Abstract
The purple photosynthetic bacterium Rhodospirillum centenum is capable of forming swarm colonies that rapidly migrate toward or away from light, depending on the wavelength of excitation. To identify components specific for photoperception, we conducted mini-Tn5-mediated mutagenesis and screened approximately 23,000 transposition events for mutants that failed to respond to either continuous illumination or to a step down in light intensity. A majority of the ca. 250 mutants identified lost the ability to form motile swarm cells on an agar surface. These cells appeared to contain defects in the synthesis or assembly of surface-induced lateral flagella. Another large fraction of mutants that were unresponsive to light were shown to be defective in the formation of a functional photosynthetic apparatus. Several photosensory mutants also were obtained with defects in the perception and transmission of light signals. Twelve mutants in this class were shown to contain disruptions in a chemotaxis operon, and five mutants contained disruptions of components unique to photoperception. It was shown that screening for photosensory defective R. centenum swarm colonies is an effective method for genetic dissection of the mechanism of light sensing in eubacteria.
Behavioral change in response to alterations in the quality and quantity of light in the environment is a ubiquitous trait among motile photosynthetic bacteria. Three distinct types of responses to light have been described in the literature (14, 19, 36, 37). The scotophobic response (fear of darkness) is characterized by a tumbling, stop, or reversal that occurs when a swimming bacterium experiences a temporal, or spatial, step down in light intensity. Photokinesis describes an alteration in the rate of motility caused by differences in light intensity. A phototactic response, which has been studied most extensively in algae and cyanobacteria, involves an oriented movement of a cell toward or away from a light source (19). An important distinction is that the direction of irradiation is not relevant to scotophobic or photokinetic responses, whereas it is a critical determinant in phototaxis. Thus, phototactic organisms are uniquely capable of migrating towards a light source, irrespective of whether they are going up or down a gradient of light intensity (37).
The various photosensory behaviors exhibited by anoxygenic photosynthetic bacteria have been studied mainly by physiological and biochemical tests, with little supporting genetic data (3, 4, 8, 9, 13, 16, 27, 38). The few genetic tests that have been undertaken have demonstrated that mutations which functionally impair the photosystem also disrupt the ability of cells to respond to light (3, 20). This indicates that a product of photosynthesis, such as the generation of proton motive force or photosynthesis-driven electron transfer, is most likely the signal that controls photosensory behavior, rather than direct absorption of light by a chromophore-containing receptor. This conclusion is supported by recent physiological studies which have shown that specific inhibitors of cyclic photosynthesis-driven electron transport inhibit photosensory behavior in Rhodobacter sphaeroides (13, 16) and Rhodospirillum centenum (38). By using a site-directed mutational approach, we have shown that the scotophobic and phototactic responses of the purple nonsulfur photosynthetic bacterium R. centenum involve components of the chemotaxis phosphorylation cascade (25, 26). This suggests that a sensor of photosynthetic activity may have features similar to that of chemoreceptors. However, which component of the photosynthesis electron transfer chain is being sensed and what is actually sensing alterations in electron transfer are unknown.
To identify components responsible for prokaryotic behavioral responses to light, it is essential that techniques be developed for the isolation of mutants that are specifically defective in photosensory behavior. One of the reasons why screens for photosensory mutants have not been developed is the inherent difficulty of assaying for photosensory behavior. Until recently, screening for such mutants involved the onerous task of microscopically assaying individual cells from liquid-grown cultures for a response to a step up or down in light intensity. Since statistically meaningful results require that multiple cells be assayed, this “brute force” approach is infeasible. A significant advance in the isolation of prokaryotic photosensory mutants was recently provided by our observation that colonies of the purple photosynthetic bacterium R. centenum are capable of macroscopic phototactic motility (36, 37). Cells of R. centenum are dimorphic, existing in liquid medium as swim cells bearing a single polar flagellum or as hyperflagellated swarm cells on solid surfaces (36, 37). A unique feature of R. centenum swarming colonies is that they are capable of migrating rapidly (up to 75 mm/h) toward an infrared light source or away from a visible light source (36, 37). This behavior allows us to rapidly screen for mutants that are deficient in photosensory responses by simply assaying colonies for aberrant light-directed migration. In this study, we have utilized mini-Tn5-mediated mutagenesis to isolate numerous mutants that exhibit defects in light-directed motility. The phenotypes of specific classes of mutants provide some unique observations on photosensory behavior, as well as on the mechanism of swim cell to swarm cell differentiation.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Wild-type swarming strain R. centenum SW (ATCC 51521) is the parental strain used in this study (12, 37). Escherichia coli S17-1(λ pir)/pUTmini-Tn5Sm/Sp has been described previously (10). R. centenum strains that were selected by transposition mutagenesis in this study are listed in Table 1.
TABLE 1.
Representative R. centenum mutants used in this study
| Strain (no. of isolates) | Photosynthetic growth | Flagellaa
|
Motilityb
|
Phototaxisc
|
Scotophobia
|
Comment(s) (reference) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Polar | Lateral | Liquid | Plate | Visible | Infrared | Liquid | Plate | |||
| Photosynthesis mutants | ||||||||||
| ZJA4-28 (10) | No | + | + | Mot | Mot | None | None | None | None | Reaction center mutant |
| ZJPM3 (20) | Yes | + | + | Mot | Mot | Normal | Normal | Normal | Normal | Carotenoid mutantd |
| ZJPM1 (22) | No | + | + | Mot | Mot | None | None | None | None | Bacteriochlorophyll mutante |
| ZJB9-3 (3) | No | + | + | Mot | Mot | None | None | None | None | Electron transport mutant, petA disruption (24) |
| Flagellar (SFr) mutants | ||||||||||
| ZJH3-9 (46) | Yes | + | + | Mot | Nonmot | None | None | Normal | None | |
| ZJG9-9 (23) | Yes | + | ↓ | Mot | Nonmot | None | None | Normal | None | |
| ZJP246 (35) | Yes | − | + | Nonmot | Nonmot | None | None | None | None | |
| ZJJ8-7 (3) | Yes | + | + | Nonmot | Nonmot | None | None | None | None | |
| ZJP65 (18) | Yes | + | − | Mot | Nonmot | None | None | Normal | None | Lateral flgI disruption (39) |
| ZJF6-27 (35) | Yes | − | − | Nonmot | Nonmot | None | None | None | None | |
| Chemotaxis mutants ZJP1177 (12) | Yes | + | + | Mot | Mot | None | None | None | None | |
| Photoreceptor mutants | ||||||||||
| ZJF6-4 (2) | Yes | + | + | Mot | Mot | None | None | None | None | |
| ZJF7-13 (1) | Yes | + | + | Mot | Mot | Normal | Reduced | None | None | |
| ZJH7-24 (1) | Yes | + | + | Mot | Mot | Reduced | Reduced | None | None | |
| YB300-3 (1) | Yes | + | + | Mot | Mot | Reduced | None | None | None | |
| Morphology mutants | ||||||||||
| YB600-1 (9) | Yes | + | + | Mot | Mot | Aberrant | Aberrantf | Normal | Normal | Hyperswarmerf |
| BR2-68 (1) | Yes | + | + | Mot | Mot | Faster | Faster | Normal | Normal | Superdriverf |
| BR2-39 (1) | Yes | + | + | Mot | Mot | Faster | Reduced | Normal | Normal | Superdriverf |
Results based on Western blot analyses with anti-lateral-flagellum serum. +, wild-type level of flagella. −, no flagellum detected. ↓, less than 50% of the wild-type level of lateral flagella as judged visually from Western blot results.
