Abstract
BACKGROUND
Inflammatory bowel disease (IBD) is an idiopathic intestinal disease with various levels and trends in different countries and regions. Understanding the current burden and trends of IBD in various geographical locations is essential to establish effective strategies for prevention and treatment. We report the average annual percentage change (AAPC) and estimated annual percentage change (EAPC) in age-standardized rates (ASR) of IBD in different regions based on the Global Burden of Disease (GBD) study from 1990-2019, and the relationships between IBD and the human development index (HDI) and socio-demographic index (SDI). The prevalence trends of IBD were predicted by gender from 2019-2039.
AIM
To comprehensively investigate IBD data, providing further insights into the management of this chronic disease.
METHODS
We collected the information on the incidence of IBD from the GBD study from 1990-2019 to calculate the AAPC and EAPC in ASR of IBD in different regions. The relationships between IBD, HDI, and SDI were analyzed. The Nordpred and Bayesian age-period-cohort models were used to predict the prevalence trends of IBD by gender from 2019-2039, and the reliability of the results was validated. Statistics of all the data in this study were performed using R software (version 4.2.1).
RESULTS
North America consistently had the highest IBD ASR, while Oceania consistently had the lowest. East Asia had the fastest average annual growth in ASR (2.54%), whereas Central Europe had the fastest decline (1.38%). Countries with a low age-standardized incidence rates in 1990 showed faster growth in IBD while there was no significant correlation in 2019. Additionally, IBD increased faster in countries with a low age-standardized death rates in 1990, whereas the opposite was true in 2019. Analysis of SDI and IBD ASR showed that countries with a high SDI generally had a higher IBD ASR. Finally, the projections showed a declining trend in the incidence of IBD from 2019-2039, but a gradual increase in the number of cases.
CONCLUSION
As the global population increases and ages, early monitoring and prevention of IBD is important to reduce the disease burden, especially in countries with a high incidence of IBD.
Keywords: Inflammatory bowel disease, Incidence, Average annual percentage change, Age-standardized rates, Prevalence trends, Global Burden of Disease
Core Tip: This study comprehensively analyzed the burden of inflammatory bowel disease (IBD) from 1990 to 2019 at the global, regional, and national levels. The association and significance of various demographic indicators were analyzed in different areas. Furthermore, the number and incidence rate of IBD for the next twenty years (from 2019 to 2039) were predicted and validated based on the R software. This study provides new hypotheses for the management of IBD to alleviate the global burden of this chronic disease.
INTRODUCTION
Inflammatory bowel disease (IBD) is an idiopathic, chronic, intestinal inflammatory condition that includes Crohn’s disease and ulcerative colitis. It is a relapsing and life-threatening disease with increasing incidence and prevalence worldwide[1,2]. Typically, IBD has a tendency for recurrence and remission, with exacerbations characterized by diarrhea, abdominal pain, and enterorrhagia. The treatment can generally relieve symptoms effectively, but does not cure the patient completely[3]. A considerable proportion of patients diagnosed with IBD ultimately required surgical intervention and experienced various complications, including extraintestinal manifestations, and complications inherent to the disease itself, such as strictures, fistulas, and abscesses[4]. This caused significant suffering for patients and affected their overall quality of life. Although the exact causes of IBD remain unclear, some common features have been observed in IBD susceptibility factors, including genetic predisposition, dysbiosis of the gut microbiota, environmental triggers, and immune abnormalities[5]. Furthermore, due to recurrent episodes of chronic intestinal inflammation, patients with IBD are at a high risk of developing colorectal cancer and extraintestinal malignancies[6,7], which is a negative factor that contributes to poor outcomes. Despite the emergence of novel biologics and therapeutic approaches for the treatment of IBD that has mitigated the potential adverse effects of traditional treatments such as corticosteroids and immunosuppressants, the clinical needs of patients have not been fully met due to the complexity and clinical heterogeneity of IBD[8,9]. Currently, new strategies that have focused on IBD personalized treatment, microbiota research, immunotherapy, digital health and remote monitoring, and collaborative disease research are expected to expand the prevention and treatment options for IBD[10-14].
IBD initially emerged in Western countries and is now considered a Western disease. Over the past few decades, the incidence of IBD has increased rapidly in developing countries in Asia, Eastern Europe, and Africa. However, in developed Western countries that previously had high prevalence of IBD, the incidence of IBD is now stabilizing[15]. The changing trends are parallel to alterations in dietary habits, such as the introduction of processed foods, increased intake of sugar and fat, excessive use of antibiotics, and overall improvements in sanitation[16]. Meanwhile, with the rapid development of economy, the increase in psychological issues and socio-economic burdens is also correlated with IBD[17]. This comprehensive analysis of the global burden and trends of IBD from 1990 to 2019 was conducted to determine its incidence, prevalence, and prognosis in different regions to provide researchers with valuable insights into the etiology of the disease. By comprehensively understanding the specific disease burden of IBD in different regions, future healthcare policies, medical resource allocations, and healthcare service systems can be established to satisfy the health needs of the population. According to the Global Burden of Disease (GBD) 2019, we reported the burden of IBD in 204 countries and territories from 1990-2019, including the age-standardized rates (ASR), average annual percentage change (AAPC), and estimated annual percentage change (EAPC). The relationship between the socio-economic status of the regions and incidence and mortality of IBD were also analyzed. Additionally, the global number of IBD cases, incidence, and prevalence trends for the next 20 years (2019-2039) were predicted. The aim of our study is to comprehensively investigate and give adequate attention to the IBD data, providing further insights into the IBD trends to guide future policy-making efforts in alleviating the global burden of IBD.
MATERIALS AND METHODS
Data sources
Data regarding the incidence, mortality, and age-standardized incidence rates (ASIR) for IBD in 204 countries and territories from 1990-2019 were extracted from the Global Health Data Exchange Query Tool (http://ghdx.healthdata.org/gbd-results-tool)[18]. We selected “all countries or territories” or “all GBD regions” from the database as the location, “inflammatory bowel disease” as the cause, “death” and “incidence” as the measure, “number”, “percent” and “rate” as metric, and selected “female”, “male” and “both” as gender. For age, in addition to “all ages” and “age-standardized,” the following age groups were also used: “< 1 year”, “1-4 years”, “5-9 years”, “10-14 years”, “15-19 years”, “20-24 years”, “25-29 years”, “30-34 years”, “35-39 years”, “40-44 years”, “45-49 years”, “50-54 years”, “55-59 years”, “60-64 years”, “65-69 years”, “70-74 years”, “75-79 years”, “80-84 years”, “85-89 years”, “90-94 years”, and “95+ years”. Based on the above criteria, we finally selected the data from 1990-2019 for follow-up analysis.
Country-level human development index (HDI) data were collected from the World Bank to explore the correlation between HDI and IBD (https://hdr.undp.org/data-center/human-development-index#/indicies/HDI). Finally, to further predict the future development of IBD, global population estimates (2017-2100) were obtained from the GBD database (https://ghdx.healthdata.org/record/ihme-data/global-population-forecasts-2017-2100).
Statistical analysis
The ASR, EAPC, and AAPC were used to quantify the trends in the incidence of IBD. As the included data were from multi-group populations of different age groups and changed over time, the data must be standardized and analyzed to avoid errors. The ASR was used as the main indicator for estimating disease burden, and its calculation formula is as follows: ASR = ΣiA ai wi / ΣiA wi × 100000, where i represents the ith age group, and ai and wi represent the age-specific ratio of each age group and the standard population of the world, respectively. The EAPC is a widely used summary of the ASR trends over a certain period. The regression line is fitted to the natural logarithm of the ratio, y = α + βx + e, where y = ln (ASR) and x = calendar year. EAPC is calculated as 100 × [exp(β)-1], and its 95% confidence interval (CI) was obtained from the linear regression model[19]. Similarly, the AAPC was calculated using Joinpoint software (version 4.9.1.0, https://surveillance.cancer.gov/joinpoint) to measure the trends in IBD from 1990 to 2019, calculated as follows: AAPC = {exp (Σwibi / Σwi) - 1} × 100, where bi is the slope coefficient for each segment in the desired year range, and wi is the length of each segment in the year range. Next, the relationships between the EAPCs, ASRs (1990 and 2019), and HDI (2022) in various countries were determined. In addition, a hierarchical clustering analysis was used to cluster the EAPCs of morbidity and mortality, and countries with similar trends in EAPCs were identified. Additionally, socio-demographic index (SDI) data from 1950-2019 from the GBD database (https://ghdx.healthdata.org/record/ihme-data/gbd-2019-socio-demographic-index-sdi-1950-2019) were used to analyze the relationship between the socioeconomic development status of regions and countries and the age-standardized incidence and mortality of IBD. The value of the SDI ranges from 0 to 1, with 0 being the worst and 1 being the best. This index consists of the total fertility rate of women under 25 years of age, the average education level of individuals aged 15 years and older, and the lagging distribution of per capita income. In addition, the country’s economic development level was divided according to the quintiles of the SDI value into low, low-medium, medium, high-medium, and high SDI.
