Abstract
Backgrounds/Aims
Liver organoids have emerged as a powerful tool for studying liver biology and disease and for developing new therapies and regenerative medicine approaches. For organoid culture, Matrigel, a type of extracellular matrix, is the most commonly used material. However, Matrigel cannot be used for clinical applications due to the presence of unknown proteins that can cause immune rejection, batch-to-batch variability, and angiogenesis.
Methods
To obtain human primary hepatocytes (hPHs), we performed 2 steps collagenase liver perfusion protocol. We treated three small molecules cocktails (A83-01, CHIR99021, and HGF) for reprogramming the hPHs into human chemically derived hepatic progenitors (hCdHs) and used hCdHs to generate liver organoids.
Results
In this study, we report the generation of liver organoids in a collagen scaffold using hCdHs. In comparison with adult liver (or primary hepatocyte)-derived organoids with collagen scaffold (hALO_C), hCdH-derived organoids in a collagen scaffold (hCdHO_C) showed a 10-fold increase in organoid generation efficiency with higher expression of liver- or liver progenitor-specific markers. Moreover, we demonstrated that hCdHO_C could differentiate into hepatic organoids (hCdHO_C_DM), indicating the potential of these organoids as a platform for drug screening.
Conclusions
Overall, our study highlights the potential of hCdHO_C as a tool for liver research and presents a new approach for generating liver organoids using hCdHs with a collagen scaffold.
Keywords: Human primary hepatocytes (hPHs), Human chemically derived hepatic progenitors (hCdHs), Collagen organoid
INTRODUCTION
Organoids are three-dimensional (3D) cell culture models that closely mimic the architecture and function of organs or tissues in vivo [1]. These models are typically derived from stem or progenitor cells cultured in a specialized environment that can promote their differentiation into various cell types. Organoids can be created for a wide range of tissues and organs, including the liver, lung, kidney, brain, and other tissues [2-6]. Moreover, organoids provide a more physiologically relevant environment for studying tissue biology and disease than traditional two-dimensional (2D) cell cultures [7]. In 2D cultures, cells are grown on a flat surface, whereas organoids are grown in a 3D matrix that more closely resembles the extracellular matrix (ECM) found in vivo [8]. This allows for the acquisition of more accurate and representative data in various research applications.
However, as organoids require Matrigel as a scaffold, organoids from Matrigel cannot be used for clinical applications [9]. Matrigel is a commonly used ECM to create 3D cell culture models, including organoids [10,11]. Although Matrigel has several advantages, such as its ability to promote cell growth and differentiation, it may contain unknown proteins that can cause side effects such as batch-to-batch variability, angiogenesis, and immune rejection when used in clinical applications [12-17]. Matrigel is derived from a tumor. Thus, foreign proteins could trigger an immune response in the recipient [18]. This can lead to rejection of transplanted cells or tissues, which can compromise the success of transplant.
Collagen organoids, also known as collagen-based organoids, are excellent replacements for Matrigel organoids for clinical use [19]. These organoids are 3D cell culture systems using collagen as ECM, which is found in various tissues in the human body. Collagen is a fibrous protein that can provide structural support and a more physiologically relevant environment for cells as it closely mimics the ECM in vivo [19,20]. This can contribute to more accurate and representative data in drug discovery, disease modeling, and clinical applications [19].
Human primary hepatocytes (hPHs) are known to have a low proliferative potential. They can easily losing their morphology. Thus, hPHs maintenance and proliferation in vitro are still challenging [21]. We have previously reported the generation of human chemically derived hepatic progenitors (hCdHs) using a combination of three small molecules, i.e., A83-01, CHIR99021, and hepatocyte growth factor (HGF) [22]. Here, we generated organoids using hCdHs and a collagen scaffold. We demonstrated the significantly higher generation efficiency of hCdH-derived organoids in a collagen scaffold (hCdHO_C). These organoids were better than hPH-derived organoids with collagen scaffold (hALO_C) in terms of organoid features.
