study aimed to evaluate the antinociceptive effect of probiotics in neuropathic pain and oxidative biomarkers’ responsiveness to the probiotic treatment.
Methods:
Using chronic constriction injury (CCI) of the rats' sciatic nerve, neuropathic pain was induced. Investigating the analgesic effect of the probiotics mixture, 40 male rats were randomly assigned to 4 groups (n=10 for each): Sham-operated (SM), and CCI model rats orally received 1 mL saline (CS), or 100 mg/kg gabapentin (CG) or 1 mL probiotics mixture (CP) Lactobacillus plantarum, Lactobacillus delbrueckii, Lactobacillus acidophilus, Lactobacillus rhamnosus, and Bifidobacterium bifidum (109 CFU of each) daily. Using behavioral tests, the pain was assessed on days 1, 4, 7, 14, and 21 of the study. Finally, the biochemical evaluation of sciatic nerve tissue was done.
Results:
Probiotics decreased cold and mechanic allodynia and thermal hyperalgesia. Reducing lipid peroxidation levels and increasing total antioxidant capacity, superoxide dismutase (SOD) and glutathione peroxidase (GPx) activity were also significant in the probiotics group.
Conclusion:
These findings suggest that probiotics have analgesic effects on the CCI model of neuropathic pain via increasing the antioxidant capacity of the rats’ sciatic nerve.
Highlights
Oral probiotics mixture diminishes cold and mechanical allodynia in chronic constriction injury (CCI) rats.
Probiotics mixture reduces thermal hyperalgesia in CCI rats as well as gabapentine.
Balancing in oxidant/anti-oxidant system is key pathway in neuropathic pain reduction.
Plain Language Summary
Currently, neuropathic pain is an underestimated socioeconomic health problem affecting millions of people worldwide. Neuropathic pain occurs when a health condition affects the nerves that carry sensations to the brain. With neuropathic pain, the pain isn’t typically triggered by an event or injury. Instead, the body sends pain signals to the brain unprompted. Neuropathic pain tends to get worse over time. As neuropathic pain could result from an imbalance in the oxidative/antioxidative system, antioxidant supplementation may be a treatment option. Oxidative stress is known to result in the occurrence of molecular mechanisms of diabetes, atherosclerosis, inflammatory bowel disease, and damage to the heart, brain, or transplanted organs. Probiotics are made of good live bacteria and/or yeasts that naturally live in the body and have various health benefits. Consumption of probiotics alone or foods supplemented with probiotics may reduce cell oxidative damage. Incorporating probiotics in foods can provide an excellent strategy to supply dietary antioxidants. This study subjected rats to sciatic nerve ligation to induce neuropathic pain. The oral probiotics mixture was administered for 21 days post-surgery. The result showed that the probiotics mixture administration could reduce oxidative stress and pain in neuropathic pain rats. Therefore, probiotics can prevent and treat many systemic diseases in animal and human studies.
Keywords: Neuropathic pain, Chronic constriction injury, Probiotics, Antioxidants, Allodynia, Hyperalgesias, Rats
1. Introduction
Neuropathic pain is a chronic nervous disorder that causes injury to the peripheral or central nervous systems ( Jensen & Finnerup, 2014). In general, neuropathic pain causes even minor stimulation and activation of the nervous system to produce a potentially severe and painful sensation ( Honoré et al., 2011). Different physiopathology, such as infections, cord injuries, nerve inflammation, and metabolic disorders have been suggested as critical factors leading to neuropathic pain ( Colloca et al., 2017; Murnion, 2018). Also, increased pro-inflammatory cytokines and accumulation of oxidative stress lead to neuropathic pain ( Shim et al., 2019). Despite recent studies, neuropathic pain treatments are a complex challenge, and the exact mechanism related to it has not been explored until now. So, finding novel medicines against neuropathic pain is necessary.
Chronic constriction injury (CCI) is a standard animal model to study the behavioral features and pathological systems of neuropathic pain ( De Vry et al., 2004). Studies’ results have shown high levels of reactive oxygen species (ROS) in the spinal cord of neuropathic pain animal models ( Bittar et al., 2017; Gao et al., 2007). Pathological stress situations cause mitochondrial fission and induce additional ROS production, significantly helping neuropathic and inflammatory pain development ( Guo et al., 2013). Besides, we now know ROS mediates both neuropathic pain and the analgesic effect of antioxidants in animal models ( Kim et al., 2010; Yowtak et al., 2011). Naik and colleagues illustrated malondialdehyde (MDA), xanthine oxidase, and glutathione decrease neuropathic pain (sciatic nerve damage) in rats ( Naik et al., 2006). Several studies also reported that chronic constriction-induced sciatic nerve hurt could reduce antioxidant enzyme activities and increase the MDA level in the spinal cord ( Pathak et al., 2014; Xu et al., 2014; Zhao et al., 2014). It has been shown that antioxidant treatment effectively decreases neuropathic pain by free radical clearing ( Mao et al., 2009).
Probiotics supplements are a group of nonpathogenic live bacteria of the gut which, when used in enough amounts, beneficially impact the host human or animal by ameliorating their intestinal microbial equilibrium ( Liang et al., 2015). Emerging studies demonstrate that the microbiota via a complex series of highly communicating apparatuses and host–microbiome symbiosis may help adjust multiple neurochemical and neurometabolic pathways ( Bravo et al., 2012; Douglas-Escobar et al., 2013). D’Souza et al. have shown that probiotics prevent oxidative stress by decreasing inflammation and increasing antioxidant enzymes, for instance, glutathione peroxidase and superoxide dismutase ( D’Souza et al., 2010). Shen et al. showed that Bifidobacterium effectively cleans free radicals, increases anti-oxidative enzyme activities, and reduces MDA content and monoamine oxidase activity in serums and livers of aging mice (Shen et al., 2010). Amdekar and Singh clearly showed that Lactobacillus rhamnosus inhibits carrageenan-induced paw edema and reduces the acetic acid-induced writhings by down-regulating pro-inflammatory cytokines (Amdekar & Singh, 2016). In another study, these authors reported that Lactobacillus casei and Lactobacillus acidophilus have a powerful protective effect against rheumatoid arthritis because of their anti-inflammatory and anti-oxidant properties ( Amdekar et al., 2013).
