Abstract
Objective:
This study aimed to evaluate patients with relapsed/refractory multiple myeloma (RRMM) who underwent daratumumab (DARA) therapy.
Materials and Methods:
This multicenter retrospective study included 134 patients who underwent at least two courses of DARA from February 1, 2018, to April 15, 2022. Epidemiological, disease, and treatment characteristics of patients and treatment-related side effects were evaluated. Survival analysis was performed.
Results:
The median age at the start of DARA was 60 (range: 35-88), with 56 patients (41.8%) being female and 48 (58.2%) being male. The median time to initiation of DARA and the median follow-up time were 41.2 (5.1-223) and 5.7 (2.1-24.1) months, respectively. The overall response rate after DARA therapy was 75 (55.9%), and very good partial response or better was observed in 48 (35.8%) patients. Overall survival (OS) and progression-free survival (PFS) for all patients were 11.6 (7.8-15.5) and 8.0 (5.1-10.9) months, respectively. OS was higher for patients undergoing treatment with DARA and bortezomib-dexamethasone (DARA-Vd) compared to those undergoing treatment with DARA and lenalidomide-dexamethasone (DARA-Rd) (16.9 vs. 8.3 months; p=0.014). Among patients undergoing DARA-Rd, PFS was higher in those without extramedullary disease compared to those with extramedullary disease (not achieved vs. 3.7 months; odds ratio: 3.4; p<0.001). The median number of prior therapies was 3 (1-8). Initiation of DARA therapy in the early period provided an advantage for OS and PFS, although it was statistically insignificant. Infusion-related reactions were observed in 18 (13.4%) patients. All reactions occurred during the first infusion and most reactions were of grade 1 or 2 (94.5%). The frequency of neutropenia and thrombocytopenia was higher in the DARA-Rd group (61.9% vs. 24.7%, p<0.001 and 42.9% vs. 15.7%, p<0.001).
Conclusion:
Our study provides real-life data in terms of DARA therapy for patients with RRMM and supports the early initiation of DARA therapy.
Keywords: Daratumumab, Relapsed/refractory multiple myeloma, Real-world data
Abstract
Amaç:
Bu çalışmanın amacı, relaps/refrakter multipl myelom (RRMM) tanısı ile daratumumab (DARA) kullanan hastaların değerlendirilmesidir.
Gereç ve Yöntemler:
Çalışma, çok merkezli ve retrospektif olarak tasarlandı. 01.02.2018-15.04.2022 tarifleri arasında en az iki kür DARA kullanmış olan 134 hasta çalışmaya dahil edildi. Hastaların epidemiyolojik, hastalık ve tedavi ile ilişkili özellikleri ve tedavi ilişkili yan etkileri değerlendirildi. Sağ kalım analizleri yapıldı.
Bulgular:
DARA tedavisine başlama yaşının ortancası 60 (35-88) olup, hastaların 56’sı (%41,8) kadın ve 48’i (%58,2) erkekti. DARA tedavisine başlama ve takip sürelerinin ortanca değerleri sırasıyla 41,2 (5,1-223) ve 5,7 (2,1-24,1) aydı. DARA tedavisi sonrası genel yanıt oranı hastaların 75’inde (%55,9) ve çok iyi kısmi yanıt veya daha iyisi hastaların 48’inde (%35,8) gözlendi. Tüm hastalar için genel sağkalım (OS) ve progresyonsuz sağkalım (PFS) sırasıyla 11,6 (7,8-15,5) ve 8,0 (5,1-10,9) aydı. DARA ve bortezomib-deksametazon (DARA-Vd) ile tedavi gören hastalarda OS, DARA ve lenalidomid-deksametazon (DARA-Rd) ile tedavi görenlere göre daha yüksek bulundu (sırasıyla 16,9 ve 8,3 ay; p=0,014). DARA-Rd tedavisi gören hastalar arasında, ekstramedüller hastalığı olmayanlarda PFS, ekstramedüller hastalığı olanlara göre daha yüksekti (NA’ya karşılık 3,7 ay; OR: 3,4; p<0,001). Önceki tedavilerin ortanca sayısı 3 (1-8) idi. DARA tedavisine erken dönemde başlamanın OS ve PFS için bir avantaj sağladığı, ancak istatistiksel olarak anlamlı olmadığı görüldü. İnfüzyonla ilişkili reaksiyonlar 18 (%13,4) hastada gözlendi. Tüm reaksiyonlar ilk infüzyon sırasında meydana geldi ve reaksiyonların çoğu 1 veya 2. derecedeydi (%94,5). Nötropeni ve trombositopeni sıklığı DARA-Rd grubunda daha yüksekti (%61,9’a karşı %24,7, p<0,001 ve %42,9’a karşı %15,7, p<0,001).
Sonuç:
Çalışmamız, RRMM hastalarında DARA kullanımıyla ilişkin gerek yaşam verisi niteliğini taşımaktadır ve DARA’nın erken dönemde kullanılmasını destekler niteliktedir.
