Highlights
-
•
Disruptive behavior is associated with resting-state functional connectivity (rsFC) alterations in several brain regions implicated in emotion regulation, decision-making, and moral-related behaviors.
-
•
Overlapping and distinct rsFC alterations were identified for aggression subtypes.
-
•
Our findings underscored the importance of comorbid ADHD and anxiety symptoms to detect aggression-related rsFC alterations in youths.
Keywords: Reactive aggression, Proactive aggression, Callous-unemotional traits, Resting-state fMRI, Children, Adolescents
Abstract
Background
Disruptive behavior in children and adolescents can manifest as reactive aggression and proactive aggression and is modulated by callous-unemotional traits and other comorbidities. Neural correlates of these aggression dimensions or subtypes and comorbid symptoms remain largely unknown. This multi-center study investigated the relationship between resting state functional connectivity (rsFC) and aggression subtypes considering comorbidities.
Methods
The large sample of children and adolescents aged 8–18 years (n = 207; mean age = 13.30±2.60 years, 150 males) included 118 cases with disruptive behavior (80 with Oppositional Defiant Disorder and/or Conduct Disorder) and 89 controls. Attention-deficit/hyperactivity disorder (ADHD) and anxiety symptom scores were analyzed as covariates when assessing group differences and dimensional aggression effects on hypothesis-free global and local voxel-to-voxel whole-brain rsFC based on functional magnetic resonance imaging at 3 Tesla.
Results
Compared to controls, the cases demonstrated altered rsFC in frontal areas, when anxiety but not ADHD symptoms were controlled for. For cases, reactive and proactive aggression scores were related to global and local rsFC in the central gyrus and precuneus, regions linked to aggression-related impairments. Callous-unemotional trait severity was correlated with ICC in the inferior and middle temporal regions implicated in empathy, emotion, and reward processing. Most observed aggression subtype-specific patterns could only be identified when ADHD and anxiety were controlled for.
Conclusions
This study clarifies that hypothesis-free brain connectivity measures can disentangle distinct though overlapping dimensions of aggression in youths. Moreover, our results highlight the importance of considering comorbid symptoms to detect aggression-related rsFC alterations in youths.
1. Introduction
Severe aggressive behavior in children and adolescents is a main characteristic of DSM-5 aggression-related disorders (American Psychiatric Association, 2013). Among them, conduct disorder (CD) is defined as a persistent violation of rules, norms, and rights, including physical and psychological aggressive behaviors. Oppositional defiant disorder (ODD) refers to a pattern of angry, argumentative, and vindictive behavior. Brain studies have associated CD and ODD with frontal, striatal, and limbic functional alterations (Blair et al., 2018). Notably, the combination of childhood CD and comorbid attention-deficit/hyperactivity disorder (ADHD) is thought to be linked to persistent antisocial behavior (Frick, 2016, Moffitt, 2017, Moffitt et al., 2002). In addition to ADHD (Loeber et al., 2000), comorbid anxiety (Frick, 2012, Frick et al., 2014) also seems crucial for subtyping aggression at a psychophysiological level (Fanti, 2018).These above observations underline the relevance of considering symptomatology co-occurring with aggression and suggest that brain-related measures may mark aspects of these juvenile disorders.
Resting-state functional magnetic resonance imaging (rs-fMRI) allows for the detection of intrinsic activity and functional synchronizations in brain regions during the absence of goal-directed behaviors (i.e., task-free mental state) (Fox et al., 2015, Greicius et al., 2003, Raichle et al., 2001). This well-established approach has provided important insights into biological psychiatry in general and, specifically, in relation to aggression-related disorders. For instance, a recent coordinate-based meta-analysis (Dugré and Potvin, 2021) showed that individuals with antisocial behavior exhibited abnormal resting-state functional connectivity (rsFC) in frontal and parietal regions constituting the default-mode network (DMN), which confirms the findings of previous empirical work conducted in male adolescents with CD (Lu et al., 2017a, Lu et al., 2015, Zhou et al., 2016). However, altered rsFC was not only restricted to the DMN but identified also in limbic (Cao et al., 2018, Wu et al., 2017, Zhou et al., 2015), motor (Cao et al., 2018, Dugré and Potvin, 2023, Lu et al., 2021a), cerebellar (Cao et al., 2019, Cao et al., 2018, Wu et al., 2017), and visual regions (Lu et al., 2021a, Lu et al., 2017b, Lu et al., 2015, Pu et al., 2017, Zhou et al., 2016). Furthermore, these alterations encompassed both decreased (Lu et al., 2017b, Lu et al., 2017a, Zhou et al., 2016) and increased (Lu et al., 2017a, Pu et al., 2017, Uytun et al., 2017) rsFC patterns compared to controls, especially in the DMN. These studies, however, have been mostly restricted to case–control comparisons in male adolescents with CD and further limited by their small sample sizes. Research on the diverse aggression-related conditions and subclinical profiles beyond CD and ODD diagnoses is also necessary to characterize the functional brain organization underlying disruptive behavior across a spectrum.
Aggression is a heterogeneous phenotype and has frequently been subdivided into reactive aggression (RA) and proactive aggression (PA) behaviors (Dodge and Coie, 1987, Romero-Martínez et al., 2022, Vitiello and Stoff, 1997). RA is described as perceiving the behavior of others as a provocation or threat and reacting aggressively (Smeets et al., 2017), while PA is characterized by planned, instrumental aggressive behaviors. A recent systematic review of RA and PA reported morphological differences in the amygdala and temporal regions as well as higher medial prefrontal cortex activation in both aggression subtypes alongside several distinct morphological and brain activation alterations (Romero-Martínez et al., 2022). However, most of the studies they included were conducted in adults, and neural correlates of RA and PA in disruptive children and adolescents remain underexamined. To the best of our knowledge, only two previous studies have examined RA- and PA-related neural differences using functional connectivity in children and adolescents (Ibrahim et al., 2022, Werhahn et al., 2021). Ibrahim et al. (2022) employed connectome-based predictive modeling during an implicit face perception task and identified largely similar patterns of network connectivity for PA and RA in children (alongside with some distinct alterations). In our previous study, we had investigated rsFC differences related to PA and RA in children and adolescents with disruptive behavior disorder using a seed-based approach (Werhahn et al., 2021). Our results showed that both subdimensions were associated with overlapping as well as distinct effects on rsFC. Altered rsFC between the left amygdala and precuneus (see also Wong et al., 2019 for an ALE meta-analysis on aggression) was observed in both aggression subtypes, whereas RA was further associated with rsFC differences between parietal, temporal, occipital, and limbic regions. However, these findings were limited to the connectivity pattern of a few regions of interest selected a priori. A global approach will offer the advantage of elucidating potential RA- and PA-related rsFC differences across the whole brain
Callous-unemotional (CU) traits are thought to be important modifiers of aggression and may further shape the manifestation of aggression. These traits, characterized by limited prosocial emotions, have been added to the DSM-5 (American Psychiatric Association, 2013) to further specify CD. They were also shown to be associated with a reduced treatment response and poorer clinical outcomes (Bakker et al., 2017, Wilkinson et al., 2016). Moreover, CU traits are linked to impaired empathy (Blair et al., 2014, Ciucci et al., 2015), neurocognitive dysfunctions in emotion and reward learning processes (Reidy et al., 2017), and adult psychopathy (Frick et al., 2014, Frick and White, 2008). There is also evidence for CU traits being linked to both RA and PA (Pechorro et al., 2017) or PA alone (Urben et al., 2018). Importantly, youths with CU and CU-related traits but various levels of anxiety differ in their behavioral characteristics (Fanti et al., 2013, Kimonis et al., 2012). Prior investigations showed that male adolescents with CU traits exhibited altered amygdala sub-regional rsFC (Aghajani et al., 2016, Aghajani et al., 2017) and reduced amygdala efficiency (Jiang et al., 2021) compared to controls. Correlational studies conducted with large samples further demonstrated that CU traits were linked to rsFC abnormalities in the DMN and attention networks (Umbach and Tottenham, 2021, Werhahn et al., 2021) as well as disrupted connectivity between DMN and attention networks (Pu et al., 2017, Winters et al., 2023). However, these studies were also biased in terms of a priori selection of regions or parcellation maps. In addition, despite anxiety commonly co-occurring with prominent behavioral and psychophysiological effects (Dadds et al., 2018, Fanti et al., 2013, Guelker et al., 2014) and interacting with psychopathy levels (Motzkin et al., 2011), knowledge on rsFC correlates of CU traits is still scarce, and the impact of comorbid anxiety is unknown.