Motility of each mutant strain was inspected under a microscope in CENS medium (liquid) or on CENS–0.8% agar plates. Mot, motile. Nonmot, nonmotile.
Based on phototactic colony migration assays. None, no colony migration observed. Normal, colony migration comparable to that of the wild-type strain. Reduced, slower migration rate with the wild-type strain as the control.
Phototactic colony migration was observed only under anaerobic condition. Under aerobic conditions, illumination kills carotenoid mutant cells due to photo-oxidative damage.
Photosystem and photopigments were determined by scanning the absorption of a cell suspension in 30% bovine serum albumin at wavelengths of 400 to 900 nm with a spectrophotometer (Beckman DU 640).
See Results for details.
All of the E. coli strains used were cultured at 37°C in Luria broth. Antibiotics were added, when appropriate, at the following concentrations: ampicillin, 150 μg/ml; spectinomycin, 50 μg/ml; gentamicin, 10 μg/ml; kanamycin, 40 μg/ml. R. centenum strains were grown either photosynthetically in CENS medium (44) at 37°C with illumination by 60-W tungsten Lumiline bulbs or aerobically in PYVS or CENS medium at 37°C (37). Assays of colony phototaxis toward infrared light and away from visible light were performed as described previously (25, 37).
Recombinant DNA techniques.
Restriction and other DNA modification enzymes were purchased from New England Biolabs and used in accordance with the vendor’s instructions. Chromosomal DNA was prepared by a previously described protocol (40). For Southern analyses, the SmaI fragment including a large portion of the R. centenum chemotaxis gene operon (25, 26) was purified from an agarose gel with a GeneClean II kit (Bio 101, Inc., Vista, Calif.) and internally labeled with DIG High Prime labeling and detection starter kit I (Boehringer Mannheim, Indianapolis, Ind.). Hybridization was performed in accordance with the manufacturer’s instructions. The results were scanned with an Epson ES-1200C scanner attached to a Power Mac 7600/120 running Adobe Photoshop 3.0 (Adobe Systems Incorporated). Notice that the spectinomycin resistance (Spr) interposon does not contain an ApaI site, but transposition introduces two new SmaI restriction sites, so SmaI and ApaI digestions would reveal the presence or absence of the interposon in the chemotaxis gene cluster or its promoter region in these mutants. Mutants showing wild-type restriction patterns in both digestions are free of the interposon in this region.
Conjugation and transposition of the Mini-Tn5 interposon.
Mini-Tn5ΩSp was delivered to wild-type R. centenum via conjugation with S17-1(λ pir)/pUTmini-Tn5Sm/Sp. The suicide plasmid pUTmini-Tn5Sm/Sp contains the transposase gene located in cis outside of the transposable element, thus preventing secondary transposition events once the plasmid is lost from the cell (10). pUTmini-Tn5Sm/Sp also contains an incomplete replicon from plasmid R6K, and thus its replication is dependent on the pir gene product (π protein) that is provided in trans on the chromosome of E. coli strains such as S17-1(λ pir) or SM10 (λ pir) (42). The interposon was introduced via a membrane filter mating procedure (5, 47, 48). Usually, 5 × 109 R. centenum cells were applied to each filter unit (Nalgene no. 245-0045). After mating for 12 to 16 h at room temperature or for 4 to 5 h at 42°C, cells were collected by washing with 4 ml of phosphate-buffered saline (PBS; containing 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.7 mM KH2PO4 at pH 7.2) and 200-μl aliquots of resuspended cells were spread on either CENS plates for photosynthetic growth or PYVS plates for aerobic dark growth. Plates were supplemented with 10 μg of spectinomycin per ml for selection of the transposition event and 40 μg of kanamycin per ml for counterselection of the E. coli donor (note that R. centenum is naturally resistant to kanamycin). Spr transconjugants were identified after 48 to 72 h of incubation.
Isolation of mutants defective in the phototactic or scotophobic response.
In a macroscopic approach, Spr strains from mini-Tn5 mutagenesis were screened for photosensory defects by transferring individual colonies with sterile toothpicks to square PYVS–0.8% agar plates containing kanamycin. Alternatively, strains were grown in liquid medium and concentrated 10- to 20-fold, and 5 to 10 μl of cell concentrate was spotted onto assay plates. Phototaxis assays were performed for 12 to 24 h with unilateral light sources as previously described (26, 34, 37). In a microscopic approach, photosensory perception was tested by inspection of the cell motility of an individual swarming colony with a Nikon OPTIPHOT-2 microscope equipped with a 40× lens, a Sony 3CCD camera, and a Sony Trinitron monitor. The microscope beam was intercepted with a cutoff filter that allows light with wavelengths above 550 nm to pass through (37), and the colony was then challenged with a fourfold step down in light intensity by suddenly inserting a neutral-density filter into the light path. In both assays, wild-type R. centenum SW served as a control.
Generation of polyclonal antisera to polar and lateral flagella.
