Abstract
Background
In two pivotal phase 3 trials, up to 24 weeks of treatment with elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) was efficacious and safe in patients with cystic fibrosis (CF) ≥12 years of age who have at least one F508del allele. The aim of this study is to assess long-term safety and efficacy of ELX/TEZ/IVA in these patients.
Methods
In this phase 3, open-label, single-arm extension study, participants with F508del–minimal function (from a 24-week parent study; n=399) or F508del–F508del (from a 4-week parent study; n=107) genotypes receive ELX/TEZ/IVA at the same dose (ELX 200 mg once daily, TEZ 100 mg once daily and IVA 150 mg every 12 h). The primary end-point is safety and tolerability. A prespecified interim analysis was conducted when the last participant reached the Week 144 visit.
Results
At the Week 144 interim analysis, mean duration of exposure to ELX/TEZ/IVA in the extension study was 151.1 weeks. Exposure-adjusted rates of adverse events (AEs) (586.6 events per 100 participant-years) and serious AEs (22.4 events per 100 participant-years) were lower than in the ELX/TEZ/IVA treatment group in the 24-week parent study (1096.0 and 36.9 events per 100 participant-years, respectively); most participants had AEs classified as mild (16.4% of participants) or moderate (60.3% of participants) in severity. 14 participants (2.8%) had AEs that led to treatment discontinuation. Following initiation of ELX/TEZ/IVA, participants had increases in forced expiratory volume in 1 s (FEV1) percentage predicted, Cystic Fibrosis Questionnaire-Revised respiratory domain score and body mass index, and had decreases in sweat chloride concentration and pulmonary exacerbation rates that were maintained over the interim analysis period. The mean annualised rate of change in FEV1 % pred was +0.07 (95% CI −0.12–0.26) percentage points among the participants.
Conclusions
ELX/TEZ/IVA was generally safe and well tolerated, with a safety profile consistent with the 24-week parent study. Participants had sustained improvements in lung function, respiratory symptoms, CF transmembrane conductance regulator function, pulmonary exacerbation rates and nutritional status. These results support the favourable safety profile and durable, disease-modifying clinical benefits of ELX/TEZ/IVA.
Tweetable abstract
This 144-week interim analysis of an open-label extension study in participants who completed the ELX/TEZ/IVA pivotal studies supports the favourable safety profile and durable, disease-modifying clinical benefits of ELX/TEZ/IVA https://bit.ly/3PLRfbD
Introduction
Cystic fibrosis (CF), a life-shortening autosomal recessive disease that affects more than 100 000 adults and children worldwide [1–4], is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, which decrease the quantity and/or function of the CFTR protein, an anion channel present at the surface of a variety of epithelial cells, leading to impaired transport of chloride and bicarbonate [2, 3, 5]. These molecular defects manifest clinically in respiratory, pancreatic, hepatic and gastrointestinal dysfunction [6].
Ivacaftor (IVA) is a CFTR potentiator that augments the impaired CFTR gating associated with some CFTR mutations [7]. Treatment with IVA led to improvements in forced expiratory volume in 1 s (FEV1) percentage predicted, Cystic Fibrosis Questionnaire-Revised (CFQ-R) respiratory domain score and sweat chloride concentration, along with increases in weight and reductions in the rate of pulmonary exacerbations, in patients with at least one CFTR gating mutation [8–10]. An open-label extension study of IVA showed that improvements in lung function, weight and pulmonary exacerbations were maintained for up to 144 weeks, and another study reported an annual rate of lung function decline nearly 50% lower in patients receiving IVA than in those receiving only symptomatic treatment [10, 11]. These results established that a CFTR modulator can provide effective, long-term, disease-modifying treatment for CF.
Elexacaftor (ELX) and tezacaftor (TEZ) are CFTR correctors that act via complementary mechanisms to ameliorate the CFTR protein processing and trafficking defects intrinsic to the F508del mutation [12]. F508del is the most common CFTR mutation; up to 90% of people with CF have at least one F508del allele [13]. The combination of TEZ and IVA improved lung function and decreased sweat chloride concentration in patients homozygous for F508del (F/F) [14, 15]. TEZ/IVA resulted in improvements that were generally maintained for up to 96 weeks in an open-label extension study; patients had an annual rate of lung function decline that was 61.5% slower than that seen among patients who were not taking CFTR modulators [16].
The efficacy and safety of the triple combination of ELX, TEZ and IVA (ELX/TEZ/IVA) were established in two phase 3 pivotal trials in people with CF ≥12 years of age who were heterozygous for F508del and a minimal function mutation (F/MF) or had the F/F genotype [17–19]. In both trials, ELX/TEZ/IVA treatment led to significant improvements in FEV1 % pred, CFQ-R respiratory domain score and sweat chloride concentration. In patients with the F/F genotype, these improvements were superior to those seen with TEZ/IVA. To assess the long-term safety and efficacy of extended ELX/TEZ/IVA use, a 192-week open-label extension study was initiated in participants who completed one of these pivotal studies. Here, we report results from the Week 144 interim analysis of this extension study.
Methods
Participants, trial design and oversight
Study VX17-445-105 (Study 445-105; ClinicalTrials.gov: NCT03525574) is a phase 3, multicentre, open-label extension study that enrolled participants ≥12 years of age with CF and either F/MF (from Study VX17-445-102 (Study 445-102; ClinicalTrials.gov: NCT03525444)) or F/F (from Study VX17-445-103 (Study 445-103; ClinicalTrials.gov: NCT03525548)) genotypes. To enrol in this extension study, participants must have completed study drug treatment or completed study visits up to the last scheduled visit of the treatment period in parent Study 445-102 or 445-103. For a complete list of inclusion and exclusion criteria, and additional details on study design, dosing and statistical analysis, see the supplementary material.
The extension study is designed to evaluate long-term safety and efficacy of ELX/TEZ/IVA over a 192-week treatment period (supplementary figure S1). All participants regardless of parent study assignment receive ELX/TEZ/IVA at the same dose level that was evaluated in the parent studies (ELX 200 mg once daily, TEZ 100 mg once daily and IVA 150 mg every 12 h). An interim analysis with prespecified analyses was conducted after the last patient completed the Week 144 visit.
The trial was designed by Vertex Pharmaceuticals Incorporated in collaboration with the authors. Informed consent was provided by all participants or their parent or legal guardian; assent was obtained from patients in accordance with local requirements. Safety was monitored by an independent data safety monitoring committee, and data collection and analysis were performed by Vertex Pharmaceuticals in collaboration with the authors and the VX17-445-105 Study Group. All authors had full access to the trial data, critically reviewed the manuscript and approved it for submission. Investigators vouch for the accuracy and completeness of the data generated at their respective sites, and the investigators and Vertex Pharmaceuticals vouch for the fidelity of the trial to the protocol.
Due to this study overlapping with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, a global protocol addendum was implemented. Participant access to study drug therapy and collection of safety data were prioritised. Measures were implemented in accordance with country and local regulations, as well as site-level considerations, and included, as applicable, remote consent, shipment of study drug to the participant's home, virtual study visits conducted by site personnel via teleconference, home nursing visits for blood draws, in-home assessments and remote monitoring.
The clinical trial protocol, SARS-CoV-2-related protocol addendum and informed consent forms were approved by independent ethics committees for each region or site, as required by local regulations.
Outcome measures
The primary end-point of the study is safety and tolerability as assessed by adverse events (AEs), clinical laboratory values, ECGs, vital signs and pulse oximetry. Secondary end-points include absolute changes in FEV1 % pred, sweat chloride concentration, CFQ-R respiratory domain score (range 0–100; higher scores indicate higher patient-reported quality of life with regard to respiratory symptoms) and body mass index (BMI) from parent study baseline, as well as number of pulmonary exacerbations. The rate of lung function change was determined by calculating the annualised rate of change of FEV1 % pred in a post hoc analysis.
