Abstract
BACKGROUND
There is no consensus on the usage of extended criteria donor (ECD) grafts in liver transplantation (LT) for acute-on-chronic liver failure (ACLF) patients.
AIM
To summarize the experience of using ECD livers in ACLF-LT.
METHODS
A retrospective cohort study was conducted, enrolling patients who underwent LT at the First Affiliated Hospital of Sun Yat-Sen University from January 2015 to November 2021. The patients were divided into ECD and non-ECD groups for analysis.
RESULTS
A total of 145 recipients were enrolled in this study, of which ECD and non-ECD recipients accounted for 53.8% and 46.2%, respectively. Donation after cardiac death (DCD) recipients accounted for the minority compared with donation after brain death (DBD) recipients (16.6% vs 83.4%). Neither overall survival nor graft survival significantly differed between ECD and non-ECD and DCD and DBD recipients. ECD grafts were associated with a significantly higher incidence of early allograft dysfunction (EAD) than non-ECD grafts (67.9% vs 41.8%, P = 0.002). Postoperative outcomes between DCD and DBD recipients were comparable (P > 0.05). ECD graft (P = 0.009), anhepatic phase (P = 0.034) and recipient gamma glutamyltransferase (P = 0.016) were independent risk factors for EAD. Recipient preoperative number of extrahepatic organ failures > 2 (P = 0.015) and intraoperative blood loss (P = 0.000) were independent predictors of poor post-LT survival.
CONCLUSION
Although related to a higher risk of EAD, ECD grafts can be safely used in ACLF-LT. The main factors affecting post-LT survival in ACLF patients are their own severe preoperative disease and intraoperative blood loss.
Keywords: Extended criteria donor, Acute-on-chronic liver failure, Liver transplantation
Core Tip: This manuscript is intended to summarize a Chinese single center experience of using extended criteria donor (ECD) grafts in liver transplantation (LT) for acute-on-chronic liver failure (ACLF) patients. In this paper, we found that under ECD grafts are associated with a higher risk of early allograft dysfunction than non-ECD grafts but can be safely used in ACLF recipients as they do not affect post-LT survival. The main factors affecting the prognosis of ACLF recipients are the severity of their own preoperative disease and intraoperative blood loss.
INTRODUCTION
Acute-on-chronic liver failure (ACLF) is a complex clinical syndrome characterized by the failure of extrahepatic organ(s) that has an extremely high short-term mortality and a 90-d transplant-free mortality above 50%[1]. Liver transplantation (LT) is the only curative treatment option for various end-stage liver diseases and has been reported to bring strong survival benefits to ACLF patients[2]. However, the current supply of acceptable donor livers is far from sufficient to meet the demands of the growing number of recipients. In an effort to reduce waiting list mortality, extended criteria donor (ECD) livers, also known as marginal livers, are increasingly being used in LT[3,4].
Usually, ECD livers are mainly defined as livers from donors with advanced age, macrovesicular steatosis, donation after cardiac death (DCD), and other unfavorable characteristics that indicate suboptimal quality[5,6]. The use of livers with ECD in ACLF-LT remains controversial. On the one hand, ECD livers were historically considered to be related to poor graft function and even poor survival; on the other hand, although transplanted with ECD grafts, recipients with high model for end-stage liver disease (MELD) scores or severe ACLF also obtained strong survival benefits, with 1-year post-LT survival rates reaching 78.1%[7,8]. The impact of the increased use of ECD grafts in ACLF patients needs to be further researched[9].
To our knowledge, there are still no studies published based on experiences at a Chinese hospital. In this study, we aimed to investigate the perioperative and long-term outcomes of ACLF patients in terms of whether they were ECD or non-ECD recipients.
MATERIALS AND METHODS
Patients
A retrospective cohort study was conducted. We recruited patients who underwent LT at the First Affiliated Hospital of Sun Yat-Sen University from January 2015 to November 2021 for our study. The inclusion criteria were as follows: Underwent LT; met the ACLF diagnostic criteria; and age ≥ 18 years. The exclusion criteria were complicated with hepatocellular carcinoma or other hepatobiliary cancer, combined transplantation with other organ (s), cases of ischemia-free LT (IFLT)[10] in a prospective randomized controlled study (registration number: ChiCTR1900021158) conducted during the same period, living donor LT, and incomplete medical records. Of note, there is still no unified definition of ACLF. Considering the unique epidemiological background in our country in which ACLF is mainly caused by hepatitis B virus infection, we adopted the diagnostic criteria recently proposed by the Chinese Group on the Study of Severe Hepatitis B[11] in this study; that is, regardless of the presence of cirrhosis, patients with chronic hepatitis B, total bilirubin (TB) ≥ 12 mg/dL and international normalized ratio ≥ 1.5 should be diagnosed with ACLF.
Donor and recipient clinical characteristics
The clinical parameters of both donors and recipients were extracted from electronic medical records. The baseline characteristics of recipients were based on the last examination before LT. The severity of ACLF was measured by the MELD score and the number of extrahepatic organ failures (OFs) at the time before LT. Extrahepatic OF was defined by previous reports and included kidney[12], coagulation[13], circulatory system[14], respiratory system[15], and hepatic encephalopathy[16]. The baseline characteristics of donors were based on the last examination before organ procurement. There is still no precise definition of an ECD liver; with reference to previous reports[5,6] and the experience of our center, ECD was defined in this study as meeting any of the following criteria: Age > 65 years, body mass index (BMI) > 30 kg/m2, macrovesicular steatosis ≥ 30%, serum sodium > 165 mmol/L, serum alanine aminotransferase (ALT)/aspartate aminotransferase (AST) > 120 U/L, serum TB > 51 μmol/L, cold-ischemia time (CIT) > 12 ho, split, DCD.