Polar flagella were isolated from a 2-liter culture of early-log-phase R. centenum cells grown photosynthetically in CENS medium. Lateral flagella were isolated from swarm cells by gently suspending cell paste from 30 0.8% agar PYVS square (9 by 9 cm) plates in liquid PYVS medium with a glass rod. The flagella were then harvested as described previously (43). Partially purified and concentrated flagellar samples were then mixed with sodium dodecyl sulfate (SDS) loading buffer and boiled for 5 min before being loaded onto a preparative 12% denaturing polyacrylamide gel (SDS-polyacrylamide gel electrophoresis). After electrophoresis, the gels were stained with ice-cold 0.5 M KCl, the predominant bands were excised from the gels, and the gel slices were crushed between two glass plates and stored at −70°C. Immunization and sampling of antisera from rabbits were subsequently performed by a commercial facility (Cocalico Biologicals, Reamstown, Pa.). Polyclonal antisera and preimmune sera were tested by immunoblot assay (see below) with samples from wild-type and mutant R. centenum strains that are unable to synthesize flagella as determined by a flagellar stain kit (Carr-Scarborough Microbiologicals, Decatur, Ga.). The polyclonal antiserum raised against lateral flagella cross-reacts with the polar flagellum. However, the antiserum against the polar flagellum does not cross-react with the lateral flagella and also displays a very high background in an immunoblot assay. Consequently, lateral flagellar antiserum was used to detect both flagellar types in the immunoblots.
Immunoblot assays.
Immunoblot (Western blot) assays were performed as previously described (21) with polyclonal anti-lateral-flagellum serum. Swarm cell flagellar samples were collected from cells that were grown on 0.8% agar plates and then gently rinsed off the agar surface with 5 ml of PBS buffer. For both swim and swarm cell samples, about 5 ml of cells between 50 and 80 Klett photometric units (red filter no. 66) were centrifuged and the cell pellet was resuspended in 500 μl of PBS buffer supplemented with 25 mM EDTA–1 mM phenylmethylsulfonyl fluoride and then vortexed for 2 min. The cells were pelleted by centrifugation, and sheared flagella that were present in the supernatant were subjected to standard procedures for SDS-gel electrophoresis and Western transfer to nitrocellulose membranes (Schleicher & Schuell, Keene, N.H.). After the transfer, membranes were blocked with 5% (wt/vol) nonfat dry milk in PBS buffer plus 0.1% (vol/vol) Tween 20 for 1 h at 24°C. The primary antiserum was added to the blocking mixture at dilutions of 1:10,000 to 1:20,000 and incubation proceeded for an additional 2 h at 24°C; afterwards, the membranes were washed three times in PBS-Tween buffer and then incubated with a 1:10,000 dilution of a goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Amersham) in PBS-Tween buffer for 1 h at 24°C. Finally, the membrane was washed three times in PBS-Tween buffer and the results were visualized by development with an ECL kit (Amersham).
Chemotaxis capillary assay.
Chemotaxis measurement was performed as previously described (26), with a few modifications. In addition to pyruvate and acetate, we determined that serine also serves as a strong chemoattractant for R. centenum. Therefore, 10 mM serine in CTX buffer (26) was used for all assays. Also, nonmotile strain ZJJ8-7 (Table 1), which still synthesizes a polar flagellum in broth, was used as a “background” control for subtraction of numbers of cells that adhered to glass capillary tube nonspecifically. Assays were carried out for 1 h at room temperature instead of at 42°C. The response to serine is similarly reported as the ratio of the number of cells entering a 1-μl capillary tube with serine to the number of cells entering the tube with buffer only (26).
RESULTS
Mini-Tn5 transposon mutagenesis of R. centenum swarm cells.
Mini-Tn5 transposition mutagenesis was used to study loci involved in light-directed colony motility. We chose a mini-Tn5 transposon since it exhibits a relatively high transposition frequency in diverse gram-negative bacteria and integrates into the host chromosome with little sequence specificity (7). Interrupting alleles with an antibiotic resistance marker also facilitates cloning and sequence characterization of genes by direct selection of the transposon. We used a derivative of mini-Tn5 that contains the omega spectinomycin interposon (ΩSp) cassette, since earlier experiments (24) indicated that spontaneous Spr occurs infrequently in R. centenum, and it had previously been useful in the isolation of photosynthesis mutants of R. centenum (46). Southern blot analysis of a number of strains that were derived from independent transposition events have indicated that the transposon integrated as a single event in random locations (24, 39).
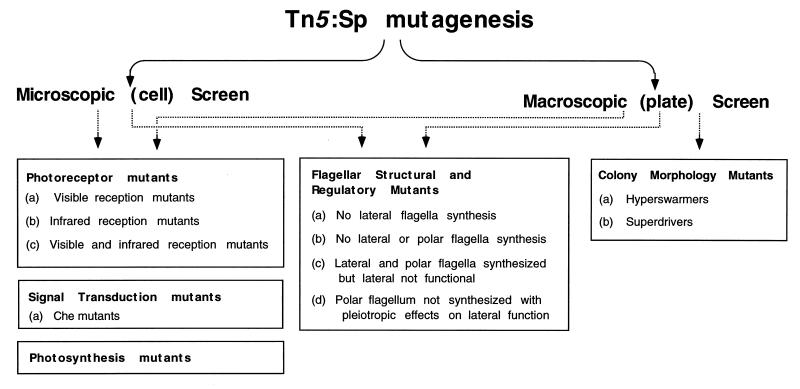
We screened for mutants that were defective in photosensory behavior by using two approaches (Fig. 1). With a macroscopic approach, we assayed colonies for defects in the ability to move toward or away from light. As discussed in detail below, this assay yielded photosensory mutants, as well as mutants exhibiting altered swarm cell behavior, such as those that migrated slower or faster or showed irregular swarm colony morphology. In a separate assay, which we term the microscopic approach, we directly observed the motility responses of individual cells in swarm colonies by using a microscope equipped with a 40× objective lens. As discussed above, when liquid-grown photosynthetic bacteria encounter a sudden reduction in light intensity, they exhibit a characteristic scotophobic response observed as a reversal, stop, or tumble. Microscopic observation of R. centenum swarm cell colonies on an agar plate indicated that virtually all of the cells in the colony are highly motile and that they also exhibit a scotophobic response. Specifically, when swarm cells were challenged with a step down in light intensity, we observed a consistent 3- to 5-s freeze of all cell movement in the colony, followed by resumption of movement at a lower rate of motility. We interpret the light-induced pause of motility to be the result of a tumbling response. This response is followed by resumption of smooth swimming at a lower speed, a photokinetic effect. Thus, by direct microscopic inspection of swarm cell responses to alterations in light, we are able to screen for mutants altered in photosensory behavior, as well as for mutants that display a general defect in swarm cell motility. The microscopic approach proved to be less labor intensive than direct observation of colony migration across an agar surface, and thus, the former assay was used for most of our screens. Overall, approximately 1,300 colonies were assayed for defects in light-driven motility by the macroscopic approach and approximately 22,000 colonies were assayed by the microscopic approach.