Statistical analysis
Analyses of safety and efficacy included all participants who received at least one dose of ELX/TEZ/IVA in the extension study. For all efficacy end-points, data for participants with F/MF genotypes (parent Study 445-102) were analysed separately from those for participants with the F/F genotype (parent Study 445-103). The main analyses were based on data collected up to the date the last participant reached the Week 144 visit (i.e. the data lock date). The main analyses of safety were based on all events collected up to the data lock date and included both in-clinic and at-home assessments. The main analyses for the efficacy end-points included data collected up to Week 144 and all subsequent scheduled or unscheduled visits (Extended Week 144).
The Extended Week 144 visit is an extension to include the Week 144 visit and all subsequent scheduled or unscheduled visits. For participants who have Week 144 visit assessments, the Week 144 assessment was used. For participants whose Week 144 visit assessment was missing, the most recent visit assessment beyond Week 144, if available, was used.
Analysis of FEV1 % pred, sweat chloride concentration and BMI used only in-clinic data, while CFQ-R respiratory domain score used both in-clinic and at-home assessments.
Absolute change from baseline in FEV1 % pred, sweat chloride concentration, CFQ-R respiratory domain score and BMI were analysed using a mixed-effects model for repeated measures. For participants transitioning from Study 445-102, the model included treatment group (as randomised in the parent study), visit and treatment-by-visit interaction as fixed effects with continuous baseline FEV1 % pred from the parent study, age at screening for the parent study (<18 versus ≥18 years) and sex (male versus female) as covariates. For participants transitioning from Study 445-103, the analysis was similar with the exception that sex was not a covariate in the model. Number of pulmonary exacerbations was analysed using a negative binomial regression model, which was the same as in the respective parent study.
We conducted a post hoc analysis for the annualised rate of change of FEV1 % pred in participants with the F/MF and the F/F genotypes, as well as a pooled analysis that included all participants. The annualised rate of change in FEV1 % pred was estimated using a linear mixed-effects model, excluding measures during the first 21 days of ELX/TEZ/IVA treatment to avoid inclusion of acute lung function improvement.
Results
Population
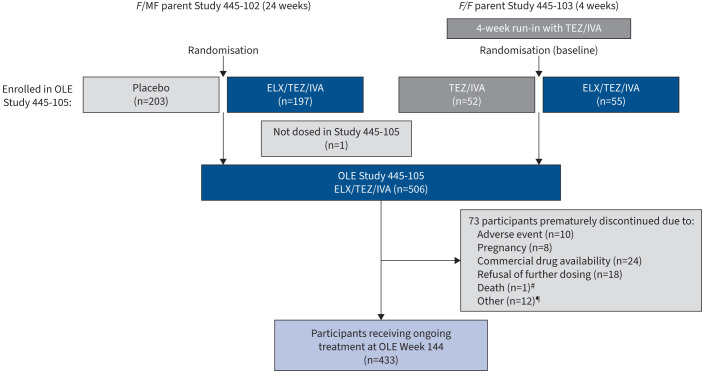
The trial was conducted at 110 sites in North America, Australia and Europe. Overall, 506 participants (n=399 with F/MF genotypes and n=107 with F/F genotype) entered the open-label extension and received at least one dose of ELX/TEZ/IVA, representing 99.2% of participants in the parent studies. Additional detail on participant disposition is provided in figure 1. Participant demographics and clinical characteristics at baseline are provided in table 1 and supplementary table S1. The mean±sd exposure to ELX/TEZ/IVA during the interim analysis period of this trial was 151.1±33.7 weeks. A total of 433 participants were still receiving treatment with ELX/TEZ/IVA in this extension study at Week 144; 73 participants discontinued, with the majority either changing to commercial ELX/TEZ/IVA (n=24), refusing further dosing (n=18) or having AEs that led to treatment discontinuation (n=10) (see figure 1 for all participant dispositions).
FIGURE 1.
Participant disposition diagram. F: F508del; MF: minimal function; TEZ/IVA: tezacaftor/ivacaftor; OLE: open-label extension; ELX/TEZ/IVA: elexacaftor/tezacaftor/ivacaftor. #: one participant died during the study due to an adverse event of accidental oxycodone toxicity that was considered by the investigator to be unrelated to study drug; ¶: other reasons for discontinuation included physician decision (n=2), requirement of prohibited medication (n=2), loss to follow-up (n=1), noncompliance with study drug (n=1), other noncompliance (n=2) and not specified (n=4).
TABLE 1.
Demographics and clinical characteristics of participants at baseline#
| Subgroup of participants from parent Study 445-102 (F/MF genotypes) |
Subgroup of participants from parent Study 445-103
(F/F genotype) |
All participants in Study 445-105 | |||
|
Placebo
(n=203) |
ELX/TEZ/IVA
(n=196) |
TEZ/IVA
(n=52) |
ELX/TEZ/IVA
(n=55) |
ELX/TEZ/IVA
(n=506) |
|
| Female | 98 (48.3) | 94 (48.0) | 28 (53.8) | 31 (56.4) | 251 (49.6) |
| Age, years | 26.8±11.3 | 25.7±9.7 | 27.9±10.8 | 28.8±11.5 | 26.7±10.7 |
| Age group at screening visit | |||||
| ≥12– <18 years | 60 (29.6) | 55 (28.1) | 14 (26.9) | 16 (29.1) | 145 (28.7) |
| ≥18 years | 143 (70.4) | 141 (71.9) | 38 (73.1) | 39 (70.9) | 361 (71.3) |
| Ethnicity | |||||
| Hispanic or Latino | 12 (5.9) | 4 (2.0) | 3 (5.8) | 2 (3.6) | 21 (4.2) |
| Not Hispanic or Latino | 175 (86.2) | 184 (93.9) | 49 (94.2) | 52 (94.5) | 460 (90.9) |
| Not collected per local regulations | 16 (7.9) | 8 (4.1) | 0 | 1 (1.8) | 25 (4.9) |
| Race¶ | |||||
| White | 184 (90.6) | 183 (93.4) | 52 (100.0) | 54 (98.2) | 473 (93.5) |
| Black or African American | 2 (1.0) | 4 (2.0) | 0 (0) | 0 (0) | 6 (1.2) |
| Asian | 1 (0.5) | 0 (0) | 0 (0) | 0 (0) | 1 (0.2) |
| American Indian or Alaska Native | 1 (0.5) | 0 (0) | 0 (0) | 0 (0) | 1 (0.2) |
| Other | 1 (0.5) | 2 (1.0) | 0 (0) | 0 (0) | 3 (0.6) |
| Not collected per local regulations | 16 (7.9) | 8 (4.1) | 0 (0) | 1 (1.8) | 25 (4.9) |
| Geographical region | |||||
| North America | 120 (59.1) | 117 (59.7) | 33 (63.5) | 34 (61.8) | 304 (60.1) |
| Europe and Australia | 83 (40.9) | 79 (40.3) | 19 (36.5) | 21 (38.2) | 202 (39.9) |
| FEV1 % pred, percentage points | 61.3±15.5 | 61.4±14.9 | 60.2±14.4 | 61.6±15.4 | 61.2±15.1 |
| FEV1 % pred category+ | |||||
| <40 | 16 (7.9) | 18 (9.2) | 4 (7.7) | 6 (10.9) | 44 (8.7) |
| ≥40– <70 | 120 (59.1) | 112 (57.1) | 34 (65.4) | 31 (56.4) | 297 (58.7) |
| ≥70– ≤90 | 62 (30.5) | 65 (33.2) | 14 (26.9) | 18 (32.7) | 159 (31.4) |
| >90 | 5 (2.5) | 1 (0.5) | 0 (0) | 0 (0) | 6 (1.2) |
| Sweat chloride concentration, mmol·L−1 | 102.9±9.8 | 102.4±11.9 | 90.0±12.3 | 91.4±11.0 | 100.1±12.0§ |
| CFQ-R respiratory domain score, points | 70.0±17.8 | 68.2±16.8 | 72.6±17.9 | 70.6±16.2 | 69.6±17.2 |
| BMI, kg·m−2 | 21.31±3.14 | 21.53±3.08 | 21.88±4.12 | 21.75±3.19 | 21.50±3.23 |
Data are presented as n (%) or mean±sd. F: F508del; MF: minimal function; ELX/TEZ/IVA: elexacaftor/tezacaftor/ivacaftor; TEZ/IVA: tezacaftor/ivacaftor; FEV1: forced expiratory volume in 1 s; CFQ-R: Cystic Fibrosis Questionnaire-Revised; BMI: body mass index. #: demographics and baseline characteristics of the full analysis set, which was defined as all enrolled participants who received at least one dose of study drug in the open-label extension study (Study 445-105). Baseline characteristics are based on data obtained at parent study baseline, which was defined as the most recent nonmissing measurement before the first dose of study drug in the parent study treatment period (Study 445-102 or Study 445-103). Baseline in Study 445-103 was assessed after a 4-week run-in period with TEZ/IVA. ¶: the race categories may sum to >100% due to participants being able to indicate more than one race. +: although those eligible for enrolment were required to have FEV1 ≥40% predicted at screening, there were some participants who had decreases to <40% predicted by baseline. §: the baseline mean sweat chloride value is a composite from participants with F/MF and F/F genotypes with different baseline values and is weighted towards the F/MF baseline due to the larger number of participants in this genotype subgroup (n=399 F/MF; n=107 F/F).