Outcomes
The primary endpoint events of interest were graft survival (from LT to re-LT or death) and overall survival (OS), from LT to death. The patients were followed up until December 2021. The secondary outcomes mainly included rates of early allograft dysfunction (EAD)[17], acute kidney injury (AKI)[17] and other perioperative characteristics [intraoperative blood loss/transfusion, operative time, and intensive care unit (ICU) stay].
Statistical analysis
The patients were divided into ECD and non-ECD recipient groups for analysis. As the main factor of concern in ECD, DCD recipients were also analyzed as a subgroup for comparison with donation after brain death (DBD) recipients. Statistical analyses were conducted using SPSS (version 23.0, IBM). Continuous measurement data are expressed as the mean ± SD, and differences between groups were detected by Student’s t test. Enumeration data are expressed as numbers (percentages), and differences were detected by the χ2 test. OS and graft survival were calculated by the Kaplan-Meier method and compared through the log-rank test. Univariate and multivariate logistic/Cox regression analyses were performed to identify the risk factors and independent risk factors for EAD/OS, and variables showing univariate significance or considered clinically relevant were entered into multivariate analysis[18]. Statistical significance was defined as P < 0.05.
RESULTS
Baseline characteristics of recipients
Ultimately, 145 patients were enrolled in our study, of whom 78 (53.8%) were in the ECD group and 67 (46.2%) were in the non-ECD group (Table 1). The severity of ACLF was quantified by the MELD score and OFs, and both quantitative tools showed no significant difference in the severity of the preoperative disease between these two groups (P = 0.579 and 0.547 and 0.591 and 0.547, respectively). Other demographic indicators, such as age, sex, blood type, and preoperative biochemical tests, also proved to have nonsignificant differences (P > 0.05). In addition, the numbers of IFLT cases were approximately similar in both groups (P = 0.170).
Table 1.
Baseline characteristics of recipients, n (%)
|
|
ECD (n = 78)
|
Non-ECD (n = 67)
|
P value
|
| MELD | 30.73 ± 5.96 | 30.19 ± 5.60 | 0.579 |
| MELD | 0.547 | ||
| > 30 | 40 (51.3) | 31 (46.3) | |
| ≤ 30 | 38 (48.7) | 36 (53.7) | |
| OFs | 0.591 | ||
| 0 | 25 (32.1) | 28 (41.8) | |
| 1 | 33 (42.3) | 20 (29.9) | |
| 2 | 11 (14.1) | 9 (13.4) | |
| 3 | 7 (9) | 8 (11.9) | |
| 4 | 2 (2.6) | 2 (3) | |
| OFs | 0.547 | ||
| > 2 | 9 (11.5) | 10 (14.9) | |
| ≤ 2 | 69 (88.5) | 57 (85.1) | |
| Age (yr) | 0.365 | ||
| > 60 | 4 (5.1) | 6 (9) | |
| ≤ 60 | 74 (94.9) | 61 (91) | |
| Sex | 0.730 | ||
| Male | 69 (88.5) | 58 (86.6) | |
| Female | 9 (11.5) | 9 (13.4) | |
| BMI (kg/m2) | 0.611 | ||
| > 30 | 5 (6.4) | 3 (4.5) | |
| ≤ 30 | 73 (93.6) | 64 (95.5) | |
| Blood group | 0.411 | ||
| O | 29 (37.2) | 28 (41.8) | |
| A | 24 (30.8) | 20 (29.9) | |
| B | 22 (28.2) | 13 (19.4) | |
| AB | 3 (3.8) | 6 (9) | |
| WBC (× 109/L) | 6.84 ± 3.56 | 6.35 ± 3.52 | 0.411 |
| N/L | 6.27 ± 6.34 | 7.00 ± 6.12 | 0.482 |
| Hb (g/L) | 94.27 ± 22.29 | 89.45 ± 19.06 | 0.167 |
| GGT (U/L) | 49.51 ± 26.39 | 50.12 ± 32.49 | 0.901 |
| ALB (g/L) | 36.87 ± 4.94 | 38.37 ± 4.99 | 0.071 |
| ALT (U/L) | 85.33 ± 96.36 | 60.88 ± 75.02 | 0.088 |
| AST (U/L) | 114.47 ± 103.80 | 92.42 ± 82.42 | 0.163 |
| PLT (× 109/L) | 63.50 ± 33.83 | 62.49 ± 38.57 | 0.867 |
| Fib (g/L) | 1.02 ± 0.47 | 1.12 ± 0.43 | 0.176 |
| IFLT | 2 (2.6) | 5 (7.5) | 0.170 |
MELD: Model for end-stage liver disease; ECD: Extended criteria donor; OFs: Organ failures; BMI: Body mass index; WBC: White blood cell; Hb: Hemoglobin; GGT: Gamma glutamyltransferase; ALB: Albumin; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; PLT: Platelets; Fib: Fibrinogen; IFLT: Ischemia-free liver transplantation.
