Abstract
Kaposiform lymphangiomatosis (KLA) is a rare and complex lymphatic anomaly associated with substantial morbidity and mortality. It features diffuse and multifocal malformed lymphatic channels, often leading to diagnostic difficulties due to its uncommon occurrence and non-specific clinical presentation. This case report emphasizes the crucial role of expert radiologists in accurately diagnosing a challenging KLA case initially mistaken for a neoplasm.
Keywords: Lymphatic anomalies, Computer tomography, Magnetic resonance, MTOR-inhibitors, Kaposiform lymphangiomatosis
Introduction
Kaposiform lymphangiomatosis (KLA) is a rare disorder characterized by diffuse proliferation of abnormal, dilated lymphatics with uncertain prevalence and typical onset in childhood. It is associated with significant morbidity and a poor prognosis. While the exact cause and mechanisms remain elusive, dysregulated signaling pathways have been implicated. Treatment options are limited, with sirolimus and steroids commonly used. This case report aims to shed light on the challenges of diagnosing KLA by presenting the case of a 43-year-old female with a history of chylothorax and severe disseminated intravascular coagulopathy (DIC), initially misdiagnosed with myelodysplastic syndrome (MDS). This report reviews imaging findings of diffuse lymphangiomatosis and underscores the importance of considering rare primary lymphatic disorders in cases of chronic DIC and atypical clinical presentations when supported by characteristic imaging findings.
Case report
In summary, this 43-year-old female presented with transfusion-dependent DIC, shortness of breath, and generalized malaise. Extensive work-up for infectious and malignant etiologies was negative. Imaging showed a diffuse low-density infiltrative process throughout the pleura, pericardium, gall bladder wall, and subcutaneous tissues consistent with lymphangiomatosis. She has been treated for KLA with appropriate therapy response.
Initial presentation
A 43-year-old female with a one-year history of restrictive lung disease and remote history of idiopathic bilateral chylothorax status post-talc pleurodesis 20 years prior presented to an outside emergency department after an episode of shortness of breath, myalgias, and generalized fatigue.
During her visit, she was found to be severely thrombocytopenic and in disseminated intravascular coagulopathy (DIC) requiring transfusions. A computed tomography (CT) scan revealed enhancing nodularity along the pleural and peritoneal cavities with infiltrative processes around the pericardium and mediastinum, raising concerns for neoplasm (initial CT not pictured). The patient was discharged home and told to follow up with hematology.
During the following 6 months, the patient underwent extensive diagnostic workup to assess for infectious, malignant, and autoimmune causes, including numerous laboratory tests (HIV, B-HCG, CEA, CA-19-9, Lactate dehydrogenase, ANA, among others) but all were unrevealing. During this work-up, a bone marrow biopsy revealed mild hypercellularity and the presence of pleomorphic megakaryocytes leading to a presumptive diagnosis of myelodysplastic syndrome (MDS) but this was eventually ruled out through flow cytometry, and fluorescence in situ hybridization (FISH). Meanwhile, the patient's condition continued deteriorating, necessitating multiple ED visits during which DIC findings were frequently observed.
Subsequent presentation and radiologic diagnosis
Approximately 6 months after the initial presentation, the patient presented to our hospital for severe and refractory transfusion-dependent DIC. Chest radiograph revealed bilateral pleural thickening and interlobular septal thickening, initially thought to be loculated bilateral pleural effusions and interstitial edema (Fig. 1). Contrast-enhanced CT showed low-density infiltration of the pleura, pericardium, gall bladder wall, and flank unchanged from her initial outside CT scan 6 months earlier (Fig. 2). Thin enhancing septations were noted in the pleural infiltrative process. Magnetic resonance (MR) was performed for further characterization, displaying fluid signal with thin septations and delayed enhancement in the involved areas consistent with extensive lymphangiomatosis (Figs. 3 and 4).