MATERIALS AND METHODS
Isolation of human primary hepatocytes
Human liver tissues were obtained from two donors operated on at Hanyang University Medical Center (Supplementary Table 1). The isolation of hPHs followed a previously published method [22]. In brief, hPHs were isolated using a two-step collagenase perfusion procedure as follows: 1) washing human liver fragment with Tris-EDTA buffer (Sigma) using a perfusion pump (BT100-1F, Dongbang Hitech); and 2) digesting it with 165 units/mL collagenase solution including calcium chloride solution (Sigma). Subsequently, the digested human liver fragment was minced and filtered with a 100 μm strainer (Corning) to remove tissue debris. After centrifugation, hPHs were purified by 25% Percoll gradient centrifugation and seeded on a collagen-coated plate (STEMCELL Technologies) in William’s E Media (Gibco).
The study was approved by the Institutional Review Board of the Hanyang University (IRB No. HYUH201711012020-HE001). Written informed consent was obtained from all participants.
Generation of human chemically derived hepatic progenitors
hPHs were cultured with reprogramming medium consisting of DMEM/F12 (Gibco) supplemented with 1% fetal bovine serum (FBS; Gibco), 10 mM nicotinamide (Sigma), 1% insulin-transferrin-selenium (Gibco), 60 μM beta-mercaptoethanol (Sigma), 1% penicillin/streptomycin (Gibco), 0.1 μM dexamethasone (Sigma), and 20 ng/mL epidermal growth factor (EGF) (Peprotech). We added three small molecules, i.e., 20 ng/mL HGF (Peprotech), 3 μM CHIR99021 (STEMCELL Technologies), and 4 μM A83-01 (Gibco), according to the protocol described by Kim et al. [22]. The medium of the hCdHs was changed every 2 days. When cell confluency reached 80%, sub-culture was performed. For the sub-culture, hCdHs were detached with TrypLE solution (Gibco) and washed with DMEM. After centrifugation, 5 × 105 hCdHs were re-seeded onto a collagen-coated 100 mm plate.
Immunostaining
All samples of hCdHs and hCdHO_C were fixed with 4% paraformaldehyde for 20 min at room temperature (RT) or overnight at 4°C. Samples were washed with phosphate-buffered saline (PBS), permeabilized in 0.25% Triton X-100 (Sigma) for 10 min, and washed three times with PBS. A blocking solution containing 3% bovine serum albumin (BSA) (without IgG) and protease (Jackson ImmunoResearch) was added to samples and incubated at RT for 1 h. After washing with a staining solution including 10x PBS (Welgene), 1% BSA (without IgG), protease (Jackson ImmunoResearch), and 10% sodium azide (Sigma) three times, samples were then incubated with primary antibodies at 4°C overnight. On the next day, samples were washed three times with a staining solution. Secondary antibodies conjugated to 488 (green) and 546 or 594 (red) dyes were then added, followed by 1 h of incubation at RT. After washing with the staining solution thrice and PBS twice, samples were imaged with a confocal microscope. All antibodies used in this study are listed in Supplementary Table 2. Nuclei were co-stained with Hoechst 33342 (1:10,000, Molecular Probes). All cells were imaged using a TCS SP5 confocal microscope (Leica).
Real-time quantitative polymerase chain reaction
Cell pellets were incubated with TRIzol reagent (Ambion) and chloroform to isolate total RNA. RNA purity and concentration were determined with a NanoDrop machine using 1 μg/μL sample for cDNA synthesis. A Transcriptor First Strand cDNA Synthesis Kit (Roche) was used for reverse transcription. For real-time quantitative polymerase chain reaction (RT-qPCR), 1 µg of cDNA and 10 µg of quantitative PCR PreMix (Dyne Bio) were used. PCR conditions were as follows: 40 cycles of 95°C for 20 sec and 60°C for 40 sec. All quantitative data are presented as mean ± standard error of the mean (SEM) with p-values. Statistical significance was evaluated by two-tailed t-test with significance set at p < 0.05, p < 0.01, and p < 0.001. Data from at least three different experiments were used for each case. All primer sequences are listed in Supplementary Table 3.
