Abstract
Vascular tumors of the liver are mesenchymal lesions from endothelial cells. They range from common benign lesions such as haemangioma, intermediate tumors like Kaposi sarcoma, and perivascular epithelioid cell tumor to malignant tumors such as hepatic epithelioid hemangioendothelioma and hepatic angiosarcoma in adults. Pediatric vascular tumors of the liver also include benign, locally aggressive, borderline, and malignant masses with haemangiomas being the most common benign tumors and epithelioid hemangioendothelioma being an uncommon pediatric malignancy. The list of these lesions is completed by nodular regenerative hyperplasia, solitary fibrous tumour, and hepatic small vessel neoplasms (HSVN). Some of these tumors are uncommon and rare. This review article aimed to enumerate hepatic vascular tumors along with their imaging, histopathology, molecular findings for accurate diagnosis that can result in better management.
Keywords: Liver, Vascular tumour, Diagnostic
INTRODUCTION
The liver parenchyma comprises of epithelial components and mesenchymal components such as blood vessels and adipose tissues. Vascular tumors can be formed from endothelial cells of vascular channels. They range from common benign tumors such as haemangiomas and intermediate entities such as Kaposi sarcoma (KS) and PEComas to malignant tumors such as hepatic epithelioid hemangioendothelioma (HEHE) and hepatic angiosarcoma [1,2]. They also include tumors like solitary fibrous tumour (SFT) and hepatic small vessel neoplasms (HSVN). Haemangiomas are the most common pediatric vascular tumors of the liver. They are different from adult hepatic haemangiomas [3].
As most of these tumors are uncommon, their diagnostic approach and treatment modalities are ambiguous and non-standardised [4,5]. Early and accurate diagnosis will help us better manage these rare tumors.
BENIGN MASSES AND TUMORS
Adult hepatic haemangioma
Hepatic haemangiomas in adults and pediatric patients are different entities. Hepatic haemangiomas in adults are slow-flow venous malformations. They are not actual neoplasms/tumors according to the International Society for Study of Vascular Anomalies (ISSVA) classification [6]. They are the most common liver lesions with a prevalence of 3%–20%. They might contribute up to 70% of all benign liver tumors. They can be single or multiple. They can present at any age with a strong female predilection (female-to-male ratio: 4:1 to 5:1) [7,8].
They are usually asymptomatic. They are detected as incidental findings on imaging. Among patients who develop symptoms, vague abdominal pain is the most common symptom. Haemangiomas of more than 5 cm in size are called giant haemangiomas [1]. They rarely present as an abdominal lump. They can cause compression of surrounding structures depending on their location. They might cause gastric compression and early satiety when present in the left lobe of liver, compression of inferior vena cava and limb edema when present in the right or caudate lobe, and biliary or portal vein compression leading to jaundice or portal hypertension when present in a central location. Haemangiomas might have intra-lesional thrombosis with subsequent inflammation, hyalinization, and fibrosis that can increase pain and constitutional symptoms such as fever, weight loss, and increased erythrocyte sedimentation rate. However, they usually have a normal leucocyte count. Giant haemangiomas may rarely present as a generalized consumptive coagulopathy syndrome (Kasabach-Merrit syndrome) due to intra-lesional thrombus and fibrinolysis leading to disseminated intravascular coagulation and thrombocytopenia [8].
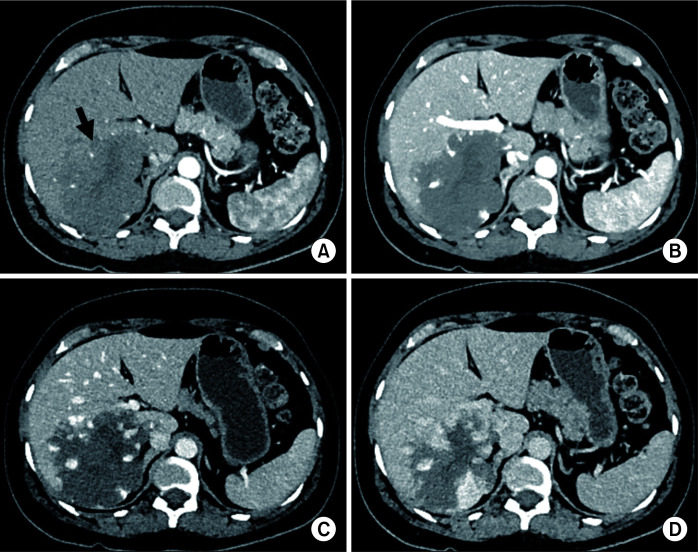
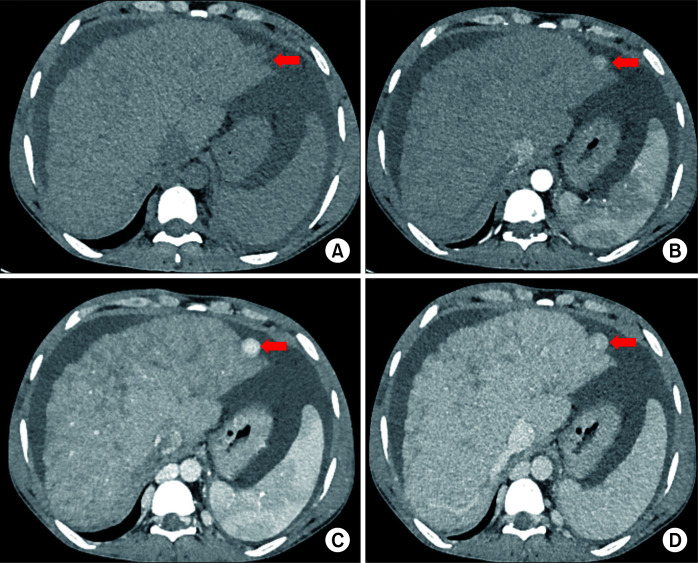
On ultrasonography (USG), haemangiomas appear as sharp homogeneous hyperechoic masses. On contrast-enhanced ultrasound (CEUS), these lesions may show peripheral enhancement in arterial phase and become isoechoic in later phases. On contrast-enhanced computed tomography (CECT), they appear as lesions with peripheral, globular enhancement in arterial phase with progressive central enhancement and retention of contrast in delayed phases. On magnetic resonance imaging (MRI), they appear as hypointense on T1 and very hyperintense on T2 weighted images. On contrast-enhanced MRI (CEMRI), a progressive enhancement pattern is similar to CECT. A few haemangiomas might show atypical features on imaging. Giant haemangiomas may show atypical features on imaging due to thrombosis, hyalinization, fibrosis, cystic degeneration, or calcifications. A subset of small haemangiomas may be rapidly-filling completely on arterial phase, mimicking hepatocellular carcinoma (HCC). However, they usually retain contrast on delayed phases. Haemangiomas in background of cirrhosis might have more fibrosis. They might be difficult to differentiate from HCC. Haemangioma is usually confidently diagnosed by imaging with ultrasound/multiphasic CT/MRI. Very rarely, a biopsy may be needed to confirm the diagnosis of atypical haemangiomas [8-10]. A giant hemangiomas and multiple hemngiomas are shown in Fig. 1 and 2.
Fig. 1.
Multiphasic contrast-enhanced computed tomography (CT) in a 52-year-old male patient showing a giant hepatic haemangioma (arrow in A). Axial images in (A) arterial, (B) portal venous, (C) hepatic venous, and (D) 3-minute delayed phases show typical peripheral discontinuous nodular enhancement with progressive centripetal filling of contrast. Note that the density of nodular contrast in the lesion matches with the aorta (blood pool).