Safety
ELX/TEZ/IVA treatment was generally safe and well tolerated. During this 144-week interim analysis period, 98.8% of participants had at least one AE. Most participants had AEs that were classified as mild (16.4%) or moderate (60.3%) in severity (table 2). The most common AEs reported in participants were infective pulmonary exacerbation (44.5%), cough (41.9%), headache (32.8%), oropharyngeal pain (28.9%) and nasopharyngitis (26.7%). Serious AEs were reported in 154 participants (30.4%). 14 participants (2.8%) discontinued treatment. This included 10 participants with discontinuations attributed to AEs, two participants with discontinuations attributed to physician decision due to AEs, one participant who died during the study due to an AE of accidental oxycodone toxicity that was considered by the investigator to be unrelated to study drug, and one participant who had an AE prior to the start of this extension study and was not dosed in the extension study. The AEs leading to treatment discontinuation in the remaining participants were elevated transaminases (n=6), hepatic encephalopathy in a participant with a medical history of cirrhosis and portal hypertension (n=1), rash (n=1), depression (n=1), myalgia (n=1) and anorexia nervosa (n=1), as well as a participant with tinnitus, sinus discomfort and dizziness (n=1), all of which resolved, and recurrence of postural orthostatic tachycardia syndrome (n=1) in one participant with a prior medical history, which was not considered related to study drug and had not resolved as of the data cut-off date for this analysis.
TABLE 2.
Adverse events (AEs)#
| Parent Study 445-102 ¶ | Study 445-105 Week 144 interim analysis | |||||
|
Placebo
(n=201) Mean exposure 23.7 weeks |
ELX/TEZ/IVA
(n=202) Mean exposure 23.6 weeks |
ELX/TEZ/IVA
(n=506) Mean exposure 151.1 weeks |
||||
| Participants, n (%) | Events per 100 participant-years | Participants, n (%) | Events per 100 participant-years | Participants, n (%) | Events per 100 participant-years | |
| Any AE | 193 (96.0) | 1287.96 | 188 (93.1) | 1096.01 | 500 (98.8) | 586.55 |
| AEs by maximum severity | ||||||
| Mild | 53 (26.4) | NA | 67 (33.2) | NA | 83 (16.4) | NA |
| Moderate | 125 (62.2) | NA | 102 (50.5) | NA | 305 (60.3) | NA |
| Severe | 14 (7.0) | NA | 19 (9.4) | NA | 106 (20.9) | NA |
| Life threatening | 1 (0.5) | NA | 0 (0) | NA | 6 (1.2) | NA |
| Serious AEs | 42 (20.9) | 67.05 | 28 (13.9) | 36.93 | 154 (30.4) | 22.42 |
| AEs leading to treatment discontinuation | 0 (0) | 0 | 2 (1.0) | 2.99 | 14 (2.8) | 1.82 |
| AEs leading to death | 0 (0) | 0 | 0 (0) | 0 | 1 (0.2)+ | 0.06 |
| AEs leading to treatment interruption | 10 (5.0) | 14.01 | 19 (9.4) | 25.95 | 49 (9.7) | 6.20 |
| Most common AEs § | ||||||
| Infective pulmonary exacerbation of cystic fibrosis | 95 (47.3) | 181.13 | 44 (21.8) | 64.88 | 225 (44.5) | 37.40 |
| Cough | 77 (38.3) | 113.08 | 34 (16.8) | 38.93 | 212 (41.9) | 30.63 |
| Headache | 30 (14.9) | 42.03 | 35 (17.3) | 48.91 | 166 (32.8) | 18.29 |
| Oropharyngeal pain | 25 (12.4) | 26.02 | 20 (9.9) | 26.95 | 146 (28.9) | 16.85 |
| Nasopharyngitis | 26 (12.9) | 34.03 | 22 (10.9) | 29.95 | 135 (26.7) | 17.04 |
| Pyrexia | 19 (9.5) | 25.02 | 17 (8.4) | 17.97 | 134 (26.5) | 12.59 |
| Sputum increased | 39 (19.4) | 47.03 | 40 (19.8) | 46.91 | 120 (23.7) | 12.09 |
| Upper respiratory tract infection | 22 (10.9) | 26.02 | 24 (11.9) | 29.95 | 111 (21.9) | 11.65 |
| Nasal congestion | 15 (7.5) | 18.01 | 19 (9.4) | 20.96 | 106 (20.9) | 10.08 |
| Fatigue | 20 (10.0) | 22.02 | 9 (4.5) | 8.98 | 104 (20.6) | 11.21 |
| COVID-19 | 0 (0) | 0 | 0 (0) | 0 | 99 (19.6) | 7.70 |
| Nausea | 14 (7.0) | 17.01 | 16 (7.9) | 15.97 | 84 (16.6) | 7.77 |
| Diarrhoea | 14 (7.0) | 23.02 | 26 (12.9) | 31.94 | 80 (15.8) | 6.64 |
| Haemoptysis | 28 (13.9) | 42.03 | 11 (5.4) | 11.98 | 78 (15.4) | 12.03 |
| Vaccination complication | 0 (0) | 0 | 0 (0) | 0 | 78 (15.4) | 9.52 |
Data are presented as n (%). ELX/TEZ/IVA: elexacaftor/tezacaftor/ivacaftor; COVID-19: coronavirus disease 2019; NA: not applicable. #: a participant with multiple events within a category was counted only once in that category; ¶: the safety profile of ELX/TEZ/IVA was based on the 24-week, placebo-controlled, F508del–minimal function parent study; +: there was one death in the 144-week interim analysis of Study 445-105 which was due to accidental oxycodone toxicity and was not considered to be related to study drug; §: the most common AEs that occurred in ≥15% of participants in Study 445-102 or the interim analysis of Study 445-105 (listing is according to the preferred term; Medical Dictionary for Regulatory Activities version 24.1 (www.meddra.org)).