It appears that gut flora and probiotics can influence issues such as oxidative stress and neuroinflammation, seen in neuropathic pain. So, in the present study, we described for the first time the effect of probiotic mixture administration on neuropathic pain and oxidative stress factors in a rat model of chronic constriction injury of the sciatic nerve.
2. Materials and Methods
Animals
This experimental study was performed on healthy and young male Wistar rats. Animals were housed in a temperature- and humidity-controlled animal house on a 12 hours light-dark cycle. Food and water were available ad libitum. The rats were distributed into four groups (n=10 in each): Sham-operated (SM) and CCI-operated groups that received saline (0.9%) (CS), probiotics mixture (CP), or gabapentin (CG).
Surgery
The CCI of the sciatic nerve model was induced as described earlier ( Bennett & Xie, 1988). The rats were anesthetized with ketamine/xylazine (100/10 mg/kg, intraperitoneal) (Alfasan, The Netherlands). The hairs of the middle area of the left leg thigh were removed. Near the trochanter, just distal to the branching site of the posterior bicep’s semi-tendinosus nerve, the common sciatic nerve was uncovered and detached from the surrounding tissue. Four loose ligatures with a 4.0 chromic gut were tied around the nerve with 1 mm spacing so that the circulation through the superficial epineuria vasculature was not interrupted. In the SM animals, the sciatic nerve was bared, and the wound was closed without ligation. All the animals were housed in an individual cages after the surgery.
Drug supplementation
The probiotic powder was a mixture of Lactobacillus plantarum, Lactobacillus delbrueckii, L. acidophilus, L. rhamnosus, and Bifidobacterium bifidum (Probiotics International Ltd, United Kingdom). Each gram of supplement contained a total CFU of 2×109.The probiotic suspension was made by adding 500 mg of the probiotics mixture to 1 mL saline and supplementation was administered through intragastric gavage ( Hadidi Zavareh et al., 2020) lasting for 21 days post-surgery. Gabapentin dose was 100 mg/kg and administered through intragastric gavage 30 minutes before the behavioral tests ( Amin et al., 2018).
Behavioral studies
Behavioral tests were performed by a blind person on post-surgery days 1, 4, 7, 14, and 21 as follows:
Mechanical allodynia (von-Frey test)
This test was done to evaluate withdrawal to an innocuous mechanical stimulus that consisted of von-Frey filament (ranging from 2 to 60 g, Stoelting Inc., Wood Dale, IL). The rats were placed in a chamber with a mesh floor and given 15 minutes to adapt. Each hair was applied to the left hind paw’s mild plantar until the rat withdraws its paw or the hair is bent. The stimulation was repeated three times with a 1 min inter-stimulus interval. The withdrawal threshold was the smallest hair size that stimulated at least two withdrawal responses to the three hair stimulus repetitions.
Cold allodynia (acetone test)
Acetone spray test (evaporation-evoked cooling) was used to assess the cold sensitivity. Rats were placed in a chamber with a mesh floor. Using a propylene tube attached to the syringe, one drop of acetone was squirted into the hind paw’s plantar surface. The acetone application was repeated five times at 5 min intervals, and the frequency of withdrawal response was reported as a percentage.
Thermal hyperalgesia (hot-plate test)
To evaluate thermal hyperalgesia, a radiant heat (temperature: 52°C) was applied under the mid-plantar surface using plantar test apparatus (UgoBasile, Varese, Italy), and paw withdrawal latency was measured. The times (seconds) between heat application and paw withdrawal were considered the latency. To avoid tissue damage, a cut-off time of 22 sec was used. Three measurements were made on each paw, and the minimum interval of the stimulus was about 5 min. Hyperalgesia score was reported as the mean of withdrawal latency.
Tissue collection
The rats were euthanized on day 21 post-surgery after the behavioral tests were completed, and the 1 cm around the ligation of the left sciatic nerve was promptly cut and snap-frozen in liquid nitrogen. The samples were then placed in a freezer at −70°C for biochemical evaluation.
Biochemical studies
Evaluating oxidative stress parameters, the sciatic nerve tissue was homogenized with PBS buffer and centrifuged for 10 minutes at 3000 g. Thiobarbituric acid (TBA) reaction was used to determine the MDA content, the end-product of lipid peroxidation. The amount of 0.5 mL of homogenate supernatant was mixed with 1 mL trichloroacetic acid (20%) and centrifuged at 2500 g for 10 minutes. Then, 1 mL of TBA 0.067% was added to the 0.5 mL of the supernatant. The resulting mixture was heated at 100°C for 25 minutes and centrifuged at 3000 g after chilling in ice. Finally, a spectrophotometer was used to measure the absorbance of the transparent supernatant at 532 nm. The amount of MDA was expressed as nmol/mg protein. The modified Lowry method ( Wilson & Walker, 2010) was used to determine the protein content of the samples. The FRAP method was used to assess total antioxidant capacity (TAC) in the rat’s sciatic nerve. This method’s basis was the ability of the homogenized tissue to reduce ferric ions to ferrous ions. FRAP reaction mixture was prepared by mixing acetate buffer (300.0 mmol/L), TPTZ solution (10 mmol/L), and FeCl3 solution (20 mmol/L) in a 10:1:1 ratio. Then, 200 μL of this solution was mixed vigorously with 10 μL of the sample, and the maximum light absorption of the complex was measured at 593 nm. Using standard laboratory ELISA kits (ZellBio, Germany), superoxide dismutase (SOD) and glutathione peroxidase (GPx) activity were measured.