FIG. 1.
Flow chart depicting the categories of R. centenum mutants isolated in this study.
The frequency of transposition events that produced some form of aberrant response to light was a surprisingly high 1 in 20 assayed colonies. Assuming that R. centenum has a typical genome size of ∼4,000 genes, this frequency indicates that as many as 200 genes may be involved in the swarm cell response to light. Although this appears to be a large number of loci, the results below demonstrate that disruption of any number of genes involved in swim cell to swarm cell differentiation, synthesis of a functional bacterial photosystem, signal transduction events, or light perception results in observable defects in photosensory behavior (Fig. 1 and Table 1 show the categories of mutants that have been obtained in our screens). Consequently, the challenge is not to obtain mutants that do not respond to light but to place them correctly into appropriate categories. Below are detailed phenotypic descriptions of mutants that have been obtained.
Photosynthesis mutants.
One category of mutants that was isolated early in our study included mutants with disrupted photosynthetic growth capability. Like most nonsulfur purple photosynthetic bacteria, R. centenum is capable of heterotrophic growth aerobically in the dark, as well as photosynthetic growth under anaerobic conditions. However, this species also exhibits the unusual capability of synthesizing a functional photosystem, as well as exhibiting photosensory behavior, when growing aerobically as a heterotroph (46). To facilitate genetic manipulation, our initial screens involved selection for Spr transconjugants under heterotrophic conditions prior to assaying for defects in photosensory behavior. Under these conditions, a large fraction of transconjugants that were observed to be defective in photosensing actually had mutations that disrupted photosynthetic growth capabilities (Table 1). Included were numerous pigment biosynthesis mutants that are deficient in the synthesis of bacteriochlorophyll (strain ZJPM1) and mutants that fail to synthesize a functional reaction center (B800) (strain ZJA4-28). Sequence analysis indicates that the photosynthesis-defective mutant ZJB9-3 has a Tn5 insertion in the pet operon, which codes for structural polypeptides of the cytochrome bc1 complex (24). This indicates that both the infrared- and visible-light responses measure some component of photosynthesis-driven electron transport. To reduce the background frequency of photosynthesis mutants, our later screens for photosensory mutants incorporated strict anaerobic photosynthetic growth conditions during drug selection of the transposon.
Flagellar-biosynthesis mutants.
A second large class of mutants was those that are defective in solid-surface motility, which we have termed SFr mutants for surface-frozen mutants. To our surprise, about 1 in every 25 transconjugants exhibited no solid-surface motility, implying that a large number of genes are involved in this process. SFr mutants could potentially have such diverse functional defects as the inability to induce swarm cell differentiation, disruption in lateral flagellar synthesis or assembly, a defect in lateral flagellar rotation, or a defect in the production of a surface lubricant.
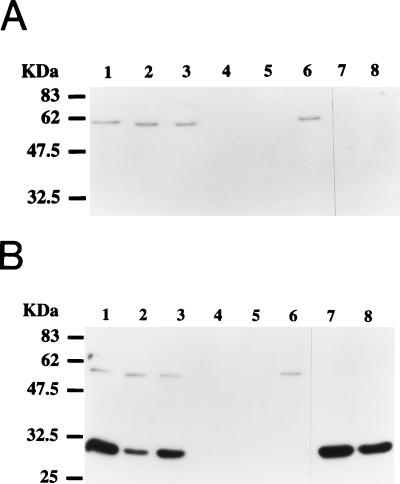
We performed detailed analysis of approximately 160 SFr mutants from our collection to categorize potential defects (Table 1). The mutants were assayed for motility when grown in liquid medium and examined for the presence or absence of polar and lateral flagella by Western blot analysis with a polyclonal antiserum that cross-reacts with both flagellar types (Fig. 2). We observed that, similar to Vibrio parahaemolyticus (1, 31, 32), wild-type R. centenum cells synthesize different flagellar types. Specifically, wild-type swim cells synthesize a polar flagellum comprising a 58-kDa flagellin subunit (Fig. 2A, lane 1), whereas wild-type swarm cells synthesize the same polar flagellum, as well as a large number of additional lateral flagella comprising 32-kDa flagellin subunits (Fig. 2B, lane 1). Thus, synthesis of the polar flagellum is constitutive, irrespective of the presence or absence of surface-induced lateral flagella. The various mutants that are defective in surface motility can be grouped into the following six classes. One class (46 isolates), represented by Western blot analysis of strains ZJH3-9 and ZJH3-26 (Fig. 2, lanes 2 and 3, respectively), shows the normal polar-flagellum pattern in liquid medium and agar-solidified medium, as well as normal surface-induced lateral-flagellum synthesis. Motility is observed in liquid medium, indicating normal rotation of the polar flagellum, and yet this class of mutants is completely defective in solid-surface motility. A loss of surface motility can arise as a consequence of a defect in lateral-flagellar rotation or synthesis of surface surfactants such as exopolysaccharides (18, 45) or serrawettin-like substances (28).
FIG. 2.
Western blot analyses of the flagellar types from nonswarming mutants. (A) Flagellar samples collected from cells grown in liquid medium. (B) Flagellar samples collected from cells grown on 0.8% agar plates. Lanes: 1, wild-type R. centenum; 2, ZJH3-9; 3, ZJH3-26; 4, ZJF6-27; 5, ZJH8-3; 6, YB280; 7, ZJP246; 8, ZJG8-11. Protein molecular size markers are labelled on the left.
The second class of mutants (23 isolates), represented by mutant ZJG9-9 (Table 1), synthesizes quantitatively fewer lateral flagella than do wild-type cells, indicating that the mutants may harbor defects in the induction of lateral-flagellar synthesis (24).
The third class (35 isolates), represented by mutants ZJP246 and ZJG8-11 (Fig. 2, lanes 7 and 8, respectively; Table 1), fail to synthesize a polar flagellum under our tested condition. Interestingly, these mutants still exhibit normal surface-dependent induction of lateral-flagellum synthesis, indicating that induction of lateral-flagellum synthesis does not involve the presence of a polar flagellum. Normal regulation of lateral-flagellum synthesis in these mutants is distinctly different from that reported for polar-flagellum mutants of V. parahaemolyticus, which exhibit a phenotype of constitutive synthesis of lateral flagella (31). Despite the fact that these mutants still induce lateral-flagellum synthesis, polar-flagellum mutants exhibit a defect in surface motility.
