Abstract
Significance:
Chronic skin wounds are a significant health problem around the world, often leading to amputation and even death. Although persistent inflammation is a hallmark of these poorly healing wounds, few available therapies have been designed to target inflammation. In this review, we summarize available evidence of the role of the NOD-like receptor pyrin domain containing 3 (NLRP3) inflammasome in impaired wound healing and describe strategies to inhibit the inflammasome to improve wound healing.
Recent Advances:
The NLRP3 inflammasome plays an important physiological role in skin wound healing, during which transient inflammasome activity contributes to both epidermal and dermal healing. In contrast, sustained activity of the NLRP3 inflammasome leads to impaired epidermal and dermal healing associated with diabetes. Of importance, preclinical studies have demonstrated that inhibiting the NLRP3 inflammasome-induced resolution of inflammation, increased granulation tissue formation and collagen deposition, and accelerated reepithelialization and wound closure.
Critical Issues:
NLRP3 inflammasome inhibitors have appealing potential for translation into therapies for chronic wounds. Although preclinical studies have shown promising results, there is a need for human/clinical studies to evaluate dosing formulations, potential therapeutic effects, dose–response relationships, and possible side effects.
Future Directions:
Among strategies to inhibit the NLRP3 inflammasome, glyburide, metformin, peroxisome proliferator-activated receptor agonists, and the dipeptidyl peptidase 4 inhibitor saxagliptin appear to be closest to clinical translation, as these drugs are already Food and Drug Administration approved for other indications. Future clinical studies are needed to develop topical formulations of these drugs, and to assess the safety and efficacy of these inhibitors, to improve healing of chronic wounds.
Keywords: wound healing, inflammation, diabetes, chronic wounds, NLRP3 inflammasome, macrophage

Timothy J. Koh, PhD
SCOPE AND SIGNIFICANCE
Chronic skin wounds are a significant health problem around the world, often leading to amputation and even death. Although a persistent inflammatory response is a hallmark of these poorly healing wounds, few available therapies have been designed to target inflammation.
The NOD-like receptor pyrin domain containing 3 (NLRP3) inflammasome is a multiprotein complex that plays an important role in initiating and propagating inflammatory responses. In this review, we summarize available evidence of the role of the NLRP3 inflammasome in both physiological and impaired wound healing and describe strategies to inhibit the inflammasome to improve healing of chronic wounds.
TRANSLATIONAL RELEVANCE
A number of publications have demonstrated the central role of the NLRP3 inflammasome in chronic inflammation and impaired wound healing associated with diabetes. Preclinical studies have demonstrated that inhibiting the NLRP3 inflammasome-induced resolution of inflammation, increased granulation tissue formation and collagen deposition, and accelerated reepithelialization and wound closure. There is now a need for translation to human/clinical studies to evaluate dosing formulations, potential therapeutic effects, dose–response relationship, and possible side effects.
CLINICAL RELEVANCE
Despite the socioeconomic impact of chronic wounds, the causes of impaired healing are not well understood and current treatments are often ineffective. Successful translation of NLRP3 inhibitors into clinical therapies would lead to tools for mitigating chronic inflammation in wounds, and improving healing in conditions such as diabetes. In turn, this could reduce downstream complications such as amputation and death.
INTRODUCTION
Wound healing restores architecture and function of tissues and organs after damage, and thus is critical for survival of all organisms. Wound healing involves sequential but overlapping phases of hemostasis, inflammation, proliferation, and remodeling, and each phase involves complex interactions between different cell types. For skin, this involves inflammatory cells, endothelial cells, fibroblasts, and keratinocytes working together to restore tissue structure and function.1
A number of studies have highlighted the critical role of the inflammatory response, orchestrated by macrophages (Mp), in the wound healing response. The Mp response is important not only for the removal of debris and killing pathogens but also the recruitment, proliferation, and activity of other cell types.2,3 A number of publications demonstrate that Mp produce proinflammatory factors early during healing and then change phenotype to release anti-inflammatory and prohealing factors thus carrying out multiple essential functions in the healing process.3–5
Chronic wounds are nonhealing wounds in which the physiological healing process is impaired, usually correlated with a disease or condition, such as diabetes mellitus (DM).6 DM is the most common metabolic disorder throughout the world, affecting 463 million people in 2019. In the United States, 25% of diabetic population suffer with chronic wounds, which represents one of the major causes of nontraumatic lower limb amputations with a postoperative mortality of 9–18% over the past 15 years.7 Despite its socioeconomic impact, the underlying causes of impaired healing remain poorly understood and effective treatments are lacking.
A common characteristic of chronic wounds in DM is a sustained inflammatory response, with persistent accumulation of proinflammatory Mp.2,6 This results in sustained production of proinflammatory cytokines and proteases and reduced production of growth factors, leading to decreased angiogenesis and granulation tissue formation besides impaired closure. Thus, DM disrupts the timely progression through the stages of healing, leaving the wound in a chronic inflammatory state.6
The NLRP3 inflammasome is a multiprotein complex that plays an important role in initiating and propagating inflammatory responses, through its role in post-translational processing of proinflammatory cytokines such as interleukin 1 beta (IL-1β) and interleukin 18 (IL-18).8,9 Activation of the NLRP3 inflammasome is thought to first require assembly of the multiprotein complex, and then activation of its effector enzyme caspase-1, which is responsible for cleaving and activating the pro-IL-1β and IL-18 into their active forms to be released.8,9
Although the NLRP3 inflammasome plays important physiological roles in infection control and cytokine production, sustained activation can result in chronic inflammation and pathology, including impaired wound healing associated with DM.10 As such, NLRP3 inflammasome signaling may represent a target for novel therapies to mitigate complications associated with chronic inflammation, such as those associated with DM.
This review will discuss published findings on the role of NLRP3 inflammasome in regulating the inflammatory response and progression of physiological skin wound healing as well as its role in impaired skin wound healing, with an emphasis in its role in Mp function. We will also review publications on potential novel therapies for chronic wounds targeting the NLRP3 inflammasome.
NLRP3 INFLAMMASOME AND THE INFLAMMATORY RESPONSE
Many pathways contribute to the inflammatory response and inflammatory cells can be activated by a variety of signals including pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs).
Examples of PAMPs include pathogen-associated particles such as lipopolysaccharide (LPS), found in the surface of gram-negative bacteria; lipoteichoic acids, found in gram-positive bacteria; and peptidoglycans found in either gram-positive or gram-negative bacteria. DAMPs include a variety of endogenous molecules released in conditions of cellular damage or stress, such as extracellular nucleotides (adenosine triphosphate [ATP]/adenosine diphosphate), high mobility group box-1, and serum amyloid A. Inflammatory cells recognize these signals through pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), Rig-I like receptors, and NLRs.
In general, these PRRs initiate signaling cascades (e.g., TAK1, IKK, IRAK4, and MAPK) that converge in the regulation of cytokines and chemokines transcription.8,9,11 Of importance, for the purpose of this review, inflammasomes are multiprotein complexes contained in PRRs family that induce inflammation in response to microbes and/or tissue damage, and the best characterized is the NLRP3 inflammasome. Other inflammasomes that are known to contribute to inflammatory responses include NLRP1, NLRP6, NLRC4, and AIM2.12 However, the focus of this review is the NLRP3 inflammasome and in-depth discussion of other inflammasomes is outside the scope of this review.