Using seed-based rsFC, we previously identified altered connectivity of the DMN and salience network regions with frontal, parietal, and occipital regions in children and adolescents with disruptive behavior disorder (Werhahn et al., 2021). In this present study, we extend our prior work by investigating unconstrained voxel-to-voxel rsFC to provide complementary and more comprehensive insights into whole-brain rsFC alterations related to disruptive behavior in this large sample of children and adolescents. Specifically, we examined the rsFC patterns in relation to RA and PA dimensions as well as CU traits, and simultaneously considered co-occurring ADHD and anxiety symptoms in the analyses. We focused on two parameters, namely global and local brain rsFC. On a global level, the intrinsic connectivity contrast (ICC) was computed to measure network centrality by calculating the global strength of connectivity for each voxel with other voxels in the rest of the brain (Martuzzi et al., 2011). This method has the advantage of circumventing the need for a priori definitions of regions of interest. ICC has been applied in prior studies (Browndyke et al., 2018, Cassady et al., 2018, Vatansever et al., 2017, Walpola et al., 2017) and has recently been combined with a measure of local coherence (Browndyke et al., 2018), i.e., integrated local correlation (ILC) (Deshpande et al., 2009). Both methods reveal complementary information on brain topology from a hemodynamic response. Based on our previous findings (Werhahn et al., 2021), we hypothesized that we would find (1) between-group differences in rsFC in frontal regions, (2) RA- and PA-related rsFC differences in the precuneus, (3) rsFC alterations of CU traits beyond (para-)limbic regions, and (4) these alterations would be partly modulated by ADHD and anxiety symptoms.
2. Methods and materials
2.1. Participants
For the present study, 118 children and adolescents (males, n = 99) aged 8–18 years (mean age = 13.23, SD = 2.68) diagnosed with ODD and/or CD and/or exhibiting clinically relevant aggression scores along with 89 healthy controls were included (Werhahn et al., 2021). Cases were recruited from resident hospitals, ambulatories, and eligible schools at nine sites in Europe within the framework of the joint EU-MATRICS and EU-Aggressotype projects. Clinically relevant aggression was defined as aggression scores in the clinical range (T >70) according to the Child Behavior Checklist (CBCL), Youth Self Report (YSR), or Teacher Report Form (TRF) (Achenbach, 1991). Exclusion criteria for cases were a primary DSM-diagnosis of depression, anxiety, psychosis, or bipolar disorder for cases and, for the typically developed comparison group, a DSM-diagnosis or clinically relevant aggression score in the CBCL, TRF, or YSR. Furthermore, participants were excluded in cases of contraindications for MRI scanning (i.e., braces, metal implants), insufficient language skills, or an IQ below 80 (Wechsler, 2003). Parents or legal representatives of all participants gave written informed consent. The participating sites obtained ethical approval separately from their local ethics committees. Further information on the study sample is provided in Supplementary Material S1.
2.2. Clinical assessments
To be included in the study, cases had to meet a DSM -diagnosis of CD and/or ODD according to the semi-structured interview Kiddie-Schedule for Affective Disorders and Schizophrenia, present and lifetime version (K-SADS-PL) (Kaufman et al., 1997) or a clinically relevant score (T >70) on the aggression or rule-breaking behavior subscale of the CBCL, YSR, or TRF (Achenbach, 1991). The assessment of these clinical questionnaires and the K-SADS-PL by trained (clinical) psychologists or interns also in the typically developed comparison group ensured the absence of a DSM-diagnosis or clinically relevant aggression scores. Anxiety symptoms were measured using the YSR, as internalizing problems including anxiety have been shown to exhibit higher validity (Leung et al., 2006) and reliability (Gomez et al., 2014) compared to the CBCL and TRF. The self-report Reactive-Proactive Questionnaire (RPQ) was used to measure the frequency of RA and PA symptoms (Raine et al., 2006). RA and PA symptoms within cases were strongly correlated in our sample (r = 0.63, p < 0.001), as previously reported (Cima et al., 2013, Naaijen et al., 2020). CU traits were assessed by means of the parent-reported version of the Inventory of Callous-Unemotional traits (ICU) (Essau et al., 2006), as it seems to quantify these traits better than the self-reported version (Docherty et al., 2017). To assess comorbid ADHD symptoms, parents answered the SNAP-IV (Swanson, 1992), with the inattention and hyperactivity/impulsivity subdomains being used in the present study. Four subscales of the Wechsler Intelligence Scale for Children (Block Design, Similarities, Vocabulary, Picture Completion) (Wechsler, 2003) enabled the estimation of an IQ to ensure sufficient intellectual and cognitive functioning (For more details about assessment tools and study procedure, see Supplementary Material S2).
2.3. Image acquisition
MRI scanning took place at nine sites across Europe, with six sites using Siemens 3T scanners, two sites using Philips 3T scanners, and one site using a GE 3T scanner. Following a T1-weighted structural scan, a T2*-weighted whole-brain echo planar imaging (EPI) resting state sequence (TR 2.45 s or less, with at least 32 slices) with an average acquisition time of 8 min 25 sec was performed (Supplementary Table S2). Participants were instructed to lie still, look at a white crosshair presented against a black background, and not think about anything in particular (Supplementary Material S2). More information on the scanning parameters is provided in the Supplementary Material S3 (Table S1 and Table S2).
2.4. Preprocessing
Functional magnetic resonance imaging (fMRI) data were preprocessed using SPM version 12 (https://www.fil.ion.ucl.ac.uk/spm). After realignment and unwarping (Andersson et al., 2001) and subsequent slice timing correction, multi-echo images were weighted by their echo time (TE) using MATLAB (https://www.mathworks.com, MathWorks, Natick, MA, USA). All rs-fMRI data were normalized to the Montreal Neurological Institute template brain to reduce variability across subjects (Calhoun et al., 2017) using the SPM-based functional connectivity CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012) in MATLAB. Functional scans were smoothed with a Gaussian 6 mm full width at half-maximum kernel. Functional outliers exceeding three standard deviations from the mean intensity across blood-oxygen-level-dependent (BOLD) time series data and exceeding 0.5 mm composite scan-to-scan motion were identified with the Artifact Detection Toolbox (ART, https://www.nitrc.org/projects/artifact_detect) within CONN. Ten cases were excluded from further analysis, owing to their functional scans exceeding 5 % of the highest mean root mean square (RMS) framewise displacement (FD) (cut-off = 0.95 RMS-FD), the threshold applied in prior studies of adolescents with ADHD (Von Rhein et al., 2016). Moreover, 23 cases and 3 controls were further excluded owing to missing scans (n = 2) or insufficient quality of the structural (n = 10) or functional (n = 14) scans. In the denoising step, the aCompCor strategy (Behzadi et al., 2007) as implemented in CONN, was applied to reduce physiological and motion-related noise (Whitfield-Gabrieli and Nieto-Castanon, 2012) and to improve interpretability of the resulting correlation patterns (Chai et al., 2012, Saad et al., 2013). Following linear detrending, BOLD time series data were band-pass filtered between 0.008 and 0.09 Hz (Fox et al., 2005).
2.5. Functional connectivity analyses
Voxel-to-voxel covariance matrices for each subject were calculated in the first-level analysis, which further served to compute ICC (Martuzzi et al., 2011) and ILC (Deshpande et al., 2009) indices. ICC reflects the squared sum of mean connections of a given voxel with all the remaining voxels in the brain. ILC reflects the average correlation between a given voxel and its neighboring voxels. Compared to other local coherence approaches, ILC is independent of image resolution and a predefinition of the neighborhood is unnecessary. ILC also seems to be tissue-specific and largely independent of physiological noise. An 8-mm convolution kernel was applied to determine ILC bounds. In the second-level analysis, random-effects analysis of covariance was employed to conduct between-group comparisons. Correlation coefficients were z-transformed. First, case–control group comparisons were conducted using a two-sample t-test in CONN for both ICC and ILC measures. Second, linear regression analyses were conducted in CONN to test for associations of ICC and ILC with RA, PA, and CU scores separately. All regression analyses were performed at the whole-brain level and within the cases only. All analyses included study sites (8 dummy-coded variables), parent-reported ADHD symptoms (Broulidakis et al., 2016), and self-reported anxiety levels (Gomez et al., 2014, Leung et al., 2006, Motzkin et al., 2011) as covariates of no interest, given their crucial role in behavioral studies on aggression (Dadds et al., 2018, Fanti et al., 2013, Frick, 2016, Guelker et al., 2014). Additional sensitivity analyses including further covariates are provided in Supplementary Material S4. For all results, an uncorrected height threshold of p < 0.001 combined with a false-discovery-rate (FDR)-corrected cluster threshold of p < 0.05 was applied.
2.6. Exploratory analyses
In addition to the primary analyses regressing the RA and PA scores, we also explored the impact of general aggression (RPQ total score) as well as the effects of RA and PA while simultaneously controlling for the variance in PA and RA, respectively (as in Naaijen et al., 2020 for structural MRI analyses of the current study). This type of analysis relates to potential suppressor effects of other variables described previously in the literature on child/adolescent aggression (Ibrahim et al., 2021, Ibrahim et al., 2019, Lozier et al., 2014, Sebastian et al., 2014).
To address the heterogeneity of CU traits, we conducted exploratory analyses for CU subdimensions (callousness, uncaring, and unemotional). Finally, we performed additional analyses to examine the main effects of RA, PA, and general aggression on rsFC while controlling for CU traits.