The fourth class of mutants, represented by three isolates, ZJJ8-7, ZJN608, and ZJP1310 (Table 1), synthesizes a polar flagellum that is defective in rotation (synthesis of a polar flagellum was determined by Western blot and flagellar-stain analyses, and lack of rotation was determined by the observed lack of motility of liquid-grown swim cells). As in the case of cells which lack synthesis of a polar flagellum, these mutants are also defective in surface motility.
The fifth class of mutants (35 isolates), represented by strains ZJF6-27 and ZJH8-3 (Fig. 2, lanes 4 and 5; Table 1), fails to synthesize either lateral or polar flagella. This phenotype indicates that the polar- and lateral-flagellum types must have some common regulatory or structural components.
The sixth class (18 isolates), represented by mutant YB280 (Fig. 2, lane 6; Table 1), has normal polar-flagellum synthesis and normal liquid motility but no surface-induced lateral-flagellum synthesis. This class either has mutations that disrupt structural components of the lateral flagellum or defects in regulatory circuits that are responsible for surface-induced lateral-flagellum synthesis.
Signal transduction (che) mutants.
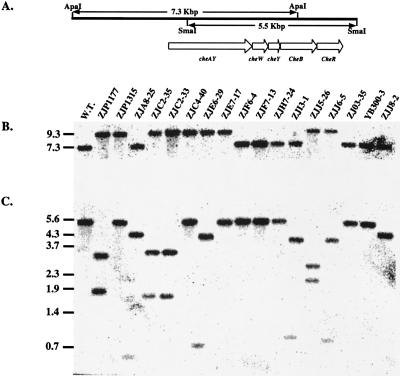
From our screen, we isolated 17 mutants, represented in Table 1 by strain ZJP1177, that exhibit normal photosynthetic growth capabilities, as well as normal induction of lateral flagella on a solid surface. Microscopic analysis indicated that swarm cells of this mutant class are actively motile on an agar surface; however, unlike wild-type cells, they fail to exhibit the characteristic freeze of movement when given a step down in light intensity. Swim cells from a majority of these mutants displayed a biased smooth-swimming pattern and also failed to exhibit the scotophobic tumbling response when challenged with a step down in light intensity. Macroscopically, colonies of these mutants also failed to migrate toward or away from light. The phenotypes manifested by these mutants are virtually identical to those of smooth-swimming chemotaxis mutants that we previously described (26). Southern blot analysis using a che gene probe subsequently demonstrated that 12 of the 17 isolates contained a mini-Tn5 insertion in the che gene cluster (Fig. 3, mutants ZJP1177, ZJP1315, ZJA8-25, ZJC2-35, ZJC2-33, ZJC4-20, ZJE6-29, ZJE7-17, ZJI3-1, ZJJ5-26, ZJJ6-5, and ZJJ8-2). The remaining five isolates, which do not contain a mini-Tn5 insertion in the che gene cluster, are discussed in the section below.
FIG. 3.
Southern blot analysis of genomic DNAs from scotophobic and phototactic mutants by probing with an SmaI fragment of the R. centenum chemotaxis gene cluster. (A) Diagram of the che gene cluster and flanking sequences along with relevant restriction sites. (B) Genomic DNA samples digested with ApaI. (C) Genomic DNA samples digested with SmaI. Lengths of DNA markers and fragments are marked in kilobase pairs on the left. W.T., wild-type R. centenum sample. ZJP1177, ZJP1315, ZJA8-25, ZJC2-35, ZJC2-33, ZJC4-20, ZJE6-29, ZJE7-17, ZJF6-4, ZJF7-13, ZJH7-24, ZJI3-1, ZJJ5-26, ZJJ6-5, ZJO3-35, YB300-3, and ZJJ8-2 are samples from the corresponding mutant strains. The loading order in panel C is identical to that in panel B.
Photoperception mutants.
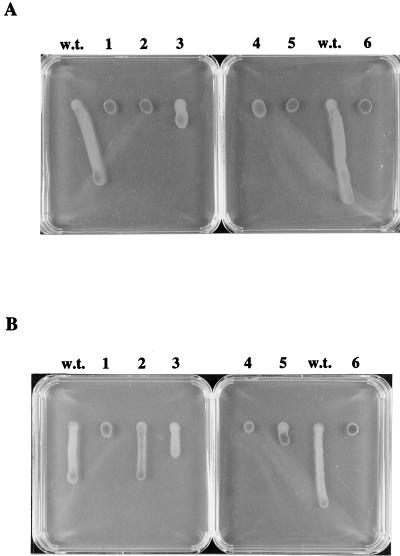
As discussed above, five isolates (Fig. 3, strains ZJF6-4, ZJF7-13, ZJF7-24, ZJO3-35, and YB300-3) were obtained which exhibited defects in the scotophobic response when challenged with a step down in infrared light intensity similar to that observed with che mutants (Table 1). One important observation provided by Southern analysis is that these mutants contain an intact che operon, which was characterized by us previously (25, 26). Furthermore, chemotaxis to serine (Fig. 4) or pyruvate (data not shown) indicated that, with the exception of YB300-3, which has a general growth defect, these mutants are not defective in chemotaxis per se, compared to the che gene cluster deletion mutant (25). As such, each of the mutations potentially disrupts components of the light perception and/or light signal transduction pathway or down regulates the functions of these components. The results of light-driven colony motility assays of these five mutants (Fig. 5) demonstrated that mutants ZJF6-4 and ZJO3-35 are defective in both infrared- and visible-light phototactic responses; mutant ZJF7-13 displays no infrared-light response; mutant ZJH7-24 has attenuated infrared- and visible-light responses (particularly the infrared-light response); and mutant YB300-3 has a diminished visible-light response and a completely defective infrared-light response.
FIG. 4.
Chemotaxis capillary assays for photoperception mutants. The vertical axis represents the relative ratio (see Materials and Methods). Columns: a, wild-type R. centenum strain; b, ZJF6-4; c, ZJF7-13; d, ZJH7-24; e, ZJO3-35; f, R. centenum cheΔ mutant (26).
FIG. 5.
Phototactic colony migration assays of isolated mutants. (A) Positive swarm colony phototaxis toward an infrared-light source. (B) Negative phototaxis away from a visible-light source. Lanes: w.t., wild-type R. centenum; 1, ZJF6-4; 2, ZJF7-13; 3, ZJH7-24; 4, ZJO3-35; 5, YB300-3; 6, cheΔ mutant.