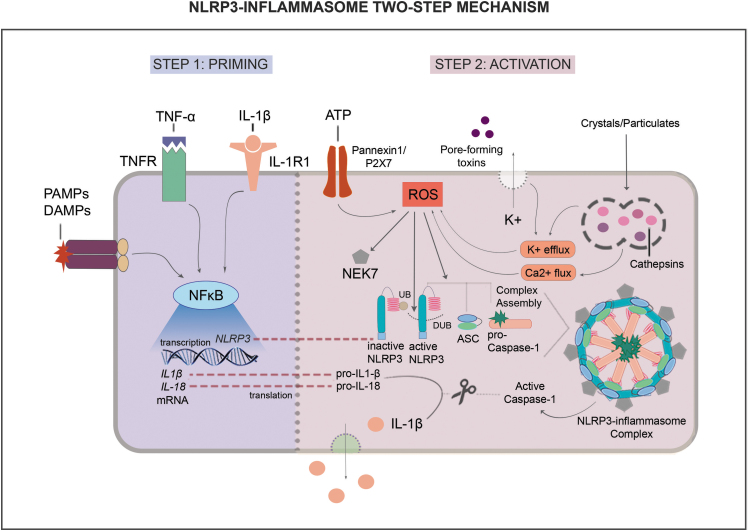
The NLRP3 inflammasome multiprotein complex includes NLRP3, apoptosis-associated speck-like protein (ASC), NIMA-related kinase 7 (NEK7), and pro-caspase-1, which can interact with an array of activators and inhibitors (Fig. 1).13
Figure 1.
NLRP3-inflammasome two-step mechanism. The NLRP3 inflammasome is activated through a two-step mechanism. In step 1, called priming, NF-κB signaling is induced by IL-1β, TNF-α, DAMPs, and PAMPs through specific receptors, leading to transcription of NLRP3 components and its effectors IL-1β and IL-18. In step 2, called activation, ATP, pore-forming toxins, crystals/particulates and other cell metabolism stresses induce ROS formation. In turn, ROS contribute to the assembly of active NLRP3, ASC, and pro-caspase-1, inducing NEK7 and deubiquitination of NLRP3. This multiprotein complex then activates caspase-1, which in turn, cleaves pro-IL-1β and pro-IL-18 into active IL-1β and IL-18 and these cytokines are then released into extracellular space. ASC, apoptosis-associated speck-like protein; ATP, adenosine triphosphate; DAMPs, damage-associated molecular patterns; DUB, deubiquitinase; IL-1β, interleukin 1 beta; IL-18, interleukin 18; NEK7, NIMA-related kinase 7; NF-κB, nuclear factor kappa B; NLRP3, NOD-like receptor pyrin domain containing 3; PAMPs, pathogen-associated molecular patterns; ROS, reactive oxygen species; TNF-α, tumor necrosis factor alpha; UB, ubiquitin.
Expression and activity of this complex is induced by both microbial and nonmicrobial stimuli in a two-step mechanism, called priming and activation. The priming step involves the upregulation of inflammasome components (e.g., NLRP3) and effectors (e.g., pro-IL-1β). DAMPs, PAMPs, and inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and IL-1β can induce NLRP3 inflammasome components and effectors through TLR/nuclear factor kappa B (NF-κB) signaling (Fig. 1).9,11
The activation step can involve different triggers, including extracellular ATP engaging with Pannexin1/P2X7 receptor, pore-forming toxins and crystals inducing ion influx/efflux (e.g., calcium, potassium and chloride), and increased mitochondrial reactive oxygen species (ROS) production, all contributing to inflammasome complex assembly and subsequent activity (Fig. 1).
Factors such as endoplasmic reticulum stress, mitochondrial dysfunction, and frustrated phagocytosis also contribute to increase ROS production and, consequently, promote NLRP3 inflammasome activity. Following complex assembly, pro-caspase-1 is cleaved to form active caspase-1. In turn, caspase-1 cleaves the pro-IL-1β and IL-18 into their active forms. Detailed information on NLRP3 inflammasome signaling can be found in excellent recent reviews.8,9,14–18
Recent studies have elucidated mechanisms by which NF-κB and ROS pathways contribute to NLRP3 inflammasome activity.14 TLR/NF-κB signaling can induce deubiquitinases (DUBs) in a ROS-dependent manner, and DUBs in turn can promote NLRP3 complex assembly.17,19 ROS may also induce assembly of NLRP3, ASC, and pro-caspase-1 through induction of NEK7, a kinase that acts downstream of potassium efflux participating in the assembly process and in the inflammasome activation.15,18
In addition, in response to PAMPs and DAMPs, different cell types including Mp produce TNF-α, amplifying the response to extracellular ATP through P2X7 receptor, inducing potassium efflux and, consequently, ROS formation.14,20 TNF-α also activates the NF-κB pathway, which in turn induces the transcription of NLRP3 inflammasome components.
Moreover, neutrophil extracellular traps (NETs), which play an important role in host defense, interact with Mp activating NF-κB and thus contributing to NLRP3 inflammasome activation.21,22 Therefore, TNF-α production, NF-κB induction, and ROS formation contribute to the transcription of inflammasome components and complex assembly, as well as to a positive feedback loop promoting NLRP3 inflammasome activity.
In addition to the many factors that promote NLRP3 inflammasome activity, endogenous proteins/regulators also can inhibit activity. Pyrin-only proteins (POPs), CARD-only proteins (COPs), and proteinase inhibitor 9 are NLRP3 inflammasome inhibitors.23 POP1 and POP2 regulate NLRP3-inflammasome activation inhibiting NLRP3–ASC interaction and preventing the complex assembly, in primary human Mp and in murine models of peritonitis.24
Furthermore, POP1 and POP2 dampen exaggerated inflammatory responses and increase survival rate in murine models of bacterial infection and LPS sepsis, decreasing expression of proinflammatory cytokines.25 In turn, COPs limit inflammasome activation by competing with pro-caspase-1 in NLRP3 complex assembly and thus inhibiting formation of active caspase-1 in COS-7, 293T and HEK293 cells.26 Finally, proteinase inhibitor 9 is a serine proteinase inhibitor that can directly inhibit caspase-1 activity, thus reducing IL-1β activation.27
The best described effectors of the NLRP3 inflammasome are IL-1 family members IL-1β and IL-18, whereas IL-33 has been suggested to be another effector. IL-1β is a proinflammatory cytokine that signals through the IL-1 receptor type 1 (IL-1R). IL-1β can act to amplify inflammatory responses, contributing to the increased expression of other inflammatory cytokines and chemokines, cyclooxygenase type 2, adhesion molecules, and nitric oxide synthase, and playing an important role in the pathophysiology of different inflammatory diseases, including DM.28
IL-1β induces a proinflammatory phenotype in Mp, stimulating production of chemokine ligand 2 (CCL-2), VCAM-1, and TNF-α.29 IL-18 is known for its ability to stimulate interferon-γ production in T cells and NK cells, and is a major mediator of Th1 responses, through the IL-18 receptor.30,31 IL-33 was first described as an inducer of immune responses activating T cells and mast cells, besides mediating Th2 responses. New evidence indicates that IL-33 activates innate lymphoid cells, Treg cells, and NK cells, through the receptor IL-1RL1.32
The NLRP3 inflammasome has been linked with several inflammatory disorders, including atherosclerosis, Parkinson's, Alzheimer's diseases, obesity, and DM.16 Accumulating evidence highlights a central role for the NLRP3 inflammasome in obesity-induced insulin resistance, with adipose tissue Mp in obese and diabetic individuals exhibiting increased levels and activity of the NLRP3 inflammasome.33
The NLRP3 inflammasome has been shown to play a significant role in DM pathophysiology, contributing to impaired glucose and insulin sensitivity. In the next section, we present evidence that the NLRP3 inflammasome may also constitute part of the positive feedback loop sustaining inflammation in chronic wounds, leading to impaired skin wound healing in DM.
