Abstract
Traumatic brain injury (TBI) is known to affect the physiology of neural circuits in several brain regions, which can contribute to behavioral changes after injury. Disordered sleep is a behavior that is often seen after TBI, but there is little research into how injury affects the circuitry that contributes to disrupted sleep regulation. Orexin/hypocretin neurons (hereafter referred to as orexin neurons) located in the lateral hypothalamus normally stabilize wakefulness in healthy animals and have been suggested as a source of dysregulated sleep behavior. Despite this, few studies have examined how TBI affects orexin neuron circuitry. Further, almost no animal studies of orexin neurons after TBI have included female animals. Here, we address these gaps by studying changes to orexin physiology using ex vivo acute brain slices and whole–cell patch clamp recording. We hypothesized that orexin neurons would have reduced afferent excitatory activity after injury. Ultimately, this hypothesis was supported but there were additional physiological changes that occurred that we did not originally hypothesize. We studied physiological properties in orexin neurons approximately 1 week after mild traumatic brain injury (mTBI) in 6–8-week-old male and female mice. mTBI was performed with a lateral fluid percussion injury between 1.4 and 1.6 atmospheres. Mild TBI increased the size of action potential afterhyperpolarization in orexin neurons from female mice, but not male mice and reduced the action potential threshold in male mice, but not in female mice. Mild TBI reduced afferent excitatory activity and increased afferent inhibitory activity onto orexin neurons. Alterations in afferent excitatory activity occurred in different parameters in male and female animals. The increased afferent inhibitory activity after injury is more pronounced in recordings from female animals. Our results indicate that mTBI changes the physiology of orexin neuron circuitry and that these changes are not the same in male and female animals.
Keywords: electrophysiology, hypocretin, mice, mild traumatic brain injury, orexin, sex differences
Introduction
Traumatic brain injury (TBI) is very common; in the United States alone, an estimated 2.5 million emergency department visits are due to TBIs each year.1 Mild TBI (mTBI) accounts for 75% of these cases, representing a significant number of patients.2,3 There are a variety of negative symptoms associated with TBI and mTBI, including cognitive dysfunction, memory impairments, and mood disorders.4,5 Previous research has shown that TBI can have a profound effect on the neural circuitry that underlies these negative symptoms.6–13 However, there are some behavioral symptoms that have not been well studied from a physiological perspective. Sleep disorders are a common symptom after TBI; approximately 30-70% of TBI patients will experience sleep disorders after injury.14–18 There are known neural circuits that regulate sleep19 but relatively little work has investigated how TBI affects those circuits.
The physiology of orexin/hypocretin neurons is at the heart of sleep/wake regulation. Orexin/hypocretin (hereafter referred to as orexin) is a neuropeptide produced by neurons in the lateral hypothalamus (LH).20,21 Reduced levels of orexin cause narcolepsy22–25 or hypersomnias26 and injecting orexin neuropeptide into the brain causes wakefulness,27,28 showing it is necessary and sufficient to promote wake behavior. In normal animals, orexin neurons stabilize wakefulness by activating wake-promoting neurons29,30 and it is thought that orexin neurons regulate an animal's arousal threshold.31,32 28-50% of TBI patients report disorders of hypersomnolence as part of their sleep disruptions after injury16,33–36 and it is believed that orexin dysregulation may be a source of this excessive sleepiness after TBI.
There is evidence that orexin is disrupted after TBI. Studies have shown that TBI patients have reduced orexin neuropeptide in their cerebral spinal fluid (CSF).37 This reduction can last 6 months after injury17 and correlates with increased excessive daytime sleepiness. In rodents, there is reduced orexin neuropeptide in the cerebral spinal fluid of the hypothalamus immediately following TBI.38 Some studies suggest that it is a reduction of the number of orexin neurons that explains these decreases in orexin.38–43 However, the study that found decreased orexin in the CSF of animals after injury did not observe a decrease in the number of orexin neurons meaning that these two factors do not necessarily correlate with one another.38
Additionally, our lab reported sleep disturbances after mTBI even when the number of orexin neuron numbers did not decrease,44 which means that the number of orexin neurons is not the only consideration after injury. Our lab used cFos as a marker of neuronal activity to demonstrate that the activity of orexin neurons is reduced after mTBI, not the number of cells. This shows that it is important to consider orexin neuron function after injury rather than only counting the number of orexin neurons.
Only two previously published studies have examined of the activity of orexin neurons after TBI. The first was the previously mentioned study, Lim and colleagues.44 The other reported that TBI reduces presynaptic glutamate in dendritic afferents onto orexin neurons.45 The current study expands on this work and uses electrophysiological techniques together with a transgenic orexin EGFP mouse to determine the mechanism of altered orexin neuronal function after mTBI.
Importantly, sleep is different in males and females.46,47 Female sex is considered a risk factor for sleep disorders after TBI.48 Previous studies report that TBI induces changes in sleep in both male38–41,44,49–56 and female animals.55,57,58 Despite this, little research on orexin after TBI has been done in female animals. Previously, it has been demonstrated that disruptions in homeostasis, such as stress59 can affect orexin neurons in a sex dependent manner. Sex-dependent changes in orexin could occur with TBI as well since injury disrupts homeostasis. Therefore, this study specifically examines orexin physiology of males and females after mTBI. Based on the two previous functional studies of orexin neurons after injury, we hypothesized that excitatory afferent activity onto orexin neurons would be reduced after mild traumatic brain injury.
Methods
Animals
All experiments were performed in accordance with protocols approved by the Children's Hospital of Philadelphia's Institutional Animal Care and Use Committee (IACUC; Protocol 0694) and the guidelines established by the U.S. Public Health Service's Guide for the Care and Use of Laboratory Animals. Animals were given access to ad libitum food and water. A total of 79 animals had data analyzed for these experiments. 41 Males, 19 Sham and 22 Injured; 35 Females, 17 Sham and 18 Injured, and 3 Male Naïve animals only used for neurobiotin filling and no other data collection. Mice were between 6-8 weeks old at the time of injury. Animals were bred in-house to contain EGFP on the prepro-orexin promoter.60 Each mouse was genotyped to check for the Orexin-EGFP marker prior to injury and recording. Female animals were checked for estrus cycle stage on the day of sacrifice. There were not enough animals to appropriately compare between estrus cycle stages (Supplementary Table S1); therefore, the female animals were analyzed as one group. For all experiments, the experimenter was blinded to the injury condition of the animal until after analysis of the data.
Surgical procedures in preparation for lateral fluid percussion injury
Animals were anesthetized with a mixture of ketamine (2.6 mg/kg) and xylazine (0.16 mg/kg) via intraperitoneal injection. Animals were given meloxicam (2 mg/kg) as an analgesic prior to surgery. Once fully anesthetized, animals were placed in a stereotaxic frame (Stoelting, Wood Dale, IL, USA), the scalp was incised and pulled away to fully expose the right parietal bone. An ultra-thin Teflon disk, (3-mm diameter) was glued to the skull with Vetbond (3M, St. Paul, MN, USA) between lambda and bregma sutures, and between the sagittal suture and the lateral ridge over the right hemisphere. Guided by the Teflon disk, a trephine was used to perform a 3-mm diameter craniectomy over the right parietal area. Following craniectomy, a Luer-lock needle hub (3-mm inner diameter) was secured above the skull opening with superglue (Loctite, Düsseldorf, Germany) and dental acrylic (Stoelting), filled with saline and capped. Lastly, animals were removed from stereotaxis, placed on a heating pad until fully recovered from anesthesia as assessed by the animal's spontaneous ambulation. Once the animals can walk on their own, they are then returned to their respective home cage. Animals were monitored for 24 h after surgery.
Lateral fluid percussion injury
Twenty-four hours following craniectomy, animals were placed under isoflurane anesthesia (2% oxygen in 500 mL/min) in a chamber and respiration was visually monitored until animals reached a surgical plane of anesthesia (one respiration per 2 sec). At this point, animals were removed from isoflurane, the needle hub was refilled with saline and connected to the fluid percussion injury device (Department of Biomedical Engineering, Virginia Commonwealth University, Richmond, VA, USA, and Custom Design and Fabrication, Sanston, VA, USA) via high-pressure tubing. The animal was placed onto a heating pad on its left side and upon resumption of normal breathing pattern but before sensitivity to stimulation, the injury was induced by a 20-msec pulse of saline onto the intact dura. The pressure transduced onto the dura was monitored with a pressure transducer connected to an oscilloscope, with injury severity ranging between 1.4 and 1.6 atmospheres. This is considered a mild injury. Immediately after injury, the hub was removed from the skull and the animal was placed in a supine position to assess righting reflex. After righting, the animal was subjected to inhaled isoflurane to suture the scalp.
Animals were allowed to recover on a heating pad until mobile, at which point they were returned to their home cage. Animals were given meloxicam post-surgery (5 mg/kg) for pain relief. Sham animals underwent all surgical procedures and anesthesia, including attachment to the fluid percussion injury device, with exclusion of the actual fluid pulse. Average righting times and standard deviations for animals in seconds: Shams: 41.39 ± 31.77; Injured: 275.97 ± 160.53. Divided by sex, the righting times were not significantly different: Male Sham: 30.29 ± 10.76; Male Injured: 233.12 ± 166.50; Female Sham: 50.53 ± 40.02; Female Injured: 321.5 ± 145.3. Injured animals that showed no delay in righting time were not included in the analysis. The injury used is classified as mild, so animals with hemorrhaging, or severe motor dysfunction after injury were not included in the analysis.
Slicing details
All animals used for electrophysiological recordings were sacrificed 6-10 days after the injury or sham-injury. Animals were anesthetized with inhaled isoflurane in a bell jar, decapitated and the brain was removed into an ice-cold bath of sucrose cutting solution. Animals were sacrificed between zeitgeber time (ZT) ZT4 - ZT5 after lights-on in the animal facility, between 10 a.m. and 11 a.m. Concentrations of the components of the sucrose cutting solution are the following (all in mM): Sucrose 202, NaHCO3 26, KCl 3, MgCl2 1, Glucose 10, NaH2PO4 1.25, CaCl2 2. Slices were cut on a vibratome (Leica VT1200S, Deer Park, IL, USA) at 350 μm and slices were cut at a speed of 0.06 mm/sec and an amplitude of oscillation of 1.0 mm. Slices were then incubated at 35°C in artificial cerebral spinal fluid (aCSF) for at least 1 h prior to recording. Artificial CSF was comprised of the following (all in mM): NaCl 130, NaHCO3 26, KCl 3, MgCl2 1, Glucose 10, NaH2PO4 1.25, CaCl2 2. Artificial CSF and sucrose solutions were prepared with an osmolality between 290-310 mmol/kg and were constantly bubbled with a carbogen gas mixture (95% oxygen, 5% carbon dioxide; AirGas, Radnor Township, PA, USA).