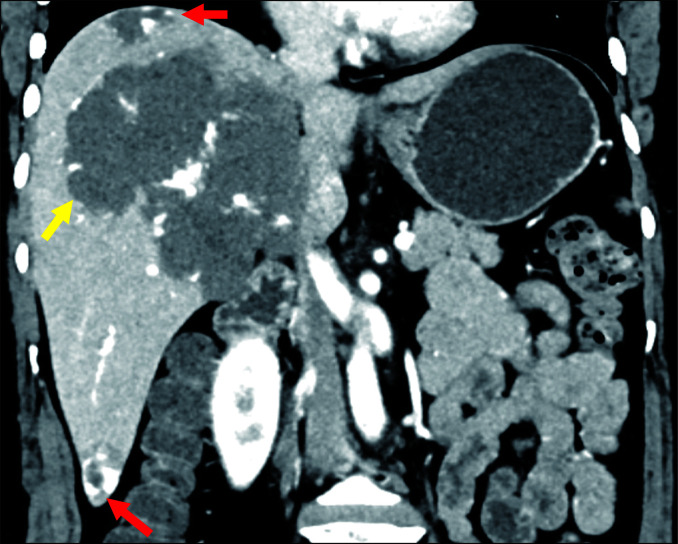
Fig. 2.
Multiple hepatic haemangiomas on contrast-enhanced computed tomography (CT) in portal venous phase in a 42-year-old female. The coronal image shows two small haemangiomas (red arrows) and one giant haemangioma (yellow arrow).
Microscopically, they can be typified into cavernous, capillary, and sclerosing [7,11]. Cavernous haemangioma are large dilated spaces lined by endothelial cells. They are the most common type. Giant haemangiomas differ only in size by being more than 5 cm with the same histopathology [1]. Capillary haemangiomas comprise of small caliber capillaries. Sclerosing haemangiomas are fibrosed and sclerosed with degenerative changes. Cavernous haemangiomas also regress to sclerosing haemangiomas if they are asymptomatic for a long period [12].
Diffuse haemangiomas in liver (haemangiomatosis) might be associated with some syndromes such as Osler-Weber-Rendu disease [13] and medications such as estrogen [14] and metoclopramide [15] with a female predilection. These may present with abdominal pain, distension, weight loss, fever, night sweats, and jaundice. These lesions are ill- defined. They may present with complications such as disseminated intravascular coagulation (Kasabach-Merritt syndrome) [16]. Major differential diagnoses on histopathology are HEHE and hepatic hemangiosarcoma (HHS) [17]. On histopathology, haemangiomatosis will present as an ill-defined mass of capillaries while HEHE will show cords and strands of cells in a myxohyaline stroma and HHS will show highly pleomorphic cells without any vasoformation [2].
As most haemangiomas are asymptomatic, they need no treatment or follow-up imaging. When they produce symptoms or complications, surgical resection or enucleation is the treatment of choice. A pre-operative angioembolization of the feeder vessels may help reduce intra-operative bleeding. When the location is not feasible for surgical treatment or if the patient is not a good operative candidate, angioembolization may be offered. Haemangioma rupture spontaneously or after blunt trauma is exceptionally rare. It has a high mortality [8].
Pediatric hepatic haemangiomas
Haemangiomas are the most common benign tumors in the pediatric age group. They are benign proliferative vascular tumors originating from endothelial cells. They are the most common benign vascular tumors of infancy, accounting for 12% of all childhood hepatic tumors with a male-to-female ratio of 1:2 [18-20]. Cutaneous haemangiomas are the most common, followed by liver as the second most common site. Liver haemangioma can be congenital haemangioma (CH) which is present at birth. It starts to develop in fetal life or infantile haemangioma (IH) which is rarely present at birth. CH usually presents as a single lesion. It is completely formed at birth. It generally does not grow after birth. It can be further subdivided into three types: 1) rapidly involuting congenital haemangioma (RICH), the most common subtype, which involutes before the age of 14 months; 2) non-involuting congenital haemangioma (NICH), which does not regress and may grow with growth of child; 3) partially involuting congenital haemangioma (PICH). CH is glucose transporter-1 (GLUT1) negative while IH is GLUT1 positive [18,21].
Infantile hepatic haemangiomas (IHH) are the commonest tumors of infancy, more common than CH [18]. IHH can be further subdivided into focal, multifocal, and diffuse types with nearly half of all cases being associated with cutaneous haemangiomas. Focal type is typically solitary. It is a heterogeneous mass with dilated vascular channels with or without calcifications. Multifocal and diffuse types may grow to a large size and cause peripheral arteriovenous (AV) shunting. The classical triad for clinical diagnosis of IHH includes palpable liver, cardiac failure, and cutaneous haemangiomas in an infant. Most of the IHH lesions grow during the first year of life and then spontaneously regress by 5–7 years [9,18,19]. These patients with multifocal and diffuse types may present with cardiac failure, liver failure, consumption coagulopathy (Kasabach-Merritt syndrome), hypothyroidism, or abdominal compartment syndrome.
On USG, IHH may present as a complex liver mass, single or multiple, ranging from hypoechoic to hyperechoic with large draining hepatic veins due to intra-lesional AV shunting and high blood flow. On multiphasic CT/MRI, early nodular peripheral enhancement and delayed progression to the center of the lesion, similar to adult haemangiomas, are seen. In larger tumors, there is central hypodensity due to either fibrosis, hemorrhage, or necrosis. The aorta typically has a decreased caliber distal to the origin of the hepatic mass, indicating large blood supply to the tumour. On MRI, IHHs are hypointense on T1-weighted images and hyperintense on T2-weighted images. Lesions with significant AV shunting and blood pooling may show flow voids on T2-weighted images [9,10]. Cases of pediatric hemengioma have been depicted in Fig. 3 and 4.
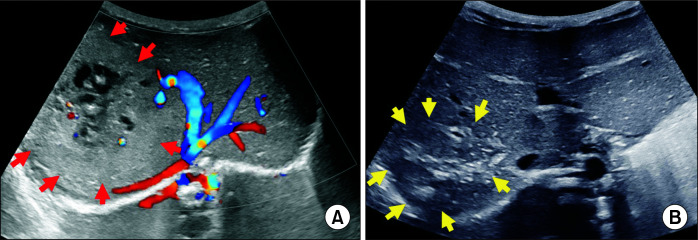
Fig. 3.
Ultrasound images in an infant with congenital hepatic haemangioma. (A) Colour doppler image in a 19-day-old neonate shows a large hyperechoic mass with internal cystic areas and vascularity. Lesion is delineated by red arrows. Mass is causing splaying of hepatic veins. (B) Follow-up ultrasound at the age of 15 months shows a significant decrease in the size of the mass lesion with increased hyperechoic fibrous component. Mass is delineated by yellow arrows.
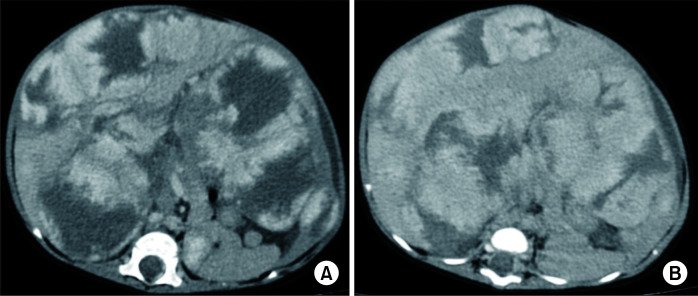
Fig. 4.
Contrast-enhanced computed tomography (CT) images in a 28-day-old female neonate showing diffuse infantile hepatic haemangiomas who presented with abdominal distension. Axial images in (A) portal venous, (B) 3-minute delayed phases show typical peripheral nodular enhancement with progressive centripetal filling of contrast in delayed phase. Intervening normal liver parenchyma is seen between lesions.
