Abstract
Aim
To compare and evaluate the regenerative potential of blood clots and platelet-rich fibrin (PRF) in IYNPT based on the revised American Academy of Endodontics (AAE) clinical considerations for regenerative endodontics 2016.
Materials and methods
A total of 20 patients (7–12 years) with immature young necrotic permanent teeth were included and irrigation and disinfection were done using the revised AAE protocol. Teeth were randomly categorized into PRF scaffolding and conventional bleeding technique. The cases were followed up for 1, 3, and 6 months for clinical and radiographic evaluation.
Result
At 6 months there was no significant difference between the groups in terms of clinical healing and periapical healing. A significant statistical difference was noted at the end of 6 months with respect to apical closure within the PRF group. A significant difference was seen in the increase in dentin thickness between groups with PRF showing more increase.
Conclusion
The PRF scaffold can be used as it induces the regenerative potential of stem cells at the apex.
How to cite this article
Prakash AJ, Naik SV, Attiguppe P. Comparative Evaluation of the Regenerative Potential of Blood Clot and Platelet-rich Fibrin in Young Permanent Teeth Based on the Revised American Academy of Endodontics Clinical Considerations for Regenerative Procedure: 2016. Int J Clin Pediatr Dent 2023;16(S-2):S149–S154.
Keywords: Advanced platelet-rich fibrin, American Academy of Endodontics regenerative protocol 2016, Blood clot, Necrotic young permanent teeth, Stem cells of apical papilla
Introduction
Endodontic management of young permanent teeth poses a significant challenge due to the lack of natural apical constriction and thin dentinal walls which makes endodontic treatment consisting of chemomechanical preparation and obturation to achieve a hermetic seal arduous.1 The clinical protocol followed for immature necrotic teeth was apexification, that is, to induce an apical barrier using an intracanal medicament traditionally calcium hydroxide was used to disinfect the canal space and help induce an apical barrier over time. The treatment outcomes depended on patient compliance due to extended treatment time and follow-up required for constant changes of calcium hydroxide dressing.2 The current single-visit apexification involving mineral trioxide aggregate (MTA) had a lot of advantages over calcium hydroxide, it had the superior sealing ability and biocompatibility and could stimulate cytokine release to promote hard tissue formation. Its osteoconductive properties, ability to set in the presence of blood and tissue fluids, shorter treatment time, lesser failures, and predictable time for apical closure made it the material of choice for the management of necrotic young permanent teeth. But, the MTA which had taken the world of endodontics by storm is not without limitations especially when used for apexification. The major limitation was that it neither reinforced the root canal dentin nor did it lead to the induction of root formation to attain an apical closure.3
Recently, regenerative endodontics have evolved as a new treatment protocol on the basis of the idea of revascularization with its origin in an experiment conducted by Ostby in 1960. The rationale, behind this procedure was the ability of the tissue in the periapical region of nonvital teeth to regenerate pulp, reinforce the tooth by increasing root dimensions, and restore the functional property, thereby eliminating the disadvantages of apexification using calcium hydroxide and MTA.4,5 With the advent of advances in biological sciences the scientific community could study the concepts associated with stem cells and growth factors more precisely and comprehensively as they were able to isolate and understand the stem cells of apical papilla (SCAP) and embedded growth factors in dentin mesh, which in fact lead to the understanding that it is SCAP, growth factor and scaffolds that form the edifice of regenerative endodontics.
Regenerative endodontic treatment generally relies on three main aspects chemical debridement, disinfection, and a scaffold. Unlike conventional endodontic therapy, it becomes imperative on our part that the disinfection protocols provide a completely disinfected root canal space free of microorganisms and at the same time cause minimum cytotoxicity to SCAP and maintain their capacity to multiply and differentiate.6,7
Since there is a paucity of in vivo studies on regenerative endodontic procedures following the Current American Academy of Endodontics (AAE) clinical considerations for regenerative endodontics this study was formulated to assess and compare the regenerative potential of the two autologous scaffold systems blood clot and platelet-rich fibrin (PRF).