The exposure-adjusted rates of AEs and serious AEs in the extension study were lower than those seen in participants with F/MF genotypes in the active arm of the 24-week parent study that forms the basis of the ELX/TEZ/IVA safety profile (table 2 and supplementary tables S2 and S3). The overall exposure-adjusted rate of AEs in the 144-week interim analysis was 586.6 events per 100 participant-years compared with 1288.0 events per 100 participant-years for participants in the placebo arm of the parent study.
Consistent with previous clinical trials of ELX/TEZ/IVA [17, 18], data related to aminotransferases, rash, creatine kinase and blood pressure were reviewed. Elevated levels of alanine aminotransferase and/or aspartate aminotransferase greater than three, five and eight times the upper limit of normal were reported in 57 (11.3%), 32 (6.3%) and 11 (2.2%) participants, respectively (supplementary table S4). Two participants (0.4%) had levels of alanine aminotransferase and/or aspartate aminotransferase greater than three times the upper limit of normal concurrent with bilirubin greater than two times the upper limit of normal: one participant had Gilbert's syndrome and elevated total bilirubin (mostly indirect) throughout the study, and the other participant had concurrent elevations that resolved with study drug discontinuation. 82 participants (16.2%) had AEs of elevated transaminases; in six participants (1.2%) the AE was considered serious. 21 participants (4.2%) interrupted treatment due to AEs of elevated transaminases and six participants (1.2%) discontinued. 82 participants (16.2%) had rash events, of whom 33 were male and 49 were female. Two participants (0.4%) had rash events that were considered serious (supplementary table S5). One participant discontinued due to an AE of rash. 65 participants (12.8%) had AEs of blood creatine phosphokinase increased, one of whom had a serious AE that resolved without change in study drug dosing (supplementary table S6). Three participants (0.6%) had AEs of rhabdomyolysis that presented with blood creatine kinase elevations; none had symptoms consistent with rhabdomyolysis syndrome, and before onset, all three participants had exercised. One participant (0.2%) discontinued due to an AE of blood creatine phosphokinase increased and alanine aminotransferase increased. The exposure-adjusted rates of AEs of transaminase elevations, rash and creatine kinase elevations were lower than those in the 24-week parent study. The mean±sd systolic and diastolic blood pressures increased by 3.9±12.5 and 2.6±9.0 mmHg, respectively, from initiation of ELX/TEZ/IVA treatment (supplementary table S7). 16 participants (3.2%) had AEs related to elevated blood pressure. One participant with type 2 diabetes, chronic kidney disease and a history of hypertension had a serious AE of hypertensive urgency which was not considered related to study drug and did not require change in ELX/TEZ/IVA dosing. All other AEs of elevated blood pressure were nonserious and did not require change in ELX/TEZ/IVA dosing; seven of the 16 participants required medication for elevated blood pressure (supplementary table S8). Other clinical or laboratory assessments did not reveal any notable safety findings.
Efficacy
F508del–minimal function genotypes
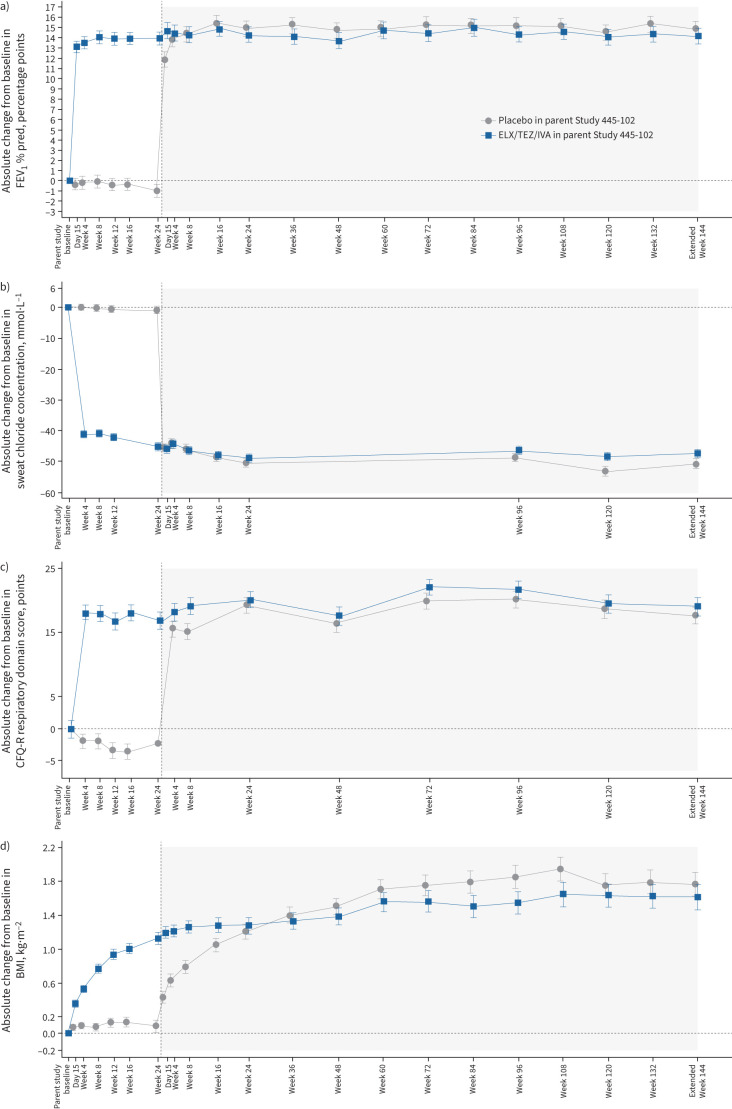
At Extended Week 144, there was a mean absolute increase in FEV1 % pred from parent study baseline of 14.8 (95% CI 13.3–16.3) percentage points for participants who previously received placebo in the parent study (n=161) and 14.1 (95% CI 12.6–15.6) percentage points for participants who previously received ELX/TEZ/IVA (n=166) (table 3 and figure 2a). The annualised rate of pulmonary exacerbations was 0.20 (95% CI 0.16–0.24) (table 3). At Extended Week 144, the mean absolute change in sweat chloride concentration from parent study baseline was −50.5 (95% CI −53.4– −47.7) mmol·L−1 for participants who previously received placebo in the parent study (n=146) and −47.2 (95% CI −49.9– −44.4) mmol·L−1 for participants who previously received ELX/TEZ/IVA (n=160) (table 3 and figure 2b). The mean absolute change in CFQ-R respiratory domain score from parent study baseline was 17.6 (95% CI 14.9–20.2) points for participants who previously received placebo in the parent study (n=175) and 19.1 (95% CI 16.4–21.8) points for participants who previously received ELX/TEZ/IVA (n=171) (table 3 and figure 2c). The mean absolute change in BMI was 1.76 (95% CI 1.48–2.05) kg·m−2 from parent study baseline for participants who previously received placebo in the parent study (n=167) and 1.61 (95% CI 1.32–1.90) kg·m−2 for participants who previously received ELX/TEZ/IVA (n=169) (table 3 and figure 2d).
TABLE 3.