Microscopically, they can be divided into two types: type 1 and type 2. Type 1 is the most common type. It shows proliferation of small, irregular capillary-like vascular spaces lined by bland or plump endothelial cells separated by variable connective tissue. Extramedullary hematopoiesis and entrapped hepatocytes might be seen in the periphery of the lesion. Type 2 lesion is more uncommon. It is aggressive, comprising of irregular branching vascular structures lined by pleomorphic, hyperchromatic endothelial cells, frequent mitotic activity. Although Type 2 is pleomorphic, it has better prognosis. HEHE differs from IHH in having intracellular vascular lumina with luminal red blood cells (RBCs) [2]. IHH is immunopositive for factor VIII related antigen, CD31, CD34, and GLUT1 [22].
Treatment of asymptomatic IHH is conservative as most will involute and regress spontaneously. Symptomatic IHH are treated with propranolol and medical therapy of cardiac failure if present. If medical therapy fails, then angioembolization of the IHH, surgical ligation of hepatic artery, and surgical resection of the lesion might be done [18,19].
Nodular regenerative hyperplasia
Nodular regenerative hyperplasia (NRH) is a rare condition of the liver that can cause portal hypertension. It comprises 0.7% to 2.6% of hepatic lesions with a mean age of presentation at 40 years [1]. These vasculopathies are among causes of non-cirrhotic portal hypertension. Common etiologies of NRH are myelo- and lympho-proliferative diseases, autoimmune diseases, inflammatory conditions, immunodeficiency disorders, primary biliary cholangitis, Budd-Chiari syndrome, collagen-vascular diseases, and congenital and acquired hepatic macrovascular abnormalities (Rendu-Osler-Weber, Abernethy malformation). These may also occur after chemotherapy such as highly active anti-retroviral therapy (HAART), platinum-based chemotherapy, thiopurines, azathioprine, and thioguanine [23]. NRH develops due to obstructive vasculopathy or secondary damage to sinusoids. They are usually asymptomatic until the lesion becomes large enough to cause portal hypertension. They present as small tan white nodules without perinodular fibrosis. A case has been depicted in case Fig. 5. On microscopic examination, these lesions comprise of diffuse nodules of hyperplastic hepatocytes around the portal tract with centrilobular atrophy of hepatocytes. Due to compression of the central veins, sinusoids become congested. Regenerative hepatocytes have clear, vacuolated cytoplasm along with cholestasis. They are 2–3 plates thick while adjacent hepatocytes are usually one plate thick. There is no evidence of fibrosis or extramedullary haematopoiesis in the liver parenchyma [24]. Liver transplant may be one of the treatment options when significant complications develop secondary to portal hypertension [1].
Fig. 5.
Multiphasic contrast-enhanced computed tomography (CT) in a 45-year-old male patient showing a large regenerative nodule in the background of Budd-Chiari syndrome. Axial images in (A) non-contrast, (B) arterial, (C) portal venous, and (D) hepatic venous phases show a well-defined nodule in segment II (red arrow). Nodule is iso-hypoattenuating in non-contrast CT (A), showing arterial enhancement (B) which homogeneously progresses in portal venous phase and becomes isoattenuating in hepatic venous phase (D) with the background parenchyma. Hepatomegaly, heterogeneous reticular enhancement of liver, splenomegaly, prominent azygous system, and ascites are features of Budd-Chiari syndrome seen in this case due to supra hepatic inferior vena cava (IVC) stenosis.
LOCALLY AGGRESSIVE OR BORDERLINE MALIGNANT TUMORS
Kaposi sarcoma
KS is a low-grade mesenchymal tumour of blood vessels. It is lymphatic. It primarily involves skin. It can be disseminated to other organs. It has four variants, namely classic KS, endemic (African) KS, iatrogenic (organ transplant-related) KS, and acquired immunodeficiency syndrome (AIDS)-related KS. Hepatic KS is most commonly associated with AIDS or organ transplantation. It is usually detected on autopsy. Its clinical features such as nausea, abdominal bloating, lethargy, and fever are usually non-specific and related to systemic diseases. Hepatic KS appears as hepatomegaly with hyperechoic bands and nodules along peripheral branches of portal veinson ultrasound and hypodense nodules in hilar, periportal or capsular in location on CT with delayed enhancement. On MRI, they display high signal on in-phase T1-weighted images and low signal on out-of-phase images due to intracellular fat. They are isointense on T2-weighted images [25,26].
On histopathology, these tumors are typically perivascular with main concentration around peripheral portal branches. There is presence of nodules with macrovesicular steatosis and the perinodular area showing presence of small vessels. Nodules are comprised of spindle shaped cells with intervening slit like or sieve-like blood vessels [2]. Intracytoplasmic hyaline globules, extravasated RBCs and hemosiderin laden macrophages are common findings. These tumors are typically immunopositive for HHV-8, showing a stippled nuclear staining pattern. They are also positive for ERG, CD34, CD31, and D2-40 [2]. However, they are negative for myoid, melanocytic, and epithelial markers [2].
Perivascular epithelioid cell tumour (PEComa)
Perivascular epithelioid cell tumors (PEComas) are a family of mesenchymal tumors composed of distinctive perivascular epithelioid cells (PECs). They are rare tumors with more frequency in females and a mean age of presentation at 45 years. They can present as painless masses at a wide range of anatomical sites. Most of them are asymptomatic, but may present with non-specific symptoms such as nausea, abdominal bloating, indigestion, and loss of appetite.
In AML, macroscopic fat (may be more than 50% of the tumour) can be detected on CT or MRI. In rest of PEComas, radiological findings are non-specific with absence of macroscopic fat [27,28]. Hypervascularity and arterio-venous connections or dysmorphic vessels in or surrounding lesions are hallmarks of PEComas on imaging [27,29].
These are rare tumors arising from perivascular endothelial cells. They show myoid and melanocytic differentiation [30]. They comprise of angiomyolipoma (AML), epithelioid AML, and lymphangioleiomyomatosis [2]. Hepatic AML are very uncommon. They often present in association with Tuberous Sclerosis resulting from loss of heterozygosity in TSC2 locus [2]. They are also seen in association with TFE3 gene rearrangement [2]. Grossly, these are well circumscribed with a fibrous or fleshy cut surface. Microscopically, these tumors are comprised of nests, trabeculae, or sheet like architecture of cells that are epithelioid to spindle with granular eosinophilic to clear cytoplasm [2]. They are perivascular in orientation. They are immunopositive for HMB45, Melan A, MITF, SMA, Desmin, and h-Caldesmon [30]. These tumors have uncertain malignant potential with clinically malignant tumors metastasizing to liver, lymph nodes, and bone [2].
Solitary fibrous tumour
SFT is a rare soft tissue tumour. Its incidence in the liver is less than 1% [1]. It is seen in the 5th to 6th decade of life as a solitary, well-defined lesion often with haemorrhage and cystic degeneration [31].
SFT was previously known as hemangiopericytoma. It is a tumour arising from pericytes which lie near endothelial cells of blood vessels. Liver involvement might be primary or metastatic. Primary hepatic SFTs are very rare with only a few case reports. They are usually seen in elderly females. They are mostly benign with progressive growth. However, a few cases of malignant potential have also been reported [32]. These patients may remain asymptomatic or may present with acute abdomen in case the tumour ruptures. Their clinical features are generally nonspecific, such as vomiting, weight loss, post prandial fullness, abdominal distension, anorexia, and fatigue. A few cases of hypoglycemia due to secondary secretion of insulin-like growth factor-2 (IGF-2) by tumour cells have been reported [32,33].
SFTs are well-defined hypoto hyperechoic masses on USG and hypodense on non-contrast CT. They show minimal to heterogenous enhancement on CECT. MRI is the imaging modality of choice that shows a hypointense mass on T1-weighted and an iso-hypointense mass on T2-weighted images due to a high content of fibrous collagen tissue and hypocellularity. Hyperintensity of T2-weighted images can be due to cystic or myxoid degeneration, vascular structures, and hypercellular areas. On CE-MRI, there is a typical intense heterogeneous enhancement which persists into the late phase [34].