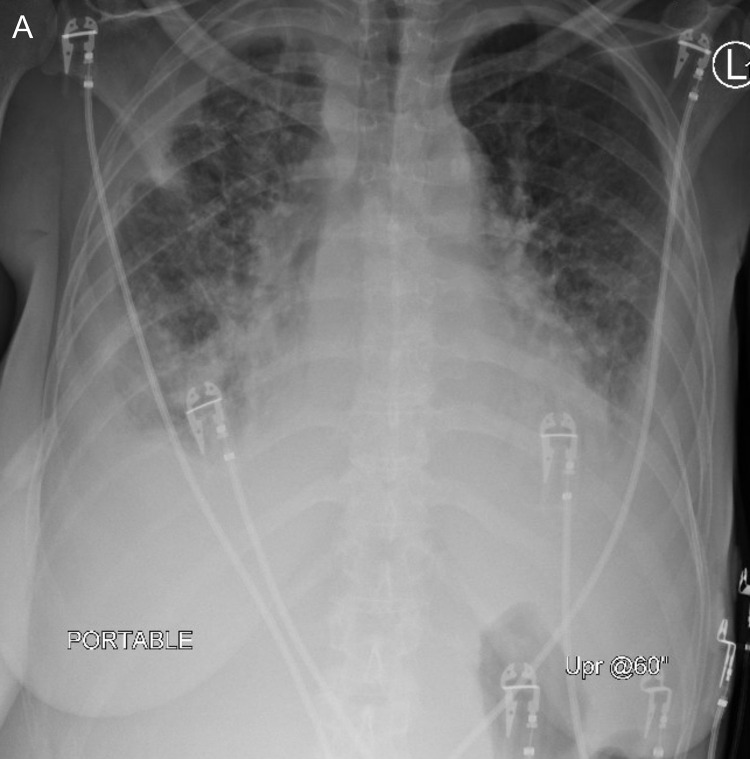
Fig. 1.
Chest radiograph at the time of admission to our hospital revealed bilateral pleural thickening and interstitial thickening initially thought to be loculated bilateral pleural effusions and pulmonary edema.
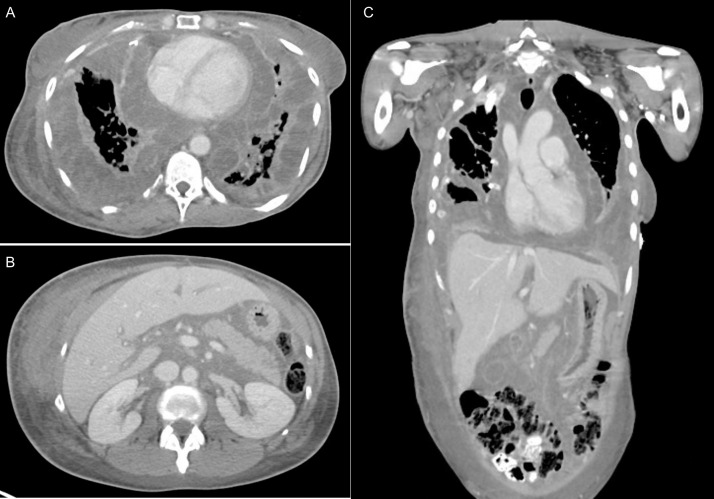
Fig. 2.
Contrast enhanced CT at the time of admission to our hospital showed low density infiltrative soft tissue with thin-enhancing septations with diffuse involvement of the pleura and pericardium (A), right flank and mesentery (B), and gallbladder wall (C).
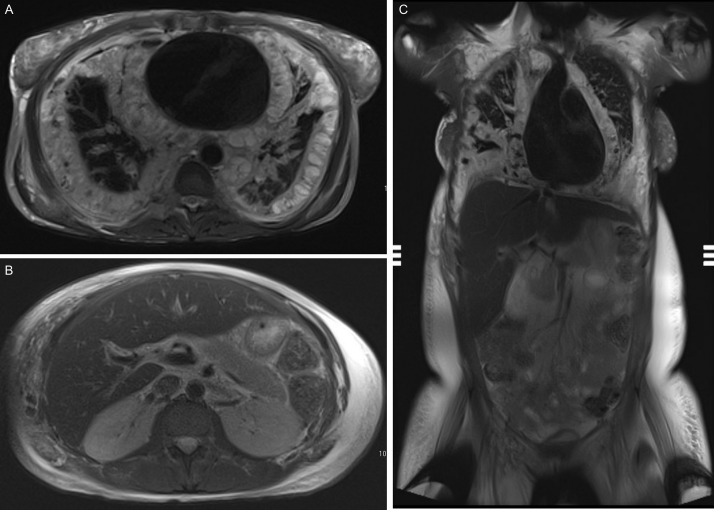
Fig. 3.