Statistical analysis
All of the data was presented as Mean±SEM. Repeated measures analysis of variance (ANOVA) with Tukey post hoc was used to analyze data of behavior tests. The two-way ANOVA test was used to analyze the biochemical assays, followed by Tukey’s test. GraphPad Prism software, version 7.05 was used for all statistical analyses, and P<0.05 was considered a significant difference.
3. Results
Behavioral tests of neuropathic pain
The results of our study revealed that sciatic nerve ligation does not hurt motor activity, and the rats were healthy and well-groomed. The paw gesture of the surgical paw was somewhat altered, but this did not interfere with the rats’ usual life.
Mechanical allodynia
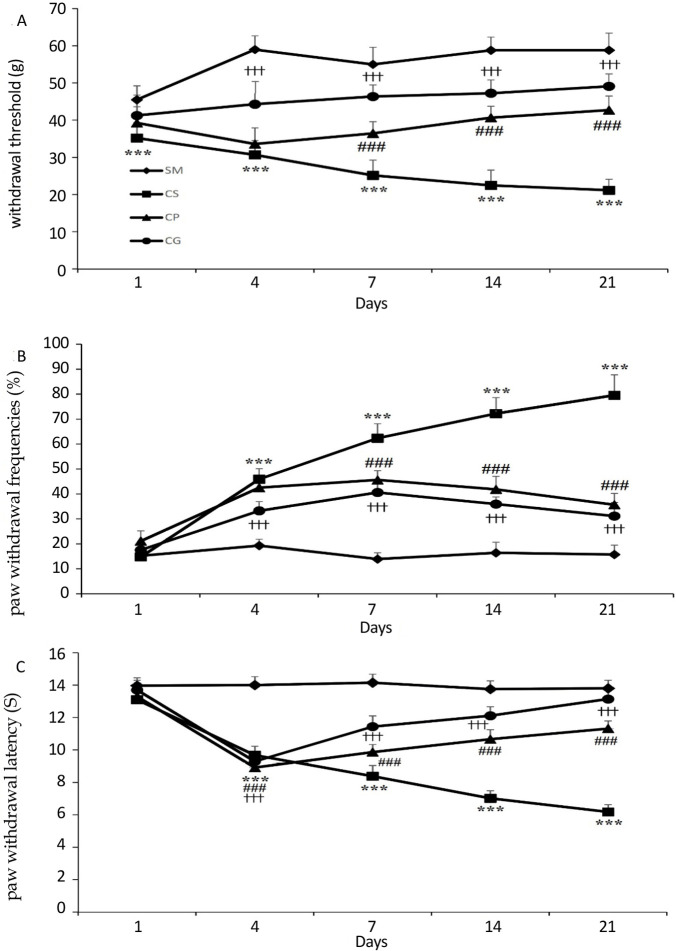
In comparison to the SM group, partial sciatic nerve ligation resulted in a substantial reduction in the ipsilateral paw withdrawal threshold (P<0.001). It could be said that the ipsi-lateral hind paw became so sensitive to stimulation as a result of partial sciatic ligation that it responded to a 2 g von-Frey filament (Figure 1A). Data analysis also showed that on all days of the experiment, the difference between the CG and CS groups was significant (P<0.001). Probiotics administration significantly increased the withdrawal threshold of the ipsilateral paw from day 7 of the experiment compared to the CS group (P<0.001). No statistical difference was evident between the withdrawal threshold of CP and CG offspring.
Figure 1.
A) The mechanical withdrawal threshold (g) in response to stimuli applied via von-Frey filament in CCI rats treated by the probiotics mixture
B) The graphs show the withdrawal frequencies following using cold stimuli (acetone) to the rats’ hind paw
C) The thermal withdrawal latency (S) in response to applying noxious radiant heat to the CCI rats’ hind paw
The behavioral responses were determined on days 1, 3, 7, 14, and 21 post-surgery. Treatment by probiotics mixture for 21 days significantly reduced elevated cold and mechanical allodynia and thermal hyperalgesia in rats’ model of chronic constriction injury of the sciatic nerve. Results are expressed as Mean±SEM and n=10 in all groups.
***P<0.001 the CS group vs. the SM group,###P<0.001 the CS group vs. the CP group, †††P<0.001 the CS group vs. the CG group.
Cold allodynia
As shown in Figure 1B, the ipsilateral paw of nerve-ligated animals (the CS rats) was more sensitive to acetone application than the SM rats (P<0.001). Also, the sham operation did not cause any change in the nociceptive response of rats’ paws. Gabapentin, as an effective treatment for neuropathic pain, significantly reduced withdrawal frequency compared to the CS group (P<0.001). Also, on days 7, 14, and 21 post-surgery, probiotics administration lowered withdrawal frequency significantly more than the CS group (P<0.001). The probiotics treatment reduced the withdrawal frequency of the CP animals to a gabapentin treatment level so that no significant was evident between the CP and CG groups (P=0.213).
Thermal hyperalgesia
On days 4, 7, 14, and 21 after surgery, partial sciatic nerve ligation reduced ipsilateral paw withdrawal latency to the thermal stimulus compared to the SM group (P<0.001). Treatment with gabapentin increased the withdrawal threshold on days 7, 14, and 21 post-surgery than the CS group (P<0.001). The experimental group’s withdrawal latency also increased significantly by probiotics treatment compared to the CS group (P<0.001), although not to the degree of gabapentin treatment (Figure 1C).