Electrophysiological recording and recording parameters
After incubation, slices were moved to a slice chamber (Warner Instruments RC-26, Holliston, MA, USA) superfused with aCSF and kept at 29.6°C with an inline heater (Warner Instruments TC-324B). Patch electrodes with resistances of 2-8 MΩ were pulled from borosilicate glass (1B150F-4; World Precision Instruments, Sarasota, FL, USA). Series resistance was monitored throughout the experiment and recordings were not used if a cell exceeded 35 MΩ in series resistance. Series resistance was compensated for at 70%. All recordings were made using a Multiclamp 700B (Molecular Devices, Palo Alto, CA, USA) sampled at 20 kHz, filtered at 3 kHz.
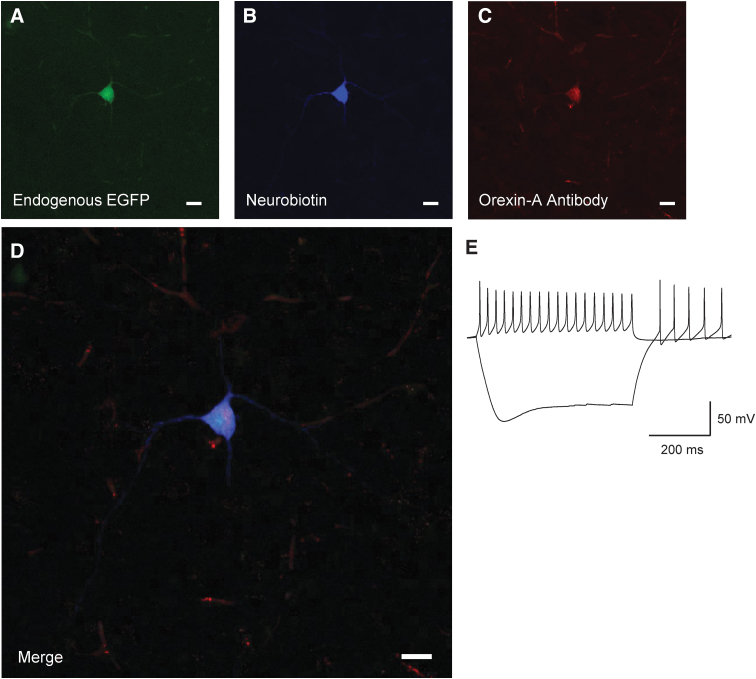
Orexin neurons were initially identified using 2% neurobiotin (Vector Laboratories, Newark, CA, USA) in K-Gluconate internal solution (n = 9 cells; Fig. 1 as an example). After the accuracy of our EGFP label was confirmed, neurons were identified using EGFP at the time of recording. All orexin neurons recorded were selected from the side ipsilateral to the brain injury.
FIG. 1.
Identification of orexin/hypocretin neurons. Post hoc neuronal staining after recording in Orexin-EGFP mice. EGFP Neuron (A) co-stained with (B) neurobiotin, and (C) orexin-A antibody. (D) Merged image of endogenous EGFP fluorescence, neurobiotin, and orexin -A antibody. Scale bars at 20 μm. (E) Electrophysiological properties of recorded orexin neuron. Sag current during hyperpolarization and non-accommodating action potentials as previously reported for orexin neurons and assist in confirming the identity of the recorded cell. Sample traces show -200 pA and 100 pA current injection.
Passive membrane properties, action potential properties, and excitatory post-synaptic currents (EPSC) recordings were collected with a K-Gluconate based internal pipette solution. Composition of the K-Gluconate internal solution was (all in mM): K Gluconate 125, KCl 5, EGTA 1.1, HEPES 10, MgCl2 1, Mg-ATP 5, Na-GTP 0.5. Inhibitory post-synaptic current (IPSC) recordings were collected with KCl based internal pipette solution to achieve isotonic chloride recordings. Composition of the KCl internal solution was: KCl 145, EGTA 1.1, HEPES 10, MgCl2 1 Mg-ATP 2, Na-GTP 0.5. In both internals, KOH was used to titrate pH between 7.2 and 7.3. The osmolality of both internals was between 280 and 290 mmol/kg. All measurements described in this study were compensated for the liquid junction potentials of the internal solutions estimated at 14 mV for the K-Gluconate Internal and 3.4 mV for the KCl Internal (Clampex; Molecular Devices).
To isolate excitatory events, EPSCs were recorded in the presence of the Gamma-Aminobutyric Acid (GABA) receptor antagonist (-)- bicuculline methiodide (Abcam, 30 μM, Cambridge, UK). To isolate inhibitory events, IPSCs were recorded in the presence of the N-methyl-D-aspartate (NMDA) channel antagonist DL-2-amino-5-phosphonopentanoic acid sodium salt (DL-AP5, sodium salt; Abcam, 50 μM, Cambridge, U.K.) and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) channel antagonist (1,2,3,4-Tetrahydro-7-nitro-2,3-dioxoquinoxaline-6-carbonitrile disodium; DS-CNQX; Abcam, 20 μM, Cambridge, UK). To isolate miniature EPSCs and IPSCs, events were recorded in the presence of the sodium channel antagonist tetrodotoxin citrate (TTX; Abcam, 0.4 μM, Cambridge, U.K.). To test drug efficacy and determine current identity in orexin neurons, IPSCs were blocked with bicuculline and EPSCs were blocked with DS-CNQX and DL-AP5 and no currents were observed (n = 10 cells, data not shown).
Recording measurements
Intrinsic membrane properties and action potential properties were recorded in current clamp. Resting membrane potential was determined by reading the membrane voltage in current clamp between 1-5 sec after cell break in. To measure action potential (AP) firing frequency versus injected current, cells were injected with current steps from -200 pA to 200 pA in increments of 25 pA for a duration of 500 msec. The frequency of AP firing was determined by counting the number of APs in the first 100 msec of the 500 msec pulse during each current step. Input resistance was determined by using the change in voltage during the -100 pA step and calculated with Ohm's law, . Other AP properties were measured using an AP during the 0 pA pulse when there was no exogenous current injection and the cell was allowed to fire spontaneously. The threshold of AP firing was found by taking the first derivative (dV/dt) of the AP and measuring where the AP exceeded 10 mV/msec.61 The afterhyperpolarization size was determined by the value in mV between threshold and the minimum value reached during the after-hyperpolarization. Sag ratio was determined by using the -200 pA current pulse. The size of the voltage drop from baseline to the peak of the sag was compared with the size of the voltage drop from baseline to steady state after the sag. The sag ratio was calculated with the formula: .
All voltage clamp recordings were made while holding cells at -67 mV after adjusting for the internal solution's liquid junction potential. Synaptic events were determined via the Template Search algorithm in Clampfit 11.1 (Molecular Devices). Current events with a 10-90% rise time greater than 1.5 msec were excluded from the analysis. MATLAB (2021a, MathWorks, Natick, MA, USA) code was used to randomly select 75 current events per cell to ensure equal weighting of cells in the cumulative probability histogram analysis. All events from all cells in one group (e.g., Sham vs. Injured) were then pooled to create a cumulative probability histogram. Statistical analyses were performed in Prism 9 (GraphPad, San Diego, CA, USA).
Staining to confirm orexin/hypocretin identity after recording
For cells filled with 2% neurobiotin, post hoc staining was used to confirm the identity of the EGFP neurons. Post-recording, slices were fixed in 4% paraformaldehyde overnight. Slices were washed four times with 0.01M phosphate-buffered saline (PBS) and then blocked in blocking solution of 10% bovine serum albumin, NGS, 0.3% Triton-X and PBS for 3 h. All washes were done with 0.01M PBS. After blocking, slices were washed and incubated in Mouse-On-Mouse Blocker (1:40 concentration; Vector Labs, Newark, CA, USA) for 2 h at room temperature. Slices were washed and Orexin-A Antibody (1:1000; KK09, Santa Cruz Laboratories, Dallas, TX, USA) was added to incubate overnight at room temperature. Slices were washed and incubated with 594 goat-anti rabbit (1:200) and 405 Dylight Conjugated Streptavidin (1:300; Jackson ImmunoResearch, West Grove, PA, USA) at room temperature for 3 h and then at 4°C overnight. Slices were washed and mounted in Fluoromount-G (Southern Biotech, Birmingham, AL, USA) and imaged with a Leica Sp8 confocal microscope (Leica, Deer Park, IL, USA).
Statistical analysis
Statistical tests for current clamp recordings including intrinsic properties and membrane potential properties included unpaired Student's t-tests comparing means of these properties, or mixed-effects analysis where appropriate. For these tests, values of p < 0.05 were considered significant (Table 1). For voltage clamp recordings, Kolmogorov-Smirnov tests were performed on the pooled cumulative probability histograms and compared between sham and injured groups. Significant differences in distributions were set at a threshold p value of p < 0.001 or less to be considered significant due to the large number of events (Table 2).
Table 1.
Current Clamp Values
| |
Male animals |
Female animals |
Test used | ||
|---|---|---|---|---|---|
| Property | Sham | Injured | Sham | Injured | |
| Resting membrane potential | -51.03 mV (1.553) |
-53.95 mV (1.669) |
-54.45 mV (1.718) |
-53.44 mV (2.121) |
Unpaired two-tailed t-test |
| p = 0.2087 | p = 0.7168 | ||||
| Firing threshold | -38.22 mV (1.062) |
-41.14 mV (0.9561) |
-40.08 mV (1.263) |
-41.04 (1.196) |
Unpaired two-tailed t-test |
| p = 0.0473* | p = 0.5834 | ||||
| Afterhyperpolarization | 23.97 mV (0.6719) |
24.43 mV (0.7701) |
23.12 mV (0.8982) |
25.68 mV (0.7872) |
Unpaired two-tailed t-test |
| p = 0.6593 | p = 0.0409* | ||||
| Resistance | 606.6 MΩ (28.21) |
562.8 MΩ (36.89) |
519.0 MΩ (31.16) |
532.8 MΩ (27.28) |
Unpaired two-tailed t-test |
| p = 0.3620 | p = 0.7422 | ||||
| Rise slope | 55.72 mV/msec (5.878) |
55.87 mV/msec (6.180) |
61.33 mV/msec (10.84) |
85.41 mV/msec (14.04) |
Unpaired two-tailed t-test |
| p = 0.9867 | p = 0.1915 | ||||
| Fall slope | -29.35 mV/msec (2.027) |
-28.71 mV/msec (2.100) |
-32.37 mV/msec (3.544) |
-38.35 mV/msec (4.391) |
Unpaired two-tailed t-test |
| p = 0.8293 | p = 0.3038 | ||||
| Sag ratio | 15.95 (1.984) |
14.96 (2.122) |
15.05 (2.247) |
20.66 (2.189) |
Unpaired two-tailed t-test |
| p = 0.6683 | p = 0.0853 | ||||
| Action potentials in 60 sec | 4.003 Hz (0.3505) |
4.519 Hz (0.5086) |
5.300 Hz (0.9256) |
4.025 Hz (0.5979) |
Unpaired two-tailed t-test |
| p = 0.4055 | p = 0.2459 | ||||
| Firing frequency as a function of current injection. Measured as # of AP's in 100 msec. The p value is comparison between injury groups | p = 0.3214 | p = 0.2651 | Mixed effects analysis | ||
Bolded data indicates a significant finding.