This finding indicates that there is no significant bias between these two groups in the allocation and usage of ECD grafts, and the probability of being assigned an ECD liver is approximately equal for patients with severe or mild ACLF.
Baseline characteristics of donor livers
Compared with non-ECD, ECD accounted for more than half of the total grafts [Table 2, 67 (46.2%) vs 78 (53.8%)]. The specific types of ECD grafts are shown in Table 2. Liver grafts were defined as ECD mainly because ALT/AST was greater than 120 U/L (29.5%/46.2%), followed by DCD (30.8%) and high serum sodium (17.9%). A total of 11.5% of grafts were classified as ECD due to BMI > 30 kg/m2, and 9% were classified due to TB > 51 μmol/L. Notably, macrovesicular steatosis (2.6%), advanced age (1.3%), prolonged CIT (6.4%), and split (1.3%) only accounted for a very small proportion of ECD livers.
Table 2.
Baseline characteristics of donor livers, n (%)
|
|
ECD (n = 78)
|
Non-ECD (n = 67)
|
P value
|
| DCD/DBD | 24 (30.8)/54 (69.2) | 0/ 67 (100) | 0.000 |
| Split | 1 (1.3) | 0 | 0.352 |
| Macrovesicular steatosis | 0.187 | ||
| ≥ 30 | 2 (2.6) | 0 | |
| < 30 | 76 (97.4) | 67 (100) | |
| Age (yr) | 0.352 | ||
| > 65 | 1 (1.3) | 0 | |
| ≤ 65 | 77 (98.7) | 67 (100) | |
| BMI (kg/m2) | 0.004 | ||
| > 30 | 9 (11.5) | 0 | |
| ≤ 30 | 69 (88.5) | 67 (100) | |
| Na (mmol/L) | 0.000 | ||
| > 165 | 14 (17.9) | 0 | |
| ≤ 165 | 64 (82.1) | 67 (100) | |
| ALT (U/L) | 0.000 | ||
| > 120 | 23 (29.5) | 0 | |
| ≤ 120 | 55 (70.5) | 67 (100) | |
| AST (U/L) | 0.000 | ||
| > 120 | 36 (46.2) | 0 | |
| ≤ 120 | 42 (53.8) | 67 (100) | |
| TB (μmol/L) | 0.012 | ||
| > 51 | 7 (9.0) | 0 | |
| ≤ 51 | 71 (91.0) | 67 (100) | |
| CIT (h) | 0.035 | ||
| > 12 | 5 (6.4) | 0 | |
| ≤ 12 | 73 (93.6) | 67 (100) | |
| Reason of death | 0.969 | ||
| Trauma | 41 (52.6) | 35 (52.2) | |
| Other | 37 (47.4) | 32 (47.8) |
DCD: Donation after cardiac death; DBD: Donation after brain death; ECD: Extended criteria donor; BMI: Body mass index; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TB: Total bilirubin; CIT: Cold-ischemia time.
Primary outcomes
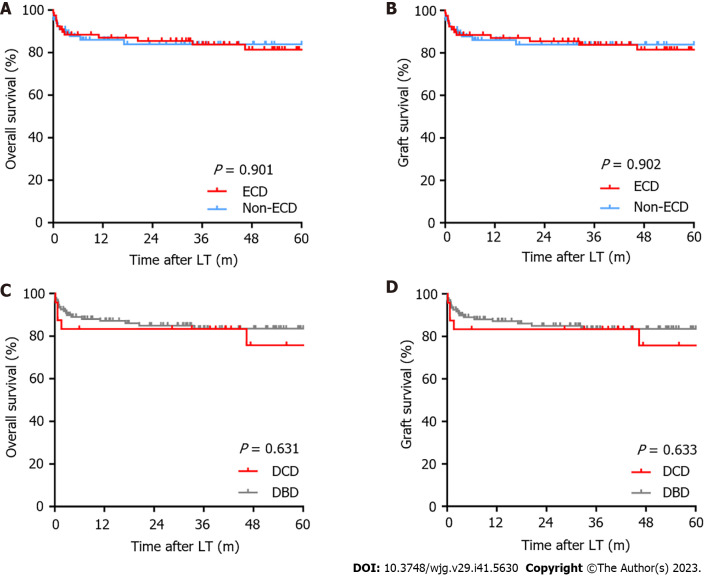
During the follow-up period, only one patient underwent retransplantation. Neither OS nor graft survival significantly differed between patients in the ECD and non-ECD groups and in the DCD and DBD groups (Figure 1). In ECD vs non-ECD recipients, the 1-, 3-, and 5-year OS rates were 87.0%, 83.7%, and 81.4% vs 86.0%, 83.8% and 83.8% (P = 0.901), respectively, and the 1-, 3-, and 5-year graft survival rates were 87.0%, 83.8% and 81.5% vs 86.0%, 83.8% and 83.8% (P = 0.902). In DCD and DBD recipients, the 1-, 3-, and 5-year OS rates were equal to the graft survival rates (83.3%, 83.3%, and 75.8% vs 87.1%, 83.5% and 83.5%, respectively) (P = 0.631 and 0.633, respectively).
Figure 1.
Kaplan-Meier analysis. A: Overall survival between extended criteria donor (ECD) and non-ECD groups; B: Graft survival between ECD and non-ECD groups; C: Overall survival between donation after cardiac death (DCD) and donation after brain death (DBD) groups; D: Graft survival between DCD and DBD groups. LT: Liver transplantation; ECD: Extended criteria donor; DCD: Donation after cardiac death; DBD: Donation after brain death.