Oxidative stress factors
Lipid peroxidation
We used calorimetry to investigate the variations in MDA levels in the study groups. All the treatments affected the level of malondialdehyde in different groups, as indicated in Figure 2A (FF(3, 36)=63.573, P<0.0001). According to the obtained results, the MDA level was significantly higher in the CS group than in the SM group (P<0.001). When compared to the CS group, receiving probiotics or gabapentin resulted in a significant reduction in MDA levels (P<0.001). It is noticeable that there was no significant difference between MDA levels in probiotics and gabapentin-treated groups (P=0.157).
Figure 2.
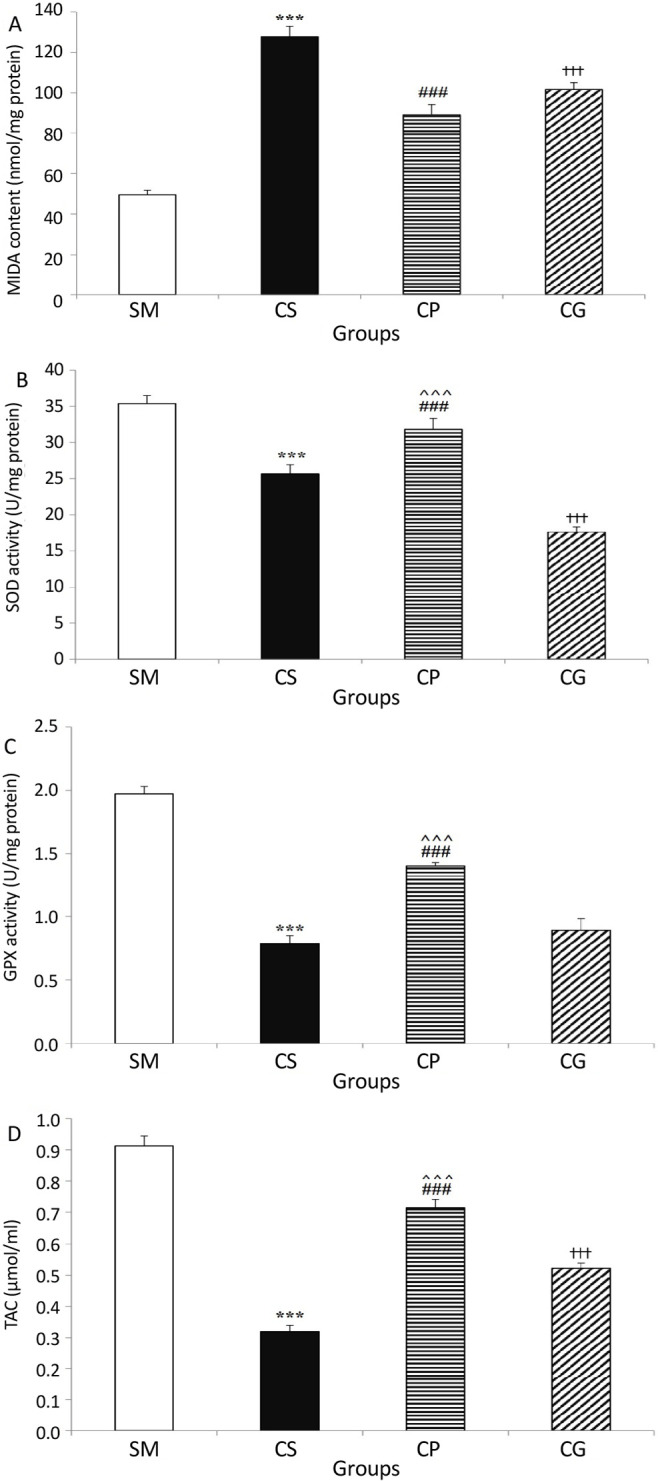
A) The MDA level, B) SOD activity, C) GPx activity, D) TAC activity in animals’ sciatic nerve tissue in all groups of the study
Induction of neuropathic pain in rats’ sciatic nerve caused elevation in the MDA level and reduction in the SOD and GPx activity and total antioxidant capacity in nerve tissue. Treatment with a probiotic mixture for three weeks reversed these changes significantly. Values are expressed as Mean±SEM and n=10 in all groups.
***P<0.001 the CS group vs. the SM group, ###P<0.001 the CS group vs. the CP group, †††P<0.001 the CS group vs. the CG group, ^^^P<0.001 the CP group vs. the CG group.
SOD and GPx enzyme activities
According to the two-way ANOVA results, there was a significant difference between SOD and GPx activity levels in the study groups (F(3, 36)=42.398, P<0.0001 and F(3, 36)=253.749, P<0.0001, respectively). There was also a significant reduction in SOD and GPx activity in the CCI-operated group that received saline in comparison to the SM group (P<0.001 for both comparisons). As shown in Figure 2B, the administration of a probiotics mixture increased, and gabapentin intake decreased the activity of SOD activity in CCI-model rats significantly (P<0.001 for both comparisons). The GPx activity elevated in CCI-operated rats after probiotics mixture in-take (P<0.001) and administration of gabapentin did not change the GPx activity (Figure 2C).
Total antioxidant capacity
Data analysis showed a statistical difference between TAC levels in the sciatic nerve tissues of the study’s groups (F(3, 36)=118.265, P<0.0001). The TAC level in the sciatic nerve tissue was significantly lower in the CS group (0.32±0.02 μmol/mL) than in the SM group (0.91±0.03 μmol/mL, P<0.001). Moreover, the TAC level in the rats’ sciatic nerve (0.72±0.03 μmol/mL, P<0.001) was significantly elevated by treatment with a probiotics mixture. On the other hand, probiotics significantly enhanced the total antioxidant capacity (P<0.001). Also, the TAC level in the sciatic nerve tissues of the gabapentin-received rats was significantly more than the CS group (P<0.001).