In short, the NLRP3 inflammasome is a key driver of inflammation, playing essential role in the innate immune system response to pathogens and tissue damage. However, dysregulated NLRP3 inflammasome activation can drive persistent inflammation, which has been implicated in a host of inflammatory disorders, contributing to the progression of several diseases.
NLRP3 INFLAMMASOME IN PHYSIOLOGICAL SKIN WOUND HEALING
The NLRP3 inflammasome plays both physiological and pathological roles in skin wound healing (Figs. 2 and 3). During wound healing, the NLRP3 inflammasome is expressed in a number of different wound cells, but Mo/Mp are the primary producers of the NLRP3 inflammasome effector IL-1β, a cytokine that plays a central role in many inflammatory responses, including skin wound healing (Fig. 2).34,35 Another NLRP3 inflammasome effector IL-18 has also been reported to increase early in inflammatory responses following tissue injury; however, little is known about the role of IL-18 in skin wound healing.30,36
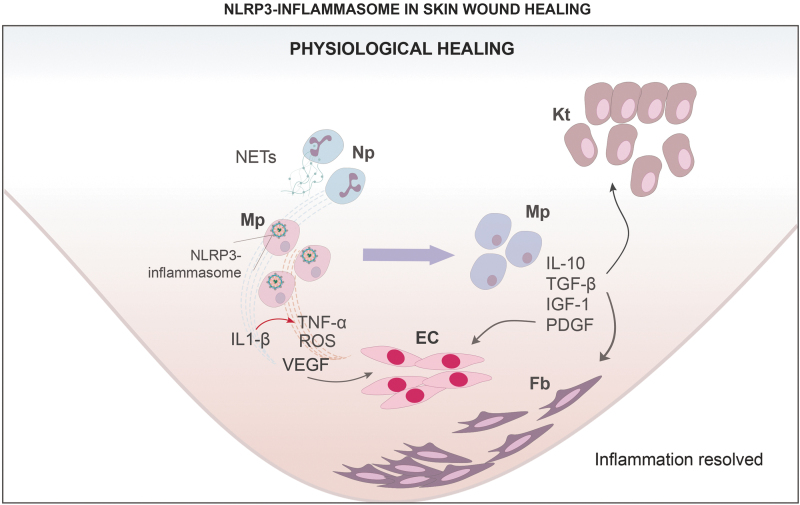
Figure 2.
NLRP3-inflammasome in skin wound healing: physiological healing. In physiological healing, the early inflammatory response is characterized by expression of proinflammatory factors IL-1β, TNF-α, and ROS produced by classically activated Mp in part by NLRP3 inflammasome signaling. Induction of NLRP3 inflammasome activity in proinflammatory Mp is stimulated by NETs, and by IL-1β, TNF-α, and ROS. Proinflammatory Mp also expresses VEGF, stimulating EC and angiogenesis. During the late inflammatory response, Mp transition to noninflammatory phenotype, expressing anti-inflammatory cytokine IL-10 and growth factors TGF-β, IGF-1, and PDG-F. These factors contribute to recruitment, proliferation, and maturation of Kt, Fb, and EC, promoting granulation tissue formation, and collagen deposition. EC, endothelial cells; Fb, fibroblasts; IGF-1, insulin-like growth factor 1; IL-10, interleukin 10; Kt, keratinocytes; Mp, macrophages; NETs, neutrophil extracellular traps; TGF-β, transforming growth factor beta; VEGF, vascular endothelial growth factor.
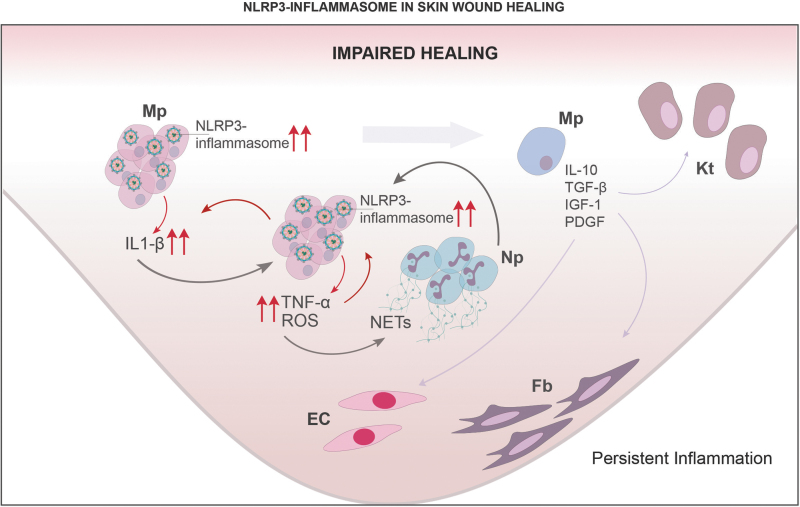
Figure 3.
NLRP3-inflammasome in skin wound healing: impaired healing. In impaired healing, a chronic inflammatory response is characterized by expression of proinflammatory markers IL-1β, TNF-α, and ROS produced by proinflammatory Mp in part by NLRP3 inflammasome signaling. NETs, IL-1β, TNF-α, and ROS, all contribute to a proinflammatory feedback loop that sustains the inflammatory response. Mp remain stuck in a proinflammatory phenotype, compromising expression of anti-inflammatory cytokine IL-10 and growth factors TGF-β, IGF-1, and PDGF. Dysregulated inflammation thus leads to reduced recruitment, proliferation and maturation of other cell types involved in skin wound healing such as Kt, Fb and EC, compromising angiogenesis, granulation tissue formation, and collagen deposition.
Our laboratory investigated the role of the NLRP3 inflammasome in the early skin wound healing process using NLRP3 and caspase-1-deficient mice.34 Both NLRP3 and caspase-1-deficient mice exhibited reduced IL-1β and TNF-α levels and reduced accumulation of neutrophils (Np) and Mp early in wound healing, indicating a blunted early inflammatory response. Associated with this blunted inflammatory response, NLRP3-deficient and caspase-1-deficient mice exhibited delayed reepithelialization, impaired angiogenesis, and granulation tissue formation.34
In addition, topical treatment of wounds in NLRP3-deficient mice with IL-1β partially rescued reepithelialization and granulation tissue formation, supporting the hypothesis that the NLRP3/IL-1β pathway is important for efficient wound healing.34 Similar results were reported in a subsequent study on skin wound healing in NLRP3 and ASC-deficient mice.37
Consistent with these findings, burn injury increased expression of NLRP3, IL-1β, and IL-18 early after injury, both in mice and in humans.38 In NLRP3-deficient mice, burn wounds exhibited reduced expression of IL-6, CCL-2, vascular endothelial growth factor (VEGF), FGF-2, transforming growth factor beta (TGF-β), and reduced Mp accumulation, associated with impaired healing.
Of interest, systemic treatment with the antidiabetic drug glyburide, which inhibits the NLRP3 inflammasome, mimicked the effects of NLRP-3 deficiency, reducing wound inflammation, and impairing healing if given early after burn injury.38 But when treatment was initiated after the initial inflammatory response, NLRP3 inhibition did not compromise wound healing.38
In addition, whereas compressive loading increased levels of NLRP3, ASC, caspase-1, and IL-1β in skin explants from young donors, such induction was not observed in skin explants from old donors.39 This impaired load-induced NLRP3 inflammasome expression may correlate with increased risk of pressure ulcer development in older individuals. These data support the hypothesis that insufficient activity of NLRP3 inflammasome compromise wound inflammation and healing in diverse contexts.