Materials and Methods
It was an experimental in vivo study, the calculated sample size was 20 based on the previous studies at a level of significance at 5% and power of study at 80%.
After obtaining approval from the Institutional Review Board on 24th November 2017 with Ref.No.BDC/EXAM/383/2017–18 the patients visiting the Department of Pedodontics and Preventive Dentistry, Bapuji Dental College and Hospital, Davangere, Karnataka, India, who fulfilled the following inclusion criteria were inducted into the study.
Around 7–9 years with immature necrotic young permanent teeth (INYPT) secondary to trauma or caries.
Preoperative radiograph should show no periapical radiolucency of >10 mm and the apical foramen should be more or equal to 1 mm.
There should be no signs of external or internal resorption.8
Written informed consent was obtained from the parent/guardian of the children who satisfied the eligibility criteria before starting the treatment.
First Appointment
Local anesthesia was administered and the teeth to be treated were isolated with rubber dam.
After preparing an access cavity the irrigation protocol as per AAE recommendation, that is, gentle and copious irrigation with 1.5% sodium hypochlorite with side vented closed needle (20 mL/canal, 5 minutes) was carried out.
Canals were dried and calcium hydroxide mixed with saline was given as a dressing and the access cavity was sealed, the patient was recalled after 4 weeks.
Second Appointment
Plain local anesthetic (LA) was administered.
Irrigation was done using saline and 20 mL of 17% ethylenediamine-tetraacetic acid (EDTA) was used as the last irrigant.
Group I (Blood Clot)
The blood clot was formed till the level of cementoenamel junction (CEJ) by inducing bleeding through over-instrumentation of a 15 k-file 2 mm beyond the apex. The stability of the blood clot was assessed with a paper point (Fig. 1).
In order to prevent the degradation of the blood clot it was sealed with a collagen plug and the canal orifice was sealed with 3–4 mm of MTA followed by glass ionomer cement (GIC) to seal the access cavity.9
Fig. 1.
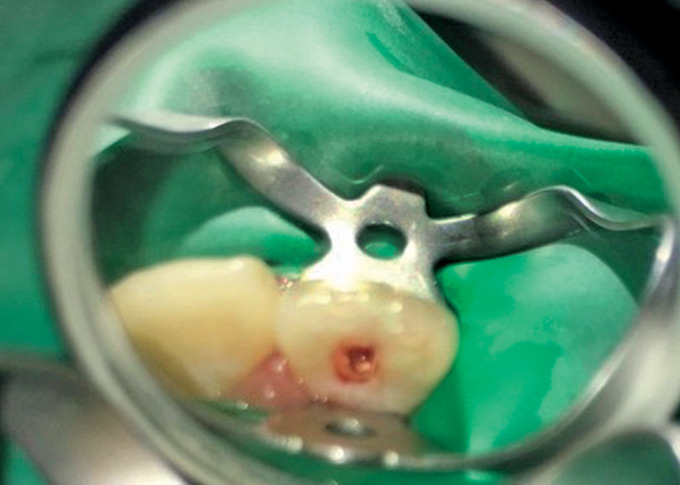
Representative photograph of inducing bleeding (group I)
Group II (PRF)
In order to obtain PRF clot 10 mL blood was withdrawn from the patient and was centrifuged at 3000 for a span of 10 minutes.
The PRF clot was separated from the red blood cell (RBC) base at the bottom, acellular platelet poor plasma (PPP) the supernatant which was then compressed with a sterilized gauze to obtain the PRF membrane.
The PRF membrane was cut into small pieces loaded into a spinal needle and condensed into the canal (Fig. 2).
It was sealed with a 3–4 mm fast-setting MTA and the access cavity was sealed with GIC.
A digital radiograph was taken. The radiograph was standardized using the long cone technique and saved as a JPEG image.10
Fig. 2.