Secondary efficacy end-points (F508del–minimal function (F/MF) genotypes)
| Parent Study 445-102 (F/MF genotypes) through Week 24 |
Study 445-105 at Extended Week 144
(F/MF genotypes) |
|||
|
Placebo
(n=203) |
ELX/TEZ/IVA
(n=200) |
Placebo
→ ELX/TEZ/IVA (n=203) |
ELX/TEZ/IVA
→ ELX/TEZ/IVA (n=196) |
|
| Absolute change in FEV1 % pred, percentage points | −0.4 (−1.5–0.7) | 13.9 (12.8–15.0) | 14.8 (13.3–16.3) n=161 |
14.1 (12.6–15.6) n=166 |
| Absolute change in sweat chloride concentration, mmol·L−1 | −0.4 (−2.2–1.4) | −42.2 (−44.0– −40.4) | −50.5 (−53.4– −47.7) n=146 |
−47.2 (−49.9– −44.4) n=160 |
| Absolute change in CFQ-R respiratory domain score, points | −2.7 (−4.6 to −0.8) | 17.5 (15.6–19.5) | 17.6 (14.9–20.2) n=175 |
19.1 (16.4–21.8) n=171 |
| Absolute change in BMI, kg·m−2 | 0.09 (−0.05–0.22)# | 1.13 (0.99–1.26)# | 1.76 (1.48–2.05) n=167 |
1.61 (1.32–1.90) n=169 |
| Estimated pulmonary exacerbation event rate per 48 weeks | 0.98 | 0.37 | 0.20 (0.16–0.24)¶ | |
Date are presented as least squares mean absolute change (95% CI) from baseline of the parent study, except for pulmonary exacerbation event rate (95% CI). ELX/TEZ/IVA: elexacaftor/tezacaftor/ivacaftor; FEV1: forced expiratory volume in 1 s; CFQ-R: Cystic Fibrosis Questionnaire-Revised; BMI: body mass index. #: for BMI, parent study result represents mean absolute change from baseline at Week 24; ¶: calculated from cumulative ELX/TEZ/IVA exposure in the parent study (Study 445-102) and in the extension study (Study 445-105).
FIGURE 2.
Absolute change from baseline in a) forced expiratory volume in 1 s (FEV1) percentage predicted, b) sweat chloride concentration, c) Cystic Fibrosis Questionnaire-Revised (CFQ-R) respiratory domain score and d) body mass index (BMI) in participants with F508del–minimal function genotypes by study visit, each based on a mixed-effects model for repeated measures. Data presented are least squares means, with I-bars indicating standard error of the mean. White shading corresponds to the parent study (Study 445-102) and grey shading corresponds to the extension study. CFQ-R respiratory domain scores (c) are normalised to a 100-point range, with higher scores indicating higher patient-reported quality of life with regard to respiratory symptoms. The minimal clinically important difference for CFQ-R respiratory domain score is 4 points. ELX/TEZ/IVA: elexacaftor/tezacaftor/ivacaftor.
F508del–F508del genotype
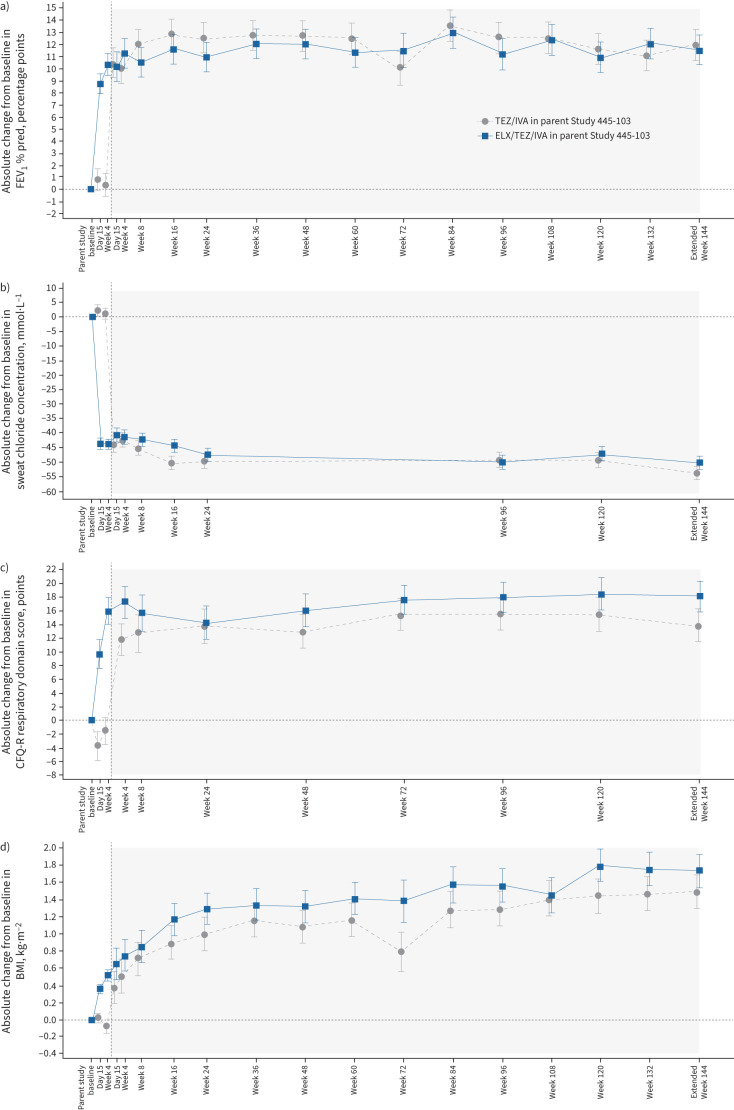
At Extended Week 144, the mean absolute change in FEV1 % pred from parent study baseline was 12.0 (95% CI 9.5–14.5) percentage points for participants who previously received TEZ/IVA in the parent study (n=44) and 11.6 (95% CI 9.1–14.0) percentage points for participants who previously received ELX/TEZ/IVA (n=48) (table 4 and figure 3a). The annualised rate of pulmonary exacerbations was 0.18 (95% CI 0.12–0.26) (table 4). At Extended Week 144, the mean absolute change in sweat chloride concentration from parent study baseline was −53.4 (95% CI −57.7– −49.0) mmol·L−1 for participants who previously received TEZ/IVA in the parent study (n=42) and −49.9 (95% CI −54.1– −45.7) mmol·L−1 for participants who previously received ELX/TEZ/IVA (n=47) (table 4 and figure 3b). The mean absolute change in CFQ-R respiratory domain score from parent study baseline was 13.9 (95% CI 9.2–18.6) points for participants who previously received TEZ/IVA in the parent study (n=45) and 18.2 (95% CI 13.6–22.7) points for participants who previously received ELX/TEZ/IVA (n=48) (table 4 and figure 3c). The mean absolute change in BMI was 1.50 (95% CI 1.11–1.89) kg·m−2 from parent study baseline in participants who previously received TEZ/IVA in the parent study (n=44) and 1.74 (95% CI 1.36–2.12) kg·m−2 in participants who previously received ELX/TEZ/IVA (n=48) (table 4 and figure 3d).
TABLE 4.