T2-weighted MR sequences at the same levels and planes as CT in Figure 2. The infiltrative process seen is fluid signal with thin septations with diffuse involvement of the pleura and pericardium (A), mesentery and flank (B), and gallbladder wall (C).
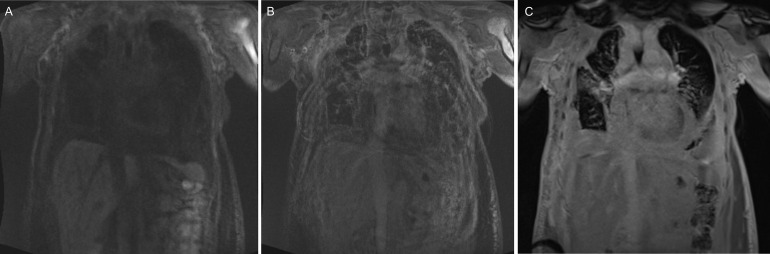
Fig. 4.
Pre- and postcontrast fat-saturated T1-weighted MR images. (A) Precontrast coronal images of the chest and abdomen show intrinsic T1 hyperintensity of the infiltration around the pleura, pericardium, flank, and mesentery. (B) 4-minute postcontrast images show enhancement of the thin septations, but fill-in of the fluid. (C) 10-minute postcontrast images show delayed enhancement of the entire structure including fill-in of the fluid component, consistent with a lymphatic malformation like lymphangiomatosis.
In a multi-disciplinary vascular malformation discussion, the patient's combined history of chylothorax, DIC, and specific imaging findings were collectively deemed consistent with the rare primary lymphatic disorder, KLA. The patient was discharged on a regimen of sirolimus and steroids. One year after starting therapy, there was significant radiologic and clinical improvement in her disease, with resolution of DIC and improvement in her restrictive lung disease (Fig. 5).
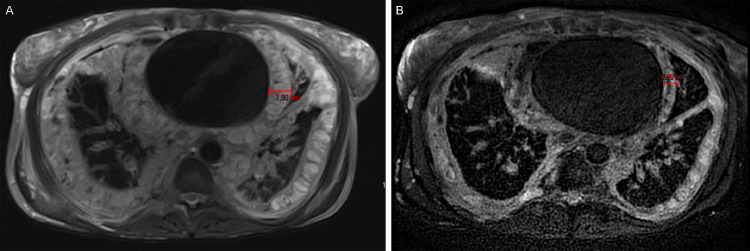
Fig. 5.
Axial T2 weighted images through the chest at the time of diagnosis (A) and after 1 year of therapy with sirolimus and steroids (B). There is a marked reduction in the burden of lymphangiomatosis with thinning of the pleural (1.8-0.9 cm) and pericardial involvement.
Discussion
Epidemiology
KLA is an uncommon vascular condition that was initially documented in 2014 and subsequently classified by The International Society for the Study of Vascular Anomalies (ISSVA) as a subset of generalized lymphatic anomaly (GLA). However, the precise categorization of KLA remains a subject of active debate, as some proponents argue that KLA exhibits tumor-like characteristics [1,2]. The prevalence of KLA is still uncertain due to its rarity [1]. Various studies have reported an average age of symptom onset ranging from 6 to 8 years, although a limited number of cases have reported an onset of symptoms during early adulthood [1], [2], [3]. KLA is linked to substantial morbidity and an overall unfavorable prognosis, with studies indicating a 5-year survival rate that is generally poor, ranging from 50% to 71% [1], [2], [3]. No sex predilection has been observed [2].
Etiopathology
The exact etiopathology of KLA remains unclear, but significant research efforts have been dedicated to unraveling its molecular underpinnings, particularly focusing on genetic factors and serum biomarkers. Exome sequencing studies have revealed somatic mutations in the regulation of canonical pathways MAPK and PI3K/AKT/MTOR, which are crucial for cellular angiogenesis, and when mutated, tumorigenesis [4], [5], [6], [7], [8]. Several cases in the literature have shown marked improvement in patients with KLA following treatment with Sirolimus, an MTOR pathway inhibitor, such as in our patient [9], [10], [11]. Furthermore, elevated levels of angiogenic factor angiopoietin-2 have been reported in patients with KLA [8,12,13]. Although its significance remains elusive, it has the potential to serve as a biomarker for therapeutic monitoring and diagnostic purposes [12,13].