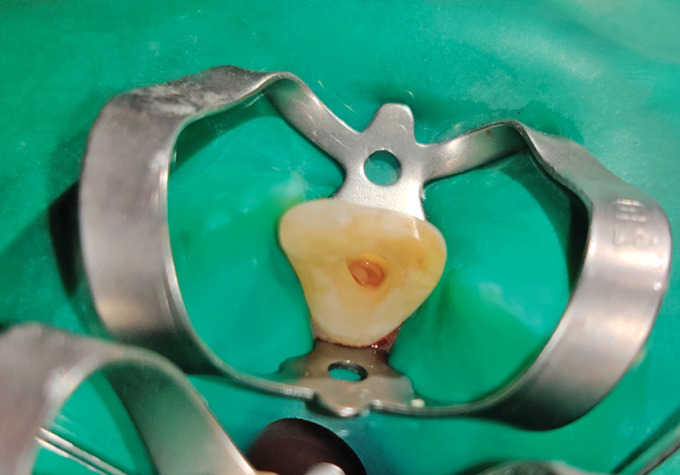
Representative photograph of placement of PRF membrane (group II)
Evaluation
The cases were followed up at 1, 3, and 6 months interval to assess clinical outcome using Miller's criteria11 (Table 1) and radiographic outcome (Figs 3 and 4) were assessed for periapical healing (Ostravik's criteria)12 (Table 2). Apical response (Chen et al.)12 (Table 3) and root dimensions, that is, increase in root length and dentin thickness (Bose et al.)13 (Figs 5A and B).
Table 1.
Miller's criteria for mobility
| Score | Criteria |
|---|---|
| Score 0 | Normal physiologic mobility |
| Score 1 | Mobility <1 mm |
| Score 2 | Mobility >1 mm |
| Score 3 | Mobility >1 mm in the horizontal direction with vertical depressability |
Figs 3A and B.
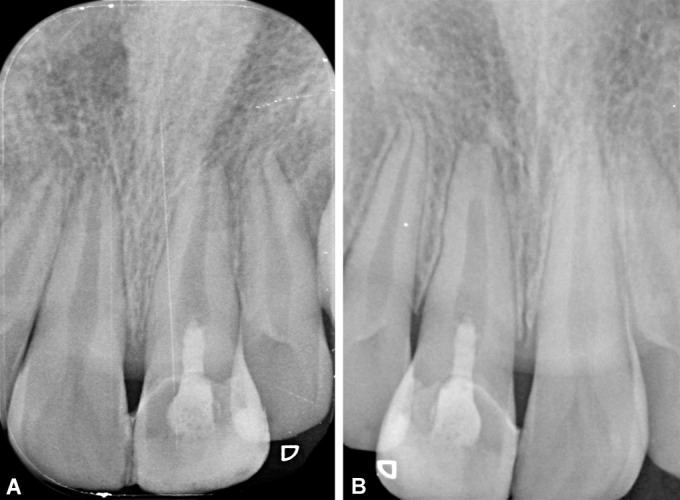
Representative radiographs of group I (blood clot). (A) 1 month; (B) 6 months
Figs 4A and B.
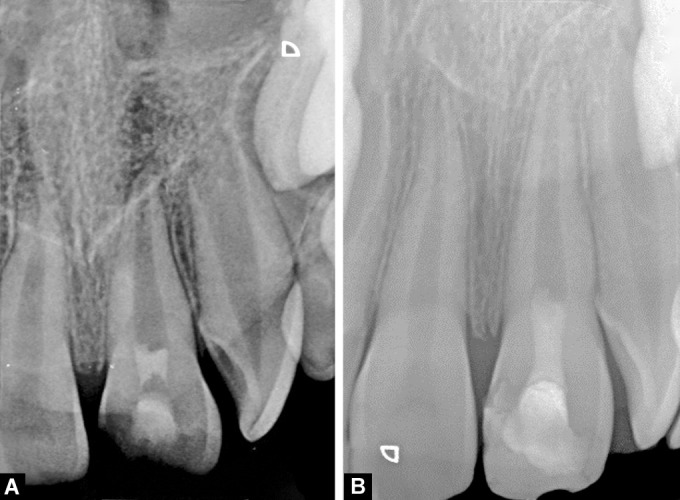
Representative radiographs of group II (PRF). (A) 1 month; (B) 6 months
Table 2.