Introduction
Multiple myeloma (MM) is a relatively rare type of cancer, accounting for approximately 2% of all malignancies and 10% of all hematologic malignancies. The median age of patients with MM is >65 years, and it occurs slightly more often among men than women [1]. Even though decent response and survival rates have been achieved with the usage of proteasome inhibitors (PIs) and immunomodulatory drugs (IMiDs) together with the usage of autologous stem cell transplantation (ASCT) as a mainstay of treatment, MM continues to be considered a relapsing and incurable disease [2]. Over the last 20 years of malignancy treatment, monoclonal antibodies have been developed as target-specific treatments and promising results have been observed in cases of lymphoma and solid organ cancers [3]. In recent years, there have been studies of monoclonal antibodies in the treatment of MM, as well. Daratumumab (DARA) was the first monoclonal antibody developed against CD38 expressed on the plasma cell surface. DARA has antibody- and complement-mediated cytotoxic effects via CD38 and antibody-dependent phagocytotic effects [4]. It has also been reported to cause the elimination of immunosuppressor cells carrying CD38, thus exerting an immunomodulatory effect [5]. In patients with relapsed/refractory MM (RRMM), 3-year overall survival (OS) with DARA monotherapy is 36.5% and median progression-free survival (PFS) is 15 months [6]. In prospective comparative studies, survival and response rates were also in favor of patients who underwent DARA treatment. The addition of DARA to the bortezomib-dexamethasone (Vd) combination increased PFS from 7.2 to 16.7 months [7]. Compared to the lenalidomide-dexamethasone (Rd) combination alone, the PFS of the patients in the DARA-Rd arm was also significantly higher at 45 months versus 17.5 months. Overall response rates were respectively 83% and 93% for DARA-Vd and DARA-Rd [8].
In this study, we report the clinical outcomes of RRMM patients treated with DARA-based therapy.
Materials and Methods
This study was a multicenter retrospective study including 11 centers and their patients with RRMM who had received at least two cycles of DARA-based chemotherapy from February 1, 2018, to April 15, 2022. Epidemiological characteristics (age and gender), disease-related characteristics [stage, MM subtype, genetic characteristics, risk group, presence of lytic lesions, and extramedullary disease (EMD) status], number of previous treatments, duration of DARA treatment, best response achieved with DARA, hematological and non-hematological side effects, progression, and survival status were recorded. For response assessment, the International Myeloma Working Group Response Criteria were used in all centers [2]. Adverse effects were evaluated as per the Common Terminology Criteria for Adverse Events (CTCAE, version 5). This study was approved by the Necmettin Erbakan University Meram Faculty of Medicine’s Drugs and Non-Medical Devices Research Ethics Committee with approval number 2022/3761.
Since most patients received DARA-Rd or DARA-Vd, comparisons were performed between those groups. For DARA-Rd treatment, DARA was administered at 16 mg/kg on days 1, 8, 15, and 22 for 8 weeks during cycles 1 and 2; once every 2 weeks during cycles 3-6; and every four weeks thereafter. Lenalidomide was administered at 25 mg/day on days 1-21 of each cycle and dexamethasone was administered at 40 mg weekly. For DARA-Vd treatment, DARA was administered at 16 mg/kg on days 1, 8, and 15 during cycles 1-3; once every 3 weeks on day 1 during cycles 4-8; and once every 4 weeks until progression. Bortezomib was administered at 1.3 mg/m2 on days 1, 4, 8, and 11 during cycles 1-8 while dexamethasone was administered at 20 mg/day on days 1, 2, 4, 5, 8, 9, 11, and 12 during cycles 1-8.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 23. The distribution of continuous numerical variables was analyzed with the Kolmogorov-Smirnov test. Descriptive variables were presented as mean ± standard deviation or median (minimum-maximum) according to the distribution analysis. Categorical variables were presented as percentages and compared using the chi-square test. Survival was evaluated with the Kaplan-Meier method and comparisons were performed using the log-rank test. Values of p<0.05 were considered statistically significant.
Results
A total of 134 patients were included in this study. The median patient age was 60 (35-88) years and 78 (58.2%) of the patients were men.
Epidemiological and disease-related basic clinical features are presented in Table 1. The median time from diagnosis to initiation of DARA was 41.2 (5.1-223) months, and the median follow-up time after DARA was 5.7 (2.1-24.1) months. The median number of previous therapy lines was 3 (1-8). For 51 (38.1%) patients, DARA was initiated with ≥4 lines. In the analysis of the DARA protocols, 89 (66.4%) of the patients had received DARA-Vd, 42 (31.3%) had received DARA-Rd, and 3 (2.2%) had been treated with other protocols (2 patients: DARA monotherapy; 1 patient: DARA-VRD). The best response to DARA therapy was achieved after a median of three cycles, and very good partial response or better (≥VGPR) was achieved by 48 (35.8%) patients. Treatment-related data are presented in Table 2.
Table 1. Epidemiological and baseline clinical characteristics (n=134).