4. Discussion
The CCI model is a common model that shows the number of symptoms seen in human cases that are associated with neuropathic pain syndromes ( Bennett & Xie, 1988). Considering our results, intragastric gavage of a probiotic mixture of L. plantarum, L. delbrueckii, L. acidophilus, L. rhamnosus, and B. bifidum lasting for 21 days post-surgery has anti-allodynic and anti-hyperalgesic effects compared to the SM rats. In this study, four days after chronic constriction injury of the sciatic nerve rats demonstrated a high level of hyperalgesia against the thermal and mechanical stimulus. Our results also indicated that probiotics reduce allodynia and weaken cold and heat hyperalgesia in the CCI model rats. These findings are consistent with previous findings that reported L. casei and L. acidophilus have potent anti-inflammatory and analgesic properties ( Amdekar et al., 2012; Amdekar et al., 2013; Amdekar et al., 2011).
Scientists have shown that there are more than 1000 species and 7000 strains, including bacteria, yeast, and viruses, in the gut, which is named the microbiome (Huttenhower et al., 2012). The gut microbiome variety in patients with pelvic pain syndrome is significantly lesser than that in controls ( Shoskes et al., 2016). Huang and et al. reported that oral administration of Lactobacillus reuteri LR06 or Bifidobacterium BL5b supplement does not reduce pain in CCI model animals ( Huang et al., 2019). The various probiotic mixtures and severity of the disease are the possible causes of the difference between the results of the two studies. Nevertheless, there is only a little research on the relationship between the microbiota of the gut and neuropathic pain, to the best of our knowledge.
Several studies have shown high levels of reactive oxygen species (ROS) in the spinal cord of neuropathic pain animal models ( Bittar et al., 2017; Gao et al., 2007). Previous studies showed that ROS mediates both neuropathic pain and the analgesic effect of antioxidants in animal models ( Kim et al., 2010; Yowtak et al., 2011). Under pathological stress, mitochondrial fission induces high-level ROS production that remarkably contributes to neuropathic and inflammatory pain ( Guo et al., 2013). Besides, studies’ results have shown high ROS levels in the spinal cord of animal models of neuropathic pain can affect pain transmission ( Bittar et al., 2017; Gao et al., 2007) and that ROS might be a key factor in neuropathic pain pathogenesis. As discussed, an extreme and sustained increase in ROS production and oxidative stress play a role in neuropathic pain development ( Naik et al., 2006). From this aspect, it seems that the analgesic effect of antioxidants on neuropathic pain treatment is useful.
Probiotics have been used to prevent and treat many systemic diseases in both animal and human studies, including rheumatoid arthritis ( de Oliveira et al., 2017) and systemic lupus erythematosus ( Esmaeili et al., 2017). The probable mechanism of probiotics treatment’s effects may comprise the elimination of pathogenic microorganisms and immune system modulation ( Kwon et al., 2013). Our findings illustrated that the chronic constriction-induced sciatic nerve hurt reduces antioxidant enzyme activities and increases the MDA level in the sciatic nerve tissue. Also, oral bacteriotherapy to these animals decreased the MDA level and increased antioxidant (TAC, GSH, and SOD) factors. Several studies indicated that probiotic bacteria have considerable antioxidant abilities in vivo and in vitro ( Bittar et al., 2017; Shen et al., 2011). The strain antioxidant attributes of lactic acid bacteria (LAB) are well-known and show antioxidant capability ( Gaisawat et al., 2019). However, the number of their abilities and the mechanisms that they act is different ( Lin & Yen, 1999). Probiotics appear to act as antioxidants through a variety of pathways, namely the production of antioxidant enzymes like SOD, GPx, CAT, and metabolites like lactate ( Mishra et al., 2015; Wang et al., 2017). Moreover, probiotics suppress pathogen growth by modulating gut microbiota and acting as indirect antioxidants by diminishing the bioavailability of elements such as iron ( Azcárate-Peril et al., 2011).
It is indicated that LAB can resist ROS, including peroxide radicals ( Stecchini et al., 2001), superoxide anions, and hydroxyl radicals ( Kullisaar et al., 2002). In humans, L. rhamnosus show intense antioxidant activity in situations of high physical stress. So, athletes who are subjected to oxidative stress may profit from the capability of L. rhamnosus to enhance antioxidant levels and counteract the effects of ROS ( Martarelli et al., 2011). Moreover, probiotics can also provoke the host’s anti-oxidant system and increase antioxidants’ activities efficiently ( Wang et al., 2017). Wang and colleagues have shown L. fermentum supplementation increases serum SOD and GPx and hepatic CAT, muscle SOD, Cu- and Zn-SOD in pigs compared to the control group ( Wang et al., 2009). Thus, the present study strains may possess similar antioxidant mechanisms to produce analgesic effects on CCI-induced neuropathy.
In conclusion, our results suggest that oral consumption of a probiotics mixture for 3 weeks has analgesic effects on the CCI model of neuropathic pain via decreasing MDA content and increasing SOD and GPx activity and also the total antioxidant capacity of the rats’ sciatic nerve.
Ethical Considerations
Compliance with ethical guidelines
Animal handling and all the experiments were done according to the guides of the Ethical Committee of Kashan University of Medical Science (Code: IR.KAUMS.MEDNT.REC.1396.104) and also the Directive 2010/63/EU on the protection of animals used for scientific purposes.
Funding
This study was financially supported by Deputy of Research and Technology, Kashan University of Medical Sciences (Grant No.: 96201, that was assigned to Sayyed Alireza Talaei).
Authors' contributions
Conceptualization, supervision, and data analysis: Sayyed Alireza Talaei; Data collection: Mohammad Shabani, Elham Hasanpour, Mojgan Mohammadifar and Fereshteh Bahmani; Methodology and data analysis: Fatemeh Aghighi; Writing draft, reviewing & editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Alireza Abed for feedback on the manuscript and Mehdi Haghighat for technical assistance.