We also obtained an interesting mutant, YB72, which exhibits a normal scotophobic response as measured microscopically. However, light-directed colony migration was not observed (39).
Colony morphology mutants.
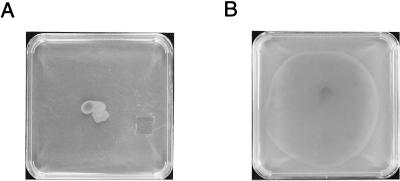
Another class of mutants exhibited pleiotropic effects on swarm colony morphology. These mutants do not appear to have defects in light perception per se but, instead, display altered light-driven colony motility as a result of aberrant swarm cell behavior. One frequently obtained type of morphology mutants is hyperswarmers, represented by strain YB600-1 in Fig. 6. Hyperswarmer mutants quickly spread across the surface of the agar medium, in contrast to the more discrete colony formation exhibited by the wild-type strain (Fig. 6). Variations exist among this class of mutants, with some strains forming very thin layers of cells and others forming thick layers. Additional isolates form ruffled edges rather than smooth edges. Another type of morphology mutants, which we categorize as superdrivers, is represented by mutants BR2-68 and BR2-39. BR2-68 exhibits consistently faster colony motility in response to both visible light and infrared light, whereas BR2-39 has a reduced response to infrared light and an enhanced response to visible light (39).
FIG. 6.
Swarming morphology of wild-type R. centenum versus that of hyperswarming mutant YB600-1. The plates were spotted with equal numbers of cells and incubated in the dark for 40 h at 42°C on PYVS–0.8% agar plates. (A) Wild-type R. centenum. (B) Transposon mutant YB600-1.
DISCUSSION
This study demonstrates that light-directed swarm colony motility is a very complex process involving numerous (possibly 200) genetic loci. They include genes involved in swarm cell differentiation, synthesis of the lateral flagella, and synthesis of a functional photosystem, as well as those involved in light perception and sensory transduction. As shown by our results, it is relatively easy to obtain mutants that exhibit defects in light-driven swarm colony motility. The difficulty is in properly analyzing and classifying the numerous mutants that are obtained. Despite these hurdles, we were successful in obtaining and characterizing a number of strains that give us a unique perspective on the complexities involved in R. centenum swarm cell differentiation and how these cells perceive and respond to light signals.
Motility-defective isolates providing insights into swarm cell differentiation.
Even though surface-dependent swarm cell differentiation is known to occur in many species (22, 23), little is known about the mechanisms used to sense a solid surface. The best-understood example is V. parahaemolyticus, which has a pattern of swarm cell differentiation very similar to that of R. centenum (31, 32). As observed with R. centenum, V. parahaemolyticus swim cells have a single, sheathed polar flagellum, whereas swarm cells contain additional, numerous, unsheathed lateral flagella. Genetic studies of V. parahaemolyticus have demonstrated that loss of polar-flagellum synthesis results in constitutive (liquid and solid) synthesis of lateral flagella (31). This observation and other experimental results have led to a model proposing that impeded movement of the polar flagellum generates a signal that leads to induction of lateral-flagellum synthesis. Transmission of a signal regarding polar-flagellum rotation appears to involve the che gene products, since che mutants of V. parahaemolyticus are deficient in the induction of lateral-flagellum synthesis (41). Our mutational analysis of R. centenum clearly indicates that swarm cell differentiation involves a distinctly different mechanism. This is evidenced by the isolation of mutants that fail to synthesize a polar flagellum but which are still capable of undergoing normal swim cell to swarm cell differentiation when placed on a solid surface. We have also observed that disruption of the che operon has no effect on swarm cell differentiation (26). Thus, it appears that R. centenum has a mechanism of sensing a solid surface that does not involve synthesis or rotation of the polar flagellum. Indeed, some mutants in our collection that fail to synthesize lateral flagella could potentially contain defects in a surface sensor receptor or in transmission of a signal from a surface receptor. Additional sequence and expression analysis of this class of mutations needs to be undertaken to further subgroup these mutants into those that contain defects in structural components of the lateral flagellum versus those that contain defects in the regulation of the induction of lateral-flagellum synthesis.
No mutants of V. parahaemolyticus missing both the polar and lateral flagella have been isolated (29, 30, 32, 33). In contrast, we have obtained numerous mutants of R. centenum that lack both flagellar types (Table 1). This phenotype suggests that lateral and polar flagella in R. centenum have structural and/or regulatory factors in common (such as a commonly utilized sigma factor). Another possibility is that lateral- and polar-flagellum genes are cotranscribed in one or more large operons. If so, integration of the mini-Tn5 transposon, which contains flanking transcription termination sites, would produce polarity effects on the synthesis of both flagellar types. At this early stage of our structural analyses, we do know (24, 39) that at least two structural components of the lateral flagella are not utilized by the polar flagellum. Specifically, lateral and polar flagella have separate flagellin polypeptides, as well as different subunits of FlgI, which is a component of the P-ring of the flagellar basal body. Future sequence analysis of loci that are disrupted in mutants that fail to synthesize one or both flagellar types should sort out whether or not common subunits and/or regulatory factors are, indeed, shared.
Another surprising observation is that a number of polar-flagellum mutants were isolated that are defective in lateral-flagellum rotation. This suggests that these two flagellar types have motor or switch subunits in common. An argument against common motor or switch components is that we have isolated mutants that are defective in lateral-flagellum rotation but have normal polar-flagellum rotation. Thus, even more complex scenarios must be considered, such as communication of polar-flagellum rotation to the lateral flagella. For example, perhaps rotation of the polar flagellum is needed as a nucleation point for the formation of a lateral-flagellar bundle. It should also be noted that since we screened for cells that are defective in surface motility, we do not know whether it is possible to obtain mutants that are defective in polar-flagellum rotation but are still capable of surface motility. A separate screen for nonmotile swim cells has to be undertaken to address this issue. Finally, the involvement of the polar flagellum in swarm cell motility has also been observed in V. parahaemolyticus, in which mutations that disrupt polar-flagellum synthesis result in swarm cells that exhibit aberrant “wobbly” surface motility (31). This indicates that the presence of a polar flagellum is also important for proper lateral-flagellum function in this species.
Photoperception and signal transduction.