Periapical healing (Ostravik's criteria)
| Score | Criteria |
|---|---|
| 1 | Normal periapical structures |
| 2 | Small changes in bone structure |
| 3 | Changes in bone structure with some mineral loss |
| 4 | Periodontitis with well-defined radiolucent areas |
| 5 | Severe periodontitis with exacerbating features |
Table 3.
Criteria for apical response (Chen et al.)
| Score | Criteria |
|---|---|
| Type 1 | Increased thickening of the canal walls and continued root maturation |
| Type 2 | No significant continuation of root development with the root apex becoming blunt and closed |
| Type 3 | Continued root development with the apical foramen remaining open |
| Type 4 | Severe calcification (obliteration) of the canal space |
| Type 5 | A hard tissue barrier formed in the canal between the coronal MTA plug and the root apex |
Figs 5A and B.
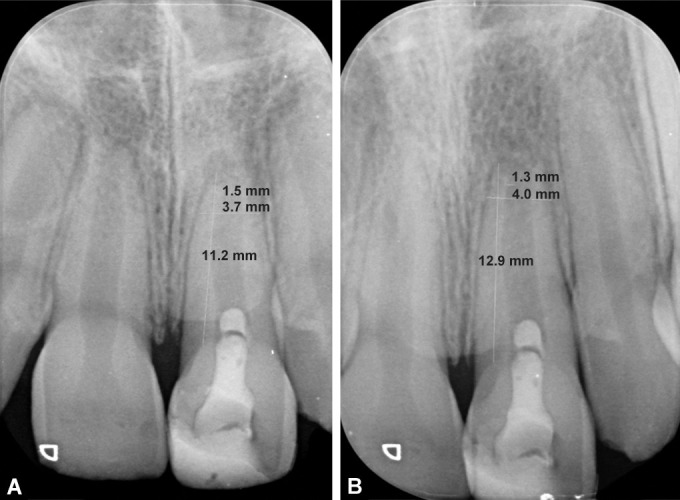
Representative radiographs of measurement of root dimensions using radiovisiography (RVG) application at baseline i.e. immediately after posttreatemnt (A) and at 6 months (B)
Results
The results were analyzed using the Wilcoxon signed-rank test and Mann–Whitney U test for intergroup, Friedman test for intragroup, student's t-test for intragroup and intergroup change in root length and dentin thickness with the help of computer software, that is, Statistical Package for the Social Sciences version 16 and p < 0. 05 was considered as statistically significant.
There was no significant difference seen with respect to clinical outcome, or periapical healing index at intergroup comparison with respect to both scaffold groups at follow-up periods (Table 4 and Fig. 6).
There was a significant difference noted with respect to apical response scores at 1, 3, and 6 months on intragroup comparison of group II with a p-value = 0.039* (Table 5).
On intragroup comparison of the change in root length and dentin thickness at baseline, that is, immediately posttreatment and at 6 months in groups I and II were found to be highly significant with a p-value of 0.001** and 0.000** for group I and 0.000** and 0.000**for group II, respectively.
On intergroup comparison, a highly significant statistical difference was seen for the difference in change for dentin thickness at baseline (i.e., immediately posttreatment) and at 6 months with a p-value of 0.019* (Table 6 and Fig. 7).
Table 4.
Descriptive statistics showing intergroup comparison between group I (blood clot) and group II (PRF) for periapical healing and apical response at 1, 3, and 6 months, respectively
| Index | Median group I | Median group II | Z value | Mann–Whitney U test | p-value groups I vs II |
|---|---|---|---|---|---|
| Periapical healing 1 month | 1 | 1 | −1.373 | 21.000 | 0.463 |
| 3 months | 1 | 1 | −1.369 | 21.000 | 0.463 |
| 6 months | 1 | 1 | 0.000 | 32.000 | 1.000 |
| Apical response | |||||
| 1 month | 3 | 1.5 | −0.766 | 25.500 | 0.505 |
| 3 months | 3 | 1.5 | −0.520 | 27.500 | 0.645 |
| 6 months | 3 | 1 | −0.717 | 26.000 | 00.574 |
SD denotes standard deviation; p-value represents the probability value p < 0.05 is considered significant
Fig. 6.