3. Results
3.1. Behavioral results
Sample characteristics are summarized in detail in Table 1 (see also Werhahn et al., 2021 and Supplementary Material Table S3). Forty-eight cases had a diagnosis of ODD, twenty-five cases of CD and ODD, and seven cases of CD. Seventy-seven cases presented with scores in the clinical range (T > 70) on the aggression or rule-breaking behavior subscale of the CBCL, and forty-one cases scored in the clinical range on both subscales. Thirty-eight cases had an aggression score in the clinical range but no DSM diagnosis. These cases showed comparable RA values (M = 12.67, SD = 5.62) and CU traits (M = 33.61, SD = 10.01) and lower PA scores (M = 3.91, SD = 4.00) compared to cases with a DSM diagnosis (RA, M = 12.50, SD = 4.87; PA, M = 5.27, SD = 5.37; CU traits, M = 33.71, SD = 10.25). Most participants presented with comorbid ADHD symptoms (n = 103), while 66 cases had anxiety problems. Seventy cases were receiving medication. Aggression dimensions (RA, PA, CU traits) correlated with comorbidities of interest (ADHD inattention and hyperactivity/impulsivity, anxiety) in cases only for RA with anxiety symptoms (r = 0.32, p = 0.004), and for CU traits with ADHD inattention (r = 0.32, p = 0.001). Further associations among sample characteristics are reported in Supplementary Material Table S4.
Table 1.
Sample characteristics.
| Cases (n = 118) | Controls (n = 89) | |
|---|---|---|
| Age (Mean ± SD) | 13.23±2.68 | 13.40±2.49 |
| Sex, m/f (N) a | 99/19 | 51/38 |
| IQ (Mean ± SD) a | 100.78±11.00 | 106.64±10.42 |
| Handedness, left/right (N) | 16/95 | 10/77 |
| CD plus ODD Diagnosis (N) | 25 | |
| ODD Diagnosis (N) | 48 | |
| CD Diagnosis (N) | 7 | |
| ADHD Diagnosis (N) | 29 | |
| CBCL – Aggression T-score (Mean ± SD) | 74.46±10.10 | |
| CBCL – Rule-breaking T-score (Mean ± SD) | 69.00±12.14 | |
| SNAP-IV – Inattention (Mean ± SD) b | 15.47±6.06 | 2.00±4.11 |
| SNAP-IV – Hyperactivity/Impulsivity (Mean ± SD) b | 12.00±5.95 | 1.00±3.17 |
| YSR – Anxiety Problems (Mean ± SD) a | 3.00±2.26 | 2.00±2.04 |
| ICU – Total Score (Mean ± SD) b | 33.68±10.16 | 21.00±8.70 |
| RPQ – Reactive Aggression (Mean ± SD) b | 12.55±5.09 | 5.00±3.48 |
| RPQ – Proactive Aggression (Mean ± SD) b | 3.00±5.01 | 0.82±1.45 |
| Medication use (N) Stimulants Neuroleptics- Antidepressants |
70 52 182 |
- - -- |
Values are means with standard deviations or counts. Abbreviations: IQ, intelligence quotient; CD, conduct disorder; ODD, oppositional defiant disorder; ADHD, attention-deficit hyperactivity disorder; CBCL, Child Behavior Checklist; SNAP-IV, Swanson, Nolan and Pelham Teacher and Parent Rating Scale; YSR, Youth Self Report; ICU, Inventory of Callous-Unemotional Traits, parent report; RPQ, Reactive and Proactive Aggression Questionnaire; RMS-FD, root mean square framewise displacement. Diagnoses are derived from the Kiddie-Schedule for Affective Disorders and Schizophrenia, present and lifetime version. Medication use was assessed from parental reports. Significant differences between groups are indicated by lowercase letters. ap < 0.01, bp < 0.001.
3.2. Voxel-to-voxel functional connectivity
Analyses of group differences yielded significant results only after exclusion of ADHD symptoms as a covariate (i.e. controlling only for site and anxiety symptoms; Supplementary Table S5). Cases showed lower ILC in a cluster including the bilateral frontal pole extending to the medial frontal cortex (t(142) = -6.12, p-FDR < 0.05; x = 4, y = 66, z = -18). Moreover, cases demonstrated higher ICC in a cluster including the right occipital pole (t(142) = 5.19, p-FDR < 0.05; x = 26, y = -102, z = 2). The ILC effect survived additional controlling for age, sex, IQ, and medication, but not handedness (t(131) = -5.67, p-FDR < 0.05; x = -10, y = 56, z = –22). The ICC effect survived additionally controlling for age and sex (t(140) = 5.35, p-FDR < 0.05; x = 26, y = -102, z = 2).
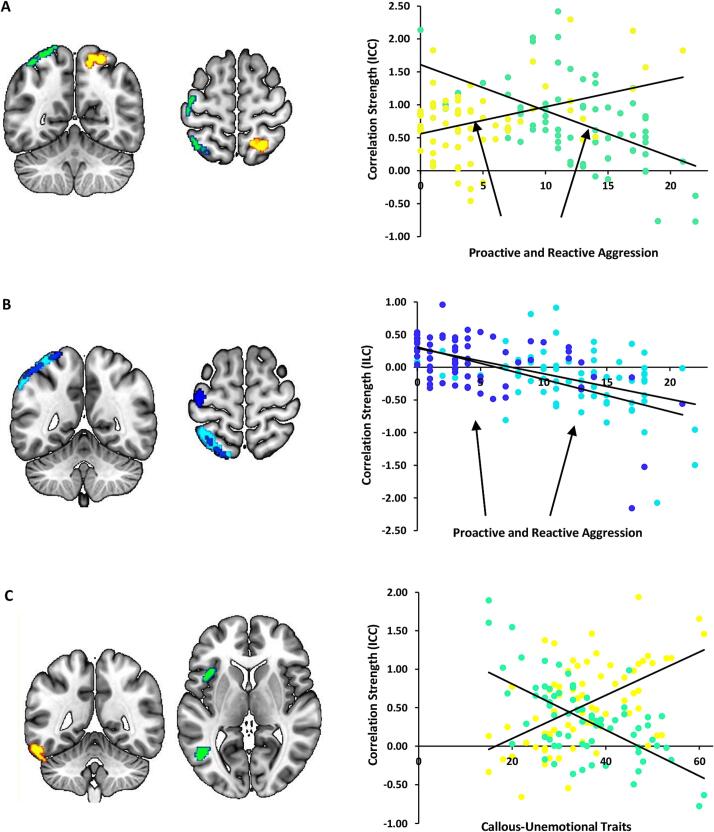
The analysis with aggression scores showed that with higher levels of RA, ICC was decreased in the left superior parietal lobe and lateral occipital cortex in the left hemisphere, while, with higher levels of PA, ICC increased in these regions in the right hemisphere. The RA-related cluster extended to the left central gyrus, while the PA-related cluster extended to the precuneus (all p-FDR < 0.05; Fig. 1A, Supplementary Table S6). Moreover, with increasing levels of both RA and PA symptoms, ILC was decreased in left hemispheric clusters, including superior portions of the parietal lobe and lateral occipital cortex along with the supramarginal gyrus, extending to the angular gyrus. The PA-related cluster extended to the left central gyrus (all p-FDR < 0.05; Fig. 1B, Supplementary Table S7). Furthermore, with higher levels of CU traits, ICC was increased in a left hemispheric cluster including the inferior temporal gyrus and the cerebellum. In contrast, ICC was decreased in left hemispheric clusters that extended from the inferior temporal gyrus and middle temporal gyrus and inferior lateral occipital cortex to frontal and central opercular areas, including the insula lobe (all p-FDR < 0.05; Fig. 1C, see Supplementary Table S6).
Fig. 1.
Whole-brain voxel-to-voxel connectivity correlates of reactive (RA) and proactive (PA) aggression as well as callous-unemotional (CU) traits. A. Higher RA and PA scores were associated with decreased versus increased intrinsic correlation contrast (ICC) in the parietal lobe and occipital cortex, colored in green and yellow, respectively. Moreover, a cluster in the left central gyrus exhibited RA-specific decreased ICC. B. Higher RA and PA scores were associated with decreased integrated local correlation (ILC) in regions colored in light and dark blue, respectively. Additionally, a region in the left central gyrus exhibited a PA-specific decrease in ILC. C. Higher CU traits were positively associated with ICC in the left inferior temporal gyrus (yellow), while a negative association was evident for the medial and inferior temporal gyrus and frontal and central opercular regions in the left hemisphere (green). ICC and ILC values (y-axis) are plotted against scores of RA and PA and CU traits (x-axis). The z-values are Fisher-transformed correlation coefficients, averaged across all observed voxels in cases comprising more than one cluster. The statistical threshold is p < 0.001, with a false discovery rate (FDR) cluster-level correction (p < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Exploratory analyses
We identified a negative association between general aggression and ILC in the superior parietal lobule, supramarginal gyrus, pre-central/post-central gyrus, and superior frontal gyrus (Supplementary Table S7). No significant association was identified between general aggression and ICC.