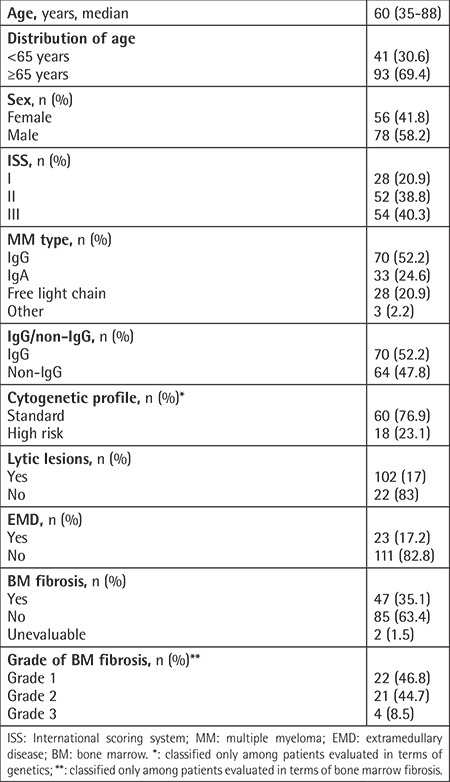
Table 2. Previous therapy and daratumumab-related features (n=134).
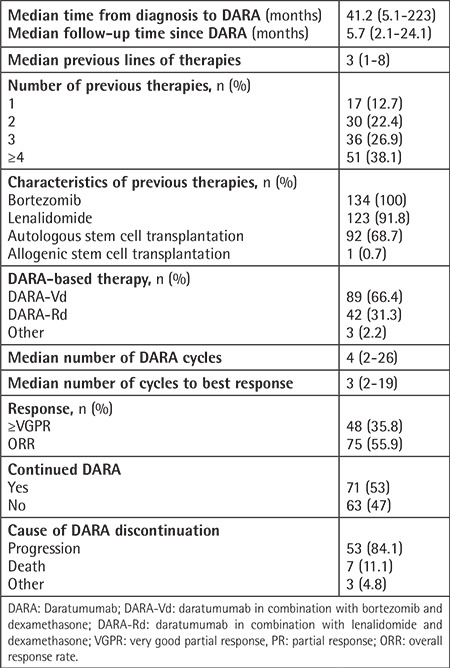
The results of genetic analysis were available for 78 (58.2%) patients, 51 (65.3%) of whom had a normal karyotype. The most common genetic features were del13q (5%) and 1q gain (5%).
Survival Analysis
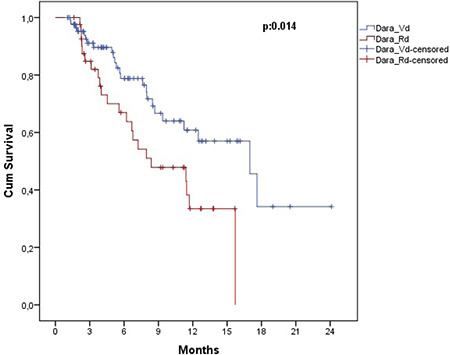
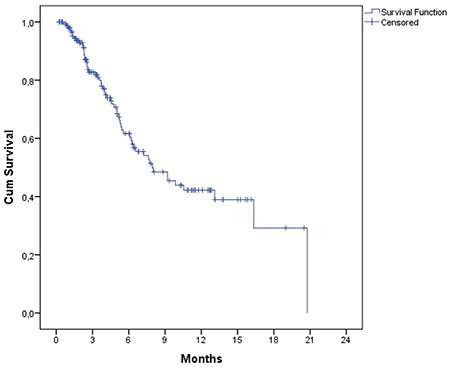
The estimated OS and PFS were 11.6 (7.8-15.5) months and 8.0 (5.1-10.9) months, respectively (Figures 1 and 2). Table 3 presents the survival outcomes of all patients based on clinical and epidemiological characteristics. The OS of the patients who received DARA-Vd was significantly higher than that of those who received DARA-Rd (16.9 vs. 8.3 months; p=0.014) (Figure 3). The patients who received DARA-Vd and DARA-Rd and the subgroup analysis of survival outcomes are presented in Tables 4 and 5. Among the patients who received DARA-Rd, PFS was higher among those without EMD than those with EMD (not achieved vs. 3.7 months; odds ratio: 3.4; p<0.001) (Figure 4).
Figure 1.
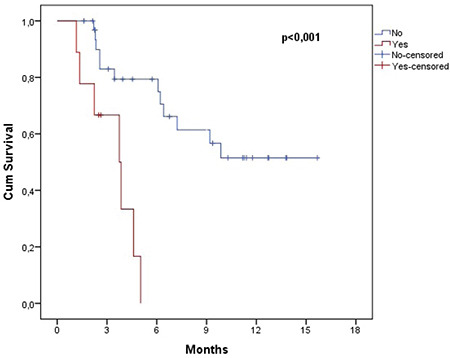
Overall survival of all patients.
Figure 2.
Progression-free survival of all patients.
Table 3. Overall and progression-free survival in subgroups for all patients (n=134).
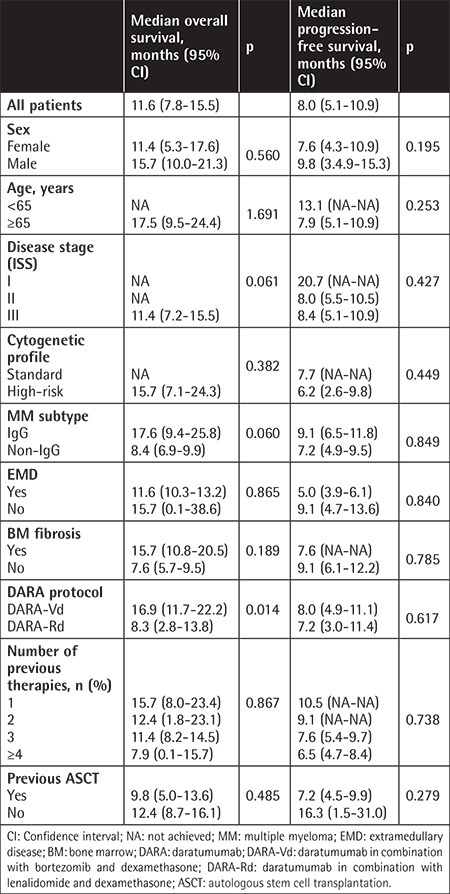
Figure 3.