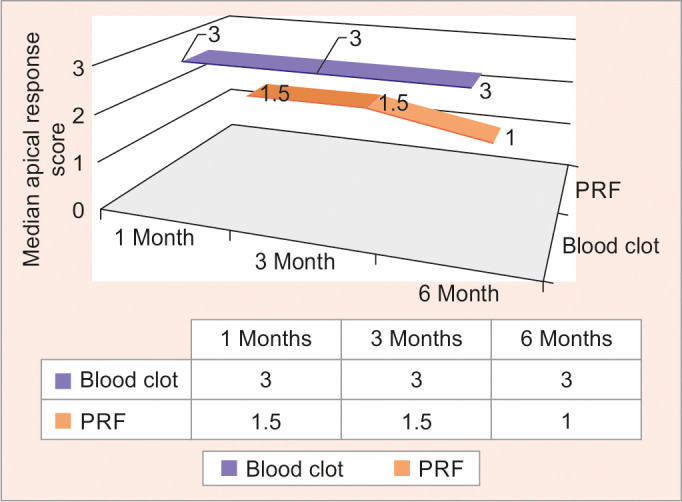
Graphical representation of the intergroup comparison of groups I and II for apical response at 1, 3, and 6 months, respectively
Table 5.
Descriptive statistics showing intragroup comparison of group II (PRF) for apical response at 1, 3, and 6 months, respectively
| Apical response | Mean ± SD | Chi-square test | p-value |
|---|---|---|---|
| 1 month | 1.6250 ± 0.91613 | 6.500 | 0.039* |
| 3 months | 1.875 ± 0.99103 | ||
| 6 months | 1.0000 ± 0.00000 |
SD denotes standard deviation; p-value represents the probability value p < 0.05 is considered significant
Table 6.
Descriptive statistics showing a comparison between the change in root length and dentin thickness at baseline (immediately posttreatment) and at 6 months between groups I and II
| Parameter | Blood clot Mean ± SD | PRF Mean ± SD | t-value | p-value |
|---|---|---|---|---|
| Root length | 0.7000 ± 0.3545 | 0.7875 ± 0.2587 | −0.564 | 0.582 |
| Dentin thickness | 0.2875 ± 0.1126 | 0.4500 ± 0.1309 | −2.66 | 0.019* |
SD denotes standard deviation; p-value represents the probability value p < 0.05 is considered significant
Fig. 7.
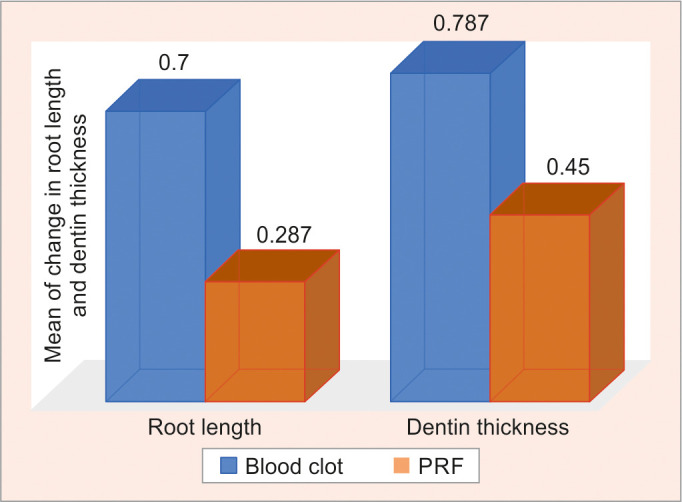
Graphical representation showing a comparison between the change in root length and dentin thickness at baseline (immediately posttreatment) and at 6 months between groups
Discussion
Regenerative endodontics since, its inception has undergone a dynamic change with a burst of case series, in vitro and in vivo studies reporting success under various treatment protocols with respect to the use of various irrigation, disinfection, and scaffold system. Since the failure cases aren't reported frequently it is very difficult to ascertain the correct causal-effect relationship between the practiced protocols and success and hence are of low level of evidence. Since the practitioners should always follow the best evidenced clinical protocols for all treatments AAE reacted to the ongoing inconsistencies in procedures and came up with a position statement on regenerative endodontics based on the best available research evidence, these are revised every three years.14 Current AAE clinical considerations recommend the use of 1.5% sodium hypochlorite and 17% EDTA as irrigants for regenerative endodontic procedures.