Secondary outcomes
ECD recipients demonstrated a significantly higher postoperative EAD incidence than non-ECD recipients (67.9% vs 41.8%, P = 0.002). Except for EAD, there were no significant differences (P > 0.05) in the other secondary endpoints between these two groups (Table 3).
Table 3.
Secondary outcomes of recipients between extended criteria donor and non-extended criteria donor grafts, n (%)
|
|
ECD (n = 78)
|
Non-ECD (n = 67)
|
P value
|
| EAD | 53 (67.9) | 28 (41.8) | 0.002 |
| AKI | 34 (43.6) | 30 (44.8) | 0.886 |
| Blood loss (mL) | 2.55 ± 1.70 | 2.82 ± 2.93 | 0.489 |
| RBC transfused (U) | 7.89 ± 3.93 | 9.13 ± 6.18 | 1.148 |
| Plasma transfused (U) | 9.77 ± 4.28 | 10.23 ± 4.51 | 0.534 |
| Operative time (h) | 7.48 ± 1.23 | 7.35 ± 1.37 | 0.554 |
| ICU stay (d) | 4.68 ± 4.95 | 4.63 ± 6.27 | 0.958 |
EAD: Early allograft dysfunction; ECD: Extended criteria donor; AKI: Acute kidney injury; ICU: Intensive care unit; RBC: Red blood cell.
Then, we divided the patients into DCD and DBD groups, and all secondary endpoints, including EAD, AKI, blood loss and other perioperative indicators, showed no significant differences (Table 4, P > 0.05).
Table 4.
Secondary outcomes of recipients between donation after cardiac death and donation after brain death grafts, n (%)
|
|
DCD (n = 24)
|
DBD (n = 121)
|
P value
|
| EAD | 17 (70.8) | 64 (52.9) | 0.106 |
| AKI | 11 (45.8) | 53 (43.8) | 0.855 |
| Blood loss (mL) | 2.53 ± 1.81 | 2.70 ± 2.44 | 0.747 |
| RBC transfused (U) | 8.61 ± 3.77 | 8.43 ± 5.35 | 0.876 |
| Plasma transfused (U) | 9.17 ± 5.03 | 10.14 ± 4.24 | 0.319 |
| Operative time (h) | 7.67 ± 1.46 | 7.37 ± 1.26 | 0.298 |
| ICU stay (d) | 5.25 ± 5.00 | 4.54 ± 5.70 | 0.571 |
DCD: Donation after cardiac death; DBD: Donation after brain death; ECD: Extended criteria donor; AKI: Acute kidney injury; ICU: Intensive care unit; RBC: Red blood cell.
Identification of independent risk factors for EAD and OS
We enrolled all potential clinical parameters in univariable and multivariable logistic regression analyses to identify risk factors and independent risk factors for EAD. The results showed that ECD graft (P = 0.002), anhepatic phase (P = 0.003), operation time (P = 0.005) and recipient gamma glutamyltransferase (GGT), (P = 0.027) were risk factors for EAD. Then, ECD graft (P = 0.009), anhepatic phase (P = 0.034) and recipient GGT (P = 0.016) were shown to be independently associated with EAD (Table 5).
Table 5.
Univariable and multivariable logistic analysis of risk factors for early allograft dysfunction
| Variables | OR |
Univariate analysis
|
OR |
Multivariate analysis
|
||
|
HR (95%CI)
|
P value
|
HR (95%CI)
|
P value
|
|||
| Donor characteristics | ||||||
| HBV (positive vs negative) | 1.076 | 0.522-2.220 | 0.843 | |||
| Death of trauma (yes vs no) | 1.331 | 0.689-2.568 | 0.395 | |||
| ECD (yes vs no) | 2.953 | 1.497-5.826 | 0.002 | 2.712 | 1.286-5.720 | 0.009 |
| Operation characteristics | ||||||
| Blood loss (L) | 1.080 | 0.925-1.262 | 0.330 | |||
| Anhepatic phase (min) | 1.036 | 1.012-1.060 | 0.003 | 1.031 | 1.002-1.060 | 0.034 |
| Operation time (h) | 1.487 | 1.124-1.967 | 0.005 | 1.271 | 0.915-1.767 | 0.153 |
| IFLT (yes vs no) | 0.121 | 0.014-1.031 | 0.053 | 0.114 | 0.011-1.218 | 0.072 |
| Recipient characteristics | ||||||
| BMI (> 30 vs ≤ 30) | 0.779 | 0.187-3.244 | 0.732 | |||
| MELD (> 30 vs ≤30) | 1.455 | 0.753-2.812 | 0.265 | |||
| OFs (> 2 vs ≤ 2) | 0.861 | 0.327-2.264 | 0.761 | |||
| WBC (× 109/L) | 1.039 | 0.945-1.142 | 0.432 | |||
| N/L | 0.997 | 0.946-1.051 | 0.906 | |||
| Hb (g/L) | 1.006 | 0.990-1.022 | 0.444 | |||
| GGT (U/L) | 1.015 | 1.002-1.029 | 0.027 | 1.017 | 1.003-1.032 | 0.016 |
| ALB (g/L) | 1.052 | 0.984-1.126 | 0.139 | |||
| ALT (U/L) | 1.002 | 0.998-1.006 | 0.288 | |||
| AST (U/L) | 1.001 | 0.997-1.004 | 0.650 | |||
| PLT (× 109/L) | 1.001 | 0.992-1.010 | 0.800 | |||
| Fib (g/L) | 0.692 | 0.332-1.441 | 0.325 | |||
OR: Odds ratio; CI: Confidence interval; HBV: Hepatitis B virus; ECD: Extended criteria donor; BMI: Body mass index; IFLT: Ischemia-free liver transplantation; MELD: Model for end-stage liver disease; OFs: Organ failures; WBC: White blood cell; Hb: Hemoglobin; GGT: Gamma glutamyltransferase; ALB: Albumin; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; PLT: Platelets; Fib: Fibrinogen.