Overall survival of patients receiving DARA-Vd versus DARA-Rd.
DARA-Vd: Daratumumab in combination with bortezomib and dexamethasone; DARA-Rd: daratumumab in combination with lenalidomide and dexamethasone.
Table 4. Overall and progression-free survival in subgroups for DARA-Vd (n=89).
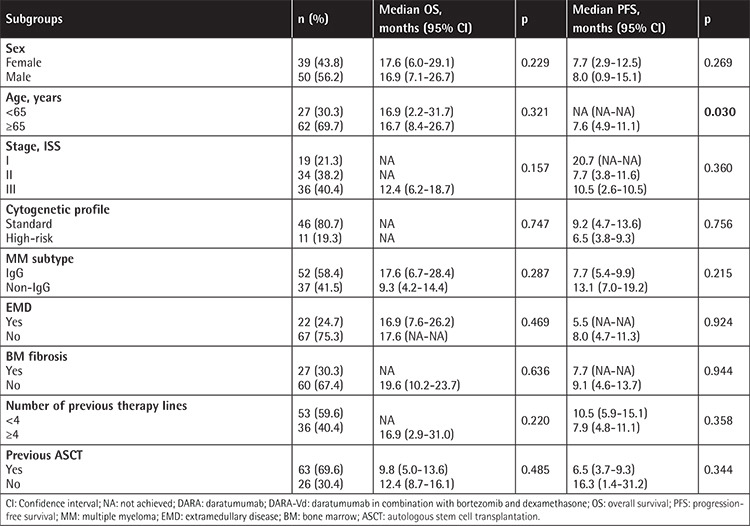
Table 5. Overall and progression-free survival in subgroups for DARA-Rd (n=42).
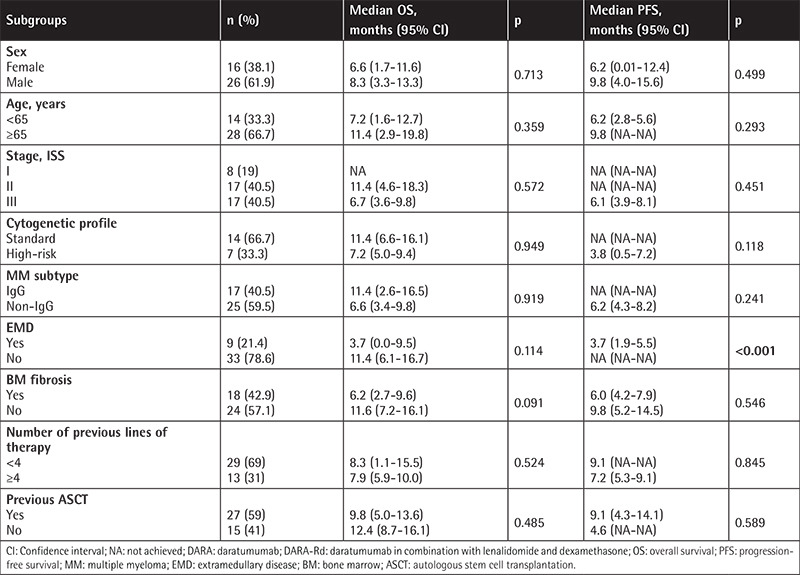
Figure 4.
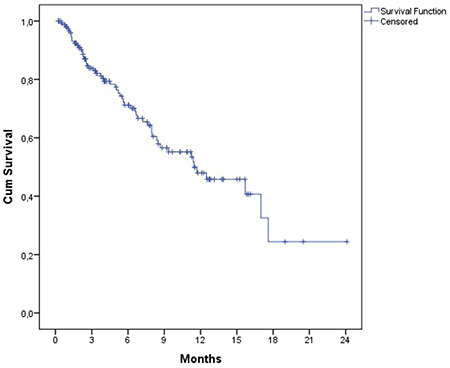
Progression-free survival of DARA-Rd patients with (+) and without (-) extramedullary disease.
DARA-Rd: Daratumumab in combination with lenalidomide and dexamethasone.
For 63 (47%) of the patients, DARA therapy was discontinued, as detailed in Table 2. Reasons other than death or progression were present for two patients who achieved VGPR and were switched to autologous stem cell transplantation and one patient who voluntarily discontinued treatment.