We additionally examined the suppressor effect of aggression dimensions on ICC and ILC. Our results revealed a significant negative association between RA and ICC in the right pre-central/post-central gyrus when controlling for the effect of PA (Supplementary Fig. S2), and a significant positive association between PA and ICC in the left lateral frontal cortex and supplementary motor area when controlling for the effect of RA (Supplementary Fig. S2). For ILC analyses, the previously identified RA and PA main effects became statistically non-significant when controlled for PA and RA, respectively (Supplementary Fig. S3). We found similar results for the main effect of RA and PA when controlling for CU traits (Supplementary Tables S8 and S9, Supplementary Figs. S4 and S5).
Exploratory analyses for CU subdimensions are provided in Supplementary Tables S6 and S7. Among these three subdimensions, only the relationship with the uncaring facet showed significant effects. Positive associations were found in the pre-/post-central gyrus for ICC, and a negative association was found in the cerebellum for ILC. These effects did not overlap with those observed for the CU total score.
4. Discussion
The present study advances previous research on the neural basis of aggression using whole brain rsFC analyses without any particular a priori regions in a large sample of children and adolescents. Comorbid anxiety and ADHD symptoms were taken into account as possible modulators of aggressive dimensions. Between-group differences were detectable only after removing the control for ADHD symptoms, resulting in voxel-wise rsFC (ICC/ILC) findings in frontal clusters, which are typically implicated in cognitive control. Both RA and PA were correlated with voxel-wise rsFC alterations in superior parts of the parietal lobe, which are linked to attentional control. Our analyses also yielded disparate patterns for RA and PA in the left central gyrus and/or precuneus, which are implicated in aggression and cognitive control. Distinct CU trait-specific ICC associations were observed in temporal and cerebellar regions, which are known to mediate fear, morality, and reward processing.
Importantly, aggression subtype-specific rsFC findings were evident after additionally controlling for both ADHD and anxiety symptoms in the dimensional analyses. This suggests that these symptoms may have modulatory effects that can obscure aggression subtype-specific patterns. This further underlines the importance of considering both ADHD and anxiety in research on aggression. The crucial role of ADHD (Broulidakis et al., 2016, Uytun et al., 2017) and anxiety (Motzkin et al., 2011) in subtyping aggression is also substantiated by previous psychophysiological experiments (Fanti, 2018). Additionally, studies focused on aggressive behavior showed a correlation between anxiety symptoms and RA and PA (Fite et al., 2014, Fung et al., 2015) or RA symptoms alone (Marsee et al., 2008, Vitaro et al., 2002), as in our sample. In other studies, RA has been linked to anxiety and ADHD problems(Smeets et al., 2017) and internalizing symptoms (Fite et al., 2014). Multiple therapeutic components may be incorporated to relieve RA symptoms, including addressing anxiety, emotional problems, and hostile attributional biases, as RA symptoms may be manifestation of threat-circuit hypersensitivity (Blair et al., 2014). On the other hand, CU traits seem typically associated with reduced anxiety (Eisenbarth et al., 2016, Frick et al., 2014). Individuals with CU traits and co-occurring high levels of anxiety might thus represent a distinct phenotype with different clinical outcomes (Fanti et al., 2013) and neural correlates (Sethi et al., 2018). Moreover, within cases, we found generally low correlations of RA and PA levels and CU traits with ADHD and anxiety levels, which might further indicate heterogeneous underlying profiles associated with these forms of aggression. Overall, our results emphasize the value of differentiating between aggression subtypes and considering comorbid anxiety and ADHD symptoms. A similar conclusion may be reached from the case–control analysis. In our group analysis, cases exhibited rsFC differences in the medial frontal cortex compared to controls, conforming with our previous seed-based rsFC results (Werhahn et al., 2021), but only without controlling for ADHD symptoms. This suggests that ADHD symptoms affected prefrontal connectivity, consistent with the important role of the frontal lobe in ADHD and cognitive control (Ridderinkhof et al., 2004). The importance of considering anxiety levels in this context is in line with task-based fMRI studies in antisocial youths (Byrd et al., 2014), although the modulatory role of ADHD was not confirmed in previous studies (Broulidakis et al., 2016, Werhahn et al., 2021).
Our results suggest overlapping rsFC alteration patterns of RA and PA in brain regions involved in attention, decision-making, emotions, and CU-related traits. Thus, RA- and PA-related ICC was decreased in clusters including superior parts of the parietal lobe and lateral occipital cortex, however, in different hemispheres. These brain areas have been implicated in previous aggression-related rsFC studies. Decreased ILC was observed in the angular gyrus, where altered rsFC was reported also with antisocial personality disorder (Tang et al., 2016), and CU-related traits (Espinoza et al., 2018). Activity of the angular gyrus as part of the DMN has been associated with CU-related traits during emotional processing (Anderson et al., 2017) and with moral behaviors (Boccia et al., 2017, Fumagalli and Priori, 2012, Pujol et al., 2012). The lateral occipital cortex has been implicated in reduced functional activity during rest in adolescents with CD. The superior parietal lobe has been linked to attentional control (Bisley and Goldberg, 2010), learning from reward and punishment (Finger et al., 2011), and fearful expressions (Marsh et al., 2008, Peraza et al., 2015).
In line with behavioral studies (Fite et al., 2010, Marsee et al., 2011, Marsee and Frick, 2007) and our previous imaging study (Werhahn et al., 2021), we found distinct rsFC patterns associated with RA and PA symptoms. They involved the precuneus and central gyrus, which were previously linked to different forms of aggressive behavior and cognitive control-related processes known to be impaired in disruptive behavior (Blair et al., 2018). For example, adolescents with CD showed decreased postcentral gyrus rsFC (Cao et al., 2018, Lu et al., 2021a, Lu et al., 2021b, Lu et al., 2015). The precuneus, as part of the DMN, has been linked to self-reflection (Cavanna, 2007) and moral reasoning (Boccia et al., 2017), but also cognitive control and aggressive interactions (Fanning et al., 2017). In contrast with the neural findings delineated in this study and the correlation between CU traits and both RA and PA symptoms (Feilhauer et al., 2012, Pechorro et al., 2017), RA- and PA-related rsFC did not overlap with CU trait-related patterns. The strength of connectivity between a seed in the salience network projecting to the precuneus and central gyrus was previously found to be associated with CU sub-dimensions (Werhahn et al., 2021). Moreover, aberrant rsFC of the precuneus has been linked to impulsivity (Lu et al., 2017a), ADHD, and severe temper outbursts (Roy et al., 2018), but also to CU-related traits (Cao et al., 2019). During rest, the precuneus has previously been observed to show altered functional connectivity in male adolescents with CD (Zhou et al., 2016) and in male juvenile offenders (Chen et al., 2015) as well as in antisocial (Tang et al., 2016, 2013) and psychopathic adults (Motzkin et al., 2011, Philippi et al., 2015). Differences in rsFC of the precentral gyrus have also been reported in psychopathic adults (Espinoza et al., 2018, Tang et al., 2016).
In this study, CU traits were associated with connectivity measures in the left hemispheric inferior temporal gyrus, middle temporal gyrus, opercular regions, and the cerebellum. These findings align with previous studies using other connectivity methods, in which male adolescents also exhibited CU-related differences in the inferior temporal gyrus and middle temporal gyrus (Aghajani et al., 2016, Thijssen and Kiehl, 2017) as well as CD-related diminished activity in the right middle temporal gyrus (Wu et al., 2017), suggesting altered neural synchronization in these regions. The role of the middle temporal gyrus in this context has been further substantiated by the fact that this region exhibited the highest discriminating power among the DMN nodes to distinguish individuals with antisocial personality disorder from controls, while analysis of the cerebellum enabled the best differentiation overall (Tang et al., 2013). Additionally, other studies reported decreased regional homogeneity (a measure of local brain coherence) in the middle temporal gyrus of adults with antisocial personality disorder (Tang et al., 2013) and CU-specific rsFC decreases in prison inmates (Espinoza et al., 2018). Cerebellar and opercular regions have been implicated in rsFC alterations in adults with psychopathy (Philippi et al., 2015), and juvenile offenders (Aghajani et al., 2016). There is also evidence for the engagement of the left middle temporal gyrus in the theory of mind (Bzdok et al., 2012) and the CU-level-dependent fear response (Sebastian et al., 2014). The inferior temporal gyrus and middle temporal gyrus are also implicated in moral reasoning (Boccia et al., 2017), emotions (Alegria et al., 2016, Anderson et al., 2017), and reward processes (Crowley et al., 2010) along with the bilateral cerebellum (Veroude et al., 2016). Furthermore, exploratory analyses on the CU subdimensions only revealed an association between the uncaring facet and rsFC pattern. This effect did not overlap with the effect of CU total scores, supporting dimensional heterogeneity of CU traits. However, our results are exploratory in nature, and future studies can examine differential rsFC patterns in relation to CU subgroups, similar to prior work focused on brain structure (Ibrahim et al., 2021). This approach may help in identifying subtype-specific associations in rsFC.