Microscopic examination reveals that SFT has hypocellular and hypercellular areas with cellular areas being comprised of spindle cells in a patternless arrangement interspersed with staghorn vessels. It has been found that 50% of SFTs show atypia and increased mitotic activity as features of malignancy [31]. On immune-histochemistry, these tumors are positive for CD 34 and STAT-6. They show molecular alterations in the form of somatic mutations between NAB2 and STAT6 genes [2,31]. Aggressive surgery is the treatment of choice [35].
MALIGNANT TUMORS
Hepatic epithelioid hemangioendothelioma
HEHE is a rare malignant and locally aggressive hepatic neoplasm that develops mainly in adults. It comprises of less than 1% of all vascular tumors [1]. It presents commonly between 30 and 50 years of age with a male-to-female ratio of 1:2 [9]. It has shared features of haemangioma and angiosarcoma. It has metastatic potential. However, it is less aggressive than primary angiosarcoma [36]. HEHE is usually incidentally detected, but may present with pain abdomen, jaundice, weight loss, and liver failure in advanced cases due to parenchymal replacement by tumour [9,19].
HEHEs may appear on imaging as solitary nodule or multiple nodules or diffuse confluent masses involving both lobes [36,37]. Solitary nodules are small, usually in a subcapsular location. Multiple nodules may be large in subcapsularor deep locations. They are usually associated with capsular retraction [9]. Multiple nodules then grow to coalesce and form large confluent masses. The active infiltrative tumour is usually at the edge of the mass. There might be fibrosis, hyalinisation, or calcification in the central part [20,38].
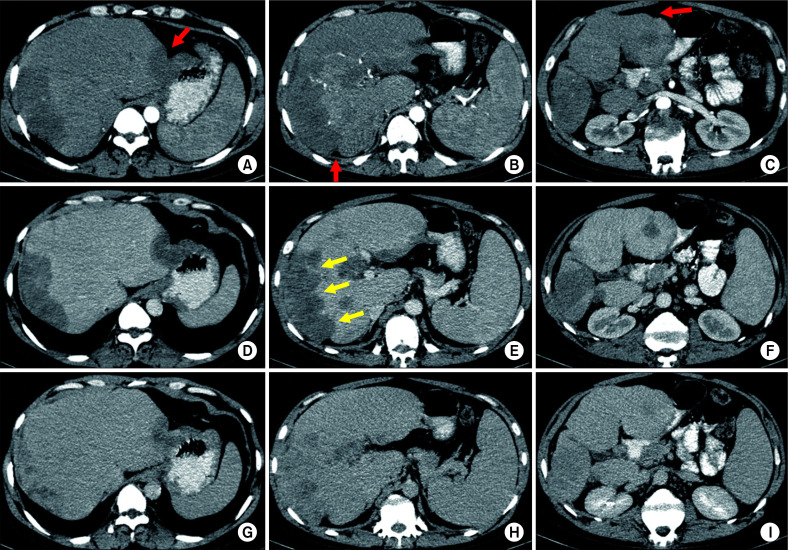
They are usually hypoechoic on ultrasound. On plain CT, they are hypodense to normal. Upon contrast administration, they become isodense. Hence, plain CT may be more reliable to estimate the extent of lesion. Depending on the extent of hyalinization, fibrosis, and so on within the tumour, the contrast enhancement may also be heterogeneous. On MRI, they are hypointense on T1- and hyperintense on T2-weighted images. A few signs such as “Target sign” and “Lollipop sign” have been described on imaging, more discernible on MRI. On CE-MRI, peripheral enhancement with delayed central enhancement may be seen. These tumors commonly show hepatic vein or portal vein invasion [9,38,39]. These may metastasize to lymph nodes and lungs [2,40]. Fig. 6 shows a case of HEHE.
Fig. 6.
Multiple hepatic epitheloid hemangioendotheliomas on multiphasic contrast-enhanced computed tomography (CT) (all axial images at three different levels) in a 35-year-old male patient. (A, B, C) Arterial phase, (D, E, F) venous phase, (G, H, I) 3-minute delayed phase images showing multiple peripheral subcapsular lesions with progressive enhancement. Lesions are causing subcapsular retraction (red arrows) leading to a nodular outline of the liver. Some lesions are coalescing to form confluent masses along the right lobe (yellow arrows in E).
According to recent WHO classification of soft tissue tumors, they can be classified into two groups: WWTR1-CAMTA1 rearranged tumors and YAP1-TFE3 rearranged tumors [2]. Microscopically, WWTR1-CAMTA1 rearranged tumors show cords, strands, or small nests of large endothelial cells with abundant eosinophilic cytoplasm embedded in a myxohyaline stroma with cells having vesicular, round to oval sometimes indented nuclei. A few tumour cells may even show intracytoplasmic vacuoles with erythrocytes [2]. YAP-TFE3 rearranged tumors have solid nests or pseudo alveolar arrangement of epithelioid cells enmeshed in a fibrous stroma with tumour cells having abundant, densely eosinophilic cytoplasm, although intracytoplasmic vacuoles are rare [2]. These tumors are immunopositive for ERG, CD31, podoplanin (D2-40), FLI1, von Willebrand factor, CAMTA1 in WWTR1-CAMTA1 rearranged tumors, and TFE3 in YAP-TFE3 rearranged [41-43]. They are negative for S100P, SOX10, Desmin, and EMA [40]. Angiosarcoma is the major differential diagnosis. It can be differentiated on the basis of ill-formed vasoformation with pleomorphic cells. It lacks the above-mentioned mutations. HEHEs, although extremely rare, can also present in pediatric population at around 12 years of age. They share similar histopathological features as those of adults [3].
Surgical management, either resection or liver transplantation, is the treatment of choice and the only modality that can lead to a 5-year survival rate of 75%–80%. Only 10% lesions are diagnosed early. They are amenable to resection. Majority undergo liver transplantation. Extrahepatic disease may be present in one-third of patients at presentation. However, surgical management still gives good results and prolongs survival [9,19]. There is no role of chemotherapy or radiotherapy.
Hepatic angiosarcoma
Hepatic angiosarcoma or hemangiosarcoma is a rare aggressive malignant tumour arising from endothelial cells. It is the most common primary sarcoma of the liver, contributing to 1% of all liver tumors in adults. It is more common in elderly men, with a male-to-female ratio of 3:1 [9,20]. History of previous exposure to Thorotrast, vinyl chloride, arsenicals, and androgenous steroids has been found in 25%–40% cases [20,44]. Clinically, it presents with generalized weakness, weight loss, abdominal pain, anemia, or hepatomegaly. About half of patients may present with liver failure with jaundice, splenomegaly, hepatic encephalopathy, or ascites. Thrombocytopenia (Kasabach-Merrit syndrome) and rupture with acute hemoperitoneum are rare presentations.
If it is associated with previous thorotrast exposure, metallic density in ‘network fashion’ in upper abdominal masses (liver, spleen or mesenteric lymph nodes) is noted on plain radiographs or CT [45]. On USG, it presents as single or multiple hyperechoic lesions with variable echogenicity due to hemorrhages. On CT, the reticular pattern of thorotrast deposition in liver and spleen with its characteristic circumferential deposition in the periphery of the nodule is noted. In cases other than thorotrast exposure, on plain CT, it usually presents as single or multiple hypodense masses (hyperdense if hemorrhagic) which show arterial enhancement on contrast injection that persists during portal and delayed phases. The pattern of contrast enhancement may be centripetal similar to a haemangioma or a reverse haemangioma pattern. On MRI, it is hypointense on T1-weighted images and predominantly heterogeneously hyperintense on T2-weighted images [46]. The inhomogeneity on T2-weighted images differentiates angiosarcoma from haemangiomas. As thorotrast does not produce any signal on MRI, its presence can easily be missed. On CE-MRI, enhancement patterns are similar to CECT. As imaging findings of angiosarcoma overlap with other vascular tumors of liver, the only reliable finding to diagnose it on imaging is its rapid growth on serial imaging studies. Percutaneous trucut biopsy is contraindicated due to the risk of bleeding. Fine needle aspiration cytology may be done for diagnosis [9].