Secondary efficacy end-points (F508del–F508del (F/F) genotype)
|
Parent Study 445-103 (F/F genotype)
at Week 4 # |
Study 445-105 at Extended Week 144
(F/F genotype) |
|||
|
TEZ/IVA
(n=52) |
ELX/TEZ/IVA
(n=55) |
TEZ/IVA
→ ELX/TEZ/IVA (n=52) |
ELX/TEZ/IVA
→ ELX/TEZ/IVA (n=55) |
|
| Absolute change in FEV1 % pred, percentage points | 0.4 (−1.4–2.3) | 10.4 (8.6–12.2) | 12.0 (9.5–14.5) n=44 |
11.6 (9.1–14.0) n=48 |
| Absolute change in sweat chloride concentration, mmol·L−1 | 1.7 (−1.9–5.3) | −43.4 (−46.9– −40.0) | −53.4 (−57.7– −49.0) n=42 |
−49.9 (−54.1– −45.7) n=47 |
| Absolute change in CFQ-R respiratory domain score, points | −1.4 (−5.4–2.6) | 16.0 (12.1–19.9) | 13.9 (9.2–18.6) n=45 |
18.2 (13.6–22.7) n=48 |
| Absolute change in BMI, kg·m−2 | −0.07 (−0.21–0.06) | 0.53 (0.39–0.66) | 1.50 (1.11–1.89) n=44 |
1.74 (1.36–2.12) n=48 |
| Estimated pulmonary exacerbation event rate per 48 weeks ¶ | NA | NA | 0.18 (0.12–0.26) | |
Date are presented as least squares mean absolute change (95% CI) from baseline of the parent study, except for pulmonary exacerbation event rate (95% CI). TEZ/IVA: tezacaftor/ivacaftor; ELX/TEZ/IVA: elexacaftor/tezacaftor/ivacaftor; FEV1: forced expiratory volume in 1 s; CFQ-R: Cystic Fibrosis Questionnaire-Revised; BMI: body mass index; NA: not available. #: participants in Study 445-103 had a 4-week TEZ/IVA run-in period prior to baseline; ¶: calculated from cumulative ELX/TEZ/IVA exposure in the parent study (Study 445-103) and in the extension study (Study 445-105).
FIGURE 3.
Absolute change from baseline in a) forced expiratory volume in 1 s (FEV1) percentage predicted, b) sweat chloride concentration, c) Cystic Fibrosis Questionnaire-Revised (CFQ-R) respiratory domain score and d) body mass index (BMI) in participants with the F508del–F508del genotype by study visit, each based on a mixed-effects model for repeated measures. Data presented are least squares means, with I-bars indicating standard error of the mean. White shading corresponds to the parent study (Study 445-103) and grey shading corresponds to the extension study. CFQ-R respiratory domain scores (c) are normalised to a 100-point range, with higher scores indicating higher patient-reported quality of life with regard to respiratory symptoms. The minimal clinically important difference for CFQ-R respiratory domain score is 4 points. TEZ/IVA: tezacaftor/ivacaftor; ELX/TEZ/IVA: elexacaftor/tezacaftor/ivacaftor.
Annualised rate of change in FEV1 % pred
In an analysis assessing lung function change over time, the annualised rate of change in FEV1 % pred was +0.08 (95% CI −0.14–0.30) percentage points for participants with F/MF genotypes and +0.03 (95% CI −0.33–0.39) percentage points for participants with the F/F genotype (supplementary table S9). As the rate of change was similar for both genotype groups, a pooled analysis of the annualised rate of change in FEV1 % pred that included all participants was conducted. In this pooled analysis, the annualised rate of change in FEV1 % pred was +0.07 (95% CI −0.12–0.26) percentage points (supplementary table S9).
Discussion
In this extension study of ELX/TEZ/IVA in participants ≥12 years of age with at least one F508del allele, ELX/TEZ/IVA treatment was generally safe and well tolerated, and led to sustained and clinically meaningful improvements in lung function, respiratory symptoms, CFTR function and nutritional status. Participants also had lower rates of pulmonary exacerbations than seen with previous CFTR modulator regimens. Because people with CF are likely to require long-term treatment with ELX/TEZ/IVA or similar CFTR modulator regimens, safety and efficacy data spanning several years, as demonstrated in this study, are crucial for making informed patient care decisions.
At the time of this interim analysis, 433 participants of the 506 originally enrolled remained in the study; 73 participants discontinued, with the majority either changing to commercial ELX/TEZ/IVA (n=24), refusing further dosing (n=18) or having AEs that led to treatment discontinuation (n=10). Overall, participants had a mean exposure to ELX/TEZ/IVA of 151.1 weeks. For most participants, AEs were mild or moderate in severity and generally consistent with common manifestations of CF. The overall exposure-adjusted rates of AEs and serious AEs were lower than those in the 24-week parent study. Data related to transaminase elevations, rash events, creatine kinase elevations and blood pressure, as well as AEs leading to treatment discontinuation, were generally consistent with the established safety profile based on the 24-week parent study. Rash events were mainly nonserious, manageable and occurred more frequently in female participants than male participants. Consistent with the 24-week parent study, participants had small increases in both systolic and diastolic blood pressure. In the current extension study, one participant with a medical history of depression had a nonserious AE of depression that resolved after treatment discontinuation. Overall, the incidences of AEs of depression and anxiety were 7.1% and 6.3%, respectively, during this 144-week treatment period, equal to 2.69 and 2.63 events per 100 participant-years, respectively, and were generally consistent with the rates seen in previous interim analyses as well as in the pivotal 24-week parent study. In an integrated analysis of clinical trials of CFTR modulators, the exposure-adjusted rate of depression-related AEs was 3.32 events per 100 participant-years in the pooled ELX/TEZ/IVA group (n=1711; 3857 participant-years of exposure) and 3.24 events per 100 participant-years in the pooled placebo group (n=1369; 709 participant-years of exposure) (supplementary table S10). It is important to note that previous studies found symptoms of depression were elevated in people with CF, with Quittner et al. [20] reporting that among 6088 patients with CF, 5–19% of adolescents and 13–29% of adults had elevated symptoms of depression. Our results suggest that participants taking ELX/TEZ/IVA in these clinical trials have rates of depression-related AEs consistent with background. The overall safety results demonstrate a favourable long-term safety profile for ELX/TEZ/IVA.
In the pivotal Studies 445-102 and 445-103, participants who received ELX/TEZ/IVA had rapid and statistically significant increases in FEV1 % pred and CFQ-R respiratory domain score along with significant decreases in sweat chloride concentration [17, 18]. For participants who received the control regimen in these parent studies, ELX/TEZ/IVA treatment in the extension study led to increases in FEV1 % pred and CFQ-R respiratory domain scores and decreases in sweat chloride concentration that were consistent with those seen in participants who received ELX/TEZ/IVA in the parent studies. Crucially, all these improvements in efficacy outcome measures were sustained through the end of the 144-week interim analysis period, establishing the durability of treatment response and disease-modifying benefits with ELX/TEZ/IVA.
Given the robust and durable improvements in FEV1 % pred seen in this interim analysis, we sought to further quantify the impact of ELX/TEZ/IVA treatment on CF lung disease progression by conducting an analysis of the rate of change in lung function over time. Previous studies with IVA and TEZ/IVA showed that lung function declines more slowly in people with CF treated with IVA or TEZ/IVA than in those not taking CFTR modulators (by nearly 50% and 61.5%, respectively), indicating that these therapies can modify CF disease progression by slowing lung function decline [11, 16]. In contrast, through Week 144 of this extension study, the pooled participant population had a mean annual increase in FEV1 % pred of 0.07 (95% CI −0.12–0.26) percentage points, indicating that across a CF population with at least one F508del allele, there was no mean loss of pulmonary function over 144 weeks of ELX/TEZ/IVA treatment. These results raise for the first time the potential that a CFTR modulator therapy could halt loss of lung function in patients with CF over an extended period of time.
Participants taking ELX/TEZ/IVA in the parent studies had steady and sustained increases in BMI [17, 18]. While the mean BMI for each genotype group was within the normal range at the respective parent study baselines, it is important to understand long-term impacts of ELX/TEZ/IVA on nutritional status [21]. Overall, the mean BMI increased rapidly during the first 24 weeks of treatment with ELX/TEZ/IVA with smaller increases and slight variations during the remainder of the interim analysis period. Importantly, at Week 144 mean BMI remained in the normal range. This result suggests ELX/TEZ/IVA generally leads to rapid improvement in nutritional status which is then maintained over time.