Clinical presentation
KLA predominantly affects the mediastinum, lungs, and pleura [1,2,14]. Clinical presentations commonly manifest with dyspnea and cough, often accompanied by pleural effusions [1,2,14]. KLA also involves osseous structures, leading to pain and an increased risk of fractures [15]. Notably, DIC, characterized by coagulopathy and thrombocytopenia frequently complicate KLA, resulting in bleeding and the development of hemorrhagic pericardial and pleural effusions [1,2,16]. Tissue swelling and spleen involvement are also observed [14,17,18] Mortality in KLA is typically associated with hemorrhage and cardiorespiratory failure [2,19].
Pathology
The diagnosis of KLA often encounters delays due to the heterogeneity of symptoms and the rarity of the condition [1,2]. Pathology and imaging play crucial roles in establishing the diagnosis. Histologically, KLA is typically characterized by diffuse proliferation of abnormal, dilated lymphatics and small fascicles of hemosiderin-laden spindled lymphatic endothelial cells, exhibiting the absence of cellular atypia and a lack of prominent mitoses [2,20]. While pathology provides valuable insights for diagnosing KLA, biopsy procedures may be deferred in certain patients due to significant coagulopathy and thrombocytopenia [1].
Imaging findings
Imaging plays a critical role in the diagnosis of KLA. Although the diagnosis can be easily overlooked by trained individuals, there are distinct features identifiable in imaging studies that should raise suspicion for KLA and other vascular anomalies. On imaging, the most affected regions include the mediastinum, hilum, lungs, pleura, bones, and spleen [2,21]. Plain radiographs have limited utility and typically show nonspecific changes in the lung parenchyma and pleural thickening that mimics pleural effusions. However, both plain radiographs and computed tomography (CT) scans can be valuable for assessing bone insults which often manifest in KLA as osteolytic lesions with sparing of the cortical bone, predominantly affecting the vertebral bodies and ribs [2,15,21]. CT scans of the thoracic cavity may demonstrate infiltrative low-density soft tissue thickening following the lymphatic distribution of the bronchovascular bundles. Additionally, segmental interlobular septal thickening, parenchymal ground-glass opacities, and mediastinal fat infiltration can be observed [1,21]. Pleural and pericardial low-density infiltrative soft tissue or loculated effusions, which may exhibit serosanguineous, chylous, or mixed characteristics, are commonly detected on CT scans [1,21]. Abdominal CT scans can reveal organomegaly, retroperitoneal low-density infiltrative soft tissue commonly involving the retrocrural region, and hypodense cystic lesions in the spleen [16,21]. Magnetic resonance imaging (MRI) demonstrates a similar pattern to CT scans, with fluid signal infiltrating along the pleura and pericardium, and spleen cystic lesions with delayed post-contrast enhancement [21]. Osseous abnormalities infiltrating the bone marrow can be visualized as fluid signal infiltrative lesions with delayed enhancement on MRI [15,21]. Magnetic resonance lymphangiography (MRL) can also be employed to evaluate the extent of lymphatic vessel abnormalities by assessing central lymphatic channels, ductal dilation, tortuosity, and flow patterns [22].
Differential diagnosis
The differential diagnosis of KLA comprises conditions such as central conducting lymphatic anomaly (CCLA), Gorham-Stout disease (GSD), and Kaposiform hemangioendothelioma (KHE). However, due to its rarity, overlapping symptoms, and heterogeneity, KLA is often initially confused with more common conditions like pneumonia, neoplasm, or rheumatologic disease [1,10]. CCLA is primarily characterized by radiological evidence of impaired lymphatic drainage resulting in central ductal dilation [1,10,23]. Conversely KLA displays pronounced mediastinal disease, without significant dilation of the central lymphatic channels [1], [2], [3],10,17]. GSD is hallmarked by prominent skeletal involvement, with characteristic findings of trabecular osteolysis and cortical bone loss, unlike KLA, which spares cortical bone [10,14,15,23]. KHE typically manifests as a unifocal, infiltrative, enhancing mass in the trunk, while KLA tends to exhibit diffuse and multifocal involvement in the thoracic and extra-thoracic regions [2,10,21,24]. Collectively, a detailed description of structures involved in all imaging modalities is critical for accurate diagnosis of KLA.