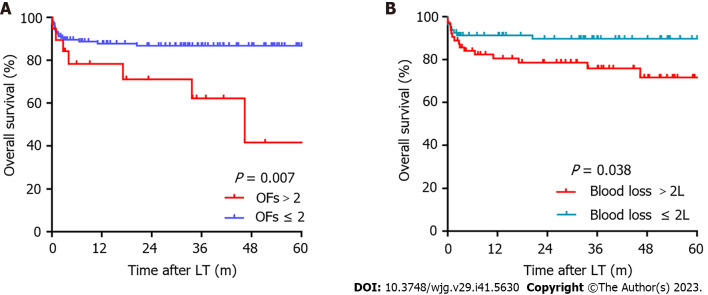
All potential factors that may be related to OS were included in the Cox regression analysis. Univariable analysis showed that blood loss (P = 0.000), EAD (P = 0.048), and OFs > 2 (P = 0.011) were risk factors for OS; then, multivariable analysis further demonstrated that blood loss (P = 0.000) and OFs > 2 (P = 0.015) were independent risk factors for OS (Table 6). Patients complicated with preoperative OFs > 2 (P = 0.007) or intraoperative blood loss > 2 L (median = 2 L, P = 0.038) were associated with significantly poorer post-LT survival (Figure 2).
Table 6.
Univariable and multivariable Cox analysis of risk factors for overall survival
| Variables | OR |
Univariate analysis
|
OR |
Multivariate analysis
|
||
|
HR (95%CI)
|
P value
|
HR (95%CI)
|
P value
|
|||
| Donor characteristics | ||||||
| HBV (positive vs negative) | 1.478 | 0.646-3.383 | 0.355 | |||
| Death of trauma (yes vs no) | 0.848 | 0.379-1.898 | 0.688 | |||
| ECD (yes vs no) | 1.053 | 0.465-2.386 | 0.901 | |||
| No. of ECD features (≥ 2 vs < 2) | 0.840 | 0.279-2.531 | 0.757 | |||
| Operation characteristics | ||||||
| Blood loss (L) | 1.271 | 1.137-1.421 | 0.000 | 1.276 | 1.123-1.449 | 0.000 |
| Anhepatic phase (min) | 1.009 | 0.989-1.028 | 0.381 | |||
| Operation time (h) | 1.002 | 0.746-1.345 | 0.990 | |||
| IFLT (yes vs no) | 0.822 | 0.111-6.107 | 0.848 | |||
| EAD (yes vs no) | 2.726 | 1.009-7.365 | 0.048 | 2.481 | 0.914-6.737 | 0.075 |
| AKI (yes vs no) | 1.882 | 0.830-4.269 | 0.130 | |||
| Recipient characteristics | ||||||
| BMI (> 30 vs ≤ 30) | 0.831 | 0.112-6.166 | 0.856 | |||
| MELD (> 30 vs ≤ 30) | 1.528 | 0.678-3.444 | 0.307 | |||
| OFs (> 2 vs ≤ 2) | 3.191 | 1.309-7.780 | 0.011 | 3.042 | 1.245-7.432 | 0.015 |
| WBC (× 109/L) | 1.007 | 0.897-1.129 | 0.912 | |||
| N/L | 1.040 | 0.992-1.090 | 0.102 | |||
| Hb (g/L) | 0.984 | 0.963-1.005 | 0.128 | |||
| GGT (U/L) | 0.986 | 0.968-1.005 | 0.142 | |||
| ALB (g/L) | 1.044 | 0.963-1.131 | 0.300 | |||
| ALT (U/L) | 0.992 | 0.984-1.001 | 0.070 | |||
| AST (U/L) | 0.998 | 0.992-1.004 | 0.463 | |||
| PLT (× 109/L) | 0.999 | 0.987-1.010 | 0.813 | |||
| Fib (g/L) | 0.874 | 0.345-2.211 | 0.775 | |||
OR: Odds ratio; CI: Confidence interval; HBV: Hepatitis B virus; ECD: Extended criteria donor; EAD: Early allograft dysfunction; BMI: Body mass index; IFLT: Ischemia-free liver transplantation; AKI: Acute kidney injury; MELD: Model for end-stage liver disease; OFs: Organ failures; WBC: White blood cell; Hb: Hemoglobin; GGT: Gamma glutamyltransferase; ALB: Albumin; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; PLT: Platelets; Fib: Fibrinogen.
Figure 2.
Kaplan-Meier analysis of overall survival. A: Organ failures; B: Blood loss. OFs: Organ failures.