NLRP3 INFLAMMASOME IN IMPAIRED SKIN WOUND HEALING
In contrast to the positive role for NLRP3 inflammasome in physiological skin wound healing, a number of reports demonstrate that NLRP3 inflammasome contributes to the chronic inflammation and impaired healing in diabetic wounds (Fig. 3). Our laboratory reported that Mo/Mp isolated from chronic wounds in diabetic patients and poorly healing wounds in diabetic db/db mice exhibit a proinflammatory phenotype that included enhanced expression of NLRP3 inflammasome components and downstream effectors IL-1β and IL-18, but reduced expression of inflammasome inhibitors proteinase inhibitor-9 and caspase-12.36
The wound environment appears to contribute to this proinflammatory Mo/Mp phenotype through ROS-mediated activation of NLRP3 inflammasome, because wound conditioned medium induces a similar proinflammatory phenotype in both cultured human and mouse Mp and antioxidants N-acetylcysteine and diphenyleneiodonium chloride inhibit inflammasome activity in these cells.36
Our group also demonstrated that an IL-1β blocking antibody, and the NLRP3 inflammasome inhibitors YVAD and glyburide, downregulate the inflammasome activity and the proinflammatory Mo/Mp phenotype induced by wound conditioned medium in vitro or in wounds of diabetic db/db mice.40 Furthermore, transferring bone marrow of NLRP3-deficient or caspase-1-deficient mice to diabetic db/db mice downregulated inflammation, increased levels of IGF-1, and TGF-β in wounds and improved wound healing in the diabetic mice.36
Other groups have reported similar findings, including increased expression of NLRP3, caspase-1, ASC, IL-1β, and IL-18 in poorly healing wounds of diabetic db/db mice and streptozocin (STZ)-treated mice and rats.41,42 One of these studies highlighted a central role of IL-1β/IL-1R axis in impaired healing of diabetic db/db mice, showing that an IL-1R antagonist dampened inflammation and accelerated reepithelialization in these mice.42
Other studies have elucidated the role of ROS and NETs in persistent activation of the NLRP3 inflammasome through CXCR2 and TLR signaling through NF-κB during impaired wound healing in diabetic humans, mice, and rats.21,43 Of importance, NETs digestion by local application of DNAse accelerated wound closure in diabetic rats, and systemic treatment with ROS inhibitor N-acetylcysteine or inflammasome inhibitor BAY 11-7082 improved healing of incisional wounds in db/db mice or high-fat diet (HFD) fed + STZ-injected rats.44,45
These studies contrast with a study on mice fed a HFD for 10 weeks, which showed elevated baseline levels of NLRP3 components in skin but impaired induction of these components after wounding, indicating that the NLRP3 inflammasome may be affected differently by states of insulin resistance versus full blown diabetes.46 In another report, components of the NLRP3 inflammasome have been found to be induced by compressive loading of skin explants from young but not old donors and thus could contribute to pathophysiology of pressure ulcers.39
In summary, although the NLRP3 inflammasome appears to play a critical role in the inflammatory response during physiological wound healing, sustained activation of the NLRP3 inflammasome appears to contribute to the chronic inflammation and impaired healing in both human and rodent diabetic wounds.
NOVEL STRATEGIES FOR TARGETING NLRP3 INFLAMMASOME IN CHRONIC WOUNDS
Because of the central role of the NLRP3 inflammasome in chronic inflammation and impaired wound healing associated with diabetes, research on NLRP3-inflammasome inhibitors has significant potential for translation into therapies for chronic wounds. Strategies reported so far include direct inhibitors that target inflammasome components, effectors or upstream/downstream signaling and inhibitors that have an indirect and less well understood effect on inflammasome activity. In this section, we review preclinical studies focusing in NLRP3 inflammasome as a target to improve healing of diabetic wounds. NLRP3 inflammasome inhibitors and mechanisms of action are illustrated in Fig. 4 and summarized in Table 1.
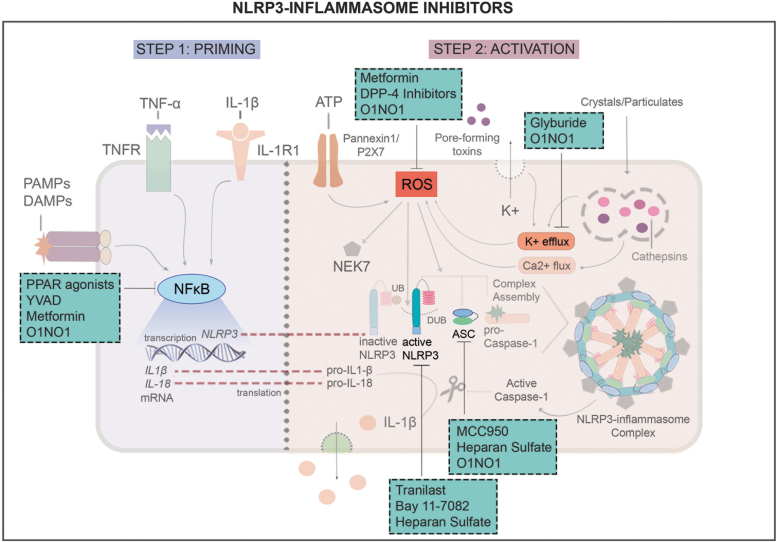
Figure 4.
NLRP3 inflammasome inhibitors and mechanisms of action. Various compounds have been shown to target different aspects of NLRP3 inflammasome activation. Compounds inhibiting ROS: metformin and DPP-4 inhibitors. Compounds inhibiting K+ efflux: glyburide. Compounds inhibiting ASC/NLRP3 oligomerization: MCC950 and heparan sulfate. Compounds inhibiting active NLRP3, upon binding to NACHT domain: Tranilast, Bay 11-7082, and heparan sulfate. Compounds inhibiting NF-κB: PPAR agonists, YVAD, and metformin. DPP-4, dipeptidyl peptidase 4; PPAR, peroxisome proliferator-activated receptor.
Table 1.
NOD-like receptor pyrin domain containing 3 inflammasome inhibitors investigated in the context of diabetic wound healing
| Inhibitor | Mechanism | Study Type | Reference |
|---|---|---|---|
| Glyburide | Closure of potassium channel | In vitro, in vivo | 36,47 |
| Tranilast | NLRP3 NACHT domain binding | In vivo, clinical | 48–50 |
| YVAD | NF-κB activity limitation | In vitro, in vivo | 36,50 |
| MCC950 | NLRP3–ASC oligomerization blocking | In vitro, in vivo | 51–53 |
| Bay 11-7082 | NLRP3 NACHT domain binding | In vitro, in vivo | 41,45,54 |
| PPAR agonists | NF-κB activity limitation | In vitro, in vivo | 55,56 |
| ON1O1 | Limitation of NF-κB activity, ROS, NLRP3–ASC oligomerization and potassium efflux | In vitro, in vivo, clinical | 57–59 |
| Metformin | NF-κB activity limitation and ROS inhibition | In vitro, in vivo | 61–64 |
| DPP-4 inhibitors | ROS inhibition | In vivo, clinical | 65–70 |
| Heparan sulfate | NLRP3 and ASC downregulation | In vitro, in vivo | 71–73 |
ASC, apoptosis-associated speck-like protein; DPP-4, dipeptidyl peptidase 4; NF-κB, nuclear factor kappa B; NLRP3, NOD-like receptor pyrin domain containing 3; PPAR, peroxisome proliferator-activated receptor; ROS, reactive oxygen species.