References
- Amdekar S., Roy P., Singh V., Kumar A., Singh R., Sharma P. (2012). Anti-inflammatory activity of lactobacillus on carrageenan-induced paw edema in male Wistar rats. International Journal of Inflammation, 2012, 752015. [DOI: 10.1155/2012/752015] [PMID https://www.ncbi.nlm.nih.gov/pubmed/22518342] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3299308] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdekar S., Singh V. (2016). Studies on anti-inflammatory and analgesic properties of Lactobacillus rhamnosus in experimental animal models. Journal of Complementary and Integrative Medicine, 13(2), 145–150. [DOI: 10.1515/jcim-2015-0087] [PMID https://www.ncbi.nlm.nih.gov/pubmed/27078675] [DOI] [PubMed] [Google Scholar]
- Amdekar S., Singh V., Kumar A., Sharma P., Singh R. (2013). Lactobacillus casei and Lactobacillus acidophilus regulate inflammatory pathway and improve antioxidant status in collagen-induced arthritic rats. Journal of Interferon & Cytokine Research, 33(1), 1–8. [DOI: 10.1089/jir.2012.0034] [PMID https://www.ncbi.nlm.nih.gov/pubmed/23030670] [DOI] [PubMed] [Google Scholar]
- Amdekar S., Singh V., Singh R., Sharma P., Keshav P., Kumar A. (2011). Lactobacillus casei reduces the inflammatory joint damage associated with collagen-induced arthritis (CIA) by reducing the pro-inflammatory cytokines. Journal of Clinical Immunology, 31(2), 147–154. [DOI: 10.1007/s10875-010-9457-7] [PMID https://www.ncbi.nlm.nih.gov/pubmed/20838859] [DOI] [PubMed] [Google Scholar]
- Amin B., Noorani R., Razavi B. M., Hosseinzadeh H. (2018). The effect of ethanolic extract of Lippia citriodora on rats with chronic constriction injury of neuropathic pain. Cell Journal (Yakhteh), 19(4), 528–536. [Link ] https://www.celljournal.org/article_250460.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcárate-Peril M. A., Sikes M., Bruno-Bárcena J. M. (2011). The intestinal microbiota, gastrointestinal environment and colorectal cancer: A putative role for probiotics in prevention of colorectal cancer? American Journal of Physiology-Gastrointestinal and Liver Physiology, 301(3), G401–G424. [DOI: 10.1152/ajpgi.00110.2011] [PMID https://www.ncbi.nlm.nih.gov/pubmed/21700901] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3774253] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G. J., Xie Y. K. (1988). A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain, 33(1), 87–107. [DOI: 10.1016/0304-3959(88)90209-6] [PMID https://www.ncbi.nlm.nih.gov/pubmed/2837713] [DOI] [PubMed] [Google Scholar]
- Bittar A., Jun J., La J. H., Wang J., Leem J. W., Chung J. M. (2017). Reactive oxygen species affect spinal cell type-specific synaptic plasticity in a model of neuropathic pain. Pain, 158(11), 2137–2146. [DOI: 10.1097/j.pain.0000000000001014] [PMID https://www.ncbi.nlm.nih.gov/pubmed/28708760] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5963270] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo J. A., Julio-Pieper M., Forsythe P., Kunze W., Dinan T. G., Bienenstock J., et al. (2012). Communication between gastrointestinal bacteria and the nervous system. Current Opinion in Pharmacology, 12(6), 667–672. [DOI: 10.1016/j.coph.2012.09.010] [PMID https://www.ncbi.nlm.nih.gov/pubmed/23041079] [DOI] [PubMed] [Google Scholar]
- Colloca L., Ludman T., Bouhassira D., Baron R., Dickenson A. H., Yarnitsky D., et al. (2017). Neuropathic pain. Nature Reviews Disease Primers, 3, 17002. [DOI: 10.1038/nrdp.2017.2] [PMID https://www.ncbi.nlm.nih.gov/pubmed/28205574] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5371025] [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza A., Fordjour L., Ahmad A., Cai C., Kumar D., Valencia G., et al. (2010). Effects of probiotics, prebiotics, and synbiotics on messenger RNA expression of caveolin-1, NOS, and genes regulating oxidative stress in the terminal ileum of formula-fed neonatal rats. Pediatric Research, 67(5), 526–531. [DOI: 10.1203/PDR.0b013e3181d4ff2b] [PMID https://www.ncbi.nlm.nih.gov/pubmed/20101198] [DOI] [PubMed] [Google Scholar]
- de Oliveira G. L. V., Leite A. Z., Higuchi B. S., Gonzaga M. I., Mariano V. S. (2017). Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology, 152(1), 1–12. [DOI: 10.1111/imm.12765] [PMID https://www.ncbi.nlm.nih.gov/pubmed/28556916] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5543467] [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vry J., Kuhl E., Franken-Kunkel P., Eckel G. (2004). Pharmacological characterization of the chronic constriction injury model of neuropathic pain. European Journal of Pharmacology, 491(2–3), 137–148. [DOI: 10.1016/j.ejphar.2004.03.051] [PMID https://www.ncbi.nlm.nih.gov/pubmed/15140630] [DOI] [PubMed] [Google Scholar]
- Douglas-Escobar M., Elliott E., Neu J. (2013). Effect of intestinal microbial ecology on the developing brain. JAMA Pediatrics, 167(4), 374–379. [DOI: 10.1001/jamapediatrics.2013.497] [PMID https://www.ncbi.nlm.nih.gov/pubmed/23400224] [DOI] [PubMed] [Google Scholar]