DISCUSSION
The impact of the increased use of ECD grafts in ACLF patients has not yet been evaluated well[9]. To our knowledge, this is the first report from China that summarizes the experiences of using ECD grafts in ACLF-LT patients.
In our study, both OS and graft survival between ECD and non-ECD recipients and DCD and DBD recipients were not significantly different. This was approximately consistent with the conclusions of recent studies based on adult recipients (regardless of primary disease)[19] and high-acuity patients (MELD ≥ 35)[20]. For severe ACLF recipients[8], a marginal donor liver (donor risk index[21] above 1.7) was considered an independent risk factor for 1-year post-LT survival. However, as the authors pointed out in this article, although transplanted with marginal livers, it was clear that patients still obtain strong survival benefits, with 1-year post-LT survival rates reaching 78.1%. In our study, ECD recipients had a 5-year post-LT survival rate above 80%. Compared with the poor prognosis of 90-d transplant-free mortality above 50%[1], ECD grafts undoubtedly provide an important life-saving option for ACLF patients. Moreover, refusing ECD and continuing to wait for an ideal graft means a prolonged waiting time, which also means an increased risk of worse preoperative disease and higher post-LT mortality[22]. Consequently, it may be better for ACLF patients to accept an existing ECD graft rather than waiting for a prospective ideal liver.
In our opinion, the reason why there was no significant difference in survival between ECD and non-ECD patients was mainly due to the inevitable selection bias in clinical practice. As shown in Table 2, advanced age, prolonged CIT and macrovesicular steatosis, which have been widely recognized as the strongest prognostic risk factors[23], only accounted for 1.3%, 6.4% and 2.6% of our ECD grafts, respectively. This indicates that the ECD grafts actually adopted in our clinical practice may be relatively safe, and those grafts empirically judged as "high risk" were abandoned. Nevertheless, ECD recipients still showed a significantly higher incidence of EAD than non-ECD recipients.
ECD grafts, anhepatic phase and recipient GGT were proven to be significantly associated with EAD in our further research. The importance of shortening the anhepatic phase in transplantation is self-evident and has been proven by previous studies[24,25]. Our research emphasized the importance of surgical techniques once again. Traditionally, high serum GGT has been considered a biomarker of hepatobiliary diseases. Recent studies have shown its predictive role in carcinogenesis, tumor progression and many other life-threatening diseases[26]. The potential of donor GGT in predicting EAD[27] and graft survival[28] has also been reported, but few studies have focused on its role in recipients. Our study found that a high preoperative recipient serum GGT level was significantly correlated with post-LT EAD but did not affect survival. This may be due to its critical role in the modulation of redox equilibria[29], and high serum GGT may reflect worse preoperative disease in recipients. At the same time, it should be noted that the determinants of prognosis in LT are numerous and complex.
Our analysis showed that only preoperative recipient OFs and intraoperative blood loss were independently associated with OS. This finding indicates that under our current ECD experience, the post-LT survival of ACLF patients mainly depends on the severity of their own preoperative disease and intraoperative conditions. The MELD score is widely accepted as a tool to quantify the severity of end-stage liver disease and to allocate donor livers. However, in recent years, a growing number of studies have found that MELD or MELD-Na underestimates the severity of ACLF, mainly because it fails to capture the two key pathophysiological features of ACLF: Extrahepatic OF and systemic inflammation[30,31]. Our study also showed that OFs may reflect the severity of ACLF more accurately than MELD.
There are limitations in our study. The first is that the boundary of ECD remains undetermined. It should be recognized that the definition of ECD is not a simple concept of yes or no but should be linearly quantified. The application value of the Donor Risk Index[21] in China is limited due to unique ethnic characteristics. What is the safe boundary of an acceptable ECD graft? Unfortunately, we have not been able to establish a quantitative formula thus far in our country, and this will be the focus of our future research. Second, our results need to be further confirmed by a larger sample study.
In conclusion, our experience suggests that ECD grafts are associated with a higher risk of EAD than non-ECD grafts but can be safely used in ACLF recipients, as they do not affect post-LT survival. The main factors affecting the prognosis of ACLF recipients are the severity of their own preoperative disease and intraoperative blood loss.
CONCLUSION
In conclusion, our experience suggests that ECD grafts are associated with a higher risk of EAD than non-ECD grafts but can be safely used in ACLF recipients as they do not affect post-LT survival. The main factors affecting the prognosis of ACLF recipients are the severity of their own preoperative disease and intraoperative blood loss.
ARTICLE HIGHLIGHTS
Research background
There is no consensus on the usage of extended criteria donor (ECD) grafts in liver transplantation (LT) for acute-on-chronic liver failure (ACLF) patients.
Research motivation
It was intended to summarize the experience of using ECD livers in ACLF-LT.
Research objectives
Our study aimed to summarize the experience of using ECD livers in ACLF-LT, and to provide reference for clinical practice.
Research methods
We conducted a retrospective cohort study to analyze outcomes between ECD and non-ECD recipients.
Research results
There was no significant difference (P > 0.05) in survival between ECD and non-ECD recipients after LT, although ECD grafts were associated with a significantly higher incidence of early allograft dysfunction. The most important factors affecting post-LT survival of ACLF patients were extrahepatic organ failures (OFs) > 2 (P = 0.015) and intraoperative blood loss (P = 0.000).
