To the Editor,
Patients with acute myeloid leukemia (AML) are at high risk of invasive fungal infections (IFIs) due to profound and prolonged neutropenia as a result of induction chemotherapy protocols [1]. Invasive aspergillosis (IA) became the most common form of IFI after the introduction of fluconazole prophylaxis [1]. The serum galactomannan (GM) assay has emerged as an important diagnostic tool for the early detection of IA. This study aimed to determine the accuracy of the serum GM test and the effect of the revised 2020 European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSGERC) definitions on IA incidence in children with AML.
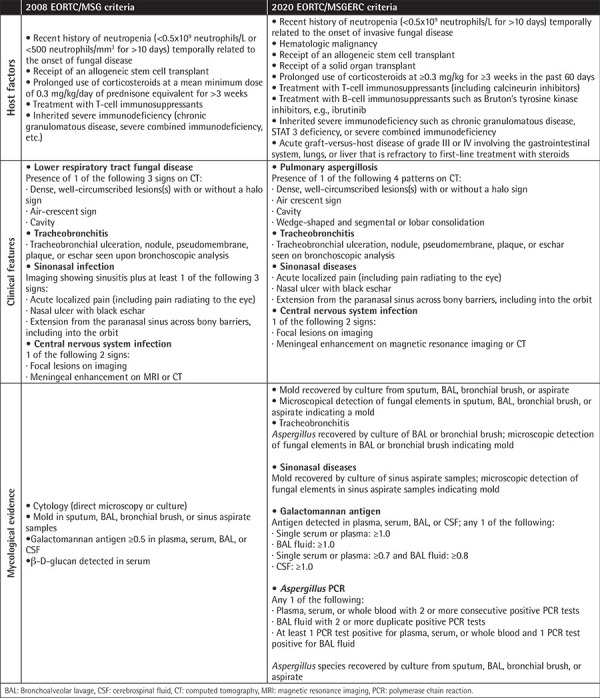
We retrospectively reviewed the cases of 71 pediatric patients treated for AML between January 2005 and December 2022 in the Pediatric Hematology Department of Ege University. IA was defined according to the 2008 EORTC/MSG criteria and compared with the new 2020 criteria [2,3]. GM testing was conducted twice a week during the neutropenia period. True-positive antigenemia was defined as a positive GM test with the diagnosis of proven or probable IA (Table 1).
Table 1. Comparison of 2008 EORTC/MSG and 2020 EORTC/MSGERC criteria for probable invasive aspergillosis.
Thirty-six of the patients were male (50.7%) and 35 (49.3%) were female. The median age was 55 months (range: 1-214 months). Thirty-three (46.4%) of the patients were classified as being of high risk. A total of 275 febrile neutropenia episodes were evaluated and IA was thought to account for 11.2% of them. Probable and possible IA events were 2.54% and 8.7%, respectively. There were no proven IA events. Since 2013, 47 (66%) patients had received mold-active antifungal prophylaxis with voriconazole and a significant decrease in the rate of IA was achieved with this prophylaxis [19 (6.9%) attacks before the pre-prophylaxis period, 12 (4.3%) attacks after prophylaxis began; p=0.015].
A total of 1528 serum samples from 65 patients with at least one serum GM result were analyzed. The median number of GM tests was 16 (range: 2-89). Thirty-one (47.6%) patients had positive GM antigenemia, which corresponded to 5.1% (n=79) of all serum samples. GM was truly positive in 16.1% of these cases.
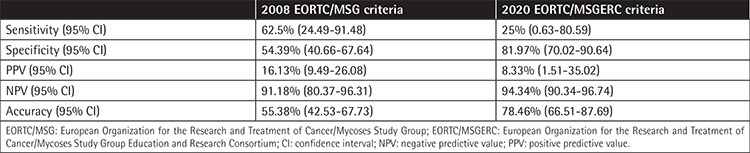
According to the 2008 EORTC/MSG criteria, the sensitivity of the serum GM test was 62.5% [95% confidence interval (CI): 24.49-91.48%] and the specificity was 54.39% (95% CI: 40.66-67.64%) with a positive predictive value of 16.13% (95% CI: 9.49-26.08%), a negative predictive value of 91.18% (95% CI: 80.37-96.31%), and accuracy of 55.38% (95% CI: 42.53-67.73%) for proven/probable IA.