Finally, the Nordpred package of R software (version 4.2.1) was used to predict the number, incidence, and ASR of IBD from 2019-2039 by sex. To verify the reliability of the predicted results, the Bayesian age-period-cohort (BAPC) and Integrated nested Laplace approximation packages of the R software were used for analysis. All statistical analyses were conducted using R software (version 4.2.1). The level of significance was set at P < 0.05.
RESULTS
Global disease burden of IBD
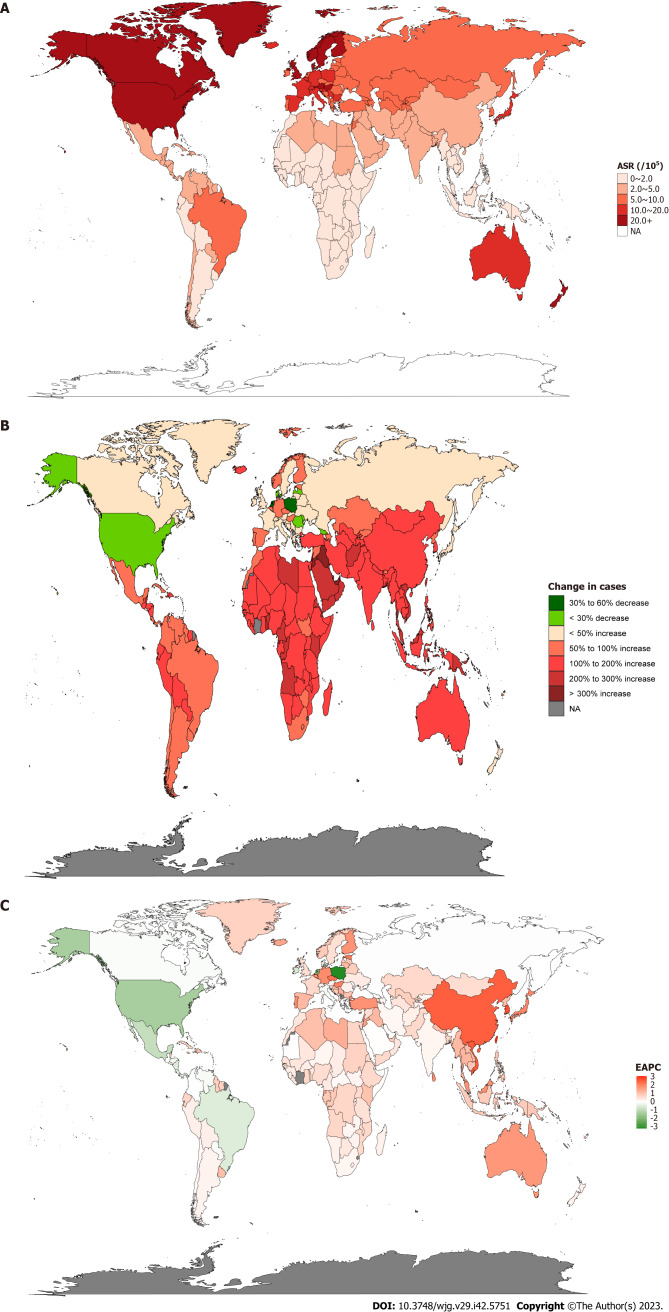
After normalizing the data for IBD incidence, Canada [37 per 100000, 95% uncertainty interval (UI): 35.6-38.3] had the highest ASR followed by Norway (36.6 per 100000, 95%UI: 31.7-42.1). Meanwhile, Timor-Leste, Cambodia, Maldives, Papua New Guinea, Laos, and Thailand had the lowest ASR (all 0.5 per 100000, 95%UI: 0.4-0.6) (Figure 1A and Supplementary Table 1). Furthermore, we counted the incidence of IBD in each country in 1990 and 2019 to analyze the changes in IBD. It was found that the highest incidence rate increase in Qatar was 862.7 % from 13.0 in 1990 to 125.1 in 2019. However, Poland saw the most pronounced decline, from 14031.4 in 1990 to 5805.2 in 2019, a drop of 58.6% (Figure 1B and Supplementary Table 1). In order to better reflect the changes in the incidence of IBD, the results of EAPC analysis based on ASR showed that Taiwan (China)’s ASR increased the fastest, with an average annual rate of 3.07%, while Poland’s ASR decreased the fastest, with an average annual rate of 3.19% (Figure 1C and Supplementary Table 1). In addition, the United States of America had the highest incidence of IBD in both 1990 and 2019 (1990: 96513.1 per 100000, 95%UI: 83182-112506.7; 2019: 85388 per 100000, 95%UI: 77956.1-94653.4), followed by China (1990: 17221.4 per 100000, 95%UI: 14326.5-20399.5; 2019: 51462 per 100000, 95%UI: 43933-60474.5) (Supplementary Table 2). The results obtained from the AAPC analysis were consistent with the EAPC analysis. The fastest-growing ASR was observed in Taiwan (China), with an average annual growth rate of 3.107% (95%CI: 2.939-3.274), whereas Poland’s ASR declined the fastest, with an average annual average of 3.198% (95%CI: -3.325 to -3.071) (Supplementary Table 2).
Figure 1.
Inflammatory bowel disease burden in 204 countries and territories worldwide. A: Inflammatory bowel disease (IBD) age standardized rate (ASR) in 2019; B: Changes in the incidence of IBD from 1990-2019; C: Estimated annual percent change analysis of IBD ASR from 1990-2019. IBD: Inflammatory bowel disease; ASR: Age-standardized rate; EAPC: Estimated annual percent change.
Through the analysis of ASR in different regions, it was found that the regions with the highest incidence of IBD in 1990 and 2019 were both High-income North America (1990: 34.9 per 100000, 95%UI: 30.6-40; 2019: 24.5 per 100000, 95%UI: 22.6-26.8) and North America (1990: 34.9 per 100000, 95%UI: 30.6-40; 2019: 24.5 per 100000, 95%UI: 22.6-26.8); at the same time, the Oceania region had the lowest ASR (1990: 0.4 per 100000, 95%UI: 0.4-0.5; 2019: 0.6 per 100000, 95%UI: 0.5-0.7). However, the EAPC analysis found that the ASR in the East Asia region had the fastest average annual growth rate of 2.54% (95%CI: 2.4-2.68), while the Central Europe region had the fastest decline, with an average annual ASR decline of 1.38% (95%CI: -1.75 to -1.01) (Table 1).
Table 1.
Incidence and age-standardized incidence of inflammatory bowel disease in various regions of the world from 1990-2019 and temporal trends from 1990-2019
| Region |
1990
|
2019
|
1990-2019
|
||
|
Incident cases, No. (95%UI)
|
ASR per 100000, No. (95%UI)
|
Incident cases, No. (95%UI)
|
ASR per 100000, No. (95%UI)
|
EAPC, No. (95%CI)
|
|
| Africa | 6350.4 (5449.9-7409.6) | 1.4 (1.2-1.6) | 16261.3 (13488.9-19647.2) | 1.6 (1.4-2) | 0.41 (0.35-0.47) |
| African Region | 3832.8 (3242.9-4587.4) | 1.1 (1-1.3) | 10258.1 (8583.6-12449.3) | 1.3 (1.1-1.6) | 0.52 (0.46-0.57) |
| America | 121311.1 (106225.2-139803.3) | 17.8 (15.6-20.4) | 122924.6 (112507.2-135597.1) | 11.1 (10.1-12.2) | -1.3 (-1.64 to -0.96) |
| Andean Latin America | 472.1 (401.7-549.7) | 1.6 (1.4-1.9) | 1128.2 (974.6-1314.4) | 1.8 (1.6-2.1) | -0.05 (-0.28-0.19) |
| Asia | 64767.7 (54186.2-76818.5) | 2.3 (2-2.8) | 145560.7 (124960.3-170895) | 2.9 (2.5-3.4) | 0.78 (0.69-0.86) |
| Australasia | 2979 (2555.6-3481.3) | 13.6 (11.6-15.8) | 6446.3 (5754.6-7211.5) | 20 (17.8-22.6) | 0.94 (0.73-1.15) |
| Caribbean | 676.8 (580.4-796) | 2.1 (1.8-2.5) | 1311.8 (1121-1571.1) | 2.6 (2.2-3.1) | 0.49 (0.34-0.63) |
| Central Asia | 3514.1 (2995-4146.2) | 6 (5.1-7.1) | 6461.3 (5487.2-7636.7) | 6.9 (5.9-8.1) | 0.5 (0.46-0.53) |
| Central Europe | 20996.8 (18082.3-24169.9) | 15.8 (13.6-18.2) | 14808.9 (13442.8-16390.5) | 11.6 (10.5-12.8) | -1.38 (-1.75 to -1.01) |