Assessment of Adverse Effects
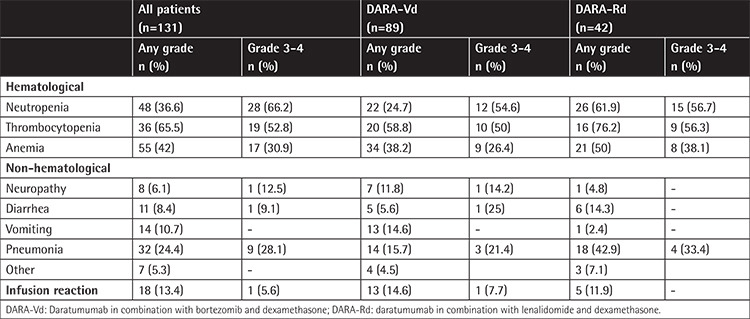
The assessment of adverse effects in patients who received DARA-Vd and DARA-Rd is presented in Table 6. Infusion-related reactions (IRRs) were noted in 18 (13.4%) patients, and all IRRs were reported during the first cycle of therapy. No patients experienced reactions in later cycles. Reactions of grade 1, 2, and 3 were observed in 14 (77.8%), 3 (16.7%), and 1 (5.6%) of the patients, respectively. The frequency of IRRs was similar in the DARA-Vd and DARA-Rd treatment groups (13.4% vs. 11.9%, p=0.675). However, the frequencies of neutropenia and pneumonia were significantly higher in the DARA-Rd group than the DARA-Vd group (neutropenia: 61.9% vs. 24.7%, odds ratio: 4.9; 95% CI: 2.25-10.87, p<0.001; pneumonia: 42.9% vs. 15.7%, odds ratio: 4.0, 95% CI: 1.74-9.27, p=0.001). Although not statistically significant, the DARA-Rd group also had more cases of thrombocytopenia at 58.8% compared to 76.2% in the DARA-Vd group (p=0.188).
Table 6. Adverse events.
Discussion
DARA has been commercially available in Türkiye since December 29, 2017. In May 2020, the Social Security Institution authorized the use of DARA as monotherapy or in combination with Vd or Rd for patients who have received at least one treatment cycle with a PI and IMiD. The use of DARA as monotherapy or in combination at an earlier stage is possible with special permission from the Social Security Institution. Considering this standard practice in our country, our study can be considered as falling within the scope of real-world data as it is a multicenter and retrospective study.
Retrospective data on DARA therapy primarily reflect patients who have received DARA monotherapy. Among such studies, the case series reported by the GIMEMA Lazio group included 68 patients, a Hungarian study included 48 patients, and early access program results from Türkiye included 42 patients [9,10,11]. The most extensive study to date involving real-world data was performed by the Canadian Myeloma Research Data Group and included data on patients undergoing DARA monotherapy and DARA-pomalidomide-dexamethasone (DARA-Pd) combination therapy [12]. In our study, patients receiving DARA-Pd were not reported and the number of patients receiving DARA monotherapy and DARA-VRD was two and one, respectively.
The initial studies on DARA combination therapy for patients with RRMM were the CASTOR (DARA-Vd) and POLLUX (DARA-Rd) studies. The epidemiological characteristics of the patients in our study are similar to those in these foundational studies. In the CASTOR and POLLUX studies, PFS was 16.7 and 45 months, respectively [7,8]. In a study of 47 patients who received DARA-Vd, Harvanová et al. [13] reported that PFS was 10 months with a median follow-up time of 8 months. Szabo et al. [14] found that the time to transition to a new therapy was 16.1 months for DARA when combined with different IMiDs; that result was better compared to combination therapy with PIs and monotherapy. In our study, PFS was inferior for all patients and combination groups compared to earlier studies. In contrast, OS was notably longer for patients treated with DARA-Vd compared to DARA-Rd. Additionally, the PFS in the DARA-Vd group was better in patients aged <65 years of age compared to those aged ≥65 years, in contrast to the CASTOR study [7]. In our study, the median follow-up time for DARA was 5.7 (2.1-24.1) months, shorter than in other retrospective studies. Compared to other combination studies, this shorter duration undermines the strength of our study’s survival results. In contrast, in a study evaluating 42 RRMM patients who received DARA monotherapy in Türkiye, the median follow-up duration was 6 months, similar to the present study. That study included patients who received at least 3 lines of treatment; the median number of previous therapy lines was 5.5 and the median PFS was 5.5 months. The fact that the median PFS was higher in our study supports the claim that combination therapies could be superior to monotherapy even if they are administered as earlier lines of therapy [11].
The rates of patients with ≥VGPR in the CASTOR and POLLUX studies were 59.2% and 75.8%, respectively. In our study, the ≥VGPR rate was lower (35.8%) than those previously reported; however, it was similar to rates reported in other retrospective studies [13,14]. The median number of previous therapy lines was 1 (1-11) in the POLLUX study and 2 (1-9) in the CASTOR study. These studies highlighted the fact that DARA is more effective when used earlier on [7,8]. In a retrospective Canadian study, the median PFS for patients with previous numbers of therapy lines of 1, 2, and ≥3 was 23.5, 12.8, and 7.0 months, respectively. The decrease in survival rate with lines of therapy was statistically significant [12]. In the study conducted by Harvanová et al. [13], PFS was longer in patients who received 2 lines of previous therapy compared to ≥2 lines of therapy. In a more recent retrospective analysis of 30 patients who received DARA-Rd and 4 patients who received DARA-Vd, the overall response rate and VGPR rate were 88% and 44%, respectively. At a median follow-up of 16 months, the 12-month PFS and OS rates were 78% and 86.5%, respectively. In this study, 85% of patients received DARA as second-line therapy [15]. In our study, the median number of previous therapy lines was 3 (1-8) and over 50% of patients received DARA with ≥4 lines of therapy. Although statistically insignificant, it was observed that as the number of previous therapy lines increased, survival rates decreased. In this regard, our results reinforce the finding that earlier DARA therapy could be more beneficial.