In addition to obtaining information on swarm cell differentiation, this study also established that light-directed colony motility can be exploited to obtain mutants that are specifically defective in photosensory perception. To our knowledge, this investigation provides the first extensive genetic screen for bacterial mutations that exhibit altered photosensory behavior. From analysis of isolated mutants, it is evident that part of the signal(s) for both positive and negative phototactic responses involves light-driven photosynthetic electron transport. Movement toward infrared light is most likely a response to increased energy conversion caused by efficient utilization of infrared light to drive photosynthesis (3, 13, 16, 20, 37). Movement away from visible light is somewhat more complex, since visible light is also utilized by the photosystem to drive photosynthesis. Thus, the negative response must measure photosynthetic electron transport, as well as an additional component of the light spectrum. The isolation of mutants ZJF6-4 and ZJO3-35, which have normal chemotaxis but no visible- or infrared-light phototaxis, indicates that the signal transduction pathways for both responses converge. Presumably, these pathways converge at a receptor or transducer that communicates with the chemotaxis cascade, since a number of che gene mutations were also obtained which affect phototaxis, as well as chemotaxis (25, 26).
Morphology mutants.
Our ability to obtain colony morphology mutants that are crippled in light-driven colony migration suggests that cell-cell communication or production of a surface surfactant is a factor in swarm motility. Multicellular cooperative behavior is widespread in prokaryotes and is involved in processes such as fruiting body formation in Myxococcus xanthus and swarming differentiation. In swarming, perhaps the best-understood systems are those of Proteus mirabilis (2, 6, 17) and Serratia marcescens (11, 15, 35). Mutants of these species which exhibit altered swarming patterns have been isolated. Most of the morphology mutants that we obtained in this study tend to form sheets of cells rapidly across the surfaces of the plates rather than forming discrete colonies. Western blot and flagellum-staining analyses suggest that the hyperswarmer mutants have the normal complement of flagella per cell, so we suspect that this phenotype could be caused by alterations in exopolysaccharide synthesis or surface surfactant production (18, 28, 45). More extensive macroscopic screening for colony morphology mutants can be undertaken to address the nature and number of loci involved in potential cell-cell communication events that are involved in light-driven swarm colony motility.
ACKNOWLEDGMENTS
We thank members of the Photosynthetic Bacteria Group for helpful comments.
This work was supported in part by a grant from the U.S. National Science Foundation (IBN-9303836), as well as by a National Science Foundation postdoctoral fellowship awarded to B.G.R.
REFERENCES
- 1.Allen R, Bauman P. Structure and arrangements of flagella in species of the genus Beneckea and Photobacterium fischeri. J Bacteriol. 1971;107:295–302. doi: 10.1128/jb.107.1.295-302.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison C, Hughes C. Closely linked genetic loci required for swarm cell differentiation and multicellular migration by Proteus mirabilis. Mol Microbiol. 1991;5:1975–1982. doi: 10.1111/j.1365-2958.1991.tb00819.x. [DOI] [PubMed] [Google Scholar]
- 3.Armitage J P, Evans M C W. The reaction center in the phototactic and chemotactic responses of Rhodopseudomonas sphaeroides. FEMS Microbiol Lett. 1981;11:89–92. [Google Scholar]
- 4.Armitage J P, Ingham C, Evans M C W. Role of proton motive force in phototactic and aerotactic responses of Rhodopseudomonas sphaeroides. J Bacteriol. 1985;161:967–972. doi: 10.1128/jb.161.3.967-972.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai Y. Characterization of phototactic mutants produced by mini-Tn5 transposon mutagenesis. M.S. thesis. Bloomington: Indiana University; 1995. [Google Scholar]
- 6.Belas R, Erskine D, Flaherty D. Proteus mirabilis mutants defective in swarmer cell differentiation and multicellular behavior. J Bacteriol. 1991;173:6279–6288. doi: 10.1128/jb.173.19.6279-6288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg D E. Transposon Tn5. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C: American Society for Microbiology; 1989. pp. 185–210. [Google Scholar]
- 8.Clayton R K. Studies in the phototaxis of Rhodospirillum rubrum. I. Action spectrum, growth in green light and Weber-law adherence. Arch Microbiol. 1953;19:107–124. doi: 10.1007/BF00446395. [DOI] [PubMed] [Google Scholar]
- 9.Clayton R K. Studies in the phototaxis of Rhodospirillum rubrum. III. Quantitative relations between stimulus and response. Arch Microbiol. 1953;19:141–165. doi: 10.1007/BF00446397. [DOI] [PubMed] [Google Scholar]
- 10.de Lorenzo V, Herrero M, Jakubzik U, Timmis K N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172:6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elberl L, Winson M K, Sternberg C, Stewart G S A B, Christiansen G, Chhabra S R, Bycroft B, Williams P, Molin S, Givskov M. Involvement of N-aceyl-l-homoserine lactone autoinducers in controlling the multicellular behavior of Serratia liquefaciens. Mol Microbiol. 1996;20:127–136. doi: 10.1111/j.1365-2958.1996.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 12.Favinger J, Stadtwald R, Gest H. Rhodospirillum centenum, sp. nov., a thermotolerant cyst-forming anoxygenic photosynthetic bacterium. Antonie Leeuwenhoek. 1989;55:291–296. doi: 10.1007/BF00393857. [DOI] [PubMed] [Google Scholar]
- 13.Gauden D E, Armitage J P. Electron transport-dependent taxis in Rhodobacter sphaeroides. J Bacteriol. 1995;177:5853–5859. doi: 10.1128/jb.177.20.5853-5859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gest H. Phototaxis and other sensory phenomena in purple photosynthetic bacteria. FEMS Microbiol Rev. 1995;16:287–294. [Google Scholar]
- 15.Givskov M, Eberl L, Christiansen G, Benedik M J, Molin S. Induction of phospholipase and flagellar synthesis in Serratia liquefaciens is controlled by expression of the flagellar master operon flhD. Mol Microbiol. 1995;15:445–454. doi: 10.1111/j.1365-2958.1995.tb02258.x. [DOI] [PubMed] [Google Scholar]
- 16.Grishanin R N, Gauden D E, Armitage J P. Photoresponses in Rhodobacter sphaeroides: role of photosynthetic electron transport. J Bacteriol. 1997;179:24–30. doi: 10.1128/jb.179.1.24-30.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gygi D, Bailey M J, Allison C, Hughes C. Requirement for FlhA in flagella assembly and swarm cell differentiation by Proteus mirabilis. Mol Microbiol. 1995;15:761–769. doi: 10.1111/j.1365-2958.1995.tb02383.x. [DOI] [PubMed] [Google Scholar]
- 18.Gygi D, Rahman M M, Lai H-C, Carlson R, Guard-Peter J, Hughes C. A cell-surface polysaccharide that facilitates rapid population migration by differentiated swarm cells of Proteus mirabilis. Mol Microbiol. 1995;17:1167–1175. doi: 10.1111/j.1365-2958.1995.mmi_17061167.x. [DOI] [PubMed] [Google Scholar]
- 19.Häder D P. Photosensory behavior in procaryotes. Microbiol Rev. 1987;51:1–21. doi: 10.1128/mr.51.1.1-21.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harayama S, Iino T. Phototaxis and membrane potential in the photosynthetic bacterium Rhodospirillum rubrum. J Bacteriol. 1977;131:34–41. doi: 10.1128/jb.131.1.34-41.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 22.Harshsy R M. Bees aren’t the only ones: swarming in gram-negative bacteria. Mol Microbiol. 1994;13:389–394. doi: 10.1111/j.1365-2958.1994.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 23.Harshey R M, Matsuyama T. Dimorphic transition in Escherichia coli and Salmonella typhimurium: surface-induced differentiation into hyperflagellated swarmer cells. Proc Natl Acad Sci USA. 1994;91:8631–8635. doi: 10.1073/pnas.91.18.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang, Z.-Y., and C. E. Bauer. Unpublished data.