The present study fell into the T2 domain of the spectrum of clinical and translational research for designing and interpreting studies on regenerative endodontics, that is. experimental research with control over the conditions but with a smaller sample size as it addressed the question of clinical and radiographic success.15
Bioengineering is the concept on which regenerative endodontics is based on the interplay orchestration of stem cells, growth factors, and scaffolds. Since regenerative endodontics is a process based on cell homing strategies, it becomes essential to create a biomimetic microenvironment for stem cells to retain their “stemness,” that is, to be viable to replicate and proliferate. This specialized microenvironment concept was termed a stem cell niche by Schofield. The biological basis of regenerative endodontics is that SCAP retains its potential to regenerate even in an event of necrosis of pulp as Hertwig's epithelial root sheath is believed to be the signaling transcription factor for SCAP and is unaffected even in the case of pulpal necrosis for an extended period of time. The undifferentiated mesenchymal cells that constitute SCAP are considered to be a source of primary odontoblasts which lay down root dentin. Hence, it became imperative to employ irrigation and disinfection protocols that would disinfect the canal space and at the same time be congenial to SCAP and retain its viability.15–17
The AAE recommends the use of 1.5% sodium hypochlorite followed by EDTA as irrigation protocol. Sodium hypochlorite which possesses strong antimicrobial and proteolytic activity but, the concentration of sodium hypochlorite should be adequate for appropriate debridement while being conducive to the undifferentiated cells of the apical papilla. Martin et al. concluded that 1.5% sodium hypochlorite had maintained the vitality of SCAP and at the same time was adequate as a disinfectant. EDTA acts as a chelating agent to remove the smear layer which allows for better penetration and wettability of root canal irrigants as well as intracanal medicaments.6 Zhao et al. and Pang et al. demonstrated in their study that EDTA plays a vital role as it releases the growth factors which were embedded in dentin during dentinogenesis like transforming growth factor- β1 (TGF-β1) which in turn enhances the stimulation and proliferation of mesenchymal stem cells (MSCs) by upregulation of the expression of dentin sialophosphoprotein and dentin matrix protein 1.17,18 Pang et al., had conducted an in vitro study to assess the number of growth factors which are released following the current irrigation protocol proposed by AAE, that is, 1.5% sodium hypochlorite when followed by EDTA it was proven that this protocol enhanced release of TGF-β1to a concentration of 2–90 ng/mL and its also cited in various studies that TGF-β1 was capable of inducing cell migration as it acts as chemokine which helps in binding of SCAP to dentin matrix thereby enhancing regenerative endodontic success by cell homing procedure.18
To achieve a complete purification of canal space irrigation should be followed by an intracanal medicament, AAE recommends the use of calcium hydroxide or triple antibiotic paste (TAP) as an intracanal medicament. Ruparel et al. in their study on TAP used at clinical concentration and calcium hydroxide, had shown that TAP was detrimental to SCAP whereas the use of calcium hydroxide showed a higher expression of phosphorylated extracellular kinases indicating stem cell viability and proliferation.19 Althmuriay in his study had concluded that the TAP and double antibiotic paste does hamper the survival of SCAP whereas calcium hydroxide didn't have any detrimental effect on SCAP. Moreover, calcium hydroxide has been shown to solubilize and release TGF-β1 embedded in dentin which encourages mesenchymal stem cell proliferation and accentuates cell homing procedure.20