| Central Latin America | 3967.5 (3401.5-4617.8) | 3.2 (2.7-3.7) | 6753.6 (5833.6-7825.9) | 2.6 (2.3-3.1) | -0.7 (-0.86 to -0.54) |
| Central Sub-Saharan Africa | 381.4 (318.5-468.6) | 1 (0.9-1.3) | 1149.7 (954.9-1399.8) | 1.3 (1.1-1.5) | 0.64 (0.59-0.69) |
| Commonwealth High Income | 26861.8 (24786.3-29244.5) | 22.3 (20.6-24.2) | 37806 (34948.3-41071.2) | 24.5 (22.6-26.6) | 0.42 (0.33-0.52) |
| Commonwealth Low Income | 1939.8 (1595.5-2362) | 1.4 (1.1-1.7) | 5032.5 (4152.5-6067.4) | 1.6 (1.4-2) | 0.61 (0.56-0.66) |
| Commonwealth Middle Income | 18395 (15168.4-22494.1) | 2 (1.7-2.4) | 39643.1 (33048-48435) | 2.1 (1.8-2.6) | 0.44 (0.3-0.58) |
| East Asia | 17567.3 (14626.9-20802.4) | 1.4 (1.2-1.7) | 52318.8 (44680.4-61454.8) | 3 (2.5-3.4) | 2.54 (2.4-2.68) |
| East Asia & Pacific - WB | 40537 (34343.4-47828.7) | 2.3 (2-2.7) | 90853.2 (79210-104418.9) | 3.4 (2.9-3.9) | 1.16 (1.06-1.26) |
| Eastern Europe | 18149.7 (15600.1-21209.1) | 7.1 (6.1-8.3) | 19499.8 (16838-22671) | 7.3 (6.3-8.5) | 0.07 (-0.01-0.16) |
| Eastern Mediterranean Region | 6925.3 (5871.5-8131.4) | 2.5 (2.1-2.9) | 18957.5 (15653.5-22992.9) | 2.9 (2.4-3.5) | 0.54 (0.49-0.59) |
| Eastern Sub-Saharan Africa | 1092.2 (908-1315.3) | 0.9 (0.8-1.1) | 2937.7 (2448.6-3538.4) | 1 (0.9-1.3) | 0.55 (0.49-0.61) |
| Europe | 100967 (89900.9-113361.1) | 11.5 (10.3-13) | 119525.9 (108023.4-132990.6) | 12.5 (11.3-14) | 0.18 (0.11-0.26) |
| Europe & Central Asia - WB | 103166.8 (91767-115892) | 11.2 (10-12.7) | 124122.3 (111829.4-138334.3) | 12 (10.8-13.5) | 0.13 (0.06-0.2) |
| European Region | 103678.4 (92211.4-116480.8) | 11.2 (10-12.6) | 125055.7 (112703.8-139366) | 12 (10.8-13.4) | 0.12 (0.05-0.2) |
| High-income Asia Pacific | 18105.1 (15391.4-21421.8) | 9.2 (7.9-10.9) | 27083.8 (23870.3-30621.1) | 14.9 (13.1-16.9) | 1.1 (0.85-1.36) |
| High-income North America | 107616.3 (94146.8-123884.4) | 34.9 (30.6-40) | 99611.1 (91945.2-108999.8) | 24.5 (22.6-26.8) | -0.82 (-1.18 to -0.47) |
| Latin America & Caribbean - WB | 13797.5 (11970.2-15892.3) | 3.8 (3.3-4.3) | 23456.2 (20522.6-26876.2) | 3.4 (3-3.9) | -0.41 (-0.47 to -0.35) |
| Middle East & North Africa - WB | 5823.7 (5004.6-6721.7) | 2.9 (2.5-3.4) | 15826.6 (13170.5-19047.6) | 3.4 (2.9-4.1) | 0.5 (0.43-0.58) |
| North Africa and Middle East | 8106.7 (7039.5-9203.5) | 2.9 (2.6-3.4) | 22722.3 (19277.1-26951.9) | 3.7 (3.2-4.5) | 0.62 (0.53-0.72) |
| North America | 107604.1 (94136.5-123870) | 34.9 (30.6-40) | 99596.5 (91933-108983) | 24.5 (22.6-26.8) | -0.82 (-1.18 to -0.47) |
| Oceania | 22.5 (18.3-26.8) | 0.4 (0.4-0.5) | 63.2 (51.8-76.5) | 0.6 (0.5-0.7) | 0.87 (0.77-0.98) |
| Region of the Americas | 121311.1 (106225.2-139803.3) | 17.8 (15.6-20.4) | 122924.6 (112507.2-135597.1) | 11.1 (10.1-12.2) | -1.3 (-1.64 to -0.96) |
| South-East Asia Region | 18276.9 (15080.6-22303.3) | 1.7 (1.4-2.1) | 38664.5 (32256.8-47052.4) | 1.9 (1.6-2.3) | 0.57 (0.42-0.73) |
| South Asia | 18439.4 (15168.6-22581.9) | 2.2 (1.8-2.6) | 39704.7 (32943.4-48453.5) | 2.3 (1.9-2.8) | 0.47 (0.32-0.62) |
| South Asia - WB | 18738.6 (15412.5-22942.5) | 2.1 (1.8-2.6) | 40659.5 (33741.9-49588.8) | 2.3 (1.9-2.8) | 0.49 (0.34-0.64) |
| Southeast Asia | 1882 (1527.1-2270.3) | 0.5 (0.4-0.6) | 4980 (4181.8-5972.3) | 0.7 (0.6-0.8) | 1.45 (1.28-1.62) |
| Southern Latin America | 879.4 (741.6-1029.7) | 1.8 (1.5-2.1) | 1382 (1177.4-1619.7) | 1.9 (1.6-2.3) | 0.24 (0.15-0.33) |
| Southern Sub-Saharan Africa | 503.7 (422.7-607.5) | 1.3 (1.1-1.6) | 988.7 (837.3-1188) | 1.4 (1.2-1.6) | 0.13 (0.07-0.2) |
| Sub-Saharan Africa - WB | 3626.5 (3031.8-4397.1) | 1.1 (0.9-1.3) | 9635 (8053-11630.3) | 1.2 (1-1.5) | 0.42 (0.34-0.5) |
| Tropical Latin America | 7825.7 (6858.8-8975.6) | 6.1 (5.4-6.9) | 12927.7 (11333.6-14873.9) | 5.2 (4.6-6) | -0.55 (-0.59 to -0.51) |
| Western Europe | 59080.5 (53732.6-65528.7) | 14 (12.7-15.5) | 78644.7 (71611.1-86708.9) | 16.9 (15.3-18.7) | 0.6 (0.56-0.65) |
| Western Pacific Region | 39273.1 (33180.5-46324.7) | 2.6 (2.2-3.1) | 88120.1 (76953.2-101217.2) | 4 (3.5-4.5) | 1.25 (1.15-1.35) |
| Western Sub-Saharan Africa | 1313.7 (1101.9-1598.6) | 1 (0.9-1.2) | 3628.3 (3028.5-4400.8) | 1.2 (1-1.4) | 0.36 (0.22-0.49) |
| World Bank High Income | 206040 (182079.4-234728.1) | 18.8 (16.6-21.4) | 225597.7 (206227.4-249141.7) | 17.1 (15.6-18.9) | -0.24 (-0.39 to -0.08) |
| World Bank Low Income | 2388.3 (1998.4-2891.8) | 1 (0.9-1.3) | 6306.6 (5252.9-7655.6) | 1.2 (1.1-1.5) | 0.56 (0.52-0.6) |
| World Bank Lower Middle Income | 30142.4 (25192.1-36034.9) | 2 (1.7-2.4) | 63401.3 (53275.5-76198.6) | 2.1 (1.8-2.6) | 0.29 (0.2-0.37) |
| World Bank Upper Middle Income | 54916.9 (47252.2-63473.1) | 2.8 (2.5-3.3) | 109097 (94356.3-126861) | 3.5 (3.1-4.1) | 0.74 (0.67-0.8) |
ASR: Age standardized rate; CI: Confidence interval; EAPC: Estimated annual percentage change; UI: Uncertainty interval.