- Esmaeili S. A., Mahmoudi M., Momtazi A. A., Sahebkar A., Doulabi H., Rastin M. (2017). Tolerogenic probiotics: Potential immunoregulators in Systemic Lupus Erythematosus. Journal of Cellular Physiology, 232(8), 1994–2007. [DOI: 10.1002/jcp.25748] [PMID https://www.ncbi.nlm.nih.gov/pubmed/27996081] [DOI] [PubMed] [Google Scholar]
- Gaisawat M. B., Iskandar M. M., MacPherson C. W., Tompkins T. A., Kubow S. (2019). Probiotic supplementation is associated with increased antioxidant capacity and copper chelation in C. difficile-infected fecal water. Nutrients, 11(9), 2007. [DOI: 10.3390/nu11092007] [PMID https://www.ncbi.nlm.nih.gov/pubmed/31454897] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6769851] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Kim H. K., Mo Chung J., Chung K. (2007). Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain, 131(3), 262–271. [DOI: 10.1016/j.pain.2007.01.011] [PMID https://www.ncbi.nlm.nih.gov/pubmed/17317010] [PMCID] http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2048490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B. L., Sui B. D., Wang X. Y., Wei Y. Y., Huang J., Chen J., et al. (2013). Significant changes in mitochondrial distribution in different pain models of mice. Mitochondrion, 13(4), 292–297. [DOI: 10.1016/j.mito.2013.03.007] [PMID https://www.ncbi.nlm.nih.gov/pubmed/23542162] [DOI] [PubMed] [Google Scholar]
- Hadidi Zavareh A. H., Haji Khani R., Pakpour B., Soheili M., Salami M. (2020). Probiotic treatment differentially affects the behavioral and electrophysiological aspects in ethanol exposed animals. Iranian Journal of Basic Medical Sciences, 23(6), 776–780. [PMID https://pubmed.ncbi.nlm.nih.gov/32695294/] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré P. H., Basnet A., Eljaja L., Kristensen P., Andersen L. M., Neustrup S., et al. (2011). Neuropathic pain models in the development of analgesic drugs. Scandinavian Journal of Pain, 2(4), 172–177. [DOI: 10.1016/j.sjpain.2011.06.003] [PMID https://www.ncbi.nlm.nih.gov/pubmed/29913750] [DOI] [PubMed] [Google Scholar]
- Huang J., Zhang C., Wang J., Guo Q., Zou W. (2019). Oral Lactobacillus reuteri LR06 or Bifidobacterium BL5b supplement do not produce analgesic effects on neuropathic and inflammatory pain in rats. Brain and Behavior, 9(4), e01260. [DOI: 10.1002/brb3.1260] [PMID https://www.ncbi.nlm.nih.gov/pubmed/30839179] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6456777] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium . (2012). Structure, function and diversity of the healthy human microbiome. Nature, 486(7402), 207–214. [DOI: 10.1038/nature11234] [PMID https://www.ncbi.nlm.nih.gov/pubmed/22699609] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3564958] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T. S., Finnerup N. B. (2014). Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. The Lancet. Neurology, 13(9), 924–935. [DOI: 10.1016/S1474-4422(14)70102-4] [PMID https://www.ncbi.nlm.nih.gov/pubmed/25142459] [DOI] [PubMed] [Google Scholar]
- Kim H. K., Zhang Y. P., Gwak Y. S., Abdi S. (2010). Phenyl N-tert-butylnitrone, a free radical scavenger, reduces mechanical allodynia in chemotherapy-induced neuropathic pain in rats. Anesthesiology, 112(2), 432–439. [DOI: 10.1097/ALN.0b013e3181ca31bd] [PMID https://www.ncbi.nlm.nih.gov/pubmed/20068451] [DOI] [PubMed] [Google Scholar]
- Kullisaar T., Zilmer M., Mikelsaar M., Vihalemm T., Annuk H., Kairane C., et al. (2002). Two antioxidative lactobacilli strains as promising probiotics. International Journal of Food Microbiology, 72(3), 215–224. [DOI: 10.1016/S0168-1605(01)00674-2] [PMID https://www.ncbi.nlm.nih.gov/pubmed/11845820] [DOI] [PubMed] [Google Scholar]
- Kwon H. K., Kim G. C., Kim Y., Hwang W., Jash A., Sahoo A., et al. (2013). Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clinical Immunology, 146(3), 217–227. [DOI: 10.1016/j.clim.2013.01.001] [PMID https://www.ncbi.nlm.nih.gov/pubmed/23416238] [DOI] [PubMed] [Google Scholar]
- Liang S., Wang T., Hu X., Luo J., Li W., Wu X., et al. (2015). Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience, 310, 561–577. [DOI: 10.1016/j.neuroscience.2015.09.033] [PMID https://www.ncbi.nlm.nih.gov/pubmed/26408987] [DOI] [PubMed] [Google Scholar]
- Lin M. Y., Yen C. L. (1999). Antioxidative ability of lactic acid bacteria. Journal of Agricultural and Food Chemistry, 47(4), 1460–1466. [DOI: 10.1021/jf981149l] [PMID https://www.ncbi.nlm.nih.gov/pubmed/10563999] [DOI] [PubMed] [Google Scholar]
- Mao Y. F., Yan N., Xu H., Sun J. H., Xiong Y. C., Deng X. M. (2009). Edaravone, a free radical scavenger, is effective on neuropathic pain in rats. Brain Research, 1248, 68–75. [DOI: 10.1016/j.brainres.2008.10.073] [PMID https://www.ncbi.nlm.nih.gov/pubmed/19028459] [DOI] [PubMed] [Google Scholar]
- Martarelli D., Verdenelli M. C., Scuri S., Cocchioni M., Silvi S., Cecchini C., et al. (2011). Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Current Microbiology, 62(6), 1689–1696. [DOI: 10.1007/s00284-011-9915-3] [PMID https://www.ncbi.nlm.nih.gov/pubmed/21400082] [DOI] [PubMed] [Google Scholar]