Our multi-center study included a large sample of both male and female child and adolescent cases and controls. The study was not restricted to the inclusion of conduct disorder, given that CU traits are present in only a minority of children with CD (Frick, 2016). Moreover, we focused on RA and PA aggression symptoms and CU traits, applied unrestricted whole-brain analysis of rsFC, and controlled for ADHD and anxiety symptoms. Our results might enable conclusions about aggression subtypes of aggressive youths, with potential implications for clinical practice. The study also has limitations. Some results may have been influenced by data heterogeneity owing to data collection at different sites. Yet, the large sample size and the multi-center design increases the generalizability of our findings, and study site was accounted for as a covariate in each analysis. However, including site as a covariate of no interest in the analysis may have caused some loss of power. Furthermore, the 38 cases without a DSM diagnosis exhibited lower PA scores, however, the sub-analysis yielded comparable aggression subtype-specific associations. Lastly, although we included participants from both sexes, our sample predominantly consisted of males. This might restrict the generalizability of our results, although we found similar effects when controlled for sex (see Supplementary Material).
5. Conclusions
The current study showed both distinct and partially overlapping brain connectivity patterns of aggression subtypes and their modifying factors. Considering comorbid symptoms seems crucial for this process. The identified brain regions have been implicated in emotion regulation and moral-related behaviors. As a future research endeavor, investigating task-based alterations for the aggression subtypes could enhance their biological understanding. A transfer of aggression subtype-specific knowledge to clinical practice should be encouraged by tailoring existing therapeutic approaches (Umbach et al., 2015, Wilkinson et al., 2016). In these applications, neurobiological interventions, such as real-time fMRI/arousal-biofeedback training (Aggensteiner et al., 2023) or transcranial direct current stimulation (Chan et al., 2022), may become promising treatment strategies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This project has received funding from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement 602805 (Aggressotype) and no 603016 (MATRICS). This manuscript reflects only the authors’ views, and the European Union is not liable for any use that may be made of the information contained herein.
The authors express their gratitude to all participating families and gratefully acknowledge the contributions of Alexander Roth, Julian Djamil Fagir, Yu Jin Ressel, Noemi Signer, Aline Pfister, Georg Grön, and Kathrin Brändle.
The present work is unrelated to the above grants and relationships. The authors do not have potential conflicts of interest.
Declaration of competing interest
TB has served in an advisory or consultancy role for Actelion, Hexal Pharma, Lilly, Lundbeck, Medice, Neurim Pharmaceuticals, Novartis, and Shire; he received conference support or speaker’s fees from Lilly, Medice, Novartis, and Shire. He has also been involved in clinical trials conducted by Shire and Viforpharma and has received royalties from Hogrefe, Kohlhammer, CIP Medien, and Oxford University Press. CA has been a consultant to or has received honoraria or grants from Acadia, Ambrosseti, Gedeon Richter, Janssen Cilag, Lundbeck, Merck, Otsuka, Roche, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion, and Takeda. DB served as an unpaid scientific advisor for an EU-funded neurofeedback trial unrelated to the present work. BF receives funding from a personal Vici grant from the Dutch Organisation for Scientific Research (Grant 016 130 669) and the Dutch National Science Agenda for the NWANeurolabNL project (Grant 400 17 602). She received educational speaking fees from Shire and Medicine. S.W. has received in the last 5 years royalties from Thieme, Hogrefe, Kohlhammer, Springer, and Beltz. In 2023, she received speakers honorary from Takeda and Medice. Her work was supported in the last years by the Swiss National Science Foundation (SNF), diff. EU FP7s, HSM Hochspezialisierte Medizin of the Kanton Zurich, Switzerland, Bfarm Germany, ZInEP, Hartmann Müller Stiftung, Olga Mayenfisch, Gertrud Thalmann, Vontobel, Unicentia, Erika Schwarz, Heuberg Fonds, National Government of Health (BAG), Gesundheitsförderung Schweiz, and Horizon Europe. Outside professional activities and interests are declared on the web page of the University of Zurich (https://www.uzh.ch/prof/ssl-dir/interessenbindungen/client/web/).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2023.103542.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Achenbach, T.M., 1991. Integrative guide for the 1991 CBCL/4-18, YSR, and TRF profiles. Burlington, VT, USA.
- Aggensteiner, P., Hohmann, S., Heintz, S., Franke, B., 2023. Randomized Controlled Trial of Individualized Arousal-Biofeedback for children and adolescents with Disruptive Behavior Disorders (DBD). https://doi.org/https://doi.org/10.21203/rs.3.rs-2872518/v1. [DOI] [PMC free article] [PubMed]
- Aghajani M., Colins O.F., Klapwijk E.T., Veer I.M., Andershed H., Popma A., van der Wee N.J., Vermeiren R.R.J.M. Dissociable relations between amygdala subregional networks and psychopathy trait dimensions in conduct-disordered juvenile offenders. Hum. Brain Mapp. 2016;37 doi: 10.1002/hbm.23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajani M., Klapwijk E.T., van der Wee N.J., Veer I.M., Rombouts S.A.R.B., Boon A.E., van Beelen P., Popma A., Vermeiren R.R.J.M., Colins O.F. Disorganized Amygdala Networks in Conduct-Disordered Juvenile Offenders With Callous-Unemotional Traits. Biol. Psychiatry. 2017;82 doi: 10.1016/j.biopsych.2016.05.017. [DOI] [PubMed] [Google Scholar]
- Alegria A.A., Radua J., Rubia K. Meta-analysis of fmri studies of disruptive behavior disorders. Am. J. Psychiatry. 2016;173 doi: 10.1176/appi.ajp.2016.15081089. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Pub; 2013. Diagnostic and statistical manual of mental disorders (DSM-5®) [Google Scholar]
- Anderson N.E., Steele V.R., Maurer J.M., Rao V., Koenigs M.R., Decety J., Kosson D.S., Calhoun V.D., Kiehl K.A. Differentiating emotional processing and attention in psychopathy with functional neuroimaging. Cogn. Affect. Behav. Neurosci. 2017;17 doi: 10.3758/s13415-016-0493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L.R., Hutton C., Ashburner J., Turner R., Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13 doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Bakker M.J., Greven C.U., Buitelaar J.K., Glennon J.C. Practitioner Review: Psychological treatments for children and adolescents with conduct disorder problems – a systematic review and meta-analysis. J. Child Psychol. Psychiatry Allied Discip. 2017 doi: 10.1111/jcpp.12590. [DOI] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37 doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley J.W., Goldberg M.E. Attention, intention, and priority in the parietal lobe. Annu. Rev. Neurosci. 2010 doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J.R., Leibenluft E., Pine D.S. Conduct Disorder and Callous-Unemotional Traits in Youth. N. Engl. J. Med. 2014;371 doi: 10.1056/nejmra1315612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J.R., Veroude K., Buitelaar J.K. Neuro-cognitive system dysfunction and symptom sets: A review of fMRI studies in youth with conduct problems. Neurosci. Biobehav. Rev. 2018 doi: 10.1016/j.neubiorev.2016.10.022. [DOI] [PubMed] [Google Scholar]
- Boccia M., Dacquino C., Piccardi L., Cordellieri P., Guariglia C., Ferlazzo F., Ferracuti S., Giannini A.M. Neural foundation of human moral reasoning: an ALE meta-analysis about the role of personal perspective. Brain Imaging Behav. 2017 doi: 10.1007/s11682-016-9505-x. [DOI] [PubMed] [Google Scholar]
- Broulidakis M.J., Fairchild G., Sully K., Blumensath T., Darekar A., Sonuga-Barke E.J.S. Reduced Default Mode Connectivity in Adolescents With Conduct Disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55 doi: 10.1016/j.jaac.2016.05.021. [DOI] [PubMed] [Google Scholar]