Angiosarcomas are the most common malignant mesenchymal tumors of the liver comprising 2% of primary hepatic tumors with a predominantly male occurrence. They present commonly in the 6th to 7th decade [47-49]. On histopathology, these tumors have features ranging from bland cells having vasoformation to sheets and cords of pleomorphic cells without any vasoformation [2]. These tumour cells are plump, pleomorphic, and mitotically active. They can be spindle-shaped, polygonal, epithelioid, and primitive round cells, forming papillae or solid nests within vascular lumina [2]. Intratumoural haemorrhage can also be seen. These tumors are immunopositive for CD31, CD34, ERG, FLI1, VEGF, and factor VIII but negative for HHV8 ruling out KS [2]. Pediatric angiosarcomas are very rare hepatic tumors having similar biopsy features as those of adults, presenting more commonly in females at around 40 months of age with abdominal distention and pain [3]. These tumors show upregulation of vascular specific receptor tyrosine kinases such as TIE1, KDR, TEK, and FLT [50]. Surgical management and adjuvant chemotherapy are treatments of choice for a better survival [51]. Antiangiogenic therapy, recombinant therapy, and immunotherapy have been tried with or without chemotherapy, showing highly debatable results [31].
Angiosarcoma is an aggressive tumour with an extremely poor prognosis. Most patients present with advanced tumors involving both lobes of liver. They usually die within six months of diagnosis. Up to 50% may also have extrahepatic metastases to lung, bone, or spleen [20]. Rarely, if the lesion is localised to one lobe, surgical resection may be done. Palliative angioembolisation may be done for symptomatic relief or in cases of tumour rupture with hemoperitoneum. Liver transplantation is contraindicated due to its aggressive nature and early recurrence post-transplant [9,19].
Hepatic small vessel neoplasm
HSVN is a recently identified rare vascular tumour of liver that shows features of both angiosarcoma and cavernous haemangioma. It has an infiltrative growth pattern with minimal cytological atypia and mitotic activity [52]. It occurs mainly in men, with a mean age of 54 years and a size of 2.1 cm [53]. It is usually asymptomatic. It can cause epigastric fullness when large. Its imaging features are non-specific, showing strong enhancement on arterial phase with the highest enhancement on portal phase and equivocal findings on delayed phases on CT and diffusion-weighted MRI [54,55]. The final diagnosis is usually made on immunohistochemistry and Ki-67 proliferation index.
HSVNs are different from haemangiomas in having infiltrative borders. They are not as malignant as angiosarcomas as they do not have atypical cells or high mitotic activity [56]. Joseph et al. [57] have suggested that these tumors may harbour GNAQ, GNA11, or GNA14 mutations. These tumors have uncertain malignant potential. They present as poorly defined haemorrhagic tumors. Microscopically, they comprise of infiltrative neoplasm having irregular vessels lined by flat to oval endothelial cells showing hobnailing [23]. These tumors are immunopositive for CD31, CD34, and FLI-1 [31,56]. Their major differential diagnosis is angiosarcoma. Both entities can be diagnosed and differentiated on the basis of GLUT-1, p53, Ki-67, and c-myc expression. HSVN has higher Ki-67 than haemangiomas. However, if it is more than 10%, then angiosarcomas should be suspected [31]. Immunopositivity for GLUT-1, p53, and c-mycgoes in favour of angiosarcoma [31]. Surgical resection is curative. No recurrence has been reported [8,53].
CONCLUSION
There are not many vascular tumors of the liver. They are definitely uncommon and difficult to discern other than typical haemangiomas. A correct diagnosis is essential to decide about the management and prognosis. All vascular tumors of liver that can be diagnosed on histopathology and treated accordingly are summarized in Table 1-3 [1-3,11,12,16,21-23,30,31,35-37,40,41,47-51,53,56-69].
Table 1.
Summary of hepatic vascular lesions
| Entities | Inc (%) | Age | Sex | Presentation | Gross | Microscopic examination | IHC | Molecular | Mx | Articles |
|---|---|---|---|---|---|---|---|---|---|---|
| Hemangioma | 1–6 | Children to adults | Common in females | If large or long standing Thrombosis, post traumatic bleeding or pressure related symptoms | Well circumscribed; > 5 cm: Giant hemangioma | Cavernous hemangioma: Most common; large dilated spaces lined by endothelial cells. | CD 31, CD 34, ERG, FLI-1, Factor VIII | Nothing specific | Resection if large | Lerut and Iesari [1], Thampy et al. [11], Miyaki et al. [12], Kristidis et al. [21], Yoon et al. [58], Brouwers et al. [59] |
| Hemangiomatosis | Rare | Children to adult | Common in females | Kasabach-Merritt syndrome | Ill-defined | Similar to hemangiomas | Similar to hemangiomas | Nothing specific | Liver transplant | Lerut and Iesari [1], Thampy et al. [11], Maeda et al. [16], Lazăr et al. [31] |
| HEHE | < 1 | 30–50 years | Common in females | Mostly asymptomatic | Asymptomatic masses but can coalesce into large masses having whitish firm surfaces |
WWTR1-CAMTA1 rearranged tumors: cords, strands or small nests of large endothelial cells with abundant eosinophilic cytoplasm embedded in a myxohyaline stroma with cells having vesicular, round to oval sometimes indented nuclei. YAP-TFE3 rearranged tumors: Solid nests or pseudo alveolar arrangement of epithelioid cells enmeshed in a fibrous stroma with tumor cells have abundant, densely eosinophilic cytoplasm. |
ERG, CD31, podoplanin (D2-40), FLI1, von Willebrand factor, CAMTA1 in WWTR1-CAMTA1 rearranged tumors and TFE3 in YAP-TFE3 rearranged. Negative for S100P, SOX10, Desmin and EMA. | WWTR1-CAMTA1 rearrangemnet; YAP-TFE3 rearrangement | Wide local excision | Lerut and Iesari [1], Bannoura and Putra [3], Miller et al. [36], Da Ines et al. [37], Lau et al. [40], Flucke et al. [41], Lazăr et al. [31] |
| Angiosarcoma | 2 | 60–70 years | Common in males | Portal hypertension/liver failure | Ill-defined, multifocal | Features ranging from bland cells having vasoformation to sheets and cords of pleomorphic cell without any vasoformation.2 Tumour cells are plump, pleomorphic and mitotically active. Can be spindle shaped, polygonal, epithelioid and primitive round cells, forming papillae or solid nests within vascular lumina. | Positive: CD31, CD34, ERG, FLI1, VEGF and factor VIII. Negative: HHV-8 |
Upregulation of TIE1, KDR, TEK and FLT | Surgical resectionand adjuvant chemotherapy | WHO [2], Lazăr et al. [31], Yasir and Torbenson [47], Mehrabi et al. [48], Li et al. [49], Omiyale and Carton [50], Huang et al. [51] |