Collagen organoid culture
hPHs and hCdHs (1 × 104 cells) were mixed with 50 μL of collagen solution consisting of collagen I (Gibco), auto-cleaved distilled water, and 10x DMEM (Sigma) mixed at a 3:5:1 ratio following a published protocol [23]. NaOH (Sigma) was added until collagen solution appeared orange in color (pH of 7.0–7.4). Huch’s protocol [3] was used for liver organoid culture. Briefly, the culture medium was based on an expansion medium (EM): AdDMEM/F12 (Invitrogen) supplemented with 1% N2 (Gibco), 1% B27 (Gibco), 1.25 mM n-acetylcysteine (Sigma), 10 nM gastrin (Sigma), 50 ng/mL EGF (Peprotech), 100 ng/mL FGF10 (Peprotech), 25 ng/mL HGF (Peprotech), 10 mM nicotinamide (Sigma), 10% RSPO1 conditioned medium (homemade), 5 μM A83.01 (Sigma), and 10 μM FSK (Sigma). For collagen organoid culture, the first 3 days after embedding with collagen, the starting EM was supplemented with 25 ng/mL Noggin (Peprotech), 30% Wnt CM (homemade), and 10 μM Y27632 (Sigma). After 3 days, the starting EM was changed to EM without Wnt, Noggin, or Y27632. After 10 to 14 days, organoids were removed from the collagen matrix, mechanically dissociated into small fragments of organoids, and centrifuged at 1,200 rpm for 5 min at 4°C. The supernatant was then removed and transferred to a fresh collagen matrix. Organoids with collagen matrix were seeded onto a pre-warmed 24-well plate for 30 min, followed by addition of 500 μL of starting EM only to each well for 3 days. Sub-culture was performed in a 1:4–1:8 split ratio once every 7–10 days.
Doubling time assay
hCdHs or hPHs were seeded at a density of 1 × 104 cells/well with collagen solution on 24-well plates. Cell numbers were determined at 24 h and 72 h. Doubling time was calculated using the following formula: doubling time = duration*log (2)/[log(final concentration) - log(initial concentration)] (http://www.doubling-time.com/compute.php).
Hepatocyte differentiation
Organoids were cultured in EM for 7–10 days and split at a 1:6 ratio. Split organoids were then cultured in EM supplemented with BMP7 (25 ng/mL). After 3 days, EM was changed to differentiation medium (DM) (AdDMEM/F12 medium and supplemented with 1% N2, 1% B27, 50 ng/mL EGF, 10 nM gastrin, 25 ng/mL HGF, 100 ng/mL FGF19, 500 nM A83-01, 10 μM DAPT, 25 ng/mL BMP7, and 30 μM dexamethasone). DM was changed every 2–3 days for 11–13 days.
RESULTS
Generation of human chemically derived hepatic progenitors from human primary hepatocytes
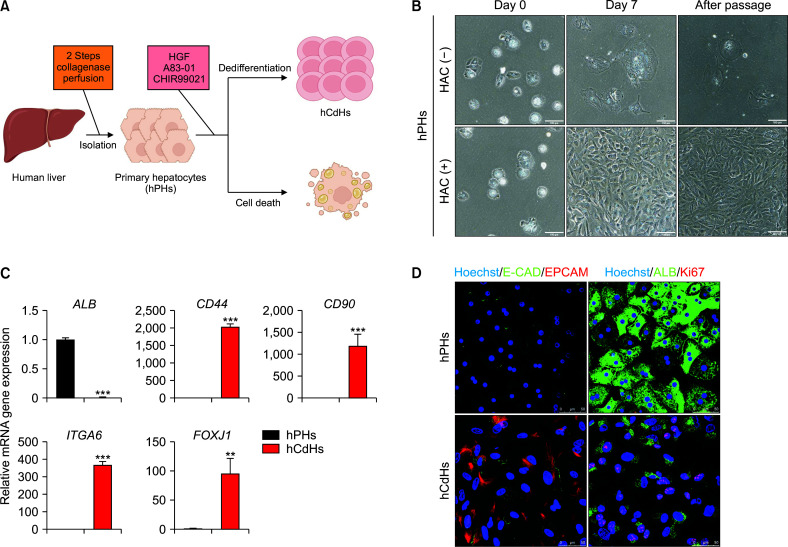