Direct inhibitors
Glyburide
Glyburide is an antidiabetic drug of the sulfonylurea class that has been investigated for treating diseases other than diabetes including inflammatory bowel disease, melioidosis, and leishmaniasis. Glyburide was shown to inhibit activation of the NLRP3 inflammasome in response to various DAMPs and PAMPs through a mechanism different from that involved in its antidiabetic activities—inflammasome inhibition does not require the cyclohexylurea group of glyburide, but does require the benzamido and sulfonyl groups for optimal inhibition.36,47
Glyburide induces closure of ATP-sensitive potassium channels, leading to inhibition of NLRP3 activation. As noted previously, treatment of skin wounds in diabetic db/db mice resulted in reduced levels of IL-1β and IL-18, consistent with reduced activity of NLRP3 inflammasome, leading to accelerated reepithelialization and increased granulation tissue formation and collagen deposition.36 Because glyburide is an established Food and Drug Administration (FDA)-approved drug with an excellent safety profile, repurposing for wound healing may be an appealing strategy.
Tranilast (N-[3′,4′-dimethoxycinnamoyl])
Tranilast is an anthranilic acid analog of a tryptophan metabolite with anti-allergic and anti-inflammatory properties. Tranilast is used clinically in Japan, South Korea, and China for treatment of keloid and hypertrophic scars.48 However, the drug is not FDA approved in the United States. The mechanism of action of Tranilast involves direct binding to the NACHT domain of NLRP3, suppressing NLRP3 inflammasome assembly and preventing activation.49,50
In diabetic rats, a combination ointment containing solid tranilast nanoparticles enhanced skin wound closure, reduced redness, and increased collagen deposition, although effects on NLRP3 inflammasome activity or components were not assessed.51 In a murine burn model, tranilast showed no significant effects on wound closure, but did decrease mast cell number, collagen production, TGF-β, and histamine levels.50 Consistent with this study, a number of reports indicate the therapeutic potential of tranilast to prevent and treat scar and keloids by reducing collagen deposition.51,52
Despite wide clinical use in Asia, further investigation is needed to reveal the potential therapeutic effects of tranilast on different aspects of healing in diabetic wounds.
Caspase-1 inhibitor YVAD
YVAD is a synthetic tetrapeptide and irreversible inhibitor of caspase-1. YVAD was found to reduce levels of IL-1β and IL-18 in skin wounds of diabetic db/db mice, consistent with NLRP3 inflammasome inhibiting activity, leading to accelerated reepithelialization and increased collagen deposition.36 Another report showed that YVAD decreases P-P38 and P-IκB expression, downregulating NF-κB activity, and limiting NLRP3-inflammasome priming in a murine acute gastric injury model.53
MCC950
MCC950 or CP-456773, a diarylsulfonylurea-containing compound, is a NLRP3 inflammasome inhibitor that is thought to block NLRP3-induced ASC oligomerization.54 The compound reduced IL-1β in murine models and cells from patients with multiple sclerosis, Cryopyrin-associated periodic syndrome, and Muckle–Wells syndrome. Treatment with MCC950 also decreased IL-1β levels and Np infiltration, and induced a prohealing Mp phenotype following injury to the cornea, resulting in accelerated reepithelialization in STZ-induced diabetic mice.55
In contrast, one study showed that topical treatment with MCC950 did not alter skin reepithelialization in skin wounds of diabetic ob/ob mice.56 Characterization of MCC950 indicated that the compound, specifically targets NLRP3, and does not inhibit the major antimicrobial inflammasomes NLRC4 and NLRP1, thus preserving anti-infection responses.
Bay 11-7082
Bay 11-7082 is an NLRP3 inflammasome activation inhibitor that targets ATPase activity in the NACHT domain of NLRP3, which is necessary for NLRP3 inflammasome assembly and activation.45 The compound is also a NF-κB inhibitor and induces phosphorylation of IκBα, thus acting both directly and indirectly on NLRP3 inflammasome.45
In diabetic db/db mice, topical treatment of skin wounds with Bay 11-7082 accelerated wound closure and reepithelialization and decreased NLRP3 inflammasome components and effectors, including caspase-1, IL-1β, and IL-18.57 In STZ-induced diabetic rats, treatment of skin wounds with Bay 11-7082 showed a similar result, increasing wound closure and decreasing NLRP3-inflammasome components such as NLRP3, ASC, caspase-1, and IL-1β.41 Despite these encouraging preclinical results, human studies appear not to have yet been performed.
Indirect inhibitors
Peroxisome proliferator-activated receptor agonists
Peroxisome proliferator-activated receptor (PPAR)-α, -γ, and -δ are transcription factors activated by lipophilic ligands, that are known for mediating metabolic processes, and also play a role in regulating inflammation. In both mouse and human Mp, IL-1β downregulated PPAR-γ and downstream targets and blocking IL-1β in diabetic mouse wounds induced upregulation of the PPAR-γ activity, indicating negative feedback between the NLRP3 inflammasome and PPAR pathways.58
Of interest, loss of PPAR-γ in Mp is sufficient to prolong wound inflammation and delay healing.58 In contrast, topical treatment of diabetic db/db mouse wounds with PPAR-γ agonists rosiglitazone or 15-deoxy-Δ-12,14-prostaglandin J2 downregulated the proinflammatory Mp phenotype characteristic of these wounds and induces a prohealing Mp phenotype, leading to an improved healing environment, increased angiogenesis and granulation tissue formation, and accelerated wound closure.58
Consistent with these findings, topical treatment of wounds in diabetic db/db mice with a novel dual PPAR-α/γ agonist showed reduced wound expression of proinflammatory IL-1β and TNF-α, and increased expression of anti-inflammatory cytokines IL-10 and TGF-β, accelerated wound closure and increased collagen deposition.59 Some PPAR agonists, such as rosiglitazone and pioglitazone, are FDA approved and clinically used for DM treatment, but with considerable side effects when orally ingested. Hence, topical treatment of diabetic wounds may provide therapeutic benefit without these systemic side effects.
ON1O1
ON1O1 is composed of two active pharmaceutical ingredients extracted from Plectranthus amboinicus (PA-F4) and Centella asiatica (S1). Although C. asiatica extract can activate PPAR-γ,60 P. amboinicus extract has been found to inhibit potassium efflux and NLRP3–ASC oligomerization, all converging to inhibit NLRP3 inflammasome activity in THP-1 cells.61
In nondiabetic Wistar rats, C. asiatica in a topical spray decreased area of full thickness wounds similar to povidone–iodine solution when compared with untreated group.62 In addition, a recent clinical trial in China reported incidence of 60.7% of complete healing after 16 weeks of daily topical treatment with ON1O1.63 However, study outcomes were limited to wound area, and inhibition of NLRP3 inflammasome activity was not assessed.