All properties and significance values recorded in current clamp of orexin/hypocretin neurons. Values reported as means with standard error of the mean.
AP, Action potential.
Table 2.
Voltage Clamp Significance
| Males sEPSC | Males mEPSC | Females sEPSC | Females mEPSC | Males sIPSC | Males mIPSC | Females sIPSC | Females mIPSC | |
|---|---|---|---|---|---|---|---|---|
| Amplitude | 0.0019 | < 0.0001 | < 0.0001 | 0.0023 | 0.2237 | 0.7223 | 0.0008 | < 0.0001 |
| Interevent interval | < 0.0001 | 0.2655 | 0.1349 | < 0.0001 | 0.0001 | 0.3927 | < 0.0001 | 0.1498 |
Bolded data indicates a significant finding.
Significance of cumulative probability histograms of orexin/hypocretin neurons recorded in voltage clamp. Values computed with the Kolmogorov-Smirnov test with a value of p < 0.001 to be considered significant.
sEPSC, spontaneous excitatory post-synaptic current; mEPSC, miniature excitatory post-synaptic current; sIPSC, spontaneous inhibitory post-synaptic current; mIPSC, miniature inhibitory post-synaptic current.
Results
Identification of orexin/hypocretin neurons for electrophysiological recording
We used transgenic mice with enhanced green fluorescent protein (EGFP) downstream of the prepro-orexin promoter to visually identify orexin neurons.60,62 To initially confirm their identity, we performed visualized whole–cell patch clamp on EGFP labeled neurons (Fig. 1), filled a subset with neurobiotin (n = 9 cells) and co-stained with orexin-A antibody (Fig. 1D) post hoc. Recorded EGFP labelled neurons show the previously established electrical properties of orexin neurons.63-65 That is, spontaneously firing action potentials, non-accommodating action potentials and a hyperpolarizing sag in response to injection of negative current (Fig. 1E).
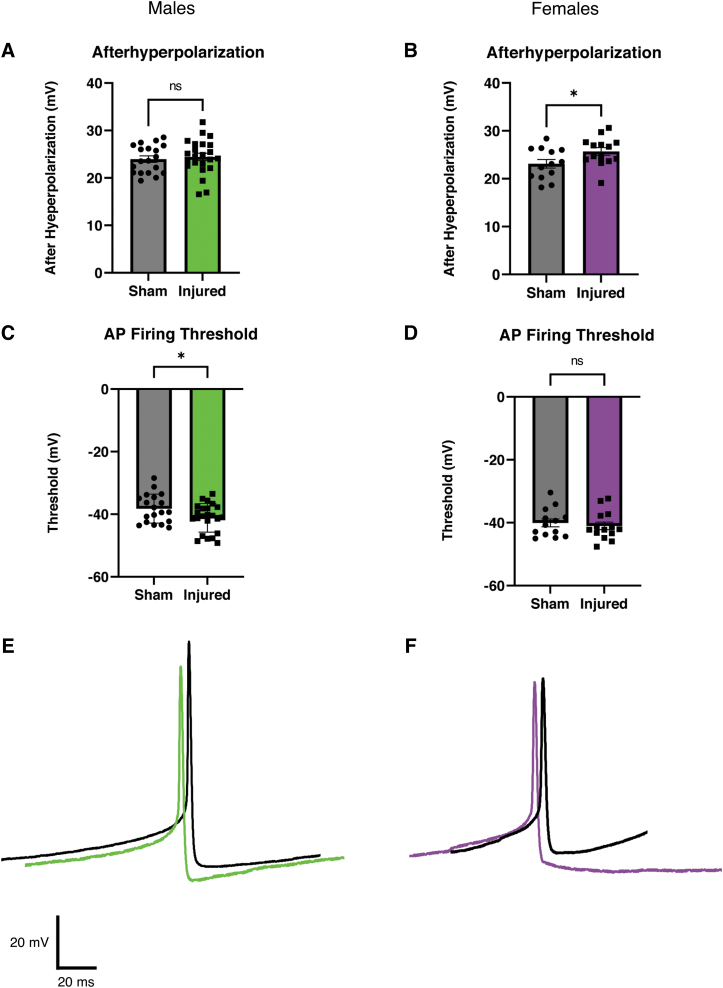
Increased afterhyperpolarization in female animals and reduced action potential firing threshold in male animals after mild traumatic brain injury
We next examined if mTBI altered the intrinsic membrane properties or action potential firing of orexin neurons (Fig. 2). In current clamp recordings from female animals, we observed a larger after hyperpolarization after mTBI (Fig. 2B). This result was not observed in neurons from injured male animals (Fig. 2A). We also observed a reduction in the action potential firing threshold in orexin neurons from male animals after mTBI (Fig 2C), but we did not see this reduction in firing threshold in neurons from female animals (Fig 2D). No other significant differences between intrinsic properties in slices from sham and injured animals were detected (Table 1; Males: Sham = 9 animals, 19 cells; Injured = 11 animals, 23 cells; Females: Sham = 7 animals, 13 cells; Injured = 8 animals, 14 cells]
FIG. 2.
Afterhyperpolarization is increased after injury in female animals but not in male animals. Action potential firing threshold is reduced after injury in male animals, but not in female animals. (A, B) Bar graphs of afterhyperpolarization in males (A) and females (B). Each point represents one orexin neuron. Graphs show means of individual cells with error bars as standard error of the means (SEMs). Males: Sham = 9 animals, 19 cells, Injured = 11 animals, 23 cells, p = 0.6593. Females: Sham = 7 animals, 13 cells, Injured = 8 animals, 14 cells, p = 0.0409* Unpaired Two tailed t-test. (C, D) Bar graphs of action potential threshold in males (C) and females (D). Each point represents one orexin neuron. Graphs show means of individual cells with error bars as SEMs. Males: Sham = 9 animals, 19 cells, Injured = 11 animals, 23 cells, p = 0.0473* Unpaired two-tailed t-test. Females: Sham = 7 animals, 13 cells, Injured = 8 animals, 14 cells, p = 0.5834 Unpaired two-tailed t-test. (E, F) Representative action potentials recorded in current clamp. Action potentials from orexin neurons from male animals (E) have a reduced action potential threshold after injury. Action potentials from orexin neurons from female animals (F) show a larger afterhyperpolarization after injury. (E) Males- Sham: Black, Injured: Green. (F) Females- Sham: Black, Injured: Purple. *p < 0.05 significance.
Orexin neurons receive local afferents from within the LH66 as well as from a variety of other brain regions.62,67,68 Due to the extensive afferent connections onto orexin neurons, we examined whether mTBI alters afferent synaptic activity onto orexin neurons.
Mild traumatic brain injury reduces afferent excitatory activity onto orexin/hypocretin neurons in both male and female mice
Orexin neurons are innervated by both excitatory and inhibitory afferents.69 Therefore, we recorded EPSCs in orexin neurons in bicuculline (30 μM) to isolate excitatory currents. We recorded both spontaneous excitatory post-synaptic currents (sEPSCs) as well as miniature excitatory post-synaptic currents (mEPSCs) where miniature excitatory events were isolated by recording in bicuculline plus tetrodotoxin (TTX; 0.4 μM).
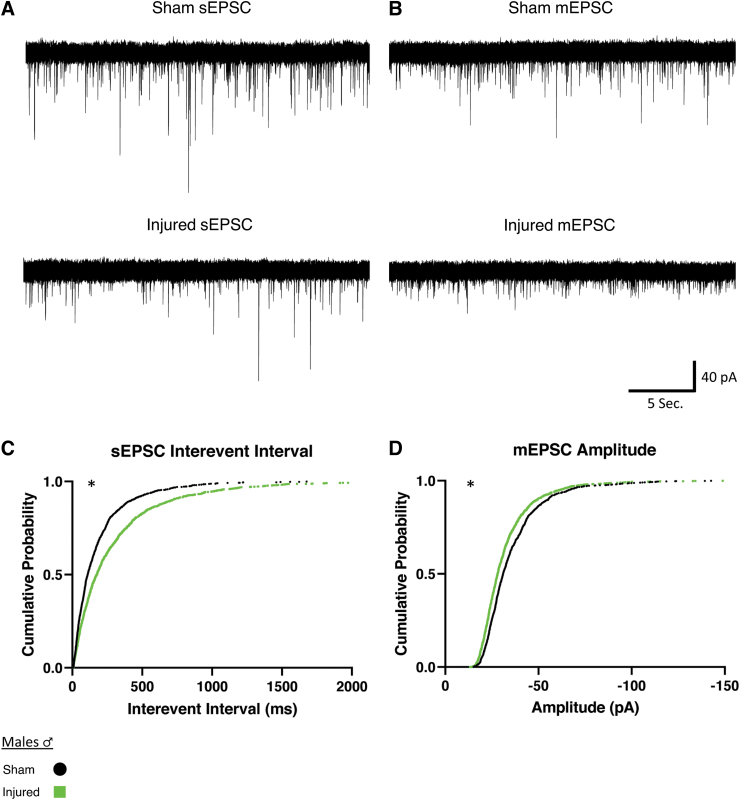
We examined if injury would affect the properties of EPSCs onto orexin neurons from male animals (Fig. 3). For sEPSCs in slices from male animals, mTBI increased the time between events, or the interevent interval, indicating a decrease in frequency of afferent excitatory activity (Fig. 3C). For mEPSCs in male animals, the amplitude was decreased after mTBI (Fig. 3D). These results match our expected hypothesis that there would be diminished afferent excitatory activity onto orexin neurons after mTBI (sEPSC Sham: n = 7 animals, 16 cells; Injured: n = 7 animals, 18 cells; mEPSC Sham: n = 5 animals, 18 cells; Injured: n = 5 animals, 17 cells).