Research conclusions
ECD grafts can be safely used in ACLF-LT, although related to a higher risk of early allograft dysfunction.
Research perspectives
Due to the unavoidable selection bias in clinical practice, there were only 2 cases of donor liver have diagnosed as macrovesicular steatosis more than 30%. This indicates that the ECD grafts actually adopted in our clinical practice may be relatively safe, and those grafts empirically judged as "high risk" were abandoned. Admittedly, this is a major limitation of our current study, and the next step will be to try to compensate it by including more cases in our study over a longer period or in conjunction with other transplant centers.
Footnotes
Institutional review board statement: The study was reviewed and approved for publication by our Institutional Reviewer.
Informed consent statement: All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrolment.
Conflict-of-interest statement: All the Authors have no conflict of interest related to the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: August 23, 2023
First decision: September 11, 2023
Article in press: October 23, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta T, India; Vij M, India S-Editor: Qu XL L-Editor: A P-Editor: Yu HG
Contributor Information
Jin-Long Gong, Department of Hepatobiliary Surgery, Hunan Provincial People's Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha 410005, Hunan Province, China; Organ Transplant Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China.
Jia Yu, Organ Transplant Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China; Department of Gastroenterology Surgery, The First Affiliated Hospital of University of South China, Hengyang 421005, Hunan Province, China.
Tie-Long Wang, Organ Transplant Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China.
Xiao-Shun He, Organ Transplant Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China.
Yun-Hua Tang, Organ Transplant Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China.
Xiao-Feng Zhu, Organ Transplant Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China. zhuxiaof@mail.sysu.edu.cn.
Data sharing statement
The datasets of the current study are available from the corresponding author upon reasonable request.
References
- 1.Arroyo V, Moreau R, Jalan R. Acute-on-Chronic Liver Failure. N Engl J Med. 2020;382:2137–2145. doi: 10.1056/NEJMra1914900. [DOI] [PubMed] [Google Scholar]
- 2.Artru F, Louvet A, Ruiz I, Levesque E, Labreuche J, Ursic-Bedoya J, Lassailly G, Dharancy S, Boleslawski E, Lebuffe G, Kipnis E, Ichai P, Coilly A, De Martin E, Antonini TM, Vibert E, Jaber S, Herrerro A, Samuel D, Duhamel A, Pageaux GP, Mathurin P, Saliba F. Liver transplantation in the most severely ill cirrhotic patients: A multicenter study in acute-on-chronic liver failure grade 3. J Hepatol. 2017;67:708–715. doi: 10.1016/j.jhep.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Zhang T, Dunson J, Kanwal F, Galvan NTN, Vierling JM, O'Mahony C, Goss JA, Rana A. Trends in Outcomes for Marginal Allografts in Liver Transplant. JAMA Surg. 2020 doi: 10.1001/jamasurg.2020.2484. [DOI] [PubMed] [Google Scholar]
- 4.Goldaracena N, Cullen JM, Kim DS, Ekser B, Halazun KJ. Expanding the donor pool for liver transplantation with marginal donors. Int J Surg. 2020;82S:30–35. doi: 10.1016/j.ijsu.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433–485. doi: 10.1016/j.jhep.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Vodkin I, Kuo A. Extended Criteria Donors in Liver Transplantation. Clin Liver Dis. 2017;21:289–301. doi: 10.1016/j.cld.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Schaubel DE, Sima CS, Goodrich NP, Feng S, Merion RM. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transplant. 2008;8:419–425. doi: 10.1111/j.1600-6143.2007.02086.x. [DOI] [PubMed] [Google Scholar]
- 8.Sundaram V, Jalan R, Wu T, Volk ML, Asrani SK, Klein AS, Wong RJ. Factors Associated with Survival of Patients With Severe Acute-On-Chronic Liver Failure Before and After Liver Transplantation. Gastroenterology. 2019;156:1381–1391.e3. doi: 10.1053/j.gastro.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Karvellas CJ, Francoz C, Weiss E. Liver Transplantation in Acute-on-chronic Liver Failure. Transplantation. 2021;105:1471–1481. doi: 10.1097/TP.0000000000003550. [DOI] [PubMed] [Google Scholar]
- 10.van Leeuwen OB, Ubbink R, de Meijer VE, Porte RJ. The first case of ischemia-free organ transplantation in humans: A proof of concept. Am J Transplant. 2018;18:2091. doi: 10.1111/ajt.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu T, Li J, Shao L, Xin J, Jiang L, Zhou Q, Shi D, Jiang J, Sun S, Jin L, Ye P, Yang L, Lu Y, Li T, Huang J, Xu X, Chen J, Hao S, Chen Y, Xin S, Gao Z, Duan Z, Han T, Wang Y, Gan J, Feng T, Pan C, Li H, Huang Y, Xie Q, Lin S, Li L Chinese Group on the Study of Severe Hepatitis B (COSSH) Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67:2181–2191. doi: 10.1136/gutjnl-2017-314641. [DOI] [PubMed] [Google Scholar]