| Kaposi sarcoma | Predominantly HIV associated | Predominantly HIV associated | Predominantly HIV associated | Indolent; Locally aggressive | Multinodular | Typically perivascular with main concentration around peripheral portal branches. Presence of nodules with macrovesicular steatosis and the perinodular area showing presence of small vessels. Nodules are comprised of spindle shaped cells with intervening slit like or sieve like blood vessels. | Typically immunopositive for HHV-8 showing stippled nuclear staining pattern | Nothing specific | Palliative | WHO [2] |
| PEComa | Rare | Common in females | Mean age 45 years | Commonly associated with tuberous sclerosis; May range from benign to malignant | Well-circumscribed with a fibrous or fleshy cut surface | Comprised of nests, trabeculae or sheet like architecture of cells which are epithelioid to spindle with granular eosinophilic to clear cytoplasm | Immunopositive for HMB45, Melan A, MITF, SMA, Desmin and h-Caldesmon. | Loss of heterozygosity in TSC2 locus. TFE3 gene rearrangement |
Benign: Resection Malignant: Chemotherapy including mTOR inhibitors [42] |
WHO [2], Folpe and Kwiatkowski [30] |
| HIHE | 20 of pediatric hepatic tumors | Common in females | Mean age 6 months | Congestive cardiac failure, hepatomegaly and failure to thrive | Solitary to multiple well circumscribed masses with red tan, soft to spongy cut surface with co-existing cavernous hemangiomas | Type 1: proliferation of small, irregular capillary-like vascular spaces lined by bland or plump endothelial cells separated by variable connective tissue. Type 2: irregular branching vascular structures lined by pleomorphic, hyperchromatic endothelial cells, frequent mitotic activity. |
Factor VIII related antigen, CD31, CD34 and GLUT1 | Can be associated with Wilms tumour | May regress; Solitary: resection Steroids, Embolization and transplant can be tried [43]. | Lerut and Iesari [1], WHO [2], Christison-Lagay et al. [60], Mo et al. [22] |
| HSVN | Recent entity | Common in males [22] | Mean age: 54 years [22] | Mostly asymptomatic | Infiltrative borders | Irregular vessels lined by flat to oval endothelial cells showing hobnailing. | CD31, CD34 and FLI-1 | GNAQ, GNA11, or GNA14 mutations | Resection and follow-up | Lazăr et al. [31], Walcott-Sapp et al. [56], Joseph et al. [57] |
| SFT | Less than 1 | Not specific | 50–60 years | Asymptomatic to acute abdomen | Solitary, well-defined lesion | Hypocellular and hypercellular areas with the cellular areas being comprised spindle cells in a patternless arrangement interspersed with staghorn vessels. | CD 34 and STAT-6 | Somatic mutations between NAB2 and STAT6 genes. | Aggressive surgery | Lerut and Iesari [1], WHO [2], Lazăr et al. [31], Adams et al. [35] |
| NRH | 0.7 to 2.6 | Not specific | Mean age: 40 years | Portal hypertension | Small nodules without cirrhosis | Diffuse nodules of hyperplastic hepatocytes around the portal tract with centrilobular atrophy of hepatocytes. Sinusoids become congested with compression of the central veins. The regenerative hepatocytes are have clear, vacuolated cytoplasm along with cholestasis and are 2–3 plates thick as compared to the adjacent hepatocytes which are usually one plate thick. No evidence of fibrosis or extramedullary hematopoiesis in the liver parenchyma. | Lerut and Iesari [1], Shastri et al. [23], WHO [2] |
HEHE, hepatic epithelioid hemangioendothelioma; PEComa, perivascular epithelioid cell tumour; HIHE, hepatic epithelioid hemangioendothelioma; HSVN, hepatic small vessel neoplasms; NRH, nodular regenerative hyperplasia; IHC, immunohistochemistry.
Table 2.
Benign and intermediate hepatic vascular tumors: a comparison among literature
| Entities | Hepatic hemangiomas | Kaposi Sarcoma | HSVN | |||||
|---|---|---|---|---|---|---|---|---|
| Bajenaru et al. [61] | Jia et al. [62] | Van Leer-Greenberg et al. [63] | Arora and Goldberg [64] | Gill et al. [53] (described first; 17 cases) | Mulholland et al. [65] | |||
| Incidence | 0.4%–20% | 0.4%–7.3% | 12-24; 34% with HIV | > 50% in GIT | Rare | 22–23 cases till date | ||
| Age | 30–50 yr | 30–50 yr | 20–40 yr | Patients with HIV | 24–83 yr | |||
| M:F | 1:4.5 to 1: 5 | 1:1.2 to 1:6 | Common in males; Africa | Males | Common in males | |||
| Treatment | Resection if symptomatic | Resection if symptomatic | HAART and chemotherapy | HAART | Resection | |||
GIT, gastrointestinal tract; HAART, highly active anti-retroviral therapy; HSVN, hepatic small vessel neoplasms.
Table 3.
Malignant hepatic vascular lesions: a comparison among literature
| Entities | Hepatic angiosarcomas | Hepatic hemangioendothelioma | |||
|---|---|---|---|---|---|
| Kumar et al. [66] | Chen et al. [67] | Kou et al. [68], Virarkar et al. [69] |
|||
| Incidence (%) | 0.1–2 | 0.1–2 | 1–2/million | ||
| Age (yr) | 60–70 | 60–70 | 30–50 | ||
| M:F | 3:1 to 4:1 | 3:1 | 2:3 | ||
| Treatment | Complete hepatic resection or radical resection | Complete hepatic resection | Surgical | ||
| Median survival | 6 mon | 6 mon | 5 yr survival: 75% | ||
Funding Statement
FUNDING None.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: SS. Data curation: SS, BT, TY, SB. Methodology: SS. Writing - original draft: all authors. Writing - review & editing: SK.
REFERENCES
- 1.Lerut J, Iesari S. Vascular tumours of the liver: a particular story. Transl Gastroenterol Hepatol. 2018;3:62. doi: 10.21037/tgh.2018.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Classification of Tumours Editorial Board, author. Soft tissue and bone tumours. 5th ed. Vol 3. International Agency for Research on Cancer; 2020. [Google Scholar]
- 3.Bannoura S, Putra J. Primary malignant vascular tumors of the liver in children: angiosarcoma and epithelioid hemangioendothelioma. World J Gastrointest Oncol. 2021;13:223–230. doi: 10.4251/wjgo.v13.i4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver, author. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Dasgupta R, Fishman SJ. ISSVA classification. Semin Pediatr Surg. 2014;23:158161. doi: 10.1053/j.sempedsurg.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Choi BY, Nguyen MH. The diagnosis and management of benign hepatic tumours. J Clin Gastroenterol. 2005;39:401–412. doi: 10.1097/01.mcg.0000159226.63037.a2. [DOI] [PubMed] [Google Scholar]
- 8.Dokmak S, Ronot M. Chapter 88A - Benign liver lesions. In: Jarnagin W, editor. Blumgart’s Surgery of the Liver, Biliary tract and Pancreas. 7th ed. Elsevier Inc.; 2023. pp. 1181–1202. [Google Scholar]
- 9.Ehman EC, Torbenson MS, Wells ML, Welch BT, Thompson SM, Garg I, et al. Hepatic tumours of vascular origin: imaging appearances. Abdom Radiol (NY) 2018;43:1978–1990. doi: 10.1007/s00261-017-1401-3. [DOI] [PubMed] [Google Scholar]
- 10.Ros PR, Erturk SM. Benign Tumours of the Liver. In: Gore RM, Levine MS, editors. Textbook of Gastrointestinal Radiology. 5th ed. Elsevier Inc.; 2021. pp. 767–787. [DOI] [Google Scholar]