FIG. 3.
Males show reduced afferent excitatory activity onto orexin/hypocretin neurons after mild traumatic brain injury. (A, B) Representative spontaneous and miniature excitatory synaptic currents recorded in orexin neurons in slices from male mice. Spontaneous excitatory post-synaptic currents (sEPSCs) (A) and miniature excitatory post-synaptic currents (mEPSCs) (B). Note the increase in sEPSC interevent interval and the decrease mEPSC amplitude. (C, D) Cumulative probability plots (75 current events per cell) corroborating the representative cell data above. Significant differences in distributions observed in interevent interval for sEPSCs (C) and amplitude for mEPSCs (D) in male animals after injury. sEPSCs were observed to have an increase of interevent interval after injury, representing a decrease in frequency of afferent excitatory signaling. Cells clamped at -67 mV and recorded in artificial cerebral spinal fluid (aCSF) containing bicuculline (30 μM) (A) or bicuculline plus TTX (0.4 μM) (B). Significance determined with Kolmogorov-Smirnov test for distributions with significance set at p < 0.001. sEPSC Sham: n = 7 animals, 16 cells, Injured: n = 7 animals, 18 cells. mEPSC Sham: n = 5 animals, 18 cells, Injured: n = 5 animals, 17 cells. *p < 0.001 significance (see Table 2).
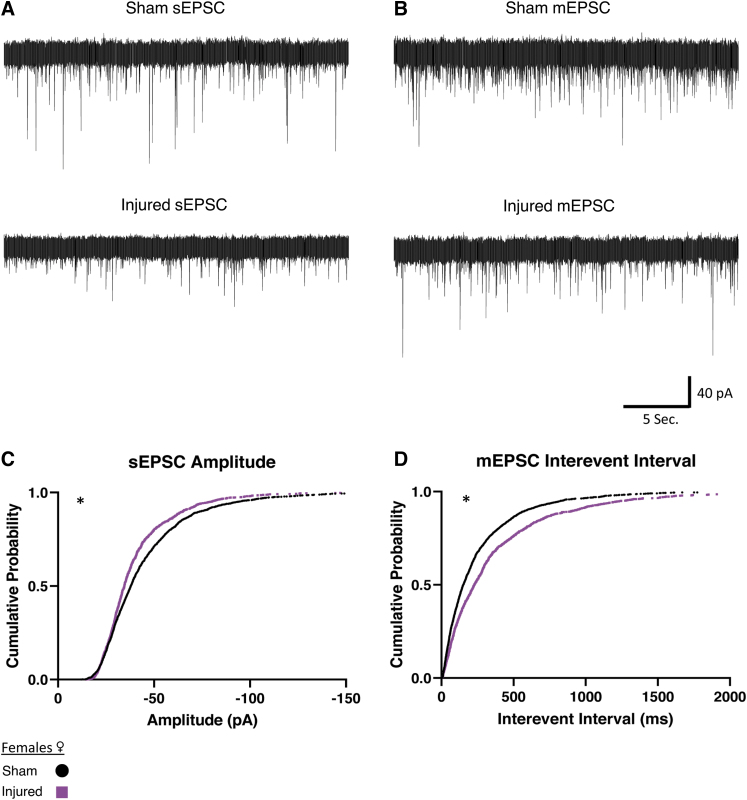
We next examined whether this reduced afferent excitatory activity after injury would hold true for female animals (Fig. 4). We observed in slices from female animals that sEPSCs decreased in amplitude after mTBI (Fig. 4C). Miniature EPSCs in orexin neurons in slices from females had an increase in interevent interval (Fig. 4D), which indicates a decrease in the frequency of miniature afferent excitatory activity. Similar to the male animals, these results indicate a reduction of afferent excitatory signaling after injury (sEPSC Sham: n = 8 animals, 29 cells, Injured: n = 5 animals, 18 cells. mEPSC Sham: n = 8 animals, 32 cells. Injured: n = 4 animals, 17 cells).
FIG. 4.
Females show reduced afferent excitatory activity onto orexin/hypocretin neurons after mild traumatic brain injury. (A, B) Representative excitatory currents recorded in orexin neurons in slices from female mice. Traces shown from spontaneous excitatory post-synaptic currents (sEPSCs) (A) and miniature excitatory post-synaptic currents (mEPSCs) (B). Note the decrease in sEPSC amplitude, and the increase in mEPSC interevent interval. (C, D) Cumulative probability plots (75 current events per cell) corroborating representative cell data above. In female mice significant differences in distributions included a decrease in sEPSC amplitude after injury (C). mEPSCs in female animals were observed to have an increase of interevent interval after injury (D), representing a decrease in the frequency of afferent excitatory signaling. Voltage clamp traces recorded in orexin neurons recorded at -67 mV in brain slices from female Sham or Injured animals. Afferent excitatory signals recorded in bicuculline (30 μM) to block inhibitory signals. Afferent excitatory miniature currents (B) recorded in the presence of bicuculline and TTX (0.4 μM). Significance determined with Kolmogorov-Smirnov test for distributions with significance set at p < 0.001. sEPSC Sham: N = 8 animals, 29 cells, Injured: n = 5 animals, 18 cells. mEPSC Sham: n = 8 animals, 32 cells. Injured: n = 4 animals, 17 cells. *p < 0.001 significance (see Table 2).
Mild traumatic brain injury increases afferent inhibitory activity onto orexin/hypocretin neurons
Since changes in inhibitory synaptic transmission could also affect orexin neuron activity, and because inhibitory regulation of orexin neurons is crucial for reducing arousal at appropriate times,70–74 we next investigated whether there were injury-induced changes in the inhibitory synaptic activity onto orexin neurons. IPSCs in orexin neurons were recorded in the presence of AP5 (50 μM) and CNQX (20 μM) to eliminate excitatory currents from NMDA and AMPA receptors respectively. We recorded both spontaneous (sIPSCs) and miniature currents (mIPSCs) where miniature events were again isolated by recording in the presence of TTX (0.4 μM).
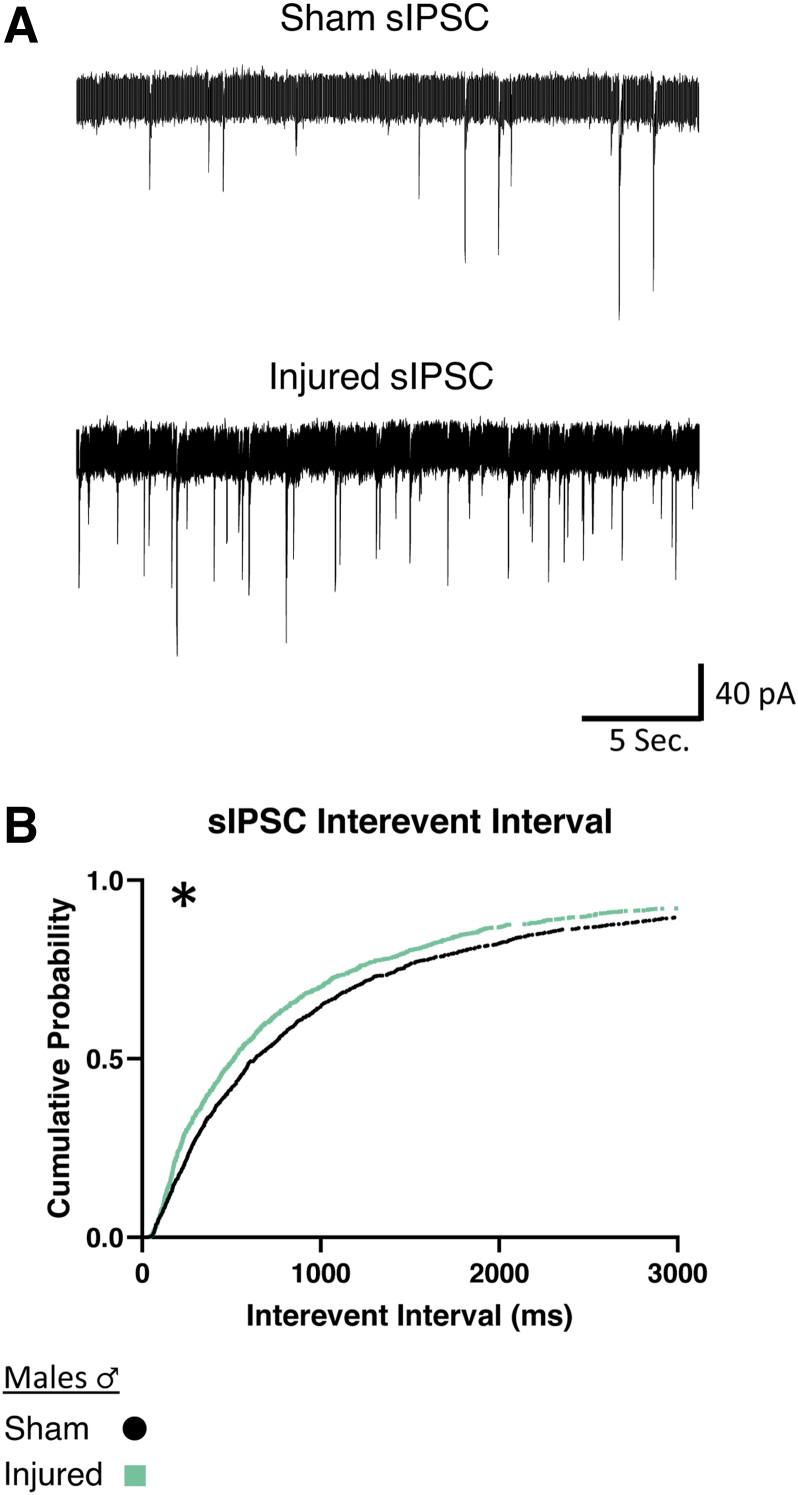
In slices from male animals, injured animals showed a decrease of the interevent interval between sIPSCs, (i.e., an increased frequency of inhibitory activity onto orexin neurons; Fig. 5B). There were no significant differences in mIPSCs in male animals after injury (Table 2; sIPSC Sham: n = 7 animals, 17 cells; Injured: n = 6 animals, 19 cells).
FIG. 5.