Stem cell homing in regenerative endodontics is orchestrated by growth factors and chemokines released during irrigation and disinfection and from the scaffolds placed into the canal space. Growth factors regulate the MSCs toward pulp regeneration hence, providing a tangible pathway towards clinical transition. In regenerative endodontics two major scaffolds are used, induction of blood clots and PRF. Lovelace et al. in their study, concluded that induction of bleeding infuses stem cells from SCAP niches into the canal space.21 PRF on the contrary, is rich in growth factors TGFβ1, vascular endothelial-derived growth factor, platelet-derived growth factor, interleukin-1, and interleukin-6 which has shown to exhibit consistent and stronger healing responses. All the growth factors present in PRF promote dentinogenesis and angiogenesis and stimulate the expression of dentin sialoprotein (DSP) which is a precursor for dentinogenesis. Moreover, PRF has a sustained release of growth factor over a long period of time with a peak release on the 14th day.22
The success of regenerative endodontic procedures is evaluated by the goals achieved, that is, a primary goal which is the elimination of symptoms and evidence of bone healing, the secondary goal is increased root wall thickness and increased root length which is desirable and the tertiary goal, that is, positive vitality testing which if achieved could prove the organization of pulp tissue. Since the present study was followed up for a period of 6 months, only the primary and secondary goals were evaluated.
The statistical analysis of the results of the present study proved that both scaffolds had shown good periapical healing as no cases were scored below 2 and it could be attributed to the irrigation and disinfection protocol employed in the present study which aims at preserving the SCAP.
It was found that type 3 response was the most common in the blood clot group, although one case had shown a type 5 response (Fig. 3B) (i.e., hard tissue barrier formed in the canal between the coronal MTA plug and the root apex) which was also a finding in previous studies using blood clot as the scaffold. Though the PRF group had shown a significant difference in apical response at 1,3, and 6 months, type 1 response was the most commonly observed response in the PRF group. This could be attributed to the superior growth factors and signaling molecules enmeshed in PRF which helps in better cell homing strategy thus helping SCAP to migrate, proliferate and differentiate into mineralization phenotypes.
In the present study in order to quantify the increase in root length and dentin thickness the method given by Bose et al., was employed. On comparative analysis of blood clot and PRF for an increase in root length at baseline (i.e., posttreatment) and at 6 months, there was no statistically significant change, but a significant difference was seen in dentin thickness at baseline (i.e., posttreatment) and at 6 months, with PRF group showing better results than blood clot group. The results of our study with respect to the increase in dentin thickness could be correlated with the study of Narang et al. in which a different irrigation protocol was followed with PRF showing increased lateral wall thickening when compared with a blood clot and PRP.23
Conclusion
In the present study employing AAE clinical recommendations for current regenerative endodontics 2016 was effective and both the groups had optimal clinical and radiographic outcomes since both groups were similar in clinical outcome, periapical healing, apical response, and increase in root length except for increased dentin thickness in PRF group.
Recommendation
The AAE clinical considerations for current regenerative endodontics 2016 advocate the use of both scaffolds blood clot and PRF. Considering the age and cooperative ability of the child and analyzing the pros and cons of both scaffold techniques clinically, blood clots can be used as a scaffold especially in pediatric endodontics as it is less invasive.