Management
Currently, there is no consensus on optimal treatment modalities for KLA. However, patients are typically managed through a combination of medical therapies, including sirolimus, often in combination with steroids [9], [10], [11]. While medical therapies are essential in managing the condition, symptomatic management targeting coagulopathy and pleural or pericardial effusions is frequently employed [1]. Splenectomy has been performed in patients with profound thrombocytopenia [2]. Additionally, patients with significant osseous involvement may receive bisphosphonates as a supplementary treatment [10,15]. However, the overall therapeutic response remains heterogeneous, and many patients ultimately experience disease progression.
Conclusion
We presented the case of a 43-year-old female with presumed chylothorax and severe DIC diagnosed with KLA through meticulous radiological evaluation and interdisciplinary collaboration. Patient was appropriately managed with sirolimus, resulting in notable clinical improvement corroborated by MRI.
This case underscores the challenging clinical nature of KLA, and the critical role of radiologist for timely diagnosis. We also highlighted KLA salient radiological features including diffuse, multifocal thoracic involvement with enhancing septations in the context of chronic respiratory symptoms, which collectively should raise a strong suspicion of KLA.
Patient consent
I hereby confirm that patient reported in the manuscript signed the informed consent/authorization for participation in research which includes the permission to use data collected in future research projects including presented case details and images used in this manuscript. No protected health information is shown in this case report.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.McDaniel CG, Adams DM, Steele KE, Hammill AM, Merrow AC, Crane JL, et al. Kaposiform lymphangiomatosis: diagnosis, pathogenesis, and treatment. Pediatr Blood Cancer. 2023;70(4) doi: 10.1002/PBC.30219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croteau SE, Kozakewich HP, Perez-Atayde AR, Fishman SJ, Alomari AI, Chaudry G, et al. Kaposiform lymphangiomatosis: a distinct aggressive lymphatic anomaly. J Pediatr. 2014;164(2):383. doi: 10.1016/J.JPEDS.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez-Atayde AR, Debelenko L, Al-Ibraheemi A, Eng W, Ruiz-Gutierrez M, O’Hare M, et al. Kaposiform lymphangiomatosis: pathologic aspects in 43 patients. Am J Surg Pathol. 2022;46(7):963–976. doi: 10.1097/PAS.0000000000001898. [DOI] [PubMed] [Google Scholar]
- 4.Barclay SF, Inman KW, Luks VL, McIntyre JB, Al-Ibraheemi A, Church AJ, et al. A somatic activating NRAS variant associated with kaposiform lymphangiomatosis. Genet Med. 2019;21(7):1517–1524. doi: 10.1038/S41436-018-0390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boscolo E, Pastura P, Glaser K, Goines J, Hammill AM, Adams DM, et al. Signaling pathways and inhibitors of cells from patients with kaposiform lymphangiomatosis. Pediatr Blood Cancer. 2019;66(8) doi: 10.1002/PBC.27790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19(3):1997. doi: 10.3892/ETM.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castellano E, Downward J. RAS Interaction with PI3K: more than just another effector pathway. Genes Cancer. 2011;2(3):261. doi: 10.1177/1947601911408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karar J, Maity A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front Mol Neurosci. 2011;4:51. doi: 10.3389/FNMOL.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Li K, Yao W, Dong K, Xiao X, Zheng S. Successful treatment of kaposiform lymphangiomatosis with sirolimus. Pediatr Blood Cancer. 2015;62(7):1291–1293. doi: 10.1002/PBC.25422. [DOI] [PubMed] [Google Scholar]