Males show increased frequency of afferent spontaneous inhibitory activity onto orexin/hypocretin neurons after mild traumatic brain injury. (A) Representative voltage clamp recordings of inhibitory currents from orexin neurons in brain slices from male mice. (B) sIPSC interevent interval cumulative probability plot was constructed from 75 current events per cell. Significant differences were observed in the interevent interval distribution for sIPSCs in male animals. The distribution had a decrease of interevent interval after injury, representing increased frequency of afferent inhibitory current. Voltage clamp traces in orexin neurons clamped at -67 mV. Afferent spontaneous inhibitory post synaptic currents (sIPSCs) recorded in AP5 (50 μM) and CNQX (20 μM) to block excitatory signals. Significance determined with Kolmogorov-Smirnov test for distributions with significance set at p < 0.001. sIPSC Sham: n = 7 animals, 17 cells, Injured: n = 6 animals, 19 cells. *p < 0.001 significance (see Table 2).
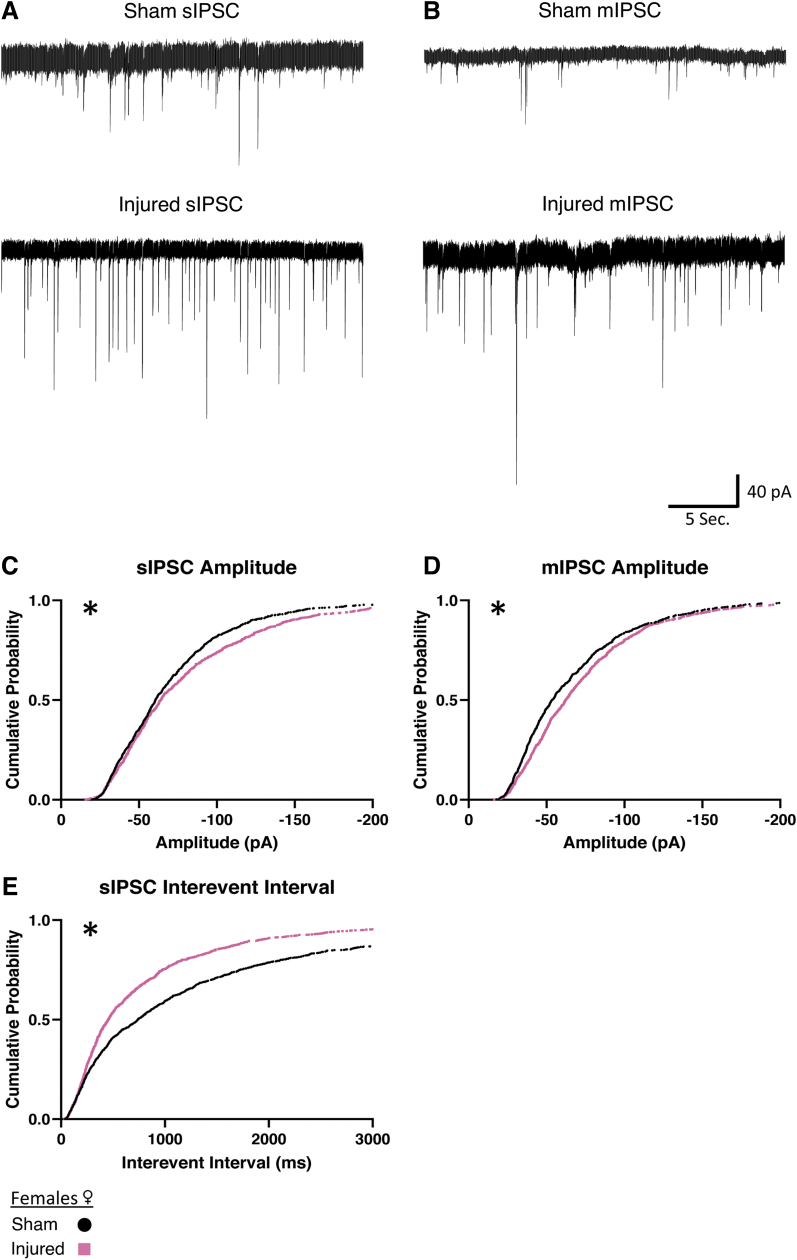
We then wanted to determine if increased afferent inhibitory activity would occur in female animals. In slices from female animals, we also found a reduction of the interevent interval in sIPSCs (Fig. 6E), indicating that injury also increases spontaneous inhibitory activity in female animals. Interestingly, after injury in female animals, both sIPSCs and mIPSCs show an increase in amplitude (Fig. 6C, 6D) that was not present in male animals (Table 2). Taken together, this data suggests that in both males and females there is an increase in afferent inhibitory activity onto orexin neurons after injury. Interestingly, the increase in inhibitory activity is more robust in slices from females (sIPSC Sham: n = 7 animals, 16 cells; Injured: n = 6 animals, 13 cells; mIPSC Sham: n = 5 animals, 13 cells; Injured: n = 6 animals, 14 cells).
FIG. 6.
Females show increased afferent inhibitory activity onto orexin/hypocretin neurons after mild traumatic brain injury. (A, B) Representative inhibitory currents. Traces shown from spontaneous inhibitory post-synaptic currents (sIPSCs) and miniature inhibitory post-synaptic currents (mIPSCs). sIPSC show both an increase in amplitude and shorter interevent interval, while for mIPSCs an increase in amplitude is observed. (C, D, E) Cumulative probability plots corroborating representative plots above, 75 current events per cell. Significant differences in sIPSC distributions observed as an increase of amplitude (C) and reduced interevent interval (E). Significant differences in mIPSC distributions observed as an increase in amplitude (D) after injury. Decreases in interevent interval represent an increase of frequency of inhibitory current onto orexin neurons after injury. Voltage clamp traces recorded in orexin neurons clamped at -67 mV. Afferent inhibitory signals recorded in AP5 (50 μM) and CNQX (20 μM) to block excitatory signals. Miniature currents (B) recorded in the presence of AP5 (50 μM), CNQX (20 μM) and TTX (0.4 μM). Significance determined with Kolmogorov-Smirnov test for distributions with significance set at p < 0.001. sIPSC Sham: n = 7 animals, 16 cells, Injured: n = 6 animals, 13 cells. mIPSC Sham: n = 5 animals, 13 cells, Injured: n = 6 animals, 14 cells. *p < 0.001 significance (see Table 2).
Discussion
While previous work has shown that mild traumatic brain injury affects orexin neurons, how injury affects their activity has not been studied in depth. This work addresses this gap in knowledge by employing whole–cell patch clamp recordings from identified orexin neurons to study the intrinsic properties and afferent synaptic activity onto orexin neurons. We found reduced afferent excitatory activity and increased inhibitory activity onto orexin neurons after injury, suggesting reduced orexin activity. These findings are consistent with others in the field that have shown that there is reduced orexin neuropeptide in CSF after injury,37,38 as well as studies of orexin neurons that showed reduced orexin neuron activity after injury.44 We observed an increase of the size of afterhyperpolarization in neurons from female animals and a reduction of action potential threshold in male animals. Injury affecting intrinsic neuronal properties is a known consequence of injury in some brain regions as alterations of action potential threshold have also been observed in neurons in layer 2/3 of the prefrontal cortex after injury.9 The changes presented in this study provide potential mechanisms for the observations of altered orexin neuron activity after TBI.
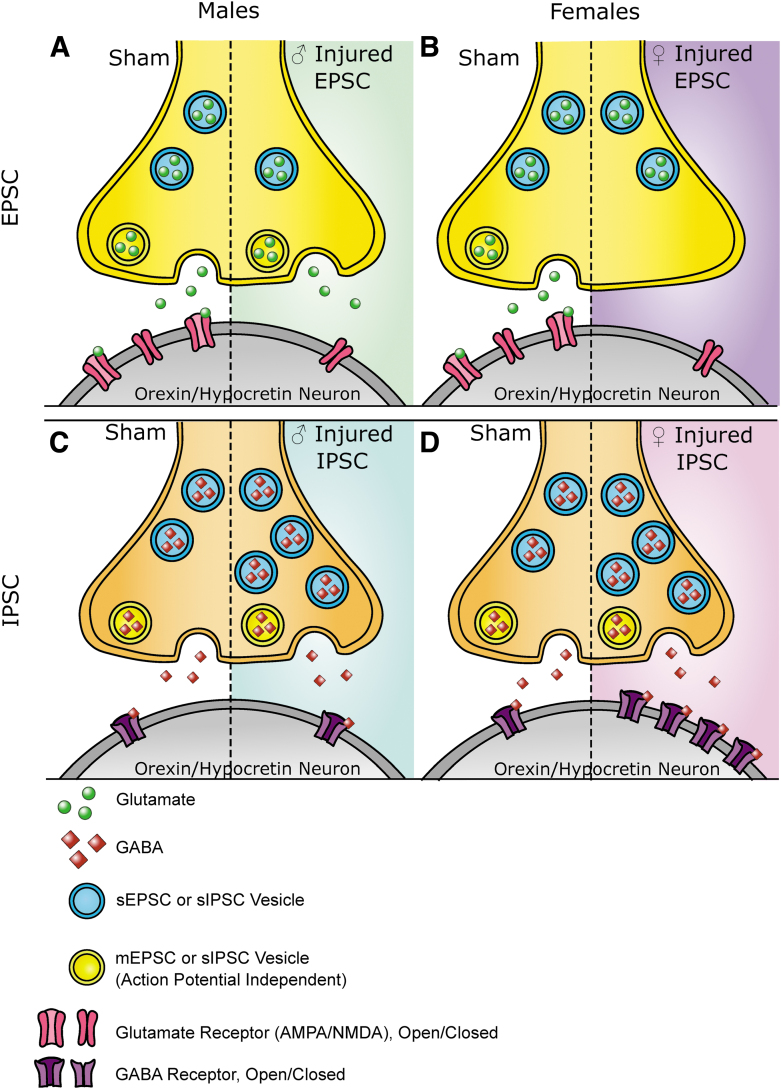
We observed that there were different changes in spontaneous and miniature synaptic current events after mTBI. Miniature events are exclusively action-potential independent, while spontaneous events include currents that are action potential dependent. It has been demonstrated that different vesicular pools can contribute to these currents.75–82 The injury-induced alterations in current activity may occur in miniature or spontaneous events, which means that mTBI may affect synaptic pools differently. In addition, it is thought that changes in the frequency of currents are related to presynaptic alterations and changes in the amplitude of currents are related to postsynaptic alterations. Together with the model that spontaneous and miniature events contribute to different vesicular pools, we can conceptualize how injury may alter synaptic activity in a mechanistic way (see Fig. 7 for a summary of these ideas).
FIG. 7.