- 11.Thampy R, Elsayes KM, Menias CO, Pickhardt PJ, Kang HC, Deshmukh SP, et al. Imaging features of rare mesenychmal liver tumours: beyond haemangiomas. Br J Radiol. 2017;90:20170373. doi: 10.1259/bjr.20170373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyaki D, Aikata H, Waki K, Murakami E, Hashimoto Y, Nagaoki Y, et al. [Significant regression of a cavernous hepatic haemangioma to a sclerosed haemangioma over 12 years: a case study] Nihon Shokakibyo Gakkai Zasshi. 2011;108:954–961. Japanese. [PubMed] [Google Scholar]
- 13.Yoo BR, Han HY, Choi SY, Kim JH. Giant cavernous haemangioma coexistent with diffuse hepatic haemangiomatosis presenting as portal vein thrombosis and hepatic lobar atrophy. Ultrasonography. 2014;33:65–70. doi: 10.14366/usg.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conter RL, Longmire WP., Jr Recurrent hepatic haemangiomas. Possible association with estrogen therapy. Ann Surg. 1988;207:115–119. doi: 10.1097/00000658-198802000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feurle GE. Arteriovenous shunting and cholestasis in hepatic haemangiomatosis associated with metoclopramide. Gastroenterology. 1990;99:258–262. doi: 10.1016/0016-5085(90)91256-6. [DOI] [PubMed] [Google Scholar]
- 16.Maeda E, Akahane M, Watadani T, Yoshioka N, Goto A, Sugawara Y, et al. Isolated hepatic haemangiomatosis in adults: report of two cases and review of the literature. Eur J Radiol Extra. 2007;61:9–14. doi: 10.1016/j.ejrex.2006.10.007. [DOI] [Google Scholar]
- 17.Chung EM, Lattin GE, Jr, Cube R, Lewis RB, Marichal-Hernández C, Shawhan R, et al. From the archives of the AFIP: pediatric liver masses: radiologic-pathologic correlation. Part 2. Malignant tumours. Radiographics. 2011;31:483–507. doi: 10.1148/rg.312105201. [DOI] [PubMed] [Google Scholar]
- 18.Hsi Dickie B, Fishman SJ, Azizkhan RG. Hepatic vascular tumours. Semin Pediatr Surg. 2014;23:168–172. doi: 10.1053/j.sempedsurg.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Bisceglie AMD. Chapter 96 - Hepatic Tumours and Cysts. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 11th ed. Elsevier Inc.; 2021. pp. 1509–1532. [Google Scholar]
- 20.Paradis V. Chapter 87 - Tumours of the liver: Pathologic aspects. In: Jarnagin W, editor. Blumgart’s Surgery of the Liver, Biliary tract and Pancreas. 7th ed. Elsevier Inc.; 2023. pp. 1152–1180. [Google Scholar]
- 21.Kristidis P, de Silva M, Howman-Giles R, Gaskin KJ. Infantile hepatic haemangioma: investigation and treatment. J Paediatr Child Health. 1991;27:57–61. doi: 10.1111/j.1440-1754.1991.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 22.Mo JQ, Dimashkieh HH, Bove KE. GLUT1 endothelial reactivity distinguishes hepatic infantile haemangioma from congenital hepatic vascular malformation with associated capillary proliferation. Hum Pathol. 2004;35:200–209. doi: 10.1016/j.humpath.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Shastri S, Dubinsky MC, Fred Poordad F, Vasiliauskas EA, Geller SA. Early nodular hyperplasia of the liver occurring with inflammatory bowel diseases in association with thioguanine therapy. Arch Pathol Lab Med. 2004;128:49–53. doi: 10.5858/2004-128-49-ENHOTL. [DOI] [PubMed] [Google Scholar]
- 24.WHO Classification of Tumours Editorial Board, author. Digestive system tumours. 5th ed. International Agency for Research on Cancer; 2019. [DOI] [Google Scholar]
- 25.Restrepo CS, Martínez S, Lemos JA, Carrillo JA, Lemos DF, Ojeda P, et al. Imaging manifestations of Kaposi sarcoma. Radiographics. 2006;26:1169–1185. doi: 10.1148/rg.264055129. [DOI] [PubMed] [Google Scholar]
- 26.Addula D, Das CJ, Kundra V. Imaging of Kaposi sarcoma. Abdom Radiol (NY) 2021;46:5297–5306. doi: 10.1007/s00261-021-03205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nie P, Wu J, Wang H, Zhou R, Sun L, Chen J, et al. Primary hepatic perivascular epithelioid cell tumours: imaging findings with histopathological correlation. Cancer Imaging. 2019;19:32. doi: 10.1186/s40644-019-0212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen HQ, Chen DF, Sun XH, Li X, Xu J, Hu XB, et al. MRI diagnosis of perivascular epithelioid cell tumour (PEComa) of the liver. Rom J Morphol Embryol. 2013;54:643–647. [PubMed] [Google Scholar]
- 29.Maebayashi T, Abe K, Aizawa T, Sakaguchi M, Ishibashi N, Abe O, et al. Improving recognition of hepatic perivascular epithelioid cell tumour: case report and literature review. World J Gastroenterol. 2015;21:5432–5441. doi: 10.3748/wjg.v21.i17.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. 2010;41:1–15. doi: 10.1016/j.humpath.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Lazăr DC, Avram MF, Romoșan I, Văcariu V, Goldiș A, Cornianu M. Malignanthepatic vascular tumours in adults: characteristics, diagnostic difficulties and currentmanagement. World J Clin Oncol. 2019;10:110–135. doi: 10.5306/wjco.v10.i3.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yugawa K, Yoshizumi T, Mano Y, Kurihara T, Yoshiya S, Takeishi K, et al. Solitary fibrous tumour in the liver: case report and literature review. Surg Case Rep. 2019;5:68. doi: 10.1186/s40792-019-0625-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan G, Horton PJ, Thyssen S, Lamarche M, Nahal A, Hill DJ, et al. Malignant transformation of a solitary fibrous tumour of the liver and intractable hypoglycemia. J Hepatobiliary Pancreat Surg. 2007;14:595–599. doi: 10.1007/s00534-007-1210-0. [DOI] [PubMed] [Google Scholar]
- 34.Bejarano-González N, García-Borobia FJ, Romaguera-Monzonís A, García-Monforte N, Falcó-Fagés J, Bella-Cueto MR, et al. Solitary fibrous tumour of the liver. Case report and review of the literature. Rev Esp Enferm Dig. 2015;107:633–639. doi: 10.17235/reed.2015.3676/2014. [DOI] [PubMed] [Google Scholar]
- 35.Adams J, Lodge JP, Parker D. Liver transplantation for metastatic hemangiopericytoma associated withhypoglycemia. Transplantation. 1999;67:488–489. doi: 10.1097/00007890-199902150-00027. [DOI] [PubMed] [Google Scholar]
- 36.Miller WJ, Dodd GD, 3rd, Federle MP, Baron RL. Epithelioid hemangioendothelioma of the liver: imaging findings with pathologic correlation. AJR Am J Roentgenol. 1992;159:53–57. doi: 10.2214/ajr.159.1.1302463. [DOI] [PubMed] [Google Scholar]
- 37.Da Ines D, Petitcolin V, Joubert-Zakeyh J, Demeocq F, Garcier JM. Epithelioid hemangioendothelioma of the liver with metastatic coeliac lymph nodes in an 11-year-old boy. Pediatr Radiol. 2010;40:1293–1296. doi: 10.1007/s00247-009-1532-y. [DOI] [PubMed] [Google Scholar]
- 38.Ros PR, Erturk SM. Malignant Tumours of the Liver. In: Gore RM, Levine MS, editors. Textbook of Gastrointestinal Radiology. 5th ed. Elsevier Inc.; 2021. pp. 788–818. [Google Scholar]
- 39.Lyburn ID, Torreggiani WC, Harris AC, Zwirewich CV, Buckley AR, Davis JE, et al. Hepatic epithelioid hemangioendothelioma: sonographic, CT, and MR imaging appearances. AJR Am J Roentgenol. 2003;180:1359–1364. doi: 10.2214/ajr.180.5.1801359. [DOI] [PubMed] [Google Scholar]