ORCID
Aarathi J Prakash https://orcid.org/0000-0003-2434-2745
Saraswathi V Naik https://orcid.org/0000-0002-3754-3880
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Mahla RS. Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol. 2016;2016(1):6940283. doi: 10.1155/2016/6940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rafter M. Apexification: a review. Dent Traumatol. 2005;21(1):1–8. doi: 10.1111/j.1600-9657.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- 3.Muhamad A, Azzaldeen A, Hanali A. Mineral trioxide aggregate (MTA) in apexification. Endodontology. 2013;25(2):97–101. doi: 10.4103/0970-7212.352338. [DOI] [Google Scholar]
- 4.Lee BN, Moon JW, Chang HS, et al. A review of the regenerative endodontic treatment procedure. Restor Dent Endod. 2015;40(3):179–187. doi: 10.5395/rde.2015.40.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostby BN. The role of the blood clot in endodontic therapy. An experimental histologic study. Acta Odontol Scand. 1961;19:324–353. [PubMed] [Google Scholar]
- 6.Martin DE, De Almeida JF, Henry MA, et al. Concentration-dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J Endod. 2014;40(1):51–55. doi: 10.1016/j.joen.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Shivashankar VY, Johns DA, Maroli RK, et al. Comparison of the effect of PRP, PRF and induced bleeding in the revascularization of teeth with necrotic pulp and open apex: a triple blind randomized clinical trial. J Clin Diagn Res. 2017;11(6):ZC34–ZC39. doi: 10.7860/JCDR/2017/22352.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Association of Endodontists[Internet]. AAE Clinical Consideration for a Regenerative Procedure. Chicago: 2016. Revised 6-8-16; [cited 2017 oct12]. Available from: [Google Scholar]
- 9.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Prabhakar AR, Rani NS, Yavagal C. Revascularization of immature necrotic teeth with platelet-rich fibrin and blood clot. Int J Oral Health Sci. 2016;6(1):4–10. [Google Scholar]
- 11.Orstavik D, Kerekes K, Eriksen HM. The periapical index: a scoring system for radiographic assessment of apical periodontitis. Endod Dent Traumatol. 1986;2(1):20–34. doi: 10.1111/j.1600-9657.1986.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen MY, Chen KL, Chen CA, et al. Responses of immature permanent teeth with infected necrotic pulp tissue and apical periodontitis/abscess to revascularization procedures. Int Endod J. 2012;45(3):294–305. doi: 10.1111/j.1365-2591.2011.01978.x. [DOI] [PubMed] [Google Scholar]
- 13.Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod. 2009;35(10):1343–1349. doi: 10.1016/j.joen.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Galler KM. Clinical procedures for revitalization: current knowledge and considerations. Int Endod J. 2016;49(10):926–936. doi: 10.1111/iej.12606. [DOI] [PubMed] [Google Scholar]
- 15.Hargreaves KM, Diogenes A, Teixeira FB. Paradigm lost: a perspective on the design and interpretation of regenerative endodontic research. J Endod. 2014;40(4 Suppl):S65–S69. doi: 10.1016/j.joen.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Hargreaves KM, Diogenes A, Teixeira FB. Treatment options: biological basis of regenerative endodontic procedures. J Endod. 2013;39(3 Suppl):S30–S43. doi: 10.1016/j.joen.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao S, Sloan AJ, Murray PE, et al. Ultrastructural localisation of TGF-beta exposure in dentine by chemical treatmentHistochem J. 2000;32(8):489–494. doi: 10.1023/a:1004100518245. [DOI] [PubMed] [Google Scholar]
- 18.Pang NS, Lee SJ, Kim E, et al. Effect of EDTA on attachment and differentiation of dental pulp stem cells. J Endod. 2014;40(6):811–817. doi: 10.1016/j.joen.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Ruparel NB, Teixeira FB, Ferraz CC, et al. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod. 2012;38(10):1372–1375. doi: 10.1016/j.joen.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Althumairy RI, Teixeira FB, Diogenes A. Effect of dentin conditioning with intracanal medicaments on survival of stem cells of apical papilla. J Endod. 2014;40(4):521–525. doi: 10.1016/j.joen.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Lovelace TW, Henry MA, Hargreaves KM, et al. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endod. 2011;37(2):133–138. doi: 10.1016/j.joen.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Galler KM, Buchalla W, Hiller KA, et al. Influence of root canal disinfectants on growth factor release from dentin. J Endod. 2015;41(3):363–368. doi: 10.1016/j.joen.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Narang I, Mittal N, Mishra N. A comparative evaluation of the blood clot, platelet-rich plasma, and platelet-rich fibrin in regeneration of necrotic immature permanent teeth: a clinical study. Contemp Clin Dent. 2015;6(1):63–68. doi: 10.4103/0976-237X.149294. [DOI] [PMC free article] [PubMed] [Google Scholar]