We first isolated hPHs from human liver tissues using a two-step perfusion method [24]. These hPHs were cultured in a reprogramming medium including with/without a small molecule cocktail of HGF, A83-01, and CHIR9902 (HAC) (Fig. 1A) as described by Kim et al. [22]. These hPHs showed poor proliferation when cultured in HAC(-) medium. However, when they were cultured in HAC(+) medium, they could expand for 7 days and maintain their proliferation capacity after sub-culture (Fig. 1B). Therefore, our reprogramming medium could dedifferentiate hPHs into hCdHs. Next, we performed RT-qPCR to examine whether hCdHs exhibited hepatic progenitor features by analyzing hepatic progenitor markers (CD44, CD90, ITGA6, and FOXJ1) and hepatic marker ALB as a negative control (Fig. 1C). As expected, compared with hPHs, hCdHs showed significantly high expression levels of hepatic progenitor markers. Moreover, we examined protein expression levels of hepatic progenitor markers E-CAD, EPCAM, and Ki67 and the hepatic marker ALB by immunocytochemistry. We observed high expression levels of hepatic progenitor markers but low expression level of the hepatic marker ALB in hCdHs with increased proliferation ability (Fig. 1D). Taken together, these results demonstrate that the presence of small molecules HAC in the medium could promote dedifferentiation into hCdHs and increase hepatic progenitor marker expression and proliferation capacity.
Fig. 1.
Generation of hCdHs. (A) Schematic of the hPHs culture procedure in vitro using human liver tissue. The viability of hPHs is dependent on the presence of HAC. (B) Bright-field image of hPHs during culture without HAC(-) or with HAC(+) medium on days 0 and 7 and after passage. Scale bar: 100 μm. (C) RT-qPCR of the hepatic marker (ALB) and hepatic progenitor markers (CD44, CD90, ITGA6, and FOXJ1) in hPHs and hCdHs. Data on the expression of each gene marker were normalized against GAPDH. Data were analyzed by a two-tailed t-test. They are presented as mean ± standard error of the mean (SEM) of three individual experiments performed in triplicate. (D) Immunocytochemistry of protein expression of hepatic progenitor markers (E-CAD, EPCAM, and Ki67) and a hepatocyte marker (ALB) in hPHs and hCdHs. hCdHs, human chemically derived hepatic progenitor cells; hPHs, human primary hepatocytes; HGF, hepatocyte growth factor. **p < 0.01, ***p < 0.001.
Characterization of human chemically derived hepatic progenitor-derived organoids in a collagen scaffold
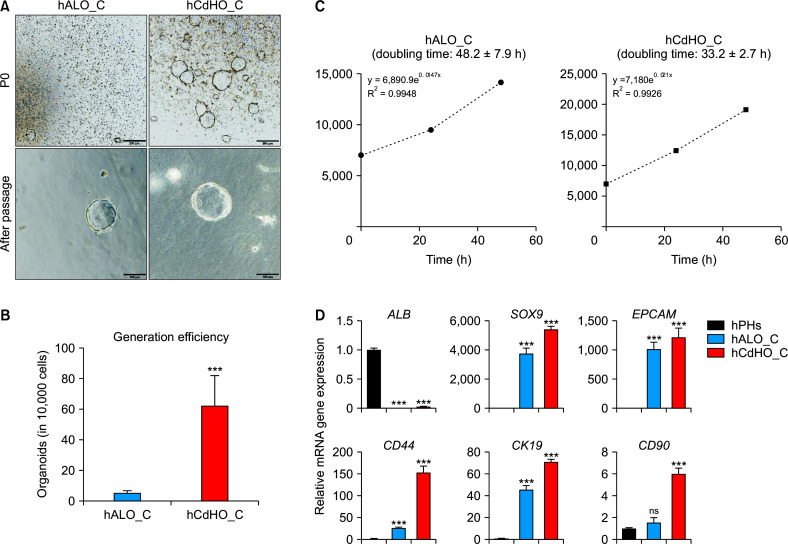