- 25.Jiang Z-Y, Bauer C E. Analysis of a chemotaxis operon from Rhodospirillum centenum. J Bacteriol. 1997;179:5712–5719. doi: 10.1128/jb.179.18.5712-5719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang Z-Y, Gest H, Bauer C E. Chemosensory and photosensory perception in purple photosynthetic bacteria utilize common signal transduction components. J Bacteriol. 1997;179:5720–5727. doi: 10.1128/jb.179.18.5720-5727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manten A. Phototaxis in the purple bacterium Rhodospirillum rubrum, and the relation between phototaxis and photosynthesis. Antonie Leeuwenhoek J Microbiol Serol. 1948;14:65–86. doi: 10.1007/BF02272681. [DOI] [PubMed] [Google Scholar]
- 28.Matsyyama T, Kaneda K, Nakagawa Y, Isa K, Harta-Hotta H, Yano I. A novel extracellular cyclic lipopeptide which promotes flagellum-dependent and -independent spreading growth of Serratia marcescens. J Bacteriol. 1992;174:1769–1776. doi: 10.1128/jb.174.6.1769-1776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarter, L. Personal communication.
- 30.McCarter L. Genetic and molecular characterization of the polar flagellum of Vibrio parahaemolyticus. J Bacteriol. 1995;177:1595–1609. doi: 10.1128/jb.177.6.1595-1609.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarter L, Hilmen M, Silverman M. Flagella dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell. 1988;54:345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- 32.McCarter L, Silverman M. Surface-induced swarmer cell differentiation of Vibrio parahaemolyticus. Mol Microbiol. 1990;4:1057–1062. doi: 10.1111/j.1365-2958.1990.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 33.McCarter L, Wright M. Identification of genes encoding components of the swarmer cell flagellar motor and propeller and a sigma factor controlling differentiation of Vibrio parahaemolyticus. J Bacteriol. 1993;175:3361–3371. doi: 10.1128/jb.175.11.3361-3371.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nickens D, Fry C J, Ragatz L, Bauer C E, Gest H. Biotype of the purple nonsulfur photosynthetic bacterium, Rhodospirillum centenum. Arch Microbiol. 1996;165:91–96. doi: 10.1007/BF00262196. [DOI] [PubMed] [Google Scholar]
- 35.O’Rear J, Alberti L, Harshey R A. Mutations that impair swarming motility in Serratia marcescens 274 include but are not limited to those affecting chemotaxis or flagellar function. J Bacteriol. 1992;174:6125–6137. doi: 10.1128/jb.174.19.6125-6137.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ragatz L, Jiang Z-Y, Bauer C E, Gest H. Phototactic purple bacteria. Nature. 1994;370:104. [Google Scholar]
- 37.Ragatz L, Jiang Z-Y, Bauer C E, Gest H. Macroscopic phototactic behavior of the purple photosynthetic bacterium Rhodospirillum centenum. Arch Microbiol. 1995;163:1–6. doi: 10.1007/BF00262196. [DOI] [PubMed] [Google Scholar]
- 38.Romagnoli S, Hochkoeppler A, Damgaard L, Zannoni D. The effect of respiration on the phototactic behavior of the purple nonsulfur bacterium Rhodospirillum centenum. Arch Microbiol. 1997;167:99–105. [PubMed] [Google Scholar]
- 39.Rushing, B. G., and C. E. Bauer. Unpublished data.
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 41.Sar N, McCarter L, Simon M, Silverman M. Chemotactic control of the two flagellar systems of Vibrio parahaemolyticus. J Bacteriol. 1990;172:334–341. doi: 10.1128/jb.172.1.334-341.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 43.Sprott G D, Koval S F, Schnaitman C A. Cell fractionation. In: Gerhardt P, Murray R G E, Wood W A, Kreig N R, editors. Methods for general and molecular bacteriology. Washington, D.C: American Society for Microbiology; 1994. pp. 72–103. [Google Scholar]
- 44.Stadtwald-Demchick R, Turner F R, Gest H. Physiological properties of the thermotolerant photosynthetic bacterium, Rhodospirillum centenum. FEMS Microbiol Lett. 1990;67:139–144. [Google Scholar]
- 45.Stahl S J, Stewart K R, Williams F D. Extracellular slime associated with Proteus mirabilis during swarming. J Bacteriol. 1983;154:930–937. doi: 10.1128/jb.154.2.930-937.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yildiz F H, Gest H, Bauer C E. Attenuated effect of oxygen on photopigment synthesis in Rhodospirillum centenum. J Bacteriol. 1991;173:5502–5506. doi: 10.1128/jb.173.17.5502-5506.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yildiz F H, Gest H, Bauer C E. Genetic analysis of photosynthesis in Rhodospirillum centenum. J Bacteriol. 1991;173:4163–4170. doi: 10.1128/jb.173.13.4163-4170.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yildiz F H, Gest H, Bauer C E. Conservation of the photosynthesis gene cluster in Rhodospirillum centenum. Mol Microbiol. 1992;6:2683–2691. doi: 10.1111/j.1365-2958.1992.tb01445.x. [DOI] [PubMed] [Google Scholar]