Cytogenetic characteristics influence prognosis and response rates in RRMM. The rate of patients with high cytogenetic risk in our study was consistent with rates reported in the literature [1]. In a study by Mohan et al. [16] that focused on patients with RRMM involving 1q gain, PFS and OS were significantly inferior compared to patients with standard risk. When combinations were compared, the time to a new therapy was longer in patients with DARA and IMiD combinations than the combination of DARA and PIs [14]. Conversely, a meta-analysis evaluated DARA combinations as first-line treatment and found no survival advantage in patients with high cytogenetic risk [17]. In our study, the results of genetic analysis were only available for 58.2% of the patients. No significant survival difference was found between the cytogenetic risk groups. However, although it was not statistically significant, survival rates were inferior among high-risk patients for both combinations.
The incidence of EMD during the course of MM ranges from 24% to 37% [18]. However, data on DARA in EMD is limited. The overall response rate (57.7%) and PFS (7.8 months) obtained in a previous study that included 188 patients who received DARA-Rd were inferior among patients with EMD compared to those without [19]. In patients with EMD, several combinations of DARA have been used. In a phase II study by Byun et al. [20], DARA was added to DCEP (dexamethasone, cyclophosphamide, etoposide, and cisplatin) for patients with RRMM and EMD. At a median follow-up of 11 months, the median PFS and OS were found to be 5 months and 10 months, respectively. Beksac et al. [21] evaluated treatment with DARA-VCD (DARA with bortezomib, cyclophosphamide, and dexamethasone) in 27 newly diagnosed and 11 first-relapse MM patients presenting with EMD. They considered the DARA-VCD regime to be effective with 75% of patients achieving ≥VGPR and 18 months of PFS. In our study, all patients had RRMM and none received DARA-VCD, DARA-DCEP, or any other combinations. Furthermore, 17% of the patients had EMD either at the time of diagnosis or during follow-up. The OS and PFS of patients with EMD were inferior, as also seen in the literature. Moreover, PFS was significantly inferior in patients with EMD in the DARA-Rd group (3.7 months vs. not achieved).
The incidence of bone marrow fibrosis (BMF) in MM at diagnosis is approximately 20%-25% [22,23]. Although the frequency of EMD is higher in fibrotic patients, the association of fibrosis with the genetic profile and prognosis remains unclear. Furthermore, fibrosis does not influence the response to PI and IMiD therapies [24,25]. The fibrosis rate in the present study was approximately 35%, which is higher than rates previously reported in the literature. Unlike other studies, we also examined the relationship between BMF and response to DARA-based therapy. Twenty-seven patients (30.3%) had BMF at diagnosis, and the response rates and survival were similar between patients with and without BMF.
The frequency of IRRs was reported to be approximately 45% in previous prospective studies [7,8]. However, in retrospective studies, it varied between 28% and 57%. In both prospective and retrospective studies, >90% of IRRs were reported during the first cycle of therapy, and the frequency of grade 3-4 responses was <10% [7,8,11]. In our study, the frequency of all grades of IRR was found to be lower than that reported in the literature. We believe the primary reason for this is the unavailability of records on IRRs. The IRR rate was similar between the DARA-Rd and DARA-Vd therapy groups; however, the frequency of neutropenia and related pneumonia was higher in the DARA-Rd group, which is consistent with findings reported in the literature.
Conclusion
The main limitations of our study include a short median follow-up time compared to the literature and insufficient documentation of adverse events, especially for IRRs. Nevertheless, considering the conditions for DARA usage in Türkiye, this study has provided real-world data by showcasing the effects of DARA therapy on patients with RRMM in terms of the counts of centers and cases.
Footnotes
Ethics
Ethics Committee Approval: This study was approved by the Necmettin Erbakan University Meram Faculty of Medicine’s Drugs and Non-Medical Devices Research Ethics Committee with approval number 2022/3761.
Informed Consent: Retrospective study.