- Browndyke J.N., Berger M., Smith P.J., Harshbarger T.B., Monge Z.A., Panchal V., Bisanar T.L., Glower D.D., Alexander J.H., Cabeza R., Welsh-Bohmer K., Newman M.F., Mathew J.P. Task-related changes in degree centrality and local coherence of the posterior cingulate cortex after major cardiac surgery in older adults. Hum. Brain Mapp. 2018;39 doi: 10.1002/hbm.23898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd A.L., Loeber R., Pardini D.A. Antisocial Behavior, Psychopathic Features and Abnormalities in Reward and Punishment Processing in Youth. Clin. Child Fam. Psychol. Rev. 2014 doi: 10.1007/s10567-013-0159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D., Schilbach L., Vogeley K., Schneider K., Laird A.R., Langner R., Eickhoff S.B. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct. Funct. 2012;217 doi: 10.1007/s00429-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D., Wager T.D., Krishnan A., Rosch K.S., Seymour K.E., Nebel M.B., Mostofsky S.H., Nyalakanai P., Kiehl K. The impact of T1 versus EPI spatial normalization templates for fMRI data analyses. Hum. Brain Mapp. 2017;38 doi: 10.1002/hbm.23737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Sun X., Dong D., Yao S., Huang B. Sex differences in spontaneous brain activity in adolescents with conduct disorder. Front. Psychol. 2018;9 doi: 10.3389/fpsyg.2018.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Li C., Zhang J., Dong D., Sun X., Yao S., Huang B., Liu J. Regional homogeneity abnormalities in early-onset and adolescent-onset conduct disorder in boys: A resting-state fMRI study. Front. Hum. Neurosci. 2019;13 doi: 10.3389/fnhum.2019.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady K., Ruitenberg M., Koppelmans V., Reuter-Lorenz P., De Dios Y., Gadd N., Wood S., Riascos Castenada R., Kofman I., Bloomberg J., Mulavara A., Seidler R. Neural predictors of sensorimotor adaptation rate and savings. Hum. Brain Mapp. 2018;39 doi: 10.1002/hbm.23924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E. The precuneus and consciousness. CNS Spectr. 2007 doi: 10.1017/S1092852900021295. [DOI] [PubMed] [Google Scholar]
- Chai X.J., Castañán A.N., Öngür D., Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59 doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, L., Simmons, C., Tillem, S., Conley, M., Brazil, I.A., Baskin-Sommers, A., 2022. Classifying Conduct Disorder Using a Biopsychosocial Model and Machine Learning Method. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. https://doi.org/10.1016/j.bpsc.2022.02.004. [DOI] [PMC free article] [PubMed]
- Chen C., Zhou J., Liu C., Witt K., Zhang Y., Jing B., Li C., Wang X., Li L. Regional homogeneity of resting-state brain abnormalities in violent juvenile offenders: A biomarker of brain immaturity? J. Neuropsychiatry Clin. Neurosci. 2015;27 doi: 10.1176/appi.neuropsych.13030044. [DOI] [PubMed] [Google Scholar]
- Cima M., Raine A., Meesters C., Popma A. Validation of the Dutch Reactive Proactive Questionnaire (RPQ): Differential Correlates of Reactive and Proactive Aggression From Childhood to Adulthood. Aggress. Behav. 2013;39 doi: 10.1002/ab.21458. [DOI] [PubMed] [Google Scholar]
- Ciucci E., Baroncelli A., Golmaryami F.N., Frick P.J. The Emotional Correlates to Callous-Unemotional Traits in Children. J. Child Fam. Stud. 2015;24 doi: 10.1007/s10826-014-0040-3. [DOI] [Google Scholar]
- Crowley T.J., Dalwani M.S., Mikulich-Gilbertson S.K., Du Y.P., Lejuez C.W., Raymond K.M., Banich M.T. Risky decisions and their consequences: Neural processing by boys with antisocial substance disorder. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadds M.R., Kimonis E.R., Schollar-Root O., Moul C., Hawes D.J. Are impairments in emotion recognition a core feature of callous-unemotional traits? Testing the primary versus secondary variants model in children. Dev. Psychopathol. 2018;30 doi: 10.1017/S0954579417000475. [DOI] [PubMed] [Google Scholar]
- Deshpande G., LaConte S., Peltier S., Hu X. Integrated local correlation: A new measure of local coherence in fMRI data. Hum. Brain Mapp. 2009;30 doi: 10.1002/hbm.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty M., Boxer P., Huesmann L.R., O’Brien M., Bushman B. Assessing Callous-Unemotional Traits in Adolescents: Determining Cutoff Scores for the Inventory of Callous and Unemotional Traits. J. Clin. Psychol. 2017;73 doi: 10.1002/jclp.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge K.A., Coie J.D. Social-Information-Processing Factors in Reactive and Proactive Aggression in Children’s Peer Groups. J. Pers. Soc. Psychol. 1987;53 doi: 10.1037/0022-3514.53.6.1146. [DOI] [PubMed] [Google Scholar]
- Dugré J.R., Potvin S. Impaired attentional and socio-Affective networks in subjects with antisocial behaviors: A meta-Analysis of resting-state functional connectivity studies. Psychol. Med. 2021 doi: 10.1017/S0033291721001525. [DOI] [PubMed] [Google Scholar]
- Dugré J.R., Potvin S. Clarifying the role of Cortico-Cortical and Amygdalo-Cortical brain dysconnectivity associated with Conduct Problems. NeuroImage Clin. 2023;37 doi: 10.1016/j.nicl.2023.103346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth H., Demetriou C.A., Kyranides M.N., Fanti K.A. Stability Subtypes of Callous-Unemotional Traits and Conduct Disorder Symptoms and Their Correlates. J. Youth Adolesc. 2016;45 doi: 10.1007/s10964-016-0520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza F.A., Vergara V.M., Reyes D., Anderson N.E., Harenski C.L., Decety J., Rachakonda S., Damaraju E., Rashid B., Miller R.L., Koenigs M., Kosson D.S., Harenski K., Kiehl K.A., Calhoun V.D. Aberrant functional network connectivity in psychopathy from a large (N = 985) forensic sample. Hum. Brain Mapp. 2018;39 doi: 10.1002/hbm.24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essau C.A., Sasagawa S., Frick P.J. Callous-unemotional traits in a community sample of adolescents. Assessment. 2006;13:454–469. doi: 10.1177/1073191106287354. [DOI] [PubMed] [Google Scholar]
- Fanning J.R., Keedy S., Berman M.E., Lee R., Coccaro E.F. Neural Correlates of Aggressive Behavior in Real Time: a Review of fMRI Studies of Laboratory Reactive Aggression. Curr. Behav. Neurosci. Reports. 2017;4 doi: 10.1007/s40473-017-0115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanti K.A. Understanding heterogeneity in conduct disorder: A review of psychophysiological studies. Neurosci. Biobehav. Rev. 2018 doi: 10.1016/j.neubiorev.2016.09.022. [DOI] [PubMed] [Google Scholar]
- Fanti K.A., Demetriou C.A., Kimonis E.R. Variants of Callous-Unemotional Conduct Problems in a Community Sample of Adolescents. J. Youth Adolesc. 2013;42 doi: 10.1007/s10964-013-9958-9. [DOI] [PubMed] [Google Scholar]
- Feilhauer J., Cima M., Arntz A. Assessing callous-unemotional traits across different groups of youths: Further cross-cultural validation of the Inventory of Callous-Unemotional Traits. Int. J. Law Psychiatry. 2012;35 doi: 10.1016/j.ijlp.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Finger E.C., Marsh A.A., Blair K.S., Reid M.E., Sims C., Ng P., Pine D.S., Blair R.J.R. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. Am. J. Psychiatry. 2011;168 doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fite P.J., Raine A., Stouthamer-Loeber M., Loeber R., Pardini D.A. Reactive and proactive aggression in adolescent males: Examining differential outcomes 10 years later in erarly adulthood. Crim. Justice Behav. 2010;37 doi: 10.1177/0093854809353051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fite P.J., Rubens S.L., Preddy T.M., Raine A., Pardini D.A. Reactive/proactive aggression and the development of internalizing problems in males: The moderating effect of parent and peer relationships. Aggress. Behav. 2014;40 doi: 10.1002/ab.21498. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. u. s. a. 2005;102 doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K.C.R., Spreng R.N., Ellamil M., Andrews-Hanna J.R., Christoff K. The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage. 2015 doi: 10.1016/j.neuroimage.2015.02.039. [DOI] [PubMed] [Google Scholar]
- Frick P.J. Developmental Pathways to Conduct Disorder: Implications for Future Directions in Research, Assessment, and Treatment. J. Clin. Child Adolesc. Psychol. 2012;41 doi: 10.1080/15374416.2012.664815. [DOI] [PubMed] [Google Scholar]
- Frick P.J. Current research on conduct disorder in children and adolescents. South African J. Psychol. 2016;46 doi: 10.1177/0081246316628455. [DOI] [Google Scholar]
- Frick P.J., Ray J.V., Thornton L.C., Kahn R.E. Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A Comprehensive Review. Psychol. Bull. 2014;140 doi: 10.1037/a0033076. [DOI] [PubMed] [Google Scholar]
- Frick P.J., White S.F. Research Review: The importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. J. Child Psychol. Psychiatry Allied Discip. 2008 doi: 10.1111/j.1469-7610.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- Fumagalli M., Priori A. Functional and clinical neuroanatomy of morality. Brain. 2012 doi: 10.1093/brain/awr334. [DOI] [PubMed] [Google Scholar]
- Fung A.L.C., Gerstein L.H., Chan Y., Engebretson J. Relationship of Aggression to Anxiety, Depression, Anger, and Empathy in Hong Kong. J. Coatings Technol. Res. 2015;12 doi: 10.1007/s10826-013-9892-1. [DOI] [Google Scholar]