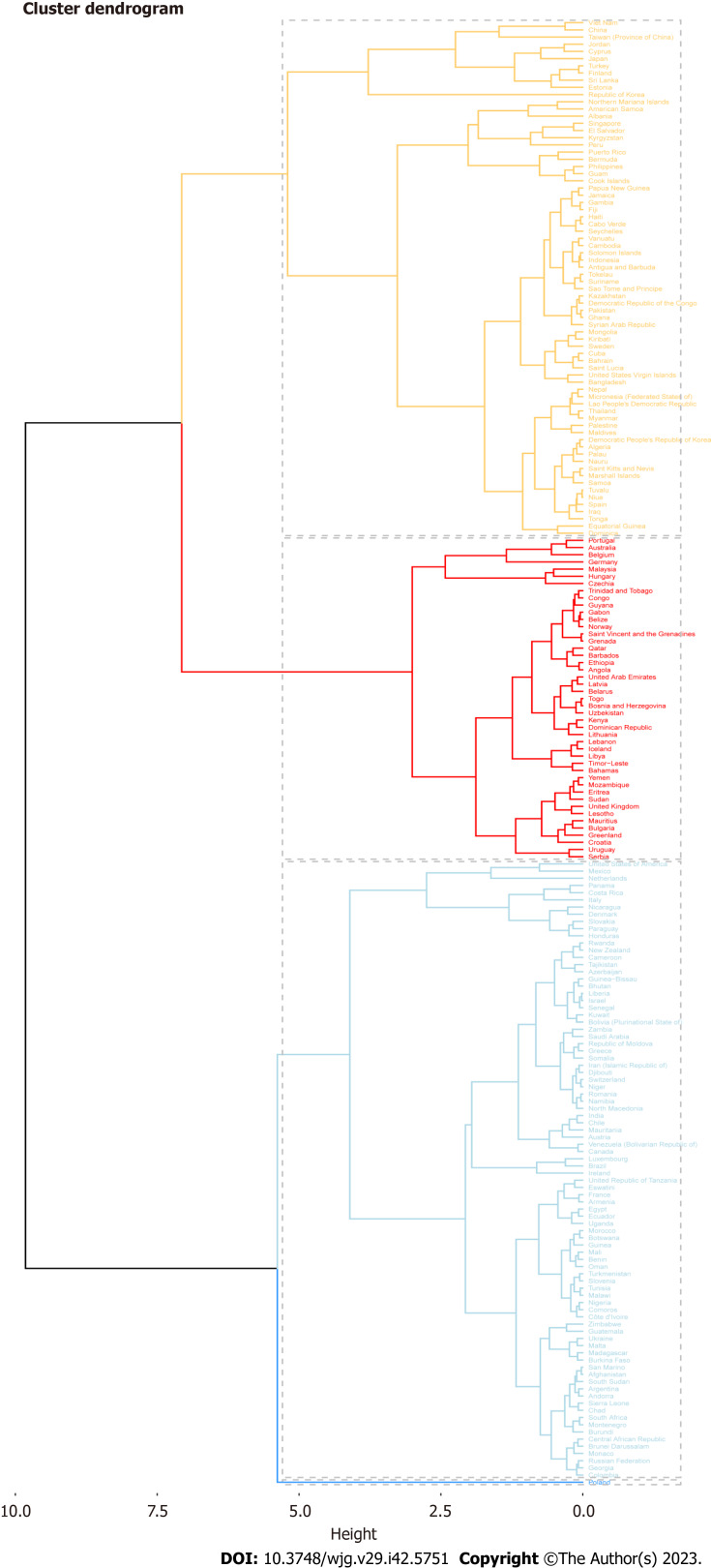
Seventy-two countries (or regions) including Vietnam, China, Taiwan (China), and Jordan were classified as the “significantly increased” group in the cluster analysis (Figure 2). Forty-five countries (or territories), including Portugal, Australia, Belgium, and Germany, were placed in the “small growth” group. Eighty-six countries (or territories), including the United States of America, Mexico, the Netherlands, and Panama, were included in the “stable or slightly declining” group. Only one country, Poland, was included in the “significant decrease” group (Supplementary Figure 1).
Figure 2.
Cluster analysis: Estimated annual percent change data combining incidence and deaths.
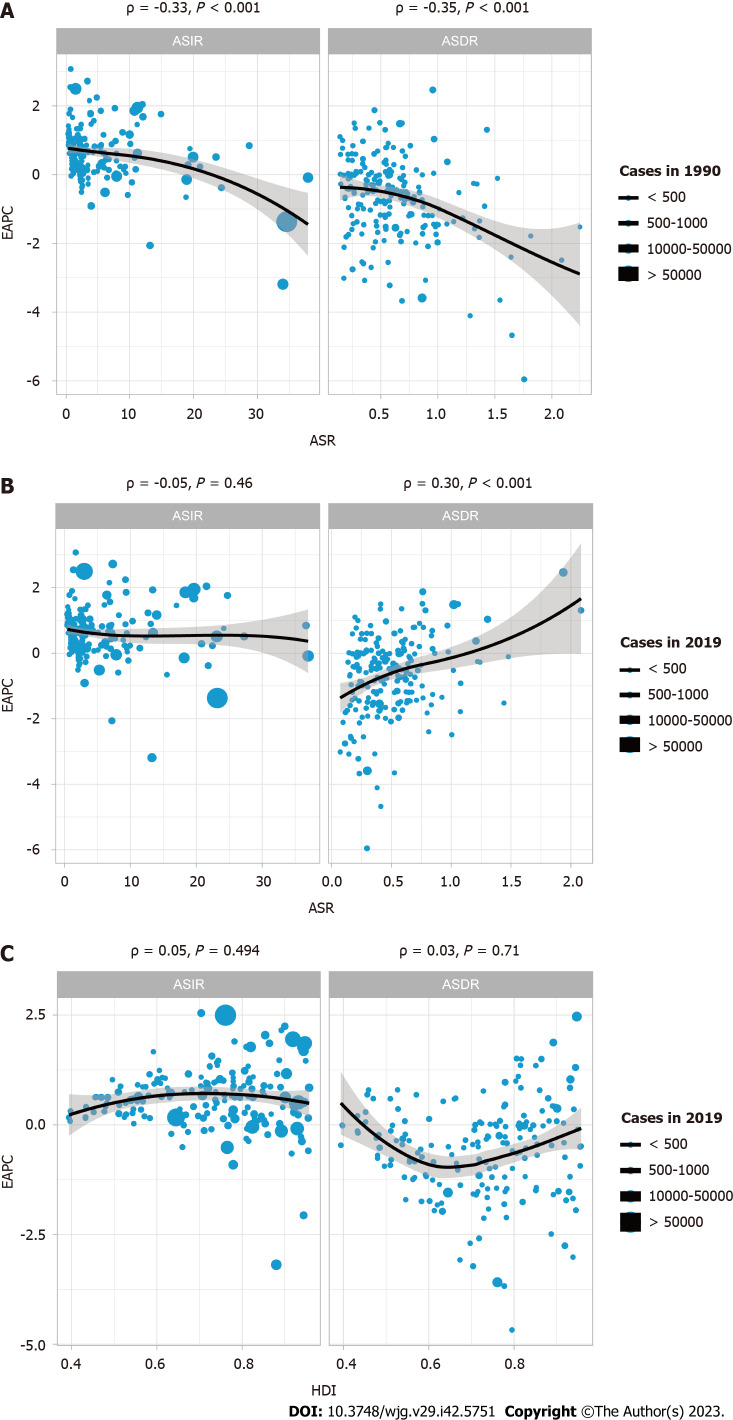
Correlation analysis of EAPC with ASR and HDI
To determine whether there was a potential association among EAPC, ASR, and HDI, a correlation analysis was performed. HDI data were obtained from Human Development Reports (http://hdr.undp.org/en/composite/HDI). As shown in Figure 3A, in 1990, the EAPC was significantly negatively correlated with both ASIR (ρ = -0.33, P < 0.001) and age-standardized death rates (ASDR) (ρ = -0.35, P < 0.001). By 2019, Figure 3B showed there was no significant correlation between EAPC and ASIR (ρ = -0.05, P = 0.46); more interestingly, at this time, EAPC and ASDR (ρ = 0.30, P < 0.001) showed a significant positive correlation. This indicates that the incidence of IBD has changed significantly over the past 30 years. In addition, the analysis found that there was no significant correlation between HDI and EAPC (both P > 0.05).
Figure 3.
Correlation of estimated annual percent change with inflammatory bowel disease age standardized rate and human development index. A: Correlation of estimated annual percent change (EAPC) with inflammatory bowel disease (IBD) age-standardized incidence rates (ASIR) and age-standardized death rates (ASDR) in 1990; B: Correlation of EAPC with IBD ASIR and ASDR in 2019; C: Correlation of EAPC with human development index in 2019. The circles in the figure represent countries, and the size of the circles represents the number of IBD cases. ρ and P values were obtained using the Pearson correlation coefficient analysis. EAPC: Estimated annual percent change; ASR: Age standardized rate; HDI: Human development index.