- 10.Adams DM, Ricci KW. Vascular anomalies: diagnosis of complicated anomalies and new medical treatment options. Hematol Oncol Clin North Am. 2019;33(3):455–470. doi: 10.1016/J.HOC.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Adams DM, Trenor CC, Hammill AM, Vinks AA, Patel MN, Chaudry G, et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics. 2016;137(2) doi: 10.1542/PEDS.2015-3257/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozeki M, Nozawa A, Kawamoto N, Fujino A, Hirakawa S, Fukao T. Potential biomarkers of kaposiform lymphangiomatosis. Pediatr Blood Cancer. 2019;66(9):e27878. doi: 10.1002/PBC.27878. [DOI] [PubMed] [Google Scholar]
- 13.Le Cras TD, Mobberley-Schuman PS, Broering M, Fei L, Trenor CC, Adams DM. Angiopoietins as serum biomarkers for lymphatic anomalies. Angiogenesis. 2017;20(1):163–173. doi: 10.1007/S10456-016-9537-2/FIGURES/6. [DOI] [PubMed] [Google Scholar]
- 14.Ozeki M, Fujino A, Matsuoka K, Nosaka S, Kuroda T, Fukao T. Clinical features and prognosis of generalized lymphatic anomaly, kaposiform lymphangiomatosis, and Gorham–Stout Disease. Pediatr Blood Cancer. 2016;63(5):832–838. doi: 10.1002/PBC.25914. [DOI] [PubMed] [Google Scholar]
- 15.Solorzano E, Alejo AL, Ball HC, Magoline J, Khalil Y, Kelly M, et al. Osteopathy in complex lymphatic anomalies. Int J Mol Sci. 2022;23(15) doi: 10.3390/IJMS23158258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato H, Ozeki M, Fukao T, Matsuo M. Chest imaging in generalized lymphatic anomaly and kaposiform lymphangiomatosis. Pediatr Int. 2018;60(7):667–668. doi: 10.1111/PED.13593. [DOI] [PubMed] [Google Scholar]
- 17.Suárez-Vilela D, Izquierdo FM, Honrado E, Díez-Tascón C. Splenic lesions and other findings in kaposiform lymphangiomatosis. Am J Surg Pathol. 2023;47(5):631–633. doi: 10.1097/PAS.0000000000002026. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes VM, Fargo JH, Saini S, Guerrera MF, Marcus L, Luchtman-Jones L, et al. Kaposiform lymphangiomatosis: unifying features of a heterogeneous disorder. Pediatr Blood Cancer. 2015;62(5):901–904. doi: 10.1002/PBC.25278. [DOI] [PubMed] [Google Scholar]
- 19.Bundy JJ, Ootaki Y, McLean TW, Hays BS, Miller M, Downing T. Thoracic duct embolization in kaposiform lymphangiomatosis. J Vasc Surg Venous Lymphat Disord. 2020;8(5):864–868. doi: 10.1016/J.JVSV.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Allen-Rhoades W, Al-Ibraheemi A, Kohorst M, Tollefson M, Hull N, Polites S, et al. Cellular variant of kaposiform lymphangiomatosis: a report of three cases, expanding the morphologic and molecular genetic spectrum of this rare entity. Hum Pathol. 2022;122:72–81. doi: 10.1016/J.HUMPATH.2022.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Goyal P, Alomari AI, Kozakewich HP, Trenor CC, 3rd, Perez-Atayde AR, Fishman SJ, et al. Imaging features of kaposiform lymphangiomatosis. Pediatr Radiol. 2016;46(9):1282–1290. doi: 10.1007/S00247-016-3611-1/FIGURES/7. [DOI] [PubMed] [Google Scholar]
- 22.Itkin M. Vascular anomalies: magnetic resonance lymphangiography and lymphatic embolization in the treatment of pulmonary complication of lymphatic malformation. Semin Intervent Radiol. 2017;34(3):294. doi: 10.1055/S-0037-1604301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iacobas I, Adams DM, Pimpalwar S, Phung T, Blei F, Burrows P, et al. Multidisciplinary guidelines for initial evaluation of complicated lymphatic anomalies-expert opinion consensus. Pediatr Blood Cancer. 2020;67(1) doi: 10.1002/PBC.28036. [DOI] [PubMed] [Google Scholar]
- 24.DeFatta RJ, Verret DJ, Adelson RT, Gomez A, Myers LL. Kaposiform hemangioendothelioma: case report and literature review. Laryngoscope. 2005;115(10):1789–1792. doi: 10.1097/01.mlg.0000176539.94515.75. [DOI] [PubMed] [Google Scholar]