Summary of findings. (A) In excitatory afferents in male animals, mild traumatic brain injury (mTBI) increases interevent interval in spontaneous events, represented by fewer presynaptic vesicles associated with spontaneous activity. Miniature events show a reduced amplitude, represented by fewer glutamatergic receptors. (B) In excitatory afferents in female animals, mTBI increases the interevent interval in miniature events, represented by fewer presynaptic vesicles associated with miniature events. Spontaneous events show reduced amplitude, represented by fewer glutamatergic receptors. (C) In inhibitory events in male animals, mTBI reduces the interevent interval in spontaneous events, represented by more spontaneous presynaptic vesicles. (D) In inhibitory events in females, mTBI reduces the interevent interval in spontaneous events, represented by more presynaptic vesicles. mTBI also increases the spontaneous and miniature event amplitudes, represented by an increase of post synaptic GABA receptors. Figure is representative of pre- and post- synaptic changes generally. Changes in the number of synapses, proteins involved in vesicle fusion, density of neurotransmitter in vesicles or channel function may also be possible but not represented here for the sake of clarity. EPSC, excitatory post-synaptic current; IPSC, inhibitory post-synaptic current; sEPSC, spontaneous excitatory post-synaptic current; mEPSC, miniature excitatory post-synaptic current; sIPSC, spontaneous inhibitory post-synaptic current; mIPSC, miniature inhibitory post-synaptic current.
Overall, the physiological alterations presented here contribute to a more mechanistic understanding of the changes to orexin circuitry after mTBI. The data presented here may also point to other changes in synapses not summarized in Figure 7, which could include injury induced changes in the number of synapses, changes in the proteins involved in vesicle fusion, altered activity of the receptors or changes in the amount of neurotransmitter within vesicles. Each of these ideas provides interesting and testable avenues for future research.
Based on previous studies, we had originally hypothesized that a majority of observed changes would come from decreased presynaptic glutamate. We did observe reduced afferent glutamatergic activity but we also observed significant increases in afferent inhibitory activity, especially in slices from female animals. The upregulation of GABAergic activity may be a result of compensatory mechanisms after mTBI. After injury, it is known that there is a transient increase in glutamate release83 and increased inhibition may be an attempt to compensate for this initial transient increase in excitatory activity to restore overall balance. Unfortunately, any potential compensation now appears maladaptive. The reduced afferent excitatory activity and increased inhibitory afferent activity were both measured approximately 1 week after injury. It appears that the increased inhibition is no longer compensating for an initial transient glutamatergic surge. Instead, increased inhibition onto orexin neurons may further reduce their activity following injury and potentially impair their ability to properly integrate signals from across the brain.
An interesting and important outcome of this study is that there are sex differences in how TBI affects orexin physiology after injury. While a majority of the injury induced alterations in the circuitry presented in this work imply reduced orexin neuron activity, the mechanisms are not the same between sexes. The circuitry may be differentially vulnerable to injury in males versus females. Previously published work regarding the effects of TBI on orexin neurons has been conducted only in male animals38–41,44,45,52,84 with just one exception, Saber and colleagues,55 who reported no change in the number of orexin neurons after injury. Due to this paucity of evidence, it has been difficult to speculate further regarding the possibility that injury could affect orexin circuitry differently between sexes. The data presented here will be important for future research and highlights specific alterations in pre- and post-synaptic sites for potential treatment of sleep disorders in men and women after mTBI.
Broadly, the orexin system is not identical between males and females. There is evidence for more prepro-orexin in female animals85 and higher levels of orexin in females.86 Another study has reported more orexin neurons in males compared with females,87 although this study was done in older rats as compared with the work reported from Taheri and colleagues.86 In either case, males and females measured at similar times and with similar techniques in the same lab show differences between sexes in the orexin neurons in the lateral hypothalamus. This is an indication that the system might be structured differently between sexes at baseline.
The orexin system in males and females also responds differently to stressors. Females have higher levels of prepro-orexin after restraint stress,88 males and females have different changes in orexin neuron synaptic structure after restraint stress,59 and female animals show a significantly greater correlation between prepro-orexin messenger RNA (mRNA) expression and corticotrophin releasing hormone, an indicator of stress, compared with males after unpredictable mild stress.89 Women with major depressive disorder have fewer orexin neurons.89 Even aging can affect the orexin system differently with one study showing that older female animals have fewer orexin neurons than males.87 This note about the orexin system differing in the sexes during aging may be particularly important since some research suggests that TBI “ages” the brain.90 Thus, the stressor of TBI may emphasize sex differences in the orexin system.
In addition to how the orexin system is different in males and females in terms of cells, circuitry, and response to stress, one can also consider whether circulating sex hormones may influence orexin responses to injury since those are also different in males and females. There is evidence that estrogens can influence the orexin system91-93; therefore, higher levels of estrogen in females may cause injury responses in the orexin system that would be different than males, where estrogen is lower. There is also interest in the female sex hormone cycling and whether that could influence outcomes in female animals. In this current study, there were not enough animals per estrus stage to appropriately compare them (Supplementary Table S1), but comparisons of orexin neurons after injury with a consideration of hormone levels or estrus cycle stage could be a potentially interesting future direction for this work.
As of now, only two published studies have studied electrophysiology to understand mechanistic changes to physiology in the hypothalamus after TBI.94,95 One of these studies did not find differences in physiology after injury94 and the other did.95 In other brain regions, there is evidence that TBI can affect brain regions and cell types differently, even in the same circuit or cells in close proximity.6–9 The structure, location, and properties of neurons within a brain region contribute to how TBI affects its physiology. The hypothalamus is a highly heterogeneous structure composed of many nuclei that perform a variety of essential homeostatic functions.96,97 TBI may very well affect each region of the hypothalamus differently. The work presented in this paper investigating the LH is part of the foundation of exploring how TBI affects the physiology of neural circuits throughout the hypothalamus.
The current study does have several limitations. This study measured the activity of orexin neurons after mTBI, but the complete circuitry of arousal and sleep is more complicated than any one cell subtype. Further investigations of the other connections within the sleep/wake circuitry could provide valuable insights into how the system is affected by injury. Additionally, while whole–cell patch clamp recording can provide detailed information regarding the summed synaptic input onto a neuron, there are still other mechanistic questions that cannot be answered with this technique. One of these questions includes the source of the afferent inputs onto orexin neurons, which could not be determined in this study. An exciting future direction of this work is to determine these potential inputs and isolate which of them are particularly susceptible to mTBI.
Conclusions
In conclusion, orexin circuitry is a critical part of the network in the brain that regulates wakefulness, but there has not been much direct study of how TBI affects this circuitry. As in other brain regions, mTBI does have an effect on the balance between excitation and inhibition in orexin circuitry. This study is among a handful that explore this phenomenon in the hypothalamus. This study is also one of the few that examines orexin neurons after TBI and shows that the physiological differences may appear similar but are not the same between sexes. Future mechanistic studies of how TBI affects neuronal function may reveal important changes in the brain after injury that could be missed with other techniques.
Transparency, Rigor, and Reproducibility Summary
This study and analysis plan were not formally preregistered. Based on extensive experience with patch clamp electrophysiology, sample size was set at a minimum of three animals and 10 cells per group. A total of 186 mice were bred for these experiments, 79 animals were the right age and genotype and had data collected for analysis. Technical animal exclusions included: hemorrhage, herniation, motor deficits, and no delay in righting time for injured animals. The experimenter performing patch clamp experiments was blinded to the animals' injury condition by the technician who performed the injury. The experimenter was blinded to injury condition until data analysis was complete. Animals were heterozygotes for Orexin-EGFP. All animals were 6-8 weeks old at the time of injury, except for one sham female that was 10 weeks old but whose data were not significantly different than other sham females. Animals were sacrificed between 6-10 days after the mild traumatic brain injury. Animals were brought from the animal facility at 9 a.m. and sacrificed between 10 and 11 a.m. on the recording day. Holding cages for the hour had bedding and water but no food provided. Females had their estrus cycle checked via vaginal saline swab at the time of sacrifice, but data from females was not divided by estrus cycle since there were not enough animals per estrus cycle stage to make appropriate comparisons (Supplementary Table S1). Recordings were not used if access resistance was above 35 MΩ or if recordings were unstable. For statistical analysis, all cells were treated as independent. Action potential frequency was treated as a repeated measure since it was done with current pulses in the same cell and a mixed model test was used. Statistical outliers were determined by the ROUT test Q = 1% and removed before final analysis. Means were used for current clamp data with an assumption of normal distribution, while medians were used for outlier tests for voltage clamp data with an assumption that the cumulative probability data is not normally distributed. Current clamp parameters were assumed as significantly different with a value of p < 0.05. Voltage clamp data was gathered by selecting 75 random currents per cell, constructing a cumulative probability histogram and comparing the distributions with the Kolmogorov-Smirnov (KS) test. Cells that did not have a minimum of 75 current events were excluded from analysis. Since 75 events per cell creates a high-powered test, the KS was not considered significant unless p < 0.001. To ensure that randomness would not produce artificial significance, code was created to repeat the 75 draws per cell and KS comparison 1000 times. If less than 60% of these draws were not significant by the KS test, the distributions were not considered significantly different. Analytic code and original data are available from the author upon request.
Supplementary Material
Acknowledgments
We would like to thank Dr. Robert Greene of UT Southwestern for graciously providing the Orexin-EGFP breeder mice with permission from Dr. Masashi Yanagisawa, creator of the mouse line, and Dr. Theresa Bjorness for helping with their transfer. We would like to thank the Intellectual and Developmental Disabilities (IDDRC) research center at CHOP/Penn and the Robinson lab for the use of the confocal microscope (NIH/NICHD P50HD105354). We would also like to thank Dr. Brian Johnson for thoughtful comments and input.
Authors' Contributions
Rebecca Somach: Conceptualization, data curation, formal analysis, software, investigation, methodology, project administration, resources, visualization, writing-original draft, writing—review and editing.
Ian Jean: Investigation, methodology, resources.
Anthony Farrugia: Investigation, methodology, resources.
Akiva Cohen: Conceptualization, funding acquisition, project administration, supervision, writing—review and editing.
Funding Information
Provided by training grant 5T32HL007953, R01 NS120099, R37HD059288 and the Children's Hospital of Philadelphia Frontier Program.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Taylor CA, Bell, JM, Breiding MJ, et al. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ 2017; 66(9):1–16; doi: 10.15585/mmwr.ss6609a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sosin DM, Sniezek J, Thurman DJ. Incidence of mild and moderate brain injury in the United States, 1991. Brain Inj 1996;10(1):47–54; doi: 10.1080/026990596124719 [DOI] [PubMed] [Google Scholar]
- 3. National Center for Injury Prevention and Control. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. 2003. Available from: https://www.cdc.gov/traumaticbraininjury/pdf/mtbireport-a.pdf [Last accessed August 10, 2023].