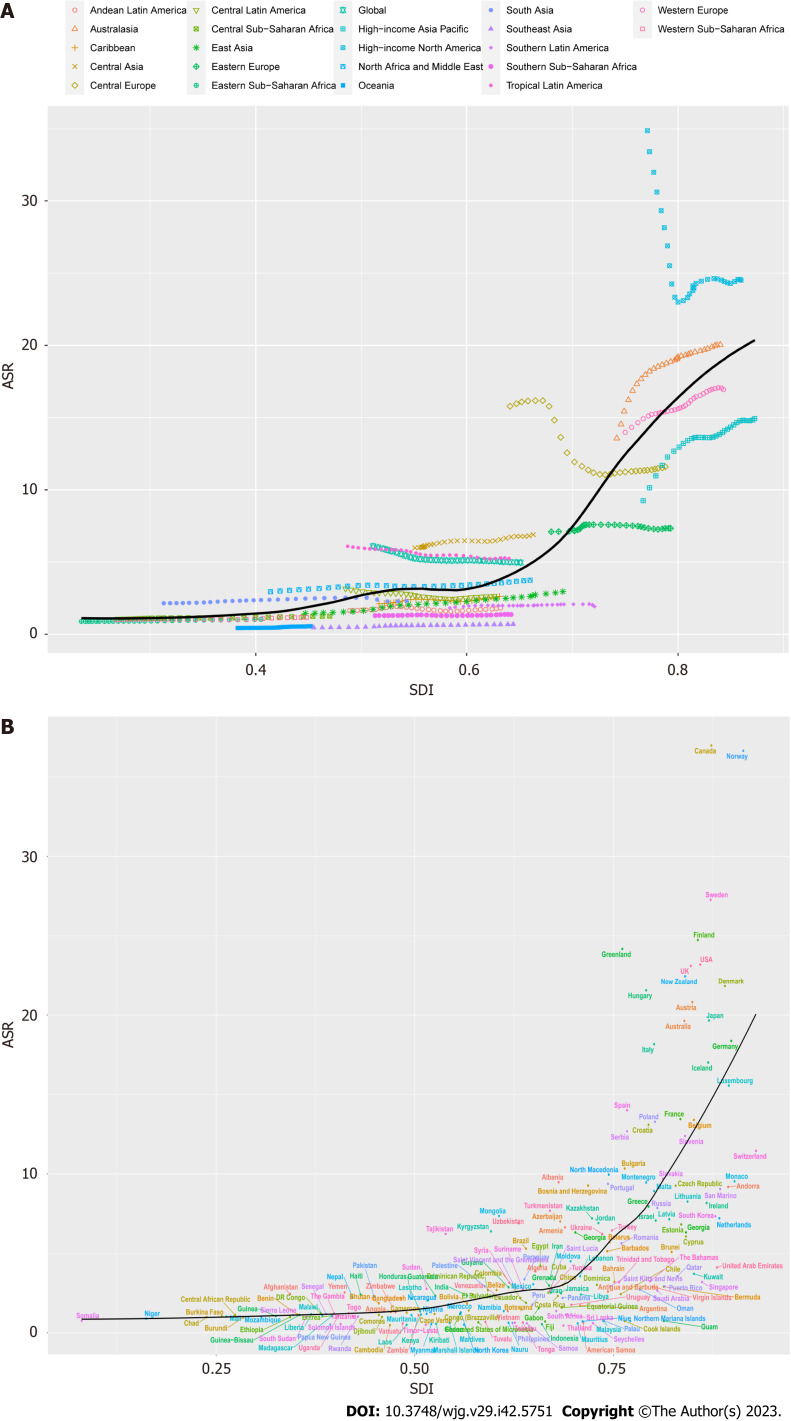
Trends of IBD ASR in various regions based on SDI
As shown in Figure 4, we investigated the relationship between ASR and SDI, and further analyzed the expected levels of SDI-based ASR at different locations. Research shows that Eastern, Western, and Central sub-Saharan Africa, East Asia, Andean Latin America, and the Caribbean are closely related to the expected ASR trends. In the mid-rear region of the SDI, the ASR is highly variable. On the one hand, some regions were much lower than expected and the ASR remained basically unchanged throughout the study period, other regions were much higher than expected with significant fluctuations in the ASR (Figure 4A). According to the analysis of each country, it is found that the ASR of IBD was significantly and positively correlated with the SDI in 2019, and the ASR of IBD in countries with higher SDI levels was also higher (Figure 4B).
Figure 4.
Trends in inflammatory bowel disease age standardized rate by sociodemographic index from 1990-2019. A: Twenty-one Global Burden of Diseases regions are shown; B: 204 countries and territories are shown. The expected values for sociodemographic indices and disease incidence based on all sites are shown as black lines. ASR: Age standardized rate; SDI: Socio-demographic index.
Prediction of global IBD incidence
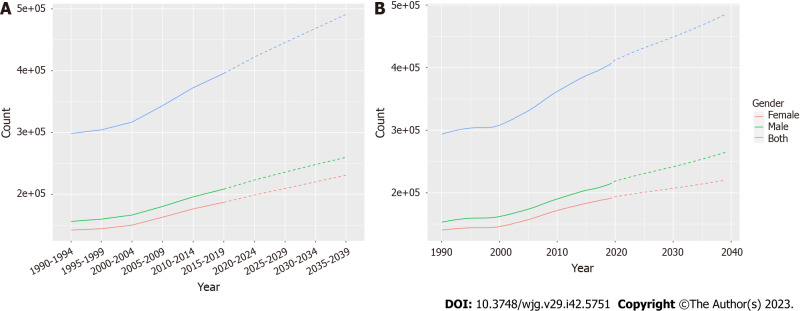
To further understand the future incidence of IBD globally, we used the Nordpred and BAPC packages of R software to predict it, respectively. The incidence of IBD in all age groups increased annually. Based on the Nordpred analysis, the total number of cases is predicted to increase from 298412 in 1990-1994 to 490887 in 2035-2039, with the incidence among men being consistently higher than that among women (Figure 5A and Supplementary Table 3). The analysis results based on the BAPC model also proved the same conclusion, and the total number of cases is predicted to increase from 298393 to 476402 with a higher incidence among males than that of females (Figure 5B and Supplementary Table 3).
Figure 5.
Statistics and predictions of the number of cases in all age groups. A: Prediction based on Nordpred model; B: Prediction based on Bayesian age-period-cohort model.
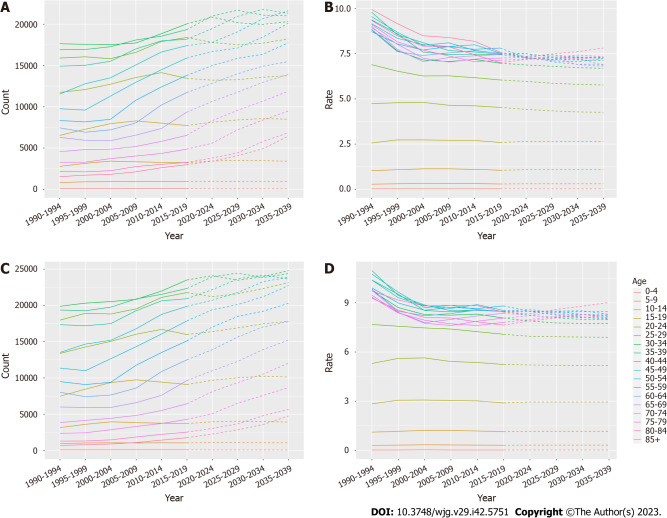
The number and incidence of IBD in each age group were analyzed. Overall, the total number of women and men with IBD showed an upward trend, but the incidence showed a downward trend, and the overall trend increase was higher in males than in females (Figure 6, Supplementary Tables 4 and 5). To assess the robustness of the predicted results, the BAPC model was used to predict the future burden of IBD. The predicted results of the BAPC model showed a consistent trend with the above results (Supplementary Figure 1, Supplementary Tables 6-8).
Figure 6.
Inflammatory bowel disease incidence from 1990-2019 and projections for the next 20 years, stratified by gender. A: Statistics and projections of the number of female patients with inflammatory bowel disease (IBD) are shown; B: Statistics and predictions of the female incidence of IBD are shown; C: Statistics and projections of the number of male patients with IBD are shown; D: Statistics and predictions of the male incidence of IBD are shown. Prediction of changes over the next 20 years are based on the Nordpred model.
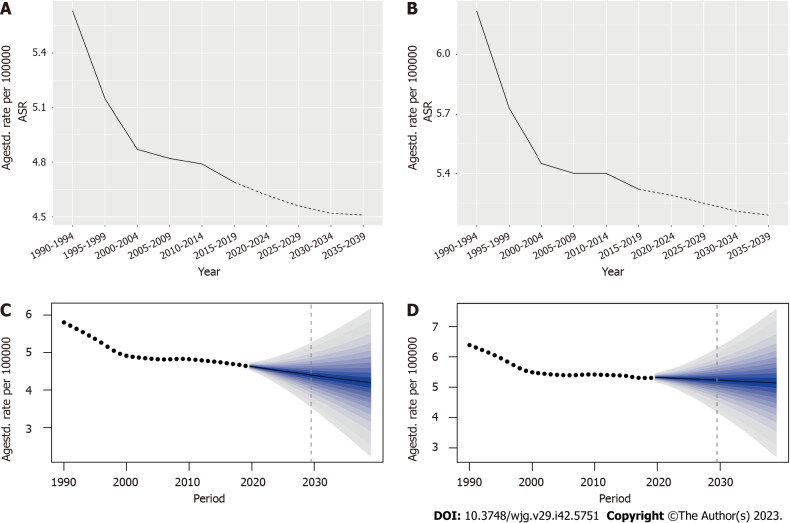
In order to better predict the future incidence of IBD, we divided the patients by gender and, analyzed and predicted the changes in the ASR of IBD. According to the Nordpred analysis, the ASR of females is predicted to decrease from 5.63 per 100000 in 1990-1994 to 4.51 in 2035-2039; and the ASR of males is predicted to decrease from 6.22 per 100000 to 5.19 during the same period (Figure 7 and Supplementary Table 9). The prediction results of the BAPC model also showed that the ASR of females decreased from (5.79983 ± 1.52e-07) per 100000 in 1990 to (4.19518 ± 1e-05) in 2039 and a decreased ASR in males from (6.38098 ± 1.64e-07) per 100000 to (5.14319 ± 1.24e-05) (Figure 7 and Supplementary Table 10). Therefore, combined with the previous analysis results for each age group (Figure 6), we can confirm that the overall incidence of IBD is declining, and the incidence of males is higher than that in females.
Figure 7.