Metformin
Metformin is an antidiabetic drug of the biguanide class and is one of the most widely used medications for DM therapy. Besides its antidiabetic activity, metformin has widely reported anti-inflammatory properties, in part by inhibiting NF-κB, which in turn could inhibit expression of NLRP3 inflammasome components and/or their activation.64 Recent studies reported that metformin may suppress NLRP3 inflammasome activity by activating AMP-activated protein kinase (AMPK) and consequently inhibiting mammalian Target Of Rapamycin (mTOR), an inducer of ROS needed for NLRP3 inflammasome activation.65
These reports showed that metformin can decrease expression of NLRP3 inflammasome components and effectors, including NLRP3, caspase-1, and IL-1β. With respect to wound healing, topical treatment with metformin in wounds in STZ-induced diabetic rats decreased NLRP3 inflammasome activity and induced alternative activation of Mp, associated with improved angiogenesis and wound healing.65 The same report showed that blocking AMPK abolished metformin effects in wound healing. In other models with diabetic and nondiabetic mice and rabbits, topical treatment with metformin improved wound healing, increasing granulation tissue, and collagen deposition.66,67
Dipeptidyl peptidase 4 inhibitors
Dipeptidyl peptidase 4 (DPP-4) is an enzyme that degrades the incretin glucagon-like peptide 1 (GLP-1). Thus, DPP-4-1 inhibitors prolong the half-life of GLP-1 and are antihyperglycemic agents widely used in DM treatment. DDP-4 inhibitors also have pleiotropic effects, including improved endothelial function, reduced thrombogenesis, improved VEGF production by HIF-1α signaling, as well as anti-inflammatory effects.68–70
Reports indicate that DDP-4 inhibitors can inhibit the NLRP3 inflammasome in Mp through inhibition of protein kinase C pathway, which may lead to reduced ROS formation and reduced NLRP3 inflammasome activity.71 In STZ-induced mice, the DPP-4 inhibitor saxaglipin accelerated wound closure and reepithelialization of skin wounds.72 In a clinical study of diabetic participants with chronic ulcers, oral treatment with the DPP-4 inhibitor saxagliptin also demonstrated improvement in healing ulcer rate and time for complete wound closure compared with a placebo group.73
As saxagliptin is an FDA-approved compound with potential results found in a clinical study, this drug may represent an attractive strategy for treating chronic diabetic wounds.
Heparan sulfate
Heparan sulfate is a glycosaminoglycan that is a structural element of the extracellular matrix (ECM) scaffold, and also regulates activities of local proteolytic enzymes, growth factors, and chemokines.
In DM, heparan sulfate levels are decreased and heparan sulfate-dependent interactions between ECM and growth factors are impaired, contributing to the persistent proinflammatory environment characteristic of diabetic wounds.74 An early study reported that heparan sulfate improves wound healing through attenuating inflammation in STZ-induced rats and further investigation provided evidence that this effect could be owing to downregulation of NLRP3 and ASC, and upregulation of the endogenous NLRP3 inflammasome inhibitors proteinase inhibitor-9 and caspase-12.75,76
In parallel, our group has found that expression of proteinase inhibitor-9 and caspase-12 in wound Mp is impaired in both db/db mice and human chronic wounds, indicating a possible target for dampening sustained activity of the inflammasome in chronic wounds.36
Other NLRP3 inflammasome inhibitors have been described in preclinical and clinical studies as strategies for treating a variety of metabolic disorders but have not yet been investigated in the context of diabetic wound healing. These inhibitors might represent interesting targets for additional preclinical studies in the future and are summarized in Table 2.
Table 2.
NOD-like receptor pyrin domain containing 3 inflammasome inhibitors currently not investigated in the context of diabetic wound healing
| Inhibitor | Mechanism | Study Type | Reference |
|---|---|---|---|
| Bot-4-one | NACHT ATPase inhibitor | In vitro, in vivo | 77 |
| CY-09 | NACHT ATPase inhibitor: binds Walker A motif | In vitro, in vivo | 78 |
| INF39 | NACHT ATPase inhibitor | In vitro, in vivo | 79 |
| MNS | NACHT ATPase inhibitor | In vitro, in vivo | 80 |
| OLT1177 | NACHT ATPase inhibitor | In vitro, in vivo, clinical | 81 |
| Oridonin | Binds NLRP3 Cys279: inhibits NLRP3-NEK7 interaction | In vitro, in vivo | 82 |
| Parthenolide | NACHT ATPase inhibitor, caspase-1 inhibitor | In vitro, in vivo | 78 |
NEK7, NIMA-related kinase 7.
SUMMARY
The NLRP3 inflammasome plays an important physiological role in skin wound healing, during which transient inflammasome activity contributes to the propagation of wound inflammation, essential for both epidermal and dermal healing. In contrast, impaired healing associated with DM is characterized by sustained activity of NLRP3 inflammasome, through persistent ROS production, sustained upregulation of TNF-α and IL-1β, and NF-κB activity. Thus, targeting NLRP3 inflammasome activity and its effectors appears to be an appealing therapeutic strategy to mitigate chronic inflammation in diabetic wounds and to improve healing.
In addition, more research is needed on the role of NLRP3 inflammasome in other types of chronic wounds. Among published studies reviewed, a variety of compounds can be used to directly or indirectly inhibit NLRP3 inflammasome activity in diabetic wounds, affecting NLRP3 inflammasome components, effectors, and/or upstream/downstream signaling, resulting in improved healing. Beneficial effects included resolution of inflammation through IL-1β downregulation, accelerated reepithelialization and wound closure, as well as increased granulation tissue formation and collagen deposition.
Among the described strategies, glyburide, metformin, PPAR agonists, and the DPP-4 inhibitor saxagliptin appear to be closest to clinical translation, as these drugs are already FDA approved for other indications. Although preclinical studies for these and other drugs have yielded promising results, there is still a need of human/clinical studies to further evaluate potential therapeutic effects, dose–response relationship, and possible side effects of such compounds to treat chronic wounds and prevent complications.
TAKE HOME MESSAGES
NLRP3 inflammasome plays important role in physiological wound healing, although sustained activity is associated with impaired healing in diabetes.
NLRP3 inflammasome inhibitors are an appealing strategy to dampen chronic inflammation and improve healing of diabetic wounds.
Human/clinical studies are needed to evaluate dosing formulations, potential therapeutic effects, dose–response relationship, and possible side effects.
Abbreviations and Acronyms
- AMPK
AMP-activated protein kinase
- ASC
apoptosis-associated speck-like protein
- ATP
adenosine triphosphate
- CCL-2
chemokine ligand 2
- COPs
CARD-only proteins
- DAMPs
damage-associated molecular patterns
- DM
diabetes mellitus
- DPP-4
dipeptidyl peptidase 4
- DUBs
deubiquitinases
- ECM
extracellular matrix
- Fb
fibroblast
- FDA
Food and Drug Administration
- FGF-2
fibroblast growth factor-2
- GLP-1
glucagon-like peptide 1
- HFD
high-fat diet
- HIF-1α
hypoxia-inducible factor 1 alpha
- IGF-1
insulin-like growth factor 1
- IL-1β
interleukin 1 beta
- IL-10
interleukin 10
- IL-18
interleukin 18
- IL-1R
IL-1 receptor type 1
- Kt
keratinocytes
- LPS
lipopolysaccharide
- Mo
monocytes
- Mp
macrophages
- NEK7
NIMA-related kinase 7
- NETs
neutrophil extracellular traps
- NF-κB
nuclear factor kappa B
- NLRP3
Nod-like receptor pyrin domain containing 3
- NLRs
nod-like receptors
- Np
neutrophil
- PAMPs
pathogen-associated molecular patterns
- POPs
pyrin-only proteins
- PPAR
peroxisome proliferator-activated receptor
- PRRs
pattern recognition receptors
- ROS
reactive oxygen species
- STZ
streptozocin
- TGF-β
transforming growth factor beta
- TLRs
Toll-like receptors
- TNF-α
tumor necrosis factor alpha
- UB
ubiquitin
- VCAM
vascular cell adhesion protein
- VEGF
vascular endothelial growth factor
ACKNOWLEDGMENTS AND FUNDING SOURCES
The authors thank Dr. Giamila Fantuzzi, University of Illinois at Chicago, for critical comments on a previous draft of the article and Ikaro Morais for comments and suggestions on figures design. This work was supported by NIGMS, Grant No. R35GM136228 to T.J.K.