- 40.Lau K, Massad M, Pollak C, Rubin C, Yeh J, Wang J, et al. Clinical patterns and outcome in epithelioid hemangioendothelioma with or without pulmonary involvement: insights from an internet registry in the study of a rare cancer. Chest. 2011;140:1312–1318. doi: 10.1378/chest.11-0039. [DOI] [PubMed] [Google Scholar]
- 41.Flucke U, Vogels RJ, de Saint Aubain Somerhausen N, Creytens DH, Riedl RG, van Gorp JM, et al. Epithelioid hemangioendothelioma: clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases. Diagn Pathol. 2014;9:131. doi: 10.1186/1746-1596-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doyle LA, Fletcher CD, Hornick JL. Nuclear expression of CAMTA1 distinguishes epithelioid hemangioendothelioma from histologic mimics. Am J Surg Pathol. 2016;40:94–102. doi: 10.1097/PAS.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 43.Antonescu CR, Le Loarer F, Mosquera JM, Sboner A, Zhang L, Chen CL, et al. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer. 2013;52:775–784. doi: 10.1002/gcc.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy DW, Rindsberg S, Friedman AC, Fishman EK, Ros PR, Radecki PD, et al. Thorotrast-induced hepatosplenic neoplasia: CT identification. AJR Am J Roentgenol. 1986;146:997–1004. doi: 10.2214/ajr.146.5.997. [DOI] [PubMed] [Google Scholar]
- 45.Zheng YW, Zhang XW, Zhang JL, Hui ZZ, Du WJ, Li RM, et al. Primary hepatic angiosarcoma and potential treatment options. J Gastroenterol Hepatol. 2014;29:906–911. doi: 10.1111/jgh.12506. [DOI] [PubMed] [Google Scholar]
- 46.Worawattanakul S, Semelka RC, Kelekis NL, Woosley JT. Angiosarcoma of the liver: MR imaging pre- and post-chemotherapy. Magn Reson Imaging. 1997;15:613–617. doi: 10.1016/S0730-725X(96)00393-1. [DOI] [PubMed] [Google Scholar]
- 47.Yasir S, Torbenson MS. Angiosarcoma of the liver: clinicopathologic features and morphologic patterns. Am J Surg Pathol. 2019;43:581–590. doi: 10.1097/PAS.0000000000001228. [DOI] [PubMed] [Google Scholar]
- 48.Mehrabi A, Kashfi A, Fonouni H, Schemmer P, Schmied BM, Hallscheidt P, et al. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer. 2006;107:2108–2121. doi: 10.1002/cncr.22225. [DOI] [PubMed] [Google Scholar]
- 49.Li DB, Si XY, Wan T, Zhou YM. A pooled analysis of treatment and prognosis of hepatic angiosarcoma in adults. Hepatobiliary Pancreat Dis Int. 2018;17:198–203. doi: 10.1016/j.hbpd.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Omiyale AO, Carton J. Clinical and pathologic features of primary angiosarcoma of the kidney. Curr Urol Rep. 2018;19:4. doi: 10.1007/s11934-018-0755-6. [DOI] [PubMed] [Google Scholar]
- 51.Huang NC, Kuo YC, Chiang JC, Hung SY, Wang HM, Hung YM, et al. Hepatic angiosarcoma may have fair survival nowadays. Medicine (Baltimore) 2015;94:e816. doi: 10.1097/MD.0000000000000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goh IY, Mulholland P, Sokolova A, Liu C, Siriwardhane M. Hepatic small vessel neoplasm - a systematic review. Ann Med Surg (Lond) 2021;72:103004. doi: 10.1016/j.amsu.2021.103004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gill RM, Buelow B, Mather C, Joseph NM, Alves V, Brunt EM, et al. Hepatic small vessel neoplasm, a rare infiltrative vascular neoplasm of uncertain malignant potential. Hum Pathol. 2016;54:143–151. doi: 10.1016/j.humpath.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewis S, Aljarallah B, Trivedi A, Thung SN. Magnetic resonance imaging of a small vessel hepatic haemangioma in a cirrhotic patient with histopathologic correlation. Clin Imaging. 2015;39:702–706. doi: 10.1016/j.clinimag.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Rangaswamy B, Minervini M, Tublin M, Sholosh B, Dasyam AK. Imaging and pathologic findings of hepatic small vessel haemangioma. Curr Probl Diagn Radiol. 2019;48:626–628. doi: 10.1067/j.cpradiol.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 56.Walcott-Sapp S, Tang E, Kakar S, Shen J, Hansen P. Resection of the largest reported hepatic small vessel neoplasm. Hum Pathol. 2018;78:159–162. doi: 10.1016/j.humpath.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Joseph NM, Brunt EM, Marginean C, Nalbantoglu I, Snover DC, Thung SN, et al. Frequent GNAQ and GNA14 mutations in hepatic small vessel neoplasm. Am J Surg Pathol. 2018;42:1201–1207. doi: 10.1097/PAS.0000000000001110. [DOI] [PubMed] [Google Scholar]
- 58.Yoon SS, Charny CK, Fong Y, Jarnagin WR, Schwartz LH, Blumgart LH, et al. Diagnosis, management, and outcomes of 115 patients with hepatic haemangioma. J Am Coll Surg. 2003;197:392–402. doi: 10.1016/S1072-7515(03)00420-4. [DOI] [PubMed] [Google Scholar]
- 59.Brouwers MA, Peeters PM, de Jong KP, Haagsma EB, Klompmaker IJ, Bijleveld CM, et al. Surgical treatment of giant haemangioma of the liver. Br J Surg. 1997;84:314–316. doi: 10.1046/j.1365-2168.1997.02534.x. [DOI] [PubMed] [Google Scholar]
- 60.Christison-Lagay ER, Burrows PE, Alomari A, Dubois J, Kozakewich HP, Lane TS, et al. Hepatic haemangiomas: subtype classification and development of a clinical practice algorithm and registry. J Pediatr Surg. 2007;42:62–67. doi: 10.1016/j.jpedsurg.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 61.Bajenaru N, Balaban V, Săvulescu F, Campeanu I, Patrascu T. Hepatic hemangioma -review- J Med Life. 2015;8 Spec Issue(Spec Issue):4–11. [PMC free article] [PubMed] [Google Scholar]
- 62.Jia K, Gao Z, Li M, Yu C. Interventional treatments for hepatic hemangioma: A state-of-the-art review. J Interv Med. 2022;5:6–9. doi: 10.1016/j.jimed.2021.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Leer-Greenberg B, Kole A, Chawla S. Hepatic Kaposi sarcoma: A case report and review of the literature. World J Hepatol. 2017;9:171–179. doi: 10.4254/wjh.v9.i4.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arora M, Goldberg EM. Kaposi sarcoma involving the gastrointestinal tract. Gastroenterol Hepatol (N Y) 2010;6:459–462. [PMC free article] [PubMed] [Google Scholar]
- 65.Mulholland P, Goh IY, Sokolova A, Liu C, Siriwardhane M. Hepatic small vessel neoplasm case report: A surveillance conundrum. Int J Surg Case Rep. 2021;81:105742. doi: 10.1016/j.ijscr.2021.105742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar A, Sharma B, Samant H. Liver Angiosarcoma [Internet] StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538224/ [PubMed] [Google Scholar]
- 67.Chen N, Yu AJS, Jung J. Primary hepatic angiosarcoma: a brief review of the literature. EMJ Hepatol. 2018;6:64–71. doi: 10.33590/emjhepatol/10314175. [DOI] [Google Scholar]
- 68.Kou K, Chen YG, Zhou JP, Sun XD, Sun DW, Li SX, et al. Hepatic epithelioid hemangioendothelioma: Update on diagnosis and therapy. World J Clin Cases. 2020;8:3978–3987. doi: 10.12998/wjcc.v8.i18.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Virarkar M, Saleh M, Diab R, Taggart M, Bhargava P, Bhosale P. Hepatic hemangioendothelioma: an update. World J Gastrointest Oncol. 2020;12:248–266. doi: 10.4251/wjgo.v12.i3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]