Statistics and predictions of inflammatory bowel disease age standardized rate for different genders. A and B: The age standardized rate (ASR) statistics and predictions based on the Nordpred model for female and male, respectively; C and D: The ASR statistics and predictions for female and male based on the Bayesian age-period-cohort model, respectively. ASR: Age standardized rate.
DISCUSSION
In this study, we conducted a comprehensive analysis on the burden of IBD at the global, regional, and national levels. The GBD 2019 report estimated that there were approximately 4.9 million cases of IBD worldwide, and the number of cases is on the rise. In our study, we found that the regions with the highest ASIR of IBD were predominantly high-income regions of North America, Canada, and Norway, which was consistent with previous findings[20,21]. Conversely, Oceania, East Timor, Cambodia, the Maldives, Papua New Guinea, Laos, and Thailand had the lowest ASR of IBD, suggesting a possible correlation among urban industrialization, environmental factors, and hygiene conditions in these areas. Additionally, different dietary habits in various countries, such as a high-fat, low-fiber, high-sugar diet or the consumption of processed foods, may contribute to the occurrence of the disease[22-24]. Moreover, the incidence of IBD is related to income level. In 2019, the ASR of IBD and the SDI were significantly and positively correlated on a global scale. Countries with higher SDI also exhibited a higher incidence of IBD, which could be explained by individuals with higher social status undergoing more routine and specialized examinations, resulting in higher diagnostic rates[25]. The delayed or limited exposure to common infectious diseases during early childhood may also account for this correlation. Consequently, in genetically susceptible individuals, the immune response underwent alterations, leading to an increased incidence of IBD[26,27]. Piovani et al[28] also reported a significant increase in the prevalence of IBD in countries with low and medium-low SDI. This shift may be attributed to increasing Westernization trends in these regions, which have introduced new environmental exposures that contributed to the increasing incidence of IBD[20,29,30]. In countries with a low SDI, disease-related premature mortality was a major component of the IBD burden, whereas in countries with a high SDI, the proportion was relatively low[28]. This may reflect variations in healthcare services, infrastructure, disease diagnoses, and awareness among different countries.
IBD has long been regarded as a disease that is primarily prevalent in industrialized, Western countries. However, the EAPC analysis included in this study revealed that the average annual growth rate of the ASR was highest in East Asia, whereas Central Europe had the most rapidly decreasing ASR. Furthermore, a significant decrease in ASR was observed in the high-income regions of North America, which was consistent with the previous reports[20,31] and indicated a noteworthy shift in the epidemiological stage of IBD in Western countries. As Western countries experienced a widespread aging population, the number of individuals diagnosed with IBD is projected to increase despite the stable or declining incidence rates of IBD[32]. Economic improvements have resulted in improvements to healthcare infrastructure, including advances in medical resources such as colonoscopy examinations. Consequently, the diagnostic rate of IBD has increased in low-income regions, leading to an increasing incidence of IBD in emerging industrialized nations such as Asia. The cluster analysis results of the current study further support this trend, revealing a significant surge in the incidence of IBD in Asian regions, such as Vietnam, China, Taiwan, and Jordan. This transformation may be attributed to urbanization, industrialization, and cultural Westernization in societies. In addition, the actual increase in IBD incidence may be due to an increased number of individuals with genetically-susceptible genes to an environment influenced by Westernization[33]. In densely populated areas, residents experience the influence of Westernization, leading to changes in diet with higher fat and refined sugar consumption. Lifestyles changes as well including increased smoking, reduced breastfeeding, greater exposure to antibiotics, and improvements in personal hygiene and sanitation, may also play a role[34]. All of these factors have contributed to the increase in the incidence of IBD, which exerted significant pressure on the populations. The reported IBD incidence varied among emerging industrialized countries in Asia and within different regions of Asian countries. For example, from 2011-2013, the IBD incidence was 1.14 per 100000 (95%CI: 0.91-1.41) in Singapore, while in India was 9.31 per 100000 (95%CI: 8.38-10.31)[35]. Overall, the highest incidences rates have been observed in India and China, as the published incidence rates are approaching the Coalescing Incidence Range in the Western world[20,35,36]. Due to the large population proportions and continuously increasing gross domestic product of the economy, IBD may become a substantial healthcare burden in these countries. Furthermore, the current study revealed that the increasing trend of IBD incidence has gradually extended to East and Southeast Asia.
While previous studies have reported that the global IBD incidence was higher in females than males[37], our analysis of IBD cases from different age groups revealed that both females and males had an increasing trend in the number of cases, but a declining trend in incidence. The overall incidence was higher in males than in females in this study. The IBD incidence predictions for the next 20 years by BAPC and Nordpred models indicate a decreasing trend in ASR for both males and females, with a higher incidence in males than in females. Based on the heterogeneity among different geographical regions, significant disparities exist in the incidence of IBD between genders. These disparities were influenced not only by biological factors but also by variations in environmental exposure and access to healthcare services[38,39]. Males exhibited more severe disease symptoms of IBD compared to females, and premature mortality confirmed that this constituted a significant portion of the burden in males[28]. Additionally, sex hormones may influence the pathogenesis of IBD and the function of the intestinal epithelial barrier[37,40]. Moreover, some certain genetic loci associated with IBD are located on the X chromosome, indicating the potential involvement of sex-related factors in the development of the disease[41].
This study revealed a continuous increase in IBD cases paired with a declining trend in the global ASIR of IBD. The population is aging over time, resulting in more elderly IBD individuals in 2020 compared to 1990. For instance, the annual increase in IBD prevalence among elderly individuals between 1999 and 2008 in Ontario, Canada was 5.2%[42]. Furthermore, in terms of life expectancy, the elderly population with IBD experienced a slightly increased risk of mortality compared to both the age-related comorbidity group (such as cardiovascular diseases and cancer) and the general control group in previous studies[43,44]. Therefore, with an aging population and extended life expectancies, healthcare systems face a significant demographic burden. While the IBD population in the Western world is expected to eventually transition into a phase of balanced disease prevalence and even decline in some regions[33]. Furthermore, there were significant variations in the ASIR among the GBD regions, and we found that East Asia exhibited the largest upward trend, while Central Europe showed the most rapid decline, which were consistent with the previous report[20].
The increasing incidence of IBD in emerging industrialized nations and the heterogeneity of reported values may be attributed to several factors. First, economic progress has improved awareness regarding IBD and provided greater access to healthcare, leading to the identification of undiagnosed cases. Second, the Westernization of society has contributed to an increase in the prevalence of IBD. With advancements in healthcare facilities, the epidemiological surveillance strategies towards electronic medical databases, and the implementation of endoscopic examinations, the detection and diagnosis of IBD have increased. Over time, there has been a steady increase in the reported incidence of IBD. However, studies have shown that age-standardized disability-adjusted life years and mortality rates had a declining trend globally[28], which might be attributed to improved treatment strategies and the implementation of patient support programs. The introduction of biological agents has significantly improved IBD treatment strategies, enhanced patient quality of life, and reduced the rates of surgical intervention[45]. Recent emerging therapies showed immense potential for the treatment of IBD, for example, the targeted adhesion molecule therapies, cytokine inhibitors, stem cell therapies, gut-brain axis modulation, and host-microbiome interaction regulation have enhanced the efficacy of IBD treatment and reduced the adverse events and overall mortality[46]. However, the cost of these biological therapies was considerably high, imposing a substantial financial burden on patients[47]. Therefore, it is crucial to focus more on the inclusion of early stage patients, optimization of drug dosage, and determination of the appropriate time for trial discontinuation to alleviate such financial burden.
This study has several strengths. First, it included a large, population-based dataset covering numerous countries and regions, including Asia. Second, the associations and significances of various demographic indicators in different areas were analyzed. We forecast the number and incidence of IBD from 2019-2039 based on the R software and validated the reliability of the results. Variations in the incidence of IBD may reflect differences in urbanization rates, living conditions, or dietary factors[48]. The data in this study may guide research targeting specific environmental risk factors for IBD and be used to determine the burden of IBD on society.
However, the study also has some limitations. First, the data obtained from the GBD 2019 is limited. As for regions with scarce data, we have to rely on predictive covariates or global trends, which leads to poor representativeness. Therefore, further population-based high-quality research is needed, especially in countries with scarce data. Second, we cannot establish a causal relationship between the population data and the incidence of IBD as they are ecological associations. We also did not investigate the dose-response effects to demonstrate whether the risk of IBD increases with the rising population size or levels of multiple exposure factors. In regions and countries with a lower SDI, patients may be less likely to be diagnosed with IBD due to limited access to healthcare, resulting in an underestimation and underreporting of the disease burden. Therefore, the reported incidence may be lower than the actual incidence in these areas. Further studies should focus on regional variations in IBD incidence, taking into account the potential influences of healthcare conditions, lifestyles, and the environment with an emphasis on the associations between IBD and certain risk factors. In conclusion, based on the global burden of IBD from 1990-2019 and the prediction for the next 20 years trend, the latest global epidemiological estimates for IBD and new hypotheses are provided in this study to improve the management of IBD and alleviate the global burden of this chronic disease.