- Mishra V., Shah C., Mokashe N., Chavan R., Yadav H., Prajapati J. (2015). Probiotics as potential antioxidants: A systematic review. Journal of Agricultural and Food Chemistry, 63(14), 3615–3626. [DOI: 10.1021/jf506326t] [PMID https://www.ncbi.nlm.nih.gov/pubmed/25808285] [DOI] [PubMed] [Google Scholar]
- Murnion B. P. (2018). Neuropathic pain: Current definition and review of drug treatment. Australian Prescriber, 41(3), 60–63. [DOI: 10.18773/austprescr.2018.022] [PMID https://www.ncbi.nlm.nih.gov/pubmed/29921999] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6003018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik A. K., Tandan S. K., Dudhgaonkar S. P., Jadhav S. H., Kataria M., Prakash V. R., et al. (2006). Role of oxidative stress in pathophysiology of peripheral neuropathy and modulation by N-acetyl-L-cysteine in rats. European Journal of Pain, 10(7), 573–579. [DOI: 10.1016/j.ejpain.2005.08.006] [PMID https://www.ncbi.nlm.nih.gov/pubmed/16214382] [DOI] [PubMed] [Google Scholar]
- Pathak N. N., Balaganur V., Lingaraju M. C., Kant V., Latief N., More A. S., et al. (2014). Atorvastatin attenuates neuropathic pain in rat neuropathy model by down-regulating oxidative damage at peripheral, spinal and supraspinal levels. Neurochemistry International, 68, 1–9. [DOI: 10.1016/j.neuint.2014.01.014] [PMID https://www.ncbi.nlm.nih.gov/pubmed/24513038] [DOI] [PubMed] [Google Scholar]
- Shen Q., Shang N., Li P. (2011). In vitro and in vivo antioxidant activity of bifidobacterium animalis 01 isolated from centenarians. Current Microbiology, 62, 1097–1103. [DOI: 10.1007/s00284-010-9827-7] [PMID https://www.ncbi.nlm.nih.gov/pubmed/21132298] [DOI] [PubMed] [Google Scholar]
- Shen Q., Shang N., Li P. (2011). In vitro and in vivo anti-oxidant activity of Bifidobacterium animalis 01 isolated from centenarians. Current Microbiology, 62(4), 1097–1103. [DOI: 10.1007/s00284-010-9827-7] [PMID https://www.ncbi.nlm.nih.gov/pubmed/21132298] [DOI] [PubMed] [Google Scholar]
- Shim H. S., Bae C., Wang J., Lee K. H., Hankerd K. M., Kim H. K., et al. (2019). Peripheral and central oxidative stress in chemotherapy-induced neuropathic pain. Molecular Pain, 15, 1744806919840098. [DOI: 10.1177/1744806919840098] [PMID https://www.ncbi.nlm.nih.gov/pubmed/30857460] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6458664] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoskes D. A., Wang H., Polackwich A. S., Tucky B., Altemus J., Eng C. (2016). Analysis of gut microbiome reveals significant differences between men with chronic prostatitis/chronic pelvic pain syndrome and controls. The Journal of Urology, 196(2), 435–441. [DOI: 10.1016/j.juro.2016.02.2959] [PMID https://www.ncbi.nlm.nih.gov/pubmed/26930255] [DOI] [PubMed] [Google Scholar]
- Stecchini M. L., Del Torre M., Munari M. (2001). Determination of peroxy radical-scavenging of lactic acid bacteria. International Journal of Food Microbiology, 64(1–2), 183–188. [DOI: 10.1016/S0168-1605(00)00456-6] [DOI] [PubMed] [Google Scholar]
- Wang A. N., Yi X. W., Yu H. F., Dong B., Qiao S. Y. (2009). Free radical scavenging activity of Lactobacillus fermentum in vitro and its antioxidative effect on growing-finishing pigs. Journal of Applied Microbiology, 107(4), 1140–1148. [DOI: 10.1111/j.1365-2672.2009.04294.x] [PMID https://www.ncbi.nlm.nih.gov/pubmed/19486423] [DOI] [PubMed] [Google Scholar]
- Wang Y., Wu Y., Wang Y., Xu H., Mei X., Yu D., et al. (2017). Antioxidant properties of probiotic bacteria. Nutrients, 9(5), 521. [DOI: 10.3390/nu9050521] [PMID https://www.ncbi.nlm.nih.gov/pubmed/28534820] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5452251] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K, Walker J. Principles and techniques of practical biochemistry. Cambridge, Cambridge University Press: 2010. [Link https://books.google.com/books/about/Principles_and_Techniques_of_Practical_B.html?id=0NvlqobpwCwC] [Google Scholar]
- Xu Y. Q., Jin S. J., Liu N., Li Y. X., Zheng J., Ma L., et al. (2014). Aloperine attenuated neuropathic pain induced by chronic constriction injury via anti-oxidation activity and suppression of the nuclear factor kappa B pathway. Biochemical and Biophysical Research Communications, 451(4), 568–573. [DOI: 10.1016/j.bbrc.2014.08.025] [PMID https://www.ncbi.nlm.nih.gov/pubmed/25128276] [DOI] [PubMed] [Google Scholar]
- Yowtak J., Lee K. Y., Kim H. Y., Wang J., Kim H. K., Chung K., et al. (2011). Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain, 152(4), 844–852. [DOI: 10.1016/j.pain.2010.12.034] [PMID https://www.ncbi.nlm.nih.gov/pubmed/21296500] [PMCID http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3108328] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B. S., Meng L. X., Ding Y. Y., Cao Y. Y. (2014). Hyper-baric oxygen treatment produces an antinociceptive response phase and inhibits astrocyte activation and inflammatory response in a rat model of neuropathic pain. Journal of Molecular Neuroscience, 53(2), 251–261. [DOI: 10.1007/s12031-013-0213-3] [PMID https://www.ncbi.nlm.nih.gov/pubmed/24390961] [DOI] [PubMed] [Google Scholar]