Authorship Contributions
Surgical and Medical Practices: A.T., A.G., A.A., E.T.D., V.G., M.A.A., M.B., S.Ç., F.K.K., A.O.U., A.D., S.D., H.A.E., C.A., F.Ö., Ş.E., M.D., M.S.D., G.S., M.M., C.U., Ö.Ç.; Concept: A.T.; Design: A.T., S.D., Ö.Ç.; Data Collection or Processing: A.T., A.G., A.A., E.T.D., V.G., M.A.A., M.B., S.Ç., F.K.K., A.O.U., A.D., S.D., H.A.E., C.A., F.Ö., Ş.E., M.D., M.S.D., G.S., M.M., C.U.; Analysis or Interpretation: A.T., S.D.; Literature Search: A.T., A.A., F.K.K., C.A.; Writing: A.T.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95:548–567. doi: 10.1002/ajh.25791. [DOI] [PubMed] [Google Scholar]
- 2.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, Munshi N, Lonial S, Bladé J, Mateos MV, Dimopoulos M, Kastritis E, Boccadoro M, Orlowski R, Goldschmidt H, Spencer A, Hou J, Chng WJ, Usmani SZ, Zamagni E, Shimizu K, Jagannath S, Johnsen HE, Terpos E, Reiman A, Kyle RA, Sonneveld P, Richardson PG, McCarthy P, Ludwig H, Chen W, Cavo M, Harousseau JL, Lentzsch S, Hillengass J, Palumbo A, Orfao A, Rajkumar SV, Miguel JS, Avet-Loiseau H. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, Vanderplas A, Zelenetz AD, Abel GA, Rodriguez MA, Nademanee A, Kaminski MS, Czuczman MS, Millenson M, Niland J, Gascoyne RD, Connors JM, Friedberg JW, Winter JN. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123:837–842. doi: 10.1182/blood-2013-09-524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morandi F, Horenstein AL, Costa F, Giuliani N, Pistoia V, Malavasi F. CD38: A target for immunotherapeutic approaches in multiple myeloma. Front Immunol. 2018;9:2722. doi: 10.3389/fimmu.2018.02722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overdijk MB, Jansen JH, Nederend M, Lammerts van Bueren JJ, Groen RW, Parren PW, Leusen JH, Boross P. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor-mediated cross-linking. J Immunol. 2016;197:807–813. doi: 10.4049/jimmunol.1501351. [DOI] [PubMed] [Google Scholar]
- 6.Usmani SZ, Nahi H, Plesner T, Weiss BM, Bahlis NJ, Belch A, Voorhees PM, Laubach JP, van de Donk NWCJ, Ahmadi T, Uhlar CM, Wang J, Feng H, Qi M, Richardson PG, Lonial S. Daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma: final results from the phase 2 GEN501 and SIRIUS trials. Lancet Haematol. 2020;7:e447–e455. doi: 10.1016/S2352-3026(20)30081-8. [DOI] [PubMed] [Google Scholar]
- 7.Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, Spicka I, Hungria V, Munder M, Mateos MV, Mark TM, Qi M, Schecter J, Amin H, Qin X, Deraedt W, Ahmadi T, Spencer A, Sonneveld P; CASTOR Investigators. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754–766. doi: 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 8.Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, Rabin N, Orlowski RZ, Komarnicki M, Suzuki K, Plesner T, Yoon SS, Ben Yehuda D, Richardson PG, Goldschmidt H, Reece D, Lisby S, Khokhar NZ, O’Rourke L, Chiu C, Qin X, Guckert M, Ahmadi T, Moreau P; POLLUX Investigators. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–1331. doi: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 9.Vozella F, Siniscalchi A, Rizzo M, Za T, Antolino G, Coppetelli U, Piciocchi A, Andriani A, Annibali O, De Rosa L, Cimino G, La Verde G, De Stefano V, Cantonetti M, di Toritto TC, Petrucci MT. Daratumumab in multiple myeloma: experience of the multiple myeloma GIMEMA Lazio group. Ann Hematol. 2021;100:1059–1063. doi: 10.1007/s00277-020-04374-y. [DOI] [PubMed] [Google Scholar]
- 10.Lovas S, Varga G, Farkas P, Masszi T, Wohner N, Bereczki Á, Adamkovich N, Borbényi Z, Szomor Á, Alizadeh H, Szaleczky E, Wolf K, Schneider T, Plander M, Szendrei T, Csacsovszki O, Csukly Z, Rajnics P, Egyed M, Nagy Z, Rejtő L, Illés Á, Mikala G, Váróczy L. Real-world data on the efficacy and safety of daratumumab treatment in Hungarian relapsed/refractory multiple myeloma patients. Int J Hematol. 2019;110:559–565. doi: 10.1007/s12185-019-02715-w. [DOI] [PubMed] [Google Scholar]
- 11.Beksac M, Aydin Y, Goker H, Turgut M, Besisik SK, Cagirgan S, Tuglular T, Vural F, Yagci M, Alacacioglu I, Aytan P, Goksoy HS, Gulbas Z, Gunes AK, Gurkan E, Hacioglu SK, Karti SS, Kaynar L, Ozdogu H, Paydas S, Solmaz S, Sonmez M, Tekgunduz E, Yildirim R, Ilhan O. Early access program results from Turkey and a literature review on daratumumab monotherapy among heavily pretreated patients with relapsed/refractory myeloma. Clin Lymphoma Myeloma Leuk. 2020;20:e474–e484. doi: 10.1016/j.clml.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 12.LeBlanc R, Mian H, Reece D, Masih-Khan E, Kardjadj M, Jimenez-Zepeda VH, McCurdy A, Song K, Sebag M, Louzada M, White D, Stakiw J, Kotb R, Reiman A, Aslam M, Gul E, Venner CP. Outcomes of daratumumab in the treatment of multiple myeloma: a retrospective cohort study from the Canadian Myeloma Research Group Database. Br J Haematol. 2022;198:93–102. doi: 10.1111/bjh.18172. [DOI] [PubMed] [Google Scholar]