CONCLUSION
This study provides insights into monitoring the burden and trends of IBD at global, regional, and national levels, which is crucial for implementing interventions to address the increasing burden of IBD. The findings revealed that IBD continues to pose a significant disease burden worldwide, particularly in regions with higher SDI, where the incidence of IBD is higher. However, in recent years, the incidence of IBD has rapidly increased in emerging industrialized and developing countries. Therefore, it is imperative to prioritize the prevention, management, and recurrence of IBD with a focus on closely-related factors such as infections, diet, biomarkers, disease treatments, lifestyle, psychology, and genetics. Emphasizing personal hygiene and health education, improving nutritional status, and implementing early treatment are essential methods to alleviate the burden of IBD. Further research is needed to determine more effective public health interventions.
ARTICLE HIGHLIGHTS
Research background
Inflammatory bowel disease (IBD) is an intestinal inflammatory disorder of unknown origin with a prevalence that varies across different countries and regions. To develop effective strategies for the prevention and treatment of IBD, a comprehensive understanding of the current burden and distinct trends in various geographical areas are crucial. There is a scarcity of research for the prediction of the global burden of IBD and future epidemiological trends.
Research motivation
Data from the Global Burden of Disease (GBD) database 2019 were used to assess the burden of IBD across 204 countries and regions using various indicators from 1990-2019. Previous studies lacked the use of specific models for forecasting future trends in IBD and did not validate the reliability of their findings. Our findings offer new insights regarding the management of IBD.
Research objectives
This study aimed to conduct a thorough investigation of IBD data and predict future epidemiological trends to provide the latest estimates of the global burden of IBD and improve the management strategies.
Research methods
The incidence data for IBD were collected from the GBD study from 1990-2019. The average annual percentage change and estimated annual percentage change (EAPC) in age-standardized rates (ASR) of IBD were calculated for various regions. The relationships among IBD, the human development index, and the socio-demographic index (SDI) were analyzed. The Nordpred and Bayesian age-period-cohort models were used to predict the prevalence trends of IBD from 2019-2039.
Research results
North America consistently maintained the highest IBD ASR, while Oceania consistently had the lowest ASR. East Asia had the fastest average annual growth in ASR (2.54%), whereas Central Europe had the most rapid decline (1.38%) in ASR. Nations that had low age-standardized incidence rates in 1990 exhibited accelerated IBD growth, although no significant correlation was observed in 2019. Additionally, IBD grew faster in countries with low age-standardized death rates in 1990, whereas the opposite was true in 2019. An examination of the SDI and IBD ASR revealed that countries with a high SDI generally exhibited a higher IBD ASR. Finally, the projections indicated a decreasing trend in IBD incidence from 2019-2039 but a gradual increase in the total number of cases.
Research conclusions
IBD poses a substantial disease burden globally, particularly in regions with higher SDI scores, which are associated with increased IBD incidence. Nevertheless, in recent years, the incidence of IBD has rapidly increased in emerging industrialized and developing nations. Therefore, it is imperative to prioritize the prevention, management, and reduction of IBD cases, with particular consideration for the associated factors.
Research perspectives
The investigation of regional variations in the incidence of IBD is required, and it is imperative to investigate the associations between IBD and various risk factors.
ACKNOWLEDGEMENTS
We are grateful to all the participants who contributed to this study. We thank the Global Burden of Disease database, where all the data is publicly available in an open access repository.
Footnotes
Institutional review board statement: The study was wavied by the IRB of the First Affiliated Hospital of Xi’an Jiaotong University due to all the data used in this study were from the Global Burden of Diseases (GBD) 2019 database. This database comprises publicly accessible data, freely available in an open-access repository, ensuring that all information used was already in the public domain.
Informed consent statement: There was no requirement to give informed consent in this study, because all the data used in this study were from the Global Burden of Diseases (GBD) 2019 database. This database comprises publicly accessible data, freely available in an open-access repository, ensuring that all information used was already in the public domain.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: July 25, 2023
First decision: September 30, 2023
Article in press: October 30, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: M'Koma AE, United States; Ulasoglu C, Turkey S-Editor: Wang JJ L-Editor: A P-Editor: Yuan YY
Contributor Information
Cheng-Jun Li, Department of Gastroenterology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710061, Shaanxi Province, China.
Yi-Kai Wang, Department of Infectious Diseases, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710004, Shaanxi Province, China.
Shun-Ming Zhang, School of Public Health, Xi’an Jiaotong University Health Science Center, Xi’an 710061, Shaanxi Province, China.
Mu-Dan Ren, Department of Gastroenterology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710061, Shaanxi Province, China.
Shui-Xiang He, Department of Gastroenterology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710061, Shaanxi Province, China. dyyyjxk@mail.xjtu.edu.cn.
Data sharing statement
Dataset can be available.
References
- 1.Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. 2019;12:113–122. doi: 10.25122/jml-2018-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J Immunol Res. 2019;2019:7247238. doi: 10.1155/2019/7247238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vilela EG, Torres HO, Martins FP, Ferrari Mde L, Andrade MM, Cunha AS. Evaluation of inflammatory activity in Crohn's disease and ulcerative colitis. World J Gastroenterol. 2012;18:872–881. doi: 10.3748/wjg.v18.i9.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen B. Interventional IBD: The Role of Endoscopist in the Multidisciplinary Team Management of IBD. Inflamm Bowel Dis. 2018;24:298–309. doi: 10.1093/ibd/izx058. [DOI] [PubMed] [Google Scholar]
- 5.Dowdell AS, Colgan SP. Metabolic Host-Microbiota Interactions in Autophagy and the Pathogenesis of Inflammatory Bowel Disease (IBD) Pharmaceuticals (Basel) 2021;14 doi: 10.3390/ph14080708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol. 2016;22:4794–4801. doi: 10.3748/wjg.v22.i20.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen N, Duricova D, Elkjaer M, Gamborg M, Munkholm P, Jess T. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am J Gastroenterol. 2010;105:1480–1487. doi: 10.1038/ajg.2009.760. [DOI] [PubMed] [Google Scholar]
- 8.Mao R, Hu PJ. The Future of IBD Therapy: Where Are We and Where Should We Go Next? Dig Dis. 2016;34:175–179. doi: 10.1159/000443135. [DOI] [PubMed] [Google Scholar]
- 9.Park J, Park S, Lee SA, Park SJ, Cheon JH. Improving the care of inflammatory bowel disease (IBD) patients: perspectives and strategies for IBD center management. Korean J Intern Med. 2021;36:1040–1048. doi: 10.3904/kjim.2021.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kammermeier J, Lamb CA, Jones KDJ, Anderson CA, Baple EL, Bolton C, Braggins H, Coulter TI, Gilmour KC, Gregory V, Hambleton S, Hartley D, Hawthorne AB, Hearn S, Laurence A, Parkes M, Russell RK, Speight RA, Travis S, Wilson DC, Uhlig HH. Genomic diagnosis and care co-ordination for monogenic inflammatory bowel disease in children and adults: consensus guideline on behalf of the British Society of Gastroenterology and British Society of Paediatric Gastroenterology, Hepatology and Nutrition. Lancet Gastroenterol Hepatol. 2023;8:271–286. doi: 10.1016/S2468-1253(22)00337-5. [DOI] [PubMed] [Google Scholar]
- 11.Qiu P, Ishimoto T, Fu L, Zhang J, Zhang Z, Liu Y. The Gut Microbiota in Inflammatory Bowel Disease. Front Cell Infect Microbiol. 2022;12:733992. doi: 10.3389/fcimb.2022.733992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catalan-Serra I, Brenna Ø. Immunotherapy in inflammatory bowel disease: Novel and emerging treatments. Hum Vaccin Immunother. 2018;14:2597–2611. doi: 10.1080/21645515.2018.1461297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen NH, Martinez I, Atreja A, Sitapati AM, Sandborn WJ, Ohno-Machado L, Singh S. Digital Health Technologies for Remote Monitoring and Management of Inflammatory Bowel Disease: A Systematic Review. Am J Gastroenterol. 2022;117:78–97. doi: 10.14309/ajg.0000000000001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argollo M, Gilardi D, Peyrin-Biroulet C, Chabot JF, Peyrin-Biroulet L, Danese S. Comorbidities in inflammatory bowel disease: a call for action. Lancet Gastroenterol Hepatol. 2019;4:643–654. doi: 10.1016/S2468-1253(19)30173-6. [DOI] [PubMed] [Google Scholar]