- Gomez R., Vance A., Gomez R.M. Analysis of the Convergent and Discriminant Validity of the CBCL, TRF, and YSR in a Clinic-Referred Sample. J. Abnorm. Child Psychol. 2014;42 doi: 10.1007/s10802-014-9879-4. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. u. s. a. 2003;100 doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelker M.D., Barry C.T., Barry T.D., Malkin M.L. Perceived positive outcomes as a mediator between adolescent callous-unemotional traits and antisocial behavior. Pers. Individ. Dif. 2014;69 doi: 10.1016/j.paid.2014.05.029. [DOI] [Google Scholar]
- Ibrahim K., Eilbott J.A., Ventola P., He G., Pelphrey K.A., McCarthy G., Sukhodolsky D.G. Reduced Amygdala-Prefrontal Functional Connectivity in Children With Autism Spectrum Disorder and Co-occurring Disruptive Behavior. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2019;4:1031–1041. doi: 10.1016/j.bpsc.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim K., Kalvin C., Li F., He G., Pelphrey K.A., McCarthy G., Sukhodolsky D.G. Sex differences in medial prefrontal and parietal cortex structure in children with disruptive behavior. Dev. Cogn. Neurosci. 2021;47 doi: 10.1016/j.dcn.2020.100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim K., Noble S., He G., Lacadie C., Crowley M.J., McCarthy G., Scheinost D., Sukhodolsky D.G. Large-scale functional brain networks of maladaptive childhood aggression identified by connectome-based predictive modeling. Mol. Psychiatry. 2022;27 doi: 10.1038/s41380-021-01317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Gao Y., Dong D., Sun X., Situ W., Yao S. Impaired global efficiency in boys with conduct disorder and high callous unemotional traits. J. Psychiatr. Res. 2021;138 doi: 10.1016/j.jpsychires.2021.04.041. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36 doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kimonis E.R., Frick P.J., Cauffman E., Goldweber A., Skeem J. Primary and secondary variants of juvenile psychopathy differ in emotional processing. Dev. Psychopathol. 2012;24 doi: 10.1017/S0954579412000557. [DOI] [PubMed] [Google Scholar]
- Leung P.W.L., Kwong S.L., Tang C.P., Ho T.P., Hung S.F., Lee C.C., Hong S.L., Chiu C.M., Liu W.S. Test-retest reliability and criterion validity of the Chinese version of CBCL, TRF, and YSR. J. Child Psychol. Psychiatry Allied Discip. 2006;47 doi: 10.1111/j.1469-7610.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- Loeber R., Burke J.D., Lahey B.B., Winters A., Zera M. Oppositional defiant and conduct disorder: A review of the past 10 years, part I. J. Am. Acad. Child Adolesc. Psychiatry. 2000 doi: 10.1097/00004583-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Lozier L.M., Cardinale E.M., Van Meter J.W., Marsh A.A. Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry. 2014;71:627–636. doi: 10.1001/jamapsychiatry.2013.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, F.M., Zhou, J.S., Zhang, J., Wang, X.P., Yuan, Z., 2017b. Disrupted small-world brain network topology in pure conduct disorder. Oncotarget 8. https://doi.org/10.18632/oncotarget.19098. [DOI] [PMC free article] [PubMed]
- Lu F., Wang M., Xu S., Chen H., Yuan Z., Luo L., Wang X., Zhang J., Dai J., Wang X., Chen H., Zhou J. Decreased interhemispheric resting-state functional connectivity in male adolescents with conduct disorder. Brain Imaging Behav. 2021;15 doi: 10.1007/s11682-020-00320-8. [DOI] [PubMed] [Google Scholar]
- Lu F., Zhao Y., He Z., Ma X., Yao X., Liu P., Wang X., Yang G., Zhou J. Altered dynamic regional homogeneity in patients with conduct disorder. Neuropsychologia. 2021;157 doi: 10.1016/j.neuropsychologia.2021.107865. [DOI] [PubMed] [Google Scholar]
- Lu F.M., Zhou J.S., Zhang J., Xiang Y.T., Liu Q., Wang X.P., Yuan Z. Functional connectivity estimated from resting-state fMRI reveals selective alterations in male adolescents with pure conduct disorder. PLoS One. 2015;10 doi: 10.1371/journal.pone.0145668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F.M., Zhou J.S., Wang X.P., Xiang Y.T., Yuan Z. Short- and long-range functional connectivity density alterations in adolescents with pure conduct disorder at resting-state. Neuroscience. 2017;351 doi: 10.1016/j.neuroscience.2017.03.040. [DOI] [PubMed] [Google Scholar]
- Marsee M.A., Weems C.F., Taylor L.K. Exploring the association between aggression and anxiety in youth: A look at aggressive subtypes, gender, and social cognition. J. Child Fam. Stud. 2008;17 doi: 10.1007/s10826-007-9154-1. [DOI] [Google Scholar]
- Marsee M.A., Barry C.T., Childs K.K., Frick P.J., Kimonis E.R., Muñoz L.C., Aucoin K.J., Fassnacht G.M., Kunimatsu M.M., Lau K.S.L. Assessing the Forms and Functions of Aggression Using Self-Report: Factor Structure and Invariance of the Peer Conflict Scale in Youths. Psychol. Assess. 2011;23 doi: 10.1037/a0023369. [DOI] [PubMed] [Google Scholar]
- Marsee M.A., Frick P.J. Exploring the cognitive and emotional correlates to proactive and reactive aggression in a sample of detained girls. J. Abnorm. Child Psychol. 2007;35 doi: 10.1007/s10802-007-9147-y. [DOI] [PubMed] [Google Scholar]
- Marsh A.A., Finger E.C., Mitchell D.G.V., Reid M.E., Sims C., Kosson D.S., Towbin K.E., Leibenluft E., Pine D.S., Blair R.J.R. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am. J. Psychiatry. 2008;165 doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Martuzzi R., Ramani R., Qiu M., Shen X., Papademetris X., Constable R.T. A whole-brain voxel based measure of intrinsic connectivity contrast reveals local changes in tissue connectivity with anesthetic without a priori assumptions on thresholds or regions of interest. Neuroimage. 2011;58 doi: 10.1016/j.neuroimage.2011.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt T.E., Caspi A., Harrington H., Milne B.J. Males on the life-course-persistent and adolescence-limited antisocial pathways: Follow-up at age 26 years. Dev. Psychopathol. 2002;14 doi: 10.1017/S0954579402001104. [DOI] [PubMed] [Google Scholar]
- Moffitt, T.E., 2017. Adolescence-limited and life-course-persistent antisocial behavior: A developmental taxonomy, in: Biosocial Theories of Crime. https://doi.org/10.4324/9781315096278-3. [PubMed]
- Motzkin J.C., Newman J.P., Kiehl K.A., Koenigs M. Reduced prefrontal connectivity in psychopathy. J. Neurosci. 2011;31 doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaijen J., Mulder L.M., Ilbegi S., de Bruijn S., Kleine-Deters R., Dietrich A., Hoekstra P.J., Marsman J.B.C., Aggensteiner P.M., Holz N.E., Boettinger B., Baumeister S., Banaschewski T., Saam M.C., Schulze M.E., U., Santosh, P.J., Sagar-Ouriaghli, I., Mastroianni, M., Castro Fornieles, J., Bargallo, N., Rosa, M., Arango, C., Penzol, M.J., Werhahn, J.E., Walitza, S., Brandeis, D., Glennon, J.C., Franke, B., Zwiers, M.P., Buitelaar, J.K., Specific cortical and subcortical alterations for reactive and proactive aggression in children and adolescents with disruptive behavior. NeuroImage Clin. 2020;27 doi: 10.1016/j.nicl.2020.102344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechorro P., Ray J.V., Gonçalves R.A., Jesus S.N. The Inventory of Callous-Unemotional Traits: Psychometric properties among referred and non-referred Portuguese female juveniles. Int. J. Law Psychiatry. 2017;54 doi: 10.1016/j.ijlp.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Peraza J., Cservenka A., Herting M.M., Nagel B.J. Atypical parietal lobe activity to subliminal faces in youth with a family history of alcoholism. Am. J. Drug Alcohol Abuse. 2015;41 doi: 10.3109/00952990.2014.953251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi C.L., Pujara M.S., Motzkin J.C., Newman J., Kiehl K.A., Koenigs M. Altered resting-state functional connectivity in cortical networks in psychopathy. J. Neurosci. 2015;35 doi: 10.1523/JNEUROSCI.5010-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu W., Luo Q., Jiang Y., Gao Y., Ming Q., Yao S. Alterations of Brain Functional Architecture Associated with Psychopathic Traits in Male Adolescents with Conduct Disorder. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-11775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J., Batalla I., Contreras-Rodríguez O., Harrison B.J., Pera V., Hernández-Ribas R., Real E., Bosa L., Soriano-Mas C., Deus J., López-Solà M., Pifarré J., Menchón J.M., Cardoner N. Breakdown in the brain network subserving moral judgment in criminal psychopathy. Soc. Cogn. Affect. Neurosci. 2012;7 doi: 10.1093/scan/nsr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. u. s. a. 2001;98 doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A., Dodge K., Loeber R., Gatzke-Kopp L., Lynam D., Reynolds C., Stouthamer-Loeber M., Liu J. The reactive-proactive aggression questionnaire: Differential correlates of reactive and proactive aggression in adolescent boys. Aggress. Behav. 2006;32 doi: 10.1002/ab.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy D.E., Krusemark E., Kosson D.S., Kearns M.C., Smith-Darden J., Kiehl K.A. The Development of Severe and Chronic Violence Among Youth: The Role of Psychopathic Traits and Reward Processing. Child Psychiatry Hum. Dev. 2017;48 doi: 10.1007/s10578-017-0720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof, K.R., Ullsperger, M., Crone, E.A., Nieuwenhuis, S., 2004. The role of the medial frontal cortex in cognitive control. Science (80-.). https://doi.org/10.1126/science.1100301. [DOI] [PubMed]