According to the 2020 EORTC/MSGERC criteria, the sensitivity was 25% (95% CI: 0.63-80.59%) and the specificity was 81.97% (95% CI: 70.02-90.64%) with a positive predictive value of 8.33% (95% CI: 1.51-35.02%), a negative predictive value of 94.34% (95% CI: 90.34-96.74%), and accuracy of 78.46% (95% CI: 66.51-87.69%) for proven/probable IA (Table 2).
Table 2. Comparison of serum galactomannan test performance in the diagnosis of invasive aspergillosis according to 2008 EORTC/MSG and 2020 EORTC/MSGERC criteria.
Acet-Öztürk et al. [4] reported that the use of 2020 EORTC/MSGERC criteria resulted in a significant 27.3% reduction in probable IA diagnoses. Similarly, with the new 2020 criteria, the specificity of the serum GM test increased and the number of patients in the probable IA group decreased by 42% (3/7 cases) with the GM test alone in our study; however, the lack of an Aspergillus PCR test was a limitation. Siopi et al. [5] reported that episodes of probable IA were reduced by 33% with GM alone and by 11% when GM + PCR were used as mycological criteria.
False positivity of the serum GM test remains a significant disadvantage. However, the contribution of our high mold-effective prophylaxis rate to the false negative rate cannot be denied.
In conclusion, diagnostic criteria should be reviewed over time and further investigations are essential for accurate diagnostic tests in the early diagnosis of IA.
Footnotes
Authorship Contributions
Surgical and Medical Practices: G.A., N.K., Ş.Ö.G., D.Y.M., D.Y.K.; Concept: G.A.; Design: G.A.; Data Collection or Processing: G.A., N.K., Ş.Ö.G.; Analysis or Interpretation: G.A., D.Y.M., D.Y.K.; Literature Search: G.A., D.Y.M.; Writing: G.A.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.Rubio PM, Sevilla J, González-Vicent M, Lassaletta A, Cuenca-Estrella M, Díaz MA, Riesco S, Madero L. Increasing incidence of invasive aspergillosis in pediatric hematology oncology patients over the last decade: a retrospective single centre study. J Pediatr Hematol Oncol. 2009;31:642–646. doi: 10.1097/MPH.0b013e3181acd956. [DOI] [PubMed] [Google Scholar]
- 2.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, Clancy CJ, Wingard JR, Lockhart SR, Groll AH, Sorrell TC, Bassetti M, Akan H, Alexander BD, Andes D, Azoulay E, Bialek R, Bradsher RW, Bretagne S, Calandra T, Caliendo AM, Castagnola E, Cruciani M, Cuenca-Estrella M, Decker CF, Desai SR, Fisher B, Harrison T, Heussel CP, Jensen HE, Kibbler CC, Kontoyiannis DP, Kullberg BJ, Lagrou K, Lamoth F, Lehrnbecher T, Loeffler J, Lortholary O, Maertens J, Marchetti O, Marr KA, Masur H, Meis JF, Morrisey CO, Nucci M, Ostrosky-Zeichner L, Pagano L, Patterson TF, Perfect JR, Racil Z, Roilides E, Ruhnke M, Prokop CS, Shoham S, Slavin MA, Stevens DA, Thompson GR, Vazquez JA, Viscoli C, Walsh TJ, Warris A, Wheat LJ, White PL, Zaoutis TE, Pappas PG. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71:1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acet-Öztürk NA, Ömer-Topçu D, Vurat-Acar K, Aydın-Güçlü Ö, Pınar İE, Demirdöğen E, Görek-Dilektaşlı A, Kazak E, Özkocaman V, Ursavas A, Akalın H, Özkalemkaş F, Ener B, Ali R. Impact of revised EORTC/MSGERC 2020 criteria on diagnosis and prognosis of invasive pulmonary aspergillosis in patients with hematological malignancies undergoing bronchoscopy. J Mycol Med. 2022;32:101304. doi: 10.1016/j.mycmed.2022.101304. [DOI] [PubMed] [Google Scholar]
- 5.Siopi M, Karakatsanis S, Roumpakis C, Korantanis K, Sambatakou H, Sipsas NV, Tsirigotis P, Pagoni M, Meletiadis J. A prospective multicenter cohort surveillance study of invasive aspergillosis in patients with hematologic malignancies in Greece: impact of the revised EORTC/MSGERC 2020 Criteria. J Fungi (Basel) 2021;7:27. doi: 10.3390/jof7010027. [DOI] [PMC free article] [PubMed] [Google Scholar]