Next, we determined whether hCdHs could generate organoids. Normally, organoids should be cultured in Matrigel, a type of ECM, for stability at RT. However, Matrigel is not a suitable option for clinical use due to its potential tumorigenicity [25]. For clinical applications, we used collagen as a scaffold for organoid culture and compared the efficiency of organoid generation from hPHs (hALO_C) or hCdHs (hCdHO_C) (Fig. 2A). Bright-field images showing organoid generation on day 10 (P0) and after passage were obtained. Interestingly, hCdHO_C demonstrated significantly higher generation efficiency (10-fold increase) than hALO_C (Fig. 2B). In addition, we measured proliferation rate by counting doubling time for hCdHO_C and hALO_C. The graph showed the rapid growth of hCdHO_C (68% greater) compared with that of hALO_C (Fig. 2C). We next examined whether hALO_C and hCdHO_C maintained their hepatic progenitor features. hALO_C and hCdHO_C showed increased expression of hepatic progenitor markers (SOX9, EPCAM, CD44, CK19, and CD90) compared with the hepatic marker ALB (Fig. 2D). However, gene expression levels of hepatic progenitor markers in hCdHO_C were higher than those in hALO_C. These results indicated that once organoids formed, both organoids showed the organoid morphology was similar but organoid generation efficiency and features were significantly high in hCdH_C. Overall, compared with hALO_C, hCdHO_C enhanced the efficiency of organoid generation and growth with high expression of hepatic progenitor markers in a collagen scaffold.
Fig. 2.
Characterization of hALO_C and hCdHO_C. (A) Bright-field images showing the generation of organoids of different origin hPHs or hCdHs at the generation stage (P0) and after sub-culture (after passage). Scale bar: 500 μm (top) or 100 μm (bottom). (B) Number of cells in hCdHO_C and hALO_C. Both hPHs or hCdHs were seeded into a 24-well cell culture plate with collagen solution. The number of cells in each organoid (hCdHO_C and hALO_C) was counted using a Nikon Eclipse Ti-e microscope. Data were obtained from triplicate experiments for a donor, hALO_C (n = 12) and hCdHO_C (n = 12). (C) Doubling time of hCdHO_C and hALO_C. Organoids were dissociated into single cells and cells were counted to analyze the doubling time for 48 h. (D) RT-qPCR of the hepatic marker (ALB) and hepatic progenitor markers (SOX9, EPCAM, CD44, CK19, and CD90) in hPHs, hALO_C, and hCdHO_C. Data on the expression of each gene marker were normalized against GAPDH. Data were analyzed by a two-tailed t-test. They are presented as mean ± standard error of the mean of three individual experiments performed in triplicate. hPHs, human primary hepatocytes; hALO_C, hPH-derived organoids with collagen scaffold; hCdHs, human chemically derived hepatic progenitors; hCdHO_C, hCdH-derived organoids in a collagen scaffold; ns, not significant. ***p < 0.001.