Reducing the number of pulmonary exacerbations is critical to averting the progressive lung function decline seen in patients with CF [22]. During the first 144 weeks of this extension study, the estimated pulmonary exacerbation rate for patients with F/MF genotypes (0.20) was lower than in the ELX/TEZ/IVA arm of the 24-week parent study (0.37) [18] and the rate of pulmonary exacerbations for participants in both genotype groups was lower than in the previous 24-week interim analysis of this extension study (0.19 versus 0.30) [23]. Because this study overlapped with the SARS-CoV-2 pandemic, restrictions on social interactions might have also reduced the incidence of pulmonary exacerbation among the study participants [24]. Nonetheless, it is clear that the reduction in pulmonary exacerbations observed in the parent study was maintained in this 144-week extension, supporting the durable benefits of ELX/TEZ/IVA.
One limitation of the current extension study is lack of direct comparator group, which limits the interpretation of safety and efficacy data. For this reason, safety and efficacy results were compared with the placebo treatment group in the parent Study 445-102. Additionally, it should be noted the participants in this extension study had to meet specific inclusion criteria for enrolment into the pivotal trials and therefore extrapolation of these results to all people with CF will likely require studies of real-world usage of ELX/TEZ/IVA. However, results from studies on the safety and efficacy of real-world use of ELX/TEZ/IVA have been consistent with the results reported here [25].
In conclusion, ELX/TEZ/IVA was generally safe and well tolerated through Week 144 of this extension study, with a safety profile consistent with the pivotal 24-week parent study. The clinically meaningful improvements in measures of lung function, respiratory symptoms, CFTR function, pulmonary exacerbation rates and nutritional status reported for patients treated with ELX/TEZ/IVA in the two pivotal phase 3 studies were maintained through this longer-term analysis period. These results, along with the finding of no mean loss in pulmonary function over 144 weeks of treatment, further support the durable and disease-modifying benefits of ELX/TEZ/IVA.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02029-2022.Supplement (844.9KB, pdf)
Shareable PDF
Acknowledgements
We thank the patients and their families for participating in this trial; all site trial investigators and coordinators for their support of the trial sites; Morgan Deng and Emily Poulin, employees of Vertex Pharmaceuticals, who may own stock or stock options in the company, for providing editorial coordination and support; and Nathan Blow of Vertex Pharmaceuticals, who may own stock or stock options in the company, for providing medical writing support under the guidance of the authors; and ArticulateScience LLC (www.articulatescience.com) for providing editorial assistance under guidance of the authors with support from Vertex Pharmaceuticals.
Footnotes
This study is registered at ClinicalTrials.gov with identifier number NCT03525574. Vertex is committed to advancing medical science and improving participant health. This includes the responsible sharing of clinical trial data with qualified researchers. Proposals for the use of these data will be reviewed by a scientific board. Approvals are at the discretion of Vertex and will be dependent on the nature of the request, the merit of the research proposed and the intended use of the data. Please contact CTDS@vrtx.com if you would like to submit a proposal or need more information.
Ethics approval: The trial was designed by Vertex Pharmaceuticals Incorporated in collaboration with the authors. Informed consent was provided by all participants or their parent or legal guardian; assent was obtained from patients in accordance with local requirements. Safety was monitored by an independent data safety monitoring committee, and data collection and analysis were performed by Vertex Pharmaceuticals in collaboration with the authors and the VX17-445-105 Study Group. All authors had full access to the trial data, critically reviewed the manuscript and approved it for submission. Investigators vouch for the accuracy and completeness of the data generated at their respective sites, and the investigators and Vertex Pharmaceuticals vouch for the fidelity of the trial to the protocol. Due to this study overlapping with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, a global protocol addendum was implemented. Participant access to study drug therapy and collection of safety data were prioritised. Measures were implemented in accordance with country and local regulations, as well as site-level considerations, and included, as applicable, remote consent, shipment of study drug to the participant's home, virtual study visits conducted by site personnel via teleconference, home nursing visits for blood draws, in-home assessments and remote monitoring. The clinical trial protocol, SARS-CoV-2-related protocol addendum and informed consent forms were approved by independent ethics committees for each region or site, as required by local regulations.
Author contributions: The study sponsor (Vertex Pharmaceuticals Incorporated) designed the protocol in collaboration with the academic authors. Site investigators collected the data, which were analysed by the sponsor. All authors had full access to the study data. C.L. Daines, E. Tullis, S.M. Moskowitz, V. Prieto-Centurion, T. Weinstock, B. Ramsey and M. Griese developed the initial draft of the manuscript, with writing assistance from the sponsor. All authors participated in subsequent revisions. All authors approved the final version submitted for publication.
This article has an editorial commentary: https://doi.org/10.1183/13993003.02026-2023
Conflicts of interest: All authors received nonfinancial support (assistance with manuscript preparation) from Nucleus Global, which received funding from Vertex Pharmaceuticals Incorporated. C.L. Daines has nothing further to disclose. E. Tullis has received consulting, speaker and travel fees from Vertex Pharmaceuticals. S. Costa serves on an advisory board for Vertex Pharmaceuticals. R.W. Linnemann serves on an advisory board and reports grants paid to her institution and consulting fees from Vertex Pharmaceuticals. M.A. Mall reports patient recruitment fees paid to his institution and advisory fees from Vertex Pharmaceuticals, consulting fees from Antabio, Arrowhead Pharmaceuticals, Boehringer Ingelheim, Enterprise Therapeutics, Santhera, Sterna Biologicals and Vertex Pharmaceuticals, speaker fees from Arrowhead Pharmaceuticals, Boehringer Ingelheim and Vertex Pharmaceuticals, travel fees from Boehringer Ingelheim and Vertex Pharmaceuticals, advisory fees from Antabio, Arrowhead Pharmaceuticals, Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotech, Santhera and Vertex Pharmaceuticals, and serves on the European Cystic Fibrosis Society (ECFS) board. E.F. McKone reports grants, lecture fees and serving on an advisory board for Vertex Pharmaceuticals, lecture fees from Roche, travel fees from A. Menarini, and serving on advisory boards for Janssen, Insmed and CF Storm. D. Polineni reports grants from Laurent Pharmaceuticals, Parion Sciences, Proteostasis Therapeutics and Vertex Pharmaceuticals, consulting fees from Vertex Pharmaceuticals, nonfinancial support for travel to investigator meeting from Vertex Pharmaceuticals, and serves on an advisory board for Sanofi. B.S. Quon reports payments paid to his institution and speaker fees from Vertex Pharmaceuticals. F.C. Ringshausen reports grants paid to his institution from Basilea Pharmaceutica, German Center for Lung Research (DZL), German Center for Infection Research (DZIF), Inhaled Antibiotics in Bronchiectasis and Cystic Fibrosis (iABC)/Innovative Medicines Initiative (IMI), European Federation of Pharmaceutical Industries and Associations (EFPIA), Insmed, Novartis and Polyphor, consulting fees from Grifols, Insmed, Parion, Shionogi and Zambon, speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Grifols, Insmed and Novartis, participation on advisory boards for Grifols, Insmed, Parion, Shionogi and