- Romero-Martínez, Á., Sarrate-Costa, C., Moya-Albiol, L., 2022. Reactive vs proactive aggression: A differential psychobiological profile? Conclusions derived from a systematic review. Neurosci. Biobehav. Rev. https://doi.org/10.1016/j.neubiorev.2022.104626. [DOI] [PubMed]
- Roy A.K., Bennett R., Posner J., Hulvershorn L., Castellanos F.X., Klein R.G. Altered intrinsic functional connectivity of the cingulate cortex in children with severe temper outbursts. Dev. Psychopathol. 2018;30 doi: 10.1017/S0954579417001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad Z.S., Reynolds R.C., Jo H.J., Gotts S.J., Chen G., Martin A., Cox R.W. Correcting brain-wide correlation differences in resting-state FMRI. Brain Connect. 2013;3 doi: 10.1089/brain.2013.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C.L., McCrory E.J., Dadds M.R., Cecil C.A.M., Lockwood P.L., Hyde Z.H., De Brito S.A., Viding E. Neural responses to fearful eyes in children with conduct problems and varying levels of callous-unemotional traits. Psychol. Med. 2014;44 doi: 10.1017/S0033291713000482. [DOI] [PubMed] [Google Scholar]
- Sethi A., McCrory E., Puetz V., Hoffmann F., Knodt A.R., Radtke S.R., Brigidi B.D., Hariri A.R., Viding E. Primary and Secondary Variants of Psychopathy in a Volunteer Sample Are Associated With Different Neurocognitive Mechanisms. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2018;3 doi: 10.1016/j.bpsc.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets K.C., Oostermeijer S., Lappenschaar M., Cohn M., van der Meer J.M.J., Popma A., Jansen L.M.C., Rommelse N.N.J., Scheepers F.E., Buitelaar J.K. Are Proactive and Reactive Aggression Meaningful Distinctions in Adolescents? A Variable- and Person-Based Approach. J. Abnorm. Child Psychol. 2017;45 doi: 10.1007/s10802-016-0149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J.M. School-based assessments and interventions for ADD students. KC Publishing. 1992 [Google Scholar]
- Tang Y., Liu W., Chen J., Liao J., Hu D., Wang W. Altered spontaneous activity in antisocial personality disorder revealed by regional homogeneity. Neuroreport. 2013;24 doi: 10.1097/WNR.0b013e3283627993. [DOI] [PubMed] [Google Scholar]
- Tang Y., Long J., Wang W., Liao J., Xie H., Zhao G., Zhang H. Aberrant functional brain connectome in people with antisocial personality disorder. Sci. Rep. 2016;6 doi: 10.1038/srep26209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen S., Kiehl K.A. Functional connectivity in incarcerated male adolescents with psychopathic traits. Psychiatry Res. - Neuroimaging. 2017;265 doi: 10.1016/j.pscychresns.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach R., Berryessa C.M., Raine A. Brain imaging research on psychopathy: Implications for punishment, prediction, and treatment in youth and adults. J. Crim. Justice. 2015;43 doi: 10.1016/j.jcrimjus.2015.04.003. [DOI] [Google Scholar]
- Umbach R.H., Tottenham N. Callous-unemotional traits and reduced default mode network connectivity within a community sample of children. Dev. Psychopathol. 2021;33 doi: 10.1017/S0954579420000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urben S., Habersaat S., Pihet S., Suter M., de Ridder J., Stéphan P. Specific Contributions of Age of Onset, Callous-Unemotional Traits and Impulsivity to Reactive and Proactive Aggression in Youths with Conduct Disorders. Psychiatr. q. 2018;89 doi: 10.1007/s11126-017-9506-y. [DOI] [PubMed] [Google Scholar]
- Uytun M.C., Karakaya E., Oztop D.B., Gengec S., Gumus K., Ozmen S., Doğanay S., Icer S., Demirci E., Ozsoy S.D. Default mode network activity and neuropsychological profile in male children and adolescents with attention deficit hyperactivity disorder and conduct disorder. Brain Imaging Behav. 2017;11 doi: 10.1007/s11682-016-9614-6. [DOI] [PubMed] [Google Scholar]
- Vatansever D., Manktelow A.E., Sahakian B.J., Menon D.K., Stamatakis E.A. Angular default mode network connectivity across working memory load. Hum. Brain Mapp. 2017;38 doi: 10.1002/hbm.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veroude K., von Rhein D., Chauvin R.J.M., van Dongen E.V., Mennes M.J.J., Franke B., Heslenfeld D.J., Oosterlaan J., Hartman C.A., Hoekstra P.J., Glennon J.C., Buitelaar J.K. The link between callous-unemotional traits and neural mechanisms of reward processing: An fMRI study. Psychiatry Res. - Neuroimaging. 2016;255 doi: 10.1016/j.pscychresns.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Vitaro F., Brendgen M., Tremblay R.E. Reactively and proactively aggressive children: Antecedent and subsequent characteristics. J. Child Psychol. Psychiatry Allied Discip. 2002;43 doi: 10.1111/1469-7610.00040. [DOI] [PubMed] [Google Scholar]
- Vitiello B., Stoff D.M. Subtypes of aggression and their relevance to child psychiatry. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36 doi: 10.1097/00004583-199703000-00008. [DOI] [PubMed] [Google Scholar]
- Von Rhein D., Oldehinkel M., Beckmann C.F., Oosterlaan J., Heslenfeld D., Hartman C.A., Hoekstra P.J., Franke B., Cools R., Buitelaar J.K., Mennes M. Aberrant local striatal functional connectivity in attention-deficit/hyperactivity disorder. J. Child Psychol. Psychiatry Allied Discip. 2016;57 doi: 10.1111/jcpp.12529. [DOI] [PubMed] [Google Scholar]
- Walpola I.C., Nest T., Roseman L., Erritzoe D., Feilding A., Nutt D.J., Carhart-Harris R.L. Altered Insula Connectivity under MDMA. Neuropsychopharmacology. 2017;42 doi: 10.1038/npp.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D., 2003. Wechsler Intelligence Scale for Children-(WISC-IV Australian). Pearson Clinical and Talent Assessment, Sydney.
- Werhahn J.E., Mohl S., Willinger D., Smigielski L., Roth A., Hofstetter C., Stämpfli P., Naaijen J., Mulder L.M., Glennon J.C., Hoekstra P.J., Dietrich A., Kleine Deters R., Aggensteiner P.M., Holz N.E., Baumeister S., Banaschewski T., Saam M.C., Schulze U.M.E., Lythgoe D.J., Sethi A., Craig M.C., Mastroianni M., Sagar-Ouriaghli I., Santosh P.J., Rosa M., Bargallo N., Castro-Fornieles J., Arango C., Penzol M.J., Zwiers M.P., Franke B., Buitelaar J.K., Walitza S., Brandeis D. Aggression subtypes relate to distinct resting state functional connectivity in children and adolescents with disruptive behavior. Eur. Child Adolesc. Psychiatry. 2021;30 doi: 10.1007/s00787-020-01601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect. 2012;2 doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wilkinson, S., Waller, R., Viding, E., 2016. Practitioner Review: Involving young people with callous unemotional traits in treatment - Does it work? A systematic review. J. Child Psychol. Psychiatry Allied Discip. https://doi.org/10.1111/jcpp.12494. [DOI] [PubMed]
- Winters D.E., Pruitt P.J., Gambin M., Fukui S., Cyders M.A., Pierce B.J., Lay K., Damoiseaux J.S. Cognitive and Affective Empathy as Indirect Paths Between Heterogeneous Depression Symptoms on Default Mode and Salience Network Connectivity in Adolescents. Child Psychiatry Hum. Dev. 2023;54 doi: 10.1007/s10578-021-01242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T.Y., Sid A., Wensing T., Eickhoff S.B., Habel U., Gur R.C., Nickl-Jockschat T. Neural networks of aggression: ALE meta-analyses on trait and elicited aggression. Brain Struct. Funct. 2019;224:133–148. doi: 10.1007/s00429-018-1765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Zhang X., Dong D., Wang X., Yao S. Altered spontaneous brain activity in adolescent boys with pure conduct disorder revealed by regional homogeneity analysis. Eur. Child Adolesc. Psychiatry. 2017;26 doi: 10.1007/s00787-017-0953-7. [DOI] [PubMed] [Google Scholar]
- Zhou J., Yao N., Fairchild G., Zhang Y., Wang X. Altered hemodynamic activity in conduct disorder: A resting-state fMRI investigation. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Yao N., Fairchild G., Cao X., Zhang Y., Xiang Y.T., Zhang L., Wang X. Disrupted default mode network connectivity in male adolescents with conduct disorder. Brain Imaging Behav. 2016;10 doi: 10.1007/s11682-015-9465-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.