Hepatic differentiation of human chemically derived hepatic progenitor-derived organoids in a collagen scaffold
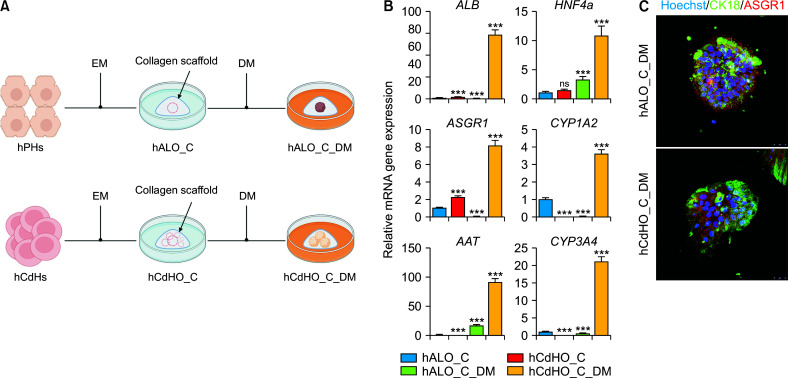
Finally, we investigated the hepatic differentiation potential of hCdHO_C, which demonstrated better differentiation ability than hALO_C in a collagen scaffold. Both hALO_C and hCdHO_C were cultured in hepatic DM for two weeks (Fig. 3A). After 2 weeks, we harvested hALO_C_DM and hCdHO_C_DM to examine their gene expression by RT-qPCR. Expression levels of mature hepatic markers (ALB, HNF4a, AAT, ASGR1, CYP1A2, and CYP3A4) of hCdHO_C were higher than those of hALO_C_DM (Fig. 3B). As expected, immunofluorescence staining showed that expression levels of mature hepatic markers (CK18 and ASGR1) of hCdHO_C_DM were consistent with results of RT-qPCR (Fig. 3C). Moreover, both hCdHO_C_DM and hALO_C_DM showed a compact morphology with loss of the lumen structure after hepatic differentiation. These results demonstrated that the hepatic differentiation capacity of hCdHO_C and expression levels of hepatic markers of hCdHO_C_DM were higher than those of hALO_C_DM in a collagen scaffold.
Fig. 3.
Hepatic differentiation of hCdHO_C (hCdHO_C_DM). (A) Schematic of hepatic differentiation with hALO_C and hCdHO_C. (B) Relative mRNA levels of hepatic markers (ALB, HNF4a, AAT, ASGR1, CK19, CYP1A2, and CYP3A4) in hALO_C, hCdHO_C, hALO_C_DM, and hCdHO_C_DM determined by RT-qPCR. Expression levels of each gene marker in all samples were normalized agasint GAPDH (triplicate experiments). (C) Immunocytochemistry of hepatic markers (CK18 and ASGR1) in hALO_C_DM and hCdHO_C_DM. Scale bar: 50 μm. hCdHs, human chemically derived hepatic progenitors; hCdHO_C, hCdH-derived organoids in a collagen scaffold; DM, differentiation medium; hALO_C, hPH-derived organoids with collagen scaffold; EM, extension medium; ns, not significant. ***p < 0.001.
DISCUSSION
Organoids are 3D structures that mimic the function and structure of organs in the human body. These structures are derived from stem cells or sometimes from tissue directly. They can be used for a broad range of applications. The generation of organoids by Barker et al. [26] has been a significant breakthrough in the field of regenerative medicine. By using stem cells, researchers can generate organoids that closely resemble the structure and function of a real tissue.
Liver organoids, which have been developed by Huch et al. [3], are also 3D structures created using stem cells or primary hepatocytes from liver tissue. They reflect the function of the human liver. These organoids offer a promising alternative for liver transplantation [27], studying liver diseases, and testing liver drugs [27-29]. The main benefit of liver organoids is their potential use in drug screening. The development of drugs for liver disease is a major challenge worldwide. Many patients die due to the lack of effective drugs. However, liver organoids can provide an alternative platform for drug testing to treat liver disease patients. This approach could help address the shortage of donor organs and improve lives of countless patients. Liver organoids can also be used to study liver diseases including hepatitis, cirrhosis, and fatty liver disease. By investigating mechanisms of these diseases using organoids, researchers can gain a better understanding of how they develop and progress. This knowledge could support the development of new treatments for these diseases. Overall, liver organoids present a novel and promising strategy for drug testing, disease modeling, and liver transplantation. Although there remain obstacles to be overcome, advantages of these organoids make them a promising option for future studies. Therefore, researchers should continue to conduct studies with liver organoids to provide patients with a better life.
In this study, we focused on the generation of collagen organoids for clinical applications using a collagen scaffold instead of Matrigel to generate organoids. When we compared the efficiency of organoid generation between collagen and Matrigel, Matrigel was better for organoid generation. However, organoids derived from Matrigel have not been used for clinical studies due to the unknown composition of Matrigel [13]. Collagen organoids represent a significant advancement in the field of 3D cell culture systems. Unlike traditional 2D cell cultures, collagen organoids provide a more physiologically relevant environment for cells as collagen closely mimics the ECM found in vivo [19,20]. These organoids are also highly customizable. They can be created using different cell types and ECM components. They can be designed to mimic specific tissue architectures and functions [30,31]. This allows researchers to generate highly specialized organoids tailored to the specific needs of their research projects.