Zambon, unpaid honoraria as co-chair of the German Bronchiectasis Registry (PROGNOSIS), and payments to his institution from AbbVie, AstraZeneca, Boehringer Ingelheim, Corbus, Celtaxsys, Insmed, Novartis, Parion, Polyphor, Vertex and Zambon; and is a steering committee member of the European Bronchiectasis Registry (EMBARC), a steering committee member of the European NTM registry (EMBARC-NTM), a core network lead in ERN-LUNG, a principal investigator for DZL, a chair of the cystic fibrosis working group of the German Respiratory Society (DGP), a steering committee member of the Group of German CF Physicians (AGAM), and co-chair of medical consultants of PCD Patient Advocacy Group (Kartagener Syndrom und Primäre Ciliäre Dyskinesie eV). S.M. Rowe reports grants paid to his institution from AbbVie, Arrowhead Pharmaceuticals, AstraZeneca, Bayer, Celtaxsys, Eloxx, Ionis Pharmaceuticals, Novartis, Proteostasis Therapeutics, Synedgen, Synspira Therapeutics, Translate Bio and Vertex Pharmaceuticals, nonfinancial support from AbbVie, Ionis Pharmaceuticals, Proteostasis Therapeutics, Renovion, Synedgen and Synspira Therapeutics, consulting fees from AbbVie, Arrowhead Pharmaceuticals, Bayer, Cystetic Medicines, Ionis Pharmaceuticals, Novartis, Renovion, Synedgen, Synspira Therapeutics and Vertex Pharmaceuticals, and serves as co-chair for the Next Generation Steering Committee on Vertex Pharmaceuticals. H. Selvadurai has nothing further to disclose. J.L. Taylor-Cousar serves on the board of trustees, clinical research executive committee, clinical research advisory board and Women's Health Research-Working Group for the US Cystic Fibrosis Foundation, serves on the scientific advisory board for Emily's Entourage, serves on the respiratory health awards working group, scientific grants review committee and clinical problems assembly programming committee for the American Thoracic Society; reports consulting fees from 4D Molecular Therapeutics, Celtaxsys, Prolarean Imaging, Protalix Biotherapeutics, Proteostasis Therapeutics and Santhera Pharmaceuticals, grants to her institution from Bayer, Celtaxsys, Eloxx Pharmaceuticals, Gilead, N30 and Proteostasis Therapeutics, speaking fees from Celtaxsys and Gilead, serves on advisory board for AbbVie, Genentech, Insmed and Novartis, and is an associate editor for the Journal of Cystic Fibrosis. N.J. Withers reports lecture fees from Vertex Pharmaceuticals and serves on an advisory board for Vertex Pharmaceuticals and Proteostasis Therapeutics. B. Ramsey reports travel fees and grants from, and serves on advisory board for Vertex Pharmaceuticals, and personal fees from Cystetic Medicines. M. Griese reports grants to his institution from Vertex Pharmaceuticals. N. Ahluwalia, S.M. Moskowitz, V. Prieto-Centurion, Y.V. Tan, S. Tian, T. Weinstock, F. Xuan and Y. Zhang are employees of Vertex and may own stock or stock options in Vertex.
Support statement: This study was funded by Vertex Pharmaceuticals, VX17-445-105. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet 2009; 373: 1891–1904. doi: 10.1016/S0140-6736(09)60327-5 [DOI] [PubMed] [Google Scholar]
- 2.Elborn JS. Cystic fibrosis. Lancet 2016; 388: 2519–2531. doi: 10.1016/S0140-6736(16)00576-6 [DOI] [PubMed] [Google Scholar]
- 3.Bell SC, Mall MA, Gutierrez H, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med 2020; 8: 65–124. doi: 10.1016/S2213-2600(19)30337-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cystic Fibrosis Foundation . About cystic fibrosis. 2023. www.cff.org/intro-cf/about-cystic-fibrosis Date last accessed: 3 March 2023.
- 5.Anderson MP, Gregory RJ, Thompson S, et al. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science 1991; 253: 202–205. doi: 10.1126/science.1712984 [DOI] [PubMed] [Google Scholar]
- 6.Ratjen F, Bell SC, Rowe SM, et al. Cystic fibrosis. Nat Rev Dis Primers 2015; 1: 15010. doi: 10.1038/nrdp.2015.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA 2009; 106: 18825–18830. doi: 10.1073/pnas.0904709106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011; 365: 1663–1672. doi: 10.1056/NEJMoa1105185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Boeck K, Munck A, Walker S, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros 2014; 13: 674–680. doi: 10.1016/j.jcf.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 10.McKone EF, Borowitz D, Drevinek P, et al. Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the Gly551Asp-CFTR mutation: a phase 3, open-label extension study (PERSIST). Lancet Respir Med 2014; 2: 902–910. doi: 10.1016/S2213-2600(14)70218-8 [DOI] [PubMed] [Google Scholar]
- 11.Sawicki GS, McKone EF, Pasta DJ, et al. Sustained benefit from ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am J Respir Crit Care Med 2015; 192: 836–842. doi: 10.1164/rccm.201503-0578OC [DOI] [PubMed] [Google Scholar]
- 12.Keating D, Marigowda G, Burr L, et al. VX-445–tezacaftor–ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med 2018; 379: 1612–1620. doi: 10.1056/NEJMoa1807120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem 2008; 77: 701–726. doi: 10.1146/annurev.biochem.75.103004.142532 [DOI] [PubMed] [Google Scholar]
- 14.Taylor-Cousar JL, Munck A, McKone EF, et al. Tezacaftor–ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med 2017; 377: 2013–2023. doi: 10.1056/NEJMoa1709846 [DOI] [PubMed] [Google Scholar]
- 15.Rowe SM, Daines C, Ringshausen FC, et al. Tezacaftor–ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med 2017; 377: 2024–2035. doi: 10.1056/NEJMoa1709847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flume PA, Biner RF, Downey DG, et al. Long-term safety and efficacy of tezacaftor-ivacaftor in individuals with cystic fibrosis aged 12 years or older who are homozygous or heterozygous for Phe508del CFTR (EXTEND): an open-label extension study. Lancet Respir Med 2021; 9: 733–746. doi: 10.1016/s2213-2600(20)30510-5 [DOI] [PubMed] [Google Scholar]
- 17.Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 2019; 394: 1940–1948. doi: 10.1016/S0140-6736(19)32597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Middleton PG, Mall MA, Drevinek P, et al. Elexacaftor–tezacaftor–ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med 2019; 381: 1809–1819. doi: 10.1056/NEJMoa1908639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor-Cousar JL, Mall MA, Ramsey BW, et al. Clinical development of triple-combination CFTR modulators for cystic fibrosis patients with one or two F508del alleles. ERJ Open Res 2019; 5: 00082-2019. doi: 10.1183/23120541.00082-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quittner AL, Goldbeck L, Abbott J, et al. Prevalence of depression and anxiety in patients with cystic fibrosis and parent caregivers: results of The International Depression Epidemiological Study across nine countries. Thorax 2014; 69: 1090–1097. doi: 10.1136/thoraxjnl-2014-205983 [DOI] [PubMed] [Google Scholar]
- 21.NHLBI Obesity Education Initiative . The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Bethesda, National Institutes of Health, 2000. [Google Scholar]
- 22.Waters V, Stanojevic S, Atenafu EG, et al. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J 2012; 40: 61–66. doi: 10.1183/09031936.00159111 [DOI] [PubMed] [Google Scholar]
- 23.Griese M, Costa S, Linnemann RW, et al. Safety and efficacy of elexacaftor/tezacaftor/ivacaftor for 24 weeks or longer in people with CF and one or more F508del alleles: interim results of an open-label phase 3 clinical trial. Am J Respir Crit Care Med 2020; 203: 381–385. doi: 10.1164/rccm.202008-3176LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel S, Thompson MD, Slaven JE, et al. Reduction of pulmonary exacerbations in young children with cystic fibrosis during the COVID-19 pandemic. Pediatr Pulmonol 2021; 56: 1271–1273. doi: 10.1002/ppul.25250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bower JK, Ahluwalia N, Sahota G, et al. Real-world safety and effectiveness of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis: interim results of a long-term registry-based study. J Cyst Fibros 2023; 22: 730–737. doi: 10.1016/j.jcf.2023.03.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02029-2022.Supplement (844.9KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02029-2022.Shareable (495.3KB, pdf)