AUTHOR DISCLOSURE AND GHOSTWRITING
The authors do not have any commercial or financial conflicts of interests to declare. The named authors wrote this article and no ghostwriters were used.
ABOUT THE AUTHORS
Jacqueline Cavalcante Silva, PhD, is a postdoctoral researcher in the Department of Kinesiology and Nutrition of the University of Illinois at Chicago. She is currently investigating the role of different cell types in physiological and impaired healing. Timothy J. Koh, PhD, is Professor in the Department of Kinesiology and Nutrition at the University of Illinois at Chicago. His laboratory focuses on the role of inflammation in tissue repair.
REFERENCES
- 1. Singer A, Clark R. Cutaneous wound healing. N Engl J Med 1999;341:738–746. [DOI] [PubMed] [Google Scholar]
- 2. Mirza R, DiPietro L, Koh T. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol 2009;175:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lucas T, Waisman A, Ranjan R, et al. . Differential roles of macrophages in diverse phases of skin repair. J Immunol 2010;184:3964–3977. [DOI] [PubMed] [Google Scholar]
- 4. Novak M, Koh T. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol 2013;183:1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daley J, Brancato S, Thomay A, Reichner J, Albina J. The phenotype of murine wound macrophages. J Leuk Biol 2010;87:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med 2006;23:594–608. [DOI] [PubMed] [Google Scholar]
- 7. International Diabetes Federation. Brussels, Belgium: IDF Diabetes Atlas, 2019. [Google Scholar]
- 8. Swanson K, Deng M, Ting J. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev 2019;19:477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci 2019;20:3328–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee H, Kim J, Kim H, Shong M, Ku B, Jo E. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 2013;62:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol 2011;22:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Broz P, Dixit V. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 2016;16:407–420. [DOI] [PubMed] [Google Scholar]
- 13. He Y, Zeng M, Yang D, Motro B, Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 2016;530:354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 2010;10:210–215. [DOI] [PubMed] [Google Scholar]
- 15. Shi H, Wang Y, Li X, et al. . NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol 2015;17:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol 2011;32:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri E. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem 2012;287:36617–36622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abais J, Xia M, Zhang Y, Boini K, Li P. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal 2015;22:1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lopez-Castejon G, Luheshi N, Compan V, et al. . Deubiquitinases regulate the activity of caspase-1 and interleukin-b secretion via assembly of the inflammasome. J Biol Chem 2013;288:2721–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Franchi L, Eigenbrod T, Nunez G. TNF-a mediate sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol 2009;183:792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu D, Yang P, Gao M, et al. . NLRP3 activation induced by neutrophil extracellular traps sustains inflammatory response in the diabetic wound. Clin Sci 2019;133:565–582. [DOI] [PubMed] [Google Scholar]
- 22. An Z, Li J, Yu J, et al. . Neutrophil extracellular traps induced by IL-8 aggravate atherosclerosis via activation NF-κB signaling in macrophages. Cell Cycle 2019;18:2928–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Indramohan M, Sthelik C, Dorfleutner A. COPs and POPs patrol inflammasome activation. J Mol Biol 2019;430:153–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Almeida L, Khare S, Misharin A, et al. . The PYRIN domain-only protein POP1 inhibits inflammasome assembly and ameliorates inflammatory disease. Immunity 2015;43:264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Periasamy S, Porter K, Atianand M, et al. . Pyrin-only protein 2 limits inflammation but improves protection against bacteria. Nat Commun 2017;8:15564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee S, Sthelik C, Reed J. COP, a caspase recruitment domain-containing protein and inhibitor of caspase-1 activation processing. J Biol Chem 2001;276:34495–34500. [DOI] [PubMed] [Google Scholar]
- 27. Annand R, Dahlen J, Sprecher C, et al. . Caspase-1 (interleukin-1beta-converting enzyme) is inhibited by the human serpin analogue proteinase inhibitor 9. Biochem J 1999;3:655–665. [PMC free article] [PubMed] [Google Scholar]
- 28. Okusawa S, Gelfand J, Ikejima T, Connolly R, Dinarello C. Interleukin-1 induces a shock-like state in rabbits: synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest 1988;81:1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dinarello C. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 2009;27:519–550. [DOI] [PubMed] [Google Scholar]
- 30. Dinarello C. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol 2007;27:98–114. [DOI] [PubMed] [Google Scholar]
- 31. Dinarello C. IL-18: a TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin immunol 1999;103:11–24. [DOI] [PubMed] [Google Scholar]
- 32. Liew F, Girard J, Turnquist H. Interleukin-33 in health and disease. Nature 2016;16:676–689. [DOI] [PubMed] [Google Scholar]
- 33. Vandanmagsar B, Youm Y, Ravussin A, et al. . The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 2011;17:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weinheimer-Haus E, Mirza R, Koh T. Nod-like receptor protein 3 inflammasome plays important role during early stages of wound healing. PLoS One 2015;10:e0119106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mirza R, Koh T. Contributions of cell subsets to cytokine production during normal and impaired wound healing. Cytokine 2015;71:409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mirza R, Fang M, Weinheimer-Haus E, Ennis W, Koh T. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes 2014;63:1103–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ito H, Kanbe A, Sakai H, Seishima M. Activation of NLRP3 signalling accelerates skin wound healing. Exp Dermatol 2017;27:80–86. [DOI] [PubMed] [Google Scholar]
- 38. Vinaik R, Abdullahi A, Barayan D, Jeschke M. NLRP3 inflammasome activity is required for wound healing after burns. Transl Res 2020;217:47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stojadinovic O, Minkiewicz J, Sawaya A, et al. . Deep tissue injury in development of pressure ulcers: a decrease of inflammasome activation and changes in human skin morphology in response to aging and mechanical load. PLoS One 2013;8:e69223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mirza RE FM, Ennis WJ, Koh TJ. Blocking Interleukin-1 induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013;62:2579–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dai J, Zhang X, Wang Y, Chen H, Chai Y. ROS-activated NLRP3 inflammasome initiates inflammation in delayed wound healing in diabetic rats. Int J Clin Exp Pathol 2017;10:9902–9909. [PMC free article] [PubMed] [Google Scholar]
- 42. Tan J, Lash B, Karami R, et al. . Restoration of the healing microenvironment in diabetic wounds with matrix-binding IL-1 receptor antagonist. Commun Biol 2021;4:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang W, Jiao J, Liu J, et al. . MFG-E8 accelerates wound healing in diabetes by regulating “NLRP3 inflammasome-neutrophil extracellular traps” axis. Cell Death Discov 2020;6:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fadini G, Menegazzo L, Rigato M, et al. . NETosis delays diabetic wound healing in mice and humans. Diabetes 2016;65:1061–1071. [DOI] [PubMed] [Google Scholar]
- 45. Juliana C, Fernandes-Alnemri T, Wu J, et al. . Anti-inflammatory compounds parthenolide and Bay 11–7082 are direct inhibitors of the inflammasome. J Biol Chem 2010;285:9792–9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eo H, Lim Y. Combined mulberry leaf and fruit extract improved early stage of cutaneous wound healing in high-fat diet-induced obese mice. J Med Food 2016;19:161–169. [DOI] [PubMed] [Google Scholar]