- 12.Ostermann M, Bellomo R, Burdmann EA, Doi K, Endre ZH, Goldstein SL, Kane-Gill SL, Liu KD, Prowle JR, Shaw AD, Srisawat N, Cheung M, Jadoul M, Winkelmayer WC, Kellum JA Conference Participants. Controversies in acute kidney injury: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2020;98:294–309. doi: 10.1016/j.kint.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437, 1437.e1. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 14.Hyland SL, Faltys M, Hüser M, Lyu X, Gumbsch T, Esteban C, Bock C, Horn M, Moor M, Rieck B, Zimmermann M, Bodenham D, Borgwardt K, Rätsch G, Merz TM. Early prediction of circulatory failure in the intensive care unit using machine learning. Nat Med. 2020;26:364–373. doi: 10.1038/s41591-020-0789-4. [DOI] [PubMed] [Google Scholar]
- 15.Fan E, Brodie D, Slutsky AS. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 16.Wijdicks EF. Hepatic Encephalopathy. N Engl J Med. 2016;375:1660–1670. doi: 10.1056/NEJMra1600561. [DOI] [PubMed] [Google Scholar]
- 17.Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, Shaked A, Christie JD. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943–949. doi: 10.1002/lt.22091. [DOI] [PubMed] [Google Scholar]
- 18.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 19.Pandya K, Sastry V, Panlilio MT, Yip TCF, Salimi S, West C, Virtue S, Wells M, Crawford M, Pulitano C, Strasser SI, McCaughan GW, Majumdar A, Liu K. Differential Impact of Extended Criteria Donors After Brain Death or Circulatory Death in Adult Liver Transplantation. Liver Transpl. 2020;26:1603–1617. doi: 10.1002/lt.25859. [DOI] [PubMed] [Google Scholar]
- 20.Guorgui J, Ito T, Younan S, Agopian VG, Dinorcia J 3rd, Farmer DG, Busuttil RW, Kaldas FM. The Utility of Extended Criteria Donor Livers in High Acuity Liver Transplant Recipients. Am Surg. 2021;87:1684–1689. doi: 10.1177/00031348211024658. [DOI] [PubMed] [Google Scholar]
- 21.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 22.Sundaram V, Kogachi S, Wong RJ, Karvellas CJ, Fortune BE, Mahmud N, Levitsky J, Rahimi RS, Jalan R. Effect of the clinical course of acute-on-chronic liver failure prior to liver transplantation on post-transplant survival. J Hepatol. 2020;72:481–488. doi: 10.1016/j.jhep.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lozanovski VJ, Khajeh E, Fonouni H, Pfeiffenberger J, von Haken R, Brenner T, Mieth M, Schirmacher P, Michalski CW, Weiss KH, Büchler MW, Mehrabi A. The impact of major extended donor criteria on graft failure and patient mortality after liver transplantation. Langenbecks Arch Surg. 2018;403:719–731. doi: 10.1007/s00423-018-1704-z. [DOI] [PubMed] [Google Scholar]
- 24.Ijtsma AJ, van der Hilst CS, de Boer MT, de Jong KP, Peeters PM, Porte RJ, Slooff MJ. The clinical relevance of the anhepatic phase during liver transplantation. Liver Transpl. 2009;15:1050–1055. doi: 10.1002/lt.21791. [DOI] [PubMed] [Google Scholar]
- 25.Buchholz BM, Gerlach UA, Chandrabalan VV, Hodson J, Gunson BK, Mergental H, Muiesan P, Isaac JR, Roberts KJ, Mirza DF, Perera MTPR. Revascularization Time in Liver Transplantation: Independent Prediction of Inferior Short- and Long-term Outcomes by Prolonged Graft Implantation. Transplantation. 2018;102:2038–2055. doi: 10.1097/TP.0000000000002263. [DOI] [PubMed] [Google Scholar]
- 26.Takemura K Board PG. Koga F. A Systematic Review of Serum γ-Glutamyltransferase as a Prognostic Biomarker in Patients with Genitourinary Cancer. Antioxidants (Basel) 2021;10 doi: 10.3390/antiox10040549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoyer DP, Paul A, Gallinat A, Molmenti EP, Reinhardt R, Minor T, Saner FH, Canbay A, Treckmann JW, Sotiropoulos GC, Mathé Z. Donor information based prediction of early allograft dysfunction and outcome in liver transplantation. Liver Int. 2015;35:156–163. doi: 10.1111/liv.12443. [DOI] [PubMed] [Google Scholar]
- 28.Capelli R, Kitano Y, Linhares M, Da Silva D, Golse N, Karam V, Sa Cunha A, Vibert E, Azoulay D, Cherqui D, Adam R, Allard MA. The prognostic significance of serum aspartate transaminase and gamma-glutamyl transferase in liver deceased donors. Transpl Int. 2021;34:2247–2256. doi: 10.1111/tri.13978. [DOI] [PubMed] [Google Scholar]
- 29.Corti A, Belcastro E, Dominici S, Maellaro E, Pompella A. The dark side of gamma-glutamyltransferase (GGT): Pathogenic effects of an 'antioxidant' enzyme. Free Radic Biol Med. 2020;160:807–819. doi: 10.1016/j.freeradbiomed.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Hernaez R, Liu Y, Kramer JR, Rana A, El-Serag HB, Kanwal F. Model for end-stage liver disease-sodium underestimates 90-day mortality risk in patients with acute-on-chronic liver failure. J Hepatol. 2020;73:1425–1433. doi: 10.1016/j.jhep.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mookerjee RP. Prognosis and Biomarkers in Acute-on-Chronic Liver Failure. Semin Liver Dis. 2016;36:127–132. doi: 10.1055/s-0036-1583200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets of the current study are available from the corresponding author upon reasonable request.