- 4. Riggio S. Traumatic brain injury and its neurobehavioral sequelae. Psychiatr Clin North Am 2010;33(4):807–819; doi: 10.1016/j.psc.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Concussion Signs and Symptoms. 2020. Available from: https://www.cdc.gov/headsup/basics/concussion_symptoms.html [Last accessed: January 4, 2023].
- 6. Folweiler KA, Xiong G, Best KM, et al. Traumatic brain injury diminishes feedforward activation of parvalbumin-expressing interneurons in the dentate gyrus. eNeuro 2020;7(6); doi: 10.1523/ENEURO.0195-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palmer CP, Metheny HE, Elkind JA, et al. Diminished amygdala activation and behavioral threat response following traumatic brain injury. Exp Neurol 2016;277:215–226; doi: 10.1016/j.expneurol.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Witgen BMM, Lifshitz J, Smith MLL, et al. Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: a systems, network and cellular evaluation. Neuroscience 2005;133(1):1–15; doi: 10.1016/j.neuroscience.2005.01.052 [DOI] [PubMed] [Google Scholar]
- 9. Smith CJ, Xiong G, Elkind JA, et al. Brain injury impairs working memory and prefrontal circuit function. Front Neurol 2015;6:240; doi: 10.3389/fneur.2015.00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lengel D, Huh JW, Barson JR, et al. Progesterone treatment following traumatic brain injury in the 11-day-old rat attenuates cognitive deficits and neuronal hyperexcitability in adolescence. Exp Neurol 2020;330:113329; doi: 10.1016/j.expneurol.2020.113329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen AS, Pfister BJ, Schwarzbach E, et al. Injury-induced alterations in CNS electrophysiology. Prog Brain Res 2007;161:143–169; doi: 10.1016/S0079-6123(06)61010-8 [DOI] [PubMed] [Google Scholar]
- 12. Gupta A, Elgammal FS, Proddutur A, et al. Decrease in tonic inhibition contributes to increase in dentate semilunar granule cell excitability after brain Injury. J Neurosci 2012;32(7):2523–2537; doi: 10.1523/JNEUROSCI.4141-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta A, Dovek L, Proddutur A, et al. Long-term effects of moderate concussive brain injury during adolescence on synaptic and tonic GABA currents in dentate granule cells and semilunar granule cells. Front Neurosci 2022;16:800733; doi: 10.3389/fnins.2022.80073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Masel BE, Scheibel RS, Kimbark T, et al. Excessive daytime sleepiness in adults with brain injuries. Arch Phys Med Rehabil 2001;82(11):1526–1532; doi: 10.1053/apmr.2001.26093 [DOI] [PubMed] [Google Scholar]
- 15. Castriotta RJ, Wilde MC, Lai JM, et al. Prevalence and consequences of sleep disorders in traumatic brain injury. J Clin Sleep Med 2007;3(4):349–356. [PMC free article] [PubMed] [Google Scholar]
- 16. Kempf J, Werth E, Kaiser PR, et al. Sleep-wake disturbances 3 years after traumatic brain injury. J Neurol Neurosurg Psychiatry 2010;81(12):1402–1405; doi: 10.1136/jnnp.2009.201913 [DOI] [PubMed] [Google Scholar]
- 17. Baumann CR, Werth E, Stocker R, et al. Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain 2007;130(7):1873–1883; doi: 10.1093/brain/awm109 [DOI] [PubMed] [Google Scholar]
- 18. Chan LG, Feinstein A. Persistent sleep disturbances independently predict poorer functional and social outcomes 1 year after mild traumatic brain injury. J Head Trauma Rehabil 2015;30(6):E67–E75; doi: 10.1097/HTR.0000000000000119 [DOI] [PubMed] [Google Scholar]
- 19. Eban-Rothschild A, Appelbaum L, de Lecea L. Neuronal mechanisms for sleep/wake regulation and modulatory drive. Neuropsychopharmacol 2018;43(5):937–952; doi: 10.1038/npp.2017.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 1998;95(1):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998;92(4):573–585; doi: 10.1016/S0092-8674(00)80949-6 [DOI] [PubMed] [Google Scholar]
- 22. Bourgin P, Zeitzer JM, Mignot E. CSF hypocretin-1 assessment in sleep and neurological disorders. Lancet Neurol 2008;7(7):649–662; doi: 10.1016/S1474-4422(08)70140-6 [DOI] [PubMed] [Google Scholar]
- 23. Mahoney CE, Cogswell A, Koralnik IJ, et al. The neurobiological basis of narcolepsy. Nat Rev Neurosci 2019;20(2):83–93; doi: 10.1038/s41583-018-0097-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000;6(9):991–997; doi: 10.1038/79690 [DOI] [PubMed] [Google Scholar]
- 25. Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron 2000;27(3):469–474; doi: 10.1016/s0896-6273(00)00058-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ebrahim IO. Hypocretin (orexin) deficiency in narcolepsy and primary hypersomnia. J Neurol Neurosurg Psychiatry 2003;74(1):127–130; doi: 10.1136/jnnp.74.1.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piper DC, Upton N, Smith MI, et al. The novel brain neuropeptide, orexin-A, modulates the sleep–wake cycle of rats. Eur J Neuroscie 2000;12(2):726–730; doi: 10.1046/J.1460-9568.2000.00919.X [DOI] [PubMed] [Google Scholar]
- 28. Hagan JJ, Leslie RA, Patel S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A 1999;96(19):10911–10916; doi: 10.1073/pnas.96.19.10911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci 2007;8(3):171–181; doi: 10.1038/nrn2092 [DOI] [PubMed] [Google Scholar]
- 30. Inutsuka A, Yamanaka A. The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions. Front Endocrinol (Lausanne) 2013;4; doi: 10.3389/fendo.2013.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Borniger JC, Lecea L de. The Hypocretin Arousal Network. In: Oxford University Encyclopedia of Neuroscience. Oxford University Press: Oxford, U.K.; 2019. [Google Scholar]
- 32. Tyree SM, Borniger JC, de Lecea L. Hypocretin as a hub for arousal and motivation. Front Neurol 2018;9:413; doi: 10.3389/fneur.2018.00413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mathias JL, Alvaro PK. Prevalence of sleep disturbances, disorders, and problems following traumatic brain injury: a meta-analysis. Sleep Medicine 2012;13(7):898–905; doi: 10.1016/j.sleep.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 34. Ouellet M-C, Beaulieu-Bonneau S, Morin CM. Sleep-wake disturbances after traumatic brain injury. Lancet Neurol 2015;14(7):746–757; doi: 10.1016/S1474-4422(15)00068-X [DOI] [PubMed] [Google Scholar]
- 35. Sandsmark DK, Elliott JE, Lim MM. Sleep-wake disturbances after traumatic brain injury: synthesis of human and animal studies. Sleep 2017;40(5):zsx044; doi: 10.1093/sleep/zsx0440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Verma A, Anand V, Verma NP. Sleep disorders in chronic traumatic brain injury. J Clin Sleep Med 2007;3(4):357–362. [PMC free article] [PubMed] [Google Scholar]
- 37. Baumann CR, Stocker R, Imhof H-G, et al. Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology 2005;65(1):147–149; doi: 10.1212/01.wnl.0000167605.02541.f2 [DOI] [PubMed] [Google Scholar]
- 38. Willie JT, Lim MM, Bennett RE, et al. Controlled cortical impact traumatic brain injury acutely disrupts wakefulness and extracellular orexin dynamics as determined by intracerebral microdialysis in mice. J Neurotrauma 2012;29(10):1908–1921; doi: 10.1089/neu.2012.2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Skopin MD, Kabadi SV, Viechweg SS, et al. Chronic decrease in wakefulness and disruption of sleep-wake behavior after experimental traumatic brain injury. J Neurotrauma 2015;32(5):289–296; doi: 10.1089/neu.2014.3664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thomasy HE, Febinger HY, Ringgold KM, et al. Hypocretinergic and cholinergic contributions to sleep-wake disturbances in a mouse model of traumatic brain injury. Neurobiol Sleep Circadian Rhythms 2017;2:71; doi: 10.1016/J.NBSCR.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thomasy HE, Opp MR. Hypocretin mediates sleep and wake disturbances in a mouse model of traumatic brain injury. J Neurotrauma 2019;36(5):802–814; doi: 10.1089/NEU.2018.5810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baumann CR, Bassetti CL, Valko PO, et al. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann Neurol 2009;66(4):555–559; doi: 10.1002/ana.21836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Valko PO, Gavrilov YV, Yamamoto M, et al. Damage to histaminergic tuberomammillary neurons and other hypothalamic neurons with traumatic brain injury. Ann Neurol 2015;77(1):177–182; doi: 10.1002/ana.24298 [DOI] [PubMed] [Google Scholar]
- 44. Lim MM, Elkind J, Xiong G, et al. Dietary therapy mitigates persistent wake deficits caused by mild traumatic brain injury. Sci Transl Med 2013;5(215):215ra173; doi: 10.1126/SCITRANSLMED.3007092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Elliott JE, De Luche SE, Churchill MJ, et al. Dietary therapy restores glutamatergic input to orexin/hypocretin neurons after traumatic brain injury in mice. Sleep 2018;41(3); doi: 10.1093/sleep/zsx212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Krishnan V, Collop NA. Gender differences in sleep disorders. Currt Opin Pulm Med 2006;12(6):383–389; doi: 10.1097/01.mcp.0000245705.69440.6a [DOI] [PubMed] [Google Scholar]
- 47. Mong JA, Cusmano DM. Sex differences in sleep: impact of biological sex and sex steroids. Philos Trans R Soc Lond B Biol Sci 2016;371(1688):20150110; doi: 10.1098/rstb.2015.0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Englander J, Bushnik T, Oggins J, et al. Fatigue after traumatic brain injury: Association with neuroendocrine, sleep, depression and other factors. Brain Inj 2010;24(12):1379–1388; doi: 10.3109/02699052.2010.523041 [DOI] [PubMed] [Google Scholar]
- 49. Hazra A, Macolino C, Elliott MB, et al. Delayed thalamic astrocytosis and disrupted sleep–wake patterns in a preclinical model of traumatic brain injury. J Neurosci Res 2014;92(11):1434–1445; doi: 10.1002/jnr.23430 [DOI] [PubMed] [Google Scholar]
- 50. Konduru SR, Isaacson JR, Lasky DJ, et al. Dual orexin antagonist normalized sleep homeostatic drive, enhanced GABAergic inhibition, and suppressed seizures after traumatic brain injury. Sleep 2022;45(12):zsac238; doi: 10.1093/sleep/zsac238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Konduru SS, Wallace EP, Pfammatter JA, et al. Sleep-wake characteristics in a mouse model of severe traumatic brain injury: relation to posttraumatic epilepsy. Epilepsia Open 2021;6(1):181–194; doi: 10.1002/epi4.12462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Noain D, Büchele F, Schreglmann SR, et al. Increased sleep need and reduction of tuberomammillary histamine neurons after rodent traumatic brain injury. J Neurotrauma 2017;35(1):85–93; doi: 10.1089/neu.2017.5067 [DOI] [PubMed] [Google Scholar]