- 47. Lamkanfi M, Mueller J, Vitari A, et al. . Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol 2009;187:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang Y, Jiang H, Chen Y, et al. . Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol Med 2018;10:e8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Suzawa H, Kikuchi S, Arai N, Koda A. The mechanism involved in the inhibitory action of tranilast on collagen biosynthesis of keloid fibroblasts. Jpn J Pharmacol 1992;60:91–96. [DOI] [PubMed] [Google Scholar]
- 50. Huang Y, Jiang H, Chen Y, et al. . Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol Med 2018;10:e8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nagai N, Ogata F, Deguchi S, Ueno A, Kawasaki N, Yoshimasa I. Combination ointment containing solid tranilast nanoparticles and dissolved sericin is efficacious for treating skin wound-healing deficits and redness in diabetic rats. Biol Pharm Bul 2017;40:444–450. [DOI] [PubMed] [Google Scholar]
- 52. Hu Z, Chen B, Li Y, et al. . Effect of tranilast on wound healing and administration time on scar hyperplasia of deep partial-thickness burn in mice [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2017;31:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang F, Wang L, Wang J, Luo P, Wang X, Xia Z. The caspase-1 inhibitor AC-YVAD-CMK attenuates acute gastric injury in mice: involvement of silencing NLRP3 inflammasome activities. Sci Rep 2016;6:24166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Coll R, Robertson A, Chae J, et al. . A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 2015;21:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang Y, Wan L, Zhang z, Li J, Qu M, Zhou Q. Topical calcitriol application promotes diabetic corneal wound healing and reinnervation through inhibiting NLRP3 inflammasome activation. Exp Eye Res 2021;209:108668. [DOI] [PubMed] [Google Scholar]
- 56. Lee J, Robertson A, Cooper M, Khosrotehrani. The small molecule NLRP3 inflammasome inhibitor MCC950 does not alter wound healing in obese mice. Int J Mol Sci 2018;19:3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bitto A, Altavilla D, Pizzino G, et al. . Inhibition of inflammasome activation improves the impaired pattern of healing in genetically diabetic mice. Br J Pharmacol 2014;171:2300–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mirza R, Fang M, Novak M, et al. . Macrophage PPARγ and impaired wound healing in type 2 diabetes. J Pathol 2015;236:433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Silva J, Pitta I, Pitta M, Koh T, Abdalla D. New peroxisome proliferator-activated receptor agonist (GQ-11) improves wound healing in diabetic mice. Adv Wound Health 2019;8:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Meeran M, Goyal S, Suchal K, Sharma C, Patil C, Ojha S. Pharmacological properties, molecular mechanisms, and pharmaceutical development of asiatic acid: a pentacyclic triterpenoid of therapeutic promise. Front Pharmacol 2018;9:892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Leu W, Chen J, Guh J. Extract from Plectranthus amboinicus inhibit maturation and release of interleukin 1β through inhibition of NF-κB nuclear translocation and NLRP3 inflammasome activation. Front Pharmacol 2019;10:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sawatdee S, Choochuay K, Chanthorn W, TS. Evaluation of the topical spray containing Centella asiatica extract and its efficacy on excision wounds in rats. Acta Pharm 2016;66:233–244. [DOI] [PubMed] [Google Scholar]
- 63. Huang Y, Lin C, Cheng N, et al. . Effect of a novel macrophage-regulating drug on wound healing in patients with diabetic foot ulcers: a randomized clinical trial. JAMA Netw Open 2021;4:e2122607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sultuybek G, Soydas T, Yenmis G. NF-κB as the mediator of metformin's effect on ageing and ageing-related diseases. Clin Exper Pharm Physiol 2019;46:413–422. [DOI] [PubMed] [Google Scholar]
- 65. Qing L, Fu J, Wu P, Zhou Z, Yu F, Tang J. Metformin induces the M2 macrophage polarization to accelerate the wound healing via regulating AMPK/mTOR/NLRP3 inflammasome singling pathway. Am J Transl Res 2019;11:655–668. [PMC free article] [PubMed] [Google Scholar]
- 66. Han X, Tao Y, Deng Y, Yu J, Sun Y, Jiang G. Metformin accelerates wound healing in type 2 diabetic db/db mice. Mol Med Rep 2017;16:8691–8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tawfeek HM, Abou-Taleb D, Badary D, Ibrahim M, Abdellatif A. Pharmaceutical, clinical, and immunohistochemical studies of metformin hydrochloride topical hydrogel for wound healing application. Arch Dermatol Res 2020;312:113–121. [DOI] [PubMed] [Google Scholar]
- 68. Sabboo A, Rathnayake A, Vangaveti V, Malabu U. Wound healing effects of dipeptidyl peptidase-4 inhibitors: an emerging concept in management of diabetic foot ulcer—a review. Diabetes Metab Syndr 2016;10:113–119. [DOI] [PubMed] [Google Scholar]
- 69. Shah Z, Kampfrath T, Deiuliis J, et al. . Long-term dipeptidylpeptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 2011;124:2338–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Matsubara J, Sugiyama S, Akiyama E, et al. . Dipeptidyl peptidase-4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti-inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circulation 2013;77:1337–1344. [DOI] [PubMed] [Google Scholar]
- 71. Dai Y, Dai D, Wang X, Ding Z, Mehta J. DPP-4 inhibitors repress NLRP3 inflammasome and interleukin-1beta via GLP-1 receptor in macrophages through protein kinase C pathway. Cardiovasc Drugs Ther 2014;28:5. [DOI] [PubMed] [Google Scholar]
- 72. Long M, Cai L, Li W, et al. . DPP-4 inhibitors improve diabetic wound healing via direct and indirect promotion of epithelial mesenchymal transition and reduction of scarring. Diabetes 2018;67:518–531. [DOI] [PubMed] [Google Scholar]
- 73. Marfella R, Sasso F, Rizzo M, et al. . Dipeptidyl peptidase 4 inhibition may facilitate healing of chronic foot ulcers in patients with type 2 diabetes. Exp Diab Res 2012;2012:892706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bame K. Heparanases: endoglycosidases that degrade heparan sulfate proteoglycans. Glycobiology 2001;11:91R–98R. [DOI] [PubMed] [Google Scholar]
- 75. Tong M, Tuk B, Shang P, et al. . Diabetes-impaired wound healing is improved by matrix therapy with heparan sulfate glycosaminoglycan mimetic OTR4120 in rats. Diabetes 2012;61:2633–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang T, Zhao J, Mei J, et al. . Heparan sulfate inhibits inflammation and improves wound healing by downregulating the NLR family pyrin domain containing 3 (NLRP3) inflammasome in diabetic rats. J Diabetes 2018;10:556–563. [DOI] [PubMed] [Google Scholar]
- 77. Shim D, Shin W, Yu S, et al. . BOT-4-one attenuates NLRP3 inflammasome activation: NLRP3 alkylation leading to the regulation of its ATPase activity and ubiquitination. Sci Rep 2017;7:15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jiang H, Chen Y, Huang W, et al. . Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med 2017;214:3219–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cocco M, Pellegrini C, Martinez-Banaclocha H, et al. . Development of an acrylate derivative targeting the NLRP3 inflammasome for the treatment of inflammatory bowel disease. J Med Chem 2017;60:3656–3671. [DOI] [PubMed] [Google Scholar]
- 80. He Y, Varadajan S, Munoz-Planillo R, et al. . 3,4-Methylenedioxy-β-nitrostyrene inhibits NLRP3 activation by blocking assembly of the inflammasome. J Biol Chem 2013;289:1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Marchetti C, Swartzelter B, Gamboni F, et al. . OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc Natl Acad Sci U S A 2018;115:E1530–E1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. He H, Jiang H, Chen Y, et al. . Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun 2018;9:2550. [DOI] [PMC free article] [PubMed] [Google Scholar]