- 13.Harvanová Ľ, Štulajterová V, Guman T, Ladická M, Hlebašková M, Chudej J, Šimek M, Štecová N, Flochová E, Kubala J, Simančíková I, Drgoňa Ľ, Vranovský A, Wild A, Mistrík M, Bátorová A. Real-world effectiveness and safety of daratumumab, bortezomib, and dexamethasone in relapsed/refractory multiple myeloma in Slovakia. Neoplasma. 2021;68:626–630. doi: 10.4149/neo_2021_201113N1223. [DOI] [PubMed] [Google Scholar]
- 14.Szabo AG, Klausen TW, Levring MB, Preiss B, Helleberg C, Breinholt MF, Hermansen E, Gjerdrum LMR, Bønløkke ST, Nielsen K, Kjeldsen E, Iversen KF, Teodorescu EM, Dokhi M, Kurt E, Strandholdt C, Andersen MK, Vangsted AJ. The real-world outcomes of multiple myeloma patients treated with daratumumab. PLoS One. 2021;16:e0258487. doi: 10.1371/journal.pone.0258487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fucci L, Gensini L, Coppetelli U, La Barbera EO, Gentile M, Fiori L, Perrone S, Cimino G. Daratumumab triplet therapies in patients with relapsed or refractory multiple myeloma: a “real world” experience. Leuk Res Rep. 2022;17:100330. doi: 10.1016/j.lrr.2022.100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohan M, Weinhold N, Schinke C, Thanedrarajan S, Rasche L, Sawyer JR, Tian E, van Rhee F, Zangari M. Daratumumab in high-risk relapsed/refractory multiple myeloma patients: adverse effect of chromosome 1q21 gain/amplification and GEP70 status on outcome. Br J Haematol. 2020;189:67–71. doi: 10.1111/bjh.16292. [DOI] [PubMed] [Google Scholar]
- 17.Premkumar V, Pan S, Lentzsch S, Bhutani D. Use of daratumumab in high risk multiple myeloma: a meta‐analysis. eJHaem. 2020;1:267–271. doi: 10.1002/jha2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhutani M, Foureau DM, Atrash S, Voorhees PM, Usmani SZ. Extramedullary multiple myeloma. Leukemia. 2020;34:1–20. doi: 10.1038/s41375-019-0660-0. [DOI] [PubMed] [Google Scholar]
- 19.Jelinek T, Sevcikova T, Zihala D, Popkova T, Kapustova V, Broskevicova L, Capkova L, Rihova L, Bezdekova R, Sevcikova S, Zidlik V, Havel M, Plonkova H, Jungova A, Minarik J, Stork M, Pour L, Pavlicek P, Spicka I, Maisnar V, Radocha J, Simicek M, Hajek R. Limited efficacy of daratumumab in multiple myeloma with extramedullary disease. Leukemia. 2022;36:288–291. doi: 10.1038/s41375-021-01343-w. [DOI] [PubMed] [Google Scholar]
- 20.Byun JM, Min CK, Kim K, Bang SM, Lee JJ, Kim JS, Yoon SS, Koh Y. Phase II trial of daratumumab with DCEP in relapsed/refractory multiple myeloma patients with extramedullary disease. J Hematol Oncol. 2022;15:150. doi: 10.1186/s13045-022-01374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beksac M, Tuglular T, Gay F, Mina R, Katodritou E, Unal A, Cavo M, Ozsan GH, van der Velden VHJ, Beverloo B, Vermeulen M, van Duin M, Cengiz G, Sevindik OG, Merante S, Manousou K, Sonneveld P, Terpos E. Efficacy of daratumumab combined with bortezomib, cyclophosphamide and dexamethasone for the treatment of multiple myeloma patients presenting with extramedullary disease: a European Myeloma Network Study (EMN19) Blood. 2022;140(Suppl 1):10177–10178. [Google Scholar]
- 22.Subramanian R, Basu D, Dutta TK. Significance of bone marrow fibrosis in multiple myeloma. Pathology. 2007;39:512–515. doi: 10.1080/00313020701570038. [DOI] [PubMed] [Google Scholar]
- 23.Sidhu G, Latif R, Liu J, Axiotis C, Khillan R, Braverman AS. Incidence of bone marrow (BM) fibrosis in multiple myeloma (MM) patients and its relationship to cytogenetic (CG) abnormalities in a minority population. Blood. 2011;118:5077. [Google Scholar]
- 24.Koshiishi M, Kawashima I, Hyuga H, Nakadate A, Matsuura M, Hosokawa E, Sakamoto Y, Suzuki J, Suzuki M, Kumagai T, Yamamoto T, Nakajima K, Tanaka M, Kirito K. Presence of bone marrow fibrosis in multiple myeloma may predict extramedullary disease. Int J Hematol. 2022;116:544–552. doi: 10.1007/s12185-022-03373-1. [DOI] [PubMed] [Google Scholar]
- 25.Geirnaert M, Howarth J, Martin K, Ricard C, Streilein S, Wasney D, Dao V, Kotb R, Rimmer E, Minuk L. A multicenter review of infusion-related reactions to daratumumab for relapsed multiple myeloma in the real world setting. J Oncol Pharm Pract. 2021;27:907–910. doi: 10.1177/1078155220967738. [DOI] [PubMed] [Google Scholar]