Although the generation of organoids without Matrigel is still challenging, Matrigel might be replaced with several candidate materials such as collagen, decellularized ECM, synthetic hydrogels, and peptide and recombinant protein matrices [17]. We attempted to combine collagen and hepatic progenitor cells for the generation of organoids, which can be adapted to clinical applications. We initially hypothesized that proliferating cells would be beneficial for the generation of organoids. However, the generation efficiency of organoids might be dependent on EPCAM expression, for which normal primary hepatocytes (hPHs) do not express [3]. To reprogram hPHs, we used a HAC(+) reprogramming medium reported previously [22] and successfully performed dedifferentiation of hPHs into hCdHs which has a high expression of EPCAM. Next, we assessed organoid generation, features, and differentiation ability of two different cell lineages (hPHs and hCdHs). We optimized the suitable potential of hydrogen (pH) in the collagen scaffold for organoid generation by following the James protocol [23]. To visually indicate the pH in the collagen scaffold, we used DMEM 10x including phenol-red, which turned yellow (acidic) or red (basic). We then adjusted the pH from 7.0 to 7.4 by adding NaOH because the initial collagen scaffold was acidic due to DMEM 10×. We confirmed the pH level with a pH paper (BTB). We observed that a pH of less than 7 (acidic) or greater than 7.6 (basic) showed disruption of its scaffold easily and displayed low organoid generation efficiency. After optimizing, we cultured both hPH- and hCdHs-collagen organoids and found that the organoid generation efficiency of hCdHO_C was higher than that of hALO_C. hCdHO_C also had higher expression levels better of liver progenitor markers than hALO_C. In addition, we examined hepatic differentiation in these organoids by measuring mRNA expression and performing immunostaining of hepatic markers. For further studies, we plan to perform several assays to determine hepatic functions of hALO_C_DM and hCdHO_C_DM, such as albumin secretion, CYP gene activation, and PAS staining, to determine whether these hepatic-induced organoids could express functional features of hepatocytes.
Taken together, these results demonstrate that both hCdHO_C and hALO_C are suitable as a liver organoid model. However, hCdHO_C is better than hALO_C in terms of large-scale culture and generation efficiency. Therefore, collagen organoids represent an exciting development in the field of 3D cell culture systems. As research in this area continues to advance, collagen organoids are likely to become an important tool for studying tissue biology, disease, and regenerative medicine.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found at https://doi.org/10.14701/ahbps.23-052.
Funding Statement
FUNDING This work was supported by grants (NRF-2022R1A2C 2004593, NRF-2021M3A9H3015390, and NRF-2022R1F1A 1073058) of the National Research Foundation (NRF) funded by the Ministry of Science and ICT (MSIT), Republic of Korea.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: JHS, DC. Data curation: MK. Methodology: ESSS, MA. Visualization: KSK, YKJ, KGL. Writing - original draft: YK. Writing - review & editing: MK.