- 53. Petraglia AL, Plog BA, Dayawansa S, et al. The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J Neurotrauma 2014;31(13):1211–1224; doi: 10.1089/neu.2013.3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rowe RK, Harrison JL, O'Hara BF, et al. Diffuse brain injury does not affect chronic sleep patterns in the mouse. Brain Inj 2014;28(4):504–510; doi: 10.3109/02699052.2014.888768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Saber M, Giordano KR, Hur Y, et al. Acute peripheral inflammation and post-traumatic sleep differ between sexes after experimental diffuse brain injury. Eur J Neurosci 2020;52(1):2791–2814; doi: 10.1111/ejn.14611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sabir M, Gaudreault P-O, Freyburger M, et al. Impact of traumatic brain injury on sleep structure, electrocorticographic activity and transcriptome in mice. Brain Behav Immun 2015;47:118–130; doi: 10.1016/j.bbi.2014.12.023 [DOI] [PubMed] [Google Scholar]
- 57. Barekat A, Gonzalez A, Mauntz RE, et al. Using drosophila as an integrated model to study mild repetitive traumatic brain injury. Sci Rep 2016;6(1):25252; doi: 10.1038/srep2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Olson E, Badder C, Sullivan S, et al. Alterations in daytime and nighttime activity in piglets after focal and diffuse brain injury. J Neurotrauma 2016;33(8):734; doi: 10.1089/NEU.2015.4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Grafe LA, Geng E, Corbett B, et al. Sex- and stress-dependent effects on dendritic morphology and spine densities in putative orexin neurons. Neuroscience 2019;418:266–278; doi: 10.1016/j.neuroscience.2019.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yamanaka A, Beuckmann CT, Willie JT, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 2003;38(5):701–713; doi: 10.1016/S0896-6273(03)00331-3 [DOI] [PubMed] [Google Scholar]
- 61. Platkiewicz J, Brette R. A Threshold equation for action potential initiation. PLoS Comput Biol 2010;6(7):e1000850; doi: 10.1371/journal.pcbi.1000850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sakurai T, Nagata R, Yamanaka A, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in Mice. Neuron 2005;46(2):297–308; doi: 10.1016/j.neuron.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 63. Eggermann E, Bayer L, Serafin M, et al. The wake-promoting hypocretin–orexin neurons are in an intrinsic state of membrane depolarization. J Neurosci 2003;23(5):1557–1562; doi: 10.1523/JNEUROSCI.23-05-01557.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yamanaka A, Muraki Y, Tsujino N, et al. Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem Biophys Res Commun 2003;303(1):120–129; doi: 10.1016/S0006-291X(03)00299-7 [DOI] [PubMed] [Google Scholar]
- 65. Li Y, Gao X-B, Sakurai T, et al. Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron—a potential mechanism for orchestrating the hypothalamic arousal system. Neuron 2002;36(6):1169–1181; doi: 10.1016/S0896-6273(02)01132-7 [DOI] [PubMed] [Google Scholar]
- 66. Burt J, Alberto CO, Parsons MP, et al. Local network regulation of orexin neurons in the lateral hypothalamus. Am J Physiol Regul Integr Comp Physiol 2011;301(3):R572–R580; doi: 10.1152/ajpregu.00674.2010 [DOI] [PubMed] [Google Scholar]
- 67. James MH, Campbell EJ, Dayas CV. Role of the orexin/hypocretin system in stress-related psychiatric disorders. Curr Top Behav Neurosci 2017;33:197–219; doi: 10.1007/7854_2016_56 [DOI] [PubMed] [Google Scholar]
- 68. Yoshida K, McCormack S, España RA, et al. Afferents to the orexin neurons of the rat brain. J Comp Neurol 2006;494(5):845–861; doi: 10.1002/cne.20859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Horvath TL, Gao X-B. Input organization and plasticity of hypocretin neurons: possible clues to obesity's association with insomnia. Cell Metab 2005;1(4):279–286; doi: 10.1016/j.cmet.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 70. Apergis-Schoute J, Iordanidou P, Faure C, et al. Optogenetic evidence for inhibitory signaling from orexin to MCH neurons via local microcircuits. J Neurosci 2015;35(14):5435–5441; doi: 10.1523/JNEUROSCI.5269-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Konadhode RR, Pelluru D, Shiromani PJ. Neurons containing orexin or melanin concentrating hormone reciprocally regulate wake and sleep. Front Syst Neurosci 2015;8; doi: 10.3389/fnsys.2014.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 2005;46(5):787–798; doi: 10.1016/j.neuron.2005.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rao Y, Lu M, Ge F, et al. Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus. J Neurosci 2008;28(37):9101–9110; doi: 10.1523/JNEUROSCI.1766-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tsunematsu T, Ueno T, Tabuchi S, et al. Optogenetic manipulation of activity and temporally controlled cell-specific ablation reveal a role for MCH neurons in sleep/wake regulation. J Neurosci 2014;34(20):6896–6909; doi: 10.1523/JNEUROSCI.5344-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Crawford DC, Kavalali ET. Molecular underpinnings of synaptic vesicle pool heterogeneity. Traffic 2015;16(4):338–364; doi: 10.1111/tra.12262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. del Castillo J, Katz B. Quantal components of the end-plate potential. J Physiol 1954;124(3):560–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Denker A, Rizzoli SO. Synaptic vesicle pools: an update. Front Synaptic Neurosci 2010;2; doi: 10.3389/fnsyn.2010.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kaeser PS, Regehr WG. Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu Rev Physiol 2014;76:333–363; doi: 10.1146/annurev-physiol-021113-170338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kaeser PS, Regehr WG. The readily releasable pool of synaptic vesicles. Curr Opin Neurobiol 2017;43:63–70; doi: 10.1016/j.conb.2016.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kavalali ET. The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci 2015;16(1):5–16; doi: 10.1038/nrn3875 [DOI] [PubMed] [Google Scholar]
- 81. Kavalali ET, Chung C, Khvotchev M, et al. Spontaneous neurotransmission: an independent pathway for neuronal signaling? Physiology 2011;26(1):45–53; doi: 10.1152/physiol.00040.2010 [DOI] [PubMed] [Google Scholar]
- 82. Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci 2005;6(1):57–69; doi: 10.1038/nrn1583 [DOI] [PubMed] [Google Scholar]
- 83. Guerriero RM, Giza CC, Rotenberg A. Glutamate and GABA imbalance following traumatic brain injury. Curr Neurol Neurosci Rep 2015;15(5):27; doi: 10.1007/s11910-015-0545-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vu PA, Tucker LB, Liu J, et al. Transient disruption of mouse home cage activities and assessment of orexin immunoreactivity following concussive- or blast-induced brain injury. Brain Res 2018;1700:138–151; doi: 10.1016/j.brainres.2018.08.034 [DOI] [PubMed] [Google Scholar]
- 85. Jöhren O, Neidert SJ, Kummer M, et al. Sexually dimorphic expression of prepro-orexin mRNA in the rat hypothalamus. Peptides 2002;23(6):1177–1180; doi: 10.1016/S0196-9781(02)00052-9 [DOI] [PubMed] [Google Scholar]
- 86. Taheri S, Mahmoodi M, Opacka-Juffry J, et al. Distribution and quantification of immunoreactive orexin A in rat tissues. FEBS Lett 1999;457(1):157–161; doi: 10.1016/S0014-5793(99)01030-3 [DOI] [PubMed] [Google Scholar]
- 87. Brownell SE, Conti B. Age-and gender-specific changes of hypocretin immunopositive neurons in C57Bl/6 mice. Neurosci Lett 2010;472(1):29–32; doi: 10.1016/j.neulet.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Grafe LA, Cornfeld A, Luz S, et al. Orexins mediate sex differences in the stress response and in cognitive flexibility. Biol Psychiatry 2017;81(8):683–692; doi: 10.1016/j.biopsych.2016.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lu J, Zhao J, Balesar R, et al. Sexually dimorphic changes of hypocretin (orexin) in depression. EBioMedicine 2017;18:311–319; doi: 10.1016/j.ebiom.2017.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cole JH, Leech R, Sharp DJ. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann Neurol 2015;77(4):571–581; doi: 10.1002/ana.24367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Deurveilher S, Cumyn EM, Peers T, et al. Estradiol replacement enhances sleep deprivation-induced c-Fos immunoreactivity in forebrain arousal regions of ovariectomized rats. American J Physiol Regul Integr Comp Physiol 2008;295(4):R1328–R1340; doi: 10.1152/ajpregu.90576.2008 [DOI] [PubMed] [Google Scholar]
- 92. Kim HJJ, Dickie SA, Laprairie RB. Estradiol-dependent hypocretinergic/orexinergic behaviors throughout the estrous cycle. Psychopharmacology 2023;240(1):15–25; doi: 10.1007/s00213-022-06296-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tenorio-Lopes L, Fournier S, Henry MS, et al. Disruption of estradiol regulation of orexin neurons: a novel mechanism in excessive ventilatory response to CO2 inhalation in a female rat model of panic disorder. Transl Psychiatry 2020;10(1):1–12; doi: 10.1038/s41398-020-01076-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Osterstock G, El Yandouzi T, Romanò N, et al. Sustained alterations of hypothalamic tanycytes during posttraumatic hypopituitarism in male mice. Endocrinology 2014;155(5):1887–1898; doi: 10.1210/en.2013-1336 [DOI] [PubMed] [Google Scholar]
- 95. Simmons S, Langlois LD, Oyola MG, et al. Blast-induced mild traumatic brain injury alterations of corticotropin-releasing factor neuronal activity in the mouse hypothalamic paraventricular nucleus. Front Synaptic Neurosci 2022;13:804898; doi: 10.3389/fnsyn.2021.804898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Saper CB. Hypothalamus. In: The Human Nervous System. Elsevier: Amsterdam, the Netherlands; 2012; pp. 548–583. [Google Scholar]
- 97. Saper CB, Lowell BB. The hypothalamus. Curr Biol 2014;24(23):R1111–R1116; doi: 10.1016/j.cub.2014.10.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.