- 15.Tavakoli P, Vollmer-Conna U, Hadzi-Pavlovic D, Grimm MC. A Review of Inflammatory Bowel Disease: A Model of Microbial, Immune and Neuropsychological Integration. Public Health Rev. 2021;42:1603990. doi: 10.3389/phrs.2021.1603990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghouri YA, Tahan V, Shen B. Secondary causes of inflammatory bowel diseases. World J Gastroenterol. 2020;26:3998–4017. doi: 10.3748/wjg.v26.i28.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knowles SR, Graff LA, Wilding H, Hewitt C, Keefer L, Mikocka-Walus A. Quality of Life in Inflammatory Bowel Disease: A Systematic Review and Meta-analyses-Part I. Inflamm Bowel Dis. 2018;24:742–751. doi: 10.1093/ibd/izx100. [DOI] [PubMed] [Google Scholar]
- 18.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Jiang Y, Yuan H, Fang Q, Cai N, Suo C, Jin L, Zhang T, Chen X. The trends in incidence of primary liver cancer caused by specific etiologies: Results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J Hepatol. 2019;70:674–683. doi: 10.1016/j.jhep.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 21.Ye Y, Manne S, Treem WR, Bennett D. Prevalence of Inflammatory Bowel Disease in Pediatric and Adult Populations: Recent Estimates From Large National Databases in the United States, 2007-2016. Inflamm Bowel Dis. 2020;26:619–625. doi: 10.1093/ibd/izz182. [DOI] [PubMed] [Google Scholar]
- 22.Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR, Fuchs CS, Willett WC, Richter JM, Chan AT. A prospective study of long-term intake of dietary fiber and risk of Crohn's disease and ulcerative colitis. Gastroenterology. 2013;145:970–977. doi: 10.1053/j.gastro.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, Kuperman Y, Harmelin A, Kolodkin-Gal I, Shapiro H, Halpern Z, Segal E, Elinav E. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–186. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 24.Ananthakrishnan AN. Environmental risk factors for inflammatory bowel diseases: a review. Dig Dis Sci. 2015;60:290–298. doi: 10.1007/s10620-014-3350-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernstein CN, Kraut A, Blanchard JF, Rawsthorne P, Yu N, Walld R. The relationship between inflammatory bowel disease and socioeconomic variables. Am J Gastroenterol. 2001;96:2117–2125. doi: 10.1111/j.1572-0241.2001.03946.x. [DOI] [PubMed] [Google Scholar]
- 26.Alexander KL, Targan SR, Elson CO 3rd. Microbiota activation and regulation of innate and adaptive immunity. Immunol Rev. 2014;260:206–220. doi: 10.1111/imr.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146:1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piovani D, Danese S, Peyrin-Biroulet L, Bonovas S. Inflammatory bowel disease: estimates from the global burden of disease 2017 study. Aliment Pharmacol Ther. 2020;51:261–270. doi: 10.1111/apt.15542. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan GG, Ng SC. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology. 2017;152:313–321.e2. doi: 10.1053/j.gastro.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 31.Zhao M, Gönczi L, Lakatos PL, Burisch J. The Burden of Inflammatory Bowel Disease in Europe in 2020. J Crohns Colitis. 2021;15:1573–1587. doi: 10.1093/ecco-jcc/jjab029. [DOI] [PubMed] [Google Scholar]
- 32.Coward S, Benchimol EI, Clement F, Hazlewood G, Kuenzig E, McBrien K, Deardon R, Panaccione R, Seow C, Windsor JW, Kaplan GG. The evolving incidence of inflammatory bowel disease: What will the future hold? J Can Assoc Gastroenterol. 2019;2:56–58. [Google Scholar]
- 33.Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18:56–66. doi: 10.1038/s41575-020-00360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng SC, Tang W, Leong RW, Chen M, Ko Y, Studd C, Niewiadomski O, Bell S, Kamm MA, de Silva HJ, Kasturiratne A, Senanayake YU, Ooi CJ, Ling KL, Ong D, Goh KL, Hilmi I, Ouyang Q, Wang YF, Hu P, Zhu Z, Zeng Z, Wu K, Wang X, Xia B, Li J, Pisespongsa P, Manatsathit S, Aniwan S, Simadibrata M, Abdullah M, Tsang SW, Wong TC, Hui AJ, Chow CM, Yu HH, Li MF, Ng KK, Ching J, Wu JC, Chan FK, Sung JJ Asia-Pacific Crohn's and Colitis Epidemiology Study ACCESS Group. Environmental risk factors in inflammatory bowel disease: a population-based case-control study in Asia-Pacific. Gut. 2015;64:1063–1071. doi: 10.1136/gutjnl-2014-307410. [DOI] [PubMed] [Google Scholar]
- 35.Ng SC, Kaplan GG, Tang W, Banerjee R, Adigopula B, Underwood FE, Tanyingoh D, Wei SC, Lin WC, Lin HH, Li J, Bell S, Niewiadomski O, Kamm MA, Zeng Z, Chen M, Hu P, Ong D, Ooi CJ, Ling KL, Miao Y, Miao J, Janaka de Silva H, Niriella M, Aniwan S, Limsrivilai J, Pisespongsa P, Wu K, Yang H, Ng KK, Yu HH, Wang Y, Ouyang Q, Abdullah M, Simadibrata M, Gunawan J, Hilmi I, Lee Goh K, Cao Q, Sheng H, Ong-Go A, Chong VH, Ching JYL, Wu JCY, Chan FKL, Sung JJY. Population Density and Risk of Inflammatory Bowel Disease: A Prospective Population-Based Study in 13 Countries or Regions in Asia-Pacific. Am J Gastroenterol. 2019;114:107–115. doi: 10.1038/s41395-018-0233-2. [DOI] [PubMed] [Google Scholar]
- 36.Kedia S, Ahuja V. Epidemiology of Inflammatory Bowel Disease in India: The Great Shift East. Inflamm Intest Dis. 2017;2:102–115. doi: 10.1159/000465522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah SC, Khalili H, Gower-Rousseau C, Olen O, Benchimol EI, Lynge E, Nielsen KR, Brassard P, Vutcovici M, Bitton A, Bernstein CN, Leddin D, Tamim H, Stefansson T, Loftus EV Jr, Moum B, Tang W, Ng SC, Gearry R, Sincic B, Bell S, Sands BE, Lakatos PL, Végh Z, Ott C, Kaplan GG, Burisch J, Colombel JF. Sex-Based Differences in Incidence of Inflammatory Bowel Diseases-Pooled Analysis of Population-Based Studies From Western Countries. Gastroenterology. 2018;155:1079–1089.e3. doi: 10.1053/j.gastro.2018.06.043. [DOI] [PubMed] [Google Scholar]
- 38.Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Lytras T, Bonovas S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology. 2019;157:647–659.e4. doi: 10.1053/j.gastro.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 39.Shannon G, Jansen M, Williams K, Cáceres C, Motta A, Odhiambo A, Eleveld A, Mannell J. Gender equality in science, medicine, and global health: where are we at and why does it matter? Lancet. 2019;393:560–569. doi: 10.1016/S0140-6736(18)33135-0. [DOI] [PubMed] [Google Scholar]
- 40.van der Giessen J, van der Woude CJ, Peppelenbosch MP, Fuhler GM. A Direct Effect of Sex Hormones on Epithelial Barrier Function in Inflammatory Bowel Disease Models. Cells. 2019;8 doi: 10.3390/cells8030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JC, Biasci D, Roberts R, Gearry RB, Mansfield JC, Ahmad T, Prescott NJ, Satsangi J, Wilson DC, Jostins L, Anderson CA UK IBD Genetics Consortium, Traherne JA, Lyons PA, Parkes M, Smith KG. Genome-wide association study identifies distinct genetic contributions to prognosis and susceptibility in Crohn's disease. Nat Genet. 2017;49:262–268. doi: 10.1038/ng.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen GC, Targownik LE, Singh H, Benchimol EI, Bitton A, Murthy SK, Bernstein CN, Lee K, Cooke-Lauder J, Kaplan GG. The Impact of Inflammatory Bowel Disease in Canada 2018: IBD in Seniors. J Can Assoc Gastroenterol. 2019;2:S68–S72. doi: 10.1093/jcag/gwy051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bitton A, Vutcovici M, Sewitch M, Suissa S, Brassard P. Mortality Trends in Crohn's Disease and Ulcerative Colitis: A Population-based Study in Québec, Canada. Inflamm Bowel Dis. 2016;22:416–423. doi: 10.1097/MIB.0000000000000608. [DOI] [PubMed] [Google Scholar]
- 44.Bernstein CN, Nugent Z, Targownik LE, Singh H, Lix LM. Predictors and risks for death in a population-based study of persons with IBD in Manitoba. Gut. 2015;64:1403–1411. doi: 10.1136/gutjnl-2014-307983. [DOI] [PubMed] [Google Scholar]
- 45.Jones JL, Nguyen GC, Benchimol EI, Bernstein CN, Bitton A, Kaplan GG, Murthy SK, Lee K, Cooke-Lauder J, Otley AR. The Impact of Inflammatory Bowel Disease in Canada 2018: Quality of Life. J Can Assoc Gastroenterol. 2019;2:S42–S48. doi: 10.1093/jcag/gwy048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higashiyama M, Hokaria R. New and Emerging Treatments for Inflammatory Bowel Disease. Digestion. 2023;104:74–81. doi: 10.1159/000527422. [DOI] [PubMed] [Google Scholar]
- 47.Marchetti M, Liberato NL. Biological therapies in Crohn's disease: are they cost-effective? A critical appraisal of model-based analyses. Expert Rev Pharmacoecon Outcomes Res. 2014;14:815–824. doi: 10.1586/14737167.2014.957682. [DOI] [PubMed] [Google Scholar]
- 48.Juyal G, Prasad P, Senapati S, Midha V, Sood A, Amre D, Juyal RC, BK T. An investigation of genome-wide studies reported susceptibility loci for ulcerative colitis shows limited replication in north Indians. PLoS One. 2011;6:e16565. doi: 10.1371/journal.pone.0016565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Dataset can be available.