REFERENCES
- 1.Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research. Nat Rev Genet. 2018;19:671–687. doi: 10.1038/s41576-018-0051-9. [DOI] [PubMed] [Google Scholar]
- 2.Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez- Morán P, et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 3.Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller AJ, Dye BR, Ferrer-Torres D, Hill DR, Overeem AW, Shea LD, et al. Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc. 2019;14:518–540. doi: 10.1038/s41596-018-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng Z, Huang B, Parvez RK, Li Y, Chen J, Vonk AC, et al. Generation of patterned kidney organoids that recapitulate the adult kidney collecting duct system from expandable ureteric bud progenitors. Nat Commun. 2021;12:3641. doi: 10.1038/s41467-021-23911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 8.Suarez-Martinez E, Suazo-Sanchez I, Celis-Romero M, Carnero A. 3D and organoid culture in research: physiology, hereditary genetic diseases and cancer. Cell Biosci. 2022;12:39. doi: 10.1186/s13578-022-00775-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, et al. Generation of human vascularized brain organoids. Neuroreport. 2018;29:588–593. doi: 10.1097/WNR.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sodunke TR, Turner KK, Caldwell SA, Mcbride KW, Reginato MJ, Noh HM. Micropatterns of Matrigel for three-dimensional epithelial cultures. Biomaterials. 2007;28:4006–4016. doi: 10.1016/j.biomaterials.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 13.Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–1890. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 14.Aisenbrey EA, Murphy WL. Synthetic alternatives to Matrigel. Nat Rev Mater. 2020;5:539–551. doi: 10.1038/s41578-020-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnaoutova I, Kleinman HK. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc. 2010;5:628–635. doi: 10.1038/nprot.2010.6. [DOI] [PubMed] [Google Scholar]
- 16.Kleinman HK, Kim K, Kang H. Matrigel uses in cell biology and for the identification of thymosin β4, a mediator of tissue regeneration. Appl Biol Chem. 2018;61:703–708. doi: 10.1007/s13765-018-0400-6. [DOI] [Google Scholar]
- 17.Kozlowski MT, Crook CJ, Ku HT. Towards organoid culture without Matrigel. Commun Biol. 2021;4:1387. doi: 10.1038/s42003-021-02910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneeberger K, Spee B, Costa P, Sachs N, Clevers H, Malda J. Converging biofabrication and organoid technologies: the next frontier in hepatic and intestinal tissue engineering? Biofabrication. 2017;9:013001. doi: 10.1088/1758-5090/aa6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaur S, Kaur I, Rawal P, Tripathi DM, Vasudevan A. Non-matrigel scaffolds for organoid cultures. Cancer Lett. 2021;504:58–66. doi: 10.1016/j.canlet.2021.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol. 2013;87:1315–1530. doi: 10.1007/s00204-013-1078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim Y, Kang K, Lee SB, Seo D, Yoon S, Kim SJ, et al. Small molecule-mediated reprogramming of human hepatocytes into bipotent progenitor cells. J Hepatol. 2019;70:97–107. doi: 10.1016/j.jhep.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Dunn JC, Tompkins RG, Yarmush ML. Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J Cell Biol. 1992;116:1043–1053. doi: 10.1083/jcb.116.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vondran FW, Katenz E, Schwartlander R, Morgul MH, Raschzok N, Gong X, et al. Isolation of primary human hepatocytes after partial hepatectomy: criteria for identification of the most promising liver specimen. Artif Organs. 2008;32:205–213. doi: 10.1111/j.1525-1594.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 25.Conboy I, Freimer J, Weisenstein L, Liu Y, Mehdipour M, Gathwala R. Comprehensive Biomaterials II. Elsevier; 2017. 6.13 Tissue Engineering of Muscle Tissue☆; pp. 216–235. [DOI] [Google Scholar]
- 26.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Sampaziotis F, Muraro D, Tysoe OC, Sawiak S, Beach TE, Godfrey EM, et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science. 2021;371:839–846. doi: 10.1126/science.aaz6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shinozawa T, Kimura M, Cai Y, Saiki N, Yoneyama Y, Ouchi R, et al. High-fidelity drug-induced liver injury screen using human pluripotent stem cell-derived organoids. Gastroenterology. 2021;160:831–846.e10. doi: 10.1053/j.gastro.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun L, Wang Y, Cen J, Ma X, Cui L, Qiu Z, et al. Modelling liver cancer initiation with organoids derived from directly reprogrammed human hepatocytes. Nat Cell Biol. 2019;21:1015–1026. doi: 10.1038/s41556-019-0359-5. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira AM, Gentile P, Chiono V, Ciardelli G. Collagen for bone tissue regeneration. Acta Biomater. 2012;8:3191–3200. doi: 10.1016/j.actbio.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Rezvani Ghomi E, Nourbakhsh N, Akbari Kenari M, Zare M, Ramakrishna S. Collagen-based biomaterials for biomedical applications. J Biomed Mater Res B Appl Biomater. 2021;109:1986–1999. doi: 10.1002/jbm.b.34881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.